4
Research and Development
Based on the discussions in Chapters 2 and 3, the committee sees potential long-term value in the use of reusable elastomeric respirators both during routine use and during public health emergencies, although it has identified several gaps that currently will impede their widespread implementation (see Chapter 5). This chapter focuses on the research and development efforts that are needed to improve reusable elastomeric respirators, beginning with a discussion of performance parameters and then examining the need for research on better understanding the airborne transmission of certain infectious diseases, cleaning and disinfection, designing for the next generation of elastomeric respirators, and informing market demand and the supply chain.
PERFORMANCE AND SIZE PARAMETERS
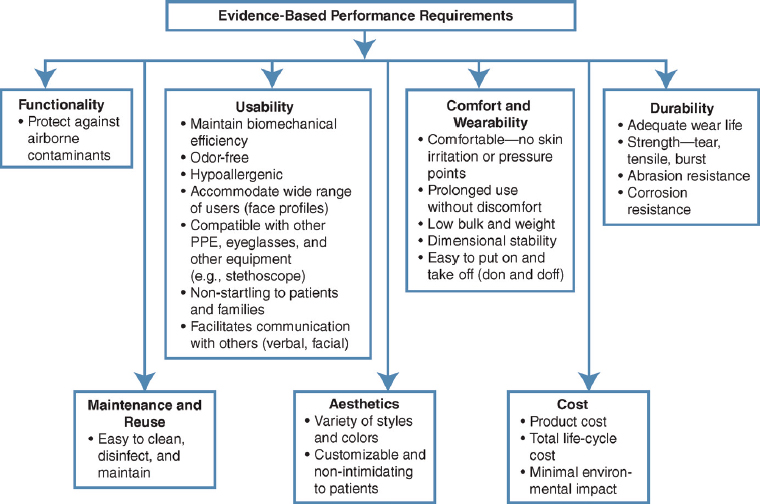
A 2008 Institute of Medicine (IOM) study proposed a comprehensive framework for the design and development of respirators and other personal protective equipment (PPE; e.g., gowns, gloves) for health care workers which was driven by evidence-based performance requirements and encompassed the three phases typically associated with a product’s life cycle: user requirements analysis, design realization, and field use and evaluation (see Figure 4-1). The framework also called for greater interac tion among end users, designers, manufacturers, and standards and certification agencies and organizations to ensure the design and realization of effective PPE. In addition to the functionality of the device (to provide the desired degree of protection), the framework focused on factors, such as
NOTE: PPE = personal protective equipment.
SOURCE: IOM, 2008.
usability, comfort, wearability, and aesthetics, that are of critical impimportance in promoting the use of the device by the health care worker. From the health care facility management’s perspective, durability, maintenance, and affordability are of importance, as shown in the figure. Adopting such a system and iterative approach would result in the development and deployment of effective reusable elastomeric respirators in the range of health care settings from patients’ homes to hospitals to long-term-care facilities.
The design of the elastomeric respirator significantly influences the compliance of health care workers who use the device to protect themselves in the clinical setting. Even the most “protective” of devices is not effective if it is not comfortable for the user and, as a result, the health care worker does not use the device. Figure 4-2 depicts key factors that determine the comfort of respirators being used in the clinical setting—specifically, physical, sensory, cognitive, and psychological factors.
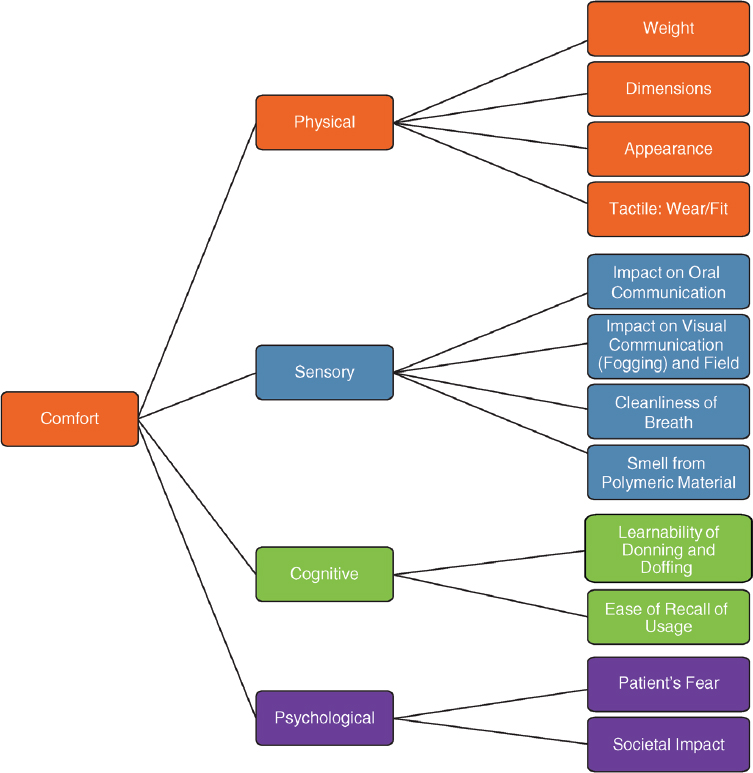
The physical factors include the weight and dimensions, the device’s aesthetic appearance, the tactile feeling at the skin–respirator interface, and the fit of the device. The generation and buildup of CO2 in the dead space of the respirator has the potential to affect the work of breathing and hence the health care worker’s comfort. As shown in the figure, the sensory factors include the impact on oral communication (i.e., speech intelligibility), the effect on visual communication (i.e., the view of lip movements on which the hearing-impaired, elderly, and many other patients depend), fogging, the effect of the device on the cleanliness of the air breathed in, and the potential discomfort from the smell of the specific elastomer used in the device.
