2
Healthy Development from Conception Through Early Childhood
INTRODUCTION TO THE SCIENCE FROM PRENATAL THROUGH EARLY CHILDHOOD
Fetal and early child development are often conceptualized as a “black box,” in which children simply learn by soaking up information, much like a sponge and water. Developmental science has demonstrated that development is, in fact, an active process and that it starts early. Thus, understanding early development, when biological systems and functions are coming “online,” is essential for ensuring that prevention, interventions, policies, and systems are responsive to the needs of children. This will provide children with the best opportunity for healthy outcomes. When the science of early development is coupled with a health equity approach to inform decision making, it provides an opportunity to improve outcomes for children and families in at-risk contexts. The goal of this chapter is to reveal core concepts of brain development and other systems relevant to understanding the impact of disparities on cognitive, social-emotional, and physical health and to describe new scientific advances in this area over the past 20 years. This information can be used by the public and policy makers to better inform effective actions for promoting healthy outcomes. (See Box 2-1 for an overview of this chapter.)
Early understanding of children’s development, as postulated by Piaget and many others in the 20th century, consisted of age-specific stages, ruled primarily by maturation and biology (Flavell, 1963). This characterization has undergone profound revisions. The 2000 publication by the National Research Council and the Institute of Medicine,
From Neurons to Neighborhoods: The Science of Early Childhood Development, provided a transformational synthesis of existing research to reveal a complexity of factors that influence development beyond previous simplified models. This landmark report integrated a wealth of scientific knowledge on early childhood development to emphasize the continuous, dynamic interaction between the environment and the biological systems of each individual across the life course. The report offered clear recommendations for policy and the future of developmental science that were organized around core principles of child development that remain accurate to this day (NRC and IOM, 2000). The report inspired new science and novel ways to communicate the science to the public, service providers, and policy makers. From Neurons to Neighborhoods
spurred research to refine the fields’ understanding of how environmental influences shape child development, even at a molecular resolution. The report motivated research to understand how health-promoting environments and responsive relationships enable a strong foundation for healthy child development (i.e., learning, adaptive behaviors, and optimal health), while chronic adversity in the absence of supporting caregivers can lead to increased risk for negative learning and health outcomes, both in childhood and well into adulthood.
In the two decades since the publication of the report, there has been a convergence of activities that led to many of the advances that will be described in this chapter. First, a wave of neurobiological studies in model systems and in humans underscored that responses to pre- and postnatal
early life stress are rooted in genetic and environmental interactions that can result in altered molecular and cellular development that impacts the assembly of circuits during sensitive periods of development (Cameron et al., 2017; McEwen and Morrison, 2013; Shonkoff and Levitt, 2010). The demonstration that certain systems involved in cognitive and emotional development are more sensitive to early disturbances that activate stress response networks, such as the frontal cortex, hippocampus, amygdala, and hypothalamic-pituitary-adrenal (HPA) axis, provided a basis for both short- and long-term functional consequences of early life stress (Chen and Baram, 2016; Shonkoff et al., 2012). Second, life course theory (Ben-Shlomo et al., 2014; Kuh et al., 2003) and the developmental origins of an adult health and disease framework (Gluckman et al., 2007; Halfon et al., 2014) have become more widely adopted. Third, a key policy statement from the American Academy of Pediatrics in 2012 emphasized the important life-span health implications of the impact of early adversity, declaring a “need for the entire medical community to focus more attention on the roots of adult diseases that originate during the prenatal and early childhood periods” (Shonkoff et al., 2012, p. e233).
Numerous research teams have clearly documented the effects and some mechanisms through which early childhood adversity affects development. Early adversity, such as maltreatment (Cicchetti, 1996; Teicher et al., 2016) and poverty (Cicchetti and Curtis, 2006; Council on Community Pediatrics, 2016; Gabrieli and Bunge, 2017; Johnson et al., 2016; McEwen and McEwen, 2017), has negative impacts on selective brain circuits and emotional control, cognitive growth, and stress responsiveness. A new discovery from multiple animal studies shows that early adversity can change the timing of critical periods of brain development (Bath et al., 2016; Cameron et al., 2017; Hensch, 2016a; Heun-Johnson and Levitt, 2018), impacting the “plasticity” of developmental processes that are driven by experiences in the life of the young child and their family (Cicchetti, 2015a,b; Cicchetti and Blender, 2006; Pollak et al., 1997) (see Box 2-2). The extensions of the original retrospective Adverse Childhood Experiences (ACEs) Study by the Centers for Disease Control and Prevention and Kaiser Permanente fostered sustained scientific interest in the long-term implications of early childhood adversity (see Chapter 3 for more information). In addition, the prospective Dunedin Multidisciplinary Health and Development (Poulton et al., 2015), the Perry Preschool longitudinal study, and the Abecedarian studies (see Chapter 7 for more information on the Perry Preschool and Abecedarian projects) added to a growing body of scientific evidence that early experiences, positive or negative, have profound long-term effects on physical, mental, and cognitive functions. The U.S. Department of Health and Human Services introduced Healthy People 2020—and, subsequently, Healthy People 2030—which elevated the importance of research on strategies to intervene in health
disparities, including among pregnant women and children, by declaring “the elimination of health disparities” as a national goal.1
Taken together, these research endeavors have generated many new questions about when and how early childhood adversity is incorporated biologically to influence long-term outcomes and about optimal approaches to address early adversity and racial/ethnic and socioeconomic health disparities. These activities, and others, have pioneered and proliferated a new field of research on the importance of prenatal and early childhood experiences for health across generations.
Beyond scientific advances, From Neurons to Neighborhoods can also be credited as the impetus for the establishment of the National Scientific Council on the Developing Child in 2003, which uses science to catalyze public and private activities to promote the healthy development of children (Center on the Developing Child at Harvard University, 2014) to “close the gaps” between what the science says and which actions will promote child well-being. Composed of subsets of members of the From Neurons to Neighborhoods committee and the MacArthur Foundation Research Network on Early Experience and Brain Development, the Council has a central objective of communicating the science to the public and policy makers. Through an ongoing collaboration with the FrameWorks Institute on applying qualitative and quantitative research on communicating the science of brain and child development, a suite of effective “explanatory metaphors” has been developed, including a taxonomy of the stress response: positive, tolerable, and toxic stress (National Scientific Council on the Developing Child, 2014; Shonkoff and Bales, 2011). The term “toxic stress” was proposed to describe “excessive or prolonged activation of the stress response systems” in the absence of buffering protection from adult caregivers (National Scientific Council on the Developing Child, 2014). Unlike positive or tolerable stress, “toxic stress” refers to biological changes in the child that can result in disruption of developing brain architecture and other maturing organs, dysregulation of metabolic processes, and excessive stress system activation. These responses can lead to increased risk for chronic diseases later in life (Shonkoff et al., 2009). These explanatory metaphors have been adopted by the American Academy of Pediatrics (Garner et al., 2012; Shonkoff and Garner, 2012) and helped the science of child development be incorporated into federal and state legislation that generated evidence-based policies (Center on the Developing Child at Harvard University, 2014; Thompson, 2016).
This chapter highlights a selection of important scientific advances since From Neurons to Neighborhoods (NRC and IOM, 2000). Next, key
___________________
1 For more information, see https://www.cdc.gov/dhdsp/hp2020.htm (accessed June 17, 2019).
processes of healthy development from conception through early childhood are described, and some of the major biological responses to stressors are summarized. The chapter concludes with a discussion of individual differences in responsiveness and susceptibility. Throughout, the chapter identifies key points in development where disparities in health by race/ethnicity or socioeconomic status (SES) can emerge, which could inform strategies for prevention-oriented interventions.
The goal of this chapter is to provide essential information about the state of the science on human development from preconception through early childhood and some of the exciting findings in the past two decades within the fields of neurobiology, social psychology, epidemiology, and others that have contributed to advancing knowledge about how and when to intervene to improve outcomes for children. All of this information needs to be read through the lens of life course theory (Ben-Shlomo and Kuh, 2002), which recognizes that early experiences influence health outcomes within and across generations. It is also important to keep in mind the interconnectedness of brain and body health, as brain activity controls peripheral systems, which in turn affect brain
structure and function. The committee provides this evidence to help “bridge the gap” between what science tells us and what actions need to be taken—by policy makers, health care providers, educators, religious leaders, parents, and others—to close disparities and improve outcomes for all children in the United States. At present, many of the new scientific advances in neuroscience are still in development, and more study is needed to apply these new findings in clinical and public health practice and to use them to inform policies. In particular, greater effort and more support is needed to develop, implement, and evaluate programs based on scientific discoveries regarding the optimal timing for interventions. The committee could not be comprehensive with regard to coverage of all scientific advances, biological processes of healthy development, biological responses to stressors, and factors associated with individual differences, as each is a complex and active area of research.
SCIENTIFIC AND TECHNOLOGICAL ADVANCEMENTS SINCE FROM NEURONS TO NEIGHBORHOODS
The big questions driving research on child development and interventions to improve well-being are the same today as they have been for decades. “What are the influences of genes and experience on child health and development?” “To what degree does the brain exhibit plasticity (i.e., change over time)?” “What are the brain circuits that control specific kinds of behaviors, and how can their functioning be influenced through experience-based interventions and/or medications?” “Why does early experience have lasting disease risk and functional effects into adulthood?” “How does a child’s environment impact development of the brain, immune system, endocrine, metabolism, and cardiovascular systems, as well as physical and mental health?” (See Box 2-3 for highlights of applications of neurobiological research in practice.)
Much more is known today than two decades ago about the process of child development, and the explosion of research reports on child development since the publication of From Neurons to Neighborhoods (NRC and IOM, 2000) is remarkable. A PubMed search using just the term “early adversity” generated a list of 2,047 research articles,2 96 percent of which were published since 2001. Advances in several related fields of study have developed simultaneously to refine understanding of child health and development and the responses that each child may have to early adverse experiences. The following section outlines a selection of major developments, including genetics, transcriptomics, epigenetics, neuroimaging, computational modeling, maternal–fetal interactions, advances
___________________
2 Accessed November 12, 2018.
in longitudinal research, and the study of social interactions and physical environments. Refer to Box 2-2 for definitions of the key terms in bold below.
Genetics
Technological advances have led to cost-effective methods for capturing whole genome sequences. This has been completed for tens of
thousands of human genomes, highlighting remarkable genetic variation among all individuals. Genome sequencing also has revealed a small but consistent set of genes, including HSP-90 and FKBP5, both of which are involved in regulating responses to stress activation through the HPA axis. Genome sequencing led to the discovery of specific sequence variations, some of which alter gene expression and are correlated with specific psychiatric disorders and stress lability. The variations in these genes contribute to individual differences in vulnerability and heterogeneity in response to early adverse experiences (Criado-Marrero et al., 2018). For complex outcomes, such as psychopathology, researchers are increasingly exploring polygenic risk (i.e., the combined contribution of many genes that influence brain development and function) rather than single gene variants that have very small contributions to risk. One idea being tested is that polygenic risk “scores” may better predict an individual’s likelihood of a given outcome (Brikell et al., 2018). Genomics has developed in tandem with experimental animal models and clinical discoveries in humans to provide opportunities to study (1) genes that control the formation and physiological maturation of neural connections; (2) the molecules responsible for the onset and termination of sensitive periods; (3) the molecular basis for experience-driven synapse formation, elimination (pruning), and stabilization; (4) regulation of inflammatory responses; and (5) regulation of hormonal activation and feedback of the stress response (Bae et al., 2015; Geschwind and Rakic, 2013; Hensch, 2016b; Sudhof, 2018; Sudhof and Malenka, 2008).
Epigenetics3
Genomics discoveries were followed rapidly by advanced methods to identify the consequences of differences in how, where, and when specific genes are expressed—that is, the epigenetic (“above the genome”) profile of specific cells (Waddington, 1942, 1957). Initial discoveries recognized that epigenetic changes are chemical signatures (National Scientific Council on the Developing Child, 2010) placed on an individual’s inherited DNA due to environmental experiences and related contributing factors (e.g., nutrition, mental and physical stressors, immune activation, environmental toxicants, enriched environments) (Goldberg et al., 2007). Once thought to be limited in scope and immutable, there are millions of sites on DNA that can be chemically modified epigenetically, and the
___________________
3 For a more in-depth discussion on epigenetics and how the environment gets under the skin, see Chapter 3 of the 2019 National Academies report The Promise of Adolescence: Realizing Opportunity for All Youth by the Committee on the Neurobiological and Socio-Behavioral Science of Adolescent Development and Its Applications (NASEM, 2019).
modifications at these sites can be gained and lost rapidly, or, in many instances, remain stable over the lifetime (Goll and Bestor, 2005). Recent studies have shown that environmental experience influences the genome with specific molecular changes, such as DNA methylation and modifications to chromatin (Goldberg et al., 2007; Heim and Binder, 2012; Kalish et al., 2014; Kumsta, 2019; Miguel et al., 2019). While evidence of these molecular changes has been observed in many studies, the process by which these changes are controlled is complex and not completely understood. Exposures and experiences can result in profound epigenetic modifications that alter the timing and location of expressed or suppressed genes (McEwen, 2017; Miguel et al., 2019). For instance, epigenetics is one mechanism that results in identical twins exhibiting distinct physical and mental traits. Preliminary data suggest that epigenetic changes may explain how early experiences get “under the skin” of individuals for whom physical and brain-related disruptions and disease risk can last a lifetime (McEwen, 2017; Miguel et al., 2019). Research shows that epigenetic changes that alter the DNA chemistry in germ cells of adult humans may be one way that experiences are passed on to the next generation through their children (Donkin et al., 2016). However, the best evidence for such intergenerational transmission comes from animal studies. Epigenetic changes occurring during preconception or during pregnancy can impact the germ cells of the offspring and result in intergenerational transmission of the parental experiences (Weber-Stadlbauer, 2017). Moreover, it has been hypothesized that germ cells of the fetus may be altered during pregnancy, meaning that the mother, and even the child when that child is grown and has children of his or her own, can pass on influences that occurred several generations previously (Bale, 2015; Rodgers et al., 2015; Rowold et al., 2017).
Transcriptomics
Keeping in mind that one’s entire genome is in every cell of the body, the study of transcriptomics uses methods to identify how particular genes are expressed over time, in specific types of cells, and under specific circumstances (Li et al., 2018). New methods facilitated the organization of a research consortium that led to a remarkably detailed, open-access atlas4 of gene expression in 16 different human brain areas during pre- and postnatal development and into adulthood, building a unique bridge between human and model system brain research (Carlyle et al., 2017; Enoch et al., 2014; Li et al., 2018; Vied et al., 2014). For example, the expression of genes responsible for producing the thousands of neuron types
___________________
4 For more information, see http://www.brainspan.org (accessed June 16, 2019).
in the brain is enriched during the first trimester of pregnancy. As development continues, many of those genes are turned off because neuron production ceases, and other genes are newly expressed that control the initial wiring and building of the unique architecture of the brain. After birth, genes are turned on to cause the rapid formation of synapses, pruning, and the signaling between neurons to process more and more complex information. The ability to relate specific events in development to the changing transcriptome has been accomplished by creating enormous databases of gene expression in the pre- and postnatal human brain and in experimental animal models. These new data offer a detailed directory of gene expression in space and time in the complex and rapidly changing developing brain, thereby providing the framework for ongoing research to determine how the interplay of genetic and environmental factors and experience alters the gene expression that contributes to typical or atypical developmental maturation.
Neuroimaging
Human and animal neuroimaging have provided the basis for a new understanding of the brain connectome, which defines the breadth and complexity of circuits that relay information between ensembles of neurons. New technologies provide unprecedented resolution of the organization of the connectome in humans, painting a far more complex picture that begins prenatally as early as the third trimester of pregnancy (Kostović et al., 2014; Krsnik et al., 2017) and continues in infancy and toddlerhood as remarkable periods of growth for the brain. Functional maturation of circuits continues throughout childhood, but connectome research, combined with genomics, has identified adolescence as a second period of dynamic changes in brain wiring.5 In humans, it is now understood that while classic diagrams of brain development present a well-delineated blueprint, there is far more variability in infants and toddlers than expected in the timing and extent of development of connectivity within the standard connectome blueprint. This basic neuroimaging research in humans has provided a foundation for determining the impact of disparities resulting from poverty and other social factors on the trajectory of brain growth and development of circuitry (Gabrieli and Bunge, 2017; Hanson et al., 2013; Johnson et al., 2016; Luby et al., 2012). Given the vast heterogeneity between individuals in terms of brain development, drawing generalizations about the overall connectome and its influence on social, emotional, and cognitive maturation remains a key research challenge.
___________________
5 For a comprehensive discussion of the developing adolescent brain, see NASEM, 2019.
Maternal and Fetal/Infant Interactions
Prenatal development is marked by a unique relationship between the mother, the fetus, and a transient organ, the placenta. Once thought of as a filtration system of cells contributed mostly by the fetus and blood vessels from both mother and fetus to control the passage of nutrients and other substances, the placenta has been shown by current genomic, cell biological, and physiological evidence to be an active partner in fetal development and influenced by maternal health. For example, maternal immune activation or persistent stress during pregnancy can alter placental cell gene expression and function (Hoffman, 2016). Depending on the stage, the placenta expresses 40 to 60 percent of the human genome and produces cytokines, hormones, neurotransmitters, and growth factors that are necessary for healthy fetal development (Gonzalez et al., 2018; Jones, 2019; Paquette et al., 2018; Zhao et al., 2019). These genes are subject to environmentally regulated epigenetic changes; exposure to toxicants, nutrient status, high stress, or infection can result in altered gene expression through epigenetic changes (Bale, 2015). Thus, while the organ clearly is involved in regulating transport of maternally produced substances, the placenta also serves as a critical resource for organ development and maturation of functions to ready the fetus for birth (Bonnin and Levitt, 2011; Bonnin et al., 2011; Goeden et al., 2016; Nugent and Bale, 2015). Developmentally, the maternal–placental–fetal relationship begins between the third and fourth week postconception through a complex process of extraembryonic cell differentiation and vascularization that results in implantation in the maternal uterus. Given that the main functional cell of the placenta, the trophoblast, is embryonic in origin and that there are thousands of genes expressed in the placenta, any gene mutation that could functionally disrupt the embryo also can impact placental function (McKay, 2011).
Intergenerational Transmission of Risk
Evidence from both animals and humans indicates that adverse social/psychological and physical exposures in one generation can alter risks for psychopathology, maladaptive behavior, and chronic disease in the next generation (i.e., the children of individuals who endured severe threat have elevated rates for psychiatric disorders and dysregulated physiology, despite an absence of direct exposure) (Boyce and Kobor, 2015; Burton and Metcalfe, 2014; Franklin et al., 2010; Matthews and Phillips, 2012; Santavirta et al., 2018; Yehuda and Bierer, 2007; Yehuda et al., 2014). For example, research has documented that (1) adolescents born to women who experienced severe child abuse are at higher risk of smoking and overweight/obesity (Roberts et al., 2014), (2) there is altered cortisol expression among
children who were in utero at the time of the 2001 World Trade Center attack in New York City (Yehuda et al., 2005), and (3) there are differences in stress reactivity in children of women affected by the Dutch Hunger Winter (Painter et al., 2006). These excess risks may emerge through a variety of mechanisms that are not mutually exclusive, such as altered parenting behaviors, lower socioeconomic position, epigenetic pathways (described above), or an altered fetal environment.
