4
Biosignature Identification and Interpretation
Traditionally, biosignatures have been defined as an object, substance, and/or pattern whose origin specifically requires a biological agent (Des Marais et al. 2003). The compendium of features listed by Des Marais et al. (2008) includes, but is not limited to the following:
- Cellular and extracellular morphologies,
- Biogenic fabrics in rocks,
- Bio-organic molecular structures,
- Chirality,
- Biogenic minerals,
- Biogenic stable isotope patterns in minerals and organic compounds,
- Atmospheric gases,
- Remotely detectable features on planetary surfaces, and
- Temporal changes in global planetary properties.
In order to qualify as biosignatures, these “features must be sufficiently complex and/or abundant so that they retain a diagnostic expression of some of life’s universal attributes” (Des Marais et al. 2008). Another essential characteristic is that their formation by nonbiological processes be highly improbable. Informational biopolymers like DNA or polypeptides, for example, would be examples of biosignatures that are highly unlikely to arise in the absence of biology.
The past 20 years has seen a major evolution in biosignature science. This evolution is summarized in the following statement from the report of the Mars 2020 Science Definition Team (Mustard et al. 2013):
The scientific significance of any potential sign of past life comes not only from the probability of life having produced it, but also from the improbability of non-biological processes producing it.
These concepts govern the selection of candidate biosignatures, which are ranked by how well they pass three criteria: reliability (i.e., a feature that is more likely to be produced by life), survivability (i.e., the ability of the biosignature to be preserved or otherwise persist in its environment), and detectability (the likelihood that the biosignature can be observed or measured) (NASEM 2017; Meadows 2017; Meadows et al. 2018b).
So far, only the Viking mission has conducted a rigorous search for in situ biosignatures on another planet, focused on metabolic indicators of life, including the search for organic compounds (Biemann et al. 1977; Klein 1978). After a hiatus of more than four decades, a NASA mission concept is being developed—a Europa lander—whose top priority is the search for life (Hand et al. 2018). This new search for life is spurred by the idea that the search for life by future missions will be likely to benefit from a broader definition of life, with the search focused on the function of biochemical processes (Chyba and Phillips 2001).
The Galileo spacecraft, while en route to Jupiter, searched for biosignatures on Earth using remote-sensing techniques. Signs of life were found during Galileo’s flyby of Earth in December 1990. Purported biosignatures on Earth comprised the detection of gases—in this case methane and oxygen—in strong thermodynamic disequilibrium (Hitchcock and Lovelock 1967). The thermodynamic disequilibrium of Earth’s atmosphere was interpreted to imply a continuous and large surface flux of both gases. Additional biosignatures included surface reflectance signals, due to vegetation, and narrow-band, pulse-modulated radio signs (Sagan et al. 1993). While abiotic processes may be identified that can produce disequilibria and narrow-band radio signals (Rennó and Ruf 2012), as a suite, the planetary characteristics identified by Galileo are strongly suggestive of an inhabited planet. Earth continues to be studied for the nature and detectability of its biosignatures, and significant work is under way to develop the telescopic capability to search for life on exoplanets. These future searches in the solar system and beyond will need to be supported by significant new research on biosignature identification and interpretation.
Since publication of the 2015 Astrobiology Strategy (NASA 2015), the field of biosignature research has advanced four major areas:
- The search for and identification of novel biosignatures, especially those that are agnostic to life’s molecular makeup or metabolism;
- A concerted effort to better understand abiosignatures (signature of abiotic processes and phenomenon), in particular those that may mimic biosignatures;
- An improved understanding of which biosignatures are most likely to survive in the environment and at what timescales of preservation; and
- The first steps toward developing a comprehensive framework that could be used to interpret potential biosignatures, abiosignatures, false positives, and false negatives, and increase confidence and consensus in interpretations.
This work has progressed in parallel for both in situ biosignatures (e.g., those preserved in rock or ice that can be searched for on the surface of Mars or Europa) and in remotely sensed, often global-scale, biosignatures that might be observed telescopically in the atmospheres or surfaces of exoplanets or detected on solar system bodies by orbiter or flyby spacecraft. For remotely sensed biosignatures, a comprehensive series of review papers on the topic of exoplanet biosignatures and future directions (Schwieterman et al. 2018; Meadows et al. 2018b; Catling et al. 2018; Walker et al. 2018; Fujii et al. 2018) was published as part of a Nexus for Exoplanet System Science (NExSS) community-wide biosignatures workshop activity (Kiang et al. 2018).
IDENTIFYING NOVEL BIOSIGNATURES TO IMPROVE RELIABILITY
The search for life is constrained by the ability to recognize life’s impact on its environment—a biosignature. Recent research has identified new ideas for both in situ and global remote sensing of biosignatures and advanced a new area of research into agnostic biosignatures. Agnostic biosignatures are those that are not tied to a particular metabolism-informational biopolymer or other characteristic of life as we know it, but which may manifest as unexpected complexity either in a system-wide alteration of a planetary environment or in preserved molecules.
The in situ search for biosignatures typically focuses on microbial life because microbes are more pervasive than multicellular organisms. Indeed, diverse and complementary indicators of biological activity, including the enantiomeric and isotopic distribution of organic compounds, are useful (e.g., Lovelock 1965, 1975). These compounds can be measured using mass spectrometry, gas chromatography–mass spectrometry, evolved gas analysis, Raman spectroscopy, and culture-based methods (e.g., Summons et al. 2008). The presence of organic compounds
does not prove the existence of life, because complex carbon-containing molecules are readily generated abiotically. Rather, a chemical biosignature is the presence of specific patterns in the abundances of selected compounds that do not typically occur via chance in reactions driven by thermodynamics alone. Microscopy can provide another line of evidence, for instance, if motile microorganisms are present in a sample (e.g., Seo et al. 2010; Ha et al. 2015; Bedrossian et al. 2017). DNA-based surveys are also being proposed for in situ searches for Earth-like biosignatures (e.g., Carr et al. 2016). Because microbes on Earth thrive in brine inclusions in sea ice, which also contain nutrients and organics in high concentration (Junge et al. 2001), brine inclusions would be an excellent target for the search for biosignatures and life in cold worlds. However, continued developments in identification of novel biosignatures for in situ life searches are still needed, including an improved understanding of potential biosignatures for icy worlds, subsurface organisms, and chemoautotrophic microorganisms.
Finding: The catalog of potential biosignatures would benefit from a systematic reevaluation and increased understanding of the nature and detectability of biosignatures, especially for in situ detection of energy-starved or otherwise sparsely distributed forms such as chemoautotrophic and subsurface life.
Novel remote-sensing biosignatures for exoplanets include, for example, the formation of hazes in anoxic environments due to methanogenic production of CH4 well above that expected from geological processes such as serpentinization (recently identified by Arney et al. 2018), a range of volatile molecules that could potentially be biosignatures (e.g., Seager et al. 2016), seasonality in gas abundances as a biosignature (Olson et al. 2018), and identification of unusual global disequilibria. While the simultaneous presence of O2 (or O3) and CH4 (or N2O) is still a compelling remote biosignature, recent research has sought to identify other potential disequilibria that might indicate life. These include the presence of ammonia on hydrogen-dominated worlds (Seager and Bains 2015), the combination of both O2 and N2 and the presence of an ocean on a habitable world (Krissansen-Totton et al. 2016), or the simultaneous presence of N2, CH4, CO2, and liquid water, and/or methane mixing ratios greater than 0.001. The latter biosignature is potentially biogenic due to the difficulty in maintaining large abiotic methane fluxes to support high methane levels in anoxic atmospheres (Krissansen-Totton et al. 2018).
While thermodynamic disequilibrium can be abiotically generated, most often by photochemical and other planetary processes, the biosignature is not the thermodynamic disequilibrium itself, but rather the fluxes inferred to drive that disequilibrium. As such, thermodynamic disequilibrium does not have to presuppose a particular metabolism and can be considered agnostic. The classic Earth O2/CH4 disequilibrium provides an example. In the context of the atmospheric composition, volcanic outgassing, and the stellar ultraviolet spectrum, O2 has a geologic lifetime of ~2 million years, while CH4 and N2O have photochemical lifetimes of ~12 and ~150 years, respectively. This means that the latter two gases, in particular, are fluxed into the atmosphere at a high rate, or else they would disappear almost immediately. If these fluxes are particularly high, they can identify a more likely biological than planetary source. This is the case of Earth’s microbially generated CH4, which is more than 60 times higher than the estimated abiotic flux from water-rock reactions on Earth (e.g., Etiope and Sherwood Lollar 2013), and photosynthetically generated O2, which has a flux many orders of magnitude higher than photochemical production of O2 for Earth, given its distance from the Sun.
AGNOSTIC BIOSIGNATURES
In addition to this expanded study of global thermodynamic disequilibrium, there have been considerable recent advances in the field of agnostic biosignatures (Johnson 2018; Johnson et al. 2018a,b). These advances explore specific frameworks and techniques for universal life detection that do not presuppose any particular molecular framework (Cronin and Walker 2016) or evolutionary endpoint (Cabrol 2016). While the current field of novel biosignature identification for in situ biosignatures focuses on structures believed to represent life as we know it—particular classes of molecules or isotopic signatures, chirality, or molecular weight patterns for fatty acids or lipids—the goal for the emerging field of agnostic biosignatures is to expand the ability to search for life as we do not know it through exploration of a broader definition of life based on activity, with less dependence on assumptions about structure and specific biogeochemistry (Johnson et al. 2018a,b). Discussion focuses on a
variety of approaches including but not limited to consideration of general molecular complexity associated with observations of matter and energy transfer, as well as conceptualization of the number of pathways by which a given molecule can be assembled and the probability of its formation in the absence of biology (Cronin and Walker 2016; Marshall et al. 2017). Statistical analysis and combinatorial chemistry including machine learning and computational models and algorithms to estimate the probability for disequilibrium are the foundation of this approach (Keefe et al. 2010; Goodwin et al. 2015). Defining molecular complexity not simply based on molecular size or type but rather on detecting assemblages of molecules that are abiotically improbable is a key approach to this research. Other approaches consider elemental and/or isotopic gradients or accumulations distinct from the surrounding environment, as well as disequilibrium redox chemistry inconsistent with the abiotic environmental baseline.
Example: From Terrestrial Genetic Code to Agnostic Biosignatures
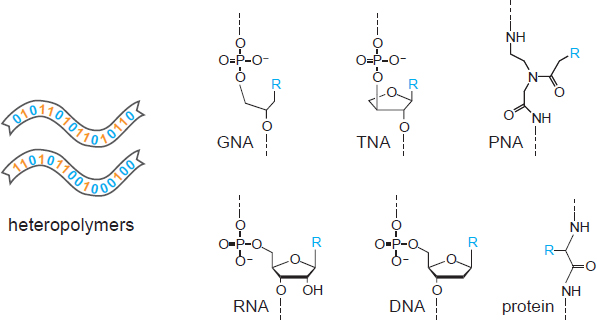
A hallmark of living systems is the perpetuation of genetic information reflecting the history of the system’s adaptive responses to the environment. For all known cellular organisms, this information is stored in nucleic acid molecules (e.g., Figure 4.1). It is also transiently represented as RNA transcripts and protein translation products. If the goal of an in situ life detection experiment is to detect Earth-like life, or terrestrial contaminants thereof (Box 6.2), then the powerful technologies of nucleic acid amplification and sequencing can be applied (Box 5.1). These technologies require minimal deviation from the canonical structure of DNA or RNA. For alternate forms of life, however, it is important to consider how to detect informational molecules more generally. Arguably, the detection of heteropolymers (i.e., polymers that contain more than one type of subunit) that have an “aperiodic” composition (Schrödinger 1944) would be strongly indicative of biological processes. Such a composition would be witnessed as the nonuniform spatial arrangement of monomeric subunits within a linear (or perhaps two- or three-dimensional) polymer (and potentially an agnostic biosignature).
