6
Methane and Biogas Waste Utilization
As described in Chapter 2, methane waste gas sources include emissions from oil and gas supply chains, which are primarily methane mixed with other low-molecular-weight hydrocarbons, and emissions from landfills, manure, sewage, and other waste management operations, which are primarily mixtures of carbon dioxide and methane (biogas). Biogas utilization has primarily focused on use of the methane content of the waste gas, so this chapter will treat biogas and methane utilization as a single topic. A few processes under development utilize both the carbon dioxide and methane content of biogas, and these will be noted as the technologies are described.
Methane utilization and utilization of methane derived from biogas fundamentally differ from carbon dioxide waste gas utilization. Carbon dioxide is a low-value, low-energy waste gas, which is often available in large quantity in single locations. Methane, in contrast, is a high-value, high-energy molecule. Because of its high value and high energy of combustion, methane recovered from waste gases is often used as a fuel and any other utilization pathways must compete against the fuel value of methane (NASEM, 2016). If utilization pathways are able to add more value to methane than its value as a fuel, then the extent of methane available to utilization processes could increase substantially. Methane is the principal constituent of natural gas, and more than 600 Tg/yr of natural gas is produced and used in the United States. Like carbon dioxide, methane utilization pathways are both chemical and biological. Unlike carbon dioxide, methane does not have mineral carbonation use pathways.
COMMERCIAL TECHNOLOGIES FOR THE CHEMICAL UTILIZATION OF METHANE
A major commercial route for the chemical use of methane is steam reforming. Steam reforming of methane is the dominant route for the commercial production of hydrogen and is a mature technology which was viewed by the committee as outside the scope of this report. Another major commercial route for the chemical use of methane, considered outside the scope of this report, is the production of synthesis gas (syngas) through methane partial oxidation. In syngas production, methane is partially oxidized to form carbon monoxide, carbon
dioxide, and water (see Eq 4-6). Through the water gas-shift reaction, the carbon dioxide and water can be transformed into molecular hydrogen and carbon dioxide, allowing the carbon monoxide–to-hydrogen ratio in the syngas to be controlled. Syngas has been used for decades as a feedstock for a variety of commercial technologies. For example, in the Fischer-Tropsch process, which has been used commercially since the 1930s, syngas is converted to liquid hydrocarbon fuels.
In contrast to steam reforming of methane and partial oxidation of methane to produce syngas, which are mature commercial technologies, dry and tri-reforming of methane are still under active development. Dry reforming of methane (Eq 4) uses carbon dioxide as a feedstock rather than the water used in steam reforming (Eq 5), and generates syngas as a 1:1 mixture of CO and H2. Although this is not the most useful ratio of CO to H2 for conversion into chemicals, dry reforming is a useful strategy for converting two greenhouse gases into a valuable chemical feedstock and is a possible use for biogas waste streams that are mixtures of methane and carbon dioxide. In 2015 Linde built a pilot reactor for dry reforming, which uses a nickel or cobalt catalyst and also feeds in a small amount of water to increase the ratio of H2 to CO. At this stage it is not clear if this technology or related systems which are in operation on a pilot scale in Japan will be commercially viable on a large scale. Dry reforming is challenging because it is highly endothermic. As a consequence it is typically performed at high temperature (> 800°C), which also reduces the thermodynamically favorable formation of coke. Noble metal catalysts show the best activity, while minimizing coke formation, but are expensive. Other options are to use noble metals to decorate nickel catalysts, to make nickel nanoparticles of a certain size, or to encapsulate the nickel catalyst, all of which significantly increase the activity of the base metal catalyst. Nevertheless, finding an economically viable catalyst that minimizes catalyst deactivation through the formation of coke remains the biggest challenge in dry reforming.
An alternative to the dry reforming of methane is tri-reforming. In tri-reforming, three reactions occur in the same reactor: dry reforming (Eq 4), steam methane reforming (Eq 5), and partial oxidation of methane (Eq 6):
| Dry reforming | CH4 + CO2 → 2 CO + 2 H2 | (Eq 4) |
| Steam methane reforming | CH4 + H2O → CO + 3 H2 | (Eq 5) |
| Partial methane oxidation | CH4 + 0.5 O2 → CO + 2 H2 | (Eq 6) |
The net reaction involves the formation of an approximately 2:1 mixture of H2 and CO from CH4, CO2, H2O, and O2. The steam reforming and dry reforming reactions are endothermic, while the partial oxidation is exothermic, which assists in providing the energy for the process. The reaction can be run at high conversions of CH4 and CO2 using nickel catalysts. The presence of H2O and O2 in the reaction mixture limits coke formation as a result of side oxidation reactions, but this is still a problem in catalysis. Current research is
exploring the use of both supports and promoters to limit coking and this along with the design of nickel catalysts with specific shapes and sizes should be a focus of research. Additionally, improvements in the selectivity of tri-reforming are required to generate a commercially viable system.
