1
Introduction
Protecting the health and safety of health care workers is vital to the health of each of us. Preparing for and responding to a future influenza pandemic or a sustained outbreak of an airborne transmissible disease requires a high-level commitment to respiratory protection for health care workers across the wide range of settings in which they work and the jobs that they perform. Keeping health care workers healthy is an ethical commitment both in terms of addressing the occupational risks faced by health care workers and of providing for the continuity of patient care and services needed to maintain the health of individuals and communities. During a public health emergency, challenges will arise concerning the availability of respiratory protective devices (i.e., respirators). In response to product shortages during the 2009 influenza pandemic, the Strategic National Stockpile distributed more than 85.1 million N95 disposable filtering facepiece respirators (sometimes referred to as N95s), which was in addition to the inventory that hospitals and other health care facilities already had in stock or had acquired through normal supply chains (NASEM, 2016b).
Reusable respirators (specifically, reusable half-facepiece elastomeric respirators) are the standard respiratory protection device used in many industries, but they are used infrequently in health care (Wizner et al., 2016; Brown et al., 2017). However, the durability and reusability of these respirators make them desirable for stockpiling for emergencies, where the need for large volumes of respirators can be anticipated. Recent experiences with various epidemics and pandemics—severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), H1N1 influenza in 2009, and Ebola in 2014—underscore the vital need to protect the health and safety of health care workers.
Even without the sort of surge in demand for respirators that is created by a pandemic, the respirator supply chain may be interrupted by other factors such as international meteorological events, changing trade policies, and conflict. The potential for a massive global surge in the demand for personal protective equipment (PPE) creates a challenge for manufacturers, suppliers, and health care leaders in planning how to meet the safety needs of their workers. Respiratory protection efforts are also critical to routine health care (e.g., care for patients with tuberculosis) and therefore, hospitals and other health care facilities are mandated in the United States to establish and maintain respiratory protection programs. The joint efforts of the facility’s infection prevention and control, occupational health and safety, and industrial hygiene programs are focused on creating a safe and healthy work and patient care environment. This report specifically focuses on one type of respirator—half-facepiece reusable elastomeric respirators1—and explores the efficacy, effectiveness, and implementation issues associated with this type of respiratory protection in both routine use and during a public health emergency.
STUDY BACKGROUND AND SCOPE
In 2005, the National Personal Protective Technology Laboratory (NPPTL) at the National Institute for Occupational Safety and Health (NIOSH) asked the National Academies of Sciences, Engineering, and Medicine to form a standing committee to provide strategic guidance in addressing PPE issues for a wide range of workers. Additionally, the National Academies have conducted a number of relevant workshops and ad hoc studies, including Preparing for an Influenza Pandemic: Personal Protective Equipment for Healthcare Workers and Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnel (IOM, 2008, 2011b).
In 2017, NPPTL and the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention requested that the National Academies conduct a study on the use of half-facepiece reusable elastomeric respirators in health care. This report is the result of that request.
___________________
1 For the purposes of this report, the term reusable elastomeric respirator will refer to the half-facepiece configuration of reusable elastomeric respirators.
To address the study’s Statement of Task (see Box 1-1), the National Academies appointed a 16-member committee with expertise in occupational health, industrial hygiene, clinical care, infection prevention and control, respiratory protection engineering and design, health care workforce development and training, health care supply distribution, and emergency preparedness. Brief biographies of each of the 16 members of the committee can be found in Appendix B.
The committee held four in-person meetings, of which the first three included public workshops and sessions with invited speakers providing their expertise on the topics relevant to the Statement of Task. The fourth and final meeting included a short, open-to-the-public session via Web conference. The agendas for the public sessions can be found in Appendix A. Additionally, the committee reviewed the published scientific literature and considered information and input provided by the public and various agencies and organizations.
GUIDING PRINCIPLES
After examining the complexities and challenges surrounding the use of reusable elastomeric respirators in health care, the committee identified the following set of principles to guide its work:
- All health care workers in all settings are important. Health care workers’ safety and good health are vital to the health of the public and patients, as well as to our economy and national security, both on a day-to-day basis and during public health emergencies and in a range of settings—from homes to hospitals and in rural to urban areas.
- The health of health care workers is an ethical imperative. Health care workers must be fully informed about risks related to respiratory infections and be supplied with methods, education, environments, and equipment for protection. In turn, individual health care workers must fulfill their responsibilities to be aware of, proficient in, and practice respiratory protection.
- Research is the essential basis for good decisions and practices. Public health crises in recent decades have been fraught with uncertainties and tensions between leadership and front-line clini-
-
cians over respiratory protection. The development of strong research data will support practical and harmonized standards, policies, and guidance.
- Effective systems and teams are the basis of safety and health efforts. Employers and clinical leadership need to work collaboratively to establish effective respiratory protection programs and together with health care workers take on the responsibilities to champion, monitor, and enforce respiratory protection. The use of respirators and other PPE is one part of an integrated set of prevention and control strategies that include engineering, regulatory, administrative, educational, work practice, and environmental measures that collectively create the operational environment necessary to protect and sustain the health and safety of health care workers.
FOCUSING ON ELASTOMERIC RESPIRATORS
As noted above, this study follows a number of studies by the National Academies on PPE for health care workers (IOM, 2006, 2008, 2009, 2011a,b; NASEM, 2017). Prior studies and workshops that have explored the issues of protecting the health and safety of health care workers have focused largely on disposable filtering facepiece respirators, powered air-purifying respirators (IOM, 2015), and a range of other protective equipment including gowns, gloves, and eye protection (IOM, 2008, 2011b). Following its Statement of Task, this study instead took an in-depth look at reusable elastomeric respirators and considered the use of these respirators both in routine health care settings and during public health emergencies.
