4
Applying Methodological Approaches to Nutrient Reference Values for Young Children and Women of Reproductive Age: An Assessment of Exemplar Nutrients
In response to its task to demonstrate the application of a methodological approach for deriving nutrient reference values (NRVs) using an analysis of an exemplar nutrient, the committee determined there was a need to address three nutrients of concern for young children and women of reproductive age, zinc, iron, and folate. This chapter summarizes the committee’s application of its proposed framework for deriving NRVs for these exemplar nutrients. The committee did not carry out an analysis for the two population subgroups for each nutrient. Rather, the three nutrients were selected to illustrate the methodological applications, applicable to the target population subgroups. The chapter begins with an overview of the key steps and core principles underlying the committee’s framework; then describes in detail its three exemplar nutrient analyses, with a focus on defining the specific method to be used to derive an NRV; and concludes with the committee’s assessment of the feasibility of harmonizing methodologies for deriving NRVs on a global scale and a discussion of the application of NRVs in low- and middle-income countries.
The committee was not tasked with actually setting NRVs for the exemplar nutrients. Additionally, because the nutrient review process applies to nutrients derived from the diet, not from supplements, although supplements are mentioned briefly in certain contexts when relevant, a review or discussion of supplements was outside the committee’s task. Finally, while the committee evaluated the strengths and weaknesses of methods currently being used to derive NRVs as they apply to the three exemplar nutrients, it was not within the committee’s scope to compare these methods or to identify which methods would be most suitable for other nutrients.
HARMONIZING THE PROCESS FOR NUTRIENT REFERENCE VALUES USING EXEMPLAR NUTRIENTS
In the previous chapter, the committee reviewed the key steps in the process of deriving NRVs as outlined in Chapter 3, Figure 3-1; then, based on its assessment of strengths and weaknesses in the methodologies for each of these steps, the committee developed a framework for establishing key NRVs. The framework, illustrated in Figure 3-4, includes four major steps to deriving key NRVs:
- Choose the appropriate tools and resources.
- Collect relevant data from the tools and other resources.
- Identify the best approach or method for the nutrient under consideration.
- Derive two key reference values, the average requirement (AR) and the tolerable upper intake level (UL).
Also in Chapter 3, the committee identified six core values as being critical to global harmonization of methodologies for setting NRVs:
- Regularly update existing NRVs.
- Manage the process with clarity and transparency.
- Assess the quality of evidence with rigor.
- Document all factors that influence the potential NRV (e.g., infection, body size).
- Determine the strength of the evidence reviewed.
- Assure that the review is complete and efficient.
As a part of this process, any uncertainties in the data need to be considered and fully described. The specific methodology applied in this chapter for deriving NRVs is based on these key steps and core values, along with consideration for the specific characteristics of the nutrient under review.
More specifically, the flow diagram illustrated in Figure 4-1 is a detailed subset of the committee’s proposed framework for deriving NRVs. This chapter describes how the committee used this subset of the proposed framework in its analyses of zinc, iron, and folate to demonstrate the feasibility of its recommendations for harmonizing methodologies for deriving NRVs on a global scale. As discussed below, zinc NRVs can only be estimated using the factorial approach; iron NRVs can be estimated using either the factorial approach or dose–response modeling, but the values derived by the factorial approach are recommended; and dose–response assessment is the preferred approach for folate. As explained in Chapter 3,
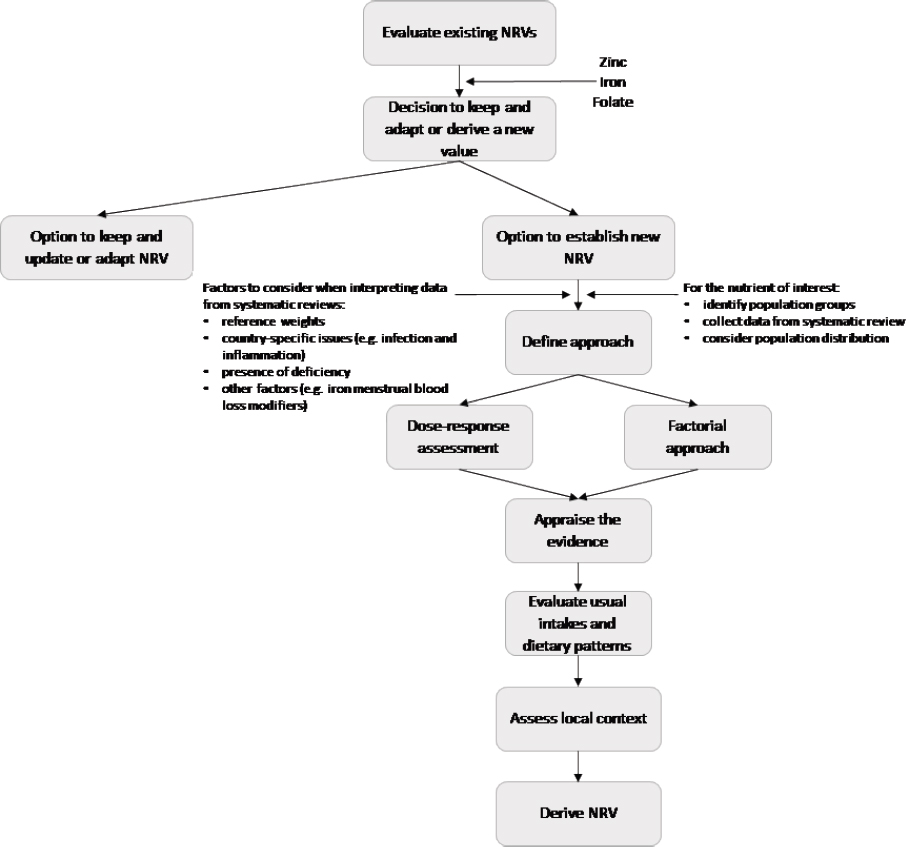
NOTES: This figure represents a detailed subset of the flow diagram for deriving NRVs shown in Figure 3-1. No actual derivation of NRVs was carried out by the committee.
the balance method is used to estimate protein and mineral requirements and is therefore not relevant to these case analyses.
For each nutrient, the case analysis begins with a statement of the problem relative to the nutrient under consideration; next, factors that influence derivation of NRVs and the strengths and weaknesses of methodologies used to derive them is discussed; finally, the derivation of ARs and ULs is discussed. The committee did not carry out analyses for both young children and women of reproductive age for all three nutrients. Rather, the three nutrients were selected to illustrate the application of the framework across age groups. In addition, the committee did not actually derive any NRVs. Lastly the committee presents its findings, conclusions, and proposed solutions for future recommendations regarding each nutrient. In
Chapter 5, the committee discusses consideration of data gaps and options for moving toward harmonization.
ZINC CASE ANALYSIS
Introduction and Statement of the Problem
Certain population subgroups, especially those living in low- and middle-income countries, are known to be potentially deficient and particularly vulnerable to the constellation of problems that present with zinc deficiency. These include infants and young children and pregnant and lactating women with increased zinc requirements who are also consuming diets that are low in zinc and/or high in phytate (i.e., cereal-based diets) (Shah et al., 2016). A number of factors contribute to risk of zinc deficiency in populations, including poor dietary quality, especially in populations with widespread zinc depletion in top soils (Roohani et al., 2013). In other instances, while diets may not be necessarily low in zinc, low bioavailability caused by dietary factors such as high phytate concentration in staple foods may be important. Indeed, many populations living in the African nations, the Eastern Mediterranean, and South and Southeast Asia frequently are at risk of zinc deficiency because of inadequate intake (Hess et al., 2009; Wessells et al., 2012). Other causes include increased zinc loss due to diarrheal infections (King et al., 2016).
Factors Influencing Zinc Requirements
A number of contextual factors affect the zinc requirement of a population. These include intake patterns of foods rich in zinc or fortified with zinc, dietary components that inhibit zinc absorption (e.g., phytates), increased dietary requirements to support the gain of new tissue during pregnancy or growth, and the presence of infections that cause impaired zinc absorption and/or increased gastrointestinal losses. Key factors are reviewed below.
Whole Body Zinc Utilization and Status
The total amount of zinc absorbed is directly related to the amount of dietary zinc; as dietary zinc increases, the total amount absorbed also increases, although the percent or fractional zinc absorption declines. Shifts in endogenous fecal losses are the primary mechanism for maintaining whole body zinc homeostasis. When the amount of dietary zinc declines, endogenous fecal losses also decline to reestablish zinc balance, at least initially. A reduction in dietary zinc causes a decline in endogenous fecal losses within
2 days. Urinary and integumental zinc losses, in contrast, vary little with shifts in dietary zinc (Hotz and Brown, 2004). If insufficient intakes persist, tissue zinc catabolism may occur to mobilize zinc from a small, vulnerable pool for the body’s needs. Plasma zinc is thought to be a component of this pool. However, a moderate reduction in dietary zinc of 3–5 mg per day reduces plasma zinc concentrations only if the limited intake is continued for several months or if endogenous losses are increased because of diarrheal disease. Even though circulating levels remain relatively stable over a wide range of zinc intakes, serum/plasma zinc concentrations are the most widely used biochemical indicator of zinc status (King et al., 2016). A more sensitive biomarker to changes in zinc intake has not been identified.
Physiological Requirements
The physiological zinc requirement is the amount of absorbed zinc required to offset all obligatory zinc losses, plus any additional amounts needed for growth, pregnancy, or lactation (Gibson et al., 2016). Certain populations, including children, adolescents, and pregnant and lactating women, are at increased risk of zinc deficiency because of their higher physiologic requirements (Roohani et al., 2013). This is true even though zinc absorption increases during pregnancy and lactation, especially if the dietary intake is low.
Because zinc is involved in many core metabolic pathways, the manifestations of its deficiency are nonspecific (King et al., 2016). In childhood, zinc deficiency retards growth (stunting), impairs cognitive function (Gogia and Sachdev, 2012; Levenson and Morris, 2011), increases the risk of recurrent infections and diarrhea (Lazzerini and Ronfani, 2012), causes hair loss, and may trigger conjunctival and eyelid inflammation. Stunting in growing children because of inadequate zinc intakes has led to its wide use in nutritional assessment (Brown et al., 2004; de Benoist et al., 2007). In adolescents and adults, zinc deficiency can reduce fertility, cause reproductive performance problems, and impair work capacity (Bernhardt et al., 2012; Kawade, 2012). In the elderly, recurrent infections may occur when zinc levels are low (Pae et al., 2012). Zinc deficiency can lead to death from complications caused by diarrhea, pneumonia, and malaria (Wazny et al., 2013).
Dietary Zinc Sources
Zinc is present in many types of food but is often associated with proteins in lean tissue. Animal source foods (i.e., the organs and flesh of mammals, fowl, fish, and crustaceans) are the richest source of absorbable zinc (King et al., 2016). Other good zinc sources include ready-to-eat cere-
als that are fortified with zinc, and certain nuts, seeds, and legumes (Shah et al., 2016). Amounts of zinc in grains and legumes are influenced by the soil zinc concentration. For example, increasing the soil zinc content via irrigation has doubled the concentration of zinc in wheat (Rosado et al., 2009).
Zinc Bioavailability
As mentioned previously, zinc absorption varies with the amount of phytate in the food; animal foods do not contain phytate, but unrefined cereals and legumes contain high amounts (Gibson, 2012). However, although the availability of zinc from diets with phytate:zinc molar ratios greater than 15 is generally low, fermentation or germination of these plant foods causes phytate to be hydrolyzed by phytase enzymes to lower levels of inositol phosphates (i.e., from IP6 to IP4 or lower) that do not inhibit zinc absorption (Gibson and Anderson, 2009). In addition, recent research has shown that dietary phytate does not have a detectable effect on zinc absorption in infants and young children; rather, the total amount of dietary zinc was found to be the primary determinant of zinc absorption (Miller et al., 2015). Further research is needed, however, to determine if the effect of phytate on zinc absorption differs in growing children compared to adults.
Animal studies conducted in the 1980s suggested that calcium could inhibit zinc absorption because of the formation of an insoluble calcium-zinc-phytate complex. However, later studies in humans confirmed that calcium did not impede zinc absorption when dietary zinc intake was adequate, regardless of whether phytate concentration was either low or high (Hunt and Beiseigel, 2009).
Generally, zinc absorption is enhanced by dietary protein (both quantity and quality). However, some individual proteins, such as casein, have a modest inhibitory effect on zinc absorption. Organic acids, such as citrate, enhance zinc absorption (Lonnerdal, 2000). Also, ethylenediaminetetra-acetic acid (EDTA) has been shown to modestly enhance zinc absorption from ZnSO4-fortified cereals, but not ZnO-fortified cereals (Brnic et al., 2014).
Infection
Zinc is required for the synthesis and function of immune regulatory proteins and for maintaining immune function (King et al., 2014). Diarrheal infections reduce zinc absorption, which further impairs immune function and the defense against infections (Wapnir, 2000). Currently, the World Health Organization (WHO) recommends that 20 mg supplemental zinc be given in conjunction with oral rehydration therapy to children being treated for diarrheal disease (WHO/UNICEF, 2004). Importantly,
supplemental zinc reduces child mortality from diarrheal disease by about 6 percent; in children over 12 months of age the effect is higher—about an 18 percent reduction in mortality (Brown et al., 2009a). Zinc insufficiency also increases the risk of acute, lower respiratory tract infections (Brown et al., 2009b).
