3
Guiding the System of Cancer Control
When thinking about the cancer control system, it is helpful to consider it as being composed of multiple levels. At the societal level, there are multiple public- and private-sector agencies, institutions, organizations, and companies, which are all relatively independent agents, pursuing their missions and interests separately, sometimes in coordination or sometimes in conflict with one another. A second level includes social networks of individuals, families, and community groups, all again pursing their own aspirations and interests. At the biological level, cancer cells adapt to medical interventions with mutations that, in effect, help the cancer “learn” how to avoid or resist the intended consequences of the interventions. To the extent that there is any form of a “command level” in cancer control, it would consist of different administrative and political entities spread across society, making decisions about resource allocation and other policies. There is competition for resources not only within the cancer control system but also among entities in that system (e.g., cardiovascular or neurodegenerative diseases) and those outside it (e.g., environmental protection, space research, and naval resources).
This chapter introduces the concept of systems engineering, which is helpful in analyzing, developing, and making decisions concerning complex adaptive systems. In particular, the systems engineering approach requires and relies on the development of effective models of the system and its states. These models are then modified and improved under study to predict and respond to a system’s likely behavior under various “futures.” With that in mind, the chapter ultimately explores some essential
ingredients for a planning and monitoring tool based on the principles of systems engineering that can be used to guide the cancer control system.
SYSTEMS ENGINEERING IN SOCIETY
The world today is full of complex systems: manufacturing systems, logistics systems, transportation systems, banking systems, and on and on. Most of them, like the cancer control system, developed over time with no overarching plan and no one entity in charge, so they tend to have many of the same challenges as the cancer control system, such as difficulty coordinating the different components, which are generally pursuing their own goals and interests. The discipline of systems engineering has evolved as a way of improving the performance of these and other large, complex systems.
Unlike most other engineering disciplines, which focus mainly on complicated systems that can be designed and understood by deconstructing them down into component parts and considering them independently, systems engineering is inherently holistic because the properties of the overall system depend on the interactions among the system’s components, meaning that all those components must be considered at once. One of the primary tasks in systems engineering design and analysis, therefore, is understanding the component parts of a system and how they interact to produce the system’s behavior. Such an analysis is generally the first step in working with a complex system, and it often involves creating a model-based simulation and testing it to see whether it accurately reproduces the system’s behavior under different constraints and parameters. Once such a simulation has been constructed, it can be used to test how the system will respond to various stimuli and changes, which in turn makes it possible to learn how to guide—not “control”—the system to a certain degree.
A Case Scenario: The National Airspace System
As one example of systems engineering in action, consider the U.S. National Airspace System, a complex adaptive system that is part of the larger global transportation system. The system consists of the airspace over the United States and nearby bodies of water, the aircraft moving through that airspace, and all the facilities, technology, organizations, and people needed to make sure that these aircraft have safe journeys—airports, radar and other navigation equipment, weather detection and forecasting systems, air traffic controllers, communication equipment, landing systems, and so on (GAO, 2015) (see Figure 3-1).
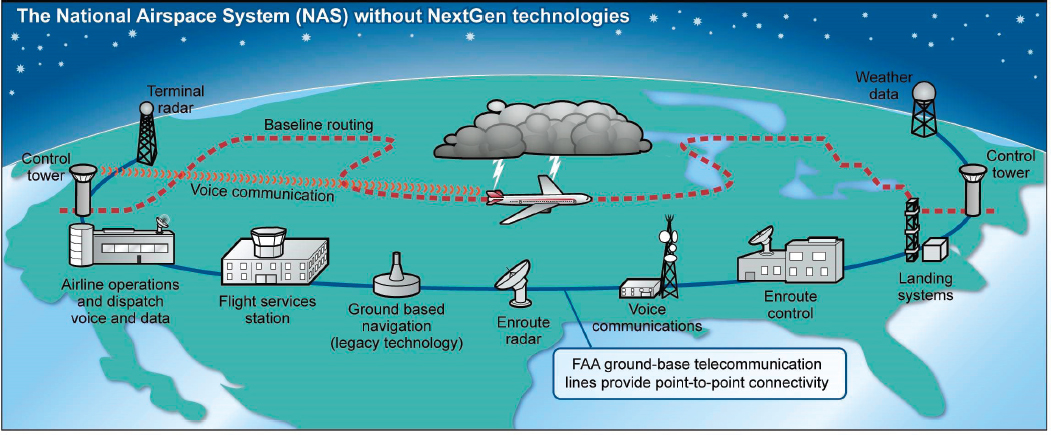
SOURCE: GAO-15-370.
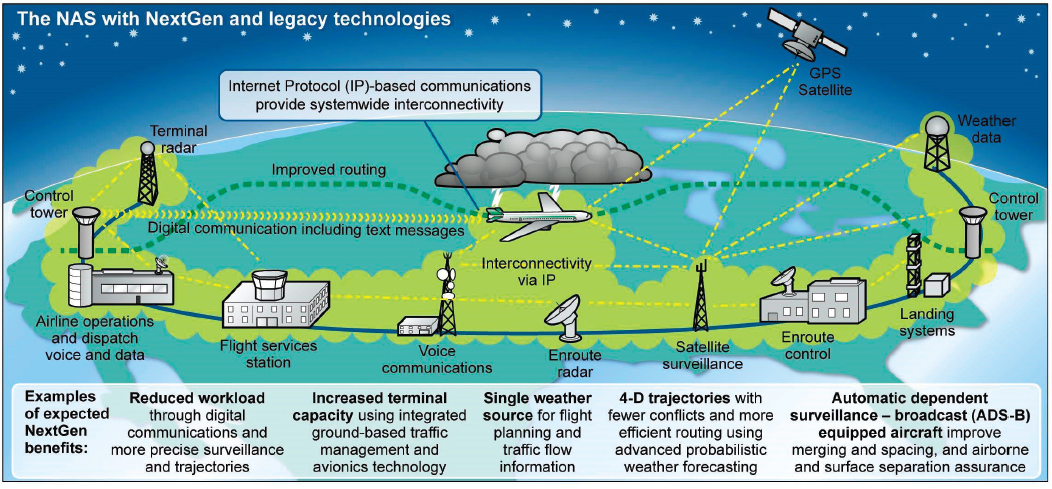
The National Airspace System is composed of many more different and smaller systems at work. Aircraft taking off from and landing at an airport with a control tower are the responsibility of a terminal radar approach control (TRACON). Once a plane climbs to about 18,000 feet, it is directed by one of 24 air route traffic control centers (ARTCCs), each responsible for a large section of the airspace over the United States (FAA, 2018). An aircraft is passed from one ARTCC to the next as it moves across the country until it approaches its destination and is handed off to the TRACON at that airport. Communication and coordination among the ARTCCs and TRACONs are crucial to monitoring all planes in U.S. airspace and to ensuring that they maintain safe distances from one another, horizontally and vertically.
