6
A Spectrum of Considerations for Pandemic Vaccines
Session 1, part B of the workshop explored major lessons related to a spectrum of challenges for pandemic vaccines based on the 2009 H1N1 influenza pandemic. Moderator Jacqueline Katz, deputy director of the Influenza Division of the U.S. Centers for Disease Control and Prevention (CDC), explained that vaccination is the most effective method for preventing influenza-related complications. However, the development and delivery of vaccines is challenging, particularly during a pandemic, because vaccines need to be available in a timely manner to mitigate the height of illness. The 2009 H1N1 influenza pandemic, as an example, illustrates the complexities and challenges related to pandemic vaccines. In the United States and in Hong Kong, the first vaccines became available at the peak of the major pandemic wave, when it was largely too late for vaccines to provide protection. Closing this timing gap, Katz said, will require improvements in vaccine development, production, and distribution. During the 2009 pandemic, for example, challenges arose at almost every step of the vaccine process: strain selection, manufacturing, potency testing, regulatory approval, and clinical trials to determine dosing. After the vaccine became available, further challenges hampered the logistics of vaccine distribution and administration; post-vaccination safety issues also arose in some countries. Many countries entirely lacked access to vaccines, and vaccine donation programs that attempted to bridge this gap in equity also faced challenges from the lack of local-level infrastructure to accept, deliver, and administer vaccines (Broadbent and Subbaro, 2011; HHS, 2012). Panelists who had experience working with the World Health Organization (WHO), private-sector companies, nonprofit organizations,
and governments shared their perspectives on progress made over the past decade and remaining challenges and opportunities for pandemic vaccines.
The first panelist, Wenqing Zhang, manager of the Global Influenza Programme at WHO, described the role of global coordination in vaccine development and focused on the Global Influenza Surveillance Response System (GISRS). Karen Midthun, senior advisor of the Center for Vaccine Innovation and Access at PATH, illustrated the regulatory considerations of vaccine development. A perspective from the pharmaceutical industry on pandemic vaccine manufacturing was provided by Clement Lewin, associate vice president of research and development strategy at Sanofi Pasteur. Bruce Gellin, president of global immunization at Sabin Vaccine Institute, focused on the importance of vaccine timing in response to a pandemic. Finally, Steven Solomon, principal legal officer at WHO, surveyed some legal considerations related to vaccine response.
GLOBAL COORDINATION IN VACCINE DEVELOPMENT: A SNAPSHOT OF THE GLOBAL INFLUENZA SURVEILLANCE AND RESPONSE SYSTEM
The past century of infectious disease outbreaks and pandemics has underscored how many population health problems, such as influenza, must include international collaboration and coordination, said Wenqing Zhang, manager of the Global Influenza Programme at WHO. The international community did not begin taking action on this front until 30 years after the 1918 pandemic, when the WHO Constitution entered into force and when WHO approved the establishment of the Global Influenza Programme in 1948. Another influenza-specific milestone was the establishment of the Global Influenza Surveillance Network in 1952, which became GISRS in 2011.
Research, innovation, and global coordination are major components of vaccine development, said Zhang. The global public health response to influenza is driven by the constant evolving nature of influenza viruses. The prevalence of the West Nile virus in birds makes the eradication of influenza impossible, and little is known about the potential for an animal influenza virus to acquire human-to-human transmissibility and to emerge as a pandemic strain. Given these barriers, Zhang said the global approach to influenza response requires an internationally coordinated, step-by-step approach that spans every stage from surveillance to preparedness and response.
WHO’s role is to enable this global collaboration, she said, with the GISRS as an essential mechanism. The GISRS network, which includes institutions from 114 countries, tests around 4 million clinical specimens each year, including around 40,000 virus specimens shared by WHO
Collaborating Centers for advanced analysis (WHO, 2019b). For a vaccine to be effective, the viruses it contains must be updated regularly. She explained how this complicated updating process is facilitated by the GISRS system, with updates conducted twice per year for seasonal vaccines and occur on an ad hoc basis for pandemic and prepandemic vaccines. She described the seasonal influenza vaccine cycle as an effective public–private partnership. In other words, the vaccine cycle occurs across a very tight time frame that requires smooth, effective, and timely interaction among key players throughout the year in order for a seasonal vaccine to be market ready before influenza season, she explained. WHO assists in this process by facilitating communication and disseminating information among all stakeholders, including antigen manufacturers, regulatory agencies, and the GISRS.
