2
Forest Health
This chapter contains the committee’s definition of forest health, which includes ecological, economic, and sociocultural factors. It summarizes the threats facing North American forests from insect pests and pathogens and introduces, as examples, the cases of four tree species affected by one or more of these pressures. These case study species are referenced throughout this report. This chapter concludes by describing the effects these threats have on forest health and ecosystem services.
DEFINING FOREST HEALTH
The committee spent much of its early deliberations discussing the term forest health. It heard a number of presentations on the topic (see Meeting 2 in Appendix B) and consulted the scientific literature (e.g., Kolb et al., 1994; Helms, 1998; Raffa et al., 2009; USDA-FS, 2009; Trumbore et al., 2015). On the basis of its information-gathering efforts, the committee agreed on the definition of forest health for this analysis as
A condition that sustains the structure, composition, processes, function, productivity, and resilience of forest ecosystems over time and space. An assessment of this condition is based on the current state of knowledge and can be influenced by human needs, cultural values, and land management objectives.
Forest structure is the horizontal and vertical distribution of plant material, including ground vegetation and dead or fallen woody material, shrubs, and understory, midstory, and overstory trees (Bennett, 2010). Structure also concerns the age distribution of the trees in the forest. Forest stands are considered even-aged if all of the trees are within the same age class. A forest with uneven-aged structure is a stand with three or more age classes (Bennett, 2010). In practice, size is often used as a proxy for age. Forest structure affects seedling growth, survival, and crown formation of trees as well as the formation of habitat niches (von Gadow et al., 2012).
Forest composition refers to the identity and frequency of plant species found in a stand or landscape, including grass, forbs, shrubs, and trees. In other words, it is the entire plant community of the forest (Moore, 2004). Forest composition, directly or indirectly, affects all other biota present.
Trees play an important role in ecological processes, that is, the cycling of water, nutrients, and energy through the ecosystem, as well as in the natural successional dynamics, that is, the changes in plant species composition and structure following a disturbance (Glitzenstein et al., 1986; Keeton and Franklin, 2005). Trees’ influence on plant species composition and structure affects in turn the other species present in the system.
Healthy forests support economic, ecological, and sociocultural functions. Economic functions relate to the quality and quantity of timber or other vegetation products and game extracted from a forest as well as revenues generated through recreational uses of the forest. Ecological functions include habitat for wildlife, maintenance of biodiversity, soil erosion control, climate regulation, flood control, and effective maintenance of water quality. Sociocultural functions concern aesthetic, spiritual, and cultural values (DeFries et al., 2005; Cooper et al., 2016).
Forest productivity refers to the net primary productivity of plants in the forest system (reflected by the difference between the carbon captured via photosynthesis and that lost via respiration) (Landsberg and Waring, 1997).
Resilience in a forest ecosystem describes its capacity to absorb a disturbance1 without a significant long-term change to the forest community functions and processes that existed before the disturbance (Holling, 1973; Millar and Stephenson, 2015; Seidl et al., 2016). For this report, resilience is specifically defined as a forest’s ability to maintain its structure, processes, and functions in the long term; however, the committee was mindful of other aspects of resilience in response to disturbance (e.g., resistance, absorption, reorganization, and transformation; Fisichelli et al., 2016). In particular, transformative resilience, that is, the capacity to change into a new system when disturbance makes the existing system untenable (Walker et al., 2004), could be of great relevance in the context of using biotechnology in forest ecosystems.
Like forests themselves, the assessment of whether a forest is healthy is not static. The assessment of the health of a forest will change not only with the evaluation of its structure, composition, processes, function, productivity, and resilience, but also with the state of knowledge about these aspects of forest health. Increasing numbers of studies are also demonstrating that climate change is also altering various aspects of forest health (Boisvenue and Running, 2006; Reyer et al., 2017; Paquette et al., 2018).
THE VALUE OF HEALTHY FORESTS
A healthy forest can be valued for the benefits it provides to humans and also for its own sake. An instrumental view of forest health takes it as a means to an end: the betterment of human welfare. In contrast, the intrinsic value of a forest does not depend on its contribution to human society (NRC, 2005). While the instrumental valuation of the forest ecosystem is framed in terms of the services it provides to humans, intrinsic value concerns the value a forest may have in itself, independent of its usefulness to human beings. Here, both perspectives on valuation are introduced.
Maintaining forest health is essential for the conservation and sustainable management of the many ecosystem services provided to humans by forests. Ecosystem services are the goods and services that are of value to people, provided wholly or in part by ecosystems (Olander et al.,
___________________
1 Natural disturbance is part of the normal functioning of a forest. Forested systems undergo successional and cyclical changes in structure and composition, which help to maintain high levels of biodiversity (Perry, 1994; Barnes and Wagner, 2004). Healthy forests may withstand natural disturbances either by being able to maintain similar properties (i.e., showing resistance) or by being able to recover many of their original properties afterward (i.e., being resilient). Land management practices can influence forest function and productivity following disturbance (Millar and Stephenson, 2015).
2015). In 2005, the Millennium Ecosystem Assessment categorized these services as provisioning, regulating, supporting, and cultural (Shvidenko et al., 2005; see Box 2-1).
Many ecosystem services that are provisioning, regulating, or supporting are biologically mediated (Burkhard and Maes, 2017). Trees help form and retain soil, cycle nutrients, and store carbon (e.g., Seidl et al., 2016). They filter and regulate the flow of water, first by intercepting rainfall in the canopy. The reduced volume and speed of the rain allows more water to be absorbed into the ground and, combined with the roots’ soil retention properties, controls flooding and reduces erosion (Ellison et al., 2017). Second, roots take up nutrients and pollutants in the subsurface water, preventing these elements from filtering into the groundwater supply. Trees improve air quality by intercepting pollutant particles (Nowak et al., 2014). Water vapor cools the surrounding environment when it evaporates from leaves. Trees buffer the landscape from the heat of the sun and the force of winds, and forests provide food and habitat for pollinators, fish, wildlife, and other organisms, as well as food, fuel, and products for humans.
Cultural ecosystem services are diverse (Milcu et al., 2013). They vary according to the intended or desired use of an ecosystem, such as recreation or creation of traditional forest products. Additionally, forests provide substantial cultural heritage or identity and spiritual, educational, and aesthetic values (Cooper et al., 2016). The values at stake may vary by individual or group. For example, some people may value mountain bike trails through a forest, whereas others may value the same area for its wildlife viewing opportunities or for a spiritual connection felt to nature when in that space. People may also place existence or nonuse value on forests simply because they wish to preserve the ecosystem or species within it (NRC, 2005).
Alongside the services they provide to humans, ecosystems such as forests may also be thought to have intrinsic value, value for their own sake. Intrinsic value, however, can be understood in different ways. Subjective intrinsic value arises from human evaluative attitudes. In the context of forests, for instance, people might intrinsically value forest ecosystems or wild animals or the perceived state of wildness itself. Objective intrinsic value describes value that is believed to exist on the basis of certain properties or features, independent of anyone’s evaluative attitudes (Sandler, 2012, 2018). If someone argues that human lives are valuable on the basis of certain properties
humans have, whether or not anyone actually values human lives, then they are defending the objective intrinsic value of human life. If someone argues that a forest ecosystem is objectively intrinsically valuable, they are maintaining that it has intrinsic value whether or not any human actually values it. Although the existence of objective intrinsic value is disputed on the ground that values must be created by valuers, the existence of objective intrinsic value in species, ecosystems, individual organisms, or all three has often been assumed or defended in conservation and environmental ethics (e.g., Soulé, 1985; Taylor, 1986; Rolston, 1988).
The relationship between intrinsic value and existence value is complex. Because existence value is based on human preference, it is clearly distinct from objective intrinsic value. Existence value and subjective intrinsic value, however, are much closer in meaning, and some definitions take existence value to be synonymous with subjective intrinsic value (e.g., Aldred, 1994). However, Davidson (2013:175) interprets existence value “as the (willingness to pay for the) benefits one derives from something’s mere existence, although one has no current or future plans for its active use.” Existence value, on this account, entails some kind of benefit or satisfaction to the valuer. Intrinsic value, on the other hand, does not imply any benefit to the valuer; rather, the existence of something with intrinsic value “exerts a moral duty on us to take it into account.” Therefore, Davidson suggests something could have intrinsic value without existence value; for example, a rat in a kitchen has intrinsic value, in that the human in the kitchen has a duty not to harm it, but presumably that person would prefer for the rat not to exist at all. Given this understanding of intrinsic value, Davidson argues that intrinsic value, though not existence value, falls outside the scope of ecosystem services because it is not in any sense about nature’s services to humans.
In this report, the committee adopts ecosystem services as the basis for assessment of the instrumental impacts of introducing a biotech tree to counter a threat to forest health. Chapter 5 presents a specific framework for defining ecosystem services in impact assessment that is compatible with regulatory decision making (discussed in Chapter 6). The impact assessment considers the potential benefits, risks, and trade-offs of the introduction of a biotech tree by evaluating expected changes in forest ecosystem services. However, the committee also believes that consideration of the intrinsic values of a healthy forest could usefully broaden the scope of public deliberations about the use of biotechnology (discussed in Chapter 7). Chapter 4 considers some of these values and the ways in which they may be affected by the introduction of a biotech tree to a forest ecosystem.
A healthy forest—that is, one in a condition that sustains the components of an ecosystem over time and space—is more likely to sustain ecosystem services of value to individuals and society. When assessing the impact of a threat (such as an invasive insect) on forest health, evaluating the effect of that threat on the biologically mediated processes and the cultural and aesthetic values of the forest ecosystem provides the basis for assessing how the provision of ecosystem services may change. When adverse effects are experienced or anticipated, alternative means of returning the forest ecosystem to health are considered, including the introduction of a biotech tree that can resist the threat. The remainder of this chapter reviews the scope of the threat from insect pests and pathogens facing North American forests and the implications of that threat for the forest ecosystem and the ecosystem services it provides.
THREATS TO FOREST HEALTH FROM INSECT PESTS AND PATHOGENS
Despite being part of the forest natural disturbance regime, outbreaks of insects and pathogens have dramatically increased in number and impact since the mid-19th century (Aukema et al., 2010; Boyd et al., 2013). The most recent national insect and disease risk assessment, conducted in 2012 by the Forest Service of the U.S. Department of Agriculture (USDA), estimated that 32.9 million
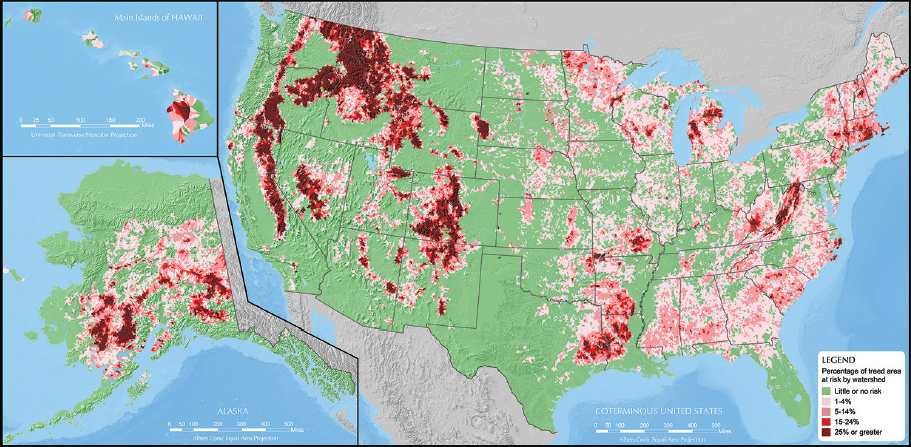
NOTES: Hectares at risk total 32.9 million. Percentage of treed area at risk by watershed: Green, little or no risk; light pink, 1–4%; dark pink, 5–14%; red, 15–24%; maroon, 25% or greater.
SOURCE: Krist et al., 2014.
hectares (81.3 million acres)—that is, almost 7 percent of all forested2 or treed3 land in the United States—were at risk of losing at least 25 percent of tree vegetation between 2013 and 2027 due to insects and diseases (Krist et al., 2014; see Figure 2-1). That assessment placed 9.4 million more hectares (23.3 million acres) at risk than was estimated in 2006 (Krist et al., 2014).
Most of these outbreaks have been caused by introduced insects and pathogens or by native species within their natural range as well as those expanding their geographic ranges due to climate change (Liebhold et al., 1995; Lovett et al., 2006; Sambaraju et al., 2012; Weed et al., 2013). Climate change is further compounding the impact of insects and pathogens by increasing abiotic stresses on trees, which may result in reduced defenses and increased susceptibility (Breshears et al., 2005; Berg et al., 2006). As a result, the impacts of insects and pathogens are among the greatest threats to forest ecosystems in North America (Moser et al., 2009; Krist et al., 2014; Lovett et al., 2016).
As the frequency of insect and pathogen outbreaks increases, forest resilience and the ecosystem services associated with forests are threatened (Millar and Stephenson, 2015; Seidl et al., 2016). The next section describes general threats posed by insects and pathogens and their interaction with climate change.
