5
Evolution in the Technology and Programmatic Landscape
TECHNOLOGY FOR SEARCHING FOR LIFE ON NEARBY EXOPLANETS
The National Aeronautics and Space Administration’s (NASA’s) Kepler mission revolutionized the field of exoplanet astrobiology by proving that rocky, potentially habitable worlds are commonplace in the Milky Way galaxy, as first prioritized by the 2001 astronomy and astrophysics decadal survey (NRC 2001). The path forward is to determine which of these worlds might be not only habitable, but perhaps even inhabited. Fortunately, even before the stunning results from Kepler, NASA and its community advisors prioritized the search for habitable, inhabited exoplanets. This has resulted in several space telescopes currently in operation, development, or planning. Ground-based astronomers and their observatory advisory bodies have similarly been preparing in anticipation of this new era of exoplanet astrobiology to make use of telescopes already in operation or undergoing upgrades, development, or planning over the next decade. Together, these telescopes continue the hunt for a convincing biosignature.
Although the Kepler mission finished its extended phase in 2017, and the follow-on Kepler K2 mission concluded this year, NASA’s follow-on space transit photometry telescope—the Transiting Exoplanet Survey Satellite (TESS)—launched successfully on April 18, 2018. During its 2-year prime mission, TESS is expected to discover at least 50 small exoplanets (i.e., Neptune-size or smaller) orbiting in the habitable zones of nearby bright M-dwarf stars (e.g., Ricker et al. 2015). Ground-based follow-on programs using high-precision Doppler spectrometers will be employed to determine the masses of these exoplanets with the goal of assessing if the targets are truly “Earth-like.”
In large part, TESS is intended to be a finder telescope for NASA’s next flagship telescope, the 6.5 m James Webb Space Telescope (JWST). JWST will use the approach pioneered by the Spitzer Space Telescope to study the atmospheres of exoplanets through precise measurements made during both transits and occultations, when delicate subtractions of the combined stellar and planetary starlight can be employed to separate the exoplanet’s atmospheric features from those of its host star. However, this approach only works for transiting planets, such as the seven transiting planets in the TRAPPIST-1 system and the exoEarths expected to be found by TESS. While the launch date has been delayed multiple times and is currently expected to be no earlier than late-March 2021, JWST is anticipated to provide unprecedented sensitivity for imaging and spectroscopy in the infrared.
For those planets that do not transit their host stars from the observer’s perspective, space-based telescopic discovery and characterization requires either an internal coronagraph or an external starshade, or, ideally, both. This is necessary to attain the high contrast needed to directly image nontransiting exoplanets. NASA’s Wide Field Infrared Survey Telescope (WFIRST), slated for launch in 2025, is baselined to carry a coronagraph that
will test and prove the technology needed for future space telescopes capable of the direct imaging of Earth-like planets. The last several years have seen substantial investment in the WFIRST coronagraph. These investments have yielded a coronagraph able to detect exoplanets that are approximately 10 million times fainter than their host stars, in spite of the nonideal obscured-pupil design of the WFIRST optical system. Coronagraphs designed for a more ideal, unobscured pupil are expected to be able to achieve considerably better performance.
Parallel to further development of internal coronagraph technology, NASA is developing the technology needed to build and deploy large (30-50 m) starshades. Starshades are external occulting devices flying in formation with the telescope and are suitable for operation with WFIRST or other future space telescopes. Decisions regarding the prioritization of these technologies will be made by the 2020 astronomy and astrophysics decadal survey (Astro 2020). In support of Astro 2020, NASA funded several planning studies for large (4-12 m) optical, space-based observatories that will offer technological options to fly as the next flagship mission following WFIRST.
The main challenge for exoplanet spectroscopy is starlight suppression. The light from stars can be 10 billion times brighter than that of a rocky planet in its habitable zone. Starlight suppression technologies, such as coronagraphs and starshades, and nulling interferometers can be used to suppress the light from an exoplanet’s host star (NASA 2018). Transmission spectroscopy, high-resolution Doppler spectroscopy, and phase-curve measurements have been used to determine the composition of exoplanets’ atmospheres (e.g., Brogi et al. 2013; Birkby et al. 2013; de Kok et al. 2013; Brogi et al. 2014). Spectroscopy and photometric measurements using the combined line from an exoplanet and its host star are fundamentally limited by stellar photon noise which limits the potential for characterization of habitable planets. Thanks to starlight suppression techniques, the planet’s light can be separated from most of the starlight, providing measurements with a significantly better signal-to-noise ratio.
Recommendation: NASA should implement high-contrast starlight suppression technologies in near-term space- and ground-based direct imaging missions.
In addition to the anticipated launch of a direct-imaging space telescope, a new generation of 30-m-class ground-based giant segmented mirror telescopes (GSMTs) will be deployed in the 2020s. These telescopes increase collecting area per telescope by an order of magnitude over the current 8 to 10 m diameter telescopes. The three-fold increase in angular resolution and improved sensitivity, combined with advances in supporting technologies, such as adaptive optics (to mitigate image degradation caused by Earth’s turbulent atmosphere), detectors, computing power, and machine learning, will provide new measurement opportunities for exoplanet observations. Two U.S.based teams, the Giant Magellan Telescope (GMT) and the Thirty Meter Telescope (TMT), plan on searching for exoplanets with their first light spectrographic instruments. The European Extremely Large Telescope (E-ELT) has a similar focus on detecting and characterizing nearby exoplanet systems.
In summary, these space- and ground-based telescopes offer significant new opportunities for studying exoplanets. Using transit spectroscopy, large observatories such as JWST and the GSMTs will have the sensitivity to characterize the atmospheres of the nearest transiting habitable planets orbiting M-dwarf stars. Using direct imaging of reflected light from the host star, ground-based GSMTs will provide the angular resolution and sensitivity to image reflected-light habitable planets orbiting nearby low-mass main-sequence stars of M- and possibly K-types (see, e.g., Kopparapu et al. 2018, for a means to estimate the number of exoplanets expected to be seen using direct-imaging missions). Spectroscopy in the near-infrared will reveal atmosphere compositions, which could yield molecules associated with biosignatures, such as atmospheric water, oxygen, methane, nitrous oxide, carbon monoxide, and carbon dioxide. High-resolution spectroscopy observations will need to calibrate their measurements against Earth’s atmosphere perturbations and will reveal valuable exoplanet dynamic features (e.g., planet orbital motion and rotation). Space-based optical telescopes, free of atmospheric turbulence, will reach the contrast level required to image the reflected light of habitable planets around Sun-like (types F-, G-, K-) stars. Direct imaging of the thermal emission of Earth-like planets orbiting the nearest A-, F-, and G-type stars will be observable with GSMTs in the mid-infrared (~7-13 µm), thanks to the exoplanets’ thermal emission, where the contrast ratio between the planet and the star is typically a thousand times greater than at optical wavelengths.
