4
Strategies for Legionella Control and Their Application in Building Water Systems
This chapter focuses on strategies for Legionella control in building water systems. Such controls should ideally begin as early as the design and commissioning phases and subsequently be applied routinely as preventative measures and, when necessary, for remedial purposes, i.e., in response to outbreak or flags raised by monitoring data. A summary of the key strategies for controlling Legionella by affecting their growth and survival (or that of their free-living amoebae hosts) is presented first. The real-world application of these strategies for Legionella control in building water systems and devices is then described. Table 4-1 summarizes which specific controls are applicable to which building water systems and devices. The chapter also discusses emerging issues, such as potential conflicts among strategies for green building design, water and energy conservation, and more prospective Legionella control strategies.
As detailed in the following sections, factors known to influence Legionella growth in water systems include temperature, disinfectant type and levels, hydraulic conditions (particularly avoiding stagnation), presence of nutrients, pipe materials, presence of distal devices, and extent of aerosol formation. Many of these factors come into play during the initial building design and commissioning stages, while others can more readily be adjusted in existing buildings. For example, in a building, the pipe sizing, the materials and devices used, and the flow conditions are determined prior to the building’s construction and are more difficult to adjust once a building is operating. Factors such as temperature, disinfectants, and distal devices can be more easily adjusted after building construction and during operation. Control of Legionella can be based on limiting not only its growth, but also the opportunities for humans to be exposed, for example by avoiding the formation of aerosols, particularly those of ideal size (less than 10 µm) for inhalation and deep deposition into the lungs. Aerosols can also be diverted, as in the case of drift eliminators on cooling towers, to reduce potential for human exposure. Additional barriers, such as point-of-use size-exclusion filters, can also be considered for immunocompromised or other sensitive populations.
TABLE 4-1 Overview of Legionella Control Strategies and Relevance of Their Application to Building and Water System Types
| Building Water Systems | Large Engineered Systems | Other Devices | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strategy | Large Institutional Buildings (page 196) | Green Buildings (page 209) | Households (page 201) | Potable Water Supply (page 192) | Wastewater Treatment (page 195) | Reclaimed Water Systems (page 194) | Cooling Towers (page 203) | Humidifiers (page 205) | Hot Tubs (page 207) |
| Temperature Control (page 167) | ✔ | ✖ (incentive is to reduce temperature) | ✔ | ? (limited options) | ? (future possibility) | ✔ | |||
| Disinfection (page 176) | ✔ | ✔ | ? (only POU UV devices) | ✔ | ? (somewhat limited) | ✔ | ✔ | ✔ | |
| Manage Hydraulics (page 182) | ✔ | ✖ (prone to low flow/stagnation) | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Nutrient Limitation (page 184) | ? (Dutch example) | ✔ | ✔ | ||||||
| Plumbing Materials (page 186) | ✔ | ✔ | ✔ | ? (limited for DS mains) | ✔ | ✔ | |||
| Distal Portion of Plumbing (page 187) | ✔ | ✖ (low-flow faucets used) | ✔ | ||||||
| Aerosol Control (page 190) | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
NOTES: ✔ = a strategy has been successfully used in a particular system; ? = a strategy could be partially used in a particular system, but there are noted limitations/considerations; ✖ = a strategy is actually being worked against in a particular type of system; blank boxes are where there is no indication that a strategy can be used in a particular system. POU UV = point-of-use ultraviolet light.
In addition to drinking, potable water is used for other critical services in buildings, especially hot tubs, spas, and Jacuzzis (collectively referred to as hot tubs), cooling towers, humidifiers, decorative features such as fountains, medical equipment, dental units, and ice machines. Although any of these water systems has the potential to grow and transmit Legionella, this discussion is limited to the premise plumbing of buildings, cooling towers, humidifiers, hot tubs, and corresponding water supplies, though some of the basic principles apply to other systems as well.
The precise target for Legionella control can be quite complex in terms of species, serotypes, strains, and corresponding virulence factors. Notably, some treatments may shift the composition of types and virulence of Legionella, which is difficult to assess and not typically measured. This chapter provides information based on the targets that are described in the available literature. Still, it is important to note that the type of Legionella detection method will also influence the perception of efficacy of various controls. The majority of well-documented case studies base their evaluation on measurements of Legionella or Legionella pneumophila using culture-based methods, which cannot detect viable but nonculturable (VBNC-like) forms. Certain control strategies like heat treatment, chlorine-based disinfectants, and copper-silver ionization are known to trigger L. pneumophila to enter a VBNC-like state (see Chapter 2, Allegra et al., 2008, 2011).
It is clear from research and practice that, in most situations, “zero” is not an achievable target for evaluating whether Legionella has effectively been controlled, for several reasons. First, some level of Legionella is common in drinking water systems in the absence of an outbreak. For example, L. pneumophila serogroup 1 was detected in nearly half of public and private cold-water taps tested in a national survey, with the mean and median concentrations being 1.97 × 103 gene copies per liter (GC/L) and 62 GC/L, respectively (Chapter 3; Donohue et al., 2014). Second, current human-health risk models indicate that a bulk water concentration much higher than “zero” (see Chapter 3; Perinel et al., 2018; Pourchez et al., 2017) is actionable and associated with transmission of Legionella into the lungs. Third, monitoring methods are limited in their ability to assess live cells and are subject to detection limits; none can confirm “zero.”
In evaluating any building water system, it is important to recognize that Legionella does not exist in isolation, but is part of a complex microbial ecosystem spanning biofilms, bulk water, and aerosols. Thousands of other species of bacteria and other microbes reside in these environments (Chapter 2; Pinto et al., 2014) and can potentially enhance or inhibit the growth of Legionella (Paranjape et al., 2019; Wang et al., 2013a). Most notoriously, free-living amoebae play a key role in amplifying Legionella and enhancing its virulence; thus, it has been suggested that effective control strategies should also target amoebae (Thomas and Ashbolt, 2011). However, such approaches that potentially tap into more precise control of the microbial ecology of premise plumbing to manage Legionella are still in their infancy. Here we seek to provide information about how various controls influence Legionella and, where possible, their free-living amoebal hosts.
FUNDAMENTAL FACTORS FOR LEGIONELLA CONTROL
Temperature
A fundamental control strategy for Legionella in buildings is to keep the hot- and cold-water systems at temperatures outside the organism’s growth range of 25°C to 43°C (see Chapter 2). Warm water leaves a water system especially vulnerable to Legionella colonization and growth. Several studies summarized in this section, across multiple scales,
countries, and building settings, demonstrate the overarching benefit of elevated temperature for Legionella control. In particular, water heater settings of greater than 60°C are a key threshold for reducing positive detection of Legionella as well as for reducing Legionnaires’ disease cases and outbreaks. Adjusting the temperature at the water heater outlet to ensure temperatures greater than 55°C to distal points1 can be highly effective in reducing the proportion of Legionella-positive swabs or water samples (Arvand et al., 2011; Blanc et al., 2005).
Temperature control strategies fall into two broad categories: preventive and curative. Preventive refers to maintenance of (1) elevated temperatures (greater than 55°C) to limit colonization and growth of Legionella across hot-water systems and (2) sufficiently cool temperatures (less than 25°C) across cold-water systems. Curative approaches, on the other hand, are somewhat varied in their application, generally involving elevating the temperature temporarily as a “heat-shock” approach. Heat shocks may be applied one time or many times, for various durations, and over a range of temperatures (60°C to 70°C). It should be noted that eradication of Legionella species (spp.) and L. pneumophila reservoirs can only be achieved at very high temperatures. Work by Epalle et al. (2015) shows that only strict thermal treatment (i.e., 70°C for 60 minutes) kills more cells and renders non-infectious all L. pneumophila strains, both environmental and clinical, but milder heat treatment shocks (60°C to 70°C for 30 minutes) do not. Recent investigations by Cervero-Arago et al. (2019) suggest that prolonged exposure to high temperature (greater than 60°C) can be efficient against both culturable and VBNC-like cells of L. pneumophila, and most importantly, that the loss of culturability after heat exposure is associated with decreased virulence and host infection.
The temperature set at the water heater is not equivalent to the temperature experienced at the tap. One controlled study demonstrated that hot water received in taps can cool to room temperature within 30 minutes (Rhoads et al., 2015a). To counteract this, large institutional buildings, such as hospitals, are required by plumbing codes to have hot-water circulation lines leading from the water heater, throughout the building, and back to the heater. This helps provide hot water on demand in distal reaches of the building and also keeps the water lines sufficiently hot to deter Legionella growth. Recirculation lines cannot reach each point of use, such that the volume of water between the recirculating pipe and the faucet or showerhead will remain stagnant between uses. Even with recirculation, temperature losses are expected throughout the piping as a function of water circulation and piping isolation. This can result in large variations of water temperatures at distal points, including temperatures that increase risk for Legionella growth (Bédard et al., 2015; Boppe et al., 2016).
None of the control strategies discussed in this chapter occur in isolation, and they all have interactive effects. In the case of temperature, the associated water-use frequency is an important factor in determining the temperature regime experienced at the tap (Rhoads et al., 2015a). Thus, efficacy of temperature control is intimately related to the hydraulics of the system. Figure 4-1 illustrates a standard hot-water system as commonly applied in large institutional buildings, including recirculating options and points where temperature control may be applied. This section focuses on the basic evidence of temperature control efficacy, while later parts of the chapter discuss specific applications in buildings and devices.
___________________
1 “Distal point” refers to the point of connection to a fixture such as a faucet, showerhead, thermal mixing valve, etc. Hence, the distal point is just upstream of the point of use. Temperature measurements at the tap are representative of conditions at distal points unless there is a thermostatic mixing valve.
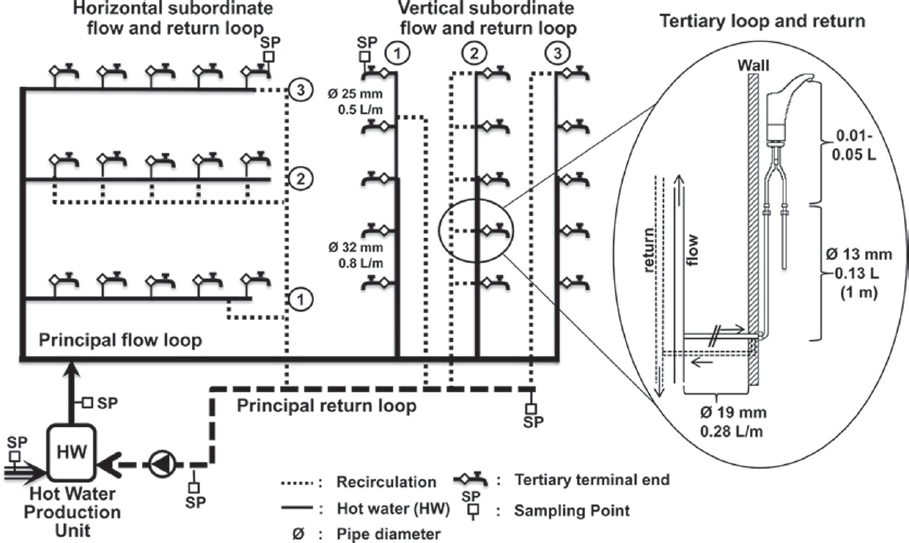
SOURCE: Bédard et al. (2015).
Impact of Temperature on Legionella in Building Water Systems
Groothuis et al. (1985) observed that when the temperature of a hot-water return line in buildings is maintained at 60°C, cultivable L. pneumophila was not observed, but when the temperature was lowered to 54°C, L. pneumophila was culturable. Similar observations have been made by others. L. pneumophila could be cultivated from a hot-water system at a hospital that maintained hot water at 43°C to 45°C, but not at a hospital where hot water was maintained at 58°C to 60°C (Plouffe et al., 1983). Apartments in the Chicago area (n = 95) that had water temperatures below 60°C in the premise plumbing were more often colonized with cultivable L. pneumophila (42 percent) than were systems with water temperatures above 60°C (7 percent) (Arnow et al., 1985). In a survey of 40 Italian hotels, hot water above 60°C in the drinking water system and above 55°C in the outlet water was protective from legionellae (Borella et al., 2005). Finally, cultivable legionellae were only isolated from drinking water in hotels (n = 385) in Greece when water temperatures were between 23.7°C and 60.3°C (Mouchtouri et al., 2007).
Table 4-2 summarizes several examples of the efficacy of thermal controls in healthcare facilities. The Hungarian study (Barna et al., 2016) in Table 4-2 is particularly illustrative of the overarching importance of thermal control of Legionella in hot-water plumbing. Over seven years, 1,809 samples were collected from healthcare facilities (n = 22), accommodation sites (n = 21), educational institutions (n = 26), office buildings (n = 10), industrial buildings (n = 35), and private residences with central (n = 26) and individual hot-water supplies (n = 26). Water temperature was found to be the most important factor in a multiple linear regression analysis of 11 system and water characteristics associated with Legionella.
TABLE 4-2 Long-term Healthcare Facility Experience Showing the Importance of Maintaining an Adequate Preventive Thermal Regime to Control Legionella
| Size of Building(s) Number of Samples Study Length | Key Findings | Reference |
|---|---|---|
| Hospital with 870 beds in Switzerland Number of samples unknown 7-year study |
|
Blanc et al. (2005) |
| 450-bed Swedish hospital with history of L. pneumophila nosocomial cases and 1991 outbreak (31 cases) |
|
Darelid et al. (2002) |
| 18 facilities in the Czech Republic 805 samples |
|
Hruba (2009) |
| 4 healthcare facilities in Germany 625 samples (316 cold and 309 hot) |
|
Arvand et al. (2011) |
| Various building types in Hungary 1,809 samples 7-year study |
|
Barna et al. (2016) |
TABLE 4-2 (continued)
| Size of Building(s) Number of Samples Study Length | Key Findings | Reference |
|---|---|---|
| Pediatric hospital with 450 beds in Quebec 46 samples |
|
Boppe et al. (2016) |
| Tertiary care hospital with 400 beds in Quebec 2 hot-water systems 64 samples from hot-water system |
|
Bédard et al. (2016) |
| Primary and tertiary hospital with 1,000 beds in France 127 sampling locations 726 samples |
|
Lecointe et al. (2018) |
In general, Table 4-2 and other reports on the efficacy of the implementation of temperature control in healthcare facilities (Bargellini et al., 2011; Lee et al., 2011; Serrano-Suarez et al., 2013) reveal moderate success. Differences among these reports most probably reflect whether the temperature set points were actually reached across the whole system, including at the outlets (e.g., faucets and showers). In most case studies, the actual application of temperature control is poorly documented, with only partial information on temperatures available for the water heater and the return line.
Indeed, thermal control is greatly improved if hydraulic deficiencies are addressed, ensuring that water temperatures greater than 55°C reach distal points, resulting in lower
positivity and concentrations of L. pneumophila using both culture and quantitative polymerase chain reaction (qPCR) methods (Blanc et al., 2005; Boppe et al., 2016; Lecointe et al., 2018). Bédard et al. (2015) showed that local deficiencies in the hydraulics of hot-water recirculation resulted in lower temperatures and elevated levels of L. pneumophila; they correlated these issues to the location where clinical cases of Legionnaires’ disease occurred. Heat-shock treatment at 70°C to remove L. pneumophila reservoirs and then maintaining temperatures above 55°C at the distal points of a large 1,000-bed hospital were highly efficient at reducing L. pneumophila to undetectable levels (using either culture methods or qPCR).
The effects of temperature on legionellosis risk are dynamic and intimately connected to the plumbing configuration and hydraulic conditions. Rhoads et al. (2015a) observed that setting the water heaters at a temperature that technically is within the inhibitory range for Legionella, in this case 51°C, can actually enrich for Legionella in distal pipes. Further, a seemingly simple matter of whether a hot-water pipe is oriented with upward or downward flow can directly affect Legionella levels close to the point of use. Indeed, since cooler water is denser, upward plumbed pipes experience convective mixing, which delivers more nutrients and pushes distal pipes back into the warm-water range conducive to Legionella growth (Rhoads et al., 2016b).
Thermal Control in Residential Hot-Water Systems
Residential water systems vary depending on the type of building, with centralized hot water generation being more common in large buildings, often with recirculation. In residences, electric or fuel-heated tanks and on-demand water heaters are commonly used, with a possibility of in-tank recirculation. Balancing the thermal and sanitary performance of domestic hot-water storage is a growing concern as energy stored in sanitary hot-water systems represents about 14.8 percent of total residential energy consumption in the United States2 and 19 percent of residential energy consumption in Canada.3
The type of water heater and the presence of storage and recirculation are critical features in determining the risk of Legionella spp. and L. pneumophila in residential hot-water systems. Electric water heaters are by design thermally stratified, with lower temperatures found in the bottom section; in contrast, oil and gas water heaters are not stratified because the heating element is located under the bottom of the tank. On-demand water heaters are discontinuous and will deliver water at a set temperature without any storage if properly sized. Many extensive field studies in American, Canadian, Danish, and German residential water systems have demonstrated the prevalence of Legionella in hot-water heaters that are thermally stratified (Alary and Joly, 1991; Dewailly and Joly, 1991; Marrie et al., 1994; Mathys et al., 2008; Stout et al., 1992; Wallet et al., 2016). In particular, Dewailly and Joly (1991) investigated 205 electric water heaters using high-volume samples (500 mL) and reported greater than 45 percent positivity for L. pneumophila serogroups 4 and 2 in the water heater sediments, while no positives were detected in 50 oil or gas water heaters sampled. They identify the major factors for positivity to be the type of water heater (electric versus gas) and the temperature at the bottom of the water heater (less than 40°C). Alary and Joly (1991) observed that 39 percent of the 178 electric water heaters sampled in the Quebec City area were positive for L. pneumophila by culture with a wide variety of serogroups present. Despite a relatively high water heater outlet temperature (56.6°C ± 0.4°C) in
___________________
2 See https://www.eia.gov/consumption/residential/data/2015.
3 See https://www.nrcan.gc.ca/energy/products/categories/water-heaters/13735.
electric water heaters, 12 percent of faucets and 16 percent of showers were positive. Noteworthy is the fact that no gas- or oil-fired water heaters operated at a higher temperature (61.5°C ± 1.1°C) had distal sites (showers and taps) that were positive for L. pneumophila. In a survey of 343 German residential water heaters with a water tank and, in some cases, recirculation, 94 percent of sites were positive for Legionella spp. in flushed samples by culture, most (93.7 percent) being L. pneumophila (Mathys et al., 2008). No positive sites were detected by culture if a temperature greater than 60°C in the main piping was maintained or if on-demand water heaters producing water with higher temperatures were used. Borella et al. (2004) found that tank size and the distance between the heater and the tap were significant factors in positivity and that different species and serotypes of Legionella were associated with different heater types.
Studies have also shown the importance of maintaining high temperatures at the distal ends of hot-water systems. In Germany, an analysis of over 30,000 water samples collected over a period of seven years (2003 to 2009) from 4,600 public buildings for compliance purposes was completed to establish the prevalence of Legionella and the conformity of hot-water systems to regulated minimum temperature requirements (Kistemann and Wasser, 2018). Overall, 15.8 percent of all samples were positive for Legionella, with positivity highest at distal sites (18.8 percent), lower in the recirculation loop (10.2 percent), and lowest in flushed samples (4.7 percent). More importantly, concentrations were higher by more than an order of magnitude at distal sites, corresponding to lower mean temperatures (47.2°C) versus temperatures found in the recirculation (54.8°C) and in the flushed samples (58.8°C). Figure 4-2 summarizes the impact of water temperature on the percentage of exceedances of the German standard of 100 colony forming units (CFU)/100 mL at distal sites, in the main
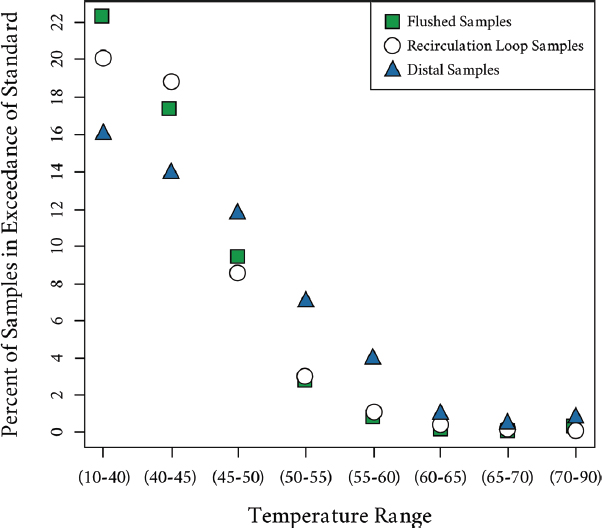
SOURCE: Kistemann and Wasser (2018).
piping, and in the recirculation loop. In the two lowest temperature classes (up to 45°C), approximately 22 percent of the samples were above the standard in the flushed samples, 20 percent in the samples from the recirculation loop, and about 15 percent at distal sites. The situation reverses when temperatures exceed 45°C, with increased prevalence at the distal sites. Even with temperatures at the outlet of 55°C to 60°C after a one-minute flush, 5 to 7 percent of the samples remain positive, while fewer positives are found in the flushed and return loop (1 to 3 percent).
Heat Shock
Temporarily elevating the temperature, or heat shock, is applied in a variety of forms and generally is intended as a temporary remedial or emergency measure, not as a preventive measure. An example would be maintaining a water temperature of at least 70°C for at least 30 minutes at each point of use for decontamination of an entire building water system. The efficacy of heat shock is controversial. For example, Temmerman et al. (2006) observed that Legionella numbers increased following system recovery from heat shock, presumably because of bacterial growth on nutrients liberated from killed cells (necrotrophic growth).
Temperature, duration, and frequency of heat shock application are certainly important factors. The efficacy of a stringent thermal shock (70°C for 30 minutes) on culturable Legionella is high in water but limited in biofilms, and most importantly, of short duration (Saby et al., 2005). Moreover, frequent heat shocks can promote the emergence of heat-resistant L. pneumophila strains, as observed in hospital water systems submitted to periodic extreme temperature (24 hours at 65°C a few times a year), while no such resistance was observed for strains isolated from the system where heat shock treatments (70°C for 30 minutes) were sparingly applied (Allegra et al., 2011).
Periodic heat shocks at 60°C were compared to a well-managed system continuously maintained at 60°C by analyzing L. pneumophila and microbiota in the water plumbing (Ji et al., 2018). Results suggest that maintaining the water system at a set point of 60°C and water use frequency are more promising for the long-term control of both the microbial community and L. pneumophila.
Heat shock should be considered as an extreme remediation measure because of such potential problems as (1) the dislodging of particles from piping walls due to thermal shock, which can subsequently cause clogging in balancing valves; (2) damage to equipment from sustained high temperatures; and (3) requirement for close supervision during the process to protect patients, staff, and visitors from scalding. Compatibility of system materials for heat shock is a key consideration. For example, faucets should be designed and constructed with materials that can withstand a superheating treatment. Each component of the system should be evaluated to determine the effect of high water temperatures on materials and equipment (e.g., thermostatic mixing valves). Mitigation measures, such as bypass, should only be considered to protect equipment that cannot withstand the specified temperature and time, since they can themselves become a reservoir for Legionella.
