To gain a better understanding of the sources of variability associated with regenerative engineering products, the first group of speakers presented three case studies for consideration. Ivonne Hernandez Schulman, a professor of clinical medicine at the University of Miami’s Miller School of Medicine, discussed variability in the use of mesenchymal stem cells for treating cardiomyopathy. Clive Svendsen, the Kerry and Simone Vickar Family Foundation Distinguished Chair in Regenerative Medicine at Cedars-Sinai Medical Center, described sources of variability in preclinical and clinical research on stem cell therapies for amyotrophic lateral sclerosis (ALS). David Stroncek, the chief of the cell processing section of the Department of Transfusion Medicine at the National Institutes of Health (NIH) Clinical Center, discussed variability in the development of cellular therapies. The session was moderated by Cato Laurencin of the Connecticut Convergence Institute for Translation in Regenerative Engineering.
It is well known that there will be variability when findings are translated into clinical practice, Laurencin said. For any given procedure there will be a range of possible outcomes. This variability can be due to differences in immunology, genetics, the sources of the materials that are used, the way the materials are applied, and even the psychology of expectations, he said. The term “clinical translation” in the definition of regenerative engineering acknowledges a range of factors, including immunology and patient biofitness, that influence the actual regenerative process in humans. The field of regenerative engineering is now embracing this variability and working to understand it, he said.
CASE STUDY 1: VARIABILITY IN THE USE OF MESENCHYMAL STEM CELLS FOR TREATING CARDIOMYOPATHY
The focus of the first case study was the use of culture-expanded cell products in clinical trials, specifically the work that Schulman and her colleagues are carrying out at the Interdisciplinary Stem Cell Institute at the University of Miami using mesenchymal stem cells to treat heart failure. The mesenchymal stem cells used are derived from bone marrow and umbilical cord, she said, and they are identified based on the expression of the cell surface antigen CD105. As Guillermo Ameer explained earlier (see
Chapter 1), sources of variability include the sex, age, and health status of the cell donors. The studies that Schulman described use both autologous mesenchymal stem cells (which have inherent variability based on the patient they are derived from) and allogeneic mesenchymal stem cells. The allogeneic cells are collected from young, healthy donors. The donors are generally males between the ages of 20 and 35 who are screened for a range of illnesses, Schulman said. She and her colleagues are now also studying cells from female donors, and there is still much to be explored about the potential differences. Similarly, allogeneic cells are currently being collected from young donors, but the ideal age range for donors is not known. Also, the sex, age, and disease process of the patient receiving the therapy all need to be considered for their potential impact on the effectiveness of the cell therapy, she said.
There is variability inherent in the manufacturing process concerning the isolation, expansion, and characterization of the cells. Once manufactured, the product should be assessed for viability and potency, ideally by testing for anti-fibrotic or anti-inflammatory properties, Schulman said.
The Mechanisms of Mesenchymal Stem Cells
Mesenchymal stem cells can differentiate into adipose, bone, and cartilage phenotypes and exert a range of other effects (Karantalis and Hare, 2015). Under different conditions, mesenchymal stem cells also have the capacity to differentiate into endothelial, cardiac, and vascular smooth muscle cells in vitro, Schulman said, although it is not clear the extent to which there is biologically significant differentiation in vivo. Mesenchymal stem cells release paracrine factors that have been shown to have anti-fibrotic, immunomodulatory, and anti-inflammatory effects in various disease processes. Mesenchymal stem cells also release exosomes, which Schulman suggested could be the mechanism that delivers the factors involved in facilitating the immune response, cell differentiation, and cell-to-cell interactions. It is also known that mesenchymal stem cells communicate with other cells through gap junctions, stimulate endogenous stem cell differentiation, and interact with cardiac progenitor cells, Schulman said.
