2
Diagnosis and Assessment of Traumatic Brain Injury
This chapter provides an overview of traumatic brain injury (TBI), including how it is defined, its mechanisms of injury, and its neuropathology. The chapter also provides a conceptual model on the recovery trajectories after TBI and intrinsic factors related to the variability in its presentation and diagnosis and in recovery from TBI. There is a discussion of the complexity of establishing a diagnosis of TBI, especially mild TBI (mTBI), the role of neuroimaging after injury, and the limitations of the current approaches. Finally, there is a discussion of which health care providers are qualified to make the diagnosis as well as the additional complexity of common co-occurring conditions in diagnosing TBI.
TRAUMATIC BRAIN INJURY
As noted in Chapter 1, traumatic brain injury is defined as an insult to the brain from an external force that leads to temporary or permanent impairment of cognitive, physical, or psychosocial function. TBI is a form of acquired brain injury, and it may be open (penetrating) or closed (non-penetrating) and can be categorized as mild, moderate, or severe, depending on the clinical presentation (Gennarelli and Graham, 2005). A TBI diagnosis is best documented at the time of injury or within the first 24 hours.
Mechanism of Injury
There are various mechanisms that can bring about a traumatic brain injury, which can result in physiologic or structural brain damage. The committee discusses those different mechanisms, which include blunt, non-penetrating TBI injury; penetrating injury; and blast injury.
Blunt, Non-Penetrating TBI
Blunt, non-penetrating TBI can result from a direct impact to the head or from rapid head acceleration or deceleration without impact. Brain injury from this mechanism has two phases. The first phase occurs as a direct result of the initiating traumatic event; the second involves a cascade of several neuropathologic processes continuing for weeks to months after the initial injury.
The primary injury phase is immediate, and its damage, which can cause death almost instantaneously, is often complete by the time emergency care is initiated. Direct impact of the brain against the bony cranial vault and shearing of neurovascular structures result in neuronal damage. Because the brain resides within a fluid-filled compartment, the movement of its cellular elements lags behind the skull during rapid deceleration. Thus, the brain will strike both anteriorly and posteriorly against the inner aspect of the skull, and a coup-countercoup lesion will result (Graham et al., 2002). If a rotational component is present—which is nearly universal in the case of blunt TBI—intracranial structures will torque and twist, resulting in excessive shear strain (i.e., stretch) (Morales et al., 2005; Smith, 2013). Neuronal axons and blood vessels are most susceptible to sheer strain due to their elongated microstructure. Thus, the primary injury phase of TBI results in damage to axons (axonal injury) and blood vessels (hemorrhage). Motor vehicle accidents are particularly injurious because of the sudden deceleration (Johnson et al., 2017).
The secondary injury phase begins immediately after the primary phase and involves a progression of axonal injury, with shifts in ionic flux leading to axonal swelling, a loss of axonal transport, and altered neurotransmission (Giza and Hovda, 2014). Mitochondrial failure results in an energy crisis for the neuron, leading to a loss of neuronal function and apoptosis (programmed cell death). This secondary phase might also involve necrosis and neuronal demyelination. A neuroinflammatory response involving microgliosis starts within hours of the injury and might continue for months or even years. TBI-induced blood–brain barrier dysfunction (BBBD) allows elements of the peripheral immune system to participate in this process. Diffuse microvascular damage combined with BBBD and a loss of autonomic regulation results in both hyper- and hypo-perfusion, contributing to ischemia and cerebral edema. The destruction of intra-axonal structures can result in abnormal accumulations of neurotoxic proteins such as beta-amyloid and phosphorylated tau. It is thought that post-TBI accumulations of those proteins in combination with persistent abnormal neuroinflammation might contribute to early-onset neurodegeneration or dementia (Giza and Kutcher, 2014; Smith, 2013).
Penetrating TBI
A TBI may be open (penetrating) or closed (non-penetrating). A penetrating TBI occurs when physical, external forces affect the brain and an object enters the brain tissue. A non-penetrating (closed) head injury is caused by an external force that produces movement of the brain within the skull.
Missile injuries, such as gunshot wounds, are a common cause of TBI, and are classified as either penetrating or perforating depending on how the missile traverses the head (Graham et al., 2000). In penetrating injuries, the object enters and lodges within the cranial cavity. Perforating injuries occur when the object traverses the cranial cavity and leaves through an exit wound. The extent of damage is governed by the shape and mass of the missile and by its direction and velocity (Morales et al., 2005). Damage is also related to the amount of energy released when the missile passes through the brain (Graham et al., 2000).
Blast-Induced TBI
Blast-induced traumatic brain injury (bTBI) has become a common type of military head injury, although non-blast mechanisms are still common in the military and civilian population (e.g., injuries from car and motorcycle accidents, athletic activities, and military physical training).
The neurologic injury from bTBI can result both from a direct shock wave effect and an indirect transfer of the shock wave through blood vessels and cerebrospinal fluid to the brain. Exposure to blast overpressure initiates a cascade of cellular pathologic processes in the brain, including damage to the microvasculature and blood–brain barrier (BBB) integrity, followed by increased BBB permeability. The breakdown of the BBB can result in brain edema and an increase in intracranial pressure, accompanied by the activation of secondary brain injury by impairing cerebral perfusion and oxygenation. In particular, the activation of oxidative mechanisms and neuroinflammation has been shown to contribute to the neurodegeneration and cell death in secondary brain injury following bTBI.
As with TBIs from other causes, bTBI may range from a severe form, which is often comorbid with polytrauma (i.e., multiple traumatic injuries, such as a TBI in addition to a serious burn or TBI and posttraumatic stress disorder [PTSD]), to the mild form, which shares symptoms or is comorbid with PTSD (discussed later in the chapter). The epidemiologic scale and complexity of bTBI and closely related neuropsychiatric conditions present significant short- and long-term challenges for the military health care system and for the Department of Veterans Affairs (VA) (Papa et al., 2015). Comorbidities often associated with TBI, including bTBI, will be discussed later in the chapter.
Neuropathology
TBI neuropathology consists of a primary injury that is a direct consequence of the traumatic insult and a secondary injury that results from a cascade of molecular and cellular events triggered by the primary injury and which leads to cell death, axonal injury, and inflammation (McKee and Daneshvar, 2015; Taylor and Gercel-Taylor, 2014). In response to tissue damage, cells release proteins into extracellular space which offers transit to body fluids, including the blood (Taylor and Gercel-Taylor, 2014). Acute responses occur as a result of the primary injury and orchestrate neuronal recovery; however, in a subset of individuals these biologic changes are related to symptoms and deficits which last beyond this period into the subacute, and for some even into the chronic, period of recovery (Taylor and Gercel-Taylor, 2014). The mechanisms that influence individual variability into recovery are not well understood and are a current research focus.
Chronic neurologic symptoms following traumatic brain injuries in military personnel are common and can include global disability, neurobehavioral impairment, and psychological comorbidities (Laskowitz and Grant, 2016). It is hypothesized that TBI and the subsequent pathogenic processes induce neurons and glial and endothelial cells to release molecules extracellularly that transit into blood (DeKosky et al., 1998). Extracellular release of molecules may occur through a breakdown of cell membranes (e.g., neurodegeneration) or via secretion as part of intercellular communication (e.g., cytokines or angiogenic factors), which likely contribute to the development and maintenance of chronic symptoms and deficits following TBIs. There is also evidence linking TBI to neurodegenerative diseases, including Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, and other types of dementia (e.g., Alzheimer’s disease and chronic traumatic encephalopathy) (Freeman and Ting, 2016; Gardner et al., 2014; Witcher et al., 2015).
ASSESSMENT OF TBI SEVERITY
During the diagnostic process, a clinician typically assesses the severity of TBI. However, the initial assessment of TBI severity does not necessarily predict the extent of disability arising from TBI. Typical approaches to determining severity early after injury include neuroimaging, assessing the presence of an altered consciousness or loss of consciousness, assessing the presence of posttraumatic amnesia, and applying the Glasgow Coma Scale score.
That score has been the gold standard of neurologic assessment of trauma patients since its development by Teasdale and Jennett in 1974 (Teasdale and Jennett, 1974). The Glasgow Coma Scale is a clinical tool designed to assess coma and impaired consciousness and is one of the most commonly used TBI severity scoring systems. Other TBI severity-classification systems grade single indicators, such as loss of consciousness and the duration of posttraumatic amnesia. The predictive value of those measures has been demonstrated (Dikmen et al., 1990; Levin, 1990, 1995; Levin et al., 1990; Sherer et al., 2008), but each may be influenced by factors unrelated to or only indirectly related to the severity of TBI (e.g., intoxication).
The severity of a TBI might range from mild to severe. There are multiple schema that have been developed by several organizations to assist in defining TBI severity (see Table of Case Definitions of Traumatic Brain Injury in Appendix F) which differ slightly from one another according to which criteria are weighed most heavily. All are similar in that those individuals with mTBI experience just a brief loss of consciousness or even an alteration of consciousness without complete loss. The Department of Defense (DoD) classifies severity using a combination of the four factors mentioned above: neuroimaging results (normal or abnormal), extent of altered or loss of consciousness (0–30 minutes, >30 minutes to <24 hours, and >24 hours), length of posttraumatic amnesia (up to 24 hours versus >24 hours), and Glasgow Coma Scale scores (using best score in first 24 hours; 13–15, 9–12, <9).
Recovery Trajectories of TBI
In this section, the committee presents a conceptual model for understanding recovery trajectories of TBI and then describes subject-level factors that might influence TBI recovery.
Within the first week of any TBI, most patients will experience a decline in function associated with a variety of symptoms. However, the degree of functional decline varies; some will be able to carry out normal daily activities such as school and work, while others will require formal rehabilitation (Eisenberg et al., 2013) (see Figure 2-1, Acute Phase). The initial severity of the brain injury is thought to have a major influence on the degree of functional decline during this phase. Indicators of injury severity include the Glasgow Coma Scale score (15 is the least severe, 3 is the most severe) and mechanism of injury (TBIs from motor vehicle collisions are typically more severe than other mechanisms). Vulnerability to neuronal injury may also influence the degree of functional decline; a prior TBI and pre-existing central nervous system diseases (e.g., dementia, multiple sclerosis, stroke) increase the risk of dysfunction during this stage (Iverson et al., 2017). Females may be more vulnerable than males due to weaker neck muscles (allowing for more head rotation and shear strain on neurons) (Collins et al., 2014)1 and reduced tensile strength of neuronal axons (Dollé et al., 2018). When symptoms are used to define function, declines from baseline also might be related to symptom reporting style.
___________________
1 The authors note that it is unclear the relative roles biophysiology, anthropomology, and sociocultural constructs play in these differences.
Although the initial severity of TBI often predicts the speed of recovery, recent research suggests that not everyone follows the same trajectory. Some recover fully, but slowly, while others never fully recover and might experience prolonged functional disability (Yeates et al., 2009) (see Figure 2-1, Subacute Phase). Factors influencing the degree of recovery during this phase include other comorbidities, resilience, cognitive reserve, and cognitive stressors such as work and school (Iverson et al., 2017; Sullivan et al., 2016). The treatments received might also influence the degree of recovery (Collins et al., 2016). It is not clear if the functional trajectory experienced in the acute phase influences the trajectory in subacute phase. Moreover, it should be emphasized that these subject-level factors account for less than one-quarter of the variance in TBI outcomes. That fact underscores their weakness as predictors of outcome and suggests that other, unmeasured factors are at play.
A single TBI of any severity can increase the risk of accelerated cognitive decline 10 or more years after the injury (Vincent et al., 2014) (see Figure 2-1, Chronic Phase). The individual’s age at the time of initial injury has a significant influence on the risk of accelerated decline (Gardner et al., 2014). Subsequent brain insults, such as those experienced during contact sports, also might influence the risk of accelerated decline (e.g., chronic traumatic encephalopathy) (Asken et al., 2017). It is not clear if the functional trajectory of either the acute or subacute post-injury phase influences that risk.
Factors Related to Variability in Presentation, Diagnosis, and Recovery of TBI
A number of factor might affect the trajectory of disability and recovery after TBI. They might affect the presentation, diagnosis, and course of TBI, which is viewed here as a progressive and chronic disease with lifetime consequences (Maas, 2016). While there is a vast literature on factors related to TBI in the general population, there are fewer studies describing associations within the military setting. Regardless, there is evidence that demographic factors, including age and sex, might influence the course, progression, and outcomes of TBI, as will the type of injury, comorbidities, and genetic predisposition. There is also evidence that differences in TBI outcomes might be related to factors involving access to care. These are briefly discussed below.
Age
While the median age of incident TBI is relatively young, especially in cases due to trauma occurring during active duty, it is highly variable. In the general population, TBI is more prevalent among those under 25 years of age and those older than 75 years (Peeters et al., 2015), with the mean age for women higher than for men. The absolute incidence of TBI among the elderly is increasing, most likely due to the greater life expectancy and mobility of older adults, with the resulting increased risk of falls (Faul and Coronado, 2015). Studies following the outcome of TBI have reported older age to be associated with greater disability in terms of both physical and cognitive function (Graham et al., 2010), with older individuals experiencing more hospitalizations and higher mortality than any other age group (Roozenbeek et al., 2013). However, not all studies have found an association between age and TBI outcomes. Age did not affect the Glasgow Outcome Scale or Disability Rating Scale in one study examining outcomes (Oppelt et al., 2018), and mixed results were seen in a second study, with no age differences found in 1-year functional outcomes, but an association of being age 80 or older observed with 1-year outcome scores on the total Quality of Life after Brain Injury (Gross and Amsler, 2018).