Scalding
The higher water temperatures (greater than 140°F/60°C) that prevent Legionella growth are associated with an increased risk of scalding and burns. Those at increased risk include young children, elderly patients (older than age 65), and those with substance-abuse disorders, physical disabilities, neurologic illness/disabilities or altered mental status. The U.S. Centers for Disease Control and Prevention (CDC) found that between 2001
TABLE 4-3 Water Temperature, Risk of Scalding/Burning, and Legionella Growth Potential
| °F | °C | Time to First-degree Burn | Time to Second-degree Burn | Legionella Growth Potential |
|---|---|---|---|---|
| <77 | <25 | No | ||
| 80 | 27 | Low | ||
| 90 | 32 | Moderate | ||
| 100 | 38 | Very high | ||
| 110 | 43 | Very high | ||
| 116 | 47 | 35 min | 45 min | Moderate |
| 122 | 50 | 1 min | 5 min | Very low |
| 131 | 55 | 5 sec | 25 sec | No |
| 140 | 60 | 2 sec | 5 sec | No |
| 149 | 65 | 1 sec | 2 sec | No |
| 154 | 68 | instantaneous | 1 sec | No |
SOURCE: Adapted from Armstrong (1978) and Klein (2018).
and 2006, adults older than 65 years made an estimated 51,700 initial visits to emergency rooms for nonfatal scald burns (CDC, 2009). Over this time period, the average was 8,620 visits per year with an estimated average annual rate of 23.8 visits per 100,000 population. Although most scalding and burn injuries in the homes are related to exposures other than hot water, such as food, cookware, and microwaved items, the risk of scalding from home premise plumbing remains important. It is difficult to tell from CDC (2009) which cases were, in fact, plumbing related. Bathtubs and showers are associated with prolonged exposure to larger body-surface areas, and therefore are particularly concerning for scalding of at-risk populations.
As shown in Table 4-3, scalding and burns are linked to water temperature and time of exposure (Armstrong, 1978; Moritz and Henriques, 1947), as is the growth potential of Legionella (Klein, 2018). The CDC, the American Academy of Pediatrics, the American Society of Sanitary Engineering Scald Awareness Task Group, and other safety-promotion organizations recommend that home hot-water heater thermostats be set at 49°C to 53°C (120°F to 130°F) to reduce scalding risks (Lukefar and Ezekial, 1994).4CDC (2009) recommends that hot-water heaters be kept below 49°C (120°F) to minimize the risk for scalding in the home. Most municipalities and state regulations recommend that home hot-water heater temperatures remain below 49°C (120°F), since most burns occur in the home and not at hospitals or rehabilitation facilities where there are more at-risk patients (CDC, 2009; Haik et al., 2007; Tung et al., 2005). Maximum allowable temperatures in hospitals and healthcare organizations are often regulated by states. Data from 39 states reported regulating maximum allowable hospital water temperature from as low as 43°C (110°F) to as high as 53°C (130°F) (Mandel et al., 1993).
Table 4-3 shows the trade-off between scald risk and the risk of Legionella growth. This table was submitted for inclusion in the 2020 Uniform Plumbing Code pending a member vote. In buildings with sensitive populations, the production and storage of hot water at greater than 60°C (140°F) will likely require the use of thermostatic mixing valves to blend
___________________
4 See http://www.asse-plumbing.org/WaterHeaterScaldHazards.pdf.
cold and hot water to appropriate temperatures at the tap. It is important for these devices to be routinely serviced and for temperature to be monitored closely (Bédard et al., 2015; Johansson et al., 2006).
Disinfection
Maintenance of a disinfectant residual can be an integral part of a building’s water management plan for control of Legionella. Disinfection methods should be paired with scheduled water testing to ensure that the system maintains a residual. Many of the disinfectants reviewed below have demonstrated at least some degree of efficacy toward management of Legionella in drinking water distribution systems and building water systems. Hence, the choice, and success, of disinfection technology will depend on additional considerations such as cost, operator training, materials (corrosion), water chemistry, system configuration, and water use patterns.
Chemical Disinfection
Chemical disinfectants, particularly oxidizing agents such as chlorine, chlorine dioxide, chloramine, and ozone, are widely used to control Legionella spp. and protozoa—both as disinfectants in drinking water distribution systems and as secondary disinfectants within buildings. The disinfectant should ideally inactivate microorganisms in the bulk water, but also penetrate and inactivate microorganisms associated with biofilms. Overall, the efficacy of disinfectants depends on the culture condition of Legionella spp. and their host protozoa and the physicochemical characteristics of the water (e.g., temperature, pH, organic carbon, hardness).
Disinfection strategies are sometimes evaluated in terms of “CT” or disinfectant concentration (measured in mg/L) multiplied by time of exposure (measured in minutes). Very high disinfectant levels (4 mg/L or more) applied for many hours might be recommended when responding to an outbreak in a hospital or nursing home but would be impractical and excessive for routine water treatment in premise plumbing. Choice of a disinfectant also needs to consider corrosion impacts on pipe materials, reliability, and safety. Because Legionella spp. can use protozoa and their cysts as a protective shield against disinfectants, it is imperative to consider the efficacy of each disinfectant for both organisms. In some systems, multiple points of application are necessary to maintain chemical residuals throughout the entire network.
Chlorine.
Chlorine is the most commonly used disinfectant by water utilities in the United States. Chlorine adversely affects the cell membrane, nucleic acids, respiration, and enzymatic activity of microbes, leading to their inactivation (Kim et al., 2002). During treatment, chlorine can be added to water as elemental chlorine (chlorine gas), sodium hypochlorite solution, or dry calcium hypochlorite. In water, chlorine exists as hypochlorous acid and hypochlorite ion, where the hypochlorous acid predominates when pH is below 7.5 and is a more effective biocide.
Generally, maintenance of a free chlorine residual in potable water systems is effective for control of Legionella spp. (Kim et al., 2002). For example, planktonic Legionella spp. resuspended in water were eliminated within three minutes by 2 mg/L free chlorine derived from sodium hypochlorite (Miyamoto et al., 2000). Mouchtouri at al. (2010) disinfected Legionella-positive cooling towers by circulating water with 5 mg free chlorine/L for five hours. Systems with pH greater than 8.0 received higher free chlorine dosages of 15 to 20 mg/L to achieve the required disinfection level; disinfection was considered successful
when samples showed concentrations less than 1 CFU/mL (103 CFU/L). Hyperchlorination with 4 to 6 mg/L decreased L. pneumophila in plumbing systems by 5 to 6 logs over six hours (Muraca et al., 1987). The decline in L. pneumophila was more rapid at 43°C than at 25°C. However, a higher dose of chlorine was required at 43°C to overcome thermal decomposition and maintain a chlorine residual of 4 to 6 mg/L. The high temperatures likely accelerated chlorine reactions with demand-causing compounds, including natural organic matter and reduced metals like iron or manganese.
The ecology of Legionella plays an important role in disinfection efficacy; whether the bacteria is shielded from the disinfectant depends on whether it is planktonic or within a protozoan trophozoite or cyst. Amoebae cysts are much more resistant to disinfection than the free-living trophozoite (De Jonckheere and Van de Voorde, 1976). Legionella spp. in protozoa cysts survived 25-fold more chlorine disinfectant than planktonic cells after 18 hours (Kilvington and Price, 1990). Dupuy et al. (2011) showed that co-culture significantly increased survival of L. pneumophila at 30°C, but not at 50°C.
Guidelines for the maintenance of continuous chlorine residuals in building premise plumbing to prevent amplification of Legionella tend to recommend residual concentrations similar to those required in drinking water distribution systems. The Allegheny County (Pennsylvania) Health Department specifies that potable water, from entering a building through to all outlets (e.g., faucets, showerheads), should maintain at least 0.3 mg/L free residual chlorine (Moore and Shelton, 2014). The California Code of Regulations, Title 22, Section 60306, requires that industrial or commercial cooling towers maintain a 0.3 to 0.7 mg/L free chlorine residual (State of California Energy Commission Staff, 2004).
Chlorination can have adverse effects on the plumbing system by making the water acidic, which in turn can make the water more corrosive to pipes, joints, fittings, and fixtures. If chemical flushing is used with hyperchlorination, these adverse effects can be more pronounced.
Chlorine Dioxide.
Unlike free chlorine, chlorine dioxide does not hydrolyze when it enters water; it remains a dissolved gas in solution. As a neutral compound, it can easily diffuse through cell membranes of microorganisms where it disrupts protein synthesis. It is typically generated on site for immediate use by slowly adding a strong acid (e.g., hypochlorous or sulfuric acid) to a sodium chloride solution.
Chlorine dioxide has been found to be more effective in penetrating biofilms than chlorine (Kim et al., 2002; Lin et al., 2011; Walker et al., 1995), and it is effective over a wider pH range (Lin et al., 2011). Loret et al. (2005) evaluated 0.5 mg/L chlorine dioxide for control of Legionella grown in biofilms in a pilot-scale premise plumbing system incubated at 30°C. Legionella populations decreased to undetected levels (less than 500 CFU/L) within six days of treatment. As with chlorine, the presence of amoebae reduces the efficacy of chlorine dioxide disinfection of Legionella (Dupuy et al., 2011). Despite the effectiveness of chlorine dioxide, it is not commonly used as a disinfectant in the distribution system due to the toxicity of the disinfectant and some of its byproducts (EPA, 1998) and the potential for objectionable odors (Dietrich et al., 1991).
There have been a handful of real-world applications of chlorine dioxide treatment of premise plumbing. Walker et al. (1995) reported elimination of Legionella spp. to below detection in a hospital water system after treatment with 50 to 80 mg/L chlorine dioxide. Srinivasan et al. (2003) evaluated the use of chlorine dioxide (0.3 to 0.5 mg/L residual) for 17 months in a hospital and found Legionella occurrence decreased from 41 percent to 4 percent in distal sites. Only L. anisa was recovered during the chlorine dioxide treatment and it was cultured from both the hot- and the cold-water systems. No cases of nosocomial Legionella infection were detected in the building with the chlorine dioxide system during
the 17-month evaluation. Marchesi et al. (2013) reported reduction in L. pneumophila contamination in three hospital hot-water (60°C) systems over a three-year period using a chlorine dioxide dose of 0.50 to 0.70 mg/L and a targeted residual of 0.3 mg/L at distal sites. Cristino et al. (2012) described use of chlorine dioxide after shock treatment to maintain 0.3 mg/L residual at the tap after 5 minutes of flushing in a hospital. Legionella counts remained acceptable (less than 103 CFU/L), and no cases of hospital-acquired legionellosis occurred during the study period. Zhang et al. (2009) reported that after installation of a chlorine dioxide system it took months to achieve a 0.11 mg/L chlorine dioxide residual within two hospital systems, but the occurrence of Legionella at hot-water taps decreased from 60 percent to less than 10 percent of sampling sites, and no cases of hospital-acquired Legionnaires’ disease were detected.
Monochloramine.
Monochloramine is formed by adding free chlorine in a solution of ammonium chloride at a chlorine-to-nitrogen molar ratio of 0.5 (pH 8.5). Disinfection with monochloramine has gained traction in the United States because the disinfectant is more stable in the distribution system, it minimizes the formation of disinfection byproducts, and it can penetrate biofilms better than free chlorine (LeChevallier et al., 1988; Lee et al., 2011; Pressman et al., 2012). Monochloramine has a lower chlorinous odor threshold than free chlorine (EPA, 1994), but it has a much lower disinfection efficacy than free chlorine (Symons, 1978) and requires a much longer contact time or higher dose if used as a primary disinfectant.
One of the challenges with using monochloramine, particularly within a building system, is properly managing the chlorine-to-ammonia ratio (4.5:1) at an optimum pH (8.3) in order to form monochloramine without stimulating nitrification within biofilms. Nitrification is a microbial growth process by which ammonia is sequentially oxidized to nitrite and nitrate. Nitrite catalyzes the decay of chloramines and can leave a system without disinfectant residual and hence even more vulnerable to bacterial regrowth. Nitrifying bacteria fare better at warmer temperatures, making nitrification a summer problem for water utilities, which often implement flushing campaigns and even temporarily convert to free chlorine. Nitrification can be even more problematic in buildings because some premise plumbing is consistently maintained at a warm temperature, there is a high surface area-to-volume ratio for biofilm formation, and stagnant conditions can be especially conducive to slow-growing autotrophic organisms like nitrifiers and stimulate further decay of chloramines (Zhang and Edwards, 2009)—all of which could potentially undermine chloramine disinfection systems in premise plumbing.
As a disinfectant in the water supply distribution system, chloramines appear to be more effective than free chlorine in reducing the overall risks from Legionella. Kool et al. (1999) examined 32 hospital-acquired (nosocomial) outbreaks of Legionnaires’ disease from 1979 to 1997 where drinking water was implicated. They found that the odds of a nosocomial Legionella outbreak were 10.2 times higher in hospitals supplied by a water system that maintained free chlorine than in those supplied by a water system using a chloramine residual. Similar results were obtained by Heffelfinger et al. (2003), who surveyed 152 hospitals with reported cases of hospital-acquired Legionnaires’ disease. Flannery et al. (2006) showed significant reductions in the occurrence of both amoeba and Legionella spp. in building plumbing systems in San Francisco after the utility converted from free chlorine to chloramines. The prevalence of amoebae decreased from 169 of 1,405 (12 percent) samples when chlorine was used to 78 of 944 (8 percent) samples collected after conversion to monochloramine. Prior to the conversion, Legionella spp. were cultured from 61 of 169 (36 percent) samples in which amoebae were present versus 291 of 1,236 (24 percent)
samples without amoebae. After conversion to monochloramine, Legionella were found in 1 of 78 (1 percent) samples containing amoebae and 8 of 866 (1 percent) samples without amoebae. Legionella occurrence was also reduced in 96 buildings in Pinellas County, Florida, when the drinking water distribution system converted from chlorine to monochloramine disinfection (Moore et al., 2006). When free chlorine was used, 20 percent of the buildings were colonized with Legionella in at least one sampling site. Within a month after chloramination, Legionella colonization was reduced by 69 percent. Monochloramine appeared to be more effective in reducing Legionella in hotels and single-family homes than in county government buildings, perhaps because of more consistent water usage.
Chloramines also appear to be more effective than chlorine when used as a treatment in buildings. Coniglio et al. (2015) studied the addition of monochloramine after two hospital hot-water systems failed to control Legionella with thermal treatment (65°C to 70°C), shock chlorination (50 mg/L free chlorine for one hour at distal sites), point-of-use filters (0.2 micron), and hydrogen peroxide (17 mg/L). Prior to chloramine treatment, 100 percent of samples were positive with L. pneumophila serogroups 3 and 6. Monochloramine treatment began at 3.0 mg/L and was then reduced to 2.0 to 2.5 mg/L after one month. Legionella was not detected during the following year except for one month when the monochloramine generator failed for 15 days. In a three-year study of monochloramine addition to a hospital in Italy, Marchesi et al. (2012, 2013) reported that a residual between 1.5 and 3.0 mg/L effectively controlled Legionella occurrence, with seven of the eight positive samples occurring within the first eight months and the eighth positive sample occurring at 15 months, when the monochloramine dose decreased below 1 mg/L.
Not all studies have been as straightforward, however. Duda et al. (2014) showed that although monochloramine concentrations of 1 to 4 mg/L significantly reduced the occurrence of Legionella in a hospital hot-water system (with the average number of positive sites declining from 53 percent to 9 percent), during certain months when nitrate, total ammonia, and pH levels were elevated, the percentage of positive samples increased, suggesting inadequate control of the chloramination process and nitrification. Legionella speciation changed from 90 percent of samples testing for L. pneumophila serogroup 1 to only 49 percent post-disinfection, while L. bozemanii occurrence increased.
The effectiveness of monochloramine is generally thought to be due to its ability to penetrate biofilms and inactivate the bacteria (Donlan et al., 2002; LeChevallier et al., 1988). Lee et al. (2011) and Pressman et al. (2012) both used microelectrodes to demonstrate that monochloramine had greater penetration into biofilms than chlorine, but this penetration did not necessarily translate to immediate loss of viability. Johnson et al. (2018) found that amoebae in five free chlorinated reclaimed water systems were mostly (50 percent to 95 percent) in the active trophozoite phase; however, in the chloraminated system, 87 percent of the mesophilic amoebae and 66 percent of the thermophilic amoebae were in the cyst phase. They hypothesized that the penetration of chloramines into the biofilm might trigger the amoebae to form cysts rather than outright kill the protozoa. Since L. pneumophila only amplifies in the trophozoite stage, it may be possible to manage Legionella risk by limiting the free-living trophozoite population. Additional research is needed to examine the precise action of monochloramine on Legionella persistence and growth within pipeline biofilms.
Ozone.
Ozone attacks unsaturated bonds of aldehydes, ketones, and carbonyl compounds (Langlais et al., 1991) and can participate in electrophilic reactions with aromatic compounds and neutrophilic reactions with many cellular components (i.e., fatty acids, carbohydrates, amino acids, proteins, nucleic acids). These reactions collectively affect the cytoplasmic membrane of bacterial cells and their protein structure as well as DNA. How-
ever, because ozone does not form a stable residual and decomposes rapidly in water, it is not typically used for building plumbing systems, but primarily to disinfect water supplies.
Several laboratory studies have evaluated ozone for inactivating Legionella (Domingue et al., 1988; Muraca et al., 1987) and amoebae cysts (Langlais and Perrine, 1986; Wickramanayake et al., 1984). There are few studies of using ozone to treat a building water system. Edelstein et al. (1982) applied continuous ozonation to the water of one wing of an unoccupied hospital building while the other wing used chlorinated tap water. The results were inconclusive, with both the ozonated and chlorinated sections having some positive results for Legionella (three of 12 samples positive for the ozone treatment, eight of 12 samples positive for the chlorine treated wings). Moreover, when the ozone was discontinued L. pneumophila regrew and reached levels similar to the pre-treatment densities. The authors noted that residual ozone at a faucet or shower would be released as a gas and could create a health hazard if inhaled.
Ultraviolet Irradiation
Ultraviolet (UV) light may not directly kill microorganisms but rather damages their DNA and proteins, which prevents them from replicating and becoming infectious. UV intensity times the duration of exposure is commonly referred to as fluence (mJ/cm2) and describes UV disinfection capability. Fluence represents the energy per unit area falling onto a surface. Maximum efficacy with UV is attained at 254 nm (Kim et al., 2002) but turbidity, natural organic matter content, and particulate matter can affect UV disinfection capability. Medium-pressure UV light sources may also generate higher wavelength UV light (268 and 286 nm) that impacts proteins more than nucleic acids (Beck et al., 2017). Because UV does not provide a residual, it is only effective at the point of treatment and is typically combined with a chemical disinfectant for distributed water to effectively control Legionella spp.
All Legionella isolates tested by Cervero-Aragó et al. (2014) required 5 to 6 mJ/cm2 UV fluence to inactivate 4 logs. However, a higher fluence was required when Legionella was co-cultured with amoeba. Muraca et al. (1987) found that UV irradiation at 30 mJ/cm2 reduced L. pneumophila by 5 log units in 20 minutes although the very high concentrations of the bacteria could have affected the UV adsorption of the suspension. Legionella inactivation requires slightly higher doses when the bacteria are exposed to light repair (i.e., DNA repair mediated by enzymes activated by visible light), but has a similar level of inactivation when either low-pressure or medium-pressure lamps are used (see Table 4-4). Notably, when amoeba co-culture was used on samples below detection using buffered charcoal yeast extract (BCYE) agar plates, VBNC-like cells were resuscitated (Grossi et al., 2018).
TABLE 4-4 UV Doses (mJ/cm2) for Inactivation of L. pneumophila
| L. pneumophila Strain | Lamp Type | 1-log | 2-log | 3-log | 4-log |
|---|---|---|---|---|---|
| Philadelphia Type 2 | LP | 0.92 | 1.84 | 2.76 | No data |
| Philadelphia 1 (no light repair) | LP | 0.5 | 1 | 1.6 | No data |
| Philadelphia 1 (with light repair) | LP | 2.3 | 3.5 | 4.6 | No data |
| Philadelphia 1 ATCC33152 | LP | 1.6 | 3.2 | 4.8 | 6.5 |
| Philadelphia 1 ATCC33152 | MP | 1.9 | 3.8 | 5.8 | 7.7 |
NOTES: LP = low-pressure lamps, which have a single output around 254 nm. MP = medium-pressure lamps, which have polychromatic output at multiple wavelengths.
SOURCES: EPA (2016a); Knudson (1985); Oguma et al. (2004).
Hence, previous reports only using plate culture to assay inactivation may overestimate actual UV inactivation, particularly for higher wavelength UV light.
Hijnen et al. (2006) reported a log reduction of Acanthamoeba spp. with 40 mJ/cm2. A 3-log inactivation of various Acanthamoeba species and Vermamoeba vermiformis was achieved with fluences of 23 to 100 mJ/cm2; the higher levels were required for cyst inactivation. Overall, inactivation of Acanthamoeba spp. and V. vermiformis required higher levels of UV compared to Giardia or Cryptosporidium (EPA, 2006).
Copper-Silver Ionization
The use of copper-silver (Cu-Ag) ionization to control Legionella in building water systems is widespread, partly because it is relatively low cost and low maintenance compared to other controls. Copper (Cu) and silver (Ag) both have biocidal activity, especially when used in combination. In ionization chambers, both metals can be ionized through electrolysis to form positively charged ions. The copper ions interact with negatively charged cell walls of Legionella spp. (and other bacteria), disrupting cell wall permeability and subsequent nutrient uptake. The copper ions penetrate the cell wall and create an entrance for silver ions, which bond with DNA, RNA, cellular proteins, and respiratory enzymes, immobilizing the cell and curtailing cell division.
Field studies constitute the majority of the published reports on the efficacy of copper-silver ionization for controlling Legionella in building plumbing systems (Blanc et al., 2005; Chen et al., 2008; Demirjian et al., 2015; Dziewulski et al., 2015; Kusnetsov et al., 2001; Liu et al., 1994, 1998; Mòdol et al., 2007; Rohr et al., 1999; States et al., 1998; Stout and Yu, 2003). These reports typically describe applying copper-silver ionization to remediate situations where Legionella have already colonized the system. Most studies have looked at the disinfection effects of these ions used together, but Lin et al. (1996) examined the effects of each ion individually. They reported 6-log reduction of L. pneumophila serogroup 1 in 2.5 hours with 0.1 mg/L copper. Similarly, a 6-log reduction L. pneumophila was obtained within six hours on exposure to a solution of 50 µg/L silver ions (Miyamoto et al., 2000). Cloutman-Green et al. (2019) reported effective Legionella management in a healthcare building hot-water system operated at 42°C (range 37°C to 44°C) supplemented with copper-silver ionization operated at 0.37/0.034 mg/L, respectively. The authors reported a reduction in energy and carbon emissions of 33 percent and 24 percent, respectively, compared to an equivalent temperature-controlled system.