In recent years, there have been ongoing concerns about the potential for a severe influenza pandemic as well as rising concerns about the emergence of other infectious diseases that may be transmitted by airborne particles. Experiences with bioterrorist attacks in the early 2000s, combined with the appearance of novel viruses with pandemic potential—H5N1, SARS, and MERS—the 2009 H1N1 influenza pandemic, and the Ebola outbreak in Western Africa in 2014, have led public health and health care institutions to examine the preparedness of respiratory protection programs for handling potentially high-consequence infections. A catastrophic event, such as the 1918 influenza pandemic, can disrupt the social networks and the basic services of a so-
ciety. Even though the mortality rate of the 1918 pandemic was just 2 percent in developed nations, public fear and the sheer volume of affected individuals rapidly overwhelmed basic services and health care facilities (Barry, 2017). The impact of future pandemics may be mitigated to some extent by modern methods of communication and surveillance; the delivery of community countermeasures ranging from social distancing to antivirals, vaccines, and antibiotics; and other modern medical technology. However, it is clear that securing access to a health care workforce that is both willing and able to work will be key to minimizing patient and health care worker morbidity and mortality. Recent experiences with the SARS and Ebola epidemics and with the 2009 H1N1 influenza pandemic, which was considered mild, demonstrate both the importance of the health care workforce in responding to a public health emergency (Murray et al., 2010; Martin, 2011; NASEM, 2016a; Le et al., 2018) and how surges in demand for disposable respirators during the emergence of a new pathogen can quickly outstrip the available supply (IOM, 2011b; Beckman et al., 2013; Patel et al., 2017). The 2003 SARS outbreak was characterized by extensive transmission occurring in a health care setting, with more than 7 out of 10 cases involving health care workers, patients, or visitors in health care institutions. In a review of the outbreak, the SARS Commission (2006) reported that in Canada health care workers accounted for 45 percent of all confirmed or suspected SARS cases transmitted in a health care setting. Other countries also experienced a high burden of cases associated with transmission in health care settings. In Singapore, health care workers accounted for more than 40 percent of all cases linked to a health care setting (Chowell et al., 2015). Shortages of respirators and an initial overwhelming of the respirator supply chain occurred during the 2009 H1N1 pandemic (Patel et al., 2017). Estimates of the demand for respirators in a future severe pandemic (on the scale of the 1918 pandemic) range from 1.7 billion to 3.5 billion disposable filtering facepiece respirators (Patel et al., 2017).
Reusable respirators may provide a possible solution for emergency situations. They also deserve consideration for use in routine health care settings where factors such as cost, time, effort, and ethics are driving efforts to deliver quality care for good value.
ETHICAL CONTEXT
Although this report is focused on one particular type of respiratory protective device—reusable elastomeric respirators—the key issues that the report grapples with are much broader and center on ensuring the safety and health of health care workers and the continuity of high-quality patient care. “Health care worker” has long been acknowledged as a profession with potentially dangerous and even life-threatening risks; as such, there is a concomitant ethical obligation to continuously make efforts to improve and ensure health care worker safety and health (see Box 1-2).
The committee emphasizes the ethical considerations that are needed in considering prevention and mitigation efforts across the full range of worker protections. During a public health emergency, such as an influenza pandemic, health care workers, their families, and their employers will be forced to address complex, ethical quandaries associated with the prioritization of workplace health and safety. A 2008 Institute of Medicine (IOM) report noted,
The expertise of healthcare workers is an integral and principal component of the response to a pandemic. Heightened work demands and increased chance of exposure to infectious agents will necessitate that healthcare workers and employers evaluate responsibilities with regard to the personal safety of the worker, his or her duty to work, and the safety and care of the employee’s family members. Discussions of these responsibilities point to the need for an ethical framework for pandemic planning that considers the balance of reciprocity, beneficence, and autonomy in decision making. (IOM, 2008, p. 23)
DEFINITIONS AND TERMINOLOGY
Health Care Workers
In the context of this report the committee has chosen to use the definition of health care worker or health care personnel provided in the 2011 IOM report Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnel. According to that report, health care personnel encompass
all workers in direct patient care and support services who are employed by private and public healthcare offices and facilities as well as those working in home healthcare and emergency medical services, including those who are self-employed. This broad definition of healthcare personnel encompasses those working in administration, patient care, and facilities upkeep, and it includes health professional students who are receiving instruction or who are working in healthcare facilities as well as volunteers trained to provide systematic, regulated, and licensed healthcare services (including emergency medical responders) (IOM, 2008, 2009). All relevant work situations with the potential for infection risk (e.g., cleaning patient rooms, delivering food) are considered part of the health care workforce. (IOM, 2011b, p. 21)
The health care field in the United States employs more than 16 million workers in a wide variety of health care facilities (see Table 1-1). Of those in the health care workforce, approximately 36 percent are employed by hospitals, 15 percent work in physician offices, 20 percent
work in nursing and residential care facilities, 8.5 percent are employed in home health care, and 20 plus percent work in other settings (BLS, 2018).
TABLE 1-1 Health Care Workers, Location of Employment
| Employment Location | May 2017 Employment |
|---|---|
| Hospitals (public and private) | 6,001,810 |
| Nursing and residential care facilities | 3,324,640 |
| Offices of physicians | 2,547,640 |
| Home health care service | 1,396,570 |
| Offices of dentists | 932,040 |
| Outpatient care centers | 880,400 |
| Offices of other health practitioners | 876,010 |
| Other ambulatory health care services | 298,580 |
| Medical and diagnostic laboratories | 266,010 |
| Total | 16,523,700 |
SOURCE: BLS, 2018.
Routine and Surge Use
This report examines two distinct circumstances in which reusable elastomeric respirators could be considered for use in health care settings—routine use and surge use. Routine use refers to those circumstances when current clinical scenarios—in the absence of notably increased clinical activity—require the use of a respirator to protect health care workers from airborne contaminants (OSHA, 2009). The potential benefits of deploying reusable elastomeric respirators in routine use include an increased familiarity among institutional and health care workers with these devices in the event of a public health emergency as well as possible improvements in fit and protection. Another factor to take into account when considering these respirators for routine use is the frequency of health care workers’ potential interactions with airborne contaminants. The number of occasions when respirators are used may vary widely among health care settings, from essentially zero in ambulatory care settings to multiple daily uses in certain specialized inpatient settings, such as hospitals dedicated to the care of patients with tuberculosis or other diseases transmitted by infectious aerosols. In high-income countries, where tuberculosis, measles, varicella, and most other airborne transmissible infections are infrequent, most respirators are worn during
aerosol-generating procedures and while caring for patients who are being “ruled out” for tuberculosis, a process that takes 1 to 3 days and where the vast majority of patients will, in fact, be ruled out. The frequency at which any individual health care worker is required to use a respirator also varies widely, even within the same institution and the same unit. For instance, a nurse or respiratory therapist assigned to care for a patient with presumed or confirmed tuberculosis may need to wear a respirator numerous times per shift for a few days, but then that individual may not need to wear one for weeks or months.
