1
Complexity: From Cells to Society
For millennia, humanity has grappled with cancer, seeking both to understand it and to control it. The first documented mention of cancer was 3,500 years ago, when an Egyptian surgeon described treatmentts, usually unsuccessful, for breast cancer. Eleven hundred years later, the Greek physician Hippocrates thought that tumors of the breast resembled crabs and referred to them as karkinos, the Greek word for crab. Later, the Romans translated the term into Latin and called the tumors “cancer,” the term we still use today. From the 15th through the 19th centuries, clinicians suggested a variety of causal explanations for cancers at different points of understanding, from “divine punishment,” to noxious substances that spread through the body, or the product of lymph fluids (Sudhakar, 2009).
In the past century, cancers have become a very prominent threat to population health, in large part because people live long enough to have a higher likelihood of developing some type of cancer. During that same 100 years, the tools available to prevent, diagnose, and treat cancer have also multiplied. Eighty years ago, the only available treatments for cancer were surgery and radiation, but today’s clinicians have many more options to treat their patients. Research over the past several decades has led to a much better understanding of the biology of cancer, albeit still incomplete, which in turn has led to rapid advances in prevention, detection, and treatments. Although benefiting many patients, these new technologies unfortunately have not mitigated the still substantial toll of cancer or led to the expected victory in the “war on cancer.” In 2018, about 600,000 people in the United States died from cancer, and about 1.7
million people received a new diagnosis of cancer (Gapstur et al., 2018). According to the National Cancer Institute’s (NCI’s) “cancer clock,” a new cancer is diagnosed in the United States approximately once every 30 seconds (NCI, 2018b).
THE SCOPE OF CANCER CONTROL
The goal of this report (see Box 1-1 for the study context) is to provide a national strategic vision for cancer control in the United States. In doing so, this report approaches “cancer control” as a much broader range of actions than is most commonly understood. The reason lies in the complex nature of cancer, which requires efforts on multiple fronts. Indeed, the history of cancer control can be seen as a gradual broadening of efforts, with the realization that each successive effort has not yet been sufficient to address the full spectrum of the cancer burden.
The earliest approach to treating cancer was exceptionally direct: excising it. One hundred years ago, this was the only option, and surgery to remove cancerous tissue did have some successes. But, in many cases, the cancer returned, often in a more aggressive version than the first time around. So in the mid-20th century, clinicians began working with radiation and drugs, generally in combination with surgery, to rid the body of the cancer. This was more successful, and clinicians gradually assembled an array of cytotoxic and molecularly targeted cancer drugs from which to choose for particular cases. More recently, clinicians have been working with immunotherapy, using the body’s immune system to attack cancer cells. These various approaches—surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy—constitute the “treatment” aspect of cancer control.
The realization in the 20th century that many cancer cases are not random occurrences but rather are the body’s response to a particular carcinogen led to a second aspect of cancer control: “prevention.” Such strategies as decreasing smoking rates and alcohol use, improving healthy food consumption, eliminating environmental carcinogens such as asbestos, and vaccinating against cancer-causing viruses such as the human papillomavirus (HPV) seek to prevent cancer from occurring in the first place.
If cancer does strike, the sooner it is detected, the better the odds are that a patient can be cured or effectively treated for some time. Thus, early detection is another important component of cancer control. Similarly, once a patient has—or is suspected to have—cancer, it is crucial to get an accurate diagnosis, as this points the way to the most appropriate treatment. The diagnostic aspect of cancer control involves physical examinations, imaging, laboratory tests on blood and other body fluids and tissues, pathological examinations of tumor tissues, and the analysis of this information to determine the likely stage and characteristics of the cancer.
Both the cancer itself and the treatment for it expose the patients and their families to all sorts of stresses—physical, financial, psychological, social, spiritual, and so on. Helping patients and their families deal with these stresses is not only the humane thing to do, but it may also influence the effectiveness of the treatment. Thus, the provision of such care has become accepted as another aspect of cancer control. Supportive services can help patients and their family members deal with a variety of psychosocial stresses, such as the depression that may accompany a cancer diagnosis or an extended course of treatment. “Palliative care” is focused on addressing the symptoms of cancer and the side effects of treatment, such as pain and nausea. It can, for instance, include the prescription of nausea-relieving drugs to help a patient deal with the side effects of chemotherapy or the use of radiation to shrink tumors that are causing pain or other symptoms, such as an incurable lung cancer triggering shortness
of breath. As medical advances have allowed individuals with cancer to survive for increasingly longer periods, such palliative care has become a major aspect of cancer control. “Hospice care” refers to supportive and palliative care provided at the end of life. It is sometimes broken out as a separate aspect of cancer control. In recent years, as more patients are surviving a cancer diagnosis for long periods of time, many efforts have also focused on “survivorship care” to address the long-term and delayed effects from cancers and cancer treatment.
From Cures to Control
Formal policy approaches to reducing the cancer burden in the United States can be tracked back to 1928, when the U.S. Congress requested the National Academy of Sciences to provide advice to the federal government for developing “a successful and practical cure for cancer.”1 Nine years later, during the presidency of Franklin Delano Roosevelt, NCI was established by the U.S. Congress primarily to show “the useful application of results” (NCI, 2018c). NCI was scaled back during World War II, but in the postwar era, renewed interest in the subject led to the expansion of cancer control efforts (Breslow, 1979).
In 1971 President Richard Nixon signed the National Cancer Act, signaling the beginning of what was termed the “war on cancer.” The act widely increased research on the biological basis of cancer, with the expectation of finding a cure. In addition to increasing federal expenditures on cancer research, the act significantly boosted the political and public profile of cancer. Since then, for instance, cancer surveillance capabilities have dramatically improved thanks to efforts of entities such as the National Program of Cancer Registries (which provides population-based cancer data for national, state, and local health planning),2 and the federal Surveillance, Epidemiology, and End Results program (which established a coordinated system of cancer registries), complemented by efforts of private companies in the technology sector (White et al., 2017). As shown in Figure 1-1, the generally upward trend in cancer death rates evident through the early 1970s did not initially abate once the federal government had declared “war on cancer.” Rather, mortality rates generally continued to increase for two decades before finally peaking around 1990. Since then, declines in age-adjusted mortality rates have been seen for specific high-incidence cancers: lung, colorectal, breast, and prostate. However, the statistics related to many other conditions, including
___________________
1 S. 3554, 70th Congress, Sess. 1 (1928).
2 This text has been revised since prepublication release.
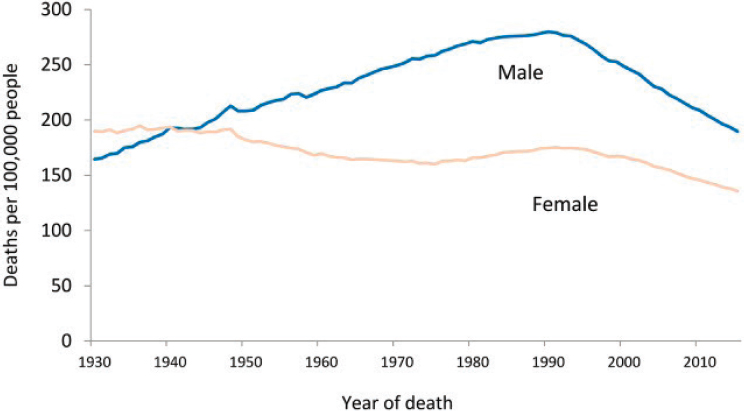
SOURCE: Siegel et al., 2018.
pancreatic, glioblastoma, and advanced metastatic cancers, have remained largely unchanged over time.
Generally speaking, the therapeutic approach to cancer has evolved from a purely “cures”3 mind-set to a broader concept of “control.” Although the general public, politicians, and many philanthropies still interpret the ultimate goal of cancer research as finding a “cure”—as indicated by the popular use of terms such as “conquests” or “moon shots” (inspired by the Apollo program)—in recent years, as noted earlier, various communities have begun to appreciate the need to find new avenues to better manage cancer as a chronic disease rather than focusing solely on elimination.
CANCER BURDEN AND DISPARITIES
Cancer control efforts face a vexing challenge due to the rapidly growing numbers of older people in the United States. As shown in
___________________
3 The term “cure” is typically used to describe an outcome of a treatment where there are no traces of the cancer and where the cancer will never come back. Clinically, the term has been used to refer to conditions for persons whose signs and symptoms of cancer are reduced for 5 years or more. This concept, of course, is difficult to apply to all cancers because even after treatment, some cancer cells may still remain in the body and can cause complications later.
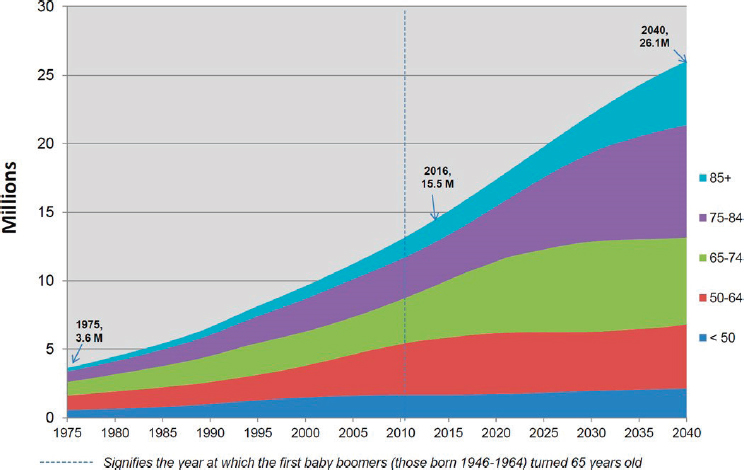
SOURCE: Bluethmann et al., 2016. Reprinted with permission.
Figure 1-2, by 2040 the number of people in the United States aged 50–64 years with cancer is expected to be four times the number in 1975. The projected growth in the number of cancer cases is even more dramatic in older groups: a 6-fold increase in the 65–74 age range, a 10-fold increase for 75–84, and a 17-fold increase in the over-85 cohort (Bluethmann et al., 2016). The situation is due to the rapidly increasing numbers of people in older age brackets and not to increases in cancer rates among them. Concomitant increases in obesity rates are also expected to contribute to increased cancer diagnoses.
The burden of cancer can be observed most directly at the individual level. That burden is physical, emotional, psychological, social, and financial, and all these aspects are important, but it is the financial burden that can be most easily calculated. Cancer care, which is most often provided in outpatient settings,4 comprises one of the largest cost components of clinical services in the United States (KFF, 2017), and expenditures on biopharmaceuticals and various care services have been growing. The financial burden of cancer care falls on a relatively small part of the
___________________
4 See Table 3: Total expenses and percent distribution for selected conditions by type of service: United States, 2014. Agency for Healthcare Research and Quality. From https://bit.ly/2NsfCyd (accessed February 15, 2019).
population, with about 72 percent of cancer expenditures attributable to 5 percent of those with cancer (Cohen, 2014). Overall, 33 percent of cancer care expenditures during treatment are borne by Medicare and Medicaid, 44 percent by private insurance, and 15 percent through other sources (e.g., veterans’ benefits), while only 4 percent of the cost of cancer care is actually paid (mainly through cost sharing) by the patients (ACS CAN, 2017). Still, that seemingly small portion can be a major burden. Nonreimbursed cost sharing for cancer care often leaves patients and their families with considerable debt as the percentage of cancer care costs fully paid for by insurance has been shrinking, and some patients may lack insurance altogether. One-third of patients undergoing cancer treatment go into debt (Banegas et al., 2016), and the costs associated with cancer care are one of the top reasons for declaring personal bankruptcy in the United States (Gilligan et al., 2018; Gordon et al., 2017; Lathan et al., 2016). Generally speaking, the economic impact of a cancer diagnosis will vary according to the features of the patient’s insurance plan as well as the patient’s ability to pay; furthermore, differences in patients’ ability to pay is a major contributor to the disparities in cancer outcomes (ASCO, 2017; Gordon et al., 2017).
The incidence of cancer among minorities is also expected to grow much faster than among whites, with the latter expected to see the highest absolute number of cancer cases (because of their larger numbers in the population) but likely to have the smallest change in incidence—about 31 percent—over the 2010–2030 period. By comparison, cancer incidence is projected to increase by 142 percent for Hispanics, 64 percent for blacks, 132 percent for Asians/Pacific Islanders, 76 percent for American Indians/Alaskan Natives, and 101 percent for multiple-race individuals (Smith et al., 2009).
Cancer control efforts also exhibit persistent disparities in mortality rates among patients of different racial and ethnic groups, socioeconomic status, and geographic location of residence (Siegel et al., 2018) (see Figure 1-3). While the black–white differences in death rates during 1975–1980 for breast cancer and colon cancer (for both males and females) were relatively small, by 1990 (or before) the mortality rates for blacks far exceeded those for whites, and significant gaps remained through 2015. For prostate cancer, the mortality rate has declined consistently over the past 20 years, but it remains significantly higher for black men than for all other racial and ethnic groups.
