3
Global Progress to Prepare for the Next Influenza Pandemic
Following the keynote, the pre-workshop event featured a series of presentations that highlighted global progress in driving science, public health, and global governance to prepare for pandemic influenza. Suzet McKinney, chief executive officer of the Illinois Medical District, moderated the panel. In the first presentation, Yoshihiro Kawaoka, professor of virology at the University of Wisconsin–Madison, focused on scientific advances—particularly in virology—that can contribute to preparing for and countering the threat of an influenza pandemic. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID) at the U.S. National Institutes of Health (NIH), explored the challenges researchers have faced and the progress they have made on the path toward developing a universal influenza vaccine. David Fidler, professor of law at Indiana University Bloomington, examined how global governance has bolstered national and international preparedness for pandemic influenza. Finally, Jacqueline Katz, deputy director of the Influenza Division at the U.S. Centers for Disease Control and Prevention (CDC), described how the One Health approach can be applied to strengthen preparedness for influenza pandemics.
SCIENTIFIC ADVANCES IN COUNTERING PANDEMIC INFLUENZA
Yoshihiro Kawaoka, professor of virology at the University of Wisconsin–Madison, began the panel presentations by exploring how scientific advances have helped counter the threat of pandemic influenza
starting at the molecular level. In particular, he traced how the expanding frontiers of science and research techniques can be used to identify the pathogenicity of influenza viruses and assess their pandemic potential in nonhuman reservoirs.
Scientific Assessment of Influenza Viruses
Kawaoka described how modern research techniques have been applied to investigate the influenza A virus that caused the 1918 influenza pandemic. The influenza A virus contains two surface proteins, hemagglutinin (HA) and neuraminidase (NA). Specific influenza strains are named based on the properties of those two proteins and are classified as H1 through H18 and as N1 through N11—all of which reassort into subtypes (e.g., H1N1, H5N1, H7N9). In 1997, researchers sequenced the entire genome of the 1918 influenza virus, and in 1999, the development of the reverse-genetics technique allowed researchers to recreate influenza viruses from cloned cDNA with the goal of shedding light on the exceptional virulence of the 1918 virus. This may inform predictions of future influenza pandemics by helping to explain how these strains emerge and what genetic features contribute to virulence in humans (Taubenberger et al., 1997, 2005; Neumann et al., 1999; Reid et al., 1999, 2000, 2002, 2004; Basler et al., 2001).
Histopathology of the 1918 Virus
Kawaoka then described how these scientific advances have been used to investigate why the 1918 virus was deadlier that other pandemic influenza strains. For example, using macaques, one study compared the pathogenicity of the 1918 pandemic virus to that of the 2001 seasonal influenza virus (Cillóniz et al., 2009).1 Within 8 days, every macaque infected with the 1918 virus had become so ill that it had to be euthanized. Histopathological studies revealed mild inflammation in the lung tissue of macaques infected with the 2001 seasonal influenza virus. However, the lungs of macaques infected with the 1918 influenza virus were filled with fluid and inflammatory cells—the same histopathology found in human victims of the 1918 strain. Kawaoka explained that the 1918 virus is unusually pathogenic because it replicates inside human lungs, and seasonal influenza viruses do not.
___________________
1 Kawaoka noted the 1918 influenza virus is the only influenza virus that is lethal in nonhuman primates.
Airborne Transmissibility of Influenza Viruses
All influenza viruses originate from wild waterfowl, said Kawaoka, but the viruses can infect many different animal species. This makes it possible to assess the pandemic potential of influenza viruses in nonhuman reservoirs. Understanding the airborne transmissibility of influenza viruses in nonhuman animals helps to determine the potential for certain strains to cause pandemic influenza in humans. He noted that researchers are particularly interested in the H5N1 and H7N9 subtypes because of their pandemic potential.
In 1996, researchers first identified the H5N1 virus in geese in southern China. In 2005, a major H5N1 outbreak among wild waterfowl in that region spread the virus to Europe and Africa. The resulting influenza outbreak caused 450 deaths among more than 850 infected people (WHO, 2014). Kawaoka explained that, to date, humans have only sporadically been infected with H5N1 from bird species, and human-to-human transmission has been limited. However, the H5N1 virus could cause a pandemic if it mutated and developed effective human-to-human airborne transmission. Researchers have used ferret studies to test the airborne transmissibility of the H5N1 virus, and although airborne transmission did not occur among ferrets, four amino-acid mutations in one of the HA genes were transmitted via respiratory droplets (Herfst et al., 2012; Imai et al., 2012).