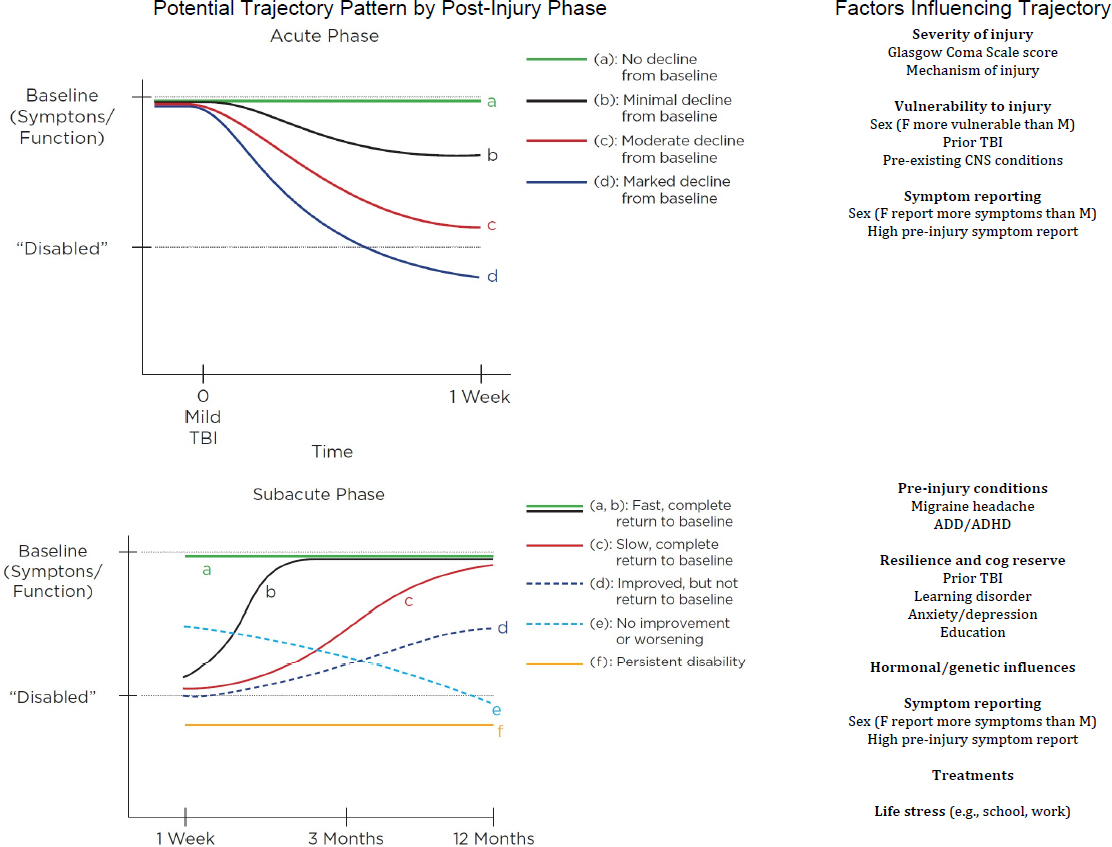
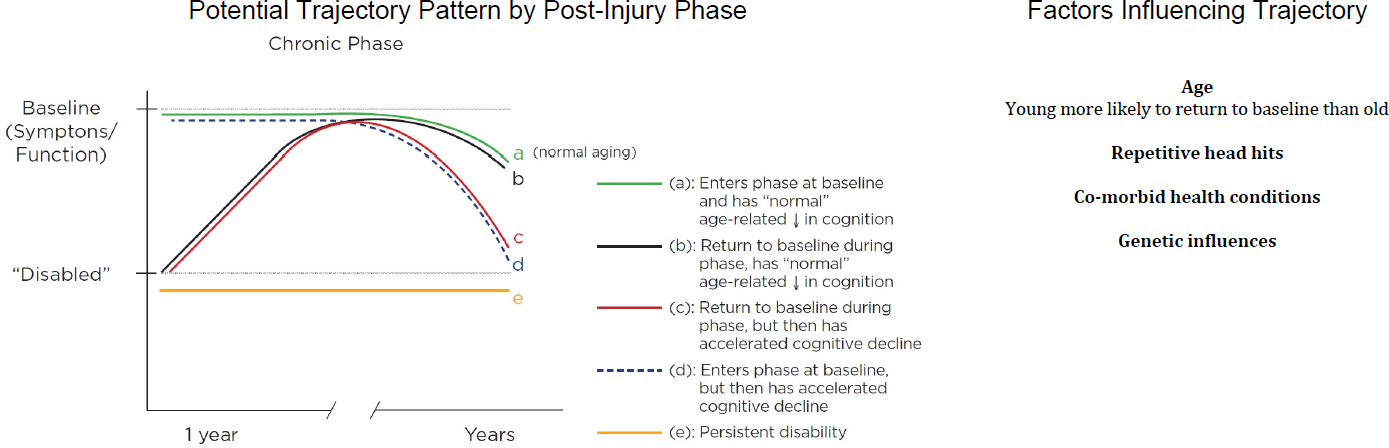
NOTES: The trajectories of disability and recovery after a single TBI are represented during the acute phase (0–1 week), the subacute phase (1 week to 3 months), and the chronic phase (3 months to >10 years). A solid line represents “typical” recovery trajectory; dashed lines represent trajectory variations. Breaks in the trajectory lines, between phases, indicate a lack of knowledge of how a trajectory in one phase influences the trajectory of the following phase. ADD/ADHD = attention deficit disorder/attention deficit hyperactivity disorder; CNS = central nervous system; F = female; M = male.
Sex
There is strong evidence that sex plays an important role in various aspects of TBI, from pathophysiology to clinical care. TBI disproportionately affects young males due to their high-risk behaviors, and with a mortality rate after TBI being four times higher in males age 20 to 24 than in females of the same age (Coronado et al., 2011). However, incidence is increasing in women as their involvement in military combat, sports, and other high-risk activities associated with TBI is increasing (Amara et al., 2014). Research on the impact of sex differences has produced variable findings.
In 2001 the Institute of Medicine acknowledged the important influence of sex on brain function (IOM, 2001). In one study, women with mTBI were found to have significantly higher odds of poor outcome than males (Bazarian et al., 2010). However, males and older adults are at increased risk of depression following TBI (Albrecht et al., 2018).
TBI may affect women of reproductive age; the stress of TBI may result in anovulation and central hypothalamic-pituitary-ovarian axis suppression (Ranganathan et al., 2016). A growing body of evidence indicates that hormones may play a role in injury susceptibility as well as recovery (Wright et al., 2014). There is evidence that high estradiol production is associated with adverse outcomes related to the extracerebral consequences of severe TBI (Rakholia et al., 2018).
Another recent study found that males, but not females, with TBI performed significantly worse than comparison participants without TBI on a dynamic task of emotional recognition abilities and that the sex difference could not be explained by lesion location, TBI severity, or other neuropsychologic variables (Rigon et al., 2016). In another study, by contrast, sex was not found to be an independent predictor for poorer outcome after severe TBI (Herrera-Melero et al., 2015).
Type of Injury
The types of TBI have been investigated, and significant differences in medical complications have been reported. For example, although penetrating injuries are less common than closed injuries in the civilian population, they are far more lethal (Santiago et al., 2012). Compared with blunt injuries, penetrating injuries are associated with higher rates of comorbidities involving the pulmonary and central nervous systems including respiratory failure, pneumonitis/pneumonia, skull fracture, cerebrospinal fluid leak, and hypotonia (Black et al., 2002). Recently, a number of studies have examined the consequences of blast as a new mechanism of brain injury. Trotter et al. (2015) reported a dose–response relationship between military blast exposure and white matter integrity and added that the number of years since the most severe blast was negatively associated with fractional anisotropy. High rates of sensory impairment, pain issues, and polytrauma were also found to be present in those injured by blasts (French, 2010). Although differences in the frequency of long-term complications between blast and non-blast TBIs have been reported, clinical presentation of blast-related injuries are difficult to track as they are classified by severity score rather than mechanism; severity scoring is associated with prognosis in clinical practice (Yamamoto et al., 2018). Unfortunately, injuries that appear to have different pathophysiologies and outcomes are managed in the same way, with perhaps not all of them being managed optimally (Santiago et al., 2012).
Comorbidities
Medical conditions associated with TBI are often diagnosed concurrent with or following the brain injury (Farmer et al., 2017; Hoge et al., 2006; Lew et al., 2007). A very comprehensive study was conducted by the RAND Corporation concerning the characteristics of non-deployed active duty service members diagnosed with mTBI, including their co-occurring symptoms and conditions (Farmer et al., 2017). In general, the rates of behavioral health diagnoses were found to be twice as high in those with a history of TBI, regardless of severity, than in those without such a history. Treatments for adjustment disorders (16 percent) and anxiety disorders (14 percent) were the most common, followed by treatment for depression; no differences were found in the rates of alcohol abuse or attention deficit disorder/attention deficit hyperactivity disorder (Farmer et al., 2017). Hoge at al. (2006), Kontos et al. (2013), and Manners et al. (2016) found that service members deployed in the Iraq war exhibited high rates of posttraumatic stress disorder associated with mTBI. However, self-reports of poorer general health, missed work days, medical visits, and a higher number of somatic and post-concussive symptoms were no longer associated with the TBI when adjusted for PTSD and depression. It is important to recognize that mental health symptoms might have causes other than TBI—specifically, causes involving pain, medication, alcohol or drug use or intoxication, or PTSD, any of which can be present either in isolation or in addition to a brain injury and can confound or complicate the diagnosis (Roozenbeek et al., 2013).
Genetic Predisposition
In the era of promoting precision medicine for the treatment of specific disease and disorders in individuals, genetic predisposition plays an important role in TBI outcomes. Understanding the impact of genetic influences on neurorecovery from TBI has the potential to provide guidance for the better individualization of prognosis and to inform the development of novel treatments, which are currently lacking (Kurowski et al., 2017). Several recent reviews and meta-analyses have been published that hint at the influence that genes may have in post-injury recovery and disability. The gene that has been most investigated to date is apolipoprotein E (ApoE), a lipid transport protein, which is recognized as an important genetic risk factor for dementia and other neurodegenerative diseases (Van Giau et al., 2015). While the ε4 allele of ApoE plays a significant role in the pathogenesis of neurodegeneration, particularly in Alzheimer’s disease, its role in other neurologic diseases has not been conclusively elucidated, as confounding factors have affected interpretation of the allele’s role (Maiti et al., 2015). A meta-analysis evaluating cognitive function and neuropsychologic domains published in 2016 concluded that that ApoE ε4 does not have a detrimental effect on cognitive performance following TBI (Padgett et al., 2016).
Several other systematic reviews have investigated the relationship between non-ApoE genes and TBI recovery. Kurowski and colleagues (2017) used a system biology–based approach to identify biologic processes over-represented with genetic variants previously implicated in clinical outcomes after TBI and attempted to identify unique genes potentially related to recovery after TBI. Their study identified genetic variants primarily involved in two biologic processes: response to injury (cell proliferation, cell death, inflammatory response, and cellular metabolism) and neurocognitive and behavioral reserve (brain development, cognition, and behavior). They concluded that novel sets of genes are implicated in the healing process following TBI, which may be important in understanding the underlying complex biologic processes important to TBI recovery (Kurowski et al., 2017). Another review of the relationship
between genetic variations and outcomes after TBI found that the tissue, cellular, and subcellular location of non-ApoE single nucleotide polymorphisms (SNPs) reported to be associated with variation in global, neuropsychiatric, and behavioral outcomes could be clustered into three types: those associated with the blood–brain barrier, neuroprotective/regulatory functions, and neuropsychiatric/degenerative groups (Zeiler et al., 2018). A review of polymorphisms associated with TBI reported two studies that found SNPs related to brain-derived neurotrophic factor to be significantly associated with concussion incidence (Panenka et al., 2017). The investigators noted that U.S. soldiers with that genotype were more likely to report a history of concussion prior to deployment and to sustain a concussion during deployment. While work in this field is just beginning, it is clear that future studies using genomic, proteomic, and epigenetic approaches to research will have a significant impact on the understanding of risk and outcomes related to TBI.
Access to Care and Disparities in Outcomes, Treatment, and Follow-Up
While it is apparent that age, sex, race, and other factors are associated with differences in TBI incidence, presentation, and severity, studies investigating the follow-up of TBI are critical to determining if and what factors might affect long-term outcomes.
