5
A Research Agenda: A Vision for the Future of Separation Science
The chemistry and technology communities have made substantial advances in separation science and technology in recent decades. However, to take advantage of advances in experimental techniques, data science, and computation and simulation, as well as to address ever-changing societal demands, key fundamental challenges in separation science must be met. In this chapter, the committee identifies its highest-priority research directions categorized in two fundamental research themes: designing separations that have high capacity, selectivity, and throughput; and understanding temporal changes that occur in separation systems. The accompanying research agenda charts a path forward to transform separation science.
SCIENTIFIC VALUE OF IMPROVED SEPARATIONS
Chemical separations are invaluable processes that underpin diverse chemical transformations and modern technologies. Virtually every product that people use—clean water, pharmaceuticals, fuel for transportation, and so on—depends on efficient chemical separations. In the past, separation-system design did not necessarily consider sustainability; results were high energy consumption, excess solvent waste, unusable byproducts, and other conditions. In the future, the design of separation systems will integrate sustainability into synthesis and use.
To create exquisitely tailored separation systems that have high selectivity, capacity, and throughput and that are robust over time, fundamental concepts must be understood. The research agenda developed by the committee addresses the fundamental research themes noted above through a series of eight research directions and possible paths forward based on a combination of experimental and theoretical approaches. The research priorities described below lay the foundation for transforming separation science, enabling reduced costs and energy use, improving human and environmental health, and advancing efficient new practices in industry.
As the committee developed its research agenda, three cross-cutting topics emerged. The first is synthesis. The novel requirements for designing new separation systems detailed in this research agenda challenge scientists’ ability to make and test the necessary materials. The ability to make molecules and assemblies of molecules that have the desired functional properties is necessary to advance separation science. However, there are synthesis challenges with making new materials in a form that can be used in a separation system. For example, polymeric or nanocomposite membranes developed and tested in laboratories are typically thick (for example, greater than 10 μm), whereas thinner (about hundreds of nanometers) selective layers are needed to produce reasonable throughputs; that requirement motivates the need for synthesis of asymmetric membranes. Furthermore, the science of synthesis itself is in a nascent state, in which many approaches are Edisonian, that is, based on trial and error. The importance of synthesis for many disciplines is clearly recognized, as seen in the exploration of the topic by funding and research communities. High-priority research directions for synthesis science have been highlighted recently (DOE, 2016) and will not be expanded on here.
Additive manufacturing might offer new opportunities to produce structured adsorbents, especially membranes that have improved performance. Demonstrations of improvements enabled by three-dimensional printing of thin-film microstructures on a nanometer scale are emerging (Zhang et al., 2018) as are uses of three-dimensional printing to create functional devices whose desirable macroscopic features might reduce fouling and improve heat and mass transfer rates (Chowdhury et al., 2018). Moreover, three-dimensional printing allows the fabrication of processing equipment to create novel ar-
chitectures, such as twisted hollow fibers (Luelf et al., 2018). The development of new materials coupled with breakthroughs in such fabrication technologies as three-dimensional printing will drive new platforms in which advanced separations can occur (Fichou and Morlock, 2017; Macdonald 2017; Kataoka et al., 2017).
The second and third cross-cutting topics are standardized systems of materials and testing protocols for separation science and the exploitation of data science to extract new knowledge and insights from large complex datasets. Those two cross-cutting topics are discussed after the eight research directions.
RESEARCH AGENDA FOR SEPARATION SCIENCE
The research agenda is divided into two research themes that require fundamental advances: designing separation systems that have high capacity, selectivity, and throughput; and understanding the temporal changes that occur in separation systems. The key problems for those themes are stated below, followed by suggested research directions. Overall, the multidisciplinary research agenda presents eight high-priority research directions and suggested approaches, each of which is critical for transforming separation science. The committee views each research direction as a recommendation for study and exploration. It chose not to set priorities for the various research directions because although advances in each will have major effects, advances in no single research direction will be sufficient to transform the field as a whole.
Theme 1: Designing Separation Systems That Have High Selectivity, Capacity, and Throughput
The goal of any chemical separation is to divide a mixture into two or more purer components. That could mean recovering a protein from a fermentation broth, quantifying the concentrations of dozens or hundreds of components of a complex mixture extracted from a natural product, or obtaining high-purity, polymer-grade ethylene from an olefin–paraffin mixture. The appropriateness of a material for a particular separation is most commonly judged by its selectivity for the desired compounds and the capacity or throughput that can be achieved. Overall, the current fundamental knowledge on how matter interacts in complex environments, including the static and dynamic properties of interfaces, is insufficient to design and manufacture highly selective, high-capacity, and high-throughput separation systems. Separation scientists use many concepts when they envision a separation system. Examples are reversible-reaction chemistry, enthalpic attractions between molecules, and differing diffusivities based on molecular size. A much fuller understanding of those concepts will enable separation systems that are more selective, that have higher capacity and higher throughput, and that can be effective for more complex mixtures and in more challenging environments.
1-A. Advance the Understanding of Complex Systems
The biggest challenge in separation science is to understand and design for complex systems. Tremendous advances have been made in increasing the capacity of a target species and in improving the selectivity of the removal of one component from a binary mixture, but separation materials seldom perform as desired in the presence of multicomponent mixtures, compounds that span a wide range of compositions, or highly dilute or highly concentrated species. The ability to understand and design for the separation of complex mixtures will be a turning point for the separations community and is a key to transforming separation science and industrial practice. The challenges associated with complex systems are described below, with approaches to meet them.
Three main challenges must be met. First, scientists must be able to predict selectivities, capacities, and throughput when they use multicomponent mixtures; a subset of this challenge is to design separation systems for selective interactions in the presence of competition. Second, scientists must be able to design separation materials for mixtures that contain species of vastly different concentrations. Third, improvements in the limits of detection continue to push the requirements for trace analysis.
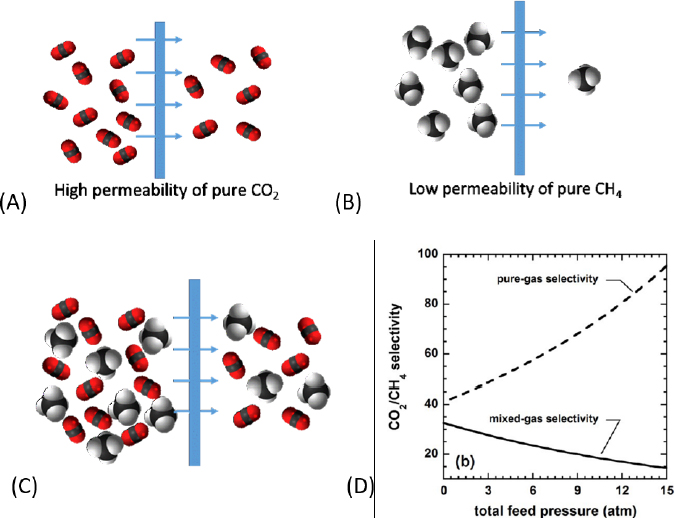
1-A-i: Development of measurement and simulation techniques for multicomponent mixtures. Many separation materials are tested with only pure components. The American Chemical Society recently highlighted that deficiency with a “virtual issue” on multicomponent phase equilibria measurements and simulation (Siepmann et al., 2018). The majority of separation-science literature focuses on measurements of pure-component adsorption isotherms, data on the solubilities of pure gases in liquid absorbents, and pure-gas permeabilities through membranes. Researchers “estimate” the selectivities, capacities, and throughput for separation of a desired mixture by using an ideal model based on the capacities and permeabilities of the components in a pure state (see Figure 5-1 for an example relevant to selectivity). The models are not always valid.
Improved methods for routine measurements of multicomponent separations are needed because the current methods are laborious. Obtaining single data points might take several hours or even days given the time that it takes for a separation system to reach equilibrium. That difficulty creates a series of practical challenges in designing efficient and accurate measurement techniques for multicomponent mixtures and has undoubtedly contributed to the lack of multicomponent data in the literature. Numerous potential separation materials need to be studied, but the number of materials already being considered precludes the study of materials for multicomponent systems, which are given lower priority because of their time-intensive nature. This committee challenges the experimental community to develop experimental techniques explicitly for multicomponent phase equilibrium and transport measurements relevant to separation science. In many cases, new techniques will not be needed; rather, existing analytical methods could be miniaturized and automated to make multicomponent measurements more accessible. Furthermore, it might be desirable to complement gaps in experimental data with benchmarked simulated data, for example, from Grand Canonical Monte Carlo simulations. Data-science methods could also be used to infer relevant missing data and ascertain uncertainties from mixed experimental-simulation datasets.
Accurate models for predicting binary and multicomponent behavior from data on single components would reduce the need for multicomponent measurements, but such models do not exist for most types of separation materials. New mixture models, however, should be developed in the vein of the Ideal Adsorbed Solution Theory (Myers and Prausnitz, 1965). A move toward more realis-
tic models is important for enhanced predictive capability and for systems in which strong intermolecular interactions become relevant. When new models are developed, they will need to be validated with multicomponent experimental data. Recent developments in experimental capabilities to characterize structures from the atomic scale to bulk scale through in situ or operando studies offer opportunities to advance the development of better multicomponent theories and models.
In many cases, molecular simulations that use existing techniques can generate information about multicomponent mixtures far faster than experiments. However, careful comparisons of results of experiments and simulations in nonideal systems are needed to establish the precision of simulation methods. Work to develop carefully controlled “model systems” in which the assumptions underlying existing and developing methods can be quantitatively tested would be valuable (see the section on the cross-cutting concept of standards at the end of this chapter). A model might, for example, consist of extremely well-characterized solid adsorbents, even if they are not the best adsorbents for any particular separation. An example of a recent such effort can be found in Nguyen et al. (2018); the material used in their work finds extensive applications in catalysis (Perego et al., 2001; den Hollander, 2002).
It will also be highly desirable to establish research efforts that account for different length and time scales of information, uncertainty associated with different models, and the complexity of system composition. Once the utility of simulation protocols for multicomponent systems has been demonstrated, aggressive use of simulations to generate fundamental data and insight on mixtures that have many (that is, many more than two) components will be of great value.
1-A-ii: Design of separation systems that can handle a large dynamic range. Often in chemical separations, analytes are present at highly varied concentrations. Separation systems must be designed to operate over a large dynamic range of concentrations if they are to identify and distinguish components at very low concentrations from those present at much higher concentrations. That requires a separation material that has excellent selectivity for the dilute component and whose capacity is not overwhelmed by the analyte present at a higher concentration. The problem is especially important in biological analysis, in which proteins span a very large concentration range and are often present within a plasma matrix. Ideally new systems would permit the analysis of all analytes in a sample without the use of preconcentration methods, which typically favor analytes at particular concentrations.
Separation science combined with sensitive detection methods, such as mass spectrometry, is an essential tool for understanding the world. As an example, preconcentration combined with chromatographic separations and mass spectrometry is commonly used in proteomics and to study human effects on flora and fauna in natural streams. Much has been learned, but there is much more to unravel. For example, oxidative-stress studies provided important evidence that mixtures of compounds (pharmaceuticals and pesticides) at low concentrations were adversely affecting natural biological systems (Escher et al., 2013). However, the concentrations of individual compounds in the mixtures are too low to detect with the most advanced methods, and the number of compounds in question is unknown. Improved preconcentration capabilities and improved separation efficiency are required to tackle such challenges. In separation methods that involve dilution, new strategies to sharpen chromatographic peaks should be developed to improve detection limits further.
1-A-iii: Trace analysis and multistep processes. Trace analyses are important in many fields of science, including the monitoring of residual pharmaceutical compounds in natural and treated water; the monitoring of pesticides, herbicides, and their metabolites in biota samples; and the analysis of similar compounds as residues on harvested food. Typically, trace analysis of targeted compounds requires multistep processing that might include preconcentration of the compounds of interest (which can reduce throughput) and selective removal of compounds that might interfere with the analysis. An example is the trace analysis of pesticide multiresidues in sediments or soils (Nannou et al., 2018).