Considering the in situ detection of informational heteropolymers more broadly, it will be essential to develop techniques that are agnostic to the detailed chemical composition of the informational heteropolymer. It has been suggested that a linear genetic polymer operating in an aqueous environment is highly likely to be a polyelectrolyte (Benner 2017). Thus, it may be advantageous to devise capture and detection methods based on the regular spacing of positively or negative charged subunits. Slight variation in the spacing of subunits might be indicative of heterogeneous composition of the polymer and perhaps the presence of genetic information content. Note that such an approach would apply not only to the genetic material itself, but also to corresponding transcription and translation products that contain a preponderance of charged subunits. It may not be possible to achieve precisely
matched spacing of complementary charges in the detector. However, even if the detector is out of frame relative to the polymer, the phase mismatch between polymer and detector will be discernable, so long as the spacing is regular.
Whether or not the genetic material is a polyelectrolyte, there are other potential approaches for determining whether it has a heteropolymeric composition. Both matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry and liquid-chromatography tandem mass spectrometry (LC-MS/MS) have been used to fingerprint proteins and other heteropolymers. The former technique requires relatively pure samples and therefore would not be suitable for in situ detection unless the materials could first be selectively captured on a surface or enriched by some other means. The latter technique relies on liquid chromatography to first separate the materials prior to mass analysis. In both cases, sample material is typically preprocessed by enzymatic digestion to generate a library of fragments that can be matched to a database of known fragments of the same material. No such database exists for the detection of previously uncharacterized heteropolymers, but the goal of in situ life-detection studies is to determine whether informational heteropolymers are present, not the particular ordering of subunits within those heteropolymers.
The generalized detection of informational heteropolymers can be accomplished using mass spectrometry methods (e.g., Arevalo et al. 2015). If the bonds between polymeric subunits undergo spontaneous cleavage in the mass spectrometer, then fragmentation is achieved “on the fly,” and the degree of fragmentation can be modulated by adjusting the voltage of the instrument. This approach has been used to detect and even sequence synthetic informational heteropolymers where the subunits are joined by labile alkoxyamine groups (Al Ouahabi et al. 2017). For an unknown heteropolymer, one cannot rely on fragmentation between subunits in the mass spectrometer. Instead it will be necessary to apply a standard set of preprocessing steps that aim to achieve partial fragmentation, then compare the spectra of intact and partially fragmented samples. The fragmentation processes might include shear force (forced flow through a narrow aperture), sonication, and acid or base hydrolysis.
The task of in situ detection of a heteropolymer of unknown composition is not an easy one. However, it provides the opportunity to detect biosignatures that are very unlikely to arise through abiotic processes. The ultimate signature of life is information, written in the language of molecules. Whether that information is the product of extinct or extant life, simple or complex life, it is a distinguishing feature that is worth pursuing. In support of these efforts, there is substantial research activity in the area of synthetic biology that considers alternative genetic polymers and functional macromolecules. There too, analytic methods are challenging, but are being addressed by various techniques that may prove applicable to in situ detection.
The above comments notwithstanding, the detection of DNA (or RNA) heteropolymers is an essential part of biosignature detection, both because the opportunity exists for a common form of life within the solar system and because the technology for such detection is highly mature. As a cautionary note, it is important to recognize that the detection of ancient DNA samples (approximately 1 million years old) is highly problematic, even for the most pristine samples on Earth. DNA is susceptible to both chemical and enzymatic degradation, making its detection and analysis even more challenging for remote instrumentation.
Agnostic biosignatures that can be detected by remote sensing may take the form of atmospheric disequilibria, as discussed previously, or the presence of complex chemical networks in a planetary atmosphere. In both cases, significant environmental context will be needed to interpret the disequilibria or chemical networks as a sign of life, including quantification of the biosignature gas and other gases in the atmosphere; knowledge of the stellar spectral energy distribution, including the ultraviolet; and photochemical and climate models that can be used to constrain the fluxes of gases required to maintain the disequilibrium. In addition to disequilibria, the community is now considering how biosignatures can be quantified using a Bayesian framework that can generalize search strategies beyond the biosignatures of known life. This Bayesian methodology will help quantitatively define the conditional probabilities and confidence of future life detection and may constrain the prior probability of life even without a positive detection (Walker et al. 2018).
Recommendation: The search for life beyond Earth requires more sophisticated frameworks for considering the potential for nonterran life; therefore, NASA should support research on novel and/or agnostic biosignatures.
SURVIVABILITY OF BIOSIGNATURES
Arguably, the question of survivability and preservation—especially on a global planetary scale, in the rock record and in the atmosphere, and on planetary timescales—that may extend to billions of years has received less attention than research into biosignature reliability and detectability. At the present time, Earth analog studies and modeling approaches appear to be two of the most practical ways to shed light on taphonomic issues—namely biosignature formation, preservation, alteration, and destruction—as they relate to planetary exploration and the search for life.
Record Bias
For the better part of Earth’s history, life was entirely microbial, devoid of mineralogical hard parts, and with little in the way of recalcitrant and preservable biopolymers. Those records of early life that do exist are fragmentary and, likely, highly biased. For example, cherts are sometimes fossiliferous, while sandstones rarely are. As a consequence, fossil hunters target cherts, sometimes to the exclusion of other lithologies, thereby leading to strong biases in the paleontological and astrobiological literature. Improved understanding of the mechanistic aspects of fossilization processes and environmental conditions that are particularly favorable for fossil formation and preservation has long been an agenda item in biosignature research with potential to uncover records that have previously been overlooked. Over the past few years, there has been an evolving landscape in terms of understanding how long habitable environments comprising lakes, seas, groundwater, and subsurface fluids might
have existed on Mars (Ehlmann and Edwards 2014; Grotzinger et al., 2015; Goudge et al. 2016; Ehlmann et al. 2016). On Earth, preservation of subsurface fluids providing a habitable subsurface environment rich in electron donors and acceptors has been recently shown to extend to more than a billion years (Holland et al. 2013; Li et al. 2016; Warr et al. 2018).
Preservational Bias
Understanding taphonomic biases is particularly important for environments and times when fossils are exclusively microscopic, such as in the Archean, and requires painstaking work. Whether addressing microbial life on the early Earth or Mars, a common assumption is that organisms that can be preserved are abundant in the environment. However, this situation is relatively rare, as shown by a recent study in which hundreds of meters of core samples had to be screened to detect a centimetric-scale horizon of brucite-calcite veins. These veins preserved biosignature lipids and microfossil remnants of a Cretaceous serpentinizing system in the seafloor near the Iberia margin (Klein et al. 2015). With all the modern tools available for studies of biosignatures for a hydrothermal ecosystem on Earth, this particular study illustrates the very difficult problem of looking for life that is not abundant. The challenges would be orders of magnitude more difficult on Mars.
One of the most interesting signatures of planetary bodies is the isotopic composition of minerals, especially with respect to carbon, hydrogen, nitrogen, oxygen, phosphorus, sulfur (CHNOPS),1 the six key biogenic elements that are incorporated into the macromolecules of life on Earth. Metabolism of organisms leads to “waste” products that are incorporated into minerals and kerogens and are potential biomarkers of past life. Indeed, the isotopic record of stable carbon isotopes in Earth’s geological record, especially in carbonates, has been used to infer the oxidation state of this planet. Similarly, the pattern of sulfur isotopes has changed over geological time on Earth and has been used to infer when the atmosphere of Earth contained ozone, a gas that cannot exist in a planetary atmosphere without molecular oxygen. Secular changes in nitrogen isotopes in kerogens can be used to infer the oxidation state in aquatic systems. However, these and other isotopic “signatures” can be overprinted by hydrothermal fluids, metamorphism, and other geological processes. Similarly, the valence state of transition metals, such as molybdenum and chromium can be diagenically altered, leading to a misinterpretation of the oxidation state of the environment at the time of deposition. Hence, it is highly desirable to perform redundant analyses of several isotopes of minerals and/or kerogens (when available) to provide more conclusive evidence of past or present life on planets and planetary bodies within the solar system.
On Earth, the preservation of ancient biosignatures is largely attenuated by tectonic processes, including the thermal metamorphism that accompanies burial, together with uplift and erosion, which can destroy them completely. Crustal fluid flow and contact with radioactive minerals are other deleterious factors. Recycling of sedimentary rock via plate tectonics has destroyed much of Earth’s early crust. Although this is perceived to be less of a factor for Mars, the cratering record indicates that volcanism has contributed to significant resurfacing. Even the large expanses of ancient rock on Earth (70 percent of the exposed surface area of continental lithosphere is Precambrian in age with approximately 14 percent of that Archean versus 86 percent Proterozoic; Goodwin 1996) have typically been heated to temperatures that confound preservation of biosignatures and abiosignatures and reset many parameters of interest.
Nonetheless, there are some locations where thermal conditions (French et al. 2015) may have been low enough on billion-year timescales for isotopic biosignatures (Li et al. 2016) and fluid components (Holland et al. 2013; Warr et al. 2018) to survive. Careful examination of such opportunities from Earth analogs (even if rare) will provide important test beds for expanding our understanding of biosignature preservation in ancient rock-hosted systems at low temperatures that may be relevant to Mars.
Studies of the sedimentary rock record on Earth have shown that favorable conditions for capturing and preserving microstructures and molecular biosignatures include rapid burial in fine-grained, detrital sediments (mudstones and shales) that experience early cementation by stable secondary phases, commonly silica and carbonate (e.g., Westall et al. 2015a,b; Knoll and Golubic 1979; Hofmann 1976) and sulfate evaporites (Westall et al. 2011;
___________________
1 Strictly speaking CHNOS, because phosphorous has only one stable isotope.
Schopf et al. 2012). These early diagenetic processes can enhance the detail of preserved fossils and textures and also reduce the permeability to crustal fluids during later burial and diagenesis, thus protecting organic matter from oxidation. Most organically preserved cellular remains observed in the Precambrian fossil record on Earth formed by these basic processes (e.g., Knoll 2003, 2012; Farmer and Des Marais 1999; Javaux et al. 2010). On Earth, such preservation is also observed in saline lake environments where evaporation forms brines. Mineral precipitation results from fluids that are characterized by a broad range of salinity and temperature conditions that are also observed on Mars (Barbieri and Stivaletta 2011).
Biosignature preservation is observed in thermal spring environments over a broad range of temperature, pH, and redox conditions (Campbell et al. 2015). Where springs deliver effluents rich in minerals (e.g., silica, carbonate, iron oxides, evaporites, and clays), organisms may be quickly entombed in the mineral deposits and preserved as a wide range of biosignature types, including cellular permineralization, millimeter- to centimeter-scale microfabrics, and microbially mediated mesoscale structures (including stromatolites) (Figure 4.2). Examples of potential hydrothermal spring deposits and evaporites and clay-rich mudstones have been identified on Mars by landed and surface missions, and an example, the “Home Plate” feature in the Columbia Hills, has been proposed as potential landing sites for Mars 2020 (Ruff and Farmer 2016).
