2
The Listed Species
Four of the listed species are discussed in detail in this chapter: the fountain darter, Texas wild rice, the Comal Springs riffle beetle (CSRB), and the San Marcos salamander. Each discussion includes a description of the organism’s life history and habitat. In addition, the biological goals and objectives found in the Habitat Conservation Plan (HCP) for each organism are given. The biological goals tend to reflect the desired population of an organism in the system, such as the number of organisms per unit area. The biological objectives usually have three components: flow requirements, generic water quality criteria, and qualitative characteristics of the organism’s desired habitat. The sections below also discuss the monitoring done for each organism by the Edwards Aquifer Authority (EAA) and its contractors.
Although there are eight listed species (seven that are endangered including one presumed extinct and one that is threatened, plus three petitioned species (see Table 1-1), this report focuses on the four species listed above for two primary reasons. First, the HCP identifies three indicator species: the fountain darter, Texas wild rice, and the CSRB. The indicator species are intended to represent the other listed species: the Comal Springs dryopid beetle, the Peck’s Cave amphipod, the Edwards Aquifer diving beetle, the Texas troglobitic water slater, the Comal Springs salamander, the San Marcos salamander, the Texas blind salamander, and the San Marcos gambusia (EARIP, 2012, p. 4-38). That is, in accordance with the 2008 U.S. Fish and Wildlife (FWS) Strategic Habitat Conservation Handbook (FWS, 2008), the plan assumes that the conservation measures developed for these three “indicator” (sometimes referred to as “sentinel”) species will be suf-
ficient to protect all of the listed species.1 The use of indicator species is felt to be an important way of containing the costs of implementing conservation measures, as well as the costs and time-consuming efforts associated with processing individual incidental take permits under the Endangered Species Act (FWS, 2013). However, because certain conservation measures found in the HCP are specific to the non-sentinel San Marcos salamander, the Committee includes this species in the detailed discussion below. Second, there is considerable overlap and redundancy among the goals and objectives for the eight species, with much less specificity provided for the species that are not discussed in detail. By focusing on the four species in Figure 1-2, the Committee was able to provide some rational bounds to its analysis. As will be evident from this chapter, much more is known about the life histories and habitat requirements of the indicator species compared to the others.
FOUNTAIN DARTER

Description and Life History
The life history of the fountain darter has been described in many documents (Schenck and Whiteside, 1977; Brandt et al., 1993; Labay and
___________________
1 The use of the CSRB as an indicator of the other ESA-listed invertebrates is problematic because of a lack of information on the beetle’s spatial distribution, range of potential habitats, and natural history. As pointed out in NRC (2015), “The degree to which the CSRB is a reliable indicator is presently not well understood nor has it been objectively tested.” Recent Applied Research projects strive to fill these knowledge gaps.
Brandt, 1994; McDonald et al., 2007; Alexander and Phillips, 2012; Becker et al., 2012; Dammeyer et al., 2013). Fountain darters live about one to two years, with adults reaching 1-2 inches in length. They have an affinity for the bottom (sand and gravel substrates) and relatively still waters and preferentially inhabit vegetation. Spawning occurs year-round with peaks in the spring and lows in the late summer and fall. Temperatures of about 22–23°C are favorable, and reproduction stops when water is warmer than 26°C. Females are batch spawners and deposit eggs in vegetation that are then fertilized with no further parental care. The young are restricted to the stream bottom until they can swim through currents. Adults show limited daily movement and are visual feeders that eat copepods, amphipods, and insect larvae using a stationary foraging style. Fountain darters are vulnerable to infection by the gill trematode (Centrocestus formosanus).
There are four fundamental processes that dictate how individual fish progress through the life cycle. The four interrelated processes are growth (which determines development), mortality, reproduction, and movement. The HCP goals are based on abundance and densities of fountain darters, and so the processes of growth and movement are only important if they affect mortality or reproduction. Otherwise, growth and movement result in the same number of individuals that are just smaller or larger or located in a different place. Available data suggest that fountain darters have a limited spatial range. Dammeyer et al. (2013) estimated a maximum displacement of 95 meters within 26 days. Such high site fidelity typically increases the risk of sudden population declines. Localized problems with water quantity and quality and with habitat can create slow and delayed movement responses of individuals and also slow colonization of new habitat if connectivity is not considered. Given the small domain of both systems, this issue is likely of secondary importance.
The population dynamics of fountain darters are based on their densities and habitat availability in the long-term biological goal (LTBG) reaches. However, the domain of interest is the entire system because that constitutes the biological unit used for listing and recovery assessment. The first National Academies report (NRC, 2015) discussed what role the LTBG reaches play in the interpretation of fountain darter population dynamics. A key question is: Can the LTBG reaches be interpreted as representative of other, unmonitored reaches and therefore be scaled up to system-level population abundances? Or do the LTBG reaches effectively constitute the major habitat available and therefore the number of fountain darters in the LTBG reaches can be viewed as the population abundance for the entire system? It is the latter; EAA treats the LTBG reaches as constituting the entire population of fountain darters when determining population abundance goals in each of the systems.
Historically, the geographic extent of fountain darters in the San Mar-
cos system was from Spring Lake to about 0.5 mi (0.8 km) past the confluence with the Blanco River, and in the Comal system from Landa Lake to the confluence with the Guadalupe River. Presently, fountain darters reside in the upper Comal River, including Landa Lake, and the San Marcos River between Spring Lake and the City of San Marcos wastewater treatment plant outfall. The HCP refers to proportional expansion as a way to scale habitat restoration from the LTBG reaches to the system; however, proportional expansion is rather vaguely described in the HCP. Recently, the EAA has done what it considers a version of proportional expansion by adding new restoration reaches to the LTBG reaches because the biological goals were not obtainable by considering only the LTBG reaches. There is now an assumption that the LTBG and restoration reaches are considered the entire system when computing fountain darter population abundance.
Biological Goals and Objectives
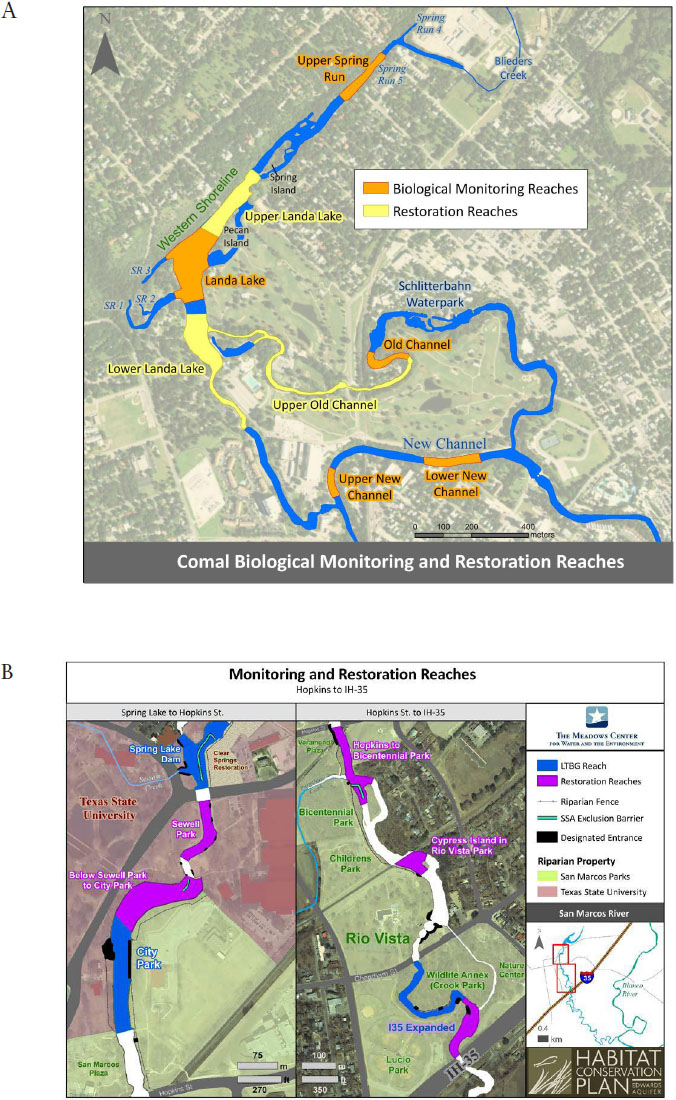
The biological goals for fountain darters are stated as a specific areal coverage by submerged aquatic vegetation (SAV) type (m2) and minimum densities of fountain darter per square meter of each SAV type (Table 2-1). SAV coverage is measured in the LTBG and restoration reaches, while fountain darter density is measured only in the LTBG reaches. Maps of the LTBG and restoration reaches are shown in Figure 2-1. When the SAV areal coverages and fountain darter densities are multiplied, these goals result in the LTBG and restoration reaches supporting about 176,000 fountain darter juveniles and adults in the Comal system and 35,000 in the San Marcos system. This method of determining population abundance is discussed further in Chapter 5.
The goals in Table 2-1 were derived primarily from data collected as part of the EAA Variable Flow Study (BIO-WEST, 2011a,b). Areal coverages of the major habitat types were roughly set to the maximum values observed during the ten-year study for each reach, with consideration given to whether the maximum coverages observed for each habitat type in each reach, some of which occurred in different years, could possibly occur concurrently within the reaches. According to the HCP (EARIP, 2012, pp. 4-2 and 4-24), fountain darter densities by SAV type were also estimated from the same Variable Flow Study as the median densities across samples, years, and reaches within each system. However, the Committee notes that in its examination of the drop-net data for fountain darters, there were few to no fountain darter densities measured for Hydrocotyle, Ludwigia, Sagittaria, and Texas wild rice in the San Marcos system and for Potamogeton in both systems. For these SAV types, which were added to the goals by amendment of the HCP in 2016, fountain darter densities were estimated based on more recent observations (BIO-WEST and Watershed Systems Group, 2016).
