Proceedings of a Workshop
| IN BRIEF | |
September 2018 |
Geologic Capture and Sequestration of Carbon
Proceedings of a Workshop—in Brief

Carbon dioxide removal (CDR) techniques, which aim to remove and sequester excess carbon from the atmosphere, have been identified as an important part of the possible responses to climate change and have been garnering increased attention.1 The Committee on Developing a Research Agenda for Carbon Dioxide Removal and Sequestration was convened to develop a detailed research and development agenda to assess the benefits, risks, and sustainable scale potential for CDR and sequestration approaches, as well as increase their commercial viability. The CDR approaches under consideration by the committee are coastal and land ecosystem management, carbon mineralization (sometimes known as accelerated weathering), bioenergy with carbon capture, direct air capture, and geologic sequestration. To aid the development of the research agenda, each approach is being examined by the committee through a series of information-gathering workshops and webinars for open discussions with relevant communities about the current state of knowledge, along with the research needs for understanding the potential of each approach and for deploying them at large scales.
Geologic carbon capture and sequestration encompasses approaches for relatively permanent storage of carbon in the Earth’s geologic formations. Carbon dioxide (CO2) that has been captured from flue gas or other waste streams as pressurized fluids can be trapped geologically through thermodynamically favorable reactions between CO2 and silicate rocks to create stable mineral carbonates. This mineralized carbon is stored permanently in silicate-bearing rocks. CO2 can also be captured as dissolved or supercritical CO2 and stored in the subsurface pore space of sedimentary rock, trapped under impermeable layers. The committee convened a webinar on November 15 and a workshop on November 28, 2017, in Palo Alto, California, to discuss carbon mineralization and subsurface storage approaches. Included in these discussions were presentations by speakers on the state of science and deployment, research and monitoring needs, potential risks, and costs of geologic capture and storage. This Proceedings of a Workshop—in Brief summarizes the presentations from both the webinar and workshop.
OVERVIEW OF GEOLOGIC CAPTURE AND SEQUESTRATION APPROACHES
Peter Kelemen from Columbia University, a member of the committee, provided an overview of the carbon mineralization process and its value for CDR. The primary reactive materials are (1) basalt, which is common in the Earth’s crust and has high porosity and permeability, but reacts slowly relative to peridotite; and (2) peridotite, which reacts faster, but is less common and has low porosity and permeability. Mineralization is a spontaneous reaction, and can be used to capture CO2 at a relatively low cost. This natural process can be exploited for CDR by increasing the interaction of the reactants (captured CO2 and reactive minerals). These reactions can occur ex situ, where the reactant minerals are brought to a CO2 source (e.g., a power plant). Typically, peridotite that has been ground into pieces to increase surface
__________________
1 National Research Council. 2015. Climate Intervention: Carbon Dioxide Removal and Reliable Sequestration. Washington, DC: The National Academies Press.
![]()
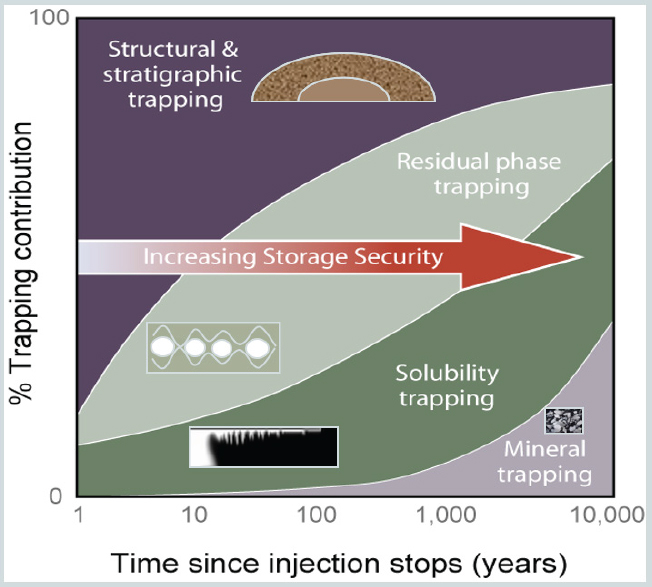
SOURCES: Presentation by Sally Benson; modified from Benson, S., P. Cook, J. Anderson, S. Bachu, H. B. Nimir, B. Basu, J. Bradshaw, G. Deguchi, J. Gale, G. von Goerne, W. Heidug, S. Holloway, R. Kamal, D. Keith, P. Lloyd, P. Rocha, B. Senior, J. Thomson, T. Torp, T. Wildenborg, M. Wilson, F. Zarlenga, and D. Zhou. 2005. Underground geological storage. In IPCC Special Report on Carbon Dioxide Capture and Storage. B. Metz, O. Davidson, H. d. Coninck, M. Loos and L. Meyer, eds. Cambridge, UK: Cambridge University Press.
area is used for these reactions, which Kelemen suggested may cost about $10/t CO2. Costs can potentially be lowered by using flue gas rather than purified CO2. In situ reactions occur when CO2 is injected into the rock. To date, in situ pilot experiments have been performed in basalt. In situ injection does not involve grinding to increase surface area, but rather relies on the permeability of the formation (high in basalt) and/or the development of cracks caused by the increasing pressure from carbonate formation (observed in peridotite). However, he noted that negative feedbacks can occur when permeability is reduced as cracks fill with carbonate. Kelemen estimated that the storage capacity for this approach is large—approximately 1 Gt CO2/km3 of rock based on existing stores of reactant. Globally, there is a 100 trillion ton capacity for storage within peridotite located 3 km deep on the continents; the seafloor could have a capacity that is one thousand times greater. Basalt is even more common than peridotite. The disadvantages that Kelemen said would need to be overcome include potentially slow kinetics; costs for mining, transport, and grinding for ex situ reactions; and negative feedbacks from permeability for in situ reactions.