Finding: Technologies for spectroscopic measurements and high-contrast direct imaging have advanced rapidly in the last decade, making possible the remote characterization of the atmospheres of nearby rocky exoplanets and enabling the search for potential biosignatures within the next two decades.
Numerous challenges remain, however. Exoplanet observation techniques need to be able to extract small signals from the noise. While large ground- and space-based telescopes will have the sensitivity and angular resolution to image and characterize exoplanets, the measurements remain challenging, especially for ground-based telescopes that have to overcome the scattering of starlight due to turbulence, absorption, and emission of gases in Earth’s atmosphere. The interpretation of the measurements will also be challenging. Better understanding of biotic and abiotic processes that could influence atmospheric signatures, especially for planets orbiting M-dwarf stars, is required. Transit spectroscopy, direct imaging, Doppler spectroscopy, and astrometry will provide a rich data set, but with a small overlap in targets. The results will likely suffer from small-number statistics. Combining this information to infer both statistical distributions and individual exoplanet properties will be very challenging.
TECHNOLOGY FOR SAMPLE-BASED LIFE DETECTION
Instrument technologies that can contribute to in situ life detection in samples have developed rapidly in the past decade as the result of, in particular, investments made by companies in the biomedicine, food security, and defense sectors. Comparatively modest investments might be sufficient to transform such instruments, including miniaturized mass spectrometers and DNA sequencers (see Box 5.1) for rapid sample analyses, into capable spacecraft hardware able to address astrobiological mission goals and/or ensure compliance with appropriate planetary protection requirements (Box 6.2). Further, the exceptional computational capability developed in the past decade allows rapid analyses of these samples onboard a spacecraft. Together, these advances increasingly facilitate in situ sample analysis that previously would require sample return to Earth.
Raman microspectroscopy would be well suited for in situ detection of organic materials and characterization of associated minerals, including hydrated phases (e.g., Wang et al. 2006). While fluorescence may hinder the identification of some minerals, Raman spectroscopy has the advantages of not requiring a great deal of sample preparation and, because it is nondestructive, allowing further analyses to be done on the same location in the sample.
Importantly, Raman spectroscopy is also a powerful tool for identifying organic matter indigenous to the metamorphosed rocks and not derived from post-metamorphic processes. Raman spectroscopy provides structural information about the organization of the aromatic skeleton of graphitic carbons (Bernard et al. 2010), which evolve systematically with increasing metamorphic grade (e.g., graphitization; Beyssac and Lazzeri 2012). A geothermometer has been calibrated based on Raman spectral characteristics of carbonaceous materials by Beyssac et al. (2002). This technique has been recently widely and successfully used to estimate the peak temperature experienced by carbonaceous materials from various lithologies and ages, including early Archean rocks (Ueno et al. 2004; Wacey et al. 2011; Sugitani et al. 2013, 2015; Morag et al. 2016; Hickman-Lewis et al. 2016; Flannery et al. 2018). For further characterization of the organic materials, synchrotron-based scanning transmission X-ray microscopy allows microscopic observations with chemical sensitivity (i.e., in situ mapping of organics within rocks at a 15 nm spatial resolution) and spectroscopic measurements of X-ray absorption that offer a precise estimation of the nitrogen-to-carbon atomic ratio of organics (Alleon et al. 2015) as well as key information about carbon and nitrogen speciation at the submicrometer scale (Alleon et al. 2016).
The field of paleogenomics holds significant promise for understanding the connections between life and the environment. Growing understanding of the last universal common ancestor and ancient genetic information will allow increasingly sophisticated tests of successful organismal and community evolutionary strategies in the context of their environments, thus becoming not just tools to study life’s innovations, but its record of environment change, as well. At the same time, synthetic biological techniques may be adapted to allow astrobiologists to reverse-engineer genes to discover their ancient function, thereby revealing their impact on the environment or their environment’s impact on them.
For extant life detection, the challenge is the absence of a single, mature flight-ready instrument capable of making in situ measurements of elemental, mineralogical, and organic composition. Of particular importance is the ability to detect long-chain informational polymers associated with replicating genetic systems. On Earth, the genomic polymers are RNA and DNA, which encode functional polymers based on RNA, proteins, and oligosaccharides. While these polymers may take a different form on other planets, informational polymers in general can be regarded as a signature of life. Information polymers are uniquely important because even surprisingly complex materials, such as amino acids, sugars, and nucleobases, can be produced by abiotic processes and have been observed in meteorites without any evidence for biosynthesis. One further requirement for such instruments would be the ability to analyze sample materials nondestructively. The destructive techniques commonly employed in current in situ analyses are excellent for determining low-level elemental and molecular composition of ingested materials, but they often lose the measurement context and create new compounds during sample processing. Although techniques for stand-off measurements are relatively mature, they frequently return nonunique information about organics.
Finding: The commercial availability of compact, low-power, RNA and DNA sequencing devices could contribute significantly to the robustness of the current portfolio of life detection technologies.
Finding: Current technology for DNA amplification and sequencing may be useful for in situ detection of terrestrial contamination and lifeforms that are closely related to terran life, but at present, they are not sufficiently agnostic to the subunit composition of an informational heteropolymer.
On Earth, one key issue for in situ biosignature research, particularly with respect to fossil biosignatures, is the mineral-scale context of the purported biosignatures. This is amply demonstrated by the approach developed for Mars 2020 (Hays et al. 2017), for which the Planetary Instrument for X-Ray Lithochemistry (Allwood et al. 2015) and the Scanning Habitable Environments with Raman and Luminescence for Organics and Chemicals (Beegle and Bhartia 2016) are able to co-locate carbon on a mineral scale. In situ compositional (mineral, carbon) and elemental analyses, in combination with optical microscopy, provides essential context for gas chromatography–mass spectrometry (GCMS) and laser desorption–mass spectrometry (LDMS) destructive analysis of organics. Thus, a key emerging technology necessary for astrobiological missions is optical microscopy. Microscopy can be used for the detection of both extant and extinct life, although the signatures of extinct (fossil) life can be enigmatic. Further, microscopy observations need to be coupled with other lines of evidence of biogenicity, such as environmental context, chemical and isotopic composition, and informational polymers that are suggestive of biological origin (Westall et al. 2015a,b).
