In addition to those sources of variability in regenerative medicine products that are associated with the patient, there are also sources of variability associated with the donors of the cells and tissues (e.g., in the tissue, dose, route of administration, culture conditions). The next workshop session explored these topics. Andrew Fesnak, an assistant professor of clinical pathology and laboratory medicine at the University of Pennsylvania Perelman School of Medicine, discussed the challenges of variability in CAR T cell manufacturing, specifically with mononuclear cell collection. George Muschler, a staff member in the Department of Biomedical Engineering at the Cleveland Clinic, reviewed select sources of variation when identifying and collecting cells for regenerative therapies. Alison Hubel, a professor of mechanical engineering at the University of Minnesota, described the importance of preservation with regard to the variability of regenerative medicine products, specifically issues associated with cryopreservation. The session was moderated by Martha Lundberg, a program director in the Division of Cardiovascular Sciences in the Advanced Technologies and Surgery Branch at the National Heart, Lung, and Blood Institute.
THE CHALLENGE OF VARIABILITY IN CAR T CELL MANUFACTURING
Mechanism of Apheresis Collection of Mononuclear Cells
CAR T cell product manufacturing involves the collection of autologous PBMCs by apheresis (see Chapter 2). “Apheresis is a continuous or semi-continuous method of isolating PBMCs based on density,” Fesnak explained. During apheresis, the cell separation occurs in real time. Blood that is removed from the patient via vascular access enters the machine and is centrifuged, which separates the blood into its components based on density. Red blood cells are the most dense components, and plasma is the least dense. White blood cells layer in density-specific bands in between the red cell and plasma layers, with granulocytes being more dense and mononuclear cells being less dense, Fesnak said. This method reliably harvests
large numbers of mononuclear cells, such as lymphocytes and monocytes, and returns granulocytes, platelets, and red blood cells to the patient. Cells for CAR T cell manufacturing are generally collected from steady state, non-mobilized donors—that is, donors who have not received granulocyte colony stimulating factor (a hematopoietic growth factor).
Mechanical apheresis dates back to the mid-1800s, Fesnak said, when a continuous-flow centrifuge was invented in Sweden to separate the cream from whole milk. The De Laval cream separator revolutionized the dairy industry, he said. A century later, in 1962, IBM engineer George Judson adapted the De Laval mechanism to create a closed system for whole blood separation. The process was intended to treat leukemia by reducing the number of circulating white blood cells, Fesnak said.
Sources of Variability in CAR T Cell Manufacturing and Downstream Effects
Current apheresis instruments are limited in their ability to resolve cell types, Fesnak said. Mononuclear cell collection via apheresis results in a product that contains T cells, B cells, monocytes, reticulocytes, and possibly blast cells, he said. If the blood flow is interrupted during apheresis (e.g., if the vascular access is compromised), the separation of cells into density-based layers can be disrupted, and the resulting product can contain other blood elements, including platelets, granulocytes, and red blood cells. Understanding these characteristics of apheresis is important for CAR T cell manufacturing because non-T cell contaminants can negatively affect the process downstream. Different cellular contaminants can, for example, inhibit T cell proliferation or selectively induce apoptosis of activated T cells, Fesnak said.
The mononuclear cell product will always be a direct reflection of the cell populations circulating in the donor at the time of collection, Fesnak said. As such, the donor is the primary driver of variability in the manufacturing of CAR T cells, and given the variability of the material retrieved from donors, standardizing the final product presents a challenge.
To better characterize CAR T cell manufacturing variability, Fesnak divided the sources of variability into pre-collection factors and collection factors. Pre-collection sources of variability include patient demographics, clinical indication, and prior treatment, which, Fesnak pointed out, are generally fixed factors. The sources of variability associated with collection include the type of access, the duration of the apheresis procedure, and how well the patient tolerates the procedure. Fesnak also listed some of the parameters that could be used to assess the impact of these sources of variability. The parameters that characterize the product include, for example, yield, purity, and collection efficiency. The parameters that characterize the cells in culture include the rates of cell loss and population doubling
and also transduction efficiency. These are just some of the many different sources of variability that can affect multiple downstream parameters, Fesnak said.