TABLE 2-1 Long-Term Biological Goals for Fountain Darters in the Comal and San Marcos Systems
| Comal Springs Fountain Darter Habitat, Submerged Aquatic Vegetation, m2 | ||||||
|---|---|---|---|---|---|---|
| Study Reach | Potamogeton | Ludwigia | Cabomba | Sagittaria | Bryophytes | Vallisneria |
| Upper Spring Run | 25 | 25 | 850 | 1,750 | ||
| Landa Lake | 25 | 900 | 500 | 2,250 | 3,950 | 12,500 |
| Old Channel | 425 | 180 | 450 | 550 | ||
| New Channel | 100 | 2,500 | 150 | |||
| TOTAL | 25 | 1,450 | 3,205 | 3,550 | 6,400 | 12,500 |
| Comal Springs Fountain Darter Median Density, number/m2 | ||||||
| Potamogeton | Ludwigia | Cabomba | Sagittaria | Bryophytes | Vallisneria | |
| 3.3 | 7 | 7 | 1 | 20 | 1 | |
| San Marcos Springs Fountain Darter Habitat, Submerged Aquatic Vegetation, m2 | ||||||
| Study Reach | Potamogeton | Ludwigia | Cabomba | Sagittaria | Hydrocotyle | Zizania |
| Spring Lake Dam | 200 | 100 | 50 | 200 | 50 | 700 |
| City Park | 1,450 | 150 | 90 | 300 | 10 | 1,750 |
| I-35 | 250 | 50 | 50 | 150 | 50 | 600 |
| TOTAL | 1,900 | 300 | 190 | 650 | 110 | 3,050 |
| San Marcos Springs Fountain Darter Median Density, number/m2 | ||||||
| Potamogeton | Ludwigia | Cabomba | Sagittaria | Hydrocotyle | Zizania | |
| 5 | 7 | 7 | 1 | 4 | 5 | |
Compliance with the biological goals is evaluated using extensive mapping of SAV coverages in the LTBG and restoration reaches and cumulative median fountain darter densities by SAV type. The use of a cumulative median density is very insensitive to year-to-year changes in fountain darter densities (Box 2-1). Indeed, there is no theoretical or statistical basis for using the cumulative median, and its insensitivity to change can create risks to understanding the status of the fountain darter.
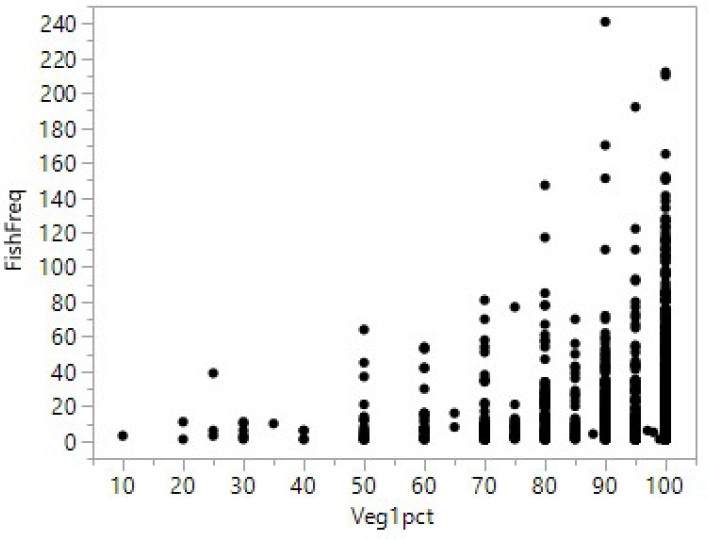
The biological objectives for fountain darters have three components: habitat, flow, and water quality. The habitat objective is to restore native vegetation. Fountain darters are associated with SAV, with different
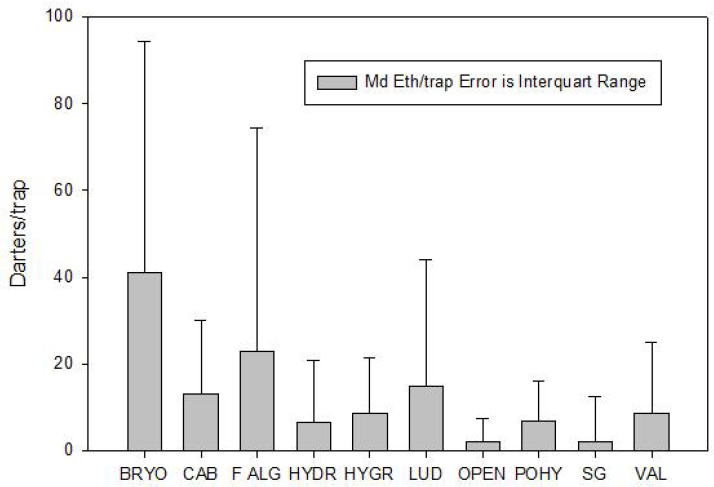
densities found among the vegetation taxa, as shown in Figures 2-2 and 2-3. Figure 2-2 is based on the drop-net sampling and uses the estimated coverage of the dominant vegetation type in each sample. It shows that greater coverage of SAV in the drop-net sample allows for higher densities of fountain darters but does not guarantee higher densities. Figure 2-3 uses the same drop-net data and shows that fountain darter densities are generally higher in some SAV types over others, with a strong preference for bryophytes and filamentous algae. However, the error bars (interquartile range) are relatively wide, suggesting that fountain darter densities vary greatly within many of the SAV types, and thus the densities overlap among many of the SAV types.
The flow component of the biological objectives is to maintain a long-term average total Comal discharge above 225 cubic feet per second (cfs) with a minimum of 30 cfs that is not to exceed six months in duration, followed by at least 80 cfs for three months. For fountain darters in the San Marcos system, the flow objective is to maintain a long-term average
total discharge above 140 cfs with a minimum of 45 cfs that is not to exceed six months in duration, followed by at least 80 cfs for three months. Maintaining certain minimum and long-term average flows is necessary to ensure that the vegetation habitat is healthy and that conditions (i.e., water velocities, depths) are conducive for growth, reproduction, and survival of the fountain darter.
The water quality component of the biological objective is to maintain surface water quality (e.g., conductivity, pH, turbidity) within 10 percent of the historical daily averages; instantaneous measures of dissolved oxygen (DO) will always exceed 4.0 mg/L, and temperatures will be cooler than 25°C. The water quality objective is intended to ensure stable conditions for the fountain darter, which are associated with successful reproduction.
Monitoring, Modeling, and Applied Research
As one of the most studied of the covered species, there are long-term monitoring datasets, population modeling, and process studies available for
the fountain darter. The monitoring information for the fountain darter has been well described (including in NRC, 2015), and the data are routinely analyzed for use in HCP Annual Reports and recently in exploratory analyses (e.g., Beaver Creek Hydrology, 2018; Perkin et al., 2018). Compared to dip-net data, the drop-net data are the best data to use to look for spatial and temporal trends in the fountain darter and for determination of fish densities (medians) by vegetation type. The drop net captures juveniles and adults. The recent development of an EAA database is a major step toward allowing for the merging of datasets and performing more integrative analyses.
An individual-based population model has been developed for the fountain darter in the LTBG reaches of both systems (NASEM, 2017). There remain some technical issues that cause the Committee to limit their consideration of the use of the model to a very specific subset of the many possible questions. EAA staff and the Committee have discussed these restrictions on using the model in detail. The population model is now being maintained by EAA staff (transferred from the model developers to EAA) and they are evaluating how to use the model to address questions about flow effects on habitat and fountain darter population dynamics. There are also process studies on fountain darters available as part of the Applied Research Program and other papers and theses.
In general, the site-specific information available to address the charge questions about the fountain darter is quite extensive and should allow for some degree of quantitative assessment. In the Committee’s experience, the availability of tools (data, models) for the fountain darter is greater than in many similar analyses of covered species elsewhere. However, the degree of analyses has been limited to mostly simple graphical presentations of the monitoring data, and the process studies are done as specific issues arise rather than as a broader strategic approach. With the recent development of a database and several projects designed to explore the data, EAA has begun to make significant progress in integrating the fountain darter data with other monitoring data. The development of the population model was very effective in forcing the synthesis and integration of the available information into a single quantitative and full life-cycle platform. The population modeling as an integration of available information and the identification of critical gaps is an opportunity that should be leveraged. Further analyses of the monitoring and process studies for the fountain darter are warranted as the HCP enters Phase 2, which provides an opportunity to focus on compliance with biological goals and redirection of restoration efforts.
TEXAS WILD RICE

Description and Life History
Texas wild rice (Zizania texana Hitchcock) is an aquatic perennial grass inhabiting only the headwaters of the San Marcos River in Hays County, Texas (Poole and Bowles, 1999). The vegetative portions of Z. texana do not stand erect; instead, its 1- to 2-m-long linear, ribbon-like leaves lie decumbently semi-submerged in the shallow streambed (Terrell, 2007). More northern-adapted species of Zizania range from Quebec southward to northern Florida and westward to Louisiana. It now appears that Z. texana is a disjunct species that was stranded during the last glacial retreat and subsequently adapted to the presently hot, dry Texas summers—by assuming a more submerged habitat than its more northerly counterparts (Xu et al., 2010, 2015).
Texas wild rice reproduces both sexually and asexually. It goes through sexual reproduction typical of most angiosperms, and has male and female portions on the same panicle, which is not submerged. Female flowers mature on the emerged culms in the river after male flowers (located on the same panicle), which promotes outcrossing. When the male pollen grains are mature, they are distributed via wind, and a small percentage land on stig-
matic surfaces of the ripe female florets and grow down the style to fertilize the ovary, forming the grain (caryopsis). Following fertilization, seeds mature when dormancy is broken and form the radicle and plumule, which develop rapidly into roots and shoots. The seeds remain viable for less than a year and do not form a “viable seed bank” in the sediments, unlike many wetland species. Thus, Texas wild rice depends entirely on new seeds being produced every year to complete the cycle of sexual reproduction. This trait makes it almost impossible to store seeds for a number of years (as is the case for maize, wheat, and rice). On the other hand, asexual reproduction typically depends on rhizome extensions derived from a single genetic source. The degree to which particular species depend on sexual versus clonal growth for recruitment, expansion, and maintenance of populations is often determined by environmental conditions (e.g., temperature and flow). The advantage to having both modes of reproduction is that if sexual reproduction fails, recruitment can continue using rhizomes. The downside of asexual reproduction is that if it persists for long periods, it can result in populations with very low diversity. Recent genetic studies (Xu et al., 2010, 2015; Wilson et al., 2017) suggest that Texas wild rice is remarkably robust, which is extremely important for its small geographic coverage over past decades.