Zhang highlighted three additional concepts that underpin effective influenza surveillance and control: timeliness, sharing, and partnership. Timeliness is important, she said, because the effectiveness of countermeasures depends on the extent to which their availability lags behind virus evolution. Sharing is relevant because an important virus strain could emerge anywhere in the world at any time, and the response to the virus must be global. Partnerships are necessary among the global teams working at every step in the process, from surveillance to preparedness and response. She said WHO works to foster collaboration among other sectors and initiatives in addition to vaccine manufacturers. GISRS plays a role in fostering trust and sharing, she added, and strengthening the GISRS system has the potential to save more lives when the next pandemic hits.
REGULATORY PATHWAYS
Karen Midthun, senior advisor of the Center for Vaccine Innovation and Access at PATH, described her experience in the United States during the 2009 pandemic as the director of the Center for Biologics Evaluation and Research (CBER) at the U.S. Food and Drug Administration (FDA). CBER has a long history of collaborating with WHO, both as a WHO Collaborating Center for biological standardization and as a WHO Essential Regulatory Laboratory for influenza vaccine. In vaccine-related matters, CBER works closely with the U.S. Department of Health and Human Services (HHS) and with HHS agencies including the Biomedical Advanced Research and Development Authority (BARDA), CDC, and the National Institutes of Health (NIH).
Midthun said that prior to the 2009 pandemic, much regulatory groundwork had already been laid: the Pandemic Influenza Plan (issued by HHS in 2005 and updated in 2017) (HHS, 2017); guidance on licensure of seasonal and pandemic influenza vaccines (issued by HHS/FDA/CBER
in 2007) (FDA, 2007a,b); and Guidelines on Regulatory Preparedness for Human Pandemic Influenza Vaccines (issued by WHO in 2007) (WHO, 2007a). After the seasonal influenza vaccine shortage in the United States in 2004, CBER used FDA’s accelerated approval program to license three additional seasonal influenza vaccines between 2005 and 2007. This contributes to pandemic responsiveness, she noted, because pandemic vaccine manufacturing is built on the seasonal vaccine infrastructure (Weir and Gruber, 2016).
Regulatory Pathways for Development of the 2009 Pandemic Vaccine
Midthun explained that some of the issues that occurred during development of the 2009 pandemic vaccine—such as the need for vaccine reference strains, reagents, and biocontainment procedures—were already expected because of insights from WHO’s pandemic preparedness planning. Other issues were not necessarily expected, such as initial low yields, difficulty making the vaccine from reassorted vaccine candidates, and difficulty making the antiserum needed for the vaccine’s potency test.1 From CBER’s perspective, Midthun said, a critical regulatory issue was finding the most expeditious pathway to make the pandemic vaccine available, preferably as a licensed product, though possibly under emergency use authorization.2 CBER determined it could license the monovalent 2009 H1N1 pandemic vaccine as a strain-change supplement to vaccine manufacturers’ existing licenses for seasonal influenza vaccines, which speeds up approval by not requiring new clinical data.3 Depending on their regulatory precedents, regulatory agencies in other countries and regions pursued different pathways. For example, the European Medicines Agency approved a core pandemic dossier and mockup for an adjuvanted H5N1 vaccine that switched the H5N1 antigen with the H1N1 antigen, as the most expeditious pathway to licensure (CHMP, 2008). Development of the 2009 pandemic vaccine involved extensive WHO-coordinated collaboration among regulatory agencies, manufacturers, and public and expert advisory
___________________
1 Potency testing is performed on vaccine lots to demonstrate the capability of the product to confer protective immunity.
2 An emergency use authorization allows for the use of an unapproved medical product or an unapproved use of an approved medical product under certain emergency circumstances declared by the HHS secretary, when there are no adequate, approved, and available alternatives.
3 This regulatory process allows new strains to be incorporated into the vaccine based on nonclinical, chemistry, and manufacturing control data; it is used every year to change the composition of seasonal influenza vaccines that target H1N1, H3N2, and B-lineage influenza viruses.
committees, Midthun said. Postmarketing safety surveillance also had to be expanded for the pandemic vaccines. Ultimately, it took 6 months to make the pandemic vaccine available, and the bulk of the licensed vaccine did not become available until after the pandemic had peaked (Al-Muharrmi, 2010).