Sally Benson from Stanford University, a member of the committee, categorized potential storage approaches in sedimentary rock into four options: (1) injection into depleted oil and gas reservoirs, (2) use of CO2 as part of enhanced oil and gas recovery, (3) injection into deep saline formations (onshore and offshore), and (4) use of CO2 in enhanced coal bed methane recovery (and possibly shale). Secondary trapping mechanisms may increase storage stability over time (see Figure 1). These mechanisms include solubility trapping, where the CO2 dissolves in water; residual gas trapping, where CO2 is trapped by capillary forces; and mineral trapping, where CO2 is mineralized (though a smaller fraction than in basalt) or adsorbs onto insoluble organic matter in shale and coal. Secondary trapping increases over time, reducing the dependence on the primary seal. Their effectiveness and progress over time is site-specific. For example, in a closed structural trap, solubility trapping and capillary trapping is minimal because CO2 does not move much through the pore space. In a hydrodynamic trap (a saline aquifer) where there is significant horizontal migration, solubility trapping is rapid, capillary trapping is extensive, and more mineralization occurs because the CO2 is continuously exposed to rock during migration. Where many reactive minerals are present, an increasing fraction of CO2 is converted to carbonate minerals slowly over time.
Benson identified a potential global storage capacity between 5,000 and 25,000 Gt CO2.2 This estimate is quite uncertain, in part because assessments consider different factors when calculating storage capacity. In addition, many prospective areas have not been studied or have characteristics that require more detailed study. Large-scale projects for capturing CO2 from anthropogenic sources and storing it underground are expanding worldwide. These projects began in the 1970s, largely as a component of enhanced oil recovery, but the Norwegian Sleipner offshore project in
__________________
2 de Coninck, H. and S. Benson. 2014. Carbon Dioxide Capture and Storage: Issues and Prospects. Annual Review of Environment and Resources 39:243–270.
the 1990s set the stage for using the underground storage approach for greenhouse gas mitigation. Benson estimated that current projects are storing approximately 19 Mt of CO2/year from anthropogenic sources. In addition, 65 Mt CO2/year is being stored as part of enhanced oil recovery efforts, which use mainly natural sources of CO2. Projects currently under construction could provide an additional 9 Mt CO2/year.
REACTION KINETICS AND MECHANICS OF CARBON MINERALIZATION
Greeshma Gadikota from the University of Wisconsin—Madison described laboratory studies undertaken to understand the chemical, morphological, and mechanical processes influencing carbon mineralization reaction kinetics. She explained that understanding CO2 storage under various conditions could clarify storage potential as well as inform optimal conditions for engineering reactions at faster rates and larger scales. The mineralization process consists of paired reactions: (1) dissolution of silicate rock surfaces into a cation-rich solution and (2) precipitation of mineral carbonates when the solution is combined with hydrated CO2. These may occur as a coupled reaction or physically separated into two phases. In a two-phase process, conditions can be tuned to optimize each reaction. For example, a lower pH is optimal for dissolution while a higher pH promotes precipitation. However, a coupled reaction is a more realistic situation for injection into geologic formations.
Geologic formations are heterogeneous in their composition and morphology. Gadikota and colleagues conducted laboratory experiments to compare the reaction rates of various minerals in single-phase reactions, which show that calcium- and magnesium-bearing silicates are the most reactive while alumino-silicates have much lower reactivity. These minerals can be made more reactive in engineered conditions. Specifically, reaction rates have been increased in the laboratory setting by using temperatures up to 185ºC, completing in a few hours reactions that would take 100 years to occur naturally. The pH can also be kept in an optimal zone by the presence of sodium bicarbonate acting as a pH buffer. The morphological result of these increased rates is an increase in grain sizes and reduced pore space. Gadikota and colleagues have engineered reactions to form magnesite, a very stable carbonate, bypassing less stable hydrous mineral stages. The mineralization reaction can be slowed (a process termed passivation) by the formation of alumino-silicates (clays), if magnesium and calcium react with silica. The alumino-silicates will also plug pore space. She found this to be a limiting factor for basalt (a mixed mineral rock) but not olivine (a magnesium-bearing silicate). Because basalt is a widely available rock type globally, she noted it is important to identify its limiting factors for carbonate formation. Olivine is also widely available in specific places, such as Oman. Gadikota noted that there is wide variability in published reaction rates, in part due to differences in sample preparation and different experimental approaches and conditions.
Gadikota addressed the possibility that carbon mineralization could result in a reduction in net CO2 emissions. She described a study that modeled how at 155ºC, olivine provided substantial CO2 reductions compared to the emissions created in the mineralization process.3 Mining industries could potentially offset their own emissions because sequestration using the calcium and magnesium-bearing waste products could outweigh the CO2 emissions.4 In comparison, power generation, iron and steel production, cement production, and aluminum production do not produce enough reactant wastes to offset their emissions. Utilization of the mineralized byproduct as construction material could offset costs associated with carbon mineralization, though she said the lack of life cycle assessments prevents full quantification of the potential benefits. She provided an estimate that grinding and milling of rock for ex situ reactions, along with chemical processing, costs about $20 to $60 per ton of rock. Assuming about 35 to 40% reactivity, the costs per ton of CO2 may range from $40 to about $125. She estimated that the value of the resulting materials for other uses might range from $5–15 per ton of rock.
Marco Mazzotti from ETH Zürich described the potential for the use of flue gas for ex situ reactions as a way to lower the costs of mineralizing carbon. While the reaction kinetics for flue gas would be slower, this process removes the cost of capture and transport required for in situ methods. To evaluate the scenario, Mazzotti compared reactions using pure CO2 to reactions with flue gas (which has a lower partial pressure than pure CO2) when characterizing (1) the dissolution kinetics of heat-activated serpentine, (2) passivation by silica, and (3) the precipitation kinetics of the carbonate produced from serpentine (hydromagnesite). He tested dissolution and precipitation as a single-step process as well as a two-step process to optimize temperature and pressure conditions. He found that the use of flue gas yields
__________________
3 Kirchofer, A., A. Brandt, S. Krevor, V. Prigiobbe, and J. Wilcox. 2012. Impact of alkalinity sources on the life-cycle energy efficiency of mineral carbonation technologies. Energy & Environmental Science 5(9):8631–8641.
4 Gadikota, G., K. Fricker, S.-H. Jang, and A.-H. Park. 2015. Carbonation of Silicate Minerals and Industrial Wastes and Their Potential Use as Sustainable Construction Materials. In Advances in CO2 Capture, Sequestration, and Conversion. F. Jin, L. He, and Y. H. Hu, eds. American Chemical Society. Pp. 295–322.

