2
Genetic and Reproductive Interventions
The genetic information of an individual organism contains a blueprint for its response to a particular stimulus. Through natural selection, responses to the surrounding environment can cause the genetic composition of a population or species to change and shift. Coral reefs have existed for hundreds of millions of years, adapting and changing as the Earth’s climate has changed (Veron, 2008). However, given the unprecedented losses in reefs caused by relatively fast changes in the Earth’s climate over the past century, many coral populations may not have the capacity to adapt via selection at a sufficiently rapid rate (Bay et al., 2017). Genetic and reproductive interventions provide an opportunity to increase genetic diversity within populations to allow them to adapt to a changing environment, or permit selection of traits that may improve the resilience of coral populations and species. Coral reefs exist over a range of gradients—of temperatures and other stresses—varying over the reef scale up to across ocean basins. This indicates there is an ability for corals to acclimate or coral populations or species to select genotypes resilient to a range of conditions. Examining the genetic underpinnings of these adaptations is key to understanding and developing genetic interventions that could be employed to increase coral resilience and persistence.
Managed selection is a precursor to other interventions described in this report; it is an approach for identifying genetically resilient coral types. Observations of responses in the natural environment is one way to identify these corals, but a growing capability in molecular tools permits differentiation between evolutionary adaptations to a particular
environment, as opposed to those individuals that are temporarily acclimated to change. The controlled and careful outcrossing of genetically distinct individual corals in a managed setting can be used to create a captive population with substantial genetic diversity. Individuals from captive propagation efforts can be released into the wild to increase the genetic diversity of native populations or by introducing resilient genotypes. There are several approaches that can be used to achieve these goals. Gamete and larval capture and seeding can improve coral reproductive success, and can also provide an opportunity for outcrossing of gametes with known beneficial genotypes. Coral cryopreservation adds the ability to preserve diversity in gametes or other stages until conditions improve and successful crosses can be made.
In the absence of naturally resilient corals, genetic manipulation may provide the opportunity to create resilient corals and coral symbionts. Discovery of the utility of CRISPR/Cas9 as a tool for creating gene drives, which create a biased system of inheritance for genes of interest, has driven interest in genetic manipulation. While CRISPR/Cas9 has been shown to be technically feasible to apply to coral, there is little knowledge regarding candidate genes on which it could operate to increase resilience nor whether it may translate to a change in phenotypes. The ability to develop resilience in corals through gene drives is limited in the near term; however, genetic manipulation also provides an approach to experimentally identify the genetic causes of individual or species-level variation in stress tolerance.
MANAGED SELECTION
What It Is
Managed selection is the detection of corals with above average stress tolerance, and the use of them in subsequent interventions such as managed breeding, symbiont and microbiome isolation and manipulation, managed relocation, or genetic manipulation. Corals can thrive over a variety of environmental conditions, from the cooler waters of high-latitude reefs such as Bermuda, Hawaii, and Tonga to the warmer waters of equatorial islands, power plant effluents, and shallow patch reefs (Coles et al., 2018; Keshavmurthy et al., 2012). Their tolerances to a range of values for parameters such as temperature, salinity, sedimentation, light, and toxicant exposure reflect the ability of individuals to acclimate or populations and communities to adapt via selection of resilient phenotypes and species (Carpenter et al., 2008; Dixon et al., 2015; Jin et al., 2016; Palumbi et al., 2014; Rose et al., 2018).
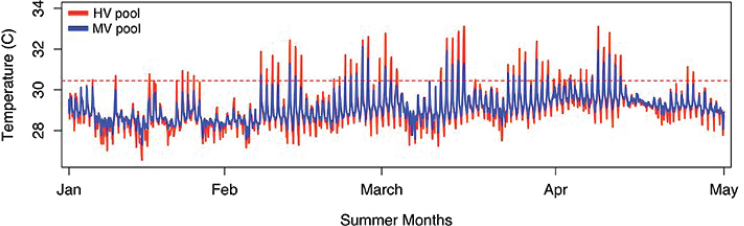
Temperature is one of the parameters of greatest concern. Some habitats and locations have steady temperatures; for example, corals in
fore-reef zones in Palau may normally experience a fairly narrow range of temperatures, from 26°C-30°C (Golbuu et al., 2007), while corals in Okinawa may be exposed to temperatures from 15°C-30°C annually (Nadaoka et al., 2001), and those in the Persian/Arabian Gulf survive temperatures up to 36°C (Hume et al., 2013). However, other habitats even within these regions can show very different temperature patterns. For example, back reef, patch reef, or harbor environments may show higher daily temperature peaks (see Figure 2.1).
One set of coral species in Nikko Bay, Palau, persists in an environment typified by elevated seawater temperatures (32°C) and reduced pH (7.9), conditions that have been predicted to be common on reefs by the year 2050 (Anthony et al., 2011; Camp et al., 2018). This bay has water retention and circulation patterns which models suggest retain coral larvae and gametes, and reduce immigration of propagules from reefs outside of the bay, suggesting the possibility of local adaptation in Nikko Bay to warm, acidic conditions (Camp et al., 2018; Golbuu et al., 2016; van Woesik et al., 2012). Other warm water habitats are embedded in the variable environmental mosaic of complex reefs at small spatial scales, and resident corals are not as isolated genetically. Overall, heat-related selection on reefs is associated with genetic differences occurring over the scale of hundreds of meters (Bay and Palumbi, 2015) or hundreds of kilometers (Dixon et al., 2015; Jin et al., 2016) at hundreds or thousands of gene loci.
Benefit and Goals
Managed selection takes advantage of the high level of genetic diversity found in many coral species, and the potential for natural selection to generate concentrations of adaptive alleles in habitats with high exposure to stressful conditions. In effect, it uses the long history of population
adaptation across the environmental mosaic of the reef as a natural laboratory for the production of genetically adapted corals. Because this natural selection has been an ongoing process, adapted populations currently exist. As the raw material for subsequent use, corals chosen through managed selection are locally available, represent native coral genotypes, and can occur in large enough numbers to represent substantial genetic diversity for other traits. Because of these advantages, managed selection is currently a main target of operation for coral restoration before other more manipulative genetic interventions can be developed.
Another advantage is that conditions imposing multiple stresses on coral populations can currently be found. Corals with genotypes resistant to multiple stressors that often co-occur, such as higher levels of sediments and toxicants, with reduced light levels and salinities, occur near watershed discharge points have the advantage of tapping into the products of natural selection that may have taken place in habitats with multiple selective pressures. Rather than needing to perform controlled crosses and expend resources for grow out, these corals are already naturally available.
How to Do It
Corals that survive stress events or that are found in areas of known elevated stressor levels are obvious targets for studies of resistant genes and tolerances and may provide good source material for intervention activities. Examples of such sites for temperature stress include (1) shallow back reef pools, reef flats, and patch reefs that heat up during daytime low tides; (2) lower latitude locations along latitudinally lengthy reefs such as the Great Barrier Reef or the Meso-American Reef; and (3) equatorial locations with high summer temperatures. There are also numerous other types of stressors for which geographic collections for resistance might be made: (1) harbors, for resistance to hydrocarbons and heavy metals (from fuel and antifouling paints); (2) offshore from agricultural sites for pesticides; and (3) locations adjacent to sewer outfalls for pharmaceuticals, nutrients, and low oxygen levels. Locations are typically assayed for stress levels (temperature, pH, and chemicals), sometimes at the scale of individual corals. Small portable, inexpensive temperature recorders have made it possible to measure temperature variation at small scales of space and time. Other stressors are more difficult and expensive to measure widely (Bahr et al., 2016; Kuffner, 2017).
A different source of information on stress tolerance is derived from monitoring the survival of individual colonies after major bleaching events. Seldom do bleaching events kill all the corals of a species on a particular reef (Depczynski et al., 2013; Glynn et al., 2001; McClanahan,
2004). Instead, there are often individuals that remain unbleached or that recover from bleaching.
Differential tolerance in corals to environmental stressors often has both a genetic component and an acclimation component (i.e., where an individual adjusts to an environment). Acclimation to stressful conditions is a widespread feature of coral populations (Bay and Palumbi, 2015; Coles and Jokiel, 1978; Jones and Berkelmens, 2010; Middlebrook et al., 2008) and as a result, phenotypic differences in corals collected from different reefs may not be entirely due to genetic or fixed features. Estimates of the amount of phenotypic variation in heat resistance due to acclimation have ranged around 50% in one study (Palumbi et al., 2014). Common garden experiments have been used to enhance the search for variation in stress tolerance that has a genetic basis in a wide variety of species (Parkinson et al., 2018).