Despite decades of investigation, numerous controversies and disagreements are a pervasive aspect of research into the fossil record of the early Earth. Perhaps the most prominent example concerns the biogenicity of microstructures found within the Apex Chert of the Pilbara Craton of Western Australia (Schopf 1993) where there has been continual questioning of the original interpretations (Brasier et al. 2002) and a series of responses that continue to the present day (Schopf and Kudryavtsev 2009; Schopf 2006; Schopf et al. 2018; Marshall et al. 2011). Given the lack of consensus, some of the oldest described microstructures fall into the categories of dubio-fossils (e.g., Wacey et al. 2016) or pseudo-fossils (Wacey et al. 2018). Other microstructures from Archean sediments from the Pilbara in Australia and Barberton in South Africa are suggested to represent biotic features (e.g., Hickman-Lewis et al. 2016). In all cases, the kerogenous structures and their encasing sediments were rapidly encapsulated in hydrothermal silica. The predominant type of microstructure identified comprises fabrics suggestive of biofilms and microbial mat fragments that contain traces of organic matter in the form of kerogen. The deformable nature of these films, their diaphanous, web-like structure, and their typical particle-trapping characteristics have been deemed difficult to produce by abiotic means. This point is balanced against the complex and uncertain setting of the cherty beds of the Apex Formation at Chinaman Creek, in which pseudo-fossils were detected (Brasier et al. 2011; Marshall et al. 2014; Hickman-Lewis et al. 2016; Westall et al. 2011, 2015a,b). Nanoscale technologies such as nanoSIMS and laser Raman microspectroscopy are proposed to provide solutions to the uncertainties about the biogenic origin of Archean microstructures (e.g., Delarue et al. 2017). There is a pressing need, however, for these to be rigorously tested on a range of materials and matrices, including well-characterized microscopic fossils, before application to controversial objects. Until then, the capabilities of these technologies require a certain level of skepticism.
The influences of post-burial processes on biosignature retention, under different conditions, are not well understood at the microscale or nanoscale, making it hard to generalize about the expected impacts on preservation bias. The deleterious effects of heating organic matter during burial or volcanism are relatively well understood, for example. Consistent with the relatively high degree of thermal metamorphism, there is no extractable organic matter preserved in Archean sediments known to have retained features that can be attributed to primary biological origins (French et al. 2015). Ancient amorphous kerogens, on the other hand, appear to be more promising archives for generating molecular biosignatures that could allow discrimination between biologically derived and abiogenic organic matter. Catalytic pyrolysis under a stream of high-pressure hydrogen (hydropyrolysis; Love et al. 1995) is one promising approach that has recently yielded evidence for the biological origin of some Archean kerogens (Duda et al. 2018). Organic matter in the 1.85 Ga old Gunflint Formation, which has experienced catagenetic temperatures of ~150°C to 170°C, still contains amide groups derived from protein compounds (Alleon et al. 2016). At a younger date, the 700 to 800 Ma old Draken Formation from Svarlbard (or Spitzbergen), which shows no sign of significant metamorphism, contains beautifully preserved carbonaceous cyanobacterial fossils with which are still associated biominerals, including pyrite, apatite, and metastable opal (Foucher and Westall 2013). The ~400 Ma old Rhynie Chert, on the other hand, is an example in which entombment in a subaerial

hydrothermal spring has led to preservation of some of the oldest terrestrial flora and the associated microbial community (Edwards et al. 2018). However, the deposit has been thermally metamorphosed to the extent that the plant remains are “coalified.” Although the silicified microbial and plant remains retain stunning morphological detail, and still contain organic matter comprising aromatic and aliphatic structures (Abbott et al. 2018), the heating regime has been so intense that no diagnostic molecular biosignatures can be confidently attributed to any specific component of the ecosystem.
Lessons from the terrestrial record can provide us with an indication of the range of organic preservation that could be detected on Mars, as well as the methods and potential difficulties involved in the analysis of the organic matter (e.g., Westall et al. 2015a; Alleon et al. 2016; Cabrol et al., 2018). Because Mars has been accumulating meteorite-borne organic matter (Flynn 1996; Benner et al. 2000), and because abiotic organic synthesis on early Mars cannot be ruled out, the presence of organic phases alone is insufficient to identify their origins. Additional textural, chemical, and isotopic indicators, as well as the contextual parameters of the system, are needed so that the potential for in situ abiotic or biotic synthesis can be evaluated. With respect to survivability, oxidative aqueous alteration and cosmic ray exposure are the primary factors over Mars’s history that are destructive to organic matter. The former is best mitigated by selecting samples for which the mineralogical and textural properties would have minimized or precluded fluid flow (Farmer and Des Marais 1999). The latter, oxidative radiation, can be mitigated through careful subsurface sampling protocols that might avoid these taphonomic effects (e.g., Gaboyer et al. 2017). New findings from the Curiosity rover have demonstrated that macromolecules, mineral interactions, and permeability reduction, which may have limited the exposure of organic compounds (including thiophenic, acomatic, and aliphatic compounds) recently to migrating fluids and gases, may all play a role in preservation of the organics recently reported for the 3.5-billion-year-old Murray Formation (Eigenbrode et al. 2018).
DETECTABILITY OF BIOSIGNATURES
Although biosignature preservation is an important consideration for molecular in situ biosignatures, “false negatives” can challenge remote-sensing and in situ biosignatures alike. False negatives occur when the environment in which the biosignature is produced or preserved is able to sufficiently suppress or overwhelm the biological signal so that it is undetectable. For remote-sensing biosignatures, the classic example of a false negative is the suppression of the rise of photosynthetically generated O2 in the early Earth atmosphere.
False Negatives and the Rise of O2
Oxygen is the best studied remote-sensing biosignature, with several false positives and their observational discriminants already identified (Meadows 2017; Schwieterman et al. 2018). Biosignatures are most robustly interpreted in the context of their environment, and the in-depth study of oxygen’s false positive and negative scenarios provides a framework for assessing future biosignature candidates (Harman et al. 2015; Meadows et al. 2018b; Catling et al. 2018). Constraints from stable isotopes and trace-element proxies indicate the evolution of oxygenic photosynthesis on Earth by at least ~3.0 Ga (Planavsky et al. 2014a), and perhaps much earlier (Rosing and Frei 2004). However, complementary isotopic constraints indicate that Earth’s atmosphere was pervasively reducing until ~2.5 Ga (Farquhar et al. 2001; Pavlov and Kasting 2002; Zahnle et al. 2006). There thus appears to have been a significant period on Earth during which oxygenic photosynthesis was present, but large amounts of O2 did not accumulate in Earth’s atmosphere. In addition, there is some evidence that after the initial accumulation of O2 in Earth’s atmosphere at ~2.3 Ga, atmospheric O2 levels remained relatively low for much of the subsequent ~2 billion years (Planavsky et al. 2014b; Cole et al. 2016; Tang et al. 2016). During this period, biogenic O2, though clearly present, may have been challenging to detect remotely given current technology (Reinhard et al. 2017).
These studies of the rise of Earth’s oxygen suggest that whether a planet develops a biogenic O2-rich atmosphere will depend on both the evolution of oxygenic photosynthesis as well as geochemical dynamics at the planetary surface that are favorable for the long-term accumulation of a large atmospheric O2 inventory (e.g., Gebauer et al. 2017). If planetary conditions are not favorable, then a false negative will occur. These dynamics will, in turn, depend on a series of planetary factors that may be challenging to constrain observationally or from
first principles. For example, heat flux from a planetary interior (as constrained by radiogenic element inventory and planet size), oxygen fugacity of the planetary mantle (as constrained by both initial chemistry and long-term recycling of materials from the surface), the degree of crustal differentiation (as constrained by both overall heat fluxes and planetary rheology), and ocean chemistry can interact to buffer atmospheric O2 to low levels, despite the presence of oxygenic photosynthesis. The ability to constrain these contextual variables via observations of the planet and star, or via modeling, may ultimately form a critical component of target selection for exoplanet biosignature searches and the diagnosis of false negatives for O2 on living planets. Similarly, they lay the foundation for critical thinking on the nature and use of contextual information for other proposed biosignatures.
False Negatives in Low-Energy Systems
Much of what we know about biosignatures is based on investigation of abundant and robust ecosystems, typically on Earth’s surface, in marine sediments, or in the relatively young (200 Ma) ocean floor subsurface (D’Hondt et al. 2009; Inagaki et al. 2015). Oligotrophic and/or electron-acceptor-limited marine sediments provide vital context about life metabolisms and rates in low-energy-flux systems (D’Hondt et al. 2009) inhabited by organisms with exceedingly slow growth rates (Trembath-Reichert et al. 2017). Additional information is increasingly being sought from other low-energy systems, including extant life in fracture-controlled systems in ancient crystalline rocks of millions to billions of years in age, where energy flux is not only low but intermittent in space and time due to processes of storage and interconnection between fractures (Lin et al. 2006; Sherwood Lollar et al. 2007, 2014). Fracture-controlled production of energy for chemolithotrophic life, and the rate of release of such energy due to fracture openings and propagation, are particularly relevant to mission planning for Mars (Onstott et al. 2006) and for ocean worlds such as Europa and Enceladus (Vance et al. 2016).
While Earth’s biological processes can provide robust signatures, if life is ephemeral in time or space, restricted to subsurface refugia or other oases, or existing under conditions of slow maintenance energy or even only as spores or other dormant or survival modes, biosignatures may be impossible to detect against the predominant abiotic baseline. The investigation of abiotic processes has sometimes been limited to the concept of establishing the abiotic (geological, chemical, physical) baseline or environmental context for biosignatures research. An emerging recent theme has been a more advanced approach that emphasizes identifying the spectrum of abiotic processes that mimic biosignatures, as well as those that may unambiguously identify abiotic processes (e.g., abiotic organic synthesis of methane or hydrocarbons). Energy flux is also a critical constraint on metabolic and biosynthetic rates as well as rates of abiotic destruction and attrition. Hence the abundance of molecules—the net result of their rates of production, accumulation, and transformation or destruction—is critical to whether or not biosignatures can accumulate to produce a detectable signature in an environment (Cabrol 2018; Cabrol et al. 2018). A classic example is the use of enantiometer excess (i.e., a preference of a system for the right- or left-handed versions of certain molecules), which may well be a biosignature, but which is consistently being interconverted abiotically to produce a more even mixture of right- and left-handed versions (a process known as racemization) and thereby erasing the signature of life. In an environment where energy flux is low, abiotic racemization may overwhelm the rate at which the biosignature is replenished by life (NASEM 2017). More generally, in oligotrophic environments, low rates of accumulation can mean the abundance of biosignatures (whether chemical, isotopic, mineralogical, or morphological) is insufficient to detect above a baseline of competing physical, chemical, or geological processes. The biological needle in an abiotic haystack will be difficult to identify and can lead to a false negative. Although less likely on Earth, some deep subsurface terrestrial habitats have been identified where such considerations are important (Sherwood Lollar et al. 2006; Moser et al. 2003), and certainly beyond Earth such scenarios may predominate.
Finding: Although suggestive of life and worthy of follow-on investigation, thermodynamic disequilibrium may result from a range of abiotic and biological processes and is therefore not always a biosignature.
Recommendation: NASA should support expanding biosignature research to address gaps in understanding biosignature preservation and the breadth of possible false positives and false negative signatures.
TOWARD A COMPREHENSIVE FRAMEWORK FOR INTEGRATION OF BIOSIGNATURES
While novel biosignature identification expands the field of search opportunities, a comprehensive framework for assessing biosignatures in the context of their environment, and the use of multiple lines of evidence, allows progressively increasing confidence in the ability to detect life. A rigorous understanding of the contextual setting provides clues that support or cast doubt on the authenticity of a biosignature, if they can be read and understood correctly. Since the 2015 Astrobiology Strategy (NASA 2015), there has been a growing realization of the importance of false positives, or abiosignatures—abiotic planetary processes that can mimic biosignatures, even for biosignatures like O2 that were previously believed to be “robust” and have no known abiotic means of production.
It has now become apparent that it is not enough to simply search for a biosignature, but an understanding of environmental processes that might result in false negatives and false positives needs to be developed to choose those environments most likely to express a robust biological signal. In some cases, a biosignature may be an enhanced abundance of an abiotic background, as discussed above, and so knowledge of the maximum feasible abiotic production of the signal also needs to be known. In addition, there is a better appreciation of the importance of combining multiple measurements to improve confidence that a given biosignature is indeed due to life. While, to a degree, the logical strategies to address such issues have been available for more than 20 years (e.g., from paleontologists contemplating how to deal with prokaryotic problematica; Hofmann 1972; Cloud 1973), these issues have been a major emerging theme in recent astrobiology discussions.
