7
Enabling Technologies and Resources
The committee collectively describes materials and processes that are required to utilize carbon waste streams at large scale (gigatons of carbon per year) as enabling technologies and resources. To accomplish a net life-cycle reduction of greenhouse gas emissions to the atmosphere, many carbon utilization technologies will require low- or zero-carbon sources of electricity, heat, and energy-carrying materials. Additionally, the waste streams will need to be purified, processed, and transported. The following chapter discusses the enabling technologies and resources required to utilize carbon waste streams.
ENABLING TECHNOLOGIES
Waste Gas Separation and Purification
Waste gas streams are heterogeneous in their composition, and this heterogeneity poses challenges for carbon utilization. Sources of carbon dioxide range from highly concentrated by-products of chemical manufacturing to relatively dilute flue gas streams from power plants with nitrogen oxide, sulfur oxide, and other contaminants. Methane in waste gas ranges from widespread biogas from waste treatment and landfills, containing contaminants such as hydrogen sulfide, oxygenated hydrocarbons, and siloxanes, to methane-contaminated air from sparse coal mine vents. Composition of the waste gas varies not only by the process generating the waste stream but also by the condition of the process (e.g., the level of activity in a coal mine or the amount of moisture available in a landfill), sometimes varying on a daily or hourly basis and sometimes in unpredicted ways. To utilize methane and carbon dioxide mixed with other gases in carbon waste streams, utilization processes must be developed that can tolerate variable and mixed streams, or waste gas separation and purification will need to be widely deployed. For many utilization technologies, which are at early stages of development, the required purity of carbon dioxide or methane is unknown. As utilization technologies develop, it will be critical to identify acceptable purity levels for carbon utilization process feedstocks that meet process requirements and minimize costs. In some cases it may be desirable to
perform separation and purification of waste gases prior to transporting carbon dioxide or methane to utilization processes because of economies of scale or other factors. In other cases it may be desirable to integrate the waste stream purification into the utilization process. Because of this uncertainty in the separation and purification needs of carbon utilization technologies, only the general need for these technologies is certain. Specific research and development needs will emerge over time. This section provides a broad overview of separation technologies and the types of research advances that might be required. Separation science is the focus of an ongoing Academies study (Committee on a Research Agenda for a New Era in Separations Science1) and, although it will not be covering specific applications like carbon waste separation, key fundamental areas will be discussed that can advance separations, regardless of application.
As discussed in Chapter 2, methane and carbon dioxide waste gases are generated in different locations, with different chemical characteristics, and with different requirements for use, leading to different needs for waste gas separations and purification. Methane waste gas streams from the oil and gas sector tend to be relatively concentrated, on the order of 70-95 percent methane, and have the fewest separation needs. Contaminants include ethane, propane, butanes, and other light hydrocarbons, and in some cases H2S or CO2.
Biogas from landfills and wastewater digesters are typically a mix of methane and CO2, at approximately 50 percent methane and 50 percent CO2 for the landfill gas, and 70 percent methane and 30 percent CO2 for the wastewater digester gas. Biogas separation needs can be approached from the methane or CO2 separation standpoint, and the biogas can also be used in a mixed state, as discussed particularly in Chapter 6. While methane waste gases associated with oil and gas production are typically relatively clean of impurities that hinder utilization, biogas sourced from landfills can include contaminants such as halogenated organic compounds, hydrogen sulfide, mercury-containing compounds, siloxanes, ketones, and sulfur-containing species and that sourced from digesters often contains problematic impurities such as ammonia, hydrogen sulfide, and a variety of organic compounds including organic sulfides, disulfides, C4 to C7 aldehydes, amines, quinoline, dimethylpyrazine, and organic acids, along with lesser amounts of C4 to C7 alcohols, ketones, aliphatic hydrocarbons, and aromatic compounds (NRC, 2003). Coal mine vents typically make up only very small percentages of methane, and face challenges in methane concentration.
Widely used cleanup technologies for methane or carbon dioxide can be generally divided into four classes: (1) chemical absorption with basic media, normally aqueous, or physical absorption in liquid media; (2) application of low temperatures (cryogenic); (3) adsorption on a solid surface, followed by later removal under a temperature and/or pressure change; and (4) membrane separation.