When first described by Albert S. Hitchcock (1933), Z. texana was reported to be thriving in the upper San Marcos River where it benefited from the springs associated with the Edwards Aquifer (Bowles and Arsuffi, 1993). Despite its modest size (approximately 11 km in length), the San Marcos system was still an excellent habitat for a rich assortment of species in the early 1930s. However, after the installation of a series of five dams, the flow regime in the river became modulated and changed the physical habitat, which some have attributed to the decline of Z. texana in the San Marcos River. For example, Power (1996) reported that Z. texana densities were highest in fast-flowing segments (0.40–0.49 m/s) compared with the moderate-flow (0.12–0.24 m/s) or slow-flow (0.05–0.12 m/s) areas that became common after the dams were built. Spring Lake Dam has effectively trapped fine-grained sediments, while sandy sediments have accumulated below it, compromising the habitat suitability for Z. texana, which prefers organic substrates between 0.8 and 2.8 percent carbon for optimal growth (Rose and Power, 2001). Regulating flow has created patchy turbidity conditions, which can impact photosynthesis of Z. texana. Unsurprisingly, Poole and Bowles (1999) found Texas wild rice to occur at sites with high water clarity. They also found that calcium and sulfur dioxide concentrations were elevated on non–wild rice transects and hypothesized that this was associated with suburban and urban runoff from the City of San Marcos and other nonpoint sources, including agriculture. More recently, the high level of recreation on the San Marcos River, including kayaking and canoeing, snorkeling, SCUBA, and tubing, has been identified as a
stressor on Texas wild rice. These activities can mechanically tear the delicate leaves of the Texas wild rice plants in meter-deep waters, such that recreation management is critical to the recovery of the species (EARIP, 2012).
Temperature is an essential consideration for Z. texana, since it is living far out of the normal range for this genus. This is a critical concern because the cool water provided by the Edwards Aquifer can keep weedy exotics from out-competing Z. texana. Plants of the genus Zizania photosynthesize via the C3 pathway, which is adapted to cooler conditions. Virtually all C3 plants utilize only the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) to fix carbon dioxide in the mesophyll of green leaves (i.e., via the Calvin cycle). When temperatures are elevated, RuBisCO makes use of oxygen by producing an unusable byproduct, glycolate, via photorespiration. Thus, Texas wild rice actually begins to lose considerable amounts of energy, particularly if internal leaf temperatures rise over 25°C (Poole and Bowles, 1999). On the other hand, C4 grasses, such as maize and sugarcane, photosynthesize under hotter conditions (25°–35°C) by capturing carbon dioxide in the mesophyll and preventing it from being respired in a back-reaction termed photorespiration (Taiz et al., 2014).
In addition to the effects of temperature, many species of SAV can readily use bicarbonate ions present in lakes and stream waters, but Z. texana cannot (Power and Doyle, 2004). Thus, weedy SAV species, such as Hydilla verticillata and Hygrophilla polysperma, would be expected to have a competitive advantage over Zizania whenever the latter becomes starved for carbon, when pH exceeds 8.5 (Wetzel and Likens, 1991). Indeed, Hydrilla verticillata can still photosynthesize when pH exceeds 10 in the freshwater portions of the Chesapeake Bay (Staver and Stevenson, 1995). On the other hand, Rose and Power (2001) have measured photosynthesis versus pH in Z. texana from the San Marcos River and found it dropped by a factor of 5, from 0.5 mg O2 per gram of tissue per hour at pH 7.5, to less than 0.1 mg O2 per gram of tissue per hour at pH 8.5. It should be noted that flow can regulate both temperature and pH in the San Marcos system, highlighting the importance of maintaining minimum flows in the San Marcos River to Texas wild rice survival.
The efficiency of carbon uptake is the foremost reason that C4 species outcompete C3 species when CO2 levels are low (and pH is high). The significance of this mechanism should not be underestimated when assessing the prospects for the long-term survival of Texas wild rice in the San Marcos River. Nonetheless, there is one physiological factor that is favorable for Texas wild rice: it can obtain a small portion of its needed CO2 from the atmosphere, and atmospheric CO2 concentrations have risen globally over 100 ppm over the last century and now are over 400 ppm (Dayton, 2016). This factor may offset the temperature and pH problems of Texas wild rice survival to some degree.
Weedy bicarbonate-utilizing SAV species (including Hydrilla) likely invaded the San Marcos system by people emptying their aquaria. These species have a competitive advantage over Z. texana and other non-bicarbonate-ion–utilizing species in colonizing openings created by disturbances. This is essentially the same scenario by which Hydrilla invaded Chesapeake Bay headwaters in the 1980s (Staver and Stevenson, 1995). Mesocosm studies containing Texas wild rice in combination with C4 plants could effectively determine the extent of competition between these species under conditions similar to those in the San Marcos River.
Biological Goals and Objectives
The biological goals for Texas wild rice are to (1) maintain a range of areal coverage in four reaches of the San Marcos River and (2) maintain a range of percentages of the coverage in those same reaches (Table 2-2). The long-term biological goals for Texas wild rice were determined by an evaluation of (1) the maximum occupied area of Texas wild rice present in the San Marcos system over time; (2) analysis of the Hardy et al. (2010) physical habitat modeling; and (3) the 1996 FWS recovery plan goals.
The biological objectives for Texas wild rice are threefold:
- To maintain flow in the San Marcos system, with a daily long-term average (50 years including Drought of Record) of 140 cfs and a minimum of 45 cfs (not to exceed six months in duration), followed by a minimum of 80 cfs for three months
- To maintain minimum areal coverage in the four river reaches during the Drought of Record (at values lower than the goals in Table 2-2)
- To establish recreation awareness, with “control” in high-quality habitat areas when flow is below 100 cfs
TABLE 2-2 Long-Term Biological Goals for Texas Wild Rice
| River Segment | Areal Coverage, m2 | Reach Percentage of Total Areal Coverage |
|---|---|---|
| Spring Lake | 1,000–1,500 | n/a |
| Spring Lake Dam to Rio Vista Dam | 5,810–9,245 | 83–66 |
| Rio Vista Dam to I-35 | 910–1,650 | 13–12 |
| Downstream of I-35 | 280–3,055 | 4–22 |
| TOTAL | 8,000–15,450 | 100 |
SOURCE: EARIP (2012).
Monitoring and Modeling
Along virtually the entire stretch of the San Marcos River, Texas wild rice is painstakingly mapped to determine the area present annually. Additionally, nonnative plant species are removed whenever possible, and Texas wild rice plants are propagated and planted in strategic areas to enhance vegetative cover in the San Marcos River system (see Chapter 4). In 2017, there were 17 new stands in the river, and there was a threefold increase in Spring Lake where SCUBA is routinely employed for monitoring and planting efforts. Since the last total vegetation sampling of SAV carried out during the 2013 drought, Texas wild rice has expanded by an estimated 7,963 m2 through planting and natural dispersal. Over the last year alone (2017) Texas wild rice coverage grew by an estimated 3,800 m2 in the river (Blanton and Associates, 2018). Every reach of the San Marcos River appears to have gained Texas wild rice.
A detailed process-driven ecosystem model was created for SAV with eventual applicability to Texas wild rice, as discussed in great detail in NASEM (2017). Unfortunately, the SAV model has not progressed to a point where it can be utilized by the Committee to address its statement of task.
COMAL SPRINGS RIFFLE BEETLE

The Comal Springs riffle beetle (CSRB), Heterelmis comalensis, was federally listed as an endangered species in 1997. The background and ecology of the CSRB are discussed in the HCP (EARIP, 2012) as well as in the two previous Committee reports (NRC, 2015; NASEM, 2017). The discussion below is meant to build on those reports and synthesize recent studies providing new biological and ecological information relevant to how the activities of the HCP will protect the CSRB and achieve its biological goals and objectives.
Description and Life History
At the initiation of the HCP, very little was understood about the life history and population biology of the CSRB, its optimal water quality conditions, and general distribution within the Comal Springs system. Hence, in an effort to better understand the habitat, ecology, and population biology of the CSRB, the Applied Research Program has focused several projects on the beetle (see Table 5-3 in NASEM, 2017, for a recent review; BIO-WEST, 2016, 2017; Nowlin et al., 2016a,b). The results of those projects inform the discussion below.
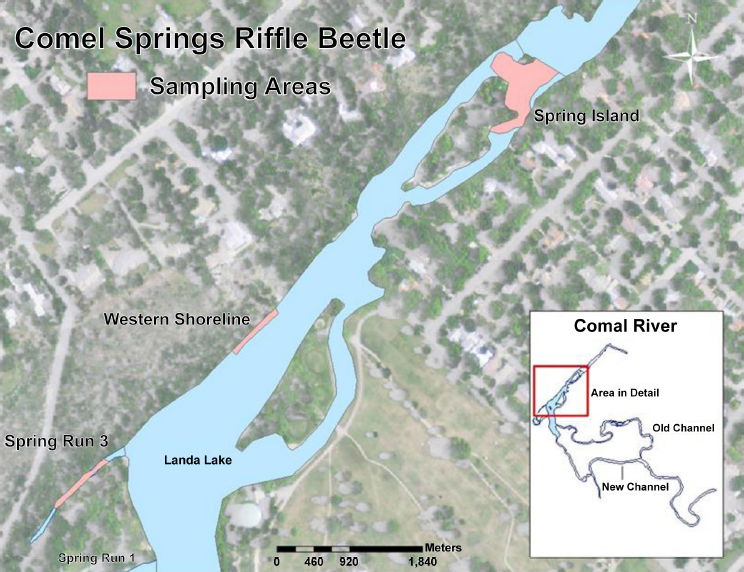
Most of the natural history knowledge about CSRB has been generated from observing and collecting beetles from three areas considered to have suitable habitat—Spring Run 3, the Western Shoreline, and the Spring Island area, although some specimens were collected from headwaters of the San Marcos River. From these studies, it was generally understood that the habitat for CSRB is adjacent to or within spring flow orifices and in areas of groundwater upwelling with silt-free substrate that is often associated with leaf and wood debris and other terrestrial plant organic matter. Populations are often found at depths of 2 to 10 cm (Bosse et al., 1988 et al.), presumably in the interstitial spaces, and associated with organic matter (Brown, 1987). The CSRB is considered to be sensitive to siltation that results from sediment runoff from riparian zones adjacent to springs (RPS Espey et al., 2014). This sensitivity may be because the plastron respiration exhibited by adults might be compromised by heavy sedimentation.