False Positives for Morphological Biosignatures
The issue of false positives is not a problem when dealing with morphologically complex multicellular organisms such as plants or animals with skeletons or biomineralization. It can be confounding, however, when the organisms comprise simple shapes like spheres or filaments with no distinct morphology or associated mineral. For example, the recent literature is replete with reports of potential biosignatures, however, a number do not ultimately withstand critical assessment (e.g., Bell et al. 2015; Djokic et al. 2017; Dodd et al. 2017; Nutman et al. 2016). The multitude of controversial biosignature reports detracts from widely accepted authentic records of Earth’s biota, and it often takes several years for critiques of problematica to appear, if they ever do. Some efforts have been made to systematically address the topic for the most ancient microscopic fossils using state-of-the-art microspectroscopic, microchemical, and imaging approaches (e.g., Westall et al. 2011; Wacey et al. 2012; Wacey 2014), but more research could be directed toward multidisciplinary approaches to discriminating between “positives” and “false positives” in all classes of biosignature.
Research into laboratory-based chemical systems (Garcia-Ruiz et al. 2002, 2003, 2009) and natural environments prone to production of microscopic objects that mimic microbes (pseudo-biosignatures) (Barge et al. 2015a,b, 2016) is an emerging field that will inform biosignature studies in general. Isotopic biosignatures and microfossils are two additional areas where incomplete knowledge or failure to recognize or understand existing work can lead to false positive findings. In the case of isotopic biosignatures, equilibrium isotopic fractionations can overlap with the kinetic fractionations induced by biological processes (Bottinga 1969; Galimov 2006). Further, kinetic fractionations associated with abiotic organic synthesis have been shown to produce fractionations of comparable scale to those produced by kinetically controlled biological processes (Horita and Berndt 1999; McCollom and Seewald 2006; Taran et al. 2007; McCollom et al. 2010). This insight has important implications for instance for resolving the origin of methane (Figure 4.3), as even on Earth, the traditional concept that biologically produced methane would be uniquely depleted in heavy carbon isotopes versus methane produced by abiotic processes has been refuted (review by Etiope and Sherwood Lollar 2013). Similarly, for both terran (Mathez et al. 1987) and meteorite studies (Steele et al. 2012), it has been demonstrated that even organic carbon with “light” carbon isotope values requires careful contextual investigation of the microstructure of minerals and fracture infillings between and across those mineral boundaries to determine the abiotic versus biotic nature of macromolecular carbon. Accordingly, abiotic explanations need to be excluded through detailed analysis of context before isotopic biosignatures are accepted as robust. Ideally, contextual evidence, including multiple and discrete biosignatures
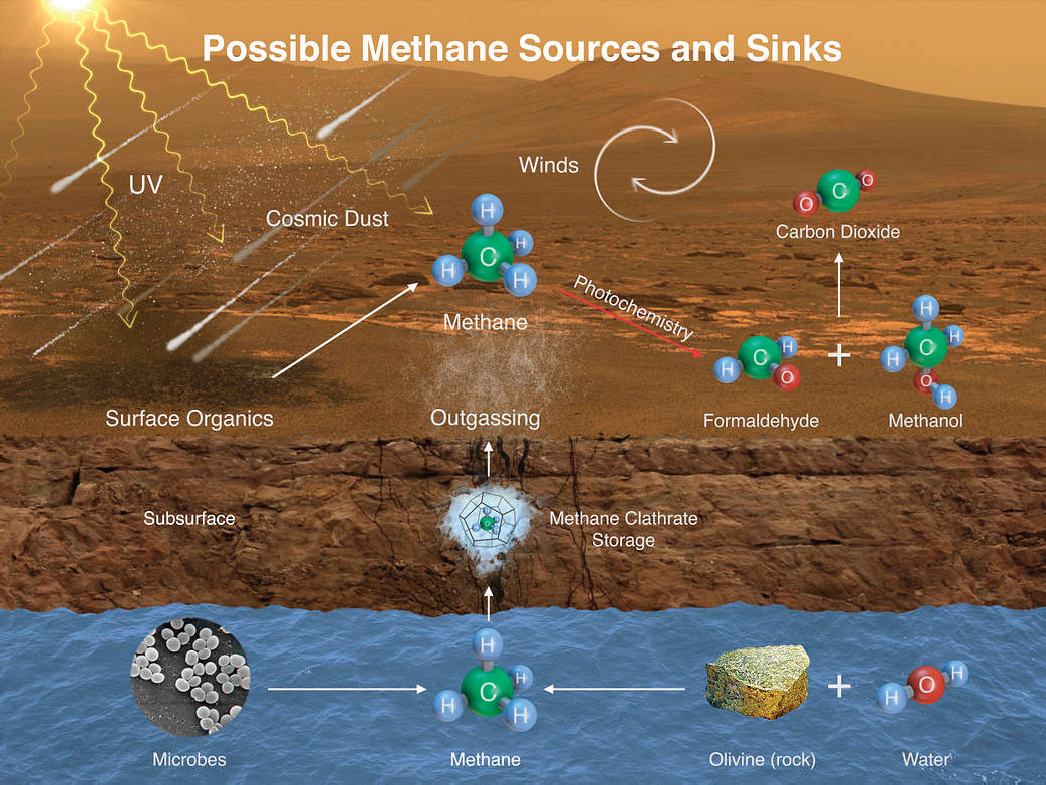
and the elimination of potential abiosignatures, would accompany claims for the “oldest life” on Earth or for life detection beyond Earth.
Finding: Reexamining controversial biosignatures from Earth’s early sedimentary rock record can provide an important test bed for biosignature assessment frameworks.
False Positives for the O2 Global Biosignature
Since publication of the 2015 Astrobiology Strategy, multiple research groups have discovered mechanisms that could produce abiotic O2 and O3 in an exoplanet’s atmosphere, especially in the atmospheres of those exoplanets orbiting M-dwarf stars. Each presents a potential false positive to different degrees. Two of the proposed mechanisms allow water to enter a planet’s stratosphere where it is photolyzed and the hydrogen atoms lost to space, resulting in O2 buildup in the planet’s upper atmosphere (Figure 4.4). Water entering the stratosphere could be enabled by loss of an ocean in a runaway greenhouse process (Luger and Barnes 2015)—a mechanism that is most effective for late-type (i.e., less massive) M-dwarf stars. Alternatively there could be a lack of noncondensable gases in the planetary atmosphere, which could affect planets orbiting stars of any spectral type (Wordsworth and Pierrehumbert 2014). The runaway mechanism could produce an O2-dominated atmosphere of hundreds of
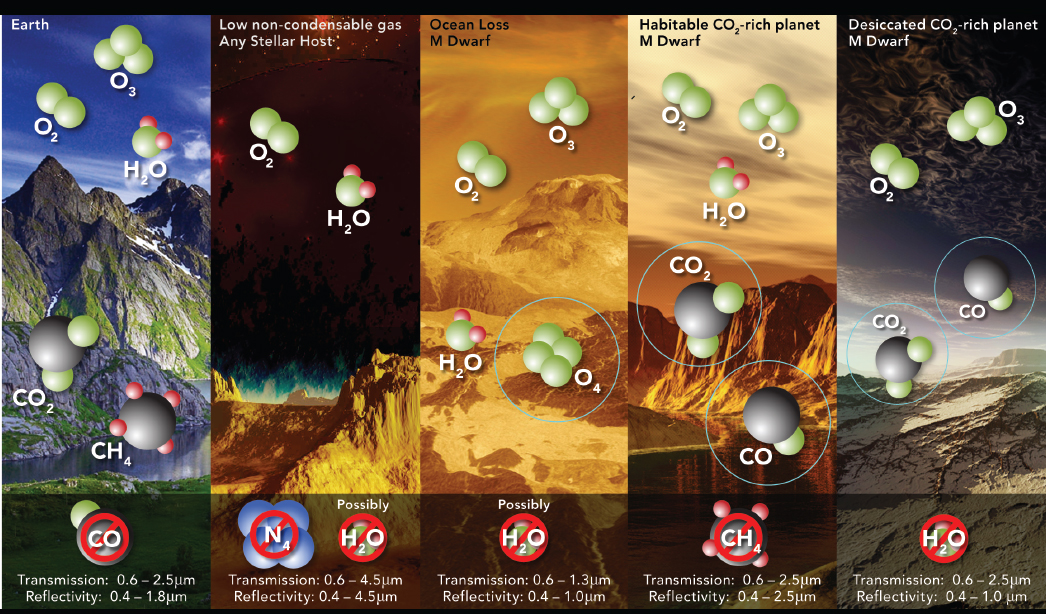
bars. The lack of noncondensable gases could potentially result in atmospheres that are ~15 percent O2. It has also been suggested that Earth-like quantities of O2 could be generated by the splitting of liquid water by a surface TiO2 photocatalyst (Narita et al. 2015).
The other major class of processes that build up abiotic O2 rely on the photolysis of CO2 and circumstances that inhibit CO2 recombination from CO and O2 (Hu et al. 2012; Tian et al. 2014; Harman et al. 2015; Gao et al. 2015). For photochemical production without atmospheric escape, O2 abundances as high as 0.2 to 6 percent are predicted, with higher values corresponding to little or no O2 sinks in the planetary environment. More realistic modeling of sinks can reduce these estimates by orders of magnitude (e.g., Domagal-Goldman et al. 2014; Harman et al. 2015). Finally, O3 may be considered a proxy for O2 in a planetary atmosphere, and large abundances of abiotic O3 may build up in the massive O2-rich atmospheres, possible after ocean loss (Meadows et al. 2018a), although in these cases large amounts of O4 will also be present. Domagal-Goldman et al. (2014) were not able to generate large abundances of O2 from CO2 photolysis for habitable planets orbiting M-dwarf stars, but did produce potentially detectable O3 column abundance as high as 10 percent of Earth’s modern abundance.
In most cases, the mechanism for abiotic production of O2 or O3 leaves a “tell,” an impact on the planetary environment that may be detectable. These indications can range from the presence of collision-induced absorption from O2 molecules that collide more frequently in dense, O2-rich post-ocean-loss atmospheres (Schwieterman et al. 2016; Meadows et al. 2018a), CO from the photolysis of CO2 (Schwieterman et al. 2016), lack of water vapor (Gao et al. 2015), lack of collisionally induced absorption from N2 (Schwieterman et al. 2015), and the absence of
reducing gases (Domagal-Goldman et al. 2014). This research has therefore identified several observations needed to search for O2 in a terrestrial planetary atmosphere and to discriminate whether that O2 is abiotic or biological in origin based on characteristics of the parent star and the planetary environment. By understanding false positive mechanisms and their discriminants, observing strategies are now being developed that incorporate biosignature detection as well as stellar characterization, searches for false positive discriminants, and other environmental characteristics that can be used to enhance the interpretation of the biosignature. Comparative planetology can help identify relevant physical and chemical processes in planetary environments that could lead to the generation of a biosignature false positive or contribute to a false negative result. A specific example would be the study of highly irradiated planets (e.g., exo-Venuses) such as GJ 1132b, a terrestrial-density planet that receives the equivalent of 19-times Earth’s solar radiation (Berta-Thompson et al. 2015). GJ 1132b could hold key information to understanding planetary processes that lead to ocean loss or to those that govern the fate of abiotic O2 generated by photolytic water loss from the planetary atmosphere. Similarly, studies of the influence of jovian planets on terrestrial water inventories, Venus catalytic chemistry (Mills et al. 2006), and the stability of CO2 photolysis on Mars-like bodies (Gao et al. 2015) all inform understanding of the likelihood and nature of potential false positives.