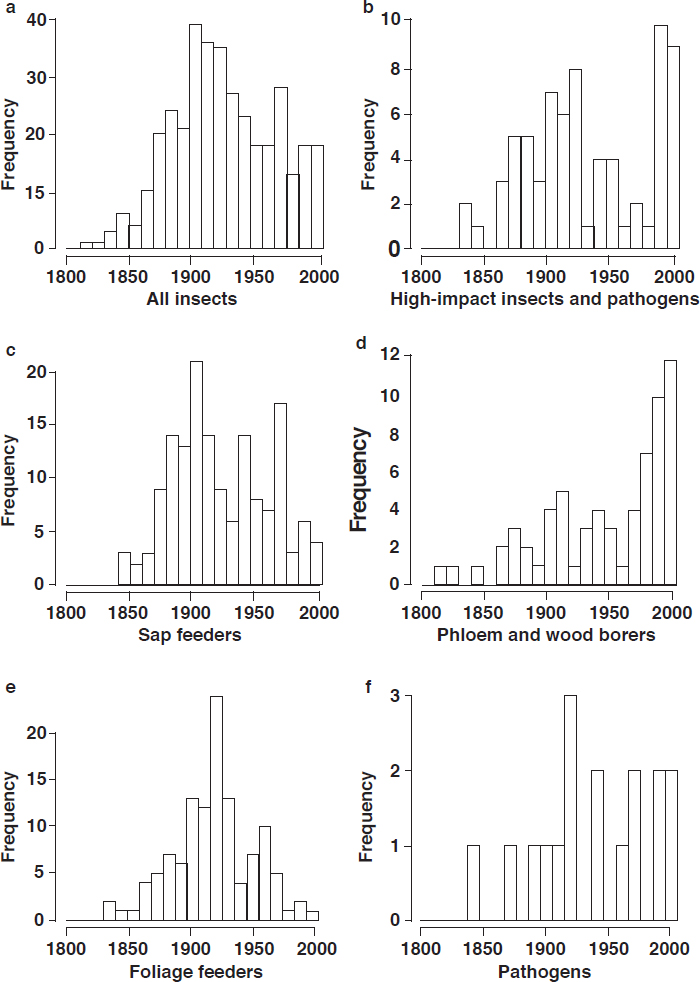
Introduced Insect Pests and Pathogens
Since the 1600s, around 450 species of insects and at least 16 species of pathogens have been introduced and become established in continental U.S. forests. Of those, 14 percent of the insects (62 species) and all of the pathogens have been classified as high-impact species (Aukema et al., 2010); that is, they cause some combination of tree mortality, canopy thinning, growth loss, defoliation, and decreased reproduction or regeneration. At least 2.5 introduced, established insect
___________________
2 Forested land contains at least 10 percent tree canopy cover.
3 Treed land is an area with measurable tree presence, including urban areas and land in the Great Plains with trees that does not meet the definition of forested land.
species have been detected each year since 1860 (Aukema et al., 2010). Given their cryptic nature and difficulties in early detection, there is little information on the rate of pathogen introduction.
Increases in human mobility and trade are the major pathways of introductions (Pyšek et al., 2010; Brockerhoff et al., 2014; Early et al., 2016). Pathogens and insect defoliators have generally been introduced with live plants (Liebhold et al., 2012). The introduction of insect borers, the most damaging group (see Box 2-2), is usually associated with wood packaging material (Aukema et al., 2010, 2011). The number of introduced borer species (including bark and ambrosia beetles) has dramatically increased since the 1990s, averaging 1.6 new introductions per year, reflecting the increased use of wood packaging materials and the growth in global trade (Haack, 2006; Aukema et al., 2010; see Figure 2-2). These introductions continue despite proactive requirements for treatment of wood pallets and shipping containers (Haack et al., 2014).
Some of these introductions have had devastating consequences in North American forests; impacts have ranged from temporary declines in population productivity to the functional extirpation of an entire species (see case study of the American chestnut, below). In many instances, the introduced insect pests and pathogens lack natural competitors, predators, parasites, or pathogens to regulate their populations (i.e., enemy release; Keane and Crawley, 2002), giving them a temporary fitness advantage that could contribute to their virulence (Hajek et al., 2016). The damage these species cause can be linked to a lack of resistance in the host tree (Herms and McCullough, 2014). Table 2-1 summarizes many of the nonnative pests threatening North American tree species.
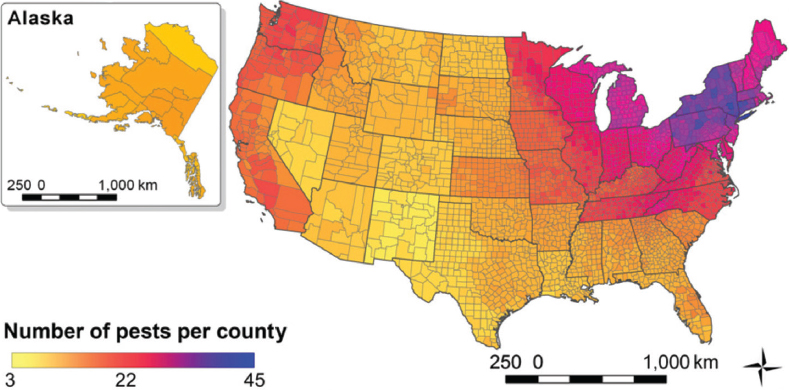
The majority of introduced insect pests and pathogens are found in the northeastern United States (Liebhold et al., 2013; see Figure 2-3). This geographic pattern likely reflects the number
SOURCE: Aukema et al., 2010.
TABLE 2-1 18 Nonnative Forest Insects and Pathogens in North America with Current or Potential Future High Impacts
| Common Name | Scientific Name | Pathway | Hosts | Impacts | Geographic Region at Risk |
|---|---|---|---|---|---|
| Established Species with High Impact | |||||
| Chestnut blight | Cryphonectria parasitica (Murrill) Barr. | Live plants | American chestnut, chinquapin | Virtually eliminated mature chestnuts | Eastern deciduous forest |
| White pine blister rust | Cronartium ribicola J.C. Fisch | Live plants | Five needle pines (section Quinquefolia in genus Pinus) | High mortality of susceptible trees in several western pine species | Continent wide; greatest impacts in West |
| Phytophthora dieback | Phytophthora cinnamomi Rands | Unknown | Many hosts including American chestnut, white oak, shortleaf pine, and Fraser fir, fruit trees | High mortality of susceptible trees | Continent wide |
| Port Orford cedar root disease | Phytophthora lateralis Tucker and Milbrath | Probably live plants | Port Orford-cedar | High mortality of trees, especially in riparian parts of its range | Klamath Mountains, California and Oregon |
| Beech bark disease (scale insect + fungus) | Cryptococcus fagisuga Lindinger + Nectria coccinea var. faginata (Pers.) Fr. | Live plants | American beech | Severely reduces mature beech; often replaced by dense thickets of root sprouts | Deciduous forests of East and Midwest |
| European gypsy moth | Lymantria dispar dispar L. | Escaped from deliberate introduction | Many hosts includes oaks, aspen, willow, and birch | Periodic outbreaks cause defoliations and can sometimes kill hosts | Deciduous forests of East and Midwest |
| Hemlock woolly adelgid | Adelges tsugae Annand | Live plants | Eastern and Carolina hemlock | High mortality in most affected stands | Appalachians, Northeast, and upper Midwest |
| Sudden oak death | Phytophthora ramorum S. Werres, A.W.A.M. de Cock | Live plants | >100 spp., especially tanoak and several western oak species; some eastern oaks vulnerable | High mortality in some vulnerable hosts (particularly tanoak); other hosts show minor impacts | Coastal California and Oregon; could potentially spread to eastern forests |
| Redbay ambrosia beetle + fungus (laurel wilt disease) | Xyleborus glabratus Eichhoff + Raffaelea lauricola Harrington and Fraedrich | Wood packaging | Numerous probable hosts including redbay and pondberry and pondspice shrubs | Predicted >90% reduction in redbay basal area within 15 yr (25 yr after first detected) | Eastern deciduous forests; greatest impacts in southeastern coastal plain |
| Common Name | Scientific Name | Pathway | Hosts | Impacts | Geographic Region at Risk |
|---|---|---|---|---|---|
| Emerald ash borer | Agrilus planipennis Fairmaire | Wood packaging | All North American ash species | Most ash trees succumb; some species of ash appear to have limited resistance | Eastern deciduous forest; riparian areas in Great Plains and West, landscape plantings continent wide |
| Dutch elm disease | Ophiostoma ulmi (Buisman) Nannf. and O. novo-ulmi Brasier; vectored by several insects including Scolytus multistriatus and S. schevyrewi | Wood products | American elm; other native elms, e.g., red or slippery elm, are more resistant | Severe impacts in urban areas; elms remain, although reduced in number and size, in riparian woodlands | Continent wide |
| Butternut canker | Sirococcus clavigignentijuglandacearum N.B. Niar, Kostichka and Kuntz | Unknown | Butternut (white walnut) | Severe mortality of butternut; greater than 80% mortality of butternut in the South | Deciduous forests of Northeast and Midwest |
| Balsam woolly adelgid | Adelges piceae Ratzeburg | Live plants | Most true fir species (Abies) in North America | Widespread impacts on firs; severe mortality of Fraser fir on southern Appalachian mountaintops and Christmas tree farms | Northeast; southern Appalachians; Northwest |
| Established, Potential for Significant Effects in the Future | |||||
| Asian longhorned beetle | Anoplophora glabripennis Motschulsky | Wood packaging | Woody vegetation in 15 families, especially maples, elms, and willows | Severe impacts possible in both urban and forest landscapes; eradication being attempted | Continent wide deciduous forests |
| Winter moth | Operophtera brumata L. | Unknown | Many species including oaks, maples, cherries | Severe impacts on hosts in southeastern New England | Eastern deciduous forest |
| Polyphagous shot hole borer and fusarium fungus | Euwallacea (sp. unknown) + Fusarium euwallacea | Unknown | >200 species attacked by insect; >100 support the fungus; hosts killed include box elder, bigleaf maple, coast live oak | High mortality levels in vulnerable hosts | Southern California hardwood forests, riparian and urban; potentially in Southeast |
| Common Name | Scientific Name | Pathway | Hosts | Impacts | Geographic Region at Risk |
|---|---|---|---|---|---|
| European woodwasp | Sirex noctilio | Probably wood packaging | Many pine species | Most important killer of pines in Southern Hemisphere; modest impacts so far in United States | All ecosystems with hard pines: Southeast, Great Lakes states, western United States |
| Not Yet Established | |||||
| Asian gypsy moth and hybrids | Lymantria dispar asiatica Vinuskovkij | Ship super structures | >600 species, including common deciduous and coniferous trees | Could have more severe impacts than European gypsy moth because it has wider host range and females fly | Continent wide |
SOURCE: Adapted from Lovett et al., 2016.
NOTE: High-impact species are those that cause some combination of tree mortality, canopy thinning, growth loss, defoliation, and decreased reproduction or regeneration.
SOURCE: Liebhold et al., 2013.
of introductions, the historically high propagule pressure, and the impact of anthropogenic disturbance on the ability of the pests to invade in this region (Liebhold et al., 2013). This distribution is also correlated with the diversity of tree species, which is higher in the eastern half of the country (Liebhold et al., 2013). Once established, the average radial rate of spread—5.2 km per year—seems to be similar for all groups of insect pests and pathogens (Liebhold et al., 2013).
Insect Pests and Pathogens Under Climate Change
Climate change is opening new opportunities for colonization by both native and introduced insect species (Harvell et al., 2002; Logan et al., 2003). Forecasted temperatures for the mid-21st century indicate decreases in the length of the cold season and the incidence of extreme cold spells (IPCC, 2013). Cold winter temperatures, cold snaps, and short growing seasons have kept many insect pest species in the United States from moving into higher elevations and more northern latitudes (Carroll et al., 2004; Esper et al., 2007; Dukes et al., 2009). However, with warmer conditions, many insects are colonizing regions that previously had been unsuitable (Williams and Liebhold, 1997; Battisti et al., 2005). In addition, changes in climate are affecting the frequency and magnitude of outbreaks of both native and introduced pests. Outbreaks are predicted to increase in frequency and magnitude in the future. In areas where cold has previously limited establishment, warmer temperatures will likely allow an increase in development and reproductive rates and survival of many insects and pathogens (Ayres and Lombardero, 2000; Bale et al., 2002). An example is the native mountain pine beetle (Dendroctonus ponderosae) outbreak in North America between 1990 and 2010, which killed millions of hectares of pines and has been estimated to be an order of magnitude larger than any previously recorded event (Meddens et al., 2012; Raffa et al., 2013). This outbreak was associated with a reduction in cold snaps (i.e., periods of four consecutive days with average temperature below −20°C (Sambaraju et al., 2012) and overall warmer summer and winter temperatures. Warmer temperatures have also allowed an expansion of the territory of the mountain pine beetle hundreds of kilometers farther north in British Columbia and movement across Alberta into jack pine forests (Pinus banksiana), where it threatens the boreal forest as an invader. Likewise, the southern pine beetle (Dendroctonus frontalis) is moving northward into new forests on the eastern coast of the United States. In Alaska, Canada, and Colorado, outbreaks of the spruce beetle (Dendroctonus rufipennis) have increased with warmer weather and drier summers (Berg et al., 2006), and the beetle’s spread has been predicted to increase as warmer conditions facilitate faster insect development (Bentz et al., 2010; see Figure 2-4).
Changes in temperature and precipitation associated with climate change may become the most influential driver of pathogen outbreaks, because these changes could simultaneously affect host susceptibility and pathogen growth, reproduction, and infection (Sturrock et al., 2012). Forecasts of future climate indicate likely changes in pathogen overwintering survival, changes in host susceptibility to pathogen attack due to other stressors (e.g., drought conditions, ozone, or damage from storms), or changes in life cycles of associated species such as insects that disperse pathogens (Dukes et al., 2009; Weed et al., 2013). However, the outcome of these changes—higher or lower virulence—will likely be site specific (Sturrock et al., 2012). For example, Phytophthora ramorum, an introduced oomycete that causes sudden oak death, may experience a decrease in favorable environmental conditions in the eastern United States, but an increase in favorable sites in the western United States (Venette and Cohen, 2006; Venette, 2009) and Europe in response to climate change (Bergot et al., 2004).