The second circumstance is surge use, defined as use in times when there is a sharply increased demand for the respirators, such as when there is a sudden or rapidly progressive influx of patients at a given point in time (Barbisch and Koenig, 2006; Welzel et al., 2010; Veneema et al., 2018). Surge use of respirators may occur in public health emergency situations, such as an influenza pandemic, in which cases of novel influenza extend beyond the epidemic curve or in other atypical outbreaks that require airborne isolation precautions (Carias et al., 2015). A health care system’s ability to handle such surges is a critical aspect of its ability to provide a safe working environment, and, unfortunately, is often an area of weakness when responding to public health emergencies or other disasters (Barbisch and Koenig, 2006; Welzel et al., 2010; Veneema et al., 2018). The committee chose to use the term “surge” to describe those urgent situations that could be of short or long duration (and could be geographically widespread or more limited in location) but where there is a critical need for respiratory protection that could go beyond the health care system’s ability to respond and would necessitate being prepared.
During a public health emergency response, protecting health care workers from infectious disease transmission is essential, given that these workers provide clinical care to those who fall ill, have a high risk of exposure, are limited in number, and need to be assured of workplace safety. Recent history demonstrates that, in the absence of advanced planning, increasing equipment supplies including PPE in the midst of a public health emergency will be challenging, if not impossible (Patel et al., 2017). Health care workers may need to use respiratory protection over an extended period of time, potentially exhausting the supply. Furthermore, the U.S. supply chain for PPE, including respirators, has only a minimal ability to provide a rapid surge in production, which will make it challenging to meet the sort of large, unexpected increases in demand that can occur during a public health emergency (Banach et al., 2017; Patel et al., 2017). During the public health responses to the 2009 H1N1
influenza pandemic and the 2014 Ebola virus epidemic, the commercial supply chain—which included manufacturers, distributors, and end users (pharmacies/hospitals) of pharmaceutical and health care products—was critical to the response. Surge needs for PPE may be determined by either an individual institution or public health officials (Banach et al., 2017). If a health care system is to be able to respond to an infectious disease catastrophic event—and consequently to minimize victims’ suffering and mortality—the health care workforce must be willing and able to work and provide care under conditions of duress. The benefits to having reusable elastomeric respirators available during surge events include potentially averting a shortage of effective respiratory protection and sending a signal to health care workers that the institution is investing in their safety and well-being. During and after a public health crisis, “the survival rates of victims will be dependent upon the ability of hospitals and ambulance services to ‘surge up’ and allocate scarce resources” (Veneema et al., 2018, p. 1).
Medical Masks and Respirators
Medical masks are loose, unfitted devices that cover the nose and the mouth of the user and provide protection for the environment from the user’s cough and exhaled secretions (see Table 1-2 for a comparison with respirators) and do not provide a face seal or require fit testing. Medical masks, which is the term used in this report to refer to both surgical masks and procedure masks (also called face masks), are not designed or approved to provide protection for the user against exposure to airborne contaminants, such as infectious aerosols. In general, medical masks may function to provide some protection by acting as an immediate physical barrier to contact with splashes and large droplets encountered in the clinical setting, but do not provide a full face seal to reduce exposure to particulate matter in the air.
A respirator is a NIOSH-approved (in the United States) device that protects the user from inhaling airborne contaminants. The proper functioning of tight-fitting respirators requires that the device be properly fitted and sealed to the face. The term respirator has a dual meaning in the health care field—either as a device that protects the user from inhaling hazardous particles (the product that is the subject of this report) or a mechanical ventilator device that maintains the respiratory functions of an intubated patient. This dual meaning of the term “respirator” has resulted in the blanket use of the term “masks” to refer to both medical
masks and respirators and has led to the blurring of the distinct uses and levels of protection provided by each device. This conflation can result in
TABLE 1-2 Comparison of Medical Masks and Respirators
| Medical Masks | Respirators | |
|---|---|---|
| Intended use | To protect the patient or others from the wearer’s expired respiratory droplets or other large droplet exposures (e.g., during wound irrigation) | To protect the wearer from inhaled exposure to hazardous airborne particles |
| Face seal requirements | Not designed to fit and seal to the face | Designed to fit and seal tightly to the face |
| Fit-testing requirements | None | Annual fit testing required |
| User seal check requirements | No user seal check possible | User seal check recommended before each use |
| Certification requirements | FDA reviews 510(k) submission and clears for marketing | Approved by NIOSH under 42 CFR 84 |
| Sizing | Generally only one size is available | Multiple sizes are available |
NOTES: Face seal, seal check, and fit-testing requirements apply only to tight-fitting respirators and not to loose-fitting PAPRs. CFR = Code of Federal Regulations; FDA = U.S. Food and Drug Administration; NIOSH = National Institute for Occupational Safety and Health; PAPR = powered air-purifying respirator.
SOURCE: Adapted from IOM, 2008.
risks to health care workers as medical masks are often more accessible than respirators, but do not provide the required protection for the user.
It is therefore important to delineate these terms and keep the terms “medical mask” and “respirator” distinct in the clinical setting. Maintaining the important distinction between these terms could be further supported though the development of easy-to-understand ratings that illustrate the protective factors and standard uses of these products (IOM, 2008).
Respirator Terminology
The public and even many users of respiratory protection often use descriptive terminology related to respiratory protective devices incorrectly. While the development of colloquial vernacular is expected, misuse of respirator-related terminology can create confusion over how different respirators and filters are identified and how they function to provide protection to the user across a variety of potentially hazardous environments. The glossary provided in the Hospital Respiratory Protection Program Toolkit is a useful reference (OSHA and NIOSH, 2015).