Finding: Characterizing the atmospheres and incident radiation fluxes for exoplanets of different sizes, compositions, and stellar irradiances is important for confident assessment of planetary habitability and biosignatures because it increases understanding of the physical and chemical processes that lead to false positives and negatives.
The Importance of Environmental Context and Multiple Lines of Evidence
Controversy enveloped the first reports of microbialites (stromatolites) deposited in rocks of the 3.49 billion-year-old Dresser Formation in the Pilbara Craton of Australia (Walter et al. 1980; Buick et al. 1981). These objects were claimed to represent Earth’s oldest signs of life until it was subsequently argued that chemical precipitation alone could explain the phenomena (Grotzinger and Rothman 1996). Further work revealed the compelling contextual association of more diverse, more widespread, and better-preserved examples of stromatolites in the ~3.45 Ga Strelley Pool Formation (Allwood et al. 2006). The depositional environment was a shallow shoreline where stromatolites have formed throughout most of Earth’s history and where they typically form today. Detailed work revealed organic layers that co-varied with stromatolite morphology, and systematic changes in their defining features with water depth (Allwood et al. 2006). Morphologic diversity correlated with evidence of changes in sedimentation, sea-floor mineral precipitation, and microbial mat development (Allwood et al. 2009). More recently, sulfur and carbon isotopic biosignatures (Bontognali et al. 2012; Flannery et al. 2018) and diverse microscopic fossils and carbonaceous objects (Sugitani at al. 2015) have added to the body of independent but complementary lines of evidence for the robustness of the Strelley Pool Formation biosignatures. Further contextual data resulted in the widespread acceptance of the Dresser Formation stromatolites as biosignatures as well (Van Kranendonk et al. 2003, 2008).
As a counter-example, filamentous objects, including haematite tubes and filaments, found in association with 3.8 billion-year-old seafloor hydrothermal vent precipitates have been proposed to be fossil remains of iron-oxidizing bacteria (Dodd et al. 2017). One particular type of bacterium identified in this study, Leptothrix sp., is a strictly aerobic iron oxidizing taxon. Not only is there no robust evidence for oxygenic photosynthesis 3.8 billion years ago, numerous geochemical proxies show that the deep ocean seafloor itself remained oxygen free until at least the Neoproterozoic Era (Lyons et al. 2014). Full ventilation was, perhaps, an even more recent phenomenon (Reinhard et al. 2013; Stolper and Keller 2018). Thus, the context of the putative fossil biosignature seems incompatible with the physiology of iron-oxidizing bacteria.
Modeling cross-comparisons and multiple lines of evidence are also extremely valuable to produce a more robust prediction for the likelihood of abiotic production of O2 as a false positive for the detection of photosynthesis. There have been disagreements in recent literature about when false positives from the photolysis of CO2 might occur. On the basis of photochemical model calculations, Hu et al. (2012) concluded that CO2-dominated worlds around Sun-like stars might build up oxygen if the outgassing rates of reduced gases are small, but Segura
et al. (2007) and Harman et al. (2015) did not find this. Disagreements on the abiotic production of O2 on CO2rich planets orbiting M-dwarf stars (Tian et al. 2014; Harman et al. 2015; Domagal-Goldman et al. 2014) have been recently resolved via model crosscomparison. Confidence in the outcomes of models of planetary processes potentially producing false positives or negatives can be greatly enhanced by intercomparison of modeling methods and results. This is especially true as advances are made in identifying mechanisms potentially generating false positives for O2 and other proposed biosignatures. The intercomparison of models is, for example, a standard practice in the climate modeling community. This work could also extend to one- and three-dimensional model comparisons to understand spatial distribution and dynamical mixing of photochemical products.
Developing a Comprehensive Framework for Biosignature Interpretation
The last 5 years have brought a rapid evolution in understanding the complexity of biosignature interpretation and an impetus for the design of more comprehensive search techniques and more rigorous standards of proof. Biosignatures, rather than isolated, specific phenomena, are now understood to occur in an environmental context in which geological, atmospheric, and stellar processes and interactions, along with the evolution of the environment, may work to enhance, suppress, or mimic biosignatures. Consequently, the interpretation of the significance of the potential biosignature, in addition to its measurement, is the most important process in life detection.
Steps toward engaging the community in the development of several systematic, progressively comprehensive frameworks are being undertaken. One example is the NASA Ladder of Life Detection (Neveu et al. 2018), which provides a community accessible spreadsheet to identify and discuss proposed in situ biosignatures and their potential false negatives and positives. The ultimate goal of the Ladder of Life Detection is to help identify sets of measurements that together discriminate between a biotic or abiotic origin for potential biosignatures with high statistical significance. For example, formalisms such as ROC (receiver-operator characteristic) curves can provide a quantitative foundation for the selection of which biosignatures to pursue in a particular setting.
An ROC curve is a classification model that is used to set threshold values for predicting outcomes, taking into account the probabilities of both false positives and false negatives. First applied to the detection of radar signals, this approach is now widely used in areas as diverse as clinical diagnostics, weather prediction, and machine learning. Such formalisms bring mathematical rigor to the search for life, but they require support by test measurements made under simulated operational conditions. It will be the task of the scientific community to make the relevant measurements of true and false biosignatures and to determine how these measurements can be combined to provide a coherent predictive model.
Recommendation: NASA should direct the community’s focus to address important gaps in understanding the breadth, probability, and distinguishing environmental contexts of abiotic phenomena that mimic biosignatures.
An analogous activity has been undertaken for exoplanet biosignatures, mediated by the community discussions at the 2016 NExSS Exoplanet Biosignatures Workshop. The template for this framework has been developed for O2, taking into account false negatives and their impact on target selection, and false positives and the observing strategy required to discriminate them from true biosignatures (Meadows et al. 2018b). Generalizing this template, Catling et al. (2018) presented a framework for biosignature assessment that allows the detection of life to be expressed as a probability. This framework uses Exo-Earth System models to simulate potential biosignature observations which are then compared with actual observations to determine the Bayesian likelihood of those data occurring for scenarios with and without life (false positives).
The Bayesian methodology provides a medium to quantitatively define conditional probabilities and confidence levels for biosignature detection. However, it also requires interdisciplinary laboratory, field, and theoretical work to place constraints on relevant likelihoods, including those emerging from stellar and planetary context, and the contingencies of evolutionary history. The Bayesian framework can also guide search strategies, including determining observational wavelengths or deciding between targeted searches or larger, lower-resolution surveys, while providing a flexible framework that is not constrained to specific metabolisms or biosignatures (Walker et al. 2018).
Recommendation: NASA should support the community in developing a comprehensive framework for assessment—including the potential for abiosignatures, false positives, and false negatives—to guide testing and evaluation of in situ and remote biosignatures.
REFERENCES
Abbott, G.D., I.W. Fletcher, S. Tardio, and E. Hack. 2018. Exploring the geochemical distribution of organic carbon in early land planets: A novel approach. Philosophical Transactions of the Royal Society B 373(1739).
Al Ouahabi, A., J.-A. Amalian, L. Charles, and J.-F. Lutz. 2017. Mass spectrometry sequencing of long digital polymers facilitated by programmed inter-byte fragmentation. Nature Communications 8:967.
Alleon, J., S. Bernard, C. Le Guilluo, J. Marin-Carbonne, S. Pont, O. Beyssac, K.D. McKeegan, and F. Robert. 2016. Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and mineralogy. Nature Communications 7:11977.
Allwood, A.C., J.P. Grotzinger, A.H. Knoll, I.W. Burch, M.S. Anderson, M.L. Coleman, and I. Kanik. 2009. Controls on development and diversity of Early Archean stromatolites. Proceedings of the National Academy of Sciences U.S.A. 106(24):9548-9555.
Allwood, A.C., M.R. Walter, B.S. Kamber, C.P. Marshall, and I.W. Burch. 2006. Stromatolite reef from the Early Archaean era of Australia. Nature 441:714-718.
Anantharaman, K., M.B. Duhaime, J.A. Breier, K.A. Wendt, B.M. Toner, and G.J. Dick. 2014. Sulfur oxidation genes in diverse deep-sea viruses. Science 344:757-760.
Arevalo, R., W. Brinkerhoff, F. van Amerom, R. Danell, V. Pinnick, X. Li, S. Getty et al. 2015. Design and demonstration of the Mars Organic Molecule Analyzer (MOMA) on the ExoMars 2018 Rover. Pp. 1-11 in 2015 IEEE Aerospace Conference. doi:10.1109/AERO.2015.7119073.
Arney, G.N., S. D. Domagal-Goldman, and V.S. Meadows. 2018. Organic haze as a biosignature in anoxic Earth-like atmospheres. Astrobiology 18:311-329.
Barbieri, R., and N. Stivaletta. 2011. Continental evaporites and the search for evidence of life on Mars. Geological Journal 46(6):513-524.
Barge, L.M., S.S.S. Cardoso, J.H.E. Cartwright, G.J.T. Cooper, L. Cronin, A. de Wit, I.J. Doloboff, et al. 2015a. From chemical gardens to chemobrionics. Chemical Reviews 115(16):8652-8703.
Barge, L.M., S.S.S. Cardoso, J.H.E. Cartwright, I.J. Doloboff, E. Flores, E. Macías-Sánchez, C.I. Sainz-Díaz, and P. Sobrón. 2016. Self-assembling iron oxyhydroxide/oxide tubular structures: Laboratory-grown and field examples from Rio Tinto. Proceedings of the Royal Society A 472(2195).
Barge, L.M., Y. Abedian, I.J. Doloboff, J.E. Nuñez, M.J. Russell, R. Kidd, and I. Kanik. 2015b. Chemical gardens as flow-through reactors simulating natural hydrothermal systems. Journal of Visualized Experiments 105:e53015.
Bedrossian, M., C. Lindersmith, and J.L. Nadeau. 2017. Digital holographic microscopy, a method for detection of microorganisms in plume samples from Enceladus and other icy worlds. Astrobiology 17(9):913-925.
Bell, E.A., P. Boehnke, T.M. Harrison, and W.L. Mao. 2015. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proceedings of the National Academy of Sciences U.S.A. 112(47):14518-14521.
Bell, P.J.L. 2009. The viral eukaryogenesis hypothesis: A key role for viruses in the emergence of eukaryotes from a prokaryotic world environment. Annals of the New York Academy of Sciences 1178:91-105.
Benner, S.A. 2017. Detecting Darwinism from molecules in the Enceladus plumes, Jupiter’s moons, and other planetary water lagoons. Astrobiology 17(9):840-851.
Benner, S.A., K.G. Devine, L.N. Matveeve, and D.H. Powell. 2000. The missing organic molecules on Mars. Proceedings of the National Academy of Sciences U.S.A. 97(6):2425-2430.
Berliner, A.J., T. Mochizuki, and K.M. Stedman. 2018. Astrovirology: Viruses at large in the universe. Astrobiology 18(2):1-17.
Berta-Thompson, Z.K., J. Irwin, D. Charbonneau, E.R. Newton, J.A. Dittmann, N. Astudillo-Defru, X. Bonfils, et al. 2015. A rocky planet transiting a nearby low-mass star. Nature 527:204-207.
Bidle, K.D., and P.G. Falkowski. 2004. Cell death in planktonic, photosynthetic microorganisms. Nature Reviews Microbiology 2:643-655.
Biemann, K., J. Oro, P. Toulmin III, L.E. Orgel, A.O. Nier, D.M. Anderson, P.G. Simmonds, et al. 1977. The search for organic substances and inorganic volatile compounds in the surface of Mars. Journal of Geophysical Research 82(28):4641-4658.
Bontognali, T.R.R., A.L. Sessions, A.C. Allwood, W.W. Fischer, J.P. Grotzinger, R.E. Summons, and J.M. Eiler. 2012. Sulfur isotopes of organic matter preserved in 3.450 billion-year-old stromatolites reveal microbial metabolism. Proceedings of the National Academy of Sciences U.S.A. 109(38):15146-15151.