Another important factor that influences the use of the device in the clinical setting relates to the cognitive factors, particularly how easy it is to learn to use the device (don, seal check, and doff) and how long it takes for an individual to be able to effectively use the device since a respirator may only be used occasionally. Finally, the psychological factors that influence the use of the device include the reaction (potential fear) of the patient upon seeing the health care worker wearing a respirator. As discussed in Chapter 2, most studies involving elastomeric respirators have been in controlled environments. Therefore, there is a critical need to assess the effects of these factors on the performance of reusable elastomeric respirators in real health care settings, both during normal times and during public health emergencies.
In addition, the absence of standard parameters for size and fit of respirators across manufacturers affects the preparedness of health care facilities for public health emergencies. A standard set of size parameters that will define the fit of respirators, similar to standard sizing parameters in clothing (e.g., waist, inseam), is needed. While one manufacturer’s approach and shape may be different from those of another manufacturer, both will fit because both have standard size parameters associated with them. In the event of a public health emergency, the transition between manufacturers’ brands would be facilitated because the same size in different brands would provide the same fit, thus reducing the time required for fit testing. Achieving standardization parameters for respirator sizes will enable different manufacturers to achieve a consistent fit, while maintaining their proprietary strengths in design and approaches to achieving respirator fit. Research is needed to serve as the basis for the development of consensus standardized parameters for the size and fit of elastomeric respirators to which all manufacturers should conform, thereby avoiding the need to do fit testing on each different manufacturer’s devices, especially during a public health emergency, when time is of the essence in saving lives. Standardization of sizing could have an important impact on the overall cost of using this equipment and its integration into a large, rapidly changing workforce.
UNDERSTANDING AIRBORNE TRANSMISSION OF INFECTIOUS PATHOGENS
An important research priority for public health preparedness and for ensuring the health and safety of health care workers is developing a thorough understanding of the transmission of infectious pathogens so that appropriate precautions can be instituted. The Centers for Disease Control and Prevention (CDC) guidelines for the use of airborne transmission precautions (that include use of a respirator) note that pathogens transmitted by the airborne route include tuberculosis, measles, severe acute respiratory syndrome (SARS), anthrax, chickenpox, and disseminated herpes zoster (CDC, 2017a,b). Exposure or potential exposure to those pathogens requires airborne precautions including the use of “a fit-tested NIOSH [National Institute for Occupational Safety and Health]-approved N95 or higher level respirator for healthcare personnel” (CDC, 2017a). For influenza and other respiratory infections, more research is needed to determine the relative contribution of airborne transmission to the spread of each disease. Influenza is used as an example in the following paragraphs to highlight the research needs.
CDC’s Healthcare Infection Control Practices Advisory Committee guidelines for the most recent 2017–2018 influenza season state that “the relative contribution of the different modes of influenza transmission is unclear” (CDC, 2018b). CDC’s guidance for respiratory protection for health care workers potentially exposed to patients with influenza varies depending on whether it is seasonal influenza or whether there is concern about the unknown virulence of a potentially pandemic influenza. For seasonal influenza, the recommendation is for droplet protection unless an aerosol-generating procedure is being carried out, in which case respiratory protection equivalent to a fitted N95 filtering facepiece respirator or equivalent N95 respirator must be used. For a potential influenza pandemic, the Healthcare Infection Control Practices Advisory Committee recommends that before entry to the patient room or care area a worker don a respirator that is “at least as protective as a fit-tested NIOSH-certified disposable N95 filtering facepiece respirator” (CDC, 2016). Research efforts continue to examine the nature of influenza transmission on the continuum between large-droplet and airborne transmission, but key questions remain (IOM, 2008, 2011). Failure to fully understand the transmission potential of an influenza virus limits the impact of policies established to limit its spread. Examples of research efforts on understanding influenza transmission include the work of Lindsley and colleagues (2012,
2015, 2016), who examined the dispersion of cough and exhalation aerosols in the health care setting as well as the size of particles with viable influenza virus. The researchers found that cough aerosols were initially carried in a plume capable of traveling across a room and exposing a health care worker to a highly concentrated aerosol. After several minutes, the aerosol particles, across a broad size range, had dispersed throughout the room, reaching everyone inside. Research gaps identified by these studies included whether influenza was transmitted to a significant degree by the inhalation of infectious aerosols, how to control and reduce the production and dispersion of potentially infectious aerosol clouds, and the possible role of larger ballistic spray droplets in disease transmission. Cowling and colleagues (2013) applied a mathematical model to data from randomized controlled trials of hand hygiene and medical masks in Hong Kong and Bangkok households. They found that airborne transmission accounted for approximately half of all transmission events and concluded that measures to reduce transmission by contact or by large droplets may not be sufficient to control influenza A virus transmission in households. Zhou and colleagues (2018) designed and evaluated a transmission chamber that separated virus-laden particles in air by their size and then studied the airborne particles that mediate influenza transmission in ferrets. Their results provide direct experimental evidence of influenza transmission via droplets and fine droplet nuclei, albeit at different efficiency.