Two important factors, race and insurance status, have been found in multiple studies to be associated with lower referral rates for rehabilitation or other follow-up treatment after TBI. A study of discharge destinations of almost 300,000 patients with moderate or severe TBI using National Trauma Data Bank data over the years 2007–2010 found that Hispanic and black patients were less likely to be discharged to higher level rehabilitation than were non-Hispanic whites (Meagher et al., 2015). In that study, the racial disparity remained even at older ages where uniform insurance coverage by Medicare existed. In a study using the same data source, racial/ethnic groups were found to be comparable in terms of injury severity score, TBI severity, and associated injuries. However, after adjusting for confounders including insurance status, non-white patients were 15 percent less likely to be placed in rehabilitation than white patients (Shafi et al., 2007). Asemota et al. (2013) directly compared insured and non-insured patients by race. They found that insured blacks, Hispanics, and Asians were less likely to be discharged to rehabilitation than insured whites. In terms of insurance coverage, all ethnicities without insurance were less likely to be discharged to rehabilitation than insured whites. Haider et al. (2008) found similar results concluding that African American, Hispanic, and uninsured patients have worse outcomes, but insurance status appears to be more strongly associated with mortality after trauma than race/ethnicity.
McQuistion et al. (2016) expanded the investigation of outcomes reporting that the uninsured were less likely to have a TBI procedure, had longer hospital stays, were more likely to die in the hospital, and were less likely to be discharged to rehabilitation than those with private insurance. In this study of more than 187,000 patients registered in the National Trauma Data Bank during 2002–2012, results by race/ethnicity varied depending on the outcome assessed. In a study of predominantly (70 percent) Hispanic patients, Hispanic ethnicity and insurance status along with markers of injury severity were predictive of discharge to rehabilitation facilities and to long-term acute care/nursing facilities (Budnick et al., 2017). In a study of almost 15,000 U.S. veterans diagnosed with TBI in 2006, Dismuke and colleagues (2015) found evidence that health care use might be a partial mediator between race/ethnicity and mortality. In that study, Hispanic veterans were found to have fewer total visits and fewer TBI clinic, neurology, rehabilitation, and other visits than non-Hispanic whites, with the only
exception to the pattern being that Hispanic veterans had more mental health visits than non-Hispanic white veterans. Similar results involving differences in outcomes or follow-up by race/ethnicity have also been published (Arango-Lasprilla and Kreutzer, 2010; Berry et al., 2010; Bowman et al., 2007; Gary et al., 2009; Perrin et al., 2014; Schiraldi et al., 2015; Shafi et al., 2007; Staudenmayer et al., 2007).
Studies of other factors that might affect TBI outcomes are limited. A recent qualitative study described rural heath disparities for TBI services (Eliacin et al., 2018). In addition to generalized findings involving inadequate access to and the unavailability of specialized, age-appropriate, and long-term health services, the researchers reported that patients experienced transportation barriers to health services which limited access to care. Those barriers tended to amplify the health disparities between rural and urban or suburban patients. The amount of “safety-net burden” of hospitals, defined as the proportion of Medicaid and uninsured patient charges that were covered by the health care facility, was also found to affect TBI outcomes (Bakhsheshian et al., 2018). High-burden hospitals had greater mortality rates and more major complications than those with lower safety-net status. In a study investigating the lifetime prevalence of TBI among 2,881 African Americans and whites, Kisser et al. (2017) found a significant three-way interaction among race, poverty status, and age. The results, which were based on individuals’ histories of TBI, indicated a higher prevalence of TBI in men, older African Americans in poverty, and younger whites in poverty.
Those studies suggest that post-TBI access to care issues, which are found to be associated with race/ethnicity, insurance coverage, and socioeconomic status, might be a primary factor in disparities in long-term outcomes in TBI patients. Preventive measures targeting the relevant TBI risk factors in those populations (Kisser et al., 2017) and policies to address systematic inequalities in access that may affect long-term functional outcomes (Shafi et al., 2007) are warranted.
EMERGING EVIDENCE ON NATURAL HISTORY OF MILD TBI
While considerable research has been conducted on mTBI, the diagnosis of mTBI and the prognosis offered after a finding of mTBI continue to be complicated and sometimes controversial. However, it is likely that three ongoing, large-scale, multi-center, observational, longitudinal studies that are now in the advanced stages of data collection, curation, and analysis will reveal new information about both the psychosocial and neurobiologic manifestations of mTBI.
Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) is a prospective, multicenter, longitudinal observational study of civilian patients with TBI presenting to 18 Level I trauma centers throughout the United States. Funded by the National Institute on Neurological Diseases and Stroke, the project recruited between 2,700–3,000 adults who were evaluated in an emergency department within 24 hours of injury and had a clinical indication to require a computed tomography (CT) scan. As a result, the sample is predominately composed of persons with mTBI; however, the full spectrum of injury severity is included. Importantly, 300 orthopedic trauma controls were also enrolled. The data collected include CT scans, advanced imaging such as magnetic resonance images (MRIs), blood biospecimens, indices of premorbid characteristics, and detailed clinical outcomes with more than 20 outcome assessments. Follow-up interviews are conducted at 2 weeks, 3 months, 6 months, and 1 year
post-injury. The research program also includes the recruitment of a friend control group without injury.
The Chronic Effects of Neurotrauma Consortium (CENC) was established in 2013 via a federal cooperative agreement responding to the National Research Action Plan for improved prevention, diagnosis, and treatment of service members and U.S. veterans with TBI. The centerpiece of CENC is a multicenter, longitudinal, observational study designed to address gaps in knowledge about who served in Operation Iraqi Freedom and Operation Enduring Freedom and experienced mTBI. Data are being collected from more than 30 academic universities, 15 VA medical centers, and 3 military treatment facilities. The study’s overarching goal is to understand the associations among chronic effects of mTBI, neurodegenerative disorders, and common comorbidities, including psychological, neurologic (i.e., memory, seizure, autonomic dysfunction, and sleep disorders), sensory (i.e., visual, auditory, and vestibular dysfunction), movement, pain (which includes headache), and cognitive and neuroendocrine disorders. CENC will collect data and conduct annual follow-up interviews on the research participants.
The National Collegiate Athletic Association and DoD established the Concussion Assessment, Research and Education (CARE) Consortium to study the natural history of clinical and neurobiologic recovery following concussion in athletes from collegiate sports and in U.S. military academy cadets. The CARE Consortium is a 30-site investigation that intends to enroll approximately 25,000 athletes and cadets, with the goal of capturing 1,200 participants who experience concussions. The project, launched in 2014, includes data collection from 30 campuses across the country. The CARE Consortium has two major components: a clinical study core, which is investigating the natural history of how symptoms of concussion manifest and evolve over time, and the advanced research core, which studies the neurobiology of concussion and repetitive head impact exposure. The CARE Consortium is also intended to provide a framework for a future longitudinal study that will examine both the intermediate and long-term effects of concussion and repetitive head impact exposure.
Each of these studies is recruiting, or has recruited, a large cohort of people from whom a broad array of imaging, biologic, clinical, and psychosocial data are being collected. Each study is designed to examine the natural course of recovery over months to years. The three studies focus on different cohorts: persons treated in civilian Level I trauma centers (TRACK-TBI), post-9/11 veterans and service members exposed to combat and experiencing an mTBI (CENC), and students engaged in collegiate sports or attending U.S. military academies (CARE Consortium). Given the breadth of characteristics being studied and the naturalistic designs, it is reasonable to expect that new insights into the manifestation and consequences of mTBI will emerge from these studies. In particular, the discovery of one or more biomarkers of the chronic effects of TBI could be an important advancement for disability determination if these indicators were sensitive to both current functional effects and vulnerability to future consequences.
ESTABLISHING A TBI DIAGNOSIS
While the previously described studies may lead to the identification of biomarkers that can confirm the diagnosis of TBI long after injury, at the present time a clinical interview and self-report using a validated screening method is considered the gold standard for determining a comprehensive lifetime history of exposure to TBI. This section will review the screening instruments used to detect potential cases of TBI, the various clinical criteria and case definitions, the limitations of the current approaches in the clinical diagnosis of mTBI, and the
role of neuroimaging in identifying patients with a potential TBI. Chapter 3 will discuss the tools specifically used by the VA to provide evidence for the disability determination for residuals of TBI.
Screening
A number of screening instruments have been developed to detect potential cases of TBI. Relying on medical records is often insufficient because many injuries are not treated, including, occasionally, even more severe injuries. Screening instruments vary in the extent to which their psychometrics have been established (e.g., Corrigan and Bogner, 2007a; Russell et al., 2013; Schneider et al., 2016; Terrio et al., 2009; Vanderploeg et al., 2012), with single-item screens tending to be the least reliable and unlikely to capture all TBIs (Diamond et al., 2007).
The primary method for screening for exposure to deployment-related TBI at the time of injury is the Military Acute Concussion Evaluation 2 (MACE 2). Because many mTBIs are not evaluated at the time of injury, injuries incurred during deployment are typically screened retroactively with the Brief Traumatic Brain Injury Screen (BTBIS) (DVBIC, 2007; Schwab et al., 2007), a four item measure that is typically completed by service members upon return from deployment as part of a comprehensive health screening. The VA’s TBI screening tool is a modified version of the Defense and Veterans Brain Injury Center’s (DVBIC’s) BTBIS. The screening tool that the VA developed from the BTBIS errs on the side of being overly inclusive in identifying veterans at risk for having a TBI (GAO, 2008). A positive screen is followed by a more comprehensive evaluation (VA, 2010). The MACE 2 and the BTBIS are described in more detail below.
Military Acute Concussion Evaluation
In the field, the MACE 2 is completed immediately post-injury—specifically, following an event that might have resulted in a TBI—to determine cognitive deficits due to mTBI (DVBIC, 2006, 2018). The major goals of the MACE 2 are to confirm the diagnosis of mTBI and to provide further assessment data by using the Standardized Assessment of Concussion (McCrea et al., 1997) to record neurocognitive deficits. The MACE 2 can be used by medics and corpsmen and can be administered within 5 minutes of injury; there are no data to support its use beyond the acute injury period (French et al., 2008). The four cognitive domains tested are orientation, immediate memory, concentration, and delayed recall. Evaluations of the validity of the MACE 2 indicate that it is able to distinguish service members who have sustained mTBI from controls and that it accurately predicts the timing of return to duty (McCrea et al., 2014). If administered more than 12 hours post-injury, however, its sensitivity and specificity are lower, and it is not considered to be of clinical utility (Coldren et al., 2010).
The MACE 2 form consists of four sections (see Appendix G):
- Concussion screening: includes a description of the injury event (event as described by the service member or a witness, observable signs, type of event, and whether there was a blow or jolt to the head) and screening questions about loss of consciousness, alteration of consciousness, and posttraumatic amnesia. Also included in the concussion screening are a checklist of symptoms and specific questions regarding medical history related to concussion, headache, migraine, depression, anxiety, and other behavioral health concerns. If the evaluator answers “yes” to the service member having both a “blow or jolt to the head” AND “any alteration of consciousness or
-
memory,” the evaluator continues with the other portions of MACE 2. In the exam summary, the evaluator reviews the symptoms checklist and marks “1 or more symptoms” or “no symptoms.” The evaluator also reviews the medical history results and marks “positive” or “negative.”
- Cognitive exam: assigns scores for orientation, immediate memory, concentration, and delayed recall. The scores are totaled out of 30 possible points and reported at the end of the MACE 2 form.
- Neurologic exam: tests for speech fluency and word finding, grip strength and pronator drift (an indicator of muscle weakness and compensation), balance and gait, normal or abnormal pupil response to light, and eye tracking. The evaluator indicates an overall response of “normal” or “abnormal.”
- Vestibular/ocular-motor screening (VOMS): tests for baseline symptoms, smooth pursuits, saccades, convergence, vestibular-ocular reflex, and visual motion sensitivity. The evaluator is instructed to consider deferring this test if the patient is overly symptomatic or a trained provider is unavailable. The evaluator scores the section as “abnormal,” “normal,” or “deferred.”
The Brief Traumatic Brain Injury Screen
The BTBIS is a one-page paper-and-pencil questionnaire designed by the DVBIC to screen for TBI in service members (DVBIC, 2007; Schwab et al., 2007). It begins with a few questions about basic demographics and deployment history over the preceding 2 years, which are followed by three questions designed to identify possible TBI (see Box 2-1). The first of those asks about any injuries received during deployment, with checkboxes indicating blast, vehicular, bullet, falls, and other as categories of injuries. The next question asks about neurologic features of TBI, including any alterations in consciousness and loss of consciousness that resulted from injuries identified by the previous question. The question also includes the categories “having symptoms of concussion afterward” and “head injury,” which are not part of the definition of TBI; those were included to provide further description of the injury for clinicians. The final question aims at identifying specific symptoms and problems that are thought to be possibly associated with a head injury or concussion. Generally, it takes about 3 to 4 minutes to complete the BTBIS. A critical review of the literature on the instrument concluded that sensitivity was poor, with 30–60 percent of cases being missed, but its specificity was acceptable (Belanger et al., 2016).
Clinical Criteria and Case Definitions
Clinical criteria provide guidance to clinicians on the specific signs, symptoms, or test results that indicate the presence of an illness, and they guide the classification of patients into diagnostic categories. Clinicians use diagnoses to manage illness, provide appropriate treatment, and predict prognosis. Case definitions are a specific type of diagnostic criteria used to define an illness, and they work well for illnesses for which the underlying pathology is understood and can be observed. Case definitions often are assessed in terms of sensitivity, or the ability to identify patients with an illness correctly.