The National Airspace System offers a clear example of the sort of communication and collaboration that must take place between the different components of a complex system if that system is to function effectively. In recent years, the National Airspace System has been modified with a set of so-called NextGen technologies (FAA, 2019a). A key improvement is the addition of satellite-based global positioning system (GPS) technologies that will be used to keep track of the planes (FAA, 2019b). Now, instead of relying on limited-range radar and handing off planes from one section to the next, the National Airspace System will be able to continuously monitor a plane’s position across the entire country. With the GPS system, aircraft also receive precise information on the positions of other aircraft near them, improving safety. It is a good example of technological improvements being used to address some of the weaknesses in a system’s operation—in this case, the limitations of using ground-based radar to keep track of thousands of planes as they fly across the country. It is worth noting that even with the installation of the NextGen technologies, the system remains complex. The improvements do not include any sort of hierarchical command system, which would be unworkable in a system this complex, but are simply making it possible for the different components of the system to all have access to more accurate information with which to make their decisions.
SYSTEMS APPROACHES IN CANCER CONTROL
The question that has prevailed in cancer control is how to achieve effectiveness and distributive equity while progressively diminishing the overall burden and costs. Thus, a promising path forward could be the adoption of a systems engineering approach—like that used with the National Airspace System—in which any sort of planning or development of strategies takes into account the entire system and its actions and interactions as a whole, instead of focusing on individual components.
This is not a novel conclusion, as many previous groups faced with similar problems—both in subsystems of cancer control and in other aspects of health and medicine—have concluded that systems analyses and approaches are the appropriate response (Hartwell et al., 2006; Huang et al., 2009; Leischow et al., 2008; Luke and Stamatakis, 2012).
A number of studies have applied—or attempted to apply—systems analysis and engineering to these individual subsystems. It is worth exploring this body of work, both because it offers lessons on how to apply systems thinking to the broad area of cancer control and also because any successful systems approach to cancer control will apply systems thinking not only to the entire system but also to its individual components considered as systems in their own right. It is also important to note that the systems engineering discussed in these various examples takes place at different levels, from the individual to the societal. Ultimately, any overarching approach to improving cancer control will have to act in a manner that is inclusive of all these different levels.
Clinical Care
Even relatively simple cancer cases can involve multiple components in the cancer care system: the patient, medical, surgical and radiation oncologists, pathologists, radiologists, hospitals, insurers, and so on. In the U.S. health care system, the involvement of so many different components may lead to less-than-effective care if coordination does not occur.
A recent case study illustrates this point (Trosman et al., 2016). The patient was a 32-year-old woman with breast cancer who was unemployed and enrolled in Medicaid. She was newly married and wanted to have children. The relevant parties were “the patient, the surgical office at a large hospital, the local oncology office, genetic counselors at the large hospital, an out-of-state genetic laboratory, a stand-alone fertility clinic, a local dental office, a local primary care provider, the psychosocial office at the large hospital, and the patient’s Medicaid insurance” (p. 1102). The case study described four different situations in which the woman was failed by the health care system because of failure of the parties to work together effectively. For instance, although the woman wished to preserve her fertility, her treatment was begun without access to fertility preservation services. In addition, a failure to get the results of genetic testing before her surgery led the woman to undergo a different procedure than she would have if she had known the results ahead of time.
The case study concluded that the system of clinical cancer care needs to be modified to take into account the existence of “task interdependence”—that is, the ways in which the outcome of one task can be influenced by the outcome of another. Too often the different components
of cancer care are treated as separate fragments with independent tasks, and improving cancer care depends on recognizing and allowing for the different ways that the components interact and, ultimately, finding ways to coordinate among those components.
Cancer Drug Delivery
Recent research in the realm of targeted drug delivery in cancer has also underscored the need for a systems approach. Drug targeting is best understood in the context of a system consisting of the tumor and the patient’s surrounding tissues, the patient’s body and health status overall, the drug or drugs being administered, the drug delivery system, and any other therapy modalities being used, such as radiation therapy (Dreher and Chilkoti, 2007). This integrated approach will be particularly important in the future because more treatments will depend on combinations of drugs in which the drug interactions are important and because emerging drug delivery technologies will make it possible to deliver drugs precisely to the tumor and avoid surrounding healthy tissue, but at the cost of having more complex delivery systems (Dreher and Chilkoti, 2007).
The development of the next generation of drug delivery systems will likely require effective interdisciplinary efforts of systems biologists, chemists, material scientists, bioengineers, pharmaceutical scientists, clinicians, and pharmacologists. The close collaboration required among these different participants will only be possible if the system is designed from the outset to recognize these interdependencies and collaboration patterns needed at different stages in the progression of delivery systems, from pre-clinical testing and clinical trials to post-approval observational studies used to generate “real-world evidence” of effectiveness. An analogy captures the point: “integration is key to getting all this to work on the therapeutic battlefield—integration of the warhead (drug) with the guidance system (targeting moiety) and with the rocket (the delivery vehicle)” (Dreher and Chilkoti, 2007). Therefore, a systems engineering approach is needed for the design of effective drug delivery systems.
Cancer Survivorship
Presently, there is substantial variation in the provision of cancer survivorship care services, and there is no standard way of evaluating that care. Because such an effort innately involves numerous participants, starting from the individual patient to insurance companies, coordinating resources and the necessary information most relevant to the patient is a perpetual challenge. A decade ago, survivorship care plans were proposed as a way to bring a basic degree of coordination (IOM, 2006), and
have become an official recommendation by the Commission on Cancer and numerous other groups. A survivorship care plan typically contains important information about the cancer type, treatments received and their potential consequences, guidance for follow-up care, and supportive resources available for patients (IOM, 2006; McCabe et al., 2013). While survivorship care plans have primarily focused on informing patients of the effects of their treatments and improving communication between patients and clinicians, recent reports have also begun to consider their shortcomings, which include mixed effectiveness in improving health outcomes of survivors, and ways to improve survivorship care delivery (Jacobsen et al., 2018).
The Systems Engineering Initiative for Patient Safety (SEIPS) has sought to improve “health outcomes, quality of care delivery, transitions of care and coordination, usability, and implementation of health information technology, as well as managing a variety of health care activities, primary care workflows, and decision support” (Tevaarwerk et al., 2018, pp. 2–3). Applying the SEIPS method to cancer survivorship care would involve analyzing the various components of care for cancer survivors, their interactions, and the workflows in the system in order to identify where breakdowns and failures of communication occur and to develop methods to improve communication and coordination among those components. Using risk modeling for service applications, electronic health records, and the design and integration of different tasks performed by clinicians and the patients themselves, the recommendations that emerge from that method could enable improvements to the care process. While this is an example of the promise of systems engineering tools in survivorship care coordination, additional opportunities could be explored and tested to improve survivorship care delivery and improve the quality of life of survivors.