Assessment of Strengths and Weaknesses in Methodologies to Determine Zinc Requirements
The committee assessed the strengths and weaknesses of all three methods for deriving NRVs (balance studies, dose–response modeling, and the factorial approach) in relation to zinc. As discussed below, the factorial approach is the preferred method for determining zinc NRVs.
Zinc Balance Studies
The Institute of Medicine (IOM) first established a recommended dietary allowance (RDA) for zinc in 1989 (NRC, 1989). The authoring committee of that first zinc RDA considered balance data, as well as the factorial approach, and found that what it considered to be the “most reliable balance studies” indicated that 12.7 mg zinc per day (mg/d) was needed from a typical U.S. mixed diet to maintain zinc status. From other data (human studies), daily endogenous zinc losses were estimated to be about 2.5 mg/d; assuming an absorption efficiency of 20 percent, the dietary requirement to replace these endogenous losses was estimated to be 12.5 mg/d, which agreed with the 12.7 mg/d estimated from balance studies. Based on these estimates, the 1989 RDA for zinc was set at 15 mg/d for men and 12 mg/d for women. However, after zinc metabolic studies showed that zinc balance could be achieved from diets as low as 4.6 mg zinc/d to over 20 mg/d (Pinna et al., 2001), subsequent NRVs for zinc have been derived using the factorial approach, not the balance method. Such a wide range makes it impossible to identify the lowest intake that replaces zinc losses while maintaining zinc functions and health.
Dose–Response Estimates
The dose–response estimate is not used to estimate zinc NRVs because the primary indicator of zinc status, plasma zinc concentrations, remains constant over a wide range of zinc intakes. Consistent with the evidence cited above, a review of studies of zinc status at the population level showed that serum zinc concentrations remained unchanged from diets providing between 3 and 60 mg zinc/d (Gibson et al., 2008). Several health outcomes
TABLE 4-1 Health Outcomes Associated with Inadequate Zinc Intakes for Various Population Subgroups
| Infants | Children and Adolescents | Pregnant and Lactating Women |
|---|---|---|
| Growth Immune response to vaccination Neurodevelopment |
Growth Immune function Cognitive function Dermatitis |
Fetus: Mother: |
SOURCE: Adapted from Lowe et al., 2013.
associated with inadequate zinc intakes in the population subgroups addressed in this report are identified in Table 4-1.
Factorial Approach
Given the aforementioned limitation of the balance method, and because there is no known biomarker of dietary zinc or a selected health outcome that is sensitive to variations in dietary zinc, the factorial approach is the only method that can be used to estimate the physiological zinc requirement. This approach requires estimating the amount of absorbed zinc needed to replace endogenous zinc losses, as well as estimating the endogenous zinc losses, including both endogenous fecal zinc (EFZ) losses and nonintestinal losses via the urine, integument (skin, hair, nails, and sweat), menstrual flow in women, and semen in men. As stated previously, total nonintestinal losses are constant over a wide range of zinc intake (4–25 mg/d) (IOM, 2001). In contrast, EFZ losses vary with the amount of zinc absorbed and are a major component of zinc homeostasis. Table 4-2 shows the estimated EFZ losses in adult men and women as determined by four different authoritative bodies.
Derivation of Zinc Nutrient Reference Values
Here, different authoritative bodies’ derivations of zinc ARs and recommended intakes (RIs) are explained for adult men and women because this group includes women of reproductive age and also to illustrate certain details of the methodology for deriving ARs and RIs that can be applied across population subgroups (i.e., infants and children, and pregnant and lactating women). Additionally, the derivation of ULs are discussed.
TABLE 4-2 Comparison of Estimates of Endogenous Losses Used in Factorial Modeling for Zinc in Adult Men and Women
| WHO | IOM | IZiNCG | EFSA | |
|---|---|---|---|---|
| Adult Men | ||||
| Body weight (kg) | 65 | 75 | 65 | 72.7 |
| Total nonintestinal endogenous losses (mg) | 0.60 | 1.27 | 1.15 | 1.14 |
| Endogenous fecal losses (mg) | 0.80 | 2.57 | 1.54 | 2.40 |
| Total endogenous losses (mg) | 1.40 | 3.84 | 2.69 | 3.54 |
| Adult Women | ||||
| Body weight (kg) | 55 | 65 | 55 | 59.1 |
| Total nonintestinal endogenous losses (mg) | 0.50 | 1.00 | 0.80 | 0.63 |
| Endogenous fecal losses (mg) | 0.50 | 2.30 | 1.06 | 2.30 |
| Total endogenous losses (mg) | 1.00 | 3.30 | 1.86 | 2.93 |
NOTE: EFSA = European Food Safety Authority; IOM = Institute of Medicine; IZiNCG = International Zinc Nutrition Consultative Group; WHO = World Health Organization.
Conversion of Physiological Zinc Requirements as Determined by the Factorial Approach to ARs and RIs for Adult Men and Women
The estimated AR is the amount of dietary zinc that will replace total endogenous losses. In other words, it is the amount of dietary zinc needed to meet the physiological requirement. To convert the physiological requirements into ARs, it is necessary to determine the fractional zinc absorption (FZA) from different diets. Radioactive and stable isotopes of zinc are used to estimate the FZA. In addition, as discussed in Chapters 2 and 3, the efficiency of mineral absorption (bioavailability) is one of several factors that needs to be considered when using the factorial approach to derive an AR. Although recent research suggests phytate may not be a primary determinant of zinc absorption (Miller et al., 2015), the dietary phytate content remains a primary factor considered when determining the AR. In the case of zinc, often the phytate:zinc molar ratio is used to estimate bioavailability. For example, the International Zinc Nutrition Consultative Group (IZiNCG) estimated that the phytate:zinc molar ratio is 11 for a mixed/refined vegetarian diet, compared to 24 for an unrefined cereal-based diet (Hotz and Brown, 2004). Experimental studies cited in IZiNCG (2004) have shown that for phytate:zinc molar ratios between 4 and 18, zinc absorption is about 26 percent in males and 34 percent in females; for phytate:zinc ratios greater than 18, zinc absorption declines to 18 in males and 25 percent in females.
Using a trivariate model that examined the relationship between total absorbed zinc, total dietary zinc, and dietary phytate (see Figure 4-2), which
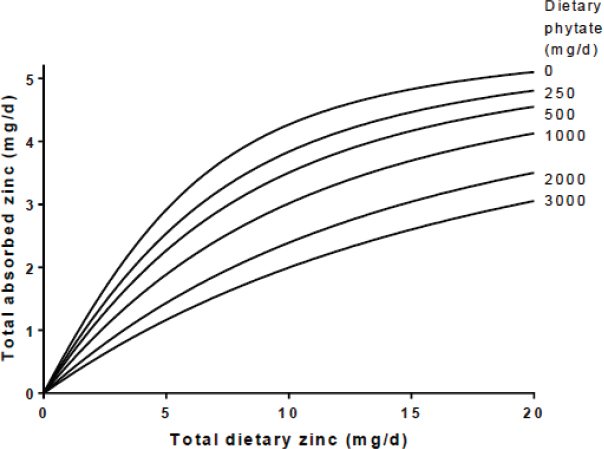
SOURCE: EFSA NDA Panel, 2014a.
itself is based on 650 individual measurements from 18 publications, the European Food Safety Authority (EFSA) estimated that a man consuming a diet with about 1,200 mg phytate/day would need to consume 12.1 mg zinc/day to meet a physiological requirement of 3.4 mg/d (EFSA NDA Panel, 2014a). However, if the phytate intake is cut in half to 600 mg/d (a phytate:zinc ratio of about 9.8), only about 10 mg of dietary zinc would need to be consumed to meet the physiological requirement. The different AR estimates for zinc for adult men and women, as determined by WHO/Food and Agriculture Organization (FAO), IZiNCG, and EFSA are listed in Table 4-3.
After an AR is determined, an RI that is sufficient to meet the needs of almost all (97–98 percent) healthy people in a particular life stage and gender group is established. The RI is the AR plus 2 standard deviations above the AR. Knowledge of the coefficient of variation (CV) in zinc requirements within a healthy population is required to determine the RI. WHO, the IOM, and IZiNCG all used different estimates to determine the RI for zinc. WHO estimated that an additional 25 percent is needed to establish an RI, based on an assumed variation in protein requirements (i.e., 12.5 percent) plus an additional 12.5 percent variation for zinc.
The IOM and IZiNCG both used an estimate of the CV of the zinc requirement. However, the IOM assumed that the coefficient of variation was 10 percent, whereas IZiNCG used 12.5 percent. EFSA took a different approach. Because multiple regression analysis showed that body weight was a strong determinant of the zinc requirement, it estimated the RI as the requirement for individuals with a body weight at the 97.5th percentile using the reference body weights of men and women. EFSA assumed a median body weight of 58.5 kg for women and 68.1 kg for men. This is equivalent to a body mass index of 22 kg/m2. The zinc RIs for adult men and women that have been established by these four authoritative bodies, as well as other national or international groups, vary widely depending on dietary phytate or bioavailability estimates. Individuals consuming diets with high zinc availability may only require about 5 mg/d, whereas those with low availability may need as much as 19 mg/d (Wessells et al., 2012).
Determination of zinc ARs and RIs for infants and children Among infants and children, the physiological zinc requirement equals the total endogenous losses plus the additional zinc required for growth. Because the need for zinc to support growth has not been measured in infants between 0 and 6 months, it can be estimated in one of two ways: (1) from the usual amount of tissue gained during this time period and assuming that the tissue contains 20 μg zinc/g tissue gained, or (2) from the amount of zinc provided in breast milk, assuming that breast milk supplies an adequate amount of zinc for growth during the first 6 months. When using the latter method, an average breast milk zinc concentration over the entire time period is used, even though it is known that the breast milk zinc concentration declines between 0 to 6 months.
WHO, the IOM, IZiNCG, and EFSA all used different assumptions for estimating the zinc needs of infants between 0 to 6 months of age. WHO based its estimate on the amount of lean tissue gained and concluded that the zinc need ranged from 0.7 to 1.3 mg/d depending on the amount of tissue gained. The IOM assumed that the zinc supplied in breast milk was an “adequate intake” over the first 5 months and that the need for zinc was 2.0 mg/d. IZiNCG also concluded that breast milk was a sufficient source of zinc for exclusively breastfed infants; however, in addition, if food is also consumed, the infant needs to absorb about 1.3 and 0.7 mg zinc/day from the food during 0 to 3 months and 3 to 5 months, respectively, to support growth. EFSA also based its estimate on the amount of breast milk consumed as well; it concluded that 2.0 mg zinc is required daily based on an average breast milk volume of 0.80 L/d and with a zinc concentration of 2.5 mg/L.
All four authoritative bodies used the factorial approach to estimate the AR for absorbed zinc for infants 6 to 12 months and children 1 to 18 years
TABLE 4-3 Estimated Physiological Requirements for Absorbed Zinc, ARs (mg/d), and for the RIs During Childhood by Age Group and Gender
| WHO | IOM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, Sex | Wt (kg) | Physiol requirements (mg/d) | AR (mg/d)a | RI (mg/d)a | Age, Sex | Wt (kg) | Physiol requirements (mg/d) | AR (mg/d) | RI (mg/d) |
| 7–12 mo | 9 | 0.84 | ––b | 2.5 | 7–12 mo | 9 | 0.84 | 2.2 | 3.0 |
| 1–3 yrs | 12 | 0.83 | 1.66 | 2.4 | 1–3 | 13 | 0.74 | 2.2 | 3.0 |
| 3–6 yrs | 17 | 0.97 | 1.94 | 2.9 | 4–8 | 22 | 1.20 | 4.0 | 5.0 |
| 6–10 yrs | 25 | 1.12 | 2.25 | 3.3 | |||||
| 10–12 M | 35 | 1.40 | 2.80 | 5.1c | 9–13 | 40 | 2.12 | 7 | 8 |
| 10–12 F | 37 | 1.26 | 2.38 | 4.3c | |||||
| 12–15 M | 48 | 1.82 | 3.65 | ||||||
| 12–15 F | 48 | 1.55 | 3.07 | ||||||
| 15–18 M | 64 | 1.97 | 3.90 | 14–18 M | 64 | 3.37 | 8.5 | 11 | |
| 15–18 F | 55 | 1.54 | 3.08 | 14–18 F | 57 | 3.02 | 7.5 | 9 | |
| WHO | IOM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, Sex | Wt (kg) | Physiol requirements (mg/d) | AR (mg/d)a | RI (mg/d)a | Age, Sex | Wt (kg) | Physiol requirements (mg/d) | AR (mg/d) | RI (mg/d) |
| 6–11 mo | 9 | 0.84 | 3 | 4 | 7–11 mo | 2.4 | 2.9 | ||
| 1–3 | 12 | 0.53 | 2 | 3 | 1–3 | 11.9 | 1.074 | 3.6 | 4.3 |
| 4–8 | 21 | 0.83 | 3 | 4 | 4–6 | 19.0 | 1.390 | 4.6 | 5.5 |
| 7–10 | 28.7 | 1.869 | 6.2 | 7.4 | |||||
| 9–13 | 38 | 1.53 | 5 | 6 | 11–14 M | 44 | 2.635 | 8.9 | 9.4 |
| 11–14 F | 45 | 2.663 | 8.9 | 9.4 | |||||
| 14–18 M | 64 | 2.52 | 8 | 10d | 15–17 M | 64 | 3.544 | 11.8 | 12.5 |
| 14–18 F | 56 | 1.98 | 7 | 9d | 15–17 F | 56 | 2.969 | 9.9 | 10.4 |
NOTE: AR = average requirement; EFSA = European Food Safety Authority; IOM = Institute of Medicine; IZiNCG = International Zinc Nutrition Consultative Group; Physiol = physiological; RI = recommended intake; WHO = World Health Organization; wt = weight.
a WHO AR and RI values included here were estimated for high bioavailability (50 percent).
b In its estimate of an AR for 7- to 12-month-old infants, WHO adjusted adult values based on metabolic rate. The resulting value, 0.6 mg/d, was less than the physiological requirement. Adjustments made by the IOM, IZiNCG, and EFSA were based on reference body weights.
c For children between 10 and 18 years of age, WHO established only two RIs, one for each sex that cover the entire age group.
d The IZiNCG RIs included here are for a mixed diet. RIs were also established by an unrefined diet.