SOURCE: Peter Kelemen
a mineralization efficiency of only 25–30%, which is likely too low to be economically viable. His future experiments will test different reactant materials, such as waste cement, which contain calcium carbonates and reduce mining costs.
The Earth has a vast supply of reactive minerals for in situ reactions, but reactions will only occur if the mineral surface is exposed to reactive fluids. Marc Spiegelman from Columbia University discussed his research on the mechanics of mineralization and the subsequent cracking and increase in reactive surface area. Mineralization reactions are known to occur naturally in the subsurface (see Figure 2) and the question Spiegelman poses is: how did it occur? Isotopic 14C dating shows that these natural, mineralized veins in some rocks have been forming for at least 50,000 years (the limit of 14C dating). At a fine scale, a rock like peridotite is composed of a mineral matrix with pore spaces that become infiltrated by water and form minerals such as serpentine or listvenite. He explained that these reactions often lead to significant increases in volume (e.g., full mineralization of olivine, a dominant mineral in peridotite, leads to a volume increase of 84%). Spiegelman noted that in nature, it appears that mineralization does cause cracking of the rock matrix, which continually enhances permeability.5
Spiegelman described theoretical models of the mechanics of natural carbon mineralization. Questions of interest to investigate with these models are how reactive fluids are transported through impermeable rocks, whether reactions lead to clogged pores or enhance cracking, whether the chemical potential energy can drive fractures, and the time scales at which these mechanisms occur. Spiegelman explained that the components of the system that a reactive cracking theory should incorporate are: (1) conservation of mass, momentum, and energy for fluids and solids; (2) thermodynamically consistent reactions; and (3) volume changes and stresses in complex rheologies. Spiegelman identified three types of models that have been developed, which have varying strengths in describing a particular aspect of cracking. Single crack reactive models describe wedgºe cracking at a micro-scale driven by oversaturated fluid. Discrete element models describe fractures caused by swelling. Coupled multi-physics continuum models describe fluid flow based on pressure gradients and reactions driven by thermodynamics. According to Spiegelman, models suggest that olivine carbonation should generate cracking, but there are significant uncertainties in the mechanics that require experimental validation and model improvement. He suggested that future research directions could include integration of full reactive cracking models, exploring feedbacks between cracking and material properties, expansion into dissolution/precipitation reactions, and experimental validation on brittle systems. Ultimately, comprehensive understanding will help determine the practicality of reactive cracking mechanisms for permanent carbon storage.
Chris Spiers from Utrecht University discussed the feasibility of in situ peridotite carbonation based on the possibility of reaction-driven fracture formation. Mineralization of peridotite creates carbonates and silicates that take up substantially more volume than the peridotite. In theory, the increase in volume increases stress and opens fractures, which increase the surface area available to react with additional injected CO2, thus repeating the cycle by creating new carbonates and silicates that open new fractures. Spiers described experiments to test this theoretical cycle, which focused on: (1) whether olivine dissolution rates in fractures are fast enough to provide sufficient magnesium and iron reactants, (2) how much stress is generated by precipitation in fractures, and (3) whether the stress can cause ongo-
__________________
5 Kelemen, P. and J. Matter. 2008. In situ carbonation of peridotite for CO2 storage. Proceedings of the National Academy of Sciences 105(45):17295–17300.
ing fracturing. Experiments by Spiers and collaborators showed that the rate of dissolution of olivine is fast enough for carbon mineralization to be feasible.6 While theoretical calculations show that the stress generated should be sufficient to create fractures, no stress was generated in 19 experiments on crushed or ground peridotite under a range of temperature, pressure, and duration conditions (one additional sample did produce stress, but the team was unable to reproduce the effect in subsequent experiments).7 Instead, it appears that mineralization caused the pores to clog or heal, cutting off the supply of CO2 except by diffusion. Calculations based on diffusion rates suggest that the timescale at which a new 1 m length fracture might appear is about 300 to 6,000 years. However, models and experiments from other investigators suggest that reaction-driven expansion can produce fractures.8 Spiers suggested that it is important to find out what causes carbonation of peridotite under various natural conditions, and to test other means (e.g., hydration) to promote reaction-driven fracture.
LABORATORY AND FIELD DEMONSTRATION OF CARBON MINERALIZATION IN BASALT
The capability for storing carbon in basaltic formations has been demonstrated in field experiments. Todd Schaef from Pacific Northwest National Laboratory described laboratory and field demonstrations of the capability for effective carbon mineralization and sequestration in basalts. He explained that the advantage of storing carbon in basalts is their favorable morphology, mineralogy, and worldwide distribution. Flood basalt forms in layers from lava flows; each layer has a porous flow top and thick impermeable interflow zones that can serve as a caprock between permeable layers. The Columbia River Basalt, a potential reservoir for storage in North America, covers 150 km2 and is up to 5 km thick. The mineralogy of the basalt is very reactive, particularly the glass basalt matrix. Laboratory experiments conducted by Schaef and colleagues on crushed Newark Basin basalt resulted in complete coverage by carbonates in 2.5 years when exposed to aqueous-dissolved CO2 (at 100 Bars, 100ºC). Conducting the experiments at a higher temperature and pressure resulted in morphological changes of the carbonates into long fibers and globules. Changes in carbonate chemistry also resulted in the transition of products from calcite to ankerite. Schaef described additional experiments on the influence of varying amounts of water on mineralization of supercritical CO2, particularly the threshold temperatures, reaction rates, and reaction products.