Educational attainment is also known to affect cancer outcomes. In 2014 the risk of death for adults (aged 25–74) who had no more than a high school education was much higher than for those with at least a college education (for all major types of cancers except brain and other central nervous system tumors). There are also notable differences at the
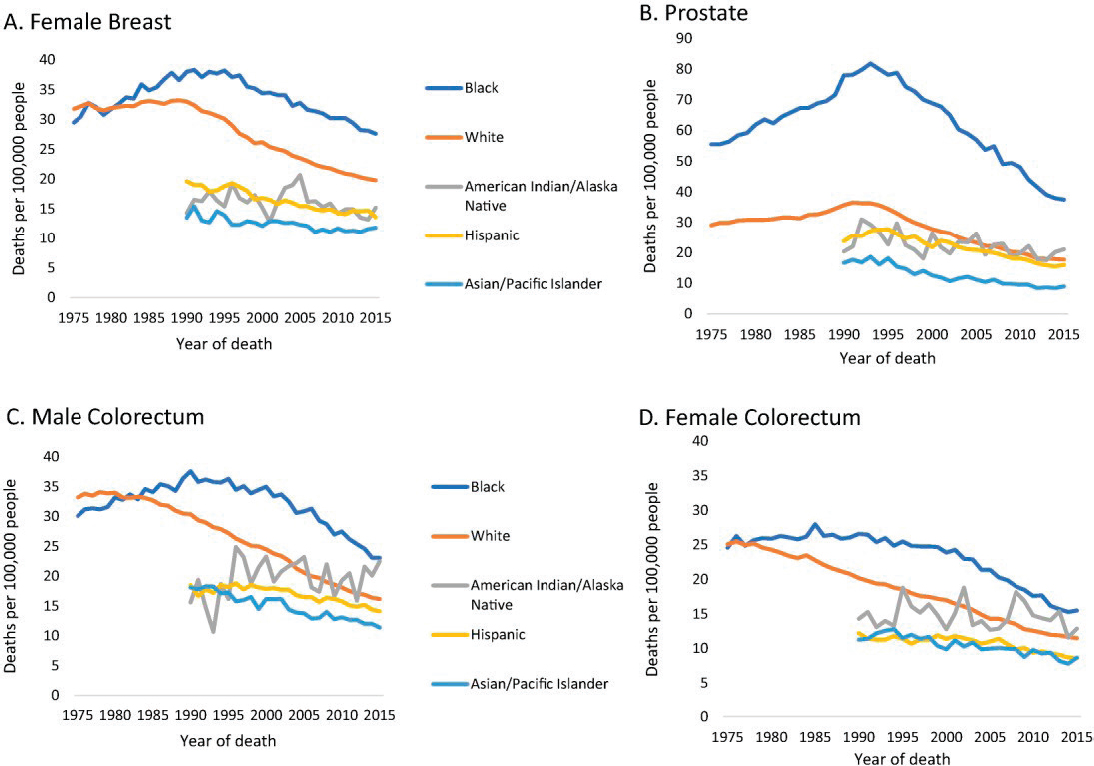
SOURCE: Siegel et al., 2018.
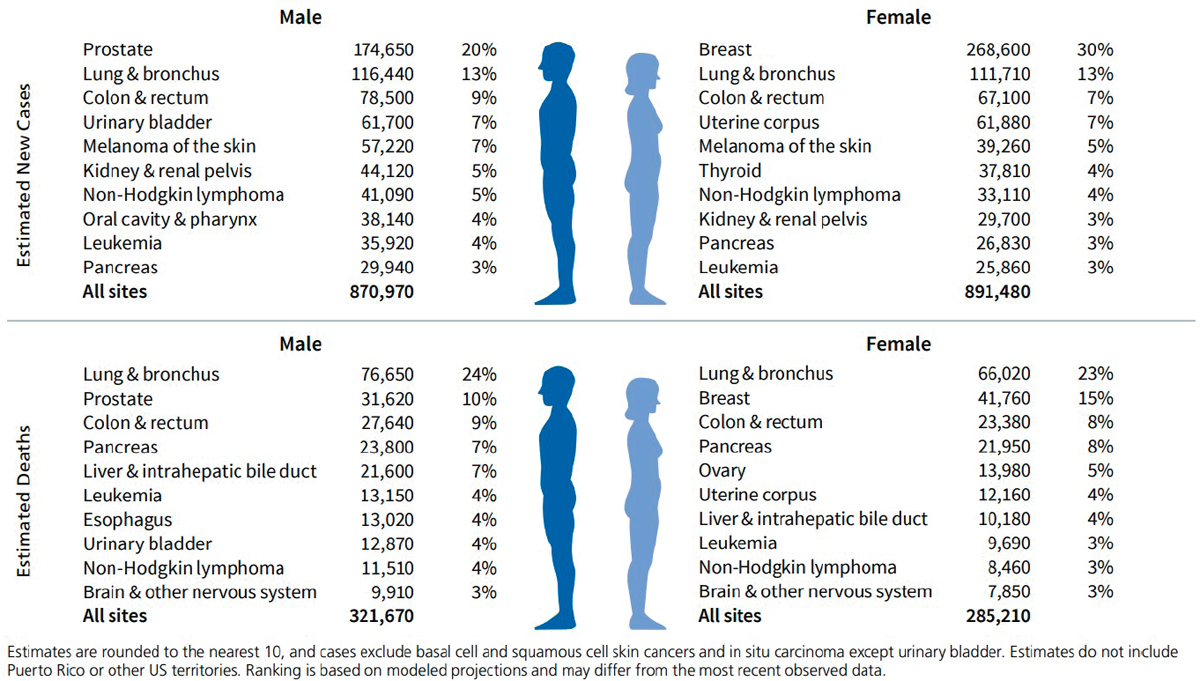
state and local levels in mortality rates for certain cancers, such as breast, colorectal, and lung. A classic example is the established link between smoking and lung cancer mortality rates at the state level, as shown in Figure 1-4. There are also important differences between men and women in the types of cancer they are likely to die from, as shown in Figure 1-5.
DIFFICULT TRADE-OFFS
The economic and clinical dimensions of cancer control present difficult resource allocation debates. One obvious issue is how to allocate health care
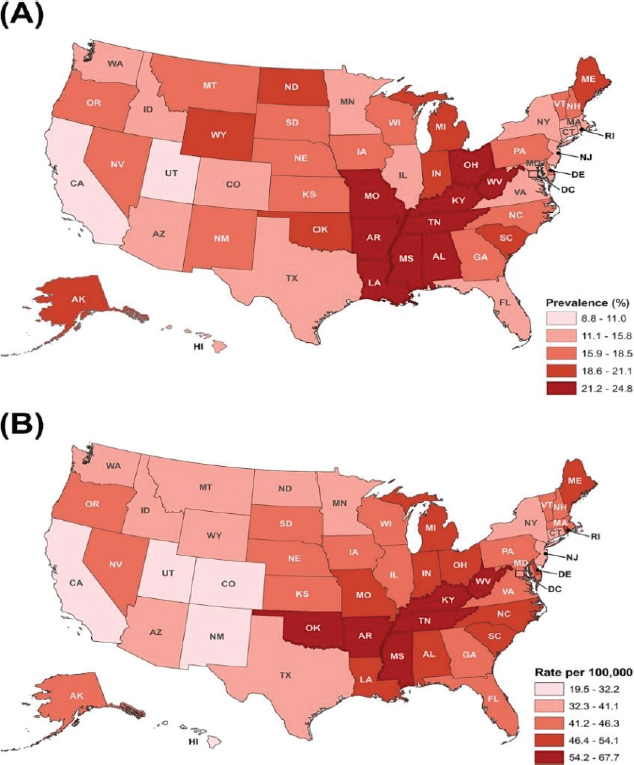
SOURCE: Siegel et al., 2018.
SOURCE: Siegel et al., 2019.
spending among cancer and other conditions such as neurodegenerative and cardiovascular conditions, diabetes, opioid addiction, or acute epidemics. These needs, in turn, have to be evaluated against broader public service priorities such as the military, environmental protection, prison system, state and national parks, water supply, transportation systems, and other diverse forms of services. Between 2000 and 2014, the proportion of state budgets spent on Medicaid increased from 19 to 26 percent, forcing decreases in the proportions spent on other public programs (Joffe, 2015).
The “iron triangle” refers to access, quality, and cost containment in health and medicine (Kissick, 1994). Improvements are possible in one or two of these areas, but they will usually be at the expense of the third (Kissick, 1994; Lehman, 2015). The same observation applies to cancer control or to any of its individual components, from awareness of risks to providing hospice care for patients and bereavement support for families. This implies that there are inevitably trade-offs to be made when making decisions about cancer control investments.
For example, the median annual cost of a new cancer drug launched in 2017 had grown to more than $150,000 (IQVIA, 2018), and spending on prescription drugs, especially novel classes of targeted therapeutics and immunotherapies, has been among the chief contributors to rising national health expenditures in recent years (Martin et al., 2016; NASEM, 2018a). Cost figures of these sorts inevitably lead to discussions about how to best allocate finite resources in cancer control. For instance, the individuals who may benefit years from now from resources devoted to cancer prevention will be different from the individuals facing the need now for treatment of a diagnosed malignancy, which will often involve multiple therapeutic regimens. The people who could potentially benefit from cancer prevention are not readily identified when the investment is made, whereas there are identified patients who can directly benefit from care today. Moreover, there has been reluctance in the United States to adopt any scheme for stratification of access to care based on objective analyses of economic and clinical performance that might impose potential limits on care, a subject of intense political and media debate.
Cancer patients may face their own trade-offs, often having to decide between cancer treatment costs and meeting their other obligations, including other medical care (in addition to cancer care), mortgage, food, and other family expenses. Despite the fact that 96 percent of newly diagnosed patients with cancer have health insurance (Soni et al., 2018), the cost sharing poses practical challenges for patients whose expensive treatments are not completely covered by their insurers (Bernard et al., 2011; Claxton et al., 2018). The “financial toxicity” and the resulting psychological stresses associated with the exorbitant costs of cancer care are now a clinically recognized phenomenon (IOM, 2008; Meeker et al., 2016).
One study also reported food insecurity rates among cancer patients in New York City that were five times the state average (Gany et al., 2014). Clinicians have been hitherto reluctant to discuss the cost of care with their patients, and in particular they may tend to avoid direct discussion of the likelihood of successful treatment outcome in patients with advanced cancer. None of these questions is straightforward to answer.
THE COMPLEXITY OF CANCERS AND CANCER CONTROL
Cancers arise in different organs and progress and evolve in different time frames and trajectories. They involve diverse patterns of underlying clonal diversification and metastatic risk and have significant variation in their responsiveness to different classes of anticancer drugs. The etiology, taxonomy, and progression of different cancers are highly complex. Understanding this complexity is the first step to being able to transform cancer control efforts to achieve more effective outcomes at a lower cost for both society and individuals.
Classifications
Cancers vary widely from patient to patient, differing in the types of tissue affected, their causes and underlying biological mechanisms, their prognosis, and the most effective type of treatment. More than 200 different types of cancers—encompassing a vast diversity of malignant conditions—have been identified in humans. Each of these types of cancer itself has several constituent subtypes, yielding potentially several hundred more different types of cancers (Song et al., 2015).
Cancers are typically classified by the anatomic tissues or organs where they arise. For example, colon cancer is the result of malignant cell proliferation in the colon. But this pattern of naming does, at times, result in one term referring to many different conditions. For instance, “skin cancer” can refer to basal cell carcinoma, the most common form, which is relatively slow growing; the localized, low-grade squamous cell cancer, for which long-term survival may be nearly 100 percent; the more deadly melanoma; or several other less common types. The medical profession has followed this tissue-specific pattern in the way it divides itself into different specialties for the treatment of cancer (e.g., gynecologic oncology, genitourinary oncology), and specializing in such a way has allowed clinicians to hone their technical skills relative to a specific organ system. “Cancer staging” has also been used to classify cancers based on the tumor size, involvement of lymph nodes, and whether the cancer has spread to distant areas of the body (Edge and Compton, 2010).
Advances in genomics profiling and other molecular methods (e.g., epigenetics, proteomics, transcriptomics—collectively referred to as “multi-omics”) are enabling a new molecular taxonomy for cancer (Chen et al., 2015; Idikio, 2011; Song et al., 2015). This reclassification of cancers could help provide refined insights into the underlying molecular pathologies in different cancer subtypes, which in turn could help clinicians in selecting treatment regimens tailored to individual patients. Another way of looking at cancers is by age: some clinicians and clinics specialize in pediatric cancers, while others specialize in treating cancers in adolescents, young adults, adults, and the elderly. There are a variety of other types of possible classifications as well. Those interested in public health might consider classifying cancers according to their ability to be detected early by screening (if that early detection could potentially lead to reduced mortality) or based on their associations with infections (e.g., cancers related to HPV or to hepatitis B and C). Some have also suggested classifying cancers based on modifiable risk factors (such as smoking, alcohol use, diet, and tanning beds).
Each of these approaches to cancer classification has its strengths and weaknesses. None is sufficient, on its own, for addressing all the complexities inherent in cancer control. That will require a wider, more comprehensive understanding of cancers and their burdens in place of the prevailing reductionist approach of narrowly understanding aspects of specific cancers. The challenge for those interested in reducing the cancer burden is to recognize that every approach to grouping cancers has limits and to develop strategies that can incorporate novel and effective ways of classifying, understanding, preventing, diagnosing, and treating cancer in order to gain the most comprehensive view possible—a subject that is discussed in detail in the latter sections of this report.
Representative Risk Factors
Another factor contributing to the difficulty of reducing cancer burden is the wide variety of things that can increase the risk of different types of cancer. This complicates efforts to predict and prevent it, to treat it, and to survive it. In brief, behavioral factors such as tobacco use, alcohol use, and certain dietary choices known to increase the risk of cancer are things that individuals have a certain amount of control over (WCRF/AICR, 2018). In recent years, the role of viruses and other infectious agents in increasing risk for certain cancers has also been established (as in hepatitis B and hepatocellular carcinoma) or better understood, as has the role of vaccines in curbing particular forms of cancers. HPV increases the risk of several types of cancer, most notably cervical cancer in women and cancer of the mouth and throat in both sexes (IARC, 2007). Vaccines
that protect against the most common versions of HPV are available, but vaccination rates are lower than anticipated, for a variety of reasons (Attia et al., 2018; Dorell et al., 2011; Holman et al., 2014; PCP, 2018). Genetic mutations are also known to play a role in predisposition to certain cancers. The role of BRCA gene mutations in breast cancer is well known, but there are many others that affect a small portion of the population (Kuchenbaecker et al., 2017).
Environmental factors continue to play an important role in cancer as well (Hiatt and Brody, 2018; Jagai et al., 2017). For example, a recent review concluded that 16 percent of cancer deaths worldwide—and 36 percent of lung cancer deaths—can be attributed to environmental factors (Pruss-Ustun et al., 2017). Although there is still some debate about the precise estimates (Israel, 2010; PCP, 2010), there is little doubt that environmental exposures play an important role in the development of some cancers. Environmental sources of carcinogen exposures include indoor and outdoor air pollution and radon gas in buildings (IOM, 1999, 2002). Similarly, occupational exposures to carcinogens in mining, construction, manufacturing, and refining industries include asbestos, benzene, cadmium, chromium, arsenic, formaldehyde, and polycyclic aromatic hydrocarbons (IOM, 2006a). Agricultural workers may be exposed to carcinogenic herbicides and pesticides (Damalas and Eleftherohorinos, 2011). Although federal regulations have led to the removal of many of the worst carcinogens from the environment and workplace, others still remain, particularly in manufacturing and agriculture (Reuben, 2010).