SOURCE: Brown et al., 2004.
(see Table 4-3). Estimates of total endogenous losses were determined by extrapolation from adult values, based on metabolic weight (WHO) or reference body weights (the IOM, IZiNCG, EFSA) (extrapolation is explained in Chapter 3). To estimate the amount of zinc needed to synthesize new tissue, the IOM, IZiNCG, and EFSA assumed that new tissue, irrespective of whether it was lean or adipose tissue, had a zinc concentration of 20 μg/g tissue.
WHO assumed a zinc concentration of 30 μg/g wet tissue weight in infants and children up to 10 years of age, and 23 μg/g thereafter. The estimated amount of new tissue gained varied slightly among the four authoritative bodies, which accounts for the differences in the estimated physiological requirements. As shown in Table 4-3, in general, the estimated physiological requirement ranged from less than 1 to 1.9 mg/d up to 10 years of age. Between 10 and 18 years of age, the physiological requirement ranges from about 1.5 to 3.5mg/d. The values for males tend to be higher than the values for females.
Also shown in Table 4-3, after adjusting for the bioavailability of dietary zinc, among 7–12-month-old infants, the AR estimates by the IOM, IZiNCG, and EFSA range from 2.2 to 3 mg/d, which translates to an RI of 3 to 4 mg/d.
AR estimates range from 2.2 to 3.6 mg/d, and the RI estimates range from 3 to 4.3 mg/d for 1–3 years of age. After 3 years of age, AR estimates increase to about 2 to 4 mg/d during the preschool years (4–8 years of age), and the RI range increases to 4 to 5.5 mg/day. Among school-age children from 7–13 years of age, AR estimates range from 7 to 9 mg/d, which translates to an RI of 6 to 9.4 mg/day. For adolescents aged 14–18 years, AR estimates range from 8 to 11 mg/d, with RI estimates ranging from about 10 to 14 mg/d.
WHO estimates of RI shown in Table 4-3 are based on high zinc bioavailability (50 percent). Not shown in the table, WHO estimated the RIs from diets with moderate (30 percent) and low (15 percent) bioavailability as well; for 7–12-month-old infants, the RIs are 2.5, 4.1, and 8.4 mg zinc/d (WHO/FAO, 2004).
Recently, zinc absorption and retention was studied in young children between 1 and 3 years of age from low-income countries who are subsisting on cereal-based diets that have been fortified with zinc. Normally, FZA declines with increased amounts of zinc in the diet. That was not observed, however, when zinc intakes were increased in toddlers by feeding biofortified cereals or micronutrient powders. Thus, increasing dietary zinc intakes from zinc supplements or fortified foods may improve tissue zinc levels and growth in vulnerable populations subsisting on cereal-based diets (Ariff, 2014; Chomba, 2015).
Also of relevance, previous studies have shown that supplemental iron
reduces zinc absorption (Chung et al., 2002; O’Brien et al., 2000). However, a recent study of 6-month-old, nonanemic infants showed that the addition of iron to micronutrient powders did not reduce zinc absorption (Esamai et al., 2014). This is an encouraging finding as iron and zinc deficiencies tend to coexist in infants and toddlers. As previously mentioned, another important recent finding is the absence of a negative effect of dietary phytate on zinc absorption in infants and young children (Miller et al., 2015), which raises questions about using the dietary phytate:zinc molar ratios to predict dietary zinc absorption and, therefore, the zinc AR.
Determination of the Zinc AR and RI for Pregnant and Lactating Women
Table 4-4 summarizes WHO, the IOM, IZiNCG, and EFSA estimates of ARs and RIs for zinc for pregnant and lactating women. The physiological zinc requirement for pregnant women can be estimated from the amount of zinc retained throughout pregnancy. WHO estimated that 100 mg of zinc is retained during pregnancy (WHO/FAO, 2004). No adaptations in zinc absorption or excretion were considered. Both WHO and the IOM determined the amount of zinc needed per trimester according to differences in the rates of fetal growth (IOM, 2001; WHO/FAO, 2004). Alternatively, IZiNCG used a single value of 0.7 mg/d as the amount of zinc retained throughout pregnancy based on the recognition that this rate of zinc retention overestimates the needs in the first and second trimester. EFSA used 0.4 mg/d as a lower average rate of retention over the four quarters of pregnancy. To meet these additional needs, the IOM and IZiNCG estimated that the zinc AR is 9.5 or 8 mg/d for women over 19 years of age, with an RI of 11 or 10 mg/d. Not shown in Table 4-4, but of note, the ARs are higher for pregnant adolescents, 10.5 (the IOM) or 12 (IZiNCG) mg/d, based on evidence that these young women may have additional needs to support any continued growth. The IOM and IZiNCG estimates of RI for pregnant adolescents are 13 and 15 mg zinc/day, respectively.
Estimating the additional zinc needed for lactation requires taking into consideration breast milk zinc concentration. Breast milk zinc appears to be relatively similar among women with different zinc intakes, owing to the redistribution of tissue zinc from uterus involution, and the release of zinc to maternal tissues resulting from the decline in blood volume during early lactation. However, breast milk volume, and thus breast milk zinc concentrations, do change over time. WHO recommended an additional 1.4 mg/d of zinc from 0 to 3 months and 0.5 mg/d from 6 to 12 months. The IOM, IZiNCG, and EFSA made one recommendation to be applied throughout lactation, recognizing that the actual need varies with time. For example, EFSA recommended an additional 1.1 mg/d of zinc, based on the assump-
TABLE 4-4 Zinc Average Requirement (AR) and Recommended Intake (RI) During Pregnancy and Lactation by WHO, the IOM, IZiNCG, and EFSA
| WHOa | IOMb | IZiNCGb | EFSAb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy | Lactation | Pregnancy | Lactation | Pregnancy | Lactation | Pregnancy | Lactation | ||||||||
| AR | RI | AR | RI | AR | RI | AR | RI | AR | RI | AR | RI | AR | RI | AR | RI |
| — | 3.4 | — | 5.8 | 9.5 | 11 | 10.4 | 12 | 8.0 | 10 | 7.0 | 9 | +1.3 | +1.6 | +2.4 | +2.9 |
| 4.2 | 5.3 | ||||||||||||||
| 6.0 | 4.3 | ||||||||||||||
NOTE: EFSA = European Food Safety Authority; IOM = Institute of Medicine; IZiNCG = International Zinc Nutrition Consultative Group; WHO = World Health Organization.
a WHO made RI recommendations for each trimester and for 0–3, 3–6, and 6–12 months lactation.
b The IOM, IZiNCG, and EFSA made a single recommendation for pregnancy and lactation.
tion that the mean increases in physiological requirement are 1.5 mg/d for the first trimester, 1.37 mg/d for the second trimester, and 0.86 mg/d for the third trimester (EFSA NDA Panel, 2014a).
EFSA NDA Panel (2014a) also estimated RIs for nonpregnant and nonlactating women consuming different levels of phytate. The additional zinc needed for pregnancy or lactation is added to the RI based on the amount of phytate normally consumed. For example, 9.3 mg of zinc per day is the RI for women of reproductive age who are consuming 600 mg of phytate per day. To meet the needs for pregnancy, 1.6 mg of additional zinc should be added (i.e., 9.3 + 1.6 = 10.9 mg zinc/d); for lactation, 2.9 mg of additional zinc is added (i.e., 9.3 + 2.9 = 12.2 mg zinc/d).
Safe Upper Levels of Intake
As described in Chapter 3, the UL is defined as the highest daily intake level of a nutrient that is likely to pose no risk of adverse health effects for almost all individuals. The physiological basis for the zinc UL is the effect of high doses of supplemental zinc (50 mg zinc/d) on the activity of erythrocyte copper-zinc superoxide dismutase. The IOM set a UL of 40 mg zinc/d as an intake with no observed adverse effect level (NOAEL) for adult men and women. A value of 4.0 mg/d was set for infants 0–6 months of age; 7 mg/d for 1- to 3-year-old children and 12 mg/d for 4- to 8-year-old children. IZiNCG adopted the IOM UL for adults. Given that U.S. children frequently have zinc intakes above the IOM’s UL without any evidence of adverse effects, IZiNCG set a NOAEL of 6 mg zinc/d for 6- to 11-year-old children and 8 mg/d for 1- to 3-year-old children. EFSA did not derive a UL for any life stage group.
Alternative Approaches to Determining Zinc Requirements
Another approach for assessing the adequacy of dietary zinc intake could be the effect of changes in zinc intakes on selected biochemical and/or functional markers of zinc adequacy. However, as discussed previously, at present, there are no quantitative zinc biomarkers available that reflect changes in the zinc status of an individual with changes in intake. IZiNCG and the Biomarkers of Nutrition for Development (BOND) both recommended serum/plasma zinc levels as one of the best biomarkers of zinc status currently available (Brown et al., 2004; King et al., 2016). But, as stated previously, plasma zinc concentrations remain constant over a range of dietary zinc intakes from 4 to 60 mg/d (Gibson et al., 2008). Thus, they cannot be used to derive ARs or RIs.
Other biomarkers that have been used to assess zinc status include, but are not limited to, erythrocyte zinc levels, urinary zinc excretion, hair zinc
levels, and alkaline phosphatase activity (Lowe et al., 2009). Unfortunately, these biomarkers are also limited in their sensitivity and/or specificity for assessing whole body zinc status, and thus, would not be useful for estimating dietary zinc requirements. In population studies, the prevalence of stunting is among the most widely used biomarkers for assessing zinc status, along with dietary intake and serum zinc levels (King et al., 2016; Roohani et al., 2013). Stunting is the preferred functional marker by physicians, and it is generally responsive to the administration of supplemental zinc (Hotz and Brown, 2001; Roohani et al., 2013). Other nutrients can also affect stunting.
FINDINGS AND CONCLUSIONS FOR ZINC
- There is no available sensitive, specific biomarker of zinc nutrition. Although severe dietary zinc restriction (i.e., diets providing less than 1 mg zinc/day) causes a marked, rapid decline in plasma zinc, it can take weeks for levels to return to baseline upon zinc repletion. Plasma zinc concentrations seem to be more responsive to zinc supplementation than to added zinc from food; supplements have been shown to cause prompt increases in plasma zinc concentrations irrespective of dietary zinc intake (King et al., 2016). Surveys show that plasma zinc concentrations remain relatively stable over a wide range of less restricted zinc intakes (i.e., from about 4 to 60 mg of zinc per day) (Gibson et al., 2008). Although linear growth has been recommended as a functional indicator of zinc status, since low height- or length-for-age has been shown to be responsive to supplemental zinc (Hotz and Brown, 2001), as with plasma zinc concentrations, the response to additional dietary zinc is not as strong as the response to supplemental zinc.
- Among adults, dietary phytate is thought to be a major determinant of zinc absorption. Thus, the phytate:zinc molar ratio is used to calculate a population’s dietary zinc requirements. EFSA initiated this approach in its 2014 recommendations (EFSA NDA Panel, 2014a). However, a recent study failed to find an effect of phytate on zinc absorption in young children and in pregnant or lactating women (Miller et al., 2015).
- Since zinc is generally associated with proteins in body cells and tissues, it is important to determine whether dietary zinc recommendations need to be matched to the amount and type of protein in the diet (i.e., animal or vegetable). When developing zinc supplementation or fortification programs, it is generally assumed that all zinc organic and inorganic salts are equally absorbed (Hotz et al.,
-
2005). However, the bioavailability of zinc–amino acid complexes may be used more effectively if certain amino acids are functioning as ligands (Wapnir and Stiel, 1986).
- Survey data suggest that growing children are particularly vulnerable to developing zinc deficiency and are more prone to develop gastrointestinal or pulmonary zinc infections as a result. The physiology underlying this increased vulnerability in children is unknown. Possibly, children lack tissue or cellular reserves to draw on when diet is marginal. Alternatively, their immune systems are less well developed than adults.