___________________
1 Available at http://nas-sites.org/dels/studies/separations/ (accessed August 1, 2018).
Within each of these categories are a number of specific processes, distinguished by the solvent, sorbent, or membrane used; processing conditions; and resulting purity of the desired products(s). Selection of a specific process is highly dependent on the specific gas stream to be treated and the conditions required for the purified gas stream. Each cleanup system has unique features, advantages, and disadvantages which include energy consumption, capital costs, and the production of by-product streams of contaminated cleanup media for disposal.
Chemical Absorption with Basic Solutions
A wide variety of amine solutions have been used to absorb carbon dioxide. Once the carbon dioxide is absorbed, the amine-containing solution must be regenerated or stripped to remove and recover the carbon dioxide so that the solution can be reused. Elevated pressure and low temperatures aid the absorption, while the opposite conditions—higher temperatures and lower pressure—are used to remove the carbon dioxide. Regeneration of the aqueous amine solution is energy intensive. Common problems for these systems include corrosion of the mechanical systems and degradation of the solution over time. Scrubbing technology was developed initially to remove acidic gases from natural gas and to purify hydrogen and synthesis gas streams. In these cases the feed gas is typically available at elevated pressure. In many CO2 waste gases, such as flue gas from a power plant or cement kiln, the gas stream is only available at slightly above atmospheric pressure. Additional energy would be required to raise the gas pressure to a level sufficient for effective treatment. In addition, many of the CO2-containing waste streams also contain oxygen, which accelerates amine degradation.
Physical Absorption with Solvent
If the waste gas stream is available at sufficient pressure, a physical solvent can be used in which the gas dissolves (but does not chemically react). The gas-rich solvent is circulated to a low-pressure vessel where the dissolved gas is released and removed. Several well-developed commercial systems have been used extensively, particularly for purifying high-pressure hydrogen streams and synthesis gas. Purisol® (using N-methyl-2-pyrrolidone), Rectisol® (using methanol), and Selexol® (using a mixture of glycol ethers) are typical examples. An advantage of such systems is that they do not require heat to regenerate the solvent, they have less corrosion and solvent degradation, and they have smaller waste streams for disposal. However, they produce a lower-pressure product gas than the feed stream, which may or may not be desirable, depending on the application.
Low-Temperature Purification
Low-temperature purification is an effective means to produce a very-high-purity (>99 percent) CO2 or methane stream. The process is energy intensive, resulting in high costs.
In addition, other compounds in a typical waste stream such as water and hydrocarbons can freeze, plugging the cryogenic equipment and interfering with the separation.
Adsorption on Solid Media
Molecular sieves have been developed and commercialized to separate a wide variety of components in mixtures, including CO2. The precise sieve composition and pore size is tailored to capture a target gas molecule and remove it from a mixture. The most common application of this technology is called pressure swing adsorption (PSA). PSA has been applied to hydrogen purification from steam-methane reforming, among other chemicals, producing a very pure hydrogen product and a carbon dioxide stream. In many cases, multiple adsorbents are used within the same vessel to remove other minor impurities such as water and ammonia. The hydrogen recovery depends on the desired purity and the nature of other contaminants. Carbon dioxide waste streams pose additional challenges for this technology since they are typically available at modest pressure so additional compression may be required. A variation of PSA is a temperature swing adsorption process in which the solid is heated to remove the adsorbed component rather than changing the pressure.
Membrane Separation Processes
A variety of porous membrane materials have been developed over the past 40 years for gas-phase separations. Current materials include a multitude of polymers with specific functional groups attached. The first commercial process developed by Monsanto in the 1980s for purifying hydrogen, now owned and licensed by Air Products,2 is currently used in a number of hydrogen manufacturing plants. Membrane separation depends on maintaining a pressure and concentration differential on the two sides of the membrane to provide a driving force for the separation. Membrane systems suffer from relatively poor mechanical strength and sensitivity to impurities in the feed stream. The challenges for future, large-scale use of membrane separations remain (1) developing membrane materials that exhibit high permeability (throughput) while maintaining high selectivity and (2) improved mechanical strength.