The 2008 IOM study recognized this “paucity of data on influenza transmission and the importance of this knowledge in refining prevention and mitigation strategies, particularly for pandemic influenza” and recommended the initiation and support of a global influenza research network (IOM, 2008, p. 1), an important need that still remains. A fundamental understanding of airborne transmission of infectious diseases will make more likely the proper selection and use of respiratory protection and facilitate the design of the next generation of respiratory protection devices, including reusable elastomeric respirators.
CLEANING AND DISINFECTION RESEARCH
Safe and effective cleaning and disinfection is a prerequisite for the reuse of elastomeric respirators. Additionally, these maintenance processes should not affect the fit and performance of the respirators. The U.S. Food and Drug Administration (FDA) provides a set of guidelines for reprocessing medical devices in health care settings, which can serve as a
valuable reference for designers and manufacturers of reusable elastomeric respirators. According to FDA, cleaning is “the physical removal of soil and contaminants from an item to the extent necessary for further processing or for the intended use.” Disinfection is “a process that destroys pathogens and other microorganisms by physical or chemical means” (FDA, 2015, p. 32). Sterilization is “a validated process used to render product free from viable microorganisms” (FDA, 2015, p. 33). While sterilization is ideal, it would likely require the use of a centralized processing facility, which has its own logistical, design, and safety challenges. For example, the high temperatures used during centralized processing may cause damage to the respirator (Bessesen et al., 2015; Lawrence et al., 2017). Alternative sterilization processes that do not require high temperatures or damage material integrity are being explored, including the use of ethylene oxide, vaporized hydrogen peroxide, and ultraviolet radiation (Subhash et al., 2014; Bessessen et al., 2015; Lawrence et al., 2017). These alternatives would also most likely require a centralized reprocessing system (Subhash et al., 2014). Another alternative is the design of specialized, hospital-grade washers that can handle multiple respirators at a time.
Studies have looked more extensively at cleaning and disinfecting agents for filtering facepiece respirators than for elastomeric respirators. For example, Heimbuch and colleagues (2011) evaluated three different energetic methods—microwave-generated steam, moist heat incubation, and ultraviolet germicidal irradiation—against H1N1 influenza-contaminated disposable filtering facepiece respirators. All three methods demonstrated substantial reductions in viable virus. Mills and colleagues (2018) built on this study and evaluated the efficiency of an ultraviolet germicidal irradiation decontamination method on 15 models of NIOSH-approved filtering facepiece respirators. Twelve samples of each of the 15 models were contaminated with H1N1 influenza (facepiece and strap) and then covered with a soiling agent—artificial saliva or artificial skin oil. For each soiling agent, three contaminated respirators were treated with 1 J/cm2 (joule/square centimeter) ultraviolet germicidal irradiation for approximately 1 minute, while the three other contaminated respirators remained untreated. All contaminated surfaces were cut out, and the virus was extracted. Viable influenza virus was quantified using a median tissue culture infectious dose assay. Significant reductions in influenza viability for both soiling conditions were observed on the facepieces from 12 of 15 models of filtering facepiece respirators and the straps from seven of those models. This study showed that decontamination and reuse using ultraviolet germicidal irradiation could be effective for filtering facepiece respirators.
A viable ultraviolet method for regular use on elastomeric respirators in a health care facility needs to be developed and implemented.