Transmitted light microscopy is particularly useful; however, it requires making sections of rock thin enough (several tens of microns thick) to allow the transmission of light and observation by a microscope. Normally, but not always (depending on the nature of the material to be sectioned), water is used during the sawing process. New methods are being developed to prepare thin sections without the use of water and with minimal energy requirements for utilization not only in in situ space missions but also in terrestrial laboratories, such as extraterrestrial sample curation facilities and others (Foucher et al. 2017). Such an instrument can be coupled with Raman and laser-induced breakdown spectroscopy (LIBS) to provide organic and elemental analysis. The addition of an infrared spectrometer could also be envisaged. This kind of instrument would provide essential contextual information from drill samples that are also used for organic biosignature organic analysis by GCMS and LDMS, as on the ExoMars 2020 rover (Goesmann et al. 2017; Vago et al. 2017).
Fortunately, recent advances have demonstrated that high resolution microscopes are increasingly able to be adapted to planetary landers or rovers. For example, submicrometer-resolution optical microscopes are now capable of autonomous operation (Nadeau et al. 2018). Holographic microscopes are capable of imaging relatively large volumes and do not require focusing, both of which are important for spaceflight instruments. In addition, they are capable of detecting transparent objects without the need for staining. An additional promising method employed in biological studies on Earth uses fluorescent labeling with dyes followed by high-resolution light microscopic imaging (Nadeau et al. 2018). Commonly used dyes produce low background fluorescence and signals that increase
upon binding to chemical targets, facilitating their detection. Fluorescent imaging also increases effective spatial resolution by allowing microbial features that are unresolved, such as flagella, to be observed. Dyes, however, can produce ambiguous results because they may also bind to mineral particles, organics, and even complex materials such as amino acids, sugars, and nucleobases that can be produced by abiotic processes. Microfluidic platforms provide a means of moving samples between detection systems and can operate in combination with fluorescence microscopy to search for microorganisms and to study their response to different agents (Ricco et al. 2018). Advancements in low-mass and robust optical systems, such as single focal-plane systems, imaging samples in low light, and imaging samples at full volume are evolving technologies.
Finding: New technologies for microscale and nanoscale analyses combining optical microscopy, Raman spectroscopy, laser-induced breakdown spectroscopy, infrared, and other interrogatory methods offer promise for advancing the detection of and confidence in microscale biosignatures.
The interpretation of biogenicity needs to be based on observation of a complementary suite of biosignatures. Technologies and techniques for the characterization of organic materials are most useful if able to detect not only informational polymers, which are currently known to be unique to life, but also simple polycyclic aromatic hydrocarbons and shorter-chained molecules. These materials form the backbone of biochemistry and, even in the absence of biologic processes, may contain contextual information about the environment. Microstructures (fossil or extant) and even motility, detectable by advanced microscopic techniques, may provide lines of direct evidence for life. Traditional lander and rover payloads further characterize the context in which possible biosignatures are discovered. These analyses together provide the multiple lines of evidence needed to create a convincing case for a purported biosignature. Notably, just as would be the case on Earth, these analyses will require a full suite of laboratory instruments.
Finding: In situ detection of life is best advanced by integrated suites of instruments or single instruments that permit multiple analytical techniques, including nondestructive approaches, to be applied to the same materials.
Finding: It is important that science requirements drive sample handling technologies—including ingestion and nondestructive sample preparation and analysis—rather than off-the-shelf engineering solutions or ease of implementation.
PROGRAMMATIC CHALLENGES AND OPPORTUNITIES
Challenges to a focused portfolio of life-detection technologies also stem from programmatic elements. Such elements identified by the committee include a risk-averse environment that in some cases may stifle the selection of high-risk, high-payoff instrumentation and missions, relaxed specification for proposer-derived success criteria, and the need for innovative instruments and complex sample handling systems. Perhaps even more importantly, the planetary exploration program has remained deeply rooted in the nonbiological sciences dating back to the Apollo sample analysis program and the early Mariner flyby missions to Mars and Mercury. Long-standing questions about the possibility of life on Mars led to the biology experiments on the Viking landers in the latter half of the 1970s, but planetary exploration thereafter returned to its geologic roots. The NASA Origins Program of the late 1990s and early 2000s, stimulated in part by the possibility of evidence for life in a martian meteorite, brought questions of life back to the center stage of planetary exploration. In the years since, astrobiology has developed, with strong NASA support, into a vibrant interdisciplinary field characterized by integrative, systems-level thinking about how a planet transforms from nonliving to living and how life and a planet coevolve.
For the past two decades, questions focused on planets from geological and astrobiological perspectives have largely overlapped in instrument and measurement requirements. Planning, implementation, and operations of planetary exploration missions with astrobiological objectives have tended to be more strongly defined by geological perspectives than by astrobiology-focused strategies. Deepening insights appear to be leading toward some divergence, however. For example, biosignature detection increasingly requires specialized instrumentation such
as macro- to microscale imaging, spectral imaging, mass spectrometry and, potentially, nucleotide sequencing technologies. Further, operational requirements for astrobiology-focused investigations may be better achieved with a grid-based approach in which an area is divided into individual nodes for surveying, rather than by traditional geological traverses along a planned path. For example, the grid approach was key to the Curiosity rover discovery of an ancient habitable environment at Yellowknife Bay in Gale Crater. The opportunity to apply this approach, however, resulted mainly from mission operational constraints that prevented beginning the planned geological traverse shortly after landing. It has thus become imperative to more fully integrate astrobiological thinking and objectives into mission planning, implementation, and operations.
Current mission-selection processes emphasize a need for low risk in technological readiness levels, which is tied to spacecraft engineering as opposed to science. Current NASA instrument evaluation and selection policies tend to favor low risk technologies, which in some cases adversely impacts scientific payoff. This inhibits development and selection of potentially game-changing life-detection technologies, for example. Currently, a high-risk science activity may be defined as recognizing carbon, which is a proven engineering capability. Detecting carbon of any type in situ (as recently demonstrated for Mars by Eigenbrode et al. 2018)—that is, directly in regolith or in rocks—is extremely difficult. Nevertheless, detecting and characterizing carbon has enormous scientific potential because it could unambiguously confirm one or more of the following:
- The presence of one of the main ingredients of life on an extraterrestrial body, and
- The presence of biogenic carbon with particular compositional and structural patterns as compared to abiogenic carbon.
At the same time, instruments suitable for environments in which life-detection activities may be relevant—such as Europa, Titan, or astrobiological targets on Mars—are rare. The lunar, asteroid, and low-Earth orbit environments are significantly different from what in situ life-detection instruments would encounter on rocky or icy planets in the solar system, and there is lower science need for such techniques to be developed for these more easily accessible environments. There is renewed need for development of instruments with high science potential, even in the face of high development risk.