Waste Gas Transportation and Delivery
The sources of carbon waste streams and other required inputs for waste stream utilization are often not co-located. In the United States, the Greenhouse Gas Reporting Program (GHGRP) hosted by the Environmental Protection Agency (EPA) tracks facilities emitting greenhouse gases over 25,000 metric tons of carbon dioxide equivalent (MMT CO2e), which includes many facilities that are sources of carbon waste streams. Figure 7-1 depicts the loca-
___________________
2 Available at http://www.airproducts.com/Products/Gases/supply-options/prism-membranes.aspx (accessed August 1, 2018).
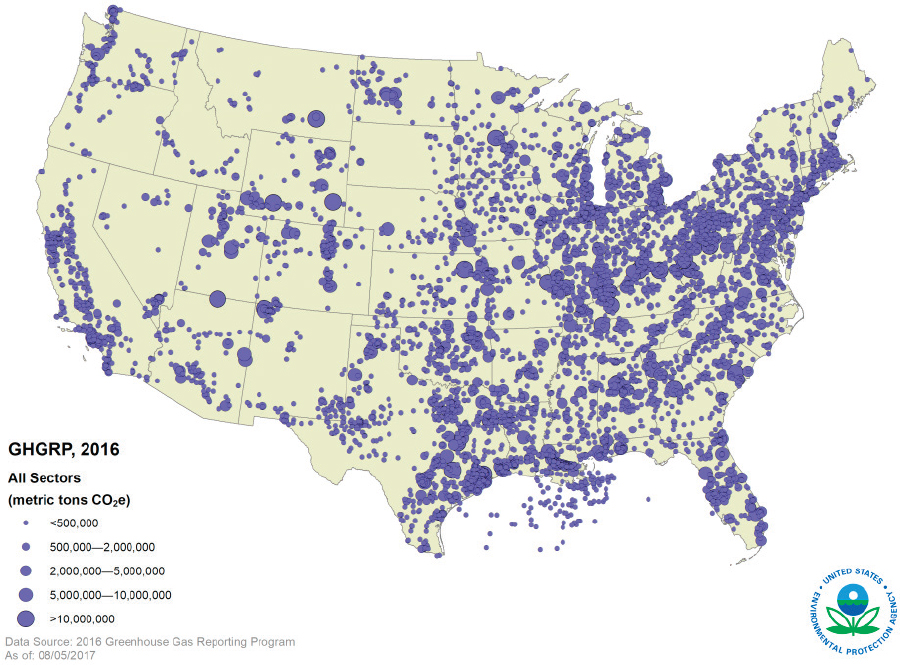
tion and total reported emissions from each facility that reported directly to GHGRP. Waste carbon is emitted by a variety of sectors, including power plants, petroleum and natural gas systems, refineries, chemical facilities, and waste management facilities. The size and distribution of these waste carbon sources and proximity to infrastructure must be considered to enable local and nonlocal utilization. It will be important to also consider the locations of courses and utilization technologies when constructing new pipelines to transport carbon waste gas streams.