June and Dziewulski (2018) provide an excellent review of copper-silver ionization for the inactivation of Legionella. The review suggests that there have been mixed results when considering the efficacy and reliability of copper-silver ionization for controlling Legionella. Copper-silver ionization is slower acting compared to other disinfectants and more dependent on water chemistry (e.g., pH, total dissolved solids or TDS), as the silver can precipitate in the presence of high dissolved solid concentrations, becoming unavailable for disinfection. Legionella can be protected from copper and silver ions when associated with biofilms or amoebae, and the potential for Legionella to develop resistance to copper and silver ions has been suggested (EPA, 2016a). Indeed, dominant sequence types of L. pneumophila isolated from two hospitals’ hot-water systems with and without copper-silver ionization have been shown to be highly resistant to copper (Prévost et al., 2017). The development of resistance to copper and silver may be a concern in ensuring the long-term efficacy of copper-silver ionization. Longitudinal case studies report that copper-silver ionization can become ineffective for the control of Legionella in biofilms and water in large existing healthcare facilities (Blanc et al., 2005; Rohr et al., 1999). A further concern is that bacteria
that develop resistance to heavy metals may also develop antibiotic resistance (Chen et al., 2015), although additional research is needed to determine whether there is an increase in antibiotic resistance in water treated with copper-silver ionization. June and Dziewulski (2018) suggest approaches for improving copper-silver ionization efficacy and reliability, including increasing the dissolved oxygen and sodium content of the treated water, applying copper and silver ions in combination with other disinfectants, and using copper and silver ions at higher temperatures.
Other Disinfecting Agents
Bromine behaves similarly to chlorine, existing in water as hypobromous acid to form HOBr and OBr− depending on the pH (Kim et al., 2002). Bromine has generally less efficacy against Legionella spp. compared to chlorine. Bromine, iodine, and iodophore are variously effective against Acanthamoeba culbertsoni and Naegleria fowleri cysts (De Jonckheere and Van de Voorde, 1976). Although used for potable water disinfection in some emergency instances, use of bromine, iodine, or hydrogen peroxide in water supply distribution systems and building water systems is not widely practiced.
Peracetic acid is thought to disinfect by impacting lipoproteins in the cell membrane (Rossoni and Gaylarde, 2000). Unlike chlorine and hydrogen peroxide, its potency is not greatly compromised by organic matter or enzymes (Baldry et al., 1991), and it has acceptable potency at neutral pH and can be effective for biofilms (Rossoni and Gaylarde, 2000). However, peracetic acid has had limited use within building plumbing systems.
Non-oxidizing biocides such as BNPD (2-bromo-2-nitropropane-1, 3-diol), glutaraldehyde, guanidines, dithiocarbamates, isothiazolin, halogenated amides such as DBNPA (di-bromo-nitrilo-propionamide), halogenated glycols such as bronopol (2-bromo-2-nitroproprionamide), and some quaternary ammonium compounds are commonly used in cooling towers (Kim et al., 2002). Among non-oxidizing biocides, glutaraldehyde, DBNPA, isothialozin, and bromopol were found to be effective against Legionella to varying degrees (Kim et al., 2002). The biocides MBC-115 [a quaternary ammonium comprised of poly(oxyethylene (dimethyliminio) ethylene (dimethyliminio) ethylene dichloride)] and MBC-215 (an isothiazine derivative of a mixture of 5-chloro-2-methyl-4-isothiazolon-3-one and 2-methyl-4-isothiazolin) have been widely used in cooling towers to control Legionella spp. Berk et al. (1998) found the efficacy of both compounds on Legionella spp. to be poor, although this may have been due to the presence of amoebae. Barker et al. (1993) found that the antiseptics polyhexamethylene bioguanide and benzisothiazolone were ineffective against L. pneumophila grown with A. polyphaga compared to L. pneumophila pure cultures. Both biocides attack the bacteria cell membrane; amoebae proteins coating Legionella may have conferred biocide resistance. Miller and Simpson (1999) reaffirmed the resistant nature of protozoa cysts to disinfection with some of these alternative compounds.
Manage Hydraulics
Appropriate hydraulic system design and maintenance are essential for effective Legionella control. In particular, hydraulics are essential to maintaining and delivering water at an inhibitory temperature as well as distributing disinfectants throughout the building. Recent guidelines following years of mandatory Legionella control in Europe stress the need to properly manage hydraulics to ensure homogeneous temperature and biocidal control in all areas of the hot-water system, including balancing under varying demand (Centre Scientifique et Technique du Bâtiment, 2012; HSE, 2013). Construction and operational
standards for buildings often specify minimizing stagnation (e.g., via recirculation loops, elimination of hydraulic and physical dead ends).
In many cases, differences among reports on the efficacy of thermal control on Legionella probably reflect whether the temperature set points were hydraulically achieved across the whole system, including at the outlets (faucets and showers). For example, a single piece of deficient equipment such as backflow preventers on a single mixing valve can influence the hot-water temperature distribution within an entire building wing, causing hot-water temperature to decrease in those sectors (Boppe et al., 2016). The presence of stagnation caused by dead legs, inadequate system hydraulic balancing, or lack of occupancy also reduces the disinfectant efficiency in these areas. As a global recommendation, extended periods of stagnation and the presence of dead legs should be avoided. To reach this goal, minimum water velocity should be maintained at all times within the recirculation pipes. The Centre Scientifique et Technique du Bâtiment (CSTB, 2012) proposes maintaining the highest value between 0.2 m/s and the velocity required to maintain heat loss below 5°C.
Flushing to Control Distal Growth
Flushing of water can have significant benefits in terms of water quality and more specifically Legionella levels. Flushing can reduce total cell counts in premise plumbing by dislodging loose deposits and biofilm, which tend to harbor higher levels of heavy metals, Aeromonas, adenosine triphosphate or ATP (indicator of biological activity), and Legionella as judged by operational taxonomic units quantified by amplicon sequencing (Liu et al., 2017). Flushing systematically reduces total and viable bacterial cells and heterotrophic plate counts in large buildings (Bédard et al., 2018; Lautenschlager et al., 2010), and in most instances will lower the concentrations of L. pneumophila concentrations in household and hospital taps (Bédard et al., 2019; Cristina et al., 2014). Lipphaus et al. (2014) found that flushing reduced total cell counts by flow cytometry in infrequently used cold-water hospital taps, but had a less pronounced effect on hot-water taps. Periodical flushing of water is particularly useful to prevent colonization and limit the growth of Legionella at the distal sites of cold- and hot-water systems. Manual flushing is recommended in guidance and is widely used during building commissioning or after periods of vacancy (e.g., weekends, vacations).
There is no consensus on the optimal flushing frequency to prevent Legionella. Several guidance documents recommend weekly flushing of low-use faucets and showers (e.g., ECDC, 2017; HSE, 2013). A much higher flushing frequency was suggested by Totaro et al. (2018)—a study done in an Italian hospital that was experiencing elevated L. pneumophila positivity and concentrations, despite optimal temperature control and on-site addition of chlorine dioxide. Five dead-end locations and the main return loop were all positive for L. pneumophila serogroups 3 and 10–14 (concentrations ranging from 8 × 103 to 1.3 × 105 CFU/L) before the installation of time-flow taps. Operating the five time-flow taps for one minute every six hours (64 L per day) slightly decreased the Legionella concentrations. After further increasing the flushing frequency to one minute every two hours (192 L per day), no positives were observed. These findings suggest that implementing automated periodic flushing may be necessary if hydraulic corrective actions such as the elimination of dead legs and the balancing of flows cannot be implemented.
Storage facilities and dead-end pipes where water velocities and turnover can be very low are locations that are more susceptible to biofilm development. Sediments can accumulate in areas of low flow, increasing disinfection demand and promoting bacterial growth. Stratification caused by warm water temperatures can prevent adequate mixing. Inlet–outlet configurations can result in “last in, first out” flow patterns in which older water
never leaves the storage tank, causing stagnation, dissipation of disinfectant residuals, and microbial growth. Increasing the frequency of storage tank cleaning will minimize sediment accumulation and help control biofilms.
Relationship Between Flow Rates and Biofilm Formation in Pipes
Higher flow rates and turbulence can reduce biofilm formation (Donlan et al., 1994; Kirisits et al., 2007). At lower residence time, the erosion of cells on the surface due to higher shear force and enhanced diffusion of disinfectant within a thinner boundary layer are factors suggested to explain the effect of flow dynamics on biofilm formation (Donlan et al., 1994). A study in which biofilms were first established under laminar or turbulent flow looked at the effect of unsteady hydraulic conditions on the biological quality of the drinking water (Manuel et al., 2010). Once the biofilm was established, periods of stagnation promoted bacterial accumulation for both the planktonic and biofilm bacteria. These cells were carried away once the flow was resumed, increasing the bacterial concentration in drinking water. Similarly, the ratio of L. pneumophila cell detachment from biofilm following exposure to 0.1, 0.3, and 0.7 m/s was found to increase with flow velocity (Shen et al., 2015). Initial adherence of L. pneumophila strains to an existing biofilm was conducted in quasi-stagnant conditions (0.007 m/s) prior to exposure to water flow. The same trends were observed both in smooth and rough biofilm, although L. pneumophila adhesion was enhanced by biofilm roughness. This enrichment was attributed to increased interception of the suspended L. pneumophila in flowing water on biofilm surface (Shen et al., 2015).
Dissimilar results have been found by others. The impact of turbulent, transition, and laminar flow on existing and newly formed biofilm was investigated by Tsagkari and Sloan (2018). They found that turbulent flow did not reduce biofilms; instead, biofilm thickness and density increased under turbulent flow conditions equivalent to 0.25 m/s in a 30.3-mm diameter pipe. Another key parameter is the surface-to-volume (S/V) ratio, which fundamentally drives the relative amount of surface area available to colonize and overall biomass production potential for pipes (Tsvetanova and Hoekstra, 2012). The authors observed a significant effect of S/V ratio on the planktonic biomass, with concentrations 4 to 14 times higher with higher S/V ratios. Premise plumbing piping usually has a small diameter and thus a larger S/V ratio than the distribution system.
***
There are few methodologies available to assess, in detail, hydraulically deficient areas within an existing water system. CSTB (2012) suggests investigating common causes such as valve obstructions (leading to stagnation or reduced water velocity within the return loop), type of control elements installed, re-circulation pump design and operation, and the lack of balance between the different secondary flow and return loops. Given the intimate relationship between temperature and hydraulics, temperature is not only a very effective proxy for residence time, but also relatively easy and inexpensive to monitor (Bédard et al., 2015). Systems that fail to maintain control temperatures at the point of use despite adequate water heater temperatures are considered at risk and hydraulically deficient.
Nutrient Control
An indirect strategy for management of Legionella in building water systems could be controlling biofilms, which are the food source for free-living protozoa (Characklis and
Marshall, 1990; LeChevallier et al., 2011; NRC, 2006). One of the most common ways to control biofilms is to limit nutrients in the water—a strategy used by some western European countries that also tend to distribute potable water with little or no disinfectant residual (Bartels, 2018; Exner, 2018). Hence, much of the work investigating the effect of limiting organic carbon on biofilm growth, and hence on Legionella, has been conducted in The Netherlands.
A substantial portion of the organic carbon present in drinking water is derived from complex natural organic matter (e.g., from decaying leaves), a form that cannot be directly utilized by microorganisms. Thus, a direct measurement of total organic carbon does not indicate the fraction that is actually bioavailable to drinking water microbes. Instead, bio-assays have been developed to directly measure the biodegradable fraction of organic carbon in the water, specifically the assimilable organic carbon (AOC) and biodegradable dissolved organic carbon (BDOC) assays. Organic carbon levels in U.S. drinking water supplies typically average 100 µg/L for AOC (ranging from 50 to 250 µg/L) and 0.3 mg/L for BDOC (ranging from 0 to 1.0 mg/L); surface water supplies have higher levels of biodegradable organic matter than groundwater supplies (LeChevallier et al., 1996; Volk and LeChevallier, 2000).
In terms of setting nutrient limits for water exiting a drinking water treatment plant, only extremely low levels of AOC (less than 50 µg/L) have been observed to have a measurable effect on downstream numbers of total bacteria as judged by heterotrophic plate counts (HPCs) or ATP (LeChevallier et al., 1991). Much lower AOC levels of 5 to 10 µg/L were associated with lower L. pneumophila levels in Dutch drinking water distribution systems (van der Kooij and van der Wielen, 2014). The same research group also observed a strong correlation among AOC, biofilm concentration, and L. pneumophila growth, with no growth observed at AOC levels below 1 µg/L (van der Kooij et al., 2017). Similarly, Learbuch et al. (2019) treated water with a pilot reverse-osmosis system and subsequent remineralisation to obtain very low AOC levels and showed that the water did not support growth of L. pneumophia. On the other hand, Williams et al. (2015) performed extensive bench-scale tests in simulated glass water heaters with spiked AOC levels ranging from 0 to 15,000 µg/L over 17 months and could find no correlation with Legionella concentration, although total bacterial numbers by HPCs did correlate.
It is important to recognize that such low AOC levels can be very difficult to achieve and maintain in drinking water because AOC can be generated in water mains and by the bacteria native to the plumbing. Dai et al. (2018) conducted a bench-scale study of controlled, replicated simulated glass water heaters representing a range of premise plumbing conditions that were fed biofiltered water (to simulate the AOC removal process used at water treatment plants or in whole-house filters). Although biofiltering the water substantially reduced the TOC and 16S rRNA gene copy numbers, there was no measurable effect on Legionella gene copy numbers. Instead, the individual plumbing conditions, such as the presence of iron corrosion sediments, nitrification, or cross-linked polyethylene (PEX) pipe material leaching organic carbon, dominated the effects on the microbial community composition and, in some cases, Legionella.
Iron Corrosion and Inorganic Nutrients
Much of U.S. water distribution systems consist of century-old unlined iron mains, which are beyond their designed lifespan and subject to substantial corrosion as well as intrusion during water main breaks. Corrosion of pipe surfaces provides not only a habitat for bacterial proliferation and protection from chlorine disinfectant residuals but also a source
of nutrients. Aerobic microbial respiration consumes oxygen, resulting in a reduced redox environment that can accelerate corrosion and produce a disinfectant demand. Corrosion of pipe surfaces and deposition of corrosion products can also create tubercles and surface roughness that protect biofilm organisms from hydraulic shear (Characklis and Marshall, 1990). The resulting turbulent flow can help transport nutrients and detritus, further enhancing the biofilm environment.
Growth of certain microbes is also promoted by other inorganic substances that can serve as electron donors or acceptors including methane, ferrous iron, reduced sulfur compounds, hydrogen gas, manganese, ammonia, and nitrite. These substances can stimulate autotrophs to fix organic carbon into the system, leading to more bacterial cells and associated organic matter. The accumulation of organic carbon and reduced inorganic compounds (e.g., iron, nitrite, sulfides) in biofilms can create a disinfectant demand that protects the attached microbes from being inactivated. In particular, iron-oxidizing bacteria oxidize ferrous iron to produce ferric iron oxides. Not only is iron a known nutrient for Legionella, but it also reacts with chlorine, thereby increasing microbial risk by removing the disinfectant residual.
Plumbing Materials
Plumbing materials are an important factor to consider in Legionella control. Common plumbing materials in buildings include copper, iron, and numerous plastics, with cross-linked PEX and cross-linked polyvinyl chloride (PVC) being particularly suitable for hot-water plumbing because of their tolerance of higher temperatures. Each pipe material will influence the building-level water chemistry and shape the biofilms that colonize premise plumbing in a unique manner (Ji et al., 2015). Being able to identify a pipe material that most effectively limits proliferation of Legionella for a given water chemistry and building type would be valuable as a passive barrier. It is important to recognize that water chemistry varies regionally, seasonally, and as dictated by various upstream water treatment processes (Dai et al., 2018), making it difficult to predict how incoming water will react with different pipe materials.
Although copper pipe has well-known antimicrobial properties, it does not universally control Legionella. Indeed, copper has been associated with decreased, increased, and comparable numbers of Legionella relative to other pipe materials (Rhoads et al., 2017b). As described in Chapter 2, the age of copper pipe, temperature, pH, and general water chemistry influence the dissolution chemistry and overall antimicrobial action of copper toward Legionella. The composition of the biofilm community also matters, e.g., interactive effects of amoebae and copper appear to favor survival of Legionella (Buse et al., 2017; Ji et al., 2017). Thus, it is clear that copper pipe cannot be the sole agent to control Legionella; other microbiological, chemical, and site-specific factors needs to be considered.
PEX and other heat-tolerant flexible polymeric plastic materials have gained popularity for their ease of use for hot-water plumbing. These materials, however, are well known to leach organic carbon and can stimulate bacterial growth (Proctor et al., 2018). In particular, flexible pipe materials commonly employed to plumb showerheads are especially vulnerable to biofilm formation and microbial growth, producing total bacterial cell counts ranging from 106 (PE-Xc—applied as a rigid control plastic) to 108 (PVC-P) cells/cm2 of hose (Proctor et al., 2016). A comprehensive comparison of six different shower pipe materials indicated that these materials had a profound influence on the microbial community composition, including the occurrence of genera containing Legionella and other pathogens (Proctor et al., 2016). However, interestingly, Legionella operational taxonomic unit were lower when total bacterial cell counts were higher, suggesting Legionella were
out-competed. An eradication strategy based on this probiotic concept is discussed later in this chapter.
Iron pipe is extremely vulnerable to biofilm formation, partly because of its susceptibility to corrosion. Even without corrosion and with depleted AOC and sufficient chlorine residual, iron is highly prone to biofilm build-up compared to other materials, such as PVC (Camper, 1996). Iron pipes also support a more diverse microbial population than do PVC pipes (Dai et al., 2018; Norton and LeChevallier, 2000). While no longer used in modern buildings, legacy iron pipe remains common in older buildings, water mains, and service lines. One major survey found that cast iron pipes comprise an estimated 38 percent of water distribution system pipes in the United States (McNeill and Edwards, 2001). Even in modern systems built without iron, other sources, such as steel components in water heaters, can elevate iron levels in water. When iron components corrode, they not only release iron into the water, but also accelerate the decay of disinfectants (Zhang and Edwards, 2009; Zhang et al., 2010). Depletion of disinfectant residuals by iron will leave downstream components vulnerable to microbial regrowth. Depletion of chlorine in general (Zahran et al., 2018) and by iron corrosion specifically (Rhoads et al., 2017a) has been hypothesized to account for the Legionnaires’ disease outbreak that occurred when corrosive water was distributed in Flint, Michigan. Thus, addressing the problem of legacy iron pipe is a critical engineering control to consider for Legionella. In 2012, the American Water Works Association estimated that it would cost $455 billion to replace just the cast iron pipe in U.S. distribution systems (AWWA, 2012). In the meantime, awareness of the presence of iron pipes and other components and practicing appropriate corrosion control, e.g., through orthophosphate addition federally mandated by the Lead and Copper Rule, are key to reducing this potential risk factor for Legionella growth in premise plumbing.
Finally, other plumbing materials besides the pipes themselves can potentially influence Legionella. For example, certain pipe gaskets and elastic sealants (containing polyamide and silicone) can be a source of nutrients for bacterial proliferation (Colbourne et al., 1984).
Managing the Distal Portion of the Plumbing
Managing the distal portion of premise plumbing is the last opportunity to control Legionella risk in building water systems. The distal section between the main piping of a building and the point of use has a number of unique features that are favorable to biofilm and Legionella growth. Unlike the main and secondary piping, the distal section immediately upflow of the point of use may include numerous components such as faucets, showerheads, thermostatic valves, backflow valves, interconnection piping, and aerators. Because of all these components, the materials found at distal sites vary extensively compared to the main premise plumbing system. In addition, the smaller diameter piping and correspondingly larger surface-to-volume ratios at distal sites provide niches for biofilm growth. These sites are also subject to recurring stagnation, which hinders the maintenance of control measures such as temperature or residual disinfectants. Together, these factors create opportunities for Legionella to thrive at distal sites.
There is strong evidence that concentrations of Legionella in the distal sites of premise plumbing can be significantly higher than in the more centralized sections of the premise plumbing of a building. Using monitoring data required by German regulations, a large investigation in Cologne focusing mostly on residential buildings revealed that 32.7 percent (223 of 712) of samples were positive for Legionella spp. (Kruse et al., 2016), with most positive detections (63.9 percent) found only at distal sites, rather than in the central recirculation system. Similarly, a large Italian database of regulatory sampling results for the
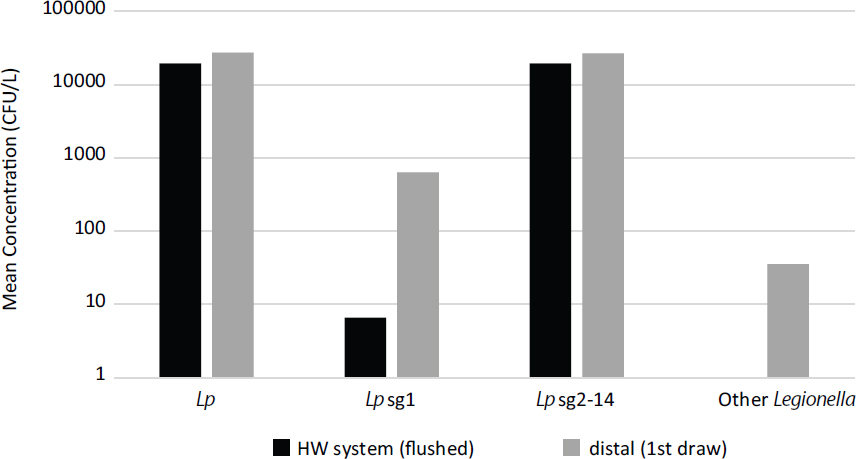
SOURCE: Cristina et al. (2014).
monitoring of Legionella spp. and L. pneumophila in hospitals in first-draw and flushed samples was analyzed by Cristina et al. (2014), who found high average concentrations of various Legionella strains and species both in the main hot-water plumbing and in first-draw samples at taps. As shown in Figure 4-3, significant amplification was noted for L. pneumophila serogroup 1 and other Legionella in first-draw samples, which specifically measure concentrations at the distal sites.
Biofilm growth and Legionella proliferation at distal sites can be prevented through various actions. Small diameter piping in the distal portion of premise plumbing can minimize water volumes and their age. Water circulation can be maximized by a combination of improved design (e.g., limiting the number of outlets) and preventive flushing procedures. The use of biostable materials (see previous section on plumbing materials) and minimization of the surface area available for biofilm growth should also be considered when selecting any distal devices, including faucets and flow-reduction aerators. Finally the use of thermostatic valves, which provide surfaces for biofilm growth at temperatures optimal for Legionella, should be carefully weighed against the risk of scalding and only used when justified on a risk basis. In cases where the premise plumbing is compromised, corrective action can be taken by installing point-of-use filtration barriers or flash disinfection devices.