Schaef described the basalt carbon sequestration pilot project located in Wallula, Washington. He emphasized that a key component of the project was engaging the local community to communicate information about the project, especially because it is located near a densely populated area. Baseline seismic monitoring was conducted in 2007 to characterize the subsurface and ensure its suitability for carbon storage (e.g., absence of disqualifying faults). Drilling began in 2009 and a permit to begin injection was issued in 2011. Approximately 1,000 Mt of CO2 were injected in July and August 2013. Air and soil monitoring was conducted until July 2015. Final well characterization surveys in 2015 consisted of wireline surveys, sidewall coring, and hydrologic testing. Observations of water saturation from the wireline surveys show that water was replaced by CO2 in two of the three injection zones (the third zone had lower permeability). The thermal signature of the heated CO2 also persisted for 22 months. Hydraulic tests indicate that the formation still has some compressibility, suggesting that unreacted CO2 may remain. Groundwater samples show that total dissolved solids, alkalinity, calcium, and magnesium increased post-injection. Sidewall cores obtained post-injection contain nodules of carbonate filling the pore space composed of ankerite, a carbonate that does not occur naturally in the rock and is isotopically distinct from the native calcite (see Figure 3). Examination of the ankerite’s layers show that they are initially dominated by calcium but become iron-rich as precipitation progresses, which is to be expected as more cations are dissolved. Schaef noted that there was no indication that pore space became impermeable, though more time may be needed to make this assessment. He concluded by stating that the Wallula field demonstration validated laboratory predictions that carbonation in basalt with supercritical CO2 could occur on relatively short time scales (on the order of a few years).
Edda Aradóttir from Reykjavík Energy described CarbFix, an industrial scale demonstration of carbon sequestration in basalt in Iceland. The CarbFix project injects CO2 into the Hellisheidi geothermal field, which is observed to
__________________
6 van Noort, R., C. J. Spiers, M. R. Drury, M. T. Kandianis. 2013. Peridotite dissolution and carbonation rates at fracture surfaces under conditions relevant for in situ mineralization of CO2. Geochimica et Cosmochimica Acta 106:1–24.
7 van Noort, R., T. Wolterbeek, M. Drury, M. Kandianis, and C. Spiers. 2017. The Force of Crystallization and Fracture Propagation during In-Situ Carbonation of Peridotite. Minerals 7(10):190.
8 Zhu, W., F. Fusseis, H. Lisabeth, T. Xing, X. Xiao, V. De Andrade, and S.-I. Karato. 2016. Experimental evidence of reaction-induced fracturing during olivine carbonation. Geophysical Research Letters 43(18):9535–9543.

SOURCES: Presentation by Todd Schaef; Reprinted with permission from McGrail, B., H. Schaef, F. Spane, J. Cliff, O. Qafoku, J. Horner, C. Thompson, A. Owen, and C. Sullivan. 2016. Field Validation of Supercritical CO2 Reactivity with Basalts. Environmental Science & Technology Letters 4(1):6–10. Copyright 2016 American Chemical Society.
be storing an estimated 1,650 Mt CO2 naturally,9 an amount that is 750 times greater than Iceland’s anthropogenic emissions. An initial pilot test was located near a geothermal power plant, which captured 2% of its waste stream. The industrial scale site initiated operations in 2014; it is co-located with the power plant and uses existing infrastructure for injection and monitoring. CarbFix is currently capturing and injecting one-third of the power plant’s CO2, and also has the added benefit of capturing two-thirds of the hydrogen sulfide emissions. The injection field is shallow (400–800 m) and does not have an impermeable caprock layer; to avoid leakage issues that would arise with the use of supercritical CO2, dissolved CO2 is used. This requires 25 tons of water per ton of CO2 injected. Reykjavík Energy injects about 25 million tons of geothermal brine into the rock each year at more than 700 m in depth and 250ºC. The current injection flow path is estimated to have a storage capacity of about 5 Mt CO2. Estimates of the storage potential for the whole field vary, with a median value of 697 Mt CO2. Rather than storage space or access to CO2, Aradóttir identified the limiting factor for injection as the water needed to inject the CO2. She cited work showing that the storage capacity of the rift zone of Iceland is 1,000–2,500 Gt CO2.10 Globally, 5% of the continents have basaltic terrains,11 while the capacity of ocean ridges is estimated to be 100,000–250,000 Gt CO2 (surpassing the 18,500 Gt CO2 of excess carbon) with the advantage of being a readily available supply of water.
Aradóttir described the methods used to verify and quantify carbon mineralization progress in CarbFix. Tracers used to track the arrival of the injection at a monitoring well show that a small percentage of the flow arrives quickly (56–58 days), with the remaining arriving at a slower rate (peaks at 350 and 406 days). There is also an immediate drop in pH and rise in dissolved inorganic carbon when the injection plume arrives. Surface and atmospheric measurements of CO2 show no sign of leakage, indicating complete dissolution of the CO2. The results of the pilot phase show that more than 95% of the injected CO2 was mineralized in 2 years. The submersible pump was clogged with calcite that contained the 14C tracer in 1.5 years, indicating that the injected carbon was being mineralized. Though this clogging indicates permeability could be reduced within a couple years of injection, Aradóttir noted that this depends on the permeability of the reservoir; in this case, natural seismic activity causes cracking that can create new reactive surfaces. Similar results are found in the upscaled project, and it appears most is mineralized within a year. Mass balance calculations show a loss of carbon from the plume, most easily explained by carbon precipitation since no leakage has been detected. Calcite was the only carbonate directly identified in the pilot phase, though they expect a range of carbonates based on reactive path modeling. Aradóttir described future plans to evaluate the large amount of data that has been collected, including seismic data. CarbFix has cost $25 million since its inception in 2007, inclusive of all activities from planning to implementation, and most
__________________
9 Weise F., T. Fridriksson, and H. Ármannsson. 2008. CO2 Fixation by Calcite in High-temperature Geothermal Systems in Iceland. Tech. Rep., ISOR−2008/003, Iceland Geosurvey. www.os.is/gogn/Skyrslur/ISOR-2008/ISOR-2008-003.pdf.
10 Snæbjörnsdóttir, S., F. Wiese, T. Fridriksson, H. Ármansson, G. Einarsson, and S. Gislason. 2014. CO2 storage potential of basaltic rocks in Iceland and the oceanic ridges. Energy Procedia 63:4585–4600.
11 Dessert, C., B. Dupré, J. Gaillardet, L. François, and C. Allègre. 2003. Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chemical Geology 202(3-4):257–273.
of this is the cost of building the initial infrastructure. This comes to a cost per injection of $1,900/t CO2 if all stages of the project are considered. The operating cost from capture to monitoring operations are about $30/t CO2. The added benefit of capturing sulfur could lower the per ton cost of CO2 storage.