Kawaoka expressed that the H7N9 virus is another pathogen of concern. It was first identified in China in 2013, has since infected many humans each winter, and has reached a total of 1,567 confirmed cases as of 2018 (FAO, 2018). When the Chinese government began vaccinating poultry in September 2017, the number of human cases of H7N9 dropped dramatically. However, between 2013 and 2016, H7N9 viruses mutated from nonpathogenicity in chickens to high pathogenicity in chickens; humans have since become infected with the highly pathogenic form. Researchers have evaluated the airborne transmissibility of both the low-pathogenic and highly pathogenic forms of the H7N9 virus. One study used ferrets to compare the airborne transmissibility of the low-pathogenic H7N9 virus, the H5N1 virus, and the 2009 pandemic influenza virus. The 2009 virus was transmitted via respiratory droplets among all three animal pairs in the study, the H5N1 virus was not transmitted in any of the animal pairs without mutation, and the low-pathogenic H7N9 virus was transmitted via respiratory droplets in one of the three animal pairs (Watanabe et al., 2013). Growing concern among researchers emerged with the publication of results from another ferret study, which found that the highly pathogenic H7N9 virus was also highly transmissible among ferrets via respiratory droplets; the highly pathogenic H7N9 virus was transmitted
in three of four animal pairs in the study, and the virus was found in the brains of infected ferrets (Imai et al., 2017).
Scientific Advances in Influenza Control
Kawaoka continued his presentation by discussing how recent scientific advances have been made in attempts to develop anti-influenza drugs and more efficacious vaccines. For example, certain antiviral drugs for influenza called neuraminidase inhibitors (such as oseltamivir) have been created to target neuraminidase, the three-dimensional protein on the surface of the virus. Another new anti-influenza drug, a polymerase inhibitor named baloxivir morboxil and marketed as Xofluza, has also been approved by the U.S. Food and Drug Administration in October 2018. However, Kawaoka said, the need for better antiviral compounds was underscored by the emergence of the oseltamivir-resistant H1N1 virus during 2007 and 2008 and its global spread during 2008 and 2009. Kawaoka suggested prioritizing further scientific research into the biology of the influenza virus in order to develop more efficacious countermeasures.
PROGRESS TOWARD A UNIVERSAL INFLUENZA VACCINE
Anthony Fauci, director of NIAID at NIH, presented a case for the need to develop a universal influenza vaccine and sketched a pathway for making that vaccine a reality. To contextualize the need for a universal influenza vaccine, Fauci identified several challenges related to the current state of influenza vaccinology and explained how they would be ameliorated by the development of a universal influenza vaccine (see Box 3-1).
Challenges in Influenza Vaccinology
Fauci noted that current seasonal influenza vaccines are not consistently effective. According to estimates by CDC, the effectiveness of seasonal influenza vaccines peaked at 60 percent in 2010 and 2011 but dipped as low as 10 percent in 2004 and 2005 (see Figure 3-1) (CDC, 2018d). He said that the need to improve seasonal vaccines is illustrated by the 2017–2018 seasonal-influenza season, which was the worst on record in recent years: There were 80,000 reported deaths,2 almost 1 million hospitalizations, and around 49 million symptomatic illnesses (CDC, 2018c). During this season, vaccine effectiveness reached only 40 percent overall and was as low as 25 percent for the circulating H3N2 strain.
___________________
2 The typical number of influenza deaths in a season ranges between 12,000 and 56,000.
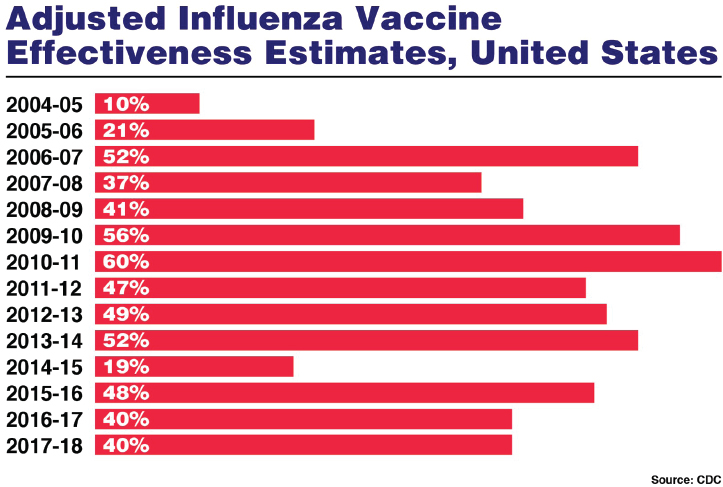
SOURCES: Fauci presentation, November 26, 2018; CDC, 2018d.
Fauci explained that when influenza pandemics occur, post hoc development of vaccines is often ineffective in response to the pandemic. During the 2009 H1N1 pandemic, for example, the first human outbreak occurred in March and began to spread worldwide by April. Egg-based vaccines take approximately 6 months to develop and produce, so public health officials presumed that the vaccine would be ready by the typical peak of influenza season in December and January. The vaccine was barely available when the pandemic peaked, and by the time vaccine doses were finally ready to administer, the pandemic had waned; only 90 million doses were used out of the 162 million doses that were produced.
