4
Disease-Induced Deficiency and Conditionally Essential Nutrients in Disease
Session 4 was moderated by Bernadette Marriott, Professor in the Departments of Medicine and Psychiatry at the Medical University of South Carolina and a Planning Committee Member. In the first presentation, Claudia R. Morris, Associate Professor of Pediatrics and Emergency Medicine at the Emory University School of Medicine, discussed arginine as a conditionally essential nutrient, using sickle cell anemia and surgery as case examples. The second presenter was Angus Scrimgeour, a nutritional biochemist in the Military Nutrition Division at the U.S. Army Research Institute of Environmental Medicine. His presentation focused on pathophysiological mechanisms and potential nutrient needs in traumatic brain injury (TBI). In the third presentation, Jesse Gregory, Professor of Food Science and Human Nutrition at the University of Florida discussed the impact on nutrient requirements of metabolic turnover, inflammation, and redistribution. The session closed with a moderated panel discussion and question and answer session with workshop participants.
ARGININE AS AN EXAMPLE OF A CONDITIONALLY ESSENTIAL NUTRIENT: SICKLE CELL ANEMIA AND SURGERY1
Morris began her remarks by defining conditionally essential amino acids as amino acids that become indispensable under stress and critical illness when the capacity of endogenous synthesis is surpassed. Arginine
___________________
1 This section summarizes information presented by Claudia Morris.
is one such amino acid, and Morris said that she would discuss sickle cell disease as an example of a condition that is associated with an acquired arginine deficiency.
What Is Arginine?
Morris explained that arginine is synthesized through the intestinal–renal axis and becomes essential under conditions of stress. It is found naturally in the diet; meat, dairy, seafood, nuts, and even watermelon are good sources. Arginine is a nutritional supplement with low toxicity and is the obligate substrate for nitric oxide production, a potent vasodilator that is important in regulating blood pressure. Arginine is converted to nitric oxide through the actions of enzymes, the nitric oxide synthases (NOSs). In addition to regulating vasodilatory tone, arginine also has an effect on platelet aggregation and in the immune response. It is a signaling molecule and can have some antioxidant effects. Arginine is also the substrate for arginase, an important enzyme in the urea cycle. This enzyme has two mammalian isoforms, arginase 1, which is cytosolic, and arginase 2, which is mitochondrial. Arginase is present in most cell types, including the red blood cell. Arginase is also upregulated in inflammation induced by cytokines, and ultimately competes with NOS for its common substrate of arginine. In the presence of arginase, arginine is converted to ornithine and urea. Ornithine and arginine use the same amino acid transporter, CAT-1 and CAT-2, so when ornithine levels rise, cellular uptake of arginine is inhibited, depending on how high the ornithine levels are. This could ultimately translate to decreased arginine and nitric oxide bioavailability. This means, said Morris, that arginine bioavailability cannot be derived by looking only at plasma arginine because other factors come into play, such as the presence of other amino acids that compete for intracellular transport.
Morris then showed a slide summarizing the arginine metabolome (see Figure 4-1). In many conditions of inflammation NOS is upregulated, yet paradoxically, nitric oxide production is decreased. It is known from the sickle cell mouse model that NOS, although it is upregulated, does not function properly and it can become uncoupled, where it produces superoxide in lieu of nitric oxide. This results in a dysfunctional arginine-to-nitric oxide pathway. This leads to substrate competition from arginase, which converts arginine to ornithine and pushes down the proliferative pathway to create prolines and polyamines. This is a pathway toward vascular smooth muscle proliferation, airway remodeling, and lung fibrosis. These structural changes are seen in the lungs of patients who develop pulmonary hypertension, a common comorbidity in sickle cell disease.