Risk factors can interact to increase or decrease the likelihood of developing cancer in various ways, complicating cancer control efforts. For example, environmental exposure to radon gas is much more likely to lead to lung cancer in people who smoke than in those who do not (Méndez et al., 2011). Cancer-causing pollutants are also often found at higher levels in areas of lower socioeconomic status. Research on epigenetics, the mechanism through which some of these exposures affect the expression of cancer-causing genes, has enhanced understanding of cancer risk factors as well.
THE “CONTINUUM” OF CANCER CONTROL
In an attempt to bring some logical structure to the many different components of cancer control, the concept of a “continuum,” illustrated in Figure 1-6, was developed (IOM, 2013a; NCI, 2018a). The continuum consists of a half dozen steps in cancer control that typically follow one another in a linear fashion: prevention, screening, diagnosis, treatment, survivorship, and hospice care, with palliative and supportive care cutting across these steps (IOM, 2013a). Despite the ideal that these various
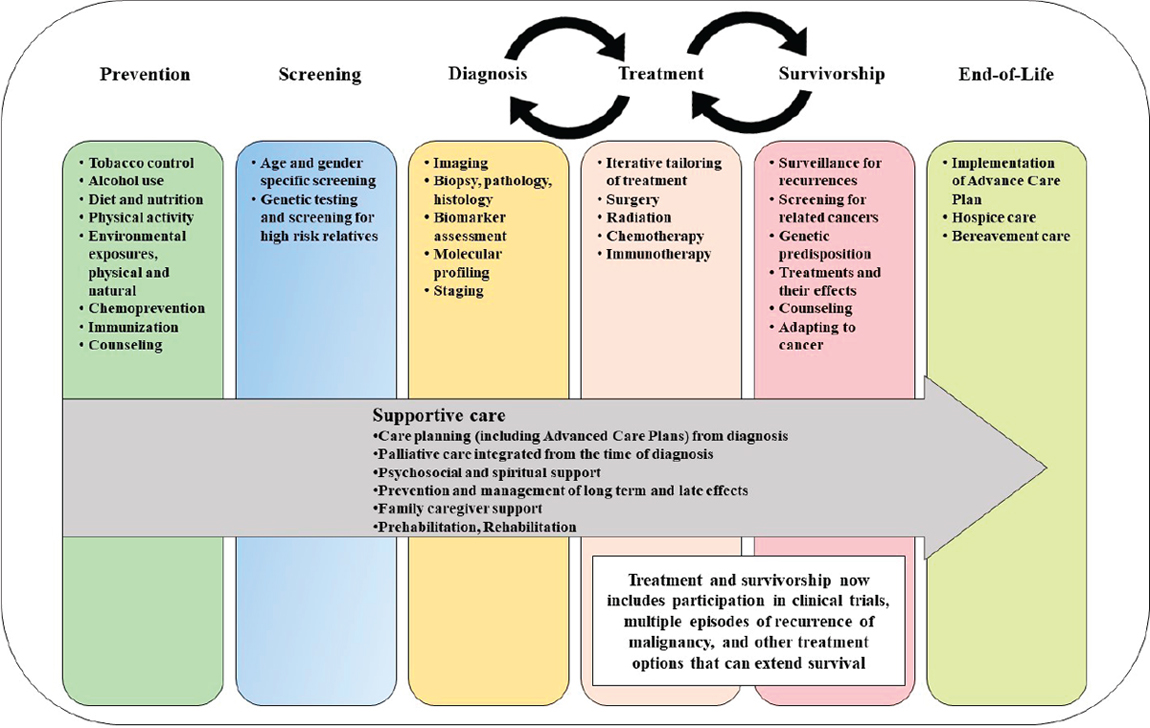
SOURCES: Adapted from IOM, 2013a; NCI, 2018a.
steps should be connected, in practice it is often the case that specialists focus on the individual components separately, with little connectivity between them. As an example, survivorship is included in the continuum in recognition of the growing numbers of people alive with a history of cancer and of the specialized care needed to address the excess morbidity in these individuals. To emphasize the often episodic and recurring nature of treatment, the continuum now envisions a two-way relationship between treatment and survivorship (as people in the surviving population may transition back into treatment, and vice versa), similar to the coupling between diagnostics and treatments. Rehabilitation was once a specific phase, but it is now folded into treatment and survivorship care.
The components of “prevention” (the first block in the continuum) have expanded, starting with an increased awareness of various health-related behaviors that can be modified to reduce cancer risk and then with advances in chemoprevention, immunization, and prophylactic surgery, as in mastectomy in women at high risk for breast cancer (see Box 1-2).
Components of “screening” (the second block in the continuum) now benefit from significant advances in genetic testing for high-risk relatives. Similar technological advances have also changed the profile of “diagnosis” (the third block in the continuum) to include improved imaging technologies and laboratory tests, including molecular profiling and biomarker identification. Surgery, radiation therapy, chemotherapy, targeted therapies, and immunotherapies have all played a significant role in advancing life-extending treatments5 (the fourth block in the continuum; see
___________________
5 A 2018 opinion survey from the American Society of Clinical Oncology noted that nearly 4 in 10 people in the United States believe that cancer can be “cured solely” through alternative therapies (ASCO, 2018). Additionally, users of alternative therapies say such therapies relieve them of side effects caused by their treatment and allow them to have control in their treatment (Bardia et al., 2006; Snyderman and Weil, 2002). Despite the growing use and awareness of these complementary and integrative health interventions—currently a $34 billion market in the United States (NCCIH, 2018)—very little analysis has been focused on the safety, standardization, regulatory, legal, and payment mechanisms for these approaches.
Box 1-3). In recent years, the ability to generate multi-omic information and the availability of advanced computational tools has fostered a growing emphasis on precision medicine approaches for cancer treatments.
Treatments are coupled with the next (fifth) block in the continuum, “survivorship,”6 a topic that has garnered more appreciation in recent decades (Brown and de Graaf, 2013; Gibson et al., 2016). Survivorship also includes surveillance for recurrence and attention to acute and chronic effects of treatments, which can affect a patient’s quality of life (see Box 1-4). This spectrum also captures a strong coupling between “treatment” and “survivorship.” Cancer care has now progressed to the point that
___________________
6 There is no uniform or universally accepted definition of who a cancer survivor is (IOM, 2006b). Attempts to define this term are commonly based on factors such as the stage of the disease, the progression of the disease across the different phases of the continuum, and the outcome of the disease after treatment (Marzorati et al., 2017). Furthermore, the heterogeneous nature of cancers—and the differing outcomes—makes it very complicated to understand when survivorship begins. Many consider survivorship as beginning at the time cancer is identified in the body and continuing through the remaining years of life. Others believe that survivorship stretches from cancer treatment until cancer recurrence or end of life. This term “survivor” was expanded in the national action plan for cancer survivorship to refer to “those people who have been diagnosed with cancer and the people in their lives who are affected by their diagnosis, including family members, friends, and caregivers” (NCI, 2019).
many survivors live long enough to experience secondary or related cancers or a recurrence of their initial cancer. Cancer survivorship is often accompanied by long-term or delayed effects of treatment. The sixth block of the continuum is hospice care (IOM, 2015), which can be used for the greatest benefit if health care providers have a good system of timely referral of patients to hospice care, rather than days or weeks prior to death. Despite the desire of most patients to die at home, many often spend their final days in clinical settings. Early and ongoing conversations about end-of-life care between patients and their clinicians have shown to be beneficial for both patients and their families (Epstein et al., 2017; Wright et al., 2008).
The total cost of cancer care delivered in the last weeks or days of life is substantial (Wang et al., 2016; Zhang et al., 2009). Much of this care, however, may not be consistent with patients’ wishes. Aggressive cancer treatment delivered in the last days of life has also been associated with poor quality of life and a worsened quality of death (Prigerson et al., 2015). Many recent analyses and efforts have focused on expanding the use of advance directives, enhanced spiritual and psychosocial counseling, and clinician education to improve communication with dying patients and their families (El-Jawahri et al., 2018; Riedel et al., 2017).
The continuum also recognizes the fact that patients and families require extensive support, as indicated by the shaded arrow cutting across the blocks of the continuum. A major shift in cancer control has been the important focus now being placed on palliative care to address symptoms and side effects (including delayed effects) from the time of diagnosis, coupled with psychosocial and spiritual support. Palliative care is considered to be most effective when it is implemented early in the course of illness and its use is communicated and applied across all stages of diagnosis, treatment, survivorship, and disease recurrence (Ahluwalia et al., 2018; Ferrell et al., 2017; Gaertner et al., 2017). Recent guidelines have stated that the comprehensive assessment and management of symptoms is as important as attending to social, spiritual, and cultural considerations at the time of a person’s death (Ahluwalia et al., 2018; El-Jawahri et al., 2018; NCHPC, 2018). The needs of family caregivers are also increasingly a subject of consideration (Lobb et al., 2015).
The continuum has been acknowledged as an oversimplification of both the biology of cancer and the clinical services needed (IOM, 2006b). The segmented phases shown in the continuum are not discrete. For example, a colonoscopy offers a valuable point of prevention via both the detection and the removal of precancerous polyps. Although external forces influence each component of the continuum (e.g., the role of neighborhood or even larger communities), their importance is rarely acknowledged in research and clinical practice (Stange et al., 2012).
Second, the continuum may not yet fully address the needs of cancer survivors. One report estimates that 80 percent of children diagnosed with cancer will now become long-term survivors (Campo et al., 2011), but these children have a substantially elevated risk of developing a second malignant neoplasm and other chronic health conditions (Bowers et al., 2013). Survivorship is thus not an end point (after which an individual is “cured” of cancer) but rather an ongoing process that may require lifestyle
changes, ongoing medical care, and treatment and support for reemerging complications as well as financial matters of care. The continuum can thus be criticized for engendering an unrealistic expectation of how individuals ultimately progress through cancer care; misperceptions of cancer care as a time-limited event rather than as an ongoing life experience can be detrimental in how cancer risk factors (and prevention) are communicated to the public and to the survivor community in particular.
Finally, each step in the cancer control continuum involves a wide range of participants, including patients; the patients’ families, caregivers, and communities; clinicians; health systems; and insurers. The dilemma is that the contributions of these different constituencies are typically not well coordinated and may have widely varied incentives and conflicts of interests. The cancer control continuum, however, still provides a baseline to discuss the evolution of a national strategic vision for cancer control, with Table 1-1 providing a set of representative knowns and unknowns in practice.
SOCIAL COSTS AND CONSEQUENCES
To appraise the costs and economic consequences of future cancer control efforts, it is important to understand the current magnitude of the aggregate cancer cost burden in the United States, including spending on cancer control. Addressing this daunting question is all the more challenging because of the complex nature of the disease, the multiplicity of cancer control activities under way, and the absence of organized efforts to identify, collect, and aggregate total spending from all agencies, organizations, and firms engaged in cancer control. The economic cost of cancer comes in many forms. There are the cancer-related health care costs across the cancer continuum (prevention, diagnosis, treatment, survivorship, and end of life); resources devoted to allied support services (including by the nonprofit sector); expenditures on research (both government and industry); and spending on wellness-promotion activities (by firms across the economy and by individuals seeking to stay cancer free). In addition, both the premature mortality and excess morbidity attributable to cancer impose significant productivity costs from a societal perspective (and, in
TABLE 1-1 Some Knowns and Some Unknowns Across the Practices of the Cancer Control Continuum
| Interventions | Some Knowns | Some Unknowns |
|---|---|---|
| Prevention |
|
|
| Screening, Detection, and Diagnostics |
|
|
| Interventions | Some Knowns | Some Unknowns |
|---|---|---|
| Treatment |
|
|
| Survivorship |
|
|
| Palliative Care and End-of-Life Care |
|
|
parallel, lead to lost or reduced incomes for individuals and families affected by cancer).
In what follows, a range of published data sources and modeling assumptions are used to arrive at a rough estimate of the total annual economic cost of cancer in the United States, from the broadest national perspective. The focus first is on cancer-related direct medical costs and indirect (productivity) costs. Then order-of-magnitude estimates are derived for additional public- and private-sector spending that may not be routinely reported (or even computed) but are clearly aimed at enhancing cancer control efforts.
First, regarding direct medical costs, NCI has projected that the net cost of cancer care for the United States in 2020 could be $173 billion (Mariotto et al., 2011) under specific assumptions about trends in cancer incidence, survival, annual increases in the cost of care, and changes in the size and age structure of the U.S. population over time. This projection includes expenditures associated with diagnosis, treatment, survivorship, and end of life—but not for screening/early detection nor for primary prevention, so in that sense can be regarded as a conservative projection of total cancer-related direct medical costs.7
___________________
7 Based on data from another recent analysis of total medical care spending in the United States (Dieleman et al., 2016), one could derive (in several steps and under certain assumptions) an alternative projection of cancer-related direct medical costs for 2020 of about $147 billion. The study by Dieleman and colleagues and the analyses by NCI were both systematically executed though relying on a different mixture of data sources. NCI results are employed here, in part because they are directly derived 2020 cost projections, based on statistical modeling analyses that took into account projected changes over time (from 2010 through 2020) in cancer incidence, survival, the cost of care, and the size and age structure of the population.
While there are evidently no published estimates of the total annual cost of cancer screening/early detection in the United States, recent studies focused on individual cancer types may be informative. For example, one analysis using multiple data sources (principally the Behavioral Risk Factor Surveillance System Survey and Breast Cancer Surveillance Consortium data) and modeling assumptions estimated the annual total cost of breast cancer screening to be $7.8 billion (O’Donoghue et al., 2014). Another analysis by Gross and colleagues (2013) using different data sources (principally SEER-Medicare) estimated the annual costs of breast cancer screening-related procedures (screening plus workup) to be $1.08 billion (2009 dollars) for the fee-for-service Medicare population only. Considering that national-level estimates of the total cost of cancer screening should also encompass the other major screen-detectable cancers (colorectal, cervical, prostate, and lung, at the least), should include the entire pertinent sub-populations (as suggested by current guidelines), and should be stated in 2020 dollars, it is clear that aggregate cancer screening costs in the United States currently run in the billions of dollars. Inclusion of all pertinent screening/early detection costs would push total projected direct medical costs for cancer in 2020 from $173 billion toward $200 billion—precisely by how much remains to be determined. The same general conclusion holds for the national-level costs of primary prevention activities undertaken by individuals (for which there are no aggregate estimates at the moment).