An assessment of peripheral blood drawn on the day of apheresis collection found that blood counts varied by clinical indication. Samples were compared from patients with pancreatic cancer, ovarian cancer, or mesothelioma; glioblastoma; multiple myeloma; chronic lymphocytic leukemia (CLL); acute lymphocytic leukemia (ALL); and lymphoma. Among the findings was that patients with CLL tended to have lymphocytosis (increased lymphocytes), and patients with lymphoma tended to have lymphopenia (low levels of lymphocytes), which, Fesnak noted, was consistent with the two diseases.
The mononuclear cell products derived from patients with CLL and ALL tended to have high total mononuclear cell counts, whereas the products from patients with lymphoma had much lower total mononuclear cell counts, Fesnak said. An analysis by flow cytometry for CD3-positive T cells (needed for CAR T cell manufacturing) revealed that the percentage of CD3-positive cells in the mononuclear cell product in patients with ALL and lymphoma varied widely. And an analysis of the impact of clinical indication on manufacturing success suggests that the lowest success rate is associated with manufacturing products from cells from lymphoma patients. In summary, Fesnak said, “because mononuclear cell product content varies by indication, impact on manufacturing success may be indication specific.”
Mitigating Variability
A manufacturing process was designed to sequentially reduce variability stepwise throughout the manufacturing process of CAR T cells, Fesnak said. The process has a number of steps designed to shed non-T cells and enrich the population for T cells, with the final steps involving cell culture and T cell–specific activation and expansion. The goal is for the final product to be as standardized and pure as possible. This process is effective, Fesnak said, but it is also inefficient and can be unpredictable. The final purity can depend on which non-T cell contaminants were present at the start. For example, he said, granulocytes can be easily removed using a Ficoll density gradient, but monocytes do not separate out from lymphocytes on Ficoll. This means, Fesnak said, that understanding both the T cell and contaminant non-T cell populations is essential.
Some of the mitigation strategies might seem straightforward, but there are limitations to be aware of, Fesnak said. For example, if there are too few cells it might seem to be an obvious solution to simply run the cell collection apheresis for longer. However, Fesnak said, the longer patients stay
on the apheresis machine, the more likely they are to experience procedural intolerance. In addition, there are diminishing returns with each subsequent passage of total blood volume through the collection process. Too many non-T cells might suggest the need for better enrichment, but Fesnak said that the availability of GMP-grade reagents and techniques for enrichment is currently limited. He also said that achieving higher purity often comes at the cost of a lower yield. The challenge is to find the optimal balance of purity and yield. Finally, he said that even a highly pure T cell population can contain suboptimal T cells because these patients have often experienced years of cytotoxic chemotherapy. One mitigation approach could be to collect autologous cells earlier in the disease process, or one could consider using an allogeneic donor. However, Fesnak said, there is currently no infrastructure to prophylactically collect and store these patients’ cells. Using allogeneic CAR T cells has additional risks, including graft versus host disease and rejection of the cells.
Closing Remarks
In closing, Fesnak reiterated that mononuclear cell products will always reflect the donor at the moment the donor is undergoing apheresis. Donor variability drives mononuclear cell product variability, which in turn drives variability in the manufacturing process. There are many sources of variability that affect the downstream parameters of the process and the product. Sequential processing to reduce variability at each step is effective at reducing overall variability, but it is not particularly efficient. Finally, he said, there are limitations to current mitigation strategies.
IDENTIFYING AND PROCESSING CELLS FOR REGENERATIVE THERAPIES
The body continually loses and regenerates tissue to maintain health over the course of a lifetime, Muschler said, and that process creates a variety of cell populations at various stages of development. Because variability is expected, he added, there is an opportunity to understand and manage, rather than simply reduce, variation. The rate of loss and renewal varies by tissue, with age, and disease state. Skin cells on the face, for example, turn over about every 2 weeks, he said, while red blood cells average 120 days.