Challenges and Progress in Pandemic Vaccines
Midthun said that the experience during the 2009 H1N1 influenza pandemic underscored several gaps in vaccine development and production, such as the need for faster identification of higher-yield vaccine candidates and for alternative potency assays. In the decade since, she noted that significant progress has been made on both those fronts. The 2009 experience also highlighted the need to develop alternative vaccines through newer technologies for more rapid manufacturing, Midthun added, including (1) cell-based and recombinant hemagglutinin influenza vaccines, (2) adjuvanted vaccines that provide for antigen-sparing, and (3) a universal influenza vaccine that provides broad and durable protection against a swath of influenza strains. FDA has since licensed a cell-based seasonal influenza vaccine, a recombinant hemagglutinin seasonal vaccine, an adjuvanted influenza vaccine for seasonal use, and an adjuvanted H5N1 vaccine. In the longer term, she noted, a universal influenza vaccine would provide the best preparedness for both seasonal and pandemic influenza, but this vaccine remains a work in progress (see Chapter 3 for more on opportunities and challenges related to universal influenza vaccines).
Seasonal influenza vaccine manufacturing capacity has increased worldwide since 2009, Midthun commented, which in turn has strengthened global capacity to manufacture, evaluate, and regulate vaccines. WHO has a prequalification process for vaccines, medicines, and devices already licensed or approved by various national regulatory authorities. This process allows countries to introduce vaccines on a more streamlined basis if they choose to do so. In response to the 2014–2016 Ebola outbreak in West Africa, WHO established a procedure for an emergency use assessment and listing, and a revised version is currently under way (WHO, 2015). This procedure could be a useful mechanism for making unapproved products (including vaccines) widely available in a pandemic setting, she suggested. However, the ultimate decision to use an unapproved product is made at the national level, she said, which requires having adequate infrastructure to allow such decisions to be made and supported.
MANUFACTURING CAPACITY AND PRODUCTION
Clement Lewin, associate vice president of research and development strategy at Sanofi Pasteur, provided a perspective from the pharmaceutical industry on manufacturing pandemic vaccines. He said that for the pharmaceutical industry, a pandemic is not business as usual; the industry is committed to contributing to the response and preventing the consequences of disease. Sanofi Pasteur, as one of the largest manufacturers of influenza vaccines using both egg-based and recombinant manufacturing technologies, and other vaccine manufacturers view pandemic preparedness not as a business opportunity, but as a public health challenge to which they can contribute, he stated.
To prepare for potential pandemics, Sanofi Pasteur is ready to produce as many influenza vaccines as possible and then to collaborate with public and private partners to distribute those vaccines. During the 2009 pandemic, for example, Sanofi made a substantial effort to make the vaccine available in 6 months, said Lewin. Currently, the company is working to improve its manufacturing processes by collaborating closely with BARDA and with other organizations in pandemic preparedness efforts. He added that Sanofi Pasteur maintains a year-round supply of eggs to ensure that pandemic production capacity is available when needed. The company also has experience in producing pandemic vaccines that can be tested both with and without adjuvant. Lewin was hopeful that forthcoming clinical data for H7N9 and H5N1 vaccines would help ensure that pandemic production capacity is available.
Lewin said Sanofi Pasteur works closely with WHO and other public health authorities on pandemic influenza activities, including vaccine development, licensing, and distribution. The 2009 pandemic demonstrated the importance of a history of collaboration among the vaccine manufacturing industry and government sectors. In 2009, Sanofi Pasteur’s prompt response was enabled by existing relationships with the government and by proactive investments made by both sectors. He explained that for vaccine manufacturers, the distribution process for pandemic vaccines is unusual because the distribution is determined by public health authorities based on their prioritization of certain populations. The pharmaceutical industry is partnering with national and supranational authorities on pandemic preparedness and response, he said, and the Pandemic Influenza Preparedness (PIP) Framework adopted in 2011 strengthens Sanofi Pasteur’s ability to respond to a pandemic (see Chapter 7 for more on the PIP Framework).