More selective preconcentration methods and separation methods with the ability to separate a large number of compounds at lower detection limits are needed. Preconcentration and separation methods need to be capable of tolerating complex matrices and be generally applicable for use with numerous solvent systems. They should exhibit low analyte carryover, which is especially important in trace-level determination. Preconcentration methods need to be readily adapted or configurable for use with various separation approaches.
Another example is the wide use of progestin compounds in human and veterinary therapies. Recent studies have shown that concentrations of progestin metabolites were over 60% higher than the concentrations of the parent compounds in sewage effluent and river samples (Shen et al., 2018). More insight could be gained at lower detection limits through research in multistep separation processes.
1-B. Explore the Entire Array of Thermodynamic and Kinetic Mechanisms
To improve selectivity, capacity, or throughput in separation processes, separation scientists could explore a broader range of multiple forces, entropic strategies, and cooperative binding mechanisms; a wider array of chemi-
cal transformations; and new ways to determine and control differential rates of species transport. Each topic is described in more detail below. The many thermodynamic and kinetic parameters that determine a system’s free energy are sometimes referred to as the free-energy landscape. For example, enthalpic or entropic contributions can alter local configurations, and interfacial characteristics can act as templates for metastable structures in a separation system (see Figure 5-2).
1-B-i: Explore a broader variety of multiple forces, entropic strategies, and cooperative binding mechanisms. Separation scientists can tune the free energy of the system by using a multitude of forces—such as dispersion or van der Waals forces, dipolar forces, dipole-induced dipole forces, hydrogen bonding, hydrophobic interactions, and electrostatic interactions—and entropy, which is the basis of size-exclusion chromatography. Using those forces and entropy can drive phase separation, the formation of micelles and microemulsions, and structuring on length scales from atomic to macroscopic.
Many of today’s separation technologies take advantage of a single force, but separation scientists are starting to explore the use of multiple forces or a combination of enthalpic and entropic effects to facilitate particular separations (see Figure 5-3). For example, in addition to the well-understood role of metal–complexant, near-neighbor enthalpic contributions to selectivity in metal-ion separation, solvent prestructuring into biphasic or multiscale architectures has recently been implicated as influential in both selectivity and capacity (Kunz et al., 2012; Ellis et al., 2014; Poirot et al., 2016; Duhamet et al., 2017; Ballesteros-Gomez et al., 2018; Karmakar et al., 2018). Recognized as an important synthetic challenge, the design and formulation of multiscale, hierarchical structur-
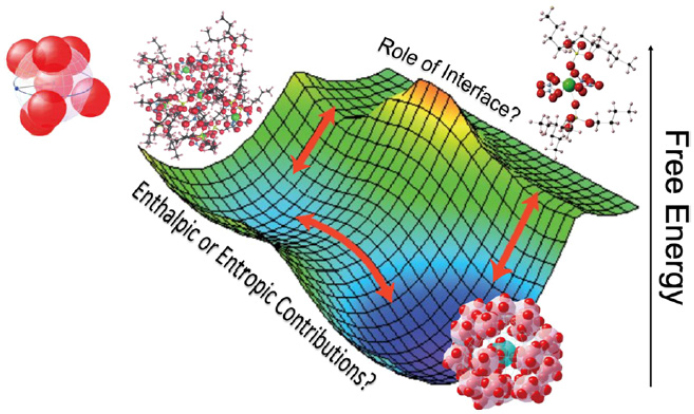
ing within separation systems constitute a subset of more broadly defined studies in soft-matter chemistry (Backov, 2006; Mann, 2009), which need to be exploited in separation science.
An excellent example of the use of multiple thermodynamic forces is the development of ionic liquids that can take advantage of multiple solvation interactions (Anderson et al., 2002) and the application of the multiple interactions in the development of stationary phases for chromatography. Other examples are the use of selective binding and packing in the design of crystallization agents (see Box 5-1) and the use of the enthalpy and entropy of reaction of an ionic liquid with CO2 (see Box 5-2).
Nature uses multiple forces to perform key functions. Biological systems are often required to select a targeted species from chemically similar constituents in
a mixture in which there is little opportunity to control environmental conditions. Furthermore, there is often the need to select from energetically equivalent species—a dilemma that is overcome by moving to a receptor-mediated process that depends on structurally enhanced mechanisms for selectivity. Such an approach transforms a single-stage energy-driven separation mechanism into a multistage, structurally driven one. For example, ferric iron is selected for transport through a cell wall by a bilobal protein that requires both lobes to be in a closed conformation that can be realized only through stringent, cooperative processes (Sun et al., 1999).
Realization of a nature-inspired multistage approach requires the design of hierarchical structures that are able to amplify, on each stage or length scale, the small differences of the preceding stage and thereby build a unique conformation that involves complexing agents recognizable by a receptor site. The design of such a complex requires a detailed description, at the molecular level, of structural variations that can then be successively amplified to distinguish the complex from similar constituents in the mixture and to capitalize on the difference through distinguishing receptors. The global free-energy landscape needs to be considered in the development of that strategy.
One important strategy used by nature is cooperativity, the core principle that underlies molecular recognition: multiple binding groups act in concert to provide a binding site that complements the electronic and geometric properties of a guest species. Scientists have had some success in arranging matter in that way, but they now recognize that full mastery of cooperative effects for selectivity by design requires manipulating the environment at much greater distances than the first shell of binding groups. A future vision includes precise control of packing effects, solvation, the role of modifiers, and the structuring of molecular assemblies. Furthermore, how cooperative binding effects might be coupled with a functional transport system and binding-release cycling remains to be understood and exploited. Triggering binding and release would be, in essence, manipulating cooperativity by using external forces.
The development of experimental methods for measuring the many potential forces between species and separation materials directly can greatly facilitate progress. The techniques include use of a surface-forces apparatus for measuring protein interactions with membranes (Koehler et al., 1997, 2000), atomic force microscopy to measure split intein binding for use as fusion proteins in bioprocess affinity column separations (Wood, et al., 2000; Sorci, et al., 2013), and laser tweezers and other optical force measurement devices for measuring particle–particle interactions for analytical electrochemical and other separations (Aveyard et al., 2002; Jonáš and Zemánek, 2008).
From a modeling and simulation perspective, one can consider the free-energy landscape on which a separation process occurs and how, overlaying statistical mechanics, the ensemble of enthalpic and entropic effects influences the total free-energy and separation outcomes. One important need is the development of theoretical methods for accurately treating short-range and long-range forces on an equal footing. Long-range forces can be estimated by using continuum approaches, provided that the forces are not sensitive to specific organizational effects of the media (media organization changes in multicomponent mixtures), but those organizational effects might be exactly what are needed to facilitate a separation. The ultimate goal, which is increasingly possible with increasing computing power, should be explicit incorporation of all forces in a simulation to test those effects.
It might be effective to consider landscapes associated with the bulk mixture to be separated and how knowledge of the landscapes can control speciation (what chemical species are present). If relevant, the landscape associated with interfacial reactivity might also be mapped (see Research Direction 1-C). Finally, the landscape associated with the speciation of the separated products might be mapped. This divide-and-conquer strategy allows the appropriate modeling and simulation method to be applied on different time and length scales. To map the global thermodynamic landscape, a variety of methods have been used successfully in other fields (such as synthesis science) and include stochastic Monte Carlo–based algorithms, genetic algorithms, and deterministic accelerated molecular dynamics and related methods. With a global landscape in hand, it is possible to delve deeply into specific regions relevant to the separation of interest and to understand the balance of enthalpic and entropic contributions.
Provision of a theoretical description of the complex energy landscape is a major challenge. Much opportunity exists to create new algorithms that can cross time and length scales and accurately predict barriers on the surface and ensemble outcomes at the molecular level. To map the free-energy landscape, one must simultaneously apply theory, computation, and in situ characterization. Research and development in those fields is needed so that modeling and simulation can be used more effectively to explore the free-energy landscape of separation processes.
1-B-ii: Design for recycle and reuse. In exploring the free-energy landscape, special attention needs to be paid to identifying regions of steep gradients, for example, where the chemical potential of a species changes substantially in response to small changes in some operational variable. That is because in most separation processes, separation materials are designed to be reused for economic and sustainability reasons. There are exceptions, such as in the separation of critical materials (for example, precious
metals) or when the separation material serves additional purposes, for example, in the later chemical conversion of the target species. Nonetheless, the separation materials must be regenerated or cycled in most cases. When the mechanism that conveys the selectivity is a reaction (see 1-B-iii), it means that the reaction must be reversible. When cycling is necessary, the important characteristics are not just the selectivity and capacity for the target species in the first contact of the mixture with the separation material but the working capacity and resulting selectivity after multiple full cycles.
An important part of the design is the “trigger” or field that will cycle the separation medium, that is, the independent variable in the thermodynamic landscape. Traditionally, such triggers have been temperature, pressure, and pH. Other triggers have recently started to be used for the cycling of separation materials, as described in Chapter 3, including light, electric and magnetic fields, and microwaves (Li and Hill, 2017; Mao et al., 2018). Figure 5-5 shows an example that uses light. The external stimuli to facilitate cycling are forms of energy. More important, some of the nontraditional triggers require the use of electric energy, which should be evaluated with respect to the equivalent heat energy required.
Irreversibilities in the cycling of separation materials can be challenging. For example, if a liquid absorbent becomes more viscous as it absorbs the species of interest, reversing the process—desorbing the species of interest—might be much more difficult than the absorption, and this can result in unwanted irreversibilities and decreases in working capacity and selectivity. A common condition that limits the recovery of a target species after separation is the stability of the bound complex, inasmuch as energy applied to release the analyte can compromise the separation material. Recognizing such irreversibilities is important in considering the full free-energy landscape in the design of separation systems that have high selectivity, capacity, and throughput.
1-B-iii: Explore a wide array of chemical transformations to facilitate separations. Selective reaction chemistry and the structural transformations of molecules or materials provide enhanced selectivity, the removal of endogenous interferences, or the improvement of isomer separations. One example of a successful chemical transformation is the removal of CO2 after combustion processes (see Box 5-3). A wide array of chemical transformations have the potential to facilitate both large-scale and small-scale separations, and current technology has only begun to explore the possibilities. A combination of theory and experiment could predict the full ensemble of viable chemical transformations under different conditions. The global free-energy landscape described in 1-B-i
could be used to predict reaction rates and identify reversible reactions to use in separations.
Another promising technique is microkinetic modeling, which has been developed and used extensively and successfully in the catalysis community (Broadbelt and Snurr, 2000). Accurate measurements of thermodynamic properties of adsorption or binding can be combined to design a reaction network that includes the ensemble of simultaneously occurring chemical reactions in a separation process.
In chromatographic separations, reactive chromatography for complex-mixture analyses might have value as an additional means of providing selectivity. For example, human breath typically includes as many as 3,000 compounds; some of them that are present at low concentrations (parts per trillion to parts per million) might be biomarkers of diseases, such as cancers and diabetes (Pereira et al., 2015). Selective preconcentration of potential biomarkers is essential. Selective preconcentration of trace amounts of aldehydes in breath is facilitated by solid-phase microextraction on-fiber reactions with o-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine (PFBHA), which forms oximes that can be sensitively measured with mass spectrometry (Poli et al., 2010). Similarly early-stage diabetes can be detected through the quantitation of acetone in breath by using on-fiber reactions of acetone with PFBHA to form an oxime (Deng et al., 2004).
1-B-iv: Determine and control rates of species transport. Many separations are based on the different rates of transport of different species. The most obvious example is membrane separations, in which selectivity and throughput depend on the diffusivity of various species through the membrane. Any separation process that is limited by mass transfer (such as absorption and liquid–liquid extraction) can be affected by the mass-transfer rates of different species. Different rates of species transport across interfaces is especially important, so it is discussed separately in the next subsection (1-C).