Challenges of Thermostatic Mixing Valves and Electronic Faucets
Electronically activated faucets and thermostatic mixing valves increase Legionella risk because they provide surfaces for biofilm growth and water at ideal temperatures (42°C to 49°C) for Legionella. Thermostatic mixing valves, mixing manual faucets, and electronic faucets are complex devices composed of various combinations of synthetic, organic, and metal-based materials, often with multiple nooks and crevices where biofilm and Legionella can proliferate.
Used mainly in showers and faucets to prevent scalding, thermostatic mixing valves combine hot and cold water to achieve a set temperature that can be adjusted to protect users. There is limited information available on the impact of thermostatic mixing valves on the prevalence of Legionella at the point of use. In The Netherlands, thermostatic mixing valves in hotels and hospitals previously found positive for Legionella spp. were investigated in detail (van Hoof et al., 2014). Biofilm swabs and water samples (cold, hot, and mixed) were collected from two types of thermostatic mixing valves, and Legionella was quantified both by culture and qPCR. In seven instances, Legionella spp. were detected in at least one sample, with swab samples taken from rubber components of the valves showing the highest concentrations, which is in agreement with the high potential of rubbers to support growth of L. pneumophila (Niedeveld et al., 1986).
The interplay among materials, water quality, and temperature was investigated at the pilot scale by testing the impact of shower-faucet materials and iron-rust deposits on the growth of L. anisa in the absence of any chlorine residual (van der Lugt, 2017). Three types of shower faucets were tested: a faucet with a stainless-steel 304 housing and a ceramic mixer, a brass housing with a ceramic mixer, and a brass thermostatic mixing valve faucet. Increasing levels of positivity were observed for the stainless-steel faucets (14.3 percent), the brass (32.1 percent), and the faucet with the thermostatic mixing valve (85.7 percent), and adding iron rust deposits collected from a building water tank increased the maximum L. anisa concentrations observed. These results suggest that thermostatic valves are the faucet type most vulnerable to Legionella contamination and that iron corrosion byproducts can enhance the potential for Legionella spp. proliferation in faucets.
Several approaches can minimize the impact of thermostatic mixing valves including changing their configuration, placing them as close as possible to the point of use, avoiding all dead volumes such as bypasses, providing ready access for maintenance and cleaning, and selecting valves made of materials that do not support biofilm growth and that can withstand elevated temperatures and oxidants for disinfection. Within thermal mixing valves, integrated check valves prevent backflow into cold- or hot-water feed piping. Unfortunately, some of these check valves are susceptible to breakage and fouling. Their failure results in the mixing of cold and hot water in the piping, which leads to poor service and temperature conditions favorable to the growth of Legionella (Boppe et al., 2016). Several guidance documents specify the maintenance and even the installation of backflow valves (Castex and Houssein, 2005). Many guidelines and regulations require the use of thermostatic mixing valves only if needed based on a scalding risk assessment (e.g., Government of South Australia, 2013; HSE, 2013a).5
Electronically activated faucets have been linked to greater risk of contamination by premise plumbing pathogens, including Legionella, and have been shown to be the cause of several nosocomial outbreaks (Charron et al., 2015; Leprat et al., 2003; Moore and Walker, 2014; Yapicioglu et al., 2011). Sydnor et al. (2012) showed that nearly all electronic-eye faucets were colonized by Legionella spp. compared to only 45 percent of manual faucets. More importantly, the electronic-eye faucets were more resistant to disinfection by chlorine dioxide (Sydnor et al., 2012). Importantly, electronically activated faucets typically contain thermostatic mixing valves and flow-reducing devices such as complex aerators. Bacterial colonization of such faucets results from the tepid water temperature, type of materials used, and the lower flows typical of these devices (Charron et al., 2015).
___________________
5 See https://www.cdc.gov/legionella/wmp/monitor-water-guidance.html.
Terminal Tap Water Filters
Different types of terminal filters, often referred to as point-of-use (POU) filters, are available commercially and can be installed either at faucets or retrofitted to showerheads to prevent exposure in high-risk patient care areas. Such filters, typically of 0.2-µm porosity, provide a physical barrier to Legionella, are disposable, and are sometimes impregnated with biocides.
Many studies mention the high cost of these filters, driven by the large number of devices that may need to be installed and their relatively short life (eight to 30 days) before clogging or breakthrough (Marchesi et al., 2011; Sheffer et al., 2005; Zhou et al., 2014). In the same hospital complex in France, three POU shower filter devices showed wide ranges of use before clogging, ranging from three days to more than six months (Lecointe et al., 2010). Some reports show either low-level breakthrough or a return of contamination after one week of use (Vonberg et al., 2005) or 12 weeks (Baron et al., 2014). The time before clogging is dependent on the type of POU device and on the nature of the feed water.
In a cancer center in Pennsylvania, a new extended-life faucet filter ensured total removal of Legionella spp. for 12 weeks, exceeding the recommended period of use of 62 days, while mean concentrations at control faucets ranged from non-detect to more than 600 CFU/mL (Baron et al., 2014). A multi-layer design including two pre-filters of 30- and 1-µm porosity resulted in minimal flow restrictions and extended the life of the devices, halving the number of change-outs and associated costs. Recently, an electrically heated carbon nanotube and polymer membrane POU filter were proposed to inactivate any captured bacteria by increasing temperature on the membrane to 71°C to 83°C (Oh et al., 2019). Although this new membrane-interface POU removed 99.99 percent of L. pneumophila, further validation is warranted. Extreme care must be taken to ensure that water pressures at POU filters do not exceed manufacturer’s recommendations. Pressures in excess of ratings can cause filter media to break away and release contaminated water at the distal device.
Showers and taps have been designed and fitted with UV lamps located immediately before the outlet for microbial control and have been installed in a number of hospitals in the UK (Moore and Walker, 2014), but their efficacy remains to be seen. Using on-site UV treatment on the incoming water main was credited for avoiding any positive detects of Legionella in a new hospital and for the lack on any documented Legionnaires’ disease in the subsequent 13 years (Hall et al., 2003), although critical information about system hydraulics and other treatment was not provided.
Aerosol Formation Prevention
Aerosol formation is a critical risk factor in the transmission of legionellosis (Hamilton et al., 2018a). Therefore, preventing or reducing their formation can be an effective strategy for managing Legionella risk. Laminar flow of water is preferred, as devices that intentionally break the water stream (e.g., shower nozzles, faucet aerators, spray nozzles) can create respirable droplets less than 5 µm (see Figure 4-4; ASHRAE, 2000). Therefore, aerators should be removed from faucets to create a laminar flow (enHealth, 2015). Falkinham (2013) recommends the following to reduce aerosol exposures in the bathroom: (1) replace a showerhead with one that produces water streams (holes larger than 1-mm diameter) rather than a fine mist, (2) replace a showerhead with one that contains a microbiological filter (i.e., pore size less than 0.45-µm diameter) to reduce the proportion of aerosol droplets containing bacteria that can enter the lung, (3) open a window in the bathroom (if possible), (4) replace an inefficient fan with one that exhausts bathroom air rapidly, and (5) minimize the time that bathroom aerosols are created, for example, by shortening showers.

SOURCE: https://www.plumbingsupply.com/water-saving-low-flow-aerators.html.
Cooling towers and evaporative condensers incorporate drift eliminators to remove water droplets generated within the units (e.g., CoolClean, 2019; VisTech, 2019). The main purpose of these devices is to collect water droplets on a surface, which then directs the water back to the cooling tower. Newer design standards can reduce the drift to a maximum of 0.0005 percent of the cooling tower flow (Stodlka and Vitkovi, 2016). The humidity of the air, however, can cause larger droplets to be reduced by evaporation to 5 µm or less. At wastewater treatment plants, changes in aeration technology (e.g., use of fine bubble diffusers) or covering the aeration basins can reduce aerosol formation and transport (Prussin et al., 2017).
HOW THESE CONTROLS ARE APPLIED TO SPECIFIC SYSTEMS
The strategies discussed above can be applied in various ways to all of the major building water system types for the purpose of Legionella control. Table 4-1 provides an overview of the type of controls relevant to particular systems, categorizing them as (1) large engineered systems (potable water supply, wastewater treatment facilities, water reuse systems); (2) building water systems (large buildings, households, green buildings); and (3) other devices (cooling towers, humidifiers, hot tubs). The following sections provide an overview of how the various control strategies are or are not applied to each system in theory and in practice. Legal frameworks and guidance documents addressing these various systems will be covered in Chapter 5. In general, whether a water system presents a potential risk as a Legionella source and requires control depends on the following criteria (HSE, 2013b):
- Presence of Legionella in the system water;
- Water temperature between 20°C to 45°C;
- The system has the means to create and/or spread aerosols;
- The system stores and/or re-circulates water;
- The system is likely to contain a source of nutrients for Legionella, such as contaminants from the surroundings or from the process, including the presence of sludge, rust, scale, organic matter, or biofilm.
Public Water Supplies
Public water supply is an important consideration in Legionella management, as the characteristics of the water chemistry will vary seasonally and regionally, depending on drinking water source. The local water supply will be characterized by varying degrees of hardness, corrosivity, and nutrient content, which in turn impacts disinfectants and plumbing materials. Correspondingly, distinct microbiomes have been noted in controlled premise plumbing pipe rigs as a function of the local water chemistry (Ji et al., 2015).
In theory, public water supplies that already comply with local, state, and federal safe water regulations and implement standard practices, including maintaining a disinfectant residual, hydraulic control via routine flushing, and cleaning of storage tanks, have a strong foundation for controlling Legionella risk. The underlying statutes of these regulations and practices were developed to provide protection from a wide range of chemical and microbiological hazards. Because of cold water temperatures and the presence of a disinfectant residual, public water distribution systems are generally thought to harbor low levels of Legionella, although there are few data to support this assumption. Continued emphasis on the following elements is essential for reducing exposure to Legionella from public water supplies.
Control Options
Disinfection.
Most water utilities in the United States strive to maintain a minimum of 0.2 mg/L disinfectant residual in all parts of the pipeline system (AWWA, 2018). The voluntary Partnership for Safe Water program, for example, requires that all member systems use secondary disinfection and that “optimized” systems meet these residual disinfectant goals throughout the distribution system:
The goals are to be achieved for 95 percent of the routine readings each month, and individual routine sample sites should not have consecutive residual readings less than the residual disinfectant goal. Additionally, well-run systems specifically target areas known to experience low disinfectant residuals due to the pipe materials (e.g., unlined cast iron mains), long retention times, or water quality characteristics (e.g., organic matter, inorganic chemicals, pH, temperature). In these cases, the stability of the disinfectant residual can be increased by replacing old mains, improving the circulation within the distribution system, or improving treatment processes.
To improve control of Legionella, the U.S. Environmmental Protection Agency (EPA) has proposed to review the Surface Water Treatment Rule residual disinfectant requirement for “at least 0.2 mg/L at the point of entry and detectable in at least 95 percent of samples collected within the distribution system.”6 Several papers suggest that disinfectant residuals are lost once water starts to stagnate in premise plumbing (Bédard et al., 2018; Charron et al., 2015; Prévost et al., 1997). Additional research is needed to understand the persistence of distribution system disinfectant residuals within building plumbing. This is important not only for large buildings where it is often assumed that residuals are insufficient to affect
___________________
6 See https://www.epa.gov/dwsixyearreview/six-year-review-3-drinking-water-standards.
Legionella, but also for single-family homes and small buildings, where there is little solid information on the persistence of residuals.
Hydraulic Management.
Public water systems should have a routine program for systematically flushing and cleaning the distribution system, as over time bacterial growth can be promoted by precipitation of treatment chemicals, settling of fine silt, and corrosion products that form sediments within the pipelines. Implementation of a “uni-directional” flushing program is recommended, during which hydrants are opened near the treatment plant, and water is flushed systematically away from the plant toward the ends of the system; this approach avoids recirculating water from unflushed pipes into the cleaned sections of the system. Application of a hydraulic model is useful to ensure that adequate water pressure is maintained while achieving the targeted velocity (greater than 5 ft/s) (Friedman et al., 2002).
Hydraulic management of the distribution system is also important to avoid areas of water stagnation that can result in the loss of a disinfectant residual and the potential for regrowth. Areas of greatest concern are dead-end or dead-leg sections of pipes (e.g., at the ends of a pipeline where there is no circulation), inadequate mixing in storage reservoirs and tanks, and areas of the distribution system with poor circulation. Distribution system hydraulic models can identify these stagnant areas to evaluate options to mitigate. Water circulation is often improved by creating loops in the pipe system, avoiding closed valves, and installation of automatic flushing valves (NAS, 2006).
Like distribution system pipelines, sediments and corrosion products can accumulate in storage tanks, which require periodic inspection and cleaning. Legionella spp. have been detected by qPCR in 66.7 percent of municipal drinking water storage tank sediments from 18 sites (Lu et al., 2015). The AWWA Manual M42 (AWWA, 2013) recommends that tanks be drained and inspected at least once every three years or as required by state regulatory agencies. Periodic inspections by operators are recommended more frequently (monthly or weekly) and can be aided by drone technology to alleviate the need for a person to climb the tank. Water quality in the tank can be improved by installation of devices to ensure water circulation and to prevent stratification, stagnation, and loss of the disinfectant residual (EPA, 2002a).
Nutrient Limitation.
Nutrient limitation in public water supplies includes reducing nutrients during water treatment, corrosion control, and preventing nitrification in the distribution system. Biological filtration treatment processes (e.g., rapid sand filtration for groundwater treatment and biological active carbon filtration and slow sand filtration for surface water treatment) are pivotal for nutrient removal during drinking water treatment. Controlling corrosion of cast iron pipes in the distribution system prevents iron from leaching into the environment, which can limit growth of L. pneumophila because iron is an essential nutrient. Finally, when the chlorine-to-ammonia ratio (4.5:1) is not properly managed in chloraminated drinking water, nitrification can occur, enhancing biofilm biomass and increasing the number of protozoan hosts for L. pneumophila.
Plumbing Materials.
Most public water supply distribution systems consist of hundreds of miles of cast-iron mains, which will never be replaced in a time frame that would allow for better Legionella control. Even where plastic pipes have been installed, metal hydrants, valves, and other appurtenances remain. Pipes, valves, gaskets, coatings, and other materials that contact public drinking water supplies must be approved for use according to NSF/ANSI 61: Drinking Water System Components–Health Effects.7 Unfortunately, the NSF/ANSI 61 stan-
___________________
7 See http://www.nsf.org/services/by-industry/water-wastewater/municipal-water-treatment/nsf-ansi-standard-61.
dard does not address the microbial growth potential of materials in contact with water, unlike similar standards in Europe (Prest et al., 2016a,b; van der Kooij et al., 2003). Further, it is not simple for water utilities to change the materials already present in their distribution systems. However, NSF/ANSI 61 could implement standards to reduce microbial growth on water-contact materials so that utilities have better information in the future.
For many utilities, corrosion control is implemented in compliance with the Lead and Copper Rule (EPA, 1991). However, these procedures may not be sufficient to address corrosion of other metallic materials.
Temperature Control.
It is impractical for most public water systems to affect major changes in water temperature in their distribution systems, but there are practices that can be used by some utilities to impact water temperature. For example, intakes can be positioned below the thermocline in some raw water supplies, so that the cooler source water can be withdrawn. In some systems, warm surface waters can be blended with cooler groundwater supplies. Management of water mixing and turnover in elevated storage tanks can prevent water stratification during warm weather and help to control water temperature and disinfectant residual loss (Peter and Routledge, 2018).
Reclaimed Water Systems
Reclaimed water is municipal wastewater treated to high standards for beneficial use such as drinking water or irrigation water (EPA, 2012). This is a growing practice that presents many advantages, especially reducing water demand in arid and drought-prone regions as well as avoiding negative consequences of unintended, de facto reuse (NRC, 2012). A challenge with reclaimed water is that the level of treatment is dictated by the particular application. For direct or indirect potable reuse, the level of treatment often surpasses that for conventional drinking water treatment. In these instances, the control measures for Legionella would be similar to those outlined above for public water systems.
Reclaimed water treated for unrestricted reuse refers to non-potable water used where public access is not restricted. Water classified for unrestricted urban reuse is commonly applied for spray irrigation on parks, playgrounds, schoolyards, and residences, and for other applications such as toilet flushing, air conditioning, fire protection, construction, ornamental fountains, and other water features. Legionella has been routinely detected in many unrestricted reuse systems (Ajibode et al., 2013; Birks et al., 2004; Buse et al., 2015; Garner et al., 2018; Jjemba et al., 2010; Johnson et al., 2018). These systems typically do not maintain a disinfectant residual nor are they routinely flushed or cleaned. Jjemba et al. (2010) described the characteristics that contribute to the growth of microbes in reclaimed water distribution systems, including warm temperatures, elevated levels of biodegradable organic carbon and other nutrients, loss of disinfectant residuals, and variable use patterns that lead to stagnation and depressurization, among others. A recent survey of four reclaimed water distribution systems indicated elevated Legionella gene markers at the point of use, compared to paired potable water systems monitored in the same study (Garner et al., 2018). Brunkard et al. (2011) reported one outbreak of Legionnaires’ disease associated with use of reclaimed water at a mass-transit vehicle washing station. Hamilton et al. (2018a) reported that risks of Legionella exposure from reclaimed water used for irrigation or cooling towers could exceed 10−4 annual risk of infection for various scenarios. A review by Garner et al. (2016) highlighted that reclaimed waters are very different from traditional potable waters in terms of water quality, conveyance practices, exposure routes, and health risk. Because distinct water chemistries could place reclaimed water plumbing in uncharted
territory for Legionella control, the authors call for water quality management guidelines and regulations more specifically tailored to recycled water. Jjemba et al. (2015) reported on best management practices (BMPs) for maintaining water quality in reclaimed water systems (see Box 4-1). Many of these BMPs are similar to those mentioned for public water systems (e.g., optimizing water age, managing storage, corrosion control, biofilm control, etc.), but managing risk of inhalation, rather than ingestion, needs to be emphasized.
Treating recycled water for purposes of direct potable reuse is gaining momentum. For example, a 2 million gallon per day direct potable reuse plant in Big Spring, Texas, treats wastewater to drinking water standards via microfiltration, reverse osmosis, and UV disinfection before blending with raw drinking water sources and routing to a conventional drinking water treatment plant (Trussell et al., 2015). Given that such an approach meets current drinking water standards, there should not be any special concerns related to Legionella beyond that of a typical municipal water supply. Nonetheless, out of an abundance of caution, efforts are under way to understand how blending of direct potable reuse water with conventional water supplies and treatments may adversely affect distribution systems via corrosion and other processes (Water Research Foundation, 2018). A pilot-scale survey following incubation of a range of direct potable reuse blends from different utilities in PVC pipe over eight weeks indicated only rare detection of Legionella spp. gene markers by qPCR (Garner et al., 2019). While this result is encouraging, longer-term studies and monitoring are recommended as municipalities begin blending direct potable reuse water.
Wastewater Treatment Plants
Wastewater treatment plants, especially those with biological treatment processes, can be a source for L. pneumophila (Caicedo et al., 2019). What measures can be taken to control legionellae depends on the treatment process in the wastewater treatment plant. For example, certain aerosol-producing installations at treatment plants (e.g., air scrubbers) can be controlled by disinfection using hot steam or hypochlorite treatment (Olsen et al., 2010).
Norway is one of the few countries where control measures for aerosol-producing devices in wastewater treatment are regulated.
In most outbreaks involving wastewater treatment plants, the biological treatment process is identified as the main cause for L. pneumophila growth (Caicedo et al., 2019). Control measures that are normally taken against legionellae (e.g., thermal control, chemical disinfection) are difficult to implement in biological treatment processes because these control measures will also eradicate the microorganisms that treat the wastewater. In addition, laboratory experiments have shown that disinfection of wastewater effluent with chlorine dioxide, hydrogen peroxide, silver ions, ozone, and alkalinization did not result in reduction of cultivable legionellae (Noguiera et al., 2016). As a result, alternative control measures have been implemented at plants that have been identified as the source for an outbreak of Legionnaires’ disease or Pontiac fever. At locations where only workers became infected with L. pneumophila, workers were required to wear respirators that prevent inhaling of aerosols and/or prevent use of L. pneumophila-contaminated waters for cleaning purposes (Castor et al., 2005; Gregersen et al., 1999; Kusnetsov et al., 2010).
Different control measures have been taken at wastewater treatment plants where the biological treatment process (e.g., an aeration pond) was identified as the source for Legionella infection among residents who live in the vicinity of the plant. One example is a plant in Norway that treated wood refinement waste. As the ultimate infection control measure, this plant was shut down, but its organic content was then released into the river (Borgen et al., 2008). During a large Legionnaires’ disease outbreak in Warstein, Germany, several control measures were implemented at the biological wastewater treatment plant that was the primary source of the outbreak (Noguiera et al., 2016). UV was installed to treat the effluent before it was discharged into the river, which resulted in a 1.6- to 3.4-log reduction of legionellae in the effluent (from about 106 CFU/L to about 104 CFU/L). Second, the aerobic pre-treatment process was stopped, which resulted in a significant decrease of L. pneumophila in the wastewater (to 102 CFU/L) and in the effluent (below the detection limit). Finally, measures were taken to reduce aerosol emission from the wastewater treatment plant, although these measures were not specified. In the Netherlands, control measures at two biological treatment plants that were involved in small outbreaks of L. pneumophila focused on preventing aerosolization from the aeration ponds to the open air. This was done by successively erecting tents to cover the aerated ponds in combination with ventilation to prevent overpressure in the covering tents (Loenenbach et al., 2018).
Large Buildings
Large buildings include most hospitals and many long-term care facilities, as well as apartment complexes, hotels, offices, high rises, schools, prisons, and industrial complexes. Legionella is inherently more difficult to manage in larger building water systems because the plumbing networks are correspondingly larger and subject to more variability, making it more challenging to ensure that controls are adequately supplied throughout the building. The extended stagnation periods experienced by water in large building premise plumbing place these systems at further risk. Thus, Legionella management in large buildings tends to focus on thermal control (Bédard et al., 2015; Boppe et al., 2016) or on-site disinfection. Any controls that have been emplaced on the municipal water supply up to the property line, e.g., a minimum chlorine residual of 0.2 mg/L, are unlikely to provide reliable protection throughout a large building plumbing network.
Another challenge is that large buildings often require substantial water storage for water security purposes. However, during storage the water quality can degrade substantially,
posing problems in times of need. Large buildings also often employ potable or recycled water for other purposes, including humidifiers, landscape irrigation, decorative fountains, hot tubs, swimming pools, and cooling towers to manage extensive HVAC needs. In this section the emphasis is on piped potable water used for drinking and bathing, though general principles apply to other piped water systems. Cooling towers, humidifiers, and hot tubs are discussed in separate sections.