Respirators can be classified by a number of characteristics (see Box 1-3). The two primary respirator classes are air-purifying respirators, which use filters and cartridges to remove air contaminants (e.g., particulate matter), and air-supplying respirators, which provide the user with clean air from a separate source (OSHA, 2018). Respirator fit can also be used to classify respirators: tight-fitting respirators require fit testing and a tight seal to the face; loose-fitting respirators do not require fit testing. Filter characteristics can provide information about the nature of the respirator and its efficiency, as well as about whether the filter constitutes the entire facepiece or there are filter cartridges used (see Box 1-3).
Terms describing the design of the respirator (quarter mask, half mask, full mask) are used to indicate the extent of facial coverage. Other terms indicate whether the respirator is designed for a single use (disposable) or for multiple uses with cleaning, disinfection, and maintenance between uses (reusable).
The committee took care to be specific about the terminology for the three types of respirators most often used in health care (see Figure 1-1 and further details below and in Chapter 2). All three of these types are air-purifying respirators.
- Disposable filtering facepiece respirators: Often referred to by the health care community simply as N95s, these respirators are half-facepiece respirators in which the facepiece is formed directly from a filter material (i.e., a filtering facepiece). They are designed to be disposable after one use. These respirators may be made up of N95 filter media, but high-efficiency P100 media can also be used in this class of respirators. Fit testing is required. Although the term is lengthy, the committee wants to be clear in its descriptions and therefore chose to use the term “disposable filtering facepiece respirator.”
- Reusable elastomeric respirators: These respirators are made from elastomeric materials, which consist of long coiled polymer chains and which can withstand high elastic deformation without rupture (Cardarelli, 2008). The respirator can be cleaned, disinfected, and reused. In health care, the half-mask configuration is frequently used. These respirators have replaceable filters or cartridges and inhalation and exhalation valves. Fit testing is required. These respirators do not filter particles from exhaled breath. The committee chose to refer to this type of respirator as
- Powered air-purifying respirators (PAPRs): The PAPR uses a battery-powered blower to draw air through the filter and into a hood or facepiece. Loose-fitting models do not require fit testing. The blower units are reusable after cleaning and disinfection. By design, these models do not filter particles from exhaled breath. The committee refers to this type of respirator as a “powered air-purifying respirator,” or “PAPR.”
a “reusable elastomeric respirator” throughout this report and will use this term to refer to the half-facepiece configuration, unless otherwise specified.
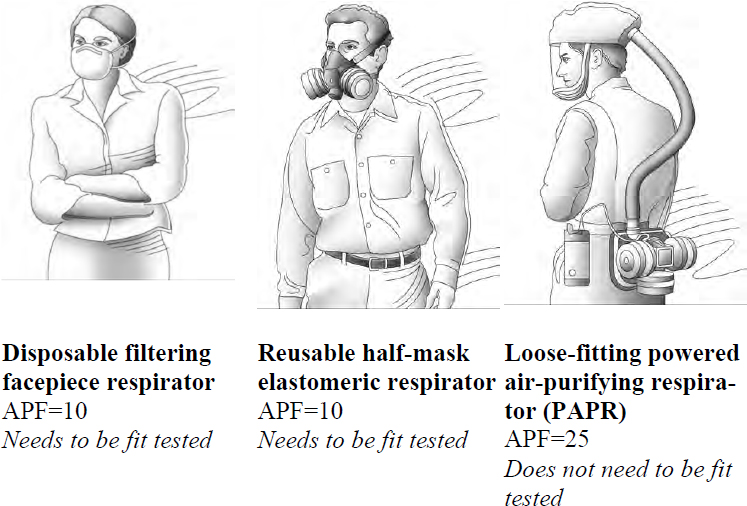
SOURCE: Adapted from OSHA, 2009.
PROTECTING THE HEALTH AND SAFETY OF HEALTH CARE WORKERS: THE RANGE OF HAZARD CONTROLS AND UNIQUE CHARACTERISTICS OF THE WORK SETTING
Protecting health care workers from workplace risks has traditionally involved a range of administrative, engineering, and environmental hazard controls designed to ensure workplace safety and to integrate into a larger system of accountability and enforcement. The overarching goals of these controls are to minimize the number of health care workers exposed, limit the intensity of exposure, and provide the best available protection (see Figure 1-2).
The first steps in protecting the health and safety of workers are hazard and exposure assessments—knowing what chemicals or other hazards are in the work environment and assessing the levels of potential exposure. However, in the health care environment these assessments are often challenging in the health care environment due to the lack of quantitative data on infectious doses and transmission routes and the dearth of ready-to-use measurement tools to assess workplace exposure. As such, it is often difficult to assess the adequacy of respirator performance and other potential control measures.
Engineering and environmental controls, such as air exchanges and negative-pressure rooms, seek to isolate and remove potentially hazardous material from the environment. Administrative controls include a range of policies and procedures that limit health care worker exposure to risk and require greater institutional and health care worker compliance; some examples of these controls are early case recognition, source control, protocol-driven clinical recognition, and early isolation of patients with suspect clinical syndromes or epidemiological risk factors. This level of control also includes the availability of PPE as well as policies and training on its proper use (Thorne et al., 2004). The correct selection and use of PPE and a consistent adherence to safety practices by individual health care workers are two other essential types of controls.