Given that some pathogen species rely on insects for their dispersal (Wingfield et al., 2016), effects of climate change on the insect populations would likely cause changes in pathogen dynamics. For example, the two fungi that cause beech bark disease (Neonectria farinata and N. ditissima) are spread by a scale insect, Cryptococcus fagisuga. The extent of the infestation had
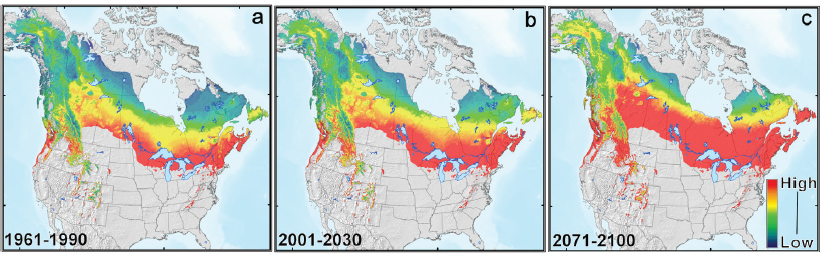
SOURCE: Bentz et al., 2010.
been restricted by cold winter temperatures, but with the onset of mild winters and dry autumns associated with climate change, both the scale and the fungi will likely move to northern latitudes and affect beech trees that had previously been shielded from the pathogen (Houston and Valentine, 1988; Stephanson and Coe, 2017).
EFFECTS OF INSECT PESTS AND PATHOGENS ON TREES AND ECOSYSTEM SERVICES
Adverse effects on forest health caused by increases in the frequency and magnitude of insect and pathogen outbreaks are already being observed and are likely to continue. This section reviews the effects on some specific tree species and genera; the feasibility of using biotechnology to address threats to these species is discussed in subsequent chapters. This section also examines more broadly the effects of insect pests and pathogens on forest health and ecosystem services.
Case Study Trees
A variety of introduced insect pests and pathogens (many included in Table 2-1) and the exacerbated pressure of some native insects and diseases facilitated by climate change threaten the long-term survival of many forest tree species native to North America. Rather than elucidating all threats, the committee decided to focus on four cases chosen by consensus and based on the following criteria:
- The severity of the threat.
- The causative agent(s) (insect, pathogen, or complex systems involving insect vectors or obligate pathogens with alternate hosts).
- The origin of the insect or pathogen (native or nonnative).
- The impact of climate instability and fire on the severity and extent of the disease or infestation.
- The ecological, economic, and cultural values of the host tree species.
- The use or potential use of the host tree species for plantation forestry.
- The efficacy or feasibility of traditional strategies to protect forest health (biological control, pesticide use, containment strategies, and selective tree breeding).
- The efficacy of gene insertion or gene-editing strategies if already in place.
- The feasibility of gene insertion or gene-editing strategies if not yet attempted or tested.
- Geographical distribution and phylogenetic position of the host species.
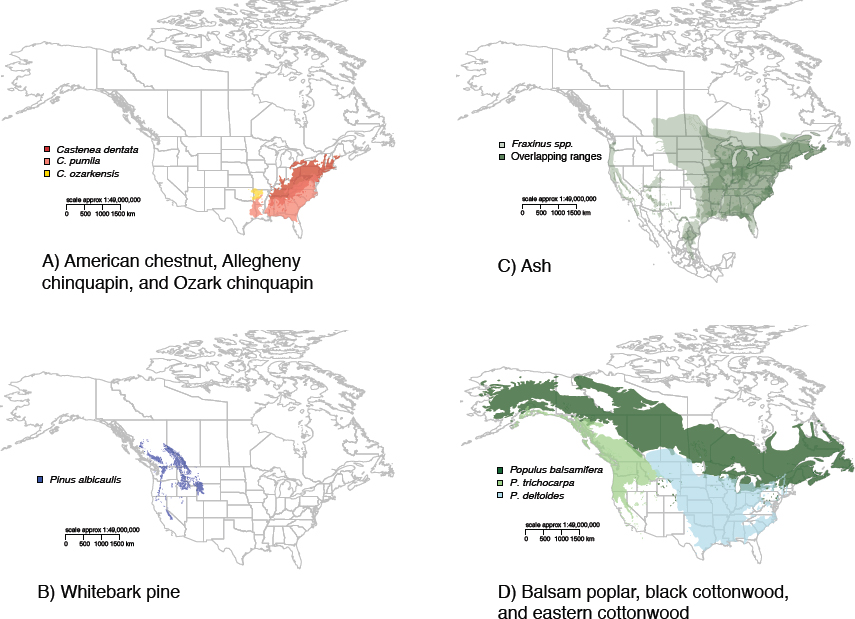
The four selected case studies—American chestnut (Castanea dentata), whitebark pine (Pinus albicaulis), ash (Fraxinus spp.), and poplar (Populus spp.)—represent a wide range of forest health problems with different combinations of characteristics in terms of the above criteria (see Table 2-2). In two cases, the committee chose specific host trees that face more than one pest pressure (American chestnut and whitebark pine). In the other two cases (ash and poplar), the committee examined the implications of a specific pest for a genus of trees. The native ranges of the major host tree species vary considerably in extent but together cover much of the United States (see Figure 2-5). Forest ecosystems, rural and urban, have all experienced negative ecological and economic impacts from tree mortality caused by the insects and pathogens examined in these studies. All of the species have clear ecological and cultural value, and all but whitebark pine have economic value. Critical for this study, the species vary in development and feasibility of a biotech solution to reduce vulnerability to the insect pest or pathogen involved. The case studies are introduced here and referenced throughout the rest of the report.
American Chestnut (Castanea dentata)
In the 19th century, the range of American chestnut extended from Maine to Mississippi along the Appalachian Mountains (Little, 1977; see Figure 2-5). American chestnuts were fast growing, and trees could reach 37 meters in height and 5 meters in diameter on favorable sites (Buttrick, 1925; Wang et al., 2013). The number of mature trees prior to the introduction of chestnut blight was estimated to be 4 billion (Detwiler, 1915), representing a major fraction of the forest biomass in many eastern forests (Braun, 1950). At some locations in the Appalachian Mountains, the American chestnut was considered to be a foundation species because of its strong influence on ecosystem structure and function (Youngs, 2000; Ellison et al., 2005a). In some regions, one in four trees in the canopy was reported to be an American chestnut (Johnson, 2013).
In 1904, American chestnuts at the Bronx Zoo in New York City died from infection by a fungal pathogen initially identified as Diaporthe parasitica but later renamed Cryphonectria parasitica. The pathogen was likely introduced on Japanese chestnuts imported to the United States as early as 1876 (Anagnostakis, 1987; Anagnostakis and Hillman, 1992).
The disease spread more or less unchecked, extending over the entire range of the American chestnut by the 1950s (see Figure 2-6). Traditional control measures, such as chemical treatments or clearing and burning, were ineffective (Stoddard and Moss, 1913). The pathogen maintained virulence over time, and almost all mature chestnuts were killed (Hepting, 1974; Russell, 1987).
The pathogen causing chestnut blight is necrotrophic, entering through small wounds in the outer bark, killing the living vascular cambium, and then developing cankers on the dead tissues. In susceptible trees, the fungus eventually girdles the branches and main stem, blocking the transfer of nutrients and resulting in tree death (Anagnostakis, 2000). In blight-tolerant Asian chestnut trees, lignified callus may surround the wound and restrict the growth of cankers; in susceptible trees, the fungus is able to overcome this resistance, leading to mortality.
In 2018, surviving chestnut trees existed mainly in shrubby growth forms that result from the formation of sprouts from the root collar. The sprouts grow for several years until they are again infected by C. parasitica and die back. Each cycle—resprout followed by fungus infection and dieback—weakens the tree until it eventually dies (Griffin, 2000). Sprouts rarely reach reproductive maturity and seeds are seldom produced (Paillet, 2002). Thus, the American chestnut persists mainly as a multistemmed shrub with only a few large chestnut trees remaining, often at the periphery of the tree’s range, presumably as “escapes” (i.e., trees that have not yet been exposed to the pathogen).
The loss of the American chestnut was devastating for rural communities that depended on the tree for food, livestock feed, and timber (Youngs, 2000; Freinkel, 2009). Equally devastating were the changes to the forest ecosystem due to the loss of a foundational species (Freinkel, 2009).
TABLE 2-2 List of Variables Considered by the Committee When Selecting Case Studies
| Variable | American Chestnut (Castanea dentata) | Whitebark Pine (Pinus albicaulis) | Ash (Fraxinus spp.) | Cottonwood (Populus trichocarpa, P. balsamifera) |
|---|---|---|---|---|
| Geographic distribution | Eastern North America | Western North American mountains | 16 species widely distributed across North America | Northern and western North America |
| Causative agent (origin) | Pathogen: chestnut blight (Cryphonectria parasitica) (nonnative) | Pathogen: Cronartium ribicola (nonnative) Insect pest: mountain pine beetle (Dendroctonus ponderosae) (native) | Insect pest: emerald ash borer (Agrilus planipennis) (nonnative) | Pathogen: Sphaerulina musiva (native to eastern species of poplar but not to northern and western species) |
| Other stressors | Pathogen: Phytophthora cinnamomi (nonnative) Insect pest: Dryocosmus kuriphilus (nonnative) | Climate change (drought), changes in fire regime | Land conversion | Land conversion, flood control |
| Urgency | High | High | High | Low |
| Alternative insect/pathogen hosts | Yes | Yes | Yes | Yes |
| Major ecological role | Yes | Yes | Yes | Yes |
| Economical values | Timber, chestnuts | None | Landscaping, timber, woodworking products | Pulp production |
| Cultural/traditional valuesa | Yes | Yes | Yes | Yes |
| Plantation forestry | Maybe | No | No | Yes |
| Potentially effective nonbiotech approaches to mitigate forest health threatsb | Hybridization (breeding) Hypovirulence | Reduced abundance of alternative hosts Selective breeding for resistance | Biocontrol (parasitoids), pesticides Selective breeding for resistance | Fungicide application Biocontrol (bacteria) |
| Biotechnological approaches in use as of 2018c | Transgenesis | None | None | Transformable with Agrobacterium |
| Potential biotechnological approachesc | Well developed | Recalcitrant | In development | Well developed |
aSee discussion in section “Social and Ethical Considerations” in Chapter 4.

Other nonnative Castanea species have been planted in urban environments or as orchard trees for commercial production of chestnuts, but they do not fill the same ecological niche as the American chestnut. Chinese chestnut (C. mollissima) and Japanese chestnut (C. crenata) are typically small trees, lacking the fast growth and tall form of American chestnut. The European chestnut (C. sativa) has a growth and form somewhat similar to American chestnut as compared to the Asian species, but the European chestnut trees growing in North America are susceptible to the same diseases as the American chestnut and are not as frost tolerant. The Asian species usually do not live as long as American chestnut. In a forest setting, the other Castanea species are not competitive; they do not grow tall enough or fast enough to compete for light against the native American chestnut or other native tree species (Wu and Raven, 1999; Fei et al., 2012). The American chestnut has lost the role it once had as a foundational species that influenced other species and ecosystem processes.
As with many trees, the American chestnut faces more than one threat. In southern Appalachia, the introduced oomycete Phytophthora cinnamomi causes black lesions on the roots, eventually killing the tree by killing the root system (Crandall et al., 1945). Trials of restoration plantings in this region reveal that P. cinnamomi persists in the soil long after the mature chestnuts die and kills the majority of planted chestnut seedlings within a few months (Rhoades et al., 2003). Asian chestnut gall wasp (Dryocosmus kuriphilus), accidentally imported on Asian chestnut cuttings in 1974 (Payne et al., 1976), attacks both Asian and American chestnuts. The galls suppress shoot growth and nut development.
American chestnut is the committee’s only case study of a species that has essentially been lost throughout its native range as of 2018. Oaks and maples have filled in for this species over much of the range and maintained some of the forest functions (Keever, 1953; Woods and Shanks, 1959; McCormick and Platt, 1980). Although acorns have replaced chestnuts as mast sources to some extent, oaks have episodic mast years, unlike the consistent, substantial annual mast produced by the American chestnut and chestnut’s relatives, the chinquapins (Castenea pumila and C. ozarkensis). Population dynamics of species dependent on the nuts were likely affected, with cascading food web impacts. At least five moth species obligate on chestnuts have gone extinct (Opler, 1978; Wagner and Van Driesche, 2010). Economies and cultures of human communities originally reliant on American chestnut products were also altered (Davis, 2006); chestnut has been identified as a cultural keystone species (sensu Garibaldi and Turner, 2004).
