2
Hazard Assessment Scoping Plan
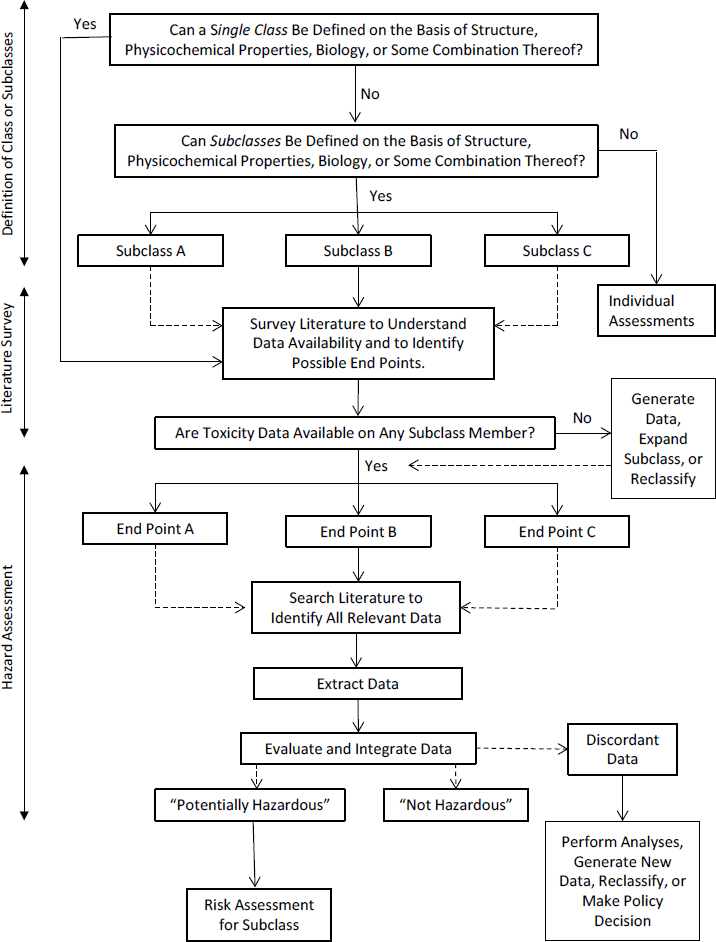
The committee developed a general strategy for using a class approach for hazard assessment, which is shown in Figure 2-1. The first step is to determine whether a class approach is appropriate for the chemicals of interest. As noted in Chapter 1, answering that question might involve determining whether subclasses need to be formed if it is not possible to treat all chemicals as a single class. If a class approach is appropriate, the second step is to survey scientific literature or databases to assess the availability of toxicity data (from human, animal, in vitro, and other relevant studies) and to identify end points to investigate. If data on any chemical for a given end point are available, the next steps are to extract, evaluate, and integrate the relevant data to reach a decision regarding potential hazard that can be applied to the entire class or subclass. Whenever possible, gaps in the data on individual chemicals should be resolved by interpolation or extrapolation of data on other members of the class or subclass. This chapter discusses the key steps in further detail and provides options for managing discordant data or addressing the no-data scenario. It concludes by discussing the implications of a class approach for risk assessment and for cost and efficiency. Chapter 3 provides examples or case studies that illustrate the committee’s general strategy for nonpolymeric, additive organohalogen flame retardants (OFRs).1
As the committee developed its scoping plan, it became clear that a multidisciplinary group is needed to execute the plan. Expertise needed includes cheminformatics, computational chemistry, computational toxicology, traditional and modern toxicology, epidemiology, and risk assessment. Furthermore, integrating the evidence at various steps will require expert judgment, and policy decisions will probably be needed to complete the assessment. For example, decisions involving what health end points to investigate, how much weight to assign a given end point, and how much uncertainty is acceptable bring value judgments into the hazard-assessment process that are beyond the scope of this report and are not discussed further here.
DETERMINE THE VIABILITY OF A CLASS APPROACH
Several methods can be used to determine whether a class approach can be applied to a chemical group to conduct a hazard or risk assessment. In this section, the committee first describes general approaches that have been used and then specifically what has been considered for flame retardants. The section concludes with the committee’s strategy for determining the viability of a class approach for an OFR hazard assessment.
Past Efforts to Assess Chemicals as Classes or Categories
The scientific and regulatory community has accepted the evaluations of chemicals as groups. For example, in the 1980s, toxic equivalency factors for dioxin-like chemicals were developed on the basis of relative potency, and this allowed assessment of these chemicals as a class (Van den Berg et al. 1998). In the late 1990s, the US Environmental Protection Agency (EPA) developed a chemical-category approach under the High-Production Volume Challenge Program (65 Fed. Reg. 81686 [December 26, 2000]). That approach allowed some extrapolation of data on tested chemicals to similar but untested chemicals as a way to reduce animal testing. And EPA assessed the cumulative risk associated with the class of cholinesterase-inhibiting pesticides in the 2000s (71 Fed. Reg. 43740 [August 2, 2006]) and then developed a framework and guidance document for cumulative risk evaluations of pesticide classes (EPA 2016). EPA is now considering chemical categories in the New Chemicals Program of the
___________________
1 The abbreviation OFRs in this report refers specifically to nonpolymeric, additive organohalogen flame retardants.
Toxic Substances Control Act (TSCA) (Henry 2017). As of December 2017, 56 chemical categories have been defined in the TSCA program, including photo-acid generators, tracer chemicals, and perfluorinated chemicals. The Consumer Product Safety Commission (CPSC) also used a class approach to assess chemicals when it convened a chronic hazard advisory panel to evaluate several phthalates (CPSC 2014).