Accurate measurement, modeling, and prediction of the rates of transport of solutes under a wide variety of conditions are needed. In general, permeation fluxes through porous and nonporous membranes are driven by gradients in chemical potential (osmosis, reverse osmosis, nanofiltration, ultrafiltration, and microfiltration) or electric potential (electrodialysis and electrophoresis). However, those gradients are not measured directly but rather are inferred from the relative concentrations of species on the feed and permeate side of the membrane. For example, with reverse osmosis of such salt solutions as seawater or brackish water transport through the membrane is thought to occur via a solution-diffusion mechanism: solute first dissolves into the polymer membrane and then diffuses down a chemical potential gradient through fluctuating spaces between the polymer backbone. Traditionally, the movements of water and salt are dictated by pressure and concentration driving forces, respectively (Baker, 2012). Another example is the transport of suspended particles (such as cells or protein aggregates) through microporous membranes where the fluid drags the particles that also interact with themselves and with the membrane pore surfaces via electrostatic, van der Waals, and other forces. The relative transport rates of fluid and particles will be different and should be modeled within the microporous membrane together with intermolecular force measurements. Those analyses will assist in the design of more effective microporous structures of the membranes.
To optimize performance and design of those materials, detailed tracking of species inside the membranes is urgently needed. It will enable membrane and adsorbent designers to form membrane pores and fluid paths through such materials structurally to maximize selectivity and capacity. Techniques that could be used for the measurement and modeling of rates of transport of different species include radioactive tracking, scanning electron microscopy for image reconstruction to obtain three-dimensional flow channels (Sundaramoorthi et al., 2016), and fluid mechanics of particle drag in the three-dimensional structure to guide experiments.
1-C. Characterize the Interface and Understand the Interfacial Forces
In separation processes, selective mass transfer often occurs across interfacial regions—the external interfaces of porous solids rather than the extended internal spaces. Although the structure and dynamics of such external interfaces are always of fundamental interest, they do not always determine the rate or selectivity of a separation process. Rates of transport of species of interest across interfaces can be much greater than rates through the bulk phases (the parts of the separation system not influenced by interfaces). In other cases, though, interfacial transport or kinetics are slow enough (compared with those in the bulk phases of the separation system) to be rate-determining. That means that the interfacial region affects the throughput or capacity of the separation process, and possibly, even selectivity. In such cases, it is critical to understand the structure and dynamics of the external interfaces and interfacial regions and to determine how they affect equilibrium and relative mass-transfer rates across the boundary. That investigation should entail examination of the role of surface roughness and imperfections (defects) as possible locations for selectivity or rate-determining behavior. Knowing whether the external interface is rate-determining for a particular separation system is critical, and Box 5-4 discusses ways to investigate this. When interfaces do influence a separation system, it might be possible to design the interfaces to accomplish particular separation tasks, ranging from kinetic resolution of the main mixture components to selective exclusion of contaminants or foulants that can compromise the robustness of the separation process (see Theme 2). Some such opportunities are discussed below.
1-C-i: Controlling interface structure and transport to enhance separations. There are many examples of technologies in which it is critical to control the structure and extent of interfaces. Specialized liquid–liquid contactors have been developed for liquid extraction to ensure high efficiency. Similarly, packing in gas–liquid absorber and stripper systems is designed to increase the interfacial area between the liquid and the gas.
Regarding extended internal interfaces, extremely high-surface area adsorbents have been developed to separate gas and liquid mixtures on the basis of selective adsorption and transport of species induced by interfacial confinement (see Box 5-5). As the pores become smaller, gas adsorbate diffusivities can vary widely and lead to the onset of selective transport mechanisms, such as Knudsen and configurational diffusion (Kärger et al., 2012).
Zeolites and metal-organic frameworks (MOFs) provide crystallographically defined high-surface area inter-
faces that can be designed to discriminate among molecules on the basis of small differences in size, shape, and chemical functionality (Jeon et al., 2017). Amorphous solids—such as porous carbons, porous oxides, polymers, and pyrolyzed polymers—offer a wider, less precisely defined, range of confinement effects that can also be used for selective separations, especially as membranes.
In addition to their extended internal interfaces, porous solids that are in contact with fluids have interfaces at their external surfaces, that is, at pore entrances. Those interfaces might be structurally and chemically unique environments that include heterogeneous sites or defects, which could affect separation outcomes. The latter interfaces, unavailable to nonporous surfaces, can also exhibit confinement effects. For example, nanometer-sized features of the external surface of some zeolites (half-pores or semi-cups) have been used as a mimic of cyclodextrin (Galletero et al., 2003). Moreover, coupling of adsorption and transport in the internal and external interfaces of porous solids can lead to cooperative effects that enhance separations, or to competing effects that can inhibit separation performance or lead to erroneous interpretations of transport and adsorption equilibria. For example, recent studies have established the importance of pore-entrance resistances in zeolites (Hibbe et al., 2011). In hierarchical (micromesoporous) zeolites, large characteristic diffusion distances resulting from confinement in micropores can be erroneously interpreted as slow diffusion (Bai et al., 2016).
One can envision the design of internal and external interfaces of porous solids for optimal coupling of transport and selectivity (and activity) to enable equilibrium or rate-based separations. Of particular interest are two-dimensional porous materials in which the internal and external interfacial fractions are similar and in nanometer-scale proximity to one another, maximizing the potential for synergism.
It is possible to functionalize the external surfaces of two-dimensional zeolites, MOFs, and covalent-organic frameworks by using functional groups or linkers that are absent or less abundant in the interior pores. That functionalization could be used to create novel ordered nanometer-sized ensembles (with adsorption sites that combine functionalized pore mouths and nonfunctional-
ized pore interiors) that could be optimized for the selective recognition, adsorption, and transport of complex molecules, such as proteins, DNA, RNA, and surfactants.
Interfaces and confinement effects can be even more important in liquid and ionic media. For example, the effects are important where charge regulation (Ninham and Parsegian, 1971) and depletion of adsorbed species depend strongly on the length scale of the confining dimensions, such as pore size and interparticle distance (Gaddam et al., 2018). An example is the case of the conformational stability of proteins during bioseparation in hydrophilic pores (Radhakrishna et al., 2013; Grimaldi et al., 2015).
Interfaces affect structure in the interfacial region, which can range from molecular to macroscopic dimensions. As a result, they can alter the solvation of different species in their vicinity (Somasundaran and Huang, 2000), selectively bind molecules that neutralize surface charge (the double layer), influence ion solvation (Barrett et al., 2016), affect the orientation of water molecules (Kocsis et al., 2018; Rock et al., 2018), trigger adsorptive cascade processes (such as surface fouling; see Theme 2), and affect the structure of adsorbed species (for example, inducing ion rearrangement and water structure). All those influences can be used to advantage in performing separations.
Interfaces can also influence fluid flow in separation systems as a result of surface structure, defects, roughness, chemistry, and flexibility. When a solid surface is present (such as a porous adsorbent or membrane), a fluid flowing above the surface exhibits no slip at solid surfaces and slip in the open regions of a porous material. That affects shear rate at the wall, re-entrainment of material deposited on the surface into the flowing fluid, and performance (selectivity and permeation flux) through porous media. Surface roughness effects are scale-dependent and represent increased surface area for possible species deposition (Han et al., 2003).
Chemical functionalization of surfaces is another tool to influence flow, adsorption, and separations. For example, brush-like surfaces that emulate villi in nature are highly flexible and have high surface areas that can be used to influence flow and adsorption. Such complex interfacial structures can affect performance in chromatography, membranes, and adsorption. Functionalized surfaces that have hydrophilic or polar molecules can have a substantial effect on selectivity and capacity because of solute adhesion and surface charge of the solid surfaces (beads, membranes and microfluidic channels).
1-C-ii: Understanding interfaces in separation systems. Experiments, theory, and molecular modeling are needed to study interfaces relevant to separation systems. A major challenge is to determine the structure of interfaces and how the molecular interactions between the two phases cause formation of the interfacial region. Defects are often a particularly important aspect of interfacial structure that are challenging but essential to probe. Interfacial regions can range from molecular to macroscopic dimensions; the former is much more challenging to detect and characterize. Interfaces can be abundant and readily observed and characterized, or they can be scarce and hidden from bulk characterization techniques. Further complicating the experimental challenge is the need to probe the interface in situ and operando, where structural evolution can affect the kinetics of mass transfer.
Historically, in separation science, interfaces have been characterized through indirect, bulk studies, at least partly because of the inherent difficulties associated with probing interfacial structure and speciation under complex operando conditions. Few molecular to mesoscale experiments have been undertaken to probe the role, influence, and effects of atomic-, molecular-, and hierarchical-scale structuring at an interface in a separation system. That situation has arisen from a lack of appropriate experimental techniques that can provide direct information on interfaces, which often are buried in solid–liquid and liquid–liquid systems and have low concentrations of the targeted species. Complicating the issue is the unknown effect of temporal and spatial dynamics; understanding these effects will require operando studies. Scientists do not know enough about the interfaces and interfacial regions that are present in separation systems.
The advent of interface-specific probes capable of measurement in realistic operating conditions makes quantitative characterization of interfacial processes possible. Examples of such probes are ambient-pressure x-ray photoelectron spectroscopy, in situ x-ray scattering techniques for structure determination at solid–liquid interfaces, sum frequency generation spectroscopy, and in situ scanning probe and atomic-force microscopies paired with spectroscopy (see Chapter 3 and Appendix C). Those advanced tools can provide information on solid and liquid interfaces that is needed to control mass transfer.
In studying an interface, the first step is to identify the species present and their concentrations by using advanced surface-specific spectroscopic tools. Do the species differ from the bulk? If so, why? What forces are at play and how do these relate to the structure at the interface? A fundamental understanding of intermolecular forces at interfaces is critical for improving the performance of capillary electrophoresis, chromatography, membrane filtration, adsorption, ion exchange, affinity multimodal systems, and others. For example, the behavior of ions near charged interfaces directly affects the resolution and speed of separation via electro-osmotic flow in capillary electrophoresis.
Theoretical models used to describe adsorption and particle–particle interactions still require essential improvements. The classic Derjaguin-Landau-VerweyOverbeek (DLVO) theory accounts for Lifshitz–van der
Waals (LW) and electrostatic double-layer (EL) interactions (Derjaguin and Landau, 1941; Verwey, 1947) but is inadequate in explaining other surface interactions, such as structural, hydration, hydrophobic, and steric forces (Israelachvili, 1982, 2011). Hydrophobic and hydration effects are considered important for apolar surfaces in bioprocessing and bioanalysis (Ma et al., 2015; Barrett et al., 2016). Lewis acid-base (LaB) interactions are considered the cause of hydrophobic attraction and hydrophilic hydration repulsion. The combination of LW with EL and LaB, called the extended DLVO theory, has been used to study the charge effects on inorganic membrane performance in a cross-flow microfiltration process (Elzo et al., 1998) and colloidal adhesion to hydrophilic membrane surfaces (Brant and Childress, 2004). Fundamental research is needed to quantify hydrophobic and other non-DLVO interactions in water for nature-inspired affinity ligands on surfaces used in bioprocessing with chromatography and membrane filtration—an important missing link in describing interfacial forces.
The second step is to understand whether there is molecular-scale ordering parallel to the interface. For example, in a liquid–solid chromatographic system involving a silyl-ether decorated silica surface, hydrophobic interactions are presented at the interface in such a way that an accompanying aqueous-based liquid might form an ordered structure near the surface. When surface silanols are present on silica, hydrogen bonding of the solvent with the surface will provide more hydrophilic interfacial regions. Characterizing the shifting on a molecular level from hydrophobic to hydrophilic interactions and the resulting topological changes in the interface would inform mechanisms to influence the efficiency of a separation.
Roughness can affect transport near surfaces, which in turn can influence separation performance. In the silyl-ether example above, physical heterogeneities, such as roughness, can cause disorder in the distribution of hydrophobic and hydrophilic regions that can work against the development of molecular-scale ordering parallel to the interface. So, it will be important to understand the roughness of an interface (or solid surface) and at what length scale. Atomic force microscopy (AFM) and similar techniques are excellent for characterizing roughness. AFM can also be used to measure forces and to discover ordering and layering near solid surfaces (Zhang et al., 2017; Appendix C).
Lastly, it is valuable to consider how the various chemical components and system conditions (such as temperature and pressure) affect an interface. One might be able to use that information to tune the properties of interfaces to enhance selectively. Alternatively, one might be able, de novo, to design separation materials that can harness the effects of system components and conditions to limit adverse effects of interfaces on separation kinetics. An example is the use of materials that exploit excluded species to precondition material interfaces to enhance selective uptake of the target and improve overall separation performance.