Whitebark Pine (Pinus albicaulis)
Whitebark pine is a high-elevation tree of the western United States and Canada (see Figure 2-7). It spans over 18o latitude and 21o longitude, but within that area it establishes only within a narrow elevational distribution extending from the subalpine to treeline (Tomback et al., 2016). The tree exhibits high phenotypic plasticity (i.e., an ability to grow in different forms in response to its environment). In open stands, it grows as a large wide-crowned tree, whereas in dense stands it takes a linear form similar to lodgepole pine. On harsh windswept ridges, it forms krummholz—dwarfed, gnarled trees that seldom reach more than 1–2 meters in height, even when hundreds of years old. In the subalpine, it sometimes grows in mixed stands, often with subalpine fir, Engelmann spruce, and lodgepole pine. In the upper extent of the subalpine and at treeline, whitebark pine is typically the only tree present (Tomback et al., 2016). It is a long-lived tree, sometimes reaching ages of 1,000 years or more (Perkins and Swetnam, 1996). It grows slowly and typically does not begin to reproduce until at least 20–30 years of age and not fully until 60 or more years (McCaughey and Tomback, 2001).
Whitebark pine is considered to be both a keystone and a foundational species. As a keystone, its presence sustains the biodiversity and function of the community of which it is part. As a foundational species, it is responsible for creating the conditions that allow the community to assemble

in the first place (Tomback et al., 2016). At the upper limits of its elevational range, whitebark pine establishes in areas too harsh to support other tree species (Weaver and Dale, 1974; Tomback and Linhart, 1990). In these places, whitebark pines provide shelter and contribute to soil development, allowing other plant species to establish (Arno and Hoff, 1990; Callaway, 1998). “Life islands” of shrubby vegetation often develop at the base of these trees, providing food and nesting habitat for birds and small mammals and stabilizing rocky slopes. Cover provided by the trees regulates snowmelt, retaining water in the subalpine for longer into the spring and supporting flows in mid and low elevations for an extended period into the summer (Farnes, 1990).
The tree is threatened by several factors including human-induced changes in fire regimes (suppression), an introduced fungal pathogen (Cronartium ribicola, the causal agent of a disease called white pine blister rust), a native bark beetle (the mountain pine beetle, Dendroctonus ponderosae), and climate change (increased drought). Individually, each threat is serious. These factors also interact, exacerbating the rate and degree of decline. Together, these threats pose an extremely complex problem for the conservation and restoration of this tree.
More than half of all whitebark pines in the northern United States and Canada are already dead. In some areas, only about 2 percent of mature (reproductive) trees remain (Kendall and Keane, 2001; Zeglen, 2002; Smith et al., 2008). Seeds are dispersed by birds in the jay family, specifically Clark’s nutcrackers (Nucifraga columbiana), that open the cones and cache the seeds for later use. Seeds in unretrieved caches germinate to produce new whitebark pines. In areas where few mature trees remain, foraging becomes inefficient and the nutcrackers reduce visitation to these sites, thus lowering the potential for regeneration (McKinney and Tomback, 2007; McKinney et al., 2009; Barringer et al., 2012).
Mortality has been most severe in the central and northern Rocky Mountains and in the coastal mountain ranges, whereas southern populations remain fairly robust primarily due to a lack of rust and beetle activity as of 2018. Canada listed whitebark pine as endangered in 2010 (COSEWIC, 2010). The tree’s status in the United States is “recommended for listing, but precluded” (USFWS, 2011). Preclusion, in this case, is based on a lack of funding and its lower priority for recovery relative to several other species. As of 2018, the tree’s status under the Endangered Species Act was under re-review, with a decision slated for 2019.
North American Ash (Fraxinus spp.)
There are 16 ash species native to North America, of which green ash (Fraxinus pennsylvanica) and white ash (F. americana) are the most widely distributed. The native range of green ash includes the Eastern Temperate, Great Plains, and Northern Forests ecoregions in North America (Omernik, 1995, 2004; CEC, 1997; Omernik and Griffith, 2014; see Figure 2-8). Although green ash grows abundantly in riparian zones in mesic temperate forests, it can persist in upland forests and seasonally dry urban environments throughout the eastern and central United States. In the Great Plains ecoregion in the western part of the range, green ash can be locally abundant in riparian zones or along ephemeral streams (Rumble and Gobeille, 1998; Lesica, 2009). Although this species occupies only 1–4 percent of the landscape in this region, green ash woodlands support a disproportionately large component of biological diversity, including migratory songbirds, gallinaceous birds, and native ungulates (Boldt et al., 1979; MacCracken and Uresk, 1984; Hodorff and Sieg, 1986; Rumble and Gobeille, 1998). Additionally, 43 native arthropod species are solely dependent on green and white ash during some part of their life cycle, and 30 additional species have only 2–3 known host plants, one of which is ash (Gandhi and Herms, 2010b).
First detected in Detroit, Michigan, and Windsor, Ontario, in 2002, the emerald ash borer (EAB, Agrilus planipennis Fairmaire [Coleoptera: Buprestidae]) poses an acute threat to all of the native ash species in North America (Herms and McCullough, 2014). The International Union for
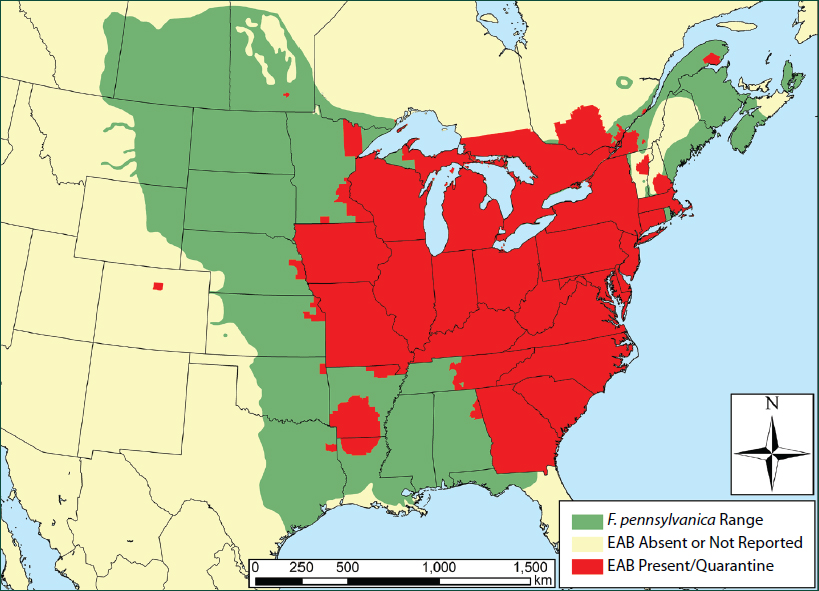
NOTES: Planting and establishment of green ash outside the native range results in emerald ash borer infestation beyond the native range of F. pennsylvanica. At the time the committee was writing its report, the U.S. Department of Agriculture was considering removing domestic quarantine regulations for EAB.
SOURCES: Data from emerald ash borer information network, http://www.emeraldashborer.info/index.php. Figure by Devin Shirley.
Conservation of Nature Red List of Threatened Species lists five North American ash species—green ash, white ash, black ash (F. nigra), pumpkin ash (F. profunda), and blue ash (F. quadrangulata)—as critically endangered due to nearly 100 percent mortality following attack, limited ability to regenerate under repeated attack, and rapid spread of the insect, largely through unintentional human agency. EAB, native to Asia, had spread to 31 states and 3 Canadian provinces as of May 2018 (see Figure 2-8).
The insect kills 99–100 percent of green ash trees in forest stands within 7 years of first detection (see Figure 2-9a) and kills urban green ash plantings as fast or faster, due to the extensive use of grafted green ash cultivars (Rebek et al., 2008; Smitley et al., 2008; Knight et al., 2012). Females oviposit in bark cracks and crevices, laying 60–80 eggs. Larvae hatch in a few weeks, feed voraciously on the phloem and other living tissues under the bark and complete four instars before overwintering as prepupae (Cappaert et al., 2005). Pupation occurs in the spring, and adults emerge starting in mid-May and continuing throughout the summer (Poland et al., 2011). EAB feeding destroys the vasculature and the tissue that forms new vessels and bark, ultimately girdling the main stem and thus killing the host (see Figure 2-9b).
Green ash, as well as the other ash species listed as critically endangered, has some capacity to regenerate from root and stump sprouts even after EAB infestation (Kashian, 2016). However, EAB also kills these resprouts, removing any mechanism for regeneration via vegetative propagation. Ash seedlings may be initially abundant after extensive mortality among adult trees (Kashian and

Witter, 2011), giving the impression that ash will recover. However, when these seedlings reach 2–3 cm in stem diameter, EAB infestation again inflicts high mortality. Ash does not have a persistent seedbank, so once mature trees are killed, it is nearly impossible for the species to reestablish itself.
The near synchronous loss of green ash has had a cascade of negative impacts, including the rapid loss of naturally occurring riparian forests, which are composed mainly of green or black ash (Gandhi and Herms, 2010a,b; Hausman et al., 2010; Kovacs et al., 2010; Knight et al., 2013), billions of dollars in tree removal cost to local governments, and the loss of a valuable utility hardwood used for cabinets, furniture, tool handles, restoration of antique cars, wooden snowshoes, guitars, and baseball bats. Five or more hawk moth species that specialize on Fraxinus are hypothesized to be at risk from the loss of ash to EAB (Wagner and Van Driesche, 2010). Thus, without effective and timely intervention, the EAB invasion threatens two of the most widely distributed hardwood species in the riparian forests of eastern North America and the most extensively used group of tree species for soil conservation, rural water management, urban green spaces, and utility woodworking as well as the species that depend on Fraxinus. It also threatens to continue its spread west, where it will likely kill western species of ash that have so far been unaffected.
Poplar (Populus spp.)
This case study presents an example of an incipient invasion of a pathogen native to forest ecosystems in eastern North America that poses a threat to an ecologically important native tree group in western North America as well as to a sector of the forest products industry. There are eight native species of Populus in North America and multiple hybrids (Cooke and Rood, 2007), but the focus of the case study is on three species: black cottonwood (P. trichocarpa), the closely related balsam poplar (P. balsamifera), and widespread eastern cottonwood (P. deltoides) (see Figure 2-5). These species are model organisms for basic research, so in some ways this tree species may represent a best-case scenario for the potential of biotechnology to prevent or mitigate a forest health crisis.
In open environments, black cottonwood is a dominant native tree in lowland riparian ecosystems in Oregon, Washington, and British Columbia (Franklin and Dyrness, 1973), where it plays essential roles in stream ecology (Pastor et al., 2014) and as habitat for birds and mammals (Kauffman and Krueger, 1984; Isaacs et al., 1993, 1996; Bryce et al., 2002). Black cottonwood populations typically become established following deposition of sand and gravel following episodic floods, resulting in bands of even-aged cohorts that line river floodplains (Braatne et al., 1996). The species produces abundant seeds with cotton-like appendages that facilitate long-distance dispersal by wind and water (Slavov et al., 2010; DiFazio et al., 2012) and enable deposition on newly created substrates following recession of floodwaters. It also spreads vegetatively by root sprouts or abscised branches, leading to the development of large clonal stands in some locations (Gom and Rood, 1999; Slavov et al., 2010). As a result, this species is critical for floodplain soil stabilization and provides habitat for other species. Black cottonwood populations have shown evidence of decline in recent decades, in part because of a loss of establishment opportunities due to flood control (Dykaar and Wigington, 2000; Braatne et al., 2007). However, extensive gallery forests of this species are still a prominent and valued component of the landscape in the Pacific Northwest.
In research, the genus Populus is widely recognized as a model for woody tree biology (Taylor, 2002; Jansson and Douglas, 2007). The genus has several desirable experimental characteristics, including a small genome (Tuskan et al., 2006), easy vegetative propagation via stem cuttings and tissue culture, ability to hybridize (Induri et al., 2012), and short generation time (Stanton et al., 2010). These features have made Populus an attractive model for applied studies focused on enhancing productivity in intensive plantation settings for pulp, biofuel, and solid wood (Dickmann, and Kuzovkina, 2014). Populus spp. have also been a primary target of basic research in the areas of physiology, ecology, and evolutionary biology. Consequently, abundant genetic and genomic
resources are available for this genus (Tuskan et al., 2006; Evans et al., 2014; Zinkgraf et al., 2016; Fahrenkrog et al., 2017).
The fungal pathogen Sphaerulina musiva (synonym, Septoria musiva) is native to eastern North America, with a historical distribution that largely mirrors that of its primary natural host, eastern cottonwood. The pathogen causes blotches and stem cankers in P. deltoides, P. balsamifera, P. trichocarpa, and hybrid Populus cultivars in North America (see Figure 2-10). The disease initially occurred primarily in natural populations of P. deltoides in the east, where it was mostly manifested as leaf spots (Waterman, 1954). However, it has since spread from eastern forests to intensively cultivated eastern plantations of native and hybrid poplars, where it commonly causes stem and branch cankers, often leading to breakage of the main stem and death of the tree (Ostry and McNabb, 1985; Dunnell et al., 2016). In the most detailed published survey of a large-scale outbreak, Strobl and Fraser (1989) documented occurrence of S. musiva canker in intensively cultivated hybrid poplar in Ontario. Within 5 years of the establishment of susceptible hybrid clones in the region, more than 150 hectares (370 acres) of plantations were affected by the disease, and 79 percent of the area planted with susceptible clones had disease outbreaks (Strobl and Fraser, 1989). This disease can clearly have rapid and devastating impacts on intensive plantations of susceptible varieties (Feau et al., 2010).