Fauci said that the current vaccinology practice of “chasing after” prepandemic influenza is costly and ineffective. For example, influenza experts projected that the 2005 H5N1 outbreak would be a major event, and the United States president at that time requested $7.1 billion for pandemic preparedness, including the development of a vaccine; however, the outbreak never reached the pandemic level in humans (CDC, 1997; Lister, 2007). In 2013, significant investment was channeled into developing, producing, and stockpiling a vaccine for the H7N9 strain. However, the H7N9 virus circulating by 2017 had mutated, and the 2013 vaccine no longer provided adequate protection. This required developers to start chasing prepandemic influenza vaccines anew (Branswell, 2017). Fauci argued that the large investments would be better invested in efforts to develop a universal vaccine rather than strain-specific ones.
Furthermore, Fauci suggested that vaccine manufacturing techniques need to transition beyond traditional egg-based methods of vaccine production and toward cell-based technologies and cutting-edge platform technologies. Once a virus is selected, egg-based vaccine development and production take about 6 months before vaccinations can begin. Given the lead time required to have a vaccine ready for winter seasonal-influenza seasons, he explained, virus selection needs to occur in February or March. However, even if the appropriate virus were selected, the virus could change during that 6-month period—which would decrease the effectiveness of the vaccine being developed. This occurred from 2014 to 2015, and the seasonal vaccine’s effectiveness only reached 19 percent (CDC, 2018b). Another challenge for egg-based vaccine-production methods is that viruses can adapt within the egg environment in ways that contribute to decreased effectiveness. For example, from 2016 to 2017, the H3N2 virus selected was a good match for the eventual seasonal virus. However, it mutated to grow more effectively in eggs, and the effectiveness of the produced vaccine was reduced.3 That same year, similar mutations were not observed in vaccines produced using cell-based production techniques.
___________________
3 The mutation occurred in an area of the protein that is the epitope for protection on the head of the HA; this compounded the effectiveness problem.
Fauci argued that a universal influenza vaccine would provide more effective protection when the next major influenza pandemic strikes (Paules and Fauci, 2018; Paules et al., 2018). However, he added that producing a universal vaccine would require advanced vaccine manufacturing techniques such as cell-based production and new platform technology. Fauci explained that researchers are currently pursuing new platforms to develop a universal influenza vaccine. These include recombinant proteins, viral vectors (e.g., cold virus/adenovirus), virus-like particles (i.e., non-infectious and devoid of genetic material), nanoparticles (with protein attached on the particle), and genetic immunization (DNA and RNA vaccines).
Pathway Toward a Universal Influenza Vaccine
Fauci sketched the pathway toward developing a universal influenza vaccine (Paules et al., 2017). The ideal universal influenza vaccine, he said, would cover all influenza strains in Group 1 and Group 2 of influenza A and potentially strains of influenza B—although this may be aspirational. He said the most practical path to a universal vaccine would be to iteratively broaden the vaccine coverage over time (see Figure 3-2).4 The first step would be to develop strain-specific vaccines able to cover all circulating strains, to develop vaccines able to cover all strains within a single subtype, and to progressively develop vaccines able to cover multiple subtypes within a single group. The penultimate iterative step in developing a universal vaccine would be developing a pan-group vaccine able to cover all strains in Group 1 or in Group 2. He noted that a vaccine able to cover H3N2 strains and all of the H1N1 strains would be very good for seasonal influenza intervention. With the appropriate group specificity, it would also be useful against pandemic influenza.
NIAID has created a network, the Collaborative Influenza Vaccine Innovation Centers, to coordinate and attract expertise to universal vaccine development efforts.5 A common research goal is a vaccine that induces a response to the part of the influenza virus that does not mutate frequently. He explained that the influenza A HA protein consists of a head region and a stem region. The target of current influenza vaccines is the head region, which is where mutations that can reduce a vaccine’s effectiveness commonly occur. To address this problem, investigators have focused on the stem region of the HA as the target of universal influenza vaccines. The stem region is similar across different influenza strains, and it is the site of relatively few mutations each season. Approaches researchers have explored
___________________
4 A recent strategic plan and research agenda for developing a universal influenza vaccine delineated the multiple approaches in detail (Erbelding et al., 2018).
5 For more information on the Collaborative, see https://www.niaid.nih.gov/grants-contracts/influenza-vaccine-research-solicitation (accessed February 25, 2019).