Molecular tools can help identify the mechanisms and responses through which hardy corals survive stress, and eventually assist in identifying those corals most likely to survive in the future when used for active reef restoration. Genomics can be used to identify genotypic diversity associated with particular habitats and novel genotypes, providing targets for restoration outcomes. This approach is easiest when heat tolerance is associated with alleles of strong effect. However, most genetic analyses of heat tolerance suggest control by many genes. For example, offspring of corals from lower latitudes of the Great Barrier Reef show intermediate heat tolerance as if many genes were involved (Dixon et al., 2015). To date, the best model of heat tolerance is one controlled by alleles at many loci, each of which has small effect. Bay and Palumbi (2015) screened populations of Acropora hyacinthus and showed results suggesting hundreds of effective loci. Positive identification of these loci is challenging because of their small individual impact. Approaches based on genome-wide association studies, which rely on hundreds of thousands to millions of markers and sufficient sample sizes to detect marker-trait associations (Visscher et al., 2017), have provided a way to detect loci in wild populations (Barson et al., 2015; Lundregan et al., 2018), including those of smaller effect (Brieuc et al., 2015; Gagnaire and Gagiotti, 2016). Such studies also pave the way for identifying individuals that have high genomic breeding values for a trait—without knowing their actual marker-trait associations—using genomic prediction based on test populations (Crossa et al., 2017). This information in turn can be used to inform actions such as managed breeding and assisted gene flow (Flanagan et al., 2018).
Proteomics might also be used in a diagnostic manner to identify the key stressors at particular sites and the genotypes able to effectively respond through protein expression (Downs et al., 2005, 2012). Transcriptomics can be used to identify gene expression, which ties back to the
genotypes exhibiting resistance and the effectiveness of the proteins being up- or down-regulated in response to specific stressors.
A few studies have been able to test the use of highly vetted stress-resistant colonies for controlled crosses and nursery grow-out (Guest et al., 2014) and to provide the raw material for candidate genes involved in stress resistance for future gene manipulation (Jin et al., 2016). A generalized procedure to identify genotypically stress-tolerant corals is to
- Survey native populations across a range of stress levels for phenotypic variation in stress response;
- Move the highest- and lowest-resilience colonies to a common garden setting for further phenotyping after acclimation to common conditions;
- Characterize colony genomes, transcriptomes, and proteomes as well as symbiont types and microbial assemblages across a range of fixed stress tolerance; and
- Conduct manipulative experiments on roles of symbionts and microbes on colony tolerance.
Current Feasibility
The ability to monitor and identify stressed sites and collect corals from these locations is straightforward. The “omics” technologies of genomics (genetics), transcriptomics (gene presence and expression), proteomics (protein expression), and metabolomics (the study of metabolites and related processes) are already available for corals, and their applications are growing and improving rapidly (e.g., Aswani et al., 2015; Devlin-Durante and Baums, 2017; Downs et al., 2012; Miller et al., 2011; Ricaurte et al., 2016; Rougée et al., 2014). Identifying biomarkers for stress resistance of individual species has proven difficult (Parkinson et al., 2018), but individual phenotyping of corals after common gardening is currently feasible in marine laboratories with running seawater systems, corals transplanted into field conditions, or in coral husbandry businesses (e.g., Muller et al., 2018). Palumbi et al. (2014) have demonstrated fixed genetic differences and patterns of gene expression in corals over a range of temperatures, with both acclimatization and adaptation occurring within coral populations.
Protein expression studies in corals have proven particularly valuable in identifying cause-and-effect relationships between stressors and responses at the cellular level, prior to outright mortality (Downs et al., 2012). Specific classes of proteins such as xenobiotic metabolizing enzymes, including Cytochromes P-450 and multiple xenobiotic resistance protein, can be assayed both qualitatively and quantitatively to
provide reliable data for identifying the contributions of individual stressors in a multistressor situation (Downs et al., 2005, 2012), supporting the design of specific and effective intervention strategies.
Potential Scale
The approach for finding “hardy corals” is broadly applicable across spatial, temporal, and taxonomic scales. Its scalability depends on how common stress-tolerant colonies are: If such corals are a rare discovery for a small number of species (e.g., Nikko Bay in Palau), the ability to use them broadly will be limited. By contrast, if they commonly occur on widespread coral habitats, such as patch reefs or back reef pools, and if these habitats house stress-tolerant corals of many species, then it may be possible to find multispecies heat-tolerant communities at many reef locations. The local availability of these corals would make their use in other interventions, including relocation and managed breeding, more feasible.
Risks
Broad scale tradeoffs are expected in the evolution of heat tolerance (Huey and Kingsolver, 1989). For example, polymorphisms in a population that confer the benefit of heat tolerance likely also confer some fitness disadvantage, otherwise they would be fixed by selection over time. The best-known example in corals is the tradeoff between the heat tolerance conferred by the symbiont genus Durusdinium (formerly Symbiodinium Clade D) and faster growth conferred by the heat-sensitive symbiont genus Cladocopium (formerly Clade C) (see Stat and Gates, 2011, for references and research history). However, recent experiments suggest that this tradeoff is less powerful in warmer waters (Cunning et al., 2015a). Other experiments have found tradeoffs between bleaching rate and disease (Shore-Maggio et al., 2018) in Hawaiian species, but Muller et al. (2018) found little relation in Caribbean staghorn corals. Tradeoffs between heat tolerance and colony growth or survival have also been harder to find; Kenkel et al. (2015) found evidence for higher transplant survival in Florida corals on native reefs but no tradeoff between heat tolerance and survival. Bay and Palumbi (2017) also found little tradeoff in growth among heat-resistant corals but suggested that strong selection for heat tolerance also selected for high transplant survival.
Risks of performing “omics” analysis on corals include further damage to corals that survive bleaching events, collecting pressure on corals in stress-tolerant populations, and mis-assignment of phenotypic variation in common garden experiments as genetic rather than epigenetic. Additionally, there are risks to the various interventions that may make
use of selected coral genotypes, such as managed breeding or relocation, described later in this report.
Limitations
A limit to this approach might be encountered if these naturally growing stress-tolerant corals cannot tolerate extreme stresses expected under reasonable climate models at the end of this century. For example, if currently available natural tolerance provides 2°C-3°C extra resilience against heat pulses, but 4°C-5°C is expected under high CO2 emission scenarios, then currently available stress tolerance may not suffice. Experiments in artificial selection classically show that phenotypic change from generation to generation slows after initial increases, even for genes under control of many additive loci. There are currently few ways of confidently predicting exactly where this asymptote will be for any coral species. In such cases, use of natural variants can be expected to extend the lifetime of current reefs, and provide raw genetic material to generate extreme stress tolerance in the future through breeding or manipulation. But natural variants alone may not suffice to generate heat tolerance needed in all future scenarios.
Infrastructure
The approach described above suggests that facilities to collect and maintain corals are required, as is equipment to monitor stress levels of local habitats. Specialized laboratory equipment is needed for the genomic, proteomic, and transcriptomic analyses.
MANAGED BREEDING
What It Is
The restoration of reefs through artificial propagation has increasingly found traction over the past decade (Hesley et al., 2017; Lirman and Schopmeyer, 2016; Rinkevich, 2014). Many such activities are focused on “coral gardening,” the propagation of coral fragments in nurseries, and their outplanting on degraded reefs. These low-cost and readily adaptable approaches may have some associated risks such as disease transmission and changes in wild population genetic diversity. However, there are examples of long-term survivorship, growth and reproduction (Lirman and Schopmeyer, 2016), and retention of genetic diversity (Drury et al., 2016) at several restoration sites. The development of infrastructures that support the culture and restoration of corals has paved the way for the
development of additional approaches based on sexual reproduction—managed breeding. These approaches range from supportive breeding within populations to hybridization between populations or species.
Managed breeding relies on the outcrossing of genetically distinct individuals, regardless of their taxonomic status. This chapter encompasses three approaches under this category that support varying goals in restoration, from increasing coral cover while preserving local genetic diversity to increasing cover by introducing individuals with novel genotypes and higher fitness. Supportive breeding seeks to enhance population size by sampling a subset of individuals from a population for captive rearing, and then releasing the captively reared offspring back into their native habitat (Ryman and Laikre, 1991). Outcrossing between populations aims to introduce novel genetic variation within a species range, following reproduction between individuals from different populations. Hybridization between species is the use of sexual reproduction to create individuals with novel genotypes that are more fit than the parental species.