Of even greater concern are reports of stem cankers caused by S. musiva in natural populations of black cottonwood in Pacific Northwest forests, where the disease is not native and was unknown until 2006 (Callan et al., 2007; Herath et al., 2016). Both P. trichocarpa and P. balsamifera show high susceptibility to this disease (LeBoldus et al., 2013; Herath et al., 2016), so the threat of a large-scale outbreak has caused substantial concern among scientists, members of the forest industry, land managers, and the public (Feau et al., 2010). Black cottonwood may be particularly

vulnerable to an outbreak of this disease. In the core of its range along rivers of northwestern North America, black cottonwood often occurs in dense, even-aged stands in climates and microsites that are characterized by abundant moisture (DiFazio et al., 2011), which could facilitate spread of the disease. Furthermore, P. trichocarpa populations are already in decline due to flood control and habitat loss (Rood and Mahoney, 1990; Dykaar and Wigington, 2000), so a disease outbreak could be particularly problematic for the long-term viability of the species.
Effects on Forest Health and Ecosystem Services
The case studies are not isolated examples of species in decline. Rather, given the rate of introductions of nonnative insect pests and pathogens and the effects of climate change on distribution and abundance of native insects and pathogens, their trajectory is likely to become the norm in North American forests. The frequency and magnitude of outbreaks and the rate of tree mortality are likely to increase. These impacts will have significant effects on forest health and ecosystem services (Dukes et al., 2009; Millar and Stephenson, 2015; Lovett et al., 2016; Liebhold et al., 2017). As outlined above, ecosystem services are generally defined as the direct and indirect contributions of ecosystems to human well-being (Braat and de Groot, 2012; see also the discussion in Chapter 5).
The most immediate effects of increased insect and pathogen activity (native and introduced) on forest health are reductions in productivity and alterations of nutrient, carbon, and water cycles (Lovett et al., 2006). In the case of extended or severe tree mortality, as in the American chestnut, substantial losses of other forest species and some ecosystem services can be expected.
The impact of increased insect pest and pathogen activity on ecosystem services is strongly linked to the proportion of the canopy affected. Increases in the effects of host-specific insects and pathogens that target dominant and keystone tree species will likely result in the most severe and long-term impacts (Ellison et al., 2005a). For example, eastern hemlock (Tsuga canadensis) dominates forest stands in its northern range and moist coves in the south. Loss of the hemlock due to the nonnative hemlock wooly adelgid (Adelges tsugae) has caused the loss of several wildlife species associated with hemlock (Tingley et al., 2002; Ellison et al., 2005b), affected soil processes (Jenkins et al., 1999), and changed local hydraulic flow (Ellison et al., 2005a). These impacts may occur even where other tree species rapidly colonize areas once occupied by hemlock (Orwig et al., 2002), as the ecosystem services provided by one species may differ from those provided by others. For example, in the Southern Appalachians, the effects of hemlock trees on stream flow and temperature sustain unique communities of salamanders, fish, and other stream invertebrate species that will be lost without hemlocks (Snyder et al., 2002).
In areas of low tree diversity, outbreaks of insect pests and pathogens can have devastating consequences for regulating and supporting services, as a large proportion of the canopy can be affected with no replacement species naturally recolonizing afterward. This is the case with whitebark pine. The ecological void created by the loss of whitebark pine (see case study above) will be vast because this species supplies numerous resources, including shelter and food to wildlife species, water regulation through snowpack retention, and soil development, which facilitates the establishment of other plant species (Arno and Hoff, 1990; Farnes, 1990; Callaway, 1998).
Intrinsic properties of the ecosystem may mediate the magnitude of the loss of ecosystem services. High-diversity forests are home to more introduced insect pests and pathogens (Liebhold et al., 2013), but the loss of one tree species in these areas may be compensated by other species. For example, even though white ash (Fraxinus americana) is a conspicuous species in eastern North American forests, it does not dominate these stands (Prasad et al., 2007-ongoing). As of 2018, EAB was causing the death of most adult white ash trees across large areas. However, the void left by the death of ash trees is rapidly being filled by other tree species, such as maples (Margulies et al., 2017). Maples likely supply some of the ecosystem services provided by ash but may not support
the biodiversity reflective of an uninvaded forest. The same was true for the eastern forest when the American chestnut declined; the replacement species do not produce the mast, timber, or stature and do not provide the cultural or spiritual values of the original forest (Davis, 2006). Additionally, while replacement species offer at least a temporary mitigation of some impacts, the continual influx of nonnative insects and pathogens could subject the replacement species themselves to impacts in the future, a factor to consider when deciding whether to try to restore species in jeopardy of extirpation.
However, even if impacts can be mostly mitigated by replacement tree species, the costs can still be substantial. Shortly after EAB was found in the United States, the U.S. Forest Service projected the lost timber value from ash trees in forested lands could be close to $280 billion (Nowak et al., 2003). Additionally, the anticipated cost of losing these species in urban settings was estimated to be between $20 billion and $60 billion (USDA-APHIS, 2003) due to loss of property value and cost of removal. Using this subset of ecosystem values, EAB is the most economically devastating invasive insect pest in North American history (Herms and McCullough, 2014).
The effects of insect pests and pathogens on individual trees have cascading impacts on populations, reducing reproduction and survival. In the most extreme cases, local extirpation of the tree species and extinction or extirpation of species dependent on the tree may result (e.g., the already mentioned extinction of five moth species with the loss of the American chestnut) (Opler, 1978; Wagner and Van Driesche, 2010). Such species-specific effects can then translate into changes in community assemblage and structure, and thus, ecosystem functionality. The loss of whitebark pine may reduce the complexity and function of high-elevation ecosystems in the west and contribute to the decline of grizzlies and other wildlife as well as ecosystem services related to water and sediment regulation. The loss of ash trees affects not only natural communities; loss of city trees has had a large effect on property values (Aukema et al., 2011). The decline of black cottonwood in the West would adversely affect riparian habitats.
CONCLUSIONS
Based on its evaluation of the scientific literature and the information it gathered from invited speakers, the committee defined forest health as a condition that sustains the structure, composition, processes, function, productivity, and resilience of forest ecosystems over time and space. An assessment of this condition is based on the current state of knowledge and can be influenced by human needs, cultural values, and land management objectives. North American forests are struggling to maintain healthy conditions because of increasing stresses, on to which outbreaks of introduced insects and pathogens and the geographic expansion of native pests due to climate change are layered. While impossible to fully isolate, the direct adverse effects of pests on forest health have significant impacts on the ecosystem services that forests provide.
Conclusion: Healthy forests provide valuable ecosystem services to humans.
The ecological processes performed by forests and the cultural and aesthetic values attached to forests are important to individuals and to society. Forests provide food and habitat for pollinators, fish, wildlife, and other organisms, as well as food, fuel, and products for humans.
Conclusion: The health of North American forests is threatened by the introduction and spread of nonnative insects and pathogens and the epidemics of native pests exacerbated by environmental stress due to climate change.
At least 62 insect species and 16 pathogens that cause tree mortality, canopy thinning, growth loss, defoliation, or decreased reproduction or regeneration have been introduced to North Amer-
ica. Some of these introductions have had devastating consequences in North American forests. Increases in human mobility and trade are likely to lead to more such introductions. Climate change is opening new opportunities for colonization by both native and introduced insect species and affecting the frequency and magnitude of outbreaks of both native and introduced pests. Outbreaks are predicted to increase in frequency and magnitude in the future.
Conclusion: Tree species in forest ecosystems, tree plantations, and urban landscapes across North America are threatened by insect pests and pathogens.
The four case study species selected by the committee—American chestnut (Castanea dentata), whitebark pine (Pinus albicaulis), ash (Fraxinus spp.), and poplar (Populus spp.)—serve as examples of diverse ecosystems and habitats that are experiencing adverse impacts from tree mortality caused by insect pests and pathogens. The American chestnut was a foundation species because of its strong influence on ecosystem structure and function and an economic resource for communities before its extirpation. Whitebark pine creates and sustains community biodiversity at high elevations. Ash woodlands support biodiversity and provide benefits to humans as a popular urban landscape tree. Black cottonwood stabilizes streambanks and provides habitat for birds and mammals; poplars are also model trees for research and an important resource for production of pulp, biofuel, and solid wood.
Conclusion: Many forest tree species are threatened by more than one insect pest or pathogen.
American chestnut, whitebark pine, ash, and poplar are just four examples of North American tree species that have been or are in danger of being extirpated. They are subject to one or more pest threats, and whitebark pine, in particular, is losing habitat to climate change. The number of (see Table 2-1) and trend in (see Figure 2-2) introduced threats and the geographic expanse of all pest threats represented by the four case study species (see Figure 2-5) suggest that native trees throughout North America are in danger of or may become subject to pest outbreaks that adversely affect forest health.
Conclusion: As the frequency of insect and pathogen outbreaks increases, many forest tree species are in jeopardy of being lost from the landscape, resulting in changes to ecosystem services.
The growth in global trade, the increase in human mobility, and the warming of the climate are all contributing to the increased pest pressure that forests now face. The magnitude of pest outbreaks may permanently change the structure, composition, processes, function, productivity, and resilience of forest ecosystems. As tree species are lost from the landscape, the species obligate to those trees will be lost as well.
REFERENCES
Aldred, J. 1994. Existence value, welfare and altruism. Environmental Values 3(4):381–402.
Anagnostakis, S.L. 1987. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 79(1):23–37.
Anagnostakis, S.L. 2000. Revitalization of the majestic chestnut: Chestnut blight disease. APSnet Feature. Available at http://www.apsnet.org/publications/apsnetfeatures/Pages/ChestnutBlightDisease.aspx. Accessed August 25, 2018.
Anagnostakis, S.L., and B. Hillman. 1992. Evolution of the chestnut tree and its blight. Arnoldia 52(2):2–10.
Arno, S.F. and R.J. Hoff. 1990. Pinus albicaulis Engelm. whitebark pine. Pp. 268–279 in Silvics of North America Volume 1, R.M. Burns and B.H. Honkala, tech. cords. Washington, DC: U.S. Government Printing Office.
Aukema, J.E., D.G. McCullough, B. Von Holle, A.M. Liebhold, K. Britton, and S.J. Frankel. 2010. Historical accumulation of nonindigenous forest pests in the continental United States. BioScience 60(11):886–897.
Aukema, J.E., B. Leung, K. Kovacs, C. Chivers, K.O. Britton, J. Englin, S.J. Frankel, R.G. Haight, T.P. Holmes, A.M. Liebhold, D.G. McCullough, and B. Von Holle. 2011. Economic impacts of non-native forest insects in the continental United States. PLoS One 6(9):e24587.
Ayres, M.P., and M.J. Lombardero. 2000. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Science of the Total Environment 262(3):263–286.
Bale, J.S., G.J. Masters, I.D. Hodkinson, C. Awmack, T.M. Bezemer, V.K. Brown, J. Butterfield, A. Buse, J.C. Coulson, J. Farrar, J.E.G. Good, R. Harrington, S. Hartley, T.H. Jones, R.L. Lindroth, M.C. Press, I. Symrnioudis, A.D. Watt, and J.B. Whittaker. 2002. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biology 8(1):1–16.
Barnes, B.V., and W.H. Wagner. 2004. Michigan Trees. Ann Arbor, MI: University of Michigan Press.
Barringer, L.E., D.F. Tomback, M.B. Wunder, and S.T. McKinney. 2012. Whitebark pine stand condition, tree abundance, and cone production as predictors of visitation by Clark’s nutcracker. PloS One 7(5):e37663.
Battisti, A., M. Stastny, S. Netherer, C. Robinet, A. Schopf, A. Roques, and S. Larsson. 2005. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecological Applications 15(6):2084–2096.
Bennett, K.P., ed. 2010. Good Forestry in the Granite State: Recommended Voluntary Forest Management Practices for New Hampshire. 2nd edition. Durham: University of New Hampshire Cooperative Extension.
Bentz, B.J., J. Régnière, C.J. Fettig, E.M. Hansen, J.L. Hayes, J.A. Hicke, R.G. Kelsey, J.F. Negrón, and S.J. Seybold. 2010. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 60(8):602–613.
Berg, E.E., J.D. Henry, C.L. Fastie, A.D. De Volder, and S.M. Matsuoka. 2006. Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: Relationship to summer temperatures and regional differences in disturbance regimes. Forest Ecology and Management 227(3):219–232.
Bergot, M., E. Cloppet, V. Pérarnaud, M. Déqué, B. Marçais, and M. Desprez-Loustau. 2004. Simulation of potential range expansion of oak disease caused by Phytophthora cinnamomi under climate change. Global Change Biology 10(9):1539–1552.
Boisvenue, C., and S.W. Running. 2006. Impacts of climate change on natural forest productivity—evidence since the middle of the 20th century. Global Change Biology 12(5):862–882.
Boldt, C.E., D.W. Uresk, and K.E. Severson. 1979. Riparian woodlands in jeopardy on Northern High Plains. Pp. 184–189 in Proceedings of the National Symposium on Strategies for Protection and Management of Floodplain Wetlands and Other Riparian Ecosystems, R.F. Johnson and J.F. McCormick, eds. Washington, DC: U.S. Forest Service.
Boyd, I.L., P.H. Freer-Smith, C.A. Gilligan, and H.C.J. Godfray. 2013. The consequence of tree pests and diseases for ecosystem services. Science 342:1235773.
Braat, L.C., and R. de Groot. 2012. The ecosystem services agenda: Bridging the worlds of natural science and economics, conservation and development, and public and private policy. Ecosystem Services 1(1):4–15.
Braatne, J.H., S.B. Rood, and P.E. Heilman. 1996. Life history, ecology, and reproduction of riparian cottonwoods in North America. Pp. 57–85 in Biology of Populus and Its Implications for Management and Conservation, R.F. Stettler, H.D. Bradshaw Jr., P.E. Heilman, and T.M. Hinckley, eds. Ottawa, ON: NRC Research Press.