Adverse experiences before and during pregnancy can influence maternal stress-related physiology, including immune activation and endocrine disruptions (Entringer, 2013; Entringer et al., 2010, 2015; Hantsoo et al., 2019; Howerton and Bale, 2012). Increasing evidence suggests that maternal immune and endocrine activity during pregnancy (including the under- or overproduction of cortisol and levels of pro- or anti-inflammatory cytokines) are associated with adverse birth outcomes (Bastek et al., 2011; Wadhwa et al., 2011), the child’s brain (Buss et al., 2017) and cognitive development (Gilman et al., 2017; Rudolph et al., 2018), and future risk for chronic disease (Entringer et al., 2013, 2015), providing support for embryonic and fetal life as sensitive periods for the intergenerational transmission of adverse maternal experiences (Buss et al., 2017; Entringer et al., 2010; Meaney, 2001). Much of this work is preliminary, and further research needs to be conducted before drawing concrete conclusions about intergenerational transmission of risk. At present, there is a limited understanding of how plastic or modifiable these intergenerational risk factors are and why some individuals are resilient to intergenerational risks whereas others are not. This is an area of active research that has elevated the importance of applying the life course approach to health and development, and by further understanding the pathways of both risk and resilience, scientists may uncover social, behavioral, or pharmacologic interventions that can interrupt the intergenerational transmission of the effects of stress on the developing embryo and fetus. Later in the chapter, the section on Biological Mechanisms of Healthy Development presents a more extensive discussion on the role of the placenta and how it can be influenced by social experiences.
Computational Methods
There has been a rapid increase in the capacity to collect and analyze enormous datasets with social and biological information. These datasets may be developed through research consortia that can involve dozens of investigators from around the world or from the linkage of administrative files, de-identified electronic health records (McGregor et al., 2013; Roden et al., 2008), and other sources (McCarty et al., 2011). In parallel, engineers and computer scientists have advanced sophisticated computational
methods, including signal processing and machine learning, to extract predictable patterns that relate to outcomes of interest (Krishnan et al., 2017; Tseng et al., 2013; Van Essen et al., 2013). For example, connectomics and genomics have depended heavily on these methods, and the rapid advances in collecting physiological data from children (electroencephalogram [EEG], eye tracking, wearable devices to monitor physical activity, sleep habits, stress, and metabolic activity) also have benefited from the advanced analytical methods that help identify patterns of maturation of brain architecture and function (Bagot et al., 2018; Frazier et al., 2018; Hosseini et al., 2016; Medland et al., 2014; O’Driscoll et al., 2013; Wee et al., 2017). These measures have been used to predict risk for behavioral and cognitive disturbances later in development. Relatedly, there is growing interest in using machine learning and predictive risk modeling methods to identify children in adverse environments and predict risk or resilience (Amrit et al., 2017; Gillingham, 2016; Schwartz et al., 2017). For example, advanced analytical methods have been applied to produce highly sensitive and specific metrics for the prospective diagnosis of fetal alcohol spectrum disorder (Zhang et al., 2019). However, there is still much more research needed to determine how well these methods may be applied to determine underlying causal mechanisms. Finally, meta-analytic approaches are using new analytical methods to synthesize the results of multiple independent studies and are now widely employed to inform evidence-based recommendations, policies, and programs for children. For example, meta-analyses have been used to summarize the effects of center-based early education (Grindal et al., 2016; Schindler et al., 2015) and parenting programs (Casillas et al., 2016; Chen and Chan, 2016).
Advances in Longitudinal Research
Investments by the National Institutes of Health, the U.S. Department of Education, numerous private foundations, and other agencies have resulted in a substantial growth in child development research using longitudinal data from prospective cohort studies with large subgroups of racial/ethnic minority children and stratified-probability samples. These studies have documented more extensively the pervasiveness of health disparities in these populations and the mechanisms by which these disparities arise. For example, the Eunice Kennedy Shriver National Institute of Child Health and Human Development supports the Fragile Families & Child Wellbeing Study, a birth cohort of nearly 5,000 children in 20 large U.S. cities that used a stratified random sample with an oversample of nonmarital births (McLanahan et al., 2003). Baseline interviews for this study occurred between 1998 and 2000, and follow-up is still ongoing. The Institute of Education Sciences, an independent evaluation arm of
the U.S. Department of Education, conducts the Early Childhood Longitudinal Study program, which has generated large representative cohorts for studies of child development for the United States, from birth onward (Tourangeau et al., 2009).
Longitudinal studies are critical to understanding both normative developmental processes and those involving deviations from the norm. These studies facilitate examination of changes across development within individuals and subgroup differences in within-person changes over time (Lerner et al., 2009). Prospective studies are critical for documenting what changes—in brain, behavior, or health—are due to normal developmental progression and what changes are caused by chronic family-level stressors, structural inequalities, and/or positive environmental stimuli. Longitudinal data, combined with advances in statistical methods for identifying underlying pathways, have made it possible to show the lasting impact of early adverse experiences and to ask questions about mediating processes that underlie the association between early childhood adversity and poor health or educational outcomes (Danese et al., 2009; Iacono et al., 2008; Poulton et al., 2015). In parallel, longitudinal data have shown that for children who have been exposed to severe adversity, such as maltreatment, providing appropriate care can reverse early damage, even to biological systems, such as brain functioning (Cicchetti and Curtis, 2006; Cicchetti and Handley, 2017).
In spite of these advances, it is important to note that U.S. investment in large birth cohort studies lags severely behind European countries. One new study, launched in 2016, is the Environmental influences on Child Health Outcomes (ECHO) program. It is a 7-year research initiative focused on understanding the effects of environmental exposures on child health and development, and it draws on existing U.S. cohorts. The cohort studies that compose ECHO collectively include approximately 50,000 children and will address environmental exposures in relation to four pediatric outcomes with high relevance to public health: pre-, peri-, and postnatal outcomes, neurodevelopment (i.e., cognition, emotion, and behavior), upper- and lower-airway function, and obesity.
Of note, many longitudinal studies of child development have been the product of interdisciplinary collaborations, which have become increasingly common. An example is the long-term follow-up of the Perry Preschool6 project, which started in the 1960s (Gramlich, 1986; Schweinhart et al., 1985). This longitudinal study of the effects of a randomized controlled trial is the result of a long-term collaboration between educational researchers and practitioners, developmental psychologists,
___________________
6 For more information, see https://highscope.org/perry-preschool-project (accessed March 29, 2019).
and economists. The latest follow-up of the intervention administered at 3 and 4 years of age still yields positive results on the life trajectory of its participants at age 40, including more positive health outcomes, improved employment trajectory, and less crime involvement (Belfield et al., 2006; Social Programs That Work, 2017). Consistent with these findings, the Carolina Abecedarian Project—a randomized study of an early childhood intervention for economically disadvantaged children with long-term follow-up—found that children randomly assigned to the early intervention displayed lower cardiovascular and metabolic risk factors in their mid-30s, with effects most pronounced for men (Campbell et al., 2014). Baby’s First Years,7 launched in 2018 and funded by both private and public grants, is a new longitudinal study that seeks to establish causal links between parental income level and brain development in very young children.
Advances in the Study of Social Interactions
Since the publication of From Neurons to Neighborhoods, researchers have introduced a number of novel topics and measurement approaches that have advanced the understanding of early development and consequences for later physical and mental health outcomes. In particular, there has been a shift of focus to the well-being of salient caregivers as “a dependent variable” in research and associated interventions. In other words, rather than continuing to report (as psychologists had over decades) that a good relationship with the primary caregiver is the single most important protective process in promoting resilience among children whose life circumstances render them at risk, there is now explicit focus on the question of what it is that helps parents maintain good parenting when they are struggling with high ongoing stress. How can we promote resilience among the adults primarily responsible for raising children in stressful life circumstances? (For reviews, see Luthar et al., 2015, and Luthar and Eisenberg, 2017.) Relatedly, intervention approaches are going beyond simply teaching parents what they should and should not do in didactic parenting classes or even imparting approaches to regulate their own affect (via mindfulness or trying to cope effectively with their own ongoing life stressors). Increasingly, programs are also seeking to provide the support that parents need to successfully negotiate the many challenges in sustaining good parenting behaviors with multiple children and across multiple decades (for examples of such programs, see Corso et al., 2015; Kaminski et al., 2013; Luthar et al., 2007, 2017).
___________________
7 For more information, see https://www.babysfirstyears.com (accessed April 18, 2019).
An emerging field of research suggests that there is a biological basis to healthy caregiver–child relationships and that caregiver–child synchrony in biological processes such as EEG or heart rate variability can help children to develop control of their behavior and emotions. The science of caregiver–child synchrony advances previous views that a child’s central nervous system is primarily responsible for developing self-regulation (Welch, 2016). This research suggests that children display dysregulated behavior due to deficient coregulation from nonoptimal caregiver relationships and that coregulation can be improved via interventions, such as the Family Nurture Intervention, developed by Martha Welch and colleagues (2014, 2015). Although there are a number of neuroendocrine changes that occur during the process of parenting, a substantial amount of this research has focused on oxytocin measures in the infant and caregiver. In addition to oxytocin’s roles in the birthing process, lactation, and maternal care in mammals, studies have demonstrated that oxytocin (and its close neuropeptide relative vasopressin) facilitates affiliative social behavior through modulation of the activity of specific forebrain circuits (Feldman and Bakermans-Kranenburg, 2017; Hammock et al., 2005; Insel, 2010b; King et al., 2016; Numan and Young, 2016). Both observational and experimental research studies suggest that oxytocin influences the synchrony between caregivers and children, and it is also associated with sensitivity of caregiving and amount of contact (Feldman and Bakermans-Kranenburg, 2017). Researchers are exploring how specific caregiver health conditions (e.g., postpartum depression) or social experiences (e.g., traumatic experiences in early childhood) influence oxytocin, other neuroendocrine hormones related to nurturing behaviors, and caregiver–infant synchrony and how interventions may target these processes to improve caregiver–child relationships.
Another major advancement is that there has been a growing appreciation for the role of culture for the development of children (Coll et al., 1996). In a study of children and families in the United States outside of the white middle class (i.e., minority families based on race/ethnicity, immigrant status, sexual orientation, and others at both SES extremes) there has been a growing emphasis on potent culturally specific risk and protective factors and processes (see Velez-Agosto et al., 2017), especially those that can be harnessed in beneficial interventions. As an example, American Psychologist devoted a special issue to marginalization, defined “as a multidimensional, dynamic, context-dependent, and diverse web of processes, rooted in power imbalance and systematically directed toward specific groups and individuals, with probabilistic implications for development” (Causadias and Umaña-Taylor, 2018). The special section contains a rich collection of articles addressing issues related to various marginalized groups, including recent immigrants and youth from different
ethnic and racial minority groups. In another recent special section in Research in Human Development, Cunningham (2019) compiled papers addressing “myths and realities associated with human development research and theorizing” encompassing
diverse perspectives on sexual minority youth, resilience and risk for youth in high-achieving schools (HASs), a reconceptualization of hostility in African American parenting styles, a critical examination of diversity and contact for students attending racial/ethnically diverse schools, and a thoughtful consideration of contextual factors associated with aggressive attitudes and prosocial behaviors in African American males. (Cunningham, 2019, p. 1)
Many of these studies move the conceptualization of “children or family at risk” to “children and family living in at-risk conditions.” The “problem” to be “fixed” is not the child and/or family but the at-risk conditions within which they live, an important paradigm shift for early identification and prevention. (On a related topic, there have also been advances in connecting racism to child and adult health outcomes; see Box 2-4.)
Advances in the Study of Physical Environments
Although the family remains a major determinant of the child’s outcomes, studies following more comprehensive theoretical models (e.g., Bronfenbrenner, 1979; Bronfenbrenner and Evans, 2000; Ferguson et al., 2013) have documented the importance of neighborhood contexts on family interactions and child development. Neighborhood context can be characterized as positive and negative, and it can reflect the physical or built environment and psychosocial characteristics. It has become increasingly common for researchers to link measures of neighborhood social and structural environments to individual-level data on children. As long as families are willing to share an address, researchers are able to draw on information from the Census and other free and proprietary data sources in order to characterize a child’s social and physical environment. When this field of research began, most studies focused on neighborhood SES (e.g., the proportion of the population in the Census tract that is below the federal poverty line), as this measure is associated with the physical infrastructure of the community, noise and pollution, amenities such as parks and groceries, and availability of health centers and early childhood education (Evans, 2004; Evans et al., 2010). However, use of neighborhood SES as a proxy for neighborhood quality and conditions does not provide sufficient information about the mechanisms of action—that is, the specific pathway through which neighborhoods influence child health. The majority of research on neighborhood SES and health is observational, but
there are a few exceptions, including the Moving to Opportunity study (Chetty et al., 2016; Ludwig et al., 2011).
The Project on Human Development in Chicago Neighborhoods (PHDCN)—an interdisciplinary study that began in 1995 and aimed to explore how families, schools, and neighborhoods influence child and adolescent development—was among the first to provide an intensive investigation of neighborhood conditions related to economic, social, organizational, political, and cultural structures and how communities change over time. The investigators conducted systematic social observations by videotaping one side of each block within the selected 80 Chicago neighborhood clusters, and they maintained observer logs about the activities of residents and the presence of detracting elements (e.g., garbage, abandoned cars). This study identified neighborhood collective efficacy and social capital as important aspects of neighborhood social organization for child and adolescent outcomes (Sampson and Raudenbush, 1997, 1999; Sampson et al., 1999).
Since PHDCN, researchers have begun to characterize neighborhood social and physical information for health research using Google Street View (Rundle et al., 2011; Rzotkiewicz et al., 2018), which has the benefits of being lower cost and more efficient, although it can be limited by resolution and availability for a given temporal period. There has also been a substantial amount of research to examine child health in relation to neighborhood violence or crime, which can be derived from police crime reports (Beck et al., 2016; Goldman-Mellor et al., 2016; McCoy et al., 2015). Some studies use surveys to assess parental perceptions of neighborhood safety (Christian et al., 2015). Other research has focused on the physical neighborhood environment, such as opportunities for physical activity, access to healthy food, density of fast food, air quality, and presence of natural environments (e.g., green space) (Gascon et al., 2016; Gorski Findling et al., 2018; McCracken et al., 2016; Sallis et al., 2018). While some studies use single measures of neighborhood context, other studies rely on more complex indexes, such as the Child Opportunity Index (Acevedo-Garcia et al., 2014, 2016). This measure—developed by diversitydatakids.org and the Kirwan Institute for the Study of Race and Ethnicity—combines 19 component indicators related to education, health and environment, and social and economic context to rank neighborhoods (defined by Census tract) to other neighborhoods within the metropolitan area (Acevedo-Garcia et al., 2014; Beck et al., 2017a,b).
BIOLOGICAL MECHANISMS OF HEALTHY DEVELOPMENT
Introduction
Development is an interplay between biology and the profound impact that experiences have on biological systems. There are basic biologically driven mechanics that involve the production and specialization of cells that serve as the ingredients to build different organ systems, such as the brain, heart, digestive system, and kidneys. These systems are ultimately responsible for performing specific functions and become specialized over different periods of developmental time. This developmental process begins prenatally, and for the most complex organ—the brain—it extends into young adulthood. The developmental process is designed to promote maximal functioning and survival of the individual. The challenges an individual faces from infancy to adulthood are numerous and varied, and through the powerful biology–experience relations, development establishes both functional and adaptive capacities in order to adjust to the environment and to future experiences. Optimal functioning and robust adaptability to the environment are signatures of healthy development. No matter which organ system or cell type is considered, a core principle of development involves the regulation of the expression of the 23,000 genes in each person’s genome at specific times, and in specific combinations, in order to achieve optimal functioning of each organ system.
It is important to re-emphasize here that the genome is composed mostly of regions of DNA that do not code for proteins but rather are responsible for using information from experiences and the environment to regulate gene expression. Gene expression is the first step toward producing proteins, which are the primary functional units of specialized cells that characterize each organ system. Thus, while all cells contain identical genetic material (DNA code), gene expression and production of proteins varies greatly between types of cells, which specialize during development to perform specific functions. Because the brain is massively complex, with billions of neurons and trillions of connections, at least 85 percent of the genome is expressed at various times and locations during development (Negi and Guda, 2017), whereas other organ systems may express far fewer genes in order to perform their functions. This orchestrated temporal and spatial expression of genes depends on maintaining healthy cellular homeostasis and accessing nutrients that serve as the building blocks for proteins and eventually organ systems. Research has shown that regulation of fundamental metabolic processes, including mitochondrial function in the brain and periphery, is essential for cellular adaptations to developmental psychosocial stress that can impact mental and physical health (Eisner et al., 2018; Picard and McEwen, 2018). The
most productive means to facilitate early healthy development include providing access to healthy nutrition, avoiding factors that can be detrimental to nutritional status for mother and fetus over the course of pregnancy, limiting chronic stress, and offering access to quality health care. Proper nutrition is needed postnatally through childhood as well. Moreover, disrupting the fetal–placental–maternal biological relationship through infection, toxicants, alcohol, nicotine, or poor diet increases risk for altering a typical, healthy developmental trajectory. Thus, improving physical environments to limit exposure risk and providing access to high-quality maternal health care during pregnancy is an additional essential ingredient for healthy development. These well-researched factors make up a strategy for viewing early development through a “prevention lens” that ultimately leads to reduced risk for later physical, mental, and cognitive disorders.
Recent studies, mostly in animal models, indicate that the developmental process starts even prior to conception (Chan et al., 2018). Certain stressors, including psychosocial factors, toxicants, or drug exposure, can impact reproductive cells through an epigenetic process that ultimately may alter the expression of the genes that are inherited by the embryo. This suggests that parental behavior may impact how a child’s genes are ultimately expressed and therefore what the child’s biology and behavior will be. This transgenerational impact on development requires studies in humans before firm conclusions are made, but the research indicates that maternal and paternal experiences prior to conception may be an important factor to consider for programs that promote family health and welfare.
The following sections outline how several biological systems, those most relevant to this committee’s report, develop between gestation and puberty and serve as a baseline for understanding how early environmental stressors and adversity affect child development and the emergence of health disparities: maternal physiological adaptations during pregnancy and the nervous, immune, endocrine, and reproductive systems. Note that in addition to psychosocial stressors in the environment of a developing child, adversity may include limited or no physical activity; exposure to toxicants, including alcohol and nicotine; and chronic disruption of bodily physiological functions, such as sleep (Feldman et al., 2014; Jones et al., 2019; McEwen and Getz, 2013; Thompson et al., 2009). Each of the following sections describes typical processes from the prenatal period through early childhood and how the system may be impacted by experiences that disrupt typical development.
Maternal Physiological Adaptations During Pregnancy
Remarkable changes take place in women during pregnancy to accommodate the needs of the developing fetus. A woman’s heart pumps out 30 to 50 percent more blood every minute, while her lungs take in more air with each deep breath, in order to deliver oxygen and nutrients to the baby (Cheung, 2013; Costantine, 2014). Her kidney functions also improve by 50 percent to filter out more waste products and toxins from the blood (Cheung and Lafayette, 2013). Her immune system is dampened to avoid rejection of the fetus, and she becomes progressively insulin resistant, which allows an increasing amount of blood glucose to stay in the bloodstream to nourish the growing fetus.