Respirators and other PPE offer direct protection for health care workers who care for patients with airborne transmissible infections. Designated individuals are included in the facility’s respiratory protection program. These individuals undergo a medical evaluation to ensure
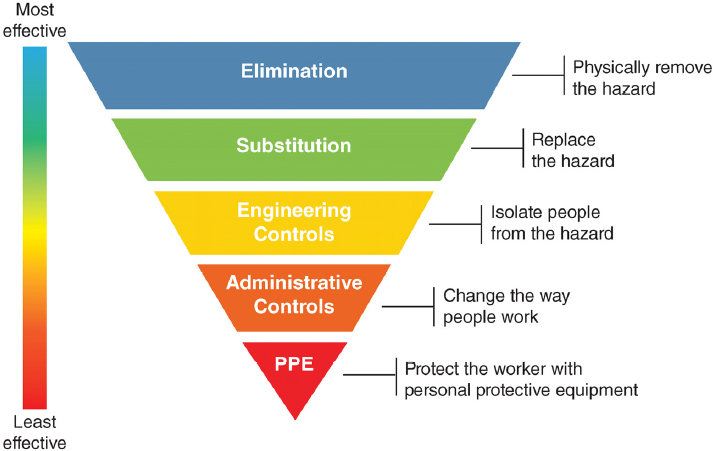
NOTE: PPE = personal protective equipment.
SOURCE: NIOSH, 2018.
they do not have conditions that would prevent respirator use (e.g., certain heart conditions, lung disease), and they are fit tested to a specific make, model, and size of respirator. They are also instructed on the proper use, maintenance, and disposal of the respirator. When caring for patients for whom it has been determined that airborne infectious isolation precautions are needed, these health care workers are expected to wear the specific respirator to which they have been fit tested and trained. In most hospitals, a loose-fitting PAPR, which does not require fit testing, is available for use during certain high-risk procedures or for staff who cannot wear a tight-fitting respirator (e.g., those who have beards).
Hospitals and health care facilities go to great lengths to integrate these control strategies into the workplace in order to create a safe and healthy working environment for the members of their workforce, whom society asks and expects to respond to a health crisis. However, these practices—led by infection prevention and control, occupational health, and industrial hygiene programs—are not consistently applied throughout health care institutions, and therefore, these protections and adequate training for their implementation are not universally available to all health care workers. Furthermore, depending on the nature of the health
care worker’s work, it may not be practical to engage the full array of controls for the prevention of transmission of airborne transmissible diseases. For example, it is often impractical for health care workers to distance themselves from the hazard, since the job requires close physical contact with the patient. Table 1-3 provides an example of the patient history and physical assessment for a patient with an airborne transmissible disease.
Furthermore, health care is unique in that the hazards facing its workers are often unknown, the workers’ responsibilities are fluid and unpredictable, and there is an expectation of human touch in providing care. Cumbersome respirators and poorly designed safety procedures can interfere with care. In addition, the consequences of exposure and subsequent infection are often inconsistent and delayed and usually cannot be clearly associated with any individual patient interaction. This can result in inaccurate perceptions of individual risk and inconsistent adherence to protective procedures.
| Control Methodology | Health Care Example | Comment |
|---|---|---|
| Elimination of the hazard | Transfer of the patient to a biological containment unit | Specialized units are a limited resource and staff providing care may still be exposed. |
| Substitution | None | Not feasible, The patient with the airborne transmissible disease needs care. |
| Engineering controls | Use of airborne infection HEPA isolation rooms; use of negative-pressure rooms | Specialized rooms are often a limited resource and limited in availability. Health care workers who staff the room and provide care will still be exposed. |
| Administrative controls | Putting a mask on a patient who is coughing and sneezing; limiting visitors | Generally only partially effective in controlling aerosol emissions. Some patients cannot tolerate a mask. |
| PPE | Use of respirators and other PPE | Challenges to respirator use include proper fit of the respirator. |
NOTE: HEPA = high-efficiency particulate air; PPE = personal protective equipment.
OVERVIEW OF RESPIRATORY PROTECTION
Respiratory Protection Programs
Respiratory protection programs are a critical component of the hazard control and prevention strategies used in health care institutions and many other workplaces to ensure worker health and safety. In the United States these programs are mandated by OSHA in workplaces “with harmful dusts, fogs, fumes, mists, gases, smokes, sprays, or vapors”2 and in which effective engineering controls are not feasible or entirely effective.
As noted in the Code of Federal Regulations:
A respirator shall be provided to each employee when such equipment is necessary to protect the health of such employee. The employer shall provide the respirators which are applicable and suitable for the purpose intended. The employer shall be responsible for the establishment and maintenance of a respiratory protection program, which shall include the requirements outlined in paragraph (c) of this section. The program shall cover each employee required by this section to use a respirator.
As outlined by OSHA, the major components of respiratory protection programs are
- A designated respiratory program administrator to oversee the program and conduct evaluations of the program’s effectiveness;
- A written respiratory program with procedures specific to the workplace;
- The provision of respirators, training, fit testing, and medical evaluations at no cost to the employee;
- Procedures for selection of an appropriate NIOSH-approved respirator;
- Medical evaluations for employees required to use respirators;
- Fit-testing procedures;
- Procedures for the use and maintenance of respirators, including the cleaning, disinfecting, storing, and disposal of respirators;
___________________
2 29 CFR 1910.134.
- The training of employees on the respiratory hazards they are facing or potentially facing in the workplace during routine and emergency situations;
- The training of employees in donning and doffing respirators and in the maintenance of the equipment; and
- Procedures for evaluating the effectiveness of the respiratory protection program.
Respiratory protection programs are often implemented by collaborations of the occupational safety and health personnel and infection prevention and control staff. Key aspects of successful respiratory protection programs go beyond simply fit testing and providing the respirator. These programs should encompass commitment to worker safety and health by health care leaders, including the health care administration; development and implementation of data-driven policies; monitoring of compliance with respirator use by management; effective education and training in all aspects of a comprehensive respiratory protection program; and thorough program evaluation done on a regular basis that leads to appropriate modification of the program (Joint Commission, 2014; OSHA and NIOSH, 2015).
Filter and Fit of Respirators
The two critical components for assessing the efficacy of an air-purifying respirator are the filter’s efficiency and the respirator’s fit.
Filter Efficiency
Air-purifying respirators require the use of a filtration system to prevent inhalation of hazardous particles by the user. The filtration media can constitute the entire facepiece (e.g., a filtering facepiece respirator) or else can be incorporated into cartridges or another filter mechanism (as on many elastomeric respirators). NIOSH classifies filters by their ability to capture a wide range of workplace hazards (see Table 1-4) and
TABLE 1-4 Efficiency Ratings for Respirator Filters
| If the Efficiency Level Is | This Means |
|---|---|
| 100 | The filter is expected to trap 99.97 particles* out of every 100. It is as efficient as a high-efficiency particulate air (HEPA) filter. |
| 99 | The filter is expected to trap 99 particles* out of every 100. |
| 95 | The filter is expected to trap 95 particles* out of every 100. |
*0.3 μm particle.