Finding: Current NASA instrument evaluation and selection policies favor low risk technologies with, in some cases, potentially low scientific payoff. This inhibits development and selection of potentially game-changing life-detection technologies.
Finding: Planning, implementation, and operations of planetary exploration missions with astrobiological objectives have tended to be more strongly defined by geological perspectives than by astrobiology-focused strategies.
Recommendation: To advance the search for life in the universe, NASA should accelerate the development and validation, in relevant environments, of mission-ready, life-detection technologies. In addition, it should integrate astrobiological expertise in all mission stages—from inception and conceptualization to planning, development, and operations.
In the current paradigm, mission teams and instrument providers define their own instrument success criteria. As a result, a wide range of instruments has claimed to address questions of habitability and further life-detection goals on the basis of the possibility of making microscopic observations and detecting and analyzing carbon and carbon-containing molecules. In some cases, this allows instruments with low science potential for astrobiology science community goals to be selected for flight on the basis of high heritage. In this way, the current instrument selection process can be biased against complex techniques with high science potential. Moreover, when proposers define their own samples and data against which the instruments are tested, it is left to members of the selection panel, who are unlikely to be life-detection experts, to decide for themselves whether a test is relevant or not. The creation of standards relevant to specific conditions, context, and instrument-detection level will aid the detection of biosignatures and life itself.
Finding: Because of possible ambiguity in proposer-defined instrument success criteria, there is inherent risk in using these to propose, evaluate, and select instruments designed to detect biosignatures, rather than using observation and measurement validation standards established by community consensus.
Many of the most challenging goals with regard to habitability and life detection cannot be achieved with a single instrument. Therefore, missions that select instruments as stand-alone or that do not allow cross-instrument requirements or that have project-wide level-1 requirements impacting science, can increase risk, and result in missed opportunities. In fact, inter-instrument tests of a mission payload are essential to demonstrate the complementarity of the payload and how the best observations from each instrument can be linked to provide the full picture (e.g., Bost et al. 2015). In the field of astrobiology, flagship missions are particularly at risk of the perception of diminished science returns. These missions require interdisciplinarity to answer systems-level questions; however, instruments are selected independently. Therefore, mission implementation can be a risk to life-detection science. The Europa Clipper mission is an example of a mission in which instruments depend on each other, in which the main mission driver, habitability, cannot be achieved with a single instrument, and in which the project team and project science group have played a crucial role in allowing for cross-instrument trades and synergy to be planned and executed. This is also the case for the European-Russian ExoMars 2020 rover mission to search for traces of life (mostly fossil but possibly extant) within the context of the geological environment.
NASA’s Astrobiology Program, which includes but is not limited to the NASA Astrobiology Institute (NAI), has been admirably forward thinking with regards to community building, fostering interdisciplinary collaboration and cooperation, and facilitating major advances in astrobiology and planetary research. The NASA Astrobiology Program has made valuable investments in training, growth, and diversification of the next generation of astrobiologists through several platforms that target early career scientists, including the Astrobiology Graduate Conference and FameLab.1 From 2012 through 2016, the NASA Astrobiology Program sponsored FameLab competitions across the United States to improve and expand the communication skills of early career scientists. During the 4 years of FameLab USA, 247 early career scientists from a broad array of disciplines and career stages benefited from the FameLab experience.
In addition, the Astrobiology Program through the NAI has provided numerous channels of support for early career scientists, including the Lewis and Clark Fund for Exploration and Field Research in Astrobiology (jointly with the American Philosophical Society); the Early Career Collaboration Fund; summer and winter school opportunities, and conference travel support. Another activity that NASA has sponsored to further interdisciplinary work in this area is a series of workshops on the theme of Comparative Climatology of Terrestrial Planets. These workshops have brought together climate scientists from across the globe to discuss progress in understanding the climates of rocky planets beyond, but still including, Earth.
Since 2015, the Astrobiology Program has also implemented a new crossdivisional research coordination network, the Nexus for Exoplanet System Science (NExSS), as a large-scale experiment in managing and catalyzing exoplanetary science that integrates the astronomical, terrestrial, planetary, and heliophysical sciences. The NExSS research coordination network provides the communications and community organization to connect and leverage research funded by several research and analysis competitions across cooperating NASA divisions, breaking down interdisciplinary and interdivisional barriers and integrating the larger scientific community into its activities. NExSS-led activities include the Upstairs-Downstairs Workshop (2016) on the impact of terrestrial planet interiors on planetary atmospheric and surface conditions, which was jointly supported by the NAI and National Science Foundation (NSF); the Exoplanet Biosignatures Workshop (2016), which produced six community scientific publications that greatly advanced understanding of the significance of false positives and agnostic biosignatures, as well as developing the comprehensive framework for biosignature assessment (Kiang et al. 2018; Schwieterman et al. 2018; Meadows et al. 2018; Catling et al. 2018; Walker et al. 2018; Fujii et al. 2018); and the Habitable Worlds Conference (2017),2 which had strong participation from Earth scientists, planetary scientists,
___________________
1 For more information about the Astrobiology Graduate Conference and FameLab see http://abgradcon.org/index.html and https://famelab.arc.nasa.gov/, respectively.
2 See agenda and abstracts for the 2017 Habitable Worlds Conference at https://www.hou.usra.edu/meetings/habitableworlds2017/pdf/program.pdf.
and heliophysicists, in addition to astronomers and exoplanet scientists. A NExSS-led community group developed a Laboratory Astrophysics Gap List to identify studies that are needed to be able to interpret exoplanet spectra (Fortney et al. 2016) and contributed numerous white papers to this National Academies of Sciences, Engineering, and Medicine study and the parallel exoplanets study. NExSS principal investigators (PIs) and their collaborators also contributed to plans for utilization of current space telescopes by bringing together the U.S. and international research communities (in almost equal numbers) to win a proposal for exoplanet characterization for JWST Early Release Science. These proposals will provide the critical initial characterization of the telescope’s performance for exoplanets, as a first step toward habitable-zone planet characterization and biosignature searches with JWST. It is also noteworthy that individual crossdivisional collaborations to study exoplanet environments are being fostered at NASA centers—for example, the Sellers Exoplanet Environments Collaboration at NASA’s Goddard Space Flight Center.3
One of the major goals of the Astrobiology Program is to advance astrobiology on NASA missions, and while the participation of astrobiologists has enabled significant progress in understanding how JWST and the future Large Ultraviolet/Optical/Infrared Surveyor (LUVOIR) and Habitable Exoplanet Observatory (HabEx) mission concepts could be used to search for signs of habitability and life on exoplanets, few solar system missions currently carry astrobiology instrumentation. While the discussion is actively changing in the planetary science community—for example, the resurgence of interest in the ocean worlds is driven by interest in the search for life—it is essential that NASA’s Astrobiology Program continues to find ways to enable cutting-edge thinking, especially that which advances exploration objectives.