The indirect costs of cancer comprise both mortality costs (the monetized value of lost productivity attributable to cancer-related premature death) and morbidity costs (the value of lost productivity attributable to cancer-related time away from work or inefficiency at work). The mortality costs of cancer for the United States in 2020, based on estimated lost wages among those in the labor force, has been projected at nearly $148 billion (Bradley et al., 2008). In an extension of their base-case model that also included the imputed value of lost productivity with respect to non-market activities (caregiving and household work), the corresponding 2020 estimate for total cancer-attributable mortality costs in the United States was about $308 billion (Bradley et al., 2008). Now, if morbidity costs are assumed to be about one-third of mortality costs—in line with calculations done for cardiovascular disease (NHLBI, 2009)—then total cancer-attributable morbidity costs for 2020 would be about $49 billion (that is, $148 billion × 0.33 because the National Heart, Lung, and Blood Institute [NHLBI] analysis was based on mortality costs that included only actual wages lost). The resulting projected mortality plus morbidity costs in the United States for 2020 come to $357 billion ($308 billion + $49 billion).
It can be calculated that government expenditures on research—basic, clinical, translation, and population science—and on field-based program implementation related directly or indirectly to cancer control sums to at least $8 billion annually (using data from the Centers for Disease Control and Prevention [CDC] and the National Institutes of Health).8 It is much more difficult to estimate the biopharmaceutical-sector investment in cancer control efforts—given the very high failure rates inherent in such high-risk investments for drugs, devices, and vaccines. Based on a recent estimate that the biopharmaceutical industry is investing about $90 billion annually on research and development (R&D) in the United States (PhRMA, 2018) and that approximately one-third of the current product pipeline is cancer related (Lloyd, 2018), a reasonable estimate of total annual R&D spending by industry on cancer is $30 billion. Total annual spending by all cancer-related nonprofits in the United States can be estimated at nearly $3 billion, based on an analysis of total reported
___________________
8 Total spending in connection with CDC’s National Center for Chronic Disease Prevention and Health Promotion for 2018 fiscal year was estimated at $1.16 billion (CDC, 2019); this included dollars allocated to the Division of Cancer Prevention and Control (DCPC), to tobacco control efforts, to a range of chronic disease prevention and control activities, and to total spending by the states on cancer prevention and control activities. Total National Institutes of Health spending on “cancer” research (basic, clinical, translational, and population sciences) for 2018 fiscal year was $6.36 billion with an additional $1.03 billion for “cancer genomics,” for a total of $7.37 billion (NIH, 2019). The total annual government expenditures on cancer control–related research and outreach by these two agencies sums up to $8.53 billion.
contributions to such nonprofits in 2017 as tracked by one of the nation’s major charity assessment organizations.9 Total spending on workplace wellness programs, aimed at reducing the incidence and consequences of cancers and many other diseases, is estimated to be $8 billion annually (Song and Baicker, 2019). And, with total annual U.S. spending on complementary and alternative medicine (CAM) currently at $34 billion, and with an estimated 12 percent of this being for cancer (John et al., 2016), total CAM spending for cancer could be nearly $4 billion. Based on these calculations, the total annual economic burden of cancer in the United States is nearly $600 billion annually,10 and could well approach $1 trillion in the years ahead given the escalating incidence of cancer in an aging society and multiple other factors, including behavioral choices.
A 2000 NCI analysis of willingness to pay (WTP) for a life year lost to cancer (Yabroff et al., 2008), which assumed that each life year lost due to cancer was worth $150,000, found a WTP of $960.6 billion (in year 2000 dollars). Recast in 2020 terms, the cost could presumably be more than $1 trillion. Based on economic theory, the WTP method is, in principle, the appropriate approach to discern the decision maker’s value for any good or service. But there are important behavioral, informational, and incentive-based challenges in identifying the “true value” of that WTP
___________________
9Charity Navigator (2019), a nonprofit organization that monitors the performance of philanthropies across the United States, has defined a general category of “Health” nonprofits, which is then broken out into four sub-categories, as listed below. For each subcategory, information is provided here on the total number of nonprofits included, the number that were deemed to be cancer-related by virtue of the brief description submitted for each organization, and the total amount of revenue (in thousands of dollars) reported for 2017 by all the cancer-related organizations in the sub-category. The focus is on total reported revenue under the assumption that over the long term, it serves to establish a ceiling on expenditures. The sub-categories are Diseases, Disorders, and Disciplines (267 nonprofits, and 39 cancer-related with total revenues $1,600,976); Patient and Family Support (308 nonprofits, and 104 cancer-related with total revenues $429,952); Treatment and Prevention Services (229 nonprofits, and 7 cancer-related with total revenues $132,600); and Medical Research (148 nonprofits, 46 cancer-related with total revenues $524,845). Summing across the sub-categories yields about $2.7 billion. Because it is highly likely that some nonprofits that are not expressly cancer-designated provide services or assistance to cancer patients and their families and because all relevant nonprofits in the nation may not be included under the Charity Navigator broad category of “Health,” a total annual estimate of $3 billion was regarded as a reasonable upper bound.
10 This rounded estimate of $600 billion was arrived at by thus summing direct medical cost ($173 billion), indirect (productivity) cost ($357 billion), government research and program implementation ($8 billion), biopharmaceutical-sector investment ($30 billion), spending by cancer-related nonprofits ($3 billion), expenditures on workplace wellness programs (8 billion), and cancer-related CAM expenditures ($4 billion)—totaling to $583 billion. Given that direct medical cost does not include spending on screening/early detection and primary prevention activities by individuals, $600 billion is arguably a conservative upper bound on the current total cost of cancer in the United States.
for health outcomes. As a practical matter, this WTP to avoid disease is not the standard basis for estimating disease burden in the United States or elsewhere.
In health and medical sectors, the cost of care and the financial incentives that can influence prescribing and other care decisions have recently become targets for health care reform efforts and reigning in costs. Participants across the health and medical systems have increasingly advocated for the concept of “high-value care” as a way to move forward on health policy matters, but this raises questions about what constitutes “high value” in cancer control, with “value” left undefined or variously defined. A narrow and commonly used definition for “value” is outcome divided by cost (Porter and Teisberg, 2006). However, outcomes for cancer are multidimensional, at best reducing to perhaps a vector for analysis rather than a single value to be divided by cost. Beyond the obvious important outcome of survival, quality of life, symptom and side effect burden, and even quality of death are important factors. Many outcomes other than survival are not well quantified for benchmarking. Nevertheless, a significant move toward paying for quality and value is under way in cancer care. Even if one knew exactly what constitutes and how best to measure both quality and value, cancer control in the clinical setting remains a multispecialty effort. To whom is an outcome attributed when multiple clinicians are involved in treating a patient’s cancer? Even if one could settle on a coherent and consistent definition of value in cancer care, how might one transition care models from a fee-for-service system (i.e., one that pays for quantity of health care procedures and interventions) to one that incentivizes clinicians to pursue “value”? These multifaceted relationships in medical care add to the existing complexity, and incentives are difficult to align because the clinicians may function as agents for patients, payers, and health care organizations (Casalino, 2001; Conrad, 2015).
Despite these uncertainties, the emphasis on “quality metrics” and “alternative payment models” such as bundled payments and accountable care organizations has grown, especially after passage of the Patient Protection and Affordable Care Act. Efforts to stimulate methods of “pay for value” were extended under the Medicare Access and CHIP (Children’s Health Insurance Program) Reauthorization Act of 2015, which embedded a penalty–reward system (Merit-Based Incentive Payment System) within the reimbursement scheme for fee-for-service clinicians receiving Medicare payments. The law also encouraged clinicians to adopt alternative payment models. The Centers for Medicare & Medicaid Services (CMS) Oncology Care Model,11 which “aims to provide higher quality, more
___________________
11 See https://innovation.cms.gov/initiatives/oncology-care (accessed February 15, 2019).
highly coordinated oncology care at the same or lower cost to Medicare,” is one such alternative payment model.
In recent years, reimbursement uncertainties for small oncology practices have prompted a high rate of vertical integration, or consolidation of independent practices into hospital systems—indeed, the rate of vertical integration is among the highest rates of all specialties (Nikpay et al., 2018). While one might expect the salaried pay structure that often accompanies those arrangements to blunt any fee-for-service, volume-driven financial motivators, in reality employers often create bonus compensations for clinicians that, at least in part, rest on productivity benchmarks of clinical volume. Salary bonuses may also include new quality metrics—some that change annually—to motivate clinicians to comply with policy changes that seek to shift toward payment for “value” rather than quantity.
A Systems Approach to Cancer Control
Cancer control policies and practices focused on the different components of cancer control continue to evolve as new evidence becomes available. This has inspired participants involved in cancer control to seek better information to guide decision making and practices with systems approaches. As an example, the learning health care system model strives to enable evidence-informed transformations across the cancer control continuum. The goals of a learning health system are to continually collect and use data to systematically integrate new knowledge into care delivery processes and to improve outcomes and motivate greater collaboration among all participants (IOM, 2007, 2013b).
Other conceptual systems frameworks have also been used to assess and improve certain aspects of cancer control efforts across the continuum. The socioecological model, for example, has been applied to identify and understand the challenges to screening and treatment of certain types of cancers in some communities in the United States (Daley et al., 2011). Variations of the socioecological model have also been used to examine the factors contributing to cancer disparities in communities with low-income residents (Paskett et al., 2016; Warnecke et al., 2008). Although these models can be useful in understanding certain aspects of cancer control, they are unable to holistically view cancer control efforts to obtain an overall perspective on the collective behavior of the numerous participants in the ecosystem.
Approaching cancer control as a system of systems would involve an integrated practice of strategies and tactics aimed at helping individuals and society deal with cancer in various ways—by avoiding it in the first place, by detecting it as early as possible, by diagnosing it accurately, by
treating it effectively, possibly by managing it as a chronic disease, by addressing the pain and other symptoms caused by the cancer and its treatment, and by addressing current and future costs. This end-to-end approach defines the scope of cancer control for this report. Instructive precedents are available from approaches to infectious diseases in which the concept of “control” as the elimination of disease has over time moved toward “outpacing” the disease and its burden. Such an approach requires the skillful integration of public education, epidemiology, surveillance, prevention, diagnostics, and treatment, reinforced by policy actions and agreements at national and international levels. Most importantly, the infectious disease community has organized its activities as an adaptive and agile framework of capabilities to respond to the shifting dynamics of pathogen evolution (Dowdle, 1998; Heymann, 2014). Cancer control efforts should be no different. Indeed, a systems approach would make it clear that the effects and costs of cancer extend far beyond this health and medical end-to-end scope and that effective cancer control needs to work at multiple levels of society, starting from the individual patient. A national strategic vision for cancer control would therefore necessarily involve technologies and markets beyond those in public health and medicine.
To overcome the limitations of the current systems-based approaches to cancer control, the committee focused on the concept of a “complex adaptive system.” This concept is described in more detail in the following sections, but in broadest terms, a complex adaptive system is a system consisting of individual entities that act to advance their own interests at many levels and that interact with one another, modifying their behavior in response to what is happening in the rest of the system.
A cell is an example of a complex adaptive system. It is complex because its functioning depends completely on the interactions among its various components, and it is adaptive because those components modify their behavior according to conditions inside and outside the cell. This complex adaptive nature of cells makes it exceptionally difficult to understand their normal functioning, much less their functioning in a cancerous state, and it also explains why it is hard to predict the effects of a particular intervention on cancerous tissue.
When dealing with living systems with many different interconnected pieces responding to one another, a push on one part of the system—say, a drug that targets a particular molecule in a cancer cell—will trigger reactionary changes in other parts of the system as it seeks to adapt to this new input. And the precise details of the system matter because small initial differences can be magnified through the interactions and emergent behaviors to produce major differences in outcome. Sometimes a detail that does not seem to have a particularly important role to play
may actually be a key factor. Thus, a treatment that works well on a cancer in a laboratory animal may not work at all in humans, or a drug that is effective on a cancer in one tissue may not work against a similar cancer in another type of tissue, or a prevention method that is widely embraced in one context may not be appropriate in another.
The U.S. cancer control system is, like cancer itself, a complex adaptive system. Each entity in the system (e.g., in public health or clinical or basic research or palliative care or survivorship services) attempts both to serve its own interests and to provide quality products and services to patients and consumers. The ultimate result, in the broader sense of cancer control, is a vast, interconnected system consisting of many different systems: clinicians, public health professionals, hospitals and other medical facilities, biopharmaceutical and medical device companies, payers, consumer technology and computing firms, research organizations, advocacy and support groups, regulatory agencies, patients and their family members, and so on. In this system, one finds a tremendous number of different plans and approaches for cancer control. There is no central command making decisions and passing orders down through a hierarchical structure to the proper entities, which then carry out the commands. Instead, the various components are mostly independent, each making its own decisions about how best to achieve its goals.
Within the federal government, NCI, CDC, CMS, the Veterans Health Administration, the Environmental Protection Agency, the Department of Defense, and the Department of the Treasury, among many more agencies, all deal with cancer control from their own individual perspectives and with their own individual goals. Each state has developed and implemented its own version of a cancer control plan. Biopharmaceutical, medical device, vaccine, and other technology firms individually research and develop products that can be used in cancer control—and that will make them a profit. Hospitals and medical practices all develop their own approaches (generally informed by national guidelines). Professional societies develop guidelines for best practices in preventing, diagnosing, and treating cancer. Insurance companies set policies for what types of cancer screening and treatment are covered under their plans. There are various degrees of coordination among these entities and approaches but no formal organizational structure.
The result is a system of systems, each chasing its own goals with the tools at its disposal. These individual components are adaptive in the sense that they respond to what is happening around them by changing their behavior so as to give themselves the best chance to meet their own goals. It is a well-acknowledged reality that with no central “control” structure—and, indeed, because the diverse independent agents that make up the cancer control system may resist efforts at reform and
may act in ways that actively undermine efforts to move the system in a certain direction—the increasingly specialized and fragmented nature of cancer control efforts debilitates the achievement of greater population health outcomes. This fragmented system enables the continued propagation of well-intentioned but often inadequate decisions and practices that reflect the system’s inability to meet the current economic demands and technological capabilities.