Patient Safety
The 2000 Institute of Medicine report To Err Is Human concluded that medical errors, which can result in patient harm or even death, are common in the health care system (IOM, 2000). Because cancer care is medically complex, it offers multiple points at which such errors can occur. A great deal of effort has been expended in exploring, classifying, and understanding the types and causes of these errors, with the goal of minimizing their occurrence. Once the various types of errors have been listed and explicated, the next step is to find ways to minimize the number and severity of the errors, and it is here that systems engineering can play a role in reducing the likelihood of miscommunication between two or more entities. Unfortunately, despite many calls for systems engineering
partnership in the redesign of health and medical systems, there has been little movement in that direction, and medical errors remain a significant concern (Carayon and Wood, 2010).
Reducing Disparities
A conceptual systems model has been used to examine the factors contributing to health disparities across the cancer continuum. Three levels have been considered for those factors—distal, intermediate, and proximal (Paskett et al., 2016; Warnecke et al., 2008). The model suggests that the distal factors, which operate at the population level, are the fundamental causes of health disparities. This is because such factors influence individuals’ health outcomes independently of the characteristics of individuals.
The distal influences include population-level social conditions, public policies, and institutional and other factors that stem from culture and social norms as well as socioeconomic status and the availability of health services. Intermediate influences include the immediate physical and social contexts as well as the social relationships in which the distal influences are experienced, such as the community or neighborhood. Proximal influences include individual genetic makeup; demographic factors such as age, health status, and race; and health behavior.
This model was applied in Delaware beginning in 2014 as part of an effort to reduce racial disparities in colorectal cancer (Grubbs et al., 2013). At the distal level of influence, the governor of Delaware legislated universal access to screening and treatment of colorectal cancer for all residents, with a statewide program that provided insurance coverage for these services for uninsured residents. At the intermediate level, the program relied extensively on nurses and care coordinators to engage and recruit underserved populations for screening as well as providing case management for patients with abnormal screening results. At the proximal level, the program collaborated with community organizations to reach African American communities and uninsured residents.
Data collected at the end of the program in 2009 indicated a reduction in colorectal cancer mortality and incidence rates among all residents of Delaware. Specifically, among African Americans, the incidence rate of colorectal cancer decreased from 66.9 per 100,000 to 44.3 per 100,000, and the mortality rate decreased from 31.2 per 100,000 to 18.0 per 100,000. Among whites, the incidence rate decreased from 58.2 per 100,000 to 43.2 per 100,000, and the mortality rate decreased from 19.5 per 100,000 to 16.9 per 100,000. In short, although the intervention lowered incidence and mortality rates among both African Americans and whites, the decreases were much greater for African Americans.
SYSTEMS ANALYSES AND SYSTEMATIC TRADE-OFFS
Making policies for complex adaptive systems is seldom straightforward. In the case of cancer control, for example, the simultaneous objectives would include maximizing survival rates, minimizing costs, maximizing quality-adjusted life years (QALYs), reducing side effects and errors, and addressing disparities and inequity. Different stakeholders have different objectives and value propositions. What payers value and prioritize may not be the same as the priorities for health care providers, commercial entities, or nonprofit organizations.
This section provides a case scenario of how a systems engineering approach can help inform policy analysis and decision making in a multilevel complex system. The goal of systems analysis is not to make the decision but to provide information about the trade-offs and help guide and achieve decision convergence on complex issues where the answers might change with time and context.
Multifactorial Analyses
Consider the case of vaccine development for cancer prevention and possibly therapy. In the past, pure health metrics (such as lives saved, infant mortality equivalents, and life years saved) or health economic measures such as cost-effectiveness (typically expressed in terms of dollars over QALYs or disability-adjusted life years [DALYs]) have been used to prioritize new vaccines for development. Cost–benefit analysis and its variant, cost-effectiveness analysis, have a rich history of guiding health policy decisions, but they also have widely recognized limits. For example, cost–benefit analysis typically poses complex ethical and political challenges by putting a specific monetary value on human life. Cost-effectiveness analysis, a close cousin of cost–benefit analysis, seeks to determine the incremental health benefits gained per incremental dollar invested. The resulting value, the incremental cost–effectiveness ratio, is compared against a cutoff value set by a decision maker (such as the £30,000-per-QALY figure used for evaluating medical interventions by the UK National Institute for Health and Care Excellence). The World Health Organization uses a cutoff of one to three times the per capita gross domestic product for cost-effectiveness calculated using DALYs (Marseille et al., 2014).
A major limitation of cost-effectiveness analyses is that a number of important considerations are left out of their calculation. These considerations include long-range issues, such as the spread of infection in a community over time; practical supply chain matters, such as a vaccine’s temperature stability or how the vaccine fits within an immunization
schedule; the herd immunity that can be achieved within a community if enough individuals are vaccinated; and higher level intergenerational issues, such as matters of equity (Phelps and Madhavan, 2017). Some recent efforts have attempted to “enhance” or “augment” cost-effectiveness analysis (Lakdawalla et al., 2018) by including such factors as scientific spillovers, the value of the hope that a disease might be cured, and gains in workforce productivity, but a major challenge remains: how to combine these various outcomes into a useful composite figure that takes into account both measurable and qualitative factors.
Those seeking to make policy decisions about cancer control face similar challenges. The traditional approach to valuing cancer control expenditures has been to use cost–benefit or cost-effectiveness analysis, but this leaves out many factors that are not easily quantified, such as the psychological stresses experienced by cancer survivors and their families or the societal costs of inequities in cancer incidence and treatment. Furthermore, different stakeholders will inevitably place different values on the various factors. Public health groups would likely place a higher value on different aspects of cancer control than cancer treatment clinics, both of which might differ from what a biopharmaceutical firm would most value. The National Institutes of Health, which is heavily focused on research, would place higher value on another set of activities, as would the Department of Agriculture or advocacy groups or a company like Google. Thus, ideally one would like to develop a systems analysis approach to evaluating cancer control activities that can weigh a variety of factors and can be personalized for different stakeholders.
A multi-attribute utility theory–based approach has been successfully prototyped in recent years for evaluating vaccine development. This approach to systems analysis makes it possible to include many different factors, each of which can have its own method of quantification. That quantification might be, for instance, a cost in dollars per QALY, a rating of public fear on a scale from 1 to 10, or even a yes/no value. Those values are all converted in such a way that their possible range is from 0 to 100, and in order to allow different vaccines to be compared, the same scales are used for all vaccines under consideration. Each vaccine is scored on each factor, that score is multiplied by a weighting value that reflects the importance of the factor, and the weighted scores are added up to get the vaccine’s total score. That total reflects the value of the vaccine under a particular set of assumptions about how important each factor is; a different set of assumptions will lead to a different total score.
Performing a cost-effectiveness analysis of a vaccine—or a cancer treatment—requires, for example, assigning a value, or “utility,” to various outcomes, such as being disease free and completely healthy or surviving the disease but with certain physical limitations. Such values can
be estimated with data from population surveys or by expert panels, but they are far from objective. Additionally, some benefits of vaccines (or particular cancer control interventions) are next to impossible to assign value to. What is the value, for instance, of removing an individual’s fear of contracting a disease or of decreasing the inequities in cancer rates and treatments?