Molecular simulations can complement experimental measurements in the characterization of interfaces. For example, Garde and his group have shown that “enhanced density fluctuations at hydrophobic–water interfaces also make those interfaces significantly more compressible than bulk water, whereas the compressibility of charge-neutral hydrophilic–water interfaces is bulk like. The density fluctuations are sensitive to interactions and respond to small changes in the chemistry of the underlying surface. Specifically, the heterogeneous surfaces show an interesting context dependence” (Acharya, 2010). That is, a hydrophilic moiety (-OH) surrounded by many hydrophobic moieties (-CH4) has a more dramatic effect on water in the vicinity than does a single hydrophobic moiety surrounded by many hydrophilic moieties. Such discoveries could have effects on the design of multimodal affinity groups for chromatography and membrane filtration and desalination.
The influence of interfaces (especially solid surfaces) on flow can be studied both indirectly (for example, by changing the interfacial area or interfacial structures) and directly with various spectroscopic techniques. Using and advancing experimental and computational tools to characterize interfaces, especially under operando conditions, will provide information that should enable control of interfacial phenomena and enhance separations.
1-D. Understand the Physical Changes That Result from External Forces
External stimuli can cause physical changes in a separation material. Conventional external influences (such as temperature and pressure) and unconventional stimuli (such as electric, magnetic, microwave, and light fields) can trigger the regeneration of separation materials, as discussed in Subsection 1-B-ii. Regeneration is accomplished by using one of those stimuli to change the affinity of the target species or analyte for the separation material, as occurs in temperature or pressure swing adsorption.
External stimulus can also induce changes in the separation material itself that affect the affinity of species for the material. An example is in the separation of nitrogen from methane with molecular gate adsorbents. That is a kinetics-based process that takes advantage of a temperature-induced change in the pore structure of microporous titanosilicates (Kuznicki et al., 1999).
Controlling structure at the angstrom to subangstrom level to separate small molecules—such as nitrogen, oxygen, CO2, and methane—remains challenging in a variety of separation systems. To take advantage of the influence of external stimuli (such as mechanical responses), they must be studied and understood. What materials exhibit
the largest changes in response to external forces? In the simplest terms, it is necessary to identify, through either experiments or simulations, materials that have large isothermal compressibilities or thermal expansion coefficients.
Understanding and predicting the net effect of the mechanical response of a separation system on capacity, selectivity, and throughput in the presence of a complex mixture remains challenging. For example, changes in the hydration level of inorganic separation materials (such as zeolites), organic materials (such as polymers), and inorganic–organic hybrids (such as MOFs) can lead to both increases and decreases in selectivity and capacity. As emphasized in Theme 1-A, understanding the effects of complex mixtures is exceedingly challenging but essential for designing effective separation systems. The situation is no different for designing separation systems based on physical changes of a separation material itself. The suggested approach is to understand the effects of complex mixtures on model systems that are chosen because of their large (for example, mechanical) response to an external variable (for example, temperature or pressure) when a single analyte is present.
Theme 2: Understanding Temporal Changes That Occur in Separation Systems
The performance of most separation materials degrades with time. Degradation is typically undesirable except in cases in which low cost materials can be developed for single-use separation technologies. Poor understanding of that degradation is one of the main reasons that industry is cautious in adoptng new materials-based separation technology for process-scale separations. Knowledge of the fundamental mechanisms that prevent long-term performance of separation materials over a spectrum of complex environments is insufficient. Understanding how separation materials and separation systems change with time involves fundamental chemistry and materials science. It is as important for this basic research agenda as is the initial design of the separation materials and systems (Theme 1).
Under some environmental conditions and over the course of time, separation materials can undergo chemical, physical, and structural changes that alter their selectivity, capacity, and throughput. The factors in and origins of those changes need to be understood and addressed to design robust separation systems to be used in complex environments. For any particular separation material, the first challenge is to identify the factors that are causing it to change with time. Three of the most common factors are changes from a nonequilibrium or metastable to an equilibrium state, chemical reactions, and interactions with non-targeted species in the mixture. Suggested research directions for those three topics and a research direction for the development of adaptive systems are described below.
2-A. Determine Changes from Nonequilibrium States That Affect the Chemical and Physical Properties of Separation Materials
Many separation materials are in either an unstable or a metastable state; both states can be classified as nonequilibrium. A metastable state is a local minimum in the free-energy landscape, where a perturbation of the system is required to cause any changes. In many such materials, the selectivity and capacity for the separation system are influenced by the chemical nature of the material (for example, dispersive interactions, hydrogen bonding, and complexation between an analyte and the material), whereas the nonequilibrium state defines the physical nature (for example, pores of various shapes and sizes). Box 5-6 provides an example involving polymer packing structure.
Spectroscopic characterization techniques, including positron annihilation lifetime spectroscopy and x-ray scattering techniques, could help to elucidate not only how the average nonequilibrium packing structure changes but how the local sizes and shapes of packing structures change with time. However, the time scales for such processes are long (often days, months, or years), and this poses an experimental challenge.
In addition to glassy polymers, such porous materials as zeolites and MOFs can undergo structural changes. The changes can be ascribed to solid-state crystalline-phase transitions, the breaking of chemical bonds, or the structural collapse of pores. The specific pathways to formation of new metastable states can be related to a variety of factors, such as the chemical composition of the porous material, removal of adsorbed molecules (such as water or templating molecules), temperature changes, and applied pressures (Cruciani, 2006; Chapman et al., 2009). In some cases, such as those described for “breathable” MOFs (for example, MIL-53), reversible metastable states can be reached as a result of temperature swings or the addition and removal of adsorbates into or from the MOF pores (Serre at al., 2002; Liiu et al., 2008). Amorphization of crystalline solids is always entropically favored, so a careful evaluation of bond enthalpies and adsorbed components must also be considered in assessing the full thermodynamic landscape and therefore the stability of porous materials.
Nonequilibrium phases must be understood better if they are to be exploited in separations. As a result of the long time scales of some transitions to equilibrium, reliable techniques for accelerated aging are needed. Mapping out the global free-energy landscape (as described in Theme 1-A) of separation materials would allow researchers to identify and consider nonequilibrium states as avenues to creation of new separation systems. Computational techniques can elucidate polymer packing structures far from equilibrium, but the slow dynamics of chain relaxations make molecular simulation of the aging process quite challenging. Overall, mechanistic studies (like those described above), coupled with theory and computation, are probably the best avenue to control of the free-energy landscape: nonequilibrium phases can be purposefully synthesized and their evolution controlled.
2-B. Determine the Identity and Rates of Fundamental Chemical Reactions That Can Change Separation Materials and How the Reactions Are Influenced by Operating Conditions
In many cases, degradation of separation systems occurs via chemical reactions (hydrolysis, radiolysis, oxidation, thermal decomposition, oligomerization, depolymerization, and so on) that irreversibly alter solid or liquid separation material. Those reactions can degrade system performance in several ways: they can slowly decrease the amount of active material present, they can make byproducts that interfere with the separation material’s performance (for example, by occupying binding sites), they can deposit products on surfaces and clog pores (discussed in Theme 2-C), and they can cause catastrophic failure of the materials, as occurs for some MOFs that hydrolyze. The rates of chemical reactions that can degrade separation systems can depend heavily on temperature, analyte and impurity concentrations (see Box 5-7), and other physical conditions.
As an example of the effect of a chemical reaction on separation performance, consider the development, over the last several decades, of new classes of porous crystalline materials that have potential applications as membranes and adsorbents. Some of the materials collapse into nonporous and amorphous structures. Sometimes, the pore collapse is purely mechanical and is brought about by applying pressure. However, it can also be caused by a reaction between components in the mixture to be separated and the porous material.
A key challenge is to understand the fundamental reactions, mechanisms, and rates that lead to pore collapse. For example, MOFs are formed by bridging organic ligands to metal cations or metal clusters. The coordination bonds between cations and ligands are reversible, and this can result in pore collapse if the bonds are chemically broken (see Box 5-8). Such reversal is related to bond strength, ligand flexibility, and the presence of various components in the mixture that compete for coordination binding sites and thereby affect the stability of bonds. This example emphasizes the fact that degradation reactions can take place in bulk (liquid or vapor) phases, on surfaces, or at interfaces. Because many separation materials are designed to have large surface areas (for example, solid adsorbents and packing for chromatographic columns), most of the reactions can take place on the surface. The reactions can be stoichiometric or catalytic.
There is a pressing need to understand the fundamental reaction pathways that control changes in the performance of separation materials. Researchers need to identify reactions and reaction products and determine reaction rates. Innovative techniques that leverage spectroscopy and other chemically sensitive probes need to be deployed to distinguish between possible reactive events (such as hydrolysis, oxidation, thermal decomposition, oligomerization, and depolymerization), especially under operando conditions. Those tools are not unique to separation scientists but rather can be adapted from other scientific fields.
If all possible degradation reactions are known and can be characterized, a reaction network model theoretically could be developed. Microkinetic modeling for catalytic and noncatalytic reaction systems is a well-developed field (Broadbelt and Snurr, 2000; Salciccioli et al.,
2011; Raybaud, 2007) that could be applied to describe changes in separation materials over time. However, as with multicomponent capacity and selectivity measurements, the time and effort required to obtain the experimental data needed as input to the models is daunting, even when quantum calculations and atomistic simulations are used to predict many of the properties.
A more tractable path forward would be to choose model systems for the type of investigation described in the previous paragraph. Those systems should be chosen to have well-characterized separation materials (for example, a solid aluminosilicate adsorbent or a relatively simple ionic liquid) and be exposed to a relatively simple binary mixture. Although even the basics of bond-breaking and bond-making are not generally understood as functions of local conditions, there has been some work on some materials relevant to separations. For example, a recent paper (Standl and Hinrichsen, 2018) reviewed detailed microkinetic modeling of catalytic olefin cracking and methanol-to-olefins that uses well-characterized zeolites, including ZSM-5, ZSM-22, and SAPO-34. If the microkinetic modeling of the degradation of these systems in the presence of even a couple of species is successful, one could develop accelerated testing protocols that might be applicable to a much wider array of separation materials. Efforts to develop well-controlled model materials or model mixtures (see the section “Cross-Cutting Concepts” below) that allow deep insight into the reactions associated with key contaminants will bring great value to the community.
As a complement to difficult and time-consuming experimental efforts, reaction-network modeling represents an important opportunity for the simulation and theory community. Simulation has the potential to determine all possible reactive pathways (degradations) that could affect the material (see Theme 1-A). One important computational challenge is to calculate barriers on energy surfaces accurately (and thus, accurately calculate reaction rates) given the high level of accuracy needed (1 kcal/mol uncertainty in an energy barrier translates to an order-of-magnitude uncertainty in reaction rate).
Moreover, predicting the distribution of potential defects (degradation products) poses a massive theoretical challenge as it depends heavily on a number of environmental factors. It might be possible to study the accumulation of deleterious species, defects on surfaces, and other degradation products, starting from an ideal medium and under ideal conditions, but accounting for the true complexity of the operational environment is currently inaccessible by theory alone. Predictive data-science methods might present an opportunity: they might use a combination of experimental and simulation data and infer the dependence of the reaction chemistry on environmental conditions, concentrations, and other factors. A challenge for computational modeling will be to describe the long-term cumulative effects of degradation as separation media evolve during extended use.
2-C. Understand the Fate of Unwanted Products
As described above, a variety of reaction products can appear in separation systems over long periods, and the fate of these products is key to understanding changes in a separation system with time. Therefore, both the detailed chemistry of the various degradation reactions and their interactions with separation materials and the relevant phase equilibria must be understood. For example, decomposition products can contaminate a gaseous product stream if they are low-volatility compounds or if they separate from the gaseous or liquid solution and agglomerate or precipitate on surfaces. Some decomposition products could result in coke formation, which is commonly observed when heterogeneous catalysts are used. If degradation of separation materials cannot be inhibited (for example, through changes in the physical condition or the use of additives), perhaps it can be tailored to result in products that do not reduce the robustness of the separation system, such as gaseous degradation products instead of coke.