A comprehensive review of maternal adaptations is beyond the scope of this report, but it is important to note that how well a woman’s body can adapt to these physiological changes during pregnancy is closely related to her overall health going into the pregnancy. For example, a healthy heart can readily adjust to the increased workload, but a diseased heart will have a much more difficult time; heart disease is a leading cause of maternal death in the United States. Pregnancy predisposes blood to clotting, but the risk of severe complications from thromboembolism and pulmonary embolus is substantially increased in women with preexisting conditions, such as hypertension, diabetes, or obesity. Pregnancy causes a physiological anemia, which can be exacerbated by ongoing iron deficiency, thereby increasing the risk of having a low birth weight baby. The insulin resistance in pregnancy is made worse if a woman enters pregnancy overweight, obese, or with preexisting diabetes mellitus; fetal exposure to excess blood glucose has been associated with adverse birth outcomes and obesity and diabetes later in life (Garcia-Vargas et al., 2012). As we will discuss in Chapter 5, advancing health equity in birth and child health outcomes first requires improving preconception health for all women.
One of the most important adaptations during pregnancy is the formation of the placenta, an organ that is responsible for controlling access of maternal-derived factors to the fetus and also serves as a source of growth factors and other molecules that support healthy fetal development. The placenta begins to form very early in pregnancy, and disruption of this process has been associated with pregnancy complications, such as intrauterine growth restriction and preeclampsia (Sharma and Sharma, 2016). At term, approximately 600–700 milliliters of blood flows through the placenta, delivering oxygen and nutrients to the baby (Wang and Zhao, 2010). The placenta also acts as a critical regulator of maternal–fetal interactions and resource allocation. For example, if a pregnant woman experiences acute stress, the placenta shields the fetus from overexposure to maternal cortisol by turning up the activity and expression of
a placental enzyme called 11b-hydroxysteroid dehydrogenase type-2 (HSD11B2), which inactivates cortisol as it passes through the placenta. However, chronic stress has been found to be associated with reduced activity and expression of placental HSD11B2, suggesting that the placenta’s built-in ability to limit fetal exposure to maternal cortisol may be diminished in the face of chronic stress (Cuffe et al., 2012; O’Donnell et al., 2012; Welberg et al., 2005).
Nervous System Development
The neurobiological processes that build the brain share some common elements with other organ systems, such as producing specialized cells (e.g., neurons and glia) with functions specific to that organ system. However, understanding of neurodevelopment lags behind the understanding of other organ systems, in large part because the brain is extraordinarily complex. Experimental studies in model systems have identified basic mechanisms and the genes that are involved in specializing areas of the developing brain into what will make up function regions, the production of a diversity of neuron types, and the initial wiring of circuits that occurs prenatally (Kast and Levitt, 2019; Kolodkin and Tessier-Lavigne, 2011). Identification of mutations in some of these same genes in humans has validated the highly conserved nature of the ingredients that are responsible for the initial brain blueprint (Doan et al., 2018; Geschwind and Rakic, 2013; Jayaraman et al., 2018; Rubenstein and Rakic, 2013). Genes and their protein products involved in later events, including the extended period of synaptogenesis (the formation of synapses), have been identified and studied experimentally (Akins and Biederer, 2006; Favuzzi and Rico, 2018; Sudhof, 2018). For all of the advances in the basic understanding of neurodevelopment, there is a knowledge gap in determining the many ways in which the environment and experience are woven into the developmental process. Figure 2-1 is a diagram showing development from the first trimester to puberty, with respect to both neurodevelopment and the development of other biological systems.
Within a few weeks of conception in the first trimester, neurons begin to be produced. By 10–12 weeks gestation, most of the neurons that make up the brain are generated (Bystron et al., 2008; Stiles, 2008). Specific types of neurons in structures like the cerebellum, hippocampus, and olfactory bulb continue to be produced prenatally. Unlike many other types of cells in the human body, once formed, neurons lose the capacity to renew themselves, even if injured (a small number of neurons in the olfactory bulb and hippocampus represent limited exceptions). Because the neurons are “born” at sites in the developing brain that are different from their final position, all neurons migrate using a combination of mechanical guides,
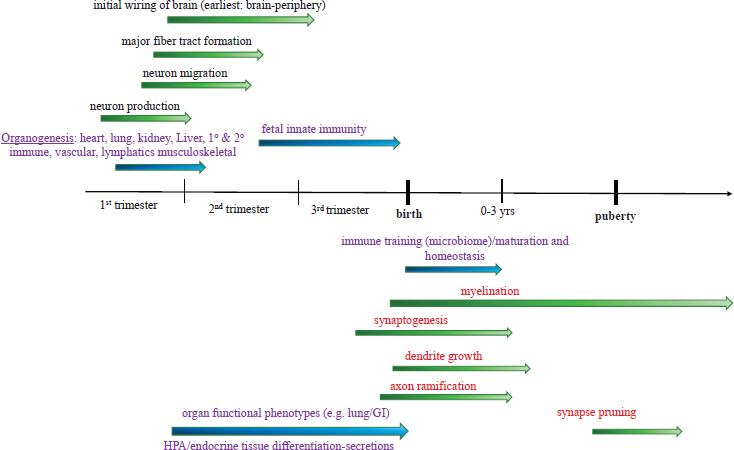
NOTES: Arrows represent time frames of approximate onset (darker shading) and completion (lighter shading) of specific developmental events for neural (green) and nonneural (blue) systems. For the neural components, above the line represents events that occur within the prenatal period, and below the line represents events that extend into postnatal periods. GI = gastrointestinal; HPA = hypothalamic-pituitary-adrenal axis.
the extended processes of radial glial cells, and molecules that serve as guidance cues. This migration process begins as soon as neurons are produced, and as the brain grows through the second trimester, the later-produced neurons take longer to reach their final position. This developmental process is further complicated because newly formed neurons not only migrate but simultaneously extend a long process—the axon—that will connect each neuron to its appropriate target. At the same time, the receiving end of the neuron—the dendrite—develops but takes much longer to reach maturity, in sync with the formation of individual neural connections—the synapses. Throughout each of these developmental stages, additional sets of genes are expressed that drive a specific process to completion. Many of the earliest born neurons will regulate peripheral organ function (e.g., heart, lungs, gastrointestinal tract). Connections are formed between these neurons and their targets in the first trimester as the targets continue to develop. By the beginning of the second trimester, long connections from the hypothalamus to the autonomic brain stem are
formed, providing the capacity for top-down regulation of what are called the sympathetic and parasympathetic nervous systems—in essence, the systems that control the “fight or flight” and “rest and digest” responses.
By the second and third trimesters, sensory systems are connected from the periphery to the brain’s cerebral cortex—the most complex part of the brain with the greatest neuronal diversity, which processes complex information from all of the senses. Toward the end of fetal development in the third trimester, the basic blueprint of neuronal connections is initiated, during a temporally extended process of synapse formation that continues through 2–3 years postnatally (Levitt and Eagleson, 2018; Silbereis, 2016). The human neocortex contains approximately 20 billion neurons, each with an average of several thousand synapses and connected with thousands of kilometers of dendrites and axons (Tang et al., 2001).
By birth, sensory systems are sufficiently wired to be able to take in information from the infant’s environment and begin to process the most salient stimuli (e.g., visual, tactile, and auditory stimuli of the primary caregiver that are experienced in temporal lockstep with satisfying the hunger, thirst, and warmth needs). The nervous system continues to build by adding more synapses, ultimately reaching its greatest synapse density during toddlerhood. Experimental studies in animals show that experiences can influence the initial organization and sheer number of synapses (Berbari et al., 2005; Turner and Greenough, 1985). In early human infant development, differences in growth parameters of gray and white matter have been shown to be influenced by experience in the human cerebral cortex (Brito and Noble, 2014; Hair et al., 2015), which assumes synaptogenesis changes due to experience. In humans, more than 1 million synapses are added per second for this 2–3-year period (Center on the Developing Child at Harvard University, n.d.-a). During early childhood, and as an individual approaches the onset of puberty, the number of synapses remains relatively stable, but this does not imply that no developmental activity is taking place. Instead, experience-driven processes impact maturation by influencing which specific genes are expressed and therefore how synapses process information. Over time, synapses that become activated more frequently and in concert with other synapses processing the same information become more stable and better at processing; those that are used less, on the other hand, are eliminated (Katz and Shatz, 1996). Remarkably, both early spontaneous and patterned activity (from sensory stimuli in the environment) influence synapse formation and stabilization (Leighton and Lohmann, 2016). In all mammalian species examined, including humans, synapse pruning (i.e., the controlled elimination of certain synapses) in the cerebral cortex begins before puberty, with a reduction of approximately 40 percent by the end of adolescence (Bourgeois, 1997). A core concept that has emerged from decades of neurobiological studies of the development
and reorganization of connectivity is that environmental factors, which include fetal, postnatal, and child experiences, regulate the expression of genetic predispositions, and these experiences can dramatically change outcomes. This underlies the mechanism through which early exposure to chronic stressors and trauma so powerfully influences developmental processes in the brain and periphery. The research also makes clear that brain development and maturation depends on experience—what Greenough labeled “experience-expectant” development (Greenough et al., 1987).
Myelination is an often overlooked developmental process. Myelination entails forming the insulation around axons and is essential for increasing the capacity for rapid and efficient transmission of information in neural circuits (Bercury and Macklin, 2015). During the entire postnatal period discussed above (Gogtay et al., 2004), the glial cells responsible for myelination (Miller et al., 2012)—the oligodendrocytes in the brain and Schwann cells in the periphery—become the most active, forming the sheath around axons that promotes more rapid signaling between neurons. Myelin is composed mostly of protein and lipids (i.e., fats) to form the ensheathing membranes; myelination is highly dependent on nutritional status, experience, and other environmental factors (McLaughlin et al., 2017). Neurons are among the most metabolically active cells in the body, so they too are highly dependent on nutritional status. Myelination begins and ends in different regions of the nervous system at different times postnatally (Yeung et al., 2014), with the axon tracts beneath the frontal lobes taking the longest; myelination in this region can extend into the third decade of life (Miller et al., 2012). The timing of myelination completion corresponds to the reduction or ending of sensory critical periods, suggesting that myelin not only facilitates improved information processing but also participates in the maturation of synapse networks. This correlation has been tested experimentally by reversing myelination, resulting in reopening the critical period (McGee et al., 2005). This suggests that for human brain regions that have long periods of circuit myelination (such as frontal-lobe-associated circuits, in which executive functions continue to develop into young adulthood), extended periods of plasticity continue (Diamond, 2013).
Plasticity
The circuits of the brain undergo initial construction prenatally and remain relatively immature until postnatal periods, when experience drives remarkable growth that is reflected in formation of extensive connections (synapses) that form at a rate of at least 1 million per second during the first 2 years (Center on the Developing Child at Harvard University, n.d.-b,c). The role of experience in the development of functional
circuits was established half a century ago in landmark studies of the visual system (reviewed by Hensch, 2016b; Hensch and Bilimoria, 2012). Binocular vision requires the use of both eyes by the infant to form an accurate representation of the visual world. This occurs during a sensitive or critical period, when there is heightened plasticity for changes in the fine details of circuits that will establish functioning for a lifetime. Since that time, neuroscience research has shown that all sensory and motor circuits, as well as those involved in social and emotional behaviors, cognition, motivation and reward, executive functioning, and even stress responsiveness, develop through experience-dependent mechanisms that involve heightened plasticity. Moreover, circuits remain open to change for different periods of time—sensory functions that are essential for the infant and toddler to perceive and respond to the environment are built first, followed by gross and fine motor and basic cognitive functions that provide the baby with opportunities to respond in more complex ways to experiences that provide input to the brain. This concept reflects the basic developmental rule that simple skills beget more complex skills, the paramount exemplar being language: from phoneme discrimination, to motor imitation, to making sounds that become part of a repertoire in which sounds become associated with objects, to objects becoming associated with words as the child begins the process of integrating language with more complex cognitive skills. It is important to emphasize that certain experiences, including physical exercise, are not necessarily limited in impact within certain periods and can be drivers of brain plasticity across the life-span. The evidence in basic and clinical research is compelling, showing effects on both physical well-being and cognitive and social-emotional capacities (Khan and Hillman, 2014; McEwen and Getz, 2013).
For all of these steps, there are heightened sensitive periods when experiences that occur over and over are the most potent. Periods have a beginning, a peak of varying length, and then a stretch of reduced plasticity when change can occur but in a far more limited fashion and with much greater effort. Think about learning a second language for the first time as an adolescent or adult. For all circuits that will be responsive to either positive or negative experiences, critical period shifts can occur that result in more rapid maturation or longer times of remaining open. Neither outcome of changes in plastic periods is beneficial. Note that genetic factors can also change circuit maturation (Heun-Johnson and Levitt, 2018), which then causes a different response to early experience, as if events were occurring in an older individual. In a model of early adversity (Bath et al., 2016), precocious maturation of molecular and behavioral measures was induced by early adversity. Disrupting neuronal metabolism in certain types of neurons prolongs the visual system’s critical period plasticity (Morishita et al., 2015). All of these experimental studies mean that the
normal periods of heightened plasticity are out of sync with the timing of when normal experiences are supposed to have optimal impact on circuit development. In humans, early adversity affects circuits that underlie specific functions in different ways (Nelson et al., 2014), with the timing of interventions (e.g., the Bucharest Early Intervention Project, which has done extensive research on the timing of placement in quality foster care after experiencing neglect in an orphanage) (Almas et al., 2018; Nelson et al., 2007; Wade et al., 2018; Zeanah et al., 2003) having a lasting effect on the quality of executive functioning, stress responsiveness, and attachment-related behaviors. Here, the impact on critical periods can only be hypothesized, but based on measures taken years later, it is likely that the timing of optimal sensitivity to experiences is changed in relation to specific functional domains.
The concept that altered neural development through genetic and experience-dependent mechanisms establishes different sensitivity to later-life adversity has been suggested for postadolescent onset psychiatric disorders (Keshavan et al., 2014) and more recently for ACEs (Danese et al., 2009; Loria et al., 2014; Morrison et al., 2017; Shalev and Belsky, 2016). The second adversity may be related to normal challenges or other life experiences that all of us endure and to which those without early adverse experiences are able to adapt. The mechanisms through which this vulnerability is read out have recently been addressed in a new set of animal studies (Peña et al., 2017). These demonstrate that there are early molecular changes in specific brain reward circuits due to early life stress that are responsible for the negative impact of later-endured juvenile social defeat (bullying) to produce depressive-like symptoms. For both human and animal studies, it is important to note that while early adverse experiences can have both acute and long-term impacts on mental and physical health, men and women may express these changes in different ways (e.g., externalizing or internalizing behaviors, respectively), which need to be accounted for in any intervention programs.
Immune System Development
The newborn immune system is shaped largely by the gut microbiome, which is initially primed by the vaginal or skin maternal microbiome during childbirth (depending on birth process) and early life, such as through breastfeeding (Miller, 2017). The gut microbiome refers to the vast collection of microbes that populate the gastrointestinal system and contribute to whole-body physiology. Molecules produced by the immune system impact various aspects of brain development, with strong evidence for mediating the formation and molecular adaptation of neural connections. The immune system also plays a role in the response of the brain
and body to early life stress. In addition to the development of neurons and their connections with the periphery, the fetus’ immune system undergoes rapid development in the first years of life and is yet another system that may be impacted by early adversity and stress (Simon et al., 2015). The innate immune system—which includes specialized cells, such as resident neutrophils, macrophages, and monocytes—develops in the fetal stage, though it is not robust enough to ward off most external pathogens. Of course, this lack of strong innate immunity may also provide the substrate for healthy microbiota to develop symbiotically with the newborn infant; the bacteria colonizing the gut, skin, and mucosa, for instance, assist in digestion and protection from other pathogens. Children’s adaptive immune systems—governed by the lymphatic system—are relatively undeveloped at the time of birth, rendering them prone to bacterial, viral, and fungal infections soon after birth. At this time, the fetal adaptive immune system is prepared for “training” by the maternal microbiome, which occurs rapidly after birth via vaginal, skin, or mammary exposure (Lynch and Pedersen, 2016). Throughout childhood, children are exposed to countless microbes that are either summarily dismissed by the immune system without notice or produce a robust immune response that primes the immune system to protect the body against similar pathogens in the future.
The immune system can be trained to cope with certain challenges. For example, the “hygiene hypothesis” suggests that the prevalence of asthma has increased dramatically in certain parts of the world where children are no longer exposed to microbes that can train the immune system (Harding, 2006). In the face of other challenges, the nervous system activates the HPA axis, which can lead to suppressed immune functioning and thus increased susceptibility to infections (Cohen et al., 2007; Thompson, 2014). When children experience sustained exposure to challenges (e.g., chronic stressors, such as poverty or maltreatment), HPA axis activity may become blunted, which can reduce inhibition of inflammation and lead to elevated levels of chronic low-grade inflammation (Koss and Gunnar, 2018; Miller et al., 2011). In the past two decades, researchers have attempted to clarify (1) the relationship between social stressors and inflammation and other immune markers in children (Slopen et al., 2012), and (2) the role of the immune system in the development of neurodevelopmental (Entringer et al., 2015; Gilman et al., 2017) and psychiatric disorders (Danese and Baldwin, 2017; O’Connor et al., 2014) and the cardiovascular and atherosclerotic disease process (Hansson and Hermansson, 2011; Pearson et al., 2003). There is now evidence from longitudinal research that inflammation tracks from childhood to adulthood (Juonala et al., 2006) and that chronic inflammation is a risk factor for a wide range of diseases, including cardiovascular diseases and depression (Dantzer et al., 2008; Libby et al., 2002).
Endocrine System Development
The endocrine system is composed of several glands that secrete hormones—signaling molecules—that reach their target organs through the circulatory system to modulate every physiological function, such as growth and development, reproduction, stress responses, and metabolism. A key characteristic of the endocrine system is that it operates on feedback loops to allow the hormones to perform a function (e.g., the “fight or flight” stress response) and then resets to be ready for the next event that may trigger an endocrine response. In all mammals, including humans, the hypothalamus and pituitary are the center of control for the endocrine system. Because they impact many stages of development, the organs of the endocrine system themselves develop early on, in the first trimester of gestation. Moreover, early in the first trimester, the placenta produces the same releasing factor proteins that are synthesized in the hypothalamus of nonpregnant women. These proteins control the production and release of hormones from the pituitary and peripheral tissues in the mother and the fetus.
Neuroendocrine hormone markers are evident by the second trimester. The endocrine systems work in concert with the autonomic nervous system to regulate organ function. Autonomic regulation can be measured in the late second trimester and throughout the third trimester and is intact and operational at the time of birth (DiPietro, 2015). The autonomic neurotransmitters norepinephrine, epinephrine, and acetylcholine and the endocrine hormones reach their targets via body circulation. Thus, they affect metabolic processes through binding to membrane protein receptors that are expressed by target cells in the brain and other organs as early as the second trimester. Related to the stress response via cortisol production, activation of the glucocorticoid receptors in the hippocampus and hypothalamus serve as classic feedback loops to reduce hormone production in order to reset the stress response capacity.