DIRECT CHEMICAL UTILIZATION OF METHANE WASTE GAS STREAMS
The direct conversion of methane into valuable products is a problem that has fascinated chemists for decades but has not resulted in the development of many industrial processes (Holmen, 2009; Karakaya and Kee, 2016; Schwach et al., 2017). Examples of commercial technologies include the conversion of methane and ammonia into hydrocyanic acid (Eq 7) (Andrussow, 1955), the production of acetylene through the partial oxidation of methane (Eq 8) (Pässler et al., 2000), and the formation of carbon disulfide via the reaction of methane with sulfur (Eq 9) (Folkins et al., 1950). The major challenge associated with methane conversion is selectivity. The C–H bonds in methane are very strong, whereas the C–H bonds in the potential products are typically weaker. Therefore, further reactions are more likely to occur with the product before all of the starting methane has been consumed. A summary of current research into the direct conversion of methane into more valuable chemicals is provided below, including an assessment of which areas of investigation should be prioritized.
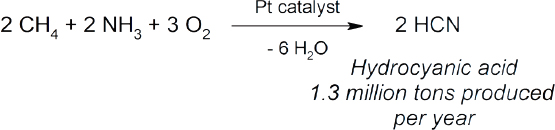 |
(Eq 7) |
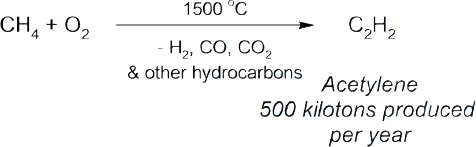 |
(Eq 8) |
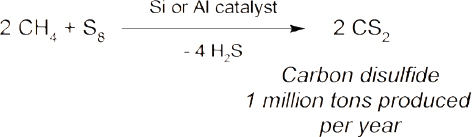 |
(Eq 9) |
Methanol
The development of catalysts for the direct conversion of methane to methanol has been a high-profile target for the homogeneous, heterogeneous, and enzymatic catalysis community for more than 50 years (Gunsalus et al., 2017; Kondratenko et al., 2017; Ravi et al., 2017; Wang et al., 2017). Despite intense research an industrial process has remained elusive. The main difficulty in direct methane conversion is that the C–H bonds in methanol are weaker than the C–H bonds in methane and preferentially react under the relatively harsh conditions required to activate the strong C–H bonds in methane. Therefore, high yields of methanol can only be achieved at low conversion. Both homogeneous and heterogenous reaction systems have been investigated. The major advantage of homogeneous systems compared to heterogeneous systems is that they can activate C–H bonds at low temperature (Gunsalus et al., 2017). However, they often require oxidants that are significantly stronger and less sustainable than O2, which is the preferred oxidant. The best yields for homogeneous systems have been achieved in systems where methanol is protected in the reaction mixture as it is formed, so that it cannot undergo further reactions. For example, Periana and co-workers oxidized methane in a concentrated solution of sulfuric acid using either a mercury- or platinum-based catalyst (Periana et al., 1993, 1998). The methanol that is formed is protected as methyl bisulfate, which is less reactive than methane. Similar strategies have used trifluoroacetic acid to protect the methanol in the form of a trifluoroacetate ester (Kao et al., 1991), but, like sulfuric acid, trifluoroacetic acid is highly corrosive, which makes it difficult to use industrially. An alternative strategy to prevent overoxidation of the methanol product is to use membranes to separate the product, but significant research into this approach is yet to be performed. At this stage the turnover frequencies, turnover numbers, and catalyst stability of the best homogeneous systems also need to be improved.