Mesenchymal Stem Cell Clinical Trials
The results of a series of phase I/II clinical trials and a phase II clinical trial of mesenchymal stem cells for the treatment of heart failure were described by Schulman (see Box 2-1). These trials have demonstrated the safety of intracardiac autologous and allogeneic mesenchymal stem cell injections, Schulman said, and there were no significant alloimmune reactions to injections of allogeneic mesenchymal stem cells. Moderate but
consistent improvement was observed in patients’ 6-minute walk distance (a measure of cardiopulmonary function) and in their Minnesota Living with Heart Failure questionnaire scores, she reported. The scar size in patients with ischemic cardiomyopathy was reduced by 30 to 50 percent, and patients with dilated cardiomyopathy had improvements in ejection fraction (a measure of cardiac function), which were greater with allogeneic mesenchymal stem cells than with autologous mesenchymal stem cells. Endothelial function (measured by flow-mediated vasodilation studies and by endothelial progenitor cell bioactivity) was improved following the injection of allogeneic mesenchymal stem cells. Finally, levels of tumor necrosis factor alpha (an inflammatory marker) were decreased in patients with dilated cardiomyopathy.
A comparison of mesenchymal stem cell injection between patients with ischemic cardiomyopathy and those with dilated cardiomyopathy indicated that patients with ischemic cardiomyopathy tended to have improvements
in their scar size reduction (less so in cardiac function) while patients with dilated cardiomyopathy had more significant improvement in cardiac function, Schulman said. In other words, all of the patients with heart failure experienced a benefit from mesenchymal stem cell injection, but the mechanisms seem to be different and to be closely related to the disease process, she said. Further studies are under way to better understand this finding.
Umbilical Cord–Derived Mesenchymal Stem Cells
Umbilical cord–derived mesenchymal stem cells are isolated from the Wharton’s jelly, a gelatinous substance within the umbilical cord. The manufacturing processes for mesenchymal stem cells vary, and in order to move cell therapies forward, the manufacturing and characterization processes need to be standardized regardless of cell source (e.g., bone marrow, umbilical cord, adipose tissue), Schulman said. As with mesenchymal stem cells derived from other sources, the umbilical cord–derived mesenchymal stem cells are extracted, processed, counted and assayed for viability, expanded, and assessed for purity (using flow cytometric analysis for specific markers such as CD105+ and CD45–). The flow cytometric analyses need to be standardized by the field, Schulman said. Of course, there is also donor-to-donor variability, she noted.
Combination Stem Cell Therapy
Studies in an animal model (pig) of ischemic cardiomyopathy found that treatment with either mesenchymal stem cells or a combination of mesenchymal stem cells and cardiac-derived progenitors reduced scar size, but the combination therapy was better at improving cardiac function (Karantalis et al., 2015). Based on the findings in the animal model, the Combination of c-kit Cells and Mesenchymal Cells: A Novel, Dual Cell Study Evaluating Regenerative Properties for Treatment in Chronic Heart Failure (CONCERT-CHF) trial was launched by the Cardiovascular Cell Therapy Research Network (funded by the National Heart, Lung, and Blood Institute) to study the combination of cardiac progenitor cells and mesenchymal stem cell in humans, Schulman said.1 CONCERT-CHF is a multi-center clinical trial across seven centers, and the cardiac-derived cells and the mesenchymal stem cells used in it are centrally processed so that their preparation is standardized across all of the centers, Schulman said.
___________________
1 For more information on the Cardiovascular Cell Therapy Research Network, see https://sph.uth.edu/research/centers/ccct/cctrn (accessed December 19, 2018). For information on the CONCERT-HF clinical trial, see https://clinicaltrials.gov/ct2/show/NCT02501811 (accessed December 16, 2018).
CASE STUDY 2: SOURCES OF VARIABILITY IN PRECLINICAL AND CLINICAL RESEARCH ON STEM CELL THERAPIES FOR ALS
A significant challenge in cell transplantation therapy is cell sourcing, Svendsen said. In treating neurological disorders, two main sources of cells are generally used for the clinical product: embryonic stem cells or iPSCs and fetal brain tissue, both of which can provide neural progenitor cells. Neural progenitor cells can differentiate into neurons, oligodendrocytes, and astrocytes. Svendsen said that it is difficult for transplanted neurons to establish connections in the adult brain efficiently (e.g., for the treatment of stroke). Remyelination with oligodendrocytes is a more practical approach (e.g., for the treatment of multiple sclerosis and spinal cord injury), he said, and astrocytes can have substantial effects on central nervous system function (e.g., for the treatment of ALS and other neurological diseases).