Benefit and Goals
Interbreeding between individuals can have positive and negative outcomes. To place these outcomes in perspective, it is helpful to view populations and species as belonging to a breeding system continuum (see Figure 2.2), where optimal effects of outcrossing are intermediate to fitness losses due to inbreeding or outbreeding depression (Allendorf and Waples, 1996; Edmands, 2007). These continua are likely to be taxon
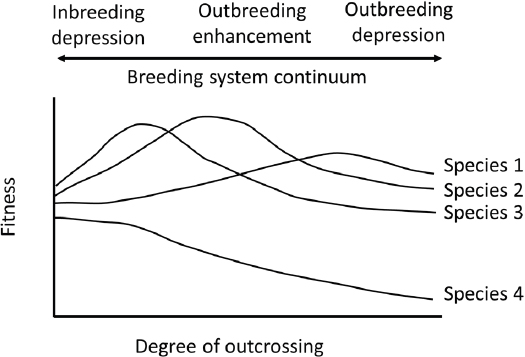
specific. Activities associated with managed breeding might be targeted at different degrees of outcrossing.
Supportive breeding within populations seeks to increase population sizes and local genetic diversity, thus improving long-term persistence. Therefore, this intervention supports recovery goals that aim to improve coral cover while maintaining genetic variation within native species.
In corals, supportive breeding may provide benefits both through captive rearing and through the release of artificially propagated individuals. Captive rearing provides a means of retaining genotypes that may be otherwise lost in wild populations (Schopmeyer et al., 2012), and can also increase genetic diversity within populations through sexual reproduction. Outplanting locally derived captive reared corals on degraded reefs may provide demographic support by augmenting wild population sizes (Ryman and Laikre, 1991). This outcome may be accompanied by an increase of genetic diversity and effective population size. An augmented population would have an increased ability to adapt to a changing environment because selection is more efficient in large populations (Falconer and Mackay, 1996; Kimura, 1983). Supportive breeding can be expected to assist population recovery if reintroduction success is high. The manipulation of gene flow can result in scenarios with beneficial outcomes, depending on the degree of domestication selection in captivity (Baskett and Waples, 2013; Ford, 2002). Supportive breeding has significant potential to address problems with low recruitment in the Caribbean (Kuffner and Toth, 2016; van Woesik et al., 2014).
Outcrossing between populations aims to increase fitness within populations, and hence population size, by introducing additional genetic variation from other populations through reproduction and gene flow. This intervention can also be used to meet recovery goals directed at increasing coral cover.
In certain scenarios, outcrossing may result in increased fitness in offspring compared to the parents (see Figure 2.2). Such an increase, or heterosis, is attributed to the masking of deleterious alleles or to greater fitness in heterozygotes compared to homozygotes. Immigrants into a small population can result in genetic rescue—an increase in population fitness due to the introduction of new alleles (Tallmon et al., 2004; Whiteley et al., 2015). For genetic rescue to occur, the offspring of immigrants would need to elevate the overall fitness of the target population, ideally accompanied with an increase in population size. The benefit of this approach is the recovery of small and fragmented populations with limited ability to adapt to a changing environment. If persistent gene flow occurs between fragmented subpopulations, the overall effective population size of the metapopulation may be increased as a whole, thus reducing
the likelihood of inbreeding, and minimizing divergence. Genetic rescue has been implemented in a small number of examples; its utility may be underappreciated in population recovery (Frankham, 2015; Whiteley et al., 2015). Relevant data on the potential for outcrossing between coral populations is limited; although self-fertilization and inbreeding in corals have been well documented (Baums, 2008), inbreeding depression has not (Baums et al., 2010). The potential for fitness improvements due to heterosis is untested in corals. However, genetic load is generally high in marine invertebrates (Plough, 2016), suggesting the potential for this intervention.
Hybridization across species aims to create novel genotypes that are more fit than the parental species that were used to create the hybrids. The use of hybrids in coral reef recovery may vary. If the goal is to increase coral cover while reducing impacts on local species diversity, then infertile hybrids may be preferred. If the goal is to increase cover and the long-term fitness of a community using individuals with novel genotypes, fertile hybrids may be preferred.
Outcrossing between species immediately creates novel genetic variation within a hybrid taxon and provides additional material for mutation, drift, and selection to act on. These processes can result in diversification and new adaptations and have played a key evolutionary role in species evolution across taxa, including corals (Richards and Hobbs, 2015; Vollmer and Palumbi, 2002; Willis et al., 2006). The potential benefit of human-mediated hybridization between species would be the development of new forms that have higher fitness than the progenitor species, which may also provide the basis for future selective breeding programs. Although typically avoided in conservation efforts, there have been increasing calls for the use of interspecific hybridization in the face of rapidly changing environments (Hamilton and Miller, 2016), because they may provide adaptive potential beyond that of the phenotypic range of the parental species and a means of preserving genomes at the risk of extinction.
How to Do It
The identification of target populations and species is a crucial first step, and depends on location and local reef restoration goals. Practically, managed breeding relies on many of the same practices for propagation of coral fragments in nurseries and their outplanting that have been developed for traditional restoration projects, some of which are described in Box 2.1.
Supportive breeding within populations would rely on species that can be readily propagated using sexual reproduction, have a high
reintroduction success, and would significantly contribute to reef building. Fitness outcomes associated with supportive breeding and restoration have been extensively studied in many marine species (Bell et al., 2005; Blaxter, 2000; Hedgecock and Coykendall, 2007; Naish et al., 2008; Waples et al., 2012), and best practices based on available science have been proposed (Waples et al., 2012). Briefly, development of an effective program relies on the maintenance of diverse populations, clear program objectives, clearly outlined broodstock management and release protocols, and long-term monitoring of enhanced populations. The use of wild-derived
individuals as broodstock for the captive population in every generation of supplementation is aimed at preventing divergence between the two and relies on processes in the natural environment to drive the ongoing evolution of the population as a whole. “Integration” of captive-reared and wild populations has gained considerable traction in the conservation of some species such as salmonids and shellfish (Waples et al., 2012). Several empirical examples support the potential use of wild broodstock in retaining genetic diversity over the short term (Ford et al., 2016; Hess et al., 2012; Waters et al., 2015, 2018), but others indicate reduced fitness
in captive-reared individuals compared to wild conspecifics (Christie et al., 2014). In corals, inadvertent selection due to domestication may occur through several mechanisms, because these species have high fecundity, high variance in reproductive success, and high mortality at settlement. Therefore, such studies provide insight into the importance of developing appropriate protocols for broodstock collection, and rearing and release strategies.
Outcrossing between populations requires prior knowledge of the fitness of individuals resulting from such crosses within the environment of the reef to be restored. Such efforts may also need to account for any future environmental changes in the target populations. Characterization of the extent of local adaptation and population structure across a species and identification of management units would provide additional information to support these endeavors. Feasibility studies on the number of individuals needed can be based on tests using laboratory studies or outplanting small numbers of crosses within the target reefs (Whiteley et al., 2015). Ideally, such tests should use more than one generation of outcrossing, and include different cross types, such as second-generation (F2) crosses and backcrosses to individuals representing the native populations.
Hybridization between species would rely on candidate species that provide viable offspring following reproduction and would use similarly well-developed protocols for within-species crosses, although the evaluation of risks to existing natural populations would likely differ. Because the intent would be to create new forms that have higher fitness than the progenitor taxa, initial field testing of hybrid performance in different target environments is essential. Testing of hybrid fertility is essential for risk assessment. In some cases, infertile hybrids may be desired simply for their potential to return habitat to a degraded reef. On the other hand, fertile hybrids may provide an opportunity to create new genotypes that are more capable of adapting to a changing environment. Determining the long-term consequences of hybrids over several generations is important to understand in this context, because in some cases fitness benefits in first-generation (F1) hybrids decrease or disappear in future generations (Burton, 1990).
Current Feasibility
Supportive breeding within populations relies on the propagation of corals using sexual over asexual methods, and success is dependent on high survivorship after reintroduction and demonstrated recruitment following outplanting (see Box 2.1). Sexual reproduction of captive broodstock has been successful in a number of species, including Acropora
tenuis (dela Cruz and Harrison, 2017; Nakamura et al., 2011; Omori, 2011), A. valida (Villanueva et al., 2012), A. millepora (Guest et al., 2014), and A. digitifera (Edwards et al., 2015).