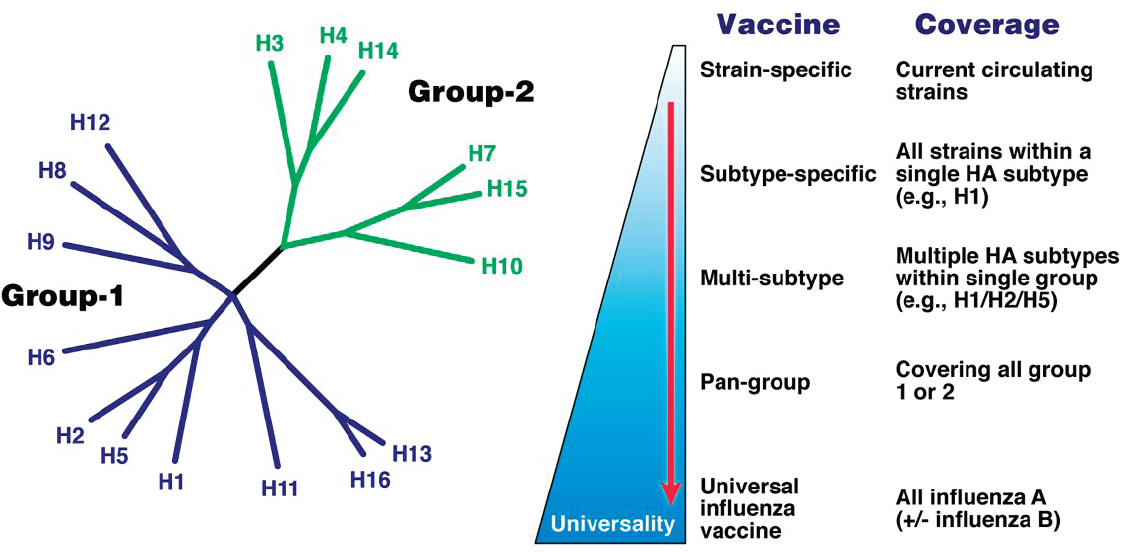
NOTE: HA = hemagglutinin.
SOURCES: Fauci presentation, November 26, 2018; Russell et al., 2008; Copyright (2008) National Academy of Sciences, U.S.A.; Paules et al., 2017; Reprinted from Immunity, Vol. 47/Issue 4, Catharine I. Paules, Hilary D. Marston, Robert W. Eisinger, David Baltimore, and Anthony S. Fauci, The pathway to a universal influenza vaccine, Pages 599–603, Copyright (2017), with permission from Elsevier.
include removing the HA head and then replacing it with an irrelevant head or placing the headless stem on a highly immunogenic nanoparticle.
A possible stumbling block to developing a universal influenza vaccine is the phenomenon of imprinting—or “original antigenic sin” (Francis, 1960). Fauci explained that a person’s immune system preferentially reverts to and responds well against the first influenza strain it encounters through either viral exposure or vaccination. For example, a person born in an era when H1N1 circulated was probably exposed to that strain first, so subsequent exposure to any other strains would induce an immune system response to the H1N1 strain. Although this does not prevent the immune system from mounting a response against the current strain, it does distract the immune response. This phenomenon explains why different influenza pandemics seem to spare different age cohorts.
GLOBAL GOVERNANCE TO BOLSTER PANDEMIC PREPAREDNESS
David Fidler, professor of law at Indiana University Bloomington, gave the next presentation on the role of global governance in bolstering preparedness for pandemic influenza. He noted that the existing set of global governance mechanisms did not directly result from the 1918 pandemic. In fact, it took roughly another 90 years before the main international legal instrument for addressing infectious diseases specifically included provisions for influenza. As a consequence, contemporary global governance structures have yet to be tested by an event as catastrophic as the 1918 influenza pandemic.
Fidler described the existing global governance infrastructure for pandemic influenza as a regime complex: a set of interlinked and overlapping institutions, rules, processes, and practices. Overall, this regime complex forms and supports a web of influenza preparedness and response activities that work at both functional and strategic levels. Functionally, governance mechanisms enable surveillance, virus sharing, scientific research, and vaccine development. Strategically, this web of activities integrates national security, economic interests, human rights, and ethical values into pandemic influenza governance. This web of governance interweaves political calculations about threats of pandemic influenza with public health capabilities to mitigate those threats. However, he noted that because it was so recently developed, this web’s sufficiency and resiliency need to be assessed in both political and public health terms.
International Health Regulations
Fidler explained that the World Health Organization (WHO) radically changed the global governance of serious health events when it revised the International Health Regulations (IHR) in 2005.6 Before this revision, the IHR reflected a narrow approach that originated in the 19th century, centered on a limited number of infectious diseases, such as cholera and the plague, for case reporting and response measures justified on public health grounds. However, this regime never included influenza as a reportable disease. The revised IHR broke away from this approach by requiring countries to notify WHO about any disease events that could constitute a public health emergency of international concern. Specifically, the revised IHR
- mandate the reporting of any case of human influenza of a new subtype B;
- require states to develop surveillance and response capacities;
- empower WHO to gather surveillance from nongovernmental authorities;
- authorize the Director-General of WHO to declare a public health emergency of international concern and to issue temporary recommendations; and
- seek to ensure the necessity of any public health measures that adversely affect trade, travel, and human rights.