The use of ozone is one of the relatively new low-temperature sterilization options for use on medical equipment and was cleared by FDA for this use in 2003 (CDC, 2008). Ozone is considered to be less harmful to bystanders and to the environment than ethylene oxide, which had been the standard for sterilization of temperature- and heat-sensitive medical equipment but was found to be associated with health risks (CDC, 2008). Following sterilization, the ozone is converted back to oxygen and water vapor. According to CDC guidelines, ozone sterilization is suitable for use with a variety of materials, including silicone, polypropylene, and polyethylene. CDC’s Healthcare Infection Control Practices Advisory Committee states, “More research is required to clarify the effectiveness and reliability of fogging, UV [ultraviolet] irradiation, and ozone mists to reduce noro-virus environmental contamination. (No recommendation/unresolved issue)” (CDC, 2008, p. 86). Rogers (2008, 2012) noted that ozone can have adverse effects on steel, brass, latex, and other polymers and that it is not recommended for plastic devices (but has been used for endoscopes), so more work needs to be done to resolve the usefulness of ozone for elastomeric disinfection. Ozone does not penetrate the device; it provides surface sterilization only. Ozone sterilizers are compact, easy to operate, and cost effective in hospital and manufacturer settings. The cycle time is 60 minutes, depending on the load and size of the chamber, which makes ozone a potential, although time-intensive, solution for a disinfection system in the health care facility. Also, the low temperatures (30–36°C) make ozone suitable for temperature-sensitive polymers. Polymers sanitized by ozone should be resistant to humidity and oxidation. Ozone is used to clean and sanitize continuous positive airway pressure (CPAP) machines (SoClean, 2018).
There are many methods that could be evaluated for the disinfection of reusable elastomeric respirators. The committee did not find a common set of guidelines or instructions for the cleaning and disinfection of elastomeric respirators. Moreover, the committee did not find a set of criteria with which to assess or evaluate the cleanliness of the device after disinfection in order to ensure its safe use. Therefore, efforts must be directed at the harmonization of the various guidelines and manufacturers’ instructions for cleaning and disinfection, which will promote the use of elastomeric respirators. FDA provides a set of guidelines for validating the cleaning process of medical devices; these guidelines could serve as a
starting point for developing a validation system for cleaning elastomeric respirators (FDA, 2015).
As discussed in Chapters 2 and 3, one of the key challenges inhibiting the widespread use of elastomeric respirators is the absence of an easy-to-use and non-burdensome method to clean and disinfect after use. In addition to identifying optimum cleaning agents, research is needed to develop systems and processes that are effective and capable of being used in health care facilities with limited space. For example, disinfection carts could be strategically placed on the floors of health care facilities so that a health care worker could, upon exiting a patient’s room, place the used respirator on one side of a cart and pick up a disinfected respirator from the other side.
NEXT GENERATION OF HEALTH CARE RESPIRATORS: RESEARCH AND DESIGN
One of the primary driving forces behind the design and development of a new generation of health care respirators is the threat of an influenza pandemic or other emerging airborne transmissible diseases (see Chapters 1 and 3). The design of the elastomeric respirator significantly influences the compliance of health care workers in using the device to protect themselves. All of the factors discussed earlier and shown in Figures 4-1 and 4-2 should be considered in the design of elastomeric respirators to enhance compliance in the clinical setting. From a manufacturer’s perspective, the design and the selection of materials are critical as they influence the comfort of the device and the other factors depicted in Figure 4-1, including functionality (the degree of protection), ease of fit testing, decontamination, and affordability. The adoption of this paradigm ensures an inclusive user-centric design, thereby enhancing compliance and the protection of health care workers.
Efforts have been in progress for more than a decade to outline the parameters for the design of new health care respirators. In 2008, Project BREATHE (Better Respiratory Equipment using Advanced Technologies for Health Care Employees) was initiated by the U.S. Department of Veterans Affairs (VA) and functioned as a working group co-chaired by staff
from the VA and CDC, with representatives from multiple federal departments and agencies1 (Radonovich et al., 2009; Gosch et al., 2013). This effort built on the 2008 IOM report’s framework and focused first on developing a detailed set of performance characteristics for health care respirators. Project BREATHE began as a federal interagency effort and later developed private-sector and academic partnerships. The project was designed to be a four-phase effort (see Box 4-1), with the first phase completed in 2009. Project BREATHE’s 2009 report listed 28 consensus recommendations for detailed performance characteristics of an ideal health care respirator (see Table 4-1) and prioritized those objectives (Radonovich et al., 2009). In a follow-up to the Project BREATHE report, several prototypes were developed, and laboratory tests were conducted. However, funding was not available for field-testing the devices.
___________________
1 Representatives on the Project BREATHE working group were from CDC (NIOSH National Personal Protective Technology Laboratory; Office for Infection Control, Division of Healthcare Quality Promotion; National Center for HIV, STD, and TB Prevention, Division of Healthcare Quality Promotion), U.S. Department of Veterans Affairs, U.S. Army Edgewood Chemical Biological Center, National Institute of Standards and Technology, National Aeronautics and Space Administration, Biomedical Advanced Research and Development Authority, and Occupational Safety and Health Administration (Gosch et al., 2013).
| Major Categories and Desirable Performance Objectives | Priority Level Designation |
|---|---|
| Perform their intended function safely and effectively | |
|
1 |
|
1 |
|
1 |
|
1 |
|
3 |
|
1 |
|
1 |
|
2 |
|
* |
| Support, not interfere, with occupational activities | |
|
1 |
|
1 |
|
2 |
|
5 |
|
2 |
| Major Categories and Desirable Performance Objectives | Priority Level Designation |
|---|---|
| Be comfortable and tolerable for the duration of wear | |
|
1 |
|
1 |
|
1 |
|
2 |
|
2 |
|
2 |
|
3 |
|
3 |
|
3 |
|
1 |
| Comply with federal standards and guidelines, state regulations, and local policies | |
|
1 |
|
1 |
|
2 |
|
2 |
NOTE: The Project BREATHE report states that priorities were assigned by the working group based on urgency and importance and consensus decisions based on a scale of 1 through 5, with 1 being the most important.