While systematic efforts at supportive breeding of coral have not been established, investigations of different release strategies of captive reared individuals point toward possible strategies that might be implemented more broadly. Efforts to date have largely focused on single generation releases, have occurred on a small scale, and have relied on local broodstock (dela Cruz and Harrison, 2017; Guest et al., 2014; Nakamura et al., 2011; Villanueva et al., 2012). Mass releases of larvae adjacent to healthy coral ecosystems in Palau were reported to have limited success post-settlement, possibly due to competition with natural recruits (Edwards et al., 2015). In contrast, larval seeding (described further in the Gamete and Larval Capture and Seeding section) in degraded areas in the northwest Philippines resulted in enhanced recruitment and increased coral cover after 3 years (dela Cruz and Harrison, 2017). Reintroduction of juveniles, rather than larvae, has potential to reduce losses due to early mortality (Nakamura et al., 2011). Given that research in this area is relatively new, most studies examined success rates within a single generation; however, natural reproduction in recruits has been reported in both larval seeding efforts (dela Cruz and Harrison, 2017) and juvenile outplanting (Guest et al., 2014). Organizations such as SECORE International are currently investigating a range of outplanting approaches with sexually produced offspring. Sexual reproduction has also been observed following outplants of nursery-grown fragments (Carne and Baums, 2016).
These initial studies indicate that approaches based on sexual reproduction of captive broodstock and release of offspring are feasible and point toward their potential in larger programs. The long-term success of supportive breeding programs over several generations has yet to be realized.
The infrastructure and protocols for outcrosses between populations would rely on those developed for supportive breeding, and therefore are technically feasible. In addition, research on transporting corals over long distances and inducing synchronous spawning has been demonstrated in some species (Craggs et al., 2017, 2018). There are few systematic efforts at investigating the fitness of within-species hybrids at different levels of divergence. However, Dixon et al. (2015) demonstrated the potential for introducing novel genetic variation into a cold thermal tolerant population of A. millepora following experimental crosses with a warm tolerant population, which in turn permitted a rapid response to heat selection within the newly created population. Additional information on population structure (Baums, 2008; Chiazzari et al., 2013; Flot et al., 2011; Forsman et al., 2015b, 2017; Suzuki and Fukami, 2012; Toonen et al.,
2011) and local adaptation in coral species (Baums et al., 2014; Pratlong et al., 2015; Sanford and Kelly, 2011) suggests that additional experimental investigations of hybrid fitness within species are feasible. Such investigations would need to carefully consider genotype-by-environment interactions in field testing (Drury et al., 2017; Dubé et al., 2017).
Direct investigation of the use of hybridization between species in coral habitat recovery is in its infancy, but several studies provide support for ongoing research. The creation of novel variation through interspecific hybridization depends on the ability to create fertile hybrids, increased hybrid fitness compared to the progenitor species, and maintenance of fitness gains across generations. There is substantial evidence that natural hybridization has played a historic role in the evolution of several coral taxa (Arrigoni et al., 2016; Budd and Pandolfi, 2010; Combosch and Vollmer, 2015; Richards and Hobbs, 2015; Willis et al., 2006), suggesting its possible role in restoring fitness as part of a deliberate intervention. Furthermore, contemporary hybridization may play an ongoing role in adaptation. For example, population declines of two Acropora species (A. cervicornis and A. palmata) in the Caribbean have been accompanied with increased incidence of their F1 hybrid A. prolifera (Fogarty, 2012). Such patterns may be especially relevant to environments that are peripheral to the species range (Budd and Pandolfi, 2010; Fogarty, 2012; Hellberg et al., 2016) that may represent near-future climate scenarios.
The ability to create viable hybrids in artificial environments varies across genera that have been studied. Hybrids between species within Ctenactis were not viable (Baird et al., 2013), whereas experimental hybrids belonging to Montipora and Platygyra were (Willis et al., 1997). Many species belonging to the genus Acropora exhibit limited pre- and postzygotic isolating mechanisms (Baird et al., 2013; Fogarty et al., 2012a, 2012b; Isomura et al., 2016; Willis et al., 1997), and thus serve as candidates for the investigation of fitness in interspecific crosses between species representing a range of divergences.
Few studies have directly investigated hybrid fitness relative to parental species. Most reports are from the genus Acropora. In the Caribbean, hybridization involves the only two species in the region, A. cervicornis and A. palmate, where F1 hybrids between the two had comparable fitness with the parental species across life history stages measured (larval survival, post-settlement survival) and higher settlement and growth rates in shallow environments (Fogarty, 2012). In the Indo-Pacific, the survival of juvenile A. millepora x A. pulchra F1 hybrids outplanted in three habitats in the Great Barrier Reef varied, with growth and survival comparable to or exceeding the nonlocal parent species (Willis et al., 2006). In the most comprehensive study to date to directly investigate the potential for hybridization in reef restoration, W. Y. Chan et al. (2018) examined the
relative fitness of hybrids and their progenitor species, using two more divergent (A. tenius and A. loripes) and two more closely related (A. sarmentosa and A. florida) cross types (five colonies per species). Survival to 28 weeks, size at 28 weeks and 1 year, photochemical efficiency, and algal symbiont uptake were measured in F1 offspring raised in ambient and elevated temperature and pCO2 environments. Generally, no significant difference between hybrid and parental performance was detected across a number of traits, and all cross types had lower size and survival in the elevated environment. However, A. sarmentosai x A. florida hybrids had higher survival and growth rates than the parental species under ambient conditions. There was also some support for the role of hybrids under changing conditions. In the elevated environment, A. tenius x A. loripes hybrid survival was greater than A. tenius, and photochemical efficiency was higher than both parental species. Hybrids representing one cross direction (female A. florida x male A. sarmentosai) had higher survival than one parental species under elevated conditions. Taken together, the results of these studies indicate that there may be circumstances under which F1 hybrid Acropora might perform better in some environments. Research in this area would benefit from systematic investigation of different cross types across a range of species divergences, increases in sample sizes, and performance testing across a variety of environments. Because research on hybrid viability is recent, the fitness consequences of interspecific hybridization beyond the F1 have yet to be directly studied. However, it appears that A. prolifera may be able to reproduce with at least one progenitor species in the natural environment, because there is evidence of unidirectional gene flow from A. palmata into A. cervicornis (Vollmer and Palumbi, 2002, 2007). There are also challenges in extrapolating these findings to other coral reef species.
Potential Scale
In corals, supportive breeding within populations has typically targeted individual reefs using few species. Outcrossing between populations and hybridization between species has been conducted within laboratory settings only. In all cases, successful introduction of captive-bred individuals would benefit corals on the reef-scale, if they are able to increase coral cover. Long-term fitness benefits and persistence of populations would be dependent on connectivity within the reef in cases where sexual reproduction and self-recruitment are desired.
Currently, supportive breeding programs based on larval releases or juvenile outplanting have been conducted as single experimental events. Ideally, populations should become self-sustaining after a limited-time captive breeding and release program; however, in other taxa, population
rebuilding has involved ongoing captive propagation and release, often for decades (Laikre et al., 2010; Naish et al., 2008). Published literature has reported the outcomes of single generation crosses both with and between species. However, if restoration goals are dependent on ongoing sexual reproduction, multigenerational studies are needed, because reductions in fitness may only be observed beyond the F1. If goals rely on sterile individuals, then fertility in hybrids would need to be investigated.
Risks
Outcrossing may result in fitness reductions, depending on the scenario (see Figure 2.2). Outbreeding depression is ascribed to two mechanisms: loss of local adaptation (extrinsic outbreeding depression) or disruption of co-adapted gene complexes (intrinsic outbreeding depression) (Templeton, 1986). In the former, individuals created as a result of outcrossing or hybridization receive only half the allelic combinations present in either parent population, and may be unsuited to one or both of the parental environments. In the latter, recombination between different parental genomes may lead to a breakdown of interactions between co-adapted loci that are inherited together and influence a fitness trait. Decrease in fitness might be observed as early as the first generation of crossing, but it may be delayed until recombination in subsequent hybrid or backcrossed generations. Outbreeding depression is predicted to be prolonged for a greater period if intrinsic mechanisms are involved: Selection is more likely to be effective in restoring favorable alleles following the loss of local adaptation than after the disruption of co-adapted loci (Edmands and Timmerman, 2003). Persistent gene flow between evolutionary divergent populations can result in loss of population structure and locally adaptive traits if hybrids are less fit, which could degrade the total genetic variation across the entire metapopulation (Spichtig and Kawecki, 2004).