The revised IHR were quickly subjected to its first influenza challenge in 2006 when Indonesia withheld its virus samples from WHO after an outbreak of H5N1 and asserted that the influenza virus-sharing system produced benefits such as access to vaccines that were mainly reaped by countries deemed more developed (see Chapter 7 for further discussions on virus sharing and benefit sharing). The revised IHR were not yet in force, and the question of whether they required countries to share influenza virus samples was contentious.
According to Fidler, WHO began negotiations to address influenza virus sharing and benefit sharing once it determined the IHR did not specifically govern that issue. The negotiations ultimately produced the Pandemic Influenza Preparedness (PIP) Framework in 2011, which, unlike the IHR, is not binding under international law. However, this innovative governance framework facilitates influenza virus sharing while also creating benefits that flow back to countries that share viruses. Fidler said the PIP
___________________
6 The International Health Regulations (2005) are a legal instrument binding all WHO member states that support the international community in the prevention of, control, and response to acute public health risks, including the spread of infectious diseases.
Framework has generally worked, but he has concerns about its future. In particular, he noted that virus sharing has declined since 2011 and that the PIP Framework has yet to be tested by a serious influenza pandemic (WHO, 2016a) (see Chapter 7 for a full list of concerns and challenges for the PIP Framework).
Fidler explained that the revised IHR were in full force in 2009 when the H1N1 pandemic provided its first real test. That pandemic was not as devastating as predicted, and the IHR generated overall benefits. However, a postpandemic review of the IHR revealed problems with country-level surveillance and response capacities and with questionable trade and travel measures (WHO, 2011). In contrast, Fidler described the 2014 Ebola outbreak in West Africa as a debacle for the IHR, and he suggested this outbreak was an example of the IHR’s inability to operate successfully during a large-scale, virulent influenza event. He said various post-Ebola efforts to strengthen the IHR are under way, but governance issues during that outbreak illustrate that the current IHR have not adequately prepared the world for a dangerous strain of influenza.
Progress in Global Influenza Governance
Fidler concluded by reflecting on whether progress has been made in global influenza governance. Despite their problems, he suggested the IHR and the PIP Framework facilitate important contributions to existing webs of governance around influenza preparedness and response. Other governance efforts support this web, such as WHO’s Global Strategic Plan to improve public health preparedness and response7 and the global activities mentioned in the pre-workshop event’s opening remarks (see Chapter 2). However, he said neither the political commitment nor the functional capacities starting at the international level currently exist to withstand a crisis. Fidler noted that the governance web for influenza preparedness and response “may turn out to be gossamer strands across the mouth of a cannon.” Strategic interest in influenza appears to regularly wax and wane as crises happen and then threats eventually fade from headlines. He underscored this pattern in the West African Ebola outbreak and the development of the revised IHR. He also drew the audience’s attention to ongoing uncertainty about the PIP Framework. He observed that the trend represents a political dynamic that handicaps functional work to build national and international preparedness and to increase response capacities. To transcend
___________________
7 WHO’s Global Strategic Plan was drafted in 2017 to meet the World Health Assembly’s request for a 5-year plan that listed global deliverables and timelines for member states to improve public health preparedness and response—in line with the IHR requirements. See http://apps.who.int/gb/ebwha/pdf_files/EB142/B142_10-en.pdf (accessed February 25, 2019).
this pattern, he said, present and future influenza governance efforts should use lessons from the 1918 pandemic—a time when no international governance around influenza existed. He also said governance efforts should be relentless about ensuring that the next severe influenza pandemic does not cause similar devastation.
A ONE HEALTH APPROACH FOR PREPAREDNESS
Jacqueline Katz, deputy director of the Influenza Division at CDC, discussed the benefits of a One Health approach for pandemic influenza preparedness.8 She explained that avian species and swine provide the reservoirs for the influenza viral genes that have contributed to past pandemics and will contribute to future ones. In the past century alone, four pandemics (those in 1918, 1957, 1968, and 2009) were caused by viruses with genes that originated from avian and/or swine species. The past three of these pandemics were caused by viruses created by reassortment—mixing genes within a common host. Katz reiterated that pandemics resulted when these viruses subsequently were able to infect humans and underwent sustained transmission in an immunologically naïve human population.
Increase in Novel Influenza A Viruses
According to Katz, the number of novel influenza A virus infections in humans has increased sharply over the past 20 years. These zoonotic infections are caused when an influenza A virus jumps from an animal source into humans; these viruses are antigenetically and genetically distinct from the seasonal influenza A viruses that circulate in humans. Ever-expanding virus subtypes have been derived either from avian or from swine species. The most recent influenza pandemic in 2009 was derived from a swine source, but that virus also contained genes of both human and avian origin. She explained that the 1997 H5N1 outbreak in Hong Kong was the first time researchers realized that a wholly avian influenza virus could infect humans, and this catalyzed the development of a One Health approach, which was able to quell the outbreak (see Box 3-2). She noted that the increasing number of zoonotic influenza A virus infections in humans will require ongoing laboratory and epidemiologic investigations carried out jointly by researchers from human- and animal-health sectors.