*Elastomerics = 2; Filtering facepieces = 5.
SOURCE: Radonovich et al., 2009.
A number of recent efforts are focusing on redesigning respirators specifically to meet the needs and demands of health care workers. These efforts include work funded by the Biomedical Advanced Research and Development Authority (BARDA) to explore a variety of options to improve health care and community access to effective respiratory protection during a public health emergency (Wallace, 2018). BARDA’s interests in innovation include the design of novel respirators, improvements to material shelf life and properties (e.g., anti-viral/bacterial), the development of processes to clean and disinfect existing respirators, and the creation of
new manufacturing techniques to eliminate production bottlenecks. One of the key assessment metrics is that the proposed new respiratory protection devices demonstrate improved features as compared with currently available products.
Work is being done on developing a reusable N95-surgical elastomeric respirator for health care workers, which would be a hybrid of the N95 filtering facepiece respirator and a half-facepiece elastomeric respirator (HHS, 2017). The goal is to have a respirator that can be reprocessed for at least 100 cycles either with a hospital autoclave or using disinfection protocols that are already widely used in a health care setting (Heimbuch, 2018). This reusable respirator would be an effective, highly protective respirator that addresses the parameters and priorities identified in the BREATHE study, meets user needs associated with its acceptability, and is cost effective per use.
Materials Selection and Design Issues
The design of the elastomeric respirator should address the key factors shown in Figure 4-2. The selection of the materials should involve such considerations as the respirator being lightweight, comfortable, aesthetically pleasing (in order to minimize any negative impact on patients, especially children), and easy to disinfect in a health care setting. Clear (transparent) materials would facilitate the visibility of the health care worker’s facial expressions and enhance communications and interactions. The health care worker should not feel claustrophobic when donning the device. Research should be carried out to identify the best polymeric materials that will meet all these performance requirements for reusable thermoplastic respirators. An example of the use of transparent materials is in the CleanSpace Ultra respirator, an air-filtering, fan-assisted positive-pressure and breath-responsive powered air-purifying respirator that provides full face protection and a panoramic field of view (CleanSpace, 2018). This respirator incorporates a speech diaphragm to enhance communication and has a built-in PortaCount adaptor to facilitate fit testing (see Figure 4-3). The NIOSH-approved device has a high-efficiency particulate air (HEPA) filter with an assigned protection factor of 1,000 (see Chapter 2).

SOURCE: Reprinted with permission from CleanSpace Technology, 2018.
Research could also be pursued into breath-assisted technologies, including the investigation of the role of positive pressure in enhancing the comfort of health care workers using elastomeric respirators. Intermittent positive-pressure breathing has been shown to increase tidal volume (Torres et al., 1960), and the incorporation of such breath-assisted technologies could be beneficial when respirators have to be used for long periods during pandemics.
One of the possible ways to minimize cross-contamination between patient visits would be to protect the filter from potential fomite contamination by using a disposable pre-filter cover on the filter cartridge, which would be discarded after a patient visit, with a new one attached. Health care facilities would need to implement doffing protocols to ensure that the goal of having the extra layer of protection is achieved and that the contaminated pre-filter cover is properly collected, handled, and discarded. Research should be conducted to explore this option as it could potentially reduce the total cost of using elastomeric respirators while ensuring the health and safety of the health care workers who use them. It is important to note that the respirator surface and straps may still be contamined with virus and will need to be cleaned and disinfected.
Individual Customization
Advancements in facial scanning and recognition technologies have made their way into everyday applications and devices, including smartphones (e.g., Apple, 2018; Perala, 2018). 3D printing is a form of rapid prototyping technology, which has led to innovative new applications in a range of fields, including biomedicine (Baskaran et al., 2016). These two state-of-the-art technologies can be used together: the face of the health care worker can be scanned to create a form-fitting frame that is customized to the individual’s facial profile. This digital design can then be transmitted to a 3D printing machine to produce the customized respirator for the health care worker (Jayaraman and Park, 2015). This formfitting, individually customized respirator with a replaceable filter has the potential to obviate extensive fit testing, thereby enhancing the health care worker’s compliance in using the device as well as reducing the learning curve in using the device. Alternatively, facial scanning could be combined with artificial intelligence systems to predict with high reliability the fit of the health care worker’s face to a specific make and model of respirator. For emergency situations, these technologies offer the opportunity to rapidly get the right size respirator to the health care workforce. Research should also be directed at elastomeric materials that can be used in 3D printing machines to produce a respirator that meets the factors shown in Figures 4-1 and 4-2, including transparency, comfort, ease of disinfection, and durability.
