2
Exploring Opportunities Afforded by Mobile Technology
The current state of assessing brain disorders is “exquisitely crude,” said William Marks, head of clinical neurology at Verily Life Sciences. The disease-specific scoring schemes used, such as the Unified Parkinson’s Disease Rating Scale, the Multiple Sclerosis Functional Composite, and the Alzheimer’s Disease Assessment Scale-Cognitive subscale are pseudo-quantitative, limited in scope, and applied in artificial settings, he said. Most importantly, he said, they often fail to consider what matters most to individuals with those conditions and their families.
Digital technologies, however, have the potential to assess objectively, quantitatively, repeatedly, and in a more natural setting the multiple aspects of a disease, including the transition from health to disease, symptom severity, progression, stability, regression, impact on daily life, and response to treatment, said Marks. Moreover, the convergence of increased cloud storage capacity and improved analytical techniques offer the potential to harness these measures for both research and clinical care, he said. If these digital advances can be married to medication development, they might even improve the delivery of different types of therapies, observed Husseini Manji.
For example, neurologists typically assess gait by observing their patients as they walk down a corridor. Digital technologies can provide complementary information about function and context by capturing more quantitative measures of gait parameters as well as other real-world parameters related to walking, such as destination and frequency of walking to a destination, said Marks. Artificial constructs such as tapping one’s finger as a measure of dexterity can be replaced by digital monitoring of changes in the ability to eat, write, or perform other daily functions in a natural environment, he said, adding that these more clinically relevant
phenotypes could enable earlier and more definitive diagnoses to guide more personalized treatment as well as a better understanding of treatment responses. In addition to passively collecting information, digital technologies can actively push out surveys and patient-reported outcome measures in a relevant manner, he said.
Although much of the emphasis so far has been on movement monitoring with accelerometers and gyroscopes, these devices are just scratching the surface, said Marks. The technology is reaching the point where gait or other types of movement can be dissected in a much more disease-specific way. Combined with geospatial activity and assessments of falls or use of assistive devices, a more complete picture of motor function can be discerned. Beyond the assessment of motor function, and because neurological and psychiatric disorders are multidimensional, digital devices lend themselves to the measurement of other domains such as sleep, cognition, speech, vision, mood, behavior, cardiovascular and autonomic function, social interactivity, and quality of life—“an amazing wealth of information that could give us insight into these diseases,” said Marks.
Currently, digital biomarkers are being incorporated into clinical trials primarily as exploratory measures, running alongside more traditional scales and measures, said Iain Simpson, senior director of digital health at IXICO. IXICO began supporting the pharmaceutical industry in clinical trials as a specialist neuroimaging clinical research organization (CRO), he said, but they have broadened into the digital biomarker field when they saw their potential of wearable devices to be used as clinical research endpoints and realized that many of the issues faced in developing digital biomarkers are similar to those encountered in imaging 5 to 10 years ago. He predicted that while digital biomarkers may eventually supplant some rating scales and other subjective measures of function, it may be more likely that they will provide complementary quantitative input to those scales. He emphasized that implementing biosensors and passive data collection into clinical trials has the potential to capture clinically important information while minimizing patient and site burden (see Figure 2-1).