The adults are presumed to be flightless, limiting the aerial dispersal of this endangered species. Interestingly, despite limited dispersal, CSRB populations are presumed to have survived the Drought of Record in the 1950s when all of the springs in the Comal system went dry for 144 days. Although it is not known how this species persisted and then recovered to repopulate the springs, this observation suggests that hyporheic habitat may act as a refuge during severe droughts. Tolerance threshold values of the CSRB to temperature and DO are generally not exceeded in the Comal springs (Nowlin et al., 2016a).
Populations of the CSRB have been reported to have overlapping gen-
erations and asynchronous emergence not associated with seasonal changes in weather (Bowles et al., 2003; Cooke, 2012). However, there has been limited information from field studies to describe the length or natural mortality of each development stage (egg, larva, pupa, and adult), or how each stage responds to changing environmental conditions, such as reduced flow. As discussed below, in new laboratory studies, researchers have now determined methods for sexing adults without dissection. This is an important step in understanding life cycle and biological characteristics, including the time of egg incubation, number of instars, length of each instar, habitat of pupation, length of metamorphosis to the adult stage, adult longevity, mating, and oviposition.
In lab experiments to determine egg oviposition preferences, BIO-WEST (2017) reported that 82 percent of eggs were laid on leaves and that egg production declined with increasing time of adult captivity. The number of eggs per pair of adults ranged, on average, from 0.3 to 17.8 and depended on both the mating substrate and adult survivorship. Egg incubation lasted an average of three weeks without diapause. It was concluded that treatment substrate (e.g., cotton, leaf, or rock) did affect hatching success, with the highest success on cotton cloth substrates. Previous studies have shown lab colony survivorship to be highly variable (BIO-WEST, 2014) and low (< 50 percent) after 60 days, even under preferred temperature and oxygen conditions (Nowlin et al., 2016a). BIO-WEST (2017) found that after the eggs hatched into larvae, CSRB development required seven instars and took approximately four months to the sixth instar and at least another four months for the seventh instar to complete development. The time to pupation has not been reported in detail. However, in a pilot laboratory study, after four months of late instar development, up to 56 percent of larvae pupated and adults were observed to emerge from puparia after about a month. The adult longevity is considered to be one year, and so the full life cycle of laboratory-reared populations is estimated to be two years (BIO-WEST, 2017). These efforts have revealed that there is substantial mortality among all life stages when raised in the laboratory. A recent tour of the U.S. Fish and Wildlife refugia facilities revealed mortality rates greater than 50 percent, leaving a level of survivorship that could jeopardize future husbandry efforts.
The lab studies associated mating, egg laying, and larval development with the presence of organic matter, such as leaves, suggesting an ecological coupling of CSRB larvae and adults with riparian-derived detritus in the Comal system. The preferred habitats are substrates that accumulate substantial amounts of coarse organic matter in the form of leaves and small branches. It is thought that these course organic food resources may also serve as habitat. In an effort to better understand this association, stable isotopes were used to identify primary food resources of the CSRB and
potentially link them to the primary habitat (Nowlin et al., 2016b). Stable isotope profiles confirmed that CSRB feed on terrestrial-derived course particulate organic matter that is often found at springheads. Importantly, these new findings indicate a trophic connection between riparian conditions and CSRB that could affect the population biology in ways relevant to HCP conservation measures. Disconnecting the CSRB from its primary food sources could negatively affect the habitat suitability and population levels of this listed species. It is unclear how the riparian minimization and mitigation (M&M) measures will affect the quantity and quality of the course particulate organic matter that accumulates in the beetles’ habitat. Better understanding this issue is an area of needed research in order to gain more confidence in the riparian restoration activities of the HCP.
Biological Goals and Objectives
The long-term biological goals for the CSRB are to (1) maintain silt-free gravel and cobble substrate in ≥ 90 percent of three areas in the Comal system and (2) maintain specific median beetle population densities (as measured by numbers per lure) in the same three areas. The areas are Spring Run 3 (≥ 20 CSRB/lure), the western shoreline of Landa Lake (≥ 15 CSRB/lure), and Spring Island (≥ 15 CSRB/lure). The locations of the monitored habitats for these biological goals are shown in Figure 2-4.
The rationale for the required beetle abundances is not well developed in the HCP (EARIP, 2012), but they appear to be greater than or equal to the median abundances observed during the EAA Variable Flow Study (BIO-WEST, 2011a). This presents a problem because during the Variable Flow Study and until 2016, the cotton-lure methodology for sampling the CSRB was without a standard operating procedure, creating uncertainty in the measured values. Regarding the biological goal of maintaining silt-free substrate, it should be noted that there have been no quantitative studies that associate variation in silt-free habitat with CSRB population estimates. A worthy project for the Applied Research Program would be to determine how sedimentation of habitat directly (e.g., habitat suitability) and indirectly (e.g., food resource changes) affects CSRB populations, and how these sedimentation rates are related to riparian buffer conditions.
As with the other listed species, the biological objectives for achieving the long-term biological goals for the CSRB have three components. The flow component of the objective is to maintain a long-term average total Comal Springs discharge above 225 cfs with a minimum of 30 cfs that is not to exceed six months in duration, followed by at least 80 cfs for three months (the same as for the fountain darter). The water quality component of the objective is to maintain water quality issuing from the spring openings within 10 percent of historical conditions at the three study locations.
The habitat component of the objective is to restore riparian habitat adjacent to spring openings to reduce siltation.
Monitoring
CSRB populations are monitored in the three study reaches of the Comal Springs system shown in Figure 2-4: Spring Run 3, the western shoreline of Landa Lake, and the Spring Island area. The populations are collected periodically each year using a cotton-lure approach that provides qualitative estimates of both larvae and adults, but cannot provide true density estimates (as discussed at length in NRC, 2015, and NASEM, 2017).2 Each lure is constructed of 200-thread-count white cloth of 60
___________________
2 A recommendation from NRC (2015) was to better quantify CSRB population densities and/or calibrate the cotton-lure method of sampling so that it could potentially be an efficient and reliable way to estimate populations. “The inability to calibrate the cotton-lure method of sampling with any real densities of the CSRB in the system is a considerable weakness, making the representativeness of this sampling approach for estimating population densities unknown and making monitoring for CSRB population estimates difficult if not impossible to achieve” (NASEM, 2017). In response, a Standard Operation Procedure for how to deploy, retrieve, and score cotton lures when collecting CSRBs was created, as discussed in the main text.
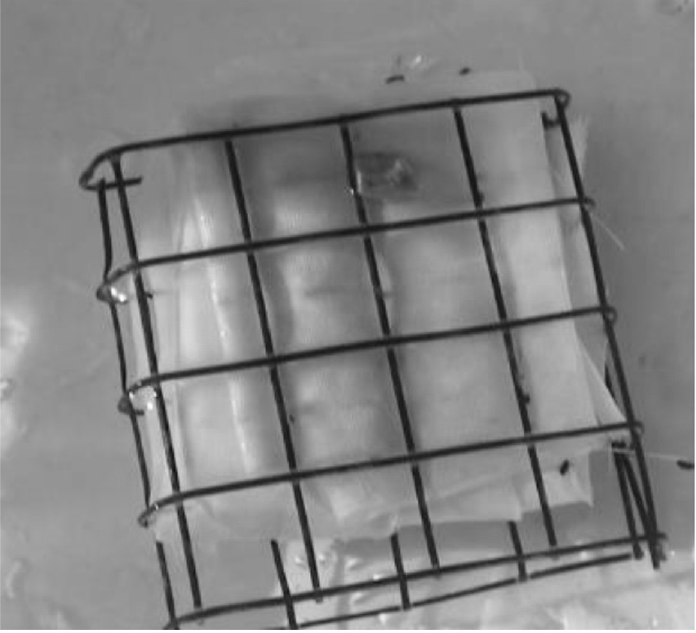
percent cotton and 40 percent polyester cut into 15- × 15-cm pieces and then folded into a 4- × 4- × 1-cm lure and placed inside a galvanized wire cage (Figure 2-5). The lures are deployed approximately 10 cm downstream of spring orifices and upwellings for passive colonization of larvae, pupae, and adults of CSRB, in addition to other invertebrates. For each monitoring event in 2016, it was reported that ten lures per study reach were placed downstream of ten individual flowing spring orifices with visible flow and then incubated for approximately four weeks (BIO-WEST, 2016).
A standard operating procedure (SOP) for the cotton lure approach was introduced during the fall 2016 sampling event. The SOP provides a
standard approach to deploy, incubate, harvest, and collect beetles from the lures, taking care to note any issues of the lures and other habitat characteristics or observations that may affect monitoring (EAHCP, 2016). All CSRB (both larvae and adults) and any Peck’s Cave amphipod or Microcylloepus pusillus are counted, and all organisms are carefully released back into the spring using mask and snorkel, although there is no mention of how well these returned organisms take hold and re-inhabit the substrate.
The sampling effort and selection of flowing spring openings for sampling are important aspects of CSRB monitoring, in that the sampling should occur twice per year and be done during the same months each year. From data provided to the Committee, it is clear that the sampling effort (in terms of frequency and number of samples per reach) is different among years, such that the number of lures used to generate the annual median values is highly variable among years. Further, the approach to spring selection for sampling should be random within each reach to avoid any bias toward certain springheads that are located in different areas of each reach. Currently within the annual biomonitoring reports, spring orifice selection is not described in any detail. Are all spring orifices mapped prior to lure deployment, and then ten openings randomly selected? Or are flowing spring openings selected in the sequence that they are found and then sampled? These are important questions that influence how the data generated from each sampling event should be analyzed and interpreted for compliance purposes.
Finally, while using median values to define the biological goals is adequate given the qualitative nature of sampling and zero-abundant data, a well-designed and articulated approach to calculating the annual median values is needed. The current method is to pool all samples from all sampling events within each reach to calculate the annual median for each reach, which could be problematic if the sampling effort is not equal and consistent from one year to the next. Based on the information provided in the most recent biomonitoring report, it is difficult to know if zero values are used in the calculation of the median.
SAN MARCOS SALAMANDER

Description and Life History
The San Marcos salamander (Eurycea nana) is a small (~40–55 mm total length), fully aquatic, obligatory paedomorphic (retaining juvenile morphology) salamander. Individuals have a dark brown back, a whitish or yellow belly, dark rings around their eyes, and rows of small spots down their sides. They retain prominent, external gills throughout life (Petranka, 1998). San Marcos salamanders are one of approximately 15 closely related species (a single monophyletic lineage) of spring- and cave-dwelling salamanders within the family Plethodontidae (lungless salamanders), and this lineage is endemic to central Texas (Chippindale et al., 2000; Chippindale, 2005).