The formation of solid phases, through agglomeration or precipitation, commonly reduces the robustness of separation materials. Solids can block the pores of solid adsorbents and membranes. The buildup of degradation products and impurities on a porous surface that has high surface area can provoke additional unforeseen catalytic reactions. Overall, the mechanistic chemistry and phase equilibria of decomposition must be understood and incorporated into the design of separation materials at an early stage of development and testing.
An important gap in understanding at the molecular level is related to nucleation and growth phenomena. Nonclassical nucleation pathways are intimately related to free-energy landscapes (see Theme 1-A) and to the differences in surface energy between polymorphs on the nanoscale. Separation processes generate high local concentrations of species that can nucleate and grow foulants. Those species can be the ones targeted for separation or can be impurities and degradation products that accumulate in the separation system. Their high concentrations can be caused, for example, by gradual accumulation from incomplete regeneration in cycling processes, by concentration gradients due to polarization in membrane processes, or by unwanted side reactions. Limiting nucleation and growth in separation materials is important in avoiding a reduction in capacity and an increase in resistance at interfaces.
The fundamentals of molecular and ionic crystal nucleation and growth, size-dependent colloidal particle aggregation in various solvents, and nucleation and growth of carbon and other deposits on surfaces and in
porous media can be studied with various techniques that are commonly used in materials synthesis. They include in situ microscopy (for example, in situ video-rate AFM and liquid-cell transmission electron microscopy, confocal microscopy, and infrared–Raman spectroscopy), light-scattering, diffraction, and other total scattering techniques. Those techniques could be adapted and extended to address nucleation-based and growth-based separation-media deactivation phenomena, which often occur under large gradients of, for example, concentration or temperature. They often have low rates compared with those of materials synthesis.
Changes in separation materials caused by solid formation are common in flow fields. Aggregation, crystallization, and flocculation driven by thermal motion are relatively slow processes, whereas much faster processes can be achieved by imposing velocity gradients through mixing and flow fields. In separation systems in which there are velocity gradients—for example, during cross-flow filtration of alpha-helix–rich globular proteins, such as human serum albumin—aggregation of suspended species can seriously affect selectivity and permeation flux (capacity) through cake build-up, pore constriction, and pore intrusion. How aggregated or nonaggregated particles perform when transported through and into porous media (convective flow above and through media, such as membranes and chromatographic beads) has hardly been studied, either experimentally or theoretically by combining computational fluid mechanics with surface interactions. Intermolecular forces between different particle types and inner porous surfaces of different materials must be understood if particle intrusion in such systems is to be predicted fully. The dynamics and physics of these processes are critical fundamental problems for separation technologies that need further research.
Nucleation and agglomeration could also be studied under flow conditions by using in situ microscopy and scattering and diffraction techniques described above. The coupling of surface chemistry or surface functionalization with fluid flow and multicomponent transport could be studied in a wide variety of conditions to identify windows of operation in which nucleation and growth are suppressed; these cases could then be analyzed quantitatively. Rate-accelerated testing that can elucidate material evolution under separation conditions could be developed on the basis of quantitative models. Corrosion, etching, and the dissolution of separation media (such as H2S corrosion of hydrogen-selective metal membranes and dissolution of silicate adsorbents in aqueous media) could also be studied by the aforementioned methods.
Another way to improve the robustness of separation materials would be to understand carryover effects. Carryover effects, sometimes referred to as memory effects, can occur in chromatographic separations and sample preparation in which samples come into contact with a high-surface-area stationary phase or sorbent bed (such as silica and alumina) and are not completely removed during a chromatographic run or elution step. Separation materials are often susceptible to interacting strongly or reacting with particular classes of molecules, which could be dilute components of the mixture to be separated or could be decomposition products. In some cases, the interactions between the solid separation material and those components are strong enough for the reaction or binding to be irreversible or not to weaken when the separation system is subjected to different conditions (such as increased ionic strength, pH, and temperature variations). The components are retained on the separation material and this results in their incomplete elution and degradation of performance of the separation material. An understanding of how degradation products (and unwanted analytes) interact with separation materials is needed. In cases in which species undergo irreversible binding with the separation material, an understanding of interfacial interactions and surface-area effects is needed to provide insight into how the binding can be weakened. In cases in which the separation media are used in sample clean-up, as in sample preparation, an understanding of the competition between the matrix components and the target analytes for the separation media is needed. Those interactions can be studied with surface spectroscopy, including x-ray photoelectron spectroscopy and solid state nuclear magnetic resonance spectroscopy.
2-D. Explore Alternative Strategies to Address Temporal Changes in Separation Systems
Separation systems will likely be exposed to a wide array of conditions. The conditions could result from normal temporal changes in the mixture that needs to be separated, from unintended exposure to the ambient environment, or from exposure to conditions that might arise spuriously in a practical application of the separation material. Examples of various conditions are the presence of air or water (vapor or liquid), extreme mixture compositions, high (or low) temperature and pressure, and unintended radiation. The studies described above—of changes in separation materials as a function of time (for example, rates of change from nonequilibrium states, reaction chemistry and kinetics, and precipitation and agglomeration)—need to include a wide variety of conditions.
Even with this additional challenge, the first strategy in the selection of separation materials might logically be to seek stability, for example, through design of the material, modification of chemical or physical conditions, or use of additives. However, such a strategy might not be possible in every case. For example, in the separation of radioactive compounds, radiolysis cannot be avoided. As an alternative, planned degradation might be appropriate,
and even desired, for reasons of environmental sustainability and, paradoxically, for stable system performance. In extreme environments, degradation might be unavoidable, so it is imperative that the breakdown products not become troublesome foulants or themselves unwanted separation agents that impart an unintended selectivity to the system.
Although degradation has typically been an afterthought in decades of empirical process development (if it was considered at all), the design of separation systems in the future could build in molecular links that serve as intended break points. To make that happen, degradation mechanisms must be determined under the punishing conditions often encountered in separations. For example, bond scission points and rates must be known or predictable in high-radiation environments. The same is true for hydrolysis and redox reactions that will occur in extreme thermal and chemical environments. The state of scientists’ ability to predict and control such chemical reactions must be brought to new levels of reliability and precision if designers of the new era of separations are to build in the behavior that is needed at the earliest stage of development rather than as a response to a failed pilot test.
Experimentally, the chemistry of extreme environments must be explored to determine degradation kinetics, mechanisms, and products. Developing the ability to predict what it means for a degradation product to be “benign” will be required. Does the degradation product build up to change the separation system or to form a new, troublesome phase? Or does it preferably wash out and flow harmlessly out of the system? As researchers transform separation science, the separation system will be designed from the outset to allow degradation products to wash out and flow harmlessly from the system.
Another strategy to address changes in a separation material is to design separation materials to be self-healing. Fundamental approaches can be drawn from parallel work in self-healing polymers, such as adding functionality capable of forming coordinative bonds to cross-link damaged materials (Gilbert et al., 2016). Some surface chemistries, such as co-deposited dopamine and alkyl silanes, can be regenerated after degradation by exposure to acids, bases, or plasma. That strategy might be useful for repairing membranes that are susceptible to surface degradation when exposed to cleaning steps required to remove foulants. Figure 5-10 presents an example of self-healing of damaged membranes for oil–water separations.
The final approach considered by the committee is the development of regeneration strategies, which might be necessary when nucleation, aggregation, and precipitation of degradation products cannot be mitigated. In such cases, information on accumulation mechanisms and on the nature of the aggregates can be used to discover strategies for removing the aggregate or precipitate or for minimizing its effect. For example, understanding the rate can point to a location where a chemical treatment (washing) or a thermal treatment can readily remove the insult without loss in integrity of the separation system.
CROSSCUTTING TOPICS
Three topics emerged throughout the development of each research theme regardless of specific research direction: the importance of synthesis science; the need for standard systems, samples, and methods; and the need to incorporate data science, molecular modeling, and simulation. Synthesis science has been heavily researched and reported on in several U.S. agency efforts and is not covered in detail in this report by the committee.
Standard Systems, Samples, and Methods
For a variety of separation processes—such as absorption, adsorption, and membrane-based separations—an ever-increasing number of new separation materials are being developed. Many are designed, synthesized, formed, and characterized in individual laboratories with different sets of characterization equipment and different experimental testing protocols. Such variation makes it challenging to compare findings easily and quickly assess reproducibility.
For more conventional separation techniques, benchmarking is relatively easy. For example, vapor–liquid, liquid–liquid and solid–liquid phase equilibria are compiled in the National Institute of Standards and Technology (NIST) ThermoLit database,1 so it is easy to identify standard systems on which there are substantial consistent data. Samples are available commercially and usually in high purity, and measurement techniques are well established.
That standardization is not evident for the many classes of solid adsorbents and membrane materials. Separation-science researchers recognize the need for standard systems, samples, and methods. Many such needs were called out in the theme descriptions above, and there have been some fledgling efforts to develop standards, as described below.
Standard Systems and Samples
NIST and the Advanced Research Projects Agency-Energy (ARPA-E) have developed the NIST/ARPA-E Database of Novel and Emerging Adsorbent Materials and the NIST Registry of Adsorbent Materials.2 The database and registry are freely accessible on line and thereby constitute a centralized archive for accessing adsorption information related to various temperatures, pressures, and gases on materials synthesized from different research groups.
The availability of this type of information is valuable for a variety of reasons. First, comparative data allow researchers to assess the fidelity of in-house synthesized materials. Second, researchers can avoid duplicating experiments (unless, of course, the purpose is validation). Finally, computational studies can leverage the large database of thermodynamic and materials information to derive fundamental structure–property relationships. Expanding the efforts to more adsorbents and other separation processes, such as membrane-based separations, would be of enormous value to the separations community.
With respect to reference materials, within the ionic-liquids community, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide is considered a benchmark. The International Association of Chemical Thermodynamics and the International Union of Pure and Applied Chemistry led a round-robin project in which researchers around the world made high-quality reference measurements on the thermodynamic and thermophysical properties of the same sample (purity verified by NIST) of this ionic liquid (Marsh et al., 2009). Creation of a reference ionic liquid means that multiple research groups can now verify the quality of their synthesis and measurements according to whether they attain the same results determined in that project.
In a related effort, the adsorption community has recently undertaken an interlaboratory study led by NIST to evaluate CO2 adsorption at 293.15 K from 1 kPa to 4.5 MPa for a well-characterized variant of the zeolite ZSM-5 (NIST Reference Material RM 8852) (Nguyen et al., 2018). This study allows the community to establish adsorption data with a 95% uncertainty interval so that laboratories can independently validate their high-pressure adsorption equipment and measurements against a standard sample. The identification of additional benchmark materials and testing protocols is recommended for the absorption and adsorption communities, especially for the development of more complex characterization efforts, such as standardizing testing protocols for various temperatures and mixed-component conditions.
Proteoforms have variations that involve amino acid sequence, glycosylation, post-translational modification, oxidation, deamidation, protein misfolding, disulfide scrambling, fragmentation, aggregation, and others, so myriad separations are needed. There has been a need for a standard reference material to facilitate development of best practices for protein analysis and to hasten the development of better analytical methods. To that end, NIST has developed a standard reference material for IgG1к: NIST Reference Material 8671, also called NISTmab (Schiel and Turner, 2018). Three volumes of an American Chemical Society symposium series describes all aspects of NISTmab; the second volume is devoted to the collaborative work of many scientists in different pharmaceutical companies to characterize NISTmab (Sciel et a., 2015).