Based on its findings for zinc, the committee came to the following conclusions:
Additional data are needed to determine if age or physiological zinc need influence the effect of phytate on zinc absorption.
Further research will be needed to determine if a modest, consistent increase in dietary zinc could reduce a child’s susceptibility to zinc deficiency by increasing the level of tissue zinc reserves or enhancing immune function. As part of this research, careful thought should be directed toward recommending a zinc intake that would achieve an increased resistance to disease.
Since the zinc physiological requirements and ARs for young children and women of reproductive age that have been made by the authoritative bodies reviewed in this report are very similar (as shown in Tables 4-2, 4-3, and 4-4), efforts should be made to consolidate these estimates globally. However, there may still be a need to set national RIs based on the dietary zinc source (i.e., bioavailability) and the average body size of the population.
Reviews addressing dietary factors influencing zinc absorption and physiological losses among various population subgroups are needed.
In reference to the four steps for setting NRVs outlined in Figure 3-4, these are the issues regarding zinc recommendations:
- Currently, systematic reviews of dietary zinc requirements are lacking.
- Limited experimental data are available for infants, young children, and pregnant and lactating women from different regions.
-
Comprehensive studies of these populations are especially needed in low- and middle-income countries.
- The factorial approach is the preferred method for estimating zinc reference values, but those data should be supplemented with dose–response modeling, which shows the effect of various zinc intakes on the growth rate of toddlers or the association between zinc intake and immune function biomarkers in children or pregnant/lactating women.
PROPOSED SOLUTIONS FOR ZINC1
Because a sensitive biomarker of zinc inadequacy is not available, the factorial method is the only feasible approach for estimating dietary zinc requirements at this time. The factorial approach involves estimating the amount of a nutrient needed to replace that lost through fecal, urine, and skin routes, either unchanged, or as a metabolite, then estimating the additional amount required to support growth, pregnancy, or lactation. Currently, all international and national groups reviewed in this report use the factorial approach for establishing zinc nutrient intake recommendations. However, quantitative data are lacking for growing children. In addition to data gaps identified and listed in the findings, including the need to continue to search for a reliable biomarker of zinc status that is more sensitive to changes in dietary zinc than plasma/serum zinc concentrations, studies are needed to help identify the potential influence of genetic polymorphisms (i.e., genetic variations) on individual dietary zinc requirements. Furthermore, the development of comprehensive models of inhibitors of zinc absorption across diverse populations would improve estimates of dietary requirements.
IRON CASE ANALYSIS
Introduction and Statement of the Problem
Iron deficiency is a common nutritional deficiency worldwide and a major cause of infant mortality (Rahman et al., 2016). Young children and women of reproductive age, especially during pregnancy, are at increased risk because of the high iron requirements for growth or the replacement of iron lost in menses. Women with high menstrual losses have a greater risk of iron deficiency, compared to women with lower menstrual losses (Harvey et al., 2005). Iron deficiency is particularly common in low-income countries because of limited intakes of animal products (which contain the
___________________
1 This text was revised after prepublication release.
more highly bioavailable heme iron, which can promote nonheme iron absorption) and the consumption of repetitive diets based on cereal grains and legumes. Cereal grain and legume-based diets are low in bioavailable iron owing to the presence of phytate and certain polyphenols that inhibit iron absorption (Hurrell and Egli, 2010). This dietary pattern leads to iron deficiency and anemia, which results in pathophysiological conditions such as low birth weight, stunted growth, delayed mental development, and impaired motor function.
Factors Influencing Requirements
As with zinc, a number of contextual factors affect the iron requirements of a population. In addition to the dietary intake pattern described above, these include the physiological state of an individual or group, environmental influences on nutrient intake, and a population’s health status. In the future, it may be important to consider the genetic background of a population as well (Stover, 2007). The key factors influencing iron requirements are whole body iron metabolism, physiological requirements dietary sources, iron bioavailability, and infection.
Whole Body Iron Utilization and Status
Iron absorption is a tightly regulated function; its efficiency is determined in the absence of disease by the size of body iron stores. The iron status of healthy populations is usually measured using serum ferritin level as a biomarker. However, infections and inflammatory disorders can increase serum ferritin concentrations even when body iron stores are depleted. Similarly, other iron biomarkers, such as soluble transferrin receptors, can be modified by chronic disease or deficiencies of other nutrients (Lynch, 2011). Thus, accurately estimating iron status can be challenging, especially in populations where infection is common.
Physiological Requirements
Physiological iron requirements are based on the amount needed to replace losses. Using data from radioisotope studies, Green et al. (1968) estimated total iron losses from skin, intestine, and the urinary tract to be, on average (among men from three different countries), 0.014 mg per kilogram of body weight per day. A more recent study used radioisotopes to measure total iron losses in women as well as men (Hunt et al., 2009). The values for men are similar to the earlier study, but in women of reproductive age the values are higher than for men because of menstrual losses, with a mean value of 0.025 mg per kilogram of body weight per day.
Dietary Patterns
The requirement for a given nutrient in a population depends on the amount of the nutrient ingested through usual dietary patterns, as well as the iron bioavailability from the diet and the presence of infections in the population (King and Garza, 2007). As stated above, habitual consumption of foods that contain such iron-binding components, that is, phytate and polyphenols, increases dietary requirements for iron. Using food preparation techniques that enhance iron absorption, such as cooking in iron pots, may increase iron intake.
Iron Bioavailability
Iron is absorbed either as heme iron, which is found in meat and fish, or as nonheme iron, as found in plant foods. Heme iron has greater bioavailability than nonheme forms. Typically, 15 to 35 percent of heme iron is absorbed, while nonheme iron absorption can range from 2 to 20 percent, depending on the type of grain or legume; the presence of other dietary components, such as phytate, which inhibits iron absorption; or iron status (Abbaspour et al., 2014). For example, at the Global Harmonization of Methodological Approaches to Nutrient Intake Recommendations workshop (NASEM, 2018), Umi Fahmida described work from Narasinga Rao et al. (1983) reporting iron absorption rates of 1.7–1.8 percent for a millet-based diet, 3.5–4.0 percent for a wheat-based diet, and 8.3–10.3 percent for a rice-based diet.
Not only do meat, fish, and poultry have high iron bioavailability because of their heme iron, but also heme iron from animal sources can enhance nonheme iron absorption from vegetable and grain sources. Also at the Global Harmonization workshop, Fahmida described data showing that iron absorption rates of 1.7 percent for a rice-based diet increased to 5.5 percent with the addition of 15 grams of fish, and to 10.1 percent with the addition of 40 grams of fish (Aung-Than-Batu et al., 1976). Grain- and vegetable-based food sources are more prevalent in low- and middle-income countries where diet, poverty, or cultural norms limit consumption of meat and fish, thus contributing to the higher prevalence of iron deficiency in those populations.
Additionally, because iron absorption depends on levels of hepcidin (a hormone that regulates iron absorption), iron bioavailability is also affected by the effects of infection and inflammatory disorders on hepcidin regulation (Nemeth and Ganz, 2009).
Infection
Seth Adu-Afarwuah, in his presentation at the Global Harmonization workshop (NASEM, 2018), explained that infections can affect nutrient requirements by decreasing food intake, impairing nutrient absorption or reabsorption, increasing absolute or direct losses of body nutrients, and altering uptake, diversion, or sequestration of body nutrients (Bresnahan and Tanumihardjo, 2014). In the case of iron, not only does poor iron status impair host defenses against infection, but the presence of infection worsens the deficiency state by triggering a physiologic down-regulation of iron absorption (Drakesmith and Prentice, 2012).
Assessment of Strengths and Weaknesses in Methodologies to Derive Iron ARs
Although attempts have been made to define a dose–response relationship between iron intake and an iron status biomarker or selected health outcome, too many uncertainties remain. The strengths and weaknesses of both approaches are discussed below.
Dose–Response Assessment
As summarized in Chapters 2 and 3, standardized systematic review methodologies are an important component of the methodological approach for deriving NRVs. In a series of systematic reviews undertaken by the European Micronutrient Recommendations Aligned (EURRECA), a large degree of heterogeneity among the study populations, iron doses, and outcome measures was found (Harvey et al., 2013). This heterogeneity precluded using meta-analyses for determining the relationship between iron intake and several selected outcomes: tiredness, physical performance, immune function, thermoregulation, restless leg syndrome, and cognitive function. Thus, it was not possible to draw conclusions about associations between iron intake and those selected outcomes. In a separate systematic review (NNR, 2004), the Nordic Council of Ministers attempted to determine the relationship between iron intake and adequate growth, development, and health maintenance (Domellof et al., 2013). A total of 55 articles were identified as relevant, and the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation system. Most of the studies focused on vulnerable groups, namely young children and women of reproductive age. There was some evidence that treatment of iron deficiency anemia improved attention and concentration in adult women.
Based on these findings on selected health outcomes, as well as other studies cited previously in this chapter on iron intake and status biomarkers (e.g., serum ferritin levels), the committee identified a number of problems in attempting to establish reference values for iron using dose–response assessment:
- Inaccurate estimates of iron intake and quantities of heme and nonheme iron in the diet
- Poor correlation between iron intake (bioavailability) and status
- Difficulty in measuring adaptive and functional responses to variations in iron intake
- Lack of sensitive and specific markers to determine iron status
- Confounding by other dietary and lifestyle factors and by responses to infection and inflammation
- Inadequate characterization of iron deficiency anemia and the relative role of iron deficiency and other causes of anemia
The Factorial Approach
In the absence of a sensitive biomarker or health outcome for defining a dose–response relationship for iron intake, the factorial approach is the preferred method for estimating physiological iron requirements. This approach involves estimating the quantity of absorbed iron needed to replace iron losses. Table 4-5 summarizes different authoritative bodies’ estimates of iron losses, bioavailability factors (used to determine the usual proportion of dietary iron absorbed), and AR for women of reproductive age.
Estimating iron losses Iron losses or needs are divided into three categories: (1) basal losses, (2) menstrual losses in women, and (3) iron accretion for pregnancy and growth in children. When calculating total loss, if the distributions of a component are not normally distributed, data from a large theoretical population with characteristics similar to the population of interest are collected and the median percentile of the distribution is used instead (e.g., see discussion of menstrual blood loss below).
Basal losses refer to the obligatory iron losses in feces, urine, sweat, and skin cell exfoliation. As mentioned previously, Green et al. (1968) measured basal iron losses using long-lived radio-labeled iron (55Fe) and calculated an average loss of about 14 mg/kg body weight per day, which is about 0.9 to 1.0 mg of iron per day. This value is corroborated by the IOM measures of whole body iron excretion (IOM, 2001). Variation in whole body iron excretion among different population subgroups is explained primarily by body weight and the magnitude of iron stores.
When menstrual iron losses are added to these basal iron losses, using
TABLE 4-5 Comparison of Factors Used in Factorial Modeling to Determine an AR for Iron in Women of Reproductive Age
| Values Used by Authoritative Bodies to Derive Iron ARs for Women of Reproductive Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| WHO/FAO (2004) | IOM (2001) | D-A-CH (2015) | NNR (2012) | Aus/NZ (2017) | NL (1992) | UK COMA (1991) | EFSA NDA Panel (2015) | |
| Premenopausal | ||||||||
| Median basal iron losses (mg/d) | 0.87 | 1.4 | 1.54 | 1.35 | 1.6 | 1.56 | 1.34 | |
| Bioavailability factor (percent) | 12–15: meat-based | 18 | 10–15 | 15 | 18 | 12 | 15 | 18 |
| 5–10: cereal-based | ||||||||
| AR (mg/d) | — | 8.1 | — | 9 | 8 | 14 | 11.4 | 7 |
| Pregnancy | ||||||||
| Total cost of pregnancy (mg) | 840 | 700–800 | 800 | 1,040 | 680 | 835 | ||
| AR (mg/d) | — | 23 (14–18 y) | — | 9 | 23 (14–18 y) | 9: 1st tri | 14.8 | 7 |
| 22 (> 18 y) | 22 (≥ 19 y) | 14: 2nd tri | ||||||
| 18: 3rd tri | ||||||||
| Lactation | ||||||||
| Milk secretion (mg/d) | 0.3 | 0.27 | 0.25–0.34 | |||||
| Bioavailability factor (percent) | — | 18 | 18 | |||||
| AR (mg/d) | — | 6.5 | — | 9 | 7 (14–18 y) | 19 | 11.4 | 7 |
| 6.5 (≥ 19 y) | ||||||||
NOTE: AR = average requirement; Aus/NZ = nutrient reference values for Australia and New Zealand; D-A-CH = Nutrition Societies of Germany, Austria, and Switzerland; EFSA = European Food Safety Authority; IOM = Institute of Medicine; NDA = nutrition, novel foods, and allergens; NL = Netherlands Food and Nutrition Council; NNR = Nordic Nutrition Recommendations; tri = trimester; UK COMA = United Kingdom Committee on Medical Aspects of Food and Nutrition Policy; WHO/FAO = World Health Organization/Food and Agriculture Organization.