Little is known about the species life history except what has been gleaned from observations and experiments with captive individuals. Although reproduction has never been observed in the wild (Fries, 2002), it is certainly aquatic. Average size of seven clutches deposited by captive females was 35 eggs, with a range of 2 to 73 (Najvar et al., 2007). In captivity, females attach eggs to aquatic moss, filamentous algae, and rocks (Chippindale and Fries, 2005). In the wild, they probably lay eggs among rocks at spring vents (Nelson, 1993). Males and females both reach sexual maturity around 20 mm (snout-vent length).
Eurycea nana are able to detect chemical cues in the water and elicit behavioral responses to these signals. For example, E. nana exposed to water containing chemical cues from predatory fish (i.e., Lepomis sp. and Micropterus salmoides) significantly reduced their activity as an antipredator response (Epp and Gabor, 2008; Davis et al., 2012). These salamanders also rely strongly on chemical cues to recognize members of the opposite sex (Thaker et al., 2006).
Because of their size and aquatic existence, San Marcos salamanders feed on small invertebrates that inhabit the SAV frequented by the salamanders. Tupa and Davis (1976) list midge larvae and pupae, as well as amphipods, as being the most commonly found food items in the gastrointestinal tracts of salamanders they collected. Sunfish, catfish, and crayfish are presumed to be the major predators of E. nana. To elude predators and avoid detection, the salamanders hide under rocks, among gravel, and in SAV.
San Marcos salamanders have an extremely limited geographic range but are locally abundant. They inhabit spring outflows throughout Spring Lake and they also occur in the San Marcos River a short distance downstream from the Spring Lake Dam spillway (with both areas being designated by the FWS [1978, 1995] as critical habitat). There are two estimates of population size for the species. Tupa and Davis (1976) estimated a population size of 20,880 based on systematic sampling at numerous sites along the north bank of Spring Lake. Density of salamanders was estimated to be 116 individuals per square meter. A second estimate of population size (Nelson, 1993) was a population of 23,000 individuals among SAV in the same area sampled by Tupa and Davis (1976). Nelson (1993) also searched for salamanders among rocky substrates at the mouth of springs throughout Spring Lake and estimated an additional 25,000 E. nana there. Salamanders associated with rocky substrates below the Spring Lake Dam spillway were estimated at around 5,200 individuals. Because the salamanders are known to hide below the surface in gravel substrate, Nelson’s estimates are thought to be conservative (FWS, 1995).
San Marcos salamanders have an extremely restricted geographic range and only occur in the area of spring outflows of the San Marcos system. Rocky substrates, especially in close proximity to spring outflows, and aquatic macrophytes are important microhabitats for San Marcos salamanders. They are most often found in Lyngyba sp. (a filamentous blue-green algae) and the aquatic moss Leptodictyum riparium (Tupa and Davis, 1976). Aquatic vegetation provides cover for the salamanders to avoid predators, and it is where the salamanders find their invertebrate prey. Salamanders also seek refuge under rocks that sit on top of sand and gravel. Areas lacking vegetation with muddy or detritus-laden substrates are not suitable habitat (FWS, 1995).
Water emanating from the San Marcos springs system is thermally
stable around 21°–22°C, and this is presumed to be the preferred water temperature for E. nana. Nonetheless, experiments conducted at the San Marcos National Fish Hatchery and Technology Center estimated the critical thermal maximum (CTmax) for the species at 36°–37°C (Berkhouse and Fries, 1995). Also noteworthy is the study by Norris et al. (1963), which compared oxygen consumption rates among three Edwards Plateau Eurycea species, including E. nana, at varying temperatures (15°, 20°, 25°, and 30°C). About half of the San Marcos salamanders tested at 30°C died after two hours of exposure.
Because San Marcos salamanders require clean, clear water to persist, ensuring adequate water quality is mentioned as a conservation concern in all documents that specifically address conservation of the species (FWS, 1996; Chippindale and Price, 2005; EARIP, 2012). Nonetheless, how changes in nutrients, organic compounds, herbicides and pesticides, as well as metals affect San Marcos salamanders is unknown and in need of study.
Maintaining adequate spring flow is identified as the main priority and conservation issue for the San Marcos salamander (FWS, 1996; Chippindale and Price, 2005; EARIP, 2012). FWS (1996) states that a spring flow rate of 60 cfs in the San Marcos system is “needed to prevent take, jeopardy, or adverse modification of critical habitat.” Nonetheless, San Marcos salamanders persisted through the 1950s during the Drought of Record when flow dropped to 46 cfs. The species likely experienced a significant population decline during this event. Adequate flow of water adjacent to spring vents in Spring Lake precludes buildup of detritus, maintains clean sand and gravel substrates, and facilitates the growth of aquatic macrophytes that provide food and shelter for San Marcos salamanders (Tupa and Davis, 1976).
Biological Goals and Objectives
The long-term biological goals for the San Marcos salamander address habitat quality and population density. The habitat goal requires that silt-free gravel and cobble substrates be maintained at the three main sites where the species has been shown to occur in the greatest densities in the past 50 years: hotel site, riverbed site, and Spring Lake Dam site (Figure 2-6). The goal is to maintain habitat quality at ≥ 90 percent of each study area. The density goal sets specific targets for median density of salamanders at each of the three sites (Table 2-3).
Designation of specific population densities at the three sampling reaches was based on data collected from 2000 to 2010 during the EAA’s Variable Flow Study (EARIP, 2012, Tables 4-26, 4-27). Densities of salamanders estimated across the three sites during this period, which included routine sampling and additional sampling during low- and high-flow events, varied considerably within and among years. Therefore, the EAA used
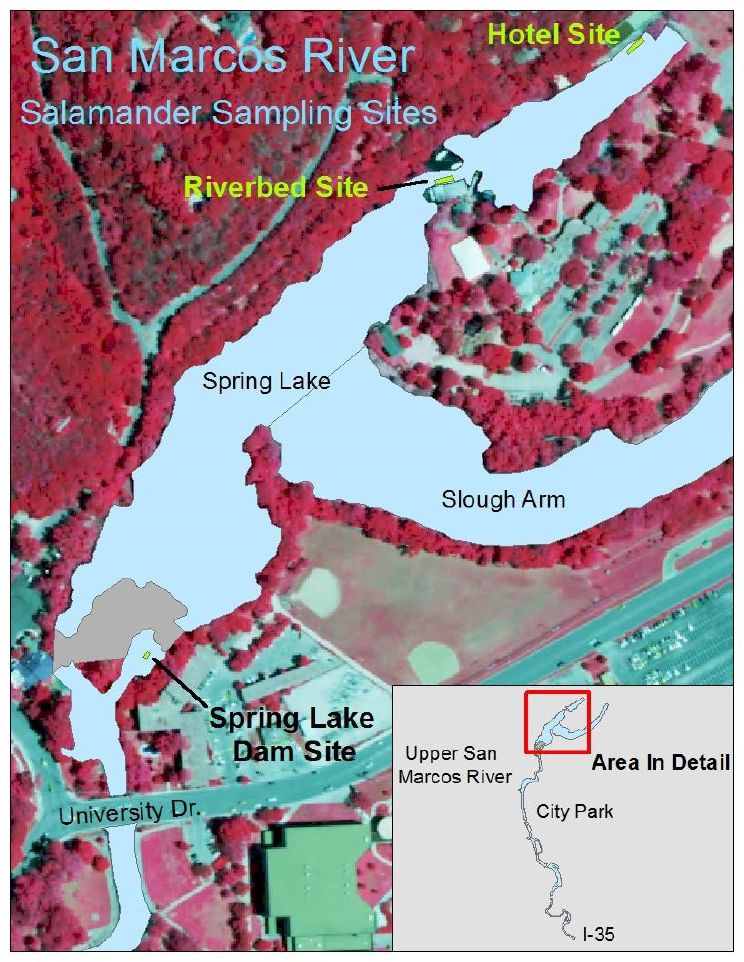
TABLE 2-3 Long-Term Biological Goals for San Marcos Salamanders
| Study Reach | Habitat Quality | Salamander Density |
|---|---|---|
| Hotel Site (Spring Lake) | Maintain ≥90% of site as silt-free gravel and cobble | ≥15 salamanders/m2 |
| Riverbed Site (Spring Lake) | ≥10 salamanders/m2 | |
| Eastern Spillway (just below Spring Lake Dam) | ≥5 salamanders/m2 | |
SOURCE: EARIP (2012).
“professional judgment” in its decision to designate median density as the population-based biological goal for the San Marcos salamander. Specific density values set for the three sites were based on data from 2000 to 2010 and seem reasonable and defensible.
Maintaining silt- and detritus-free gravel and cobble bottom substrate conditions was identified in the species recovery plans as being crucial for the persistence of the salamander (FWS, 1996). The emphasis on this specific habitat parameter at the three locations identified in the HCP is apparently based on the research of Tupa and Davis (1976) and Nelson (1993). Given the importance of SAV as cover and a source of food for San Marcos salamanders, there was some discussion of establishing SAV targets in the three sampled reaches (EARIP, 2012, p. 4-34), but this idea was dismissed in favor of the simpler target of “maintaining silt-free substrates (gravels and cobbles) over greater than or equal to 90 percent of the fixed sampling reaches.” The designation of 90 percent of the sampling reaches as silt-free appears somewhat arbitrary and a “best guess” due to the complexity of the salamander’s use of gravel substrates and macrophytes.
Three biological objectives are identified to achieve the long-term biological goals. Two of the objectives—(1) aquatic gardening at the riverbed and hotel sites and (2) regulation of human recreation activity at the spillway site—are odd because as stated they are virtually identical to certain M&M measures. A third, flow-related objective is the same as for Texas wild rice: a long-term average of 140 cfs and a minimum of 45 cfs. Note that there is no water quality component of the biological objective for San Marcos salamanders.
Monitoring and Applied Research
San Marcos salamanders have been monitored at three sample reaches (hotel area, riverbed, and eastern spillway) two to four times a year since fall 2000. Although estimated densities vary considerably within and among years, as well as among the three sampling reaches, average densities over the past 15 years are largely consistent with the LTBG density targets. Note that, like the CSRB, there is no monitoring to determine compliance with the goal of having silt-free gravel.