SOURCE: NIOSH, 2012.
provides a system for identifying those filters that are to be used in certain environmental conditions, such as when oils are present.3 The filter’s efficiency is designated by the minimum percentage of particles that are captured by the respirator at the particle size of 0.3 µm mass median aerodynamic diameter, which is considered the most difficult particle size to capture. Consequently, both smaller and larger particles are captured at a higher efficiency. An efficiency rating of 100 provides the highest level of protection for the size of particles for which the filter has been tested. The filter ratings were initially developed for industrial particulate exposures, but recent studies have demonstrated that these ratings are likely appropriate for bioaerosol exposures in health care settings (Qian et al., 1998; Rengasamy et al., 2008; Gardner et al., 2013). These studies challenged N95 and P100 filters with viruses, bacteria, and nanoparticles in order to confirm filter efficiency.
Fit
Fit testing assesses the ability of the respirator to seal around a user’s face during use. OSHA requires that tight-fitting respirators be fit tested, either qualitatively or quantitatively, before they are used. During use,
___________________
3 Oil resistance for filters (designated by an “R” or “P” preceding the efficiency rating) is important and relevant in many industrial situations. Oil is unlikely to be present in the air in the health care field, and filters suitable to the health care environment (designated by an “N” preceding the efficiency rating) are more commonly used. There is little practical difference between using an oil-resistant filter or an N filter in terms of service life or breathing resistance in a health care setting, and they can be used interchangeably, if needed.
movement of the respirator on the face is inevitable, and the ability of the respirator to conform and reseal to the face is simulated during fit testing. Talking, grimacing, bending over, and movement of the head can result in different testing results. Research has demonstrated that fit testing and the fitting characteristics of a respirator model are both associated with performance (Coffey et al., 2004; Lawrence et al., 2006). The annually required fit test and the accompanying training and feedback to the workers are essential to help the worker achieve optimal performance during actual working conditions. The fit-test passing rate allows employers to select respirators with fitting characteristics that are most likely to fit the greatest percentage of their workforce, which can help eliminate both the need for repeated fit tests and the purchasing of equipment that is unlikely to be used. This can result in substantial savings in cost and time (Lawrence et al., 2006). Respirators come in a variety of sizes in order to fit various sizes of heads and face shapes; even so, some individuals are not able to pass a fit test. Furthermore, individuals with beards, facial hair, or other facial characteristics that do not allow the user to obtain a satisfactory fit need to use a loose-fitting respirator that covers the face, often a PAPR.
Respirators Used in Health Care
Respirators are used in health care for a variety of reasons. The most prevalent reason is to protect staff from exposure to airborne transmissible diseases. Other uses in health care include protection from the chemical, biological, or radiological hazards associated with emergency response; maintenance activities (e.g., asbestos abatement, mold remediation); laboratory analysis (e.g., microbiology preparations, gross anatomy and tissue preparation); hazardous waste handling; and dealing with hazardous medications.
As part of an infection prevention and control strategy beyond the standard precautions for all patient care, the Centers for Disease Control and Prevention (CDC) has outlined transmission-based precautions that specify the type of PPE needed based on whether there are risks of contact, droplet, or airborne transmission (CDC, 2017). Precautions against airborne risks include a range of environmental controls (e.g., airborne infection isolation rooms) and administrative controls (e.g., immunization for vaccine-preventable infections, limiting health care workers who enter the room, limiting transport and movement of patients), in addition to PPE. The airborne precautions note that individuals should “use per-
sonal protective equipment (PPE) appropriately, including a fit-tested NIOSH-approved N95 or higher level respirator for healthcare personnel” (CDC, 2017) (see Figure 1-1).
Respirators are used relatively infrequently in routine health care (Brown et al., 2017); the most common uses are in emergency care and respiratory care situations. The majority of health care facilities in the United States have opted to provide their health care workers with disposable filtering facepiece respirators or PAPRs, with some limited use of reusable elastomeric respirators (Wizner et al., 2016). The committee is aware of only two health care facilities in the United States, the University of Maryland Medical Center and the Texas Center for Infectious Disease, that currently use (or recently have used) reusable elastomeric respirators either exclusively or primarily (Wizner et al., 2016; Brown et al., 2017; also see Chapter 2). However, given recent pandemic and emergent disease concerns as well as the potential for supply chain limitations, options for reusable respirators are being explored. Chapter 2 delves further into reusable respirators, but to better understand the benefits and challenges of using reusable elastomeric respirators in health care, it is useful to begin by understanding the alternatives. The next two subsections offer brief descriptions of the two main alternatives.
Disposable Filtering Facepiece Respirators
As noted earlier, the disposable filtering facepiece respirator has a filter as an integral part of the facepiece, or is composed from the filtering medium. Such a respirator has an assigned protection factor of 10, meaning that, when fit tested and used properly, it reduces the user’s exposure by 90 percent compared to unprotected exposure4 (see further discussion in Chapter 2) (Lenhart et al., 2004). Fit testing is required prior to use. These respirators are designed to be disposable, single-use items, although efforts are ongoing to determine if it is possible to extend the use (IOM, 2006; Bergman et al., 2012; Fisher and Shaffer, 2014; Zhu et al., 2014) (see Table 1-5).