Research in humans and animals shows that overexposure to stress hormones and autonomic neurotransmitters during prenatal or early postnatal development can result in a fetal programming response, which includes epigenetic changes in the genes that encode the stress hormone receptors. This process produces long-term changes in receptor expression and less robust negative feedback to limit stress hormones’ chronic effects (for examples, see Maternal and Fetal/Infant Interactions section) (Meaney and Ferguson-Smith, 2010). In contrast, high levels of maternal care documented in animal studies show that the stress response system is capable of better management of later-life stress (Meaney, 2001; Plotsky and Meaney, 1993; Plotsky et al., 2005).
Reproductive System Development
The development of the reproductive system also begins early in gestation, in the first trimester. In the first 2 months of pregnancy, differentiation between male and female gonadal development occurs, which continues into the second and third trimesters. As noted above, animal studies show that intergenerational transmission of the impact of adversity during pregnancy can influence the mother, the fetus, and, if the developing gonads are affected, the offspring of the fetus when he or she matures into a reproductive adult. Sexual differentiation is controlled primarily by levels of testosterone, estrogen, and androgen, a process that can be influenced by exogenous chemicals similar in structure to these hormones. Among women, the ovarian follicle pool develops during the prenatal period; therefore, in-utero exposures may impact the size and quality of the follicle pool and influence the timing of ovarian loss and menopause (Bleil et al., 2018). For example, studies show that in-utero cigarette smoking exposure (Strohsnitter et al., 2008), famine (Yarde et al., 2013), and extremes of birth weight (Tom et al., 2010) are associated with earlier menopause. There is also evidence to suggest that maternal metabolic factors during pregnancy, including obesity and pregnancy hyperglycemia, are also associated with the timing of puberty (Kubo et al., 2018).
There has been a trend toward an earlier age of puberty for boys and girls in the United States that is not yet fully understood, and currently 9 years of age is within the normal range for onset of puberty (Herman-Giddens et al., 1997, 2012). Adverse social experiences in early and middle childhood—including socioeconomic adversity (Hiatt et al., 2017; Kelly et al., 2017; Sun et al., 2017) and child maltreatment (Mendle et al., 2016; Noll et al., 2017), as well as exposure to endocrine-disrupting chemicals (Buttke et al., 2012)—are associated with earlier pubertal development (Ellis and Giudice, 2019). In the United States and elsewhere, the age of puberty is earlier among racial/ethnic minority children (Herman-Giddens et al., 1997, 2012; Kelly et al., 2017; Reagan et al., 2012). These patterns by early childhood adversity and race/ethnicity may have implications for health disparities into adulthood (Bleil et al., 2017; Golub et al., 2008), as earlier puberty is associated with increased risk for depression (Wang et al., 2016) and substance use (Cance et al., 2013) during adolescence and numerous chronic disease outcomes in adulthood, including diabetes, cardiovascular diseases, and cancer (Canoy et al., 2015; Day et al., 2015; Elks et al., 2013).
BIOLOGICAL MECHANISMS OF STRESS
When the brain perceives a stressor, it induces a response that calls on multiple biological systems that, in the short term, are critical for adaptation and survival—sometimes defined as the “fight or flight” response. This stress response is a normal and healthy part of human biology. The spectrum of the stress response includes positive, tolerable, and toxic stress (Center on the Developing Child at Harvard University, 2005) and is governed by a complex interplay between the severity and duration of the stressor, individual genetic differences, gene–environment interaction, family environmental factors, developmental experiences, and the availability of buffering and coping strategies and resources. Positive and tolerable stress responses are characterized by a return to homeostasis, while the toxic stress response may induce lasting changes in brain architecture and function and in organ system development. Specific endocrine, immune, and brain cell populations produce bioactive chemicals that signal through their receptor proteins, which are present on cells throughout the body. These signals control cellular metabolism and physiological activity, which, in the short term, is a positive adaptation to the stressor.
Chronic activation of the stress response system can lead to risk for short- and long-term poor health outcomes beyond the early childhood period, including dysregulation of neuroendocrine and immune system development, altered cardiovascular functioning, metabolic dysregulation related to obesity and type 2 diabetes, changes to the gut microbiome, and epigenetic modifications that alter gene expression (Black, 2003; Campbell et al., 2014; Dinan and Cryan, 2012; Taylor et al., 2011; Vaiserman, 2015). There are individual differences in risk caused by chronically activated stress response systems, due in part to variation in the intrinsic sensitivity of the child, and physiological adaptive capacities that can be impacted by supportive environments (as discussed in greater detail below).
Activation of the stress response involves circuits in the brain that are essential for threat detection (amygdala), emotional regulation (frontal cortex), neuroendocrine regulation to produce cortisol (HPA axis), feedback to shut down the stress response (hippocampus), and the vagal complex in the brain stem that sends information to peripheral organs, such as the heart, lungs, and gastrointestinal systems (Cameron, 2009;
Dedovic et al., 2009; Forsythe et al., 2014; Goodman et al., 2013). The sympatho-adreno-medullary (SAM) axis responds to stress by producing and secreting adrenaline and noradrenaline through the vascular system. Adaptive responses of the SAM axis include increased arousal, alertness, and vigilance, improved cognition, focused attention, enhanced analgesia, and inhibition of appetite, feeding, digestion, growth, reproduction, and immunity (Chrousos, 2009). Adaptive responses of the HPA axis include regulation of cognitive, behavioral, affective, cardiovascular, and immune system functioning (Kudielka and Kirschbaum, 2005; McEwen et al., 2015). See Figure 2-2 for a diagram illustrating the stress response pathway.
Dysregulation of the Stress Response
Severe or chronic activation of the stress response, in the absence of adequate caregivers who serve as buffers to the stress activation, can lead to disruption of homeostatic mechanisms and long-term changes to brain architecture and organ systems (the toxic stress response) (Shonkoff et al., 2012). Early life stress can also result in epigenetic changes that can sensitize the individual to stress and adversity, alter the development of many organ systems, and impact the response to subsequent stressors later in the life course (see Epigenetics section) (National Scientific Council on the Developing Child, 2014). Exposure to high levels of adversity in early childhood is associated with the following multisystemic disruptions, with some of these changes occurring as early as infancy (e.g., changes to brain structure), while others are not evident until later in life (e.g., hypertension):
- Neurologic: There are structural and functional changes to stress-sensitive regions of the brain (McEwen and Morrison, 2013; National Scientific Council on the Developing Child, 2014).
- Endocrine: There is excessive activation of both the SAM and HPA axes, associated with loss of feedback inhibition of the HPA axis, increased levels of corticotropin-releasing hormone, and disruption of the daily cortisol pattern, leading to abnormally lower morning cortisol levels, elevated afternoon cortisol levels, and an overall increase in cortisol exposure. Over time, excess HPA activation may recede, leading to low or deficient HPA axis response. HPA axis overactivity is associated with suppression of thyroid function. High doses of early adversity are associated with changes in reproductive function through the HPA axis’ effect on gonadotropins and/or cytokine suppression of reproductive function (Dedovic et al., 2009; Kudielka and Kirschbaum, 2005; Slopen et al., 2014).
- Immune function: There is dysregulation of neuro-endocrine-immune relations, leading to increased pro-inflammatory
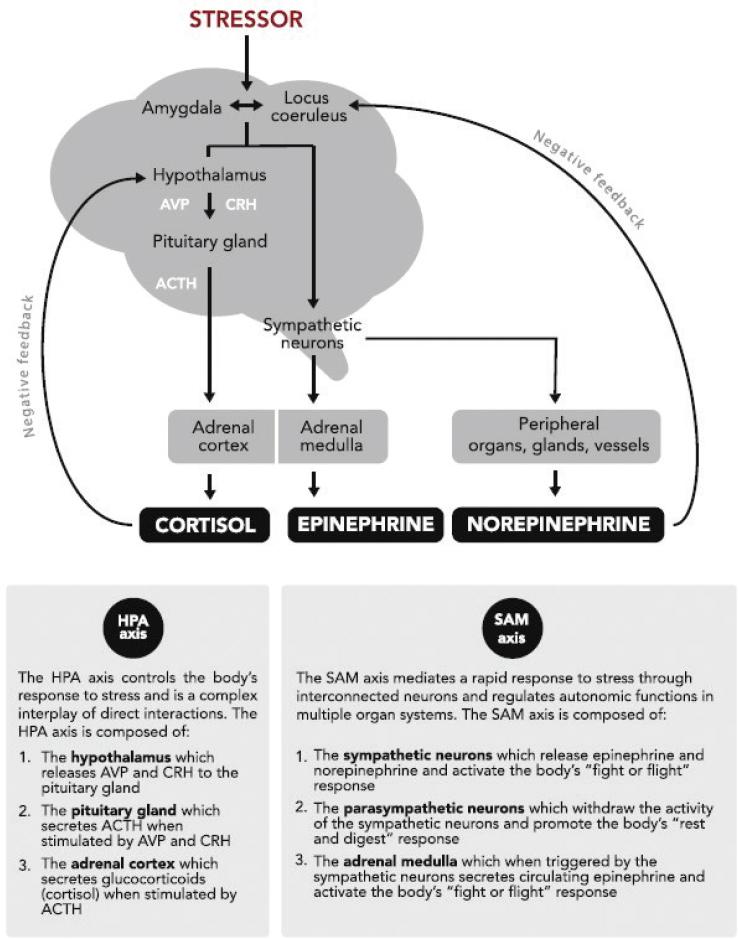
NOTE: ACTH = adrenocorticotropin hormone; AVP = arginine vasopressin; CRH = corticotropin-releasing hormone; HPA axis = hypothalamic-pituitary-adrenal axis; SAM axis = sympatho-adreno-medullary axis.
SOURCE: Bucci et al., 2016. Reprinted from Advances in Pediatrics, 63/1, Bucci et al. Toxic Stress in Children and Adolescents, 403–428, Copyright (2016), with permission from Elsevier.
- cytokines and inhibition of anti-inflammatory pathways. In addition, humoral (antibody production) and cell-mediated acquired immunity can be impaired. High levels of early adversity in childhood are associated with increased risk of autoimmune disease in adulthood (Nusslock and Miller, 2016; Padgett and Glaser, 2003).
- Cardiometabolic: There is insulin resistance, obesity, glucose intolerance, reduced control over blood lipid levels, and hypertension (Non et al., 2014; Woo Baidal et al., 2016).
Buffering the Stress Response
From a population health perspective, ameliorating the toxic stress response and understanding the biological mechanisms by which toxic stress can be prevented or mitigated is of high priority. Importantly, research suggests several mechanisms to buffer against toxic stress. One mechanism—studied in depth in animal models—is social relationships (Gunnar and Fisher, 2006). Research on resilience has clearly demonstrated that the single most important protective factor for children facing adversity is a strong, secure relationship with at least one parent; this helps foster positive outcomes across domains ranging from psychological adjustment to positive peer relationships (Cicchetti, 2013; Luthar et al., 2015; Masten, 2018; Sroufe, 2005). Moreover, a child who is securely attached to a primary caregiver is more likely to have high self-esteem, emotion regulation skills, and a positive outlook later in life (Sroufe, 2005). It also has been demonstrated that supportive relationships early in life play an important role in buffering stress responses, thereby allowing children to more easily confront stressful situations (Hostinar et al., 2014). (See Chapter 4 for an in-depth discussion on caregiver and social connections.) Some studies have shown an effect of social buffering on regulating the HPA axis, autonomic nervous system, and immune functioning, though more research is needed for more in-depth assessment of neuro-endocrine-immune, metabolic, and genetic regulatory functions (Hennessy et al., 2009; Hostinar et al., 2014; Kikusui et al., 2006).
While the mechanisms through which nurturing relationships buffer against toxic stress are likely to be complex, some studies have shown that the neuropeptide hormones oxytocin and vasopressin play important roles in affiliative social behavior (Bartz et al., 2011; Gunnar and Hostinar, 2015; Ross and Young, 2009; Ross et al., 2009a,b), though experimental work in social neuroscience is challenging (Insel, 2010a). Studies in animals and humans have shown that oxytocin and/or vasopressin play a role in social memory, pair bonding, empathy, altruism, emotion regulation, and trust (De Dreu and Kret, 2016; Donaldson and Young, 2008; Hostinar et al., 2014; Israel et al., 2012; Quirin et al., 2011; Rodrigues et al., 2009; Snowdon et al., 2010). It is thought that increased oxytocin counteracts the biological
underpinnings of toxic stress, including increased inflammation, cardiovascular dysfunction, oxidative stress, and alterations in brain structure and function (Detillion et al., 2004; Donaldson and Young, 2008; Heinrichs et al., 2003; Hostinar et al., 2014; Mantella et al., 2004). Social buffering to toxic stress, and its action through oxytocin and vasopressin (Gobrogge and Wang, 2015), is still an ongoing area of research, as is intergenerational transmission of trauma and trauma-related stress (Kim and Strathearn, 2017). For example, how such mechanisms change with development still remains to be determined (Gunnar and Hostinar, 2015). Still, this evidence suggests that interventions to create strong social relationships may ward against the long-term impacts of ACEs and other chronic adversity over the course of child development and into adulthood. More research is needed to understand other buffers against toxic stress (e.g., sleep, exercise, nutrition) and the biological mechanisms through which they operate; prefrontal cortex plasticity is one such mechanism (McEwen and Morrison, 2013).
Biological Measures of Stress in Children
In the decades since From Neurons to Neighborhoods, there have been substantial efforts to understand the biological changes that occur in response to early adversity (Danese and McEwen, 2012; NRC and IOM, 2000), at least in part inspired by the goal of developing valid, minimally invasive, low-burden measures of biological stress and neurobiological functioning in children that can be used to identify individuals at risk for poor long-term outcomes and, importantly, the impact of early life intervention (Shonkoff, 2010; Shonkoff et al., 2009). An increasing number of developmental studies with children now collect biological measures of stress, aiming to study how social experiences relate to changes in the biology of the child that may not cause immediate, gross disturbances (i.e., biological alterations that increase risk for subsequent chronic diseases) but rather set up the brain and peripheral systems for later emergence of physical and mental health problems over time. This research shows that traumatic experiences (such as child maltreatment) or prolonged exposure to chronic stress (such as poverty) can result in a chronically activated physiological stress response, which has a host of downstream consequences across biological systems. Some of this research has applied the concept of “allostasis” or “allostatic load,” which provides a method to evaluate the cumulative biological impact, or “wear and tear,” on the body as the result of a given social exposure (Danese and McEwen, 2012; McEwen, 1998). As noted in previous sections of this chapter, both animal and human research
has demonstrated that early life stress contributes to altered brain development (i.e., structure and function) and therefore to allostatic load.
Synthesis
Advances in science technologies, big data collection and information technology, and new strategies for analyzing complex data have been instrumental in rapidly advancing the science of brain and child development over the nearly 20 years since From Neurons to Neighborhoods was released to the public (NRC and IOM, 2000). Research findings have revealed the details of how early experiences are essential for building brain connections that underlie biobehavioral health, the role of genetics in development, and a new understanding of whole-child development that relies on organ systems interacting with each other and the environment to establish health and biobehavioral fitness or risk due to early influences of adversity.
- New data from longitudinal studies, combined with previous work from retrospective analyses of the early life experiences of adults, provides a compelling demonstration that toxic stress response substantially increases later-life risk for physical illnesses, including obesity and type 2 diabetes, cardiovascular disease, substance abuse, mental illness, lower educational achievement, cancer, and infectious disease. In addition to the adverse effects of toxic stress on the brain and nervous system, it also affects every other organ system in the child’s body, impacting short- and long-term health.
Individual Differences in Responsiveness and Susceptibility
Research over the past two decades clearly shows that what were previously considered to be universal developmental processes (see Chapter 4) do not occur uniformly. Relevant to the focus of this chapter, this can be due in part to variation in the development trajectory of stress-sensitive brain circuits in infancy, as well as the interplay between genes and environments that mediates the neurobiological and physical impact of positive or negative early life experiences. Thus, heterogeneity with substantial variations is often observed, such as across groups that differ by SES or ethnicity. Diversity brought about by individual and subgroup differences, based on factors such as personal experiences, individual temperament, culture, and the family and community context, can alter the pace and trajectories of developmental, biological, and psychological processes. For example, humans are predisposed to become attached to our main caregivers, but the quality of interactions between caregiver and child and other environmental conditions can contribute to the development of various types of infant attachment. To consider another example, a rich literature supports the notion that children differ in their temperament: behavioral predispositions can permeate how children react to and process environmental input. From conception, children bring differential responsiveness to—and processing of—their physical and relational context. Infants are described as difficult, inhibited or uninhibited, shy or slow to warm up (Conture et al., 2013). These dispositions carry consequences for adaptability to new environments, relational difficulties, and even long-term mental health problems (Clauss et al., 2015). One research notion from the temperament literature proposes that there are behavioral predispositions that are normative variations across individuals (Durbin and Hicks, 2014). Another theory sees the origin of these individual differences as consequences of exposure to early stress, whereby stress experienced by the mother and fetus before birth conditions the nervous system to respond, perhaps in an exaggerated way, to neutral or mild environmental input (Miller et al., 2011).
Recently, there has been an emphasis on elucidating the mechanisms underlying the differential susceptibility to the environment hypothesis. Work by both Belsky and Boyce has advanced understanding of how individual differences in how children process and react to the environment contribute to individual risk and resilience (Boyce, 2019; Bush and Boyce, 2016). Behavioral genetics research defined the contributions of both heritability and environment to the variance in the symptoms and incidence of behavioral disorder diagnoses (Plomin et al., 2001). This core principle is evident in the assessment of typical behavioral characteristics, such as affiliative social behavior. Studies of twins highlight the remarkable variability in specific traits that make up affiliative social behavior (Ebstein et al., 2010), with some elements exhibiting high (~0.7) heritability (prosocial behavior; social responsiveness) and others low (~0.3) heritability (empathy; secure attachment). In animal studies, heritability of the behaviors that make up affiliative social behavior exhibits very similar genetic contributions (Knoll et al., 2018).
These studies also showed that environmental context plays a particularly important role in modifying behavior—even in those with moderate to high heritability. As Bush and Boyce (2016) note, the accumulating biological data from studies of humans and animals have changed the concept from the long-standing diathesis stress model of sensitivity to environment to one that focuses on determining factors that confer individual differences that affect susceptibility and resilience. Stress reactivity, which had been presumed to influence behavioral outcomes in a single direction—negatively—is an important individual difference that can influence responses to different environmental contexts in either a positive or negative direction (Bush and Boyce, 2014; Ellis et al., 2011; Obradović et al., 2010). Again, context appears to be a major moderator of biological predispositions, and an ongoing topic of study is why some children adhere to healthy developmental trajectories despite profoundly adverse circumstances while others do not (Cicchetti, 2013; Masten, 2014; NASEM, 2015; Rutter, 2012; Shonkoff, 2016).