Ongoing outcrossing or gene flow may also affect the demographic properties of a population (Allendorf et al., 2001). In scenarios where outcrossing benefits population fitness as a whole, population sizes might increase because effects such as genetic drift, inbreeding depression, and reduced adaptability will diminish. In contrast, outcrossing that leads to reduced fitness can result in the opposite outcomes. Population losses can also accrue following reproduction between less fit hybrids and the parental species, if the former occur at higher frequencies (Allendorf et al., 2001; Ronce and Kirkpatrick, 2001).
Genetic risks due to human-mediated outcrossing and hybridization have been extensively documented in marine species (Waples et al., 2012). Most information comes from species that depend on sexual reproduction
exclusively and are shorter lived. Generally, the risks involved depend on the program goals, and on the accepted level of impact that captive-reared individuals have on wild populations. In all cases, risks should be evaluated with the development of genetic management plans that include clear performance indicators and monitoring plans.
The goals of supportive breeding within populations are usually aimed at increasing population sizes and recruitment rates while maintaining or restoring the genetic diversity and fitness of a target wild population (although evidence of erosion of genetic diversity in coral populations is currently minimal; van Oppen et al., 2015a). Maintaining a broad range of genotypes within a population facilitates its adaptation to a future unpredictable environment. Managing the risks depends on the ability of the rearing program to sample sufficient diversity from wild populations, retain that genetic diversity through the captive rearing programs, prevent adaptation to culture conditions, maintain an optimal effective population size in the reintroduced individuals, and ensure a high reintroduction success. Loss of genetic diversity in wild populations can occur if offspring contributions from few captive-reared families dominate (Hedgecock and Coykendall, 2007; Ryman and Laikre, 1991). Coral species are highly fertile, but with variation in reproductive success. Self-incompatibility, as well as incompatibility between genotypes has been reported (Miller et al., 2018), which means that captive rearing may result in the release of many individuals representing few families. Such releases can result in genetic homogenization, which in turn limits responses to environmental changes. Loss of fitness due to captive rearing and release can also occur through inadvertent selection. For example, high variance in reproductive success, and large nonrandom mortalities in larvae or juveniles can result in fitness losses. In a review of 266 studies on the genetic effects of captive-rearing programs, including marine invertebrates, significant losses in fitness were reported in about half of studies relative to wild populations (Araki and Schmid, 2010).
Generally, genetic effects of captive rearing can be reduced by collecting wild broodstock for every generation of culture and by moderating release sizes (Waples et al., 2012). Therefore, supportive breeding in corals can take advantage of the fact that gametes can be collected from the wild for each captive-reared cohort (see Box 2.1). In this case, it is important that divergent wild source broodstock be maintained in the natural environment.
The goals of outcrossing between populations are theoretically aimed at increasing the fitness of a wild population. Success depends on knowledge of the extent of population structure and local adaptation, and the fitness outcomes of hybridization beyond the first generation. Guidelines have been proposed for predicting the risk of outbreeding
depression in the absence of species-specific information (Frankham et al., 2011). The risks associated with captive rearing are similar to those of supportive breeding. Additional risks are associated with demographic losses to both the donor and recipient populations, due to decreased fitness in the offspring of crosses, especially following reproduction with native populations. However, in some coral populations, there may be few colonies that support natural reproduction and recruitment (Kuffner and Toth, 2016); therefore, the use of crosses between population for coral rebuilding may outweigh concerns associated with possible fitness losses.
The genetic risks of hybridization between species would vary with hybrid fertility. If fertile hybrids are desired for restoration goals, risks similarly depend on the likelihood of outbreeding depression, especially over several generations. The creation of hybrids might itself result in loss of genetic diversity through a bottleneck, caused either by the use of few individuals in initial crosses, or through the production of a wide range of novel genotypes that include less fit individuals. This bottleneck may influence genetic diversity in subsequent generations. Introduction of fertile hybrids within the same range as the parental species might result in demographic losses to the latter through reproduction. If hybrids are infertile, then losses to the parental species within the introduction range will be minimal. However, both fertile and infertile hybrids might also compete with native species. Both genetic and ecological risks would be affected on the ability to create and outplant sufficient numbers of hybrid individuals. Ecological effects such as competition may be small in situations where there is substantial degradation of the coral reef. Hybrids between native and invasive species have caused diversity declines within and among populations in a number of cases (e.g., Fitzpatrick and Shaffer, 2007; Hitt et al., 2003; Mooney and Cleland, 2001; Neira et al., 2006; Rhymer and Simberloff, 1996).
Limitations
All forms of managed breeding rely on the identification of suitable target species and populations. Within the Caribbean, there are five to seven main reef-building species (e.g., Gladfelter et al., 1978), but within the Pacific there are many more (e.g., DeVantier et al., 2006). All approaches rely on a sufficient supply of broodstock so that founder events are avoided in the captive populations and available genotypes are well represented. Because asexual reproduction may be relied on for the development of broodstock collections in coral species, this limitation may be readily addressed. On the other hand, asynchronous spawning between individuals may lead to a reduction in fertilization success or
underrepresentation of specific cross types (e.g., Craggs et al., 2017; Miller et al., 2018). This issue may be especially problematic for the development of within- and between-species crosses. Culturing relies on adequate rearing spaces to prevent loss of genetic diversity. Releases should result in sufficient numbers of individuals representing a broad array of genotypes. Recommendations for sufficient population sizes at all stages of culture vary by species (Waples et al., 2012). Goals are aimed at maximizing the effective population size while balancing realistic limitations imposed by the biology of the species, at maintaining diverse genotypes in coral culture, and at maximizing recruitment rates in the wild after release. Measuring program success by tracking the long-term fitness and reproductive success of outplants is essential, and may be challenging in scenarios where released individuals cannot be tagged. In corals, larval releases may be tracked using approaches such as age-dependent cohort analyses or by genetic-based markers if the latter are sufficiently powerful. Outplanted corals settled on substrates may be readily tracked over time.
Supportive breeding within populations relies on sexual reproduction, but asexual reproduction is known to be dominant on many reefs in the Caribbean (Miller et al., 2018), particularly those dominated by branching species (Kuffner and Toth, 2016). Within broadcast spawners in the Caribbean, many genotypes are known to be incompatible (Baums et al., 2013), but multi-genet crosses have resulted in high genetic diversity (Miller et al., 2018). There is some concern that putative broodstock sources are dependent on older, senescent colonies that have either lower viability, or are no longer adapted to prevailing or changing environmental conditions (Devlin-Durante et al., 2016; Irwin et al., 2017).
Both outcrossing between populations and hybridization between species depends on adequate field testing. Such testing should occur over more than one generation, because there are concerns about reduced fitness over more than one generation. However, depending on the growth and lifetime of the F1 generation, they may provide sufficient reef structure to support conservation goals. There is evidence that predictors for performance such as growth rate may not be sufficient over the lifetime of colonies (Edmunds, 2017) and that laboratory-based studies may not adequately predict field success (O’Donnell et al., 2018). Hybrid performance is likely to vary between field sites, and it is possible that a wide range of cross types would have to be examined for their contribution to reef building.
Infrastructure
Infrastructure for managed breeding is largely needed for the selection of propagules from the wild (including collection vessels and transport facilities), facilities for captive breeding and culturing (including running seawater systems and water quality controls), and for outplanting or larval release (see Box 2.1). This infrastructure is largely available from ongoing restoration efforts but is limited to places with marine laboratories or private coral husbandry operations. Organizations such as SECORE are developing techniques for larval rearing in in situ pools and substrates to improve settlement (Margaret Miller, presentation to committee, 2018). Well-equipped research facilities are also necessary to better understand managed breeding outcomes down through several generations.
GAMETE AND LARVAL CAPTURE AND SEEDING
What It Is
Gamete and larval capture and seeding is a specific way to enhance the natural sexual reproductive processes of corals by using natural spawning events to supply gametes for future use or larvae for settlement and laboratory growth. Corals reproduce primarily through sexual processes that result in coral planula larvae. Because corals are sessile, benthic organisms, the production of these motile propagules is essential for dispersal and population replenishment (Harrison, 2011). The vast majority of corals are broadcast spawners, releasing gametes (eggs and sperm) into the water column where fertilization and development occur (Harrison, 2011; Harrison et al., 1984; Richmond and Hunter, 1990). A few coral species brood their planula larvae following internal fertilization, and there is evidence that in some cases parthenogenesis may also occur. Larvae formed by spawned gametes take between 18 and 72 hours to fully develop to the ciliated planula stage, while brooded larvae are fully competent to settle and metamorphose upon release from the parent colony (Richmond et al., 2018). The timing of spawning events is highly predictable, allowing for the collection of gametes and larvae (dela Cruz and Harrison, 2017). Both larvae and gametes can be collected under laboratory conditions or in situ.