Numerous organizations have developed case definitions and clinical guidance for determining the diagnosis and severity of TBI (e.g., the VA and DoD, the American Psychiatric
Association) (see Appendix B). They all include similar criteria concerning which factors to consider; they vary primarily on the criteria for diagnosis of mTBI, whereas moderate to severe TBI is consistently defined as a loss of consciousness of greater than 30 minutes, posttraumatic amnesia lasting longer than 1 day, and a score on the Glasgow Coma Scale of less than 13, with or without abnormal imaging.
Department of Veterans Affairs and Department of Defense
mTBI diagnostic criteria range from observations relating to one or more of the common four factors (neuroimaging, loss of consciousness, posttraumatic amnesia, and the Glascow Coma Scale score) to the use of symptom checklists or some combination of these. The VA and DoD developed a joint clinical practice guideline for the management of concussion and mTBI (VA, 2016). These clinical practice guidelines are meant to assist in decision making rather than to prescribe a standard of care. The diagnosis of mTBI continues to be an emerging science which should be reflected in a learning system approach to the disability determination process.
The VA also provides instructions for coding any TBI using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) (see Appendix H). To ensure the most accurate and appropriate level of coding, the documentation for initial encounters must clearly state if there was a loss of consciousness due to injury based on the status of the patient at the time of injury and the duration of the loss of consciousness. If documentation does not clearly define that loss of consciousness, then an unspecified state of consciousness must be coded. Follow-up care should be coded for the sequelae of TBI using the symptom code(s) best representing the patient’s chief symptoms (VA, 2015).
American Psychiatric Association
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) addresses TBI and its neuropsychiatric sequelae with DSM’s framework for neurocognitive disorders.2 The DSM requires strict criteria for diagnosing major or mild neurocognitive disorders resulting from a TBI. These include, first, that the criteria are met for major or mild neurocognitive disorder3 and then that there is evidence of a TBI and that the neurocognitive disorder presented immediately after the TBI or immediately after the recovery of consciousness and that it persists past the acute post-injury period (APA, 2013).
TBI is defined in the DSM-5 as an impact to the head or other mechanisms of rapid movement or displacement of the brain within the skull, with one or more of the following:
- loss of consciousness,
- posttraumatic amnesia,
- disorientation and confusion, and
- neurologic signs (e.g., neuroimaging demonstrating injury; a new onset of seizures; a marked worsening of a preexisting seizure disorder; visual field cuts; anosmia [loss of smell]; hemiparesis).
___________________
2 Clinicians use the DSM to diagnose disorders affecting mood, personality, identity, cognition, etc. The DSM has been updated several times since it was first released in 1952, and it is published by the American Psychiatric Association.
3 See criteria for a major or mild neurocognitive disorder in the DSM-5 (APA, 2013).
DSM-5 distinguishes major versus mild neurocognitive disorder with evidence of a severe versus modest cognitive decline from the patient’s previous level of performance in one or more of the following cognitive domains: complex attention, executive function, learning and memory, language, perceptual-motor, and social cognition. The other distinction between major and mild neurocognitive disorder is whether the individual’s cognitive deficits interfere with his or her ability to be independent in the activities of daily living (e.g., paying bills, taking medications). In major neurocognitive disorder, there must be significant interference with the activities of daily living.
DSM-5 describes a TBI severity rating for the initial injury, which includes the standard mild, moderate, and severe TBI measures. However, DSM-5 notes that the initial severity rating of the TBI is not necessarily predictive of the severity of the resulting neurocognitive disorder. DSM-5 identifies age, a prior history of brain damage, and a history of substance abuse as factors that might impede recovery following any type of TBI. DSM-5 lists the common symptoms that further support the diagnosis of major or mild neurocognitive disorder due to traumatic brain injury. These symptoms occur with disturbances in the following areas:
- Emotional function: irritability, easy frustration, tension and anxiety, emotional lability
- Personality changes: disinhibition, apathy, suspiciousness, aggression
- Physical symptoms: headache, fatigue, sleep disorder, vertigo, tinnitus, anosmia
- Neurologic symptoms: seizures, visual disturbance, cranial nerve deficits
- Orthopedic injuries
Finally, DSM-5 states that except in cases of severe TBI, the typical course of TBI involves an improvement in the neurocognitive, neurologic and psychiatric signs and symptoms. However, some individuals will continue beyond 1 year post-injury to experience symptoms such as headaches, fatigue, depression, anxiety, and irritability and to experience post-concussive syndrome (McInnes et al., 2017), and there is evidence to suggest that a single concussion can disrupt the neurologic mechanisms underlying cognition (Xiong et al., 2014).
The World Health Organization and the National Center for Health Statistics
The World Health Organization owns and publishes the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). It is a useful tool in the classification of morbidity data for indexing health records, medical care review, and ambulatory and other health care programs as well as for basic health statistics.
The ICD-10, Clinical Modification (ICD-10-CM) is published by the U.S. government in recognition of its responsibility to promulgate this classification throughout the United States for morbidity coding (CDC, 2018). Specifically, the National Center for Health Statistics, part of the Centers of Disease Control and Prevention (CDC), is responsible for use of the ICD-10 in the United States, and it has developed the clinical modification of the classification for morbidity purposes. The ICD-10 is also used to code and classify mortality data from death certificates. The ICD-10-CM is comparable to the ICD-10. As noted on the CDC website, the term “clinical” is used to emphasize the modification’s intent.
Finally, there are many misconceptions about TBI and, particularly, about mTBI; some of these are presented in Table 2-1.
TABLE 2-1 Common Misconceptions About Traumatic Brain Injury
| Misconception | Fact |
|---|---|
| Symptoms that are not recognized immediately post-injury are not due to the TBI. | Individuals may not be able to distinguish alterations in consciousness associated with mTBI from changes in mental state due to other sources, such as sleep deprivation, acute stress, or confusion of the combat setting (Chapman and Diaz-Arrastia, 2014). |
| Sequelae that are not reported immediately post-injury are not due to the TBI. | Problems may be temporarily masked by discomfort/pain associated with polytrauma and by medications that can impede cognitive functioning. Awareness of cognitive or other changes may not emerge until the person returns to challenging situations, such as a return to duty. In addition, secondary brain injury, such as the development of a subdural hematoma, could result in the emergence of sequelae being delayed (Ghajar, 2000; Kiraly and Kiraly, 2007). |
| Symptoms from mTBI should resolve within 3 months; more chronic symptoms are likely due to psychologic factors or to secondary gains. | While many people recover from a single mild TBI quickly, some do not. Emerging research suggests that our previous expectations of recovery were too simplistic. For example, neuroinflammation has been found to persist for months post-injury and has been associated with persistent behavioral symptoms such as PTSD (Devoto et al., 2017). Repetitive TBI or subconcussive blows to the head, as commonly experienced by military personnel, can exacerbate underlying pathology and increase the likelihood of delayed or persistent symptoms (Fehily and Fitzgerald, 2017). |
| mTBIs should always be associated with mild sequelae. More severe sequelae are likely due to psychologic factors. | The severity of injury sequelae is influenced by many factors beyond the initial injury presentation. For example, changes in the structure and function of the brain may not be identified using conventional neuroimaging (Veeramuthu et al., 2015); prior injuries or compromised brain health can exacerbate the effects of new injuries (Fehily and Fitzgerald, 2017); multiple comorbid conditions can intensify the effects (Pugh et al., 2016). |
Neuroimaging
Neuroimaging plays an essential role in identifying patients with a brain (intracranial) injury, both acute injuries and, in some cases, injuries with persistent symptoms. Common imaging techniques include CT scans and MRI scans. Rapid imaging helps differentiate patients who require urgent neurosurgical intervention from those who can be monitored or sent home. When imaging is clinically indicated in the evaluation of TBI, non-contrast CT (NCCT) is the primary choice (Mutch et al., 2016). However, many patients with TBI do not show evidence of injury on CT scans, and MRI has been shown to have superior sensitivity for identifying small, focal traumatic intracranial lesions (Lee et al., 2008), and new generations of imaging technology continue to reveal abnormalities unrecognized by standard imaging.
CT Imaging
NCCT is the most common imaging technology used to assess TBI because it readily detects trauma-related fractures, hemorrhage, intracranial injury, extra-axial fluid collection, brain tissue swelling, and radio-opaque foreign bodies (e.g., shrapnel) (Jagoda, 2008; Wintermark et al., 2015). There is a consensus and evidence that NCCT should be the initial diagnostic imaging test for patients with acute moderate to severe TBI (Wintermark et al., 2015).
The early detection of expanding hemorrhage is key for rapid neurosurgical decompression, which can be lifesaving. It is recommended that all patients with a Glasgow Coma Scale (GCS) score of less than 15 get head CT scanning. For those with GCS of 15, among whom the prevalence of intracranial injury is less than 10 percent (Easter et al., 2015), clinical decision rules can be used to identify high-risk patients in need of head CT scanning. Typical post-TBI CT findings include subdural hematoma,4 epidural hematoma,5 intra parenchymal hemorrhage,6 contusion, and traumatic subarachnoid hemorrhage. Contusions and subdural hemotoma are the most common intracranial injury, followed by subarachnoid hemorrhage, and then by epidural hematoma, which are relatively uncommon.
When imaging is clinically indicated for the evaluation of acute mTBI, then NCCT is the initial choice. The advantages of CT include 24-hour availability in most emergency medical facilities, minimal imaging time, and no contraindications for patients with ferromagnetic substances (e.g., metallic foreign body or cardiac pacemakers). Following clinical screening, the majority of mTBI patients will have normal NCCTs (i.e., uncomplicated mTBI), but this is not sufficient to establish whether a patient has sustained a TBI.
MRI
Although many patients with mTBI will have normal findings on CT, that does not mean they do not have brain injury. Despite the many advantages of CT, MRI has superior sensitivity for the identification of hemorrhagic axonal injury and small contusions and has been shown to identify these lesions in patients with normal CT scans (Yuh et al., 2013). Over the past decade, there has been increasing use of MRI following the initial TBI assessment and treatment, especially in patients with persistent unexplained neurologic findings (Wintermark et al., 2015). While MRI is currently less available in the acute setting, takes longer to perform, and is more expensive than CT, newer MRI imaging techniques are advancing our understanding of TBI and will likely play a larger role in the diagnosis and management of TBI.
Advanced Imaging Techniques
Diagnosing brain injury for all levels of TBI severity is a particularly active area of research. Advanced MRI imaging techniques such as diffuse tensor imaging (DTI) might have prognostic utility in patients with TBI (Edlow et al., 2016; Yuh et al., 2014). Subtle alterations of brain tracts or fiber pathways have been visualized using DTI, which enables better imaging of the extent of early microstructural changes post-mTBI (Veeramuthu et al., 2015). DTI has
___________________
4 Subdural hematoma is an accumulation of blood above the brain but below the dura, which appears as a crescentic or concave opacity overlying the brain on CT.
5 Epidural hematoma is a traumatic accumulation of blood between the inner table of the skull and the stripped-off dural membrane.
6 Intra parenchymal hemorrhage is one form of intracerebral bleeding in which there is bleeding within brain parenchyma.
provided evidence that all TBIs, ranging from mild to severe, can result in a degree of axonal damage, with the more severe injuries showing damage to both axons and myelin (Pan et al., 2016).
Finally, positron emission tomography (PET) scans are useful for looking at brain metabolism and molecular imaging. PET scanning can reveal anomalies in TBI patients with unremarkable CT and MRI scans (Shin et al., 2017). However, the utility of PET and other advanced imaging techniques in diagnosing mTBI remains to be determined.
Limitations of Current Approaches in the Clinical Diagnosis of Mild Traumatic Brain Injury
The current methods of mTBI diagnosis rely on a report of certain symptoms at the time of injury from the person who was injured or from a witness. The immediate symptoms that indicate mTBI are a brief loss of consciousness or a period of amnesia or confusion, or both. Some definitions also include an immediate headache. There are few specific tests, such as X-ray, blood test, or CT scan, widely available to help make the diagnosis of mTBI. The Food and Drug Administration (FDA) has cleared the following tests for the prediction of intracranial hemorrhage on a head CT scan performed within 12 hours of TBI: a blood test combining glial fibrillary acidic protein and ubiquitin carboxy-terminal hydrolase L1; the Banyan Biomarkers’ brain trauma indicator, which identifies and measures the levels of two brain-specific proteins that appear in the blood within 12 hours of a brain injury when bleeding has occurred; a portable quantitative electroencephography test (BrainScope’s Ahead® 100), which provides an interpretation of the structural condition of the patient’s brain after head injury; and a portable near-infrared spectroscopy (NIRS) to detect intracranial bleeding.
Despite those options, the current widely used methods of diagnosing mTBI are far from ideal, as they can produce both false positive and false negative diagnoses. Common issues in the diagnosis of mTBI are described below.