Finding: Crossdivisional collaborations promoted by NASA’s Astrobiology Program between Earth science, astronomy, heliophysics, and planetary science have begun the task of breaking down disciplinary entrenchments and are helping the astrobiology and exoplanet communities reach their full potential.
New launch capabilities will also provide lower-cost access to Mars and beyond. Game-changing innovations in small platforms, such as CubeSats, planetary exploration drones, and ambitious, privately funded initiatives such as the laser-powered Breakthrough Starshot project,4 are opening new opportunities for space exploration. Such advances are relevant for solar system exploration, which has been severely limited by decade-long intervals between consecutive missions, combined with limited instrument flexibility requiring incremental approaches, such that mission instrumentation payloads are largely informed by results of previous missions.
DEVELOPMENTS IN DRILLING TECHNOLOGY AND SAMPLE HANDLING
Access to and sampling of the subsurface is an important strategy in the search for extant life, or for signatures of past life on planetary bodies (e.g., Skelley et al. 2005). For both rocky planets and ocean worlds, when harsh environmental conditions render their surfaces inhospitable, the subsurface provides a potential refuge, where liquid water, nutrients, and chemical energy may be available and preservation of habitat or biosignatures may be more favorable.
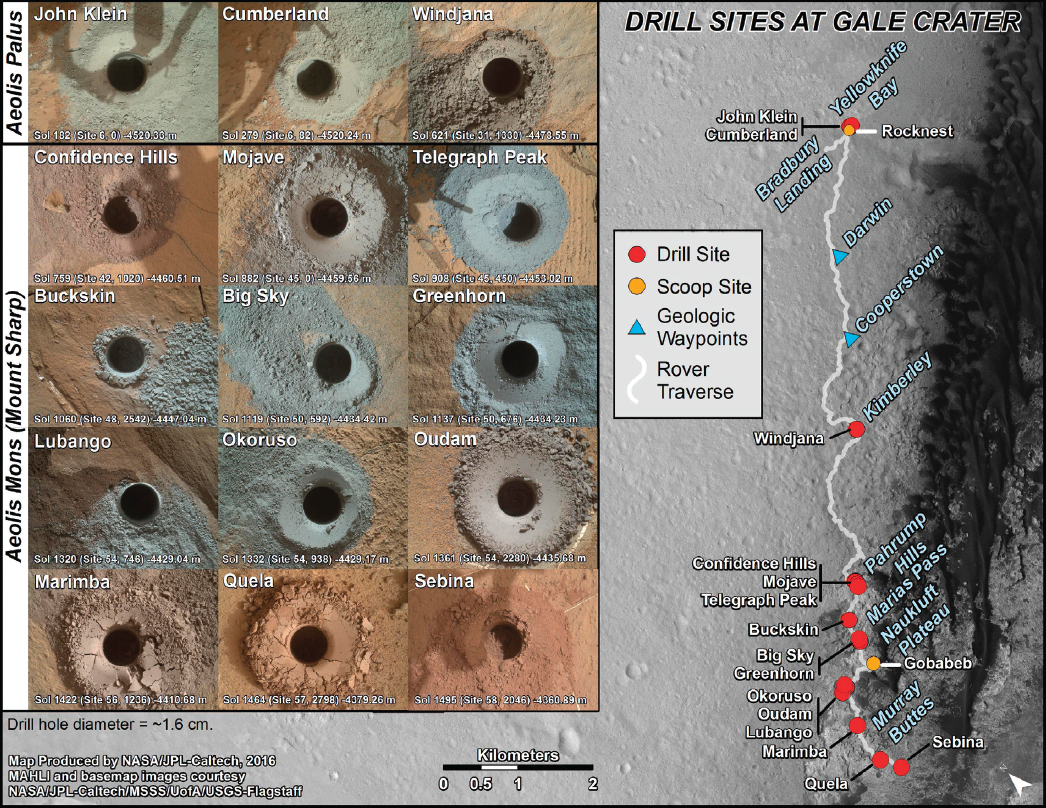
The Mars Exploration Rovers and the Curiosity rover made important discoveries using a grinder and scoop that allowed some investigation of the shallow subsurface of Mars, on a millimeter-centimeter scale at least. The discovery of the transition from gray soils to reddish material, presumably representing the transition from a reducing to oxidizing zone, was a major discovery (Figure 5.1). Following up on this, accessing Mars below the limits at which organics are seriously degraded by radiation, to 2 m as planned by ExoMars 2020 (Vago et al. 2017) and beyond 2 m, has focused attention on development of drilling capabilities. Similarly, the mission concepts for ocean worlds have focused thinking on more specialized tools, because drilling icy crust at the low-temperature surfaces is extremely difficult.
Typical drills move forward into the subsurface from the forces of large weights or via anchoring (Zacny et
___________________
3 For more about the Sellers Exoplanet Environments Collaboration, see https://science.gsfc.nasa.gov/600/seec/index.html.
4 For more information about the Breakthrogh Starshot project see https://breakthroughinitiatives.org/initiative/3.
al. 2008; Okon 2010). This is a challenge in low-gravity environments because high-mass systems are not desirable for extraterrestrial exploration. Biologically inspired solutions composed of toothed elements, such as Dual Reciprocating Drilling (DRD) systems, show promising results for drilling in certain types of planetary surfaces (Gao et al. 2007). The DRD technology consists of a pair of half cylindrical teethed elements opposing each other that reciprocate with linear motion, reducing the forces necessary for inserting the drill into the ground.
Drilling technologies that do not require large overhead forces (referred to as the weight on bit) will enable the search for in situ biosignatures in planetary subsurfaces. These technologies will need to be able to collect samples without altering their composition significantly (e.g., Kereszturi 2016). In particular, excessive heating is likely to cause chemical alteration and the release of volatiles, as demonstrated by the drill on the ExoMars 2020 rover (Vago et al. 2017). Furthermore, processing of subsurface samples obtained from drill cores needs to avoid significant physical and chemical alteration, including cementation and crosscontamination, before distribution to astrobiology science instruments (e.g., Richter and Senatore 2015). Significant progress has been made in these areas in recent years via ExoMars (Vago et al. 2017) and the Mars2020 sample caching programs (Mustard et al.