As a result of the potential ease in downstream product separation, heterogeneous catalysts have been even more widely studied than homogeneous systems for partial methane oxidation (Ravi et al., 2017). The most well-studied systems are based on molybdenum, iron, or copper. Molybdenum oxides have been studied for almost 50 years and especially when used in the form of multicomponent oxides give high selectivity to methanol due to product stabilization (Atroshchenko et al., 1965). Iron and copper systems have garnered attention as catalysts because these metals are present in the active site of different forms of the enzyme methane monooxygenase which catalyze the transformation of methane to methanol at room temperature (Figure 6-1) (Wang et al., 2017). Iron zeolites give high yields of methanol but typically use oxidants such as nitrous oxide or hydrogen peroxide, which have comparable expense to methanol. Copper zeolites give high yields of methanol using O2 as the oxidant. The product of oxidation is normally trapped in the zeolite and water is co-fed into the system along with methane to release methanol as a product. This can either be done after methane has been fed through the reactor, which results in a chemical loop rather than true
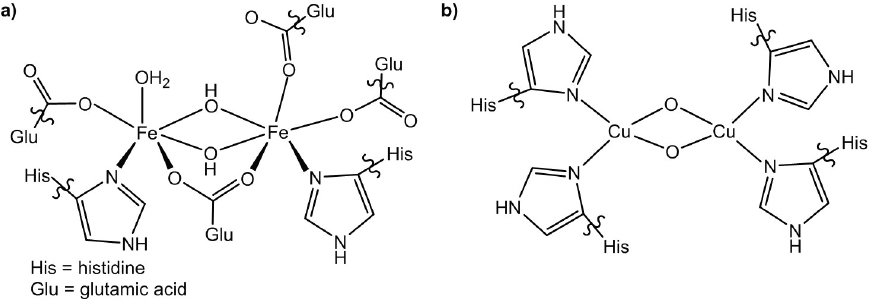
catalysis, or simultaneously. The key feature is that the trapping of the methanol in the zeolite protects it from further reaction in a similar way to trapping methanol with sulfuric acid in homogeneous systems. In general, it appears likely that, if an industrially viable system for methane conversion to methanol is to be developed, some kind of protection of the methanol product will be required to achieve high yields and conversion. This should be a focus of future research, along with fundamental catalyst design as large improvements in performance and stability are still required.
Ethylene
Research into the oxidative coupling of methane to form ethylene started in the 1980s (Holmen, 2009; Karakaya and Kee, 2016; Schwach et al., 2017). In this process methane is coupled in the presence of O2 to form ethylene and water, along with ethane and other higher-order hydrocarbons. At high temperatures (~700°C) a wide variety of different heterogeneous catalysts including systems based on Li/MgO, Fe2O3, and La2O3 can perform this reaction. At the moment this process generates significant quantities of ethane, C3 products, CO, and CO2 as well as ethylene. In particular, the formation of CO and CO2 is problematic because it is difficult to separate these products from ethylene. Recently, several catalysts that can operate at lower temperatures (~500°C) have been developed and these systems offer advantages in terms of the energy input required, and preventing coke formation (Tang et al., 2014). Nevertheless, the major problems with current catalysts are the lack of selectivity to ethylene and catalyst stability. It has been estimated that approximately a 25 percent yield of ethylene is required in a single pass for the process to be commercially viable. Given the recent advances in the development of nanomaterials with well-defined morphology and preliminary
results showing improved performance with catalysts of defined size and morphology, further research in this area is warranted.
In an alternative procedure to make ethylene, methane is heated in the absence of O2 (Holmen, 2009; Karakaya and Kee, 2016; Schwach et al., 2017). In this case the other major products are ethane and H2, but no oxygenates are formed. The ethane could be upgraded into higher-value ethylene using an oxidative dehydrogenation process. One of the major problems with this approach is that the reaction is thermodynamically unfavorable even at elevated temperatures (> 500°C) so the conversion of methane is low. Nevertheless, using a tantalum hydride catalyst supported on SiO2, 98 percent selectivity to C2 products can be achieved (at 0.05 percent conversion) (Tang et al., 2014). Another engineering problem associated with this reaction is that it is often run in two steps. First methane is exposed to the catalyst, which promotes the formation of hydrogen-deficient CHx fragments on the catalyst surface. Second, H2 is introduced, which promotes C–C bond formation and cleans the surface of the catalyst. These steps need to be performed at different temperatures, which introduces a practical challenge. Further catalyst development is also required.