Some Patients Who Experience mTBI Symptoms Cannot or Do Not Report Them
Because mTBI can result in retrospective amnesia or confusion at the time of injury, patients might not be able to recall the details of their injuries. Furthermore, within the combat setting, sleep deprivation, acute stress, sensory overload, and prolonged missions can impede a service member’s ability to recognize an alteration of consciousness, and those same complicating factors can potentially affect recovery (Chapman and Diaz-Arrastia, 2014). The recollection of the events associated with an injury might also be altered by drug or alcohol use or by preexisting dementia, both of which are common among civilians with mTBI. And two patient groups, active duty troops and athletes, might well remember injury events and symptoms but be reluctant to report them for fear of being pulled from their unit or team. Thus, given the current state of diagnosis, mTBIs are often overlooked.
A recent study found that one-third of athletes did not realize they had a concussion (Meehan et al., 2013). Patients treated in hospital settings do not fare much better. Three studies of patients with head injuries presenting to emergency departments found that concussions were missed in 56 to 89 percent of cases (De Maio et al., 2014; Delaney et al., 2005; Powell et al., 2008). Another difficulty in diagnosing mTBI occurs in the case of complex polytrauma where other injuries might appear to be more severe and the head injury is not assessed.
Some Patients Who Experience mTBI Symptoms and Report Them Might Not Have Brain Injury
A headache following a head injury might be due to a traumatically triggered migraine headache or to neck muscle strain, particularly if the headache begins hours after the injury. A brief loss of consciousness during contact sport or combat activities might be a result of severe dehydration, occult bleeding, or neurocardiogenic syncope (Williams and Bernhardt, 1995).
Some Patients Who Do Not Experience Concussion Symptoms Might Actually Have a Brain Injury
Multiple studies have found that repetitive head injury and blast exposures that do not produce immediate mTBI symptoms can result in white matter changes observable with diffuse tensor imaging (Asken et al., 2018) or in elevations of brain proteins in peripheral blood (Lucke-Wold et al., 2014). It is unclear if a single head injury not resulting in immediate mTBI symptoms demonstrates similar evidence of brain injury.
Thus, when considering the diagnosis of TBI in the clinical setting, it is important to understand the role that patient and family self-report have in providing evidence of an injury. While prospective evaluation is often able to document an initial injury, prior injuries are typically undocumented or elicited via informal methods (Corrigan and Bogner, 2007a). TBI is often confused with a variety of other conditions, including aging, depression, and emotional problems such as anxiety or posttraumatic stress disorder (Dams-O’Connor et al., 2014; Spencer et al., 2010). Even when medical records are available, a large percentage of prior injuries often do not receive recognition or medical attention (Setnik and Bazarian, 2007). Therefore, patient self-report of previous head trauma is often used in both clinical practice and research as a screening method to identify TBI. However, the most common practice of asking one or two questions to identify a history of previous TBI has been found to be inadequate in that all but the most severe or recent injuries are missed (Corrigan and Bogner, 2007b).
The problem of recall is most apparent in adults attempting to report TBI that occurred in childhood. Even when carefully cued, adults with TBI events occurring before age 4 were unable to report prior injuries 25 percent of the time even when hospitalization was involved (McKinlay et al., 2016). In general, most previously unreported childhood TBI is not recollected, although having been older at the time of injury and having experienced a more severe injury were found to increase the likelihood of remembering prior injury (McKinlay and Horwood, 2017). Failure to recognize the etiology of symptoms precludes appropriate treatment or symptom management (Yi and Dams-O’Connor, 2013).
While the literature provides evidence that self-report might be helpful for the initial screening of TBI and its symptoms, the evaluation is greatly enhanced by structured interviews using validated instruments. Furthermore, self-report does not replace the need for a clinical evaluation for TBI and comprehensive neuropsychiatric testing (Corrigan and Bogner, 2007a).
Health Care Professionals Trained to Diagnose Traumatic Brain Injury
Given the complexities in diagnosing TBI, especially mTBI, and the time that might have elapsed since the original injury, a diagnostician needs to be trained on and familiar with the standard diagnostic tools (discussed earlier in the chapter) used in making a determination of brain injury and its severity.
Currently the VA requires one of four medical specialists to diagnose TBI: a neurologist, neurosurgeon, physiatrist, or psychiatrist. The physician making the diagnosis should be familiar with the signs and symptoms of TBI, abnormal structural imaging, and abnormal physical findings on exams (such as neurologic exams). Additionally, the diagnostician should be aware of common psychological comorbidities that often present with TBI and should be prepared to refer the patient for additional evaluation. For example, in cases where the neurologic exam is normal in an individual with mTBI, a physician specializing in concussions might need to work with a psychologist as part of a team approach to ensure a comprehensive evaluation and diagnosis. Additional specialties might need to be available, as part of a team, and the physician should not hesitate to call on those team members who might assist in making the diagnosis. Thus, making a diagnosis of brain injury might include
- A detailed neurologic exam (including a headache specialist, a vision specialist, and a balance specialist to assess vestibular dysfunction),
- Brain imaging (likely MRI to look for signs of cortical or subcortical injury),
- Cognitive evaluation by a psychologist with formal training in the assessment of TBI-related cognitive and executive functioning deficits, and
- Evaluations by physical, occupational, and speech therapists to clarify the extent of the TBI and the deficits that might present, including exertional symptoms.
There are many medical/clinical specialties and subspecialties involved in making the diagnosis of a brain injury, particularly if the diagnosis occurs months to years following the injury. Clinical psychology and clinical neuropsychology, for example, are disciplines where specialized training in assessment of TBI consequences is common and documentable (Podell et al., 2010; Prince and Bruhns, 2017). Even if the sole determination is not made by a professional of one of these specialties, it is difficult to see how adequate information about cognitive consequences of TBI could be collected without a formal assessment.
Given today’s increased awareness of TBI, more medical specialties now include training in TBI within their curriculum and have continued updates concerning the current state of the science. Additionally, there are at least 18 Accreditation Council for Graduate Medical Education (ACGME) accredited brain injury fellowships (e.g., Rutgers New Jersey Medical School, New York University School of Medicine, University of Washington) that train physicians of many specialties to assist in the diagnosis, treatment, and rehabilitation of individuals diagnosed with brain injury. Thus, the VA should allow health care professionals, including non-physicians, with additional training and experience in brain injury, to make TBI diagnoses. The committee believes that it is the training and experience, not necessarily the medical specialty that renders a health care specialist capable of an accurate diagnosis.
The committee recommends that the Department of Veterans Affairs allow health care professionals who have specific traumatic brain injury (TBI) training and experience, in addition to the current required specialists, to make a TBI diagnosis. Furthermore, the committee recommends pertinent and ongoing clinical training that is up to date with the state of current knowledge regarding TBI.
The committee notes, however, that specific and ongoing clinical training does not automatically guarantee knowledge and skill acquisition. Thus, the VA should consider
implementing a mechanism to prove the success of educational initiatives through demonstration of competency in assessing and diagnosing TBI.
CO-OCCURRING TBI, PTSD, DEPRESSION, PAIN, AND SLEEP DISTURBANCE
In addition to the complexity of diagnosing TBI as described above, common co-occurring conditions including PTSD, depression, pain, and sleep disturbance may also complicate the diagnosis. TBI has been associated with behavioral health problems such as persistent pain, depression, sleep, anxiety, aggression, and impulse control and overlaps with the symptoms of PTSD (Collins et al., 2012; Stein et al., 1997). PTSD and other mental disorders are often diagnosed concurrent with or following a brain injury. Thus, a TBI evaluation is often incomplete without a skilled assessment for PTSD and other common comorbidities. PTSD and TBI share some key neuropsychologic and functional neuroanatomic characteristics, and both are associated with cognitive impairment and sleep disruption (Tanev et al., 2014). Dissociative symptoms are often observed in PTSD, and there is evidence that TBI can result in dissociative-like symptoms, such as emotional numbing, derealization, reduced awareness of surroundings, depersonalization, and amnesia (Bryant et al., 2011). Further complicating the issue of comorbidity is that TBI, PTSD, and depression are also associated with chronic pain, which similarly overlaps with those conditions (Bryant et al., 2011).
Farmer and colleagues (2017) used Military Health System electronic health record data to characterize common symptoms associated with an mTBI diagnosis in the Military Health System. The symptoms included headache, sleep dysfunction, dizziness, and balance disorders. Additionally, the report reinforced earlier findings that individuals with an mTBI diagnosis are also frequently diagnosed with behavioral health conditions such as depression and PTSD. Farmer et al. (2017) reported that behavioral health diagnoses were twice as common in those with a history of a TBI diagnosis, regardless of severity, as those without. The most common behavioral health diagnoses were adjustment disorder (16 percent) and anxiety disorder (14 percent), followed by a diagnosis of depression.
Given the significant overlapping symptoms between TBI and PTSD, differential diagnosis is difficult. Patients with TBI often meet the diagnostic criteria for PTSD on screening instruments for TBI, and vice versa. Many veterans of the wars in Iraq and Afghanistan who have experienced mTBI also have PTSD related to their combat experience (e.g., Silver et al., 2001). Numerous studies have examined the link between TBI and PTSD, and they have consistently found that veterans with positive TBI screens are more likely to have PTSD than veterans with negative TBI screens (Carlson et al., 2010; Hoge et al., 2008, 2014; Zatzick et al., 2010). Hoge et al. (2014) found that even after accounting for predeployment symptoms, prior TBI, and combat intensity, TBI was the strongest predictor of postdeployment PTSD symptoms. A study by Hoge et al. (2008) found that soldiers with mTBI, primarily those who had a loss of consciousness, were significantly more likely to report poor general health, missed workdays, medical visits, and a high number of somatic and postconcussive symptoms than were soldiers with other injuries. However, after the researchers adjusted for PTSD and depression, they found that mTBI was no longer significantly associated with those physical health outcomes or symptoms, except for headache. Thus, mTBI is strongly associated with PTSD and physical problems in soldiers returning from Iraq. Furthermore, the study found that PTSD and depression are important mediators of the relationship between mTBI and physical health problems (Hoge et al., 2008).
A recent study of veterans without TBI who had returned from Iraq and Afghanistan reported prevalence rates of 23 percent for PTSD, 17 percent to 21 percent for depression, and 7 to 15 percent for alcohol-related problems. The rates were much higher among veterans with TBI, with 89 percent having a comorbid psychiatric diagnosis, including 44 to 54 percent who had a diagnosis of PTSD and 70 percent who had pain diagnoses (Armistead-Jehle et al., 2017).
The relationships among TBI, post-concussive symptoms, anxiety, depression, PTSD, and chronic pain are complex. TBI may result in co-occurring mental and physical symptoms, mental health symptoms may exacerbate pain and other post-concussive symptoms, and symptoms may occur coincident to one another. Pain, the use of medications, alcohol or drug use or intoxication, or PTSD, which can be present either in isolation or in addition to a brain injury, can confound or complicate the diagnosis (Hoge et al., 2008, 2014; Roozenbeek et al., 2013).
SUMMARY AND RECOMMENDATION
Damage to the brain after trauma is referred to as traumatic brain injury. TBI may be blunt, non-penetrating, penetrating, or due to blast. The resulting neuropathology consists of a primary injury that is a direct consequence of the traumatic insult and a secondary injury that results from a cascade of molecular and cellular events triggered by the primary injury and that leads to cell death, axonal injury, and inflammation. According to CDC, mTBI (often referred to as a concussion) manifests initially as a brief change in mental status or unconsciousness, whereas severe TBI results in an extended period of unconsciousness or amnesia.
TBI severity is typically defined at the time of initial injury; the GCS has been the gold standard of neurologic assessment of trauma patients since its development by Teasdale and Jennett in 1974. Other TBI severity-classification systems grade single indicators, such as loss of consciousness and duration of posttraumatic amnesia. The predictive value of those measures has been demonstrated, but each may be influenced by factors unrelated to, or only indirectly related to, the severity of the TBI (e.g., intoxication). Ultimately, the severity of the injury defined initially does not necessarily predict the trajectory or natural history of TBI, as individuals diagnosed with mTBI can experience ongoing impairment.
In the absence of clear biomarkers, self-report based on a validated screening method is currently considered the gold standard for obtaining a comprehensive lifetime history of exposure to TBI. Reliance on medical records is often insufficient because many injuries are not treated, including, occasionally, even more severe injuries. Screening instruments vary in the extent to which their psychometrics have been established, with single-item screens tending to be the least reliable and least likely to capture all TBIs. Because many mTBIs are not evaluated at the time of injury, injuries incurred during deployment are typically screened retrospectively with the Brief Traumatic Brain Injury Screen (DVBIC, 2007; Schwab et al., 2007), a four-item measure that is typically completed upon return from deployment as part of a comprehensive health screening. A positive screen is followed by a more comprehensive evaluation, the VA Comprehensive TBI Evaluation (VA, 2010).
The current method of TBI diagnosis after initial injury relies on the report of certain symptoms at the time of injury from the person who was injured or from a witness. However, not all individuals who have sustained a TBI are identified at the time of the initial injury as, in the case of complex polytrauma, for example, other injuries might appear to be more severe and the head injury is not assessed, or, in the case of mTBI, the individual might not present for medical care. Furthermore, there are no current tests to help make, and perhaps document, the diagnosis
more than 24 hours after injury, although new tests have been approved by FDA for use early after injury.