Bottinga, Y. 1969. Calculated fractionation factors for carbon and hydrogen isotope exchange in the system calcite-carbon dioxide-graphite-methane-hydrogen-water vapor. Geochimica et Cosmochimica Acta 33(1):49-64.
Brasier, M.D., O.R. Green, A.P. Jephcoat, A.K. Kleppe, M.J. Van Kranendonk, J.F. Lindsay, A. Steele, and N.V. Grassineau. 2002. Questioning the evidence for Earth’s oldest fossils. Nature 416:76-81.
Brasier, M.D., O.R. Green, J.F. Lindsay, N. McLoughlin, C.A. Stoakes, A.T. Brasier, and D. Wacey. 2011. Earth’s Oldest Putative Fossil Assemblage from the ~3.5 Ga Apex Chert, Chinaman Creek, Western Australia: A Field and Petrographic Guide. 7th edition. Geological Survey of Western Australia, Perth, Australia.
Buick, R., J.S.R. Dunlop, and D.I. Groves. 1981. Stromatolite recognition in ancient rocks: An appraisal of irregularly laminated structures in an Early Archean chert-barite unit from North Pole, Western Australia. Alcheringa 5(3):161-181.
Cabrol, N.A. 2016. Alien mindscapes—A perspective on the search for extraterrestrial intelligence. Astrobiology 16(9):661-676.
Cabrol, N.S. 2018. The coevolution of life and environment on Mars: An ecosystem perspective on the robotic exploration of biosignatures. Astrobiology 18. doi:10.1089/ast.2017.1756.
Cabrol, N.A., J. Bishop, S.L. Cady, N. Hinman, J. Moersch, N. Noffke, C. Phillips, et al. 2018. “Advancing Astrobiology Through Public/Private Partnerships: The FDL Model.” White paper submitted to the Committee on an Astrobiology Science Strategy for the Search for Life in the Universe.
Campbell, K.A., B.Y. Lynee, K.M. Handley, S. Jordan, J.D. Farmer, D.M. Guido, F. Foucher, S. Turner, and R.S. Perry. 2015. Tracing biosignature preservation of geothermally silicified microbial textures into the geological record. Astrobiology 15(10):858-882.
Carr, E.C., A. Mojarro, J. Tani, S.A. Bhuttaru, M.T. Zuber, R.Doebler, M. Brown, et al. 2016. Advancing the search for extraterrestrial genomes. 2016 IEEE Aerospace Conference. doi:10.1109/AERO.2016.7500859.
Catling, D.C., J. Krissansen-Totton, N.Y. Kiang, D. Crisp, T.D. Robinson, S. DasSarma, A. Rushby, et al. 2018. Exoplanet biosignatures: A framework for their assessment. Astrobiology 18(6):709-738.
Chyba, C.F., and C.B. Phillips. 2001. Possible ecosystems and the search for life on Europa. Proceedings of the National Academy of Sciences U.S.A. 98(3):801-804.
Cloud, P. 1973. Pseudofossils: A plea for caution. Geology 1(3):123-127.
Cole, D.B., C.T. Reinhard, X. Wang, B. Gueguen, G.P. Halverson, T. Gibson, M.S.W. Hodgskiss, et al. 2016. A shale-hosted Cr isotope record of low atmospheric oxygen during the Proterozoic. Geology 44(7):555-558.
Cronin, L., and S.I. Walker. 2016. Beyond prebiotic chemistry. Science 352(6290):1174-1175.
D’Hondt, S., A.J. Spivack, R. Pockalny, T.G. Ferdelman, J.P. Fischer, J. Kallmeyer, L.J. Abrams, et al. 2009. Subseafloor sedimentary life in the South Pacific Gyre. Proceedings of the National Academy of Sciences U.S.A. 106(28):11651-11656.
Danovaro, R., A. Dell’Anno, C. Corinaldesi, M. Magagnini, R. Noble, C. Tamburini, and M. Weinbauer. 2008. Major viral impact on the functioning of benthic deep-sea ecosystems. Nature 454:1084-1087.
Delarue, F., F. Robert, K. Sugitani, R. Tartese, R. Duhamel, and S. Derenne. 2017. Investigation of the geochemical preservation of ca. 3.0 Ga permineralized and encapsulated microfossils by nanoscale secondary ion mass spectrometry. Astrobiology 17(12):1192-1202.
Des Marais, D.J., J.A. Nuth, L.J. Allamandola, A.P. Boss, J.D. Farmer, T.M. Hoehler, B.M. Jakosky, V.S. Meadows, A. Pohorille, B. Runnegar, and A.M. Spormann. 2008. The NASA Astrobiology Roadmap. Astrobiology 8:715-730.
Des Marais, D.J., L.J. Allamandola., S.A. Benner, A.P. Boss, D. Deamer, P.G. Falkowski, J.D. Farmer, et al. 2003. The NASA Astrobiology Roadmap. Astrobiology 3(2):219-235.
Djokic, T., M.J. Van Kranendonk, K.A. Campbell, M.R. Walter, and C.R. Ward. 2017. Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nature Communications 8:15263.
Dodd, M.S., D. Papineau, T. Grenne, J.F. Slack, M. Rittner, F. Pirajno, J. O’Neil, and C.T.S. Little. 2017. Evidence for early life on Earth’s oldest hydrothermal vent precipitates. Nature 543:60-64.
Domagal-Goldman, S.D., A. Segura, M.W. Claire, T.D. Robinson, and V.S. Meadows. 2014. Abiotic ozone and oxygen in atmospheres similar to prebiotic Earth. The Astrophysical Journal 792(2):90.
Duda, J.-P., V. Thiel, T. Bauersachs, H. Mißbach, M. Reinhardt, N. Schäfer, M.J. Van Kranendonk, and J. Reitner. 2018. Ideas and persepectives: Hydrothermally driven redistribution and sequestration of early Archean biomass—The “hydrothermal pump hypothesis.” Biogeosciences 15(5):1535-1548.
Edwards, D., P. Kenrick, and L. Dolan. 2018. History and contemporary significance of the Rhynie cherts—Our earliest preserved terrestrial ecosystem. Philosophical Transactions of the Royal Society B 373(1739).
Ehlmann, B.L., and C.S. Edwards. 2014. Mineralogy of the martian surface. Annual Review of Earth and Planetary Science 42:291-315.
Ehlmann, B.L., F.S. Anderson, J. Andrews-Hanna, D.C. Catling, P.R. Christensen, B.A. Cohen, C.D. Dressing, et al. 2016. The sustainability of habitability on terrestrial planets: Insights, questions, and needed measurements from Mars for understanding the evolution of Earth-like worlds. Journal of Geophysical Research 121(10):1927-1961.
Eigenbrode, J.L., R.E. Summons, A. Steele, C. Freissinet, M. Millan, R. Navaroo-Gonzálex, B. Sutter, et al. 2018. Organic matter preserved in 3-billion-year-old mudstones at Gale crater, Mars. Science 360(6393):1096-1101.
Etiope, G., and B. Sherwood Lollar. 2013. Abiotic methane on Earth. Reviews of Geophysics 51(2):276-299.
Farmer, J.D., and D.J. Des Marais. 1999. Exploring for a record of ancient martian life. Journal of Geophysical Research 104(E11):26977-26995.
Farquhar, J., J. Savarino, S. Airieau, and M.H. Thiemens. 2001. Observation of wavelength-sensitive mass-independent sulfur isotope effects during SO2 photolysis: Implications for the early atmosphere. Journal of Geophysical Research 106(E12):32829-32839.
Flannery, D.T., A.C. Allwood, R.E. Summons, K.H. Williford, W. Abbey, E.D. Matys, and N. Ferralis. 2018. Spatially-resolved isotopic study of carbon trapped in ~3.43 Ga Strelley Pool Formation stromatolites. Geochimica et Cosmochimica Acta 223:21-35.
Flynn, G.J. 1996. The delivery of organic matter from asteroids and comets to the early surface of Mars. Earth, Moon, and Planets 72(1-3):469-474.
Forterre, P. 2005. The two ages of the RNA world, and the transition to the DNA world: A story of viruses and cells. Biochimie 87:793-803.
Forterre, P., and D. Prangishvili. 2009. The great billion-year war between ribosome- and capsid-encoding organisms (cells and viruses) as the major source of evolutionary novelties. Annals of the New York Academy of Sciences 1178:65-77.
Foucher, F., and F. Westall. 2013. Raman imaging of metastable opal in carbonaceous microfossils of the 700-800 Ma old Draken Formation. Astrobiology 13(1):57-67.
French, K.L., C. Hallmann, H.M. Hope, P.L. Schoon, J.A. Zumberge, Y. Hoshino, C.A. Peters, et al. 2015. Reappraisal of hydrocarbon biomarkers in Archean rocks. Proceedings of the National Academy of Sciences U.S.A. 112:5915-5920.
Fujii, Y., D. Angerhausen, R. Deitrick, S. Domagal-Goldman, J.L. Grenfell, Y. Hori, S.R. Kane, et al. 2018. Exoplanet biosignatures: Observational prospects. Astrobiology 18(6):739-778.
Gaboyer, F., C. Le Milbeau, M. Bohmeier, P. Schwendner, T. Vannier, K. Beblo-Vranesevic, E. Rabbow, et al. 2017. Mineralization and preservation of an extremotolerant bacterium isolated from an early Mars analog environment. Scientific Reports 7:8775.
Galimov, E.M. 2006. Isotope organic geochemistry. Organic Geochemistry 37(10):1200-1262.
Gao, P., R. Hu, R.D. Robinson, C. Li, and Y.L. Yung. 2015. Stability of CO2 atmospheres on desiccated M dwarf exoplanets. The Astrophysical Journal 806(2):249.
Garcia-Ruiz, J.M., A. Carnerup, A.G. Christy, N.J. Welham, and S.T. Hyde. 2002. Morphology: An ambiguous indicator of biogenicity. Astrobiology 2(3):353-369.
Garcia-Ruiz, J.M., E. Melero-Carcia, and S.T. Hyde. 2009. Morphogenesis of self-assembled nanocrystalline materials of barium carbonate and silica. Science 323(5912):362-365.
Garcia-Ruiz, J.M., S.T. Hyde, A.M. Carnerup, A.G. Christy, M.J. Van Kranendonk, and N.J. Welham. 2003. Self-assembled silica-carbonated structures and detection of ancient microfossils. Science 302(5648):1194-1197.
Gebauer, S., J.L. Grenfell, J.W. Stock, R. Lehmann, M. Godolt, P. von Paris, and H. Rauer. 2017. Evolution of Earth-like extrasolar planetary atmospheres: assessing the atmospheres and biosphere of early Earth analog planets with a coupled atmosphere biogeochemical model. Astrobiology 17(1):27-54.
Goodwin, A.M. 1996. Principles of Precambrian Geology. Academic Press, London, UK.
Goodwin, S., A.M. Gade, M. Byrom, B. Herrera, C. Spears, E.V. Anslyn, and A.D. Ellington. 2015. Next-generation sequencing as input for chemometrics in differential sensing routines. Angewandte Chemie 127:6437-6440.
Goudge, T. A., C.I. Fassett, J.W. Head, J.F. Mustard, and K.L. Aureli. 2016. Insights into surface runoff on Mars from paleolake basin morphology and stratigraphy. Geology 44(6):419-422.
Grotzinger, J.P., and D.H. Rothman. 1996. An abiotic model for stramatolite morphogenesis. Nature 383:423-425.
Grotzinger, J.P., S. Gupta, M.C. Malin, D.M. Rubin, J. Scheiber, K. Siebach, D.Y. Summer, et al. 2015. Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars. Science 350(6257):acc7575.