Aromatics
Benzene is an important precursor for a range of aromatic compounds including ethyl benzene, 1-methylethylbenzene, cyclohexane, and nitrobenzene. Approximately 43 million tons were produced in 2012. Typically, benzene is produced from the steam cracking or dehydrogenation of naphtha, or via the disproportionation or hydrodealkylation of toluene. An efficient synthesis of benzene from methane could provide advantages over these petroleum-based routes. In 1993, the methane dehydroaromatization reaction was discovered (Wang et al., 1993). In this process methane is converted into benzene and H2. No oxygenates, including CO or CO2, are generated in this process, which means that the H2 is of suitable quality to be used directly in fuel cell applications. Unfortunately, the reaction is not thermodynamically favorable and at temperatures of around 900°C a benzene yield of approximately 12 percent is obtained, which is close to the thermodynamic yield (Tang et al., 2014). The formation of naphthalene is just as thermodynamically favorable as benzene, so it is typically observed as a by-product (Tang et al., 2014). The best catalysts are bifunctional zeolite-based systems, such as Mo-ZSM-5. The molybdenum sites are responsible for the methane activation and Brønsted acidic sites in the zeolite promote the aromatization reaction. A problem with the reaction is that a significant amount of coke is formed, which requires the catalyst to be regenerated using H2. Future research should focus on strategies to remove benzene from the product stream to drive the reaction and increase the yield and on efficient methods to regenerate the deactivated catalysts from coking.
BIOLOGICAL APPROACHES FOR UTILIZATION OF METHANE WASTE GAS STREAMS
Various methanotroph species can utilize methane as their sole carbon and energy source (Kalyuzhnaya et al., 2015). Although methane conversion can occur under aerobic and anaerobic conditions, a subset of aerobic bacteria and gamma-proteobacteria are generally the focus of metabolic engineering efforts. This bacterial group converts methane into formaldehyde which is then processed by the cell in metabolic pathways commonly used in sugar catabolism, such as glycolysis. One such pathway converts formate into CO2 which feeds into the Calvin-Bensen cycle to produce biomass. These sugar-catabolism pathways are utilized by common industrial strains, like Escherichia coli and Saccharomyces cerevisiae, that have been extensively investigated. This makes gamma-proteobacteria and similar methanotrophs more readily engineered to become a microbial production platform as more is known about the pathways.
To successfully implement methanotrophs as industrial hosts for methane conversion there are several fundamental issues that need to be addressed. The metabolic pathways downstream of methane assimilation are largely unknown or poorly understood, making metabolic engineering efforts challenging. This problem is compounded by a lack of knowledge of how carbon flux is driven and regulated in methanotrophs. Of key importance is the methane oxidation electron donors, including those involved in the first oxidative step converting methane to methanol via methane monooxygenase. For some of these issues genomic mining can be done to draw out comparisons and assumptions, but intensive experimental investigations are needed for confirmation.
In lieu of mapping methanotroph metabolism, the methane oxidation machinery could be installed into well-studied heterologous hosts, like E. coli. However, there has been no reported complete oxidative activity, with enzymes like the methane monooxygenase expressing poorly or with low activity (Balasubramanian et al., 2010; Jahng et al., 1996). This indicates significant research must be done before nonmethanotrophs can metabolize methane. One possible avenue would be high-throughput directed evolution followed by genetic screening. However, this method would most likely first require evolution to grow on methanol, then methane. Using methanotrophs for industrial production would also require extensive research, particularly into host selection, as some gamma-proteobacteria have faster growth rates while others are more readily engineered or have beneficial metabolic pathways. Whether the industrial strain will be native or heterologous, several strategies such as protein engineering are being carried out to improve the enzymes involved in methane biosynthesis.
Methane-based production faces considerable platform challenges, some common to microbial fermentation such as the risk of contamination, buildup of toxic intermediates, and the high cost of additives, like nucleic acids. Other issues are more specific, such as increased risk due to using a mixture of flammable gases, O2 and CH4. The low solubility of methane requires considerable process engineering efforts to provide large-scale microbial batches with enough carbon to grow and produce. This is especially important if the microbes are grown
solely on methane, one of the factors that would make fermentation economically viable. Despite these challenges there has been a diverse group of methane-based chemicals produced, such as sugars, alcohols, amino acids, carotenoids, epoxides, and polymers (Kalyuzhnaya et al., 2015). Some of these have been tested by companies at commercial scales, although the commercialization of these products is still limited and metabolic engineering has not been implemented in many cases.