David Goldberg from Columbia University’s Lamont-Doherty Earth Observatory described the feasibility study for large-scale CO2 storage in the offshore basalt reservoir of the Cascadia Basin. This project builds on the demonstration by the Wallula and CarbFix projects that mineralization can occur rapidly in basalt, and tests the ability to scale up to larger areas (on the order of 50 MMt CO2). Among the issues investigated in the project are CO2 source and transport options. The remote location of the offshore basin limits human inconvenience and potential damage, and the thick layer of sediment on top of it promotes the physical trapping of supercritical CO2 or buoyant dissolved CO2. The Cascadia project benefits from a wealth of existing data collected from sea floor instruments, cores, and tracer experiments on fluid flow in the crust, as well as ongoing observation and monitoring from the nearby NEPTUNE cable network. However, there are no platforms in place to monitor mineralization within the formation. Several CO2 sources are available, including power plants; refineries; and chemical, mineral, and metal production sites. Goldberg and collaborators are considering how to best group source sites to develop efficient pipeline or shipping options. Though sufficient source volume (more than 12 Mt of CO2 emissions) is available onshore, Goldberg noted that incentives would still be needed to interest CO2 producers in subsurface storage. A particular issue with this site is that it crosses an international border between Canada and the United States with no clear regulatory framework to guide the activity, and it may be inhibited by the London Convention on marine pollution. Because laboratory studies have shown that there is large variability in both reaction rates and injectivity into the basalt, Goldberg noted that site-specific sampling and experiments are being pursued at this location.
MECHANISMS OF CO2 INJECTION AND STORAGE IN SEDIMENTARY FORMATIONS
Lynn Orr from Stanford University described the possibility of sequestering CO2 in sedimentary subsurface reservoirs (see Figure 4). He highlighted the efficiency of pairing carbon sequestration with enhanced oil recovery. Oil and gas reservoirs are sealed, and more than 40 years of gas injection for enhanced oil recovery has led to a wealth of practical experience and data for flow prediction. He explained that the value of CO2 injection for oil and gas operations makes it economically viable as a CDR approach. Other reservoirs include saline aquifers and coal beds or shales. Orr identified the key characteristics of a storage site to evaluate storage potential: the CO2 propagation distance, the time it takes for CO2 to be immobilized, the fate of the CO2, and the leakage potential. In oil reservoirs, injection of CO2 into the capillary transition zone can increase net storage, and CO2 can take the place of oil that has been trapped by these forces. This capacity is dependent on site-based structure; the heterogeneity of the rock structure determines the strength of gravity forces and thus the effectiveness and capacity of capillary trapping. CO2 can also be dissolved in water and injected, and this solution
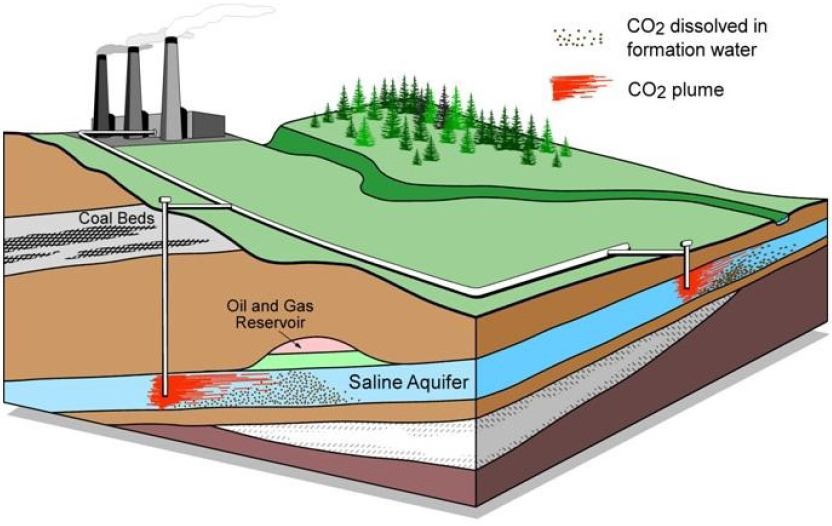
SOURCES: Presentation by Lynn Orr; Alberta Geological Survey.
could increase the strength of capillary trapping. Brine becomes denser when dissolved with CO2, which causes it to be convectively moved downward. Orr said that the combined processes of capillary trapping and dissolution are effective, but slow enough to still require an impermeable caprock. Injection into depleted natural gas reservoirs has the advantage of requiring minimal pressure management; the CO2 replaces the methane (CH4) in the reservoir, and its higher density means it can be injected beneath CH4 for enhanced gas recovery. However, he said that there is no incentive to do this while there is a low price for natural gas and no price for carbon. In coal beds and shales, CO2 can adsorb to the rock and help displace CH4.12 However, adsorption reduces permeability of the rock, interfering with natural gas extraction. Orr noted that like injection into gas reservoirs, without a carbon price, this exchange is not incentivized. Thus, he concluded that enhanced oil recovery provided the best opportunity for CO2 storage.
Jonny Rutqvist from Lawrence Berkeley National Laboratory described the mechanics and risk of induced seismicity (i.e., earthquakes) caused by pressure injections of CO2 into deep sedimentary formations. He explained that pressure changes from injected CO2 extend beyond the plume boundaries, which cause stress, strain, and deformation in an even wider area. The temperature and pressure changes near the injection well may lead to microseismicity and surface deformation, which could be useful for monitoring injection activity. At higher pressures, stress can lead to more significant fracturing and fault activation. He noted that this could occur in areas not otherwise seismically active such as the mid-continent of the United States.13 He explained that this fault reactivation could threaten CO2 storage as well as reduce public acceptance of CO2 injection; thus, it is important to consider when events will be detectable on the surface. Models of fault activation and seismicity seek to identify the pressure at which these events occur and calculate moment magnitude from the slip and the size of the rupture. A widely felt event (a magnitude of 4) would require a rupture, or fault length, size of at least 1 km. Rutqvist explained that a fault 1 km in length could be difficult to detect with seismic imaging. At the In Salah CO2 storage project in Algeria, CO2 was injected 1.8–1.9 km deep into a 20 m thick formation of relatively low permeability (nearly 1 Mt CO2/year in three wells from 2004–2011). This project experienced surface uplift of about 5 mm around each well, explained by fracture zones opening around the injection underground that were predicted by models to extend several hundred meters up from the reservoir.14 No seismic events or other strike-slip shear movements were measured at In Salah. In contrast, Rutqvist identified several carbon storage sites in the United States that have experienced pressure-induced seismic activity. He identified other factors that are important in influencing the occurrence of seismic events: stress field, existence of faults, rock properties, and injection methods (e.g., injection rates and volumes). He concluded that by managing (lowering) pressure, seismicity rates and damage to caprock could be reduced.