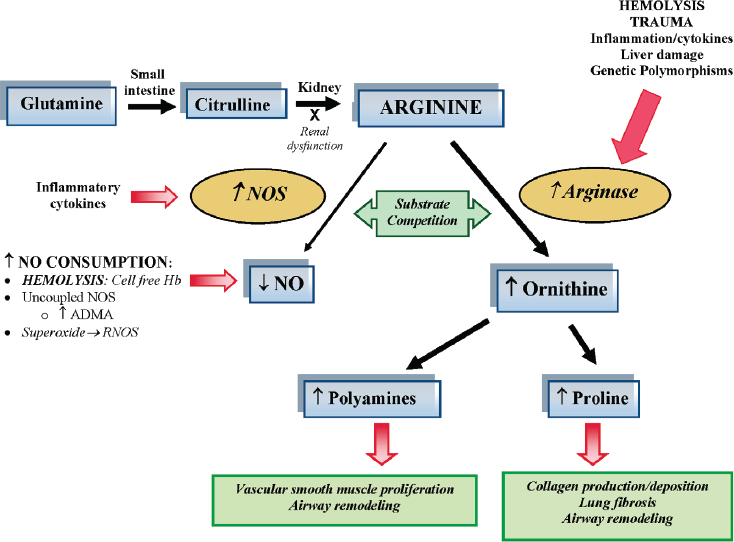
NOTES: Dietary glutamine serves as a precursor for the de novo production of arginine through the citrulline–arginine pathway. Arginine is synthesized endogenously from citrulline primarily via the intestinal–renal axis. Arginase and nitric oxide synthase (NOS) compete for arginine, their common substrate. In sickle cell disease and trauma, bioavailability of arginine and nitric oxide (NO) is decreased by several mechanisms linked to hemolysis, oxidative stress, and/or inflammation. Endothelial dysfunction resulting from NO depletion and increased levels of the downstream products of ornithine metabolism (polyamines and proline) likely contribute to the pathogenesis of lung injury, fibrosis, and pulmonary hypertension commonly seen in sickle cell disease. This disease paradigm has implications for all hemolytic processes. Red arrows denote the influence of hemolysis and inflammation on the arginine–NO pathway and the downstream consequences in the lungs. ADMA = asymmetric dimethylarginine; Hb = hemoglobin; NO = nitric oxide; NOS = nitric oxide synthase; RNOS = reactive nitrogen oxide species.
SOURCES: As presented by Claudia Morris, April 2, 2018; Morris, 2008. Republished with permission of the American Society of Hematology. From Mechanisms of vasculopathy in sickle cell disease and thalassemia, Morris, C. R., 2008: 177–185. Permission conveyed through Copyright Clearance Center, Inc.
Morris noted that glutamine just received approval from the U.S. Food and Drug Administration (FDA) in the treatment of sickle cell disease due to its ability to decrease frequency of pain and acute chest syndrome. Thinking about arginine bioavailability, Morris noted that her group has coined the term “global arginine bioavailability ratio,” a biomarker that correlates to important clinical outcomes and reflects arginine over the ratio of ornithine plus citrulline. This construct takes into account the impact of arginase, the impact on elevated ornithine levels and intracellular transport, and the effects of renal dysfunction as well as plasma arginine levels.
Sickle Cell Disease
Sickle cell disease is an autosomal recessive disease of the red blood cells that results in hemoglobin polymerization leading to a cascade of events that eventually cause decreased blood flow, tissue hypoxemia, and acute and chronic end organ damage in every organ system.
Eight percent of African Americans carry the sickle trait, explained Morris, and 1 out of 400 African Americans in the United States, or approximately 100,000 U.S. individuals, have sickle cell disease. Although it is considered an orphan disease in the United States because it affects fewer than 200,000 individuals, it affects millions of individuals worldwide.
Morris stated that a number of mechanisms of vasculopathy operate in sickle cell disease, with the depletion of nitric oxide taking center stage (see Figure 4-2).
Normally safely encapsulated within the red blood cell, the contents of the red blood cell break apart and are freed into circulation when hemolysis occurs (lactate dehydrogenase, found within the red blood cell freed into plasma during hemolysis, reflects the hemolytic rate and is a good biomarker of the hemolytic sub-phenotype of sickle cell disease). When the hemoglobin is freed into circulation, it rapidly consumes nitric oxide. The red cell also contains arginase, which when released during hemolysis rapidly consumes the obligate substrate for nitric oxide production. As the arginine level drops, the body reaches a threshold level below which NOS uncouples and produces superoxide in lieu of nitric oxide, further consuming nitric oxide and adding to the milieu of oxidative stress. Data from a large cohort of patients with sickle cell disease show the results from this cascade of events (Morris et al., 2005a). The clinical manifestations of hemolysis and decreased arginine-nitric oxide bioavailability include increased risk of stroke, renal insufficiency, priapism, leg ulcers, pulmonary hypertension, and possibly asthma.