An advantage of using embryonic stem cells and iPSCs is that the supply is limitless since cells can be expanded in culture, Svendsen said. There is, however, a risk of teratoma (a tumor made up of different types of tissue) formation, and there are issues with stability in vitro. In addition, it is challenging to get iPSCs to differentiate into fully functional cells, and cells will occasionally differentiate into a completely unexpected cell type. An advantage of using fetal tissue, he continued, is that cell differentiation has already occurred. As such, it is expected that cells from fetal tissue are more likely to be functional in the central nervous system after transplantation. However, he noted, there are ethical issues with the use of fetal tissue, and the supply is limited.
Manufacturing Human Neural Progenitors from Fetal Tissue
The method used by Svendsen’s laboratory for isolating and expanding fetal-derived cortical neural progenitor cells involves using a unique chopping technique, he explained. This technique maintains cell-to-cell contact and allows for up to 70 population doublings in culture, he said. The cells in culture mirror normal development so that early in the expansion process the cells will differentiate into neurons and, later, into astrocytes. In other words, the normal development process is used in the manufacturing process. Although there will be variation across samples, he said, the process has proven reliable with various sources of fetal tissue.
This procedure has been used under current good manufacturing practice (cGMP) for generating cells for clinical studies (Shelley et al., 2014). In this process, samples from a master cell bank are expanded using the cGMP process. Along the way, the cells are engineered to release glial cell line–derived neurotrophic factor (GDNF, a growth factor) using a lentiviral
vector. Finally, following further expansion, neural progenitor cells secreting GDNF are frozen and stored for future use in clinical trials.
Challenges
There are a variety of challenges with this manufacturing process, Svendsen said, with the most significant concern being the genetic stability of the cells during the passaging process. Artificial chromosome changes can occur during expansion, and there have been instances of trisomy 7, for example, which was found to be associated with the use of epidermal growth factor that was not sufficiently fresh. The commercial source of certain reagents was also found to affect results, he said. Another challenge involved cell viability after thawing with one large 1,200-vial batch showing only 40 percent viability after thaw (compared with a target viability of over 70 percent). It was discovered that there are process-critical steps at scale-up that affect successful freeze and thaw, Svendsen said. To address this, cells were frozen in four sub-lots. Throughout this process there was ongoing interaction with the Food and Drug Administration (FDA) to solve these challenges, he said. Ultimately, eight vials from the master cell bank were expanded to 1,200 vials of a clinical cell bank over the course of a 10-week manufacturing process. Ensuring that the manufacturing process is reproducible helps reduce variability, he said. Variability was also associated with how the final product was thawed, counted, and shipped for the patient surgical procedure, and a preparation protocol was developed to ensure consistency.
Neural Progenitor–Derived Astrocytes for ALS
ALS, also known as Lou Gehrig’s disease, leads to the death of motor neurons, paralysis, and, ultimately, the death of the patient, usually within 3 years. The cause is unknown, and there is no treatment or cure. Svendsen described an ongoing clinical study using the neural progenitor cells engineered to release GDNF.2 The cells are injected by a neurosurgeon directly into the spinal cord where the motor neurons are dying. An innovative surgical device was developed to facilitate the delivery of the cells to the spinal cord. The investigational new drug (IND) application was submitted to FDA in March 2016 for a phase I/IIa safety trial, and the first patient dose was delivered on May 3, 2017. Cells are delivered to one side of the lower lumbar spinal cord so that one leg is affected by the treatment and the other leg serves as a control. A total of 18 patients have been enrolled
___________________
2 For more information on the clinical trial of transplanted cells that express GDNF for ALS, see https://clinicaltrials.gov/ct2/show/NCT02943850 (accessed December 16, 2018).
and treated, and patients will be followed for 1 year after treatment. Thus far, there have been no serious adverse events associated with the cells and no sign of tumor formation, Svendsen reported.
The study will show whether the cells survive, secrete GDNF, and have any impact on disease progression in the treated leg, Svendsen said. There might also be data on the role of immune suppression in cell transplantation since all patients were originally immunosuppressed, although some had to discontinue due to tolerability issues. Overall, patient outcome will not be affected because the disease will continue to progress in the upper spinal cord and brain (something that the researchers make sure that the patients understand as part of the consent process). It was carefully explained to patients that they were the first patients to receive this combined cellular and gene therapy and that they would not be cured, Svendsen said. The cell therapies under investigation are still in the early stages, he added, and it is important to be very clear about this in the informed consent.