Agencies in other countries have explored evaluation of chemical groups. For example, Phase 2 of Canada’s Chemicals Management Plan in 2011–2016 included a substance-grouping initiative that used alternative approaches, such as computational and read-across methods to screen and set priorities for chemical groups. That approach was applied to azo-based and benzidine-based substances, phthalates, and others. Health Canada and Environment and Climate Change Canada (HCECCC 2017, p. 14) set a long-term goal to “move away from substance by substance assessment approach toward priority setting on emerging classes of concern”; they envision an important role for predictive toxicology.
The European Chemicals Agency (ECHA) developed an approach to assessment of chemical categories under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, which has allowed companies to use read-across within chemical
categories for chemical assessment in lieu of testing in some situations (ECHA 2009, 2010). ECHA (2010) defines a category as “substances whose physicochemical, toxicological and ecotoxicological properties are likely to be similar or follow a regular pattern as a result of structural similarity” (p. 21). Similarities might be based on a common functional group, a common precursor or breakdown products, a constant pattern of changing potency, common constituents, or chemical classes.
The Organisation for Economic Co-operation and Development (OECD) issued guidance on grouping of chemicals in 2007 and updated it in 2014 (OECD 2014). OECD defines chemical grouping as the general approach for considering more than one chemical at a given time and provides guidance on performing analogue and category approaches. The analogue approach is used when the focus is on filling gaps in data on a single chemical whereas the category approach is used for assessments of multiple chemicals. Both approaches have rationales based on common functional groups (such as aldehyde, epoxide, ester, or specific metal ion), a common mechanism, common constituents or chemical classes or similar carbon range numbers (frequently related to substances of unknown or variable composition), likelihood of common precursors or breakdown products that result in structurally similar chemicals, or an incremental and constant change throughout the category (such as a chain-length category), as is often observed in physicochemical properties (such as boiling point range) (OECD 2014). The agency’s guidance on developing categories is presented in Box 2-1.
The OECD guidance discusses interpolation and extrapolation of data within a category and the potential need to form subcategories when chemicals in a category do not align. The OECD guidance emphasizes the high level of uncertainty associated with extrapolation across an entire data-poor category on the basis of little information. It recommends category test plans, which are “designed to provide information to characterize the category as a whole rather than fill in every data point for every chemical in the category” (OECD 2014, p. 15). OECD points out that its approach to filling data gaps can increase efficiency and save animals and money. It also advises identifying all potential members of a category at the start and cautions that omitting chemicals because they are not widely used, not manufactured by particular companies, or not used for a stated purpose could introduce bias into the category (OECD 2014).
Individual companies and industry consortia have also explored ways to use read-across that is complemented by in vitro predictive screening data to evaluate hazard and risk of chemical groups. For example, an initiative co-funded by the European Commission and Cosmetics Europe published an approach to group evaluations of chemicals, set priorities among several groups for further evaluation, and stated an intention to continue to work together to develop case studies (Berggren et al. 2015).
Evaluation of the general approaches to forming chemical classes at various regulatory agencies and non-regulatory consortia reveals that there has been a growing understanding of the advantages and potential pitfalls of defining chemical hazard and risk in a class context. Acceptance of a class approach has also grown. Computational techniques, such as read-across, have greatly enhanced the ability to perform class-based assessments (Blackburn and Stuard 2014; Berggren et al. 2015). The approaches used by EPA, ECHA, OECD, and others to date, however, have defined chemical classes or categories narrowly. The examples above show that some classes have been defined solely by structure, others by a common metabolite, and still others by a mechanism of action. The large class of OFRs, in contrast, is defined by a combination of chemistry and functional use (flame retardant). The formation of such a class is outside the criteria defined by most US and international agencies so far.
One recent, more innovative approach to defining a class was described in a publication by researchers in the
EPA Office of Research and Development and the National Toxicology Program (Patlewicz et al. 2019). Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a large class of chemicals defined by structural features and chemical properties. The challenge was to identify a subset of PFAS for testing with the goals of supporting read-across within structure-based subgroups and capturing the diversity of the broader PFAS class. The researchers began with the DSSTox chemical library to identify chemicals that included relevant structural features, next narrowed the list to generate a library of PFAS, and then categorized the library into subclasses on the basis of structure. The investigators finally selected a set of 75 members of the class that represented 34 subclasses. Those substances are undergoing testing with an array of new approach methodologies (NAM).2
Past Attempts to Define Flame-Retardant Classes
There have been efforts to group OFRs in a regulatory context. For several years, EPA has been evaluating various flame retardants as “clusters” under TSCA.3 Examples of such clusters include chlorinated phosphate esters, cyclic aliphatic bromides, brominated phthalates, and the tetrabromobisphenol A cluster. EPA justified the formation of flame-retardant clusters by saying that “grouping and evaluating flame retardants with similar characteristics together, rather than individually, will help EPA to more efficiently evaluate existing data and support more informed decisions about data gaps and needs.”
The European Food Safety Authority (EFSA) published and assessed six groups of brominated flame retardants in food from 2010 to 2012. The EFSA groups included bisphenols (EFSA 2011a), phenols and derivatives (EFSA 2012a), diphenyl ethers (EFSA 2011b), alicycles (such as hexabromocyclododecanes) (EFSA 2011c), and biphenyls (EFSA 2010). It also considered other emerging and novel flame retardants (EFSA 2012b).
The Danish Environmental Protection Agency (Danish EPA) applied a more systematic class approach by using cheminformatics and quantitative structure–activity relationship (QSAR) tools to group 67 brominated flame retardants (Danish EPA 2016). A commercially available structural-feature set was applied to group chemicals. The Danish analysis resulted in 15 preliminary structural classes and seven remaining substances classified as singletons (single chemicals that had mixed modes of action that were not assignable to one of the 15 classes). The agency then focused on the category of small linear and branched alkyl alcohols for further evaluation. That category included four of the initial 67 flame retardants but was expanded to include other members of the structural category, regardless of whether they were currently used as flame retardants or even had CAS numbers. The exercise to expand the initial set resulted in 62 chemicals in the category. QSAR evaluation identified structural alerts for mutagenicity and carcinogenicity. A literature review identified three members of the category on which there were relevant experimental data and found that they had demonstrated mutagenic or genotoxic effects. The Danish EPA recommended additional steps to enhance the basis of read-across in this category, specifically inclusion of additional structural analogues outside (but structurally similar to) the category, selective additional testing of several more members of the category, and further exploration of the underlying mechanisms of action.