2013), while pilot programs to advance ice drilling capabilities have been conducted in analog environments, such as Antarctica (Talalay 2014) and the Arctic (Hansen 2018). In the latter cases, different types of drilling regimes are used—for instance, dry drilling or wet drilling using antifreeze agents, such as ethylene glycol or hot water. Recent years have seen better understanding of the challenges involved in drilling to retrieve uncontaminated biological samples on Earth, both in the deep crustal subsurface (e.g., Wilkins et al. 2014) or, for instance, into various Antarctic lakes (Siegert et al. 2016). Recent technological advances in automation and remote drilling, miniaturization, data processing, and sensor and imaging technology are also significant enabling technology supportive of subsurface investigations and sample acquisition (Stamenkovic et al. 2018).
BIG DATA ANALYSIS TECHNIQUES
Artificial intelligence (AI), or the ability of a computer to conduct inferences and other activities autonomously without human intervention, is a rapidly evolving computational science with an increasing breadth of applications. This field has spawned a range of computer algorithms, such as machine learning, that are capable of adaptively analyzing large data sets (assuming that they have been maintained in usable formats) with specific well-defined objectives (e.g., face and speech recognition). In particular, deep learning is a specific mode of machine learning that makes use of neural networks that have multiple layers of tunable parameters and learning objectives engineered to a specific purpose. Machine learning with deep-learning is expeditiously progressing in both the commercial and public sectors. In the last few years, it has begun to be incorporated in astrobiology studies. For instance, the use of machine learning with exoplanet detection has been used with Kepler data (McCauliff et al. 2015; Thompson et al. 2015; Shallue and Vanderberg 2018), exoplanet detection routines with direct imaging data (Gomez-Gonzales et al. 2016), as well as in upcoming missions like TESS (Pearson et al. 2018). In the solar system, analysis of space- and ground-based images of Mars is being advanced with machine learning routines (Rothrock et al. 2016; Wagstaff et al. 2018). Life-detection approaches for DNA sequencers for base labeling are also using deep learning techniques (e.g., Boža et al. 2017). Similarly, Bayesian frameworks in computational sciences are anticipating applications in comparative planetology and exoplanet biosignatures (e.g., Walker et al. 2017). Although machine learning analysis techniques need to be tailored and adapted for each application, there is a growing alignment between astrobiology goals and the commercial sectors that are currently heavily investing in AI development.
Finding: Rapid progress in the development of artificial intelligence machine learning algorithms has the potential to improve analysis of the large, complex data sets increasingly common to astrobiology.
REFERENCES
Alleon, J., S. Bernard, L. Remusat, and F. Robert. 2015. Estimation of nitrogen-to-carbon ratios of organics and carbon materials at the submicrometer scale. Carbon 84:290-298.
Alleon, J., S. Bernard, C. Le Guilluo, J. Marin-Carbonne, S. Pont, O. Beyssac, K.D. McKeegan, and F. Robert. 2016. Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and minerology. Nature Communications 7:11977.
Allwood, A., L. Wade, B. Clark, T. Elam, D. Flannery, M. Foote, J. Hurowitz, E. Knowles, and R. Hodyss. 2015. Texture-specific elemental analysis of rocks and soils with PIXL: The planetary instrument for X-ray lithochemistry on Mars 2020. 2015 IEEE Aerospace Conference. doi:10.1109/AERO.2015.7119099.
Beegle, L., and R. Bhartia. 2016. SHERLOC: An investigation for Mars 2020. EGU General Assembly, Vienna, Austria.
Bernard, S., K. Benzerara, O. Beyssac, and G.E. Brown, Jr. 2010. Multiscale characterization of pyritized plant tissues in blueschist facies metamorphic rocks. Geochimica et Cosmochimica Acta 74(17):5054-5068.
Beyssac, O., and M. Lazzeri. 2012. Application of Raman spectroscopy to the study of graphitic carbons in the Earth sciences. Pp. 415-454 in Raman Spectroscopy Applied to Earth Sciences and Cultural Heritage (J. Dubessy, M.-C. Caumon, and F. Rull, eds.). European Mineralogical Union and the Mineralogical Society of Great Britain and Ireland, Aberystwyth, UK.
Beyssac, O., B. Goffé, and C. Chopin. 2002. Raman spectra of carbonaceous material in metasediments: A new geothermometer. Journal of Metamorphic Geology 20:859-871.
Birkby, J.L., R.J. de Kok, M. Brogi, E.J.W. de Mooji, H. Schwarz, S. Albrecht, and I.A.G. Snellen. 2013. Detection of water absorption in the day side atmosphere of HD 189733 b using ground-based high-resolution spectroscopy at 3.2 μm. Monthly Notices of the Royal Astronomical Society: Letters 436(1):L35-L39.
Bost, N., C. Ramboz, N. LeBreton, F. Foucher, G. Lopez-Reyes, S. De Angelis, M. Josset, et al. 2015. Testing the ability of the ExoMars 2018 payload to document geological context and potential habitability on Mars. Planetary and Space Science 108:87-97.
Boža, V., B. Brejová, and T. Vinař. 2017. DeepNano: Deep recurrent neural networks for base calling in MinION nanopore reads. PLOS One 12(6): e0178751.
Brogi, M., I.A.G. Snellen, R.J. de Kok, S. Albrecht, J.L. Birkby, and E.J.W. de Mooij. 2013. Detection of molecular absorption in the dayside of exoplanet 51 Pegasi b? The Astrophysical Journal 767(1):27.
Brogi, M., R.J. de Kok, J.L. Birkby, H. Schwarz, and I.A.G. Snellen. 2014. Carbon monoxide and water vapor in the atmosphere of the non-transiting exoplanet HD 179949 b. Astronomy and Astrophysics 565:A124.
Castro-Wallace, S.L., C.Y. Chiu, K.K. John, S.E. Stahl, K.H. Rubins, A.B.R. McIntyre, J.P. Dworkin, et al. 2017. Nanopore DNA sequencing and genome assembly on the International Space Station. Scientific Reports 7:18022.
Catling, D.C., J. Krissansen-Totton, N.Y. Kiang, D. Crisp, T.D. Robinson, S. DasSarma, A. Rushby, et al. 2018. Exoplanet biosignatures: A framework for their assessment. Astrobiology 18(6): 709-738.
de Kok, R.J., M. Brogi, I.A.G. Snellen, J. Birkby, S. Albrecht, and E.J.W. de Mooji. 2013. Detection of carbon monoxide in the high-resolution day-side spectrum of the exoplanet HD 189733 b. Astronomy and Astrophysics 554:A82.
Eid, J., A. Fehr, J. Gray, K. Luong, J. Lyle, G. Otto, P. Peluso, et al. 2009. Real-time DNA sequencing from single polymerase molecules. Science 323(5910):133-138.