The basic life cycle of any tissue starts with a stem cell that periodically divides and “self-renews.” When activated, a true stem cell divides asymmetrically, producing one daughter cell, a progenitor cell, that may go on to proliferate, migrate, and differentiate into mature cells, and also a second cell that maintains the stem cell phenotype of the starting cell,
preserving this function for the future. It is expected then, that any healthy tissue will contain a range of cell populations at various stages of progenitor cell development, from stem cell through mature cell. It is important to be aware of this difference between a self-renewing stem cell and a progenitor cell, Muschler said. Cells harvested from healthy tissues likely include “hundreds of times more progenitors than true stem cells,” he said.
As an orthopedic surgeon, Muschler said, he has found that bone provides a unique model for studying the kinetics of stem and progenitor cell populations. The life span of an osteocyte (mature bone cell) is 15 to 25 years, while the life span of an osteoblast (a cell that synthesizes bone) is about 40 days. About 1 in 4 osteoblasts allow themselves to be buried by their neighbors and become osteoblasts, while about 1 in 20 osteoblasts persist as lining cells on the surface of bone after remodeling is complete.1 By studying the compartments upstream and downstream from osteoblasts, Muschler and his colleagues have been able to better understand the stages that the cells pass through (Muschler et al., 2003). For example, questions of interest include where the cells upstream from osteoblasts reside, how many there are, how they can be measured, and how they change in settings of human disease and regeneration.
Sources of Variation in Cell Sourcing and Selection
There are many sources of variation that are relevant to finding and selecting cells for regenerative therapies, Muschler said. Examples of these sources include the patient or donor; the tissue harvested and its state of health; the harvest technique; tissue transport conditions; the processing method; cell selection criteria; assay techniques; cell expansion conditions; and cell storage conditions (see Figure 4-1). Preferred cell attributes may vary depending on the preclinical model that is used for screening and on the clinical setting in which the cells will be used. Muschler discussed several topics—specifically, generalizable methods for quantitative cell and colony assaying, the benefit of automated imaging over subjective manual counting, and sampling efficiency of the harvest technique—and he briefly mentioned several others, such as clone diversity in the harvested tissue, heterogeneity of cell surface markers, and the opportunity for automated performance-based cell selection.
Assay Methods
Accurate repeatable and reproducible methods are essential for defining opportunities and value when making decisions about where and how
___________________
1 This text was revised after the prepublication release.
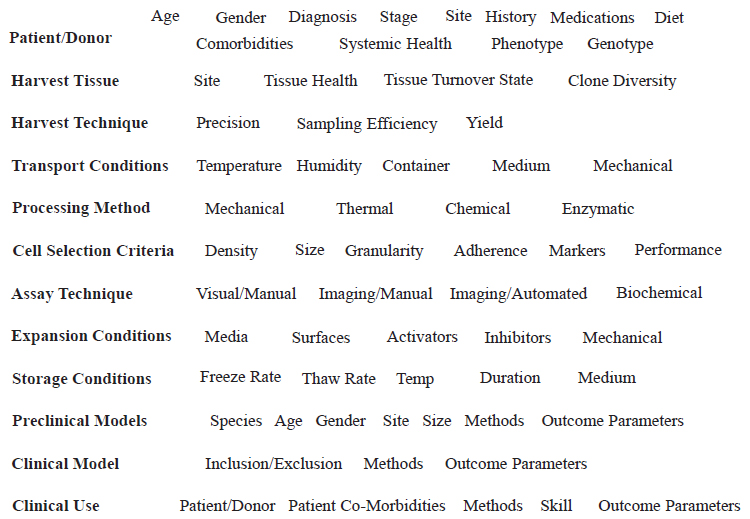
SOURCE: George Muschler, National Academies of Sciences, Engineering, and Medicine workshop presentation, October 18, 2018.
to harvest cells and effectively concentrate and select cells with preferred attributes, Muschler said. Stem and progenitors cells can be assessed based on assays of colony forming units (CFUs) under the assumption that each visible colony in a two-dimensional or three-dimensional culture system was derived from a single colony–founding stem cell or progenitor and that the performance of the progeny of that founding cell provides a measure of the intrinsic biological potential of the original founding cell. However, colony counting based on subjective manual counting can be inaccurate and is often not reproducible, Muschler said.