One major benefit of multi-attribute systems analysis is that it allows policy makers and others to consider all these sorts of factors when considering which of various policies to choose. While traditional cost-effectiveness analyses have depended primarily on factors that can be easily quantified, multi-attribute systems analysis makes it possible to take into account the factors, such as issues of disparities, religious beliefs, or concerns pertaining to privacy and individual autonomy, that do influence policy making. The simple act of including such factors in a model can be enough to bring them to the attention of policy makers and enable those individuals to think about how to weigh these factors in their decisions.
A Case Illustration: Analysis of Alternatives for Cancer Vaccines
To illustrate how this systems analysis approach might be applied to an aspect of cancer control, an analysis was conducted on a completely hypothetical yet somewhat realistic scenario for prioritizing vaccines for human papillomavirus (HPV), a sexually acquired infection linked most commonly to cervical cancer (WHO, 2019). Trade-offs related to different HPV vaccines were calculated with SMART Vaccines, the Strategic Multi-Attribute Ranking Tool for Vaccines, a software system developed by the National Academies at the request of the Department of Health and Human Services (HHS) based on multi-criteria systems analysis—specifically, multi-attribute utility theory (Phelps et al., 2017).1 SMART Vaccines was developed in response to the U.S. National Vaccine Plan issued by HHS as a dynamic, adaptive, priority-setting tool for use by a wide range of international stakeholders to systematically analyze (and compare) the options that are available and readily usable for vaccine-preventable illnesses.
The following case scenario was constructed, with the SMART Vaccines tool being used to consider three different kinds of hypothetical HPV vaccines for development or use in South Africa. More than 100 types of HPV exist, and at least 14 are known to cause cancer, particularly
___________________
1 Detailed information concerning the technical details and use of the software and data sets can be found in (IOM, 2012, 2013; IOM and NAE, 2015). The software (v1.1) is down-loadable from www.nap.edu/smartvaccines (accessed February 15, 2019).
cervical cancer, although some cancers of the vulva, vagina, penis, anus, head, and neck are also associated with HPV infection. In 2018, about 90 percent of new cervical cancer cases around the world—or 513,000 cases out of a total 570,000—were found in less developed countries (WHO, 2019). The prevalence of HPV in South Africa is known to be high. A recent survey of sexually active, HIV-negative women aged 16–22 years in two South African cities found that two-thirds of them were positive for HPV (Mbulawa et al., 2018). Vaccines against HPV 16 and HPV 18, the most prevalent cancer-causing types of HPV, have been developed, but they are not in widespread use in many countries around the world.
For the purposes of this hypothetical demonstration, the incidence of HPV in South Africa is estimated to be under 100 cases of HPV per 100,000, with a case fatality rate between 30 and 80 percent depending on the complications, which include cervical intraepithelial neoplasia and vulvar cancer. The complications differ in their severity and annual costs, which will be assumed to be between $300 and $5,500.
Now, consider a hypothetical government agency in South Africa involved in both research and product development (potentially through some private-sector partnership) trying to design a new vaccine or purchase a commercially available vaccine for HPV among three candidates, HPV-X, HPV-Y, and HPV-Z. All three candidates are intended for people over the age of 1 year and have an estimated effectiveness between 70 and 90 percent and coverage between 60 and 85 percent.
HPV-X is a single-dose vaccine offering a 25-year immunity and costing a hypothetical $13 per dose and $10 for administration per dose, and the vaccine development is expected to cost under $100 million. HPV-Y is a two-dose vaccine offering lifetime protection, costing $13 per dose, $12 for administrative expenses per dose, with development costs estimated between $100 million and $500 million. HPV-Z also confers lifetime immunity with a similar anticipated $100 million to $500 million development cost, and it costs $9 per dose and in administrative expenses, but it is a three-dose vaccine.
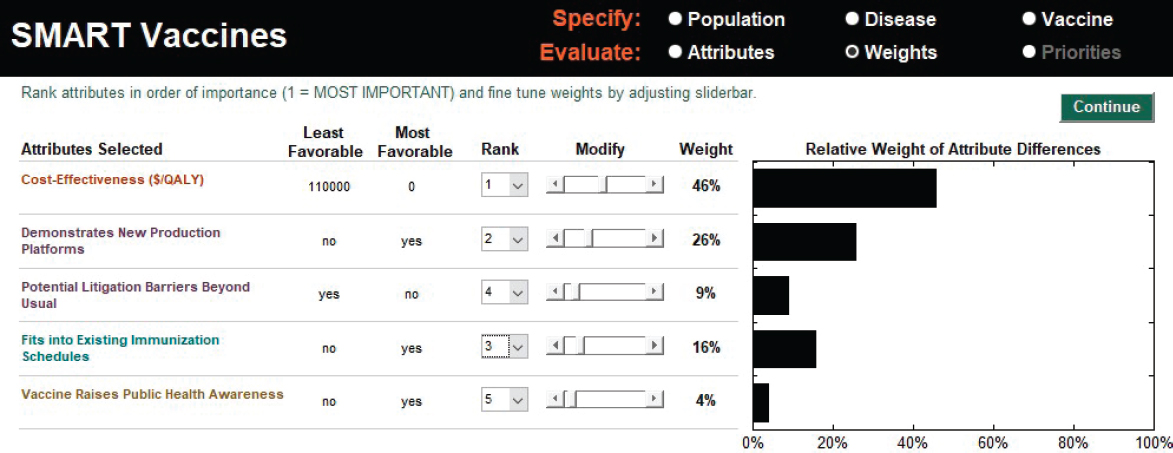
The user begins the analysis by selecting or defining the attributes of interest and then ranking them in order of importance. Of relevance to HPV priority setting are some factors selected from the collection of attributes in SMART Vaccines: cost-effectiveness (cost per QALYs); demonstration of new production platforms (this includes scientific spillovers or use of existing manufacturing approaches to produce new vaccines); potential litigation barriers beyond usual (an issue that comes to fore with HPV vaccines); fitting into the existing immunization schedule (given the comparison involving multi-dose vaccines); and raising public health awareness of HPV-related cancers (a potential collateral benefit associated with promoting safe sex practices). Next, the user determines the weights
to assign to the different attributes. The process is assisted by SMART Vaccines, which offers a suggested weighting that is based on the average of all the different possible sets of weights that are consistent with the user’s ranking of the attributes. These initial weights can be adjusted by the user with slider bars provided by SMART Vaccines. In this HPV scenario, the initial suggested weights are used. Among the attributes in this example, cost-effectiveness is the highest ranked (with a 46 percent weight), and the attribute of the vaccine raising public health awareness is lowest ranked (with a 4 percent weight) (see Figure 3-2).