___________________
8 One Health is a collaborative, multisectoral, and transdisciplinary approach—working at local, regional, national, and global levels—that seeks to achieve optimal health outcomes by recognizing the interconnection among people, animals, plants, and their shared environments (CDC, 2019b).
Influenza Viruses with Pandemic Risk
Katz said the H7N9 influenza A virus poses the greatest risk for a human pandemic. The number of H7N9 cases currently far exceeds the number of H5N1 cases worldwide (see Figure 3-3). This is likely due to a confluence of factors, including a two-fold increase in the global population of poultry, a steady increase in the number of pigs farmed globally, and a steep increase in the number of people who travel globally (UNWTO, 2018). She added that increased and improved surveillance contributes to the increasing number of cases detected. The emergence of highly pathogenic avian-influenza H7N9 viruses in 2017—not seen in previous H7N9 waves—prompted the Chinese government to introduce poultry vaccination for H7 strains (Shi et al., 2018). By controlling the virus in the poultry population, the government essentially controlled the disease in humans. Another developing concern Katz identified was the emergence of variant swine influenza viruses, which have infected humans. The largest wave thus far occurred in 2012 when 300 people were infected with H3N2 variant viruses; researchers identified a close association between these infections and children who showed swine at agricultural fairs or participated in swine events at seasonal state fairs (Bowman et al., 2014). This pattern has continued, although specific numbers vary by year. She emphasized that
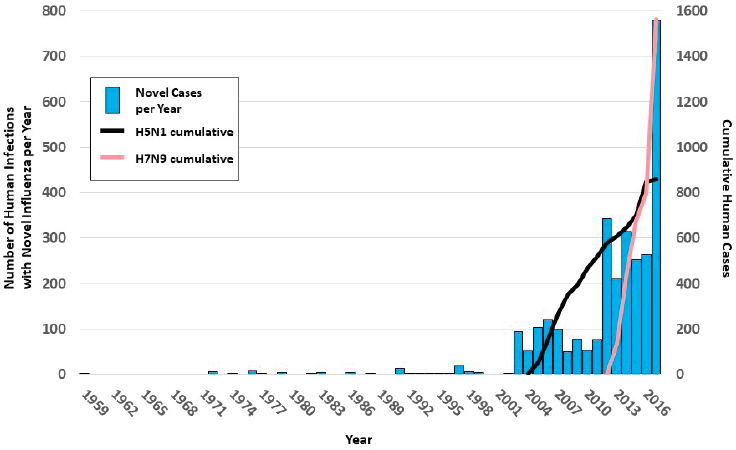
SOURCES: Katz presentation, November 26, 2018; data from Freidl et al., 2014.
strong collaboration between human- and animal-health technical groups will be needed to ensure pandemic readiness and to carry out pandemic risk assessment for H3N2 variant viruses.
World Health Organization’s Role in Tracking Viruses
Katz explained that the WHO Global Influenza Surveillance and Response System (GISRS) carries out year-round surveillance for seasonal viruses and for novel influenza A viruses that cause zoonotic infections (see Chapter 6 for more on GISRS). GISRS collaborates closely with OFFLU,9 a joint animal-influenza expert group formed by the Food and Agriculture Organization of the United Nations (FAO) and the World Organisation for Animal Health (OIE). WHO Collaboration Centers provide support for the antigenic and genetic characterization of animal viruses. This inter-sectoral collaboration creates the capacity to track viruses in animals and the emergence of zoonotic infections in humans. She noted that certain genetic markers are associated with enhanced mammal adaptation and greater transmission potential. Researchers follow these genetic markers
___________________
9 For more information on the OFFLU network, see http://www.offlu.net (accessed January 31, 2019).
in emerging viruses to determine whether they acquire additional adaptive changes.
The collaborating groups meet twice per year to analyze data about the human epidemiology of zoonotic infections, virus distribution and ecology in animals, and the joint genetic and antigenic characterization of viruses from both human and animal sources. Katz explained that these biannual analyses provide the scientific rationale for developing new candidate vaccine viruses (CVVs) against emerging and re-emerging influenza viruses that have pandemic potential. WHO Collaborating Centers and Essential Regulatory Laboratories have so far generated more than 60 CVVs against H5, H7, and H9 avian subtypes and H1 and H3 variant viruses (WHO, 2018a). Most CVVs are egg-based, inactivated vaccines that are ready for use if needed. Many have been used to produce pilot lots for human clinical vaccine trials, which have provided information about how the vaccines work in humans, including what doses would be needed and whether adjuvants would be required. She added that some of the CVVs have also been used by veterinary vaccine producers.