Thus, when considering the diagnosis of TBI in the clinical setting, it is important to understand the role that patient and family self-report have in providing evidence of injury. While prospective evaluation is often able to document an initial injury, prior injuries are typically undocumented or elicited via informal methods. Furthermore, TBI is often confused with a variety of other conditions including aging, depression, and emotional problems such as PTSD. Even when medical records are available, a large percentage of prior injuries often do not receive recognition or medical attention. Therefore, patient self-report of previous head trauma is often used in both clinical practice and research as a screening method to identify TBI.
TBI has been associated with such behavioral outcomes as depression, anxiety, aggression, and impulse control and overlaps with the symptoms of PTSD. Thus, a TBI evaluation might be incomplete unless the diagnostician is familiar with the symptoms of PTSD and other common comorbidities. PTSD and other psychiatric conditions are often diagnosed concurrent with or following a brain injury. PTSD and TBI share some pathophysiological characteristics and both are associated with cognitive impairment and sleep disruption. It is important to recognize that mental health symptoms might have causes other than TBI. These causes include pain, the use of medications, alcohol or drugs use or intoxication, or PTSD, all of which can be present either in isolation or in addition to a brain injury and, as noted, confound or complicate the diagnosis.
Given the complexities in diagnosing TBI and the time that might have elapsed since the original injury, a diagnostician needs to have experience with TBI and be trained and familiar with the state of the science in order to accurately make a determination of brain injury and its severity. In addition, there is ongoing research and new theoretical views on the trajectory of recovery after TBI, so new developments are likely forthcoming that would help providers who have training and experience with TBI to accurately diagnose TBI. Currently the VA requires one of four medical specialists to diagnose TBI: a neurologist, neurosurgeon, physiatrist, or psychiatrist. There are many specialties and subspecialties involved in making the diagnosis of a brain injury, particularly if the diagnosis occurs months to years following the injury. Universities and medical schools offer special training in brain injury to train physicians and other health care professionals with an interest in the field to assist in the diagnosis, treatment, and rehabilitation of individuals diagnosed with brain injury. Thus, the VA should consider allowing other health care professionals with experience and pertinent ongoing training in brain injury to make TBI diagnoses. The committee believes that it is the training and experience and not necessarily the specialty that renders a health care professional capable of an accurate diagnosis.
The committee recommends that the Department of Veterans Affairs allow health care professionals who have specific traumatic brain injury (TBI) training and experience, in addition to the current required specialists, to make a TBI diagnosis. Furthermore, the committee recommends pertinent and ongoing clinical training that is up to date with the state of current knowledge regarding TBI.
REFERENCES
Albrecht, J. S., L. Barbour, S. A. Abariga, V. Rao, and E. M. Perfetto. 2018. Risk of depression after traumatic brain injury in a large national sample. Journal of Neurotrauma, August 10 [Epub ahead of print].
Amara, J., K. M. Iverson, M. Krengel, T. K. Pogoda, and A. Hendricks. 2014. Anticipating the traumatic brain injury-related health care needs of women veterans after the Department of Defense change in combat assignment policy. Women’s Health Issues 24(2):e171–e176.
APA (American Psychiatric Association). 2013. Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). Arlington, VA: American Psychiatric Association.
Arango-Lasprilla, J. C., and J. S. Kreutzer. 2010. Racial and ethnic disparities in functional, psychosocial, and neurobehavioral outcomes after brain injury. Journal of Head Trauma Rehabilitation 25(2):128–136.
Armistead-Jehle, P., J. R. Soble, D. B. Cooper, and H. G. Belanger. 2017. Unique aspects of traumatic brain injury in military and veteran populations. Physical Medicine and Rehabilitation Clinics of North America 28(2):323–337.
Asemota, A. O., B. P. George, C. J. Cumpsty-Fowler, A. H. Haider, and E. B. Schneider. 2013. Race and insurance disparities in discharge to rehabilitation for patients with traumatic brain injury. Journal of Neurotrauma 30(24):2057–2065.
Asken, B. M., M. J. Sullan, S. T. DeKosky, M. S. Jaffee, and R. M. Bauer. 2017. Research gaps and controversies in chronic traumatic encephalopathy: A review. JAMA Neurology 74(10):1255–1262.
Asken, B. M., S. T. DeKosky, J. R. Clugston, M. S. Jaffee, and R. M. Bauer. 2018. Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): A systematic critical review. Brain Imaging Behavior 12(2):585–612.
Bakhsheshian, J., L. Ding, A. Tang, T. Wen, A. Patel, B. A. Strickland, R. C. Rennert, A. Amar, P. Gruen, S. Giannotta, W. J. Mack, and F. J. Attenello. 2018. Safety-net hospitals have higher complication and mortality rates in the neurosurgical management of traumatic brain injuries. World Neurosurgery 119:e284–e293.
Bazarian, J. J., B. Blyth, S. Mookerjee, H. He, and M. P. McDermott. 2010. Sex differences in outcome after mild traumatic brain injury. Journal of Neurotrauma 27(3):527–539.
Belanger, H. G., R. D. Vanderploeg, and N. Sayer. 2016. Screening for remote history of mild traumatic brain injury in VHA: A critical literature review. Journal of Head Trauma Rehabilitation 31(3):204–214.
Berry, C., E. J. Ley, J. Mirocha, and A. Salim. 2010. Race affects mortality after moderate to severe traumatic brain injury. Journal of Surgical Research 163(2):303–308.
Black, K. L., R. A. Hanks, D. L. Wood, R. D. Zafonte, N. Cullen, D. X. Cifu, J. Englander, and G. E. Francisco. 2002. Blunt versus penetrating violent traumatic brain injury: Frequency and factors associated with secondary conditions and complications. Journal of Head Trauma Rehabilitation 17(6):489–496.
Bowman, S. M., D. P. Martin, S. R. Sharar, and F. J. Zimmerman. 2007. Racial disparities in outcomes of persons with moderate to severe traumatic brain injury. Medical Care 45(7):686–690.
Bryant, R. A., M. L. O’Donnell, M. Creamer, A. C. McFarlane, and D. Silove. 2011. Posttraumatic intrusive symptoms across psychiatric disorders. Journal of Psychiatric Research 45(6):842–847.
Budnick, H. C., A. H. Tyroch, and S. A. Milan. 2017. Ethnic disparities in traumatic brain injury care referral in a Hispanic-majority population. Journal of Surgical Research 215:231–238.
Carlson, K. F., D. Nelson, R. J. Orazem, S. Nugent, D. X. Cifu, and N. A. Sayer. 2010. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. Journal of Traumatic Stress 23(1):17–24.
CDC (Centers for Disease Control and Prevention). 2018. ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification. Herndon, VA: Stylus Publishing, LLC.
Chapman, J. C., and R. Diaz-Arrastia. 2014. Military traumatic brain injury: A review. Alzheimer’s and Dementia 10(3 Suppl):S97–S104.
Coldren, R. L., M. P. Kelly, R. V. Parish, M. Dretsch, and M. L. Russell. 2010. Evaluation of the military acute concussion evaluation for use in combat operations more than 12 hours after injury. Military Medicine 175(7):477–481.
Collins, A., M. Gutièrrez-Mecinas, A. F. Trollope, and J. M. H. M. Reul. 2012. Epigenetics of stress. In Epigenetics of lifestyle. Sharjah U.A.E.: Bentham Science Publishers. Pp. 70-89.
Collins, C. L., E. N. Fletcher, S. K. Fields, L. Kluchurosky, M. K. Rohrkemper, R. D. Comstock, and R. C. Cantu. 2014. Neck strength: A protective factor reducing risk for concussion in high school sports. Journal of Primary Prevention 35(5):309–319.
Collins, M. W., A. P. Kontos, D. O. Okonkwo, J. Almquist, J. Bailes, M. Barisa, J. Bazarian, O. J. Bloom, D. L. Brody, R. Cantu, J. Cardenas, J. Clugston, R. Cohen, R. Echemendia, R. J. Elbin, R. Ellenbogen, J. Fonseca, G. Gioia, K. Guskiewicz, R. Heyer, G. Hotz, G. L. Iverson, B. Jordan, G. Manley, J. Maroon, T. McAllister, M. McCrea, A. Mucha, E. Pieroth, K. Podell, M. Pombo, T. Shetty, A. Sills, G. Solomon, D. G. Thomas, T. C. Valovich McLeod, T. Yates, and R. Zafonte. 2016. Statements of agreement from the Targeted Evaluation and Active Management (TEAM) Approaches to Treating Concussion meeting held in Pittsburgh, October 15–16, 2015. Neurosurgery 79(6):912–929.
Coronado, V. G., L. Xu, S. V. Basavaraju, L. C. McGuire, M. M. Wald, M. D. Faul, B. R. Guzman, and J. D. Hemphill. 2011. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. Morbidity and Mortality Weekly Report Surveillance Summary 60(5):1–32.
Corrigan, J. D., and J. Bogner. 2007a. Screening and identification of TBI. Journal of Head Trauma Rehabilitation 22(6):315–317.
Corrigan, J. D., and J. Bogner. 2007b. Initial reliability and validity of the Ohio State University TBI identification method. Journal of Head Trauma Rehabilitation 22(6):318–329.
Dams-O’Connor, K., J. B. Cantor, M. Brown, M. P. Dijkers, L. A. Spielman, and W. A. Gordon. 2014. Screening for traumatic brain injury: Findings and public health implications. Journal of Head Trauma Rehabilitation 29(6):479–489.
De Maio, V. J., D. O. Joseph, H. Tibbo-Valeriote, J. G. Cabanas, B. Lanier, C. H. Mann, and J. Register-Mihalik. 2014. Variability in discharge instructions and activity restrictions for patients in a children’s ED postconcussion. Pediatric Emergency Care 30(1):20–25.
DeKosky, S. T., P. M. Kochanek, R. S. Clark, J. R. Ciallella, and C. E. Dixon. 1998. Secondary injury after head trauma: Subacute and long-term mechanisms. Seminars in Clinical Neuropsychiatry 3(3):176–185.
Delaney, J. S., F. Abuzeyad, J. A. Correa, and R. Foxford. 2005. Recognition and characteristics of concussions in the emergency department population. Journal of Emergency Medicine 29(2):189–197.
Devoto, C., L. Arcurio, J. Fetta, M. Ley, T. Rodney, R. Kanefsky, and J. Gill. 2017. Inflammation relates to chronic behavioral and neurological symptoms in military personnel with traumatic brain injuries. Cell Transplantation 26(7):1169–1177.
Diamond, P. M., A. J. Harzke, P. R. Magaletta, A. G. Cummins, and R. Frankowski. 2007. Screening for traumatic brain injury in an offender sample: A first look at the reliability and validity of the traumatic brain injury questionnaire. Journal of Head Trauma Rehabilitation 22(6):330–338.
Dikmen, S., J. Machamer, N. Temkin, and A. McLean. 1990. Neuropsychological recovery in patients with moderate to severe head injury: 2 year follow-up. Journal of Clinical and Experimental Neuropsychology 12(4):507–519.
Dismuke, C. E., M. Gebregziabher, D. Yeager, and L. E. Egede. 2015. Racial/ethnic differences in combat- and non-combat-associated traumatic brain injury severity in the Veterans Health Administration: 2004–2010. American Journal of Public Health 105(8):1696–1702.
Dollé, J. P., A. Jaye, S. A. Anderson, H. Ahmadzadeh, V. B. Shenoy, and D. H. Smith. 2018. Newfound sex differences in axonal structure underlie differential outcomes from in vitro traumatic axonal injury. Experimental Neurology 300:121–134.
DVBIC (Defense and Veterans Brain Injury Center). 2006. Evaluation of traumatic brain injury: Brain potentials in diagnosis, function, and prognosis. https://dvbic.dcoe.mil/research/evaluation-traumatic-brain-injury-brain-potentials-diagnosis-function-and-prognosis (accessed August 24, 2018).
DVBIC. 2007. Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: Initial investigation of the usefulness of a short screening tool for traumatic brain injury. https://dvbic.dcoe.mil/research/screening-traumatic-brain-injury-troops-returning-deploymentafghanistan-and-iraq-initial (accessed August 24, 2018).
DVBIC. 2018. The Military Acute Concussion Evaluation 2 Pocket Card (MACE 2). https://dvbic.dcoe.mil/files/resources/dvbic_4901_mace2-pocket-card_v2.0_2018-10-23.pdf (accessed January 8, 2019).
Easter, J. S., J. S. Haukoos, W. P. Meehan, V. Novack, and J. A. Edlow. 2015. Will neuroimaging reveal a severe intracranial injury in this adult with minor head trauma? The rational clinical examination systematic review. JAMA 314(24):2672–2681.
Edlow, B. L., W. A. Copen, S. Izzy, K. Bakhadirov, A. van der Kouwe, M. B. Glenn, S. M. Greenberg, D. M. Greer, and O. Wu. 2016. Diffusion tensor imaging in acute-to-subacute traumatic brain injury: A longitudinal analysis. BMC Neurology 16(1).
Eisenberg, M. A., J. Andrea, W. Meehan, and R. Mannix. 2013. Time interval between concussions and symptom duration. Pediatrics 132(1):8–17.
Eliacin, J., S. Fortney, N. A. Rattray, and J. Kean. 2018. Access to health services for moderate to severe TBI in Indiana: Patient and caregiver perspectives. Brain Injury 32(12):1510–1517.