Salamander surveys are routinely conducted twice a year—once in spring (April–May) and again in fall (October–November)—with additional surveys conducted during periods of especially high or low flow. Considering the fact that San Marcos salamanders do not migrate and are active year-round in the thermally stable waters of the San Marcos system, sampling salamanders twice a year (spring and fall) is appropriate for monitoring. In fact, the FWS Recovery Plan (FWS, 1995) recommends sampling for salamanders only once a year. It seems unlikely that salamander detection probability varies much throughout the year, but that is an assumption that could be tested.
Visual searches are made for salamanders by trained personnel via snorkeling or SCUBA. Observers swim over historically set transects and conduct three, 5-minute timed surveys per sample area. They visually scan for salamanders and also turn over rocks (>5 cm wide) to look for animals. Salamanders are counted and substrate type is noted. Surveys are conducted in sections of each reach that are free of submerged macrophytes. Areas within or immediately adjacent to each sampling reach that are covered with dense macrophytes and algae are purposely avoided. It is possible that density estimates are inflated because of this sampling protocol; that is, it seems reasonable that experienced divers and snorkelers would learn which rocks are likely to harbor salamanders and preferentially choose those rocks to sample.
The current sampling design does not allow density estimates to be extrapolated across the entire Spring Lake area or the smaller spillway site. Therefore, there is no accurate way to get an estimate of the entire salamander population. To supplement the current sampling, an additional protocol that uses occupancy estimation could be designed for the San Marcos salamander. Such a protocol would provide estimates of proportion of area occupied as well as detection probability and allow inference of salamander population trends across more of their potential habitat within Spring Lake. A collaboration between the EAA, biologists from the FWS, and the U.S. Geological Survey Amphibian Research and Monitoring Initiative3 could be
___________________
3 See https://armi.usgs.gov/.
undertaken to design a robust occupancy estimation protocol. Furthermore, the statistical methods reviewed by Denes et al. (2015) could shed light on the extent of error associated with the current sampling protocol.
Apparently, the only EAA research activities to date focused on the San Marcos salamander are development of husbandry practices as part of the captive assurance colony Long-Term Refugia Program. The San Marcos salamander is listed as a Tier 2 priority (among four priority tiers) for additional research under the scope of work for the Long-Term Refugia Operation implemented between the EAA and the FWS on January 1, 2017. Subtask 2.3 of this scope of work directs the FWS to conduct research on the species’ physiology, environmental requirements, and life history, among others.
Since ensuring that silt-free gravel is a stated biological goal in the HCP, there is an expectation that the EAA will quantify this parameter at least annually. However, data on the extent of silt-free gravel are not presented in annual HCP or biomonitoring reports.
OTHER COVERED SPECIES
Other covered species include the Comal Springs dryopid beetle, Peck’s Cave amphipod, Edwards Aquifer diving beetle, Texas troglobitic water slater, Texas blind salamander, Comal Springs salamander, and San Marcos gambusia.
Description of the Organisms
The Comal Springs dryopid beetle (Stygoparnus comalensis) is a subterranean species inhabiting the Comal Springs system that was listed as endangered in 1997 (FWS, 1997). Comal Springs dryopid beetles are small (~3 mm), slender, reddish-brown beetles with vestigial eyes. Because of its inability to swim, the Comal Springs dryopid beetle is restricted to the headwaters of the springs and spring upwelling areas (EARIP, 2012). The subterranean nature and the habitat restriction (i.e., headwaters and upwelling areas of the spring) of the Comal Springs dryopid beetle suggest that it does not require substantial surface discharge from springs to survive and presume that spring flow (of sufficient water quality) that continually covers the spring orifice should prevent long-term detriment to the population (EARIP, 2012).
Peck’s Cave amphipod (Stygobromus pecki) is a subterranean species found in Comal and Hueco springs. This species was first described using specimens collected from Comal Springs in 1964 and 1965 (Holsinger, 1967). The Peck’s Cave amphipod was listed as endangered in 1997 (FWS, 1997). Like all members of the genus Stygobromus, Peck’s Cave amphipods
are eyeless, unpigmented, and approximately 3 mm long. It is believed that the Peck’s Cave amphipod is restricted to the headwaters of the springs and spring upwelling areas (EARIP, 2012).
The Texas blind salamander (Eurycea rathbuni, previously assigned to the genus Typhlomolge) is a relatively large (~90–135 mm total length), unpigmented, troglobitic salamander that was listed as endangered in 1967 (EARIP, 2012). Its snout is flattened and shovel-shaped, and it has small eyespots that are covered with skin. Its limbs are relatively long and thin. It has a prominent dorsal tailfin that extends from the rear legs to the tip of the tail. It is permanently aquatic and possesses enlarged reddish-colored gills (Petranka, 1998; Powell et al., 2016).
The Edwards Aquifer diving beetle (Haideoporus texanus), also known as the Texas cave diving beetle, is a small (typically less than 13 mm), blind, unpigmented, elongate, oval-shaped, and somewhat flattened member of the family Dytiscidae (Young and Longley, 1976). This species is restricted to the subterranean waters of the Edwards Aquifer in Hays and Comal Counties, where it has been collected from artesian wells and from Comal Springs (EARIP, 2012). The Edwards Aquifer diving beetle is not currently listed as endangered but is listed as under review by the FWS (2009).
The Texas troglobitic water slater (Lirceolus smithii) is a small, blind, unpigmented asellid isopod (Bowman and Longley, 1975). This species is known from two localities in Hays County—San Marcos Springs (Diversion Springs) and the artesian well that is located very close to San Marcos Springs. Specimens are rarely collected (EARIP, 2012). The Texas troglobitic water slater is not currently listed as endangered but is listed as under review by the FWS (2009).
The Comal Springs salamander (Eurycea sp.) has not been formally described as a unique species, but some authorities believe it is distinct due to restricted gene flow among other Eurycea of the Edward’s Plateau (Chippindale, 2000; Chippindale et al., 2000; Lucas et al., 2009). In their “Partial 90-Day Finding on a Petition to List 475 Species in the Southwestern United States as Threatened or Endangered with Critical Habitat,” the FWS (2009) identifies the Comal Springs salamander as Eurycea sp. 8. However, recent genetic research on the entire Eurycea complex in eastern Texas conducted at the University of Texas at Austin suggests that the salamanders inhabiting Comal Springs are a population of the Texas salamander, E. neotenes (D. Hillis, personal communication, February 6, 2018). That said, a study of gene flow among salamander habitat patches within the Comal Springs complex concluded that the Comal Springs salamander should be treated as a distinct management unit and a distinct evolutionarily significant unit for conservation purposes (Lucas et al., 2016). The Comal Springs salamander is not currently listed as endangered but is listed as “under review” by the FWS.
The San Marcos gambusia (Gambusia georgei) is a member of the family Poeciliidae. This small fish (2.5–4 cm as adults) was first described as one of three native Gambusia species in the San Marcos River by Hubbs and Peden (1969). Historically, San Marcos gambusia inhabited shaded, unsilted substrates in quiet, shallow, thermally constant, open waters adjacent to areas of flow (EARIP, 2012). The FWS designated the San Marcos River from the Highway-12 bridge downstream to just below the I-35 bridge as critical habitat for the San Marcos gambusia (FWS, 1996).
Biological Goals and Objectives
Ecological knowledge of the Comal Springs dryopid beetle, the Peck’s Cave amphipod, the Edwards Aquifer diving beetle, and the Texas troglobitic water slater is lacking, while relatively more research on the Texas blind salamander and the Comal Springs salamander is available. Regardless of available information, there are currently no HCP long-term biological goals for these species that can be addressed with biological data (Perkin et al., 2018). Therefore, the long-term biological goals for these species focus on water quality and spring flow. The water quality goal is that water quality should not exceed a 10 percent deviation (daily average) from historically recorded water quality conditions (long-term average) within the Edwards Aquifer as measured from the spring openings at Comal Springs. This includes all water quality constituents currently measured in the EAA Variable Flow Study. The HCP states that more extensive work to evaluate and assess water quality tolerances of Comal Springs dryopid beetles and Peck’s Cave amphipods will be addressed as part of the Adaptive Management Program (EARIP, 2012, p. 4-15). The current HCP Refugia program will maintain wild stock of each invertebrate and salamander species, including a genetic management plan for each, and, importantly, will allow for basic life history research that will inform capture and collection, husbandry, propagation, genetics, and reintroduction.
With respect to the spring flow requirement, it is believed that these species have the ability to retreat into subterranean refuges as spring flow declines and water levels subside into the spring vents. Nevertheless, the HCP is designed on the premise that these species require water levels adequate to support consistent spring flows. Thus, the discharge rates that are considered suitable for fountain darters (long-term average discharge of 225 cfs, minimum discharge of 30 cfs for no more than six months) are also considered suitable for these troglobitic invertebrates and salamanders (EARIP, 2012).
Because of high rates of hybridization with the western mosquitofish (Gambusia affinis), and the fact that no specimens have been collected since 1983, McKinney and Sharp (1995) concluded that the San Marcos River
gambusia was extinct. As such, there are no stated long-term biological goals for the San Marcos River gambusia.
Monitoring
The invertebrate species were monitored as part of the macroinvertebrate community drift-net surveys at four Comal Springs sources during 2003–2015 (the program continues today with modifications). The Comal Springs dryopid beetle and the Edwards Aquifer diving beetle were rarely captured in drift nets, whereas Texas troglobitic water slaters and especially Peck’s Cave amphipods were collected in high numbers during the 12-year study period. Both the Comal Springs dryopid beetle and the Peck’s Cave amphipod were considered in the recent statistical analysis of the biomonitoring datasets (Perkin et al., 2018). The primary conclusion drawn from the monitoring analysis was that both species abundance estimates were positively correlated with spring discharge rate, a pattern considered to be an artifact of higher flows forcing more animals into the drift nets. The Beaver Creek analysis documented a similar positive relationship with flow, suggesting that high flow could be related to higher DO concentrations, but the current dataset did not allow ruling out a simple artifact of higher flushing with flow. Additionally, the Beaver Creek analysis concluded that Peck’s Cave amphipods were more abundant in sampling sites with higher temperatures (western upwelling), while Comal Springs dryopid beetles were more abundant in sampling sites with cooler temperatures (Spring Runs 1 and 3) (Beaver Creek Hydrology, 2018). This analysis included additional data from seven sites in the Comal and San Marcos systems during 2013–2015, although it appears that the previous conclusion was derived using Comal Springs data only.