Design and commissioning of a large building is a key opportunity to ensure that Legionella control is prioritized, including appropriate design and implementation of hot- and cold-water systems and HVAC features. Further, large building water systems should be configured to facilitate collection of water for Legionella monitoring as well as implementation of maintenance and remediation (e.g., sampling and injection ports on hot-water lines). Hospitals or other buildings where sensitive populations are housed should be designed to facilitate remediation in the case of contamination by Legionella or other pathogens. Unfortunately, in reality the majority of existing large buildings were not designed in this manner and present numerous complex challenges for Legionella control.
Much of what has been learned to date about management of Legionella in large buildings comes from hospitals. Table 4-5 summarizes long-term hospital experience with various combinations of disinfection and thermal regimes, including long-term studies (up to ten years) with extensive monitoring to support findings. From these data, the two controls that emerge as being most broadly effective are (1) temperature set points of greater than 60°C at the water heater and greater than 55°C in the recirculation loop and (2) chloramine as an on-site disinfectant. Combining elevated temperature with addition of disinfectants yielded the best results in some cases.
Control Options
Temperature Control.
Maintaining a high water temperature (ideally greater than 60°C) in hot-water lines is the primary line of defense against Legionella in large buildings. This can be accomplished in part by installing multiple water tank heaters. Recirculating lines are also commonly employed to ensure delivery of hot water throughout the building. Recirculating lines are susceptible to heat loss and can readily fall into the ideal temperature range for Legionella growth if not maintained at a sufficiently high temperature (Brazeau and Edwards, 2013a,b,c). This can be avoided by insulating recirculating pipes (as required in Canadian building codes for large buildings) and making sure the recirculating velocities are sufficiently high. Recently, the California Exchange Commission has mandated insulation of hot-water lines (CEC, 2019). Despite the challenges mentioned above, maintaining water above 55°C across the whole system in large buildings with multiple recirculation loops can be done at relatively low cost (Bédard et al., 2015, 2016). Successful and low-cost hospital interventions have shown that poor temperature maintenance can be corrected by removing dead-end pipes, inadequate heat exchangers, and faulty thermostatic valves that can cause flow inversions and mixing with cold water (Bédard et al., 2016; Boppe et al., 2016; Lecointe et al., 2018).
Likewise, temperatures of cold-water lines can also increase into the Legionella growth range, particularly in warm climates and as water makes its way through extensive distal plumbing within the warmer building envelope. In such cases, cold-water line flushing and pipe insulation to minimize heat transfer to the cold-water piping can help. In a study of a major hospital in Germany, the cold-water lines were as contaminated with Legionella as the hot-water lines, and 35 percent were positive, even at sites where the measured temperature was less than 20°C (Arvand et al., 2011).
TABLE 4-5 Long-term Hospital Experience Using Multiple Strategies for the Control of Legionella
| Type of Facility | Permanent Regime | Shock Disinfection | Targets | Findings | References |
|---|---|---|---|---|---|
|
Hospital with 1,077 beds
9 years |
ClO2 for greater than 9 years
Chloramines – 26 weeks |
None | Legionella spp. and Mycobacterium avium complex (MAC) |
ClO2 for greater than 9 years reduced Legionella positivity to 51% but with high concentrations (greater than 105 CFU/L) remaining at some sites.
Chloramines at 2 mg/L for 26 weeks reduced all sites from greater than 103 CFU/L to non-detect but increased positivity for MAC. |
Casini et al. (2014) |
|
Hospital with four circuits (A-D)
5-18 years with Legionella contamination |
A: >55°C − 60°C
B: >65°C one day per quarter C: A&B + chlorine D: chlorine |
|
Legionella spp. and L. pneumophila |
Frequent heat-shock treatment (65°C) led to temperature resistance in some Legionella strains.
The combination of maintaining 65°C one day per quarter combined with chlorine was more efficient than repeated heat shocks at 70°C. |
Allegra et al. (2011) |
| Hospital with three wings |
ClO2 two types reactors – 1 year
Chloramines – 1 year |
ClO2: 0.6 to 0.9 mg/L
Chloramines: 2 mg/L and 3 mg/L |
L. pneumophila and Pseudomonas aeruginosa |
Using ClO2, positivity went from 100% to 57-61%.
ClO2 was less effective for L. pneumophila sg1. With chloramines, there was quick (<1 month) and large reduction in # positive sites and concentrations of L. pneumophila. 2 mg/L chloramines: <100 CFU/L for L. pneumophila; 3 mg/L chloramines: below detection limit for L. pneumophila. Both disinfectants reduced P. aeruginosa. |
Marchesi et al. (2012) |
|
Two hospitals
A: 255 beds B: 450 beds |
A: sub-optimal thermal regimes and Cu-Ag ionization
B: suboptimal thermal regime |
None | Legionella spp. L. pneumophila |
Online temperature monitoring is needed for system characterization and to use the risk framework.
The poor thermal regime resulted in greater Legionella and L. pneumophila prevalence. A framework for risk analysis using temperature profiling was developed. |
Bédard et al. (2015) |
|
16 hospitals
5 years |
Cu-Ag ionization | 50% used superheat/flush and 31% used hyperchlorination |
Legionella spp.
L. pneumophila sg1 L. pneumophila sg2-14 |
50% of sites reported zero positive samples in the years after implementation.
Only 5% of hospitals reported cases of nosocomial legionellosis, down from 100% before treatment. |
Stout and Yu (2003) |
|
Hospital with 450 beds
11 years |
Increasing temperature from 45°C to 65°C with distal temperature of 56°C–61°C for 5 minutes after outbreak | Weekly flushing of all taps and showers at 65°C for 5 minutes over 18 months |
Legionella spp.
L. pneumophila sg1 L. pneumophila sg2-14 |
There was a striking decrease from ~100% to less than 20% samples positive with lower concentrations of L. pneumophila sg1 (<500 CFU/100mL).
Increasing temperature arrested Legionnaires’ disease outbreak, but four cases occurred during 10 years even with only 5% positivity. |
Darelid et al. (2002) |
|
Two hospitals
A: 364 beds B: 672 beds 2 years |
ClO2 dosages of 0.5–0.7 mg/L in cold water | Not specified |
Legionella spp.
Heterotrophic plate counts |
Treatment reduced positivity from 60% to less than 10% and it decreased heterotrophic plate counts. However, positivity increased again after 2 years. | Zhang et al. (2009) |
| Hospital with 1,266 beds |
Target dosages of Cu/Ag 0.2/0.02 mg/L
Cu/Ag concentrations at distal sites mean 0.16/0.014 mg/L |
2 superheat/flush treatments | Legionella spp. |
Positivity in intensive care units was 14% and Chen et al. 66% after two superheat/flush treatments.
Low Cu-Ag dosages were not effective. Increased Cu-Ag dosages lowered positivity to 0-5%. There were no nosocomial cases after implementation. |
Chen et al. (2008) |
| Hospital with 765 beds |
ClO2 dose of 0.3 mg/L distal concentration for 9 years
Monochloramine at 3 mg/L for one year |
Superheat (>60°C) for 2 days, 8 times in 4 years
Hyperchlorination (20–50 mg/L) for 1-2 hours, 12 times in 8 years |
Legionella spp.
L. pneumophila |
Superheat treatment led to an insignificant, temporary reduction in positivity.
Hyperchlorination was effective for 2 months. ClO2 reduced positivity from 97% to 54% for Legionella spp., but less so for L. pneumophila. |
Marchesi et al. (2011) |
| 57 buildings |
Conversion from free chlorine (0.6 mg/L) to monochloramine (1.9 mg/L) in the distribution system
Only 13% of water heaters >60°C |
None |
L. anisa,
L. bozemanii Legionella spp. L. pneumophila sg1 and 2-14 |
Increased Legionella colonization was observed in buildings with hot-water temperature less than 50°C.
Chloramines strikingly (60% to 4%) and rapidly (<3 months) reduced the percentage of sites positive for L. anisa, L. bozemanii, Legionella spp., L. pneumophila sg1 and sg2-14. |
Flannery et al. (2006) |
Disinfection.
As mentioned previously, disinfectant residuals from the distribution system may not persist in the premise plumbing of large buildings. Hence, many hospitals and long-term care facilities in particular have found on-site disinfection to be highly beneficial. Disinfectant can be added at a constant level to manage Legionella risk, or it can be increased in response to elevated Legionella numbers or an outbreak. Low doses of disinfectant are often an effective preventative measure, but much higher doses are required for remediation purposes (see Table 4-5). Disinfection systems are typically added to hot-water lines to avoid any concerns with human consumption, as hot-water lines are not intended to produce water for ingestion, but they can be added to cold-water lines as well. Popular disinfectants for this purpose include chloramine, chlorine, copper-silver ionization, and chlorine dioxide. However, it is important to be aware of the local water chemistry, pipe materials, and other constraints of relying on such disinfectants (Rhoads et al., 2014). For example, iron plumbing components can reduce the chlorine residual by stimulating its decay. During a major Legionnaires’ disease outbreak at an Illinois veteran’s home in Quincy, reaction of on-site chlorine addition with old iron pipes delivering water throughout the campus significantly depleted chlorine residual (Rhoads et al., 2018). Copper-silver can also lose its efficacy and fail to be delivered appropriately, for example, by plating onto pipe surfaces instead of maintaining dissolved form, if installed incorrectly, or if the water chemistry is incompatible (Triantafyllidou et al., 2016; Walraven et al., 2016). Chloramine is probably the most popular on-site disinfectant, but its decay can be accelerated by nitrifying microorganisms, which happen to thrive in a similar warm temperature range as Legionella. UV can also be applied, but it may be most effective at the point of use since it does not leave a disinfectant residual.
Hydraulic Management.
Fundamental to reducing Legionella risk is managing the hydraulics of the plumbing system to ensure delivery of both hot water and disinfectant. As discussed previously, recirculating lines are commonly employed to achieve this purpose. Aside from temperature and disinfectant delivery, maintaining a low water age itself is a key aspect of hydraulic design. Installing flushing devices can help alleviate other water age issues, such as taste, odor, microbial growth, and nitrification (Nguyen et al., 2012). Dead legs and other flow anomalies must be avoided at all cost. For extremely large buildings or other situations where it is difficult to control water age, automatic flushing devices and programs may be beneficial as a routine maintenance or remedial measure. There is no consensus on the optimal frequency and duration of flushing for efficient Legionella control, but evidence clearly demonstrates that the use of frequent (one minute every two hours) automated flushing of hot-water taps with low use or poor recirculation (dead-ends) can eliminate Legionella positivity (Darelid et al., 2002; Totaro et al., 2018).
Distal Devices and Aerosol Control.
Even with maintaining water temperature and disinfectant levels in hot-water lines, it is critical to consider the mode of delivery at the fixture. Benefits and susceptibilities of various faucets and means of delivery were discussed in a previous section. Low-flow fixtures have been promoted to both conserve water and in some cases energy. However, as a consequence of their lower flow, these fixtures, primarily faucets but also showers, increase water age and restrict disinfectant levels, including the disinfection provided by elevated water temperatures. As such, low-flow fixtures present a greater risk for Legionella development in the distribution systems that feed them. Low-flow fixtures should be restricted from use in hospitals and long-term care facilities due to their high-risk occupant populations.
As previously discussed, faucets with a “hands-free” designs, including automatic sensors and foot pumps, do not reduce microbial risk (Sydnor et al., 2012). Compared to
traditional fixtures, these designs tend to have higher surface area for biofilm formation and are more conducive to Legionella growth. The same is true of the thermostatic mixing valves that produce warm water to enhance the comfort of the hand-washer. Faucet and fixture selection is a key decision, as these are typically installed as a building-wide standard.
In extreme cases where Legionella growth is uncontrolled and patient populations are extremely sensitive, size exclusion point-of-use ultrafilters can be installed. These will effectively remove Legionella from the water at the tap. However, such filters are quite costly and not a sustainable long-term solution. Selecting a device that can function with the incoming water quality, as well as proper installation and maintenance, are key to ensuring cost-effective use and efficacy (Baron et al., 2014).
Households
Households are a poorly understood source of Legionella that may contribute to the large percentage of legionellosis cases known to be sporadic (see Chapter 3; Adams et al., 2017; McClung et al., 2017; Shah et al., 2018). While water use in the home is generally more consistent than in public buildings, old piping (e.g., galvanized steel), dead legs, and low-use locations can all provide the opportunity for Legionella growth. Additionally, most home hot-water heaters are set at temperatures to limit the risk of scalding but are within the range for Legionella growth (see Table 4-3). Travel, hospitalization, home construction and remodeling, and other events that restrict water use can lead to potential opportunities for Legionella to proliferate in household water systems. Finally, there is the potential for Legionella growth in devices found in homes that are in contact with water, including humidifiers, nebulizers, and hot tubs. Given all of these potential sources of contamination, a key risk prevention strategy at the household level is communicating the risks of Legionella to immunocompromised individuals and to those who purchase devices for in-home use that could create aerosols containing Legionella.
Households may be served by a public water supply or by private wells. An estimated 13 million households rely on private wells for drinking water in the United States (U.S. Census American Housing Survey, 2017 data8), but EPA regulations do not apply to these private systems. Little information is available about occurrence of Legionella in private well water (Stojek and Dutkiewicz, 2011). Well water is not routinely disinfected, which could potentially leave well owners more susceptible to Legionella. For example, in a large field study of 255 domestic water heaters, those fed by a groundwater source distributed without any treatment were more often positive (46.3 percent) than water heaters supplied by surface water sources with residual chlorine (26.2 to 27.5 percent) (Dewailly and Joly, 1991). Most groundwater supplies in the United States would be considered low risk because of their cold temperature, but there are areas of the country where groundwater may be warm enough to support Legionella growth (Riffard et al., 2001). However, for most private systems, management of Legionella risk is mainly associated with managing the hot-water system and the devices in the home that come into contact with water and produce aerosols, as described below for typical households. Intrusion of soil and other contamination that could contain Legionella, particularly as a result of major weather events, also require attention. Well owners are given general guidance on how to remediate such intrusion events, typically by addition of bleach (i.e., chlorination) as a shock treatment. Specific guidance on Legionella control is needed for well owners, especially for immunocompromised individuals.
___________________
8 See https://www.census.gov/programs-surveys/ahs/data/interactive/ahstablecreator.html.
Control Options
Most of the strategies summarized in Table 4-1 could play a role in managing Legionella in households, though homeowners seldom implement them formally.
Hydraulic Management.
To prevent biofilm growth and exposure to Legionella, homeowners are recommended to perform several maintenance activities, including the regular flushing of sediments from hot-water tanks and cleaning of faucet aerators, showerheads, hot tubs, nebulizers, evaporative cooling fans, and humidifiers (Leoni et al., 2018). Guidance is also provided in a recent Water Research Foundation report (#4664—Customer Messaging on Plumbing System Issues) that developed materials for water utility websites.9
Because smaller-diameter pipes are found in buildings and homes, premise plumbing is particularly prone to growth of biofilm bacteria and resulting water quality problems. Although there are no ways to reduce the nutrient content of water entering premise plumbing, other strategies can be employed to control biofilm growth, including flushing pipes to reduce water age and deliver disinfectant residuals throughout the home. Indeed, one hypothesis for traveler’s associated Legionnaires’ disease is that the individual is exposed to stagnant plumbing upon returning home (Verhoef et al., 2004). After prolonged absence, flushing should be considered as a preventive measure since stagnant water may have high concentrations of bacteria including Legionella, bad taste and odor, no disinfectant residual, and elevated concentrations of metals such as copper and lead.
Temperature Control.
Elevating water heater temperatures is an obvious household intervention, though this can be restricted by building codes, which vary from state to state. For highly elevated water temperatures, the scalding risk may also not be worth the trade-off for certain elderly or less mobile individuals. Also, for many reasons delivery of hot-enough water temperatures to the point of use can be a problem in households just as it is for hospitals and hotels. Water in households typically sits stagnant during the day, and homes can be vacant for long periods during vacations. As discussed previously, the choice of hot-water heater design is also important to minimizing the risk of legionellosis.
Distal Devices.
Legionella can be amplified at distal sites such as faucets and showers in the household. Selecting faucets to minimize the potential for Legionella growth can be achieved by selecting simple designs without electronic activation and only with mixing valves if needed. If thermostatic mixing valves are justified for scald prevention, then models with the valve integrated to the body of the faucet with minimal volumes of tepid water would offer a lower risk (Charron et al., 2015).
Reverse osmosis units are not uncommon at the household level and can be installed as whole-house POU filters. Based on size exclusion, Legionella should be eliminated after passing through reverse osmosis. However, household filters, including reverse osmosis units and carbon black filters, can also remove disinfectants. Thus, if applied at the whole-house level, they could potentially leave downstream plumbing at risk of colonization. Carbon black filters, which are typically applied as faucet mounts or used in filters for water directly intended for drinking or cooking, provide surface area for microbial growth and result in elevated HPCs. A study by the World Health Organization indicated that there was no measurable human health risk associated with increased HPCs from POU filters (Hunter, 2003).
___________________
Disinfection.
On-site disinfection is likely unrealistic for most homeowners although chlorination is commonly recommended to well owners for remedial purposes. Point-of-use UV units are gaining in popularity, for example, under sinks where drinking water is drawn and as part of refrigerator-dispensed drinking water. Such units need to be evaluated in terms of efficacy for Legionella control and most effective placement.
***
No national recommendations have been developed to help protect individual households from legionellosis. However, some studies have recommended steps to limit potential exposures to Legionella (e.g., Pedro-Botet et al., 2002). Much of the effort for homeowners focuses on water temperature and water flow during periods of decreased use. Increasing the water temperature in households to 60°C (140°F) can help limit Legionella growth in home hot-water systems, but must be weighed individually against the risk of scalding and burns. Tankless, on-demand hot-water heaters may provide an opportunity to limit the amount of water that is at risk and may have higher disinfectant residuals (Brazeau and Edwards, 2013b). Prevention of water stagnation while residents are not at home, such as flushing the taps at least weekly, may help to prevent Legionella growth. Alternatively, homeowners can decrease water-heater temperatures to levels that do not promote Legionella growth when they expect to be away from home for prolonged periods; this may be less effective in areas where normal ambient summer temperatures are high. For immunocompromised or high-risk individuals, additional measures such as POU filters for sinks and showerheads can be considered (Baron et al., 2014). Use of humidifiers, particularly those using water misting, should be discouraged among higher-risk patients (Hines et al., 2014; Yiallouros et al., 2013).
Cooling Towers
Heating, ventilation, and air conditioning (HVAC) systems are designed to condition and to distribute air to provide a comfortable indoor environment. HVAC systems can be a source of Legionella infections because they have abundant water and can disseminate Legionella-contaminated aerosols (Aaron et al., 2017). Within HVAC systems the two most likely sites to harbor Legionella are the humidification and the cooling equipment. This section deals exclusively with cooling equipment, while the following section deals with humidifiers.
Evaporative heat transfer devices such as cooling towers and evaporative condensers are used to dissipate waste heat from the condenser of chillers providing air conditioning to a building. There are two basic types of evaporative heat transfer devices—a direct-contact device that exposes water directly to the cooling atmosphere, and a closed-circuit device that involves indirect contact between the heated fluid and the atmosphere. Their construction and operation are extensively detailed in documents by various organizations (such as the Cooling Technology Institute; ASHRAE, 2016).
Open and closed recirculating wet and wet/dry cooling towers may show some emissions because of drift and volatilization. Plume formation can be important in open and closed wet cooling towers when air with a high moisture content leaves the cooling tower, mixes with the atmosphere and begins to cool down. Both wet and wet/dry device types can be sources of Legionella infections due to their large use of water, their operating temperature, and their capacity to generate aerosols.
The single most important component of a cooling tower is the fill or heat-transfer surface, as different geometries and fill materials affect the heat rejection rate. Fills are
susceptible to fouling, scaling, and microbiological growth (DOE, 2011). Within the water distribution and mechanical components of cooling towers, polypropylene, acrylonitrile butadiene styrene, and fiberglass-filled nylon have largely supplanted the bronze nozzles of earlier cooling towers, and PVC and fiberglass piping have replaced most iron and steel piping. Therefore, the materials typically used now are resistant to corrosion, erosion, and microbial growth (SPX, 2009).
Cooling towers are usually situated outdoors and open to the elements. This location makes them popular for birds and bugs to live in or around and susceptible to dirt and debris carried by the wind, providing nutrient sources for microorganisms in the system (DOE, 2011). A variety of microorganisms can grow in cooling towers during the course of normal operation, which involves water temperatures ranging from 29°C to 35°C (ASHRAE, 2000). Bacteria can grow in condensers and in the cooling tower fill, while algae can grow on wet cooling tower components exposed to sunlight. Biofilms are frequently found in chiller bundles, on the surfaces of heat exchangers, and in the system’s piping (DOE, 2011).
By design, cooling towers use a significant amount of water, as they dissipate heat by evaporation. Geographic and climate concerns such as water availability or sewer usage restrictions may dictate unusually elevated water recirculation needs for the heat rejection equipment. However, the increased cycles can increase the concentrations of metals, minerals, and contaminants (SPX, 2009).
Control Options
Control options in cooling towers are somewhat limited and based primarily on the use of disinfectants to prevent microbial growth. Materials selection during cooling tower design and construction can also affect whether the tower becomes a site of Legionella amplification. A major preventive strategy when cooling towers are not in use is to recirculate the water. Finally, though not traditionally considered as a control for cooling towers, future cooling tower designs using elevated temperatures could aid Legionella prevention.
Disinfection.
Chlorination and hyperchlorination are commonly used chemical treatments to limit microbial growth in cooling towers, although numerous chemical disinfection methods have been used (Kim et al., 2002). However, these treatments generally do not completely eliminate the Legionella. If the treatments are discontinued, recolonization can occur after a lag period sometimes as short as two weeks. For example, Iervolino et al. (2017) showed the recolonization by Legionella of hyperchlorinated cooling towers can take place within weeks or months of the initial treatment. Paranjape et al. (2019) found that continuous chlorine application in a cooling tower reduced microbial diversity and promoted the presence of Pseudomonas, creating a non-permissive environment for Legionella spp.
Silver and copper ions have also been used in cooling towers to control bacterial growth (Lin et al., 2002). In a study by Martinez et al. (2004), a chlorine concentration of 0.3 parts per million (ppm or mg/L) was combined with 200 parts per billion (ppb) of silver and 1.2 ppm of copper. This method had an appreciable impact on levels of coliform bacteria, iron-related bacteria, sulfate-reducing bacteria, and slime-forming bacteria in a cooling tower.
Constant use of a single biocide can promote the establishment of a treatment-resistant microbial community in the cooling system. The typical solution for this problem is to routinely alternate between two or more biocides. However, the use and handling of toxic biocides should be evaluated to prevent overexposure of the maintenance workers and the building occupants.