CalystaTM is a UK-based sustainable products company that has developed and commercialized FeedKind® protein, a high-nutrient-density product containing 71 percent protein and 9 percent fat. This alternative feed for fish, livestock, and pet nutritional products is composed of Methylococcus capsulatus, a naturally occurring methanotroph. In this case the host organism is the product, with its biomass content the commercial application. There are several environmental benefits of FeedKind, including no agricultural land use, utilizing 77-98 percent less water than agricultural products (Cumberlege et al., 2016).
In 2017 Calysta produced more than 5 tons of FeedKind and distributed commercial samples worldwide. They announced a collaboration with Cargill to build the world’s largest gas fermentation facility on President’s Island in Memphis, Tennessee. The 37-acre modular design is projected to be built and operated in phases, with Phase 1 operational in 2019 and producing 20,000 metric tons per year and complete production occurring by 2021 producing 200,000 metric tons per year. While current FeedKind production has reached the commercial scale, improvements in metabolic engineering could further enhance this utilization pathway.
A joint venture between Calysta, industrial partner CHAIN Biotech, and the University of Nottingham’s Synthetic Biology Research Centre is working on a methanotrophic platform to convert methane into polyunsaturated fatty acids, in particular the popular supplement omega–3.1 In developmental stages are palatants, probiotics, L-amino acids, and a variety of metabolites.2 The majority of these commodities will require extensive strain improvements and product recovery engineering.
IntrexonTM has developed a commercially viable natural gas–to-liquid bioconversion platform. While Calysta used methanotrophs as the product, Intrexon is using methanotrophs to produce high-value chemicals. They circumnavigated the lack of genome and pathway knowledge by designing novel techniques for library generation and screening—technology that could help advance methanotrophic metabolic engineering.3 Intrexon has installed existing metabolic pathways into its evolved methanotroph strains and has demonstrated production of isobutanol and farnesene. Isobutanol, an energy-dense, clean-burning drop-in fuel, is the company’s initial target and scale-up model. Future products include 1,4-butanediol, a feedstock for plastic, polyester, and polyurethane production. In 2016 the synthetic biology company announced their San Francisco 500-liter pilot plant was operational and later stated
___________________
1 See http://calysta.com/2016/05/scientists-to-use-microbes-and-methane-to-create-sustainable-omega-3/.
2 See https://www.bio.org/sites/default/files/0830AM-Lori%20Giver.pdf.
3 See https://www.dna.com/userfiles/files/Engineering%20Microbes%20for%20Commercial%20Products.pdf.
that by the end of 2018 they would commercialize isobutanol production.4 However, they have since retracted their 2018 goal.
The startup company Mango MaterialsTM is focused on converting biogas streams into the biodegradable plastic polyhydroxyalkanoate (PHA) and its derivatives.5 Mango Materials chose biogas or unrefined syngas as its carbon feedstock, and methanotrophs as its host organism. They are currently working on PHAs for specific applications of textile fibers and caps and closures. Mango Materials is currently in the pilot-scale level of product development and is still in the early stages of modeling its host organism and pathways. Current research in PHA production in nonmethanotrophic organisms has the potential to help pave the way in product recovery engineering for Mango Materials, accelerating their path to commercialization.
Methanotrophs are not limited to being microbial production platforms, as they have also been used in bioremediation, biobleaching, pharmaceutical treatments, and reducing methane emissions in landfills. Generating metabolic models, studying carbon flux, and compiling fermentation data are all necessary research areas to industrialize methanotrophs.
A RESEARCH AGENDA FOR CHEMICAL AND BIOLOGICAL UTILIZATION OF METHANE AND BIOGAS
Methane and biogas utilization can be accomplished via chemical or biological approaches. While little waste gas methane is available for nonfuel chemical utilization, as compared to carbon dioxide in waste gas, research breakthroughs in methane utilization to higher-carbon-number products could displace fuel use of methane, making available very large quantities of feedstock. Few industrial processes have emerged for direct conversion of methane to nonfuel chemical products, despite decades of research. To advance methane and biogas conversion processes, more research is needed to improve existing catalysts, as well as to control the selectivity and stability of the methane-derived products to increase product yields. Additionally, separation and purification of biogas may be required depending on the desired product. For biological utilization of methane, methanotrophs have received significant attention; however, there are challenges associated with microbial fermentation, such as the risk of contamination, the buildup of toxic intermediates, and high-cost additives, such as nucleic acids. Better capability to characterize the metabolic pathways downstream of methane assimilations will create more accurate metabolic models.