In considering cancer control as a complex adaptive system, all these challenges can be understood as the natural outcome of the actions of the various individual agents in the system, each following its own strategies and interacting with other agents. Finding a set of solutions will require a similar comprehensive perspective, and this mind-set defines the foundation, principles, and approach of this report and its recommendations.
Applying a complex adaptive systems approach to cancer control—as a system of systems—would build on current models to support more robust decision making in cancer control by considering the actions and relationships among patients, families, clinicians, researchers, payers, government agencies, policy makers, and for-profit and nonprofit organizations, among others. Table 1-2 summarizes the characteristics of a complex adaptive systems approach and its implications for cancer control.
COMPLEX ADAPTIVE SYSTEMS
As described in the previous sections, the traditional approach to understanding and making changes to cancer control has usually been reductive—one element at a time. This section further examines the nature of complex adaptive systems and how they differ from complicated systems. It begins by first describing the essential difference between the “process” of cancer control and the “system” of cancer control.
“Process” Versus “System”
Throughout the rest of this report, a distinction will be made between the process and the system of cancer control: “process” will refer to what happens and how—that is, to those actions that are taken in cancer control efforts—while “system” will refer to the collection of entities and organizations that carry out the process. Thus, the cancer control continuum depicted in Figure 1-6 is describing the process of cancer control, from prevention efforts to diagnosis and treatment to hospice care, while the system of cancer control consists of all the health professionals, hospitals and other medical facilities, biopharmaceutical and device companies, payers, consumer technology and computing firms, research
TABLE 1-2 Complex Adaptive Systems Approach and Its Implications for Cancer Control
| Complex Adaptive Systems | Implications for Cancer Control |
|---|---|
| They are nonlinear and dynamic and do not inherently reach fixed equilibrium points. As a result, system behaviors may appear to be random or chaotic. | System responses often seem unpredictable and disproportionate to interventions. For example, enormous efforts are often needed to achieve small changes, while at times small improvements in treatment might lead to significant changes in clinical care. |
| They are composed of independent agents whose behaviors are based on physical, psychological, or social rules rather than the demands of system dynamics. | Stakeholders (patients, families, clinicians, suppliers, payers, regulators, etc.) respond based on their perceptions, values, and priorities, which are seldom aligned (e.g., payers might try to constrain short-term costs even when long-term health and economic benefits are easily projected). |
| Because agents’ needs or desires, reflected in their rules, are not homogeneous, their goals and behaviors are likely to conflict. In response to these conflicts or competitions, agents tend to adapt to one another’s behaviors. | Goals and behaviors of stakeholders often conflict. They may perceive these conflicts and adapt their strategies to counteract their impacts. For example, enormous resources might be devoted to advertising to convince patients of their need for products or services with questionable benefits. |
| Agents are intelligent. As they experiment and gain experience, they learn and change their behaviors accordingly. Thus, overall system behavior inherently changes over time. | As the rules of the system evolve and stakeholders understand the impacts of those rules, they might develop strategies to circumvent these rules, including lobbying to avoid rule changes or to change the rules to their benefit. |
| Adaptation and learning tend to result in self-organization. Behavior patterns emerge rather than being designed into the system. The nature of emergent behaviors may range from valuable innovations to unfortunate accidents. | Stakeholders adapt to the changing environment, learning what works and does not. These behaviors often surprise other stakeholders, who then also need to adapt. For example, industry agents might just pay penalties rather than change behaviors in response to rules in the system. |
| There is no single point of control. System behaviors are often unpredictable and uncontrollable, and no one is “in charge.” Consequently, the behaviors of complex adaptive systems can usually be more easily influenced than controlled. | The health care system is a federation of millions of entrepreneurs with no one in charge. No single entity can command change. A portfolio of motivations, incentives, and disincentives is needed but would be difficult to design and deploy, particularly if stakeholders game the process. |
organizations, advocacy and support groups, regulatory agencies, and patients and family members that are involved.
An analogy may help to make the distinction clearer. Consider modern car production. In this case, the process would be the manufacture and assembly of the individual pieces into a complete automobile. That assembly could be mapped out with a (very complicated) flowchart indicating what happens to each piece and collection of pieces as they are cut, shaped, welded, screwed, bolted, snapped, sewn, and painted. In speaking about these processes, the focus is on the actions that are taken, not who is carrying out the actions, where they are located, or what their relationships are with the others involved in the process. By contrast, the system that carries out automobile production consists of a variety of companies or divisions of companies or individual plants that manufacture the individual components and carry out the assembly of individual parts or of subassemblies.
The efficiency of the system will depend not only on the efficiency of the individual processes but also on how well the various components of the system work in unison. If, for example, the manufacturers of two different parts that were later put together failed to coordinate their efforts, then the parts might not fit together, or they might be made of two or more materials—as in composite airframes—that do not work well together. Thus, anyone who seeks to improve the manufacturing of an automobile must consider not only the processes but also the system in which those processes and standards should function.
The difference between system and process is a distinction that is often overlooked. The cancer control continuum, for example, is sometimes referred to as depicting the system of cancer control rather than the process. This is understandable because the word “system” can be used in both ways—as a collection of components that make up a whole or as a set of rules or procedures for how something is done. To avoid confusion, “system” as used in the remainder of this report, both as an organizing concept and central theme, is a collection of components that constitute a whole. In discussing cancer control, it is important to keep the distinction always in mind because improving a system is a very different challenge than improving processes, with a very different scale of economics.
“Complicated” Versus “Complex”
A key feature of the system of cancer control in the United States relates to its vast and daunting array of different interconnected pieces. Any cursory examination of the system reveals that it is extremely complicated in the sense that it has a large number of components connected in various ways. But it is more than just complicated; it is complex. Understanding
the difference between the two terms is crucial to understanding the principles and system of cancer control itself.
A classic, if lighthearted, example of a complicated system that is not complex is a Rube Goldberg device. Goldberg, a cartoonist, became famous for his drawings of exceptionally complicated devices that performed simple tasks. A typical Rube Goldberg device would have 20 or 30 elements connected one to another so that, say, flipping a switch at one end set off a series of steps—usually involving a mousetrap and a ball rolling down a chute, among other elements—that culminated in something like dropping a piece of bread into a toaster. The outrageously complicated device was amusing, but it was also very simple, as one could understand its function by looking at each of the steps in turn, thus decomposing the device. Furthermore—and this is a crucial property—a complicated yet “simple” system of this sort can be “optimized” by improving the performance of the individual pieces considered separately, with predictable gains in performance for each change in a component.
Not all systems are quite so simple, however, and not all system design and management problems can be addressed through hierarchical decomposition. In some systems, for example, decomposition may result in the loss of important information about interactions among the system’s components. And some systems consist of multiple interacting components where no one is “in charge.” That is, they are decentralized systems with no central planner to design and control the system.
Examples of complex adaptive systems include not only ecosystems and living organisms (for which the concept was initially developed) but also national economies, the military–industrial complex, the nation’s population health system, and also the cancer control system. Such a system is adaptive in the sense that the behavior of the individual entities is not fixed but rather changes over time in ways that are intended to help those entities meet their individual goals. And it is complex in that the overall performance of the system cannot be understood in terms of the behavior of the individual entities in isolation but rather is inherently the product of interactions between the different entities, which themselves are constantly changing and adapting to one another.
One straightforward example of a complex adaptive system in practice is the nation’s cargo transportation system. It consists of various types of carriers (air, rail, highway, and sea), the customers these carriers serve, unions and other organizations of transportation employees, the corporations that develop and build transportation technology (aircraft, trains, cars and trucks, ships, etc.), the government and private agencies responsible for maintaining the nation’s transportation infrastructure, various regulators responsible for transportation safety, and many other components. Again, there is no central agency controlling this system.
Government laws and regulations provide a certain amount of direction, but the overall behavior of the system is the product of many different entities pursuing their own goals and adapting their behavior in response to what is happening in the rest of the system. And again, there are emergent behaviors that are produced through the interactions of the various entities in the system, which cannot be understood by analyzing the components one at a time. A good example is the development of container shipping, which offers a way to ship cargo efficiently when it is to be carried by multiple types of carriers—ship, train, and truck. Similar ideas and complexities exist with mail and parcel delivery services, improved over the past century by the U.S. Postal Service and, in more recent decades, by global courier delivery businesses in synergy with available transportation options. Tools from complexity science and systems engineering have been applied to many other complex adaptive systems such as manufacturing, banking, air traffic control, weather prediction, homeland security, and the Internet, to name a few.
Perhaps the most important thing to note about the complex adaptive system of cancer control is that there is no hierarchical command-and-control structure, with someone at the top making decisions and issuing orders that are followed down the hierarchy; instead, there are multiple entities, each with its own goal and acting in ways that are calculated to advance those goals. Generally speaking, these various entities all agree on the general goal—to reduce the burden of cancer—but they have varying interpretations of exactly what that goal means; they have their own individual approaches, scale, and scope to attaining that goal; and they usually have additional objectives that are not necessarily related to that overarching goal. Oncologists treat the cancers that have been diagnosed in their patients and generally leave cancer prevention to other clinicians and public health professionals. Biopharmaceutical and device companies and for-profit hospitals seek to maximize returns for their shareholders while they are providing treatments for patients with cancer.
In the resulting system of systems, each participant pursues its own goals with the tools it has at its disposal. These individual components are adaptive in the sense that they respond to what is happening around them by changing their behavior so as to give themselves the best chance to meet their own goals. When necessary, the different entities communicate and coordinate with one another, but because there is no central command, there is much less coordination than found in a hierarchical system, and the different entities end up adapting their behavior in response to what other entities in the system are doing. In short, there is no such thing as a simple cause and effect in the nation’s system for cancer control, and one cannot understand the system by the traditional approach of breaking it down into its component parts and studying each of those parts. What
this means for cancer control is that it is very difficult to “optimize” the performance of the entire system using the traditional command-and-control approach. Even in the best-case scenario, with all the participating entities being well intentioned and acting in ways they judge to be ideal for cancer control, the value generated by the cancer control system will inevitably be much lower than it might be, in the sense that health outcomes may be compromised, disparities may be perpetuated, or the costs of delivering these outcomes may be excessive—or all of these.
Consider, for example, what happens when a new cancer diagnostic tool is introduced into the market (Rouse, 2000). In a simpler system, one whose components can be hierarchically decomposed, the introduction of this new technology would be expected to inevitably lead to improved cancer diagnosis and reduced cancer mortality throughout the entire system. In a complex adaptive system, however, all the entities have their own goals and strategies, and there is no one central command that can send out an order for everyone to do what is necessary to ensure that an effective technology is adopted and used. Instead, there are many different components of the system—regulation, clinical evaluation, public awareness, clinician education, coverage and reimbursement, patient preferences, and advocacy, to name a few—that need to be mobilized to do what is necessary to get the new technology adopted and used, or else the patients may not experience the benefits of the new technology. The result is that, in general, enormous investments in biomedical research will not substantially improve health care outcomes unless they are introduced with an understanding of the roles that the different components of the health and medical system play, including their interactions with one another.
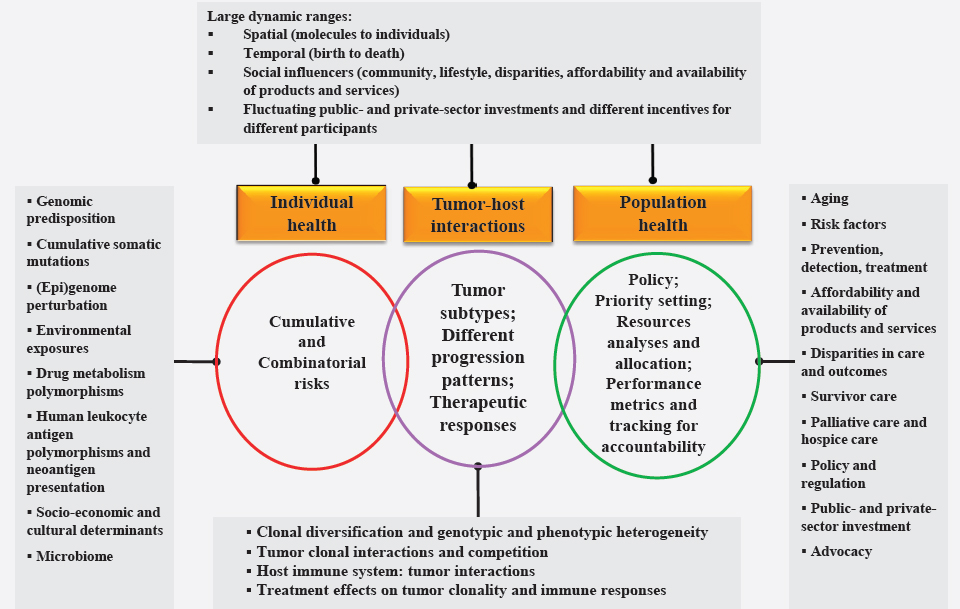
In short, the population health and clinical care system in the United States consists of what might be called a “networks of networks” or “systems of systems” that includes an enormous number of independent—and often conflicting—participants and interests, layered by organizations, specialties, regions, cancer types, and so on. A policy that seeks a particular outcome by changing one component or the behavior of one entity of the system will affect the behavior of the other entities. Therefore, the challenges of cancer control—and of health and medical care in the United States in general—need to be addressed in a different way and from a different point of view, recognizing that cancer control is a complex adaptive system, with all that this implies. Figure 1-7 is a descriptive illustration of cancer control as a complex adaptive system composed of multiple subsystems. It displays the level of complexity imposed on cancer control efforts by a range of interacting biological and environmental factors that drive cancer risk, randomness, emergence, and progression.
With all the complexities in cancer control, ultimately the central question remains: Do various forms of cancer control efforts produce adequate
return on public and private investments that are laden with risks? This central question continues to perplex the scientific, business, and policy communities and, ultimately, the taxpayers, as the expenditures in cancer control seem to uncontrollably increase each year. One way to assess the return on the investment would be to consider changes in population health outcomes attributable to cancer prevention and care strategies and to relate these changes to different types of cancer control expenditures. As noted earlier, age-adjusted cancer mortality rates have declined over the past several decades, thanks in part to new cancer screening tests, diagnostics, and treatments as well as to changes in population behaviors that have taken place. But these trends are confounded by simultaneous significant increases in the age-adjusted incidence of some cancers and several other diseases. Because cancer is not one disease, it becomes even more difficult to pinpoint which particular cancer control strategies are driving declines in cancer mortality and in what contexts of care and related services. An objective assessment of the effectiveness and cost of current forms of cancer control efforts would require complex systems analyses.