If one were to conduct a pure cost-effectiveness analysis—setting additional attributes selected for demonstration at 0—of these three HPV vaccine candidates, they are comparably cost-effective, with each achieving the high score of 100 through its cost savings or health benefits. HPV-X produces $101 per QALY, and HPV-Y and HPV-Z produce a net savings (indicated by negative) of $493 per QALY, each essentially tied at a SMART Score of 100. (The range of scores is typically 0 to 100, with 0 representing a vaccine that has no effect and 100 corresponding to a vaccine that is highly successful on all the attributes under consideration. The scores may go beyond 100 [better than the envisioned best-case scenario] or below zero [worse than the envisioned worst-case scenario] depending on their superiority or inferiority of their performance.) These numbers can easily be altered, with transparency, by changing the disease burden and vaccine profile according to the simulated or real circumstances. These changes can be made in a manner that makes it clear to the user why certain changes have occurred—a feature that is otherwise not so easily available in standard cost-effectiveness analyses, which do not capture the broader complexity of cancers and their impact on specific programs or larger aspects of social policy.
Next, the other attributes in SMART Vaccines are brought into the analysis; these include domains across health, programmatic, public concerns, and scientific and business considerations as well as intangible values. According to this hypothetical scenario, the single-dose HPV-X readily fits into the immunization schedule, does not contain potential litigation barriers beyond usual, and raises public health awareness but does not beneficially demonstrate new production platforms. HPV-Y is largely similar to HPV-X except that it requires two doses and, because of this additional dose, may pose additional litigation issues, something that also is the case with HPV-Z, the three-dose vaccine. HPV-Z offers novelty or efficiency in production methods and, because of multiple doses, does not readily fit into any existing immunization schedules, thus simultaneously presenting scientific advantages but logistical challenges. The resulting SMART Scores based on these entries are shown in Figure 3-3. Despite multiple doses and potential litigation barriers, the HPV-Z
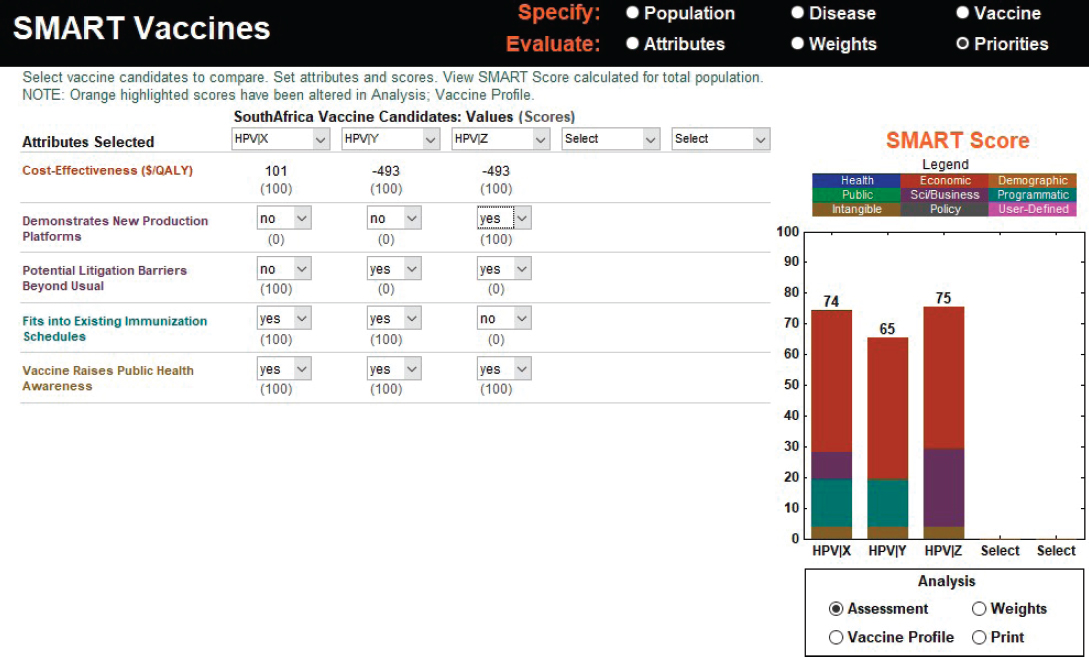
vaccine scores higher than HPV-X (at 74) and HPV-Y (at 65). With changes in weights, the results will naturally change, and sensitivity analysis can be conducted (using the “vaccine profile” button in SMART Vaccines) to reverse engineer a product profile to improve the performance of a candidate either, for example, through boosting coverage (a programmatic matter) or effectiveness (a scientific matter) or through cost reduction (an administrative and industrial manufacturing matter) or, ultimately, dosage alteration. One could conceivably work toward developing desirable hybrid attributes (for example, imagining a two-dose HPV-Z) or using a two-dose HPV-Y and supplementing it with additional social, scientific, or policy tools to increase its performance.
The results create an important discussion opportunity among participants on why a particular vaccine candidate may be scientifically better, economically preferred, or more politically feasible over another. These kinds of discussions might be particularly valuable in an interagency setting or a national or international advisory group with individuals and institutions bringing varying perspectives on issues of common interests. It is possible to envision and apply multi-criteria decision support to many aspects of population health and medicine, especially cancer control. The next section explores that prospect.
BUILDING A MULTI-LEVEL MODEL
The preceding multi-criteria systems analysis shows that it is possible to construct a decision-support model that captures an important aspect of cancer control—deciding on a vaccine strategy—and allows policy makers to consider a variety of criteria in choosing which direction to go. A key aspect of multi-criteria approaches is that it makes it possible to weigh different strategies by comparing the outcomes of those strategies on a number of different measures chosen by the user. Importantly, multicriteria approaches could also enable an expanded analysis of types of outcomes—economic, clinical, scientific, and otherwise—through different measures. Five such factors are illustrated in Figure 3-4: the strength of the evidence, the magnitude of the problem, the actions taken, barriers and facilitators, and the strategies across participant groups to determine which activities need to be improved, revamped, or terminated (Norton et al., 2018).
One could envision a similar—albeit much more complicated—“model of models” that captured the entire cancer control system and similarly made it possible to compare the outcomes of different sets of strategies as evaluated by various measures. How would one go about creating such a model? The following is one possible approach.
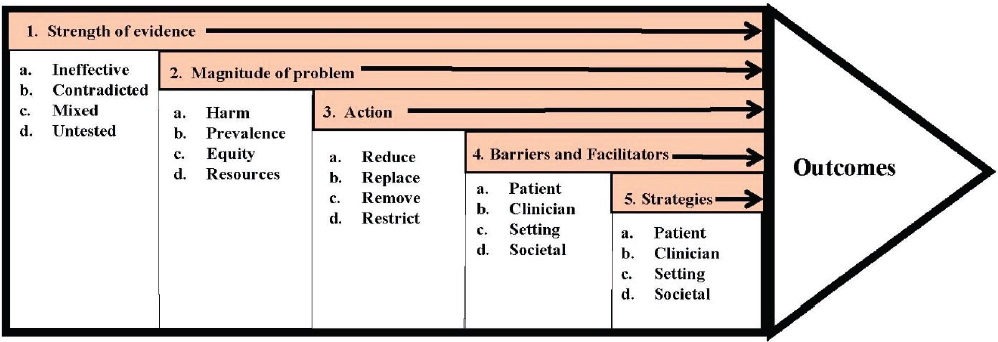
SOURCE: Adapted from Norton et al., 2018.