Carbon Dioxide Pipelines
Carbon dioxide transportation pipelines are used to move carbon dioxide from points of production (either waste gas or mineral sources) to utilization sites. There are 50 individual CO2 pipelines that have been constructed in the United States with a combined length of more than 4,500 miles (NETL, 2015). The vast majority of the CO2 pipeline system (see Figure 7-2) is dedicated to enhanced oil recovery, and a small fraction is used for other
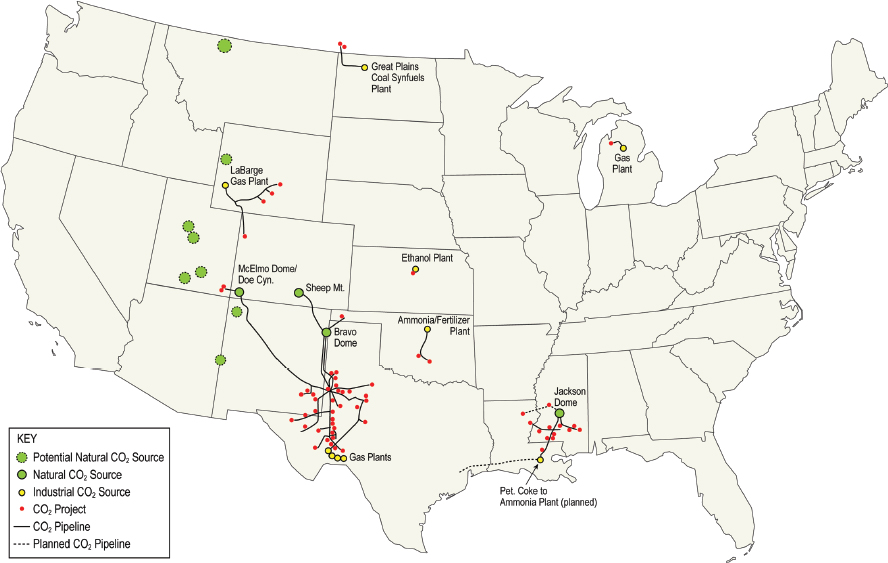
industrial uses such as the beverage industry. Roughly 80 percent of the transported CO2 is from geological sources and the remaining 20 percent from industrial sources including gas processing plants. Because of these limited sources and utilization pathways, the availability of pipeline-quality carbon dioxide is limited both in magnitude and by location.
Methane Pipelines
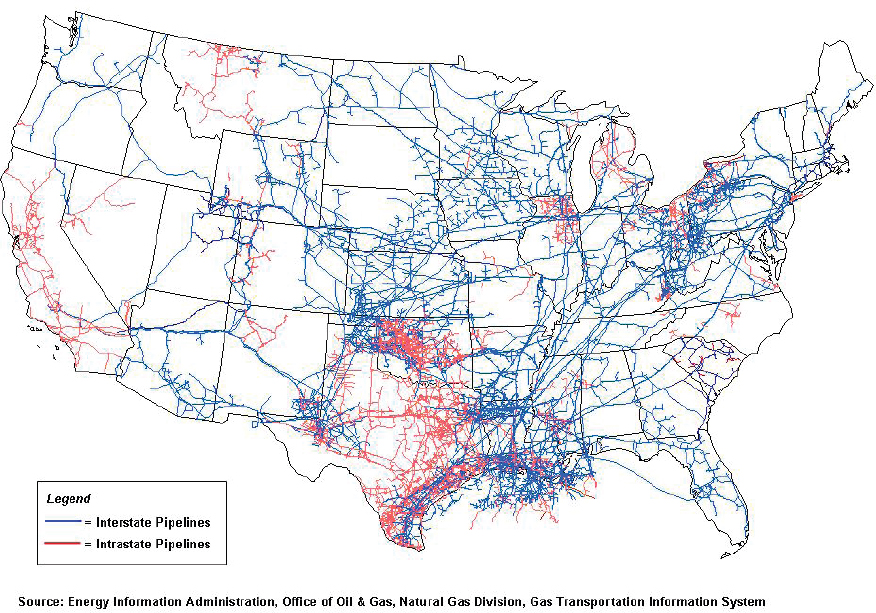
In comparison to the CO2 pipeline infrastructure, the natural gas pipeline network in the United States is very large and provides gas transportation services to each of the lower 48 states. There are three different categories of national gas pipeline systems: gathering, transmission, and distribution. Gathering pipelines transport gas from wells where it is produced to gas processing and purification and transmissions systems, which then bring the gas to storage facilities, customers, and distribution systems.
The United States has more than 320,000 miles of natural gas gathering and transmission pipelines and more than two million miles of gas distribution pipelines (see Figure 7-3). The purity of gases that are permitted to be transported is specified by pipeline operators and can vary depending on the type of pipeline and location of the pipeline (McKaskle, 2014).3
___________________
3 Available at https://www.eia.gov/todayinenergy/detail.php?id=36494 (accessed October 10, 2018).
__________________
1 Available at http://www.eia.gov/pub/oil_gas/natural_gas/analysis_publications/ngpipeline/ngpipelines_map.html (accessed October 10, 2018).