Eigenbrode, J.L., R.E. Summons, A. Steele, C. Freissinet, M. Millan, R. Navaroo-Gonzálex, B. Sutter, et al. 2018. Organics matter preserved in 3-billion-year-old mudstone at Gale crater, Mars. Science 360(6393):1096-1101.
Flannery, D.T., A.C. Allwood, R.E. Summons, K.H. Williford, W. Abbey, E.D. Matys, and N. Ferralis. 2018. Spatially-resolved isotopic study of carbon trapped in ~3.43 Ga Strelley Pool Formation stromatolites. Geochimica et Cosmochimica Acta 223:21-35.
Fortney, J.J., T.D. Robinson, S. Domagal-Goldman, D.S. Amundsen, M. Brogi, M. Claire, D. Crisp, et al. 2016. “The Need for Laboratory Work to Aid in the Understanding of Exoplanetary Atmospheres.” White paper submitted to the NASA Nexus for Exoplanet System Science (NExSS), NASA, CA.
Foucher, F., G. Guimbretière, N. Bost, and F. Westall. 2017. Petrographical and mineralogical applications of Raman Mapping. Pp. 163-180 in Raman Spectroscopy and Applications (K. Maaz, ed.). IntechOpen,, London, UK.
Fujii, Y., D. Angerhausen, R. Deitrick, S. Domagal-Goldman, J.L. Grenfell, Y. Hori, S.R. Kane, et al. 2018. Exoplanet biosignatures: Observational prospects. Astrobiology 18(6):739-778.
Gao, Y., A. Ellery, M.N. Sweeting, and J. Vincent. 2007. Bioinspired drill for planetary sampling: Literature survey, conceptual design, and feasibility study. Journal of Spacecraft and Rockets 44:703-709.
Goesmann, F., W.B. Brinckerhoff, F. Raulin, W. Goetz, R.M. Danell, S.A. Gretty, S. Siljeström, et al. 2017. The Mars Organic Molecule Analyzer (MOMA) instrument: Characterization of organic material in martian sediments. Astrobiology 17(6-7):655-685.
Gomez-Gonzalez, C.A., O. Absil, P.-A. Absil, M. Van Droogenbroeck, D. Mawet, and J. Surdej. 2016. Low-rank plus sparse decomposition for exoplanet detection in direct-imaging ADI sequences. The LLSG algorithm. Astronomy and Astrophysics 589:A54.
Hansen, S.B. 2018. “Deep Drilling with the Hans Tausen Drill.” Centre for Ice and Climate, Niels Bohn Institute. http://www.iceandclimate.nbi.ku.dk/research/drill_analysing/drilling_techniques/deep_drilling/.
Hays, L.E., H.V. Graham, D.J. Des Marais, E.M. Hausrath, B. Horgan, T.M. McCollom, M.N. Parenteau, et al. 2017. Biosignature preservation and detection in Mars analog environments. Astrobiology 17(4):363-400.
Hickman-Lewis, K., R.J. Garwood, M.D. Brasier, T. Goral, H. Jiang, N. McLoughlin, and D. Wacey. 2016. Carbonaceous microstructures from sedimentary laminated chert within the 3.46 Ga Apex Basalt, Chinaman Creek locality, Pilbara, Western Australia. Precambrian Research 278:161-178.
Jain, S., J.R. Wheeler, R. Walters, A.K. Agrawal, A. Barsic, R. Parker. 2016. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164(3):487-498.
Kereszturi, A., B. Bradak, E. Chatzitheodoridis, and G. Ujvari. 2016. Indicators and methods to understand past environments from ExoMars rover drills. Origins of Life and Evolution of Biospheres 46(4):435-454.
Kiang, N.Y., S. Domagal-Goldman, M.N. Parenteau, D.C. Catling, Y. Fujii, V.S. Meadows, E.W. Schwieterman, and S.I. Walker. 2018. Exoplanet biosignatures: At the dawn of a new era of planetary observations. Astrobiology 18(6): 619-629.
Kopparapu, R.K., E. Hébrard, R. Belikov, N.M. Batalha, G.D. Mulders, C. Stark, D. Teal, S. Domagal-Goldman, and A. Mandell. 2018, Exoplanet classification and yield estimates for direct imaging missions. Astrophysical Journal 856(2):122.
McCauliff, S.D., J.M. Jenkins, J. Catanzarite, C.J. Burke, J. L. Coughlin, J.D. Twicken, P. Tenebaum, S. Seader, J. Li, and M. Cote. 2015. Automatic classification of Kepler planetary transit candidates. The Astrophysical Journal 806(1):6.
Meadows, V.S., C.T. Reinhard, G.N. Arney, M.N. Parenteau, E.W. Schwieterman, S.D. Domagal-Goldman, A. Lincowski, et al. 2018. Exoplanet biosignatures: Understanding oxygen as a biosignature in the context of its environment. Astrobiology 18(6): 630-662.
Morag, N., K.H. Williford, K. Kitajima, P. Philippot, M.J.Van Kranendonk, K. Lepot, C. Thomazo, and J.W. Valley. 2016. Microstructure-specific carbon isotopic signatures of organic matter from ~3.5 Ga cherts of the Pilbara Craton support a biologic origin. Precambrian Research 275:429-449.
Mustard, J.F., M. Adler, A. Allwood, D.S. Bass, D.W. Beaty, J.F. Bell III, W.B. Brinckerhoff, et al. 2013. Report of the Mars 2020 Science Definition Team. Mars Exploration Program Analysis Group (MEPAG). July. http://mepag.jpl.nasa.gov/reports/MEP/Mars_2020_SDT_Report_Final.pdf.
Nadeau, J., C. Lindensmith, W. Fink, D. Schulze-Makuch, K.H. Nealson, L.M. Barge, H. Sun, J. Bowman, and I. Kanik. 2018. “Just Look!” White paper submitted to the Committee on an Astrobiology Science Strategy for the Search for Life in the Universe.
NASA (National Aeronautics and Space Administration). 2018. Technology Plan Appendix 2018. Exoplanet Exploration Program. JPL D-101271. Pasadena, CA.
Nivala, J., D.B. Marks, and M. Akeson. 2013. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nature Biotechnology 31(3):247-250.
NRC (National Research Council). 2001. Astronomy and Astrophysics in the New Millennium. The National Academies Press, Washington, DC.
Okon, A.B. 2010. Mars science laboratory drill. Proceedings of the 40th Aerospace Mechanisms Symposium. NASA/CP-2010-216272.
Pearson, K.A., L. Palafox, and C.A. Griffith. 2018. Searching for exoplanets using artificial intelligence. Monthly Notices of the Royal Astronomical Society 474(1):478-491.