Chemical-free water sterilization methods such as ozone and UV light have also been used sporadically in cooling towers. Ozone is considered to be effective against microbial contamination at a concentration of 0.2 to 1.0 mg/L. However, ozone gas is harmful to humans and must be handled carefully to avoid human overexposure. Furthermore, incorrect implementation can hamper the smooth operation of the cooling system. Of the half million or more cooling towers in the United States, it is estimated that only 300 to 1,000 use ozone. Likewise, UV has not been widely accepted for cooling tower use because of scaling of the UV system and issues arising from improper application of the technology (Rossman, 2003). The efficiency of these technologies either by themselves or in combination with other water treatments remains to be proven.
Cooling Tower Materials.
An important element in controlling biofilm growth within cooling towers are the materials selected for the construction of the heat rejection equipment. In reality, the equipment purchase specifications are mostly concerned with the performance and economics of the cooling tower operation. A study by Türetgen and Cotuk (2007) found that heterotrophic plate counts and L. pneumophila concentrations on galvanized steel were significantly higher than on six other construction materials used in a cooling tower (i.e., copper, stainless steel, polyvinyl chloride, polyethylene, polypropylene, glass). Corrosion-proof and anti-microbial resins are now being used for cooling towers (Sullivan, 2018). In selecting materials for cooling towers, not only is the bacterial growth potential of materials important, but also the performance and longevity of the materials in terms of how they are affected by the selected chemical and non-chemical treatments.
Temperature Control.
Temperature control in cooling towers is not generally considered to be an option because the temperature rise in a condenser or heat exchanger will increase the potential for calcium carbonate scaling, which can damage the fill materials. Indeed, most fill materials cannot be utilized in temperature applications greater than 49.9°C to 51.7°C (120°F to 125°F). However, given the proven efficacy of raising potable hot-water temperatures to 60°C (140°F) to control Legionella, the Committee suggests that refrigeration, HVAC, and cooling tower manufacturers collectively design and develop new systems that can operate at condenser water temperatures whereby the temperature going to the cooling tower will be greater than 60°C. In this proposed conceptual system, the condenser water temperature coming from the refrigeration equipment or chiller would be 65°C to 70°C and travel first to a reheat heat exchanger. By heating the reheat water in the heat exchanger, the water temperature would drop to 60°C before transport to the cooling tower, dropping the temperature to 55°C and then back to the refrigeration equipment or chiller. At such operating temperatures Legionella would be unlikely to survive.
Such designs would require additional energy consumption to increase the corresponding refrigerant gas pressures and temperatures to heat the condenser water to such levels. However, additional heat, in excess of the temperatures required, could be removed by a heat exchanger and used for the building’s reheat water system, increasing the overall efficiency. Finally, the creation of cooling towers that could withstand such temperature increases could potentially reduce the need for chemical biocides.
Humidification Equipment
In both residential and commercial buildings, humidification equipment uses water to cool and humidify the air. These units come in two basic types. Isothermal units such as steam humidifiers use energy to produce a steam vapor and are considered non-aerosol-
generating. On the other hand, adiabatic units allow direct contact between the water and the airstream, producing aerosols. Certain adiabatic units, such as atomizers or spray humidifiers, introduce water droplets directly into the airstream. Other adiabatic units, such as evaporative units and air washers, are considered non-aerosol-generating because the process only involves air absorbing the moisture as it passes over a pan or wetted device (ASHRAE, 2016).
Different designs of humidifiers have different levels of risk for Legionella growth (BMEC, 2009). Steam releasing-type humidifiers convert water to vapor that is then discharged into the selected space. Because of the high temperatures involved, and the fact that water droplets are not generated, this design is not considered a high risk for Legionella growth. Vaporization devices or direct evaporative coolers use a porous substrate to provide an extended surface area for water evaporation. The water is either circulated over the media or the media are rotated through a water bath. Thus, no water droplets are produced that could be contaminated with Legionella bacteria. The water used tends to be maintained at temperatures below the Legionella growth temperature range of 25°C to 43°C.
On the other hand, water spray devices such as misters, air washers, and spray humidifiers can produce aerosols through the use of ultrasonic vibrators, spinning disks, or spray nozzles. When their source water comes directly from the building’s cold-water supply or if the source water has been sent through reverse osmosis, these humidifiers can be used safely. However, when the source water is in holding tanks or in the pipes exposed to heat, the temperature of the water can reach 25°C to 43°C, a range that supports Legionella growth. Ultrasonic humidifiers and centrifugal sprays are thought to be most susceptible to Legionella contamination (BMEC, 2009). To limit the risk of legionellosis, these devices should be avoided for use in new buildings. Existing units of these types are recommended to be replaced during building renovation projects (PWGSC, 2013).
Regarding portable humidifiers, a review of the literature indicates that most of the disease transmission associated with these units is due to aerosol-producing humidifiers, i.e., ultrasonic and impeller units. Generally, the disease transmission is because the humidifiers were not properly cleaned or disinfected (Public Health Ontario, 2017). The appropriateness of allowing bedside humidifiers in institutions housing patients and residents who are more vulnerable to respiratory disease has been a topic of considerable debate.
Control Options
Disinfection.
There are limited chemical treatment options to reduce or eliminate Legionella bacteria from humidification systems. This is because the chemicals have the potential to be discharged into HVAC air distribution systems and ultimately inhaled by the building’s occupants. Water treatments such as softening and demineralization address the quality of the supply water necessary for the operation of the equipment but not the potential for microbial growth. Several Korean studies found that the use of disinfectant chemicals directly in the water of personal humidifiers has caused interstitial lung disease in children (Park et al., 2014, 2017; Pickering, 2014). Other water treatments, such as the use of UV or photochemical ozone generators instead of chemicals, have been considered (ASHRAE, 2000). Regular monitoring is needed to determine whether these treatments remain effective (HSE HSG 274, 2013c).
Temperature Control.
Water storage temperatures for all HVAC equipment are recommended to be either above, or below, the 25° to 43°C range where Legionella thrives.
Hydraulic Management.
Rigorous maintenance of humidification equipment is critical including regularly scheduled maintenance of the system, avoidance of water stagnation in the water tanks, pans, and basins, and use of water treatment where necessary. If these precautions are not feasible, the equipment must be taken out of service. Similarly, for smaller humidifier units (portable or home size), rigorous maintenance and drainage are recommended as well as appropriate cleaning and disinfection offline with suitable agents as per the manufacturer’s instructions (Public Health Ontario, 2017).
Hot Tubs and Swimming Pools
Legionella outbreaks have been caused by contaminated hot tubs (Benkel et al., 2000; Campese et al., 2010; Moore et al., 2015). Indeed, hot tubs were the third leading cause of legionellosis outbreaks among 27 investigations reported between 2000 and 2014, following potable water and cooling towers (Garrison et al., 2016). The warm water in these devices is often at the optimal growth temperature for Legionella growth (30°C to 40°C). Aerosols created by the water jets in some hot tubs can transmit the bacteria to people sitting in the units who are breathing very close to the water surface (Moore et al., 2015). Moreover, aerosols released from the water can be dispersed by air currents or ventilation systems, placing people outside the hot tub at risk for Legionella infection.
Hot-tub water is typically filtered and treated with chlorine, bromine, or ozone (Leoni et al., 2018). Although disinfection is the primary management option, the warm temperatures in hot tubs make it hard to maintain disinfectants at the levels needed to kill bacteria including Legionella. Therefore, hot tubs should be periodically inspected by health officials to ensure they are operating properly and adequately cleaned. Facility managers should check the amount of disinfectant in the water and the pH and have a regular schedule for cleaning that includes removing any films or algae from the sides of the hot tub. Filters in these units should be replaced in accordance with the manufacturer’s specifications. If Legionella is detected in a hot tub, the facility manager should follow CDC10 or American Society for Heating, Refrigerating, and Air-Conditioning Engineers guidelines for cleaning and disinfection (ASHRAE, 2015).
Although most recreational water Legionella outbreaks are linked to the warm water of hot tubs, the CDC also outlines guidance for pool operators. These include a 12-step program for prevention of recreational water illnesses, training, procedures for pool operations, and videos and guidance for the safe handling of pool chemicals.11 Facility operators should know and obey all applicable laws and regulations. If there are shower facilities associated with pools, facility managers should be cleaning and disinfecting the showerheads and faucets on a regular basis.
EMERGING OPPORTUNITIES AND UNINTENDED CONSEQUENCES
There is much still to be learned about Legionella ecology and its response to engineering controls. Currently, knowledge of built environment microbiomes is rapidly expanding (NASEM, 2017), largely driven by next-generation DNA sequencing, which promises to provide new insights. At the same time, U.S. infrastructure is aging beyond its intended lifespan and experiencing shifts in water demand, along with changes in behaviors and expectations of water consumers. Thus, the current situation presents both opportunities and challenges.
___________________
10 See http://www.cdc.gov/Legionella/downloads/hot-tub-disinfection.pdf.
11 See https://www.cdc.gov/healthywater/swimming/aquatics-professionals/index.htm.
Presently there is a major push toward advancing “green” building features in the United States, with the important goals of conserving energy, water, and materials. Water conservation features are driven by the need to reduce unsustainable water extraction, particularly as supplies experience greater pressure as a result of drought and other consequences of climate change. The need to reduce dependency on fossil fuels and limit production of greenhouse gases drives incorporation of energy-saving features, but these measures often have consequences for water systems as well. The U.S. Green Building Council (USGBC) reported that green building construction expenditures currently outpace those of general construction, with projected outlays of $224.4 billion in 2018 (USGBC, 2015). Developed by the USGBC, the Leadership in Environmental Engineering Design (LEED) certification system ascribes points for various building attributes, with “platinum” being the highest level of certification. Particularly relevant to this report are features by which such buildings can earn points for “potable water savings” and “energy savings” (USGBC 2016a,b). The following examples illustrate some of the complex and untested scenarios that can have unintended consequences and increase risk of Legionella growth in building water systems (Rhoads et al., 2015b) and highlight emerging opportunities to advance science and better understand and address such challenges.
Prebiotic and Probiotic Control of Legionella
Given that it is impossible to eradicate microbes or biofilms from engineered water systems, the possibility of intentionally shaping the kinds of microbes that colonize piped water systems to suppress pathogen growth niches has been proposed (Wang et al., 2013a). By definition, a probiotic approach would be to intentionally add such beneficial microorganisms, whereas a prebiotic approach controls the environment (e.g., water chemistry, pipe material, temperature) to favor desirable microorganisms. This exploratory concept remains to be tested and demonstrated in practice. Nonetheless, this is an interesting area for future research. As described in Chapter 2, there are several unique aspects of Legionella’s microbial ecology that lend support to the possibility of prebiotic or probiotic control.
One prebiotic approach extends from the examples of general biofilm control via nutrient reduction previously described in this chapter. Biological treatment that reduces the levels of biodegradable organic matter can help reduce the density of biofilm bacteria, and thus decrease the number of protozoan hosts available for Legionella replication. In particular, the composition of organic matter could be tailored to select for a biofilm community that is a poor food source for amoebae (Amaro et al., 2015) or for protozoa that digest Legionella (Amaro et al., 2015; Anderson et al., 2011; Maita et al., 2018). This possibility is supported by the fact that certain free-living amoebae are known to preferentially prey on certain bacteria rather than Legionella (Shaheen et al., 2019).
Alternatively, the thermal and disinfection controls described above may indirectly control Legionella by decreasing the population of free-living protozoa. Likewise, by manipulating other environmental factors such as oxygen levels, metals, organic carbon, stagnation, pipe materials, and other physicochemical and biological parameters, the ecology and life stage of free-living amoebae in water systems (and hence Legionella) could be managed. Another possibility would be to impose conditions (e.g., through nutrient deprivation, disinfection, or temperature shock) that shift free-living amoebae populations to the cyst stage, hence reducing Legionella growth potential because it is only capable of growing in trophozoites, not cysts.
One probiotic approach entails adding microbes that are a more preferred food-source for amoebae than Legionella but are non-digestible. Since the amoebae would derive little nutritional benefit from grazing on such a biofilm, their populations would decline or encyst. In particular, water systems would be supplemented with microbes that compete with
α-Proteobacteria, key prey for protozoan hosts of L. pneumophila (van der Kooij et al., 2018). Further, manipulating the types of free-living protozoa inhabiting the system presents several possibilities. For example, some amoebae are capable of digesting L. pneumophila (Amaro et al., 2015; Maita et al., 2018) or contain symbionts that do not allow ingested Legionella to replicate within the host (Maita et al., 2018; Okubo et al., 2018).
Prebiotic and probiotic approaches may be particularly attractive in the future given inherent limitations in existing engineered controls. In fact, relative resistance to both disinfectants and heat treatment are common features among Legionella and other pathogens that plague premise plumbing (Falkinham, 2015). As noted in Chapter 2, after intracellular replication in free-living protozoa, L. pneumophila can actually become more resilient to heat, oxidants, acids, osmotic pressure (Kwaik et al., 1997), biocides (Barker et al., 1992; Berk et al., 1998), and antibiotics (Barker et al., 1995; Garduño et al., 2002). Moreover, resistance to chlorine can spread among L. pneumophila on the ICE-box mobile genetic element, providing a mechanism for emergence of strains that persist in treated water (Flynn and Swanson, 2014). Thus, traditional use of disinfectants, depending on how effectively they are applied, may beneficially or detrimentally tip the microbial community balance toward one that favors Legionella. Better understanding the life stages and the ecology of free-living protozoa and Legionella in water systems could be critically important to advancing the possibility of prebiotic and probiotic control, as well as informing optimization of other more traditional engineered controls.
Unintended Consequences of Water Conservation
LEED-certified green buildings typically conserve 20 to 50 percent of potable water, although that value will rise as “off-grid” operations are adopted. To achieve water conservation goals, alternative sources of water are used for various purposes, including toilet flushing, landscaping, or even potable applications. Alternative sources include reclaimed water, greywater, and rainwater, which may present unique risks compared to traditional potable water. The other main approach to water conservation is incorporation of fixtures and appliances that use less water, such as low-flush toilets and low-flow and metered faucets. While current LEED certification does take into consideration “indoor environmental quality,” the focus is on criteria such as ventilation, thermal comfort, daylight, tobacco smoke, and avoiding volatile organic compound emitting materials, rather than water quality or Legionella. The need for these additional criteria is beginning to be recognized and would enhance the benefits of the green building movement (Cedeno-Laurent et al., 2018).
High Water Age
Deteriorating water quality due to high water age is a fundamental challenge of water storage, which many hospitals and other buildings require to ensure water security in emergency situations. For example, the Centers for Medicare & Medicaid Services (CMS) has mandated that hospitals be self-sufficient for 96 hours without essential utilities and deliverable items, including potable water. Many hospitals elected to maintain large stocks of potable water to meet the required 96-hour reserve. Any efforts to conserve water inherently increase stagnation and overall water age, both at the municipal level (i.e., main distribution system water age) and at the building level (i.e., premise plumbing water age) (Rhoads et al., 2016a). One survey found the premise plumbing water age in a typical LEED-certified healthcare suite to be eight days; it was more than six months in an off-grid office suite (Rhoads et al., 2016a). High water age has long been known to be detrimental to main distribution systems due to enhanced corrosion, development of taste and odor issues, loss of
disinfectant residual, and regrowth of microorganisms (EPA, 2002b). Increased distribution system water age can also increase water corrosivity for premise plumbing (Masters et al., 2015). A national survey indicated that there is excessive “overdesign” of water mains based on actual fixtures and flow rates (Buchberger et al., 2015), which further exacerbates water age problems at the community scale.
Once water enters the complex, high surface area and warm premise plumbing environment, such problems are only magnified. Meanwhile, the ability to compensate for lower flows is constrained by current building codes, such as mandating larger pipe sizes (Klein, 2018). A study of a newly constructed residences with green plumbing features occupied by college students noted a clear pattern of diminished water quality at the least frequently used taps (Salehi et al., 2018). In the LEED-certified healthcare suite noted above, disinfectant residual was entirely absent at all sampling points; more than 80 minutes of flushing was required before the municipal chloramine residual could be detected (Rhoads et al., 2016a). Further, the plumbing materials themselves enhanced disinfectant decay, with chloramine decay rates being 20 to 144 times faster when the well-flushed water sat in the plumbing compared to in a clean glass container. As water stagnates, it is also more often within an optimal temperature range for Legionella growth. In the LEED-certified healthcare suite, Legionella spp. gene copies were nondetectable in the incoming water supply, but were in the range of 10,000 to 100,000 GC/mL in three of the five premise plumbing sampling locations (Rhoads et al., 2016a).
Low-Flow and Metered Faucets
Lower-flow fixtures, including toilets, showerheads, and faucets labeled by EPA’s WaterSense program aim to reduce flows by at least 20 percent (EPA, 2016b). Lower flows reduce the rate at which consumers can draw water, but this can backfire because more flushing time is needed to obtain the target hot or cold temperature, depending on the application. Lower flow also pushes hydraulics into the laminar flow range, which is less effective for scouring biofilms, and can increase numbers of biofilm-associated Legionella (Liu et al., 2006). Metered faucets are very common in green buildings, only delivering a pre-determined aliquot and aiming to conserve water by incorporating electronic sensors to ensure that they are only opened when in use. Additionally, although such “hands-free” devices are intended to reduce spread of germs, ironically several studies have now confirmed that they have a propensity to grow Legionella and other pathogens, such as P. aeruginosa (Yapicioglu et al., 2011). Notably, Sydnor et al. (2012) cultured Legionella from 19 of 20 electronic faucets and only nine of 20 manual faucets co-located across three hospital units; this trend was even stronger when repeated sampling was taken into account. Further, Legionella colonizing electronic faucets were less responsive to chlorine dioxide disinfection than were Legionella in traditional faucets. Although it is not fully known why, the internal plastic components and the mixing of water create an ideal temperature for Legionella growth, which likely contributes to this problem.
Rainwater Harvesting
Collection of rainwater in cisterns is common throughout many parts of the world, particularly the rural tropics and sub-tropics, but this practice is also becoming a more intentional aspect of modern green building design elsewhere. EPA does not regulate the water quality of residential rainwater harvesting systems, but some state and local agencies do issue voluntary water quality guidelines for residential rainwater harvesting systems. Yet, natural
rainwater is not as “clean” as one might assume, as it is highly susceptible to atmospheric and rooftop sources of contamination, including bird droppings, heavy metals (Förster, 1999; Lee et al., 2010), herbicides (Bucheli et al., 1998), and pesticides (Zorbrist, 2000). The type of roof material also affects microbial water quality (Clark et al., 2019). A recent qPCR survey of Legionella in harvested rainwater tanks in Queensland, Australia encountered Legionella spp. in nearly 100 percent of tanks and L. pneumophila in 17 percent of tanks (Hamilton et al., 2017). A follow-up study in Philadelphia similarly noted qPCR detection of Legionella spp. in more than 50 percent of rooftop rainwater harvesting barrels (Hamilton et al., 2018b). Similar to findings from sediments in drinking water reservoirs (Lu et al., 2015), soil and dust are likely sources of these legionellae and associated protozoa.
Various factors associated with rainwater storage, collection, and use are likely to exacerbate potential problems with Legionella. Rainwater is characteristically low in pH and alkalinity, resulting in corrosive water whose problems were noted previously. Metal tanks are among the most frequently encountered materials (Hamilton et al., 2017) and will be directly affected by corrosion. Further, rainwater harvesting inherently entails storage, during which time typical water age problems are incurred and can be exacerbated by poor maintenance. Hamilton et al. (2017) noted in their survey of Australian tanks that 50 percent were never cleaned or desludged in their lifetime. Finally, the water savings incurred by rainwater harvesting can indirectly increase the water age within potable water plumbing. One study found that using rainwater to flush toilets resulted in a 58 percent to 80 percent reduction in potable water use, with premise plumbing water age at some taps exceeding three weeks (Nguyen et al., 2012).
Off-Grid Systems
At the extreme end of “green infrastructure” are off-grid or “net zero” buildings, which do not rely on an external water network for potable water or wastewater services (EPA, 2013). Such independence from water utilities is a primary goal of certifications such as the Living Building Challenge. The characteristics of these buildings include use of water-saving devices to reduce water consumption,12 rain-water harvesting, cisterns, on-site grey water or black water reuse, constructed wetlands, composting toilets, xeriscaping, and local aquifer recharge among other practices (Rhoads et al., 2015b). Such design configurations, however, raise a unique set of challenges and corresponding public health concerns. It is critical that these water systems be managed to control risks from Legionella and other water-related pathogens.
A recent survey estimated the premise plumbing water age of an off-grid “net zero” building to be between two to almost seven months, far exceeding that of a conventional building (Rhoads et al., 2016a). A 3,000-gallon tank for storing roof-top-harvested rainwater plus supplemented groundwater was primarily responsible for such a high water age. Disinfectant was not added to the water; rather, the water was subjected to serial filtration to 5 µm followed by a granular activated carbon (GAC) filter and UV disinfection. Legionella spp. gene markers measured by qPCR were detected throughout the system, including immediately post-treatment, in the storage tank, and in hot and cold flushed and stagnant water at 103 to 3 × 104 GC/mL (Rhoads et al., 2016a).
Because of the unique designs for off-grid buildings, each should have its own water management plan following the principles outlined in Chapter 5. Source water should be
___________________
properly filtered and disinfected, considering that even in rainwater samples Rhoads et al. (2015) reported Legionella, as measured by qPCR. The potential for extended water age means that the water management plans should address recirculation of water within the building plumbing system.
As part of the water management plan, off-grid buildings should pay close attention to keeping the hot water hot (55°C to 60°C) and the cold water cold (less than 25°C). Use of heat pumps or solar hot-water heating may result in water temperatures that are insufficient to prevent Legionella growth (Rhoads et al., 2016a). Temperatures will fluctuate on a diurnal basis and be influenced by seasonal and weather patterns if a solar heating system is not also paired with a non-solar water heater (van Amerongen et al., 2013). A review of the literature by van Amerongen et al. (2013) did not find, however, that solar heaters were more prone to Legionella detection than conventional heating systems, but they did point out that design and maintenance were important.
Flushing water lines and cleaning and inspecting storage tanks are important activities for off-grid systems, just as they are for public water systems; both should be included as part of the overall water management plan. Corrosivity of rainwater could put system components at risk and enhance conditions for Legionella.
Biowalls
Biowalls are an example of a green building feature that is gaining popularity. These walls of plants maintained in the indoor environment are advertised as a natural “botanical filter” that improves indoor air quality, helps “reduce sick building syndrome,” and saves energy by recycling internal air.13 However, a scientific literature review did not indicate that such claims have been tested. The perpetually moist environment of the biowall, along with a rich soil inoculum, maintained within a warm building envelope, could create an ideal habitat for Legionella proliferation. Further, the intentional “filtering” of air through the biowall clearly creates the potential for aerosol formation and occupant exposure. Thus, biowalls meet several criteria of a building system of concern worthy of scrutiny for its potential to be a source of Legionella exposure. Accordingly, appropriate engineered controls should be considered.