ENABLING RESOURCES
After delivering the purified waste gases to the point of utilization, other energy and material requirements are often needed to support carbon utilization. Some key enabling resources to support carbon dioxide utilization are hydrogen, electricity, and heat, each of which serve as energy carriers. Methane utilization often also requires electricity and heat. Though many processes in the chemical and biochemical industries require such inputs, they are particularly salient for carbon waste stream utilization where a net reduction of greenhouse gas emissions is a requirement.
Hydrogen Sources
There is a national-scale mismatch between the magnitude and location of hydrogen from low-carbon resources and the magnitude and location of the majority of carbon waste streams.
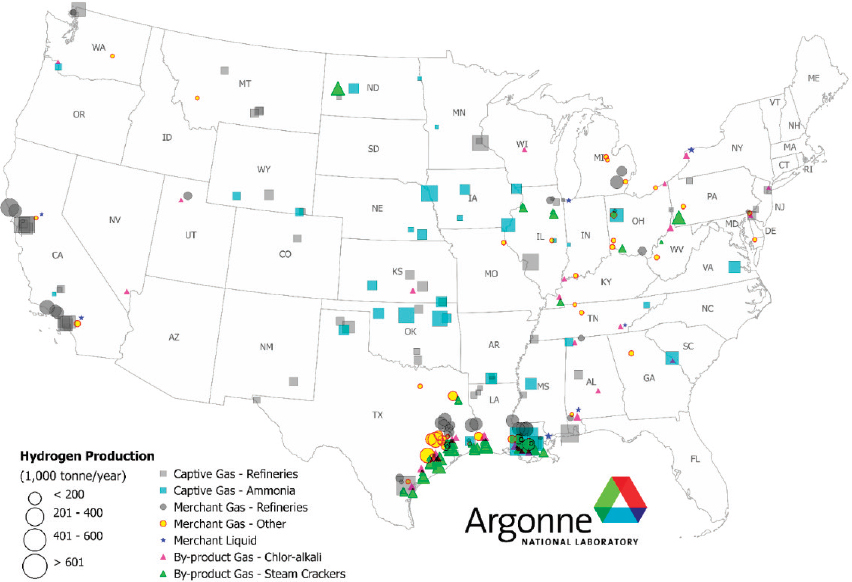
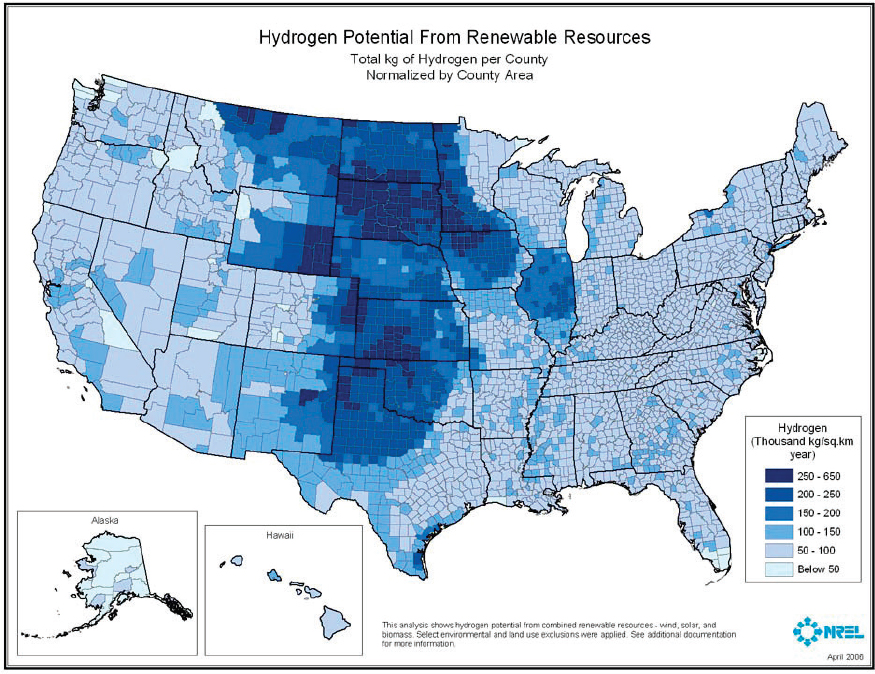
Enabling technologies for generating hydrogen and transporting hydrogen are thus integral to widespread carbon waste stream utilization. Hydrogen can be produced from a diverse range of resources. Most hydrogen is produced by the steam reforming of methane (see Figure 7-4), but to achieve net carbon utilization, hydrogen used as a reactant for carbon waste stream utilization must be produced from low-carbon resources such as wind, solar, and biomass (see Figure 7-5). Hydrogen can be produced by electrolysis from renewable electricity and water, but the cost of producing hydrogen with this route is relatively high at large scales.