To address the variability in colony counting, Muschler and his colleagues developed an automated image acquisition method. Automated imaging of large fields of view (LFOVs) can be accomplished by the capture of multiple images (often hundreds), which are then processed into one broader image of the culture dish. Through a combination of processing to remove the background (e.g., eliminating apoptotic debris, lint, dish aberrations) and identifying cells and groups of cells with specific features, CFU assays can be performed reproducibly regardless of the person performing the assay, Muschler said. This approach enables the use of robust imaging
data that are not quantifiable using manual observation (e.g., cell density, morphology, and expression of cell markers). Working with ASTM International (which develops voluntary consensus standards), Muschler and his colleagues developed a standard test method that defines key parameters, such as the necessary resolution for the images, nomenclature for identified objects, and metrics.2 Taking the method a step further, Muschler and his colleague Ed Kwee, currently of the National Institute of Standards and Technology, added robotic features for colony management (e.g., changing culture media and selecting and moving cells).
The concept and methods of the quantitative imaging and cell and colony picking that Muschler described have been applied in orthopedics and other fields. Inter- and intra-observer variation in the counting of hematopoietic stem cells were considered by Muschler’s team. Hematopoietic colonies are traditionally counted by placing the culture dish over a gridded scoring dish and counting the number of colonies in each square. In a published study, three technicians, each with at least 2 years of experience and all trained by the same person, were asked to count and define colonies by type in standardized LFOV images (Powell et al., 2007). There was surprisingly poor agreement between these expert counters on both the presence and the absence of a colony and the type of colony. There were also many colonies that were counted by the automated system that were not counted by the technicians (e.g., colonies that were too small or had an unusual phenotype).
Sampling Efficiency of the Harvest Technique
Individual samples from the same subject and tissue may also vary. Muschler described an analysis of osteoblast progenitor cells in bone marrow aspirates. Each patient had four aspirates collected. The researchers concluded that 70 percent of the variation in cell counts was due to variation across patients, and 20 percent was due to variation between aspirates for a given patient (Muschler et al., 1997).
Efficiency of the Processing Technique
Different methods of rapid clinical cell processing for increasing the concentration or prevalence of cells of interest (e.g., density separation, selective retention, magnetic separation) have been evaluated. Selective retention based on the preferential adherence of stem and progenitor cells
___________________
2 ASTM F2944–12. Standard Test Method for Automated Colony Forming Unit (CFU) Assays—Image Acquisition and Analysis Method for Enumerating and Characterizing Cells and Colonies in Culture. See http://www.astm.org/cgi-bin/resolver.cgi?F2944 (accessed December 16, 2018).
to a porous matrix can accomplish a 4- to 20-fold increase in concentration of selected cells (Caralla et al., 2012). It is possible to select clones that proliferated faster and made more bone using magnetic separation with hyaluronic acid as a marker for selection (Caralla et al., 2013). However, methods other than density separation have been slow to move into clinical development because of the high cost of getting FDA approval, Muschler said.
Clone Diversity, Markers, and Performance-Based Cell Selection
When considering the intrinsic heterogeneity of the potential cell sources for use in cell therapy, Muschler said that it is important to recognize the diversity of stem and progenitor cell compartments that may be capable of proliferating and differentiating into a desired tissue.3 Current methods of culture expansion generally fail to select the cell or clone that will ultimately contribute to the end product. Muschler evaluated the reproducibility of traditional competitive expansion for the fabrication of mesenchymal stromal cells (MSCs) from bone, bone marrow, and fat in 8 patient subjects. Analysis by flow cytometry revealed “far more variation than reported,” Muschler said. He explained that the outcome of traditional culture-expanded populations is dependent on the mix of cells in the starting product. All of the adherent culture-expanded cells expressed the traditional MSC markers, he said, but these markers are not a measure of quality. The variation in other markers associated with multipotent potential (“stem-ness”) was large (a factor of 20–1,000) (Qadan et al., 2018). Muschler said that this variation should be embraced and explored and that it will likely provide valuable information for predicting and optimizing cell performance.