Chemical Barriers to Commercialization
Stages of development and key barriers for various chemical utilization approaches for methane and biogas are shown in Figure 6-2 and Table 6-1.
___________________
4 See http://investors.dna.com/2016-03-30-Intrexon-Pilot-Plant-for-its-Methanotroph-Bioconversion-Platform-Now-Operational.
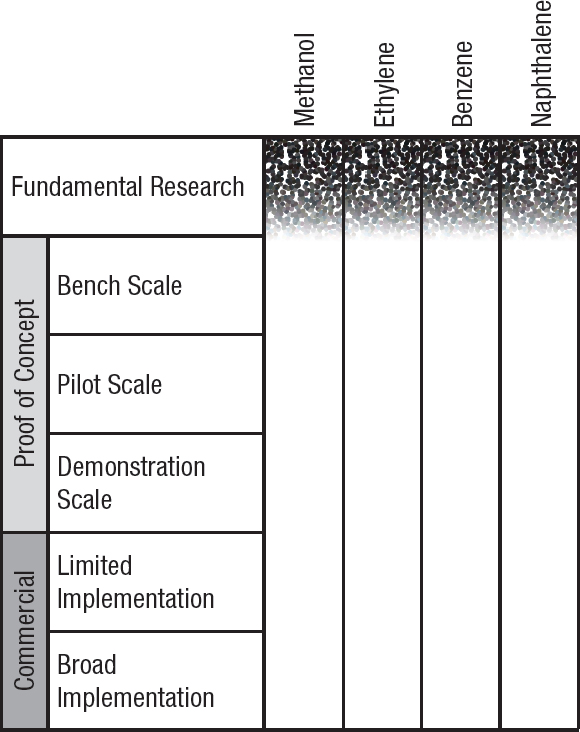
NOTE: Fundamental research is defined as observation and reporting of fundamental principles of a scientific or engineering process, and formulation of a technology concept. The three proof-of-concept stages are defined as progressively larger-scale reactions to produce product. These include bench-scale processes where critical functions are proved and components or systems are validated in a laboratory environment and at a laboratory scale. Pilot plant scale is defined as a system validated in a relevant environment and at an engineering scale. Demonstration plant scale is defined as a full-scale system demonstrated in a relevant environment. Commercial-limited is defined as an actual system operating at a stage where product is being sold in the market in limited areas with specific advantageous geographical, regulatory, or other factors. Commercial-broad is defined as an actual system operating at a stage where product is sold in the market with opportunities not limited to specifically advantaged locations (see Box 9-1).
TABLE 6-1 Key barriers for commercialization of the products from Figure 6-2.
| Product | Key Barriers |
|---|---|
| Methanol | Selectivity and stability of methane-derived product, turnover frequency and turnover numbers, catalyst stability |
| Ethylene | Reaction engineering |
| Benzene | Reducing the need for harsh reaction conditions, yield |
| Naphthalene | Reducing the need for harsh reaction conditions, yield |
Chemical Utilization Priority Research Areas
Catalyst Development
Research is needed to improve existing catalysts or discover entirely new catalysts for methane or biogas conversion. There are few examples of catalysts that are viable on a large scale and this is a high-priority research need.
System Engineering and Reactor Design
Improving the efficiency of methane conversion chemistry would be aided by the integration of catalysts with the most efficient reactor technology. For example, the integration of methane conversion catalysts with reactors that allow for the efficient removal of products which need to be formed at low conversion for thermodynamic reactions would be beneficial. Similarly, separation challenges can be mitigated by developing reactor designs which improve conversion per pass.
New Targets for Methane Conversion
Research exploring methane conversion into commodity chemicals has traditionally focused on a relatively small number of molecules, as outlined in this chapter. The identification of other nontraditional targets and subsequent catalyst development could have transformative impacts.
Biological Barriers to Commercialization
Stages of development and key barriers for various biological utilization approaches for methane and biogas are shown in Figure 6-3 and Table 6-2.