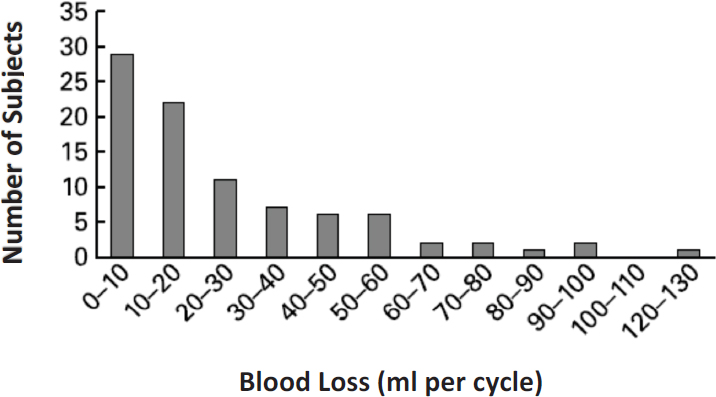
NOTE: N = 90.
SOURCE: Harvey et al., 2005.
data from Hallberg et al. (1966a,b) and Hallberg and Rossander-Hultén (1991), the estimated total iron loss increased to ~1.5 mg/d. As shown in Figure 4-3, there is wide interindividual variability in menstrual losses and a skewed distribution (Harvey et al., 2005). Thus, to obtain this estimated total of ~1.5 mg/d, menstrual blood loss was predicted from a log-normal distribution, with a predicted median of 30.9 ml/cycle, with a hemoglobin of 3.39 mg/g;2 assuming a 28-day cycle, this equates to 0.51 mg/d of menstrual loss.
The EFSA Dietetic Products, Nutrition, and Allergies (NDA) Panel (2015) took a unique approach to estimating iron requirements by using the whole-body iron loss data derived from isotope studies (Hunt et al., 2009). In premenopausal women, the 50th percentile of the model-based distribution of obligatory iron losses was 1.34 mg/day (see Table 4-5). Thus, there appears to be good agreement between this newer data from Hunt et al. (2009) and the older data generated by Green et al. (1968). The 90th, 95th, and 97.5th percentiles of iron loss were, respectively, 2.44, 2.80, and 3.13 mg iron/day.
___________________
2 Iron lost blood was calculated from the total menstrual blood loss using the equation: MIL (mg = d) = MBL (ml) × Hb (mg = ml) × 0.00334/cycle length. MIL is menstrual Fe loss, MBL is menstrual blood loss, Hb is hemoglobin, and 0.00334 is equivalent to the fraction of iron in hemoglobin at a concentration of 1 mg/ml.
As summarized in Table 4-5, there is good agreement between the values derived by different organizations for absorbed iron to replace obligatory losses of iron from women of reproductive age. Median values range from 1.4 to 1.54 mg/d, with average physiological requirement of 1.6 (for women aged 18–22 years) to 1.7 (women aged greater than 22 years) mg/d. When expressed in percentiles, the values are 1.34 (50th percentile), 2.8–2.94 (95th percentile), and 3.13–3.15 (97.5th percentile).
Estimating ARs for pregnancy Different authoritative bodies’ estimates of the total iron cost of pregnancy and ARs for pregnancy are also summarized in Table 4-5. EFSA’s estimate of an AR for pregnancy is the same estimate for premenopausal women, and WHO/FAO did not establish an AR for pregnancy. In contrast, the IOM, Australia and New Zealand, and the Netherlands all increased their estimated ARs for women during pregnancy. There are two main reasons for this disparity in iron recommendations among authoritative bodies. One is the assumption about the level of iron stores at conception. As explained below, both EFSA and WHO/FAO assumed there was a storage of iron in the liver at the beginning of pregnancy that could then be mobilized for pregnancy needs. In contrast, other authorities assumed that many women, particularly teenage girls who became pregnant, do not have sufficient iron stores, and therefore all of the additional iron has to be obtained from the diet (or supplements).
The second reason for the different recommendations is uncertainty about the degree to which the efficiency of absorption increases during pregnancy. Also explained below, both EFSA and WHO/FAO assumed an increased efficiency of absorption.
The total iron cost of pregnancy represents the iron deposited in the fetus, placenta, and umbilicus, plus the amount needed for hemoglobin mass expansion. Estimates of the iron content of the fetus and placental tissue range from 320 mg (IOM, 2001) to 450 mg (375 for the fetus and 75 for the placenta) (Hytten and Leitch, 1971). The amount of iron needed for expansion of the hemoglobin mass is thought to be about 450 to 500 mg with most of the gain occurring in the second and third trimesters. EFSA, however, in its determination of an iron intake for pregnancy, did not include an iron allocation for expanding the hemoglobin mass based on evidence that the efficiency of iron absorption increases exponentially up to about 10 mg/d during the last 6 weeks of pregnancy (Barrett et al., 1994; Whittaker et al., 1991). This increased efficiency can compensate for the higher needs, it argued, provided that adequate iron stores are present at conception. If the serum ferritin concentration is 30 μg/L at conception, then around 120 mg of stored iron can be mobilized to support the pregnancy needs.
WHO/FAO (2004) did not derive a separate iron intake requirement
for pregnancy, arguing that iron balance in pregnant women depends on the properties of the diet and on iron stores and that the requirements could be met by the increased efficiency of iron absorption (Milman, 2006). Although its estimated total iron requirements are 1,040 mg (300 mg for the fetus, 50 mg for the placenta, 450 mg for the expansion of maternal red cell mass, and 240 mg for basal iron losses), because of the assumption of sufficient iron stores, WHO/FAO’s estimated net pregnancy iron requirement is 840 mg. Similarly, the United Kingdom Committee on Medical Aspects of Food and Nutrition Policy did not give a pregnancy recommendation, nor did Nordic Nutrition Recommendations, although it did state that the physiological requirements for some women may not be satisfied during the last two trimesters of pregnancy.
Determining AR and RI for lactating women As with pregnancy, there is also inconsistency among authoritative bodies for recommendations during lactation (see Table 4-6). When using the factorial approach to calculate additional iron needed to support lactation, some agencies do not account for increased iron losses in breast milk.
Derivation of Safe Upper Levels of Intake
Here, several different authoritative bodies’ recommendations for a UL for iron, or reasons for not setting a UL, are described. First, because of the absence of more serious adverse effects (including vascular disease and cancer), the IOM (2001) based its recommendations for a UL on gastrointestinal side effects caused by high iron intakes. A lowest observed adverse effect level (LOAEL) was set based on a study in Sweden where 97 men and women were give iron supplements (60 mg/day as ferrous fumarate) in addition to an estimated dietary intake of 11 mg iron/day (Frykman et al., 1994). Most participants reported minor side effects although five withdrew because they considered the side effects (e.g., constipation) severe enough to stop taking the medication. An uncertainty factor of 1.5 was selected to account for extrapolation from an LOAEL to an NOAEL, which approximates to a UL of 45 mg/day.
Similarly, the Australia/New Zealand report based its ULs for iron on gastrointestinal symptoms. For adults, an LOAEL of 70 mg/d was set based on the same supplementation study by Frykman et al. (1994), together with median population dietary intakes (IOM, 2001). Because of the self-limiting nature of the adverse outcomes, a relatively low uncertainty factor was used to extrapolate from the LOAEL, resulting in a UL of 45 mg/d after rounding. Because of the limited data available, the same figure was applied to pregnant and lactating women.
TABLE 4-6 Comparison of Recommendations During Lactation from Different Authoritative Bodies
| Recommending Body | NRVs for Lactation |
|---|---|
| WHO/FAO, 2004 | The usual practice in WHO/FAO reports is to report only an RI, not an AR. Based on a median daily milk iron loss of 1.15 mg/d, the RI is estimated to be 10 mg/d for a dietary bioavailability of 15 percent and 30 mg/d for a bioavailability of 5 percent. |
| IOM, 2001 | Based on an average milk iron loss of 0.27 ± 0.09 mg/d and assuming a bioavailability of 18 percent, the AR was estimated at 6.5 mg/d. |
| D-A-CH, 2015 | An RI of 20 mg/d was recommended to replace losses caused by pregnancy and to meet lactation needs. No AR was reported. |
| NNR, 2012 | An AR of 9 mg/d and an RI of 15 mg/d were recommended; these are the same as those for nonpregnant and nonlactating women. |
| NL, 1992 | The AR increased to 19 mg/d to replace iron lost during birth and in milk. |
| UK COMA, 1991 | Based on an estimated milk iron loss of 0.25–0.34 mg/d. No increase in AR was recommended. |
| EFSA NDA Panel, 2015 | Assuming the total requirement for absorbed iron during the first months of lactation to be 1.3 mg/d, no increase in AR was recommended. |
NOTE: AR = average requirement; D-A-CH = Nutrition Societies of Germany, Austria, and Switzerland; EFSA = European Food Safety Authority; IOM = Institute of Medicine; NDA = nutrition, novel foods, and allergens; NL = Netherlands Food and Nutrition Council; NNR = Nordic Nutrition Recommendations; RI = recommended intake; UK COMA = United Kingdom Committee on Medical Aspects of Food and Nutrition Policy; WHO/FAO = World Health Organization/Food and Agriculture Organization.
The Nordic Council of Ministers (2012) was unable to set a quantitative iron UL based on risk of chronic disease. Instead, it set a UL of 60 mg/d, based on knowledge that a regular intake of 60 mg/d in premenopausal women may lead to iron overload. It provided further support for this value by noting that the local intestinal toxicity seen with therapeutic iron occurs in the range of 50 to 60 mg/day. Finally, EFSA reported that data were insufficient to derive a UL (EFSA NDA Panel, 2004).
FINDINGS AND CONCLUSIONS FOR IRON
Based on its review of the evidence, the committee made the following findings:
- The derivation of most values used for bioavailability is neither transparent nor evidence based. This is because data on iron bioavailability from the whole diet is limited. The one exception is the approach used by EFSA, which is based on a model in which the iron absorption from the whole (Western) diet is predicted using good quality individual data on dietary intake, serum ferritin concentrations, and calculated physiological iron requirements. The model has since been refined and updated and an interactive modeling tool published.
- The lack of agreement for reference values is caused largely by the choice of bioavailability factor used to convert physiological requirements into dietary intakes, which results from limited information on iron absorption from complete diets, as well as assumptions about storage iron at conception.
- When assessing the iron status of a population in relation to public health policies such as food fortification, it is crucial to have good quality representative data for iron intake. If the mean iron intake is below the average requirement, then evidence of iron deficiency must be supported by measures of iron status. However, because dietary iron exists in two forms, measuring dietary intake is difficult because of limited information on heme iron in food composition databases. In addition, several biomarkers of iron status (e.g., ferritin) are modified by infection, chronic disease, and deficiencies of other nutrients.
- Pregnancy is a normal physiological condition, which should not require a major change in food intake, provided the habitual diet contains all nutrients at levels that are consistent for optimal health. Many women become increasingly iron deficient or anemic during pregnancy because they do not have adequate iron stores at conception and/or their dietary iron bioavailability is insufficient to meet their needs despite the well-accepted adaptive mechanisms designed to maintain iron homeostasis.
- NRVs are derived for healthy populations, and although physiological requirements for iron should be the same for each population subgroup in every country or region, dietary iron bioavailability will depend on the local diet composition, which may change the AR.
Based on its findings, the committee came to the following conclusions for iron:
At present, the factorial approach is the only feasible option for deriving iron NRVs, and values for iron availability need to take
into account the usual dietary composition of the population, which may result in differences in NRVs, as illustrated by WHO’s approach in which it provides values for four different global scenarios.
There are several factors related to diet and lifestyle, infection, and disease that confound the association between iron intake and health outcomes, obscuring the dose–response relationship that are needed to accurately estimate reference values.
There are challenges for setting an iron UL, but the value is essential for evaluating the safety of food iron fortification and other public health programs.
PROPOSED SOLUTIONS FOR IRON
Although authoritative bodies have adopted the factorial method globally, there remains wide variation in NRVs determined for women of reproductive age, mainly attributable to different calculations used to transform physiological requirements into dietary intakes. The bioavailability model used by EFSA (Dainty et al., 2014) and subsequently updated (Fairweather-Tait et al., 2017) is the best approach to determine iron bioavailability from the whole diet. However, the approach is only applicable in low- and middle-income countries if it is appropriately adapted; in low- and middle-income countries, intakes of iron absorption inhibitors may be higher and heme iron intakes lower than in Western diets.
Additionally, many low- and middle-income countries have high burdens of infection or other widespread health concerns, such as hemoglobinopathies and thalassemia, that have an effect on iron metabolism and biomarkers of iron status. In these countries, assessing the prevalence of iron deficiency may require measuring serum ferritin and then correcting for inflammation using the most appropriate method (e.g., regression analysis).
FOLATE CASE ANALYSIS
Introduction and Statement of the Problem
The global prevalence of folate deficiency and depletion is not well documented, but data are in the process of being added to WHO’s Vitamin and Mineral Nutrition Information System (VMNIS).3 Populations in low- and middle-income countries are not necessarily at greater risk of
___________________
3 Available at http://www.who.int/vmnis/en (accessed May 16, 2018).
deficiency compared to populations in high-income countries, especially if the usual diet in the lower-income population is high in legumes and green leafy vegetables.