The importance of individual differences in development points out lessons for practice and policies in efforts to prevent developmental problems and promote positive trajectories (Shonkoff, 2017). A key fact is that universal interventions will be more or less effective depending on the individual(s) being targeted. Thus, well-designed interventions are those that are sufficiently flexible to take individual differences and heterogeneity into account. The challenge continues to be predicting what specific changes are most likely to produce the best outcomes. For example, the New Hope Project, a welfare reform demonstration based in Milwaukee, Wisconsin, was designed to raise families out of poverty, using a work-support intervention that was tailored to the specific
needs of each family (Huston et al., 2011). The intervention had multiple components that families would choose from over 3 years, and it was expected that the combination of benefits and services would increase parents’ employment, income, and use of child care and health insurance. In a report to summarize effects over 8 years (i.e., 5 years after the intervention ended), researchers concluded that work supports can positively impact low-income parents and children, even though the economic effects of employment and income were not sustained beyond the 3 years of the intervention. Importantly, positive effects for children appeared during the intervention period and extended to the 8-year follow-up.8
Another example is Filming Interactions to Nurture Development, a 10-week video-feedback program to promote acquisition of developmentally supportive parenting skills (Fisher et al., 2016; Nese et al., 2016). This behavioral training program is tailored to address individual differences in the types of challenging parenting styles via weekly structured coaching sessions, and findings suggest that it is effective for improving caregiver skills (Fisher et al., 2016).
In some cases, individuals have different responses to social or environmental stimuli as the result of age of exposure. Specific time points in development represent “sensitive periods” when risk or protective factors have a maximal influence on cognitive capacities, biology, emotions, and behavior (Ben-Shlomo and Kuh, 2002; Hertzman and Boyce, 2010; Knudsen, 2004; Zeanah et al., 2011). The strongest evidence for the heightened importance of specific periods comes from experiments using laboratory animals (Hertzman and Boyce, 2010) because it is challenging to isolate sensitive periods within observational studies in humans (given that risk factors are often continuous and may in turn elicit subsequent risks). As noted in detail above, the prenatal, postnatal, and early childhood phases are recognized as having sensitive periods that result in brain and behavioral changes. Research is beginning to elucidate how risk factors at different time points may have varying impacts. Although it is generally accepted that developmental timing can influence the effect of a social or environmental risk factor on child health, the majority of studies do not consider how risk or protective factors vary by age.
___________________
8 Although the positive effect of this personalized intervention on children’s academic performance and test scores at the 2- and 5-year follow-ups faded, at the 8-year follow-up, children in families assigned to the intervention condition were more likely to be engaged in school and to show positive social behaviors relative to those in families assigned to the control condition, and they were less likely to have to repeat a grade, to be placed in special education, to receive poor grades, or to have cynical attitudes about work (Miller et al., 2008).
CONCLUSION
With advances in science emerging from different disciplines, now more than ever, there is a major need to facilitate new public–private-nonprofit sector relations to build substantial opportunities for the fields of neuroscience, behavioral sciences, and psychology to collaborate. Therefore, the committee recommends:
This will require recruiting diverse populations, with explicit attention to addressing racial/ethnic and socioeconomic inequities in developmental outcomes. Research with these populations will require researchers and practitioners that are knowledgeable of theoretical models, measures, and the realities of these families and communities as co-investigators. For all studies that typically use expensive biological assessments (e.g., biomarkers as mediators, moderators, or outcomes) for use in interventions, researchers need to strongly consider the ability to apply measures within the context of scaled, community-based interventions to have the broadest possible impact on outcomes for children and their caregivers. For example, there is a major gap in research support for projects that combine modern, noninvasive technologies to measure key domains of brain, behavioral, and psychological development with clinical interventions that promote best outcomes for children and their caregivers.
In summary, research advances in neuroscience, immunology, endocrinology, behavior, and psychology in the past two decades provide a new depth of understanding regarding the impact of adverse experiences on developmental processes throughout the life course. Because of these advances, the fields of developmental neuroscience, behavior, and psychology are recognizing the trajectories of development in the context
of racial, ethnic, and socioeconomic health disparities. Deciphering the mechanisms through which adversity disrupts foundational developmental processes of the whole child, and the long-term impact on health, wellness, personal and community relationships, and life-span productivity across the life course, is being achieved through multidisciplinary, longitudinally designed research that has incorporated principles of human development, including individual differences and differential sensitivity to context. With this foundation, the chapters that follow provide advances in the research that address the role of macro- and micro-level variables in influencing development and outcomes.
REFERENCES
Acevedo-Garcia, D., N. McArdle, E. F. Hardy, U. I. Crisan, B. Romano, D. Norris, M. Baek, and J. Reece. 2014. The Child Opportunity Index: Improving collaboration between community development and public health. Health Affairs 33(11):1948–1957.
Acevedo-Garcia, D., N. McArdle, E. Hardy, K.-N. Dillman, J. Reece, U. I. Crisan, D. Norris, and T. L. Osypuk. 2016. Neighborhood opportunity and location affordability for low-income renter families. Housing Policy Debate 26(4–5):607–645.
Akins, M. R., and T. Biederer. 2006. Cell-cell interactions in synaptogenesis. Current Opinion in Neurobiology 16(1):83–89.
Almas, A. N., L. J. Papp, M. R. Woodbury, C. A. Nelson, C. H. Zeanah, and N. A. Fox. 2018. The impact of caregiving disruptions of previously institutionalized children on multiple outcomes in late childhood. Child Development. doi: 10.1111/cdev.13169. [Epub ahead of print.]
Amrit, C., T. Paauw, R. Aly, and M. Lavric. 2017. Identifying child abuse through text mining and machine learning. Expert Systems with Applications 88:402–418.
Bae, B. I., D. Jayaraman, and C. A. Walsh. 2015. Genetic changes shaping the human brain. Developmental Cell 32(4):423–434.
Bagot, K. S., S. A. Matthews, M. Mason, L. M. Squeglia, J. Fowler, K. Gray, M. Herting, A. May, I. Colrain, J. Godino, S. Tapert, S. Brown, and K. Patrick. 2018. Current, future and potential use of mobile and wearable technologies and social media data in the ABCD Study to increase understanding of contributors to child health. Developmental Cognitive Neuroscience 32:121–129.
Bale, T. L. 2015. Epigenetic and transgenerational reprogramming of brain development. Nature Reviews Neuroscience 16(6):332–344.
Bartz, J. A., J. Zaki, N. Bolger, and K. N. Ochsner. 2011. Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences 15(7):301–309.
Bastek, J. A., L. M. Gómez, and M. A. Elovitz. 2011. The role of inflammation and infection in preterm birth. Clinics in Perinatology 38(3):385–406.
Bath, K. G., G. Manzano-Nieves, and H. Goodwill. 2016. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones and Behavior 82:64–71.
Beck, A. F., B. Huang, P. H. Ryan, M. T. Sandel, C. Chen, and R. S. Kahn. 2016. Areas with high rates of police-reported violent crime have higher rates of childhood asthma morbidity. The Journal of Pediatrics 173:175–182.
Beck, A. F., B. Huang, K. Wheeler, N. R. Lawson, R. S. Kahn, and C. L. Riley. 2017a. The Child Opportunity Index and disparities in pediatric asthma hospitalizations across one Ohio metropolitan area, 2011–2013. The Journal of Pediatrics 190:200–206.
Beck, A. F., M. T. Sandel, P. H. Ryan, and R. S. Kahn. 2017b. Mapping neighborhood health geomarkers to clinical care decisions to promote equity in child health. Health Affairs 36(6):999–1005.
Belfield, C. R., M. Nores, S. Barnett, and L. Schweinhart. 2006. The High/Scope Perry Preschool Program cost–benefit analysis using data from the age-40 followup. Journal of Human Resources 41(1):162–190.
Ben-Shlomo, Y., and D. Kuh. 2002. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology 31(2):285–293.
Ben-Shlomo, Y., G. Mishra, and D. Kuh. 2014. Life course epidemiology. In Handbook of epidemiology, edited by W. Ahrens and I. Pigeot. New York: Springer. Pp. 1521–1549.
Berbari, E. J., E. A. Bock, A. C. Chazaro, X. Sun, and L. Sornmo. 2005. High-resolution analysis of ambulatory electrocardiograms to detect possible mechanisms of premature ventricular beats. IEEE Transactions on Biomedical Engineering 52(4):593–598.
Bercury, K. K., and W. B. Macklin. 2015. Dynamics and mechanisms of CNS myelination. Developmental Cell 32(4):447–458.
Black, P. H. 2003. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, Behavior, and Immunity 17(5):350–364.
Bleil, M. E., C. Booth-LaForce, and A. D. Benner. 2017. Race disparities in pubertal timing: Implications for cardiovascular disease risk among African American women. Population Research and Policy Review 36(5):717–738.
Bleil, M. E., P. English, J. Valle, N. F. Woods, K. D. Crowder, S. E. Gregorich, and M. I. Cedars. 2018. Is in utero exposure to maternal socioeconomic disadvantage related to offspring ovarian reserve in adulthood? Women’s Midlife Health 4(1):5.
Bonnin, A., and P. Levitt. 2011. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197:1–7.
Bonnin, A., N. Goeden, K. Chen, M. L. Wilson, J. King, J. C. Shih, R. D. Blakely, E. S. Deneris, and P. Levitt. 2011. A transient placental source of serotonin for the fetal forebrain. Nature 472(7343):347–350.
Bourgeois, J. P. 1997. Synaptogenesis, heterochrony and epigenesis in the mammalian neocortex. Acta Paediatrica 422:27–33.
Boyce, W. 2019. The orchid and the dandelion: Why some children struggle and how all can thrive. New York: Knopf.
Boyce, W. T., and M. S. Kobor. 2015. Development and the epigenome: The “synapse” of gene–environment interplay. Developmental Science 18(1):1–23.
Brikell, I., H. Larsson, Y. Lu, E. Pettersson, Q. Chen, R. Kuja-Halkola, R. Karlsson, B. B. Lahey, P. Lichtenstein, and J. Martin. 2018. The contribution of common genetic risk variants for ADHD to a general factor of childhood psychopathology. Molecular Psychiatry 1.
Brito, N. H., and K. G. Noble. 2014. Socioeconomic status and structural brain development. Frontiers in Neuroscience 8:276.
Bronfenbrenner, U. 1979. Contexts of child rearing: Problems and prospects. American Psychologist 34(10):844–850.
Bronfenbrenner, U., and G. W. Evans. 2000. Developmental science in the 21st century: Emerging questions, theoretical models, research designs and empirical findings. Social Development 9(1):115–125.
Bucci, M., S. S. Marques, D. Oh, and N. B. Harris. 2016. Toxic stress in children and adolescents. Advances in Pediatrics 63(1):403–428.
Burton, T., and N. B. Metcalfe. 2014. Can environmental conditions experienced in early life influence future generations? Proceedings of the Royal Society B: Biological Sciences 281(1785).
Bush, N. R., and T. Boyce. 2014. The contributions of early experience to biological development and sensitivity to context. In Handbook of developmental psychopathology, edited by M. Lewis and K. Rudolph. New York: Springer. Pp. 287–309.
Bush, N. R., and T. Boyce. 2016. Differential sensitivity to context: Implications for developmental psychopathology. In Developmental psychopathology, Vol. 2, edited by D. Cicchetti. Hoboken, NJ: John Wiley & Sons, Inc. Pp. 107–137.
Buss, C., S. Entringer, N. K. Moog, P. Toepfer, D. A. Fair, H. N. Simhan, C. M. Heim, and P. D. Wadhwa. 2017. Intergenerational transmission of maternal childhood maltreatment exposure: Implications for fetal brain development. Journal of the American Academy of Child and Adolescent Psychiatry 56(5):373–382.
Buttke, D. E., K. Sircar, and C. Martin. 2012. Exposures to endocrine-disrupting chemicals and age of menarche in adolescent girls in NHANES. Environmental Health Perspectives 120(11):1613–1618.
Bystron, I., C. Blakemore, and P. Rakic. 2008. Development of the human cerebral cortex: Boulder committee revisited. Nature Reviews Neuroscience 9:110.
Cameron, J. L., K. L. Eagleson, N. A. Fox, T. K. Hensch, and P. Levitt. 2017. Social origins of developmental risk for mental and physical illness. Journal of Neuroscience 37(45):10783–10791.
Cameron, O. G. 2009. Visceral brain–body information transfer. Neuroimage 47(3):787–794.
Campbell, F., G. Conti, J. J. Heckman, S. H. Moon, R. Pinto, E. Pungello, and Y. Pan. 2014. Early childhood investments substantially boost adult health. Science 343(6178):1478–1485.
Cance, J. D., S. T. Ennett, A. A. Morgan-Lopez, V. A. Foshee, and A. E. Talley. 2013. Perceived pubertal timing and recent substance use among adolescents: A longitudinal perspective. Addiction 108(10):1845–1854.
Canoy, D., V. Beral, A. Balkwill, F. L. Wright, M. E. Kroll, G. K. Reeves, J. Green, and B. J. Cairns. 2015. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation 131(3):237–244.
Carlyle, B. C., R. R. Kitchen, J. E. Kanyo, E. Z. Voss, M. Pletikos, A. M. M. Sousa, T. T. Lam, M. B. Gerstein, N. Sestan, and A. C. Nairn. 2017. A multiregional proteomic survey of the postnatal human brain. Nature Neuroscience 20(12):1787–1795.
Casillas, K. L., A. Fauchier, B. T. Derkash, and E. F. Garrido. 2016. Implementation of evidence-based home visiting programs aimed at reducing child maltreatment: A meta-analytic review. Child Abuse & Neglect 53:64–80.
Causadias, J. M., and A. J. Umaña-Taylor. 2018. Reframing marginalization and youth development: Introduction to the special issue. American Psychologist 73(6):707–712.
Center on the Developing Child at Harvard University. 2005. Excessive stress disrupts the architecture of the developing brain. Cambridge, MA: Harvard University. Pp. 1–9.
Center on the Developing Child at Harvard University. 2014. A decade of science informing policy: The story of the National Scientific Council on the Developing Child. Cambridge, MA: Harvard University.
Center on the Developing Child at Harvard University. n.d-a. Brain architecture. https://developingchild.harvard.edu/science/key-concepts/brain-architecture (accessed June 17, 2019).
Center on the Developing Child at Harvard University. n.d.-b. Five numbers to remember about early childhood development. https://pdg.grads360.org/services/PDCService.svc/GetPDCDocumentFile?fileId=16462 (accessed June 17, 2019).
Center on the Developing Child at Harvard University. n.d.-c. What is early childhood development? A guide to the science. https://developingchild.harvard.edu/guide/what-is-early-childhood-development-a-guide-to-the-science (accessed April 17, 2019).
Chan, J. C., B. M. Nugent, and T. L. Bale. 2018. Parental advisory: Maternal and paternal stress can impact offspring neurodevelopment. Biological Psychiatry 83(10):886–894.
Chen, M., and K. L. Chan. 2016. Effects of parenting programs on child maltreatment prevention: A meta-analysis. Trauma, Violence, & Abuse 17(1):88–104.
Chen, Y., and T. Z. Baram. 2016. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 41(1):197–206.
Chetty, R., N. Hendren, and L. F. Katz. 2016. The effects of exposure to better neighborhoods on children: New evidence from the Moving to Opportunity experiment. The American Economic Review 106(4):855–902.
Cheung, K. L., and R. A. Lafayette. 2013. Renal physiology of pregnancy. Advances in Chronic Kidney Disease 20(3):209–214.
Christian, H., S. R. Zubrick, S. Foster, B. Giles-Corti, F. Bull, L. Wood, M. Knuiman, S. Brinkman, S. Houghton, and B. Boruff. 2015. The influence of the neighborhood physical environment on early child health and development: A review and call for research. Health & Place 33:25–36.
Chrousos, G. P. 2009. Stress and disorders of the stress system. Nature Reviews Endocrinology 5(7):374.
Cicchetti, D. 1996. Child maltreatment: Implications for developmental theory and research. Human Development 39(1):18–39.
Cicchetti, D. 2013. Annual research review: Resilient functioning in maltreated children—past, present, and future perspectives. Journal of Child Psychology and Psychiatry 54(4):402–422.
Cicchetti, D. 2015a. Neural plasticity, sensitive periods, and psychopathology. Development and Psychopathology 27(2):319–320.
Cicchetti, D. 2015b. Preventive intervention efficacy, development, and neural plasticity. Journal of the American Academy of Child & Adolescent Psychiatry 54(2):83–85.
Cicchetti, D., and J. A. Blender. 2006. A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences 1094:248–258.
Cicchetti, D., and W. J. Curtis. 2006. The developing brain and neural plasticity: Implications for normality, psychopathology, and resilience. In Developmental psychopathology: Developmental neuroscience, 2nd ed. Vol. 2, edited by D. J. Cohen. New York: Wiley. Pp. 1–64.
Cicchetti, D., and E. D. Handley. 2017. Methylation of the glucocorticoid receptor gene, nuclear receptor subfamily 3, group c, member 1 (NR3C1), in maltreated and nonmaltreated children: Associations with behavioral undercontrol, emotional lability/negativity, and externalizing and internalizing symptoms. Development and Psychopathology 29(5):1795–1806.
Clauss, J. A., S. N. Avery, and J. U. Blackford. 2015. The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Progress in Neurobiology 127–128:23–45.
Cohen, S., D. Janicki-Deverts, and G. E. Miller. 2007. Psychological stress and disease. JAMA 298(14):1685–1687.
Coll, C. G., and L. A. Szalacha. 2004. The multiple contexts of middle childhood. Future of Children 14(2):81–97.
Collins, F. S. 2004. What we do and don’t know about “race,” “ethnicity,” genetics and health at the dawn of the genome era. Nature Genetics 36(Suppl 11):S13–S15.
Conture, E. G., E. M. Kelly, and T. A. Walden. 2013. Temperament, speech and language: An overview. Journal of Communication Disorders 46(2):125–142.
Corso, P. S., S. N. Visser, J. B. Ingels, and R. Perou. 2015. Cost-effectiveness of Legacy for Children for reducing behavioral problems and risk for ADHD among children living in poverty. Journal of Child and Adolescent Behaviour 3(5):240.
Costantine, M. M. 2014. Physiologic and pharmacokinetic changes in pregnancy. Frontiers in Pharmacology 5:65.
Council on Community Pediatrics. 2016. Poverty and child health in the United States. Pediatrics 137(4):e20160339.
Criado-Marrero, M., T. Rein, E. B. Binder, J. T. Porter, J. Koren, 3rd, and L. J. Blair. 2018. HSP90 and FKBP51: Complex regulators of psychiatric diseases. Philosophical Transactions of the Royal Society B: Biological Sciences 373(1738).
Cuffe, J. S., L. O’Sullivan, D. G. Simmons, S. T. Anderson, and K. M. Moritz. 2012. Maternal corticosterone exposure in the mouse has sex-specific effects on placental growth and MRNA expression. Endocrinology 153(11):5500–5511.
Cunningham, M. 2019. Introduction to myths and realities associated with research and theorizing for human development. Research in Human Development 16(1):1–4.
Danese, A., and J. R. Baldwin. 2017. Hidden wounds? Inflammatory links between childhood trauma and psychopathology. Annual Review of Psychology 68:517–544.
Danese, A., and B. S. McEwen. 2012. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior 106(1):29–39.