Morris and her group investigated whether increasing arginase levels would ease the pulmonary hypertension. After 5 days of oral supple-
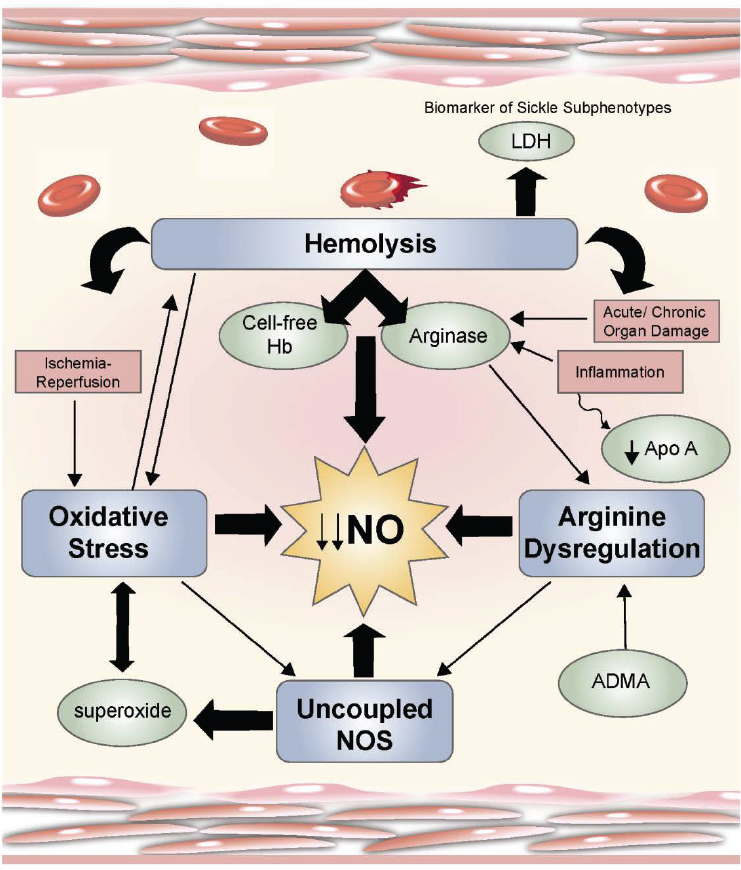
NOTE: ADMA = asymmetric dimethylarginine; Apo A = apolipoprotein A-1; Hb = hemoglobin; LDH = lactate dehydrogenase; NO = nitric oxide; NOS = nitric oxide synthase.
SOURCES: As presented by Claudia Morris, April 2, 2018; Morris, 2008. Republished with permission of the American Society of Hematology. From Mechanisms of vasculopathy in sickle cell disease and thalassemia, Morris, C. R., 2008: 177–185. Permission conveyed through Copyright Clearance Center, Inc.
mentation with arginine, significant reduction in estimated pulmonary systolic pressures were seen, similar to the ones seen with medications. They identified a similar pattern of dysregulated arginine metabolism in patients with thalassemia, another hemolytic anemia associated with a high prevalence of pulmonary hypertension, but only in the group of patients at risk of pulmonary hypertension (Morris et al., 2005b). These data suggest a role for arginine bioavailability in the development of hemolysis-associated pulmonary hypertension. Surprisingly, low arginine bioavailability was associated with worse survival, and this was reflected by the arginine-to-ornithine ratio and also the global arginine bioavailability ratio. No deaths occurred in the patients who had the highest arginine bioavailability after 5 years of follow-up (Morris et al., 2005a).
Impact of a Disease State on Nutrient Metabolism and Nutritional Status
Arginine deficiency syndromes share similarities regardless of whether they mechanistically result from trauma, hepatic disease, an immune disorder, hemolyzed red cells during hemolysis or transfusion, or clinical consequences of excess extracellular arginase. In Morris’s thinking, these syndromes fall into two baskets: endothelial dysfunction, which occurs in sickle cell disease as a model, and T-cell dysfunction, which is seen in trauma.
Arginine is essential for naïve T-cell activation. In trauma, T-cell proliferation is blunted, interferon-gamma and interleukin-2 production is inhibited, and T-lymphocyte-mediated cytotoxicity and memory response are nearly completely abolished when arginine is depleted, all of which increases the risk of infection. Providing arginine to the culture media restores T-cell function.