Marks added that in some cases, digital technologies may themselves be therapeutic, for example, by coaching a person through a treatment regimen or healthy brain activity. More granular monitoring of the trajectory of a person’s disease and their response to treatment could have the added benefit of democratizing health care, giving people more access to expert care, and facilitating population health management, he said. Indeed, mobile technologies offer the ability to engage more diverse populations, including people who typically do not participate in research either because
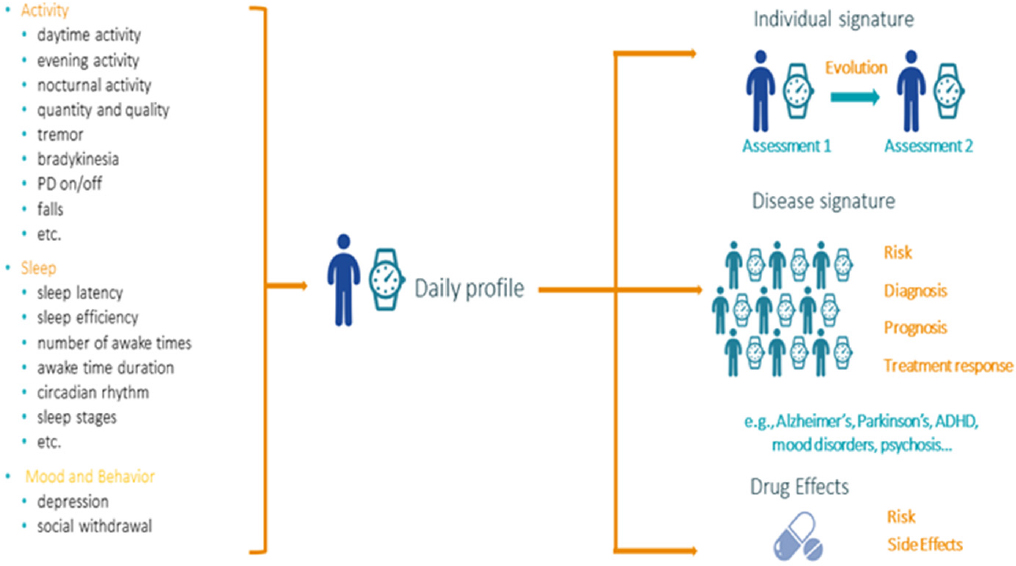
NOTE: ADHD = attention deficit hyperactivity disorder; PD = passive device.
SOURCE: Presented by Simpson, June 6, 2018.
they do not live near a clinical site, because they have mobility issues, or because they are too busy working and caring for their families, said Catherine Kopil, senior associate director of research programs at The Michael J. Fox Foundation for Parkinson’s Research (MJFF).
Some of the more obvious benefits of digital technologies are likely to be realized in the areas of early identification and objective monitoring of disease progression, with real-world measures for neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), said Manji. For neuropsychiatric disorders that are highly recurrent, such as bipolar disorder, digital technologies may also provide early warning signals of an impending cycle, switch to mania, or suicidal intent, he said. Because these recurrences and relapses may contribute to the pathogenesis of disease, Manji suggested that detecting early warning signs and intervening at that point could potentially impact disease progression (Narayan and Manji, 2016).
Manji noted that digital technologies even have the potential to monitor people when they are healthy but at risk for neurodegenerative diseases, such as people with a family history of certain diseases or those that harbor genetic mutations or genetic risk factors such as the ApoE4 gene, which increases the risk of developing AD (Corder et al., 1993). Being able to detect people in the earliest stages of a disorder not only would enable early intervention, but also the identification of appropriate candidates for clinical trials, said Manji.
Quantitative measures also can supercharge the efficiency of clinical trials through the collection of many high-resolution data points, said Marks. The increased statistical power of this high-density data may result in shorter trials with fewer participants, he said. In addition, because these assessments could be done virtually, a more diverse group of participants could be enrolled in trials.
TECHNICAL, METHODOLOGICAL, AND ETHICAL CHALLENGES
To realize the opportunities just discussed, many technological, methodological, and ethical challenges will need to be addressed, said JP Onnela; these challenges are discussed in more detail in later chapters of these proceedings. These include issues associated with the high dimensionality and noise associated with digital data, which introduce challenges related to data standardization, analytical and statistical methods, and reproducibility, he said (Chapter 3). To make digital data useful in clinical trials will also require multiple levels of validation, as well as novel approaches to organize and synthesize data so that it is useful to different audiences, said Marks (Chapters 3, 5, and 6). Developing such approaches will demand the engagement of participants, clinicians, researchers, data scientists, and regulators, he said. Moreover, a regulatory path for using digital data has yet to be developed, said Onnela, noting that data privacy and security remain substantial concerns for consumers, device developers, and regulators alike (Chapters 4 and 6). Kristen Rosati, an attorney and partner at Coppersmith Brockelman, PLC, added that an evolving web of laws and regulations present challenges to companies trying to incorporate digital measures into clinical trials (Chapter 4).




