2
Genetically Modified Nonhuman Primate Models for Neuroscience Research: Rationale and Overview of Potential Opportunities and Challenges
Science advances when the right model is available, said Robert Wurtz, scientist emeritus at the National Eye Institute, adding that the monkey is truly such a model for studying complex brain disorders. Humans and monkeys share common brain structures, including a highly developed frontal lobe that controls cognitive functions, and they display similar behaviors, said Wurtz. These commonalities enable scientists to identify circuits, examine how perturbation of those circuits affects behavior, and develop approaches to ameliorate the deficits created by that perturbation, he said.
Marmoset models, for example, have been developed using different technologies for many important human diseases, including brain disorders and diseases such as Parkinson’s disease (PD), Alzheimer’s disease, amyotrophic lateral sclerosis, stroke, and autism, said Hideyuki Okano, dean of the Keio University Graduate School of Medicine and team leader of the Laboratory for Marmoset Neural Architecture at the RIKEN Center for Brain Science. These models and others in nonhuman primates may also facilitate the development of novel and personalized treatments for neuropsychiatric disorders, said Angela Roberts, professor of behavioral neuroscience at the University of Cambridge. She predicted that in the future, chimeric models that incorporate neurons derived from induced pluripotent stem cells may enable scientists to develop better animal models of bipolar disorder and schizophrenia.
Examples of how these models are being explored in different disease states are discussed in Chapter 3.
OVERCOMING THE LIMITATIONS OF MOUSE MODELS WITH NONHUMAN PRIMATE MODELS
Although transgenic mice have revolutionized biomedical research, said Guoping Feng, they have their limitations. One key limitation is the fact that the prefrontal cortex in rodents is far less developed than in humans, which makes rodents a poor model for studying higher brain functions such as cognition, executive function, and emotion (see Figure 2-1).
The less complex (relative to human) rodent cortex results in a limited repertoire of cognitive function and this makes it difficult to interpret cognitive changes in a way that is translatable to humans, said Yoland Smith, professor of neurology and director of the neuropharmacology and neurological diseases division at the Yerkes National Primate Center of Emory University. The small size of the rodent brain also makes it difficult to translate new therapies from the bench to people, said Smith. In addition, mice have a limited life span, which makes them unsuitable for studying long-term neurodegenerative disorders.
Another limitation of mouse models for studying developmental disorders such as autism is that mice have very different social and communicative characteristics compared with humans and primates, said Feng. An example of how nonhuman primates are being used to better understand, and possibly develop, new diagnostic tests and treatments for autism is discussed in Chapter 3.
The lack of predictive animal models has contributed to numerous high-profile failures in translating preclinical success in rodents to clinical trials in humans, said Feng. Consequently, over the past 60 years, although great advances have been made in developing new treatments in other disease areas such as cardiovascular disease, there has been little progress in developing drugs that target new mechanisms in psychiatry. About 93 percent of drugs for nervous system disorders that show efficacy in rodent models fail in human clinical trials (Kola and Landis, 2004), said Karen Parker, associate professor of psychiatry and behavioral sciences at Stanford University. But rodent models do not just fail to predict human efficacy, they also fail to adequately test human safety. The sedative thalidomide, for example, was prescribed for pregnant women outside the United States in the late 1950s and early 1960s based on safety testing done only in rodents, she said, but limb deformities were seen when the drug was tested in marmosets and rhesus monkeys and the drug never received the Food and Drug Administration’s approval.
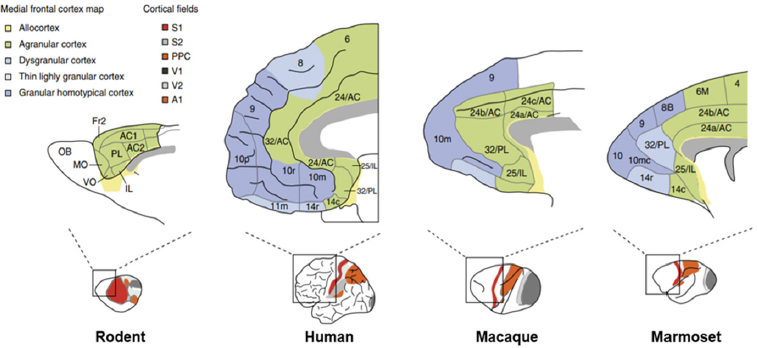
NOTE: A1 = primary auditory cortex; AC1 and AC2 = anterior cingulate cortex area 1 and 2; Fr2 = frontal area 2; IL= infralimbic cortex; MO = medial orbital cortex; OB = olfactory bulb; PL = prelimbic cortex; PPC = posterior parietal cortex; S1 and S2 = primary and secondary somatosensory cortex; V1 and V2 = primary and secondary visual cortex; VO = ventro orbital cortex.