The classical signs of severe folate deficiency include megaloblastic anemia with hypersegmented neutrophils, and an elevated mean red cell volume. However, the greatest global concern is that low folate intakes in the periconceptional period is a risk factor for infant neural tube defects (NTDs), especially in women with genetic predisposition. Supplementation and food fortification with folic acid reduce the prevalence of NTDs in women of reproductive age, especially in regions where folate status is poor and there is a high baseline prevalence of NTDs. Recognition of this issue has mobilized the global community around recommendations for folic acid requirements, supplementation, and fortification.
Factors Influencing Folate Requirements
Two key factors affect the folate requirements of a population: bioavailability and several specific genetic polymorphisms. Both of these are reviewed below.
Bioavailability
The primary dietary sources of folate are legumes, green leafy vegetables, liver (but not meat in general), and some fruits, including oranges, mangoes, and papayas (Allen, 2008). This is why poor folate status is often more common in wealthier populations than in lower income populations with higher intake of legumes and greens. Inadequate intakes of folate are less prevalent in populations where folic acid has been added to staple foods through fortification.
In foods, folates are present as polyglutamate derivatives, which are hydrolyzed by intestinal enzymes to monoglutamates prior to absorption. Most of the traditional methods for food folate analysis tend to underestimate actual folate content owing to incomplete release of the folate prior to analysis and incomplete hydrolysis of the polyglutamyl folates. Digestion with three enzymes (amylase, protease, and folate conjugase) has been shown to increase quantitative estimates of folate values in both folic acid-fortified and nonfortified cereals (Pfeiffer et al., 1997). The method should be optimized for different foods; however, it usually is not (Tamura et al., 1997). Most values are obtained from the U.S. Department of Agriculture food composition tables, but even these may be underestimates.
The bioavailability of folate from food sources is estimated to be about 50 percent, a value that is generally accepted as appropriate for all foods. However, a substantial source of folate in many populations is folic acid,
which is used in supplements and as a fortificant in cereals in at least 80 countries (Food Fortification Initiative, 2017). The estimated bioavailability of folic acid is 85 percent. Because of this difference in bioavailability between folate and folic acid, the amount of folate present in the diet is calculated as μg food folate + 1.7 × μg folic acid; the sum is expressed as dietary folate equivalents (DFEs).
Genetic Factors
As discussed by Patrick Stover at the Global Harmonization workshop (NASEM, 2018), several polymorphisms, or genetic variations, in folate metabolism can affect folate requirements. The most common is a C667T polymorphism in the gene that codes for 5, 10-methylenetetrahydrofolate reductase (MTHFR). Women with the MTHFR TT genotype have a 50 percent greater risk of an NTD birth than those with CC or CT genotypes. In some European countries up to 24 percent of the population is homozygous for this allele; thus, although EFSA did not increase the AR for folate, it did increase the coefficient of variation used to set the AI to 15 percent to reflect the genetic variability in requirements.
Methodologies to Determine Folate Requirements
A factorial method cannot be used to derive the AR because there are insufficient data on folate pool size and turnover, and because folate is metabolized into so many end products. The adequacy of folate intake can be determined, however, by its relationship to biomarkers of folate status, in other words, through dose–response assessment.
Biomarkers for Use in Folate Dose–Response Assessment
Serum or plasma folate is the most commonly used status measure in population surveys, but it reflects recent intake of the vitamin. Erythrocyte folate, also known as red blood cell (RBC) folate, serves as a more reliable indicator of longer-term status because red blood cells survive for 120 days and are not affected by short-term folate intakes (i.e., within weeks). It is also more stable in pregnancy. WHO recently recommended that RBC folate concentration be used as the sole indicator for NTD risk (Bailey and Hausman, 2018). A third folate status biomarker, plasma homocysteine level, increases with lower folate intakes and poor folate status and can be an indicator of response to folate interventions. However, homocysteine is also increased by inadequate intakes of vitamins B12, riboflavin, and B6, making it a nonspecific indicator of folate status. Because laboratory assessment can be problematic for all three biomarkers, regardless of the
biomarker used, recent sample handling and analytical guidelines should be followed, and the folate values in older population subgroups checked for their validity (Pfeiffer et al., 1997). The biomarkers used by different authoritative bodies to derive ARs for folate for women of reproductive age, and the AR value themselves, are summarized in Table 4-7. These particular authoritative bodies were selected because their recommendations were made within the last 20 years.
Several other potential markers of folate adequacy have been considered by various authoritative bodies, but they have not been used in deriving the AR. These include
- Anemia and hematological abnormalities, which have not been used because they occur only in relatively severe folate deficiency;
- Reduced risks of vascular disease, certain types of cancer, and psychiatric and mental disorders, for which the evidence was found insufficient to guide setting ARs;
- Kinetic studies of body pool size and turnover;
- Folate catabolites in urine; and
- Repletion of severe folate deficiency.
The IOM’s estimated folate requirements are based on the level of intake required to maintain normal blood levels of all three previously described biomarkers of folate status (i.e., plasma or serum folate, erythrocyte folate, and plasma homocysteine) (IOM, 1998). Cut-point values for “normal” were defined based on associations with biochemical abnormalities. The main source of data was four controlled metabolic studies in which folate was given as folate in food or as both folate in food plus folic acid supplement. In these studies the amount of intake required to maintain or restore status (after depletion) was determined (Herbert, 1962; Milne et al., 1983; O’Keefe et al., 1995; Sauberlich et al., 1987). Based on the dose–response data, 320 μg DFEs was accepted as the AR for premenopausal women (see Table 4-7). This was supported by epidemiological data showing that elevated homocysteine concentrations were more prevalent in the United States when intake was less than 280 μg/day (i.e., in the lowest four deciles). Requirements for older adults were determined to be the same as those of younger adults. WHO/FAO accepted the IOM methods and values for folate recommendations (and thus, reports an AR for this nutrient, rather than only an RI, which is the usual practice in WHO/FAO reports).
EFSA’s dose–response approach to deriving folate NRVs is based on the folate intake required to maintain serum and erythrocyte folate concentrations. As shown in Tables 4-7 and 4-8, its recommendations are lower than those of the IOM and WHO/FAO. This is because it used older studies, which showed that lower intakes were sufficient and rejected the main study
TABLE 4-7 Comparison of Factors Used in Dose–Response Modeling for Folate in Women of Reproductive Age
| WHO/FAO (O’Keefe et al., 1995) | IOM (O’Keefe et al., 1995) | D-A-CH | NNR (Herbert et al., 1962; Milne et al., 1983; Sauberlich et al., 1987) | Australia/New Zealand (O’Keefe et al., 1995) | EFSA (Milne et al., 1983; Sauberlich et al., 1987) | |
|---|---|---|---|---|---|---|
| Biomarkers: | ||||||
| RBC folate (nmol/L) | > 305 | > 305 | > 317 | — | > 340 | |
| Serum folate (nmol/L) | > 10 | > 7 | > 10 | > 6.8 | — | > 10 |
| Erythrocyte folate (nmol/L) | > 340 | |||||
| Homocysteine (μmol/L) | < 16 | < 16 | 9.3–16.3 | — | ||
| AR DFE (μg/d) | 400 | 400 | 220 | 300 | 400 | 220 |
NOTE: AR = average requirement; D-A-CH = Nutrition Societies of Germany, Austria, and Switzerland; DFE = dietary folate equivalents; EFSA = European Food Safety Authority; IOM = Institute of Medicine; NNR = Nordic Nutrition Recommendations; RBC = red blood cell; WHO/FAO = World Health Organization/Food and Agriculture Organization.
TABLE 4-8 Values Used by Authoritative Bodies to Derive ARs for Young Children and Women of Reproductive Age
| IOM | WHO/FAO | D-A-CH | Aus/NZ | EFSA | |
|---|---|---|---|---|---|
| Premenopausal women | |||||
| Intake to maintain biomarkers (DFEa μg/d) | 320 | 320 | 220 | 320 | 250 |
| Bioavailability factor (%)b | 50 | 50 | 50 | 50 | 50 |
| AR (DFE μg/d) | 320 | 320 | 220 | 320 | 250 |
| Pregnancy | |||||
| Total basal requirement (DFE μg/d) | 320 | 320 | 220 | 320 | 320 |
| AR (DFE μg/d) | 520 | 520 | 420 | 520 | — |
| Lactation | |||||
| Milk secretion (DFE μg/d) | 130 | 130 | 120 | 133 | 130 |
| AR (DFE μg/d) | 450 | 450 | 450 | 450 | 380 |
| Infants 0–6 months | |||||
| AI (DFE μg/d)c | 65 | 65 | 60 | 65 | 64 |
| IOM | WHO/FAO | D-A-CH | Aus/NZ | EFSA | |
|---|---|---|---|---|---|
| Infants 6–12 months | |||||
| Extrapolation factor | — | — | 700 kcal/d required × 12 μg folate/100 kcal milk | Weight0.75 | Weight0.75 |
| Growth factor | — | — | — | 0.3 | 0.3 |
| AR or AI (DFE μg/d) | 80 | 80 | 85 | 80 | 80 |
| Children 1–3 years | |||||
| Extrapolation factor | Weight0.75 | Weight0.75 | Weight0.75 | Weight0.75 | Weight0.75 |
| Growth factor | 0.3 | v0.3 | 0.3 | 0.3 | 0.3 |
| AR (DFE μg/d) | 120 | 120 | 79.8 (males) | 120 | 80 |
| 92 (females) | |||||
| Children 4–8 years | |||||
| Extrapolation factor | Weight0.75 | Weight0.75 | Weight0.75 | Weight0.75 | Weight0.75 |
| Growth factor | 0.15 | 0.15 | 0.15 | — | 0.38 |
| AR (DFE μg/d) | 160 | 160 | 106 F, 94 M | 160 | 110 F, 160 M |
NOTE: AI = adequate intake; AR = average requirement; Aus/NZ = Australia/New Zealand; D-A-CH = Nutrition Societies of Germany, Austria, Switzerland; EFSA = European Food Safety Authority; IOM = Institute of Medicine; WHO/FAO = World Health Organization/Food and Agriculture Organization.
a DFE = dietary folate equivalent.
b Calculated as 50 percent for food folates and 85 percent for folic acid.
c For infants aged 0 to 6 months, the folate recommendation is an AI, because of the lack of data on dose–response relationships at this age.
(O’Keefe et al., 1995) used by the IOM. Germany, Austria, and Switzerland (D-A-CH) reduced their folate ARs for adults in 2014, from 400 μg to 300 μg DFE/day, based on lack of evidence from recent randomized controlled trials that lowering homocysteine with supplements of folate and other B vitamins would reduce risk of cardiovascular disease (Krawinkel et al., 2014). The Australia/New Zealand recommendations use erythrocyte folate as the primary indicator because it reflects folate stores although serum folate and plasma homocysteine are used to support decisions on adult values.
Determination of a Folate Requirement for Infants and Children
Intake levels, bioavailability factors, and other factors used by different authoritative bodies (the IOM, WHO/FAO, D-A-CH, Australia/New Zealand, and EFSA) to derive folate ARs for young children (or AIs for infants aged 0 to 6 months) and women of reproductive age are also summarized in Table 4-8.
For infants aged 0 to 6 months, as is the case for many other nutrients, the folate recommendation is an AI, not an AR, because of the lack of data on dose–response relationships at this age. As shown in Table 4-8, AIs in most reports are around 65 μg/day, calculated as the folate concentration in milk (~85 μg/L) × milk volume (780 ml/d).
The IOM and WHO/FAO folate recommendations for infants aged 6 to 12 months are based on data derived from studies that measured intake from breast milk and/or formula, leading to an estimated intake of 80 μg/day to maintain normal levels of serum and erythrocyte folate. Australia/New Zealand and EFSA obtained the same value but by extrapolating up or down from younger infants and adults respectively, and scaling for body weight. D-A-CH also used an extrapolation method to obtain a slightly different value of 85 μg/day. The process of extrapolating NRVs from one age group to another is described in Chapter 3; methods used are listed in Appendix E.
All of the ARs for older children listed in Table 4-8 were derived by extrapolating downward from adult AR values, scaling by body weight to the 0.75 power, and assuming that maintenance requirements for folate are the same per unit body weight for children as they are for adults. A growth factor of 0.30 was added to the formula for extrapolation (see Chapter 3 and Appendix E) for children from 7 months to 3 years and growth factors of 0.15 and 0.40 for male and female children aged 4 to 18 years, respectively. The ARs are remarkably similar across NRVs from different authoritative bodies except for the lower ARs set by D-A-CH for children aged 4 to 8 years (Krawinkel et al., 2014). The D-A-CH values are based on the IOM (1998) AR and the usual growth factor of 0.15 for this age.
The reason for the lower values is unclear. The D-A-CH also gave differing AR values for male and female children.
Determination of Folate Requirements for Pregnant and Lactating Women
For pregnancy, the outcome status biomarker used by the IOM was maintenance of erythrocyte folate (IOM, 1998). Not only is this a better indicator of tissue stores than serum or plasma levels, but in pregnancy plasma folate levels are more affected by hemodilution. In addition, plasma homocysteine is generally lower during pregnancy. Using this biomarker, dose–response studies have shown that an additional 200 μg/day DFEs need to be added to the AR for nonpregnant women, for a total of 520 μg/day (Stamm and Houghton, 2013). The recommendations for pregnancy are identical across countries except for D-A-CH, which is attributable to its lower estimate for nonpregnant values.