Quantitative LFOV imaging not only enables the study of clone diversity, it also enables the strategic selection of cells for fabrication based on in vitro performance. Performance-based selection can be a powerful tool for selecting clonal populations for preferential expansion and for identifying cells and clones that are undesirable and that should be removed or depleted.
Another use of LFOV imaging is to look back in time to identify the attributes of colony-founding stem and progenitor cells using video-microscopy starting from day 1 in culture. Muschler and colleagues are now working to characterize the process of performance-based selection in the fabrication of MSCs as well as iPSCs, using data that connect early cellular attributes to downstream performance.
___________________
3 This text was revised after the prepublication release.
THE ROLE OF PRESERVATION IN THE VARIABILTY OF REGENERATIVE MEDICINE PRODUCTS
The supply chain for regenerative medicine products, Hubel said, is unique in that the source material is cells collected from a living donor (or occasionally a cadaver). The cells are processed in a different location from their collection, she said, and they are administered at yet a third location. Time and distance separate each step along the way, and the product must remain viable and functional as it traverses the supply chain to its final destination. This is in contrast to a drug or other common therapeutics that are manufactured in a factory, shipped to a pharmacy, and dispensed by a pharmacist to the patient. Because of the unique pathway that regenerative medicine products must take, preservation is critical, she said. In fact, when preserving regenerative medicine products, she said, “the process is the product.” What happens along the path influences the quality of the outcome for the patient, and understanding the scientific basis for each step in the pathway is critical for preventing poor patient outcomes.
Cryopreservation protocols can be thought of as consisting of six key steps, Hubel said: pre-freeze processing, the introduction of solution, freezing, storage, thawing, and post-thaw assessment. Variability at any of these steps can contribute to variability in the overall process. In the interest of time, Hubel focused on just three of the six steps: pre-freeze processing, storage, and post-thaw assessment.
Pre-Freeze Processing
The quality of the starting product greatly influences the quality of the final product, Hubel said. Specifically, post-thaw recovery is influenced by the pre-freeze processing. The first step for many regenerative therapies is the collection of the cells. The sources of variability in the collection process include, for example, the source of the cells, the type of container they are collected in, anticoagulants and additives, processing delays, holding temperature, and centrifugation speed. When the cells are collected via apheresis there are additional sources of variability, Hubel said, such as the anesthesia used, the collection technique, the volume collected, filtration, and cell separation.
Proper annotation is very important for being able to manage these many sources of variability, Hubel said. Biospecimens should be annotated as rigorously as possible, she said, referring participants to the Standard Pre-Analytical Code from the International Society for Biological and Envi-
ronmental Repositories and the AABB standards for their guidelines on the efficient and effective annotation of human biospecimens.4
After collection, the tissues or cells are often extensively manipulated. The processing steps can include the isolation of cells, culture and expansion, the selection of subpopulations, genetic modification, and methods that can involve many centrifugation and washing steps. “These are manipulations that can change the cells and their ability to respond to the stresses of freezing and thawing,” Hubel said. As an example, she said that hepatocytes are collected from liver tissue using an aggressive digestion process that causes the cells to become hypoxic and stressed. Culturing the cells in a highly oxygenated environment and promoting cell-to-cell contact can improve the health of the cells and improve post-thaw recovery, she said.
Another example that she offered involves the processing of mesenchymal stem cells. There are not yet standard protocols for the cultivation of mesenchymal stem cells, Hubel said, and some cultivation practices can actually alter the phenotype of the cell, often shifting some of the cells to a senescent phenotype. Both normal and altered phenotype cells survive the freezing and thawing process, which is important because the senescent cells have an undesirable, inflammatory phenotype, she said.