___________________
Chemical separations play a prominent role in monoclonal antibody (mAb) characterization. The primary sequence is determined with high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) after peptidase digestion. Sequence variations and post-translational modifications are also revealed with that method. This bottom-up approach using digestion inherently loses information about multiple variations and modifications of a given protein. A top-down method of performing HPLC-MS on intact proteins is under development (Toby et al., 2016). To assess variants from sequence and post-translational modifications that affect charge, cation and anion chromatography, capillary electrophoresis, and capillary isoelectric focusing are commonly used. Either hydrophilic interaction liquid chromatography or capillary electrophoresis can be used to analyze the glycosylation of mAbs and other proteins, and higher speed is urgently needed. Size-exclusion chromatography is used to characterize aggregation and fragmentation. MS has played an increasing role in mAb characterization. In all those examples, one could obtain the information faster with MS, but the size of mAbs, the number of proteoforms, the low resolution of current mass spectrometers, and ion suppression require the use of chemical separations for reliable characterization of mAbs. As the pharmaceutical industry wants to move quality-control measurements to the manufacturing site, where automation and simplicity of instrumentation are key, faster separation methods for intact mAbs and other proteins are desired.
The efforts described above to develop standard systems and samples for separation materials are excellent steps in the right direction. However, many more standard systems and samples are needed for different classes of solid adsorbents, liquid absorbents, and proteins. Especially critical is the need for membrane standards. Membrane-based separations could benefit greatly from a reference material for characterization. In desalination applications, polyamides formed from cross-linked trimesoyl chloride and m-phenylenediamine are the most widely used materials. However, for polymeric gas-separation membranes, no widely accepted materials have been designated as potential reference standards.
In contrast with the equilibrium absorption and adsorption characterization, many polymer membranes are formed from glassy polymers, which are nonequilibrium materials. For those samples, separation properties depend on the process by which the sample was made, and the result is a vast variety of measured performance data reported in the open literature. Therefore, it is challenging to use published data on the separation performance of these materials inasmuch as each research group uses its own methods to form polymer films. Both a common polymer in its equilibrium rubbery state and a common polymer in its glassy state should be designated as quality reference standards for membrane gas separations. For the glassy polymer, processing and film-formation conditions must be specified.
Creating these material standards would enable multiple groups to run matching experiments on the same samples processed in the same way. Comparing interlaboratory results would create standard protocols that research groups could match on custom instruments before publishing data on new materials. Interlaboratory studies could be extended to assess fundamental information on molecular diffusion and solubility data for mixed-component measurements over a variety of temperatures, pressures, and compositions. Other separation processes would also benefit from the implementation of such interlaboratory studies of fundamental separation performance.
Standard Methods
Some of the major expected fundamental advances in separation science are related to understanding of the selectivity, capacity, and robustness of new separation materials under a variety of complex conditions. However, a prerequisite for such advances is the development of common reference measurements that are broadly agreed on among the different research communities.
Within the adsorption community, commercial apparatuses for studying pure-component gas-phase adsorption are widely available. Some of the standard instruments have been used in interlaboratory studies of the ZSM-5 adsorption of CO2 described earlier (Nguyen et al., 2018). However, mixed-component systems are less common, and—because the global free-energy landscape for molecular partitioning depends on temperature, pressure, and composition effects—there remains a fundamental need to develop standard mixed-component measurements over a variety of temperatures and pressures for the adsorption community.
For membrane-based separations, the Springer Handbook of Metrology and Testing is a resource for details on standard testing methods (Czichos, et al., 2011). However, in contrast with the adsorption community, no commercial gas-permeability testing apparatus is widely available, so most experiments are performed on testing equipment built in-house by individual research groups. The designs of the instruments vary widely from group to group, and this precludes the recommendation of an instrument that can be used by multiple groups to cross-validate results. In addition, for gas separations, the community has selected a standard testing temperature of 35°C for all measurements, but there is no clear recommendation for standard pressures that need to be considered, and permeability can change substantially for membrane-based materials depending on the pressure at which an experiment is conducted. In addition to develop-
ing standards to measure permeability, there would also be considerable value in developing standard approaches for separately measuring adsorption and diffusion properties of membranes.
For those reasons, it is recommended that the community develop reference measurements to be performed on each custom-built instrument using standard reference materials to ensure quality of measurements. It is also recommended that standard pure-component measurements vary temperatures and pressures so that the full range of property sets can be assessed. As in the case of adsorption-based separations, standardized membrane-permeability measurements need to be developed for testing complex multicomponent mixtures at various temperatures and pressures. Clear definition of reference materials and testing protocols will help to improve reproducibility and measurement quality.
Recommendation: The National Institute of Standards and Technology, in cooperation with the research community, should identify materials and testing protocols for each type of separation material or system that can be used as reference standards.
Adapting Theory and Data Science for Separations
No matter the research theme, the committee found that it is critical to use advances in data science to achieve better predictive modeling. The vision for the future is to develop predictive models to advance the design of separation materials and systems. Computational methods should ultimately allow the tailoring of materials for targeted separations with desired performance and the definition of synthesis strategies to create the tailored materials.
To reach that state, the complexity and realism of models for key aspects of separations need to improve greatly. Areas in which data science and predictive modeling and simulation can help to advance separation sciences include solvation effects, solution structuring, and interfacial structure; thermodynamic-separation mechanisms and molecular-transport properties; materials evolution in complex environments (for example, trace contaminants and temperature and pressure history); the identification of synthesis paths and strategies; model validation for disordered and responsive media; and the effects of external stimuli on separation media. Although process modeling is outside the study scope, building connections between fundamental models and the ability to perform accurate process modeling has great value.
Advances in theory and modeling and in data science have caused disruptive changes in many fields, including mathematics, physics, and information science, and they will play a major role in the future of separation sciences. The concepts of theory and modeling and data science were introduced in Chapters 2 and 3, and are discussed in some detail below.
Theory and Modeling
The committee uses the term theory in this report to encompass the fundamental models of chemistry and physics and the development of the models. In the same way, modeling is associated with materials-based and applications-based studies that use the models, often to predict the properties of specific materials or processes. Because of their complexity, separation systems provide opportunities not only to develop new theories but to lead directly to new modeling methods that will also benefit related fields in soft-matter chemistry, materials chemistry, and materials science. Careful use of theory and modeling can provide insight into structural and dynamic properties of separation systems that is not readily accessible experimentally. That value does not imply, however, that theory and modeling can supplant experimental work. Rather, the greatest benefits will be realized when theory and modeling are thoughtfully integrated with complementary experiments.
Modeling on the smallest scale often focuses on the local geometric and electronic structure of a chemical and material system. The many advances that have been made and continue to be made in electronic-structure theory and quantum-chemistry methods underpin efforts on that scale. It is rarely sufficient, however, to describe separation processes solely on the scales accessible with those methods; it is necessary to incorporate methods that account for long-range forces, such as dispersion and solvation forces.
In general, adopting appropriate theories that account for interactions between species is only a starting point for modeling separation processes. The models must be combined with approaches that describe complicated energy landscapes in which characterization of the identity, connectivity, and dynamics of transitions between relevant states is often challenging (Peters, 2017). Complex landscapes cause ensembles of molecular species and system configurations. Understanding of the thermodynamic-state functions and ensemble-averaged behavior of those ensembles requires statistical-mechanic approaches.
In the theoretical and computational communities, it remains a grand challenge to understand how to propagate information across multiple time and length scales. Learning what information is essential for developing predictive models on larger scales is not trivial and is a major topic of current research. That challenge is exacerbated in separation systems in that they generally involve amorphous or liquid components, which themselves might involve hierarchical assemblies, as exemplified by amorphous porous polymers, polymeric membranes, complex surfaces, or reverse micelles. Structures that
lack long-range ordering present a prototypical scenario in which modeling might capture local configurations accurately but translate local configurations into disordered hierarchical motifs inadequately. Progress will demand synergistic efforts in experiment and theory. Determining and conceptualizing atomic-to-mesoscale correlations in noncrystalline systems with the accuracy necessary for predicting a chemical separation overlaps substantially with other fields of research, such as the modeling and prediction of soft matter in other contexts. In that sense, the complexities presented within separation science constitute an ideal framework within which to study how energy is partitioned within multifaceted, hierarchical structures.
Data Science
The ready availability of powerful algorithms and of computational resources needed for using the algorithms has justifiably created enormous interest in adapting data-science methods for use in diverse fields of science and technology. Classes of problems that are amenable to data-science techniques include regression of numerical models, classification of similarities or differences between disparate datasets, similarity matching, and uncertainty quantification. An important subset of data science is based on machine learning, in which models are developed in an automated and unsupervised way from large datasets. As in all applications of modeling, caution about the quality of input data and the domain of reliability of any resulting models is always appropriate.
The intrinsic complexity of separation materials and processes, in which chemically and structurally diverse environments are used to separate components from myriad chemical mixtures, makes separation science a field in which data science has enormous potential. As with electronic-structure or statistical-mechanics methods, the core need for the separations community is not the creation of new algorithms, although the committee does not preclude that. Instead, the greatest advances are likely to arise from efforts that deeply embed data-science approaches within the domain knowledge of separation science. Perhaps counterintuitively, the most important challenges are likely to arise from the need to curate datasets that are good enough and broad enough to allow insights into the phenomena that truly control separation processes. Tackling those issues by using computational data from high-throughput or computational modeling will be a useful starting point for such efforts because similarities in format and content of datasets are readily achievable.
The separations community will likely reap longer-term benefits when data-science efforts are extended to use experimental data. Methods that use retrospective data, perhaps identified from existing literature via text-mining or other automated methods, and methods that use apparatus or experimental protocols developed specifically to yield rich datasets will be important. As in other fields of science, there is much potential value in reporting not only the “best” experimental data but the outcomes of experiments that “failed” from the perspective of data science because diverse input data strengthen the robustness of the conclusions. In-depth collaboration between the modeling and experimental communities can reap benefits that a simple handoff of data from the latter to the former cannot achieve.
Recommendation: The research community should use data science, modeling, and simulation with experimental measurements to develop a fundamental understanding of separation materials in complex environments and on multiple scales.
ENABLING THE RESEARCH AGENDA
Achieving the research agenda presented in this chapter would provide a strong foundation for transforming separation science and hence globally reduce costs and energy use, improve human and environmental health, and advance efficient practices in industry. Many of the key research directions in the committee’s agenda were introduced in the 1987 report, which supported the need to implement the research to achieve the advances needed to move the field forward. Strategies to implement the research agenda are discussed in Chapter 6.
REFERENCES
Acharya, H., S. Vembanur, S. N. Jamadagni, and S. Garde. 2010. Mapping hydrophobicity at the nanoscale: Applications to heterogeneous surfaces and proteins. Faraday Discussions 146:353-365. doi: 10.1039/b927019a.
Anderson, J. L., J. Ding, T. Welton, and D. W. Armstrong. 2002. Characterizing ionic liquids on the basis of multiple solvation interactions. Journal of the American Chemical Society 124(47):14247–14254. doi: 10.1021/ja028156h.
Aveyard, R., B. P. Binks, J. H. Clint, P. D. Fletcher, T. S. Horozov, B. Neumann, V. N. Paunov, J. Annesley, S. W. Botchway, D. Nees, A. W. Parker, A. D. Ward, and A. N. Burgess. 2002. Measurement of long-range repulsive forces between charged particles at an oil-water interface. Physical Review Letters 88(24):246102. doi: 10.1103/PhysRevLett.88.246102.
Bachman, J. E., Z. P. Smith, T. Li, T. Xu, and J. R. Long. 2016. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal-organic framework nanocrystals. Nature Materials 15(8):845–9. doi: 10.1038/nmat4621.
Backov, R. 2006. Combining soft matter and soft chemistry: integrative chemistry towards designing novel and complex multiscale architectures. Soft Matter 2(6):452–464. doi: 10.1039/B602579J.
Bai, P., E. Haldoupis, P. J. Dauenhauer, M. Tsapatsis, and J. I. Siepmann. 2016. Understanding diffusion in hierarchical zeolites with house-of-cards nanosheets. ACS Nano 10(8):7612–7618. doi: 10.1021/acsnano.6b02856.
Baker, R. W. 2012. Membrane Technology and Applications, 3rd edition. Hoboken, NJ: John Wiley & Sons.
Ballesteros-Gomez, A., L. Lunar, M.D. Sicilia, S. Rubio. 2018. Hyphenating Supramolecular Solvents and Liquid Chromatography: Tips for Efficient Extraction and Reliable Determination of Organics. Chromatographia Ahead of Print. doi: 10.1007/s10337-018-3614-1.