Unintended Consequences of Energy Conservation
As noted above, elevated water temperature is a master variable for Legionella control in buildings. Incentives in green buildings that encourage lowering this temperature to achieve energy savings can create conditions conducive to Legionella growth (Brazeau and Edwards, 2013b). Water heating is the second largest consumer of energy in the home and, accordingly, the EPA ENERGY STAR® program recommends a lower water-heater setting of 48.8°C (120°F) (EPA, 2019). This and other similar policies are in need of critical evaluation. For example, at one point the California Energy Commission (CEC) required recirculation for hot-water lines longer than ten feet, under the assumption that this would reduce water usage by lowering the time needed to achieve target shower temperature (Brazeau and Edwards, 2011); however, head-to-head studies revealed substantial heat loss and failure to achieve target temperatures with recirculation (Brazeau and Edwards, 2013a). Current California plumbing code now requires insulation of hot-water lines to conserve heat, and recovery of heat from drains is also encouraged (CEC, 2019). Thus, there is a
___________________
need for comprehensive cost-benefit analyses of actual energy savings achieved with various types of heaters, temperature settings, and corresponding plumbing configurations versus their impacts on water quality known to present risk factors for Legionella proliferation (Brazeau and Edwards, 2011). Analysis is needed to ensure that energy savings goals are actually met, while factoring in important public health considerations.
One comprehensive hospital case study clearly illustrates the unintended consequences of implementing reduced water heater temperatures (Blanc et al., 2005). Following the implementation of energy conservation regulations, hospitals in Switzerland were required to lower their hot-water temperature from 65°C to 50°C. To minimize bacterial contamination of their hot-water plumbing, the Lausanne University Hospital first upgraded its hot-water plumbing by eliminating dead ends and improving flow patterns. A thermal-shock treatment was then conducted before implementing on-site disinfection in 1995. Two separate premise plumbing systems were treated with: (1) ozone with a residual of 0.3 mg/L and (2) copper-silver at 0.3 mg/L. After three years, the positivity for Legionella spp. remained high in ozone-treated networks (66 percent to 56 percent) and in copper-silver-treated systems (90 percent to 93 percent). Increasing the temperature to 65°C at the water heater decreased the bacterial occurrence back to acceptable levels, although some areas remained persistently positive and were associated with poor hot-water recirculation leading to temperature losses (Blanc et al., 2005).
The experience at the Lausanne University Hospital demonstrates the importance both of elevated tank temperatures and maintaining sufficiently hot delivery lines. Nonetheless, reducing the temperature at the water heater outlet and shutting down the recirculation during low-usage periods (e.g., night, weekends) remain two major targets of energy conservation. Well-documented case studies in real systems show clearly that a reduction in temperature at the water heater outlet can lead to a significant increase in the likelihood of Legionella detection and the level of contamination at the tap. Further, shutting down the recirculation during the night will create stagnant conditions for periods of eight hours or more. Even in insulated systems, water will reach the ideal temperature for Legionella growth during such long stagnation periods (Bédard et al., 2016).
Energy conservation projects that add a heat exchanger to pre-heat the water prior to the water heater have also been increasing in popularity in healthcare facilities. The installation of these devices should be carefully studied to evaluate operating conditions. The very large surface present in heat exchangers, coupled with temperatures ranging between 25°C and 43°C, provide ideal conditions for Legionella growth. Recent field investigation revealed contamination of such a heat exchanger by a L. pneumophila strain that matched clinical isolates from cases occurring a few weeks after the installation of the device (Bédard et al., 2016). Disinfecting the device on a weekly basis and determining operating conditions to minimize L. pneumophila should be mandatory in healthcare facilities.
Other options for reducing energy demand of water heating include solar heaters and on-demand heaters. Solar heaters come in various configurations, typically employing a preheat tank and taking advantage of water stratification to draw water from the top before either being used directly, feeding a traditional tank heater or on-demand water heater. This subsequently incurs less energy input to heat to the target temperature. A typical feature of solar water heaters is some level of added water storage, which takes advantage of the high heat capacity of water. Rhoads et al. (2016a) observed that the added storage incurred by a solar water heater in a net zero energy home increased the hot-water age from less than one day to between two to three days. Further, due to cloudy days, the solar pre-heat tank may essentially end up in the optimal temperature range for Legionella growth. Legionella spp. copy numbers measured by qPCR in the hot-water manifold that received the heated water
and delivered it to taps were markedly high, upward of 106 GC/mL (Rhoads et al., 2016a). On the surface, on-demand heaters could be an effective alternative, only delivering hot water where and when needed, and these devices are currently recommended by CEC (2019). However, there are many logistical constraints to their installation and use, and their benefits for Legionella control need to be more critically evaluated (Brazeau and Edwards, 2013c).
Potential Trade-Offs with Other Microbial Risks
Finally, it is important to consider whether recommendations herein intended for Legionella control could potentially have unintended consequences by favoring survival of other pathogens that are problematic in premise plumbing. A report sponsored by the Water Research Foundation (Project 4813) summarized common challenges encountered in premise plumbing that favor the proliferation of multiple pathogens, in particular P. aeruginosa, nontuberculous mycobacteria, Acanthamoeba spp., and N. fowleri (Pruden et al., 2013), though other examples include Acinetobacter baumanii and Aeromonas spp. Falkinham (2015) described several key commonalities among such organisms, including preference for biofilm environments, capacity to resist predation by protozoans, tolerance to disinfectants, and antibiotic resistance. Ideally, such commonalities could be capitalized upon to identify “silver bullet” approaches that offer protection against all pathogens that, like Legionella, are prone to proliferation in premise pluming. Indeed, efforts to reduce biofilms and amoebae hosts described in this chapter should in theory also address amoebal pathogens occurring in the plumbing. However, given that some of these organisms are markedly tolerant of disinfectants (e.g., mycobacteria), the higher doses required could pose other concerns, including generation of disinfection byproducts and selection of strains that are more tolerant of disinfectants. Also, whereas chloramines appear to be particularly effective against Legionella spp., Mycobacterium avium levels can increase when water systems are switched from chlorine to chloramine (Pryor et al., 2004; Wang et al., 2013b). Concerns have also been raised that drinking water disinfectants might inadvertently select for antibiotic-resistant bacteria, due to multifunctional or co-located antibiotic resistance genes, as was evidenced by a metagenomic-based DNA sequencing study (Shi et al., 2013). In particular, metal and antibiotic resistance traits are commonly co- or cross-selected among bacteria, begging the question of whether copper-silver ionization exerts similar effects when applied to drinking water (Chen et al., 2015). Khan et al. (2016) observed that chlorine resistance and minimum inhibitory concentration of various antibiotics positively correlated among several tap water bacterial isolates. Long-term exposure to low levels of chlorine was also recently associated with selection of antibiotic-resistant P. aeruginosa (Mao et al., 2018; Shrivastava et al., 2004) and upregulation of antibiotic resistance genes in A. baumannii (Karumathil et al., 2014).
Elevated water temperatures appear to also reduce growth of most pathogens in premise plumbing, but slightly hotter water temperatures may be necessary for mycobacteria. For example, viable mycobacteria have been observed in household water heaters, but numbers of positive heaters were substantially lower when the temperature was greater than 55°C (Falkinham, 2011). In the lab, 90 percent survival of mycobacteria was observed following exposure to 50°C for 60 minutes (Schulze-Röbbecke and Buchholtz, 1992).
Thus, there is need for research that harmonizes engineered control efforts to minimize the risk of other microbial problems, including growth, virulence, and antibiotic resistance of multiple pathogens. Ideally, selected controls for Legionella should have comprehensive benefits for control of other pathogens in water systems.
CONCLUSIONS AND RECOMMENDATIONS
For any given building water system, there are multiple strategies that can be successfully employed and should be used. Figure 4-5 provides an overview of the controls discussed in this chapter and the importance of considering their integration and applicability to various water systems. The different strategies available for controlling Legionella in water systems are feasible at different stages of a building’s life cycle, with some being feasible mainly during initial construction (such as the choice of plumbing materials) while others are implemented during ongoing operation and maintenance (such as disinfection and flushing). Table 4-6 summarizes how the various control strategies should be considered at different stages of a building’s life: design, commissioning, operations (including routine monitoring), and corrective action when necessary. It is critical to recognize that no single control strategy should be relied upon to control Legionella in building water systems, and multiple barriers are encouraged to the extent possible (Figure 4-5). Also, the effectiveness of many of the controls are interdependent, for example, optimal hydraulics are required for effective delivery of thermal and chemical disinfectant while reactivity of the plumbing materials and the water source chemistry could lead to disinfectant decay. Furthermore, as summarized in Table 4-1, not all controls are relevant to all water systems. For example, while thermal control is a primary barrier against Legionella in building systems, it cannot be applied to large engineered systems, such as wastewater treatment plants, because of the nature and scale of these systems. Other competing goals, such as commitment to water and energy savings for green building certification, must also be taken into consideration. Water management plans (discussed in detail in Chapter 5) are essential to Legionella
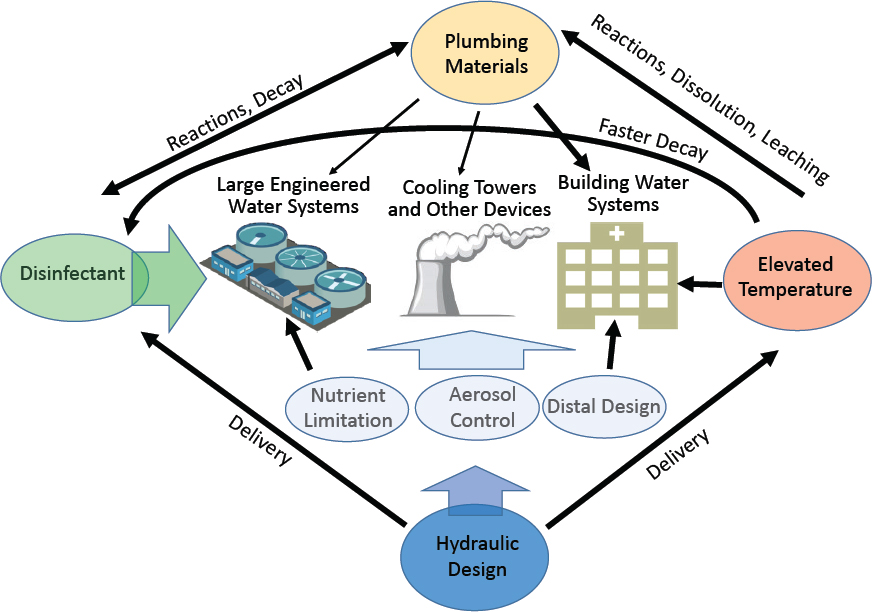
TABLE 4-6 Summary of Implementation of Various Engineering Controls and Corrective Actions at Various Stages of Building Design, Commissioning, and Operations
| Control | Design | Commissioning | Operations | Corrective Action |
|---|---|---|---|---|
| Hydraulic Control | Pipe sizing and flow distribution to minimize water age and avoid stagnation | Minimize periods of no use, preventive flushing during periods of no use | Verify hydraulic balancing | Modify hydraulics, correct deficient circulation |
| Materials Issues | Select biostable materials and ensure corrosion control | Cleaning, preventive flushing, disinfection | Verify corrosion control | Replace failing materials, eliminate corrosion byproducts |
| Source Water Quality | Consider the quality of the source water: corrosivity, nutrient content, and ability to maintain disinfectant residual | Delay filling of system during commissioning to avoid long periods without use | Verify corrosion control, disinfectant residuals, and general microbiological water quality indicators | Flush, adjust pH, hardness, corrosivity, etc. Or switch to improved water supply |
| Temperature | Minimize distance between hot-water heater and distal points, select hot-water heater, heat trace | Delay start-up of hot-water system during commissioning to avoid long periods without use | Maintain target temperatures across the system | Adjust operational temperature |
| Disinfection | Include chemical disinfection in design | Apply shock disinfection | Set disinfectant residual targets and monitor for compliance across the system | Apply shock disinfection |
| Aerosol Transmission Prevention | Select devices to minimize aerosol formation | Verify absence of Legionella in premise plumbing before occupancy | Replace devices to minimize aerosol formation | |
| Water Management Plan | Include from beginning of design | Determine and apply specific commissioning plan for Legionella control | Apply management plan | Modify management plan |
control for any water system, as they provide the opportunity to adapt and tailor the strategy to the specific system of concern and employ and integrate all applicable barriers (see Table 4-6).
Two rows in Table 4-6 do not correspond precisely to controls discussed in this chapter. First, source water quality is listed (rather than the narrower nutrient limitation), as there are important water quality considerations at each stage of a building’s life cycle and multiple control strategies will affect water quality. Second, there is a final row on water management plans for protecting a building from a Legionella outbreak because having a plan itself is a critical control. (Such plans are discussed in detail in Chapter 5.)
The conclusions and recommendations below highlight key lessons regarding Legionella control strategies for the building and device types discussed in this chapter.
For all types of buildings, hot-water heater temperature should be maintained above 60°C (140°F) and the hot-water temperature to distal points should exceed 55°C (131°F). Maintaining temperature outside Legionella’s preferred growth range is the paramount Legionella
control strategy for all buildings that provide hot water and has been proven successful by numerous longitudinal field studies. Temperature control is most effective in large, complex hot-water systems that are hydraulically balanced, with dead-end pipes removed and faulty devices that compromise the distribution of hot water identified and replaced.
There is growing evidence that, compared to free chlorine, a monochloramine residual better controls Legionella risk from building water systems, although the reasons for the improved performance are not yet clear. It is possible that amoebae trophozoites are more sensitive to monochloramine, causing the amoebae to encyst and thus preventing the proliferation of Legionella within their host. Additional research is needed to examine the precise action of monochloramine on Legionella persistence and growth within pipeline biofilms. Better understanding of the potential for nitrification in building plumbing is also required, as this reaction could negatively impact the effectiveness of a chloramine residual for Legionella management.
Additional research is needed to evaluate the potential for nutrient limitation (concentration and composition) to control Legionella growth in distribution and building water systems. These studies should examine, in full-scale drinking water systems, the impact of nutrient reduction on the concentration and composition of the microbiome in biofilms and water including amoebae growth and life stages and the subsequent effect on occurrence and decrease of pathogenic Legionella species.
New NSF/ANSI standards regarding microbial growth potential of materials are needed so that water utilities, plumbers, and building contractors can include Legionella control when making decisions about pipe material usage. Certain plastic components (e.g., PEX) tend to lead to bacterial proliferation. Iron components in distribution systems and premise plumbing should be replaced or otherwise managed with appropriate corrosion control to avoid disinfectant decay and release of iron as a nutrient for Legionella. Because of conflicting accounts in the literature about their role in Legionella growth, copper pipes cannot be relied on as a barrier to Legionella colonization and growth. More research is needed to identify circumstances when copper’s antimicrobial properties are enhanced.
There is clear evidence of Legionella amplification in the distal parts of some hot-water systems, likely due to a combination of water stagnation and loss of temperature control and disinfectant residual. Some features of distal devices such as aerators, thermostatic mixing valves, complex designs, and shower hose materials have been linked to increased prevalence of Legionella. Additional research is needed to understand the conditions in distal reaches of premise plumbing that promote the amplification of Legionella so that improved distal devices can be designed.
Research is needed on new control technologies that limit the capacity of devices and building water systems to generate aerosols, particularly those smaller than 10 microns. The formation of aerosols is an important risk factor in the transmission of Legionella. Faucets and showerheads that limit the formation of fine mists should be used in locations where high-risk individuals could be exposed (e.g., hospitals). Technologies to minimize aerosols from cooling towers should strive for the highest efficiencies, and older cooling towers should be retrofitted with newer drift eliminators that meet higher standards.
Research is needed to better understand the persistence of distribution system disinfectant residuals within building plumbing. Public water supplies that maintain a disinfectant
residual and manage hydraulics to prevent stagnation (such as through routine flushing and cleaning of storage tanks) are helping to reduce Legionella exposure from the distribution system. Nonetheless, it is unclear to what extent the disinfection residual can achieve Legionella control within premise plumbing, for both single-family homes and small buildings as well as larger buildings.
Guidance about Legionella is needed for homeowners, especially consumers from at-risk segments of the population. In particular, there is a need to identify plumbing configurations and devices that inadvertently increase risk of Legionella proliferation as well as accessible, practical control options such as flushing taps after periods of disuse. Residential water systems can benefit from most of the control strategies discussed in this chapter, yet they are almost never formally implemented because of a lack of understanding or awareness on the part of homeowners and occupants.
Low-flow fixtures should not be allowed in hospitals and long-term care facilities because of their high-risk occupant populations. Low-flow fixtures have been promoted to conserve water and in some cases energy. However, because of their lower flow, these fixtures, primarily low-flow faucets but also showers, increase water age and restrict disinfectant levels, including the disinfection provided by elevated water temperatures. As such, low-flow fixtures present a greater risk for Legionella development in the plumbing systems that feed them.
New designs are needed to help advance control of Legionella in cooling towers and humidifiers. Humidifier designs that produce water droplets within the temperature range conducive to Legionella spp. growth (such as evaporative pan, drum-type, water spray-type, sprayed coil-type humidifiers or air washers) should be avoided for use in new buildings, and existing units of these types should be replaced during building renovations. When designing and locating HVAC systems, it is important to prevent Legionella contamination and growth by considering equipment and material selection, proper drainage, and access for maintenance. Strategies relying on disinfectants should consider using alternate types of biocides at regular intervals, since bacteria can regrow in cooling towers when biocide use is infrequent and irregular. Finally, cooling tower manufacturers should collectively design new systems that can operate at condenser water temperatures whereby the temperature going to the cooling tower will be greater than 60°C.
Green buildings have exacerbated many of the problems with Legionella by lengthening water residence times (which leads to loss of disinfectant residual) and lowering hot-water temperatures in premise plumbing. Criteria for certifying green buildings, energy-conserving features, and water-conserving features should be modified to take into account risk factors for growth of Legionella and other water-based pathogens in building water systems. Substantial water conservation can still be potentially achieved while protecting public health with more overt management of water age, e.g., through routine flushing of a target fraction of the water use. Given the strong evidence that water heater settings below 60°C place a system at risk for Legionella growth, appropriate plumbing designs to conserve heat in the system may be the only reasonable path forward.
REFERENCES
Adams, D. A., K. R. Thomas, R. A. Jajosky, L. Foster, G. Baroi, P. Sharp, D. H. Onweh, A. W. Schley, and W. J. Anderson. 2017. Summary of notifiable infectious diseases and conditions—United States, 2015. Morbidity and Mortality Weekly Report 64(53):1-143.
Ajibode, O. M., C. Rock, K Bright, J. E. T. McLain, C. P. Gerba, and I. L. Pepper. 2013. Influence of residence time of reclaimed water within distribution systems on water quality. Journal of Water Reuse and Desalinization 3:185-196.
Alary, M., and J. R. Joly. 1991. Risk factors for contamination of domestic hot water systems by legionellae. Applied and Environmental Microbiology 57(8):2360-2367.
Allegra, S., F. Berger, P. Berthelot, F. Grattard, B. Pozzetto, S. Riffard. 2008. Use of flow cytometry to monitor Legionella viability. Applied and Environmental Microbiology 74(24):7813-7816.
Allegra, S., F. Grattard, F. Girardot, S. Riffard, B. Pozzetto, and P. Berthelot. 2011. Longitudinal evaluation of the efficacy of heat treatment procedures against Legionella spp. in hospital water systems by using a flow cytometric assay. Applied and Environmental Microbiology 77(4):1268-1275.
Amaro, F., W. Wang, J. A. Gilbert, O. R. Anderson, H. A. Shuman. 2015. Diverse protist grazers select for virulence-related traits in Legionella. ISME Journal 9(7):1607-1618.
Anderson, O. R., W. Wang, S. P. Faucher, K. Bi, and H. A. Shuman. 2011. A new heterolobosean amoeba Solumitrus palustris n. g., n. sp. isolated from freshwater marsh soil. Journal of Eukaryotic Microbiology 58(1):60-67.
Armstrong, P. 1978. U.S. Government Memorandum, Consumer Protection Safety Commission.
Arnow, P. M., D. Weil, and M. F. Para. 1985. Prevalence and significance of Legionella pneumophila contamination of residential hot-tap water systems. Journal of Infectious Diseases 152:145-151.
Arvand, M., K. Jungkind, and A. Hack. 2011. Contamination of the cold water distribution system of health care facilities by Legionella pneumophila: Do we know the true dimension? Eurosurveillance 16(16):9-14.
ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers). 2000. ASHRAE Guideline 12-2000. Minimizing the risk of legionellosis associated with building water systems. Atlanta, GA: ASHRAE.
ASHRAE. 2015. Standard 188 legionellosis: Risk management for building water systems. Atlanta, GA: ASHRAE.
ASHRAE. 2016. ASHRAE handbook: HVAC systems and equipment – SI Edition, S22, S40. Atlanta, GA: ASHRAE.
AWWA (American Water Works Association). 2012. Buried no longer: Confronting America’s water infrastructure challenge. Denver, CO: AWWA.
AWWA. 2013. Steel water-storage tanks. Manual M42. Denver, CO: AWWA.
AWWA. 2018. Partnership for Safe Water. https://www.awwa.org/Portals/0/files/resources/water%20utility%20management/partnership%20safe%20water/files/DistributionProgramOverview.pdf.
Baldry, M. G. C., M. S. French, and D. Slater. 1991. The activity of peracetic acid on sewage indicator bacteria and viruses. Water Science and Technology 24:353-357.
Baltimore Air Coil (BAC). 2015. Product & application handbook V5. Filtration guide, J241-J252.
Bargellini, A., I. Marchesi, E. Righi, A. Ferrari, S. Cencetti, P. Borella, and S. Rovesti. 2011. Parameters predictive of Legionella contamination in hot water systems: association with trace elements and heterotrophic plate counts. Water Research 45(6):2315-2321.
Barker, J., M. R. Brown, P. J. Collier, I. Farrell, and P. Gilbert. 1992. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: Physiological status and susceptibility to chemical inactivation. Applied and Environmental Microbiology 58:2420-2425.
Barker, J., P. A. Lambert, and M. R. Brown. 1993. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infection and Immunity 61:3505-3510.
Barker, J., H. Scaife, and M. R. Brown. 1995. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrobial Agents and Chemotherapy 39:2684-2688.
Barna, Z., M. Kadar, E. Kalman, A. Scheirich Szax, and M. Vargha. 2016. Prevalence of Legionella in premise plumbing in Hungary. Water Research 90:71-78.
Baron, J. L., T. Peters, R. Shafer, B. MacMurray, and J. E. Stout. 2014. Field evaluation of a new point-of-use faucet filter for preventing exposure to Legionella and other waterborne pathogens in health care facilities. American Journal of Infection Control 42(11):1193-1196.