This has important implications for the 10 percent of trauma patients who develop wound infections, which are the leading cause of late organ failure. Enhanced wound healing has been seen with arginine-fortified formulas given after trauma and hemorrhagic shock. The greatest benefits of arginine supplementation appear to be in surgical patients. However, Morris noted that careful patient selection is critical because some evidence also suggests harm in sepsis and following acute myocardial infarction.
Morris concluded her talk by pointing out the paucity of evidence in children with sickle cell, particularly in terms of benefits associated with adequate protein intake. She also noted that it is important to remember that nutritional deficiencies rarely occur in isolation. Patients with sickle cell disease, for example, have a multitude of nutritional deficiencies that all must be addressed.
TRAUMATIC BRAIN INJURY: PATHOPHYSIOLOGICAL MECHANISMS AND POTENTIAL NUTRIENT NEEDS2
Both soldiers and civilians experience TBIs, but they are very different traumas. Military TBIs are mainly caused by blast traumatic injury, whereas civilians generally experience non-blast injuries caused by sports or motor vehicle accidents. About 87 percent of civilian TBI injuries, Scrimgeour said, are treated in emergency departments, and as many as 52,000 will die annually from a penetrating or severe TBI. Department of Defense (DoD) data from 2000 to 2017 indicate that nearly 380,000 service members were diagnosed with a TBI and about 82 percent of these injuries were classified as mild TBI. In the future, modern imaging techniques might allow the discrimination between mild TBIs that need treatment and those that will resolve on their own within a year. Despite DoD spending more than $940 million on TBI research, no pharmacological agents have been approved for soldiers exposed to TBI.
Scrimgeour noted that the 2011 Institute of Medicine (IOM) report Nutrition and Traumatic Brain Injury provides the foundation for existing thinking about nutritional support to improve acute and subacute health outcomes in military personnel who have experienced TBI (IOM, 2011). He then discussed the following recommendations from this report that are of particular relevance to the workshop:
- Recommendation 6-1 concerns the provision of early nutrition support, particularly protein. Scrimgeour noted that soldiers who have experienced a TBI often get little or no nutritional support, or interrupted nutritional support, during the immediate period after the blast injury, during the initial care in the combat hospital, and during the medical evacuation to Germany and eventually to Walter Reed National Military Medical Center in Bethesda, Maryland.
- Recommendations 5-1 and 5-2 urge DoD to conduct dietary intake assessments in military settings and on TBI patients in medical treatment facilities. Scrimgeour acknowledged that these assessments can be done in the United States, but they are much more difficult to do overseas.
- Recommendations 6-2 and 6-3 pertain to human trials to determine appropriate levels of blood glucose following TBI and of the appropriateness of insulin therapy in treating hyperglycemia observed in soldiers exposed to TBI in inpatient settings using total parenteral nutrition. Scrimgeour stated that his group has measured blood glucose in animal models with blast injuries compared to con-
___________________
2 This section summarizes information presented by Angus Scrimgeour.
-
trols and found no change in blood glucose that would warrant intervention.
- Recommendation 9-1 concerns DoD monitoring the outcomes of a clinical trial on the effect of cytidine diphosphate (CDP)-choline on cognition and functional measures in severe, moderate, and complicated mild TBI. Scrimgeour stated that the 4-year trial was terminated early because of lack of results and that no further human trials have been conducted with CDP-choline.
- Recommendation 10-1 focused on studies to assess the use of creatine in TBI treatments, which has been shown to help sports athletes recover from muscle injury. Scrimgeour noted that no such human trials have been conducted and none are planned.
- Recommendation 13-1 states that DoD should conduct animal and human studies of omega-3 fatty acids. Scrimgeour noted that his lab is studying a docosahexaenoic acid–eicosapentaenoic acid (DHA-EPA) mix to determine whether it could help reduce neuroinflammation in brains exposed to TBI, and minimize cognitive deficits. In addition, he said several double-blind randomized controlled trials are currently looking at the effects of a mix of DHA or DHA and EPA supplementation on concussion, and these trials will have results in the coming years.
Scrimgeour then reviewed the following nutritional recommendations from the IOM report and provided an update of work in those areas:
- Zinc. Evidence indicates that in a mildly zinc-deficient state animals do not recover after exposure to blast. To date, no human studies have been done since the 2011 report was published, although preclinical studies have shown that after an initial period of total parenteral nutrition, zinc supplementation increases visceral protein mass in post-TBI patients (Cope et al., 2012).