For lactating women, a total of 450 μg/day DFE is generally accepted as adequate to replace 130 μg/day secreted in milk. As shown in Table 4-8, all of the listed authoritative bodies assumed that 120–130 μg DFE are secreted in milk daily. Concentrations of folate in milk are relatively unaffected by maternal folate status or intake so the value of 80–85 μg/L is likely applicable worldwide. However, care must be taken to release the folate from its conjugates in human milk.
Derivation of Safe Upper Levels of Intake
The UL set by the IOM at 1 mg/day, using an uncertainty factor of 5, has been adopted by other authoritative bodies. The UL for young children is adjusted downward to 300–400 μg/day of folic acid. There is no evidence that high intakes of folate from food have adverse effects; rather, these folate ULs are based on intake of folic acid from supplements and fortified foods. The main concern about high levels of folic acid intake centers on its possible exacerbation of the adverse effects of vitamin B12 deficiency, such as neurological impairment (Butterworth and Tamura, 1989). There is also evidence from vitamin B12 deficient patients that doses of folic acid greater than 5 mg/day can cause progression of neurological disorders (Dickinson, 1995).
FINDINGS AND CONCLUSIONS FOR FOLATE
Folate values in foods can vary depending on the local context. From its review of the evidence, the committee derived the following findings regarding local and contextual factors that can affect estimations of folate
levels for intake and thus the derivation of NRVs from locally available data sources.
- Measurement of food folate levels for use in the derivation of NRVs may require adjustment, and the validity of folate values available in food composition tables is of concern.
- Some food preparation procedures can cause substantial losses of folate, which is relatively unstable to heat and oxidation. From 50 to 80 percent of the folate in green vegetables, and 50 percent of the folate in legumes, is lost during boiling. When estimating the prevalence of inadequate intakes, folate values based on local recipes should be used whenever possible.
- As with food preparation, while folic acid fortification of staple and other foods does not affect the physiological requirement for folate, it will affect the amount of folate required from food sources.
- It is unlikely that local adjustments will need to be made for requirements that depend on breast milk folate concentration. Folate requirements are not affected by maternal status or intake, or bioavailability from different foods (except for the higher bioavailability of folic acid in fortified foods). Thus, adjustments are probably not necessary.
- The prevalence of the MTHFR TT genotype varies substantially across populations. In Caucasians, this genotype occurs in from 2 to 16 percent of the population, compared to 25 percent of Hispanics and a very low percentage of Africans. There appears to be large variability in prevalence across Asian populations. Compared to CC or CT genotypes, homozygosity for the T allele results in lower plasma and erythrocyte folate levels and higher plasma homocysteine levels in individuals with plasma folate levels below about 15 nmol/L.
Based on its findings, the committee came to the following conclusions about folate:
In the 1980s and 1990s, it was recognized that folate content in foods can be substantially underestimated unless the trienzyme laboratory procedure for releasing the vitamin from food was used. This likelihood of underestimation can explain discrepancies between intakes calculated from older food composition data versus more recent estimates based on measures of folate status.
While the cooking technique affects the amount of folate required from food sources, it does not affect physiological requirements.
Data on folate intake in food intake surveys must include the amounts of fortified food consumed and the levels of folic acid in that food. High folate status has emerged in a number of populations after initiating folic acid fortification programs. Although folic acid is generally regarded as not toxic, it may be a factor in neurological injury when pernicious anemia is present (Butterworth and Tamura, 1989). Thus, avoiding excessive intakes and monitoring folate status may be especially important in populations with a high prevalence of vitamin B12 deficiency since there is some evidence that a high folic acid intake may exacerbate B12 deficiency.
Because deriving an RI to cover 97.5 percent of the population depends on the variation around the AR and because populations with a higher variance will have a higher RI, a high prevalence in a population may justify a higher coefficient of variation for setting an RI, as was done in the D-A-CH recommendations.
Folate is synthesized by malarial parasites, so to avoid spuriously high values, assessment of RBC folate should not be conducted immediately after an episode of malaria with fever.
There is no evidence that deficiencies or high intakes of other micronutrients affect folate requirements.
PROPOSED SOLUTIONS FOR FOLATE
Folate recommendations for women and children are remarkably similar across countries, owing to general agreement on biomarker cut points and the relatively few factors that can affect requirements. There are no major deterrents to using existing NRVs published by authoritative bodies or modifying them for local factors.
However, the committee identified a number of data gaps for deriving new country-specific folate NRVs.
- There is a lack of validated data on the folate content of foods in low- and middle-income countries, especially cooked foods.
- The evidence for population prevalence of polymorphisms and their effect on folate requirements is increasing; however, it is not yet sufficient to use in deriving NRVs.
- The local contribution of other micronutrient deficiencies (vitamin B12, riboflavin, vitamin B6) to plasma homocysteine levels is not well understood.
- There is a need to consider local consequences of poor B12 status on NTD prevalence and its interaction with high folate intake.
OVERALL CONCLUSION
The committee came to the following overall conclusion:
From its assessment of the three nutrient case analyses described in this chapter, combined with its assessment of the process for deriving NRVs and the application of new tools to this process (discussed in Chapters 2 and 3 and throughout this chapter), the committee concludes that it is feasible to harmonize the process for deriving reference values globally.
The framework for harmonization being proposed in this report is based on four key steps (see Figure 3-4):
- Choose the appropriate tools.
- Collect the needed data.
- Identify the best approach.
- Derive the key reference values, the AR and the UL.
This framework is based on six core values:
- NRVs are regularly updated.
- The process is clear and transparent.
- The methods are rigorous and relevant.
- Factors influencing the NRV are documented.
- The strength of the evidence is determined.
- The review is complete and efficient.
APPLICATIONS OF NUTRIENT REFERENCE VALUES IN LOW- AND MIDDLE-INCOME COUNTRIES
Applications of NRVs for populations living in low- and middle-income countries include formulation of food and nutrition policies; the development of targeted intervention programs, such as food assistance or fortification programs; nutrition education; and the evaluation or monitoring of population health (NASEM, 2018). An important use of NRVs across applications is planning and assessing diets for both individuals and groups. Users of NRVs include international organizations such as WHO or FAO; nonprofit organizations; local, regional, and national governments; academic researchers; health care providers; the food industry; and the general
public. It is important that these potential users understand their derivation, particularly the AR and UL, as well as their application to public health. In the case of zinc, iron, and folate, all nutrients of particular concern for young children and women of reproductive age, bioavailability is one of a number of factors related to local context that needs to be considered when prioritizing nutrients for developing country-specific NRVs. For example, as discussed throughout this report, typical foods consumed in low- and middle-income countries may be high in phytate, which has been shown to reduce nutrient absorption. Additionally, the prevalence of micronutrient deficiencies as well as risk of chronic disease will be strong determinants for establishing country-specific NRVs.
Options and strategies to facilitate harmonization of the approaches used to derive NRVs as well as data gaps that need to be filled are discussed in the following chapter.
REFERENCES
Abbaspour, N., R. Hurrell, and R. Kelishadi. 2014. Review on iron and its importance to public health. Journal of Research in Medical Sciences 19(2):164-174.
Allen, L. H. 2008. Causes of vitamin B12 and folate deficiency. Food and Nutrition Bulletin 29:S20-S34.
Ariff, S., N. Krebs, S. Soofi, J. Westcott, A. Bhatti, F. Tabassum, and Z. A. Bhutta. 2014. Absorbed zinc and exchangeable zinc pool size are greater in Pakistani infants receiving traditional complementary foods with zinc-fortified micronutrient powder. Journal of Nutrition 144(1):20-26.
Aung-Than-Batu, Thein-Than, and Thane-Toe. 1976. Iron absorption from Southeast Asian rice-based meals. American Journal of Clinical Nutrition 29(2):219-225.
Bailey, L. B., and D. B. Hausman. 2018. Folate status in women of reproductive age as basis of neural tube defect risk assessment. Annals of the New York Academy of Sciences 1414(2018):82-95.
Barrett, J. F., P. G. Whittaker, J. G. Williams, and T. Lind. 1994. Absorption of non-haem iron from food during normal pregnancy. British Medical Journal 309:79-82.
Bernhardt, M. L., B. Y. Kong, A. M. Kim, T. V. O’Halloran, and T. K. Woodruff. 2012. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biology and Reproduction 86(4):114.
Bresnahan, K. A., and S. A. Tanumihardjo. 2014. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Advances in Nutrition 5(6):702-711.
Brnic, M., R. Wegmuller, C. Zeder, G. Senti, and R. F. Hurell. 2014. Influence of phytase, EDTA, and polyphenols on zinc absorption in adults from porridges fortified with zinc sulfate or zinc oxide. Journal of Nutrition 144(9):1467-1473.
Brown, K. H., J. A. Rivera, Z. Bhutta, R. S. Gibson, J. C. King, B. Lonnerdal, M. T. Ruel, B. Sandtrom, E. Wasantwisut, C. Hotz, D. Lopez de Romana, and J. M. Peerson. 2004. International Zinc Nutrition Consultative Group (IZiNCG) technical document 1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin 25(1 Suppl. 2):S99-S203.
Brown, K. H., S. K. Baker, and IZiNCG Steering Committee. 2009a. Galvanizing action: Conclusions and next steps for mainstreaming zinc interventions in public health programs. Food and Nutrition Bulletin 30(1):S179-S184.
Brown, K. H., J. M. Peerson, S. K. Baker, and S. Y. Hess. 2009b. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food and Nutrition Bulletin 30(1):S12-S40.
Butterworth, C. E., and T. Tamura. 1989. Folic acid safety and toxicity: A brief review. American Journal of Clinical Nutrition 50(2):353-358.
Chomba, E., C. M. Westcott, J. E. Westcott, E. M. Mpabalwani, N. F. Krebs, Z. W. Patinkin, and K. M. Hambidge. 2015. Zinc absorption from biofortified maize meets the requirements of young rural Zambian children. Journal of Nutrition 145(3):514-519.
Chung, C. S., D. A. Nagey, C. Veillon, K. Y. Patterson, R. T. Jackson, and P. B. Moser-Veillon. 2002. A single 60-mg iron dose decreases zinc absorption in lactating women. Journal of Nutrition 132(7):1903-1905.
Dainty, J. R., R. Berry, S. R. Lynch, L. J. Harvey, and S. J. Fairweather-Tait. 2014. Estimation of dietary iron bioavailability from food iron intake and iron status. PLoS ONE 9:e111824.
de Benoist, B., I. Darnton-Hill, L. Davidsson, O. Fontain, and C. Hotz. 2007. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutrition Bulletin 28(Suppl. 3):S480-S484.
Department of Health. 1991. Dietary reference values for food energy and nutrients. Report of the panel on dietary reference values of the committee on medical aspects of food policy. Report on health and social subjects 41. London HMSO.
Dickinson, C. J. 1995. Does folic acid harm people with vitamin B12 deficiency? QJM 88(5): 357-364.
Domellof, M., I. Thorsdottir, and K. Thorstensen. 2013. Health effects of different dietary iron intakes: A systematic literature review for the 5th Nordic nutrition recommendations. Food and Nutrition Research 57(1). https://doi.org/10.3402/fnr.v57i0.21667.
Drakesmith, H., and A. M. Prentice. 2012. Hepcidin and the iron-infection axis. Science 338(6108):768-772.
EFSA NDA Panel (European Food Safety Authority Panel on Dietetic Products, Nutrition, and Allergies). 2004. Scientific opinion on dietary reference values for iron. EFSA Journal 80(1-22).
EFSA NDA Panel. 2014a. Scientific opinion on dietary reference values for zinc. EFSA Journal 12(10):3844 (revised May 20, 2015).
EFSA NDA Panel. 2014b. Scientific opinion on dietary reference values for folate. EFSA Journal 12(11):3893. https://doi.org.10.2903/j.efsa.2014.
EFSA NDA Panel. 2015. Scientific opinion on dietary reference values for iron. EFSA Journal 13(10):4254. https://doi.org.10.2903/j.efsa.2015.4254.
Esamai, F., E. Liechty, J. Ikemeri, J. Westcott, J. Kemp, D. Culbertson, L. Miller, M. Hambidge, and N. Krebs. 2014. Zinc absorption from micronutrient powder is low but is not affected by iron in Kenyan infants. Nutrients 6:5636-5651.
Fairweather-Tait, S. J., A. Jennings, L. J. Harvey, R. Berry, J. Walton, and J. R. Dainty. 2017. Modeling tool for calculating dietary iron bioavailability in iron-sufficient adults. American Journal of Clinical Nutrition 105(6):1408-1414.
Food Fortification Initiative. 2017. NTD summary.http://www.ffinetwork.org/why_fortify/documents/NTD_Summary_July_2017.pdf (accessed March 2, 2018).
Frykman, E., M. Bystrom, U. Jansson, A. Edberg, and T. Hansen. 1994. Side effects of iron supplements in blood donors: Superior tolerance of heme iron. Journal of Laboratory and Clinical Medicine 123:561-564.