Ha, H.K., Y.H. Kim, H.J. Lee, B. Hwang, and H.M. Joo. 2015. Under-ice measurements of suspended particulate matters using ADCP and LISST-Holo. Ocean Science Journal 50(1):97-108.
Hand, K.P., A.E. Murray, J.B. Garvin, W.B. Brinckerhoff, B. Christner, K.E. Edgett, B. Ehlmann, et al. 2018. “Astrobiological Potential of the Europa Lander Mission Concept.” White paper submitted to the Committee on an Astrobiology Science Strategy for the Search for Life in the Universe.
Harman, C.E., E.W. Schwieterman, J.C. Schottelkotte, and J.F. Kasting. 2015. Abiotic O2 levels on planets around F, G, K, and M stars: Possible false positives for life? The Astrophysical Journal 812(2):137.
Hickman-Lewis, K., R.J. Garwood, M.D. Brasier, T. Goral, H. Jiang, N. McLoughlin, and D. Wacey. 2016. Carbonaceous microstructures from sedimentary laminated chert within the 3.46 Ga Apex Basalt, Chinaman Creek locality, Pilbara, Western Australia. Precambrian Research 278:161-178.
Hitchcock, D. R., and J.E. Lovelock. 1967. Life detection by atmospheric analysis. Icarus 7(1-3):149-159.
Hofmann, H.J. 1972. Precambrian remains in Canada: Fossils, dubiofossils, and pseudofossils. Pp. 20-30 in Geological Proceedings of the 24th International Geological Congress. International Geological Congress, Montreal, Quebec, Canada.
Hofmann, H.J. 1976. Precambrian microflora, Belcher Islands, Canada: Significance and systematics. Journal of Paleontology 50(6):1040-1073.
Holland, G., B. Sherwood Lollar, L. Li, G. Lacrampe-Couloume, G.F. Slater, and C.J. Ballentine. 2013. Deep fracture fluids isolated in the crust since the Precambrian era. Nature 497:357-360.
Horita, J., and M.E. Berndt. 1999. Abiogenic methane formation and isotopic fractionation under hydrothermal conditions. Science 285:1055-1057.
Hu, R., S. Seager, and W. Bains. 2012. Photochemistry in terrestrial exoplanet atmospheres. I. Photochemistry model and benchmark cases. The Astrophysical Journal 761(2):166.
Inagaki, F., K.U. Hinrichs, Y. Kubo, M.W. Bowles, V.B. Heuer, W.L. Hong, T. Hoshino, et al. 2015. Exploring deep microbial life in coal-bearing sediment down to ~2.5 km below the ocean floor. Science 349(6246):420-424.
Javaux, E.J., C.P. Marshall, and A. Bekker. 2010. Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 463(7283):934-938.
Johnson, S.S. 2018. “Agnostic Approaches to Life Detection.” Presentation to the Committee on An Astrobiology Science Strategy for Search for Life in the Universe, Irvine, CA, January 18.
Johnson, S.S., E.V. Anslyn, H.V. Graham, P.R. Mahaffy, and A.D. Ellington. 2018a. Fingerprinting Non-Terran Biosignatures. Astrobiology 18(7):915-922.
Johnson, S.S., H. Graham, E. Anslyn, P. Conrad, L. Cronin, A. Ellington, J. Elsila, et al. 2018b. “Agnostic Biosignatures: Towards a More Inclusive Life Detection Strategy.” White paper submitted to the Committee on an Astrobiology Science Strategy for the Search for Life in the Universe.
Junge, K., C. Krembs, J. Deming, A. Stierle, and H. Eicken. 2001. A microscopic approach to investigate bacteria under in situ conditions in sea-ice samples. Pp. 304-310 in Annals of Glaciology Volume 33: Selected Papers of the International Symposium on Sea Ice and Its Interactions with Ocean, Atmosphere, and Biosphere (M.O. Jeffries and H. Eicken, eds.). International Glaciological Society, Fairbanks, AK.
Keefe, A.D., S. Pai, and A. Ellington. 2010. Aptamers as therapeutics. Nature Reviews Drug Discovery 9:537-550.
Kiang, N.Y., S. Domagal-Goldman, M.N. Parenteau, D.C. Catling, Y. Fujii, V.S. Meadows, E.W. Schwieterman, and S.I. Walker. 2018. Exoplanet biosignatures: At the dawn of a new era of planetary observations. Astrobiology 18(6):619-629.
Klein, F., S.E. Humphris, W. Guo, F. Schubotz, E.M. Schwarzenbach, and W.D. Orsi. 2015. Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia margin. Proceedings of the National Academy of Sciences U.S.A. 112:12036-12041.
Klein, H.P. 1978. The Viking biological experiments on Mars. Icarus 34(3):666-674.
Knoll, A.H. 2003. Life on a Young Planet: The First Three Billion Years of Evolution on Earth. Princeton University Press, Princeton, NJ.
Knoll, A.H. 2012. The fossil record of microbial life. Pp. 297-314 in Fundamentals of Geobiology (A.H. Knoll, D.E. Canfield, and K.O. Konhauser, eds.). Wiley-Blackwell, Chichester.
Knoll, E.H., and S. Golubic. 1979. Anatomy and taphonomy of a Precambrian algal stromatolite. Precambrian Research 10(1-2):115-151.
Krissansen-Totton, J., D.S. Bergsman, and D.C. Catling. 2016. On detecting biospheres from chemical thermodynamic disequilibrium in planetary atmospheres. Astrobiology 16(1):39-67.
Krissansen-Totton, J., S. Olson, and D.C. Catling. 2018. Disequilibrium biosignatures over Earth history and implications for detecting exoplanet life. Science Advances 4(1):eaao5747.
Li, L., B.A. Wing, T.H. Bui, J.M. McDermott, G.F. Slater, S. Wei, G. Lacrampe-Couloume, and B. Sherwood Lollar. 2016. Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks. Nature Communications 7:13252.
Lin, L.-H., P.-L. Wang, D. Rumble, J. Lippmann-Pipke, E. Boice, L.M. Pratt, B. Sherwood Lollar, et al. 2006. Long-term sustainability of a high-energy, low-diversity crustal biome. Science 314(5798):479-482.
Love, G.D., C.E. Snape, A.D. Carr, and R.C. Houghton. 1995. Release of covalently-bound alkane biomarkers in high yields from kerogen via catalytic hydropyrolysis. Organic Geochemistry 23(10):981-986.
Lovelock, J. E. 1965. A physical basis for life detection experiments. Nature 207(4997):568-570.
Lovelock, J.E. 1975. Thermodynamics and the recognition of alien biospheres. Proceedings of the Royal Society B 189(1095):167-181.
Luger, R., and R. Barnes. 2015. Extreme water loss and abiotic O2 buildup on planets throughout the habitable zones of M dwarfs. Astrobiology 15(2):119-143.
Lyons, T.W., C.T. Reinhard, and N.J. Planavsky. 2014. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506(7488):307-315.
Marshall S.M., A.R.G. Murray, and L. Cronin. 2017. A probabilistic framework for identifying biosignatures using pathway complexity. Philosophical Transactions of the Royal Society A 375.
Marshall, A.O., J. Jehlicka, J.-N. Rouzaud, and C.P. Marshall. 2014. Multiple generations of carbonaceous material deposited in Apex chert by basin-scale pervasive hydrothermal fluid flow. Gondwana Research 25(1):284-289.
Marshall, C.P., J.R. Embry, and A.O. Marshall. 2011. Haematite pseudomicrofossils present in the 3.5-billion-year-old Apex Chert. Nature Geoscience 4:240-243.
Mathez, E.A. 1987. Carbonaceous matter in mantle xenoliths: Composition and relevance to the isotopes. Geochemica et Chosmochemica Acta 51:2339-2347.
McCollom, T.M, B. Sherwood Lollar, G. Lacrampe-Couloume, and J.S. Seewald. 2010. The influence of carbon source on abiotic organic synthesis and carbon isotope fractionation under hydrothermal conditions. Geochimica et Cosmochimica Acta 74(9):2717-2740.
McCollom, T.M., and J.S. Seewald. 2006. Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal conditions. Earth and Planetary Science Letters 243:74-84.
Meadows, V.S. 2017. Reflections on O2 as a biosignature in exoplanetary atmospheres. Astrobiology 17:1022-1052.
Meadows, V.S., G.N. Arney, E.W. Schweiterman, J. Lustig-Yaeger, A.P. Lincowski, T. Robinson, S.D. Domagal-Goldman, et al. 2018a. The habitability of Proxima Centauri b: Environmental states and observational discriminants. Astrobiology 18(2):133-189.
Meadows, V.S., C.T. Reinhard, G.N. Arney, M.N. Parenteau, E.W. Schwieterman, S.D. Domagal-Goldman, A. Lincowski, et al. 2018b. Exoplanet biosignatures: Understanding oxygen as a biosignature in the context of its environment. Astrobiology 18(6):630-662.
Mills, F.P., M. Sundaran, T.G. Slanger, M. Allen, and Y.L. Yung. 2006. Oxygen chemistry in the Venus middle atmosphere. Pp. 109-117 in Advances in Geosciences: Volume 3: Planetary Science (PS) (A. Bhardwaj, ed.). World Scientific Publishing, Singapore.
Moser, D.P., T.C. Onstott, J.K. Fredrickson, F.J. Brockman, D.L. Balkwill, G.R. Drake, S. Pfiffner, et al. 2003. Temporal shifts in the geochemistry and microbial community structure of an ultradeep mine borehole following isolation. Geomicrobiology Journal 20:517-548.
Mustard, J.F., M. Adler, A. Allwood, D.S. Bass, D.W. Beaty, J.F. Bell III, W.B. Brinckerhoff, et al. 2013. Report of the Mars 2020 Science Definition Team. Mars Exploration Program Analysis Group (MEPAG). July. http://mepag.jpl.nasa.gov/reports/MEP/Mars_2020_SDT_Report_Final.pdf.
Narita, N., T. Enomoto, S. Masaoka, and N. Kusakabe. 2015. Titania may produce abiotic oxygen atmospheres on habitable exoplanets. Scientific Reports 5:13977.
NASA (National Aeronautics and Space Administration). 2015. NASA Astrobiology Strategy 2015. NASA Astrobiology Program. Washington, D.C.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Searching for Life Across Space and Time: Proceedings of a Workshop. The National Academies Press, Washington, DC.
Neveu, M., L.E. Hays, M.A. Voytek, M.H. New, and M.D. Schulte. 2018. “The ladder of life detection.” Astrobiology 18(11):1-28.
Nutman, A.P., V.C. Bennett, C.R.L. Friend, M.J. Van Kranendonk, and A.R. Chivas. 2016. Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 537:535-538.
Olson, S.L., E.W. Schwieterman, C.T. Reinhard, A. Ridgwell, S.R. Kane, V.S. Meadows, and T.W. Lyons. 2018. Atmospheric seasonality as an exoplanet biosignature. Astrophysical Journal Letters 858(2):L14.
Onstott, T.C., D. McGown, J. Kessler, B. Sherwood Lollar, K.K. Lehmann, and S.M. Clifford. 2006. Martian CH4: Sources, flux, and detection. Astrobiology 6(2):377-395.
Pavlov, A.A., and J.F. Kasting. 2002. Mass-independent fractionation of sulfur isotopes in Archean sediments: Strong evidence for an anoxic Archean atmosphere. Astrobiology 2(1):27-41.
Peterson, B.J., and B. Fry. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology, Evolution, and Systematics 18:293-320.
Planavsky, N.J., D. Asael, A. Hofmann, C.T. Reinhard, S.V. Lalonde, A. Knudsen, X. Wang, et al. 2014a. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nature Geoscience 7:283-286.