Outside the risk-assessment context, California’s Environmental Contaminant Biomonitoring Program developed a process for defining chemical classes that combined structure and functional use (Krowech et al. 2016). That approach was adopted by the California Safer Consumer Products Program (DTSC 2013). In that context, the California Office of Environmental Health Hazard Assessment conducted a hazard identification of a class of “brominated and chlorinated chemical compounds used as flame retardants” and a separate identification of “nonhalogenated aromatic phosphates,” which also included some flame retardants (Krowech et al. 2016, p. A222). The biomonitoring hazard identification resulted in the adoption of both classes as candidate chemicals for potential regulation in consumer products. The two class identifications, however, have not been used to conduct risk assessments.
Committee Strategy to Determine Viability of Class Approach for OFRs
OFRs have several characteristics that could define them as a single class, including some physicochemical properties, their use as flame retardants, or generation of specific combustion byproducts. Those characteristics could define them as a single class for some decision contexts but are not entirely workable for conducting a hazard or risk assessment under the CPSC regulations. The committee considered the decision context and its charge and recognized the need to perform a regulatory hazard assessment that would be followed by a risk assessment focused on exposure to the consumer. Ultimately, the committee’s approach embraces the class concept, and it recommends a method to evaluate the hazard posed by OFR subclasses created on the basis of a combination of structural characteristics, physicochemical properties, and biology. The committee concludes that it is scientifically justifiable to assess OFRs by using a class approach and that extrapolation of hazard from subclass members
___________________
2 As noted in Chapter 1, NAM studies encompass computational modeling, in vitro assays in human and animal cells and tissues, and toxicity testing using alternative animal species, such as zebrafish and nematodes.
3 See https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-assessing-risks-flame-retardants.
on which there are some data to other members on which there are no data is appropriate and likely necessary to address data deficiencies.
The committee recommends that CPSC take a multistep approach to evaluating OFRs and forming subclasses. Chapter 3 illustrates how CPSC can execute this approach with details provided in Appendix B. The multistep method has the following general steps:
- Identify and characterize a “seed” set of chemicals as a working inventory of the class.
- Generate an “expanded” set of chemical analogues of the seed set on the basis of combined functional, structural, and predicted bioactivity information.
- Evaluate the similarity of the seed set to the analogues to evaluate whether the OFRs are distinguishable as a single class.
- Define subclasses for hazard evaluation.
Optimally, all chemicals of interest would fall neatly into subclasses. However, depending on the extent of heterogeneity tolerated and how the subclasses are defined, some chemicals could be outliers. The groupings of brominated flame retardants produced by the Danish EPA, for example, identified a number of chemicals that did not fit within the classification scheme. When the present committee approached grouping of OFRs, it considered various potential classifications that would generate a number of subclasses of only one or two chemicals. After considering the advantages and disadvantages of “lumping” vs “splitting” the subclasses, the committee concluded that merging the individual (or few) chemicals into the most closely related larger subclass would be the best approach to support a hazard assessment of the chemicals. That approach is recommended because the purpose of forming chemical classes is to allow evaluation of groups of chemicals; defining the classes too narrowly would generate multiple outliers or singletons and could frustrate the entire purpose of a class approach to hazard assessment. The committee therefore recommends defining chemical classes or subclasses as broadly as is feasible for the analysis.
The committee acknowledges that there are several scientifically valid approaches to forming OFR subclasses and has discussed approaches used by other organizations or agencies, such as EFSA and the Danish EPA, in the previous section. It demonstrates its approach in Chapter 3 and provides details in Appendix B. At this stage, the committee does not find that biology can be used as a primary driver for subclass formation because the experimental data available are not adequate for doing so. That approach also diminishes the advantage of a class approach in which one can extrapolate conclusions from data-rich to data-poor chemicals (that is, data are not needed for all subclass members). However, in the future, NAM data could greatly enhance subclass formation, especially if a fit-for-purpose high-throughput in vitro system that has adequate biologic coverage is developed and validated for use with OFRs. One can imagine testing all chemicals in such a system and using the data to group the chemicals of interest to improve classification methods.
Regardless of the classification method used, the committee recommends avoiding the temptation to reclassify chemicals in an iterative fashion as chemicals are added to a class or as a new dataset becomes available. Although it is tempting to use each increment of additional information to rerun the classification and refine the subclasses, such an exercise could paralyze action and make it difficult or impossible to move forward with a hazard evaluation of the class. Reclassification might be appropriate, however, if substantive new data on several class members become available or if computational models that could substantially improve classification methods emerge.
SURVEY THE LITERATURE
Once subclasses of OFRs have been formed, the next step is to survey the literature to determine the extent, range, and nature of toxicity data (human, animal, in vitro, and other relevant studies) and to identify end points that deserve investigation (NASEM 2017a; NRC 2014). The term literature is used broadly here to refer to scientific literature and databases. The survey strategy should be developed in consultation with a librarian or other information specialist and should include at least two databases.
One outcome of the survey is development of an evidence table or map that provides a descriptive or visual summary of data availability (Miake-Lye et al. 2016; NRC 2014; Wang et al. 2016). Evidence tables or maps help to identify data-rich subjects on which systematic reviews and meta-analyses might be conducted. The evidence table or map can also help to identify data gaps that might need to be addressed before a class-level hazard assessment is conducted. In other cases, the evidence table or map might help to identify one or more well-studied chemicals within an OFR subclass that can be used to anchor the hazard assessment of the subclass. Well-studied chemicals can be used to identify the hazards of concern and help to set priorities for future research on less well-studied members of the subclass. It is possible that the survey will find that no data are available to support a hazard assessment for a subclass.