Pandemic preparedness has improved since 2009, with increased capacities and new technologies; however, Lewin said that staying ahead of the epidemic curve will require continued vigilance and investment in new
technologies. From Sanofi Pasteur’s standpoint, creating demand and uptake for seasonal influenza vaccines is the best way to ensure that pandemic vaccine capacity is available when it is needed. He predicted that increased coverage of seasonal vaccines will spur industry to build sustainable mechanisms that will benefit the delivery of pandemic vaccines. Across the pharmaceutical industry, it would be cost prohibitive and infeasible to create dedicated production facilities for pandemic vaccines that are only used once per decade or more. Thus, the development of platform-based technologies would enable standard manufacturing processes and facilities to be used for the production of nonpandemic vaccines as well. Creating an integrated fill-finish plan is another important consideration, he added. For U.S. manufacturers, current filling capacity is about 150 million doses, but around 600 million doses could be needed in the event of a pandemic (MacGregor, 2018; HHS, 2019). Finally, he suggested that optimizing regulatory pathways could help ensure that new vaccines are licensed as quickly as possible.
TIMING AND DEPLOYMENT OF VACCINES
Bruce Gellin, president of global immunization at Sabin Vaccine Institute, emphasized the importance of timing in vaccine response—both time to the first dose and time to the last dose. He reiterated that the process of developing and deploying vaccines is complex and also spans surveillance, research, development, licensing, recommendations, deployment, and impact measurement. He cautioned that because the current system relies on the same manufacturing infrastructure for both seasonal and pandemic influenza vaccines, this system in the event of a pandemic threat forces a difficult decision about when to halt a given year’s seasonal vaccine production and switch over to pandemic vaccine production. This choice must be made with minimal knowledge about how the pandemic threat might unfold but with full knowledge that switching production will compromise the next year’s seasonal vaccine supply.
Donation of Vaccines and Ancillary Products
Gellin explained that donation of vaccines and ancillary products is a complex endeavor, requiring financial support, coordinated bilateral support, and legal agreements covering vaccine donation and receipt (WHO, 2012). WHO can help coordinate the deployment of donated vaccines, which involves identifying countries that are eligible to receive the donations and ensuring that recipient countries have a deployment plan, so the vaccines do not go unused, particularly during a global shortage. Legal considerations and political concerns related to vaccine donation
can contribute to delays in vaccine timing, Gellin said. He described political discussions that took place in the United States in the summer of 2009 about donating H1N1 vaccines to other countries. One camp was concerned about donating vaccines when the U.S. population was not adequately covered; the other camp was concerned about the rest of the world not having enough vaccines and believed the United States should help. In September 2009, President Obama decided that the United States would donate 10 percent of its vaccines to WHO and would allow WHO to make decisions about distribution and deployment. Delegating WHO as the impartial distributor allowed the U.S. government to avoid a political problem in donating vaccines to one country but not another.
Pandemic Vaccine Timing in 2009
The 2009 H1N1 pandemic in the United States illustrated the importance of timing, said Gellin, because the vaccine did not reach most clinics until after the virus had peaked. By the time vaccine delivery had ramped up, some countries were only receiving the vaccine deliveries while other countries had vaccination programs fully under way or even completed. Figure 6-1 depicts global monthly vaccine deliveries made through the WHO Deployment Initiative in 2009 and 2010. Several delays in the system contributed to this overall timing problem, he said. Some delays were unpredictable, such as the volcanic eruption in Iceland that disrupted air shipment of vaccines at a critical stage. Other delays were caused by customs requirements, such as those for certificates of fumigation. The volume of vaccines needed overwhelmed many existing systems, such as the cold chain. The vaccine delays in 2009 highlighted the shortcomings of a deployment system developed “on the fly,” said Gellin.