Gibson, R. S. 2012. A historical review of progress in the assessment of dietary zinc intake as an indicator of population zinc status. Advances in Nutrition 3(6):772-782.
Gibson, R., and V. P. Anderson. 2009. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food and Nutrition Bulletin 30(1):S108-S143.
Gibson, R. S., S. Y. Hess, C. Hotz, and K. H. Brown. 2008. Indicators of zinc status at the population level: A review of the evidence. British Journal of Nutrition 99(S3):S14-S23.
Gibson, R. S., J. C. King, and N. Lowe. 2016. A review of dietary zinc recommendations. Food and Nutrition Bulletin 37(4):443-460.
Gogia, S., and H. S. Sachdev. 2012. Zinc supplementation for mental and motor development in children. Cochrane Database Systematic Reviews 12:CD007991.
Green, R., R. Charlton, H. Seftel, T. Bothwell, F. Mayet, B. Adams, C. Finch, and M. Layrisse. 1968. Body iron excretion in man: A collaborative study. American Journal of Medicine 45(3):336-353.
Hallberg, L., and L. Rossander-Hultén. 1991. Iron requirements in menstruating women. American Journal of Clinical Nutrition 54(6):1047-1058.
Hallberg, L., A. M. Hogdahl, L. Nilsson, and G. Rybo. 1966a. Menstrual blood loss—A population study. Variation at different ages and attempts to define normality. Acta Obstetricia et Gynecologica Scandinavia 45(3):320-351.
Hallberg, L., A. M. Hogdahl, L. Nilsson, and G. Rybo. 1966b. Menstrual blood loss and iron deficiency. Acta Medica Scandinavia 180(5):639-650.
Harvey, L. J., C. N. Armah, J. R. Dainty, R. J. Foxall, D. John Lewis, N. J. Langford, and S. J. Fairweather-Tait. 2005. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. British Journal of Nutrition 94(4):557-564.
Harvey, L. J., C. Berti, A. Casgrain, I. Cetin, R. Collings, M. Gurinovic, M. Hermoso, L. Hooper, R. Hurst, B. Koletzko, J. Ngo, B. R. Vinas, C. Volhardt, V. Vucic, and S. J. Fairweather-Tait. 2013. EURRECA-estimating iron requirements for deriving dietary reference values. Critical Reviews in Food Science and Nutrition 53(10):1064-1076.
Herbert, V., N. Cunneen, L. Jaskiel, and C. Kapff. 1962. Minimal daily adult folate requirement. Archives of Internal Medicine 110:649-652.
Hess, S., B. Lonnerdal, C. Hotz, J. A. Rivera, and K. H. Brown. 2009. Recent advances in knowledge of zinc nutrition and human health. Food and Nutrition Bulletin 31(1):S5-S11.
Hotz, C., and K. H. Brown. 2001. Identifying populations at risk of zinc deficiency: The use of supplementation trials. Nutrition Reviews 59(3):80-88.
Hotz, C., and K. H. Brown. 2004. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin 25(1):S94-S203.
Hotz, C., J. DeHaene, L. R. Woodhouse, S. Villalpando, J. A. Rivera, and J. C. King. 2005. Zinc absorption from zinc oxide, zinc sulfate, zinc oxide + EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas. Journal of Nutrition 135(5):1102-1105.
Hunt, J. R., and J. M. Beiseigel. 2009. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. American Journal of Clinical Nutrition 89(3):839-843.
Hunt, J. R., C. A. Zito, and L. K. Johnson. 2009. Body iron excretion by healthy men and women. American Journal of Clinical Nutrition 89(6):1792-1798.
Hurrell, R., and I. Egli. 2010. Iron bioavailability and dietary reference values. American Journal of Clinical Nutrition 91(5):1461S-1467S.
Hytten, F. E., and I. Leitch. 1971. The physiology of human pregnancy, 2nd ed. Oxford, UK: Blackwell Scientific Publications.
International Zinc Nutrition Consultative Group (IZiNCG), K. H. Brown, J. A. Rivers, Z. Bhutta, R. S. Gibson, J. C. King, B. Lönnerdal, M. T. Ruel, B. Sandström, E. Wasantwisut, and C. Hotz. 2004. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin 25(1 Suppl 2):S99-S203.
IOM (Institute of Medicine). 1998. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press. https://doi.org/10.17226/6015.
IOM. 2001. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press. https://doi.org/10.17226/10026.
Kawade, R. 2012. Zinc status and its association with the health of adolescents: A review of studies in India. Global Health Action 5(1):7353.
King, J. C., and C. Garza. 2007. Harmonization of nutrient intake values. Food and Nutrition Bulletin 28(Suppl. 1):S3-S12.
King, J. C., R. J. Cousins, M. E. Shils, M. Shike, A. C. Ross, and B. Caballero. 2014. Zinc. In Modern nutrition in health and disease. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins, Pp. 189-205.
King, J. C., K. H. Brown, R. S. Gibson, N. F. Krebs, N. M. Lowe, J. H. Siekmann, and D. J. Raiten. 2016. Biomarkers of nutrition for development (BOND)-zinc review. Journal of Nutrition 146(4):858S-885S.
Krawinkel, M. B., D. Strohm, A. Weissenborn, B. Watzl, M. Eichholzer, K. Bärlocher, I. Elmadfa, E. Leschik-Bonnet, and H. Heseker. 2014. Revised D-A-CH intake recommendations for folate: How much is needed? European Journal of Clinical Nutrition 68:719-723.
Lazzerini, M., and L. Ronfani. 2008. Oral zinc for treating diarrhoea in children. Cochrane Database of Systematic Reviews 3:CD005436.
Levenson, C. W., and D. Morris. 2011. Zinc and neurogenesis: Making new neurons from development to adulthood. Advances in Nutrition 2(2):96-100.
Lonnerdal, B. 2000. Dietary factors influencing zinc absorption. Journal of Nutrition 130(5): 1378S-1383S.
Lowe, N. M., K. Fekete, and T. Decsi. 2009. Methods of assessment of zinc status in humans: A systematic review. American Journal of Clinical Nutrition 89(6):2040S-2051S.
Lowe, N. M., F. C. Dykes, A. L. Skinner, S. Patel, M. Warthon-Medina, T. Decsi, K. Fekete, O. W. Souverein, C. Dullemeijer, A. E. Cavelaars, L. Serra-Majem, M. Nissensohn, S. Bel, L. A. Moreno, M. Hermoso, C. Vollhardt, C. Berti, I. Cetin, M. Gurinovic, R. Novakovic, L. J. Harvey, R. Collings, S. Hall, and V. Moran. 2013. EURRECA—Estimating zinc requirements for deriving dietary reference values. Critical Reviews in Food Science and Nutrition 53(10):1110-1123.
Lynch, S. 2011. Case studies: Iron. American Journal of Clinical Nutrition 94(2):6735-6785.
Miller, L. V., K. Hambidge, and N. F. Krebs. 2015. Zinc absorption is not related to dietary phytate intake in infants and young children based on modeling combined data from multiple studies. Journal of Nutrition 145(8):1763-1769.
Milman, N. 2006. Iron and pregnancy—a delicate balance. Annals of Hematology 85:559-565.
Milne, D. B., W. K. Canfield, J. R. Mahalko, and H. H. Sandstead. 1983. Effect of dietary zinc on whole body surface loss of zinc: Impact on estimation of zinc retention by balance method. American Journal of Clinical Nutrition 38(2):181-186.
Narasinga Rao, B. S., C. Vijayasarathy, and T. Prabhavathi. 1983. Iron absorption from habitual diets of Indians studied by the extrinsic tag technique. Indian Journal of Medical Research 77:648-657.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2018. Global harmonization of methodological approaches to nutrient intake recommendations: Proceedings of a workshop. Washington, DC: The National Academies Press. https://doi.org/10.17226/25023.
National Health and Medical Research Council, Australian Government Department of Health and Aging, New Zealand Ministry of Health. 2006. Nutrient reference values for Australia and New Zealand. Canberra, Australia: National Health and Medical Research Council.
Nemeth, E., and T. Ganz. 2009. The role of hepcidin in iron metabolism. Acta Haematologica 122:78-86.
NNR (Nordic Nutrition Recommendations). 2004. Nordic Nutrition Recommendations, 4th ed. Nord 2004:13.
Nordic Council of Ministers. 2012. Nordic Nutrition Recommendations 2012: Integrating nutrition and physical activity, 5th ed. Copenhagen, Sweden: Nordic Council of Ministers. https://doi.org/10.6027/Nord2014-002 (accessed June 3, 2018).
NRC (National Research Council). 1989. Recommended dietary allowances, 10th ed. Washington, DC: National Academy Press. https://doi.org/10.17226/1349.
O’Brien, K. O., N. Zavaleta, L. E. Caulfield, J. Wen, and S. A. Abrams. 2000. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. Journal of Nutrition 130(9):2251-2255.
O’Keefe, C. A., L. B. Bailey, E. A. Thomas, S. A. Hofler, B. A. Davis, J. J. Cerda, and J. F. Gregory. 1995. Controlled dietary folate affects folate status in nonpregnant women. Journal of Nutrition 125:2717-2725.
Pae, M., S. N. Meydani, and D. Wu. 2012. The role of nutrition in enhancing immunity in aging. Aging Diseases 3(1):91-129.
Pfeiffer, C. M., L. M. Rogers, and J. F. Gregory. 1997. Determination of folate in cereal-grain food products using trienzyme extraction and combined affinity and reversed-phase liquid chromatography. Journal of Agriculture and Food Chemistry 45:407-413.
Pinna, K., L. R. Woodhouse, B. Sutherland, D. M. Shames, and J. C. King. 2001. Exchangeable zinc pool masses and turnover are maintained in healthy men on low zinc intakes. Journal of Nutrition 131:2288-2294.
Rahman, S., T. Ahmed, A. S. Rahman, N. Alam, A. S. Ahmed, S. Ireen, I. A. Chowdhury, P. S. Chowdhury, and S. M. Rahman. 2016. Determinants of iron status and Hb in the Bangladesh population: The role of groundwater iron. Public Health Nutrition 19(10):1862-1874.
Roohani, N., R. Hurrell, R. Kelishadi, and R. Shulin. 2013. Zinc and its importance for human health: An integrative review. Journal of Research in Medical Science 18(2):144-157.
Rosado, J. L., K. M. Hambidge, L. V. Miller, O. P. Garcia, J. Westcott, K. Gonzalez, J. Conde, C. Hotz, W. Pfeiffer, I. Ortiz-Monasterio, and N. F. Krebs. 2009. The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. Journal of Nutrition 139(10):1920-1925.
Sauberlich, H. E., M. J. Kretsch, J. H. Skala, H. L. Johnson, and P. C. Taylor. 1987. Folate requirement and metabolism in nonpregnant women. American Journal of Clinical Nutrition 46:1016-1028.
Shah, D., H. S. Sachdev, T. Gera, L. M. De-Regil, and J. P. Pena-Rosas. 2016. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population. Cochrane Database Systematic Reviews 6:CD010697.
Stamm, R. A., and L. A. Houghton. 2013. Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients 5(10):3920-3947. https://doi.org/10.3390/nu5103920.
Stover, P. J. 2007. Human nutrition and genetic variation. Food and Nutrition Bulletin 28(Suppl. 1):S101-S115.
Tamura, T., Y. Mizuno, K. E. Johnston, and R. A. Jacob. 1997. Food folate assay with protease, α-amylase, and folate conjugase treatments. Journal of Agriculture and Food Chemistry 45:135-139.
Voedingsraad, Nederlandseroedingsnorman. 1989, 1992. Voorlichtingsbureau voor de Voeding: Den Haag. Pp. 1-293.
Wapnir, R. A. 2000. Zinc deficiency, malnutrition and the gastrointestinal tract. Journal of Nutrition 130(Suppl. 5):1388S-1392S.
Wapnir, R. A., and L. Stiel. 1986. Zinc intestinal absorption in rats: Specificity of amino acids as ligands. Journal of Nutrition 116(11):2171-2179.
Wazny, K., A. Zipursky, R. Black, S. Curtis, C. Duggan, R. Guerrant, M. Levine, W. A. Petri, Jr., M. Santosham, R. Scharf, P. M. Sherman, E. Simpson, M. Young, and Z. A. Bhutta. 2013. Setting research priorities to reduce mortality and morbidity of childhood diarrhoeal disease in the next 15 years. PLoS Medicine 10(5):e1001446.
Wessels, K. R., G. M Singh., and K. H. Brown. 2012. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 7(11):e50568.
Whittaker, P., T. Lind, and J. Williams. 1991. Iron absorption during normal human pregnancy: A study using stable isotopes. British Journal of Nutrition 65(3):457-463.
WHO/FAO (World Health Organization and Food and Agriculture Organization). 2004. Vitamin and mineral requirements in human nutrition. Geneva, Switzerland: World Health Organization.
WHO/UNICEF (United Nation’s Children Fund). 2004. Clinical management of acute diarrhea. Geneva, Switzerland: World Health Organization/United Nation’s Children Fund.
















