- Curcumin. Despite the data from animal studies showing benefits of curcumin supplementation following neurotrauma (Sharma et al., 2009, 2010; Zhu et al., 2014), no human trials have been conducted.
- Resveratrol. Two animal studies have shown some benefits with the use of resveratrol in concussion treatment (Lin et al., 2014; Singleton et al., 2010), and a double-blind study at The University of Texas Southwest Medical Center has looked at 500 milligrams of resveratrol in boxers. The Texas study has ended but has not been published.
- Vitamin D. Vitamin D has shown some promise for TBI in combination with progesterone in preliminary studies (Aminmansour
et al., 2012; Tang et al., 2013, 2015). However, the large phase III ProTECT (Progesterone for the Treatment of TBI) and SyNAPse (Efficacy and Safety Study of Progesterone in Patients with Severe TBI) trials were suspended due to lack of efficacy.
To summarize, Scrimgeour stated that evidence-based nutrition guidelines are lacking. Adequate, validated biomarkers for TBI are also lacking, making it difficult to identify targets for studies. To exacerbate the situation, Scrimgeour added, an overlap exists between TBI symptoms and posttraumatic stress disorder symptoms. Additionally, polytrauma is common in soldiers, and so it is not uncommon for physicians to provide treatment for shrapnel wounds, lacerations, or dizziness, but not the TBI due to a lack of neurotherapeutics.
METABOLIC TURNOVER, INFLAMMATION, AND REDISTRIBUTION: IMPACT ON NUTRIENT REQUIREMENTS3
Because of experiments with human study participants and animal models, it was previously thought that much was known about the requirements for vitamin B6 in humans. However, Gregory stated, that has changed considerably in recent years.
In most developed countries vitamin B6 status is generally adequate. The primary biomarker used in screening studies to assess B6 status, pyridoxal phosphate (PLP), is involved in more than 160 enzymatic reactions in the body, including amino acid metabolism, gluconeogenesis, and many one-carbon metabolism reactions. The current Recommended Dietary Allowance (RDA) for B6 is based on a cutoff of 20 nanomoles per liter of circulating PLP4 (IOM, 1998). The most recent published National Health and Nutrition Examination Survey (NHANES) study that provided B6 data showed that only about 10 to 12 percent of the NHANES population of all of the different groups had less than the 20 nanomoles per liter (Pfeiffer et al., 2013).
Disease Relationships with Vitamin B6
Since the mid- to late 1990s, a number of published papers have shown a relationship between low plasma PLP and risk of vascular disease, which includes heart disease, risk of death from heart disease, stroke, and venous thrombosis (e.g., Rimm et al., 1998). Other studies also
___________________
3 This section summarizes information presented by Jesse Gregory.
4 See http://nationalacademies.org/HMD/Activities/Nutrition/SummaryDRIs/DRITables.aspx (accessed June 6, 2018).
show an association between low PLP and C-reactive protein (CRP), an inflammatory marker, and disease severity in inflammatory bowel disease (IBD) and rheumatoid arthritis, both inflammatory diseases (Sakakeeny et al., 2012). The authors observed that PLP was inversely associated with many biomarkers of inflammation, not just CRP.
Gregory also described a study in which the apparent requirement for vitamin B6 was increased due to inflammatory disease itself, as reflected by CRP (Morris et al., 2010). One explanation is that PLP can be redistributed in the body from the more kinetically mobile pools to sites of inflammation. It has been shown that approximately 70 percent of vitamin B6 is tightly bound in muscle glycogen phosphorylase. However, the other 20 or 30 percent in liver and other organs is kinetically mobile and can be relocated to sites of inflammation as needed for metabolic demands in the inflammatory response. This has been demonstrated in a rat study, which showed liver and plasma PLP levels decreased as a result of an adjuvant injection, presumably to support the inflammation (Chiang et al., 2005). A second explanation is that some acceleration of B6 catabolism may occur during inflammation, as suggested by studies showing that the ratio of pyridoxic acid in plasma to its active forms, PLP and pyridoxal, is increased. It appears then that PLP has limitations as a biomarker when inflammatory conditions exist. Other biomarkers, such as erythrocyte transaminase stimulation assay, erythrocyte PLP, and urinary pyridoxic acid also have limitations. The most effective metabolic marker is plasma cystathionine, an intermediate in the trans-sulfuration pathway that takes homocysteine to cysteine and then glutathione. The kynurenines, which are in the tryptophan pathway, have been known for many years to be altered in B6 deficiency, and the ratio of hydroxyl-kynurenine to xanthurenic acid is a good biomarker that is little influenced by inflammation. However, these alternative biomarkers require further validation.