Because the Texas blind salamander is a subterranean species that is rarely observed near the surface, except in a few caves, the EAA is not conducting any monitoring activities for this species. While it has been stated that annual monitoring would be preferred, given the cryptic natural history of the species and the difficulty encountering them, the EAA has concluded that a monitoring program for the Texas blind salamander is not practical. By contrast, Comal Springs salamanders have been monitored via visual surveys by divers or snorkelers at least twice a year since 2001. Additional surveys have been conducted during periods of especially high and low flow. Observations consist of time-constrained searches in the deeper areas of Landa Lake by biologists using SCUBA. Salamanders are also monitored by snorkelers in the following reaches of the Comal system: Spring Run 1, Spring Run 3, and the Spring Island area. During searches, biologists scan the bottom and turn over rocks. Specific locations, time, water depth, and presence/absence of SAV are noted. Numbers of Comal Springs
salamanders counted during annual surveys vary considerably among sites, years, seasons, and spring flow levels. On average, more salamanders are documented at Spring Run 1 than the other spring outflow sites. Spring Island has the fewest observations over time. As demonstrated by data collected from 2002 through 2012, absolute numbers of salamanders counted (not corrected for effort) among the spring outflow sites during any given sampling period range from 0 (Spring Island) to more than 50 at Spring Run 1 (see Table 7 in BIO-WEST, 2013). There is no active monitoring program for the San Marcos gambusia.
CONCLUSIONS AND RECOMMENDATIONS
The habitat-based approach for the biological goals for the fountain darter (fountain darter density times submerged aquatic vegetation acreage), rather than an actual measure of fish abundance, is reasonable. However, the use of the cumulative median density in determining whether the biological goals are being met is problematic because this metric is very insensitive to year-to-year changes in fountain darter densities. It is imperative that the EAA consider a metric that reflects fountain darter density in recent years (e.g., a running mean or median over the most recent four years, or similar) for each vegetation type and monitor it relative to an unchanging baseline (e.g., the cumulative median from the first ten years in the Variable Flow Study dataset, or other appropriate baseline data). The development of the fountain darter population model was very effective in integrating the available information and should be leveraged in the future. Chapter 5 further discusses approaches to ensure a resilient and sustainable fountain darter population.
The biological goals for Texas wild rice are appropriate, and this species has benefited from extensive monitoring and decades of study. The EAA and its partners have taken particular care in the overall mapping of Texas wild rice in the San Marcos River. Considerable work done over the last century has revealed the life history and physiology of Texas wild rice, which has expanded our knowledge of the genetic framework of this species and its relatives. There are still some questions about relative competition of Texas wild rice versus other native and nonnative SAV, which could be addressed with mesocosm studies.
The long-term biological goals for Comal Springs riffle beetle density should be updated during Phase 2 of the HCP to reflect more quantitative and standardized monitoring methods. The density goals were based on data derived from the Variable Flow Study, which used an unstandardized sampling methodology with no standard operating procedure. It would also be useful to conduct new CSRB studies under the Applied Research Program to better substantiate the biological goal of maintaining silt-free
habitat. Beyond the uncertainties that went into deriving these biological goals, uncertainties associated with continued population monitoring and a lack of monitoring of the effects of riparian restoration on maintaining sufficient silt-free substrate make it difficult to understand compliance. A reevaluation of how annual median values of beetle abundance are calculated for compliance purposes is needed.
Both biological goals for the San Marcos salamander—target densities in three reaches and maintenance of silt-free gravel—are reasonable and biologically justifiable. In order to meet the abundance goals, the EAA should discontinue calculating cumulative median densities, which are insensitive to temporal variation in estimates of salamander densities, and instead adopt a metric that reflects salamander density in recent years. Furthermore, the EAA needs to begin monitoring adherence to the goal of maintaining silt-free substrates. Given the considerable spatial variation in salamander abundance data and the inability to accurately estimate salamander numbers, the current sampling method could be supplemented with an additional protocol that uses occupancy estimation. It is also important to eliminate any sampler biases during salamander monitoring. Finally, the San Marcos salamander would benefit from additional studies on its life history, particularly using refugia populations, similar to what has been done for the fountain darter, CSRB, and Texas wild rice and other SAV.
The EAA should continue to collect data as possible on all other non-sentinel species with the goal of eventually being in position to test the hypothesis that the sentinel species do indeed serve as viable proxies for protecting all Edwards Aquifer species.
REFERENCES
Alexander, M. L., and C. T. Philips. 2012. Habitats used by the endangered fountain darter (Etheostoma fonticola) in the San Marcos River, Hays County, Texas. The Southwestern Naturalist 57(4):449-452.
Beaver Creek Hydrology, LLC. 2018. Statistical analysis of the San Marcos and Comal Springs aquatic ecosystems biomonitoring datasets. Edwards Aquifer Authority. http://www.eahcp.org/files/admin-records/NEPA-and-HCP/Beaver_Creek_Statistical_Analysis_of_BioMonitoring_datasets.pdf.
Becker, S., J. Lily, and C. R. Gabor. 2012. Effects of Turbidity and Visual vs. Chemical Cues on Anti-Predator Response in the Endangered Fountain Darter (Etheostoma fonticola). Ethology 118(10):994-1000.
Berkhouse, C. S., and J. N. Fries. 1995. Critical thermal maxima of juvenile and adult San Marcos salamanders (Eurycea nana). The Southwestern Naturalist 40:430-434.
BIO-WEST. 2011a. Comprehensive and critical period monitoring program to evaluate the effects of variable flow on biological resources in the Comal Springs/River Aquatic ecosystem. Final 2010 Annual Report. Edwards Aquifer Authority.
BIO-WEST. 2011b. Comprehensive and critical period monitoring program to evaluate the effects of variable flow on biological resources in the San Marcos Springs/River Aquatic ecosystem. Final 2010 Annual Report. Edwards Aquifer Authority.
BIO-WEST. 2013. Comprehensive and Critical Period Monitoring Program to Evaluate the Effects of Variable Flow on Biological Resources in the Comal Springs/River Aquatic Ecosystems. Final Report to Edwards Aquifer Authority. http://www.eahcp.org/files/admin-records/NEPA-and-HCP/Comal_Final_2012_Annual_Report_Full_(Attachment_8).pdf.
BIO-WEST. 2014. Habitat Conservation Plan Biological Monitoring Program Comal Springs/River Aquatic Ecosystem. Annual Report to Edwards Aquifer Authority.
BIO-WEST. 2016. Habitat Conservation Plan Biological Monitoring Program Comal Springs/River Aquatic Ecosystem. Annual Report to Edwards Aquifer Authority.
BIO-WEST. 2017. Comal Springs Riffle Beetle (Heterelmis comalensis): Life History and Captive Propagation Techniques. Final Report prepared for the Edwards Aquifer Authority, December 27. http://www.eahcp.org/files/admin-records/NEPA-and-HCP/BIO-WEST_CSRB.life.hist_FINAL_20180126.pdf.
BIO-WEST and Watershed Systems Group. 2016. Submerged Aquatic Vegetation Analysis and Recommendations. Edwards Aquifer Habitat Conservation Plan. Contract No. 15-7-HCP. June.
Blanton and Associates. 2018. Edwards Aquifer Habitat Conservation Plan. 2017 Annual Report. http://www.eahcp.org/files/admin-records/NEPA-and-HCP/EAHCPAnnualReport2017v3_20180313_Redlined_from_Blanton.pdf.
Bosse, L. S., D. W. Tuff, and H. P. Brown. 1988. A new species of Heterelmis from Texas (Coleoptera: Elmidae). The Southwestern Naturalist 33(2):199-203.
Bowles, D. E., and T. L. Arsuffi. 1993. Karst aquatic systems of the Edwards Plateau region of central Texas, USA: a consideration of their importance, threats to their existence, and efforts for their conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 3:317-329.
Bowles, D. E., C. B. Barr, and R. Stanford. 2003. Habitat and phenology of the endangered riffle beetle Heterelmis comalensis and a coexisting species, Microcylloepus pusillus, (Coleoptera: Elmidae) at Comal Springs, Texas, USA. Archiv für Hydrobiologie 156(3):361-383.
Bowman, T. E., and G. Longley. 1975. Redescription and assignment to the new genus Lirceolus of the Texas troglobitic water slater, Asellus smithii (Ulrich) (Crustacea: Isopoda: Asellidae). Proceedings of the Biological Society of Washington 88:489-496.
Brandt, T. M., K. G. Graves, C. S. Berkhouse, T. P. Simon, and B. G. Whiteside. 1993. Laboratory spawning and rearing of the endangered fountain darter. The Progressive Fish-Culturist 55(3):149-156.
Brown, H. P. 1987. Biology of riffle beetles. Annual Review of Entomology 32:253-273.
Chippindale, P. T. 2000. Species boundaries and species diversity in the central Texas hemidactyline plethodontid salamanders, genus Eurycea. Pp. 149-165 In: The Biology of Plethodontid Salamanders. R. C. Bruce, R. G. Jaeger, and L. D. Houck (eds.). New York: Springer Science+Business Media.
Chippindale, P. T. 2005. Eurycea rathbuni Texas Blind Salamander. Pp. 760-762 In: Amphibian Declines: The Conservation Status of United States Species. M. J. Lanoo (ed.). Berkeley: University of California Press.
Chippindale, P. T., and J. N. Fries. 2005. Eurycea nana San Marcos Salamander. Pp. 755-756 In: Amphibian Declines: The Conservation Status of United States Species. M. J. Lanoo (ed.). Berkeley: University of California Press.
Chippindale, P. T., and A. H. Price. 2005. Conservation of Texas spring and cave salamanders (Eurycea). Pp. 193-197 In: Amphibian Declines: The Conservation Status of United States Species. M. J. Lanoo (ed.). Berkeley: University of California Press.
Chippindale, P. T., A. H. Price, J. J. Wiens, and D. M. Hillis. 2000. Phylogenetic relationships and systematic revision of central Texas hemidactyline plethodontid salamanders. Herpetological Monographs 14:1-80.
Cooke, M. 2012. Natural history studies on the Comal Springs riffle beetle (Heterelmis comalensis). Doctoral dissertation, Texas State University-San Marcos.
Dammeyer, N. T., C. T. Phillips, and T. H. Bonner. 2013. Site fidelity and movement of Etheostoma fonticola with implications to endangered species management. Transactions of the American Fisheries Society 142(4):1049-1057.