The survey should also help with the development of an analysis plan. It is important that the hazard assessment be conducted in a transparent and reproducible manner, and one means of achieving that is to develop an analysis plan that documents the objectives of the hazard assessment and includes a description of the end points of inter-
est and the methods used to perform the analysis. Some approaches will need to be developed during the conduct of a class-based hazard assessment; thus, an a priori description of all methods is probably not feasible. Instead, the analysis plan can be developed iteratively and updated throughout the process with the changes documented.
Specifically, the analysis plan should clearly identify the end points to be investigated and the relevant data streams—for example, experimental animal, epidemiologic, and NAM studies—that will be considered in the analysis. The analysis plan should clearly identify the type of review, such as a systematic review (IOM 2011), that will be used. When appropriate, one or more focused research questions can be developed to guide the search for data and to develop appropriate inclusion and exclusion criteria.4 Depending on the outcome of various stages of the assessment, the analysis plan might need to be revised to document the approaches used to address data gaps and integrate the data within or across end points of interest. Ultimately, the goal of the analysis plan is to search, screen, extract, evaluate, and integrate data systematically from all relevant studies that are included in the review.
SEARCH THE LITERATURE AND EXTRACT DATA
Once the survey and analysis plan have been completed, a thorough literature search is conducted. Again, the term literature is used broadly here to refer to all scientific literature and databases that might contain any relevant data. The literature search differs from the literature survey; the literature survey was meant only to provide a broad understanding of data availability on various possible end points, whereas here, the goal is to identify all relevant studies that can potentially be used to assess a given end point for the subclass members. Different approaches to capturing the literature include systematic reviews, scoping reviews, rapid reviews, and mapping reviews (Grant and Booth 2009; Peters et al. 2015). The approach used will depend partly on data availability, time-frame, and resources, but whatever approach is selected should be transparent and reproducible and documented in the analysis plan. Specifying how the search will be conducted and documenting the results will provide assurances of the quality of the methods used both when studies are found and when they are not.
The literature search should aim to identify NAM studies in addition to traditional animal toxicity and epidemiologic studies. NAM data can be critically important in addressing human health concerns not well addressed by traditional animal toxicity studies. In drug-induced liver injury, for example, negative findings in animal studies did not predict later findings of human toxicity in clinical trials or after entry into the marketplace. The later findings led to the identification of interspecies differences—such as in immune responses and the handling of bile acids—that helped to explain some of the limitations of the animal-based predictions. The class approach proposed here would use the breadth of modern experimental and computational modeling methods to facilitate hazard assessment.
Once the relevant literature has been identified, the studies should be screened with inclusion and exclusion criteria that are specified in the analysis plan, and the data should be extracted by using consistent templates so that straightforward evaluations and comparisons can be made (NASEM 2017a; NRC 2014). The committee notes that data extraction is often resource intensive; there are often differences, for example, in chemical names, terminology, and units that further complicate the process.
EVALUATE AND INTEGRATE DATA
Once the relevant data have been extracted, the next step in the process is to evaluate and integrate them. A key concept of the class approach in contrast with the historical focus on individual chemicals is that there needs to be enough information to determine potential hazards posed by the class or subclass. The availability of epidemiologic or animal toxicity studies of at least one chemical in a subclass can provide an anchor for the hazard assessment and, if warranted, later risk-assessment steps. NAM data—for example, results of zebrafish assays, in vitro assays, and computational models—can play a useful role in demonstrating that the members of the subclass share the hazard-associated characteristics of the anchor chemical in the epidemiologic or toxicology studies (Berggren et al. 2015). For example, computational models can be used to predict whether a chemical of interest will be positive in an Ames mutagenicity assay (Hsu et al. 2016; Pandit et al. 2018). Historically, NAM data have been underused in the absence of epidemiologic or toxicity data on individual chemicals; however, they can help to characterize the subclass and might facilitate later quantitative analyses by providing information to use, for example, in a relative-potency approach.
The committee identified three determinations for an OFR subclass: potentially hazardous, not hazardous, and discordant data. The determinations are data-dependent and could change as new data on one or more members of the subclass are acquired. The “potentially hazardous” determination is consistent with CPSC in that one has reached a decision that the chemical is “toxic” as defined in the Federal Hazardous Substances Act. In such a case,
___________________
4 This step is analogous to the problem-formulation step in a systematic review (NASEM 2017a). Problem formulation is “the process of defining the scope of a problem, formulating a question about it, and establishing the assessment parameters by which the question will be answered” (NASEM 2018, p. 38).
CPSC would then conduct a risk assessment to determine whether the chemical should be considered a hazardous substance. A “not hazardous” determination indicates that the chemical does not meet the definition of toxic. A “discordant data” determination is reached when data on individual chemicals in the subclass are too heterogeneous or inconsistent to allow a determination. Data can be discordant when experimental studies provide conflicting results, for example, when there are both positive and negative studies or when effects are seen in some studies but not in others. In such cases, analyses might be needed to determine whether the discordance resulted from differences in experimental design or models or was associated with test-chemical purity, dosing regimen, or timing of dosing or observations. A final step in the process is assignment of a confidence rating (high, medium, or low) to the determination.
The committee identified four possible scenarios that are likely to occur in the conduct of a class-based hazard assessment of OFRs in light of the case examples described in Chapter 3. Scenario 1 is a subclass that has many data-rich members on which the data are concordant. The hazard determination for the subclass should be relatively straightforward. Box 2-2 provides an example of this scenario and how it could be handled.