Planavsky, N.J., C.T. Reinhard, X. Wang, D. Thompson, T. McGoldrick, R.H. Rainbird, T. Johnson, W.W. Fischer, and T.W. Lyons. 2014b. Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346(6209):635-638.
Prangishvili, D. 2013. The wonderful world of archaeal viruses. Annual Review of Microbiology 67:565-585.
Reinhard, C.T., N.J. Planavsky, L.J. Robbins, C.A. Partin, B.C. Gill, S.V. Lalonde, A. Bekker, K.O. Konhauser, and T.W. Lyons. 2013. Proterozoic ocean redox and biogeochemical stasis. Proceedings of the National Academy of Sciences U.S.A. 110(14):5357-5362.
Reinhard, C.T., S.L. Olson, E.W. Schwieterman, and T.W. Lyons. 2017. False negatives for remote life detection on ocean-bearing planets: Lessons from early Earth. Astrobiology 17(4):287-297.
Rennó, N.O., and C.S. Ruf. 2012. Comments on the search for electrostatic discharges on Mars. The Astrophysical Journal 761(2):88.
Rosing, M.T., and R. Frei. 2004. U-rich Archaean sea-floor sediments from Greenland—Indications of 3700 Ma oxygenic photosynthesis. Earth and Planetary Science Letters 217(3-4):237-244.
Roux, S., J.R. Brum, B.E. Dutilh, S. Sunagawa, M.B. Duhaime, A. Loy, B.T. Poulos, et al. 2016. Ecogenomics and potential biogeochemical impacts of globally abundant ocean viruses. Nature 537:689-693.
Ruff, S.W., and J.D. Farmer. 2016. Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nature Communications 7:13554.
Sagan, C., W.R. Thompson, R. Carlson, D. Gurnett, and C. Hord. 1993. A search for life on Earth from the Galileo spacecraft. Nature 365(6448):715.
Schopf, J.W. 1993. Microfossils of the early Archean Apex Chert: New evidence of antiquity of life. Science 260(5108):640-646.
Schopf, J.W. 2006. Fossil evidence of Archaean life. Philosophical Transactions of the Royal Society B 29(1470):869-885.
Schopf, J.W., and A.B. Kudryavtsev. 2009. Confocal laser scanning microscopy and Raman imagery of ancient microscopic fossils. Precambrian Research 173(1-4):39-49.
Schopf, J.W., J.D. Farmer, I.S. Foster, A.B. Kudryavtsev, V.A. Gallardo, and C. Espinoza. 2012. Gypsum-permineralized microfossils and their relevance to the search for life on Mars. Astrobiology 12(7):619-633.
Schopf, J.W., K. Kitajima, M.J. Spicuzza, A.B. Kudryavtsev, and J.W. Valley. 2018. SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope compositions. Proceedings of the National Academy of Sciences U.S.A. 115(1):53-58.
Schrödinger, E. 1944. What Is Life? The Physical Aspect of the Living Cell. Cambridge University Press, New York, NY.
Schulz F., N. Yutin, N. Ivanova, D.R. Ortega, T.W. Lee, J. Vierheilig, H. Daims, et al. 2017. Giant viruses with an expanded complement of translation system components. Science 356:82-85.
Schwieterman, E.D., V.S. Meadows, S.D. Domagal-Goldman, D. Deming, G.N. Arney, R. Luger, C.E. Harman, A. Misra, and R. Barnes. 2016. Identifying planetary biosignature imposters: Spectral features of CO and O4 resulting from abiotic O2/O3 production. Astrophysical Journal Letters 819(1):L13.
Schwieterman, E.W., N.Y. Kiang, M.N. Parenteau, C.E. Harman, S. DasSarma, T.M. Fisher, G.N. Arney, et al. 2018. Exoplanet biosignatures: A review of remotely detectable signs of life. Astrobiology 18(6):663-708.
Schwieterman, E.W., T.D. Robinson, V.S. Meadows, A. Misra, and S. Domagal-Goldman. 2015. Detecting and constraining N2 abundances in planetary atmospheres using collisional pairs. The Astrophysical Journal 810(1):57.
Seager, S., and W. Bains. 2015. The search for signs of life on exoplanets at the interface of chemistry and planetary science. Science Advances 1(2):e1500047.
Seager, S., W. Bains, and J.J. Petkowski. 2016. Toward a list of molecules as potential biosignature gases for the search for life on exoplanets and applications to terrestrial biochemistry. Astrobiology 16(6):465-495.
Segura, A., V.S. Meadows, J.F. Kasting, D. Crisp, and M. Cohen. 2007. Abiotic formation of O2 and O3 in high-CO2 terrestrial atmospheres. Astronomy and Astrophysics 472(2):665-679.
Seo, E.Y., T.S. Ahn, and Y.G. Zo. 2010. Agreement, precision, and accuracy of epifluorescence microscopy methods for enumeration of total bacterial numbers. Applied and Environmental Microbiology 76(6):1981-1991.
Sherwood Lollar, B., G. Lacrampe-Couloume, G.F. Slater, J. Ward, D.P. Moser, T.M. Gihring, L.-H. Lin, and T.C. Onstott. 2006. Unravelling abiogenic and biogenic sources of methane in the Earth’s deep subsurface. Chemical Geology 226(3-4):328-339.
Sherwood Lollar, B., K. Voglesonger, L.-H. Lin, G. Lacrampe-Couloume, J. Telling, T.A. Abrajano, T.C. Onstott, and L.M Pratt. 2007. Hydrogeologic controls on episodic H2 release from Precambrian fractured rocks—energy for deep subsurface life on Earth and Mars. Astrobiology 7(6):971-986.
Sherwood Lollar, B., T.C. Onstott, G. Lacrampe-Couloume, and C.J. Ballentine. 2014. The contribution of the Precambrian continental lithosphere to global H2 production. Nature 516:379-382.
Steele, A., F.M. McCubbin, M. Fries, L. Kater, N.Z. Boctor, M.L. Fogel, P.G. Conrad, et al. 2012. A reduced organic carbon component in martian basalts. Science 337:212-215.
Stolper, D.A., and C.D. Keller. 2018. A record of deep-ocean dissolved O2 from the oxidation state of iron in submarine basalts. Nature 553:323-327.
Sugitani, K., K. Mimura, M. Takeuchi, K. Lepot, S. Ito, and E.J. Javaux. 2015. Early evolution of large micro-organisms with cytological complexity revealed by microanalyses of 3.4 Ga organic-walled microfossils. Geobiology 13(6):507-521.
Summons, R.E., P. Albrecht, G. McDonald, and J.M. Moldowan. 2008. Molecular biosignatures. Pp. 133-159 in Strategies of Life Detection (O. Botta, J.L. Bada, J. Gomez-Elvira, E. Javaux, F. Selsis, and R. Summons, eds.). Space Sciences Series of ISSI, Vol. 25. Springer, Boston, MA.
Tang, D., X. Shi, X. Wang, and G. Jiang. 2016. Extremely low oxygen concentration in mid-Proterozoic shallow seawaters. Precambrian Research 276:145-157.
Taran, Y.A., G.A. Kliger, and S. Sevastianov. 2007. Carbon isotope effects in the open-system Fischer-Tropsch synthesis. Geochimica et Cosmochimica Acta 71:4474-4487.
Tian, F., K. France, J.L. Linsky, P.J.D. Mauas, and M.C. Vieytes. 2014. High stellar FUV/NUV ratio and oxygen contents in the atmospheres of potentially habitable planets. Earth and Planetary Science Letters 385:22-27.
Trembath-Reichert, E., Y. Morono, A. Ijiri, T. Hoshino, K.S. Dawson, F. Inagaki, and V.J. Orphan. 2017. Methyl-compound use and slow growth characterize microbial life in 2-km-deep subseafloor coal and shale beds. Proceedings of the National Academy of Sciences U.S.A. 114(44):E9206-E9215.
Van Kranendonk, M.J., G.E. Webb, and B.S. Kamber. 2003. Geological and trace element evidence for a marine sedimentary environment of deposition and biogenicity of 3.45 Ga stromatolitic carbonates in the Pilbara Craton, and support for a reducing Archaean ocean. Geobiology 1:91-108.
Van Kranendonk, M.J., P. Philippot, K. Lepot, S. Bodorkos, and F. Pirajno. 2008. Geological setting of Earth’s oldest fossils in the ca. 3.5 Ga Dresser Formation, Pilbara Craton, Western Australia. Precambrian Research 167(1-2):93-124.
Vance, S.D., K.P. Hand, and R.T. Pappalardo. 2016. Geophysical controls of chemical disequilibria in Europa. Geophysical Research Letters 43(10):4871-4879.
Wacey, D. 2014. In situ morphologic, elemental and isotopic analysis of Archean life. Pp. 351-365 in Modern Approaches in Solid Earth Sciences: Evolution of Archean Crust and Early Life, Vol. 7. (Y. Dilek, and H. Furnes, eds.). Springer, Dordrecht.
Wacey, D., M. Saunders, C. Kong, A. Brasier, and M. Brasier. 2016. 3.46 Ga Apex chert ‘microfossils’ reinterpreted as mineral artefacts produced during phyllosilicate exfoliation. Gondwana Research 36:296-313.
Wacey, D., N. Noffke, M. Saunders, P. Guagliardo, and D.M. Pyle. 2018. Volcanogenic pseudo-fossils from the ~3.48 Ga Dresser Formation, Pilbara, Western Australia. Astrobiology 18(5):539-555.
Wacey, D., S. Menon, L. Green, D. Gerstmann, C. Kong, N. Mcloughlin, M. Saunders, and M. Brasier. 2012. Taphonomy of very ancient microfossils from ~3400 Ma Strelley Pool Formation and ~1900 Ma Gunflint Formation: New insights using a focused ion beam. Precambrian Research 220-221:234-250.
Walker, S.I., W. Bains, L. Cronin, S. DasSarma, S. Denielache, S. Domagal-Goldman, B. Kacar, et al. 2018. Exoplanet biosignatures: Future directions. Astrobiology 18(6):779-824.
Walter, M.R., R. Buick, and J.S.R. Dunlop. 1980. Stromatolites 3,400-3,500 Myr old from the North Pole area, Western Australia. Nature 284:443-445.
Warr, O., B. Sherwood Lollar, J. Fellowes, C.N. Sutcliffe, J.M. McDermott, G. Holland, J.C. Mabry, and C.J. Ballentine. 2018. Tracing ancient hydrogeological fracture network age and compartmentalization using noble gas. Geochimica et Cosmochimica Acta 222:340-362.
Westall, F., B. Cavalazzi, L. Lemelle, Y. Marrocchi, J.N. Rouzaud, A. Simionovici, M. Salomé, et al. 2011. Implications of in situ calcification for photosynthesis in a ~3.3 Ga-old microbial biofilm from the Barberton greenstone belt, South Africa. Earth and Planetary Science Letters 310:468-479.
Westall, F., F. Foucher, N. Bost, M. Bertrand, D. Loizeau, J.L. Vago, G. Kminek, et al. 2015a. Biosignatures on Mars: What, where and how? Implications for the search for martian life. Astrobiology 15:998-1029.
Westall, F., K.A. Campbell, J.G. Bréhéret, F. Foucher, P. Gautret, A. Hubert, S. Sorieul, N. Grassineau, and D.M. Guido. 2015b. Archean (3.33 GA) microbe-sediment systems were diverse and flourished in a hydrothermal context. Geology 43(7):615-618.
Wordsworth, R., and R. Pierrehumbert. 2014. Abiotic oxygen-dominated atmospheres on terrestrial habitable zone planets. Astrophysical Journal Letters 785(2):L20.
Zahnle, K., M. Claire, and D. Catling. 2006. A loss of mass-independent fractionation in sulfur due to a Palaeoproterozoic collapse of atmospheric methane. Geobiology 4:271-283.