FINDINGS
Finding 1-1: Decades of research and development have revealed the intrinsic biological, financial, and social complexities of cancer control. Given the heterogeneity of cancers, it is not always clear whether knowledge and insights gained about one type of cancer can be applied to another type. The growth and metastasis of any type of cancer involve multiple interacting processes, so to gain a complete understanding of that cancer, one needs to comprehend not only the processes but also the ways in which they work with and against one another.
Finding 1-2: None of the methods of classifying cancers is sufficient on its own for addressing all the complexities inherent in cancer control.
Finding 1-3: Approaching cancer control efforts as if they formed a simple hierarchical “command-and-control” system is inappropriate and ineffective. The outcomes of cancer control in response to a specific change are not generally predictable because of the varied interactions, interests, and influences among its numerous participants. This makes the monitoring of the state and function of the entire “system” of cancer control difficult, especially from an economics perspective and for determining the financial, social, and other returns on investments.
Finding 1-4: The total annual economic burden of cancer in the United States is around $600 billion annually and could well approach $1 trillion in the years ahead. It is presently unclear what the current total, including hidden and
unpriced, costs of cancer control efforts are—from all forms of risk recognition and mitigation to medical treatments and palliative care, supporting research and development, investments across numerous sectors, and other forms of indirect and social expenditures.
Finding 1-5: Formidable challenges will continue to exist going forward, not only with respect to essential workforce development for reducing cancer burden and disparities but also in containing the severe financial impacts on patients and their families as well as on public and private payers.
Finding 1-6: The cancer control continuum includes activities from recognizing cancer risks to diagnosis and treatment to hospice care, but in practice the sequence of activities is not integrated effectively, with possible adverse consequences for patients, including disparities in outcomes.
Finding 1-7: The key differences between the “process” of cancer control and the “system” of cancer control are not widely appreciated. This could negatively affect the coordination needed across the activities and participants in the cancer control continuum.
Finding 1-8: A critical intellectual as well as practical challenge arises in determining the “value” of cancer control programs and interventions and how to pay for them. Value considerations are based on wide-ranging interests and perspectives without broad agreement. Cost–effectiveness ratios have been widely used for valuation in health and medical decision making, but they are known to be incomplete.
Finding 1-9: As more and more of the genetic, molecular, and biochemical details of cancers are made clear, and as diagnostics, treatments, and combination technologies become sophisticated, clinicians and other professionals are challenged to keep up with the current developments in cancer control and to effectively communicate these advances to patients and their families.
Finding 1-10: Differing philosophies can lead to differing policy judgments even in an area that is as seemingly apolitical as cancer control. Some, for example, may believe that the benefits of environmental and workforce programs are outweighed by the costs to businesses and economic competitiveness, while others may see efforts to limit smoking and alcohol consumption and to modify other behaviors as an infringement on individual rights.
Finding 1-11: Because outcome measures shape the goals of cancer control programs by implicitly defining what is important for “success,” it becomes necessary to critically and accountably analyze which outcomes are most relevant to patients.
REFERENCES
Abiola, S. E., J. Colgrove, and M. M. Mello. 2013. The politics of HPV vaccination policy formation in the United States. Journal of Health Politics, Policy and Law 38(4):645–681.
ACS CAN (American Cancer Society Cancer Action Network). 2017. The costs of cancer: Addressing patient costs. https://www.fightcancer.org/sites/default/files/Costs%20of%20Cancer%20-%20Final%20Web.pdf (accessed February 15, 2019).
Ahluwalia, S. C., C. Chen, L. Raaen, A. Motala, A. M. Walling, M. Chamberlin, C. O’Hanlon, J. Larkin, K. Lorenz, O. Akinniranye, and S. Hempel. 2018. A systematic review in support of the national consensus project clinical practice guidelines for quality palliative care, fourth edition. Journal of Pain and Symptom Management 56(6):831–870.
Allen, S. I., J. Foulds, E. Wasserman, S. Veldheer, S. Hrabovsky, J. Yingst, and G. Liu. 2018. Tobacco use among middle and high school students in Pennsylvania. Preventing Chronic Disease 15:E19.
ASCO (American Society of Clinical Oncology). 2017. The state of cancer care in America, 2017: A report by the American Society of Clinical Oncology. Journal of Oncology Practice 13(4):e353–e394.
ASCO. 2018. National survey reveals surprising number of Americans believe alternative therapies can cure cancer. https://www.asco.org/advocacy-policy/asco-in-action/national-survey-reveals-surprising-number-americans-believe (accessed February 15, 2019).
Attia, A. C., J. Wolf, and A. E. Núñez. 2018. On surmounting the barriers to HPV vaccination: We can do better. Annals of Medicine 50(3):209–225.
Backholer, K., and J. Martin. 2017. Sugar-sweetened beverage tax: The inconvenient truths. Public Health Nutrition 20(18):3225–3227.
Banegas, M. P., G. P. Guy, Jr., J. S. de Moor, D. U. Ekwueme, K. S. Virgo, E. E. Kent, S. Nutt, Z. Zheng, R. Rechis, and K. R. Yabroff. 2016. For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Affairs (Millwood) 35(1):54–61.
Bardia, A., D. L. Barton, L. J. Prokop, B. A. Bauer, and T. J. Moynihan. 2006. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: A systematic review. Journal of Clinical Oncology 24(34):5457–5464.
Bernard, D. S., S. L. Farr, and Z. Fang. 2011. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. Journal of Clinical Oncology 29(20):2821–2826.
Berrigan, D., J. A. Hipp, P. M. Hurvitz, P. James, M. M. Jankowska, J. Kerr, F. Laden, T. Leonard, R. A. McKinnon, T. M. Powell-Wiley, E. Tarlov, and S. N. Zenk. 2015. Geospatial and contextual approaches to energy balance and health. Annals of GIS 21(2):157–168.
Bluethmann, S. M., A. B. Mariotto, and J. H. Rowland. 2016. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiology Biomarkers and Prevention 25(7):1029–1036.
Booth, S. L., J. F. Sallis, C. Ritenbaugh, J. O. Hill, L. L. Birch, L. D. Frank, K. Glanz, D. A. Himmelgreen, M. Mudd, B. M. Popkin, K. A. Rickard, S. St. Jeor, and N. P. Hays. 2001. Environmental and societal factors affect food choice and physical activity: Rationale, influences, and leverage points. Nutrition Reviews 59(3 Pt. 2):S21–S39; discussion S57–S65.
Bowers, D. C., P. C. Nathan, L. Constine, C. Woodman, S. Bhatia, K. Keller, and L. Bashore. 2013. Subsequent neoplasms of the CNS among survivors of childhood cancer: A systematic review. The Lancet Oncology 14(8):e321–e328.
Bradley, C. J., K. R. Yabroff, B. Dahman, E. J. Feuer, A. Mariotto, and M. L. Brown. 2008. Productivity costs of cancer mortality in the United States: 2000–2020. JNCI: Journal of the National Cancer Institute 100(24):1763–1770.
Breslow, L. 1979. A history of cancer control in the United States, 1946–1971. DHEW Publication no. (NIH) 79-1516-9. Washington, DC: U.S. National Cancer Institute, Division of Cancer Control and Rehabilitation.
Brodersen, J., and V. D. Siersma. 2013. Long-term psychosocial consequences of false-positive screening mammography. The Annals of Family Medicine 11(2):106–115.
Brown, P., and S. de Graaf. 2013. Considering a future which may not exist: The construction of time and expectations amidst advanced-stage cancer. Health, Risk & Society 15(6–7):543–560.
Burg, M. A., G. Adorno, E. D. Lopez, V. Loerzel, K. Stein, C. Wallace, and D. K. Sharma. 2015. Current unmet needs of cancer survivors: Analysis of open-ended responses to the American Cancer Society study of cancer survivors II. Cancer 121(4):623–630.
Campo, R. A., J. H. Rowland, M. L. Irwin, P. C. Nathan, E. R. Gritz, and A. Y. Kinney. 2011. Cancer prevention after cancer: Changing the paradigm—a report from the American Society of Preventive Oncology. Cancer Epidemiology and Prevention Biomarkers 20(10):2317–2324.
Casalino, L. 2001. Managing uncertainty: Intermediate organizations as triple agents. Journal of Health Politics, Policy and Law 26(5):1055–1068.
Cataldo, J. K., S. Dubey, and J. J. Prochaska. 2010. Smoking cessation: An integral part of lung cancer treatment. Oncology 78(5–6):289–301.
CDC (Centers for Disease Control and Prevention). 2018a. Current cigarette smoking among adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (accessed February 15, 2019).
CDC. 2018b. Smoking is down, but almost 38 million American adults still smoke. https://www.cdc.gov/media/releases/2018/p0118-smoking-rates-declining.html (accessed February 15, 2019).
CDC. 2019. National Center for Chronic Disease Prevention and Health Promotion FY 2019 Operating Budget (Dollars in Thousands). https://www.cdc.gov/chronicdisease/programsimpact/budget/index.htm (accessed February 15, 2019).
Charity Navigator. 2019. Charity Categories. Health. https://www.charitynavigator.org/index.cfm?bay=search.categories&categoryid=5 (accessed February 15, 2019).
Chen, Y., J. Sun, L. C. Huang, H. Xu, and Z. Zhao. 2015. Classification of cancer primary sites using machine learning and somatic mutations. BioMed Research International 2015:491502.
Clancy, L. 2014. Reducing lung cancer and other tobacco-related cancers in Europe: Smoking cessation is the key. The Oncologist 19(1):16–20.
Claxton, G., L. Levitt, M. Rae, and B. Sawyer. 2018. Increases in cost-sharing payments continue to outpace wage growth. https://www.healthsystemtracker.org/brief/increases-in-costsharing-payments-have-far-outpaced-wage-growth/#item-start (accessed February 15, 2019).
CMS (Centers for Medicare & Medicaid Services). 2018. Oncology care model. https://innovation.cms.gov/initiatives/oncology-care (accessed February 15, 2019).
Cohen, E. E., S. J. LaMonte, N. L. Erb, K. L. Beckman, N. Sadeghi, K. A. Hutcheson, M. D. Stubblefield, D. M. Abbott, P. S. Fisher, K. D. Stein, G. H. Lyman, and M. L. Pratt-Chapman. 2016. American Cancer Society head and neck cancer survivorship care guideline. CA: A Cancer Journal for Clinicians 66(3):203–239.
Cohen, S. B. 2014. The concentration of health care expenditures and related expenses for costly medical conditions, 2012. October 2014. Statistical Brief #455. Rockville, MD: Agency for Healthcare Research and Quality. https://meps.ahrq.gov/data_files/publications/st455/stat455.pdf (accessed February 15, 2019).
Connor, J. 2017. Alcohol consumption as a cause of cancer. Addiction 112(2):222–228.
Conrad, D. A. 2015. The theory of value-based payment incentives and their application to health care. Health Services Research 50(Suppl. 2):2057–2089.
Coughlin, S., and P. Keckley. 2013. Breaking constraints: Can incentives change consumer health choices? https://www2.deloitte.com/insights/us/en/industry/health-plans/breaking-constraints.html (accessed February 15, 2019).
Cramer, J. D., J. T. Johnson, and M. L. Nilsen. 2018. Pain in head and neck cancer survivors: Prevalence, predictors, and quality-of-life impact. Otolaryngology–Head and Neck Surgery 159(5):853–858.
Daley, E., A. Alio, E. H. Anstey, R. Chandler, K. Dyer, and H. Helmy. 2011. Examining barriers to cervical cancer screening and treatment in Florida through a socio-ecological lens. Journal of Community Health 36(1):121–131.
Damalas, C. A., and I. G. Eleftherohorinos. 2011. Pesticide exposure, safety issues, and risk assessment indicators. International Journal of Environmental Research and Public Health 8(5):1402–1419.
Dieleman, J. L., R. Baral, M. Birger, A. L. Bui, A. Bulchis, A. Chapin, H. Hamavid, C. Horst, E. K. Johnson, and J. Joseph. 2016. US spending on personal health care and public health, 1996–2013. JAMA 316(24):2627–2646.
Dorell, C. G., D. Yankey, T. A. Santibanez, and L. E. Markowitz. 2011. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 128(5):830–839.
Dowdle, W. R. 1998. The principles of disease elimination and eradication. Bulletin of the World Health Organization 76(Suppl. 2):22–25.
Edge, S. B., and C. C. Compton. 2010. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology 17(6):1471–1474.
El-Jawahri, A., T. W. LeBlanc, L. J. Burns, E. Denzen, C. Meyer, L. W. Mau, E. J. Roeland, W. A. Wood, and E. Petersdorf. 2018. What do transplant physicians think about palliative care? A national survey study. Cancer 124(23):4556–4566.
El-Shami, K., K. C. Oeffinger, N. L. Erb, A. Willis, J. K. Bretsch, M. L. Pratt-Chapman, R. S. Cannady, S. L. Wong, J. Rose, A. L. Barbour, K. D. Stein, K. B. Sharpe, D. D. Brooks, and R. L. Cowens-Alvarado. 2015. American Cancer Society colorectal cancer survivorship care guidelines. CA: A Cancer Journal for Clinicians 65(6):428–455.
Epstein, R. M., P. R. Duberstein, J. J. Fenton, K. Fiscella, M. Hoerger, D. J. Tancredi, G. Xing, R. Gramling, S. Mohile, P. Franks, P. Kaesberg, S. Plumb, C. S. Cipri, R. L. Street, Jr., C. G. Shields, A. L. Back, P. Butow, A. Walczak, M. Tattersall, A. Venuti, P. Sullivan, M. Robinson, B. Hoh, L. Lewis, and R. L. Kravitz. 2017. Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: The voice randomized clinical trial. JAMA Oncology 3(1):92–100.
Ferrell, B. R., J. S. Temel, S. Temin, E. R. Alesi, T. A. Balboni, E. M. Basch, J. I. Firn, J. A. Paice, J. M. Peppercorn, T. Phillips, E. L. Stovall, C. Zimmermann, and T. J. Smith. 2017. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology 35(1):96–112.