DEMONSTRATION OF CARBON STORAGE IN DEEP SEDIMENTARY FORMATIONS
Phil Ringrose from Statoil15 and the Norwegian University of Science and Technology provided an overview of ongoing injection projects in Norway, as well as plans for future projects and storage capacity. Sleipner is an offshore injection site embedded in a gas field, and the first commercial demonstration of CO2 injection for storage. The project has helped prove the feasibility of CO2 storage—storing more than 17 Mt CO2 since 1996—and has yielded insights into monitoring techniques such as geophysical time-lapse monitoring. The monitoring program includes seismic and gravimetry surveys to verify CO2 containment, and marine surveys and chemical sampling to monitor for environmental impacts. Ringrose said he believed these activities have significantly improved understanding of CO2 storage flow processes and contribute to flow simulator physics and parameterization (e.g., TOUGH2, ECLIPSE). Snøhvit, which has stored more than 4 Mt CO2 since 2008, combines an offshore storage project with a liquid natural gas project. It uses CO2 captured onshore, requiring transport of pressurized liquid natural gas by a 150 km pipeline to the injection well. The Snøhvit site has contributed to understanding pressure-managed injection; the initial injection plan resulted in pressure buildups and an adjusted plan was guided by monitoring data on the heterogeneity of injection into the storage units. Ringrose pointed to this experience as demonstrating the value of having multiple storage options at one site.
__________________
12 Heller, R. and M. Zoback. 2014. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. Journal of Unconventional Oil and Gas Resources 8:14–24.
13 Zoback, M. and S. Gorelick. 2012. Earthquake triggering and large-scale geologic storage of carbon dioxide. Proceedings of the National Academy of Sciences 109(26):10164–10168.
14 Rutqvist, J., D. Vasco, and L. Myer. 2010. Coupled reservoir-geomechanical analysis of CO2 injection and ground deformations at In Salah, Algeria. International Journal of Greenhouse Gas Control 4(2):225–230.
15 Statoil is now known as Equinor.

SOURCE: Presentation by Don White
Ringrose described the growing geologic carbon capture and sequestration (CCS) capabilities in Norway, including plans for Smeaheia, a demonstration project in Norway supported by the Norwegian government. Compared to other possible sites, Smeaheia was selected based on its potential storage capacity (about 100 Mt), the minimal potential for leakage from legacy wells, the possibility to allow for injection from a land-based terminal, and the overall lower cost of infrastructure conversion. Plans for monitoring include surface seismic surveys, pressure gauges, downhole fibers, and targeted monitoring around wells. Ringrose described the goal of minimizing monitoring costs in the future by learning to optimize the program. In response to questions, he explained that an initial feasibility study and cost framework for capture, transport, and storage for Smeaheia estimated that the capital costs of developing the full project (three capture sources) are roughly $1.5 billion (12.6 billion kr), the annual operating cost is about $100 million (890 million kr), and the overall abatement cost would be about $160/t CO2 (1,290 kr/t CO2) for a 25 year project.16 Ringrose noted that in the future, he expects economies of scale to bring costs down.
Don White from the Geological Survey of Canada described the active injection projects in Canada. In 2017, there were three active CCS projects (Shell QUEST, Weyburn, Aquistore) as well as an additional research site (CaMI). The CaMI field research station tests and monitors shallow CO2 injections and leakage. White focused on the Aquistore project (see Figure 5), which is an active injection site storing 120 kt CO2 so far and has an ongoing research component. Aquistore is adjacent to a coal power plant that captures CO2 on-site. A seismic array of 630 geophones monitor for the migration of CO2 near the surface and induced seismicity from the injection activities. The project has a goal of improving seismic mapping. The project was monitored with surface seismic and vertical seismic surveys after 36 kt of CO2 and then 102 kt of CO2 were injected in order to track progression of CO2 in the formation. They also monitored for microseismicity because the injection zone is just above the Precambrian sandstone basement, in which they detected a fault when characterizing the site. They have not detected any induced microseismic activity to date. Other projects with basal sandstone injections have experienced detectable seismic events, so White said it was not clear if this site will be free of seismic events or if they will occur once time has passed. White noted that so far, Aquistore has stored approximately 120 kt CO2 at a cost of about $20 million, including the cost of drilling the wells, but the reservoir has an immense capacity to store around one million tons per year of additional CO2 based on the system’s rate of injection.
Jon Ennis-King from the Commonwealth Scientific and Industrial Research Organisation described experiments being conducted at the CO2CRC Otway site in Australia to understand and monitor CO2 injection. The project has been proceeding in stages since 2008, each stage consisting of injections and associated monitoring. In the first stage, 65,400 t of CO2-rich gas were injected into a depleted gas field in order to verify injection models and predictions that CO2 could be effectively stored. Pressure tests in the injection well identified the pressure buildup during injection and the location of the CO2/H2O interface over time. In addition, noble trace gas analysis of fluids in the crest well (top of the gas-trapping formation) tracked the arrival of injected gas. In the second stage, 150 t CO2 was injected in a saline aquifer to test for the occurrence of residual saturation and dissolution. The second stage of monitoring revealed that temperature, noble trace gas composition, pulsed neutron logging, and especially pressure tests provide useful information on the residual trapping of gas in saline formations, although each method has limitations. A final step of this stage is in progress, where 15,000 t of CO2 have been injected into a saline aquifer, and seismic monitoring is being used to observe the gas plume development. They found that an array of buried seismic receivers is important for improving the signal-to-noise ratio. Ennis-King noted that new technologies, such as optical fiber sensors, may enable continuous geophysical monitoring at low impact. The current stage of the project is focused on reducing the cost and footprint of
__________________
16 Gassnova. 2016. Feasibility study for full-scale CCS in Norway. https://www.gassnova.no/en/Documents/Feasibilitystudy_fullscale_CCS_Norway_2016.pdf.
monitoring using subsurface pressure, seismic, and electromagnetic techniques. Ennis-King concluded with a discussion of prospects for future carbon storage projects in Australia, noting opportunities (e.g., strong incentives to reduce emissions from burning coal, large saline storage capacity) as well as barriers (e.g., no carbon pricing, limited prospects for using CO2 in enhanced oil recovery).