Barrett, A., J. Imbrogno, G. Belfort, and P. B. Petersen. 2016. Phosphate ions affect the water structure at functionalized membrane surfaces. Langmuir 32(35):9074–9082. doi: 10.1021/acs.langmuir.6b01936.
Bhattacharyya, S., S. H. Pang, M. R. Dutzer, R. P. Lively, K. S. Walton, D. S. Sholl, and S. Nair. 2016. Interactions of SO2-containing acid gases with ZIF-8: Structural changes and mechanistic investigations. The Journal of Physical Chemistry C 120(48):27221–27229. doi: 10.1021/acs.jpcc.6b09197.
Bohlin, P., Johan Bos, Staffan Larsson, Ian Lewin, Colin Matheson, and David Milward. 1999. Survey of Existing Interactive Systems.
Brant, J. A., and A. E. Childress. 2004. Colloidal adhesion to hydrophilic membrane surfaces. Journal of Membrane Science 241(2):235–248. doi: 10.1016/j.memsci. 2004.04.036.
Broadbelt, L. J., and R. Q. Snurr. 2000. Applications of molecular modeling in heterogeneous catalysis research. Applied Catalysis A: General 200(1):23–46. doi: 10.1016/S0926-860X(00)00648-7.
Brown, A., and J. Clardy. 2018. Tiny crystals have big potential for determining structures of small molecules. Nature 564(7736):348–349. doi: 10.1038/d41586-018-07756-5.
Chapman, K. W., G. J. Halder, and P. J. Chupas. 2009. Pressure-induced amorphization and porosity modification in a metal-organic framework. Journal of the American Chemical Society 131(48):17546–17547. doi: 10.1021/ja908415z.
Chowdhury, M. R., J. Steffes, B. D. Huey, and J. R. McCutcheon. 2018. 3D printed polyamide membranes for desalination. Science 361(6403):682–686. doi: 10.1126/science.aar2122.
Clark, Aurora E. 2019. “Amphiphile-Based Complex Fluids: The Self-Assembly Ensemble as Protagonist.” ACS Central Science 5(1):10-12. doi: 10.1021/acscentsci.8b00927.
Cruciani, G. 2006. Zeolites upon heating: Factors governing their thermal stability and structural changes. Journal of Physics and Chemistry of Solids 67(9–10):1973–1994. doi: 10.1016/j.jpcs.2006.05.057.
Custelcean, R. 2018. Aqueous ion separations by selective crystallization. Presented at the 2nd Data-gathering Meeting of the Separations Committee Meeting, Irvine, CA, May 7-8, 2018.
Czichos, H., T. Saito, and L. Smith (editors). 2011. Springer Handbook of Metrology and Testing, Vol. 2. Berlin, Germany: Springer-Verlag Berlin Heidelberg. doi: 10.1007/978-3-642-16641-9.
Den Hollander, M. A., M. Wissink, M. Makkee, and J. A. Moulijn. 2002. Gasoline conversion: Reactivity towards cracking with equilibrated FCC and ZSM-5 catalysts. Applied Catalysis A: General 223(1):85–102. doi: 10.1016/S0926-860X(01)00745-1.
Deng, C., J. Zhang, X. Yu, W. Zhang, and X. Zhang. 2004. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences 810(2):269–275. doi: 10.1016/j.jchromb.2004.08.013.
Derjaguin, B., and L. D. Landau. 1941. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochimica U.R.S.S. 14:633–662.
DOE. 2016. Basic Research Needs for Synthesis Science. Basic Energy Sciences Workshop on Basic Research Needs for Synthesis Science for Energy Relevant Technology, Washington, DC, May 2016.
Donohue, M.D., B.S. Minhas, S.Y., Lee. 1989. Permeation behavior of carbon dioxide-methane mixtures in cellulose acetate membranes. Journal of Membrance Science 42(3):197-214. doi: 10.1016/S0376-7388(00)82376-5.
Duhamet, J., H. Möhwald, M. Pleines, and T. Zemb. 2017. “Self-Regulated Ion Permeation through Extraction Membranes.” Langmuir 33(38):9873-9879. doi: 10.1021/acs.langmuir.7b02256.
Ellis, R. J. 2014. Critical Exponents for Solvent Extraction Resolved Using SAXS. Journal of Physical Chemistry B 118(1):315-322. doi: 10.1021/jp408078v.
Elzo, D., I. Huisman, E. Middelink, and V. Gekas. 1998. Charge effects on inorganic membrane performance in a cross-flow microfiltration process. Colloids and Surfaces A: Physicochemical and Engineering Aspects 138(2): 145–159. doi: 10.1016/S0927-7757(96)03957-X.
Escher, B. I., C. van Daele, M. Dutt, J. Y. M. Tang, and R. Altenburger. 2013. Most oxidative stress response in water samples comes from unknown chemicals: The need for effect-based water quality trigger values. Environmetal Science & Technology 47:7002–7011. doi: 10.1021/es304793h.
Fichou, D., and G. E. Morlock. 2017. Open-source-based 3D printing of thin silica gel layers in planar chromatography. Analytical Chemistry 89(3):2116–2122. doi: 10.1021/acs.analchem.6b04813.
Flanigen, E. M., J. M. Bennett, R. W. Grose, J. P. Cohen, R. L. Patton, R. M. Kirchner, and J. V. Smith. 1978. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature 271(5645):512–516. doi: 10.1038/271512a0.
Gaddam, P., R. Grayson, and W. Ducker. 2018. Adsorption at confined interfaces. Langmuir 34(36):10469–10479. doi: 10.1021/acs.langmuir.8b01418.
Galizia, M., W. S. Chi, Z. P. Smith, T. C. Merkel, R. W. Baker, and B. D. Freeman. 2017. 50th anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 50(20):7809–7843. doi: 10.1021/acs.macromol.7b01718.
Galletero, M. S., A. Corma, B. Ferrer, V. Fornés, and H. García. 2003. Confinement effects at the external surface of delaminated zeolites (ITQ-2):An inorganic mimic of cyclodextrins. The Journal of Physical Chemistry B 107(5):1135–1141. doi: 10.1021/jp0210531.
Gilbert, Z. W., R. J. Hue, and I. A. Tonks. 2016. Catalytic formal [2+2+1] synthesis of pyrroles from alkynes and diazenes via TiII/TiIV redox catalysis. Nature Chemistry 8:63–68. doi: 10.1038/nchem.2386.
Grimaldi, J., M. Radhakrishna, S. K. Kumar, and G. Belfort. 2015. Stability of proteins on hydrophilic surfaces. Langmuir 31(3):1005–1010. doi: 10.1021/la503865b.
Han, M., A. Sethuraman, R. S. Kane, and G. Belfort. 2003. Nanometer-scale roughness having little effect on the amount or structure of adsorbed protein. Langmuir 19(23):9868–9872. doi: 10.1021/la030132g.
Harmon, H., S. Schlahta, D. Wester, J. Walker, S. Fink, and M. Thompson. 2001. Tanks Focus Area, Savannah River Site Salt Processing Project Research and Development Summary Report. Report TFA-0105. Richland, WA: Pacific Northwest National Laboratory.
Hibbe, F., C. Chmelik, L. Heinke, S. Pramanik, J. Li, D. M. Ruthven, D. Tzoulaki, and J. Kärger. 2011. The nature of surface barriers on nanoporous solids explored by microimaging of transient guest distributions. Journal of the American Chemical Society 133(9):2804–2807. doi: 10.1021/ja108625z.
Huang, Y., Y. Shan, S. Liang, X. Zhao, G. Jiang, C. Hu, J. Yang, and L. Liu. 2018. Coordinated silicon elastomer coating@fabrics with oil/water separation capabilities, outstanding durability and ultra-fast room-temperature self-healing ability. Journal of Materials Chemistry A 6(35):17156–17163. doi: 10.1039/C8TA02893A.
Israelachvili, J. N. 1982. Forces between surfaces in liquids. Advances in Colloid and Interface Science 16(1):31–47. doi: 10.1016/0001-8686(82)85004-5.
Israelachvili, J. N. 2011. Intermolecular and Surface Forces, 3rd edition. San Diego, CA: Academic Press.
Jayachandrababu, K. C., S. Bhattacharyya, Y. Chiang, D. S. Sholl, and S. Nair. 2017. Recovery of acid-gas-degraded zeolitic imidazolate frameworks by solvent-assisted crystal redemption (SACRed). ACS Applied Materials & Interfaces 9(40):34597–34602. doi: 10.1021/acsami.7b11686.
Jeon, M. Y., D. Kim, P. Kumar, P. S. Lee, N. Rangnekar, P. Bai, M. Shete, B. Elyassi, H. S. Lee, K. Narasimharao, S. N. Basahel, S. Al-Thabaiti, W. Xu, H. J. Cho, E. O. Fetisov, R. Thyagarajan, R. F. DeJaco, W. Fan, K. A. Mkhoyan, J. I. Siepmann, and M. Tsapatsis. 2017. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 543:690. doi: 10.1038/nature 21421.
Jonáš, A., and P. Zemánek. 2008. Light at work: The use of optical forces for particle manipulation, sorting, and analysis. Electrophoresis 29(24):4813–4851. doi: 10.1002/elps.200800484.
Kärger, J., D. M. Ruthven, and D. N. Theodorou. 2012. Diffusion in Nanoporous Materials. Weinheim, Germany: Wiley-VCH.
Karmakar, A., M. Duvail, M. Bley, T. Zemb, J.-F. Dufreche. 2018. Combined supramolecular and mesoscale model-ling of liquid-liquid extraction of rare earth salts. Colloids and Surfaces A 555:713-727. doi: 10.1016/j.colsur-fa.2018.07.013.
Kataoka, É. M., R. C. Murer, J. M. Santos, R. M. Carvalho, M. N. Eberlin, F. Augusto, R. J. Poppi, A. L. Gobbi, and L. W. Hantao. 2017. Simple, expendable, 3D-printed microfluidic systems for sample preparation of petroleum. Analytical Chemistry 89(6):3460–3467. doi: 10.1021/acs.analchem.6b04413.
Kocsis, I., M. Sorci, H. Vanselous, S. Murail, S. E. Sanders, E. Licsandru, Y.-M. Legrand, A. van der Lee, M. Baaden, P. B. Petersen, G. Belfort, and M. Barboiu. 2018. Oriented chiral water wires in artificial transmembrane channels. Science Advances 4(3):eaao5603. doi: 10.1126/sciadv. aao5603.
Koehler, J. A., M. Ulbricht, and G. Belfort. 1997. Intermolecular forces between proteins and polymer films with relevance to filtration. Langmuir 13(15):4162–4171. doi: 10.1021/la970010m.
Koehler, J. A., M. Ulbricht, and G. Belfort. 2000. Intermolecular forces between a protein and a hydrophilic modified polysulfone film with relevance to filtration. Langmuir 16(26):10419–10427. doi: 10.1021/la000593r.
Kunz, W., T. Zemb, and A. Harrar. 2012. Using ionic liquids to formulate microemulsions: Current state of affairs. Current Opinion in Colloid & Interfacial Science 17(4):205-211. doi: 10.1016/j.cocis.2012.03.002.
Kuznicki, S. M., V. A. Bell, I. Petrovic, and P. W. Blosser. 1999. Separation of Nitrogen from Mixtures Thereof with Methane Utilizing Barium Ecvhanged ETS-4. U.S. Patent US5989316A. Engelhard Corporation.
Lai, Z., G. Bonilla, I. Diaz, J. G. Nery, K. Sujaoti, M. A. Amat, E. Kokkoli, O. Terasaki, R. W. Thompson, M. Tsapatsis, and D. G. Vlachos. 2003. Microstructural optimization of a zeolite membrane for organic vapor separation. Science 300(5618):456–460. doi: 10.1126/science.1082169.
Lemasson, E., S. Bertin, P. Hennig, E. Lesellier, and C. West. 2017. Mixed-mode chromatography—A review. LCGC 30(6):22–33.
Li, H., and M. R. Hill. 2017. Low-energy CO2 release from metal–organic frameworks triggered by external stimuli. Accounts of Chemical Research 50(4):778–786. doi: 10.1021/acs.accounts.6b00591.