Danese, A., T. E. Moffitt, H. Harrington, B. J. Milne, G. Polanczyk, C. M. Pariante, R. Poulton, and A. Caspi. 2009. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine 163(12):1135–1143.
Dantzer, R., J. C. O’Connor, G. G. Freund, R. W. Johnson, and K. W. Kelley. 2008. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience 9(1):46–56.
Day, F. R., C. E. Elks, A. Murray, K. K. Ong, and J. R. Perry. 2015. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: The UK Biobank Study. Scientific Reports 5:11208.
De Dreu, C. K., and M. E. Kret. 2016. Oxytocin conditions intergroup relations through up-regulated in-group empathy, cooperation, conformity, and defense. Biological Psychiatry 79(3):165–173.
Dedovic, K., A. Duchesne, J. Andrews, V. Engert, and J. C. Pruessner. 2009. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage 47(3):864–871.
Detillion, C. E., T. K. S. Craft, E. R. Glasper, B. J. Prendergast, and A. C. DeVries. 2004. Social facilitation of wound healing. Psychoneuroendocrinology 29(8):1004–1011.
Diamond, A. 2013. Executive functions. Annual Review of Psychology 64(1):135–168.
Dinan, T. G., and J. F. Cryan. 2012. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 37(9):1369–1378.
DiPietro, J. A., K. A. Costigan, and K. M. Voegtline. 2015. Studies in fetal behavior: Revisited, renewed, and reimagined. Monographs of the Society for Research in Child Development 80(3):vii, 1–94.
Doan, R. N., T. Shin, and C. A. Walsh. 2018. Evolutionary changes in transcriptional regulation: Insights into human behavior and neurological conditions. Annual Review of Neuroscience 41:185–206.
Donaldson, Z. R., and L. J. Young. 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322(5903):900–904.
Donkin, I., S. Versteyhe, L. R. Ingerslev, K. Qian, M. Mechta, L. Nordkap, B. Mortensen, E. V. Appel, N. Jorgensen, V. B. Kristiansen, T. Hansen, C. T. Workman, J. R. Zierath, and R. Barres. 2016. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metabolism 23(2):369–378.
Durbin, C. E., and B. M. Hicks. 2014. Personality and psychopathology: A stagnant field in need of development. European Journal of Personality 28(4):362–386.
Ebstein, R. P., S. Israel, S. H. Chew, S. Zhong, and A. Knafo. 2010. Genetics of human social behavior. Neuron 65(6):831–844.
Eisner, V., M. Picard, and G. Hajnoczky. 2018. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nature Cell Biology 20(7):755–765.
Elks, C. E., K. K. Ong, R. A. Scott, Y. T. Van Der Schouw, J. S. Brand, P. A. Wark, P. Amiano, B. Balkau, A. Barricarte, and H. Boeing. 2013. Age at menarche and type 2 diabetes risk: The Epic-Interact study. Diabetes Care 36(11):3526–3534.
Ellis, B. J., and M. D. Giudice. 2019. Developmental adaptation to stress: An evolutionary perspective. Annual Review of Psychology 70(1):111–139.
Ellis, B. J., W. T. Boyce, J. Belsky, M. J. Bakermans-Kranenburg, and M. H. van Ijzendoorn. 2011. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology 23(1):7–28.
Enoch, M. A., A. A. Rosser, Z. Zhou, D. C. Mash, Q. Yuan, and D. Goldman. 2014. Expression of glutamatergic genes in healthy humans across 16 brain regions; altered expression in the hippocampus after chronic exposure to alcohol or cocaine. Genes, Brain and Behavior 13(8):758–768.
Entringer, S. 2013. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Current Opinion in Clinical Nutrition and Metabolic Care 16(3):320–327.
Entringer, S., C. Buss, and P. D. Wadhwa. 2010. Prenatal stress and developmental programming of human health and disease risk: Concepts and integration of empirical findings. Current Opinion in Endocrinology Diabetes and Obesity 17(6):507–516.
Entringer, S., E. S. Epel, J. Lin, C. Buss, B. Shahbaba, E. H. Blackburn, H. N. Simhan, and P. D. Wadhwa. 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. American Journal of Obstetrics and Gynecology 208(2):134.e131–134.e137.
Entringer, S., C. Buss, and P. D. Wadhwa. 2015. Prenatal stress, development, health and disease risk: A psychobiological perspective—2015 Curt Richter Award paper. Psychoneuroendocrinology 62:366–375.
Evans, G. W. 2004. The environment of childhood poverty. American Psychologist 59:77–92.
Evans, G. W., N. M. Wells, and M. Schamberg. 2010. The role of the environment in socioeconomic status and obesity. In Obesity prevention: The role of society and brain on individual behavior, edited by L. Dube, A. Bechara, A. Dagher, D. Drewnowski, J. LeBel, P. James, R. Y. Yada, and M. C. Laflamme-Sanders. New York: Academic Press. Pp. 713–726.
Favuzzi, E., and B. Rico. 2018. Molecular diversity underlying cortical excitatory and inhibitory synapse development. Current Opinion in Neurobiology 53:8–15.
Feldman, R., and M. J. Bakermans-Kranenburg. 2017. Oxytocin: A parenting hormone. Current Opinion in Psychology 15:13–18.
Feldman, R., Z. Rosenthal, and A. I. Eidelman. 2014. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biological Psychiatry 75(1):56–64.
Ferguson, K. T., R. C. Cassells, J. W. MacAllister, and G. W. Evans. 2013. The physical environment and child development: An international review. International Journal of Psychology 48(4):437–468.
Fisher, C. B., S. A. Wallace, and R. E. Fenton. 2000. Discrimination distress during adolescence. Journal of Youth and Adolescence 29(6):679–695.
Fisher, P. A., T. I. Frenkel, L. K. Noll, M. Berry, and M. Yockelson. 2016. Promoting healthy child development via a two-generation translational neuroscience framework: The Filming Interactions to Nurture Development video coaching program. Child Development Perspectives 10(4):251–256.
Flavell, J. H. 1963. The developmental psychology of Jean Piaget. Princeton, NJ: D. Van Nostrand.
Forsythe, P., J. Bienenstock, and W. A. Kunze. 2014. Vagal pathways for microbiome-brain-gut axis communication. In Microbial endocrinology: The microbiota-gut-brain axis in health and disease. Switzerland: Springer Nature. Pp. 115–133.
Foster, M. W., and R. R. Sharp. 2004. Beyond race: Towards a whole-genome perspective on human populations and genetic variation. Nature Reviews Genetics 5(10):790–796.
Franklin, T. B., H. Russig, I. C. Weiss, J. Gräff, N. Linder, A. Michalon, S. Vizi, and I. M. Mansuy. 2010. Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry 68(5):408–415.
Frazier, T. W., E. W. Klingemier, S. Parikh, L. Speer, M. S. Strauss, C. Eng, A. Y. Hardan, and E. A. Youngstrom. 2018. Development and validation of objective and quantitative eye tracking-based measures of autism risk and symptom levels. Journal of the American Academy of Child and Adolescent Psychiatry 57(11):858–866.
Gabrieli, J. D. E., and S. A. Bunge. 2017. Does poverty shape the brain? Scientific American, https://www.scientificamerican.com/article/does-poverty-shape-the-brain (accessed April 17, 2019).
García Coll, C. T. 1990. Developmental outcome of minority infants: A process-oriented look into our beginnings. Child Development 61(2):270–289.
García Coll, C., G. Lamberty, R. Jenkins, H. P. McAdoo, K. Crnic, B. H. Wasik, and H. Vazquez Garcia. 1996. An integrative model for the study of developmental competencies in minority children. Child Development 67(5):1891–1914.
Garcia-Vargas, L., S. S. Addison, R. Nistala, D. Kurukulasuriya, and J. R. Sowers. 2012. Gestational diabetes and the offspring: Implications in the development of the cardiorenal metabolic syndrome in offspring. Cardiorenal Medicine 2(2):134–142.
Garner, A. S., J. P. Shonkoff, B. S. Siegel, M. I. Dobbins, M. F. Earls, L. McGuinn, J. Pascoe, D. L. Wood, Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, and Section on Developmental and Behavioral Pediatrics. 2012. Early childhood adversity, toxic stress, and the role of the pediatrician: Translating developmental science into lifelong health. Pediatrics 129(1):e224–e231.
Gascon, M., M. Vrijheid, and M. J. Nieuwenhuijsen. 2016. The built environment and child health: An overview of current evidence. Current Environmental Health Reports 3(3):250–257.
Geschwind, D. H., and P. Rakic. 2013. Cortical evolution: Judge the brain by its cover. Neuron 80(3):633–647.
Gillingham, P. 2016. Predictive risk modelling to prevent child maltreatment and other adverse outcomes for service users: Inside the “black box” of machine learning. The British Journal of Social Work 46(4):1044–1058.
Gilman, S. E., M. Hornig, A. Ghassabian, J. Hahn, S. Cherkerzian, P. S. Albert, S. L. Buka, and J. M. Goldstein. 2017. Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proceedings of the National Academy of Sciences of the United States of America 114(26):6728–6733.
Gluckman, P. D., M. A. Hanson, and A. S. Beedle. 2007. Early life events and their consequences for later disease: A life history and evolutionary perspective. American Journal of Human Biology 19(1):1–19.
Gobrogge, K., and Z. Wang. 2015. Neuropeptidergic regulation of pair-bonding and stress buffering: Lessons from voles. Hormones and Behavior 76:91–105.
Goeden, N., J. Velasquez, K. A. Arnold, Y. Chan, B. T. Lund, G. M. Anderson, and A. Bonnin. 2016. Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. Journal of Neuroscience 36(22):6041–6049.
Gogtay, N., J. N. Giedd, L. Lusk, K. M. Hayashi, D. Greenstein, A. C. Vaituzis, T. F. Nugent, D. H. Herman, L. S. Clasen, A. W. Toga, J. L. Rapoport, and P. M. Thompson. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America 101(21):8174–8179.
Goldberg, A. D., C. D. Allis, and E. Bernstein. 2007. Epigenetics: A landscape takes shape. Cell 128(4):635–638.
Goldman-Mellor, S., C. Margerison–Zilko, K. Allen, and M. Cerda. 2016. Perceived and objectively-measured neighborhood violence and adolescent psychological distress. Journal of Urban Health 93(5):758–769.
Goll, M. G., and T. H. Bestor. 2005. Eukaryotic cytosine methyltransferases. Annual Review of Biochemistry 74:481–514.
Golub, M. S., G. W. Collman, P. M. Foster, C. A. Kimmel, E. Rajpert-De Meyts, E. O. Reiter, R. M. Sharpe, N. E. Skakkebaek, and J. Toppari. 2008. Public health implications of altered puberty timing. Pediatrics 121(Suppl 3):S218–S230.
Gonzalez, T. L., T. Sun, A. F. Koeppel, B. Lee, E. T. Wang, C. R. Farber, S. S. Rich, L. W. Sundheimer, R. A. Buttle, Y.-D. I. Chen, J. I. Rotter, S. D. Turner, J. Williams, M. O. Goodarzi, and M. D. Pisarska. 2018. Sex differences in the late first trimester human placenta transcriptome. Biology of Sex Differences 9(1):4.
Goodman, R. N., J. C. Rietschel, L.-C. Lo, M. E. Costanzo, and B. D. Hatfield. 2013. Stress, emotion regulation and cognitive performance: The predictive contributions of trait and state relative frontal EEG alpha asymmetry. International Journal of Psychophysiology 87(2):115–123.
Gorski Findling, M. T., J. A. Wolfson, E. B. Rimm, and S. N. Bleich. 2018. Differences in the neighborhood retail food environment and obesity among us children and adolescents by SNAP participation. Obesity 26(6):1063–1071.
Gramlich, E. M. 1986. Evaluation of education projects: The case of the Perry Preschool Program. Economics of Education Review 5(1):17–24.
Greenough, W. T., J. E. Black, and C. S. Wallace. 1987. Experience and brain development. Child Development 58(3):539–559.
Grindal, T., J. B. Bowne, H. Yoshikawa, H. S. Schindler, G. J. Duncan, K. Magnuson, and J. P. Shonkoff. 2016. The added impact of parenting education in early childhood education programs: A meta-analysis. Children and Youth Services Review 70:238–249.
Gunnar, M. R., and P. A. Fisher. 2006. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology 18(3):651–677.
Gunnar, M. R., and C. E. Hostinar. 2015. The social buffering of the hypothalamic–pituitary–adrenocortical axis in humans: Developmental and experiential determinants. Social Neuroscience 10(5):479–488.
Hair, N. L., J. L. Hanson, B. L. Wolfe, and S. D. Pollak. 2015. Association of child poverty, brain development, and academic achievement. JAMA Pediatrics 169(9):822–829.
Halfon, N., K. Larson, M. Lu, E. Tullis, and S. Russ. 2014. Lifecourse health development: Past, present and future. Maternal and Child Health Journal 18(2):344–365.
Hammock, E. A., M. M. Lim, H. P. Nair, and L. J. Young. 2005. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes, Brain, and Behavior 4(5):289–301.
Hanson, J. L., N. Hair, D. G. Shen, F. Shi, J. H. Gilmore, B. L. Wolfe, and S. D. Pollak. 2013. Family poverty affects the rate of human infant brain growth. PLoS ONE 8(12):e80954.
Hansson, G. K., and A. Hermansson. 2011. The immune system in atherosclerosis. Nature Immunology 12(3):204–212.
Hantsoo, L., S. Kornfield, M. C. Anguera, and C. N. Epperson. 2019. Inflammation: A proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biological Psychiatry 85(2):97–106.
Harding, A. 2006. Fernando Martinez: Seeking to solve the puzzle of asthma. The Lancet 368(9537):725.
Heard-Garris, N. J., M. Cale, L. Camaj, M. C. Hamati, and T. P. Dominguez. 2018. Transmitting trauma: A systematic review of vicarious racism and child health. Social Science & Medicine 199:230–240.
Heim, C., and E. B. Binder. 2012. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology 233(1):102–111.
Heinrichs, M., T. Baumgartner, C. Kirschbaum, and U. Ehlert. 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry 54(12):1389–1398.
Hennessy, M. B., S. Kaiser, and N. Sachser. 2009. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology 30(4):470–482.
Hensch, T. K. 2016a. Critical ingredients for brain development. Scientific American, https://www.scientificamerican.com/article/critical-ingredients-for-brain-development (accessed April 17, 2019).
Hensch, T. K. 2016b. The power of the infant brain. Scientific American 314(2):64–69.
Hensch, T. K., and P. M. Bilimoria. 2012. Re-opening windows: Manipulating critical periods for brain development. Cerebrum 11.
Herman-Giddens, M. E., E. J. Slora, R. C. Wasserman, C. J. Bourdony, M. V. Bhapkar, G. G. Koch, and C. M. Hasemeier. 1997. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the pediatric research in office settings network. Pediatrics 99(4):505–512.
Herman-Giddens, M. E., J. Steffes, D. Harris, E. Slora, M. Hussey, S. A. Dowshen, R. Wasserman, J. R. Serwint, L. Smitherman, and E. O. Reiter. 2012. Secondary sexual characteristics in boys: Data from the pediatric research in office settings network. Pediatrics 130(5):e1058–e1068.
Hertzman, C., and T. Boyce. 2010. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health 31:329–347.
Heun-Johnson, H., and P. Levitt. 2018. Differential impact of MET receptor gene interaction with early-life stress on neuronal morphology and behavior in mice. Neurobiology of Stress 8:10–20.
Hiatt, R. A., S. L. Stewart, K. S. Hoeft, L. H. Kushi, G. C. Windham, F. M. Biro, S. M. Pinney, M. S. Wolff, S. L. Teitelbaum, and D. Braithwaite. 2017. Childhood socioeconomic position and pubertal onset in a cohort of multiethnic girls: Implications for breast cancer. Cancer Epidemiology and Prevention Biomarkers 26(12):1714–1721.
Hoffman, M. C. 2016. Stress, the placenta, and fetal programming of behavior: Genes’ first encounter with the environment. American Journal of Psychiatry 173(7):655–657.
Hofmeyr, F., C. A. Groenewald, D. G. Nel, M. M. Myers, W. P. Fifer, C. Signore, G. D. Hankins, and H. J. Odendaal. 2014. Fetal heart rate patterns at 20 to 24 weeks gestation as recorded by fetal electrocardiography. Journal of Maternal-Fetal and Neonatal Medicine 27(7):714–718.
Hosseini, M., H. Soltanian-Zadeh, K. Elisevich, and D. Pompili. 2016. Cloud-based deep learning of big EEG data for epileptic seizure prediction. Paper read at 2016 IEEE Global Conference on Signal and Information Processing (GlobalSIP), December 7–9, 2016.
Hostinar, C. E., R. M. Sullivan, and M. R. Gunnar. 2014. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin 140(1):256–282.
Howerton, C. L., and T. L. Bale. 2012. Prenatal programing: At the intersection of maternal stress and immune activation. Hormones and Behavior 62(3):237–242.
Huston, A. C., A. E. Gupta, J. T. Walker, C. J. Dowsett, S. R. Epps, A. E. Imes, and V. C. McLoyd. 2011. The long-term effects on children and adolescents of a policy providing work supports for low-income parents. Journal of Policy Analysis and Management 30(4):729–754.
Iacono, W. G., S. M. Malone, and M. McGue. 2008. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology 4:325–348.
Insel, T. R. 2010. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron 65(6):768–779.
IOM aqnd NRC (Institute of Medicine and National Research Council). 2015. Transforming the workforce for children birth through age 8: A unifying foundation. Washington, DC: The National Academies Press.
Israel, S., O. Weisel, R. P. Ebstein, and G. Bornstein. 2012. Oxytocin, but not vasopressin, increases both parochial and universal altruism. Psychoneuroendocrinology 37(8):1341–1344.
Jayaraman, D., B. I. Bae, and C. A. Walsh. 2018. The genetics of primary microcephaly. Annual Review of Genomics and Human Genetics 19:177–200.
Johnson, S. B., J. L. Riis, and K. G. Noble. 2016. State of the art review: Poverty and the developing brain. Pediatrics 137(4):e20153075.
Jones, C. E., R. A. Opel, M. E. Kaiser, A. Q. Chau, J. R. Quintana, M. A. Nipper, D. A. Finn, E. A. D. Hammock, and M. M. Lim. 2019. Early-life sleep disruption increases parvalbumin in primary somatosensory cortex and impairs social bonding in prairie voles. Science Advances 5(1):1–11.
Jones, S. 2019. Encyclopedia of cells in early pregnancy. Nature Biotechnology 37:26.
Juonala, M., J. S. Viikari, T. Ronnemaa, L. Taittonen, J. Marniemi, and O. T. Raitakari. 2006. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. Arteriosclerosis, Thrombosis, and Vascular Biology 26(8):1883–1888.
Kalish, J. M., C. Jiang, and M. S. Bartolomei. 2014. Epigenetics and imprinting in human disease. The International Journal of Developmental Biology 58(2–4):291–298.
Kaminski, J. W., R. Perou, S. N. Visser, K. G. Scott, L. Beckwith, J. Howard, D. C. Smith, and M. L. Danielson. 2013. Behavioral and socioemotional outcomes through age 5 years of the Legacy for Children public health approach to improving developmental outcomes among children born into poverty. American Journal of Public Health 103(6):1058–1066.