RESEARCH AND MONITORING FOR RELIABLE GEOLOGIC STORAGE
Susan Hovorka from the University of Texas at Austin outlined her suggested research needs for CO2 injection, though she emphasized that a lot of knowledge has already been gained. First, she identified the need for improved understanding of when and why failures (e.g., blowouts, leakage) occur. Anecdotal stories indicate that most failures are due to operational errors, and have not had major consequences. Controlled release experiments can provide experience in how to respond to a blowout or identify leakage; some shallow experiments have been conducted but Hovorka noted that less has been done to work on controlled release from deeper layers. The second research need she identified was to better identify low risk, high volume capacity reservoirs. She explained that assessments of subsurface pore space have shown that volume is not a limiting factor for carbon storage. Rather, the main limiting factor is whether the pore space is suitable to use based on its pressure limitations. The Maximum Allowable Surface Injection Pressure is conventionally used for determining injection pressure limits, and she suggested that the calculation could be improved by incorporating the geomechanics of the storage area under consideration. A particular issue with identifying this limit is identifying the boundary condition of the reservoir. She suggested that one way to deal with the difficulty in identifying boundary conditions is to find large capacity, high permeability reservoirs; the near offshore seabed, which is covered in a thick sediment layer transported to the ocean by rivers, may be such a high capacity area. She recognized that work has been done in the North Sea to identify and characterize these large offshore reservoirs, though it is not clear how well this characterization can be applied to other areas. She identified several ongoing injection field tests that may improve this understanding of storage capacity. Lastly, Hovorka identified the need to improve the “model-monitoring loop,” where monitoring programs are designed to inform and improve models of the system. The mismatch between model predictions and monitoring data is caused by the heterogeneity of the geology, which cannot be completely characterized. The goal of the models, she added, should be to describe the short and long-term response of the geosystem to planned injection. Models can identify unacceptable responses (e.g., leakage caused by a plume migrating too far), while monitoring data can test whether unacceptable trends are occurring and improve the model.
Tom Daley from Lawrence Berkeley National Laboratory expanded on the goals and components of a monitoring plan. He explained that a monitoring plan is going to be driven by a balance of its costs and benefits, though he acknowledged that these could be hard to quantify. He identified the goals of monitoring as being directed toward performance evaluation (e.g., injection and growth of plume are progressing as expected/modeled), regulatory compliance, risk reduction, and public assurance (e.g., monitoring private water wells). Daley distinguished between site characterization, which is typically thought of as a static snapshot of a system, and monitoring, which captures its changes and dynamics. However, he noted that site characterizations are iterated as the system changes. Monitoring can consist of direct observations of an activity, such as pressure and geochemical sampling, or can consist of observations of indirect changes such as ground deformation or electrical conductivity. Daley identified seismic surveys as a major component of CO2 sequestration monitoring, which measures wave velocity and correlates it with gas saturation. Models of the relationship to CO2 saturation rely on many assumptions about uniformity of the rock and fluids, which limit accuracy. Thus, Daley identified improved characterization of this relationship as a research need. The calculation varies depending on location-dependent factors, such as rock physics, brine properties, anisotropy, dissolution into brine, and the presence of any other gas (e.g., CH4), which can lead to a huge range in estimated saturation.17 A complicating factor is that the porosity and granular microstructure of the rock matrix changes as CO2 saturation increases.
Daley provided an overview of this suggested research and monitoring needs. He emphasized the need for field experiments at large (Gt) scales and for integrating multi-scale experiments at a site. He pointed out that some large-scale projects, like In Salah, conducted large-scale monitoring via satellite and surface seismic platforms, but missed some of the detailed measurements from boreholes, which are important for direct pressure monitoring. He suggested performing field experiments in shallow groundwater dissolved CO2 injections, intermediate depth gas-phase injections, and deep fault injections of supercritical CO2. An important field experiment he identified is to test fault and fracture
__________________
17 Daley, T., J. Ajo-Franklin, and C. Doughty. 2011. Constraining the reservoir model of an injected CO2 plume with crosswell CASSM at the Frio-II brine pilot. International Journal of Greenhouse Gas Control 5(4):1022–1030.
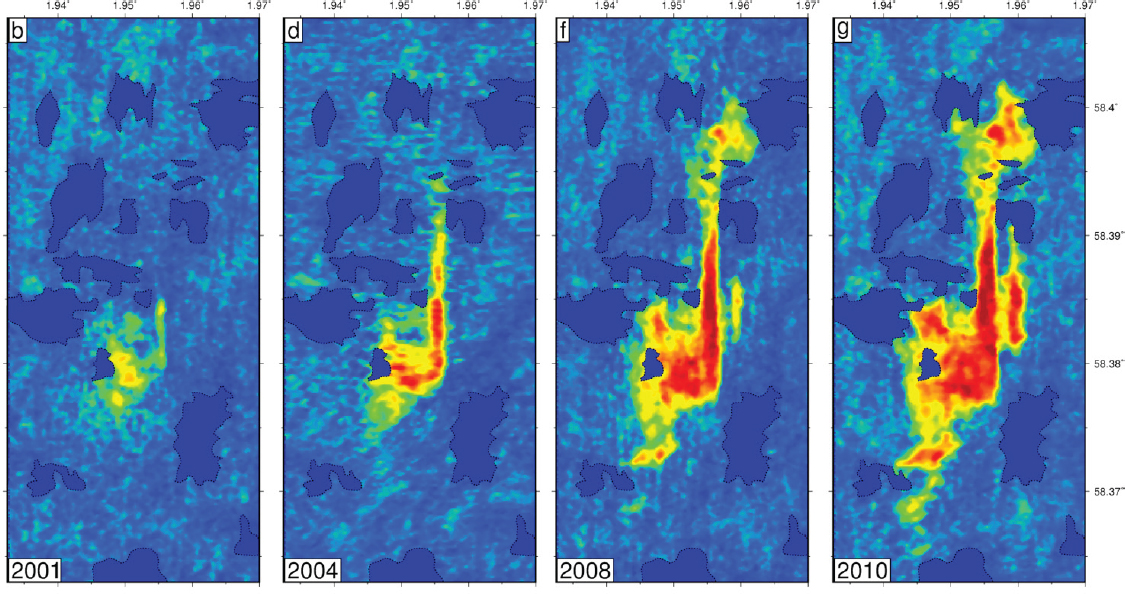
SOURCE: Presentation by Jerome Neufeld
leakage detection methods, and consider how these problems will be mitigated if they occur. Daley also suggested that multi-physics measurements, beyond reliance on seismicity, would help improve saturation estimates. Seismicity is good at detecting lower CO2 saturations, and may be better for monitoring along the plume boundary and detecting leakage. However, conductivity is a better measure at higher saturations, and may be better for monitoring the injection plume.18 Daley concluded with thoughts on the potential for reducing monitoring costs while maintaining continuous monitoring programs, including the identification of the optimal number and placement of monitoring wells and the development of new monitoring technology such as fiber optic sensing.