Approach to One Health in the United States
Katz described how the One Health approach has been applied specifically to influenza in the United States. Since 2007, human infection with novel influenza A viruses has been a nationally notifiable disease; such cases trigger an investigation by CDC and/or state health officials. Samples of any associated animal infections are sent to the U.S. Department of Agriculture (USDA) National Veterinary Services Laboratories for characterization. CDC and USDA collaborate by exchanging gene sequencing data and epidemiologic information about both human and animal viruses; they have also co-developed protocols for notification of and response to potential zoonotic events detected in either sector. Finally, CDC and USDA have jointly developed educational and guidance materials about variant influenza viruses and the outbreak-investigation process for both animal-sector producers and the public, including targeted materials for 4-H youth development and mentoring groups.
According to Katz, the expansion of next-generation sequencing capacity has strengthened One Health surveillance and readiness in the United States. Field tests of new mobile technology for influenza sequence analysis (e.g., the Oxford Nanopore MinION) were conducted at the World Pork Expo, a major hub for influenza virus transmission among swine. Real-time genetic sequencing indicated that swine viruses from the Expo were similar to human viruses detected at state fairs months later. Katz suggested that this technology could potentially allow CVVs to be prepared in advance from genetic sequences found in swine earlier in the season; it
could also be used to carry out real-time sequencing in a human outbreak setting.
Global Challenges for the One Health Approach
Katz considered some global challenges for the One Health approach to influenza preparedness and response. Because of trade and export implications, surveillance and/or reporting of influenza in animals is insufficient in many regions of the world. Sharing influenza specimens and viruses with pandemic potential is a complex process that involves different regulations across multiple sectors and countries, and this sometimes delays risk assessment and response timeliness. Katz also noted that the PIP Framework does not include animal influenza viruses, which adds to the list of concerns Fidler outlined earlier about the PIP Framework.
Finally, Katz characterized CDC’s Influenza Risk Assessment Tool (IRAT) as a global health evaluation tool to prioritize pandemic preparedness activities. It is used to assess potential pandemics for the risk of novel influenza-virus emergence in humans, including human-to-human transmission, and of public health impact and its severity.10 Furthermore, it can enhance readiness activities related to the development, stockpiling, and deployment of diagnostics, reagents, vaccines, and antivirals. The tool employs a One Health approach in which CDC experts work closely with USDA agricultural experts.11 Of the risk assessments conducted in recent years, H7N9 continues to pose the highest risk both for emergence and for impact, Katz reiterated. It is followed closely by H5 subtype viruses.
DISCUSSION
Suzet McKinney, executive director and chief executive officer of the Illinois Medical District, summarized her key points from the panelists’ presentations:
- Kawaoka built a case not only for science to provide insight into the biology of influenza viruses but also for research and response communities to focus on lessons learned from prior pandemics and the threats posed by viruses such as H5N1 and H7N9.
___________________
10 IRAT evaluates 10 elements to develop a risk assessment score. Virus-related elements are genomic variation, receptor binding, transmission in laboratory animals, and antivirals and other treatment options. Population-related elements are existing population immunity, disease severity and pathogenesis, and antigenic relationship to vaccine candidates. Ecology-related elements are global geographic distribution, infection in animals, human risk of infection, and human infections and transmission.
11 For more information on IRAT, see https://www.cdc.gov/flu/pandemic-resources/nationalstrategy/risk-assessment.htm (accessed January 31, 2019).
- Fauci emphasized that existing seasonal influenza vaccines are not consistently effective because they are often based on predictions about which prominent strains should be included. Difficulties around this process are compounded by an additional challenge: rapid virus mutation. He underscored the necessity of a shift toward new technologies for vaccine production—ones that enable vaccines to anticipate rapid mutations and that engender a more proactive approach to preparedness.
- Fidler explained that the IHR and the PIP Framework are situated within webs of activities that integrate public health capability, economics, and pandemic influenza preparedness, but these activities should also be viewed through a political lens to optimize their effectiveness in a crisis.
- From the One Health perspective, Katz described several ongoing global health challenges associated with preparedness, including insufficient surveillance and complexities around virus sharing, and explained ongoing efforts to assess those global risks and to move preparedness forward.
To start the discussion, McKinney asked Kawaoka about the current state of global readiness to identify new viruses with severe pathogenicity prior to an outbreak. Kawaoka replied that influenza pathogenicity is driven by the specific traits of each virus, but immunity in each affected population affects the size of the outbreak. For instance, the impact of the 2009 H1N1 pandemic is often considered to be relatively low, but the virus affected children more than it affected older people, who tended to have preexisting immunity. Kawaoka said that experimental settings can help researchers identify or predict the pathogenicity of a virus, but they cannot be used to predict the magnitude of an outbreak, which is shaped by this population-immunity factor.