Liu, Y., J.-H. Her, A. Dailly, A. J. Ramirez-Cuesta, D. A. Neumann, and C. M. Brown. 2008. Reversible structural transition in MIL-53 with large temperature hysteresis. Journal of the American Chemical Society 130(35):11813–11818. doi: 10.1021/ja803669w.
Luelf, T., D. Rall, D. Wypysek, M. Wiese, T. Femmer, C. Bremer, J. U. Michaelis, and M. Wessling. 2018. 3D-printed rotating spinnerets create membranes with a twist. Journal of Membrane Science 555:7–19. doi: 10.1016/j. memsci.2018.03.026.
Lyndon R., K. Konstas, R. A. Evans, D. J. Keddie, M. R. Hill, B. P. Ladewig. 2015. Tunable photodynamic switching of DArE@PAF@1 for carbon capture. Advanced Functional Materials 25(28):4405–4411. doi: 10.1002/adfm.201502069.
Ma, C. D., C. Wang, C. Acevedo-Vélez, S. H. Gellman, and N. L. Abbott. 2015. Modulation of hydrophobic interactions by proximally immobilized ions. Nature 517:347. doi: 10.1038/nature14018.
Macdonald, N. P., S. A. Currivan, L. Tedone, and B. Paull. 2017. Direct production of microstructured surfaces for planar chromatography using 3D printing. Analytical Chemistry 89(4):2457–2463. doi: 10.1021/acs. analchem.6b04546.
Mann, S. 2009. Self-assembly and transformation of hybrid nano-objects and nanostructures under equilibrium and non-equilibrium conditions. Nature Materials 8:781. doi: 10.1038/nmat2496.
Mao, X., W. Tian, Y. Ren, D. Chen, S. E. Curtis, M. T. Buss, G. C. Rutledge, and T. A. Hatton. 2018. Energetically efficient electrochemically tunable affinity separation using multicomponent polymeric nanostructures for water treatment. Energy & Environmental Science 11(10):2954–2963. doi: 10.1039/C8EE02000K.
Marsh, K. N., J. F. Brennecke, R. D. Chirico, M. Frenkel, A. Heintz, J. W. Magee, C. J. Peters, L. P. N. Rebelo, and K. R. Seddon. 2009. Thermodynamic and thermophysical properties of the reference ionic liquid: 1-hexyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]amide (including mixtures). Part 1. Experimental methods and results (IUPAC technical report). Pure and Applied Chemistry 81(5):781–790.
Myers, A. L., and J. M. Prausnitz. 1965. Thermodynamics of mixed-gas adsorption. AIChE Journal 11(1):121-127. doi: 10.1002/aic.690110125.
Nannou, C. I., V. I. Boti, and T. A. Albanis. 2018. Trace analysis of pesticide residues in sediments using liquid chromatography–high-resolution Orbitrap mass spectrometry. Analytical and Bioanalytical Chemistry 410(7):1977-1989. doi: 10.1007/s00216-018-0864-6.
National Academies. 2000. Alternatives for High-Level Waste Salt Processing at the Savannah River Site. Washington, DC: The National Academies Press.
Nguyen, H. G. T., L. Espinal, R. D. van Zee, M. Thommes, B. Toman, M. S. L. Hudson, E. Mangano, S. Brandani, D. P. Broom, M. J. Benham, K. Cychosz, P. Bertier, F. Yang, B. M. Krooss, R. L. Siegelman, M. Hakuman, K. Nakai, A. D. Ebner, L. Erden, J. A. Ritter, A. Moran, O. Talu, Y. Huang, K. S. Walton, P. Billemont, and G. De Weireld. 2018. A reference high-pressure CO2 adsorption isotherm for ammonium ZSM-5 zeolite: Results of an interlaboratory study. Adsorption 24(6):531–539. doi: 10.1007/s10450-018-9958-x.
Ninham, B. W., and V. A. Parsegian. 1971. Electrostatic potential between surfaces bearing ionizable groups in ionic equilibrium with physiologic saline solution. Journal of Theoretical Biology 31(3):405–428. doi: 10.1016/0022-5193(71)90019-1.
Perego, C., A. Carati, P. Ingallina, M. A. Mantegazza, and G. Bellussi. 2001. Production of titanium containing molecular sieves and their application in catalysis. Applied Catalysis A: General 221(1):63–72. doi: 10.1016/S0926-860X(01)00797-9.
Pereira, J., P. Porto-Figueira, C. Cavaco, K. Taunk, S. Ra-pole, R. Dhakne, H. Nagarajaram, and J. S. Camara. 2015. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 5(1):3–55. doi: 10.3390/metabo5010003.
Peters, B. 2017. Reaction rate and theory and rare event simulations. United States:Elsevier B.V.
Poirot, R., X. Le Goff, O. Diat, D. Bourgeois, D. Meyer. 2016. Metal recognition driven by weak interactions: A case study in solvent extraction. ChemPhysChem 17(14): 2112-2117. doi: 10.1002/cphc.201600305.
Poli, D., M. Goldoni, M. Corradi, O. Acampa, P. Carbognani, E. Internullo, A. Casalini, and A. Mutti. 2010. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences 878(27):2643–2651. doi: 10.1016/j.jchromb.2010.01.022.
Radhakrishna, M., J. Grimaldi, G. Belfort, and S. K. Kumar. 2013. Stability of proteins inside a hydrophobic cavity. Langmuir 29(28):8922–8928. doi: 10.1021/la4014784.
Raybaud, P. 2007. Understanding and predicting improved sulfide catalysts: Insights from first principles modeling. Applied Catalysis A: General 322:76–91. doi: 10.1016/j. apcata.2007.01.005.
Salciccioli, M., M. Stamatakis, S. Caratzoulas, and D. G. Vlachos. 2011. A review of multiscale modeling of metal-catalyzed reactions: Mechanism development for complexity and emergent behavior. Chemical Engineering Science 66(19):4319–4355. doi: 10.1016/j.ces.2011.05.050.
Schiel, J. E., O. V. Borisov, and D. L. Davis (editors). 2015. State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study. ACS Symposium Series Vol. 1201. Washington, DC: American Chemical Society.
Schiel, J. E., and A. Turner. 2018. The NISTmAb reference material 8671 lifecycle management and quality plan. Analytical and Bioanalytical Chemistry 410(8):2067–2078. doi: 10.1007/s00216-017-0844-2.
Seo, S., M. A. DeSilva, H. Xia, and J. F. Brennecke. 2015. Effect of cation on physical properties and CO2 solubility for phosphonium-based ionic liquids with 2-cyanopyrro-lide anions. Journal of Physical Chemistry B 119:11807–11814. doi: 10.1021/acs.jpcb.5b05733.
Serre, C., F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër, and G. Férey. 2002. Very large breathing effect in the first nanoporous chromium(III)based solids: MIL-53 or CrIII(OH)·{O2C−C6H4−CO2} {HO2C−C6H4−CO2H}x·H2Oy. Journal of the American Chemical Society 124(45):13519–13526. doi: 10.1021/ja0276974.
Shen, X., H. Chang, D. Sun, L. Wang, and F. Wu. 2018. Trace analysis of 61 natural and synthetic progestins in river water and sewage effluents by ultra-high performance liquid chromatography–tandem mass spectrometry. Water Research 133:142–152. doi: 10.1016/j.wa-tres.2018.01.030.
Siepmann J. I., J. F. Brennecke, D. T. Allen, M. T. Klein, P. E. Savage, G. C. Schatz, and F. M. Winnik. 2018. Journal of Chemical and Engineering Data 63(10):3651–3651 doi: 10.1021/acs.jced.8b00842.
Smith, Z. P., R. R. Tiwari, M. E. Dose, K. L. Gleason, T. M. Murphy, D. F. Sanders, G. Gunawan, L. M. Robeson, D. R. Paul, and B. D. Freeman. 2014. Influence of diffusivity and sorption on helium and hydrogen separations in hydrocarbon, silicon, and fluorocarbon-based polymers. Macromolecules 47(9):3170–3184. doi: 10.1021/ma402521h.
Somasundaran, P., and L. Huang. 2000. Adsorption/Aggregation of surfactants and their mixtures at solid–liquid interfaces. Advances in Colloid and Interface Science 88(1):179–208. doi: 10.1016/S0001-8686(00)00 044-0.
Sorci, M., B. Dassa, H. Liu, G. Anand, A. K. Dutta, S. Pietrokovski, M. Belfort, and G. Belfort. 2013. Oriented covalent immobilization of antibodies for measurement of intermolecular binding forces between zipper-like contact surfaces of split inteins. Analytical Chemistry 85(12):6080–6088. doi: 10.1021/ac400949t.
Srinivasan, K., M. Sorci, L. Sejergaard, S. Ranjan, G. Belfort, and S. M. Cramer. 2018. Protein binding kinetics in multimodal systems: Implications for protein separations. Analytical Chemistry 90(4):2609–2617. doi: 10.1021/acs.analchem.7b04158.
Standl, S., and O. Hinrichsen. 2018. Kinetic modeling of catalytic olefin cracking and methanol-to-olefins (MTO) over zeolites: A review. Catalysts 8(12):626. doi: 10.3390/catal8120626.
Sun, H., H. Li, and P.J. Sadler. 1999. Transferrin as a metal ion mediator. Chemical Reviews 99(9):2817-2842. doi: 10.1021/cr980430w.
Sundaramoorthi, G., M. Hadwiger, M. Ben-Romdhane, A. R. Behzad, P. Madhavan, and S. P. Nunes. 2016. 3D membrane imaging and porosity visualization. Industrial & Engineering Chemistry Research 55(12):3689–3695. doi: 10.1021/acs.iecr.6b00387.
Tiwari, R. R., Z. P. Smith, H. Lin, B. D. Freeman, and D. R. Paul. 2014. Gas permeation in thin films of “high free-volume” glassy perfluoropolymers: Part I. Physical aging. Polymer 55(22):5788–5800. doi: 10.1016/j.poly-mer.2014.09.022.
Toby, T. K., L. Fornelli, and N. L. Kelleher. 2016. Progress in top-down proteomics and the analysis of proteoforms. Annual Review of Analytical Chemistry 9(1):499–519. doi: 10.1146/annurev-anchem-071015-041550.
Verwey, E. J. W. 1947. Theory of the stability of lyophobic colloids. The Journal of Physical and Colloid Chemistry 51(3):631–636. doi: 10.1021/j150453a001.
Walker, D. D., M. J. Barnes, C. L. Crawford, R. A. Peterson, R. F. Swingle, and S. D. Fink. 1998. In-tank precipitation with tetraphenylborate: Recent process and research results. In Science and Technology for Disposal of Radioactive Tank Wastes, edited by W. W. Schultz and N. J. Lombardo. New York: Plenum Press, 219–230.
Wood, D. W., V. Derbyshire, W. Wu, M. Chartrain, M. Belfort, and G. Belfort. 2000. Optimized single-step affinity purification with a self-cleaving intein applied to human acidic fibroblast growth factor. Biotechnology Progress 16(6):1055–63. doi: 10.1021/bp0000858.
Yin, X., Y. Wang, X. Bai, Y. Wang, L. Chen, C. Xiao, J. Diwu, S. Du, Z. Chai, T.E. Albrecht-Schmitt, and S. Wang. 2017. Rare earth separations by selective borate crystallization. Nature Communications 8:14438.
Zhang, F., Y. Ma, J. Liao, V. Breedveld, and R. P. Lively. 2018. Solution-based 3D printing of polymers of intrinsic microporosity. Macromolecular Rapid Communications 39(13):1800274. doi: 10.1002/marc.201800274.
Zhang, X., Z. Shen, J. Liu, S. N. Kerisit, M. E. Bowden, M. L. Sushko, J. J. De Yoreo, and K. M. Rosso. 2017. Direction-specific interaction forces underlying zinc oxide crystal growth by oriented attachment. Nature Communications 8(1):835. doi: 10.1038/s41467-017-0084.




