Jerome Neufeld from the University of Cambridge described the monitoring programs employed at conventional CO2 storage sites, with the example of monitoring the offshore Sleipner site. Repeated seismic surveys conducted at the Sleipner site since 1994 show that the CO2 plume has been spreading. Seismic reflectance is used to assess CO2 volume (see Figure 6); time-lapse seismic data measures areal extent and formation topography (based on reflectance travel time) to estimate thickness, and resolves volume down to 0.5 to 1 m resolution. Vertically equilibrated models allow volume to be estimated by monitoring area increases, which are influenced by hydrostatic pressure and topography of the caprock. Topographic measurements, such as identification of submarine channels, also provide further insight into flow paths and the long-term stability of CO2. Neufeld expanded on how the stability of CO2 can be understood and improved by secondary trapping mechanisms. Dissolution and capillary trapping, the dominant hydrodynamic traps for CO2, can be incorporated into models of buoyancy-driven spreading. Additionally, storage integrity can be managed by better understanding pressure plume and the potential for induced seismicity. He concluded that there is an opportunity to increase the rate of trapping by understanding the influence of heterogeneity on enhancing capillary trapping.
Mike Celia from Princeton University discussed monitoring efforts to quantify leakage and its influence on storage efficiency. Oil and gas fields are prospective locations for CO2 injection, but they also contain old wells that could be potential sources of leakage. In North America, well density can reach six wells per square kilometer. Celia suggested the need for a risk assessment to evaluate the risks and benefits of using these locations for CO2 storage. Evaluation of the leakage potential requires characterizing not only the injection area, but also the entire vertical stack of the formation and the properties of the wells. Celia and collaborators developed a two-phased model that depicts how the pressure and CO2 plume moves through the formation. They performed a Monte Carlo simulation on a range of formation geology and well properties.19 Field measurements of the permeability of the wells provide a statistical range for the model, including data from sidewall coring, pressure tests, and direct measurements of CO2 flux. The model indicates that leakage of gas is likely to occur for less than 0.02% of injected CO2 mass, and is even less likely for brine leakage. Celia concluded that the benefits of carbon storage far outweigh the small leakage risk. However, he noted that additional field measurements, as well as improved abilities to monitor for leakage, are desired.
__________________
18 Xue, Z., J. Kim, S. Mito, K. Kitamura, and T. Matsuoka. 2009. Detecting and monitoring CO2 with P-wave velocity and resistivity from both laboratory and field scales. Society of Petroleum Engineers SPE-126885-MS. https://doi.org/10.2118/126885-MS.
19 Nordbotten, J. and M. Celia. 2011. Geological Storage of CO2: Modeling Approaches for Large-Scale Simulation. doi:10.1002/9781118137086.
DISCLAIMER: This Proceedings of a Workshop—in Brief was prepared by Emily Twigg as a factual summary of what occurred at the meeting. The statements made are those of the rapporteur or individual meeting participants and do not necessarily represent the views of all meeting participants; the planning committee; or the National Academies of Sciences, Engineering, and Medicine.
REVIEWERS: To ensure that it meets institutional standards for quality and objectivity, this Proceedings of a Workshop—in Brief was reviewed by Ruben Juanes, Massachusetts Institute of Technology; Peter Kelemen, Columbia University; Matt Lucas, Center for Carbon Removal; Wen-lu Zhu, University of Maryland.
Committee for Developing a Research Agenda for Carbon Dioxide Removal and Reliable Sequestration: Stephen Pacala (NAS) (Chair), Princeton University; Mahdi Al-Kaisi, Iowa State University; Mark Barteau (NAE), University of Michigan; Erica Belmont, University of Wyoming; Sally Benson, Stanford University; Richard Birdsey, Woods Hole Research Center; Dane Boysen, Cyclotron Road; Riley Duren, Jet Propulsion Laboratory; Charles Hopkinson, University of Georgia; Christopher Jones, Georgia Institute of Technology; Peter Kelemen (NAS), Columbia University; Annie Levasseur, International Reference Centre for the Life Cycle of Products, Processes and Services (CIRAIG); Keith Paustian, Colorado State University; Jianwu (Jim) Tang, Marine Biological Laboratory; Tiffany Troxler, Florida International University; Michael Wara, Stanford Law School; Jennifer Wilcox, Colorado School of Mines.
SPONSORS: This activity was supported by the U.S. Department of Energy, the National Oceanic and Atmospheric Administration, the U.S. Environmental Protection Agency, the U.S. Geological Survey, the V. Kann Rasmussen Foundation, the Linden Trust for Conservation, and Incite Labs, with support from the National Academy of Sciences’ Arthur L. Day Fund.
For a record of presentations and additional information regarding the workshop, including the statement of task for this study, visit http://nas-sites.org/dels/studies/cdr.
Suggested citation: National Academies of Sciences, Engineering, and Medicine. 2018. Geologic Capture and Sequestration of Carbon: Proceedings of a Workshop—in Brief. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/25210.
Division on Earth and Life Studies

Copyright 2018 by the National Academy of Sciences. All rights reserved.