Braatne, J.H., R. Jamieson, K.M. Gill, and S.B. Rood. 2007. Instream flows and the decline of riparian cottonwoods along the Yakima River, Washington, USA. River Research and Applications 23(3):247–267.
Braun, E.L. 1950. Deciduous Forests of Eastern North America. New York: Hafner.
Breshears, D.D., N.S. Cobb, P.M. Rich, K.P. Price, C.D. Allen, R.G. Balice, W.H. Romme, J.H. Kastens, M.L. Floyd, J. Belnap, J.J. Anderson, O.B. Myers, and C.W. Meyer. 2005. Regional vegetation die-off in response to global-change-type drought. Proceedings of the National Academy of Sciences of the United States of America 102(42):15144–15148.
Brockerhoff, E.G., M. Kimberley, A.M. Liebhold, R.A. Haack, and J.F. Cavey. 2014. Predicting how altering propagule pressure changes establishment rates of biological invaders across species pools. Ecology 95(3):594–601.
Bryce, S.A., R.M. Hughes, and P.R. Kaufmann. 2002. Development of a bird integrity index: Using bird assemblages as indicators of riparian condition. Environmental Management 30(2):294–310.
Burkhard, B., and J. Maes, eds. 2017. Mapping Ecosystem Services. Sofia: Pensoft.
Buttrick, P.L. 1925. Chestnut in North Carolina. Pp. 6–10 in Chestnut and the Chestnut Blight in North Carolina. North Carolina Geological and Economic Survey: Economic Paper 56.
Callan, B., I. Leal, B. Foord, J.J. Dennis, and C. van Oosten. 2007. Septoria musiva isolated from cankered stems in hybrid poplar stool beds, Fraser Valley, British Columbia. North American Fungi 2(7):1–9.
Callaway, R.M. 1998. Competition and facilitation on elevation gradients in subalpine forests of the northern Rocky Mountains, USA. Oikos 82(3):561–573.
Cappaert, D., D.G. McCullough, T.M Poland, and N.W. Siegert. 2005. Emerald ash borer in North America: A research and regulatory challenge. American Entomologist 51(3):152–165.
Carroll, A.L., S.W. Taylor, J. Régnière, and L. Safranyik. 2004. Effects of climate change on range expansion by the mountain pine beetle in British Columbia. Pp. 223–232 in Mountain Pine Beetle Symposium: Challenges and Solutions, 30–31 October 2003, Kelowna, British Columbia, T.L. Shore, J.E. Brooks, and J.E. Stone, eds. Victoria, BC: Natural Resources Canada.
Castlebury, L.A., A.Y. Rossman, and A.S. Hyten. 2006. Phylogenetic relationships of Neonectria/Cylindrocarpon on Fagus in North America. Canadian Journal of Botany 84(9):1417–1433.
CEC (Commission for Environmental Cooperation). 1997. Ecological Regions of North America: Toward a Common Perspective. Montreal: CEC Secretariat.
Cooke, J.E., and S.B. Rood. 2007. Trees of the people: The growing science of poplars in Canada and worldwide. Botany 85(12):1103–1110.
Cooper, N., E. Brady, H. Steen, and R. Bryce. 2016. Aesthetic and spiritual values of ecosystems: Recognising the ontological and axiological plurality of cultural ecosystem “services.” Ecosystem Services 21(Part B):218–229.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2010. COSEWIC Assessment and Status Report on the Whitebark Pine Pinus albicaulis in Canada. Ottawa: COSEWIC Secretariat.
Crandall, B.S., G.F. Gravatt, and M.M. Ryan. 1945. Root disease of Castanea species and some coniferous and broadleaf nursery stocks, caused by Phytophthora cinnamomi. Phytopathology 35:162–180.
Davidson, M.D. 2013. On the relation between ecosystem services, intrinsic value, existence value and economic valuation. Ecological Economics 95:171–177.
Davis, D.E. 2006. Historical significance of the American chestnut to Appalachian culture and ecology. Pp. 53–60 in Restoration of American Chestnut to Forest Lands, K.C. Steiner and J.E. Carlson, eds. Washington, DC: U.S. Department of the Interior.
DeFries, R., S. Pagiola, W.L. Adamowicz, H.R. Akçakaya, A. Arcenas, S. Babu, D. Balk, U. Confalonieri, W. Cramer, F. Falconí, S. Fritz, R. Green, E. Gutiérrez-Espeleta, K. Hamilton, R. Kane, J. Latham, E. Matthews, T. Ricketts, T.X. Yue. 2005. Analytical approaches for assessing ecosystem condition and human well-being. Pp. 37–71 in Ecosystems and Human Well-being: Current State and Trends, Volume 1, R. Hassan, R. Scholes, and N. Ash, eds. Washington, DC: Island Press.
Detwiler, S. 1915. The American chestnut tree. American Forestry 21(262):957–960.
Dickmann, D.I., and J. Kuzovkina. 2014. Poplars and willows of the world, with emphasis on silviculturally important species. Pp. 8–91 in Poplars and Willows: Trees for Society and the Environment, J.G. Isebrands and J. Richardson, eds. Wallingford, UK: CABI.
DiFazio, S.P., G.T. Slavov, and C.P. Joshi. 2011. Populus: A premier pioneer system for plant genomics. Pp. 1–28 in Genetics, Genomics and Breeding of Poplar, S.P. DiFazio, S. Joshi, and C. Cole, eds. Enfield, NH: Science Publishers.
DiFazio, S.P., S. Leonardi, G.T. Slavov, S.L. Garman, W.T. Adams, and S.H. Strauss. 2012. Gene flow and simulation of transgene dispersal from hybrid poplar plantations. New Phytologist 193(4):903–915.
Dukes, J.S., J. Pontius, D. Orwig, J.R. Garnas, V.L. Rodgers, N. Brazee, B. Cooke, K.A. Theoharides, E.E. Stange, R. Harrington, J. Ehrenfeld, J. Gurevitch, M. Lerdau, K. Stinson, R. Wick, and M. Ayres. 2009. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 39(2):231–248.
Dunnell, K.L., B. Berguson, B. McMahon, and J.M. LeBoldus. 2016. Variation in resistance of Populus nigra to Sphaerulina musiva in the North-Central United States. Plant Disease 100(2):287–291.
Dykaar, B.B., and P.J. Wigington. 2000. Floodplain formation and cottonwood colonization patterns on the Willamette River, Oregon, USA. Environmental Management 25(1):87–104.
Early, R., B.A. Bradley, J.S. Dukes, J.J. Lawler, J.D. Olden, D.M. Blumenthal, P. Gonzalez, E.D. Grosholz, I. Ibañez, L.P. Miller, C.J.B. Sorte, and A.J. Tatem. 2016. Global threats from invasive alien species in the twenty-first century and national response capacities. Nature Communications 7:12485.
Ellison, A.M., M.S. Bank, B.D. Clinton, E.A. Colburn, K. Elliott, C.R. Ford, D.R. Foster, B.D. Kloeppel, J.D. Knoepp, G.M. Lovett, J. Mohan, D.A. Orwig, N.L. Rodenhouse, W.V. Sobczak, K.A. Stinson, J.K. Stone, C.M. Swan, J. Thompson, B. Von Holle, and J.R. Webster. 2005a. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Frontiers in Ecology and the Environment 3(9):479–486.
Ellison, A.M., J. Chen, D. Díaz, C. Kammerer-Burnham, and M. Lau. 2005b. Changes in ant community structure and composition associated with hemlock decline in New England. Pp. 280–289 in Proceedings of the 3rd Symposium on Hemlock Woolly Adelgid in the Eastern United States, B. Onken and R. Reardon R, compilers. Morgantown, WV: U.S. Forest Service.
Ellison, D., C.E. Morris, B. Locatelli, D. Sheil, J. Cohen, D. Murdiyarso, V. Gutierrez, M. Van Noordwijk, I.F. Creed, J. Pokorny, and D. Gaveau. 2017. Trees, forests and water: Cool insights for a hot world. Global Environmental Change 43:51–61.
Esper, J., U. Büntgen, D.C. Frank, D. Nievergelt, and A. Liebhold. 2007. 1200 years of regular outbreaks in alpine insects. Proceedings of the Royal Society of London B: Biological Sciences 274(1610):671–679.
Evans, L.M., G.T. Slavov, E. Rodgers-Melnick, J. Martin, P. Ranjan, W. Muchero, A.M. Brunner, W. Schackwitz, L. Gunter, J.-G. Chen, G.A. Tuskan, and S.P. DiFazio. 2014. Population genomics of Populus trichocarpa identifies signatures of selection and adaptive trait associations. Nature Genetics 46(10):1089–1096.
Fahrenkrog, A.M., L.G. Neves, M.F. Resende, A.I. Vazquez, G. Campos, C. Dervinis, R. Sykes, M. Davis, R. Davenport, W.B. Barbazuk, and M. Kirst. 2017. Genome wide association study reveals putative regulators of bioenergy traits in Populus deltoides. New Phytologist 213(2):799–811.
Farnes, P.E. 1990. SNOTEL and snow course data: Describing the hydrology of whitebark pine ecosystems. Pp. 302–305 in Proceedings—Symposium on Whitebark Pine Ecosystems: Ecology and Management of a High-mountain Resource. Ogden, UT: USDA-FS.
Feau, N., M.-J. Mottet, P. Périnet, R.C. Hamelin, and L. Bernier. 2010. Recent advances related to poplar leaf spot and canker caused by Septoria musiva. Canadian Journal of Plant Pathology 32(2):122–134.
Fei, S., L. Liang, F.L. Paillet, K.C. Steiner, J. Fang, Z. Shen, Z. Wang, and F.V. Hebard. 2012. Modelling chestnut biogeography for American chestnut restoration. Diversity and Distributions 18(8):754–768.
Fisichelli, N.A., G.W. Schuurman, and C.H. Hoffman. 2016. Is “resilience” maladaptive? Towards an accurate lexicon for climate change adaptation. Environmental Management 57(4):753–758.
Franklin, J.F., and C.T. Dyrness. 1973. Natural Vegetation of Oregon and Washington. Portland, OR: U.S. Forest Service.
Freinkel, S. 2009. American Chestnut: The Life, Death, and Rebirth of a Perfect Tree. Berkeley, CA: University of California Press.
Gandhi, K.J.K., and D.A. Herms. 2010a. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biological Invasions 12(2):389–405.
Gandhi, K.J.K., and D.A. Herms. 2010b. North American arthropods at risk due to widespread Fraxinus mortality caused by the alien emerald ash borer. Biological Invasions 12(6):1839–1846.
Garibaldi, A., and N. Turner. 2004. Cultural keystone species: Implications for ecological conservation and restoration. Ecology and Society 9(3).
Glitzenstein, J.S., P.A. Harcombe, and D.R. Streng. 1986. Disturbance, succession, and maintenance of species diversity in an east Texas forest. Ecological Monographs 56(3):243–258.
Gom, L.A., and S.B. Rood. 1999. Patterns of clonal occurrence in a mature cottonwood grove along the Oldman River, Alberta. Canadian Journal of Botany 77(8):1095–1105.
Griffin, G.J. 2000. Blight control and restoration of the American chestnut. Journal of Forestry 98(2):22–27.
Haack, R. 2006. Exotic bark- and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Canadian Journal of Forest Research 36(2):269–288.
Haack, R.A., K.O. Britton, E.G. Brockerhoff, J.F. Cavey, L.J. Garrett, M. Kimberley, F. Lowenstein, A. Nuding, L.J. Olson, J. Turner, and K.N. Vasilaky. 2014. Effectiveness of the International Phytosanitary Standard ISPM No. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS One 9(5):e96611.
Hajek, A.E., B.P. Hurley, M. Kenis, J.R. Garnas, S.J. Bush, M.J. Wingfield, J.C. Van Lenteren, and M.J. Cock. 2016. Exotic biological control agents: A solution or contribution to arthropod invasions? Biological Invasions 18(4):953–969.
Harvell, C.D., C.E. Mitchell, J.R. Ward, S. Atltizer, A.P. Dobson, R.S. Ostfeld, and M.D. Samuel. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296(5576):2158–2162.
Hausman, C.E., J.F. Jaeger, and O.J. Rocha. 2010. Impacts of the emerald ash borer (EAB) eradication and tree mortality: Potential for a secondary spread of invasive plant species. Biological Invasions 12(7):2013–2023.
Helms, J.A. 1998. The Dictionary of Forestry. Bethesda, MD: Society of American Foresters.
Hepting, G.H. 1974. Death of the American chestnut. Forest & Conservation History 18(3):60–67.
Herath, P., S. Beauseigle, B. Dhillon, D.I. Ojeda, G. Bilodeau, N. Isabel, M.C. Gros-Louis, H. Kope, S. Zeglen, R.C. Hamelin, and N. Feau. 2016. Anthropogenic signature in the incidence and distribution of an emerging pathogen of poplars. Biological Invasions 18(4):1147–1161.
Herms, D.A., and D.G. McCullough. 2014. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annual Review of Entomology 59:13–30.
Hodorff, R.A., and C.H. Sieg. 1986. Bird species associated with green ash woodlands in the Slim Buttes, South Dakota. South Dakota Bird Notes 38(3):56–60.
Holling, C.S. 1973. Resilience and stability of ecological systems. Annual Review of Ecology and Systematics 4(1):1–23.
Houston, D.R., and H.T. Valentine. 1988. Beech bark disease: The temporal pattern of cankering in aftermath forests of Maine. Canadian Journal of Forest Research 18(1):38–42.