Benefit and Goals
Gamete and larval capture and seeding can be used to augment other interventions described in this report, but it also has its own advantages. Advances in coral propagation, including gamete and larval capture and
rearing, support efforts for reef restoration (Barton et al., 2017; Chamberland et al., 2017). The benefits of gamete and larval collection include
- Enhanced levels of fertilization of coral eggs compared to rates that would occur in nature, producing large quantities of larval seed material;
- Targeting of desirable genotypes or species for selective seeding efforts;
- Providing the material for controlled crosses of gametes to select for resistance attributes;
- Producing larvae for manipulations, including chimeric coral colonies and hybrids (described in the Managed Breeding section on hybridization between species); and
- Obtaining “clean” larvae devoid of zooxanthellae in species that have horizontal transmission of algal symbionts, to allow for selective infection with specific types (described in the Algal Symbiont Manipulation section in Chapter 3).
In light of the downward trajectory in live coral cover of many reefs, gamete and larval capture efforts can be of particular value for reefs where populations have declined and the Allee effect—the decrease in gamete density and subsequent fertilization of eggs—occurs. This approach is appropriate for Acropora in the Caribbean and Atlantic, where natural recruitment levels have been extremely low and populations of adults are at low densities and patchy (Baums et al., 2005; Williams et al., 2008). These tools can support the goal to achieve population sizes that are eventually self-sustaining.
How to Do It
For laboratory-based efforts, coral adults can be collected from the field just prior to predicted spawning. For many corals, egg-sperm bundles are released, which float to the water’s surface. They can be collected using containers with fine mesh (e.g., 10 μm), which traps eggs and allows sperm to pass through. By separating the sperm and eggs, their density can be controlled and selective seeding or crosses made (dela Cruz and Harrison, 2017; Pollock et al., 2017a).
In the field, there are a number of ways of collecting gametes. Tents of fine mesh can be placed over gravid colonies, with a float and chamber at the top (Sharp et al., 2010; see Figure 2.3). As egg-sperm bundles are released, they float and can be captured. These can then be crossed with gametes from another colony of the same species, which has been shown to yield higher fertilization rates than efforts at self-crossing in colonies that are simultaneous hermaphrodites (Heyward and Babcock, 1986).

Following development, fully competent coral larvae can be seeded directly onto reefs using mesh enclosures, they can be transferred to reefs on settlement plates from controlled recruitment efforts, or released onto appropriate substrata en masse, particularly in cavities, cracks, and crevices where the larvae are likely to be retained and recruited (dela Cruz and Harrison, 2017).
Current Feasibility
The technologies and techniques for gamete collection and fertilization have already been developed, although they require improvement to achieve the appropriate scale to make this approach a viable intervention. Additional efforts and experiments on larval distribution and reef seeding are needed, as few successes have been reported to date. One recent experiment demonstrated that successful in-tank, closed-system
spawning is possible with careful replication of seasonal temperature, lunar cycle, and photoperiod conditions, with a high degree of variability in success across species (Craggs et al., 2017). While some studies found low levels of success with seeding trials (Edwards et al., 2015), others suggest this remains a promising option for interventions (dela Cruz and Harrison, 2017). Recent advances in cryopreservation of gametes is a critical step forward in allowing for gene flow among distant populations (Hagedorn et al., 2017).
Potential Scale
Presently, the spatial scale for gamete collection is at the level of reefs over hundreds of meters, and for reseeding at tens of meters. This can represent millions of gametes and translate to thousands of recruits and considering mortality, tens to hundreds of mature coral colonies. For most reef-building corals used in such efforts, where the creation of habitat and rugosity are the key targets, spawning may occur only once per year for a single or a few days. Some of the planulating species have extended periods of larval production, from several months to year round. Acroporid corals are good species due to their branching and table morphologies, high growth rates, and wide distributions, but these often have very limited spawning periods.
Risks
There are few environmental risks when using locally sourced corals as studies have found most coral reefs are self-seeding. Although coral planulae may remain competent for weeks and even months, efforts to collect gametes and raise larvae for seeding the reef of origin can be considered simply facilitation and augmentation of natural processes. If gamete and larval capture and seeding is used to support other interventions, the risks of those interventions apply. The risks for selecting resistant genotypes or species for supportive breeding and hybridization are discussed in the Managed Breeding section in this chapter. Introducing larvae from distant reefs raises the same issues discussed in the Managed Relocation section (see Chapter 4).
Limitations
Limitations are mostly related to infrastructure, labor costs, and species availability. Labor for collecting gametes and larvae is intensive and can be costly. The approach is also limited by the availability and identification of preferred phenotypes, genotypes, or species in an area. Finally,
for species in which larvae and recruits acquire their algal symbionts from the environment (horizontal transmission), there is no guarantee the most suitable zooxanthellae will be available for laboratory-raised stock.
Infrastructure
There is a range of infrastructure needs, depending on the goals, objectives, and scales of interventions. Localized seeding projects can be undertaken with limited resources, including small boats, buckets, plankton nets, and inflatable pools. The more complex efforts of managed breeding and controlled zooxanthellae infections require well-equipped laboratories, flowing seawater systems, and supplies commonly associated with aquaculture facilities.
CORAL CRYOPRESERVATION
What It Is
Coral cryopreservation is the process by which gametes, embryos, or other living materials are frozen in such a way that they remain viable after being thawed. Much of the effort for corals has focused on gamete cryopreservation, particularly sperm (for which methods are better developed) (Hagedorn et al., 2017; Viyakarn et al., 2018). There have been some efforts to test methods to cryopreserve embryonic material (Hagedorn et al., 2006, 2012) and adult tissues (Feuillassier et al., 2014a, 2014b). Algal symbionts from three coral species have also been cryopreserved (Hagedorn et al., 2015).
Benefit and Goals
Living corals are continuing to decline, and as a result, there is a risk of losing the genetic variability at the population and species level that will be essential for restoration of coral reefs, both in the short term under compromised conditions and over the longer term when environmental conditions hopefully improve. Cryopreserved material can also be used to later increase genetic variation in critically endangered species, as was done, for example, with the recovery of black-footed ferrets (Howard et al., 2016). The rationale for long-term storage of frozen material as an insurance policy is the same as that which exists for other conservation-based cryopreservation efforts, such as seed banks (although it should be recognized that as conditions change the variants common today may not
be fit in the future). However, cryopreserved material can also be used for assisted gene flow and for research purposes, and thus should not be viewed simply as a last-ditch effort to prevent extinction (Hagedorn et al., 2017). In addition, cryopreservation of gametes (e.g., sperm) allows for fertilization between species that in nature do not live close together or that spawn at different times.
How to Do It
The chief challenge with cryopreservation is avoiding damage to cells caused by ice formation during freezing. To avoid ice damage, cryoprotectants such as dimethyl sulfoxide and propylene glycol are introduced during the cooling process. Optimizing the process requires empirical testing of cooling rates (which may be relatively slow or ultrafast, resulting in vitrification), thawing rates, and types and concentrations of cryoprotectants (Hagedorn and Carter, 2016; Hagedorn et al., 2012; Viyakarn et al., 2018).
Current Feasibility
Several algal symbionts and sperm from at least 16 species of coral have been successfully cryopreserved (Hagedorn and Carter, 2016; Hagedorn et al., 2017), as have embryonic cells (Hagedorn et al., 2012). There have also been preliminary tests of methods for larvae (Hagedorn et al., 2006) and small pieces of adult corals (Feuillassier et al., 2014a, 2014b) but these have not yet achieved success. Creation of viable embryos through fertilization of eggs with cryopreserved sperm is feasible now (Hagedorn et al., 2017), but other approaches are still in the developmental stage.
Potential Scale
Cryopreservation is a delicate process and requires careful technique in order to ensure that material is viable once thawed. It is thus done at the scale of a single individual organism or pool of gametes (that is, it is not currently feasible to bulk preserve large amounts of adult coral). However, once frozen, material can be transported to essentially anywhere. Hagedorn et al. (2017) found that sperm frozen for up to 2 years was as viable as sperm frozen for less than 1 month, suggesting that when done well, cryopreserved material can be stored for years and remain viable, although very long-term tests of storage efficacy have not been done.