Foulds, J. 2015. Use of electronic cigarettes by adolescents. Journal of Adolescent Health 57(6):569–570.
Frederick, N. N., S. L. Bober, L. Berwick, M. Tower, and L. B. Kenney. 2017. Preparing childhood cancer survivors for transition to adult care: The young adult perspective. Pediatric Blood & Cancer 64(10):e26544.
Gaertner, J., W. Siemens, J. J. Meerpohl, G. Antes, C. Meffert, C. Xander, S. Stock, D. Mueller, G. Schwarzer, and G. Becker. 2017. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: Systematic review and meta-analysis. British Medical Journal 357:j2925.
Gany, F., T. Lee, J. Ramirez, D. Massie, A. Moran, M. Crist, T. McNish, G. Winkel, and J. C. Leng. 2014. Do our patients have enough to eat?: Food insecurity among urban low-income cancer patients. Journal of Health Care for the Poor and Underserved 25(3):1153–1168.
Gapstur, S. M., J. M. Drope, E. J. Jacobs, L. R. Teras, M. L. McCullough, C. E. Douglas, A. V. Patel, R. C. Wender, and O. W. Brawley. 2018. A blueprint for the primary prevention of cancer: Targeting established, modifiable risk factors. CA: A Cancer Journal for Clinicians 68(6):446–470.
Gibson, A. F., L. D’Cruz, M. Janda, V. L. Beesley, R. E. Neale, and I. J. Rowlands. 2016. Beyond survivorship? A discursive analysis of how people with pancreatic cancer negotiate identity transitions in their health. Journal of Health Psychology 21(12):3060–3071.
Gilligan, A. M., D. S. Alberts, D. J. Roe, and G. H. Skrepnek. 2018. Death or debt? National estimates of financial toxicity in persons with newly-diagnosed cancer. American Journal of Medicine 131(10):1187–1199.
Glantz, S. A., and E. D. Balbach. 2000. Tobacco war: Inside the California battles. Berkeley, CA: University of California Press.
Gonzalez, C. A., and E. Riboli. 2006. Diet and cancer prevention: Where we are, where we are going. Nutrition and Cancer 56(2):225–231.
Gordon, L. G., K. M. D. Merollini, A. Lowe, and R. J. Chan. 2017. A systematic review of financial toxicity among cancer survivors: We can’t pay the co-pay. Patient 10(3):295–309.
Griswold, M. G., N. Fullman, C. Hawley, N. Arian, S. R. Zimsen, H. D. Tymeson, V. Venkateswaran, A. D. Tapp, M. H. Forouzanfar, and J. S. Salama. 2018. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet 392(10152):1015–1035.
Gross, C. P., J. B. Long, J. S. Ross, M. M. Abu-Khalaf, R. Wang, B. K. Killelea, H. T. Gold, A. B. Chagpar, and X. Ma. 2013. The cost of breast cancer screening in the Medicare population. JAMA Internal Medicine 173(3):220–226.
Guadagnolo, B. A., J. Huo, T. A. Buchholz, and D. G. Petereit. 2014. Disparities in hospice utilization among American Indian Medicare beneficiaries dying of cancer. Ethnicity & Disease 24(4):393–398.
Guadagnolo, B. A., K. P. Liao, S. H. Giordano, L. S. Elting, and Y. C. Shih. 2015. Variation in intensity and costs of care by payer and race for patients dying of cancer in texas: An analysis of registry-linked Medicaid, Medicare, and dually eligible claims data. Medical Care 53(7):591–598.
Guerard, E. J., G. Nightingale, K. Bellizzi, P. Burhenn, A. Rosko, A. S. Artz, B. Korc-Grodzicki, B. Canin, W. Dale, and B. Ferrell. 2016. Survivorship care for older adults with cancer: U13 conference report. Journal of Geriatric Oncology 7(4):305–312.
Hagenaars, L. L., P. P. T. Jeurissen, and N. S. Klazinga. 2017. The taxation of unhealthy energy-dense foods (EDFs) and sugar-sweetened beverages (SSBs): An overview of patterns observed in the policy content and policy context of 13 case studies. Health Policy 121(8):887–894.
Hall, M. T., K. T. Simms, J. B. Lew, M. A. Smith, J. M. Brotherton, M. Saville, I. H. Frazer, and K. Canfell. 2018. The projected timeframe until cervical cancer elimination in Australia: A modelling study. Lancet Public Health 4(1):e19–e27.
Hanna, N. H., and L. H. Einhorn. 2014. Testicular cancer—discoveries and updates. New England Journal of Medicine 371(21):2005–2016.
Hardy, D., W. Chan, C. C. Liu, J. N. Cormier, R. Xia, E. Bruera, and X. L. Du. 2011. Racial disparities in the use of hospice services according to geographic residence and socioeconomic status in an elderly cohort with nonsmall cell lung cancer. Cancer 117(7):1506–1515.
Heymann, D. L. 2014. Control of communicable diseases manual, 20th ed. Washington, DC: American Public Health Association.
Hiatt, R. A., and J. G. Brody. 2018. Environmental determinants of breast cancer. Annual Review of Public Health 39:113–133.
Holman, D. M., V. Benard, K. B. Roland, M. Watson, N. Liddon, and S. Stokley. 2014. Barriers to human papillomavirus vaccination among US adolescents: A systematic review of the literature. JAMA Pediatrics 168(1):76–82.
Huang, Y., J. Pomeranz, P. Wilde, S. Capewell, T. Gaziano, M. O’Flaherty, R. Kersh, L. Whitsel, D. Mozaffarian, and R. Micha. 2018. Adoption and design of emerging dietary policies to improve cardiometabolic health in the US. Current Atherosclerosis Reports 20(5):25.
IARC (International Agency for Research on Cancer) Working Group on the Evaluation of Carcinogenic Risks to Humans. 2007. Meeting, World Health Organization and International Agency for Research on Cancer. Human papillomaviruses (Vol. 90). Lyon, France: World Health Organization.
Idikio, H. A. 2011. Human cancer classification: A systems biology-based model integrating morphology, cancer stem cells, proteomics, and genomics. Journal of Cancer 2:107–115.
IOM (Institute of Medicine). 1999. Health effects of exposure to radon: BEIR VI. Washington, DC: National Academy Press.
IOM. 2002. Estimating the public health benefits of proposed air pollution regulations. Washington, DC: The National Academies Press.
IOM. 2003. Childhood cancer survivorship: Improving care and quality of life. Washington, DC: The National Academies Press.
IOM. 2006a. Asbestos: Selected cancers. Washington, DC: The National Academies Press.
IOM. 2006b. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press.
IOM. 2007. The Learning Healthcare System: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2008. Cancer care for the whole patient: Meeting psychosocial health needs. Washington, DC: National Academies Press.
IOM. 2013a. Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: The National Academies Press.
IOM. 2013b. Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academies Press.
IOM. 2015. Dying in America: Improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press.
IQVIA Institute for Human Data Science. 2018. Global oncology trends 2018. Parsippany, NJ: IQVIA.
Islami, F., A. Goding Sauer, K. D. Miller, R. L. Siegel, S. A. Fedewa, E. J. Jacobs, M. L. McCullough, A. V. Patel, J. Ma, I. Soerjomataram, W. D. Flanders, O. W. Brawley, S. M. Gapstur, and A. Jemal. 2018. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: A Cancer Journal for Clinicians 68(1):31–54.
Israel, B. 2010. How many cancers are caused by the environment? https://www.scientificamerican.com/article/how-many-cancers-are-caused-by-the-environment (accessed February 15, 2019).
Jacobs, E. J., C. C. Newton, B. D. Carter, D. Feskanich, N. D. Freedman, R. L. Prentice, and W. D. Flanders. 2015. What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Annals of Epidemiology 25(3):179–182.
Jacobsen, P. B., A. P. DeRosa, T. O. Henderson, D. K. Mayer, C. S. Moskowitz, E. D. Paskett, and J. H. Rowland. 2018. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. Journal of Clinical Oncology 36(20):2088–2100.
Jagai, J. S., L. C. Messer, K. M. Rappazzo, C. L. Gray, S. C. Grabich, and D. T. Lobdell. 2017. County-level cumulative environmental quality associated with cancer incidence. Cancer 123(15):2901–2908.
Jawerth, N. 2018. Killing more cancer cells than ever before: A new era in radiotherapy. IAEA Bulletin. P. 5.
Joffe, M. 2015. Long-term trends in medicaid spending by the states. https://www.mercatus.org/system/files/Joffe-State-Medicaid-Spending.pdf (accessed February 15, 2019).
John, G. M., D. L. Hershman, L. Falci, Z. Shi, W.-Y. Tsai, and H. Greenlee. 2016. Complementary and alternative medicine use among US cancer survivors. Journal of Cancer Survivorship 10(5):850–864.
Kakushadze, Z., R. Raghubanshi, and W. Yu. 2017. Estimating cost savings from early cancer diagnosis. Data 2(3):30.
Kenney, L. B., P. Melvin, L. N. Fishman, J. O’Sullivan-Oliveira, G. S. Sawicki, S. Ziniel, L. Diller, and S. M. Fernandes. 2017. Transition and transfer of childhood cancer survivors to adult care: A national survey of pediatric oncologists. Pediatric Blood & Cancer 64(2):346–352.
Kerr, J., C. Anderson, and S. M. Lippman. 2017. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. The Lancet Oncology 18(8):e457–e471.
KFF (Kaiser Family Foundation). 2017. How much does the U.S. spend to treat different diseases? https://www.healthsystemtracker.org/chart-collection/much-u-s-spend-treatdifferent-diseases/#item-start (accessed February 15, 2019).
Kiadaliri, A. A., J. Jarl, G. Gavriilidis, and U.-G. Gerdtham. 2013. Alcohol drinking cessation and the risk of laryngeal and pharyngeal cancers: A systematic review and meta-analysis. PLoS ONE 8(3):e58158.
Kissick, W. L. 1994. Medicine’s dilemmas: Infinite needs versus finite resources. New Haven, CT: Yale University Press.
Kline, R. M., N. K. Arora, C. J. Bradley, E. R. Brauer, D. L. Graves, N. B. Lunsford, M. S. McCabe, S. F. Nasso, L. Nekhlyudov, J. H. Rowland, R. M. Schear, and P. A. Ganz. 2018. Long-term survivorship care after cancer treatment—summary of a 2017 National Cancer Policy Forum workshop. Journal of the National Cancer Institute 110(12):1300–1310.
Koury, J., M. Lucero, C. Cato, L. Chang, J. Geiger, D. Henry, J. Hernandez, F. Hung, P. Kaur, G. Teskey, and A. Tran. 2018. Immunotherapies: Exploiting the immune system for cancer treatment. Journal of Immunology Research 2018:9585614.
Kuchenbaecker, K. B., J. L. Hopper, D. R. Barnes, K.-A. Phillips, T. M. Mooij, M.-J. Roos-Blom, S. Jervis, F. E. Van Leeuwen, R. L. Milne, and N. Andrieu. 2017. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23):2402–2416.
Lathan, C. S., A. Cronin, R. Tucker-Seeley, S. Y. Zafar, J. Z. Ayanian, and D. Schrag. 2016. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. Journal of Clinical Oncology 34(15):1732–1740.
Lehman, E. P. 2015. The health care “iron triangle” and the patient protection and Affordable Care Act. Cleveland Clinic Journal of Medicine 82(2):73–80.
Li, C. 2012. Biomarkers to distinguish aggressive cancers from non-aggressive or non-progressing cancer. https://edrn.nci.nih.gov/protocols/362-biomarkers-to-distinguish-aggressive-cancers-from (accessed February 15, 2019).
Liu, C., Y. Yang, and Y. Wu. 2018. Recent advances in exosomal protein detection via liquid biopsy biosensors for cancer screening, diagnosis, and prognosis. AAPS Journal 20(2):41.
Liu, G., E. Wasserman, L. Kong, and J. Foulds. 2017. A comparison of nicotine dependence among exclusive e-cigarette and cigarette users in the path study. Preventive Medicine 104:86–91.
Lloyd, I. Pharma R&D Annual Review 2018. https://pharmaintelligence.informa.com/resources/product-content/pharma-rd-annual-review-2018 (accessed February 15, 2019).
Lobb, E. A., J. Lacey, J. Kearsley, W. Liauw, L. White, and A. Hosie. 2015. Living with advanced cancer and an uncertain disease trajectory: An emerging patient population in palliative care? BMJ Supportive & Palliative Care 5(4):352–357.
LoConte, N. K., A. M. Brewster, J. S. Kaur, J. K. Merrill, and A. J. Alberg. 2018. Alcohol and cancer: A statement of the American Society of Clinical Oncology. Journal of Clinical Oncology 36(1):83–93.
Lortet-Tieulent, J., A. Goding Sauer, R. L. Siegel, K. D. Miller, F. Islami, S. A. Fedewa, E. J. Jacobs, and A. Jemal. 2016. State-level cancer mortality attributable to cigarette smoking in the United States. JAMA Internal Medicine 176(12):1792–1798.
Lowy, D. R., and J. T. Schiller. 2012. Reducing HPV-associated cancer globally. Cancer Prevention Research 5(1):18–23.
Lunze, K., and M. K. Paasche-Orlow. 2013. Financial incentives for healthy behavior: Ethical safeguards for behavioral economics. American Journal of Preventive Medicine 44(6):659–665.
Mariotto, A. B., K. Robin Yabroff, Y. Shao, E. J. Feuer, and M. L. Brown. 2011. Projections of the cost of cancer care in the United States: 2010–2020. Journal of the National Cancer Institute 103(2):117–128.
Martin, A. B., M. Hartman, J. Benson, and A. Catlin. 2016. National health spending in 2014: Faster growth driven by coverage expansion and prescription drug spending. Health Affairs 35(1):150–160.
Martinez, M. E., J. R. Marshall, and E. Giovannucci. 2008. Diet and cancer prevention: The roles of observation and experimentation. Nature Reviews Cancer 8(9):694–703.
Marzorati, C., S. Riva, and G. Pravettoni. 2017. Who is a cancer survivor? A systematic review of published definitions. Journal of Cancer Education 32(2):228–237.