Farmer, C. M., H. Krull, T. W. Concannon, M. Simmons, F. Pillemer, T. Ruder, A. Parker, M. P. Purohit, L. Hiatt, B. S. Batorsky, and K. A. Hepner. 2017. Understanding treatment of mild traumatic brain injury in the military health system. RAND Health Quarterly 6(2):11.
Faul, M., and V. Coronado. 2015. Chapter 1: Epidemiology of traumatic brain injury. In J. Grafman and A. M. Salazar (eds.), Handbook of Clinical Neurology, Vol. 127. New York: Elsevier. Pp. 3–13.
Fehily, B., and M. Fitzgerald. 2017. Repeated mild traumatic brain injury: Potential mechanisms of damage. Cell Transplantation 26(7):1131–1155.
Freeman, L. C., and J. P.-Y. Ting. 2016. The pathogenic role of the inflammasome in neurodegenerative diseases. Journal of Neurochemistry 136(S1):29–38.
French, L. M. 2010. Military traumatic brain injury: An examination of important differences. Annals of the New York Academy of Sciences 1208:38–45.
French, L., M. McCrae, and M. Baggett. 2008. The Military Acute Concussion Evaluation (MACE). Journal of Special Operations Medicine 8:68–74.
GAO (Government Accountability Office). 2008. VA health care: Injury screening and evaluation implemented for OEF/OIF veterans, but challenges remain. https://www.gao.gov/new.items/d08276.pdf (accessed January 11, 2019).
Gardner, R. C., J. F. Burke, J. Nettiksimmons, A. Kaup, D. E. Barnes, and K. Yaffe. 2014. Dementia risk after traumatic brain injury vs. nonbrain trauma: The role of age and severity. JAMA Neurology 71(12):1490–1497.
Gennarelli, T. A., and D. I. Graham. 2005. Neuropathology. In Textbook of traumatic brain injury., edited by J. M. Silver, T. W. McAllister and Y. S. C. Washington DC: American Psychiatric Publishing, Inc. Pp. 27 - 50.
Gary, K. W., J. C. Arango-Lasprilla, J. M. Ketchum, J. S. Kreutzer, A. Copolillo, T. A. Novack, and A. Jha. 2009. Racial differences in employment outcome after traumatic brain injury at 1, 2, and 5 years postinjury. Archives of Physical Medicine and Rehabilitation 90(10):1699–1707.
Ghajar, J. 2000. Traumatic brain injury. Lancet 356(9233):923–929.
Giza, C. C., and D. A. Hovda. 2014. The new neurometabolic cascade of concussion. Neurosurgery 75(Suppl 4):S24–S33.
Giza, C. C., and J. S. Kutcher. 2014. An introduction to sports concussions. Continuum 20:1545–1551.
Graham, D. I., T. K. McIntosh, W. L. Maxwell, and J. A. Nicoll. 2000. Recent advances in neurotrauma. Journal of Neuropathology & Experimental Neurology 59(8):641–651.
Graham, D. I., T. A. Gennarelli, and T. K. McIntosh. 2002. Trauma. In Greenfield’s Neuropathology, edited by D. I. Graham and P.L. Lantos. London: Arnold. Pp. 823–898.
Graham, J. E., D. M. Radice-Neumann, T. A. Reistetter, F. M. Hammond, M. Dijkers, and C. V. Granger. 2010. Influence of sex and age on inpatient rehabilitation outcomes among older adults with traumatic brain injury. Archives of Physical Medicine and Rehabilitation 91(1):43–50.
Gross, T., and F. Amsler. 2018. One-year outcome following brain injury: A comparison of younger versus elderly major trauma patients. Archives of Orthopaedic and Trauma Surgery 138(10):1375–1387.
Haider, A. H., D. C. Chang, D. T. Efron, E. R. Haut, M. Crandall, and E. E. Cornwell III. 2008. Race and insurance status as risk factors for trauma mortality. Archives of Surgery 143(10):945–949.
Herrera-Melero, M. C., J. J. Egea-Guerrero, A. Vilches-Arenas, M. D. Rincon-Ferrari, J. M. Flores-Cordero, J. Leon-Carrion, and F. Murillo-Cabezas. 2015. Acute predictors for mortality after severe TBI in Spain: Gender differences and clinical data. Brain Injury 29(12):1439–1444.
Hoge, C. W., J. L. Auchterlonie, and C. S. Milliken. 2006. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 295(9):1023–1032.
Hoge, C. W., D. McGurk, J. L. Thomas, A. L. Cox, C. C. Engel, and C. A. Castro. 2008. Mild traumatic brain injury in U.S. soldiers returning from Iraq. New England Journal of Medicine 358(5):453–463.
Hoge, C. W., C. A. Castro, K. A. Yurgil, D. A. Barkauskas, J. J. Vasterling, C. M. Nievergelt, G. E. Larson, N. J. Schork, B. T. Litz, W. P. Nash, and D. G. Baker. 2014. Treatment of generalized war-related health concerns: Placing TBI and PTSD in context. JAMA 312(16):1685–1686.
IOM (Institute of Medicine). 2001. Exploring the biological contributions to human health: Does sex matter? Washington, DC: National Academy Press.
Iverson, G. L., A. J. Gardner, D. P. Terry, J. L. Ponsford, A. K. Sills, D. K. Broshek, and G. S. Solomon. 2017. Predictors of clinical recovery from concussion: A systematic review. British Journal of Sports Medicine 51(12):941–948.
Jagoda, A. S., J. J. Bazarian, J. J. Bruns, Jr., S. V. Cantrill, A. D. Gean, P. K. Howard, J. Ghajar, S. Riggio, D. W. Wright, R. L. Wears, A. Bakshy, P. Burgess, M. M. Wald, and R. R. Whitson. 2008. Clinical policy: Neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Annals of Emergency Medicine 52(6):714–748.
Johnson, V. E., W. Stewart, J. D. Arena, and D. H. Smith. 2017. Traumatic brain injury as a trigger of neurodegeneration. Advances in Neurobiology 15:383–400.
Kiraly, M., and S. J. Kiraly. 2007. Traumatic brain injury and delayed sequelae: A review—traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. Scientific World Journal 7:1768–1776.
Kisser, J., S. R. Waldstein, M. K. Evans, and A. B. Zonderman. 2017. Lifetime prevalence of traumatic brain injury in a demographically diverse community sample. Brain Injury 31(5):620–623.
Kontos, A. P., R. S. Kotwal, R. J. Elbin, R. H. Lutz, R. D. Forsten, P. J. Benson, and K. M. Guskiewicz. 2013. Residual effects of combat-related mild traumatic brain injury. Journal of Neurotrauma 30(8):680–686.
Kurowski, B. G., A. Treble-Barna, A. J. Pitzer, S. L. Wade, L. J. Martin, R. S. Chima, and A. Jegga. 2017. Applying systems biology methodology to identify genetic factors possibly associated with recovery after traumatic brain injury. Journal of Neurotrauma 34(14):2280–2290.
Laskowitz, D., and G. Grant. 2016. Frontiers in neuroscience. In Translational research in traumatic brain injury, edited by D. Laskowitz and G. Grant. Boca Raton, FL: CRC Press/Taylor and Francis Group.
Lee, H., M. Wintermark, A. D. Gean, J. Ghajar, G. T. Manley, and P. Mukherjee. 2008. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. Journal of Neurotrauma 25(9):1049–1056.
Levin, H. S. 1990. Memory deficit after closed head injury. Journal of Clinical and Experimental Neuropsychology 12(1):129–153.
Levin, H. S. 1995. Prediction of recovery from traumatic brain injury. Journal of Neurotrauma 12(5):913-922.
Levin, H. S., H. E. Gary, Jr., H. M. Eisenberg, R. M. Ruff, J. T. Barth, J. Kreutzer, W. M. High, Jr., S. Portman, M. A. Foulkes, J. A. Jane, A. Marmarou, and L. F. Marshall. 1990. Neurobehavioral outcome 1 year after severe head injury. Experience of the traumatic coma data bank. Journal of Neurosurgery 73(5):699–709.
Lew, H. L., J. H. Poole, R. D. Vanderploeg, G. L. Goodrich, S. Dekelboum, S. B. Guillory, B. Sigford, and D. X. Cifu. 2007. Program development and defining characteristics of returning military in a VA polytrauma network site. Journal of Rehabilitation Research and Development 44(7):1027–1034.
Lucke-Wold, B. P., R. C. Turner, A. F. Logsdon, J. E. Bailes, J. D. Huber, and C. L. Rosen. 2014. Linking traumatic brain injury to chronic traumatic encephalopathy: Identification of potential mechanisms leading to neurofibrillary tangle development. Journal of Neurotrauma 31(13):1129–1138.
Maas, A. 2016. Traumatic brain injury: Changing concepts and approaches. Chinese Journal of Traumatology—English Edition 19(1):3–6.
Maiti, T. K., S. Konar, S. Bir, P. Kalakoti, P. Bollam, and A. Nanda. 2015. Role of apolipoprotein E polymorphism as a prognostic marker in traumatic brain injury and neurodegenerative disease: A critical review. Neurosurgery Focus 39(5).
Manners, J. L., R. D. Forsten, R. S. Kotwal, R. J. Elbin, M. W. Collins, and A. P. Kontos. 2016. Role of pre-morbid factors and exposure to blast mild traumatic brain injury on post-traumatic stress in United States military personnel. Journal of Neurotrauma 33(19):1796–1801.
McCrea, M., J. P. Kelly, J. Kluge, B. Ackley, and C. Randolph. 1997. Standardized assessment of concussion in football players. Neurology 48(3):586–588.
McCrea, M., K. Guskiewicz, S. Doncevic, K. Helmick, J. Kennedy, C. Boyd, S. Asmussen, K. W. Ahn, Y. Wang, J. Hoelzle, and M. Jaffee. 2014. Day of injury cognitive performance on the Military Acute Concussion Evaluation (MACE) by U.S. military service members in OEF/OIF. Military Medicine 179(9):990–997.
McInnes, K., C. L. Friesen, D. E. MacKenzie, D. A. Westwood, and S. G. Boe. 2017. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: A scoping review. PLOS ONE 12(4):e0174847.
McKee, A. C., and D. H. Daneshvar. 2015. The neuropathology of traumatic brain injury. In Handbook of clinical neurology. Vol. 127, edited by J. Grafman and A. M. Salazar: Elsevier. Pp. 45–66.
McKinlay, A., and L. J. Horwood. 2017. The accuracy of adult recall for early mild traumatic brain injury. Disability and Rehabilitation 39(13):1296–1299.
McKinlay, A., L. J. Horwood, and D. M. Fergusson. 2016. Accuracy of self-report as a method of screening for lifetime occurrence of traumatic brain injury events that resulted in hospitalization. Journal of the International Neuropsychological Society 22(7):717–723.
McQuistion, K., T. Zens, H. S. Jung, M. Beems, G. Leverson, A. Liepert, J. Scarborough, and S. Agarwal. 2016. Insurance status and race affect treatment and outcome of traumatic brain injury. Journal of Surgical Research 205(2):261–271.
Meagher, A. D., C. A. Beadles, J. Doorey, and A. G. Charles. 2015. Racial and ethnic disparities in discharge to rehabilitation following traumatic brain injury. Journal of Neurosurgery 122(3):595–601.
Meehan, W. P., R. C. Mannix, M. J. Oêbrien, and M. W. Collins. 2013. The prevalence of undiagnosed concussions in athletes. Clinical Journal of Sport Medicine 23(5):339–342.
Morales, D. M., N. Marklund, D. Lebold, H. J. Thompson, A. Pitkanen, W. L. Maxwell, L. Longhi, H. Laurer, M. Maegele, E. Neugebauer, D. I. Graham, N. Stocchetti, and T. K. McIntosh. 2005. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience 136(4):971–989.
Mutch, C. A., J. F. Talbott, and A. Gean. 2016. Imaging evaluation of acute traumatic brain injury. Neurosurgery Clinics of North America 27(4):409–439.
Oppelt, K., D. Hahnlein, J. Boschert, M. Kuffer, P. A. Grutzner, M. Munzberg, and M. Kreinest. 2018. Influence of demographic factors and clinical status parameters on long-term neurological, psychological and vegetative outcome following traumatic brain injury. Brain Injury 32(12):1500–1509.
Padgett, C. R., M. J. Summers, and C. E. Skilbeck. 2016. Is ApoE ε4 associated with poorer cognitive outcome following traumatic brain injury? A meta-analysis. Neuropsychology 30(7):775–790.
Pan, J., I. D. Connolly, S. Dangelmajer, J. Kintzing, A. L. Ho, and G. Grant. 2016. Sports-related brain injuries: Connecting pathology to diagnosis. Neurosurgical Focus 40(4):E14.
Panenka, W. J., A. J. Gardner, M. N. Dretsch, G. C. Crynen, F. C. Crawford, and G. L. Iverson. 2017. Systematic review of genetic risk factors for sustaining a mild traumatic brain injury. Journal of Neurotrauma 34(13):2093–2099.
Papa, L., D. Edwards, and M. Ramia. 2015. Frontiers in neuroengineering. In Brain neurotrauma: Molecular, neuropsychological, and rehabilitation aspects, edited by F. H. Kobeissy. Boca Raton, FL: CRC Press/Taylor & Francis © 2015 by Taylor & Francis Group, LLC.