WORLD HEALTH ORGANIZATION’S ROLE AND LEGAL CONSIDERATIONS IN VACCINE RESPONSE
Steven Solomon, principal legal officer at WHO, explored legal considerations relevant to international vaccine response, including governance, equity, and vaccine donations. He began by explaining that from political and legal perspectives, ministers of health have the most power to respond and communicate during a pandemic; ministers of health also have the power to establish global norms through WHO. The touchstone for ministers of health is the WHO Constitution, which posits the definition of health but also establishes the principle of equity (WHO, 2006). From the broadest perspective, the fundamental objective of WHO is to ensure the highest possible level of health for all people. WHO’s mandate is principally to respond and furnish aid in emergencies, but more importantly, he said
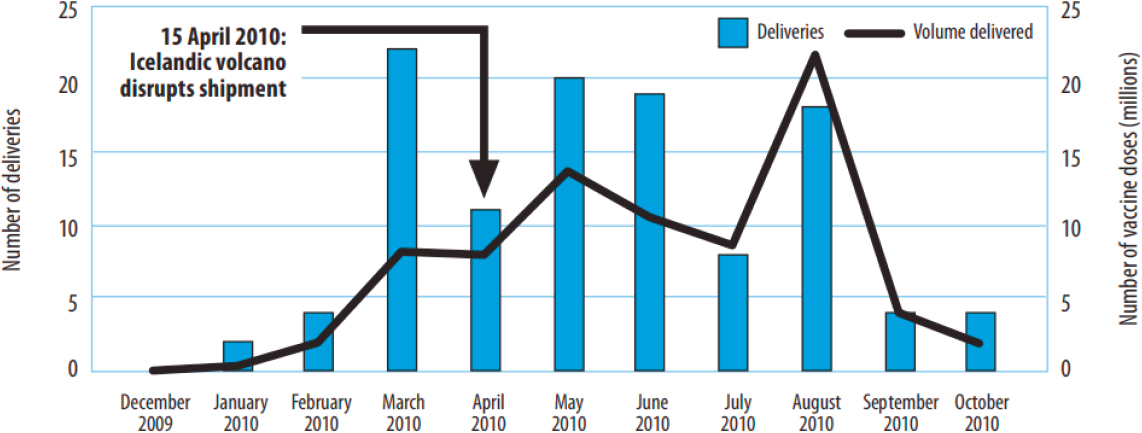
SOURCES: Gellin presentation, November 27, 2018; reprinted from Report on the WHO Pandemic A(H1N1) Vaccine Deployment Initiative, Page 20, Copyright (2012).
WHO can also assist governments in strengthening health services prior to a crisis. WHO executes this by working with all stakeholders through coordination, collaboration, and cooperation. The revised International Health Regulations (IHR) have driven some progress since coming into force in 2005; for example, improvements have been seen in core capacity building, emergency committee functioning, and providing information to WHO. However, more work is needed, he argued, to improve financing in order to support implementation, compliance, access to pathogens, benefit sharing, and awareness of the regulations.
Legal Context for Vaccine Donation
Solomon laid out a set of complex and time-consuming procedural steps between countries and WHO that may contribute to delays in vaccine timing during a pandemic. Despite the time-consuming nature of each step, they still must happen quickly during a crisis. First, WHO carries out its own mandatory prequalification review of new vaccines (WHO, 2019a). Then, WHO conducts an eligibility assessment to prioritize potential recipient countries by evaluating their domestic capacity to produce vaccines and their ability to purchase vaccines commercially. The WHO Director-General then sends letters containing complicated legal terms and conditions to ministers of health in potential recipient countries, Solomon described, to which the ministers must process and respond to confirm interest. Next, ministers must sign the terms and conditions and develop a national deployment plan to submit to WHO, which assesses the plan and assists in finalizing it. Contract law related to liability and indemnification can delay the process, said Solomon. The terms and conditions for recipient countries are couched in “legalese,” but WHO provides support in navigating the language. According to Solomon, a more pressing issue is the indemnification clause, which requires recipient countries to pay the legal fees if the donor is sued. This clause needs to be addressed because it is not feasible for many under-resourced and vulnerable countries, he said.
In 2009, the legal complexities were dealt with relatively quickly with good uptake from potential recipients—95 countries were ultimately deemed eligible potential recipients, and 77 countries developed the national deployment plan and received vaccines. However, the major problem was that, in many cases, countries’ national deployment plans were unable to accommodate the necessary speed of operation. Solomon suggested three approaches that could improve the speed and effectiveness of the process from WHO’s perspective:
- Using clearer ‘legalese’ that is translated into a broader range of languages (beyond WHO’s official languages);
- Prediscussing, preagreeing, and prepositioning the terms and conditions before a crisis; and
- Considering a global insurance mechanism for adverse events, particularly for vulnerable countries.
DISCUSSION
After the presentations, Jacqueline Katz, deputy director of the Influenza Division of CDC, remarked that the importance of timing in a pandemic and the importance of coordinating public–private partnerships were factors highlighted by all panelists. She noted that the latter already occurs annually for seasonal influenza through existing relationships and established expectations. However, because every strain is unique in a pandemic situation, a unique set of problems arises and needs to be addressed. Katz began the discussion with follow-up questions for the panelists and then opened the session for public audience engagement.