Both customization and standardized size parameters (discussed earlier in this chapter) offer opportunities to explore and improve respirator fit.
Sensor Research
A sensor is a device that responds to a physical stimulus (e.g., heat, light, sound, pressure, or motion) by producing an impulse or signal, which can be measured and used to trigger an action in the device. One of the challenges in the use of respirators (including reusable elastomeric respirators) is fit testing, which is essential to balance the degree of comfort with the degree of protection. In part, the user must “feel” that the fit is “right,” a subjective assessment that is not always comforting for the user. To alleviate concerns about fit, one possible innovation would be to incorporate strain gauge sensors into the respirator in order to provide a numerical value that will reflect the degree of fit. Knowledge of such an objective
value could help reassure health care workers that they have “full” protection so that they can focus their attention on the task at hand rather than worrying about the fit of the respirator. Research will be needed to incorporate such strain gauge sensors into respirators, but once they have been incorporated into a respirator, they could also be used to continuously monitor the respirator’s pressure and communicate any changes that might threaten the safety of the health care worker. The data could also be used with a control system to “adjust” the straps as needed—similar to how Nike’s HyperAdapt shoe lacing system works (Eden, 2016)—thus creating a closed-loop automated system that ensures the health and safety of the health care worker during regular use.
Another avenue to explore would be sensors capable of monitoring the pressure at the skin–respirator interface and providing an objective assessment of the fit. Chromogenic materials change color and transparency in response to temperature, voltage, pressure, or light (see, e.g., Fraunhofer, 2018). They can also be controlled by external stimuli. FujiFilm Prescale is an example of this type of sensor (FujiFilm, 2018).
RESEARCH TO INFORM AN UNDERSTANDING OF MARKET DEMAND AND THE SUPPLY CHAIN
The relative lack of innovation in respirators for use by health care workers, particularly reusable elastomeric respirators, can be attributed in large part to the absence of a demand for these devices in the market. Health care workers have traditionally used filtering facepiece respirators that are disposable and thereby simplify many of the operational logistics for health care facilities implementing a respiratory protection program. The absence of a “guaranteed volume” of elastomeric respirators for manufacturers in anticipation of a pandemic introduces another uncertainty in the supply chain, making it difficult for manufacturers to make a business case in support of the required investments in time, expertise, infrastructure, and funding to meet the potential for a demand surge. Consequently, there have been minimal efforts to design and commission the sorts of innovative materials and manufacturing techniques for elastomeric respirators that could facilitate a rapid production ramp-up in the event of a demand surge due to a pandemic or other public health crisis requiring respiratory protection. Research on innovations in preparedness planning and in surge manufacturing regarding respirators is needed.
Furthermore, consideration could be given to developing the data analytics and a more networked approach to tracking the use and supplies of respirators which could be of great benefit in a public health crisis (Wizner et al., 2018). Additionally, insights could be gained from the work of pharmacy benefits managers in administering prescription drug plans for organizations and individuals with health insurance from a variety of sources; the managers’ primary objective is to reduce the overall cost of medicines and hence total health care costs. They negotiate the price of drugs with pharmaceutical companies, and the procurement volume gives them leverage in the negotiations. While there are differing perspectives on the role of pharmacy benefits managers in achieving real health care cost savings, it is worth investigating how the “network model of buyers” might be applied to respirators, for several reasons:
- The aggregation of respirator demands in each health care facility (regionally and nationally) would provide a better overall demand estimate, which would provide the guidance that manufacturers are seeking to make investment decisions;
- The purchasing power that comes from buying large volumes of respirators could provide better negotiating power in reducing the cost of respirators for health care facilities; and
- The real-time “heat map” of available (and needed) respirator inventories in health care facilities around the nation would facilitate the rapid transfer of supplies to areas with outbreaks, thereby augmenting the supply chain and helping ensure the safety of those health care workers.
Furthermore, linking a respirator inventory heat map to CDC’s FluView (CDC, 2018a; see Figure 4-4) and analyzing the data from these two sources (as well as additional tools developed to address other airborne transmissible diseases) could help in assessing respirator inventories relative to the needs of health care facilities. The insights from carrying out this type of data analytics over several years could be potentially valuable in creating and maintaining an optimum stockpile of respirators (and potentially other types of personal protective equipment) in the nation.
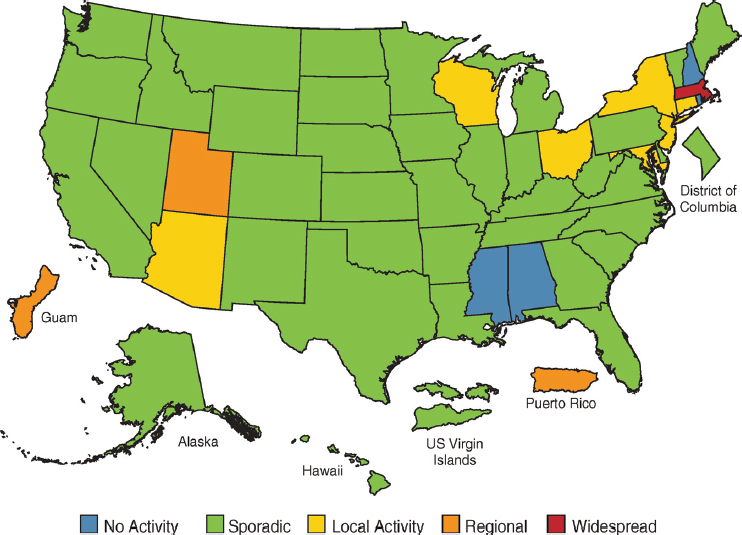
SOURCE: CDC, 2018a.