Storage
After pre-freeze processing, a cryopreservation solution is introduced, and the cells are frozen and stored. The shelf life of the product is affected by the storage practices used. Any time that someone enters a cryogenic storage dewar, pulls out a rack, removes a box from that rack, opens the box, and removes their vial of interest, that picked vial and all of the “innocent vials” in the box that will be returned to the dewar experience a transient warming. Innocent vials might stay in cryostorage for years and could experience hundreds or even thousands of transient warming event cycles before they are removed for use, Hubel said. Transient warming events can lead to sub-lethal damage to the cells, she continued, and being exposed to too many warming events can ultimately prove lethal. Using the rate of apoptosis (programmed cell death) as a biomarker makes it possible to monitor the condition of the cells and provides an indication of the storage conditions, she said.
There are several approaches that can be used to limit this type of variability, Hubel said. First, those working in the cell repository must be properly trained. While this might seem obvious, she said, she has observed
___________________
4 For more information on annotation standards, see https://www.isber.org/page/SPREC (accessed December 16, 2018) and http://www.aabb.org/sa/Pages/Standards-Portal.aspx (accessed December 16, 2018).
people pulling out racks from cryostorage and then being distracted while the boxes sit out. Another approach is to limit access and the frequency of access to the cryostorage (e.g., once per day or once per week). There is also now commercially available robotic technology that can retrieve a sample without subjecting innocent vials to transient warming, she said.
Post-Thaw Assessment
Once a sample is removed from storage, it is generally shipped to where it will be thawed and then used. Post-thaw assessment is essential in order to characterize the cells before further processing. “It is extremely easy to do post-thaw assessment poorly,” Hubel emphasized. In particular, assessing the viability of a cell that has been frozen and thawed is different from assessing the viability of cells pre-freeze, she said. Most cell viability assays interact with the cell membrane, but the membrane of a cell that has been frozen and thawed is structurally different, she continued. This results in discrepancies in viability measurements between fresh and thawed cells.
Hubel described four main sources of variation in the post-thaw assessment of cells. The first is failing to calculate cell loss due to lysis. A thawed vial contains cells that are intact and viable, cells that are intact but dead, and the remnants of cells that have lysed. This third population is often ignored and can result in an artificial bias toward a higher viability count, she said. The next source is failure to account for the effects of post-thaw apoptosis. Viability post-thaw can vary over time, Hubel said, and viability assays should be timed consistently. Post-thaw apoptosis of immune cells, for example, can result in losses of up to 50 percent, she said. The third source of variability is the failure to optimize assays for frozen and thawed cells. A classic case example is hematopoietic stem cells, she said. There are many published, peer-reviewed papers that describe the number of recovered CD34-positive cells post-thaw as being more than 100 percent of the original number. Stem cells are clearly not proliferating in liquid nitrogen, she said. Instead, what is happening is that the cells are being counted using flow cytometry protocols that have not been optimized for frozen and thawed cells. Forward and side scatter of frozen cells by flow cytometry (indicative of the size and internal complexity, respectively) is distinctly different from the forward and side scatter of a cell that has not been frozen and thawed. Finally, using a single measure, or a measure that does not correlate with function, can lead to variability in post-thaw assessment. In summary, Hubel said, “seemingly subtle changes can have a profound effect on post-thaw recovery.”
Additional Variability Concerns
Two additional points regarding variability were raised by Hubel for consideration. The first was related to a study of the controlled rate freezing of a batch of 40 vials versus a batch of 80 vials. Not surprisingly, she said, there was less variability in temperature in the freezer during the freezing of the 40-vial batch. It is a simple matter of heat transfer, she explained, and larger batches will naturally have greater variability in temperature in the controlled rate freezer chamber. On one hand, it is desirable to perform quality and safety control testing on larger batches of vials and thereby reduce costs; on the other hand, larger batches can encounter more variability during the freezing process.