Successes and Challenges with GISRS in the 2009 Response
First, Katz asked Zhang to elaborate on the performance of GISRS during the 2009 H1N1 influenza pandemic. Zhang explained that many components were executed well. The capacity of GISRS had been built over previous decades, so virus detection went smoothly, surveillance was sufficient, and genetic sequence data were shared efficiently. Timing was fortuitous because the pandemic was detected and declared in early April, a period when annual seasonal vaccine production had already finished in the southern hemisphere and had not yet started in the northern hemisphere. This averted the dilemma about whether to stop seasonal vaccine production in order to start the production of a pandemic vaccine. The process of developing the candidate vaccine virus in WHO Collaborating Centers also went well, she added, and the H1N1 pandemic vaccine yield was better than the average yield of the seasonal vaccine. She said that in terms of surveillance, enough evidence was available to determine which at-risk groups should receive the vaccine in the first round.
However, GISRS also faced challenges in 2009. Zhang noted that the response was complicated by fixed pandemic phases based on pandemic preparedness planning. The phases called for action that needed to be triggered during each pandemic phase. Biocontainment recommendations were another challenge. At the time of the outbreak, the virus was already circulating in the community and causing mild disease. However, Biosafety Level 2 Plus was still required, but this level did not exist in most manufacturing plants at the time. In terms of regulatory capacity, many recipient countries did not have adequate mechanisms in place to receive donated
vaccines. Policy-related challenges to deployment also arose because other seasonal viruses were co-circulating toward the end of the pandemic, but people were still using the monovalent H1N1 vaccine.
Improved Potency Assays
Katz asked Midthun to describe the current state of development for improved potency assays, which can help with the timing issues related to pandemic vaccine development. Midthun replied that BARDA has established an influenza vaccine improvement plan in collaboration with CBER, CDC, and NIH. A consortium has also been established with WHO Essential Regulatory Laboratories and with manufacturers to test different potency assays. Progress has been made in finding assays with apparent potential, but questions remain about how quickly the necessary monoclonal antibodies can be developed. Further work is needed to find more suitable candidates that have rapid or preexisting reagents, she said, which would preclude the need to develop strain-specific antisera when the virus is being manufactured. Midthun noted that the current approach involves searching for new potency assays for traditional inactivated influenza vaccines; however, with new types of vaccines already in the development pipeline, it may not be feasible to assume that a single potency assay will be appropriate for all new vaccines.
Increasing Fill-Finish Capacity
Katz called on Lewin to discuss more about the gap in fill-finish capacity in the United States. Lewin said that installed manufacturing capacity will be key to providing surge capacity in pandemic response since the process of increasing vaccine development capacity is lengthy. For example, companies tend to fill vaccines in prefilled syringes, as demanded by the market using a fill-finish strategy based on what they currently sell—which is a capacity of about 150 million doses in the United States (although not all doses are filled in the United States). If a pandemic occurred, about 600 million doses (two for every American) would probably need to be filled in multi-dose vials, as determined by BARDA and other agencies (MacGregor, 2018). Finding the requisite capacity to fill and finish vaccines requires a technology transfer licensed by FDA, which in turn enables the available capacity to be used when it is needed. These issues need to be considered now, he said. Even if the bulk could be produced, it would need to be filled as quickly as possible to avoid creating a bottleneck, he added. This typically involves engaging contract manufacturing organizations that can fill-finish, he noted, and that requires a reservation fee as well as investment in the necessary technology. Katz said that syringes were a major gap identified
during a recent pandemic exercise carried out by CDC and BARDA—even with sufficient vaccine capacity, not enough syringes would be available to administer the vaccine. Lewin added that manufacturers plan their supply chains for seasonal vaccine production, but there are ongoing initiatives to improve pandemic manufacturing preparedness. Gellin remarked that it could be beneficial to involve logistics experts in planning.
WHO’s Prequalification Process and Eligibility Requirements
Katz asked Solomon to elaborate on WHO’s prequalification process and its eligibility requirements for potential recipients of donor vaccines. Solomon said prequalification is mandatory for all vaccines because many member states lack their own national regulatory authorities and rely exclusively on WHO’s prequalification process (which could take as little as 1 day to as many as 20 days). Eligibility for donated vaccines is determined by domestic production capability—which so few countries have—and by a country’s capacity to purchase vaccines on the commercial market. Most of the global vaccine supply is precontracted, so most countries do not meet the latter criteria, either. Solomon said that these eligibility criteria underscore the importance of the PIP Framework’s benefit-sharing scheme and of the 10 percent commitment from countries.