Kast, R. J., and P. Levitt. 2019. Precision in the development of neocortical architecture: From progenitors to cortical networks. Progress in Neurobiology 175:77–95.
Katz, L. C., and C. J. Shatz. 1996. Synaptic activity and the construction of cortical circuits. Science 274(5290):1133–1138.
Kelly, Y., A. Zilanawala, A. Sacker, R. Hiatt, and R. Viner. 2017. Early puberty in 11-year-old girls: Millennium cohort study findings. Archives of Disease in Childhood 102(3):232–237.
Keshavan, M., J. Giedd, J. Lau, D. A. Lewis, and T. Paus. 2014. Changes in the adolescent brain and the pathophysiology of psychotic disorders. The Lancet Psychiatry 1(7):549–558.
Khan, N. A., and C. H. Hillman. 2014. The relation of childhood physical activity and aerobic fitness to brain function and cognition: A review. Pediatric Exercise Science 26(2):138–146.
Kikusui, T., J. T. Winslow, and Y. Mori. 2006. Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences 361(1476):2215–2228.
Kim, S., and L. Strathearn. 2017. Trauma, mothering, and intergenerational transmission: A synthesis of behavioral and oxytocin research. The Psychoanalytic Study of the Child 70(1):200–223.
King, L. B., H. Walum, K. Inoue, N. W. Eyrich, and L. J. Young. 2016. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biological Psychiatry 80(2):160–169.
Knoll, A. T., K. Jiang, and P. Levitt. 2018. Quantitative trait locus mapping and analysis of heritable variation in affiliative social behavior and co-occurring traits. Genes, Brain, and Behavior 17(5):e12431.
Knudsen, E. I. 2004. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience 16(8):1412–1425.
Kolodkin, A. L., and M. Tessier-Lavigne. 2011. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harbor Perspectives in Biology 3(6).
Koss, K. J., and M. R. Gunnar. 2018. Annual research review: Early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry 59(4):327–346.
Kostović, I., N. Jovanov-Milošević, M. Radoš, G. Sedmak, V. Benjak, M. Kostović-Srzentić, L. Vasung, M. Ćuljat, M. Radoš, P. Hüppi, and M. Judaš. 2014. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Structure and Function 219(1):231–253.
Krieger, N. 2012. Methods for the scientific study of discrimination and health: An ecosocial approach. American Journal of Public Health 102(5):936–944.
Krieger, N., D. Carney, K. Lancaster, P. D. Waterman, A. Kosheleva, and M. Banaji. 2010. Combining explicit and implicit measures of racial discrimination in health research. American Journal of Public Health 100(8):1485–1492.
Krishnan, M. L., Z. Wang, P. Aljabar, G. Ball, G. Mirza, A. Saxena, S. J. Counsell, J. V. Hajnal, G. Montana, and A. D. Edwards. 2017. Machine learning shows association between genetic variability in PPARG and cerebral connectivity in preterm infants. Proceedings of the National Academy of Sciences of the United States of America 114(52):13744–13749.
Krsnik, Ž., V. Majić, L. Vasung, H. Huang, and I. Kostović. 2017. Growth of thalamocortical fibers to the somatosensory cortex in the human fetal brain. Frontiers in Neuroscience 11:233.
Kubo, A., J. Deardorff, C. A. Laurent, A. Ferrara, L. C. Greenspan, C. P. Quesenberry, and L. H. Kushi. 2018. Associations between maternal obesity and pregnancy hyperglycemia and timing of puberty onset in adolescent girls: A population-based study. American Journal of Epidemiology 187(7):1362–1369.
Kudielka, B. M., and C. Kirschbaum. 2005. Sex differences in HPA axis responses to stress: A review. Biological Psychology 69(1):113–132.
Kuh, D., Y. Ben-Shlomo, J. Lynch, J. Hallqvist, and C. Power. 2003. Life course epidemiology. Journal of Epidemiology and Community Health 57(10):778–783.
Kumsta, R. 2019. The role of epigenetics for understanding mental health difficulties and its implications for psychotherapy research. Psychology and Psychotherapy: Theory, Research and Practice 92(2):190–207.
Leighton, A. H., and C. Lohmann. 2016. The wiring of developing sensory circuits—from patterned spontaneous activity to synaptic plasticity mechanisms. Frontiers in Neural Circuits 10:71.
Lerner, R. M., S. J. Schwartz, and E. Phelps. 2009. Problematics of time and timing in the Longitudinal Study of Human Development: Theoretical and methodological issues. Human Development 52(1):44–68.
Levitt, P., and K. L. Eagleson. 2018. The ingredients of healthy brain and child development. Washington University Journal of Law and Policy 57.
Li, M., G. Santpere, Y. Imamura Kawasawa, O. V. Evgrafov, F. O. Gulden, S. Pochareddy, S. M. Sunkin, Z. Li, Y. Shin, Y. Zhu, A. M. M. Sousa, D. M. Werling, R. R. Kitchen, H. J. Kang, M. Pletikos, J. Choi, S. Muchnik, X. Xu, D. Wang, B. Lorente-Galdos, S. Liu, P. Giusti-Rodriguez, H. Won, C. A. de Leeuw, A. F. Pardinas, M. Hu, F. Jin, Y. Li, M. J. Owen, M. C. O’Donovan, J. T. R. Walters, D. Posthuma, M. A. Reimers, P. Levitt, D. R. Weinberger, T. M. Hyde, J. E. Kleinman, D. H. Geschwind, M. J. Hawrylycz, M. W. State, S. J. Sanders, P. F. Sullivan, M. B. Gerstein, E. S. Lein, J. A. Knowles, and N. Sestan. 2018. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362(6420).
Libby, P., P. M. Ridker, and A. Maseri. 2002. Inflammation and atherosclerosis. Circulation 105(9):1135–1143.
Loria, A. S., D. H. Ho, and J. S. Pollock. 2014. A mechanistic look at the effects of adversity early in life on cardiovascular disease risk during adulthood. Acta Physiologica 210(2):277–287.
Luby, J. L., D. M. Barch, A. Belden, M. S. Gaffrey, R. Tillman, C. Babb, T. Nishino, H. Suzuki, and K. N. Botteron. 2012. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proceedings of the National Academy of Sciences of the United States of America 109(8):2854–2859.
Ludwig, J., L. Sanbonmatsu, L. Gennetian, E. Adam, G. J. Duncan, L. F. Katz, R. C. Kessler, J. R. Kling, S. T. Lindau, R. C. Whitaker, and T. W. McDade. 2011. Neighborhoods, obesity, and diabetes—a randomized social experiment. New England Journal of Medicine 365(16):1509–1519.
Luthar, S. S., and N. Eisenberg. 2017. Resilient adaptation among at-risk children: Harnessing science toward maximizing salutary environments. Child Development 88(2):337–349.
Luthar, S. S., N. E. Suchman, and M. Altomare. 2007. Relational psychotherapy mothers’ group: A randomized clinical trial for substance abusing mothers. Development and Psychopathology 19(1):243–261.
Luthar, S., E. J. Crossman, and P. J. Small. 2015. Resilience and adversity. In Handbook of child psychology and developmental science, 7th ed. Vol. 3, edited by R. M. Lerner and M. E. Lamb. New York: Wiley. Pp. 247–286.
Luthar, S. S., A. Curlee, S. J. Tye, J. C. Engelman, and C. M. Stonnington. 2017. Fostering resilience among mothers under stress: “Authentic connections groups” for medical professionals. Women’s Health Issues 27(3):382–390.
Lynch, S. V., and O. Pedersen. 2016. The human intestinal microbiome in health and disease. New England Journal of Medicine 375(24):2369–2379.
Mantella, R. C., R. R. Vollmer, L. Rinaman, X. Li, and J. A. Amico. 2004. Enhanced corticosterone concentrations and attenuated FOS expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 287(6):1494–1504.
Masten, A. S. 2014. Global perspectives on resilience in children and youth. Child Development 85(1):6–20.
Masten, A. S. 2018. Resilience theory and research on children and families: Past, present, and promise. Journal of Family Theory & Review 10(1):12–31.
Matthews, S. G., and D. I. Phillips. 2012. Transgenerational inheritance of stress pathology. Experimental Neurology 233(1):95–101.
McCarty, C. A., R. L. Chisholm, C. G. Chute, I. J. Kullo, G. P. Jarvik, E. B. Larson, R. Li, D. R. Masys, M. D. Ritchie, D. M. Roden, J. P. Struewing, and W. A. Wolf. 2011. The eMERGE network: A consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Medical Genomics 4:13.
McCoy, D. C., C. C. Raver, and P. Sharkey. 2015. Children’s cognitive performance and selective attention following recent community violence. Journal of Health and Social Behavior 56(1):19–36.
McCracken, D. S., D. A. Allen, and A. J. Gow. 2016. Associations between urban greenspace and health-related quality of life in children. Preventive Medicine Reports 3:211–221.
McEwen, B. S. 1998. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences 840(1):33–44.
McEwen, B. S. 2017. Allostasis and the epigenetics of brain and body health over the life course: The brain on stress. JAMA Psychiatry 74(6):551–552.
McEwen, B. S., and L. Getz. 2013. Lifetime experiences, the brain and personalized medicine: An integrative perspective. Metabolism 62(Suppl 1):S20–S26.
McEwen, C. A., and B. S. McEwen. 2017. Social structure, adversity, toxic stress, and intergenerational poverty: An early childhood model. Annual Review of Sociology 43(1):445–472.
McEwen, B. S., and J. H. Morrison. 2013. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79(1):16–29.
McEwen, B. S., N. P. Bowles, J. D. Gray, M. N. Hill, R. G. Hunter, I. N. Karatsoreos, and C. Nasca. 2015. Mechanisms of stress in the brain. Nature Neuroscience 18(10):1353–1363.
McGee, A. W., Y. Yang, Q. S. Fischer, N. W. Daw, and S. M. Strittmatter. 2005. Experience-driven plasticity of visual cortex limited by myelin and NOGO receptor. Science 309(5744):2222–2226.
McGregor, T. L., S. L. Van Driest, K. B. Brothers, E. A. Bowton, L. J. Muglia, and D. M. Roden. 2013. Inclusion of pediatric samples in an opt-out biorepository linking DNA to de-identified medical records: Pediatric BioVU. Clinical Pharmacology & Therapeutics 93(2):204–211.
McKay, R. 2011. Developmental biology: Remarkable role for the placenta. Nature 472(7343):298–299.
McLanahan, S., I. Garfinkel, N. Reichman, J. Teitler, M. Carlson, and C. N. Audigier. 2003. The Fragile Families and Child Wellbeing Study: Baseline national report. Princeton, NJ: Center for Research on Child Wellbeing, Princeton University.
McLaughlin, K. A., M. A. Sheridan, and C. A. Nelson. 2017. Neglect as a violation of species-expectant experience: Neurodevelopmental consequences. Biological Psychiatry 82(7):462–471.
McLoyd, V. C. 1990. The impact of economic hardship on black families and children: Psychological distress, parenting, and socioemotional development. Child Development 61:311–346.
Meaney, M. J. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience 24(1):1161–1192.
Meaney, M. J., and A. C. Ferguson-Smith. 2010. Epigenetic regulation of the neural transcriptome: The meaning of the marks. Nature Neuroscience 13(11):1313–1318.
Medland, S. E., N. Jahanshad, B. M. Neale, and P. M. Thompson. 2014. Whole-genome analyses of whole-brain data: Working within an expanded search space. Nature Neuroscience 17(6):791–800.
Mendle, J., R. M. Ryan, and K. M. McKone. 2016. Early childhood maltreatment and pubertal development: Replication in a population-based sample. Journal of Research on Adolescence 26(3):595–602.
Miguel, P. M., L. O. Pereira, P. P. Silveira, and M. J. Meaney. 2019. Early environmental influences on the development of children’s brain structure and function. Developmental Medicine & Child Neurology. doi: 10.1111/dmcn.14182. [Epub ahead of print.]
Miller, C., A. C. Huston, G. J. Duncan, V. C. McLoyd, and T. S. Weisner. 2008. New hope for the working poor: Effects after eight years for families and children. New York: MDRC.
Miller, D. J., T. Duka, C. D. Stimpson, S. J. Schapiro, W. B. Baze, M. J. McArthur, A. J. Fobbs, A. M. M. Sousa, N. Šestan, D. E. Wildman, L. Lipovich, C. W. Kuzawa, P. R. Hof, and C. C. Sherwood. 2012. Prolonged myelination in human neocortical evolution. Proceedings of the National Academy of Sciences of the United States of America 109(41):16480–16485.
Miller, E. M. 2017. Beyond passive immunity: Breastfeeding, milk and collaborative mother-infant immune systems. In Breastfeeding, edited by C. Tomori, A. E. L. Palmquist, and E. A. Quinn. Abindgon, UK: Routledge. Pp. 50–63.
Miller, G. E., E. Chen, and K. J. Parker. 2011. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin 137(6):959–997.
Morishita, H., J. H. Cabungcal, Y. Chen, K. Q. Do, and T. K. Hensch. 2015. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biological Psychiatry 78(6):396–402.
Morrison, K. E., C. N. Epperson, M. D. Sammel, G. Ewing, J. S. Podcasy, L. Hantsoo, D. R. Kim, and T. L. Bale. 2017. Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biological Psychiatry 81(8):693–701.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. The promise of adolescence: Realizing opportunity for all youth. Washington, DC: The National Academies Press.
National Scientific Council on the Developing Child. 2010. Early experiences can alter gene expression and affect long-term development. Working paper no. 10. Cambridge, MA: Center on the Developing Child at Harvard University.
National Scientific Council on the Developing Child. 2014. Excessive stress disrupts the architecture of the brain. Working paper no. 3. Cambridge, MA: Center on the Developing Child at Harvard University.
Negi, S. K., and C. Guda. 2017. Global gene expression profiling of healthy human brain and its application in studying neurological disorders. Scientific Reports 7(1):897.
Nelson, C. A., 3rd, C. H. Zeanah, N. A. Fox, P. J. Marshall, A. T. Smyke, and D. Guthrie. 2007. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention project. Science 318(5858):1937–1940.
Nelson, C. A., N. A. Fox, and C. Zeanah. 2014. Romania’s abandoned children. Deprivation, brain development, and the struggle for recovery. Cambridge, MA: Harvard University Press.
Nese, R. N. T., C. M. Anderson, T. Ruppert, and P. A. Fisher. 2016. Effects of a video feedback parent training program during child welfare visitation. Children and Youth Services Review 71:266–276.
Noll, J. G., P. K. Trickett, J. D. Long, S. Negriff, E. J. Susman, I. Shalev, J. C. Li, and F. W. Putnam. 2017. Childhood sexual abuse and early timing of puberty. Journal of Adolescent Health 60(1):65–71.
Non, A. L., M. Rewak, I. Kawachi, S. E. Gilman, E. B. Loucks, A. A. Appleton, J. C. Roman, S. L. Buka, and L. D. Kubzansky. 2014. Childhood social disadvantage, cardiometabolic risk, and chronic disease in adulthood. American Journal of Epidemiology 180(3):263–271.
NRC and IOM. 2000. From neurons to neighborhoods: The science of early childhood development. Washington, DC: National Academy Press.
Nugent, B. M., and T. L. Bale. 2015. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Frontiers in Neuroendocrinology 39:28–37.
Numan, M., and L. J. Young. 2016. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior 77:98–112.
Nusslock, R., and G. E. Miller. 2016. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry 80(1):23–32.
Obradović, J., N. R. Bush, J. Stamperdahl, N. E. Adler, and W. T. Boyce. 2010. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development 81(1):270–289.
O’Connor, T. G., J. A. Moynihan, and M. T. Caserta. 2014. Annual research review: The neuroinflammation hypothesis for stress and psychopathology in children—developmental psychoneuroimmunology. Journal of Child Psychology and Psychiatry 55(6):615–631.
O’Donnell, K. J., A. Bugge Jensen, L. Freeman, N. Khalife, T. G. O’Connor, and V. Glover. 2012. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology 37(6):818–826.
O’Driscoll, A., J. Daugelaite, and R. D. Sleator. 2013. “Big data,” Hadoop and cloud computing in genomics. Journal of Biomedical Informatics 46(5):774–781.
Ogbu, J. U. 1981. Racism and health I: Pathways and scientific evidence. Child Development 52(2):413–429.
Pachter, L. M., and C. G. Coll. 2009. Racism and child health: A review of the literature and future directions. Journal of Developmental and Behavioral Pediatrics 30(3):255–263.
Pachter, L. M., L. A. Szalacha, B. A. Bernstein, and C. G. Coll. 2010. Perceptions of Racism in Children and Youth (PRACY): Properties of a self-report instrument for research on children’s health and development. Ethnicity & Health 15(1):33–46.
Padgett, D. A., and R. Glaser. 2003. How stress influences the immune response. Trends in Immunology 24(8):444–448.
Pager, D., and H. Shepherd. 2008. The sociology of discrimination: Racial discrimination in employment, housing, credit, and consumer markets. Annual Review of Sociology 34:181–209.
Painter, R. C., S. R. de Rooij, P. M. Bossuyt, D. I. Phillips, C. Osmond, D. J. Barker, O. P. Bleker, and T. J. Roseboom. 2006. Blood pressure response to psychological stressors in adults after prenatal exposure to the Dutch famine. Journal of Hypertension 24(9):1771–1778.
Paquette, A. G., H. M. Brockway, N. D. Price, and L. J. Muglia. 2017. Comparative transcriptomic analysis of human placentae at term and preterm delivery. Biology of Reproduction 98(1):89–101.
Paradies, Y., J. Ben, N. Denson, A. Elias, N. Priest, A. Pieterse, A. Gupta, M. Kelaher, and G. Gee. 2015. Racism as a determinant of health: A systematic review and meta-analysis. PLoS ONE 10(9):e0138511.
Pearson, T. A., G. A. Mensah, R. W. Alexander, J. L. Anderson, R. O. Cannon, M. Criqui, Y. Y. Fadl, S. P. Fortmann, Y. Hong, G. L. Myers, N. Rifai, S. C. Smith, K. Taubert, R. P. Tracy, and F. Vinicor. 2003. Markers of inflammation and cardiovascular disease application to clinical and public health practice—a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107(3):499–511.
Peña, C. J., H. G. Kronman, D. M. Walker, H. M. Cates, R. C. Bagot, I. Purushothaman, O. Issler, Y.-H. E. Loh, T. Leong, D. D. Kiraly, E. Goodman, R. L. Neve, L. Shen, and E. J. Nestler. 2017. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356:1185–1188.
Picard, M., and B. S. McEwen. 2018. Psychological stress and mitochondria: A conceptual framework. Psychosomatic Medicine 80(2):126–140.
Plomin, R., K. Asbury, and J. Dunn. 2001. Why are children in the same family so different? Nonshared environment a decade later. The Canadian Journal of Psychiatry 46(3):225–233.
Plotsky, P. M., and M. J. Meaney. 1993. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) MRNA, median eminence CRF content and stress-induced release in adult rats. Brain Research: Molecular Brain Research 18(3):195–200.