Astrocytes derived from fetal neural progenitor cells might be used to treat many other disease targets, including Parkinson’s disease, Huntington’s disease, retinitis pigmentosa, stroke, and Alzheimer’s disease. Although they are a single product, astrocytes are important in all of these diseases, Svendsen said, and preclinical or clinical research is under way in all of these areas.
Transitioning to iPSC-Derived Products
Transitioning to the use of iPSCs would help to avoid the ethical issues surrounding the use of fetal tissue. Currently, Svendsen and his colleagues are working on the production and differentiation of clinical-grade iPSCs from the whole blood of patients with ALS. This requires good manufacturing practices (GMPs), good certificates of analysis for the cells, and good standard operating procedures (SOPs), he said. A significant concern for iPSCs is cytogenetic stability since about 24 percent of the cell lines could have an abnormal karyotype. Svendsen described an approach to producing iPSCs from non-expanded peripheral blood mononuclear cells that reduces the percentage of abnormal karyotypes to 1.6 percent. These iPSCs can then be made into “EZ-spheres,” which are neural progenitors that are similar to fetal-derived neural progenitors (Ebert et al., 2013).
Challenges
A main challenge to transitioning to iPSC-derived neural cell products, Svendsen reiterated, is the genetic stability of the iPSC lines. In response to two questions, Svendsen emphasized that it is important to karyotype the
iPSCs every 3 or 4 weeks or when they are being expanded in order to check for chromosomal abnormalities. If the karyotype is found to be abnormal, that line will be stopped and not used again.
There are also challenges to manufacturing at scale and with cGMP, Svendsen said. The consistency of the differentiation from iPSCs is an issue. It will also be necessary to demonstrate that the iPSC product has close clinical equivalency to a fetal-derived product, he said. His team is currently working with Space Tango and the National Aeronautics and Space Administration on experiments in the International Space Station to study the effects of zero gravity on iPSC growth and differentiation.
Closing Remarks
In conclusion, Svendsen emphasized the need for attention to process and to the development of SOPs when developing cell products from human fetal brain tissue for administration to animals and patients. Cells are constantly changing as they proliferate in culture, and this requires constant attention, he said. The transition to iPSC technology is under way, he said, but he reiterated that it will be important to ensure stability and reduce variability as well as to implement reliable differentiation protocols.
CASE STUDY 3: VARIABILITY IN THE DEVELOPMENT OF CAR T CELL THERAPY
The Center for Cellular Engineering is a core processing laboratory at the NIH Clinical Center. It manufactures a range of products for investigators, including cells for cancer immunotherapy, regenerative medicine, and gene therapy for inherited diseases, Stroncek said. Chimeric antigen receptor (CAR) T cells are autologous T cells that are genetically engineered to express a specific receptor that binds to an antigen on a tumor cell. The receptor construct contains a single-chain variable fraction of a monoclonal antibody, Stroncek said, adding that he would be discussing CAR T cells that target specifically CD19 or CD22 cell surface proteins. The construct also contains a co-stimulatory molecule and a T cell signaling portion, he said.
Manufacturing of CAR T cells involves the collection of autologous peripheral blood mononuclear cells (PBMCs) by apheresis, isolation of T cells, T cell stimulation, transduction (gene transfer), T cell expansion, harvest, and product testing before infusion back into the patient (see Figure 2-1). The process takes about 10 days and is relatively simple compared with manufacturing iPSCs, Stroncek said, although there are challenges at the T cell expansion and T cell transduction stages of the manufacturing process. Because the density of monocytes is similar to that of lymphocytes,
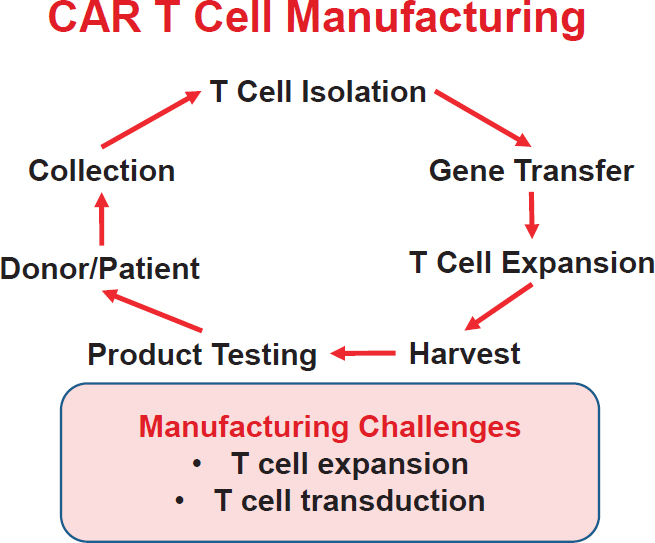
NOTE: CAR = chimeric antigen receptor.