The Levels of Cancer Control
As discussed earlier, the cancer control system spans a number of levels, and thus any model of the cancer control system will inevitably be a multi-level model with a combination of individual models that capture various aspects of the overall system. In recent years, a number of frameworks for modeling complex social systems, such as higher education, medical services, and population health, have been proposed (Rouse et al., 2019). One conceptual description centers on integrating several models that had been suggested for understanding the roles of various factors—social, psychological, political, economic, and biological—in determining health and health disparities (Kaplan, 2004). This concept follows that general pattern by describing a multilevel model of cancer control. In particular, the general model proposed here has four levels, each of which can be modeled separately and then linked with the others to produce a full model (Madhavan et al., 2018) (see Figure 3-5).
The levels of the model are population ecosystem, or society; system structure, or organization; delivery operations, or processes; and service interactions, or people. Modeling each of these levels is a separate challenge. The organizational level, for example, is described by the microeconomics of resource allocation, with decision theory and behavioral economics used to model the behavior of individuals involved in the organizations. The society level is described mainly by macroeconomics. In use, the model would be set up to be interactive so that a user could change the values of various parameters and observe the results; those results would typically be shown through some sort of visualization that would allow the user to make sense of the complex behavior of the system.
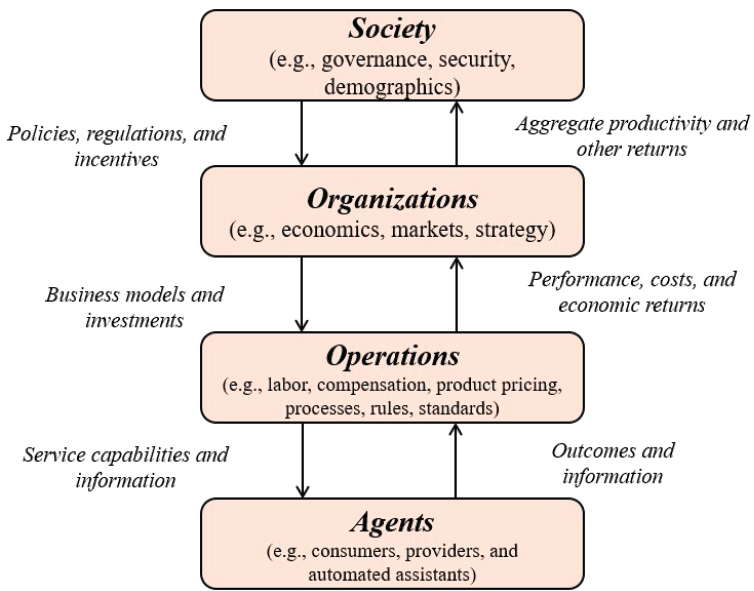
SOURCE: Madhavan et al., 2018.
A Family of Models
A model of the U.S. cancer control system would serve the same sort of purpose as a multi-criteria, multi-level model, just on a much larger scale. That is, it would allow stakeholders to see the likely outcome of various competing options, and, in so doing, it would help stakeholders clarify their values and priorities.
In building such a model, it will be possible—and desirable—to draw on the large number of existing models of various aspects of the cancer control system. For example, the researchers who are part of the Cancer Intervention and Surveillance Modeling Network (CISNET) have built a number of models predicting the outcomes of specific types of cancer depending on various factors, such as screening rates or the development of new treatments. One group of CISNET researchers, for example, created a model that projected the rates of smoking over the next 50 years and
used that to predict what the annual incidence rates and death rates from lung cancer will be up through 2065 (Jeon et al., 2018). There are CISNET models for certain types of cancer. They can be used to predict incidence rates and deaths (among other factors), given the appropriate inputs, such as individual behaviors (smoking, vaccination), technological factors (the development of new treatments or drugs), and economic factors (the cost of treatment, for example).
There are many other types of existing models that could also be used in building a model of the U.S. cancer control system. Various population-level models can be used to project different aspects of the population in coming years, such as the numbers of people and their ages living in rural, suburban, and urban areas in 2040 or the rates of obesity by age and sex in 2025. There are also models that can forecast rates of various types of relevant behavior, such as the vaccination rates for different groups of people or the rates of compliance with recommended treatment followup. While it is not likely that every important aspect of the cancer control system has been modeled or has a model that can be applied to it, that is true for most of them.
Technical challenges will certainly arise when one starts to combine these models to create, say, one model or a suite of linked core models of the entire cancer control system. To begin with, there is wide variation in how detailed the models are and how well they work. Some are very basic models designed to capture large-scale trends, while others have much more detailed projections. Some are well validated in a variety of settings, while others have not been tested nearly so thoroughly. A key challenge will come from the variations in how the different models define the variables they use and the data they rely on. If the different models are to fit together to form a single model of the cancer control system, they will all need to use the same variables and rely on the same data, which means that it will be necessary to first settle on the data standards that will be used for the model and then modify the individual models to use those standards. Once that is done, the individual models will need to be verified and validated with the new standards and data formats. The resulting family of models will provide the “raw materials” that will be assembled to create the overarching model of the cancer control system.
As the models are combined, new capabilities will appear. For example, a behavioral model that predicts the effectiveness of antismoking campaigns in getting people to stop smoking could be combined with an epidemiological model of lung cancer as a function of smoking rates to make it possible to see what effects an increase in funding for antismoking campaigns would have on lung cancer rates over the next several decades. Or projections of immigration from countries where the smoking rate is
higher could be used to sharpen the forecasts of how many people living in the United States are likely to smoke in 2030 or 2040.
The perceptive reader will have noticed a disconnect between this description of a family of models used to capture the cancer control system and the concept of cancer control being a complex adaptive system, which by its very nature cannot be decomposed into component parts that can be analyzed individually. How then could such a family of models accurately capture the behavior of the cancer control system?
The answer has several parts. First, the fact that cancer control is a complex adaptive system does not imply that none of its component parts can be modeled individually. They can—as long as it is known what the inputs from the rest of the system are. The complexity arises because of the feedback loops among the components: component A affects component B affects component C, which in turn affects component M, which links back to component A. So whatever model one designs will have to accurately reflect how the different components interact with and affect one another, but that is a separate issue from whether the individual components can be effectively modeled. In addition, there are likely to be cases where the interactions between components, while present, are insignificant enough that they can be ignored by the model or added in as a correction factor toward the end of a simulation run. And in cases where the interaction between components is particularly strong, it would likely make sense to model them together instead of modeling them separately and later adding the interactions.
In short, while the interactions between components will certainly need to be captured in the overall model, a family of models that capture individual elements of the cancer control system will form the foundation of the overall model.
GUIDANCE SYSTEMS FOR CANCER CONTROL
The ultimate goal of assembling such a family of models will be to create a system that can be used not just to make predictions about the performance of the cancer control system under various scenarios but ultimately to guide the cancer control system. As noted earlier, the choice of the verb “guide” instead of a word like “control” or “direct” is deliberate. As a complex adaptive system, cancer control cannot be directed. It can, however, be “influenced” or “guided” in such a way that it moves in a desired direction—becoming more efficient, for example, or more equitable. Developing such a guidance system will be a major undertaking (and is far beyond the scope of this report to provide a construction blueprint). However, it is possible to discuss what some of the system’s characteristics and features might be.