Induri, B.R., D.R. Ellis, G.T. Slavov, T. Yin, X. Zhang, W. Muchero, G.A. Tuskan, and S.P DiFazio. 2012. Identification of quantitative trait loci and candidate genes for cadmium tolerance in populus. Tree Physiology 32(5):626–638.
IPCC (Intergovernmental Panel on Climate Change). 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. T.F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley, eds. Cambridge and New York: Cambridge University Press.
Isaacs, F.B., R. Goggans, R.G. Anthony, and T. Bryan. 1993. Habits of bald eagles wintering along the Crooked River, Oregon. Northwest Science 67(2):55–62.
Isaacs, F.B., R.G. Anthony, M. Vander Heyden, C.D. Miller, and W. Weatherford. 1996. Habits of bald eagles wintering along the upper John Day River, Oregon. Northwest Science 70(1):1–9.
Jansson, S., and C.J. Douglas. 2007. Populus: A model system for plant biology. Annual Review of Plant Biology 58:435–458.
Jenkins, J., J.D. Aber, and C.D. Canham. 1999. Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests. Canadian Journal of Forest Research 29(5):630–645.
Johnson, R. 2013. Growing Chestnut Trees and Hope in Western North Carolina. Available at https://www.fs.usda.gov/detail/nfsnc/home/?cid=STELPRDB5439130. Accessed September 15, 2018.
Kashian, D.M. 2016. Sprouting and seed production may promote persistence of green ash in the presence of the emerald ash borer. Ecosphere 7(4):e01332.
Kashian, D.M., and J.A. Witter. 2011. Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. Forest Ecology and Management 261(3):480–488.
Kauffman, J.B., and W.C. Krueger. 1984. Livestock impacts on riparian ecosystems and streamside management implications... a review. Journal of Range Management 37(5):430–438.
Keane, R.M., and M.J. Crawley. 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution 17(4):164–170.
Keeton, W.S., and J.F. Franklin. 2005. Do remnant old growth trees accelerate rates of succession in mature Douglas-fir forests? Ecological Monographs 75(1):103–118.
Keever, C. 1953. Present composition of some stands of the former oak-chestnut forest in the southern Blue Ridge Mountains. Ecology 34(1):44–54.
Kendall, K.C., and Keane, R.E. 2001. Whitebark pine decline: Infection, mortality, and population trends. Pp. 221–242 in Whitebark Pine Communities: Ecology and Restoration, D.F. Tomback, S.F. Arno, and R.E. Keane, eds. Washington, DC: Island Press.
Kendra, P.E., W.S. Montgomery, J. Niogret, and N.D. Epsky. 2013. An uncertain future for American Lauraceae: A lethal threat from redbay ambrosia beetle and laurel wilt disease (a review). American Journal of Plant Sciences 4(3):727–738.
Knight, K.S., D. Herms, R. Plumb, E. Sawyer, D. Spalink, E. Pisarczyk, B. Wiggin, R. Kappler, and K. Menard. 2012. Dynamics of surviving ash (Fraxinus spp.) populations in areas long infested by emerald ash borer (Agrilus planipennis). Pp. 143–152 in Proceedings of the 4th International Workshop on Genetics of Host–Parasite Interactions in Forestry, R.A. Sniezko, A.D. Yanchuk, J.T. Kliejunas, K.M. Palmieri, J.M. Alexander, and S.J. Frankels, eds. Albany, CA: U.S. Forest Service.
Knight, K.S., J.P. Brown, and R.P. Long. 2013. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biological Invasions 15(2):371–383.
Kolb, T.E., M.R. Wagner, and W.W. Covington. 1994. Forest health from different perspectives. Pp. 5–13 in Forest Health Through Silviculture: Proceedings of the 1995 National Silviculture Workshop, L.G. Eskew, comp. Fort Collins, CO: U.S. Forest Service.
Kovacs, K.F., R.G. Haight, D.G. McCullough, R.J. Mercader, N.W. Siegert, and A.M. Liebhold. 2010. Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecological Economics 69(3):569–578.
Krist, F.J., J.R. Ellenwood, M.E. Woods, A.J. McMahan, J.P. Cowardin, D.E. Ryerson, F.J. Sapio, M.O. Zweifler, and S.A. Romero. 2014. 2013–2027 National Insect and Disease Forest Risk Assessment. Fort Collins, CO: U.S. Forest Service.
Landsberg, J.J., and R.H. Waring. 1997. A generalised model of forest productivity using simplified concepts of radiation-use efficiency, carbon balance and partitioning. Forest Ecology and Management 95(3):209–228.
Latijnhouwers, M., P.J.G.M. de Wit, and F. Govers. 2003. Oomycetes and fungi: Similar weaponry to attack plants. Trends in Microbiology 11(10):462–469.
LeBoldus, J.M., N. Isabel, K.D. Floate, P. Blenis, and B.R. Thomas. 2013. Testing the “hybrid susceptibility” and “phenological sink” hypotheses using the P. balsamifera–P. deltoides hybrid zone and Septoria leaf spot [Septoria musiva]. PLoS One 8(12):e84437.
Lesica, P. 2009. Can regeneration of green ash (Fraxinus pennsylvanica) be restored in declining woodlands in eastern Montana? Rangeland Ecology & Management 62(6):564–571.
Liebhold, A.M., W.L. MacDonald, D. Bergdahl, and V.C. Mastro. 1995. Invasion by exotic forest pests: A threat to forest ecosystems. Forest Science Monographs 30:1–49.
Liebhold, A.M., E.G. Brockerhoff, L.J. Garrett, J.L. Parke, and K.O. Britton. 2012. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Frontiers in Ecology and the Environment 10(3):135–143.
Liebhold, A.M., D.G. McCullough, L.M. Blackburn, S.J. Frankel, B. Von Holle, and J.E. Aukema. 2013. A highly aggregated geographical distribution of forest pest invasions in the USA. Diversity and Distributions 19(9):1208–1216.
Liebhold, A.M., E.G. Brockerhoff, S. Kalisz, M.A. Nuñez, D.A. Wardle, and M.J. Wingfield. 2017. Biological invasions in forest ecosystems. Biological Invasions 19(11):3437–3458.
Little, E.L., Jr., 1977. Atlas of United States Trees, Volume 4, Minor Eastern Hardwoods. Washington, DC: U.S. Department of Agriculture.
Logan, J.A., J. Régnière, and J.A. Powell. 2003. Assessing the impacts of global warming on forest pest dynamics. Frontiers in Ecology and the Environment 1(3):130–137.
Lovett, G.M., C.D. Canham, M.A. Arthur, R.D. Fitzhugh, and K.C. Weathers. 2006. Forest ecosystem responses to exotic pests and pathogens in eastern North America. BioScience 56(5):395–405.
Lovett, G.M., M. Weiss, A.M. Liebhold, T.P. Holmes, B. Leung, K.F. Lambert, D.A. Orwig, F.T. Campbell, J. Rosenthal, D.G. McCullough, R. Wildova, M.P. Ayres, C.D. Canham, D.R. Foster, S.L. LaDeau, and T. Weldy. 2016. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecological Applications 26(5):1437–1455.
Ludwig, D., D.D. Jones, and C.S. Holling. 1978. Qualitative analysis of insect outbreak systems: The spruce budworm and forest. Journal of Animal Ecology 47(1):315–332.
MacCracken, J.G., and D.W. Uresk. 1984. Big game habitat use in southeastern Montana. The Prairie Naturalist 16(3):135–139.
Margulies, E., L. Bauer, and I. Ibáñez. 2017. Buying time: Preliminary assessment of biocontrol in the recovery of native forest vegetation in the aftermath of the invasive emerald ash borer. Forests 8(10):369.
McCaughey, W.W., and D.F. Tomback. 2001. The natural regeneration process. Pp. 105–120 in Whitebark Pine Communities: Ecology and Restoration, D.F. Tomback, S.F. Arno, and R.E. Keane, eds. Washington, DC: Island Press.
McCormick, J.F., and R.B. Platt. 1980. Recovery of an Appalachian forest following the chestnut blight or Catherine Keever-you were right! American Midland Naturalist 104(2):264–273.
McKinney, S.T., and D.F. Tomback. 2007. The influence of white pine blister rust on seed dispersal in whitebark pine. Canadian Journal of Forest Research 37(6):1044–1057.
McKinney, S.T., C.E. Fiedler, and D.F. Tomback. 2009. Invasive pathogen threatens bird–pine mutualism: Implications for sustaining a high-elevation ecosystem. Ecological Applications 19(3):597–607.
Meddens, A.J., J.A. Hicke, and C.A. Fergusen. 2012. Spatiotemporal patterns of observed bark beetle-caused tree mortality in British Columbia and the western United States. Ecological Applications 22(7):1876–1891.
Milcu, A.I., J. Hanspach, D. Abson, and J. Fischer 2013. Cultural ecosystem services: A literature review and prospects for future research. Ecology and Society 18(3):44.
Millar, C.I., and N.L. Stephenson. 2015. Temperate forest health in an era of emerging megadisturbance. Science 349(6250):823–826.
Moore, R. 2004. Forest vegetation. Pp. 3.2-1–3.2-84 in Final Environmental Impact Statement for Forest Plan Revision, Chippewa National Forest, Superior National Forest. Available at https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/fsm91_048435.pdf. Accessed August 23, 2018.
Moser, W.K., E.L. Barnard, R.F. Billings, S.J. Crocker, M.E. Dix, A.N. Gray, G.G. Ice, M.S. Kim, R. Reid, S.U. Rodman, and W.H. McWilliams. 2009. Impacts of nonnative invasive species on US forests and recommendations for policy and management. Journal of Forestry 107(6):320–327.
Nowak, D., D. Crane, J. Stevens, and J. Walton. 2003. Potential Damage from Emerald Ash Borer. Syracuse, NY: U.S. Forest Service.
Nowak, D. J., S. Hirabayashi, A. Bodine, and E. Greenfield. 2014. Tree and forest effects on air quality and human health in the United States. Environmental Pollution 193:119–129.
NRC (National Research Council). 2005. Valuing Ecosystem Services: Toward Better Environmental Decision-Making. Washington, DC: The National Academies Press.
Olander, L., R.J. Johnston, H. Tallis, J. Kagan, L. Maguire, S. Polasky, D. Urban, J. Boyd, L. Wainger, M. Palmer. 2015. Best Practices for Integrating Ecosystem Services into Federal Decisionmaking. Durham, NC: National Ecosystem Services Partnership, Duke University.
Omernik, J.M. 1995. Ecoregions: A spatial framework for environmental management. Pp. 49–62 in Biological Assessment and Criteria: Tools for Water Resource Planning and Decision Making. Boca Raton, FL: Lewis.
Omernik, J.M. 2004. Perspectives on the nature and definition of ecological regions. Environmental Management 34(1):S27–S38.
Omernik, J.M., and G.E. Griffith. 2014. Ecoregions of the conterminous United States: Evolution of a hierarchical spatial framework. Environmental Management 54(6):1249–1266.
Opler, P.A., 1978. Insects of American chestnut: Possible importance and conservation concern. Pp. 83–85 in Proceedings of the American Chestnut Symposium, W.L. MacDonald, F.C. Cech, J. Luchok, and C. Smith, eds. Morgantown: West Virginia University.
Orwig, D.A., D.R. Foster, and D.L. Mausel. 2002. Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. Journal of Biogeography 29(10–11):1475–1487.
Ostry, M.E., and H.S. McNabb, Jr. 1985. Susceptibility of Populus species and hybrids to disease in the north central United States. Plant Disease 69(9):755–757.
Paillet, F.L. 2002. Chestnut: History and ecology of a transformed species. Journal of Biogeography 29(10–11):1517–1530.
Paquette, A., J. Vayreda, L. Coll, C. Messier, and J. Retana. 2018. Climate change could negate positive tree diversity effects on forest productivity: A study across five climate types in Spain and Canada. Ecosystems 21(5):960–970.
Pastor, A., Z.G. Compson, P. Dijkstra, J.L. Riera, E. Martí, F. Sabater, B.A. Hungate, and J.C. Marks. 2014. Stream carbon and nitrogen supplements during leaf litter decomposition: Contrasting patterns for two foundation species. Oecologia 176(4):1111–1112.
Payne, J.A., R.A. Green, and C.D. Lester. 1976. New nut pest: An Oriental chestnut gall wasp in North America. Annual Report of the Northern Nut Growers 67:83–86.
Perkins, D.L., and T.W. Swetnam. 1996. A dendroecological assessment of whitebark pine in the Sawtooth–Salmon River region, Idaho. Canadian Journal of Forest Research 26(12):2123–2133.
Perry, D.A. 1994. Forest Ecosystems. Baltimore, MD: John Hopkins University Press.
Poland, T.M., D.G. McCullough, and A.C. Anulewicz. 2011. Evaluation of double-decker traps for emerald ash borer (Coleoptera: Buprestidae). Journal of Economic Entomology 104(2):517–531.
Prasad, A.M., L.R. Iverson, S. Matthews, and M. Peters. 2007-ongoing. A Climate Change Atlas for 134 Forest Tree Species of the Eastern United States [database]. Available at http://www.nrs.fs.fed.us/atlas/tree. Accessed December 8, 2018.
Pyšek, P., V. Jarosik, P.E. Hulme, I. Kuhn, J. Wild, M. Arianoutsou, S. Bacher, F. Chiron, V. Didziulis, F. Essl, P. Genovesi, F. Gherardi, M. Hejda, S. Kark, P.W. Lambdon, M.-L. Desprez-Loustau, W. Nentwig, J. Pergl, K. Poboljsaj, W. Rabitsch, A. Roques, D.B. Roy, S. Shirley, W. Solarz, M. Vila, and M. Winter. 2010. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proceedings of the National Academy of Sciences of the United States of America 107(27):12157–12162.