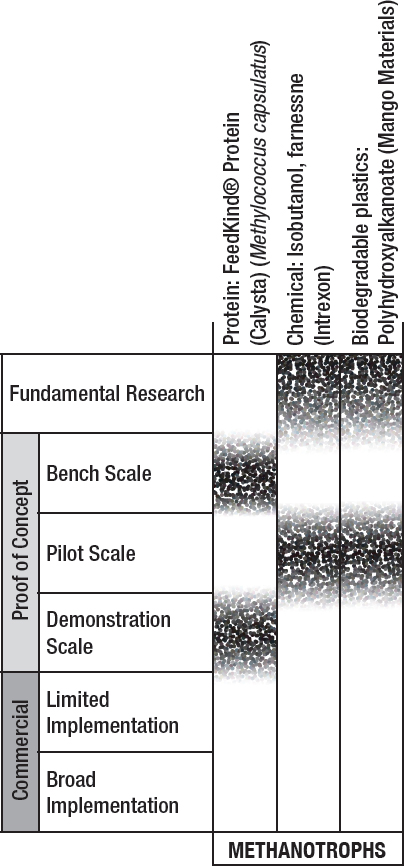
NOTE: Fundamental research is defined as observation and reporting of fundamental principles of a scientific or engineering process, and formulation of a technology concept. The three proof-of-concept stages are defined as progressively larger-scale reactions to produce product. These include bench-scale processes where critical functions are proved and components or systems are validated in a laboratory environment and at a laboratory scale. Pilot plant scale is defined as a system validated in a relevant environment and at an engineering scale. Demonstration plant scale is defined as a full-scale system demonstrated in a relevant environment. Commercial-limited is defined as an actual system operating at a stage where product is being sold in the market in limited areas with specific advantageous geographical, regulatory, or other factors. Commercial-broad is defined as an actual system operating at a stage where product is sold in the market with opportunities not limited to specifically advantaged locations (see Box 9-1).
TABLE 6-2 Key barriers for commercialization of the products from Figure 6-3.
| Platform | Product | Key Barriers |
|---|---|---|
| Methanotrophs | Protein: FeedKind® Protein (Calysta) (Methylococcus capsulatus) | Social acceptability of GMO limits additional genetic engineering |
| Chemical: Isobutanol, farnesene (Intrexon) | Scalability | |
| Biodegradable plastics: Polyhydroxyalkanoate (Mango Materials) | Scalability, product recovery | |
Biological Utilization Priority Research Areas
Bioreactor and Cultivation Optimization
Technologies concerning system design for efficient methane solvation, mass transfer, and delivery, as well as dewatering, harvesting and product isolation, and water and nutrient management and recycling remain primary challenges at scale and merit additional research efforts. Improved culture monitoring technologies will also be essential for scale-up applications. Advancement of methane utilization methods may require novel bioreactor design in order to incorporate new feedstocks or hybrid fermentative systems and improve safety. Reactors that capture and reuse unused methane should be explored, as well as pairing reactors with technologies that utilize CO2.
Genome-Scale Modeling and Metabolic Efficiency
In-depth computational modeling, genetic manipulation, biochemical validation, and fermentative demonstration will be required to create more accurate metabolic models and improve metabolic flux, including methane uptake and incorporation, efficiency, metabolic streamlining, and product accumulation. Research is needed to characterize metabolic pathways downstream of methane assimilations. These pathways are currently unknown or poorly understood.
Bioprospecting
The identification and characterization of new species with unique attributes, such as methane uptake, product profiles, conversion efficiency, and environmental tolerance, will be necessary to facilitate biomass production in diverse geographic locations and produce select products.
Valorization of Co-Products
Given the robust efficiencies of microalgae for protein and oil production, continued studies and certification processes will be required to valorize co-products for feed and food applications.
Genetic Tools
Genetic tools for engineering of nonphotosynthetic biomass organisms remain underdeveloped. Expansion of tools for genetic incorporation, selectable markers, promoter elements, protein folding and stability, and posttranslational control will be needed to develop robust platforms.
Products
Opportunities may be found in the development of new products for unmet needs in commodity and specialty chemicals as well as nontraditional products.
REFERENCES
Andrussow, L. 1955. Blausäuresynthese und die schnell verlaufenden katalytischen Prozesse in strömenden Gasen. Chemie Ingenieur Technik 27(8-9):469-472.
Atroshchenko, V. I., Z. M. Shchedrinskaya, and N. A. Gavrya. 1965. Catalysts for the oxidation of natural gas to formaldehyde and methanol. Zhurnal Prikladnoi Khimii 38:643-649.