Keiji Fukuda, director and clinical professor, School of Public Health, The University of Hong Kong, then asked the panel about ethical and safety concerns related to research that involves dangerous viruses. Fauci replied that oversight systems have improved during recent years. In the United States, the federal government exercises oversight only on federally funded work, but it has no control over research funded elsewhere. He suggested that difficult decisions about certain types of research—those related to naturally occurring virus mutations, for example—must be formally assessed to determine risks versus benefits to society. Fauci asserted that creating something that would never occur in nature is the only type of experiment that should be completely disallowed. Katz added that federally funded scientific research is subjected to careful and thorough risk–benefit analyses to ensure that the benefits of public health research outweigh the
risks. Ed You, supervisory special agent at the Weapons of Mass Destruction Directorate at the Federal Bureau of Investigation (FBI), commented that the government’s response to biological threats is multi-pronged: FBI works with partners, including CDC, USDA, state and local law enforcement, public health representatives, and state veterinary programs, to assess any unusual outbreaks and to determine if they are naturally occurring or intentionally released. At the same time, FBI is charged with protecting sensitive research and ensuring its security, as well as with protecting the people who conduct that work and their related institutions.
William Buchta, president of the American College of Occupational and Environmental Medicine, asked panelists how to preemptively create a geopolitical framework for equitably distributing the universal vaccine (when it becomes available) in a way that prevents hoarding. From a governance point of view, said Fidler, ensuring universal access would require a lengthy and complicated process that would probably not eliminate concerns about hoarding or guarantee that political promises will be fulfilled during a crisis event. Fauci added his prediction that hoarding would not be an issue if a truly universal influenza vaccine became available because mass influenza-vaccination campaigns would become as standard as mass campaigns for polio, measles, and smallpox. He noted that hoarding only occurs when there is an outbreak during which resources are limited.
Pia MacDonald, senior director of applied public health research at RTI International, asked the panelists where geographically animal surveillance should be prioritized in order for it to improve as a whole. Fauci remarked that animal surveillance needs to improve across the world, including in the United States. He noted that more robust animal surveillance would have detected swine influenza in the United States several months before the first human cases occurred. Katz said that resources for animal surveillance are far more limited than for public health surveillance in many countries, including the United States, because it is not a priority. She said, “If no one is looking for it, no one will know whether it is there.” She noted that if a virus is discovered in a country, that discovery may have negative implications for trade and export. She maintained that governments worldwide need to recognize the importance of animal surveillance in pandemic preparedness and to fund it commensurately.
Laurie Garrett, science journalist and founder of the Anthropos Initiative, asked the panelists about a notable tension between the potential for live-poultry sales in Asian markets to potentiate outbreaks and the negative environmental consequences of introducing a cultural shift toward selling processed, refrigerated poultry. She added that carbon dioxide emissions would likely skyrocket if every person in Asia had a personal refrigerator. Katz noted that people in many parts of China express strong cultural preferences for fresh poultry, which would make this type of
cultural shift difficult in the short term, but she added that changes in mitigation practices are occurring slowly in Mainland China. She was less optimistic about the prospect of introducing such cultural measures in other parts of Asia. Kawaoka said that culture is very difficult to change, so it is more feasible to introduce risk-mitigation strategies in order to change the system incrementally over a longer period of time. For example, officials in Hong Kong have implemented measures to increase surveillance for influenza viruses, to limit the saturation of viruses in wild poultry markets, and to eliminate more virus-prone bird species from the system than they currently do. Katz added that multiple layers of mitigations can be put into place, such as the measures taken in Hong Kong’s live-poultry markets to separate the general public from people who are involved in the slaughter process and who are therefore more likely to be exposed to viruses. From a political standpoint, Fidler said that influencing cultural behavior change is better suited to a regional rather than a global approach. Efforts to cooperate within Asian organizations initiated at the regional level tend to be more effective in creating functional, nonbinding approaches and for sharing best practices.
Regarding the topic of global collaboration and governance, Jerri Husch, a sociologist formerly with WHO, asked how stakeholders could move from talking about implementing interagency collaboration to making it a reality through dealing with its practical complexities. Fauci replied that the Global Health Security Network and the Global Health Security Agenda both represent progress in that regard. They are broadly aimed at improving surveillance and communication but also at facilitating real-time data sharing during a disease outbreak. Fidler said that international governance mechanisms are currently focused on the implications for genetic sequencing data, which are already being shared globally in real time through the IHR and the PIP Framework. He suggested that governance regimes should build information sharing into the design of rules and institutions so that collaboration would be integrated into the machinery of bureaucracy. Katz added that this work would require persistence and engagement from multiple sectors.
To conclude, Fukuda asked Fidler which area of global governance should be prioritized for improvement. Fidler suggested that instead of creating new global frameworks or initiatives, efforts should focus on existing components of the regime complex to improve and integrate governance operations. He said, “The concentration of political capital and economic capital should be put forward in ways that allow the incremental ratcheting up of resilience in the key pieces of the governance that are already in place—IHR, PIP Framework, and Nagoya.” He added that these governance mechanisms need to be integrated in a way that does not resurrect the viral-sovereignty controversy each time a dangerous outbreak occurs.
This page intentionally left blank.




