Mayne, S. T., M. C. Playdon, and C. L. Rock. 2016. Diet, nutrition, and cancer: Past, present and future. Nature Reviews Clinical Oncology 13(8):504.
McCullough, M. L., and E. L. Giovannucci. 2004. Diet and cancer prevention. Oncogene 23(38):6349–6364.
Meeker, C. R., D. M. Geynisman, B. L. Egleston, M. J. Hall, K. Y. Mechanic, M. Bilusic, E. R. Plimack, L. P. Martin, M. von Mehren, B. Lewis, and Y. N. Wong. 2016. Relationships among financial distress, emotional distress, and overall distress in insured patients with cancer. Journal of Oncology Practice 12(7):e755–e764.
Méndez, D., O. Alshanqeety, K. E. Warner, P. M. Lantz, and P. N. Courant. 2011. The impact of declining smoking on radon-related lung cancer in the United States. American Journal of Public Health 101(2):310–314.
Miller, K. D., R. L. Siegel, C. C. Lin, A. B. Mariotto, J. L. Kramer, J. H. Rowland, K. D. Stein, R. Alteri, and A. Jemal. 2016. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians 66(4):271–289.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Driving action and progress on obesity prevention and treatment: Proceedings of a workshop. Washington DC: The National Academies Press.
NASEM. 2018a. Making medicines affordable: A national imperative. Washington, DC: The National Academies Press.
NASEM. 2018b. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press.
NCCIH (National Center for Complementary and Integrative Health). 2018. The use of complementary and alternative medicine in the United States: Cost data. https://nccih.nih.gov/news/camstats/costs/costdatafs.htm (accessed February 15, 2019).
NCCN (National Comprehensive Cancer Network). 2018. Survivorship guidelines. http://www.nccn.org/professionals/physician_gls/default.aspx#survivorship (accessed February 15, 2019).
NCHPC (National Coalition for Hospice and Palliative Care). 2018. Clinical practice guidelines for quality palliative care, 4th ed. Richmond, VA: National Coalition for Hospice and Palliative Care.
NCI (National Cancer Institute). 2018a. Cancer control continuum. https://cancercontrol.cancer.gov/od/continuum.html (accessed February 15, 2019).
NCI. 2018b. Cancer facts & the war on cancer. https://training.seer.cancer.gov/disease/war (accessed February 15, 2019).
NCI. 2018c. National Cancer Act of 1937. https://www.cancer.gov/about-nci/legislative/history/national-cancer-act-1937 (accessed February 15, 2019).
NCI. 2019. Definitions. Survivorship. https://cancercontrol.cancer.gov/ocs/statistics/definitions.html (accessed February 15, 2019).
NHLBI (National Heart, Lung, and Blood Institute). 2009. NHLBI fact book fiscal year 2008. Bethesda, MD. https://www.nhlbi.nih.gov/files/docs/factbook/FactBook2008.pdf (accessed February 15, 2019).
NIH (National Institutes of Health). 2018. Fact sheets—cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=75 (accessed February 15, 2019).
NIH. 2019. Estimates of funding for various research, condition, and disease categories. https://report.nih.gov/categorical_spending.aspx (accessed February 15, 2019).
Nikpay, S. S., M. R. Richards, and D. Penson. 2018. Hospital-physician consolidation accelerated in the past decade in cardiology, oncology. Health Affairs 37(7):1123–1127.
O’Donoghue, C., M. Eklund, E. M. Ozanne, and L. J. Esserman. 2014. Aggregate cost of mammography screening in the United States: Comparison of current practice and advocated guidelines. Annals of Internal Medicine 160(3):145–153.
O’Dowd, E. L., and D. R. Baldwin. 2017. Lung cancer screening—low dose CT for lung cancer screening: Recent trial results and next steps. The British Journal of Radiology 90(1079):20170460.
Ogilvie, G. S., F. Phan, H. N. Pedersen, S. R. Dobson, M. Naus, and E. M. Saewyc. 2018. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003–2013). Canadian Medical Association Journal 190(41):E1221–E1226.
Olstad, D. L., R. Ancilotto, M. Teychenne, L. M. Minaker, D. R. Taber, K. D. Raine, C. I. J. Nykiforuk, and K. Ball. 2017. Can targeted policies reduce obesity and improve obesity-related behaviours in socioeconomically disadvantaged populations? A systematic review. Obesity Reviews 18(7):791–807.
Owen, N., G. N. Healy, C. E. Matthews, and D. W. Dunstan. 2010. Too much sitting: The population health science of sedentary behavior. Exercise and Sport Sciences Reviews 38(3):105–113.
Paskett, E., B. Thompson, A. S. Ammerman, A. N. Ortega, J. Marsteller, and D. Richardson. 2016. Multilevel interventions to address health disparities show promise in improving population health. Health Affairs 35(8):1429–1434.
PCP (President’s Cancer Panel). 2010. Reducing environmental cancer risk: What we can do now. https://deainfo.nci.nih.gov/advisory/pcp/annualreports/pcp08-09rpt/pcp_report_08-09_508.pdf (accessed February 15, 2019).
PCP. 2018. HPV vaccination for cancer prevention: Progress, opportunities, and a renewed call to action: A report to the president of the United States from the chair of the President’s Cancer Panel. https://prescancerpanel.cancer.gov/report/hpvupdate/pdf/PresCancerPanel_HPVUpdate_Nov2018.pdf (accessed February 15, 2019).
PhRMA (Pharmaceutical Research and Manufacturers of America). 2018. 2018 Profile: Biopharmaceutical research industry. http://phrma-docs.phrma.org/industryprofile/2018/pdfs/2018_IndustryProfile_Brochure.pdf (accessed February 15, 2019).
Pollack, L. A., W. Adamache, A. B. Ryerson, C. R. Eheman, and L. C. Richardson. 2009. Care of long-term cancer survivors: Physicians seen by medicare enrollees surviving longer than 5 years. Cancer 115(22):5284–5295.
Porter, M. E., and E. O. Teisberg. 2006. Redefining health care: Creating value-based competition on results. Boston, MA: Harvard Business Press.
Prigerson, H. G., Y. Bao, M. A. Shah, M. E. Paulk, T. W. LeBlanc, B. J. Schneider, M. M. Garrido, M. C. Reid, D. A. Berlin, K. B. Adelson, A. I. Neugut, and P. K. Maciejewski. 2015. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncology 1(6):778–784.
Pruss-Ustun, A., J. Wolf, C. Corvalan, T. Neville, R. Bos, and M. Neira. 2017. Diseases due to unhealthy environments: An updated estimate of the global burden of disease attributable to environmental determinants of health. Journal of Public Health 39(3):464–475.
Reuben, S. H. 2010. Reducing environmental cancer risk: What we can do now. 2008–2009 Annual Report, President’s Cancer Panel. https://deainfo.nci.nih.gov/advisory/pcp/annualreports/pcp08-09rpt/pcp_report_08-09_508.pdf (accessed February 15, 2019).
Riedel, R. F., K. Slusser, S. Power, C. A. Jones, T. W. LeBlanc, A. H. Kamal, D. Desai, D. Allen, Y. Yu, S. Wolf, and A. N. Galanos. 2017. Improvements in patient and health system outcomes using an integrated oncology and palliative medicine approach on a solid tumor inpatient service. Journal of Oncology Practice 13(9):e738–e748.
Riley, G. F., A. L. Potosky, C. N. Klabunde, J. L. Warren, and R. Ballard-Barbash. 1999. Stage at diagnosis and treatment patterns among older women with breast cancer: An HMO and fee-for-service comparison. JAMA 281(8):720–726.
Rosenberg, S. A., and N. P. Restifo. 2015. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68.
Rosenthal, E. T., B. Evans, J. Kidd, K. Brown, H. Gorringe, M. van Orman, and S. Manley. 2017. Increased identification of candidates for high-risk breast cancer screening through expanded genetic testing. Journal of the American College of Radiology 14(4):561–568.
Rouse, W. B. 2000. Managing complexity: Disease control as a complex adaptive system. Information-Knowledge-Systems Management 2(2):143–165.
Rubinstein, E. B., W. L. Miller, S. V. Hudson, J. Howard, D. O’Malley, J. Tsui, H. S. Lee, A. Bator, and B. F. Crabtree. 2017. Cancer survivorship care in advanced primary care practices: A qualitative study of challenges and opportunities. JAMA Internal Medicine 177(12):1726–1732.
Runowicz, C. D., C. R. Leach, N. L. Henry, K. S. Henry, H. T. Mackey, R. L. Cowens-Alvarado, R. S. Cannady, M. L. Pratt-Chapman, S. B. Edge, L. A. Jacobs, A. Hurria, L. B. Marks, S. J. LaMonte, E. Warner, G. H. Lyman, and P. A. Ganz. 2016. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. Journal of Clinical Oncology 34(6):611–635.
Sallis, J. F., R. B. Cervero, W. Ascher, K. A. Henderson, M. K. Kraft, and J. Kerr. 2006. An ecological approach to creating active living communities. Annual Review of Public Health 27:297–322.
Siegel, R. L., A. Jemal, R. C. Wender, T. Gansler, J. Ma, and O. W. Brawley. 2018. An assessment of progress in cancer control. CA: A Cancer Journal for Clinicians 68(5):329–339.
Siegel, R. L., K. D. Miller, and A. Jemal. 2019. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians 69(1):7.
Singh, T., R. A. Arrazola, C. G. Corey, C. G. Husten, L. J. Neff, D. M. Homa, and B. A. King. 2016. Tobacco use among middle and high school students—United States, 2011–2015. Morbidity and Mortality Weekly Report 65(14):361–367.
Skolarus, T. A., A. M. Wolf, N. L. Erb, D. D. Brooks, B. M. Rivers, W. Underwood III, A. L. Salner, M. J. Zelefsky, J. B. Aragon Ching, and S. F. Slovin. 2014. American Cancer Society prostate cancer survivorship care guidelines. CA: A Cancer Journal for Clinicians 64(4):225–249.
Smith, B. D., G. L. Smith, A. Hurria, G. N. Hortobagyi, and T. A. Buchholz. 2009. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. Journal of Clinical Oncology 27(17):2758–2765.
Smith, R. A., K. S. Andrews, D. Brooks, S. A. Fedewa, D. Manassaram-Baptiste, D. Saslow, O. W. Brawley, and R. C. Wender. 2018. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians 68(4):297–316.
Snyderman, R., and A. T. Weil. 2002. Integrative medicine: Bringing medicine back to its roots. Archives of Internal Medicine 162(4):395–397.
Song, Q., S. D. Merajver, and J. Z. Li. 2015. Cancer classification in the genomic era: Five contemporary problems. Human Genomics 9:27.
Song, Z., and K. Baicker. 2019. Effect of a workplace wellness program on employee health and economic outcomes: A randomized clinical trial. JAMA 321(15):1491–1501.
Soni, A., L. M. Sabik, K. Simon, and B. D. Sommers. 2018. Changes in insurance coverage among cancer patients under the Affordable Care Act. JAMA Oncology 4(1):122–124.
Spallarossa, P., G. Meliota, C. Brunelli, E. Arboscello, P. Ameri, C. C. Dessalvi, F. Grossi, M. Deidda, D. Mele, and M. Sarocchi. 2018. Potential cardiac risk of immune-checkpoint blockade as anticancer treatment: What we know, what we do not know, and what we can do to prevent adverse effects. Medicinal Research Reviews 38(5):1447–1468.
Stange, K. C., E. S. Breslau, A. J. Dietrich, and R. E. Glasgow. 2012. State-of-the-art and future directions in multilevel interventions across the cancer control continuum. Journal of the National Cancer Institute Monographs 2012(44):20–31.
Steward, W., and K. Brown. 2013. Cancer chemoprevention: A rapidly evolving field. British Journal of Cancer 109(1):1.
Sudhakar, A. 2009. History of cancer, ancient and modern treatment methods. Journal of Cancer Science and Therapy 1(2):1–4.
Thom, B., A. H. Boekhout, S. Corcoran, R. Adsuar, K. C. Oeffinger, and M. S. McCabe. 2019. Advanced practice providers and survivorship care: They can deliver. Journal of Oncology Practice 15(3):e230–e237.
Wang, S. Y., J. Hall, C. E. Pollack, K. Adelson, A. J. Davidoff, J. B. Long, and C. P. Gross. 2016. Associations between end-of-life cancer care patterns and medicare expenditures. Journal of the National Comprehensive Cancer Network 14(8):1001–1008.
Warnecke, R. B., A. Oh, N. Breen, S. Gehlert, E. Paskett, K. L. Tucker, N. Lurie, T. Rebbeck, J. Goodwin, and J. Flack. 2008. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Health 98(9):1608–1615.
WCRF/AICR (World Cancer Research Fund/American Institute for Cancer Research). 2018. Diet, nutrition, physical activity and cancer: A global perspective. Continuous Update Project Expert Report 2018. https://www.wcrf.org/sites/default/files/Summarythird-expert-report.pdf (accessed February 15, 2019).
Welch, H. G., P. C. Prorok, A. J. O’Malley, and B. S. Kramer. 2016. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. New England Journal of Medicine 375(15):1438–1447.
White, M. C., F. Babcock, N. S. Hayes, A. B. Mariotto, F. L. Wong, B. A. Kohler, and H. K. Weir. 2017. The history and use of cancer registry data by public health cancer control programs in the United States. Cancer 123(Suppl. 24):4969–4976.
Wright, A. A., B. Zhang, A. Ray, J. W. Mack, E. Trice, T. Balboni, S. L. Mitchell, V. A. Jackson, S. D. Block, P. K. Maciejewski, and H. G. Prigerson. 2008. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300(14):1665–1673.
Yabroff, K. R., C. J. Bradley, A. B. Mariotto, M. L. Brown, and E. J. Feuer. 2008. Estimates and projections of value of life lost from cancer deaths in the United States. JNCI: Journal of the National Cancer Institute 100(24):1755–1762.
Zhang, B., A. A. Wright, H. A. Huskamp, M. E. Nilsson, M. L. Maciejewski, C. C. Earle, S. D. Block, P. K. Maciejewski, and H. G. Prigerson. 2009. Health care costs in the last week of life: Associations with end-of-life conversations. Archives of Internal Medicine 169(5):480–488.




















