Balasubramanian, R., S. M. Smith, S. Rwat, L. A. Yatsunyk, T. L. Stemmier, and A. C. Rosenzweig. 2010. Oxidation of methane by a biological dicopper centre. Nature 465(7294):115-119.
Cumberlege, T., T. Blenkinsopp, and J. Clark (for the Carbon Trust). 2016. Assessment of Environmental Impact of FeedKind Protein. 26 pp. Available at https://www.carbontrust.com/media/672719/calysta-feedkind.pdf (accessed October 10, 2018).
Folkins, H. O., E. Miller, and H. Hennig. 1950. Carbon disulfide from natural gas and sulfur: Reaction of methane and sulfur over a silica gel catalyst. Industrial & Engineering Chemistry 42(11):2202-2207.
Gunsalus, N. J., A. Koppaka, S. H. Park, S. M. Bischof, B. G. Hashiguchi, and R. A. Periana. 2017. Homogeneous functionalization of methane. Chemical Reviews 117(13):8521-8573.
Holmen, A. 2009. Direct conversion of methane to fuels and chemicals. Catalysis Today 142(1):2-8.
Jahng, D., C. S. Kim, R. S. Hanson, and T. K. Wood. 1996. Optimization of trichloroethylene degradation using soluble methane monooxygenase of Methylosinus trichosporium OB3b expressed in recombinant bacteria. Biotechnology and Bioengineering 51(3):349-359.
Kalyuzhnaya, M. G., A. W. Puri, and M. E. Lidstrom. 2015. Metabolic engineering in methanotrophic bacteria. Metabolic Engineering 29:142-152.
Kao, L. C., A. C. Hutson, and A. Sen. 1991. Low-temperature, palladium(II)-catalyzed, solution-phase oxidation of methane to methanol derivative. Journal of the American Chemical Society 113(2):700-701.
Karakaya, C., and R. J. Kee. 2016. Progress in the direct catalytic conversion of methane to fuels and chemicals. Progress in Energy and Combustion Science 55(Supplement C):60-97.
Kondratenko, E. V., T. Peppel, D. Seeburg, V. A. Kondratenko, N. Kalevaru, A. Martin, and S. Wohlrab. 2017. Methane conversion into different hydrocarbons or oxygenates: Current statues and future perspectives in catalyst development and reactor operation. Catalysis Science & Technology 7:366-381.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2016. The Changing Landscape of Hydrocarbon Feedstocks for Chemical Production: Implications for Catalysis: Proceedings of a Workshop. Washington, DC: The National Academies Press. doi: 10.17226/23555.
Pässler, P., W. Hefner, K. Buckl, H. Meinass, A. Meiswinkel, H.-J. Wernicke, G. Ebersberg, R. Müller, J. Bässler, H. Behringer, and D. Mayer. 2000. Acetylene. In Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
Periana, R. A., D. J. Taube, E. R. Evitt, D. G. Loffler, P. R. Wentrcek, G. Voss, and T. Masuda. 1993. A mercury-catalyzed, high-yield system for the oxidation of methane to methanol. Science 259(5093):340-343.
Periana, R. A., D. J. Taube, S. Gamble, H. Taube, T. Satoh, and H. Fujii. 1998. Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. Science 280(5363):560-564.
Ravi, M., M. Ranocchiari, and J. A. van Bokhoven. 2017. The direct catalytic oxidation of methane to methanol—a critical assessment. Angewandte Chemie International Edition 56(52):16464-16483.
Schwach, P., X. Pan, and X. Bao. 2017. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects. Chemical Reviews 117(13):8497-8520.
Tang, P., Q. Zhu, Z. Wu, and D. Ma. 2014. Methane activation: The past and future. Energy & Environmental Science 7(8):2580-2591.
Wang, L., L. Tao, M. Xie, G. Xu, J. Huang, and Y. Xu. 1993. Dehydrogenation and aromatization of methane under non-oxidizing conditions. Catalysis Letters 21(1-2):35-41.
Wang, V. C. C., S. Maji, P. P. Y. Chen, H. K. Lee, S. S. F. Yu, and S. I. Chan. 2017. Alkane oxidation: Methane monooxygenases, related enzymes, and their biomimetics. Chemical Reviews 117(13):8574-8621.
This page intentionally left blank.
