SOURCES: Presented by Guoping Feng, October 4, 2018; adapted from Kaiser and Feng, 2015.
OPPORTUNITIES PROVIDED BY NONHUMAN PRIMATE MODELS TO FOSTER NEUROSCIENCE RESEARCH
Nonhuman primate models enable enhanced understanding of behavior and higher cortical function, potentially better model neurodegenerative and neuropsychiatric disorders, and can be used to advance therapeutic development, including developing personalized treatments, testing drugs for safety before their first use in humans, and facilitating biomarker discovery. Feng and many others have focused their efforts on two types of models: the common marmoset and the macaque. The common marmoset has the advantage of a short life span (approximately 14 years in captivity), which can be particularly useful when studying late-onset diseases such as PD, said Feng. In contrast, macaques have brains that are bigger and evolutionarily closer to humans and thus may be useful to study higher brain function and neurodevelopmental disorders, but their
longer life span (approximately 30 years in captivity for Cynomolgus macaques) would necessitate extremely long studies.
Feng said this has all been made possible by the development of CRISPR genome editing technology,1 which allows researchers to manipulate nearly any cell type or any organism (Sander and Joung, 2014). The most efficient genome editing approach is to make deletion mutants (knock-outs), said Feng, but to understand how genetic mutations or variations contribute to disease requires inserting or modifying genes (knock-ins), which is far less efficient. Tools for knocking-in genes efficiently have long been available in mice, but not in nonhuman primates, said Feng. His lab and many others have been working to develop these technologies. For example, they have recently used CRISPR to insert Cre recombinase into the paravalbumin (PV) gene locus in macaque embryos with about 60–70 percent efficiency. Cre recombinase is an enzyme that enables site-specific recombination events. Because PV neurons have been implicated in many disorders, Feng said this approach would provide a tool that would allow neuroscientists to probe and manipulate PV neuron function in the macaque. His team is also generating opsin knock-in monkeys, which will open the door to optogenetic approaches that have proved so valuable in increasing understanding of neural connectivity and circuit function.
CHALLENGES OF GENETICALLY MODIFIED NONHUMAN PRIMATE MODELS
While there are a number of opportunities afforded by the use of genetically modified nonhuman primate models for neuroscience research, challenges remain related to logistics and feasibility, selecting the appropriate primate species to study a specific disorder or disease, developing tools to bridge translation findings from nonhuman primates to humans, and exploring ethical considerations. These are briefly introduced here, and discussed in greater detail in later chapters.
___________________
1 Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) genome editing is a cost-effective, quick, and efficient technique that enables scientists to make targeted changes in the genome of living cells.
Addressing Infrastructure, Logistics, and Feasibility Issues
Generating, housing, and caring for sufficient numbers of nonhuman primates to support the range of research activities anticipated for these models will be difficult, said Smith. Parker noted that nonhuman primate colonies need to be housed in environments that provide naturalistic outdoor settings with species-typical enrichments and native temperatures as well as complex social conditions, including species-typical complex social groupings.2 The costs to maintain such a colony over a long period could be substantial, said Smith.
Breeding and obtaining sufficient numbers of animals also presents challenges. Cynomolgus macaques are continuous breeders with a 160-day gestation period and take 3.5 years to reach sexual maturity, typically producing singletons with each birth. Marmosets are also continuous breeders with a 140-day gestation period. They mature in only 1 to 1.5 years and give birth twice per year, producing twins or triplets with each birth. Nonseasonal breeders allow scientists to generate genetically modified animal models year-round through assisted reproductive technology.
Homozygous animals, while desirable for many types of studies aimed at understanding gene function, can take a long time to breed. Feng and colleagues have recently developed a new technology that dramatically increases the homozygous rate of knock-ins to about 50–80 percent with only one injection. This approach could dramatically shorten the time required to obtain homozygous animals, he said. If they can get this approach to work in neurons or somatic cells, Feng said he thinks it may have great potential in gene therapy. Infrastructure, logistics, and feasibility issues are discussed further in Chapter 6.