Perrin, P. B., D. Krch, M. Sutter, D. J. Snipes, J. C. Arango-Lasprilla, S. A. Kolakowsky-Hayner, J. Wright, and A. Lequerica. 2014. Racial/ethnic disparities in mental health over the first 2 years after traumatic brain injury: A model systems study. Archives of Physical Medicine and Rehabilitation 95(12):2288–2295.
Peeters, W., R. van den Brande, S. Polinder, A. Brazinova, E. W. Steyerberg, H. F. Lingsma, and A. I. R. Maas. 2015. Epidemiology of traumatic brain injury in Europe. Acta Neurochirurgica (Wien) 157(10):1683–1696.
Podell, K., K. Gifford, D. Bougakov, and E. Goldberg. 2010. Neuropsychological assessment in traumatic brain injury. The Psychiatric Clinics of North America 33(4):855–876.
Powell, J. M., J. V. Ferraro, S. S. Dikmen, N. R. Temkin, and K. R. Bell. 2008. Accuracy of mild traumatic brain injury diagnosis. Archives of Physical Medicine and Rehabilitation 89(8):1550–1555.
Prince, C., and M. E. Bruhns. 2017. Evaluation and treatment of mild traumatic brain injury: The role of neuropsychology. Brain Sciences 7(8).
Pugh, M. J., E. P. Finley, C. P. Wang, L. A. Copeland, C. A. Jaramillo, A. A. Swan, C. A. Elnitsky, L. K. Leykum, E. M. Mortensen, B. A. Eapen, P. H. Noel, J. A. Pugh, and The demenTia Research And Care Clinic (TRACC) Research Team. 2016. A retrospective cohort study of comorbidity trajectories associated with traumatic brain injury in veterans of the Iraq and Afghanistan wars. Brain Injury 30(12):1481–1490.
Rakholia, M. V., R. G. Kumar, B. M. Oh, P. R. Ranganathan, S. L. Berga, P. M. Kochanek, and A. K. Wagner. 2018. Systemic estrone production and injury-induced sex hormone steroidogenesis after severe traumatic brain injury: A prognostic indicator of traumatic brain injury-related mortality. Journal of Neurotrauma, August 24 [Epub ahead of print].
Ranganathan, P., R. G. Kumar, K. Davis, E. H. McCullough, S. L. Berga, and A. K. Wagner. 2016. Longitudinal sex and stress hormone profiles among reproductive age and post-menopausal women after severe TBI: A case series analysis. Brain Injury 30(4):452-461.
Rigon, A., L. Turkstra, B. Mutlu, and M. Duff. 2016. The female advantage: Sex as a possible protective factor against emotion recognition impairment following traumatic brain injury. Cognitive, Affective, & Behavioral Neuroscience 16(5):866–875.
Roozenbeek, B., A. I. Maas, and D. K. Menon. 2013. Changing patterns in the epidemiology of traumatic brain injury. Nature Reviews Neurology 9(4):231–236.
Russell, L. M., M. D. Devore, S. M. Barnes, J. E. Forster, T. A. Hostetter, A. E. Montgomery, R. Casey, V. Kane, and L. A. Brenner. 2013. Challenges associated with screening for traumatic brain injury among U.S. veterans seeking homeless services. American Journal of Public Health 103(Suppl 2):S211–S212.
Santiago, L. A., B. C. Oh, P. K. Dash, J. B. Holcomb, and C. E. Wade. 2012. A clinical comparison of penetrating and blunt traumatic brain injuries. Brain Injury 26(2):107–125.
Schiraldi, M., C. G. Patil, D. Mukherjee, B. Ugiliweneza, M. Nuño, S. P. Lad, and M. Boakye. 2015. Effect of insurance and racial disparities on outcomes in traumatic brain injury. Journal of Neurological Surgery, Part A: Central European Neurosurgery 76(3):224–232.
Schneider, A. L., T. A. Hostetter, B. Y. Homaifar, J. E. Forster, J. H. Olson-Madden, B. B. Matarazzo, J. Huggins, and L. A. Brenner. 2016. Responses to traumatic brain injury screening questions and suicide attempts among those seeking Veterans Health Administration mental health services. Frontiers in Psychiatry 7:59.
Schwab, K. A., G. Baker, B. Ivins, M. Sluss-Tiller, W. Lux, and D. Warden. 2006. The Brief Traumatic Brain Injury Screen (BTBIS): Investigating the validity of a self-report instrument for detecting traumatic brain injury (TBI) in troops returning from deployment in Afghanistan and Iraq. Neurology 66(5)(Suppl 2):A235.
Schwab, K. A., B. Ivins, G. Cramer, W. Johnson, M. Sluss-Tiller, K. Kiley, W. Lux, and D. Warden. 2007. Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: Initial investigation of the usefulness of a short screening tool for traumatic brain injury. Journal of Head Trauma Rehabilitation 22(6):377–389.
Setnik, L., and J. J. Bazarian. 2007. The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Injury 21(1):1–9.
Shafi, S., C. Marquez de la Plata, R. Diaz-Arrastia, K. Shipman, M. Carlile, H. Frankel, J. Parks, and L. M. Gentilello. 2007. Racial disparities in long-term functional outcome after traumatic brain injury. Journal of Trauma 63(6):1263–1270.
Sherer, M., S. A. Yablon, R. Nakase-Richardson, and T. G. Nick. 2008. Effect of severity of posttraumatic confusion and its constituent symptoms on outcome after traumatic brain injury. Archives of Physical Medicine and Rehabilitation 89(1):42–47.
Shin, S. S., J. W. Bales, C. Edward Dixon, and M. Hwang. 2017. Structural imaging of mild traumatic brain injury may not be enough: Overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging and Behavior 11(2):591–610.
Silver, J. M., R. Kramer, S. Greenwald, and M. Weissman. 2001. The association between head injuries and psychiatric disorders: Findings from the New Haven NIMH epidemiologic catchment area study. Brain Injury 15(11):935–945.
Smith, D. H. 2013. Diagnosing concussion. Canadian Medical Association Journal 185(18):1602.
Spencer, R. J., L. L. Drag, S. J. Walker, and L. A. Bieliauskas. 2010. Self-reported cognitive symptoms following mild traumatic brain injury are poorly associated with neuropsychological performance in OIF/OEF veterans. Journal of Rehabilitation Research and Development 47(6):521–530.
Staudenmayer, K. L., R. Diaz-Arrastia, A. de Oliveira, L. M. Gentilello, and S. Shafi. 2007. Ethnic disparities in long-term functional outcomes after traumatic brain injury. Journal of Trauma 63(6):1364–1369.
Stein, M. B., J. R. Walker, A. L. Hazen, and D. R. Forde. 1997. Full and partial posttraumatic stress disorder: Findings from a community survey. American Journal of Psychiatry 154(8):1114–1119.
Sullivan, K. A., C. B. Kempe, S. L. Edmed, and G. A. Bonanno. 2016. Resilience and other possible outcomes after mild traumatic brain injury: A systematic review. Neuropsychology Review 26(2):173–185.
Tanev, K. S., K. Z. Pentel, M. A. Kredlow, and M. E. Charney. 2014. PTSD and TBI co-morbidity: Scope, clinical presentation and treatment options. Brain Injury 28(3):261–270.
Taylor, D. D., and C. Gercel-Taylor. 2014. Exosome platform for diagnosis and monitoring of traumatic brain injury. Philosophical Transactions of the Royal Society B: Biological Sciences 369(1652).
Teasdale, G., and B. Jennett. 1974. Assessment of coma and impaired consciousness. A practical scale. Lancet 2(7872):81–84.
Terrio, H., L. A. Brenner, B. J. Ivins, J. M. Cho, K. Helmick, K. Schwab, K. Scally, R. Bretthauer, and D. Warden. 2009. Traumatic brain injury screening: Preliminary findings in a U.S. Army brigade combat team. Journal of Head Trauma Rehabilitation 24(1):14–23.
Trotter, B. B., M. E. Robinson, W. P. Milberg, R. E. McGlinchey, and D. H. Salat. 2015. Military blast exposure, ageing and white matter integrity. Brain 138(8):2278–2292.
VA (Department of Veterans Affairs). 2010. Screening and evaluation of possible traumatic brain injury in Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) veterans. VHA Directive 2010-012. Washington, DC: Department of Veterans Affairs.
VA. 2015. Traumatic brain injury (TBI) instruments user manual. Washington DC: Department of Veterans Affairs.
VA. 2016. VA/DOD clinical practice guideline for the management of concussion-mild traumatic brain injury. Washington, DC: Department of Veterans Affairs.
Van Giau, V., S. S. A. An, E. Bagyinszky, and S. Y. Kim. 2015. Gene panels and primers for next generation sequencing studies on neurodegenerative disorders. Molecular and Cellular Toxicology 11(2):89–143.
Vanderploeg, R. D., S. Groer, and H. G. Belanger. 2012. Initial developmental process of a VA semistructured clinical interview for TBI identification. Journal of Rehabilitation Research and Development 49(4):545–556.
Veeramuthu, V., V. Narayanan, T. L. Kuo, L. Delano-Wood, K. Chinna, M. W. Bondi, V. Waran, D. Ganesan, and N. Ramli. 2015. Diffusion tensor imaging parameters in mild traumatic brain injury and its correlation with early neuropsychological impairment: A longitudinal study. Journal of Neurotrauma 32(19):1497–1509.
Vincent, A. S., T. M. Roebuck-Spencer, and A. Cernich. 2014. Cognitive changes and dementia risk after traumatic brain injury: Implications for aging military personnel. Alzheimer’s and Dementia 10(3 Suppl):S174–S187.
Williams, C. C., and D. T. Bernhardt. 1995. Syncope in athletes. Sports Medicine 19(3):223–234.
Wintermark, M., P. C. Sanelli, Y. Anzai, A. J. Tsiouris, C. T. Whitlow, T. J. Druzgal, A. D. Gean, Y. W. Lui, A. M. Norbash, C. Raji, D. W. Wright, and M. Zeineh. 2015. Imaging evidence and recommendations for traumatic brain injury: Advanced neuro- and neurovascular imaging techniques. American Journal of Neuroradiology 36(2):E1–E11.
Witcher, K. G., D. S. Eiferman, and J. P. Godbout. 2015. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends in Neuroscience 38(10):609–620.
Wright, D. W., T. R. Espinoza, L. H. Merck, J. J. Ratcliff, A. Backster, and D. G. Stein. 2014. Gender differences in neurological emergencies, part II: A consensus summary and research agenda on traumatic brain injury. Academic Emergency Medicine 21(12):1414–1420.
Xiong, K., Y. Zhu, Y. Zhang, Z. Yin, J. Zhang, M. Qiu, and W. Zhang. 2014. White matter integrity and cognition in mild traumatic brain injury following motor vehicle accident. Brain Research 1591(1):86–92.
Yamamoto, S., D. S. DeWitt, and D. S. Prough. 2018. Impact and blast traumatic brain injury: Implications for therapy. Molecules 23(2):E245.
Yeates, K. O., H. G. Taylor, J. Rusin, B. Bangert, A. Dietrich, K. Nuss, M. Wright, D. S. Nagin, and B. L. Jones. 2009. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics 123(3):735–743.
Yi, A., and K. Dams-O’Connor. 2013. Psychosocial functioning in older adults with traumatic brain injury. NeuroRehabilitation 32(2):267–273.
Yuh, E. L., P. Mukherjee, H. F. Lingsma, J. K. Yue, A. R. Ferguson, W. A. Gordon, A. B. Valadka, D. M. Schnyer, D. O. Okonkwo, A. I. R. Maas, and G. T. Manley. 2013. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Annals of Neurology 73(2):224–235.
Yuh, E. L., S. R. Cooper, P. Mukherjee, J. K. Yue, H. F. Lingsma, W. A. Gordon, A. B. Valadka, D. O. Okonkwo, D. M. Schnyer, M. J. Vassar, A. I. Maas, G. T. Manley, S. S. Casey, M. Cheong, K. Dams-O’Connor, A. J. Hricik, T. Inoue, D. K. Menon, D. J. Morabito, J. L. Pacheco, A. M. Puccio, and T. K. Sinha. 2014. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: A TRACK-TBI study. Journal of Neurotrauma 31(17):1457–1477.
Zatzick, D. F., F. P. Rivara, G. J. Jurkovich, C. W. Hoge, J. Wang, M. Y. Fan, J. Russo, S. G. Trusz, A. Nathens, and E. J. Mackenzie. 2010. Multisite investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Archives of General Psychiatry 67(12):1291–1300.
Zeiler, F. A., C. McFadyen, V. F. J. Newcombe, A. Synnot, E. L. Donoghue, S. Ripatti, E. W. Steyerberg, R. L. Gruen, T. W. McAllister, J. Rosand, A. Palotie, A. I. R. Maas, and D. K. Menon. 2018. Genetic influences on patient-oriented outcomes in traumatic brain injury: A living systematic review of non-apolipoprotein E single-nucleotide polymorphisms. Journal of Neurotrauma, August 10 [Epub ahead of print].


