In Scenario 2, there are no relevant data on any subclass member that can be used to conduct the hazard assessment. The lack of data should not imply that an OFR subclass is not hazardous. For this scenario, the committee identified the following options:
- Option 2-1: Generate toxicity data for the subclass. The committee recommends a tiered approach that is described in Box 2-3.
- Option 2-2: Expand the analysis beyond the set of chemicals that were identified as OFRs (that is, the seed chemicals used to form the subclass). Toxicity data on structurally related chemicals could be used to inform the hazard assessment of an OFR subclass.
- Option 2-3: Reclassify the subclass so that data-poor members are distributed among other data-rich subclasses. Many OFRs have multiple functional groups and could go into multiple subclasses; reclassification might help to minimize the number of data-poor categories. Confidence in the reclassification can be increased if concordant biologic responses are seen among the members of the newly expanded subclasses, for example, if additional data show a common mechanism of action or effect.
In Scenario 3, coherent data on one or two chemicals are sufficient for evaluations but there are few or no data on the remaining members. However, the few data available might suggest that the members of the subclass have similar biologic activity, in which case the committee identified the following possible options:
- Option 3-1: Make a science-based policy decision, for example, to classify the group as potentially hazardous on the basis of the data-rich chemicals in the subclass.
- Option 3-2: Use the data-rich chemicals to serve as an anchor as suggested above and extrapolate or interpolate to other chemicals in the group.
- Option 3-3: Generate toxicity data on data-poor group members to the extent that satisfactory confidence is gained; testing could involve NAM studies, targeted animal testing, or a combination thereof.
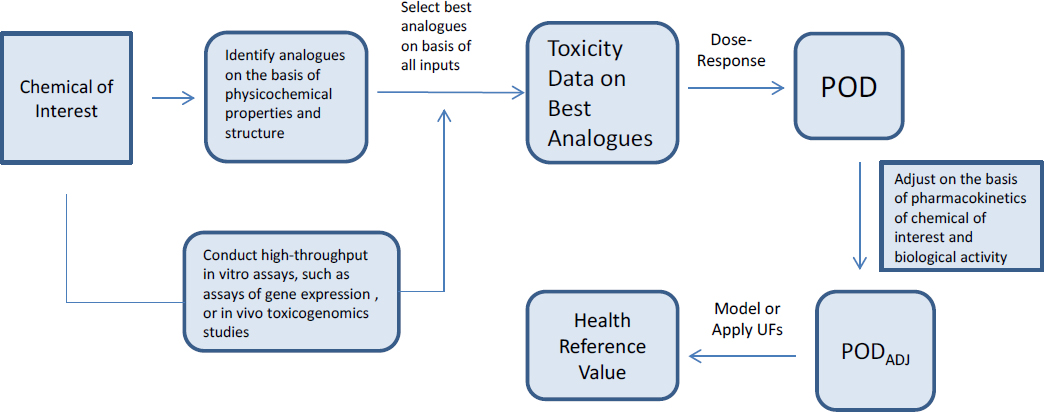
The committee notes that various NAMs could be used to increase confidence that group members are biologically similar for the end point of interest. That approach is similar to the one suggested for individual chemical assessments that use read-across in the report Using 21st Century Science to Improve Risk-Related Evaluations (NASEM 2017b) as illustrated in Figure 2-2.
Scenario 4 is the most difficult to address. There are data on some chemicals in the subclass and few or no data on other group members, and the available data are too heterogeneous or inconsistent on biologic activity so that a discordant-data designation is reached. The committee identified the following possible options, which are discussed in further detail in Chapter 3:
- Option 4-1: Make a policy decision, for example, to extend the most conservative conclusion regarding hazard to the subclass.
- Option 4-2: Reclassify members to improve their biologic similarity; generate data to increase confidence that reclassification has resulted in biologically similar members.
- Option 4-3: Perform analyses to explain the discordance and allow the assessment to move forward.
- Option 4-4: Generate new data that could increase clarity and the scientific basis for a decision.
INTEGRATING A HAZARD ASSESSMENT THAT USES A CLASS APPROACH INTO RISK ASSESSMENT
The committee has proposed a class approach to hazard assessment that represents a reinterpretation and augmentation of the traditional hazard assessment of individual chemicals. Using the proposed class approach will have implications for the later steps in the risk-assessment process—dose–response assessment, exposure assessment, and risk characterization. Although it is beyond the scope of this report to recommend how those steps should be implemented, the committee recognizes the need to integrate them with the class approach for hazard assessment.
Dose–Response Assessment
Dose–response assessments have generally been developed for a chemical by using in vivo toxicity data that adequately demonstrate the adverse effect being evaluated. Often, the analysis uses the no-observed-adverse-effect level, the lowest observed-adverse-effect level, or a benchmark dose curve-fitting approach to obtain a point of departure (POD). The POD is further adjusted by using default uncertainty factors or data-derived factors to account for interspecies extrapolation, human variability, or data deficiencies. Other extrapolation approaches have generally been applied for cancer but will not be discussed further here. Implementation of the class approach could consider several existing dose–response methods given the availability of data or potentially develop new ones.
- Surrogate chemical. Select or derive the appropriate dose–response value, such as an acceptable daily intake, for the most toxic chemical on which data are adequate as the subclass surrogate. Use that value in the risk characterization of all subclass members. Note that, absent data on multiple chemicals, it might not be certain that the ones on which data are available are the most toxic; NAM data might help to address this issue.
- Multiple surrogate chemicals. If data are available on more than one chemical in the subclass, the remainder of the subclass could be evaluated, for example, by assuming that they are similar to the most toxic chemicals in the subclass.