Plotsky, P., K. Thrivikraman, C. Nemeroff, C. Caldji, S. Sharma, and M. Meaney. 2005. Long-term consequences of neonatal rearing on central corticotropin–releasing factor systems in adult male rat offspring. Neuropsycho-pharmacology 30(12):2192–2204.
Pollak, S. D., D. Cicchetti, R. Klorman, and J. T. Brumaghim. 1997. Cognitive brain event-related potentials and emotion processing in maltreated children. Child Development 68(5):773–787.
Poulton, R., T. E. Moffitt, and P. A. Silva. 2015. The Dunedin Multidisciplinary Health and Development Study: Overview of the first 40 years, with an eye to the future. Social Psychiatry and Psychiatric Epidemiology 50(5):679–693.
Priest, N., Y. Paradies, M. Stevens, and R. Bailie. 2012. Exploring relationships between racism, housing and child illness in remote indigenous communities. Journal of Epidemiology and Community Health 66(5):440–447.
Priest, N., Y. Paradies, B. Trenerry, M. Truong, S. Karlsen, and Y. Kelly. 2013. A systematic review of studies examining the relationship between reported racism and health and wellbeing for children and young people. Social Science & Medicine 95:115–127.
Quirin, M., J. Kuhl, and R. Düsing. 2011. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology 36(6):898–904.
Reagan, P. B., P. J. Salsberry, M. Z. Fang, W. P. Gardner, and K. Pajer. 2012. African-American/white differences in the age of menarche: Accounting for the difference. Social Science & Medicine (1982) 75(7):1263–1270.
Roberts, A. L., S. Galea, S. B. Austin, H. L. Corliss, M. A. Williams, and K. C. Koenen. 2014. Women’s experience of abuse in childhood and their children’s smoking and overweight. American Journal of Preventive Medicine 46(3):249–258.
Roden, D. M., J. M. Pulley, M. A. Basford, G. R. Bernard, E. W. Clayton, J. R. Balser, and D. R. Masys. 2008. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clinical Pharmacology & Therapeutics 84(3):362–369.
Rodgers, A. B., C. P. Morgan, N. A. Leu, and T. L. Bale. 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proceedings of the National Academy of Sciences of the United States of America 112(44):13699–13704.
Rodrigues, S. M., L. R. Saslow, N. Garcia, O. P. John, and D. Keltner. 2009. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America 106(50):21437–21441.
Ross, H. E., and L. J. Young. 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology 30(4):534–547.
Ross, H. E., C. D. Cole, Y. Smith, I. D. Neumann, R. Landgraf, A. Z. Murphy, and L. J. Young. 2009a. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162(4):892–903.
Ross, H. E., S. M. Freeman, L. L. Spiegel, X. Ren, E. F. Terwilliger, and L. J. Young. 2009b. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. Journal of Neuroscience 29(5):1312–1318.
Rowold, E. D., L. Schulze, S. Van der Auwera, and H. J. Grabe. 2017. Paternal transmission of early life traumatization through epigenetics: Do fathers play a role? Medical Hypotheses 109:59–64.
Rubenstein, J., and P. Rakic. 2013. Patterning and cell type specification in the developing CNS and PNS, Vol. 1, Comprehensive developmental neuroscience. Amsterdam, the Netherlands: Academic Press.
Rudolph, M. D., A. M. Graham, E. Feczko, O. Miranda-Dominguez, J. M. Rasmussen, R. Nardos, S. Entringer, P. D. Wadhwa, C. Buss, and D. A. Fair. 2018. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nature Neuroscience 21(5):765–772.
Rundle, A. G., M. D. Bader, C. A. Richards, K. M. Neckerman, and J. O. Teitler. 2011. Using Google Street View to audit neighborhood environments. American Journal of Preventive Medicine 40(1):94–100.
Rutter, M. 2012. Resilience as a dynamic concept. Development and Psychopathology 24(2):335–344.
Rzotkiewicz, A., A. L. Pearson, B. V. Dougherty, A. Shortridge, and N. Wilson. 2018. Systematic review of the use of Google Street View in health research: Major themes, strengths, weaknesses and possibilities for future research. Health & Place 52:240–246.
Sallis, J. F., T. L. Conway, K. L. Cain, J. A. Carlson, L. D. Frank, J. Kerr, K. Glanz, J. E. Chapman, and B. E. Saelens. 2018. Neighborhood built environment and socioeconomic status in relation to physical activity, sedentary behavior, and weight status of adolescents. Preventive Medicine 110:47–54.
Sampson, J., J. D. Morenoff, and F. Earls. 1999. Beyond social capital: Spatial dynamics of collective efficacy for children. American Sociological Review 64:633–660.
Sampson, R. J., and S. W. Raudenbush. 1997. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science 277(5328):918.
Sampson, R. J., and S. W. Raudenbush. 1999. Systematic social observation of public spaces: A new look at disorder in urban neighborhoods. American Journal of Sociology 105(3):603–651.
Santavirta, T., N. Santavirta, and S. E. Gilman. 2018. Association of the World War II Finnish evacuation of children with psychiatric hospitalization in the next generation. JAMA Psychiatry 75(1):21–27.
Schindler, H. S., J. Kholoptseva, S. S. Oh, H. Yoshikawa, G. J. Duncan, K. A. Magnuson, and J. P. Shonkoff. 2015. Maximizing the potential of early childhood education to prevent externalizing behavior problems: A meta-analysis. Journal of School Psychology 53(3):243–263.
Schwartz, I. M., P. York, E. Nowakowski-Sims, and A. Ramos-Hernandez. 2017. Predictive and prescriptive analytics, machine learning and child welfare risk assessment: The Broward County experience. Children and Youth Services Review 81:309–320.
Schweinhart, L. J., J. R. Berrueta-Clement, W. S. Barnett, A. S. Epstein, and D. P. Weikart. 1985. Effects of the Perry Preschool Program on youths through age 19: A summary. Topics in Early Childhood Special Education 5(2):26–35.
Shalev, I., and J. Belsky. 2016. Early-life stress and reproductive cost: A two-hit developmental model of accelerated aging? Medical Hypotheses 90:41–47.
Sharma, D., S. Shastri, and P. Sharma. 2016. Intrauterine growth restriction: Antenatal and postnatal aspects. Clinical medicine insights. Pediatrics 10:67–83.
Shonkoff, J. P. 2010. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development 81(1):357–367.
Shonkoff, J. P. 2016. Capitalizing on advances in science to reduce the health consequences of early childhood adversity. JAMA Pediatrics 170(10):1003–1007.
Shonkoff, J. P. 2017. Rethinking the definition of evidence-based interventions to promote early childhood development. Pediatrics 140(6):e20173136.
Shonkoff, J. P., and S. N. Bales. 2011. Science does not speak for itself: Translating child development research for the public and its policymakers. Child Development 82(1):17–32.
Shonkoff, J. P., and A. S. Garner. 2012. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129(1):e232–e246.
Shonkoff, J. P., and P. Levitt. 2010. Neuroscience and the future of early childhood policy: Moving from why to what and how. Neuron 67(5):689–691.
Shonkoff, J. P., W. T. Boyce, and B. S. McEwen. 2009. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA 301(21):2252–2259.
Silbereis, J. C., S. Pochareddy, Y. Zhu, M. Li, and N. Sestan. 2016. The cellular and molecular landscapes of the developing human central nervous system. Neuron 89(2):248–268.
Simon, A. K., G. A. Hollander, and A. McMichael. 2015. Evolution of the immune system in humans from infancy to old age. Proceedings of the Royal Society B: Biological Sciences 282(1821):1–9.
Slopen, N., K. C. Koenen, and L. D. Kubzansky. 2012. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: A systematic review. Brain, Behavior, and Immunity 26.
Slopen, N., K. A. McLaughlin, and J. P. Shonkoff. 2014. Interventions to improve cortisol regulation in children: A systematic review. Pediatrics 133(2):312–326.
Slopen, N., G. Strizich, S. Hua, L. C. Gallo, D. H. Chae, N. Priest, M. J. Gurka, S. I. Bangdiwala, J. I. Bravin, E. C. Chambers, M. L. Daviglus, M. M. Llabre, M. R. Carnethon, and C. R. Isasi. 2019. Maternal experiences of ethnic discrimination and child cardiometabolic outcomes in the study of Latino (SOL) youth. Annals of Epidemiology 34:52–57.
Snowdon, C. T., B. A. Pieper, C. Y. Boe, K. A. Cronin, A. V. Kurian, and T. E. Ziegler. 2010. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Hormones and Behavior 58(4):614–618.
Social Programs That Work. 2017. Social programs that work review: Evidence summary for the Perry Preschool Project. https://evidencebasedprograms.org/document/perry-preschool-project-evidence-summary (accessed March 29, 2019).
Spencer, M. B., and C. Markstrom-Adams. 1990. Identity processes among racial and ethnic minority children in America. Child Development 61(2):290–310.
Sroufe, L. A. 2005. Attachment and development: A prospective, longitudinal study from birth to adulthood. Attachment & Human Development 7(4):349–367.
Stein, G. L., R. G. Gonzales, C. G. Coll, and J. I. Prandoni. 2016. Latinos in rural, new immigrant destinations: A modification of the integrative model of child development. In Rural ethnic minority youth and families in the United States: Theory, research, and applications. Advancing responsible adolescent development, edited by R. J. R. Levesque. Cham, Switzerland: Springer International Publishing. Pp. 37–56.
Stiles, J. 2008. The fundamentals of brain development: Integrating nature and nurture. Cambridge, MA: Harvard University Press.
Strohsnitter, W. C., E. E. Hatch, M. Hyer, R. Troisi, R. H. Kaufman, S. J. Robboy, J. R. Palmer, L. Titus-Ernstoff, D. Anderson, and R. N. Hoover. 2008. The association between in utero cigarette smoke exposure and age at menopause. American Journal of Epidemiology 167(6):727–733.
Sudhof, T. C. 2018. Towards an understanding of synapse formation. Neuron 100(2):276–293.
Sudhof, T. C., and R. C. Malenka. 2008. Understanding synapses: Past, present, and future. Neuron 60(3):469–476.
Sun, Y., F. K. Mensah, P. Azzopardi, G. C. Patton, and M. Wake. 2017. Childhood social disadvantage and pubertal timing: A national birth cohort from Australia. Pediatrics 139(6):e20164099.
Tang, Y., J. R. Nyengaard, D. M. De Groot, and H. J. Gundersen. 2001. Total regional and global number of synapses in the human brain neocortex. Synapse 41(3):258–273.
Taylor, S. E., B. M. Way, and T. E. Seeman. 2011. Early adversity and adult health outcomes. Development and Psychopathology 23(3):939–954.
Teicher, M. H., J. A. Samson, C. M. Anderson, and K. Ohashi. 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience 17(10):652.
Thompson, B. L., P. Levitt, and G. D. Stanwood. 2009. Prenatal exposure to drugs: Effects on brain development and implications for policy and education. Nature Reviews Neuroscience 10(4):303–312.
Thompson, R. A. 2014. Stress and child development. The Future of Children 24(1):41–59.
Thompson, R. A. 2016. What more has been learned? The science of early childhood development 15 years after Neurons to Neighborhoods. Zero to Three 36(3):18–24.
Tom, S. E., R. Cooper, D. Kuh, J. M. Guralnik, R. Hardy, and C. Power. 2010. Fetal environment and early age at natural menopause in a British birth cohort study. Human Reproduction 25(3):791–798.
Tourangeau, K., C. Nord, T. Lê, A. G. Sorongon, and M. Najarian. 2009. Early Childhood Longitudinal Study, kindergarten class of 1998–99 (ECLS-K): Combined user’s manual for the ECLS-K eighth-grade and K–8 full sample data files and electronic codebooks. NCES 2009-004. National Center for Education Statistics.
Tran, A. G. T. T. 2014. Family contexts: Parental experiences of discrimination and child mental health. American Journal of Community Psychology 53(1–2):37–46.
Tseng, P. H., I. G. Cameron, G. Pari, J. N. Reynolds, D. P. Munoz, and L. Itti. 2013. High-throughput classification of clinical populations from natural viewing eye movements. Journal of Neurology 260(1):275–284.
Turner, A. M., and W. T. Greenough. 1985. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Research 329(1–2):195–203.
Vaiserman, A. M. 2015. Epigenetic programming by early-life stress: Evidence from human populations. Developmental Dynamics 244(3):254–265.
Van Essen, D. C., S. M. Smith, D. M. Barch, T. E. Behrens, E. Yacoub, and K. Ugurbil. 2013. The WU-minn Human Connectome Project: An overview. Neuroimage 80:62–79.
Velez-Agosto, N. M., J. G. Soto-Crespo, M. Vizcarrondo-Oppenheimer, S. Vega-Molina, and C. García Coll. 2017. Bronfenbrenner’s bioecological theory revision: Moving culture from the macro into the micro. Perspectives on Psychological Science 12(5):900–910.
Vied, C. M., F. Freudenberg, Y. Wang, A. A. Raposo, D. Feng, and R. S. Nowakowski. 2014. A multi-resource data integration approach: Identification of candidate genes regulating cell proliferation during neocortical development. Frontiers in Neuroscience 8:257.
Waddington, C. H. 1942. The epigenotype. Endeavor 1:18–20.
Waddington, C. H. 1957. The strategy of the genes; a discussion of some aspects of theoretical biology. London, UK: Allen & Unwin.
Wade, M., N. A. Fox, C. H. Zeanah, and C. A. Nelson. 2018. Effect of foster care intervention on trajectories of general and specific psychopathology among children with histories of institutional rearing: A randomized clinical trial. JAMA Psychiatry 75(11):1137–1145.
Wadhwa, P. D., S. Entringer, C. Buss, and M. C. Lu. 2011. The contribution of maternal stress to preterm birth: Issues and considerations. Clinics in Perinatology 38(3):351–384.
Wang, H., S. L. Lin, G. M. Leung, and C. M. Schooling. 2016. Age at onset of puberty and adolescent depression: “Children of 1997” birth cohort. Pediatrics 137(6):e20153231.
Wang, Y., and S. Zhao. 2010. Vascular biology of the placenta. Colloquium Series on Integrated Systems Physiology: From Molecule to Function 2(1):1–98.
Weber-Stadlbauer, U. 2017. Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders. Translational Psychiatry 7(5):e1113.
Wee, C. Y., T. A. Tuan, B. F. Broekman, M. Y. Ong, Y. S. Chong, K. Kwek, L. P. Shek, S. M. Saw, P. D. Gluckman, M. V. Fortier, M. J. Meaney, and A. Qiu. 2017. Neonatal neural networks predict children behavioral profiles later in life. Human Brain Mapping 38(3):1362–1373.
Welberg, L. A., K. V. Thrivikraman, and P. M. Plotsky. 2005. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. Journal of Endocrinology 186(3):R7–R12.
Welch, M. G. 2016. Calming cycle theory: The role of visceral/autonomic learning in early mother and infant/child behaviour and development. Acta Paediatrica 105(11):1266–1274.
Welch, M. G., M. M. Myers, P. G. Grieve, J. R. Isler, W. P. Fifer, R. Sahni, M. A. Hofer, J. Austin, R. J. Ludwig, and R. I. Stark. 2014. Electroencephalographic activity of preterm infants is increased by family nurture intervention: A randomized controlled trial in the NICU. Clinical Neurophysiology 125(4):675–684.
Welch, M. G., M. R. Firestein, J. Austin, A. A. Hane, R. I. Stark, M. A. Hofer, M. Garland, S. B. Glickstein, S. A. Brunelli, R. J. Ludwig, and M. M. Myers. 2015. Family nurture intervention in the neonatal intensive care unit improves social-relatedness, attention, and neurodevelopment of preterm infants at 18 months in a randomized controlled trial. Journal of Child Psychology and Psychiatry 56(11):1202–1211.
Williams, D. R., and S. A. Mohammed. 2009. Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine 32(1):20–47.
Williams, D. R., and S. A. Mohammed. 2013. Racism and health I: Pathways and scientific evidence. American Behavioral Scientist 57(8).
Williams, D. R., Y. Yan, J. S. Jackson, and N. B. Anderson. 1997. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology 2(3):335–351.
Williams, D. R., J. A. Lawrence, and B. A. Davis. 2019. Racism and health: Evidence and needed research. Annual Review of Public Health 40(1):105–125.
Woo Baidal, J. A., L. M. Locks, E. R. Cheng, T. L. Blake-Lamb, M. E. Perkins, and E. M. Taveras. 2016. Risk factors for childhood obesity in the first 1,000 days: A systematic review. American Journal of Preventive Medicine 50(6):761–779.
Yarde, F., F. Broekmans, K. Van der Pal-de Bruin, Y. Schönbeck, E. Te Velde, A. Stein, and L. Lumey. 2013. Prenatal famine, birthweight, reproductive performance and age at menopause: The Dutch Hunger Winter Families Study. Human Reproduction 28(12):3328–3336.
Yehuda, R., and L. M. Bierer. 2007. Transgenerational transmission of cortisol and PTSD risk. In Stress hormones and post traumatic stress disorder: Basic studies and clinical perspectives, Vol. 167, edited by E. R. DeKloet and E. Vermetten. Amsterdam, the Netherlands: Elsevier Science. Pp. 121–135.
Yehuda, R., S. M. Engel, S. R. Brand, J. Seckl, S. M. Marcus, and G. S. Berkowitz. 2005. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. The Journal of Clinical Endocrinology & Metabolism 90(7):4115–4118.
Yehuda, R., N. P. Daskalakis, A. Lehrner, F. Desarnaud, H. N. Bader, I. Makotkine, J. D. Flory, L. M. Bierer, and M. J. Meaney. 2014. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. American Journal of Psychiatry 171(8):872–880.
Yeung, M. S., S. Zdunek, O. Bergmann, S. Bernard, M. Salehpour, K. Alkass, S. Perl, J. Tisdale, G. Possnert, L. Brundin, H. Druid, and J. Frisen. 2014. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159(4):766–774.
Yudell, M., D. Roberts, R. DeSalle, and S. Tishkoff. 2016. Taking race out of human genetics. Science 351(6273):564–565.
Zeanah, C. H., C. A. Nelson, N. A. Fox, A. T. Smyke, P. Marshall, S. W. Parker, and S. Koga. 2003. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology 15(4):885–907.
Zeanah, C. H., M. R. Gunnar, R. B. McCall, J. M. Kreppner, and N. A. Fox. 2011. VI. Sensitive periods. Monographs of the Society for Research in Child Development 76(4):147–162.
Zhang, C., A. Paolozza, P. H. Tseng, J. N. Reynolds, D. P. Munoz, and L. Itti. 2019. Detection of children/youth with fetal alcohol spectrum disorder through eye movement, psychometric, and neuroimaging data. Frontiers in Neurology 10:80.
Zhao, L., X. Zheng, J. Liu, R. Zheng, R. Yang, Y. Wang, and L. Sun. 2019. The placental transcriptome of the first-trimester placenta is affected by in vitro fertilization and embryo transfer. Reproductive Biology and Endocrinology 17(1):50.
































