Raffa, K.F., B. Aukema, B.J. Bentz, A. Carroll, N. Erbilgin, D.A. Herms, J.A. Hicke, R.W. Hofstetter, S. Katovich, B.S. Lindgren, J. Logan, W. Mattson. A.S. Munson, D.J. Robison, D.L. Six, P.C. Tobin, P.A. Townsend, and K.F. Wallin. 2009. A literal use of “forest health” safeguards against misuse and misapplication. Journal of Forestry 107(5):276–277
Raffa, K.F., E.N. Powell, and P.A. Townsend. 2013. Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proceedings of the National Academy of Sciences of the United States of America 110(6):2193–2198.
Rebek, E.J., D.A. Herms, and D.R. Smitley. 2008. Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian ash (Fraxinus spp.). Environmental Entomology 37(1):242–246.
Reyer, C.P., S. Bathgate, K. Blennow, J.G. Borges, H. Bugmann, S. Delzon, S.P. Faias, J. Garcia-Gonzalo, B. Gardiner, J.R. Gonzalez-Olabarria, C. Gracia, J.G. Hernández, S. Kellomäki, K. Kramer, M.J. Lexer, M. Linder, E. van der Maaten, M. Maroschek, B. Muys, B. Nicoll, M. Pahali, J.H.N. Palma, J.A. Paulo, H. Peltola, T. Pukkala, W. Rammer, D. Ray, S. Sabaté, M.-J. Schelhaas, R. Seidl, C. Temperli, M. Tomé, R. Yousefpour, N.E. Zimmermann, and M. Hanewinkel. 2017. Are forest disturbances amplifying or canceling out climate change-induced productivity changes in European forests? Environmental Research Letters 12(3):034027.
Rhoades, C.C., S.L. Brosi, A.J. Dattilo, and P. Vincelli. 2003. Effect of soil compaction and moisture on incidence of phytophthora root rot on American chestnut (Castanea dentata) seedlings. Forest Ecology and Management 184(1):47–54.
Rolston, H. 1988. Environmental Ethics: Duties to and Values in the Natural World. Philadelphia, PA: Temple University Press.
Rood, S.B., and J.M. Mahoney. 1990. Collapse of riparian poplar forests downstream from dams in western prairies: Probable causes and prospects for mitigation. Environmental Management 14(4):451–464.
Rumble, M.A., and J.E. Gobeille. 1998. Bird community relationships to succession in green ash (Fraxinus pennsylvanica) woodlands. The American Midland Naturalist 140(2):372–381.
Russell, E.W.B. 1987. Pre-blight distribution of Castanea dentata (Marsh.) Borkh. Bulletin of the Torrey Botanical Club 114(2):183–190.
Sambaraju, K.R., A.L. Carroll, J. Zhu, K. Stahl, R.D. Moore, and B.H. Aukema. 2012. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 35(3):211–223.
Sandler, R. 2012. Intrinsic value, ecology, and conservation. Nature Education Knowledge 3(10):4.
Sandler, R. 2018. Forest biotechnology: Environmental ethics perspectives. Webinar presentation to the National Academies of Sciences, Engineering, and Medicine Committee on the Potential for Biotechnology to Address Forest Health, March 12.
Seidl, R., T.A. Spies, D.L. Peterson, S.L. Stephens, and J.A. Hicke. 2016. Searching for resilience: Addressing the impacts of changing disturbance regimes on forest ecosystem services. Journal of Applied Ecology 53(1):120–129.
Shvidenko, A., C.V. Barber, and R. Persson. 2005. Forest and woodland systems. Pp. 587–621 in Ecosystems and Human Well-being: Current State and Trends, Volume 1, R. Hassan, R. Scholes, and N. Ash, eds. Washington, DC: Island Press.
Slavov, G.T., S. Leonardi, W.T. Adams, S.H. Strauss, and S.P. DiFazio. 2010. Population substructure in continuous and fragmented stands of Populus trichocarpa. Heredity 105(4):348–357.
Smith, C.M., B. Wilson, S. Rasheed, and B. Shepheard. 2008. Whitebark pine and white pine blister rust in the Rocky Mountains of Canada and northern Montana. Canadian Journal of Forest Research 38(5):982–995.
Smitley, D., T. Davis, and E. Rebek. 2008. Progression of ash canopy thinning and dieback outward from the initial infestation of emerald ash borer (Coleoptera: Buprestidae) in southeastern Michigan. Journal of Economic Entomology 101(5):1643–1650.
Snyder, C.D., J.A. Young, D.P. Lemarié, and D. Smith 2002. Influence of eastern hemlock (Tsuga canadensis) forests on aquatic invertebrate assemblages in headwater streams. Canadian Journal of Fisheries and Aquatic Sciences 59(2):262–275.
Soulé, M.E. 1985. What is conservation biology? Bioscience 35(11):727–734.
Stanton, B.J., D.B. Neale, and S. Li. 2010. Populus breeding: From the classical to the genomic approach. Pp. 309–348 in Genetics and Genomics of Populus, S. Jansson, R. Bhalerao, and A. Groover, eds. New York: Springer.
Stephanson, C.A., and N.R. Coe. 2017. Impacts of beech bark disease and climate change on American beech. Forests 8(5):155.
Stoddard, E.M., and A.E. Moss. 1913. The Chestnut Bark Disease, Bulletin 178. New Haven: The Connecticut Agricultural Experiment Station.
Strobl, S., and K. Fraser. 1989. Incidence of Septoria canker of hybrid poplars in eastern Ontario. Canadian Plant Disease Survey 69(2):109–112.
Sturrock, R.N., S.J. Frankel, A.V. Brown, P E. Hennon, J.T. Kliejunas, K.J. Lewis, J J. Worrall, and A.J. Woods. 2012. Climate change and forest diseases: Using today’s knowledge to address future challenges. Forest Systems 21(2):329–336.
Taylor, G. 2002. Populus: Arabidopsis for forestry. Do we need a model tree? Annals of Botany 90(6):681–689.
Taylor, P. 1986. Respect for Nature. Princeton, NJ: Princeton University Press.
Tingley, M.W., D.A. Orwig, R. Field, and G. Motzkin. 2002. Avian response to removal of a forest dominant: Consequences of hemlock woolly adelgid infestations. Journal of Biogeography 29(10–11):1505–1516.
Tomback, D.F., and Y.B. Linhart. 1990. The evolution of bird-dispersed pines. Evolutionary Ecology 4(3):185–219.
Tomback, D.F., L.M. Resler, R.E. Keane, E.R. Pansing, A.J. Andrade, and A.C. Wagner. 2016. Community structure, biodiversity, and ecosystem services in treeline whitebark pine communities: Potential impacts from a non-native pathogen. Forests 7(1):21.
Trumbore, S., P. Brando, and H. Hartmann. 2015. Forest health and global change. Science 349(6250):814–818.
Tuskan, G.A., S. DiFazio, S. Jansson, J. Bohlmann, I. Grigoriev, U. Hellsten, N. Putnam, S. Ralph, S. Rombauts, A. Salamov, J. Schein, L. Sterck. A. Aerts, R.R. Bhalerao, R.P. Bhalerao, D. Blaudez, W. Boerjan, A. Brun, A. Brunner, V. Busov, M. Campbell, J. Carlson, M. Chalot, J. Chapman, G.-L. Chen, D. Cooper, P.M. Coutinho, J. Couturier, S. Covert, Q. Cronk, R. Cunningham, J. Davis, S. Degroeve, A. Déjardin, C. DePamphilis, J. Detter, B. Dirks, I. Dubchak, S. Duplessis, J. Ehlting, B. Ellis, K. Gendler, D. Goodstein, M. Gribskov, J. Grimwood, A. Groover, L. Gunter, B. Hamberger, B. Heize, Y. Helaruitta, B. Henrissat, D. Holligan, R. Holt, W. Huang, N. Islam-Faridi, S. Jones, M. Jones-Rhodes, R. Jorgensen, C. Joshi, J. Kangasjärvi, J. Karlsson, C. Kelleher, R. Kirkpatrick, M. Kirst, A. Kohler, U. Kalluri, F. Larimer, J. Leebens-Mack, J.-C. Leplé, P. Locascio, Y. Lou, S. Lucas, F. Martin, B. Montanini, C. Napoli, D.R. Nelson, C. Nelson, K. Nieminen, O. Nilsson, V. Pereda, G. Peter, R. Phillippe, G. Pilate, A. Poliakov, J. Razumovskaya, P. Richardson, C. Rinaldi, K. Ritland, P. Rouzé, D. Rayboy, J. Schmutz, J. Schrader, B. Segerman, H. Shin, A. Siddiqui, F. Sterky, A. Terry, C.-J. Tsai, E. Uberbacher, P. Unneberg, J. Vahala, K. Wall, S. Wessler, G. Yang, T. Yin, C. Douglas, M. Marra, G. Sandberg, Y. Van de Peer, and D. Rokhsar. 2006. The genome of black cottonwood, Populus trichocarpa (Yorr. & Gray). Science 313(5793):1596–1604.
USDA-APHIS (U.S. Department of Agriculture’s Animal and Plant Health Inspection Service). 2003. Emerald ash borer; Quarantine and regulations. Federal Register 68:59082–59091. Available at https://www.federalregister.gov/articles/2003/10/14/03-25881/emerald-ashborer-quarantine-and-regulations. Accessed June 29, 2018.
USDA-FS (U.S. Department of Agriculture’s Forest Service). 2009. Forest Health Protection Business Plan. Arlington, VA: USDA-FS.
USFWS (U.S. Fish & Wildlife Service). 2011. Endangered and threatened wildlife and plants: 12-month finding on a petition to list Pinus albicaulis as endangered or threatened with critical habitat. Federal Register 76:42631–42654.
Venette, R.C. 2009. Implication of global climate change on the distribution and activity of Phytophthora ramorum. Pp. 58–59 in Proceedings of the 20th U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and Other Invasive Species, 2009, K.A. McManus and K.W. Gottschalk, eds. Newtown Square, PA: U.S. Forest Service.
Venette, R.C., and S.D. Cohen. 2006. Potential climatic suitability for establishment of Phytophthora ramorum within the contiguous United States. Forest Ecology and Management 231:18–26.
von Gadow, K., C.Y. Zhang, X.H. Zhao, C. Wehenkel, J. Corral-Rivas, A. Pommerening, M. Korol, S. Myklush, G.Y. Hui, and A. Kiviste. 2012. Forest structure and diversity. Pp. 29–84 in Continuous Cover Forestry, T. Pukkala and K. von Gadow, eds. New York: Springer.
Wagner, D.L., and R.G. Van Driesche. 2010. Threats posed to rare or endangered insects by invasions of nonnative species. Annual Review of Entomology 55:547–568.
Walker, B., C.S. Holling, S.R. Carpenter, and A. Kinzig. 2004. Resilience, adaptability, and transformability in social-ecological systems. Ecology and Society 9(2):5.
Wang, G.G., B.O. Knapp, S.L. Clark, and B.T. Mudder. 2013. The Silvics of Castanea dentata (Marsh.) Borkh., American Chestnut, Fagaceae (Beech Family). Asheville, NC: U.S. Forest Service.
Waterman, A.M. 1954. Septoria Canker of Poplars in the United States. Washington, DC: U.S. Department of Agriculture.
Weaver, T., and D. Dale. 1974. Pinus albicaulis in central Montana: Environment, vegetation and production. The American Midland Naturalist 92(1):222–230.
Weed, A.S., M.P. Ayres, and J.A. Hicke. 2013. Consequences of climate change for biotic disturbances in North American forests. Ecological Monographs 83(4):441–470.
Williams, D.W., and A. Liebhold. 1997. Latitudinal shifts in spruce budworm (Lepidoptera: Tortricidae) outbreaks and spruce-fir forest distrbutions with climate change. Acta Phytophathologica et Entomologica Hungarica 32(1–2):205–215.
Wingfield, M.J., J.R. Garnas, A. Hajek, B.P. Hurley, Z.W. de Beer, and S.J. Taerum. 2016. Novel and co-evolved associations between insects and microorganisms as drivers of forest pestilence. Biological Invasions 18(4):1045–1056.
Woods, F.W., and R.E. Shanks. 1959. Natural replacement of chestnut by other species in the Great Smoky Mountains National Park. Ecology 40(3):349–361.
Wu, Z., and P. Raven. 1999. Flora of China Vol 4. Beijing and St. Louis, MO: Science Press and Missouri Botanical Garden Press.
Youngs, R.L. 2000. “A right smart little jolt”: Loss of the chestnut and a way of life. Journal of Forestry 98(2):17–21.
Zeglen, S. 2002. Whitebark pine and white pine blister rust in British Columbia, Canada. Canadian Journal of Forest Research 32(7):1265–1274.
Zinkgraf, M., K. Haiby, M.C. Lieberman, L. Comai, I.M. Henry, and A. Groover. 2016. Creation and genomic analysis of irradiation hybrids in Populus. Current Protocols in Plant Biology 1(2):431–450.
Zvereva, E.L., V. Lanta, and M.V. Kozlov. 2010. Effects of sap-feeding insect herbivores on growth and reproduction of woody plants: A meta-analysis of experimental studies. Oecologia 163(4):949–960.


