- Relative potency factors or toxic equivalents. Dose–response approaches have been implemented or proposed for a few classes of chemicals, such as polycyclic aromatic hydrocarbons and dioxins, furans, and polychlorinated biphenyls (EPA 1993, 2010; Van den Berg et al. 2006). Those approaches evaluate the toxicity of the class members compared with a selected surrogate class member, which does not have to be the most toxic. In vivo or in vitro studies have been used to characterize class members relative to the surrogate. Scalar values based on the selected study (such as receptor binding affinity relative to the surrogate) are then used to adjust the toxicity value for the surrogate chemical to provide toxicity values for each chemical. If the chemicals all act through the same molecular target, such as a single receptor or enzyme, approaches that characterize the relative interactions
-
with that target might be feasible. If the chemicals produce the same overall effect (for example, an antiandrogenic effect) but there are several targets (for example, binding to androgen receptor or decreasing testosterone or dihydrotestosterone synthesis), evaluating the relative activity at the molecular-interaction level might not be appropriate. Alternatively, when there are multiple targets, in vivo studies that assess the apical toxicity resulting from the overall pathway might be necessary; the studies potentially could be in nonmammalian organisms that respond to that pathway. When multiple toxicity end points are evaluated for subclass members, relative potency factors or toxic equivalents could be specific to particular end point, as when different mechanisms are involved.
- NAM studies and in vitro–in vivo extrapolation. NAM studies can also be informative for dose–response assessments. The alternative assays often use multiple concentrations that allow estimates of such measures as the concentration that produces 50% activity (AC50). They can also define the highest tested concentration at which no effect is observed. Negative findings might be useful in demonstrating that there is a lack of response in a pathway (as opposed to an absence of testing) or in comparing chemicals in a class. The in vitro assays provide information on media concentrations, so in vitro to in vivo extrapolation is often needed to estimate doses that are typically needed for exposure assessment. The extrapolation can be performed with a variety of pharmacokinetic analysis methods that might be associated with greater uncertainty in the estimates when more assumptions are required because of data limitations. Comparisons of predicted exposures and activity in alternative assays can provide a path for decision-making, including suggestions as to what additional data would be most valuable (Wambaugh et al. 2018). Application of these types of data and analytic approaches is a major focus of contemporary toxicology and risk assessment and reflects strong recommendations from the National Academies (NASEM 2015, 2017b).
Exposure Assessment
Several aspects of exposure assessment might need to be addressed to integrate the class approach into risk assessment.
- Availability of data on each subclass member would need to be assessed. If no data are available, predictive models could be used to estimate chemical exposures.
- Aggregate exposure by different routes (oral, dermal, and inhalation) would need to be determined in the context of expected human exposures to the products under consideration.
- Cumulative exposure to the subclass members needs to be evaluated, although this might be more important in the risk characterization than in the exposure assessment itself. Because hazard would be assessed for each subclass, each chemical in the subclass that is present in a product would be expected to contribute to the adverse outcome evaluated for that subclass.
- Exposures to members of the subclass in products not under consideration for a specific regulatory action should be included. An approach like the relative source contribution applied to drinking water risk assessments by EPA and states is a useful model (Gadagbui et al. 2012).
Risk Characterization
Use of a class approach for hazard assessment would also be expected to involve adjustments of risk characterization. Issues or approaches that seem likely to arise include the following:
- Single subclass. Combining dose–response and exposure assessments for a single subclass would be expected to address the combined exposure to all members of the subclass and allow evaluation of the risk of each kind of toxicity that was considered in the subclass hazard assessment. It is worth noting that even if exposures to individual chemicals are considered to be below a level of concern, consideration of the contributions from co-exposure to all members of the subclass could indicate a concern. Consideration of the cumulative and aggregate risk posed by all chemicals evaluated has a long history in site-specific risk assessments (for example, federal and state Superfund sites) or analyses that rely on relative potency factors.
- Multiple subclasses. When common toxicities are shared among subclasses, it might be necessary to evaluate the risks posed by all constituents of multiple subclasses. Considering the cumulative risk associated with multiple subclasses could also provide a basis for determinations related to the entire class.
IMPROVED EFFICIENCY AND COST-EFFECTIVENESS
A class approach will likely result in increases in efficiency and decreases in cost compared with the traditional approach of evaluating individual chemicals. The magni-
tude of the improvements or savings will depend on several factors, including the class or subclass size, the level of confidence needed to make a decision, the number of data gaps that need to be filled, and the effect of policy decisions. Broader classes or subclasses would generally improve efficiency and reduce costs.
As discussed above, the committee encourages the use of NAM studies to fill data gaps. The costs, resources, and time required to obtain data using the alternative methods are often orders of magnitude less than would be needed for conducting traditional toxicology studies for each chemical. Adopting NAM data to evaluate hazards posed by OFRs would also further the goal of refining, reducing, and replacing animals while providing data on more chemicals in a timely manner. The use of both NAM studies and the traditional toxicology data can permit the setting of priorities for future research and thereby improve efficiency and reduce cost.
Using a class approach to hazard assessment can also expedite evaluations of new and emerging members of a subclass. That approach can also provide input to industry as alternative products are considered. For example, in the case of OFRs, a company making a decision about ingredients in its products might consider the attributes of the broadest possible class and then decide to avoid the entire class because of its environmental persistence, production of toxic halogenated compounds when burned, or potential for toxicity.
CONCLUSIONS
In this chapter, the committee has described a scoping plan that can be used to conduct a class-based hazard assessment of the OFRs. The committee acknowledges that there will be challenges with its application particularly in the regulatory setting. Approaches that extrapolate data from one chemical to another (read-across approaches) have struggled to gain regulatory acceptance. Using 21st Century Science to Improve Risk-Related Evaluations (NASEM 2017b), however, highlights those approaches for conducting hazard and risk assessments of data-poor chemicals and identifies them as being underused. The present committee also recognizes that there will be no relevant data on many chemicals, and CPSC will need to make difficult decisions regarding what type of data it will accept and what quantity of data will be needed to support decisions with the desired confidence. Those decisions will affect the cost and time required to generate the necessary data. If CPSC decides that only traditional rodent studies are appropriate and that some data are needed on all chemicals, data generation will be extremely expensive and take decades to complete. And although NAM data represent tremendous time and cost savings, their use in the regulatory setting is still limited even though their use has been encouraged by authoritative bodies (NASEM 2015, 2017b). Finally, the committee notes that forming classes and conducting read-across requires expertise that is not widely available (Ball et al. 2016). Although the challenges to a class approach might appear daunting, the alternative—individual assessments of hundreds of chemicals—is unrealistic. The only possible practical approach for a set of chemicals as large as the OFRs is a class approach.