SOURCE: David Stroncek, National Academies of Sciences, Engineering, and Medicine workshop presentation, October 18, 2018.
the composition of the PBMC concentrate after apheresis averages about 70 percent lymphocytes, 20 percent monocytes, and 10 percent granulocytes, which Stroncek said can be a problem in some patients. As such, manufacturing processes generally include a step to enrich the product for lymphocytes; the simplest way to do this is density gradient separation. The lymphocyte-rich fraction is then collected, and cells are stimulated with anti-CD3 and interleukin (IL)-2 for the transduction and expansion steps. An alternative method is to select for lymphocytes in the PBMC concentrate with magnetic beads coated with anti-CD3 and anti-CD28. Although the purpose of the beads is to stimulate expansion of the T cells, he said, a magnet can be used to pull the T cells out of the mixture because the antiCD3 on the beads binds the T cells, a process that works well in healthy subjects. Then the T cell mixture is transduced and expanded.
There was wide variation in the cell counts from the first 28 CD19CAR T cell products manufactured in the core processing laboratory,
Stroncek said. Some patients had up to 3 billion cells produced, while others had none or only a few million. Of the 28 patients, 4 did not have sufficient numbers of transduced T cells to meet the release criteria. Upon further investigation, it became clear that the PBMC concentrates from those four patients had higher proportions of monocytes (averaging 39 percent, versus an average of 15 percent monocytes in PBMC concentrates from the 24 patients meeting dose requirements). Because monocytes non-specifically bind to the magnetic beads, they are then carried forward along with the T cells to the expansion step. In cancer patients, Stroncek explained, the monocytes can act as myeloid-derived suppressor cells, releasing inhibitors of T cell expansion (Stroncek et al., 2016). In addition, when monocytes are bound to the CD3/CD28 beads, the beads are then unavailable to bind to and stimulate the T cells. Stroncek and his colleagues tested three methods to remove monocytes from the PBMC concentrate before the manufacturing of the CD19-CAR T cell products: counter-flow elutriation, T cell–specific monoclonal antibody–coated beads, and antibody-bead-selected cells plus adherence of monocytes to plastic. Ultimately, elutriation was chosen for its ease of use, success rate, and high yields.
Enrichment and elutriation were less suitable for the manufacture of CD22-CAR T cell products, Stroncek said. Instead, PBMC concentrates were enriched for T cells using CD4 and CD8 monoclonal antibodies conjugated to magnetic beads and a commercial GMP cell separation system (Miltenyi CliniMACS® Plus). The purity of the final product was high, but it also appeared that the process had an impact on potency, Stroncek said. Patients receiving the T cell–selected CD22-CAR T product showed a higher incidence of cytokine release syndrome (higher levels of IL-6, IL-10, and ferritin). To compensate for this, the dose of CAR T cells infused was reduced. It was found, however, that T cell selection might actually enhance CAR T cell expansion in vivo, and the clinical results were similar even though the dose was reduced. In summary, Stroncek said, there is variability in the PBMC concentrates collected by apheresis for use as the starting material for CAR T cell manufacturing, and this can lead to variability in T cell expansion. Different methods can be used to enrich the concentrates for T cell expansion, and some of these methods appear to affect CAR T cell potency.