___________________
4 Assigned protection factor means the workplace level of respiratory protection that a respirator or class of respirators is expected to provide to employees when the employer implements a continuing, effective respiratory protection program (29 CFR 1910.134).
| Strengths | Limitations |
|---|---|
|
|
Powered Air-Purifying Respirators (PAPRs)
The PAPR is an air-purifying respirator with a powered blower. The PAPR’s assigned protection factor is 25 for devices equipped with a loose-fitting facepiece, helmet, or hood and 1,000 for tight-fitting facepiece models and some hooded models (when these hooded models are tested and identified by the manufacturer as performing at a level of protection of 1,000 or greater) (OSHA, 2009). This means that the PAPR provides greater protection than a disposable filtering facepiece respirator. In health care, the loose-fitting PAPR style with a hood, head cover, or
loose-fitting facepiece is preferred over a tight-fitting mask that may be used in other industries. Loose-fitting PAPRs do not require fit testing or other user characteristics such as a clean shaven face. Although PAPRs provide superior respiratory protection compared with disposable filtering facepiece and reusable elastomeric respirators, these battery-powered respirators may have physiologic and ergonomic impacts from the weight and noise of the devices (Lenhart et al., 2004; IOM, 2015). Additionally, charged batteries are needed to maintain equipment operation. The initial costs of PAPRs are substantially greater than those of reusable elastomeric respirators (discussed in Chapter 3) (see Table 1-6).
TABLE 1-6 Strengths and Limitations of Powered Air-Purifying Respirators for Use in Health Care
| Strengths | Limitations |
|---|---|
|
|
OVERVIEW OF THE REPORT
This report covers the breadth of the committee’s Statement of Task. Chapter 1 includes the report’s guiding principles, ethical context, terminology, and background. Chapter 2 discusses elastomeric respirators in depth, with a focus on studies on efficacy, use, and disinfection. Implementation issues regarding reusable elastomeric respirators are discussed in Chapter 3, with topics ranging from fit testing to emergency stockpiling. In Chapter 4, the focus is on research needs, particularly on improving the design and effectiveness of elastomeric respirators for health care workers, with an emphasis on the issues that arise in patient care. The
report concludes in Chapter 5 with the committee’s conclusions and recommendations that explore the feasibility and benefits of the use of elastomeric respirators in health care during routine use and during public health emergencies, with recommended actions for next steps.
REFERENCES
Banach, D. B., B. L. Johnston, D. Al-Zubeidi, A. H. Bartlett, S. C. Bleasdale, V. M. Deloney, K. B. Enfield, J. A. Guzman-Cottrill, C. Lowe, L. Ostrosky-Zeichner, K. J. Popovich, P. K. Patel, K. Ravin, T. Rowe, E. S. Shenoy, R. Stienecker, P. K. Tosh, and K. K. Trivedi. 2017. Outbreak response and incident management: SHEA guidance and resources for healthcare epidemiologists in United States acute-care hospitals. Infection Control & Hospital Epidemiology 38(12):1393–1419.
Barbisch, D. F., and K. L. Koenig. 2006. Understanding surge capacity: Essential elements. Academic Emergency Medicine 13(11):1098–1102.
Barry, J. M. 2017. How the horrific 1918 flu spread across America. Smithsonian Magazine, November. https://www.smithsonianmag.com/history.com/history/journal-plague-year-180965222 (accessed August 9, 2018).
Beckman, S., B. Materna, S. Goldmacher, J. Zipprich, M. D’Alessandro, D. Novak, and R. Harrison. 2013. Evaluation of respiratory protection programs and practices in California hospitals during the 2009–2010 H1N1 influenza pandemic. American Journal of Infection Control 41(11):1024–1031.
Bergman, M. S., D. J. Viscusi, Z. Zhuang, A. J. Palmiero, J. B. Powell, and R. E. Shaffer. 2012. Impact of multiple consecutive donnings on filtering facepiece respirator fit. American Journal of Infection Control 40(4):375–380.
BLS (Bureau of Labor Statistics). 2018. May 2017 national industry-specific occupational employment and wage estimates: NAICS 621000—Ambulatory health care services. https://www.bls.gov/oes/current/naics3_621000.htm (accessed July 27, 2018).
Brown, L. M., B. Rogers, K. Buckheit, and J. P. Curran. 2017. Evaluation of 9 health care organizations’ respiratory protection programs and respiratory protective device practices: Implications for adoption of elastomerics. American Journal of Infection Control 46(3):350–352.
Cardarelli, F. 2008. Polymers and elastomerics. In Materials handbook: A concise desktop reference. 2nd ed. New York: Springer. Pp. 691–750.
Carias, C., G. Rainisch, M. Shankar, B. B. Adhikari, D. L. Swerdlow, W. A. Bower, S. K. Pillai, M. I. Meltzer, and L. M. Koonin. 2015. Potential
demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clinical Infectious Diseases 60:42–51.
CDC (Centers for Disease Control and Prevention). 2017. Transmission-based precautions. https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html (accessed August 10, 2018).
Chowell, G., F. Abdirizak, S. Lee, J. Lee, E. Jung, H. Nishiura, and C. Viboud. 2015. Transmission characteristics of MERS and SARS in the healthcare setting: A comparative study. BMC Medicine 13(1):210.
Coffey, C. C., R. B. Lawrence, D. L. Campbell, and P. A. Jensen. 2004. Fitting characteristics of eighteen N95 filtering-facepiece respirators. Journal of Occupational and Environmental Hygiene 1(4):262–271.
Fisher, E. M., and R. E. Shaffer. 2014. Commentary considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings. Journal of Occupational and Environmental Hygiene 11(8):D115–D128.
Gardner, P. D., J. P. Eshbaugh, S. D. Harpest, A. W. Richardson, and K. C. Hofacre. 2013. Viable viral efficiency of N95 and P100 respirator filters at constant and cyclic flow. Journal of Occupational and Environmental Hygiene 10(10):564–572.
IOM (Institute of Medicine). 2006. Reusability of facemasks during an influenza pandemic: Facing the flu. Washington, DC: The National Academies Press.
IOM. 2008. Preparing for an influenza pandemic: Personal protective equipment for healthcare workers. Washington, DC: The National Academies Press.
IOM. 2009. Respiratory protection for healthcare workers in the workplace against novel H1N1 influenza A: A letter report. Washington, DC: The National Academies Press.
IOM. 2011a. Occupational health nurses and respiratory protection: Improving education and training: Letter report. Washington, DC: The National Academies Press.
IOM. 2011b. Preventing transmission of pandemic influenza and other viral respiratory diseases: Personal protective equipment for healthcare personnel: Update 2010. Washington, DC: The National Academies Press.