Ricco, A.J., M.B. Wilhelm, R.C. Quinn, A. Davila, and D.J. Harrison. 2018. “The Critical Enabling Role of Integrated Microfluidic Systems in the Search for Life: Key Challenges, Recent Progress, Path Forward.” White paper submitted to the Committee on an Astrobiology Science Strategy for the Search for Life in the Universe.
Richter, L., and C. Senatore. 2015. Special issue on planetary rovers and machine-regolith interactions. Journal of Terra-mechanics 62:3.
Ricker, G.R., J.N. Winn, R. Vanderspek, D.W. Latham, G.A. Bakos, J.L. Bean, Z. Berta-Thompson, et al. 2015. Transiting Exoplanet Survey Satellite (TESS). Journal of Astronomical Telescopes, Instruments, and Systems 1(1):014003.
Rothrock, B., J. Papon, R. Kennedy, M. Ono, M. Heverly, and C. Cunningham. 2016. “SPOC: Deep Learning-based Terrain Classification for Mars Rover Missions.” Presentation to the American Institute of Aeronautics and Astronautics SPACE Forum, September 15, AAIA 2016-5539.
Schwieterman, E.W., N.Y. Kiang, M.N. Parenteau, C.E. Harman, S. DasSarma, T.M. Fisher, G.N. Arney, et al. 2018. Exoplanet biosignatures: A review of remotely detectable signs of life. Astrobiology 18(6): 663-708.
Shallue, C.J., and A. Vanderburg. 2018. Identifying exoplanets with deep learning: A five-planet resonant chain around Kepler-80 and an eighth planet around Kepler-90. The Astronomical Journal 155(2):94.
Siegert, M.J., J.C. Priscu, I.A. Alekhina, J. L. Wadham, and W.B. Lyons. 2014. Antartic subglacial lake exploration: First results and future plans. Philosophical Transactions of the Royal Society A 374(2059).
Skelley, A.M., J.R. Scherer, A.D. Aubrey, W.H. Grover, R.H.C. Ivester, P. Ehrenfreund, F.J. Grunthaner, J.L. Bada, and R.A. Mathies. 2005. Development and evaluation of a microdevice for amino acid biomarker detection and analysis on Mars. Proceedings of the National Academy of Sciences U.S.A. 102(4):1041-1046.
Stamenkovic, V., J. Barross, D. Beaty, L. Beegle, M.S. Bell, J.G. Blank, D. Breuer, et al. 2018. “Mars Subsurface Access: From Sounding to Drilling.” White paper submitted to the Committee on an Astrobiology Science Strategy for the Search for Life in the Universe.
Sugitani, K., K. Mimura, M. Takeuchi, K. Lepot, S. Ito, and E.J. Javaux. 2015. Early evolution of large micro-organisms with cytological complexity revealed by microanalyses of 3.4 Ga organic-walled microfossils. Geobiology 13(6):507-521.
Sugitani, K., K. Mimura, T. Nagaoka, K. Lepot, and M. Takeuchi. 2013. Microfossil assemblage from the 3400 Ma Strelley Pool Formation in the Pilbara Craton, Western Australia: Results form a new locality. Precambrian Research 226:59-74.
Talalay, P.G. 2017. Perspectives for development of ice-core drilling technology: A discussion. Annals of Glaciology 55(68):339-350.
Thompson, S.E., F. Mullally, J. Coughlin, J.L. Christiansen, C.E. Henze, M.R. Haas, and C.J. Burke. 2015. A machine learning technique to identify transit shaped signals. The Astrophysical Journal 812(1):46.
Ueno, Y., H. Yoskioka, S. Maruyama, and Y. Isozaki. 2004. Carbon isotopes and petrography of kerogens in ~3.5-Ga hydrothermal silica dikes in the North Pole area, Western Australia. Geochimica et Cosmochimica Acta 68(3):573-589.
Vago, J.L., F. Westall, Pasteur Instrument Teams, Landing Site Selection Working Group, and Other Contributors. 2017. Habitability on early Mars and the search for biosignatures with ExoMars Rover. Astrobiology 17(6):471-510.
Wacey, D., M.R. Kilburn, M. Saunders, J. Cliff, and M.D. Brasier. 2011. Microfossils of sulphur-metabolizing cells in 3.4-bil-lion-year-old rocks of Western Australia. Nature Geoscience 4:698-702.
Wagstaff, K., Y. Lu, A. Stanboli, K. Grimes, T. Gowda, and J. Padams. 2018. “Deep Mars: CNN Classification of Mars Imagery for the PDS Imaging Atlas.” Presentation to the Thirtieth AAAI Conference on Innovative Applications of Artificial Intelligence, February 2-7.
Walker, S.I., W. Bains, L. Cronin, S. DasSarma, S. Danielache, S. Domagal-Goldman, B. Kacar, et al. 2017. “Exoplanet Biosignatures: Future Directions.” White paper submitted to the NASA NExSS Exoplanet Biosignatures Workshop, arXiv:1705.08071.
Walker, S.I., W. Bains, L. Cronin, S. DasSarma, S. Denielache, S. Domagal-Goldman, B. Kacar, et al. 2018. Exoplanet biosignatures: Future directions. Astrobiology 18(6):779-824.
Wang, A., J.J. Freeman, B.L. Jolliff, and I.-M. Chou. 2006. Sulfates on Mars: A systematic Raman spectroscopic study of hydration states of magnesium sulfates. Geochimica et Cosmochimica Acta 70(24):6118-6135.
Westall, F., F. Foucher, N. Bost, M. Bertrand, D. Loizeau, J.L. Vago, G. Kminek, et al. 2015a. Biosignatures on Mars: What, where and how? Implications for the search for martian life. Astrobiology 15:998-1029.
Westall, F., K.A. Campbell, J.G. Bréhéret, F. Foucher, P. Gautret, A. Hubert, S. Sorieul, N. Grassineau, and D.M. Guido. 2015b. Archean (3.33 Ga) microbe-sediment systems were diverse and flourished in a hydrothermal context. Geology 43(7):615-618.
Wilkins, M.J., R.A. Daly, P.J. Mouser, R. Trexler, S. Sharma, D.R. Cole, K.C. Wrighton, et al. 2014. Trends and future challenges in sampling the deep terrestrial biosphere. Frontiers in Microbiology 5:481.
Zacny, K., M. Bar-Cohen, G. Brennan, G. Briggs, K. Cooper, B. Davis, D. Dolgin, et al. 2008. Drilling systems for extraterrestrial subsurface exploration. Astrobiology 8(3):665-706.