In summary, PLP is a very good biomarker for healthy people, but its responsiveness to inflammation raises questions about its value in assessing populations and in developing an RDA for B6, particularly because inflammation can be prevalent in the general population. The ratios of tryptophan catabolites do seem like an encouraging approach to developing a biomarker that is less sensitive to inflammation, but validation in terms of cutoffs, adequacy, and deficiency is needed.
MODERATED PANEL DISCUSSION AND Q&A
In response to a question related to L-arginine and wound healing, Morris replied that in adults, decreased mortality and morbidity are seen in some cohorts of patients who were fed arginine-fortified formulas. This is particularly true for surgical patients, although, she added, colleagues
conducting these studies have found it difficult to convince surgeons to embrace this sort of nutritional intervention even when presented with rigorous evidence from clinical trials. Unfortunately, little data are available in children. Although it makes sense to think about therapeutics that involve arginase inhibitors, she said, given the critical roles of arginase in health, drugs that inhibit an enzyme that is so important must be pursued with great care.
Arginine and Glutamine
Morris replied to a question regarding FDA-approved glutamine. She noted that this is the first FDA-approved “drug” for children and only the second for adults with sickle cell disease but that its mechanism of action is still unknown. A number of pathways, including decreasing oxidative stress through NADPH (nicotinamide adenine dinucleotide phosphate), would make sense for sickle cell disease. Morris’s thinking is that the glutamine is working through arginine, because glutamine is an arginine pro-drug. Arginine deficiency in sickle cell disease is associated with pulmonary hypertension, leg ulcers, and acute vaso-occlusive pain. These complications improve with arginine supplementation in clinical trials. She added that she has some pharmacokinetics data showing that a single dose of oral glutamine results in a significant increase in arginine bioavailability in patients with sickle cell disease. That suggests, she said, that glutamine does convert to arginine in sickle cell disease, which will ultimately improve arginine bioavailability both in plasma and within the red blood cell.
Responding to a question about the safety of arginine, Morris agreed there is reason for clinicians to be concerned because of the sepsis studies with arginine, but she emphasized that the nature of the patient population is important. Arginine is safe for sickle cell patients, most of whom are not septic but are arginine deficient. Sickle cell patients metabolize arginine differently than do normal controls, and arginine metabolism varies even in the same patient at steady-state compared to situations of acute illness including vaso-occlusive pain episodes. Ultimately, patients with sickle cell disease have a variable clinical spectrum of severity making treatment more complicated; sickle cell patients cannot all be treated in the same way.
Arginine and Trauma Recovery
To a question related to the use of omega-3 and arginine combinations that can get past the blood–brain barrier (BBB) and provide neuroprotective effects, Scrimgeour replied that his group has developed effec-
tive delivery systems to get omega-3 (specifically, DHA and EPA) and vitamin D into rats, with confirmation that these nutrients do cross the BBB. He also highlighted the distinction between non-blast TBI, common in sports and motor vehicle accidents, where the injury is restricted to the brain, and blast TBI, where the whole body is exposed, and intestinal damage frequently occurs.
Many soldiers also experience burn injuries. The animal models for burns are not optimal, and none of the current work conducted by the Army in San Antonio, Texas, is examining nutritional interventions. Zinc is leached out in the burn exudate, but studies on zinc supplements in burned animals and burned soldiers have not yet been conducted.
Limitations of Animal Models
On the limitations of animal models used to study TBI in soldiers, Scrimgeour responded that, contrary to humans with a rough (gyrencephalic) surface, the rat brain has a smooth (lissencephalic) surface and a different cortical bone thickness. He noted that multiple concerns exist about translating rat findings into humans, particularly in drug studies. For example, he said a Walter Reed lab has had very good success in drug studies repairing cognitive deficits as well as biochemical repair in the rat model, but it has not been possible to translate any of these findings into humans. It was noted that the same is true for IBD, in that animal models of IBD have terrible prognostic markers of therapeutics in human biology.