Hydrogen can be produced (1) at or near the site of use in distributed production, (2) at large facilities and then delivered to the point of use in central production, or (3) at intermediate-scale facilities located in close proximity (25-100 miles) to the point of use in semicentral production (see Figure 7-6). Distributed production is a term used when small units of hydrogen are produced where it is needed. Centralized production is a term used to describe large central hydrogen production facilities (50,000 kg/day) and oftentimes requires more capital investment as well as substantial hydrogen transport and delivery infrastructure. Semicentral production descibes intermediate-size hydrogen production facilities (5,000-50,000 kg/day)
located in close proximity to the point of use. These facilities minimize hydrogen transport costs and infrastructure.4
Electricity
Converting carbon dioxide to fuels and chemicals can require a large amount of energy. Reducing carbon dioxide emissions through carbon dioxide utilization will only be possible if the electricity and, in some cases, the thermal energy inputs are from renewable or low-carbon resources. The reduction of costs for renewable sources such as solar and wind has enabled low-cost, near-zero-carbon electricity abundant in volume and over large areas (see
___________________
4 Available at https://energy.gov/eere/fuelcells/central-versus-distributed-hydrogen-production (accessed August 2, 2018).
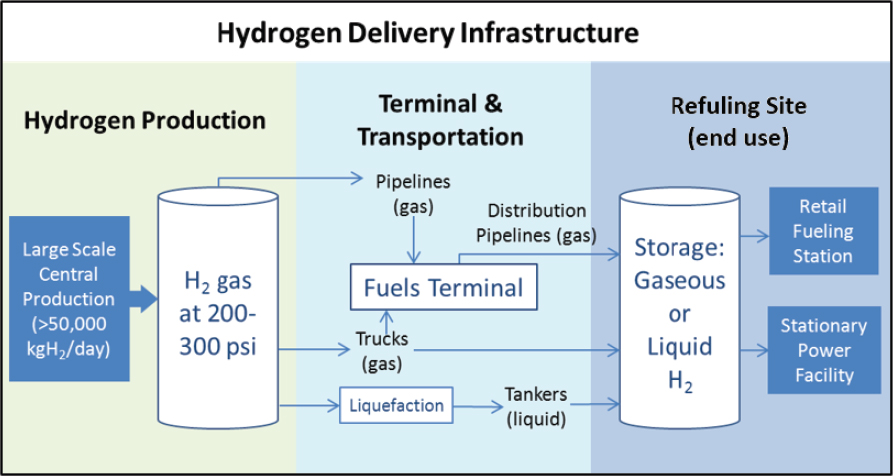
Figure 7-5). In some markets, electricity is available, at times, at very low or even negative prices.5 When low-carbon energy sources are highly variable in their output, such as for solar and wind resources, compensating for intermittency can impose additional costs on carbon utilization processes. Many facilities will use electricity from the electric grid, which will mix the sources of electricity and will reduce dependence on any one generation source. A deeper discussion of assessing emissions impacts of carbon utilization technologies is available in Chapter 8.