Selecting the Right Model to Study a Disease
The selection of primate species depends on the disease being studied and the questions one seeks to answer, said Smith. Rhesus monkeys, for example, have much larger brains, enabling investigators to target and manipulate sufficient numbers of neurons needed to apply and translate new
___________________
2 For an in-depth discussion about care, use, and welfare issues, see the forthcoming proceedings from the complementary workshop on the Care, Use, and Welfare of Marmosets as Animal Models for Gene Editing–Based Biomedical Research, hosted on October 21–22, 2018, by the National Academies’ Institute for Laboratory Animal Research. For more information, see http://nas-sites.org/ilar-roundtable/roundtable-activities/care-use-andwelfare-of-marmosets-as-animal-models-for-gene-editing-based-biomedical-research.
neurogenic, optogenetic, chimeric therapy, and gene therapy techniques. Rhesus monkeys also have a broad and sophisticated cognitive repertoire and can learn extremely complex tasks, said Smith. Feng added that rhesus physiology has been extensively studied and well characterized.
Marmosets also have advantages related to scale, said Smith, because they breed faster, thus enabling the generation of many more animals in a shorter time, and they also have a shorter life span. Marmosets also have emerged as good models of social behavior (Miller et al., 2016) and the marmoset genome has been completely sequenced (Marmoset Genome and Analysis, 2014).
Many factors need to be considered in developing and selecting the appropriate model for studies, said Parker. For example, modeling a neurodevelopmental condition like autism must be done in a younger animal to see onset of the behavioral phenotype, whereas for dementia, the phenotype begins in late adulthood, she said. Prevalence also should be considered, she said. Autism is much more common in males than in females, while depression is more common in females; ideally, the animal model will show a similar sex-biased pattern. Does the animal share the human disease (homolgous modeling) or only a part of the phenotype (endophenotypic modeling)? Is there a similarity in behavior or appearance between the model and human condition (face validity); is there a similarity in the underlying cause of disease (construct validity); and does the animal’s response to treatment reflect what will happen in a person (predictive validity)? Are behavioral readouts ecologically informed, that is, does the animal exhibit them under natural circumstances?
These various factors can be leveraged to identify a range of models that may be appropriate, said Parker. For example, to study autism, she and her colleagues have taken three approaches: (1) a behavior first approach using models with face validity in that they exhibit naturally occurring social deficits; (2) a genetics first approach with construct validity that uses homologous pathogenic variants, selective breeding, and/or genome engineering; and (3) a candidate signaling pathway approach with construct validity based on homologous biomarker profiles (see Figure 2-2). Parker’s work in autism is discussed in more detail in Chapters 3 and 4.
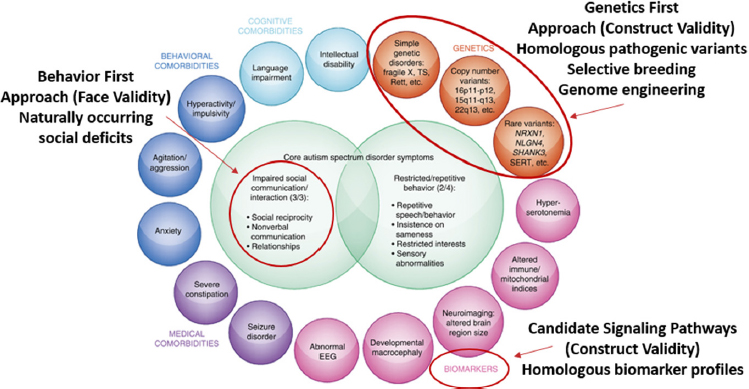
NOTE: EEG = electroencephalography.
SOURCES: Presented by Karen Parker, October 4, 2018; adapted from Veenstra-VanderWeele and Blakely, 2012.
Developing Tools to Translate Findings from Nonhuman Primates to Humans
In order to use nonhuman primate models to better understand the underlying psychological and neurobiological causes of psychiatric symptoms and develop new treatments, a range of primate behavioral tests are needed that can assess different aspects of cognition and emotion and translate easily to the human clinical condition, said Roberts. This is discussed in more detail in Chapter 4.
Exploring Ethical Considerations
The justification for using genetically modified nonhuman primate models comes down to the limitations of doing certain kinds of research in humans or in other research models, said Jeffrey Kahn, the Andreas C. Dracopoulos director of the Johns Hopkins Berman Institute of Bioethics. This means, he said, that other models should be considered before using
this “valuable and valued resource.” Then, if research on nonhuman primates is determined to be justified, oversight by people with specialized expertise is needed, said Kahn. Ethical issues related to the use of nonhuman primate models are further explored in Chapter 5.