Risks
The material being frozen and subsequently thawed has not otherwise been manipulated. For this reason, the risks of cryopreservation per se are minimal, apart from the fact that reliance on cryopreserved material creates vulnerabilities if for some reason the thawed material is later found to be inviable. However, cryopreservation will by definition not include genetic combinations that develop over time in response to changing conditions. More seriously, transport of cryopreserved material for use in other locations could potentially carry the risk of transferring other organisms including potential pathogens.
Limitations
Currently, coral cryopreservation is done at small scales as laboratory experiments and is largely restricted to sperm. Even in these cases, only a tiny fraction of extant diversity has been cryopreserved (16 coral species from the Caribbean, Hawaii, and the Great Barrier Reef; Hagedorn et al., 2017). Also, in the case of sperm, cryopreservation currently decreases fertilization success by about 50%, so it is important to start with fresh, highly mobile sperm (Hagedorn et al., 2017). Finally, scientists doing cryopreservation work are often not in close contact with initiatives actively engaged in restoration (Hagedorn et al., 2017).
Infrastructure
The infrastructure needed is widely available as cryopreservation has been developed for other organisms, including plants, agricultural and aquacultural animals, and humans; primarily this involves specialized temperature-control equipment so that precise rates of freezing and thawing can be achieved. Storage facilities for frozen material are also needed; for large-scale efforts this involves frozen biorepositories such as the Taronga Zoo’s CryoDiversity Bank currently being used for coral.
GENETIC MANIPULATION
What It Is
A novel way forward to potentially “design” more resilient corals than currently exist in nature relies on the use of genetic manipulation methods. Genetic manipulation is the direct alteration of the genome of an individual organism, which might be the coral or its algal symbiont. Modern laboratory-based approaches to genetically modify an organism involve genome editing through zinc finger nucleases, transcription
activator-like effector nucleases, and CRISPR/Cas9 gene editing (Gaj et al., 2013). Modern genetic manipulation systems contrast with classical genetics, which usually relies on identification of naturally occurring variants or the use of mutagens to generate novel phenotypes, which are identified by screening assays. Modern genetic tools can be used to insert gene drives, which use novel genetic constructs to create a biased system of inheritance by enhancing passage of a selected genotype to offspring (NASEM, 2016a). More broadly, genetic manipulation may be used as a tool to significantly expand knowledge about the genetic underpinnings of coral biology by allowing for detailed studies to understand the function of particular genes of interest.
The massive current interest in genetic manipulation is fueled by developments in CRISPR/Cas9-based genome editing, and now transcriptome editing (Konermann et al., 2018), that can be applied to a wide variety of organisms to generate loss-of-function mutations or to modify existing genes down to the single-nucleotide level (Doudna and Charpentier, 2014). CRISPR/Cas9 gene editing allows deletion, addition, or modification of existing genes and has been readily implemented in a wide variety of organisms such as yeast (DiCarlo et al., 2013), rockcress (Feng et al., 2013), and fruit fly (Gratz et al., 2013). The potential application of this RNA-programmed approach to genetic modification was described in a landmark article by Jinek et al. (2012).
Benefit and Goals
The goals of genetic manipulation are to alter specific genes in corals, their symbionts, or their associated microbiome in order to manufacture higher levels of stress resilience than can be found in nature. These goals depend on transformation technology to introduce genes and genetic constructs in corals or their symbionts, alteration of the coral or symbiont genomes in defined ways, and growth of genetically homogeneous colonies with the ability to pass these altered genes on to the next generation. If a genetically diverse population of a coral species is targeted with CRISPR/Cas9 methods, it may be possible to maintain the standing genetic variation at nontarget loci while propagating desirable traits into the environment. CRISPR/Cas9 substantially simplifies the process of genome editing because it relies on a very short RNA-coding region for target specificity (Doudna and Charpentier, 2014; Ran et al., 2013).
An additional goal is to use genetic manipulation to experimentally test hypotheses about the susceptibility of corals to stress and to identify the genetic causes of individual- or species-level variation in stress tolerance. This can also be applied to the algal symbiont, which plays a significant role in the bleaching response in corals. The major goal of
symbiont genetic manipulation will be to uncover the rules underlying the algal symbiont response to reactive oxygen species or thermal stress (e.g., antioxidant genes such as iron-type superoxide dismutase) that can be manipulated (e.g., over-expressed) to reduce bleaching susceptibility in colonies. If genetically modified symbionts can be generated that are hardened to environmental stress, they could be introduced into coral animals to protect them in the natural environment.
How to Do It
CRISPR/Cas9 is the only tool to date that has been used to directly alter a coral genome. The essential components of the CRISPR gene-editing system are a short noncoding guide RNA (gRNA) and the Cas9 protein. The gRNA is designed to be homologous to the specific genomic location of interest. This homology guides the gRNA/Cas9 protein complex to that genomic location where the Cas9 protein induces a break in the DNA, where insertions or deletions can be made as the break is repaired by the cell’s repair machinery (Li et al., 2013). Homologous sequences present elsewhere in the genome, on extra-chromosomal elements, or on foreign oligonucleotides can invade the Cas9-cut DNA and allow the incorporation of foreign sequences at the target site (Li et al., 2013).
CRISPR/Cas9 in Corals
A single paper on CRISPR/Cas9-based genetic manipulation in corals has been published (Cleves et al., 2018) and it provided preliminary proof-of-concept data using the model species, Acropora millepora. These authors targeted the genes encoding fibroblast growth factor 1a, green fluorescent protein, and red fluorescent protein. Fertilized eggs of A. millepora were injected with appropriate single guide (sg)RNA/Cas9 complexes. The results showed partial deletion mutation induction in 50% of larvae. All of the successfully altered larvae had deletion mutations that were heterozygous genetic mixtures of the wild type and several kinds of altered genes. Although the prospect of raising homozygous mutants through genetic crosses is challenging, the introduction into wild populations of beneficial alleles in heterozygous individuals may be sufficient to increase coral resilience. The generation of homozygotes would of course be very important to understand gene function but may not be necessary to improve coral health. Given these uncertainties, it is currently unclear how genome editing will impact coral research and restoration. Nonetheless, a lot was learned through this pioneering study that could contribute
to a long-term goal to generate genetically modified lines that are stable, adapted, and environmentally resilient.
CRISPR/Cas9 in Dinoflagellate Symbionts
Transformation protocols (e.g., for transgene expression) and CRISPR/Cas9-based genetic methods are also being developed for the dinoflagellate symbiont of corals (Levin et al., 2017), but no publications have yet resulted from the work. Potential targets for modification include genes such as superoxide dismutase, ascorbate peroxidase, and other antioxidants that respond to thermal stress (Wietheger et al., 2018).
CRISPR/Cas9 in Bacterial Components of the Coral Holobiont
Genetic engineering of the coral microbiome using CRISPR/Cas9 methods is another potentially powerful approach to improving coral resilience. However, CRISPR-induced double-strand breaks (i.e., both strands of the DNA duplex are broken) are lethal in bacteria because of the low efficiency of the repair pathway (known as non-homologous end joining) in these taxa. This has led researchers to engineer bacteriophage-derived recombination proteins in model species such as Escherichia coli to provide the needed function. These recombination-mediated genetic engineering (recombineering) methods are rapidly developing (Li et al., 2016). There is potential, therefore, to engineer traits into bacteria that can be beneficial for coral fitness (i.e., enhanced antioxidant activity), and then inoculate these bacteria into the coral microbiome. However, such approaches have not yet been tested, and require a far greater understanding of the coral microbiome and the ability to specifically manipulate the microbiome and maintain these shifts and conferred benefits (see the Microbiome Manipulation section in Chapter 3).
Current Feasibility
The basic mechanism of genetic manipulation with CRISPR/Cas9 has been demonstrated in corals (Cleves et al., 2018) and is well known as a tool in basic developmental biology of other cnidarians (Ikmi et al., 2014). To date, success using CRISPR/Cas9 in corals has been limited to mosaic creation of deleted gene segments in early larvae. No demonstration of altered phenotypes from manipulation and no demonstration of incorporation of manipulated genes into an adult coral have yet been published. A key area of future focus should be shortening the generation time in corals, perhaps using mutagenesis or identifying culture modifications
that enhance the rate of development, as has been done in Arabidopsis (Ochatt and Sangwan, 2008).