Heat
High-temperature (>900ºC) heat can be used to convert CO2 into value-added products. The classic example is dry reforming of methane (see Chapter 6) via the following reaction:
| CH4 + CO2 ↔ 2 CO + 2 H2 (ΔH0298K = +247 kJ mol–1) | (Eq 10) |
This reaction is highly endothermic with a ΔH value of +247 kJ/mol. Similarly, the reverse water gas-shift reaction (Eq 11) is mildly endothermic, with a ΔH value of +41 kJ/mol:
___________________
5 FUTURE Act at 45Q(f)(2), available at https://www.congress.gov/bill/115th-congress/senate-bill/1535/text (accessed August 2, 2018).
| CO2 + H2 ⇌ CO + H2O (ΔH0298K = +41 kJ mol–1) | (Eq 11) |
The dry reforming reaction is more relevant for conversion of CO2 into syngas as it does not require any hydrogen. The high-temperature heat needed for the dry reforming reaction can potentially be obtained from a number of industrial sources. Nuclear power plants can provide relatively inexpensive low-carbon waste heat at high temperatures, but this will require locating the CO2 conversion process in the vicinity of a nuclear plant, which may not be always possible. There have been recent advancements to couple the export power, heat, and hydrogen of small modular nuclear reactors with evolving advanced manufacturing technologies for further energy and carbon savings.
Other sources of this waste heat could be exhaust gases from gas turbines, electric arc furnaces in the steel industry, aluminum melting furnaces, glass furnaces, and other sources. Most oil refineries and petrochemical plants also have some waste heat available. Generally, some of this waste heat is recovered in recuperators to produce steam and/or power, but the efficiency of this conversion is quite low. Also, some of these waste exhaust gases from the steel and glass industries contain fine particulates and/or condensable vapors which may be difficult to handle without significant capital equipment.
FINDINGS
A variety of enabling technologies and resources are required for widespread carbon utilization with net greenhouse gas emissions reduction. These include separation and purification, transport, and low-carbon sources of heat, electricity, and energy-carrying materials such as hydrogen. Because the needs of carbon utilization processes are still emerging, a specific research agenda for these enabling technologies and resources is unclear. Therefore, as carbon utilization technologies emerge, the integration of the research agenda for carbon utilization and enabling technologies and resources must be considered.
Finding 7-1 Depending on the carbon utilization technology, enabling technologies and infrastructure such as waste gas cleanup, extensive energy inputs, or inputs of reactive gases (e.g., hydrogen) and co-location of waste streams may be required.
Finding 7-2 The development and deployment of systems for carbon utilization will require research advances and deployment of enabling technologies and infrastructure for energy and other resources, in addition to advances in the conversion of carbon waste to useful products.
REFERENCES
EPA (U.S. Environmental Protection Agency). 2016. Greenhouse Gas Reporting Program Data Sets. Available at https://www.epa.gov/ghgreporting/ghg-reporting-program-data-sets (accessed August 2, 2018).
McKaskle, R. 2014. A new look at impurities in CO2 for EOR and their consequences. Presented at the 20th Annual CO2 Flooding Conference. December 11-12, 2014. Midland, Texas.
NETL (National Energy Technology Laboratory). 2015. Cost and Performance Baseline for Fossil Energy Plants Volume 1a: Bituminous Coal (PC) and Natural Gas to Electricity Revision 3. U.S. Department of Energy. DOE/NETL-2015/1723. Available at https://www.netl.doe.gov/energy-analyses/temp/CostandPerformanceBaselineforFossilEnergyPlantsVolume1aBitCoalPCandNaturalGastoElectRev3_070615.pdf (accessed October 10, 2018).
NRC (National Research Council). 2003. Air Emissions from Animal Feeding Operations: Current Knowledge, Future Needs. Washington, DC: The National Academies Press.
US DRIVE (U.S. Driving Research and Innovation for Vehicle Efficiency and Energy Sustainability). 2013. Hydrogen Delivery Technical Team Roadmap. Available at https://www.energy.gov/sites/prod/files/2014/02/f8/hdtt_roadmap_june2013.pdf (accessed June 4, 2018).