Davis, D. R., K. J. Epp, and C. R. Gabor. 2012. Predator generalization decreases the effect of introduced predators in the San Marcos salamander, Eurycea nana. Ethology 118:1191-1197.
Dayton, L. 2016. Atmospheric carbon dioxide soars past crucial milestone. Science [May 16]; DOI: 10.1126/science.aaf5722.
Denes, F. V., L. F. Silveira, and S. R. Beissinger. 2015. Estimating abundance of unmarked animal populations: Accounting for imperfect detection and other sources of zero inflation. Methods in Ecology and Evolution 6:543-556.
EAHCP (Edwards Aquifer Habitat Conservation Plan). 2016. Comal Springs Riffle Beetle Cotton Lure SOP. http://www.eahcp.org/files/uploads/CSRB_SOP_Final.pdf.
EARIP (Edwards Aquifer Recovery Implementation Program). 2012. Edwards Aquifer Recovery Implementation Program Habitat Conservation Plan. http://www.eahcp.org/files/uploads/Final%20HCP%20November%202012.pdf.
Epp, K. J., and C. R. Gabor. 2008. Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology 114:607-615.
Fries, J. N. 2002. Upwelling flow velocity preferences of captive adult San Marcos salamanders. North American Journal of Aquaculture 64:113-116.
Furl, C. 2017. Conservation Measures, Biological Objectives, Biological Goals. Presentation to the National Academies’ Committee to Review the Edwards Aquifer Habitat Conservation Plan, October 2.
FWS (U.S. Fish and Wildlife Service). 1978. Proposed Listing and Critical Habitat Determination for a Fish and Salamander. Federal Register 43:30316-30319.
FWS. 1995. San Marcos/Comal (Revised) Recovery Plan. Albuquerque, New Mexico. pp. x + 93 with 28 pages of appendices.
FWS. 1996. San Marcos and Comal Springs and associated aquatic ecosystems (revised) recovery plan. Department of Interior, Fish and Wildlife Service.
FWS. 1997. 50 CFR Part 17: Endangered and threatened wildlife and plants; final rule to list three aquatic invertebrates in Comal and Hays counties, TX, as endangered. Federal Register 62(243):66295.
FWS. 2008. Strategic Habitat Conservation Handbook: A Guide to Implementing the Technical Elements of Strategic Habitat Conservation (Version 1.0). U.S. Fish and Wildlife Service. Report from the National Technical Assistance Team. February 11, 2008.
FWS. 2009. Part 3 Department of the Interior, Fish and Wildlife Service Endangered and threatened wildlife and plants; partial 90-day finding on a petition to list 475 species in the southwestern United States as threatened or endangered with critical habitat; proposed rule. 74 74 Fed. Reg. 66866-66905 (December 16, 2009).
FWS. 2013. News Release: Service Approves Edwards Aquifer Recovery Implementation Program’s Incidental Take Permit. Department of Interior. Fish and Wildlife Service.
Hardy, T. B., K. Kollaus, and K. Tower. 2010. Evaluation of the Proposed Edwards Aquifer Recovery Implementation Program Drought of Record Minimum Flow Regimes in the Comal and San Marcos River Systems. River Systems Institute, Texas State University.
Hitchcock, A. S. 1933. New Species and new names of grasses from Texas. Journal of the Washington Academy of Science 23:449-456.
Holsinger, J. R. 1967. Systematics, speciation, and distribution of the subterranean amphipod genus Stygonectes (Gammaridae). Bulletin—United States National Museum 259:1-176.
Hubbs, C., and A. E. Peden. 1969. Gambusia georgei sp. nov. from San Marcos, Texas. Copeia 2:357-364.
Labay, A. A., and T. M. Brandt. 1994. Predation by Cyclops vernalis on Florida largemouth bass and fountain darter larvae. The Progressive Fish-Culturist 56(1):37-39.
Lucas, L. K., Z. Compert, J. R. Ott, and C. C. Nice. 2009. Geographic and genetic isolation in spring-associated Eurycea salamanders endemic to the Edwards Plateau region of Texas. Conservation Genetics 10:1309-1319.
Lucas, L. K., Z. Compert, J. R. Gibson, K. L. Bell, C. A. Buerkle, and C. C. Nice. 2016. Pervasive gene flow across critical habitat for four narrowly endemic, sympatric taxa. Freshwater Biology 61:933-946.
McDonald, D. L., T. H. Bonner, E. L. Oborny Jr., and T. M. Brandt. 2007. Effects of fluctuating temperatures and gill parasites on reproduction of the fountain darter, Etheostoma fonticola. Journal of Freshwater Ecology 22(2):311-318.
McKinney, D. C. and J. M. Sharp. 1995. Springflow augmentation of Comal Springs and San Marcos Springs, Texas: Phase I - feasibility study. Center for Research in Water Resources Technical Report CRWR 247. Bureau of Engineering Research, University of Texas at Austin, TX.
Najvar, P. A., J. N. Fries, and J. T. Baccus. 2007. Fecundity of San Marcos salamanders in captivity. The Southwestern Naturalist 52:145-147.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Review of the Edwards Aquifer Habitat Conservation Plan: Report 2. Washington, DC: The National Academies Press.
Nelson, J. 1993. Population Size, Distribution, and Life History of Eurycea nana in the San Marcos River. M.S. Thesis, Texas State University.
Norris, W. E., Jr., P. A. Grandy, and W. K. Davis. 1963. Comparative studies of the oxygen consumption of three species of neotenic salamanders as influenced by temperature, body size, and oxygen tension. The Biological Bulletin 125:523-533.
Nowlin, W. H., D. Hahn, P. Nair, and F. Alfano. 2016a. Evaluation of the trophic status and functional feeding group status of the Comal Springs Riffle Beetle. EAHCP Project No. 148-15-HCP. http://www.eahcp.org/documents/Nowlin%20et%20al.%202017%20CSRB%20FFG.PDF.
Nowlin, W. H., P. Nair, and B. Schwartz. 2016b. Final Report. Evaluation of the long-term elevated temperature and low dissolved oxygen tolerances of larvae and adult Comal Springs riffle beetle. EAHCP Project No. 146-15-HCP. http://www.eahcp.org/files/uploads/Nowlin_et_al._2017_EAA_2016_Applied_Research_-_CSRB_Long_Term_Temp_and_D....pdf.
NRC (National Research Council). 2015. Review of the Edwards Aquifer Habitat Conservation Plan: Report 1. Washington, DC: The National Academies Press.
Perkin, J. S., E. Kosnicki, and J. Jackson. 2018. Analysis of the Comal Springs and San Marcos Springs long-term monitoring dataset. Edwards Aquifer Authority.
Petranka, J. W. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington, DC.
Poole, J., and D. E. Bowles. 1999. Habitat characterization of Texas wild-rice (Zizania texana Hitchcock), an endangered aquatic macrophyte from the San Marcos River, TX, USA. Aquatic Conservation: Marine and Freshwater Ecosystems 9:291-302.
Powell, R., R. Conant, and J. T. Collins. 2016. Peterson Field Guide to Reptiles and Amphibians of Eastern and Central North America, Fourth Edition New York: Houghton Mifflin Harcourt.
Power, P. 1996. Effects of current velocity and substrate composition on growth of Texas wild rice (Zizania texana). Aquatic Botany 55:199-204.
Power, P., and R. D. Doyle. 2004. Carbon use by the endangered Texas Wild Rice (Zizania texana, Poaceae). SIDA Contributions in Botany 21(1):389-398.
Rose, F., and P. J. Power. 2001. Continued Maintenance, Reintroduction and Research on Zizania texana (Texas Wildrice). Grant E#3, Report to Texas Parks and Wildlife.
RPS Espey, HEI, and BIO-WEST. 2014. 2014 Final Report for Riffle Beetle Habitat Restoration Spring Run 3 and Landa Lake Shoreline. Prepared for City of New Braunfels. December 22, 2014.
Schenck, J. R., and B. G. Whiteside. 1977. Food habits and feeding behavior of the fountain darter, Etheostoma fonticola (Osteichthyes: Percidae). The Southwestern Naturalist 21(4):487-492.
Staver, L. W., and J. C. Stevenson. 1995. The impacts of the exotic species Hydrilla verticillata on the shallows in Chesapeake Bay. Pp 364-370 in Toward a Sustainable Coastal Watershed: The Chesapeake Experiment. Proceedings of a Conference. Shady Side MD.
Taiz, L., E. Zieger, I. A. Møller, and A. Murphy, A. 2014. Plant Physiology and Development, sixth edition. Sunderland, Massachusetts: Sinauer Associates.
Terrell, E. E. 2007. Zizania. Pp. 47-51, In: Flora of North America, Vol. 24. UK: Oxford University Press.
Thaker, M., C. R. Gabor, and J. E. Fries. 2006. Sensory cues for conspecific associations in aquatic San Marcos salamanders. Herpetologica 62:151-155.
Tupa, D. D., and W. L. Davis. 1976. Population dynamics of the San Marcos salamander, Eurycea nana Bishop. Texas Journal of Science 27:179-195.
Wetzel, R. G., and G. E. Likens. 1991. Limnological Analysis, 2nd ed. New York: Springer.
Wilson, W. D., J. T. Hutchinson, and K. G. Ostrand. 2017. Genetic diversity assessment of in situ and ex situ Texas wild rice (Zizania texana) populations, an endangered plant. Aquatic Botany 136:212–219.
Xu, X., C. Walters, M. F. Antoin, M. L. Alexander, S. Lutz, S. Ge, and J. Wen. 2010. Phylogeny and biogeography of the eastern Asian–North American disjunct wild-rice genus (Zizania L., Poaceae). Molecular Phylogenetics and Evolution 55(3):1008-1017.
Xu, X.-W., J.-W. Wu, M.-X. Qi, Q.-X. Lu, P. F. Lee, S. Lutz, S. Ge, and J. Wen. 2015. Comparative phylogeography of the wild rice genus Zizania (Poaceae) in Eastern Asia and North America. American Journal of Botany 102(2):239–247.
Young, F. N., and G. Longley. 1976. A new subterranean aquatic beetle from Texas (Coleoptera: Dytiscidae-Hydroporinae). Annals of the Entomological Society of America 69:787-792.






