Using Technology to Understand Brain Biochemistry
The discussion highlighted that current technologies to understand how the brain is metabolically responding to a TBI, such as computed tomography scans, are not very sophisticated and that newer types of technology, such as 7T magnets used for functional magnetic resonance imaging and spectral imaging of the brain, could be applied. Although, currently, examinations of biomarkers of TBI can be done only after death, brain imaging technology and today’s efforts in neuroscience may allow investigators to understand the human brain while people are still alive. In addition, there is the possibility of doing electroencephalography in the field for both athletes and soldiers.
REFERENCES
Aminmansour, B., H. Nikbakht, A. Ghorbani, M. Rezvani, P. Rahmani, M. Torkashvand, M. Nourian, and M. Moradi. 2012. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: A randomized clinical trial with placebo group. Advanced Biomedical Research 1:58.
Chiang, E. P., D. E. Smith, J. Selhub, G. Dallal, Y. C. Wang, and R. Roubenoff. 2005. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis Research & Therapy 7(6):R1254–R1262.
Cope, E. C., D. R. Morris, and C. W. Levenson. 2012. Improving treatments and outcomes: An emerging role for zinc in traumatic brain injury. Nutrition Reviews 70(7):410–413.
IOM (Institute of Medicine). 1998. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press.
IOM. 2011. Nutrition and traumatic brain injury: Improving acute and subacute health outcomes in military personnel. Washington, DC: The National Academies Press.
Lin, C. J., T. H. Chen, L. Y. Yang, and C. M. Shih. 2014. Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death & Disease 5:e1147.
Morris, C. R. 2008. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology American Society of Hematology Education Program 177–185.
Morris, C. R., G. J. Kato, M. Poljakovic, X. Wang, W. C. Blackwelder, V. Sachdev, S. L. Hazen, E. P. Vichinsky, S. M. Morris, Jr., and M. T. Gladwin. 2005a. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294(1):81–90.
Morris, C. R., F. A. Kuypers, G. J. Kato, L. Lavrisha, S. Larkin, T. Singer, and E. P. Vichinsky. 2005b. Hemolysis-associated pulmonary hypertension in thalassemia. Annals of the New York Academy of Sciences 1054:481–485.
Morris, M. S., L. Sakakeeny, P. F. Jacques, M. F. Picciano, and J. Selhub. 2010. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. The Journal of Nutrition 140(1):103–110.
Pfeiffer, C. M., M. R. Sternberg, R. L. Schleicher, B. M. Haynes, M. E. Rybak, and J. L. Pirkle. 2013. The CDC’s Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population is a valuable tool for researchers and policy makers. The Journal of Nutrition 143(6):938S–947S.
Rimm, E. B., W. C. Willett, F. B. Hu, L. Sampson, G. A. Colditz, J. E. Manson, C. Hennekens, and M. J. Stampfer. 1998. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 279(5):359–364.
Sakakeeny, L., R. Roubenoff, M. Obin, J. D. Fontes, E. J. Benjamin, Y. Bujanover, P. F. Jacques, and J. Selhub. 2012. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. The Journal of Nutrition 142(7):1280–1285.
Sharma, S., Y. Zhuang, Z. Ying, A. Wu, and F. Gomez-Pinilla. 2009. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience 161(4):1037–1044.
Sharma, S., Z. Ying, and F. Gomez-Pinilla. 2010. A pyrazole curcumin derivative restores membrane homeostasis disrupted after brain trauma. Experimental Neurology 226(1):191–199.
Singleton, R. H., H. Q. Yan, W. Fellows-Mayle, and C. E. Dixon. 2010. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. Journal of Neurotrauma 27(6):1091–1099.
Tang, H., F. Hua, J. Wang, I. Sayeed, X. Wang, Z. Chen, S. Yousuf, F. Atif, and D. G. Stein. 2013. Progesterone and vitamin D: Improvement after traumatic brain injury in middle-aged rats. Hormones and Behavior 64(3):527–538.
Tang, H., F. Hua, J. Wang, S. Yousuf, F. Atif, I. Sayeed, and D. G. Stein. 2015. Progesterone and vitamin D combination therapy modulates inflammatory response after traumatic brain injury. Brain Injury 1–10.
Zhu, H. T., C. Bian, J. C. Yuan, W. H. Chu, X. Xiang, F. Chen, C. S. Wang, H. Feng, and J. K. Lin. 2014. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway in experimental traumatic brain injury. Journal of Neuroinflammation 11:59.