Feasibility for enhancing coral resilience will be dependent on the identification of clear gene targets hypothesized to be able to alter coral resilience through changes to a single gene or multiple genes in the same or different pathways. There are a wide variety of gene expression changes that have been documented during acclimation and adaptation to high-temperature conditions (Barshis et al., 2013), and a large number of changes in transcription factors likely to have multiple downstream effects soon after heat exposure (Traylor-Knowles et al., 2017). Pivotal pathways that might be important in switching from heat resistance to bleaching include the genes for heat shock proteins controlled by the heat shock factor (Louis et al., 2017), genes that control the unfolded protein response (Ruiz-Jones and Palumbi, 2017), genes associated with reactive oxygen species (ROS) production (Oakley and Davy, 2018), and others. However, differences between populations in resilience-related genes suggest that heat resistance is a multilocus phenotype controlled by tens or hundreds of genes (Bay and Palumbi, 2015; Dixon et al., 2015), so simple target genes with large temperature effects are not documented to date. One exception is a single allele discovered by Jin et al. (2016) to have a strong association with the oxidative state, potentially mediating ROS damage during symbiotic breakdown. CRISPR/Cas9 could be used as a technique to test this hypothesis as a starting point in further development. A potential avenue for identifying target genes for genetic manipulation is through the use of gene co-expression networks (an analysis to identify genes with similar expression pattern). These methods can help identify “hubs” of gene expression that impact downstream traits such as the cell cycle and stress response (Stuart et al., 2003; van Dam et al., 2018). These hub genes offer opportunities, for example through over-expression, to potentially enhance complex phenotypes such as the coral thermal stress response.
Manipulations of coral dinoflagellate symbionts appear to be less feasible. Ten Lohuis and Miller (1998) reported genetic transformation of Symbiodinium cells, but later attempts have not successfully repeated this result (Levin et al., 2017). A variety of problems have been proposed to explain the large hurdle of Symbiodinium transformation, including the thick cell wall, the atypical chromosomal structure, and the large evolutionary divergence of Symbiodinium to other tractable algal systems. In addition, as in corals, the best genes to target for Symbiodinium manipulation have not yet been identified.
Potential Scale
Initial trials of genetically modified corals or symbionts would occur at the individual colony or culture level. Once a modification was successfully accomplished, it would be individually tested for improvements in resilience. Thereafter, the modification is expected to be propagated through clonal growth or sexual reproduction into a population of corals that would be planted into a nursery population. Once the propagated genetic modification was tested for high stress tolerance, larger groups could be generated. Each phase of this amplification is likely to take 3-7 years, particularly if growth of corals to sexual reproduction is involved. Asexual propagation (e.g., repeated fragmentation) of genetically modified resilient corals might decrease the amount of time.
These changes would apply to a single modified gene. If modification of more than one gene was deemed necessary—and tests of heat tolerance suggest multiple loci are involved—then multiple modifications might need to be done in series. Alternatively, if done in separate lines, these distinct modifications would need to be combined in a single zygote through rounds of sexual reproduction. Because serial modification, or combination of separate lines through gamete fusion, would require sexual reproduction, each stage would take 3-7 years (e.g., Kojis and Quinn, 1981). This timeframe would slow deployment of multigene adaptive manipulations and reduce the number of modifications that could be rapidly deployed. These steps would apply to a single species, and would need to be completed separately, perhaps with separate genetic target loci, in different species.
Modifications to symbionts may be easier to scale up. If a symbiont with broad ability to colonize many coral species were to be conferred with a gene for heat tolerance, it might be able to be added to the holobiome of many different adult coral colonies or multiple species quickly if the original heat-sensitive symbiont could be replaced, because 85% of corals recruit the symbiont algae each generation.
Risk
The application of gene drives in field conditions is still controversial. This is due to ethical considerations and potential negative ecosystem impacts resulting from the release of genetically modified organisms that may spread undesirable traits among natural populations (e.g., NASEM, 2016a; Pugh, 2016). Unintended consequences are virtually impossible to predict a priori. For example, the gene drive could inadvertently impact other characteristics, such as the ability to transmit or withstand pathogens or other stressors (Kuzma and Rawls, 2016). Another potential risk
is drive resistance that can result from mutations that block cutting by the CRISPR nuclease (Noble et al., 2018). Resistance can arise from standing genetic variation at the drive locus or because the drive mechanism is not perfectly efficient and is predicted to prevent drive fixation in wild populations. The accidental release of gene-drive organisms by scientists poses unknown risks at this time (Callaway, 2017; Esvelt and Gemmell, 2017; NASEM, 2016a). Finally, an unintended risk of gene drives is the slow pace of development with coral models. Given that field deployment of modified individuals may take up to a decade, this method will not address immediate risks to corals and therefore be less effective in the short-term.
Polizzi et al. (2018) identified 13 potential risk factors associated with gene drives. Several of these may be relevant in the coral context, as noted below:
- Unexpected gene transfer: Efforts would be made to prevent the unwanted transfer of genes to other species, but it cannot be ruled out.
- Evolution: Resistance may be conferred in the short term and organisms may behave as expected, but it is impossible to plan and mitigate for the unexpected.
- “We don’t fully understand”: Although the technology is rapidly advancing, there are still many known unknowns (e.g., extent of off-target [nonspecific] genome editing), and an unknown number of unknown unknowns.
- Ecosystem effects are challenging to predict: Changing a coral community has many uncertainties and predicting how those changes will interact with wider ecosystems is virtually impossible.
Limitations
The discussion of methods, feasibility, and scale listed above reveal a host of limitations of the current technology of gene manipulation. Summarizing from the above:
- Current CRISPR/Cas9 technology has been demonstrated in corals but not symbionts. In addition, generation of homogeneously modified corals, and the delivery of these genes to offspring has not yet been successfully accomplished.
- Gene targets for coral or symbiont manipulation are not clear. However, gene expression followed by gene manipulation methods are powerful ways of testing which genes are the best to
-
manipulate. Such hypothesis testing will be crucial but time consuming.
- The small number of stress-resistance genes with large phenotypic effects reduces the likelihood that a single gene manipulation will provide substantial stress tolerance. Instead, current knowledge of the genetic architecture of coral stress genes suggests that multiple gene manipulations may be necessary. Such multiple manipulations are within the ability of current gene technology and animal husbandry protocols, but are time consuming.
These limitations are similar to those facing plans to genetically modify other animals introduced into the wild (salmon, mosquitoes, etc.), but are in some cases more severe because of the need to manage multiple genes and multiple species.
This approach is limited in its ability to develop resilience in corals in the near term due to the timeframe of research and development needed. An incremental approach where one species (i.e., a well-characterized “model” coral) is used to manipulate a small number of genes may be an appropriate initial plan. This initial plan would not save a substantial number of reefs by itself, but over a period of 10 years might lay the groundwork for other genes and species to be more quickly modified.
Infrastructure
Research infrastructure Marine laboratories with access to test corals, husbandry facilities, long-term growout laboratories that can be used to generate sexually mature colonies, good molecular biology laboratories, microscopy, symbiont culture facilities, and other facilities would need to be available for each species and each ocean region.
Bioinformatics infrastructure Genetic manipulation requires a well-known genome sequence for each species used, the ability to probe genome changes, and the ability to follow phenotypic, genotypic, epigenetic, and genetic changes across multiple generations.
Collaborative infrastructure It may be that a single laboratory at a single location will be able to achieve the initial success at manipulating one coral species at one gene, with resulting heritability and phenotypic change. However, it is most likely that it will take a consortium of laboratories across many countries to achieve multispecies, multigene success. This collaborative effort would require parallel facilities and parallel approaches in different nations, and would take advantage of the different coral spawning opportunities in different parts of the world
and different husbandry facilities. Different laboratories may need to test different genes for their ability to confer increased stress tolerance, with the idea that negative results would be considered a strong benefit to the consortium as a whole. A successful example of such an initiative is The Arabidopsis Information Resource (https://www.arabidopsis.org) where a wide variety of genetic and genomic resources are stored and shared for a model plant species. A parallel, coordinated effort can lead to the creation of a model coral to establish fundamental knowledge about its biology and genetics that can be used to widen analyses to a plethora of other important species. The value of having a well-annotated, manually curated, and high-quality genomic and genetic resource for a coral species cannot be overstated in terms of supporting all downstream genetic and ecological manipulations.




