A Systems Architecture for Cancer Control
The first step must be to understand the present system, its principal components, and their interactions. These components will include various governmental bodies and agencies that set cancer-related policy, research and funding organizations, clinical research entities at hospitals and universities, the biopharmaceutical and device industry, the health insurance industry, professional organizations, individual patients and their families, and advocacy groups, among others. Any model that captures this system will be exceedingly complicated and will not decompose into separate pieces that can be understood in isolation. It will include multiple subsystems—hospitals and clinicians, researchers, the insurance industry, government regulators, patient advocacy and support groups, and biopharmaceutical companies—and it will include multiple levels, from the biological to the societal.
Some of the concepts for such a multi-level modeling effort already exist. A 2012 article, for example, identified seven levels at which cancer care could be influenced: the individual patients, family and social supports, the clinical team, the clinical practice setting, the local community, the state health policy environment, and the national health policy environment (Taplin et al., 2012). These are different from, but closely related to, the four levels described earlier that are envisioned for use in analyzing and advancing the cancer control system.
Planning and Policy Setting
With the model established, the next step will be to create a simulation that can mimic the behavior of the system and then test that simulation for accuracy. One way to do that is to look to the recent past and see whether the system can “predict” what happened or “forecast” possible futures. Can it, for instance, accurately predict the response of the entire system to the introduction of a new technology, such as an effective drug? Can it accurately model the behavior of hospitals and clinics when a major change in insurance policies occurs?
A main purpose of the simulation is to show how the performance of the system changes as a result of specific modifications. The simulation requires continual updates with the most recent data so that it reflects current information. This monitoring will make it possible to detect trends and to test the accuracy of forecasts. If the simulation is to be useful in predicting how various changes will affect the system’s performance, it should certainly be able to predict outcomes in the near term without any major changes in the system. In addition, the system should be able to learn by comparing its forecasts with what actually happens. Figure 3-6
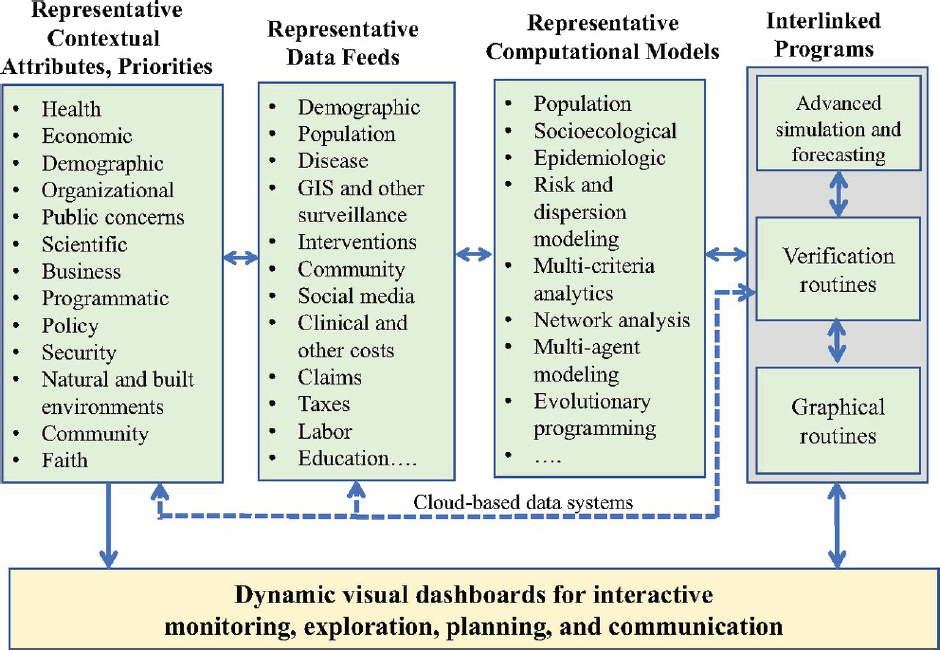
SOURCE: Madhavan et al., 2018.
shows a generic population health systems architecture, customizable for cancer control efforts, that blends contextual attributes and priorities with necessary data feeds from different channels operated on by different computational models that ultimately produce a dynamic visual dashboard to track, plan for, and initiate joint action among multiple constituents.
Construction of such a population health systems architecture will make it possible to run, evaluate, and compare different scenarios and outcomes to aid in policy making. For example, what will happen to cancer rates over the next two decades under different funding allocations between prevention efforts and complex treatment regimens with or without constraints on the use and cost of treatment for different tumor classes and subtypes?
One of the most useful aspects of such a system architecture would be the ability to see what the collective demands are at any particular point and what happens when coordinated changes are made to various components of the cancer control system in response to those demands or in advance of any potential changes in health. There is a growing agreement across various stakeholders that, to be effective, changes made to
a system such as cancer control will need to be made at multiple levels simultaneously (Taplin et al., 2012), but there currently is no capability of determining what effects a set of changes carried out at multiple levels is likely to have.
Finally, once such a comprehensive model of models has been developed, policy makers could use it to zero in on a set of changes necessary to guide the cancer control system in a desired direction. These could be changes in the allocation of funding among research, public health initiatives, training of medical personnel, and so on; they could be new regulations covering hospitals and medical practices or insurance coverage; they could be modifications to pharmaceutical patent policies; or they could be any other changes the government could initiate that would affect the cancer control system. Once the changes and the related assumptions had been put in place, the system would monitor the effects of those changes and use that feedback to sharpen future simulations and develop a learning process.
FINDINGS
Finding 3-1: Looking ahead, complex systems analytics made possible by the convergence of modern computational technologies—including biotechnologies, nanotechnologies, communication, social media, cognitive, sensor technologies, and other systems engineering tools—could materially amplify new insights, capabilities, and competencies necessary for advancing cancer control and potentially reducing costs for the United States.
Finding 3-2: Systems engineering tools have been effectively applied to design, guide, influence, and improve complex adaptive systems in multiple industrial and other settings across society. Some previous analyses involving clinical care and survivorship services have recognized the role of systems engineering approaches to offer both operational insights and prospects for effective cancer control.
Finding 3-3: Multi-criteria systems analyses offer promise to transcend the narrow analytical tools used across different aspects of health and medical policies and could support individual and group decision making at multiple levels, including critically reviewing which activities need to be initiated, improved, scaled, or discontinued as cancer control efforts evolve.
Finding 3-4: Developing a guidance system to fully understand the influences and impacts on a national cancer control plan requires a comprehensive planning and monitoring tool or a set of linked tools able to integrate different data and
perspectives, blend modeling and simulation capabilities, and produce dynamic visuals for interactive monitoring, exploration, planning, and communication.