NURTURING INNOVATION
Innovations in the development of the next generation of elastomeric respirators designed for health care workers are needed at multiple stages of the supply chain and also in the implementation process. Partnerships will be needed to create the incentives for manufacturers to move beyond the focus on disposable filtering facepiece respirators to reusable respirators. In addition to innovations in respirator products, innovations will also be needed in the training and education of health care workers regarding respiratory protection and in ensuring that workers are knowledgeable about the risks of airborne diseases and act to mitigate those risks. Specific recommendations for research are discussed in Chapter 5.
REFERENCES
Apple. 2018. About FaceID advanced technology. https://support.apple.com/enus/ht208108 (accessed September 10, 2018).
Baskaran, V., G. Štrkalj, M. Štrkalj, and A. Di Ieva. 2016. Current applications and future perspectives of the use of 3D printing in anatomical training and neurosurgery. Frontiers in Neuroanatomy 10:69.
Bessesen, M. T., J. C. Adams, L. Radonovich, and J. Anderson. 2015. Disinfection of reusable elastomeric respirators by health care workers: A feasibility study and development of standard operating procedure. American Journal of Infection Control 43(12):629–634.
CDC (Centers for Disease Control and Prevention). 2008. Disinfection and sterilization. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html (accessed August 29, 2018).
CDC. 2016. Interim guidance for infection control within healthcare settings when caring for confirmed cases, probable cases, and cases under investigation for infection with novel influenza A viruses associated with severe disease. https://www.cdc.gov/flu/avianflu/novel-flu-infection-control.htm (accessed August 28, 2018).
CDC. 2017a. Transmission-based precautions. https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html (accessed October 11, 2018).
CDC. 2017b. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings (updated 2017). https://www.cdc.gov/infectioncontrol/pdf/guidelines/isolation-guidelines.pdf (acessed October 22, 2018).
CDC. 2018a. Weekly U.S. map: Influenza summary update. https://www.cdc.gov/flu/weekly/usmap.htm (accessed August 29, 2018).
CDC. 2018b. Prevention strategies for the seasonal influenza in healthcare settings. https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. (accessed August 28, 2018).
CleanSpace. 2018. CleanSpace Ultra. https://cleanspacetechnology.com/us/cleanspace-ultra (accessed August 31, 2018).
Cowling, B. J., D. K. Ip, V. J. Fang, P. Suntarattiwong, S. J. Olsen, J. Levy, T. M. Uyeki, G. M. Leung, J. S. Malik Peiris, T. Chotpitayasunondh, H. Nishiura, and M. Simmerman. 2013. Aerosol transmission is an important mode of influenza A virus spread. Nature Communications 4:1935.
Eden, S. 2016. The secret lab where Nike invented the power-lacing shoe of our dreams. Wired, October. https://www.wired.com/2016/09/nike-self-lacing-design-hyperadapt (accessed August 31, 2018).
FDA (U.S. Food and Drug Administration). 2015. Reprocessing medical devices in health care settings: Validation methods and labeling guidance for industry and Food and Drug Administration staff. https://www.fda.gov/downloads/medicaldevices/.../ucm253010.pdf (accessed August 30, 2018).
Fraunhofer. 2018. Chromogenic polymers. https://www.iap.fraunhofer.de/en/research/functional_polymer_systems/chromogenic_polymers.html (accessed August 31, 2018).
FujiFilm. 2018. FujiFilm Prescale. http://www.sensorprod.com/index.php (accessed August 31, 2018).
Gosch, M. E., R. E. Shaffer, A. E. Eagan, R. J. Roberge, V. J. Davey, and L. J. Radonovich, Jr. 2013. B95: A new respirator for health care personnel. American Journal of Infection Control 41:1224–1230.
Heimbuch, B. K. 2018. Presentation at the March 22, 2018, public meeting of the National Academies of Sciences, Engineering, and Medicine Committee on the Use of Elastomeric Respirators in Health Care. Washington, DC. http://nationalacademies.org/hmd/~/media/Files/Activity%20Files/Workforce/ElastomericRespirators/Meeting%202/Heimbuch.pdf (accessed September 17, 2018).
Heimbuch, B. K, W. H. Wallace, K. Kinney, A. E. Lumley, C. Y. Wu, M. H. Woo, and J. D. Wonder. 2011. A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. American Journal of Infection Control 39:e1–e9.