IOM. 2015. The use and effectiveness of powered air purifying respirators in health care: Workshop summary. Washington, DC: The National Academies Press.
Joint Commission. 2014. Implementing hospital respiratory protection programs: Strategies from the field. Oakbrook Terrace, IL: The Joint Commission. https://www.jointcommission.org/assets/1/18/Implementing_Hospital_RPP_2-19-15.pdf (accessed August 14, 2018).
Lawrence, R. B., M. G. Duling, C. A. Calvert, and C. C. Coffey. 2006. Comparison of performance of three different types of respiratory protection devices. Journal of Occupational and Environmental Hygiene 3(9):465–474.
Le, A. B., S. A. Buehler, P. M. Maniscalco, P. Lane, L. E. Rupp, E. Ernest, D. Von Seggern, K. West, J. J. Herstein, K. C. Jelden, E. L. Beam, S. G. Gibbs, and J. J. Lowe. 2018. Determining training and education needs pertaining to highly infectious disease preparedness and response: A gap analysis survey of U.S. emergency medical services practitioners. American Journal of Infection Control 46(3):246–252.
Lenhart, S. W., T. Seitz, D. Trout, and N. Bollinger. 2004. Issues affecting respirator selection for workers exposed to infectious aerosols: Emphasis on healthcare settings. Applied Biosafety 9(1):20–36.
Martin, S. D. 2011. Nurses’ ability and willingness to work during pandemic flu. Journal of Nursing Management 19(1):98–108.
Murray, M., J. Grant, E. Bryce, P. Chilton, and L. Forrester. 2010. Facial protective equipment, personnel, and pandemics: Impact of the pandemic (H1N1) 2009 virus on personnel and use of facial protective equipment. Infection Control and Hospital Epidemiology 31(10):1011–1016.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2016a. Global health risk framework: Resilient and sustainable health systems to respond to global infectious disease outbreaks: Workshop summary. Washington, DC: The National Academies Press.
NASEM. 2016b. The Strategic National Stockpile: Origin, policy foundations, and federal context. In The nation’s medical countermeasure stockpile: Opportunities to improve the efficiency, effectiveness, and sustainability of the CDC Strategic National Stockpile: Workshop summary. Washington, DC: The National Academies Press. Pp. 9–30.
NASEM. 2017. Integration of FDA and NIOSH processes used to evaluate respiratory protective devices for health care workers: Proceedings of a workshop. Washington, DC: The National Academies Press.
NIOSH (National Institute for Occupational Safety and Health). 2012. Respirator fact sheet. https://www.cdc.gov/niosh/npptl/topics/respirators/factsheets/respsars.html (accessed October 29, 2018).
NIOSH. 2018. Hierarchy of controls. https://www.cdc.gov/niosh/topics/hierarchy/default.html (accessed September 13, 2018).
OSHA (Occupational Safety and Health Administration). 2009. Assigned protection factors for the revised respiratory protection standard. https://www.osha.gov/Publications/3352-APF-respirators.html (accessed February 8, 2018).
OSHA. 2018. Respirator types. https://www.osha.gov/video/respiratory_protection/resptypes_transcript.html (accessed August 10, 2018).
OSHA and NIOSH. 2015. Hospital respiratory protection program toolkit: Resources for respirator program administrators. https://www.cdc.gov/niosh/docs/2015-117/pdfs/2015-117.pdf?id=10.26616/NIOSHPUB2015117 (accessed August 16, 2018).
Patel, A., M. M. D’Alessandro, K. J. Ireland, W. G. Burel, E. B. Wencil, and S. A. Rasmussen. 2017. Personal protective equipment supply chain: Lessons
learned from recent public health emergency responses. Health Security 15(3):244–252.
Qian, Y., K. Willeke, S. A. Grinshpun, J. Donnelly, and C. C. Coffey. 1998. Performance of N95 respirators: Filtration efficiency for airborne microbial and inert particles. American Industrial Hygiene Association Journal 59(2):128–132.
Ramazzini, B. 1713. De morbis artificum (Diseases of workers). Translated by W. C. Wright. Chicago: University of Chicago Press.
Rengasamy, S., W. P. King, B. C. Eimer, and R. E. Shaffer. 2008. Filtration performance of NIOSH-approved N95 and P100 filtering facepiece respirators against 4- to 30-nanometer-size nanoparticles. Journal of Occupational and Environmental Hygiene 5(9):556–564.
SARS (Severe Acute Respiratory Syndrome) Commission. 2006. The SARS Commission final report: Spring of fear. Toronto, ON: Commission to Investigate the Introduction and Spread of SARS in Ontario. http://www.archives.gov.on.ca/en/e_records/sars/report (accessed October 24, 2018).
Thorne, C. D., S. Khozin, and M. A. McDiarmid. 2004. Using the hierarchy of control technologies to improve healthcare facility infection control: Lessons from severe acute respiratory syndrome. Journal of Occupational and Environmental Medicine 46(7):613–622.
Veenema, T. G., F. Boland, D. Patton, T. O’Connor, Z. Moore, and S. Schneider-Firestone. 2018. Analysis of emergency health care workforce and service readiness for a mass casualty event in the Republic of Ireland. Disaster Medicine and Public Health Preparedness 1–13.
Welzel, T. B., K. L. Koenig, T. Bey, and E. Visser. 2010. Effect of hospital staff surge capacity on preparedness for a conventional mass casualty event. Western Journal of Emergency Medicine 11(2):189–196.
Wizner, K., L. Stradtman, D. Novak, and R. Shaffer. 2016. Prevalence of respiratory protective devices in U.S. health care facilities: Implications for emergency preparedness. Workplace Health & Safety 64(8):359–368.
Zhu, J. H., S. J. Lee, D. Y. Wang, and H. Lee. 2014. Effects of long-duration wearing of N95 respirator and surgical facemask: A pilot study. Journal of Lung, Pulmonary & Respiratory Research 1(4):00021.
This page intentionally left blank.






