Surveillance During Vaccination
Matt Zahn, medical director of the Division of Epidemiology and Assessment of the Orange County Health Care Agency in California, asked about gathering surveillance data after a decision is made to vaccinate. Midthun said that FDA licenses a vaccine based on clinical trials that show its effectiveness against a confirmed influenza disease; the vaccines are adjusted as new strains evolve. Katz added that clinical studies are used to monitor vaccines, and CDC monitors the effectiveness and safety of vaccines in real-world situations. From a domestic standpoint, Gellin emphasized the importance of a highly robust safety system coupled with transparency about safety concerns. When the 1976 vaccine was linked to Guillain-Barré syndrome, for example, the crisis was addressed by leveraging every available government system, by using immunization registries in new ways, and by convening monthly meetings of the National Vaccine Advisory Committee to assess data and to ensure transparency. Gellin said the aim was to put the best-possible system in place for surveillance while also being able to communicate effectively with the public to allay concerns. The system was eventually de-escalated, but it was built on existing government processes and infrastructure that could be ramped up again. Global surveillance is another matter, Gellin noted, because it requires
distinguishing between a causal and temporal event and determining the appropriate response or compensation.
Progress in Vaccine Preparedness Since 2009
Peter Daszak, president of EcoHealth Alliance, asked about the current prospects for beating the epidemic curve in the event of a pandemic that was similar to H1N1 but that involved a new, rapidly moving virus and a vaccine that must be started from scratch. Gellin replied that BARDA is looking at applying new technologies to compress the development and deployment time frame, but current technology is not sufficiently advanced to beat the first viral wave; he said this highlights the need for a universal influenza vaccine. Solomon remarked that the PIP Framework contributes to progress on the global level because it has precontracted almost 400 million pandemic vaccine doses with preagreements for the terms and conditions. Lewin said that the licensed adjuvants, recombinant vaccines, and cell-culture vaccines currently available would help to decrease the timeline slightly, but a 6-week response timeframe is not yet feasible. A substantial improvement, Lewin noted, would require significant investment in technological advancements that may take decades to develop. Zhang added that WHO introduced a Pandemic Influenza Severity Assessment after 2009, which enables more granular evaluation of a disease if an outbreak occurs. Information from that assessment could enable vaccine manufacturers to devote a proportion of their production process to the influenza monovalent vaccine while continuing to produce the seasonal vaccine. Since 2009, she noted, progress that includes regulatory, logistical, and deployment elements has been made in national pandemic-preparedness planning.
Keiji Fukuda, director and clinical professor, School of Public Health, The University of Hong Kong, commented that by the time the vaccine was available in 2009, it was already clear that the impact and mortality were relatively milder than people had feared; technical processes proceeded smoothly because the operation was an intensification of systems already in place. After that point, the process was more ad hoc and thus more difficult. If the pandemic had been more severe than it was, he said, it is unclear whether the system would have fragmented or would have collaborated effectively. Gellin said that the urgency of a severe outbreak may have curtailed some process timelines, but not significantly; many systems had already collapsed at that point. Gellin surmised that a more severe pandemic would have affected U.S. political decisions about donating vaccines to other countries; in that case, the government would have considered taking vaccines away from U.S. citizens to be problematic. Zhang noted that in the past decade, specific guidance related to some key decisions (e.g., whether to switch vaccine production from seasonal to pandemic) has been
developed, and it could be communicated to a population prior to a pandemic. She said such guidance might reassure the public that evidence-based decisions are being made to ensure the best possible public health outcomes.
WHO’s Health Emergencies Programme
Finally, Gabrielle Fitzgerald, founder and chief executive officer of Panorama, asked how preparedness for pandemic influenza relates to WHO’s Health Emergencies Programme, which was established after the Ebola outbreak. Zhang explained that WHO’s seasonal influenza program is housed within the Health Emergencies Programme because it is a persistent, annual health threat. Because a country’s entire health system is the platform for any response, building capacity to respond to seasonal influenza improves preparedness for pandemics of influenza or for any other disease. Solomon added that lessons from the 2009 pandemic have been applied to the Health Emergencies Programme, but the tools and resources to apply those lessons beyond influenza may not currently exist. For example, the IHR lack a financing mechanism, have compliance issues, and do not mandate access to pathogens and their genetic sequence data.
This page intentionally left blank.
