REFERENCES
Ball, N., M.T.D. Cronin, J. Shen, K. Blackburn, E.D. Booth, M. Bouhifd, E. Donley, L. Egnash, C. Hastings, D.R. Juberg, A. Kleensang, N. Kleinstreuer, E.D. Kroese, A.C. Lee, T. Luechtefeld, A. Maertens, S. Marty, J.M. Naciff, J. Palmer, D. Pamies, M. Penman, A.N. Richarz, D.P. Russo, S.B. Stuard, G. Patlewicz, B. van Ravenzwaay, S. Wu, H. Zhu, and T. Hartung. 2016. Toward good read-across practice (GRAP) guidance. ALTEX 33(2):149-166.
Berggren, E., P. Amcoff, R. Benigni, K. Blackburn, E. Carney, M. Cronin, H. Deluyker, F. Gautier, R.S. Judson, G.E. Kass, D. Keller, D. Knight, W. Lilienblum, C. Mahony, I Rusyn, T. Schultz, M. Schwarz, G. Schüürmann, A. White, J Burton, A.M. Lostia, S. Munn, and A. Worth. 2015. Chemical safety assessment using read-across: assessing the use of novel testing methods to strengthen the evidence base for decision making. Environ. Health Perspect. 123(12):1232-1240.
Blackburn, K., and S.B. Stuard. 2014. A framework to facilitate consistent characterization of read across uncertainty. Regul. Toxicol. Pharmacol. 68(3):353-362.
Collins, F.S., G.M. Gray, and J.R. Bucher. 2008. Transforming environmental health protection. Science 319(5865):906-907.
CPSC (US Consumer Product Safety Commission). 2014. Report to the US Consumer Product Safety Commission by the Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives. Directorate for Health Sciences, CPSC [online]. Available: https://www.cpsc.gov/s3fs-public/CHAP-REPORT-FINAL%20(1).pdf [accessed February 11, 2019].
Danish EPA (Environmental Protection Agency). 2016. Category approach for selected brominated flame retardants: Preliminary structural grouping of brominated flame retardants. Environmental project No. 1872. Copenhagen, Denmark: Danish Environmental Protection Agency [online]. Available: https://www2.mst.dk/Udgiv/publications/2016/07/978-87-93435-90-2.pdf [accessed September 25, 2018].
DTSC (Department of Toxic Substances Control). 2013. Safer Consumer Product Regulations, Department of Toxic Substances Control Reference Number: R-2011-02. Available: http://www.dtsc.ca.gov/LawsRegsPolicies/Regs/upload/Text-of-Final-Safer-Consumer-Products-Regulations-2.pdf [accessed 25 July 2018].
ECHA (European Chemicals Agency). 2009. Use of read-across & categories under REACH. ECHA webinar presentation on December 10, 2009. Available: https://echa.europa.eu/documents/10162/13566/read_across_and_categories_tatiana_netzeva_echa_en.pdf [accessed February 14, 2019].
ECHA. 2010. Evaluation under REACH: Progress report 2009. ECHA-10-R-001-EN. Helsinki, Finland: ECHA [online]. Available: https://echa.europa.eu/documents/10162/13628/progress_report_2009_en.pdf/889db80c-90ff-4a79-9046-91798fc677f7 [accessed February 11, 2019].
EFSA (European Food Safety Authority). 2010. Scientific opinion on polybrominated biphenyls (PBBs) in food. EFSA Journal 8(10):1789 [online]. Available: www.efsa.europa.eu/efsajournal [accessed September 25, 2018].
EFSA. 2011a. Scientific opinion on tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA Journal 9(12):2477 (updated 2013) [online]. Available: www.efsa.europa.eu/efsajournal [accessed September 25, 2018].
EFSA. 2011b. Scientific opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA Journal 9(5):2156 [online]. Available: www.efsa.europa.eu/efsajournal [accessed September 25, 2018].
EFSA. 2011c. Scientific opinion on hexabromocyclododecanes (HBCDDs) in food. EFSA Journal 9(7):2296 [online]. Available: www.efsa.europa.eu/efsajournal [accessed September 25, 2018].
EFSA. 2012a. Scientific opinion on brominated flame retardants (BFRs) in food: Brominated phenols and their derivatives. EFSA Journal 10(4):2634. Available: www.efsa.europa.eu/efsajournal [accessed September 25, 2018].
EFSA. 2012b. Scientific opinion on emerging and novel brominated flame retardants (BFRs) in food. EFSA Journal 10(10):2908 [online]. Available: www.efsa.europa.eu/efsajournal [accessed September 25, 2018].
EPA (US Environmental Protection Agency). 1993. Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons. EPA/600/R-93/089. Washington, DC: Office of Research and Development, EPA [online]. Available: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=49732 [accessed February 11, 2019].
EPA. 2010. Recommended toxicity equivalence factors (TEFs) for human health risk assessments of 2,3,7,8-tet-rachlorodibenzo-p-dioxin and dioxin-like compounds. EPA/100/R-10/005. Washington, DC: Office of the ScienceAdvisor RiskAssessment Forum, EPA[online].Available: https://www.epa.gov/sites/production/files/2013-09/documents/tefs-for-dioxin-epa-00-r-10-005-final.pdf [accessed February 11, 2019].