REFERENCES
Carayon, P., and K. E. Wood. 2010. Patient safety—the role of human factors and systems engineering. Studies in Health Technology and Informatics 153:23–46.
Dreher, M. R., and A. Chilkoti. 2007. Toward a systems engineering approach to cancer drug delivery. Journal of the National Cancer Institute 99(13):983–985.
FAA (Federal Aviation Administration). 2018. Administrator’s fact book: December 2018. https://www.faa.gov/news/media/2018_Administrators_Fact_Book.pdf (accessed February 15, 2019).
FAA. 2019a. Modernization of U.S. Airspace. https://www.faa.gov/nextgen (accessed February 15, 2019).
FAA. 2019b. Performance based navigation in the operation. https://www.faa.gov/nextgen/how_nextgen_works/new_technology/pbn/in_depth (accessed February 15, 2019).
GAO (Government Acountability Office). 2015. Air traffic control: FAA needs a more comprehensive approach to address cybersecurity as agency transitions to NextGen. GAO-15-370. April. Washington, DC: U.S. Government Accountability Office.
Grubbs, S. S., B. N. Polite, J. Carney Jr, W. Bowser, J. Rogers, N. Katurakes, P. Hess, and E. D. Paskett. 2013. Eliminating racial disparities in colorectal cancer in the real world: It took a village. Journal of Clinical Oncology 31(16):1928.
Hartwell, L., D. Mankoff, A. Paulovich, S. Ramsey, and E. Swisher. 2006. Cancer biomarkers: A systems approach. Nature Biotechnology 24(8):905.
Huang, T. T., A. Drewnowski, S. K. Kumanyika, and T. A. Glass. 2009. A systems-oriented multilevel framework for addressing obesity in the 21st century. Preventing Chronic Disease 6(3).
IOM (Institute of Medicine). 2000. To err is human: Building a safer health system. Washington, DC: National Academy Press.
IOM. 2006. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press.
IOM. 2012. Ranking vaccines: A prioritization framework: Phase I: Demonstration of concept and a software blueprint. Washington, DC: The National Academies Press.
IOM. 2013. Ranking vaccines: A prioritization software tool: Phase II: Prototype of a decision-support system. Washington, DC: The National Academies Press.
IOM and NAE (Institute of Medicine and National Academy of Engineering). 2015. Ranking vaccines: Applications of a prioritization software tool: Phase III: Use case studies and data framework. Washington, DC: The National Academies Press.
Jacobsen, P. B., A. P. DeRosa, T. O. Henderson, D. K. Mayer, C. S. Moskowitz, E. D. Paskett, and J. H. Rowland. 2018. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. Journal of Clinical Oncology 36(20):2088–2100.
Jeon, J., T. R. Holford, D. T. Levy, E. J. Feuer, P. Cao, J. Tam, L. Clarke, J. Clarke, C. Y. Kong, and R. Meza. 2018. Smoking and lung cancer mortality in the united states from 2015 to 2065: A comparative modeling approach. Annals of Internal Medicine 169(10):684–693.
Kaplan, G. A. 2004. What’s wrong with social epidemiology, and how can we make it better? Epidemiologic Reviews 26(1):124–135.
Lakdawalla, D. N., J. A. Doshi, L. P. Garrison, C. E. Phelps, A. Basu, and P. M. Danzon. 2018. Defining elements of value in health care—a health economics approach: An ISPOR special task force report [3]. Value in Health 21(2):131–139.
Leischow, S. J., A. Best, W. M. Trochim, P. I. Clark, R. S. Gallagher, S. E. Marcus, and E. Matthews. 2008. Systems thinking to improve the public’s health. American Journal of Preventive Medicine 35(2):S196–S203.
Luke, D. A., and K. A. Stamatakis. 2012. Systems science methods in public health: Dynamics, networks, and agents. Annual Review of Public Health 33:357–376.
Madhavan, G., C. E. Phelps, W. B. Rouse, and R. Rappuoli. 2018. Vision for a systems architecture to integrate and transform population health. Proceedings of the National Academy of Sciences 115(50):12595–12602.
Marseille, E., B. Larson, D. S. Kazi, J. G. Kahn, and S. Rosen. 2014. Thresholds for the cost-effectiveness of interventions: Alternative approaches. Bulletin of the World Health Organization 93:118–124.
Mbulawa, Z. Z., C. van Schalkwyk, N.-C. Hu, T. L. Meiring, S. Barnabas, S. Dabee, H. Jaspan, J.-M. Kriek, S. Z. Jaumdally, and E. Muller. 2018. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS ONE 13(1):e0190166.
McCabe, M. S., S. Bhatia, K. C. Oeffinger, G. H. Reaman, C. Tyne, D. S. Wollins, and M. M. Hudson. 2013. American Society of Clinical Oncology statement: Achieving high-quality cancer survivorship care. Journal of Clinical Oncology 31(5):631.
Norton, W. E., D. A. Chambers, and B. S. Kramer. 2018. Conceptualizing de-implementation in cancer care delivery. Journal of Clinical Oncology 37(2):93–96.
Paskett, E., B. Thompson, A. S. Ammerman, A. N. Ortega, J. Marsteller, and D. Richardson. 2016. Multilevel interventions to address health disparities show promise in improving population health. Health Affairs 35(8):1429–1434.
Phelps, C. E., and G. Madhavan. 2017. Using multicriteria approaches to assess the value of health care. Value in Health 20(2):251–255.
Phelps, C. E., G. Madhavan, and B. Gellin. 2017. Planning and priority setting for vaccine development and immunization. Vaccine 35:A50–A56.
Rouse, W. B., K. M. Pepe, and M. M. Johns. 2019. Population health as a network of services: Integration of health, education, and social services. Handbook of Service Science 2:589–618.
Taplin, S. H., R. Anhang Price, H. M. Edwards, M. K. Foster, E. S. Breslau, V. Chollette, I. Prabhu Das, S. B. Clauser, M. L. Fennell, and J. Zapka. 2012. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. Journal of the National Cancer Institute. Monographs 2012(44):2–10.
Tevaarwerk, A. J., J. R. Klemp, G. J. van Londen, B. W. Hesse, and M. E. Sesto. 2018. Moving beyond static survivorship care plans: A systems engineering approach to population health management for cancer survivors. Cancer 124(22):4292–4300.
Trosman, J. R., R. C. Carlos, M. A. Simon, D. L. Madden, W. J. Gradishar, A. B. Benson III, B. D. Rapkin, E. S. Weiss, I. F. Gareen, and L. I. Wagner. 2016. Care for a patient with cancer as a project: Management of complex task interdependence in cancer care delivery. Journal of Oncology Practice 12(11):1101–1113.
Warnecke, R. B., A. Oh, N. Breen, S. Gehlert, E. Paskett, K. L. Tucker, N. Lurie, T. Rebbeck, J. Goodwin, and J. Flack. 2008. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Health 98(9):1608–1615.
WHO (World Health Organization). 2019. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed February 15, 2019).


