The second concern Hubel raised was protocol drift. It is not uncommon to hear some variation of “we do it this way because that is the way we do it,” she said. In fact, many organizations do not understand why they are following a protocol a particular way. This lack of understanding can lead to minor alterations in protocols that can have a large impact. A common example, she said, involves checking the liquid nitrogen dewar. A protocol may say to check the dewar every day and log the status. After checking every day for 6 months with no problems, someone may then decide it can be checked every other day . . . then once per week . . . then every 2 weeks. Everything is fine, Hubel said, until the day that the dewar thaws and the product is lost. The main ways to manage protocol drift are to have protocols in place, proper training, auditing, and proficiency testing, she said. Human-based sources of variability can only be eliminated or reduced through having quality systems in place.
Resources
In closing, Hubel referred participants to the resources available from the University of Minnesota’s Biopreservation Core Resource (BioCoR).5 These include short courses on best practices in preservation, a monthly newsletter, a library, hands-on training, and other materials.
DISCUSSION
Cryopreservation
In some cases, there may be alternatives to cryopreservation that could be used in situations when the time between collection and manufacturing is very short, Hubel said in the discussion session. Umbilical cord blood is
___________________
5 Available at https://www.biocor.umn.edu (accessed December 16, 2018).
generally shipped fresh to a location where it is banked, she said, and it is well known what is needed to maintain viability at 4°C during transport. Cord blood is typically young, healthy material, she noted. Less is known about the short-term storage requirements of regenerative medicine products, and there is an opportunity for future research, she said, especially on the mechanisms behind the loss of cells during short-term storage (e.g., effects of sedimentation, reactive oxygen species).
The impact of starting the cryopreservation process with cells or tissues that have already been frozen is not clear. Because patient material is collected at a range of different locations, it might be necessary to freeze cells right after apheresis, Hubel said. Any additional freezing step will contribute to losses in viability. At this time there are no published studies on the downstream effects on the final product. In some cases, Fesnak suggested, cryopreservation following apheresis might be beneficial. He described a case where multiple fresh apheresis samples destined for CAR T cell manufacturing were not successfully cultured (no growth of T cells). Small-scale aliquots of leftover cryopreserved apheresis product were then thawed and cultured, and there was T cell growth. There were several differences identified between the fresh and frozen samples, and Fesnak specifically noted that the myeloid-derived suppressor cell population was absent after cryopreservation, as were red blood cells.
Epigenetic differences may play a role in variability, Fesnak said, and this role will be a very important issue to address. Another workshop participant postulated that some of the variations observed during cell culture or the freezing and thawing process might be influenced by epigenetic changes. Hubel mentioned the epigenetic changes resulting from exposure to dimethyl sulfoxide (used in cryopreservation). The preservation process is selective for cells that are resistant to cryopreservation, she noted. This process-related selection may have clinical consequences, as some studies have shown that some types of committed progenitor cells are depleted by freezing.
Cell Surface Markers
It will be important to study the distribution of protein markers on a cell—whether clustered or separated—and whether variability in this distribution matters for the function of the cell, Hubel said, but no one at her institution has yet studied this issue in frozen and thawed cells. Cell markers looked at by Muschler’s team have been associated with culture-expanded cells, he said, noting that there are challenges in using flow cytometry to identify cell populations that constitute 1 percent or less of the total population. After they had run more than 100 early samples, Muschler said that his team was still very dissatisfied in terms of being able to standardize
the flow cytometric analysis, and they chose not to continue. Instead, they focused on finding and characterizing the founding cell of the adherent colony, as discussed above. The markers used for characterization were the traditional mesenchymal stem cell markers that are expressed in culture-expanded cells (CD105, CD73, and CD90). Muschler said that his team found that expressing one or more of those markers had a positive predictive value of only 7 percent with regard to whether a given cell would create a colony. There were large variations in marker expression by the founding cells, he said, and it might be that the markers are useful for sorting cells by flow cytometry and for enriching cells but that the presence of the markers might not be predictive of the patient outcome.
This page intentionally left blank.
