EPA. 2016. Pesticide cumulative risk assessment: framework for screening analysis purpose. Washington, DC: Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention, EPA [online]. Available: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/pesticide-cumulative-risk-assessment-framework [accessed February 11, 2019].
Gadagbui, B., J. Patterson, A. Rak, R.S. Kutzman, G. Reddy, and M.S. Johnson. 2012. Development of a relative source contribution factor for drinking water criteria: The case of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Hum. Ecol. Risk Assess. 18(2):338-354.
Grant, M.J. and A. Booth. 2009. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info. Libr. J. 26(2):91-108.
HCECCC (Health Canada and Environment and Climate Change Canada). 2017. Canada’s chemical management plan: Approaches to prioritization and to streamline assessments. Presentation by Health Canada and Environment and Climate Change Canada. Available: https://www.epa.gov/sites/production/files/2017-12/documents/us_epa_cmp_deck_-_december_2017_v4.pdf [accessed February 14, 2019].
Henry, T.R. 2017. Chemical categories: Progress implementing changes to the new chemicals review program under the amended TSCA. Presentation by T. Henry, Risk Assessment Division, Office of Pollution Prevention and Toxics, EPA, at Public Meeting on Progress Implementing Changes to the New Chemicals Review Program under the Amended TSCA, Washington, DC, December 6, 2017 [online]. Available: https://www.epa.gov/sites/production/files/2017-12/documents/presentation_4_and_5_-_categories_sustainable_futures_december_6th_pub.pdf [accessed February 14, 2019].
Hsu, K.H., B.H. Su, Y.S. Tu, O.A. Lin, and Y.J. Tseng. 2016. Mutagenicity in a molecule: Identification of core structural features of mutagenicity using a scaffold analysis. PLoS One 11(2):e0148900.
IOM (Institute of Medicine). 2011. Finding what Works in Health Care: Standards for Systematic Reviews. Washington, DC: The National Academies Press.
Krowech, G., S. Hoover, L. Plummer, M. Sandy, L. Zeise, and G. Solomon. 2016. Identifying chemical groups for biomonitoring. Environ. Health Perspect. 124(12):A219-A226.
Miake-Lye, I.M., S. Hempel, R. Shanman, and P.G. Shekelle. 2016. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst. Rev. 5:28.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2015. Application of Modern Toxicology Approaches for Predicting Acute Toxicity for Chemical Defense. Washington, DC: The National Academies Press.
NASEM. 2017a. Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals. Washington, DC: The National Academies Press.
NASEM. 2017b. Using 21st Century Science to Improve Risk-Related Evaluations. Washington, DC: The National Academies Press.
NASEM. 2018. Review of Report and Approach to Evaluating Long-Term Health Effects in Army Test Subjects. Washington, DC: The National Academies Press.
NRC (National Research Council). 2014. Review of EPA’s Integrated Risk Information System (IRIS) Process. Washington, DC: The National Academies Press.
OECD (Organisation for Economic Co-operation and Development). 2014. Guidance on grouping of chemicals, Second Edition. Series on Testing & Assessment, No. 194. Paris, France: Environment Directorate, Organization for Economic Co-operation and Development [online]. Available: http://www.oecd.org/publications/guidance-on-grouping-of-chemicals-second-edition-9789264274679-en.htm [accessed February 11, 2019].
Pandit, S., A. Dhawan, and R. Parthasarathi. 2018. Emerging computational methods for predicting chemically induced mutagenicity. Pp. 161-176 in Mutagenicity: Assays and Applications, A. Kumar, V.N. Dobrovolsky, A. Dhawan, and R. Shanker, eds. London, United Kingdom: Elsevier Inc.
Patlewicz, G., A.M. Richard, A.J. Williams, C.M. Grulke, R. Sams, J. Lambert, P.D. Noyes, M.J. DeVito, R.N. Hines, M. Strynar, A. Guiseppi-Elie, and R.S. Thomas. 2019. A chemical category-based prioritization approach for selecting 75 per- and polyfluoroalkyl substances (PFAS) for tiered toxicity and toxicokinetic testing. Environ. Health Perspect. 127(1):014501-1 to 014501-5.
Peters, M.D.J., C.M. Godfrey, H. Khalil, P. McInerney, D. Parker, and C.B. Soares. 2015. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 13(3):141-146.
Van den Berg, M., L. Birnbaum, A.T. Bosveld, B. Brunström, P. Cook, M. Feeley, J.P. Geisy, A. Hanberg, R. Hasegawa, S.W. Kennedy, T. Kubiak, J.C. Larsen, F.X. van Leeuwen, A.K. Liem, C. Nolt, R.E. Peterson, L. Poellinger, S. Safe, D. Schrenk, D. Tillitt, M. Tysklind, M. Younes, F. Waern, and T. Zachareweski. 1998. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 106(12):775-792.
Van den Berg, M., L.S. Birnbaum, M. Denison, M. De Vito, W. Farland, M. Feeley, H. Fiedler, H. Hakansson, A. Hanberg, L. Haws, M. Rose, S. Safe, D. Schrenk, C. Tohyama, A. Tritscher, J. Tuomisto, M. Tysklind, N. Walker, and R.E. Peterson. 2006. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 93(2):223-241.
Wambaugh, J.F., M.F. Hughes, C.L. Ring, D.K. MacMillan, J. Ford, T.R. Fennell, S.R. Black, R.W. Snyder, N.S. Sipes, B.A. Wetmore, J. Westerhout, R.W. Setzer, R.G. Pearce, J.E. Simmons, and R.S. Thomas. 2018. Evaluating in vitro-in vivo extrapolation of toxicokinetics. Toxicol. Sci. 163(1):152-169.
Wang, D.D., M. Shams-White, O.J. Bright, J.S. Parrott, and M. Chung. 2016. Creating a literature database of low-calorie sweeteners and health studies: evidence mapping. BMC Med. Res. Methodol. 16:1.