HHS (U.S. Department of Health and Human Services). 2017. HHS pursues reusable respirator to better protect medical providers during pandemics. https://www.phe.gov/Preparedness/news/Pages/reusable-respirator-2Oct2017.aspx (accessed July 2, 2018).
IOM (Institute of Medicine). 2008. Preparing for an influenza pandemic: Personal protective equipment for healthcare workers. Washington, DC: The National Academies Press.
IOM. 2011. Preventing transmission of pandemic influenza and other viral respiratory diseases: Personal protective equipment for healthcare personnel: Update 2010. Washington, DC: The National Academies Press.
Jayaraman, S., and S. Park. 2015. Respiratory protection device. U.S. Patent Application Number 14/734,630, filed June 15, 2015.
Lawrence, C., D. A. Harnish, M. Sandoval-Powers, D. Mills, M. Bergman, and B. K. Heimbuch. 2017. Assessment of half-mask elastomeric respirator and powered air-purifying respirator reprocessing for an influenza pandemic. American Journal of Infection Control 45(12):1324–1330.
Lindsley, W. G., W. P. King, R. E. Thewlis, J. S. Reynolds, K. Panday, G. Cao, and J. V. Szalajda. 2012. Dispersion and exposure to a cough-generated aerosol in a simulated medical examination room. Journal of Occupational and Environmental Hygiene 9(12):681–690.
Lindsley, W. G., J. D. Noti, F. M. Blachere, R. E. Thewlis, S. B. Martin, S. Othumpangat, B. Noorbakhsh, W. T. Goldsmith, A. Vishnu, J. E. Palmer, K. E. Clark, and D. H. Beezhold. 2015. Viable influenza A virus in airborne particles from human coughs. Journal of Occupational and Environmental Hygiene 12(2):107–113.
Lindsley, W. G., F. M. Blachere, D. H. Beezhold, R. E. Thewlis, B. Noorbakhsh, S. Othumpangat, W. T. Goldsmith, C. M. McMillen, M. E. Andrew, C. N. Burrell, and J. D. Noti. 2016. Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza and Other Respiratory Viruses 10(5):404–413.
Mills, D., D. A. Harnish, C. Lawrence, M. Sandoval-Powers, and B. K. Heimbuch. 2018. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. American Journal of Infection Control 46(7):e49–e55.
Perala, A. 2018. Samsung Galaxy S10 to sport 3D facial recognition and in-display fingerprint scanning. https://mobileidworld.com/samsung-galaxy-s10-biometrics-906262 (accessed September 11, 2018).
Radonovich, L. R., A. Baig, R. E. Shaffer, R. Roberge, A. Levinson, D. F. Doerr, and V. Davey. 2009. (PROJECT B.R.E.A.T.H.E): A report of an interagency working group of the U.S. federal government. https://www.cdc.gov/niosh/npptl/hospresptoolkit/pdfs/ProjectBREATHE-final-report-508.pdf (accessed January 24, 2019).
Rogers, W. J. 2008. Sterilisation of polymer health care products. Shropshire, UK: Smithers Rapra Press.
Rogers, W. J. 2012. Sterilisation techniques for polymers. In S. Lerouge and A. Simmons (eds.). Sterilisation of biomedical materials and medical devices. Sawston, UK: Woodhead Publishing. Pp. 151–211.
SoClean. 2018. SoClean. https://www.soclean.com/soclean (accessed September 11, 2018).
Subhash, S. S., M. Cavaiuolo, L. J. Radonovich, A. Eagan, M. L. Lee, S. Campbell, and R. A. Martinello. 2014. Effectiveness of common healthcare disinfectants against H1N1 influenza virus on reusable elastomeric respirators. Infection Control and Hospital Epidemiology 35(7):894–897.
Torres, G., H. Lyons, and P. Emerson. 1960. The effects of intermittent positive pressure breathing on the interpulmonary distribution of inspired air. American Journal of Medicine 29:946–954.
Wallace, R. 2018. Presentation at the May 22, 2018, public meeting of the National Academies of Sciences, Engineering, and Medicine Committee on the Use of Elastomeric Respirators in Health Care. Washington, DC.
Wizner, K., L. Radonovich, A. Bell, C. Oke, and M. Yarbrough. 2018. Feasibility assessment of a new surveillance tool for respiratory protective devices used in healthcare. Journal of the International Society for Respiratory Protection 35(1):26–35.
Zhou, J., J. Wei, K.-T. Choy, S. F. Sia, D. K. Rowlands, D. Yu, C.-Y. Wu, W. G. Lindsley, B. J. Cowling, J. McDevitt, M. Peiris, Y. Li, and H.-L. Yen. 2018. Defining the sizes of airborne particles that mediate influenza transmission in ferrets. Proceedings of the National Academy of Sciences of the United States of America 15(10):E2386–E2392.
This page intentionally left blank.






