DISCUSSION
The Value of Embracing and Learning from Variability
The panelists were asked to comment on variability across the spectrum, from preclinical and small animal experiments to primate studies
and clinical studies in humans. Most procedures are scaled up in animal models using cells from healthy subjects first, Stroncek said. Variability inevitably surfaces when cells from patients are then used. It is important, he said, to embrace this variability and to work to understand it—how, for example, it affects potency of the product and if there is an impact on clinical outcomes. There are key differences between allogeneic and autologous therapies, Svendsen said. With allogeneic therapy, patients are treated with an identical product. To reduce the variability in their clinical trial, patients who are at the same stage of ALS disease are enrolled, he said.
Variability in the context of developing regenerative engineering therapies is both a problem and an asset, a workshop participant said, raising the issue of study design. If a study treating multiple patients with a single donor population is successful, there is no guarantee that it can be reproduced, the participant observed. On the other hand, if that study fails, it is impossible to know whether the failure was due to an ineffective strategy or to an inappropriate donor. If a trial is designed so that each patient receives a product from one donor, it is not possible to determine whether the results are related to the patient or the product. The studies performed by Schulman and her colleagues thus far have been phase I, with between 30 and 70 patients in each, she said, and they have not been large enough to address these questions. It was also not possible to discern whether patient age or sex was a factor in response to the treatment. Large clinical trials, with many patients and significant funding, would be needed to address these questions, she said. Much of the work is being done in animal models, and clearly, animal cells are very different from human cells.
An 18-patient study using cells derived from fetal cortex tissue from a master cell bank from a single donor was recently completed by Svendsen’s group. Two to three vials of cells from a master cell bank can be expanded to 1,200 vials of product, he said, and he estimated that nearly 1,000 patients could be treated with product from this one fetal tissue sample. One main issue with the expansion process, he reiterated, is chromosomal stability. He suggested that using cells from a single donor in a trial could help answer some of the questions of variability as all of the patients would receive the same product. A grant application to study the lot-to-lot variability of mesenchymal stem cells is being developed by Schulman and her colleagues.
Cell Characterization
The issue of cell characterization for both autologous and allogeneic products was brought up by another workshop participant. Even when cells express the same surface markers (e.g., markers of neural progenitor cells), deep characterization by RNA sequencing (transcriptome analysis) shows
that cells from different donors are widely variable and that some donors have multiple populations, the participant noted. As such, surface markers do not necessarily ensure that one has the same cell population across patients. Analysis of the secretome, metabolome, and transcriptome could be used to confirm the characteristics of the cell and correlate characteristics with function. There is a need to move from markers toward functional or performance-based screening, Ameer said, but performance-based metrics would need to be adapted for use in high-throughput systems. There are challenges with developing physiologically relevant functional assays for complex diseases, such as ALS, for which the underlying mechanisms are not well understood. Markers are still very important for isolating cells, Schulman said. She acknowledged that mesenchymal stem cells are very heterogeneous, and she noted the importance of potency assays. The populations that are needed will likely be disease-specific, she said. Potency will also be variable across batches of product, Svendsen said, and it is not known what impact that differences in genetic sequence might have in terms of patient outcome. High-throughput analysis of the transcriptome has been very helpful, Stroncek said, adding that mesenchymal stem cells made in different laboratories with slightly different techniques have been shown to have highly variable transcriptomes. In some cases, he said, those cells had differences in their ability to form bone.
Genome Modification by Lentivirus
For regenerative medicine products in which lentivirus has been used for genome modification, a participant asked, how might that affect a clinical trial if only high CAR-expressing T cells or high GDNF-expressing stem cells were used? For the CAR T cell clinical trials, Stroncek said, vector copy number and expression level are considered. Levels tend to peak over several weeks and then decline. In some cases CAR T cells are no longer detectable after 1 year, and in other cases they persist at low levels, he said. The lentivirus vector integrates into the genome of the transduced stem cells, Svendsen said. Because it was very complicated to determine how many insertion sites there were, his research team chose to focus on qualifying the product (e.g., the quantity of GDNF released). FDA was interested in the construct and any tumorgenicity associated with the construct (there was none), he said, but it did not require whole-genome sequencing of the product. At this point, it is not known which insertion site is best for lentivirus, and so, pragmatically speaking, if the product is safe and effective it should be moved forward through development, Svendsen said. Safety is paramount, as these astrocytes are injected directly into the spinal cord and are integrated into the nervous system where they remain for a long time.
This page intentionally left blank.