Bartels, A. 2018. Legionella Regulations and the Impact in The Netherlands. Presentation at the third meeting of the NASEM Committee on Management of Legionella in Water Systems. July 30, 2018. Woods Hole, MA.
Beck, S. E., H. Ryu, L. A. Boczek, J. L. Cashdollar, K. M. Jeanis, J. S. Rosenblum, O. R. Lawal, and K. G. Linden. 2017. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Research 109:207-216.
Bédard, E., S. Fey, D. Charron, C. Lalancette, P. Cantin, P. Dolce, C. Laferriere, E. Deziel, and M. Prévost. 2015. Temperature diagnostic to identify high risk areas and optimize Legionella pneumophila surveillance in hot water distribution systems. Water Research 71:244-256.
Bédard, E., S. Levesque, P. Martin, L. Pinsonneault, K. Paranjape, C. Lalancette, C.-É. Dolcé, M. Villion, L. Valiquette, S. P. Faucher and M. Prévost. 2016. Energy conservation and the promotion of Legionella pneumophila growth: The probable role of heat exchangers in a nosocomial outbreak. Infection Control and Hospital Epidemiology 37(12):1475-1480.
Bédard, E., C. Laferrière, E. Déziel, and M. Prévost. 2018. Impact of stagnation and sampling volume on water microbial quality monitoring in large buildings. PLoS ONE. https://doi.org/10.1371/journal.pone.0199429.
Bédard, E., K. Paranjape, C. Lalancette, M. Villion, C. Quach, C. Laferrière, S. P. Faucher, and M. Prévost. 2019. Legionella pneumophila levels and sequence-type distribution in hospital hot water samples from faucets to connecting pipes. Water Research 156:277-286.
Benkel, D. H., E. M. McClure, D. Woolard, J. V. Rullan, G. B. Miller, S. R. Jenkins, J. H. Hershey, R. F. Benson, J. M. Pruckler, and E. W. Brown. 2000. Outbreak of Legionnaires’ disease associated with a display whirlpool spa. International Journal of Epidemiology 29:1092-1098.
Berk, S. G., R. S. Ting, G. W. Turner, and R. J. Ashburn. 1998. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Applied and Environmental Microbiology 64:279-286.
Birks, R., J. Colbourne, S. Hill, and R. Hobson. 2004. Microbiological water quality in a large in-building, water recycling facility. Water Science and Technology 50:165-172.
Blanc, D. S., P. Carrara, G. Zanetti, and P. Francioli. 2005. Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: Seven years of experience in a university teaching hospital. Hospital Infection 60:69-72.
BMEC (Building Management Education Centre). 2009. Guideline for prevention of Legionnaires’ disease. 3rd Version. Japan.
Boppe, I., E. Bedard, C. Taillandier, D. Lecellier, M.-A. Nantel-Gauvin, M. Villion, C. Laferriere, and M. Prévost. 2016. Investigative approach to improve hot water system hydraulics through temperature monitoring to reduce building environmental quality hazard associated to Legionella. Building and Environment 108:230-239.
Borella, P., M. T. Montagna, V. Romano-Spica, S. Stampi, G. Stancanelli, M. Triassi, R. Neglia, I. Marchesi, G. Fantuzzi, D. Tatò, and C. Napoli. 2004. Legionella infection risk from domestic hot water. Emerging Infectious Diseases 10(3):457.
Borella, P., M. T. Montagna, S. Stampi, G. Stancanelli, V. Romano-Spica, M. Triassi, I. Marchesi, A. Bargellini, D. Tatò, C. Napoli, F. Zanetti, E. Leoni, M. Moro, S. Scaltriti, G. Ribera D’Alcalà, R. Santarpia, and S. Boccia. 2005. Legionella contamination in hot water of Italian hotels. Applied and Environmental Microbiology 71(10):5805-5813.
Borgen, K., I. Aaberge, Ø. Werner-Johansen, K. Gjøsund, B. Størsrud, S. Haugsten, K. Nygård, T. Krogh, E. A. Høiby, D. A. Caugant, A. Kanestrøm, Ø. Simonsen, and H. Blystad. 2008. A cluster of Legionnaires’ disease linked to an industrial plant in southeast Norway, June–July 2008. Eurosurveillance 13(38):18985.
Brazeau, R. H., and M. A. Edwards. 2011. A review of the sustainability of residential hot water infrastructure: Public health, environmental impacts, and consumer drivers. Journal of Green Building 6(4):77-95.
Brazeau, R. H., and M. A. Edwards. 2013a. Optimization of electric hot water recirculation systems for comfort, energy and public health. Journal of Green Building 8(2):73-89.
Brazeau, R. H., and M. A. Edwards. 2013b. Role of hot water system design on factors influential to pathogen regrowth: Temperature, chlorine residual, hydrogen evolution, and sediment. Environmental Engineering Science 30(10):617-627.
Brazeau, R. H., and M. A. Edwards. 2013c. Water and energy savings from on-demand and hot water recirculating systems. Journal of Green Building 8(1):75-89.
Brunkard, J. M., Elizabeth Ailes, V. A. Roberts, V. Hill, E. D. Hilborn, G. F. Craun, A. Rajasingham, A. Kahler, L. Garrison, L. Hicks, J. Carpenter, T. J. Wade, M. J. Beach, and J. S. Yoder. 2011. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2007–2008. Morbidity and Mortality Weekly Report 60(12):38-75.
Buchberger, S., T. Omaghomi, T. Wolfe, J. Hewitt, and D. Cole. 2015. Peak water demand study: Probability estimates for efficient fixtures in single and multifamily residential buildings. Mokena, Illinois: IAPMO.
Bucheli, T. D., S. R. Müller, A. Voegelin, R.P. Schwarzenbach. 1998. Bituminous roof sealing membranes as major sources of the herbicide (R,S)-mecoprop in roof runoff waters: potential contamination of groundwater and surface waters. Environmental Science and Technology 32(22):3465-3471.
Buse, H. Y., J. Lu, and N. J. Ashbolt. 2015. Exposure to synthetic gray water inhibits amoeba encystation and alters expression of Legionella pneumophila virulence genes. Applied and Environmental Microbiology 81:630-639.
Buse, H. Y., P. Ji, V. Gomez-Alvarez, A. Pruden, M. A. Edwards, and N. J. Ashbolt. 2017. Effect of temperature and colonization of Legionella pneumophila and Vermamoeba vermiformis on bacterial community composition of copper drinking water biofilms. Microbial Biotechnology 88(2):280-295.
Caicedo, C., K. H. Rosenwinkel, M. Exner, W. Verstraete, R. Suchenwirth, P. Hartemann, and R. Nogueira. 2019. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: Review. Water Research 149:21-34.
Camper, A. K. 1996. Factors limiting microbial growth in the distribution system: Laboratory and pilot-scale experiments. Denver, CO: AWWA Research Foundation and American Water Works Association.
Campese, C., D. Roche, C. Clement, F. Fierobe, S. Jarraud, P. de Waelle, H. Perrin, and D. Che. 2010. Cluster of Legionnaires’ disease associated with a public whirlpool spa, France, April–May 2010. Eurosurveillance 15:pii=19602.
Casini, B., A. Buzzigoli, M. L. Cristina, A. M. Spagnolo, P. Del Giudice, S. Brusaferro, A. Poscia, U. Moscato, P. Valentini, A. Baggiani, and G. Privitera. 2014. Long-term effects of hospital water network disinfection on Legionella and other waterborne bacteria in an Italian university hospital. Infection Control and Hospital Epidemiology 35(3):293-299.
Castex, J., and D. Houssin, Eds. 2005. L’eau dans les établissements de santé. Eau et Santé, Guide technique, H2O. France, Ministère de la Santé et des Solidarités. http://nosobase.chu-lyon.fr/Reglementation/2005/guide_eau_etabs.pdf.
Castor, M. L., M. L. Castor, E. A. Wagstrom, R. N. Danila, K. E. Smith, T. S. Naimi, J. M. Besser, K. A. Peacock, B. A. Juni, J. M. Hunt, J. M. Bartkus, S. R. Kirkhorn, and R. Lynfield. 2005. An outbreak of Pontiac fever with respiratory distress among workers performing high-pressure cleaning at a sugar-beet processing plant. Journal of Infectious Diseases 191(9):1530-1537.
CDC (Centers for Disease Control and Prevention). 2009. Nonfatal scald-related burns among adults aged ≥65 years—United States, 2001–2006. Morbidity and Mortality Weekly Report 58(36):993-996.
CEC (California Energy Commission). 2019. Residential compliance manual for the 2019 building energy efficiency standards. https://www.energy.ca.gov/2018publications/CEC-400-2018-017/CEC-400-2018-017-CMF.pdf.
Cedeno-Laurent, J. G., A. Williams, P. MacNaughton, X. Cao, E. Eitland, J. Spengler, and J. Allen. 2018. Building evidence for health: green buildings, current science, and future challenges. Annual Review of Public Health 39:291-308.
Cervero-Aragó, S., R. Sommer, and R. M Araujo. 2014. Effect of UV irradiation (253.7 nm) on free Legionella and Legionella associated with its amoebae hosts. Water Research 67:299-309.
Cervero-Aragó, S., B. Schrammel, E. Dietersdorfer, R. Sommer, C. Lück, J. Walochnik, and A. Kirschner. 2019. Viability and infectivity of viable-but-nonculturable Legionella pneumophila strains induced at high temperatures. Water Research 158:268-279.
Characklis, W. G., and K. C. Marshall. 1990. Biofilms. New York: Wiley.
Charron, D., E. Bédard, C. Lalancette, C. Laferrière, and M. Prévost. 2015. Impact of electronic faucets and water quality on the occurrence of Pseudomonas aeruginosa in water: a multi-hospital study. Infection Control and Hospital Epidemiology January:1-9. doi:10.1017/ice.2014.46.
Chen, S., X. Li, G. Sun, Y. Zhang, J. Su, and J. Ye. 2015. Heavy metal induced antibiotic resistance in bacterium LSJC7. International Journal of Molecular Sciences 16(10):23390-23404.
Chen, Y. S., Y. E. Lin, Y. C. Liu, W. K. Huang, H. Y. Shih, S. R. Wann, S. S. Lee, H. C. Tsai, C. H. Li, H. L. Chao, C. M. Ke, H. H. Lu, and C. L. Chang. 2008. Efficacy of point-of-entry copper–silver ionization system in eradicating Legionella pneumophila in a tropical tertiary care hospital: Implications for hospitals contaminated with Legionella in both hot and cold water. Hospital Infection 68:152-158.
Clark, G. G., R. Jamal, and J. Weidhaas. 2019. Roofing material and irrigation frequency influence microbial risk from consuming homegrown lettuce irrigated with harvested rainwater. Science of the Total Environment 651:1011-1019.
Cloutman-Green, E., V. L. Barbosa, D. Jimenez, D. Wong, H. Dunn, B. Needham, L. Ciric, and J. C. Hartley. 2019. Controlling Legionella pneumophila in water systems at reduced hot water temperatures with copper and silver ionization. American Journal of Infection Control 47(7):761-766.
Colbourne, J. S., D. J. Pratt, M. G. Smith, S. P. Fisher-Hoch, and D. Harper. 1984. Water fittings as sources of Legionella pneumophila in a hospital plumbing system. Lancet 323(8370):210-213.
Coniglio, M. A., N. Andolfi, G. Faro, M. B. Pellegrino, A. Sgalambro, G. D’Aquila, A. Spina, and S. Melada. 2015. Continuous disinfection by monochloramine on domestic hot water system of health-care facilities for the control of Legionella contamination in Italy. Journal of Health Science 3:11-17.
CoolClean. 2019. http://www.coolclean.com.au/wp-content/uploads/2017/02/Legionella-and-the-role-of-DriftEliminators.pdf.
Cristina, M. L., A. M. Spagnolo, B. Casini, A. Baggiani, P. Del Giudice, S. Brusaferro, A. Poscia, U. Moscato, F. Perdelli, and P. Orlando. 2014. The impact of aerators on water contamination by emerging gram-negative opportunists in at-risk hospital departments. Infection Control and Hospital Epidemiology 35(2):122-129.
Cristino, S., P. P. Legnani, and E. Leoni. 2012. Plan for the control of Legionella infections in long-term care facilities: Role of environmental monitoring. International Journal of Hygiene and Environmental Health 215:279-285.
CSTB (Centre Scientifique et Technique du Bâtiment). 2012. Guide technique - Maîtrise du risque de développement des légionelles dans les réseaux d’eau chaude sanitaire - Défaillances et préconisations. https://solidarites-sante.gouv.fr/IMG/pdf/guide_maitrise_legionelles_reseaux_interieurs.pdf.
Dai, D., C. R. Proctor, K. Williams, M. A. Edwards, and A. Pruden. 2018. Mediation of effects of biofiltration on regrowth, Legionella pneumophila, and microbial community structure by hot water plumbing conditions. Environmental Science: Water Research and Technology 4:183-194.
Darelid, J., S. Löfgren, and B.-E. Malmvall. 2002. Control of nosocomial Legionnaires’ disease by keeping the circulating hot water temperature above 55°C: Experience from a 10-year surveillance programme in a district general hospital. Journal of Hospital Infection 50(3):213-219.
De Jonckheere, J., and H. Van de Voorde. 1976. Differences in destruction of cysts of pathogenic and non-pathogenic Naegleria and Acanthamoeba by chlorine. Applied and Environmental Microbiology 31:294-297.
Demirjian, A., C. E. Lucas, L. E. Garrison, N. A. Kozak-Muiznieks, S. States, E. W. Brown, J. M. Wortham, A. Beaudoin, M. L. Casey, C. Marriott, A. M. Ludwig, A. F. Sonel, R. R. Muder, and L. A. Hicks. 2015. The importance of clinical surveillance in detecting Legionnaires’ disease with a Legionella disinfection system—Pennsylvania, 2011–2012. Clinical Infectious Disease 60:1596-1602.
Dietrich, A. M., R. Hoehn, and C. E. Via. 1991. Taste and odor problems associated with chlorine dioxide. Denver, CO: Water Research Foundation and AWWA.
DOE (U.S. Department of Energy). 2011. Cooling towers: Understanding key components of cooling towers and how to improve water efficiency. DOE/PNNL-SA-75820. US DOE Energy Efficiency & Renewable Energy, Federal Energy Management Program.
Domingue, E. L., R. L. Tyndall, W. R. Mayberry, and O. C. Pancorbo. 1988. Effects of three oxidizing biocides on Legionella pneumophila serogroup 1. Applied and Environmental Microbiology 54(3):741-747.
Donlan, R. M., W. O. Pipes, and T. L. Yohe. 1994. Biofilm formation on cast iron substrata in water distribution systems. Water Research 28(6):1497-1503.
Donlan, R., R. Murga, J. Carpenter, E. Brown, R. Besser, and B. Fields. 2002. Monochloramine disinfection of biofilm-associated Legionella pneumophila in a potable water model system. In: Legionella. R. Marre, Y. A. Kwaik, and C. Bartlett (eds.). Washington, DC: American Society for Microbiology.
Donohue, M. J., K. O’Connell, S. J. Vesper, J. H. Mistry, D. King, M. Kostich, and S. Pfaller. 2014. Widespread molecular detection of Legionella pneumophila serogroup 1 in cold water taps across the United States. Environmental Science and Technology 48(6):3145-3152.
Duda, S., S. Kandiah, J. E. Stout, J. L. Baron, M. Yassin, M. Fabrizio, J. Ferrelli, R. Hariri, M. M. Wagener, J. Goepfert, J. Bond, J. Hannigan, and D. Rogers. 2014. Evaluation of a new monochloramine generation system for controlling Legionella in building hot water systems. Infection Control and Hospital Epidemiology 35(11):1356-1363.
Dupuy, M., S. Mazoua, F. Berne, C. Bodet, N. Garrec, P. Herbelin, F. Menard-Szczebara, S. Oberti, M. H. Rodier, S. Soreau, F. Wallet, and Y. Héchard. 2011. Efficiency of water disinfectants against Legionella pneumophila and Acanthamoeba. Water Research 45:1087-1094.
Dziewulski, D. M., E. Ingles, N. Codru, J. Strepelis, and D. Schoonmaker-Bopp. 2015. Use of copper–silver ionization for the control of legionellae in alkaline environments at health care facilities. American Journal of Infection Control 43(9):971-976.
ECDC (European Centre for Disease Prevention and Control) and European Working Group for Legionella Infections (EWGLI). 2017. European technical guidelines for the prevention, control and investigation, of infections caused by Legionella species, p. 125.
Edelstein, P. H., R. E. Whittaker, R. L. Kreiling, and C. L. Howell. 1982. Efficacy of ozone in eradication of Legionella pneumophila from hospital plumbing fixtures. Applied and Environmental Microbiology 44(6):1330-1334.
enHealth. 2015. Guidelines for Legionella control in the operation and maintenance of water distribution systems in health and aged care facilities. Australian Government, Canberra.
EPA. (U.S. Environmental Protection Agency). 1991. Maximum contaminant level goals and national primary drinking water regulations for lead and copper; final rule. Federal Register 56(110):26460-26564.
EPA. 1994. Drinking water criteria document for chloramines. ECAO-CIN-D002. Washington, DC: EPA.
EPA. 1998. National primary drinking water regulations; disinfectants and disinfection byproducts; final rule. 63 FR 69390. Washington, DC: EPA.
EPA. 2002a. Finished water storage facilities. Washington, DC: EPA Office of Water, Office of Ground Water and Drinking Water, Distribution System Issue Paper.
EPA. 2002b. Effects of water age on distribution system water quality. Cincinnati, OH: EPA Office of Water, Office of Ground Water and Drinking Water.
EPA. 2006. Ultraviolet disinfection guidance manual for the final long term 2 Enhanced Surface Water Treatment Rule. EPA 815-R-06-007. Washington, DC: EPA.
EPA. 2012. Guidelines for water reuse. EPA/600/R-12/618. Cincinnati, OH: EPA Office of Research and Development, National Risk Management Research Laboratory.
EPA. 2013. Sustainable design and green building toolkit for local governments. EPA 904B10001. Washington, DC: EPA.
EPA. 2016a. Technologies for Legionella control in premise plumbing systems: Scientific literature review. Washington, DC: EPA.
EPA. 2016b. WaterSense® Program Guidelines Version 5.3. https://www.epa.gov/watersense/program-guidelines.
EPA. 2019. U.S. EPA ENERGY STAR. https://www.energystar.gov/campaign/waysToSave.
Epalle, T., F. Girardot, S. Allegra, C. Maurice-Blanc, O. Garraud, and S. Riffard. 2015. Viable but not culturable forms of Legionella pneumophila generated after heat shock treatment are infectious for macrophage-like and alveolar epithelial cells after resuscitation on Acanthamoeba polyphaga. Microbial Ecology 69(1):215-224.
Exner, M. 2018. Legionella Regulations and the Impact in Germany. Presentation at the 3rd meeting of the NASEM Committee on Management of Legionella in Water Systems. July 30, 2018. Woods Hole, MA.
Falkinham, J. O. 2011. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerging Infectious Diseases 17:419-424.
Falkinham, J. O. 2013. Reducing human exposure to Mycobacterium avium. Annals of the American Thoracic Society 10(4):378-382.
Falkinham, J. O. 2015. Common features of opportunistic premise plumbing pathogens. International Journal of Environmental Research and Public Health 12(5):4533-4545.
Flannery, B., L. B. Gelling, D. J. Vugia, J. M. Weintraub, J. J. Salerno, M. J. Conroy, V. A. Stevens, C. E. Rose, M. R. Moore, B. S. Fields, and R. E. Besser. 2006. Reducing Legionella colonization of water systems with monochloramine. Emerging Infectious Diseases 12(4):588-596.
Flynn, K. J., and M. S. Swanson. 2014. Integrative conjugative element ICE-box confers oxidative stress resistance to Legionella pneumophila in vitro and in macrophages. mBio 5(3):e01091-14.
Förster, J. 1999. Variability of roof runoff quality. Water Science and Technology 39(5):137-144.
Friedman, M., G. J. Kirmeyer, and E. Antoun. 2002. Developing and implementing a distribution system flushing program. Journal of the American Water Works Association 94 (7):48-56.
Garduño, R. A., E. Garduño, M. Hiltz, and P. S. Hoffman. 2002. Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infection and Immunity 70:6273-6283.
Garner, E. D., N. Zhu, L. E. Strom, M. A. Edwards, and A. Pruden. 2016. A human exposome framework for guiding risk management and holistic assessment of recycled water quality. Environmental Science: Water Research and Technology 2:580-598.
Garner, E., J. McLain, J. Bowers, D. M. Engelthaler, M. A. Edwards, and A. Pruden. 2018. Microbial ecology and water chemistry impact regrowth of opportunistic pathogens in full-scale reclaimed water distribution systems. Environmental Science and Technology 52(16):9056-9068.
Garner, E., M. Inyang, E. Garvey, J. Parks, C. Glover, A. Grimaldi, E. Dickenson, J. Sutherland, A. Salveson, M. A. Edwards, and A. Pruden. 2019. Impact of blending for direct potable reuse on premise plumbing microbial ecology and regrowth of opportunistic pathogens and antibiotic resistant bacteria. Water Research 151:75-86.
Garrison, L. E., J. M. Kunz, L. A. Cooley, M. R. Moore. C. Lucas, S. Schrag, J. Sarisky, and C. G. Whitney 2016. Vital signs: deficiencies in environmental control identified in outbreaks of Legionnaires’ disease—North America, 2000–2014. Morbidity and Mortality Weekly Report 65:576-584.
Government of South Australia. 2013. Guidelines for the control of Legionella in manufactured water systems in South Australia. Rundle, Australia: Health Protection Programs, Public Health Services, Public Health and Clinical Systems, Department for Health and Aging.
Gregersen, P., K. Grunnet, S. A. Uldum, B. H. Andersen, and H. Madsen. 1999. Pontiac fever at a sewage treatment plant in the food industry. Scandinavian Journal of Work, Environment and Health 25(3):291-295.
Groothuis, D. G., H. R. Veenendaal, and H. L. Dijkstra. 1985. Influence of temperature on the number of Legionella pneumophila in hot water systems. Journal of Applied Bacteriology 59:529-536.
Grossi, M., R. Dey, and N. Ashbolt. 2018. Searching for activity markers that approximate (VBNC) Legionella pneumophila infectivity in amoeba after ultraviolet (UV) irradiation. Water 10(9). doi.org/10.3390/w10091219.
Haik, J., A. Liran, A. Tessone, A. Givon, A. Orenstein, and K. Peleg. 2007. Burns in Israel: Demographic, etiologic and clinical trends, 1997–2003. Israel Medical Association Journal 9:659-662.
Hall, K. K., E. T. Giannetta, S. I. Getchell-White, L. J. Durbin, and B. M. Farr. 2003. Ultraviolet light disinfection of hospital water for preventing nosocomial Legionella infection: A 13-year follow-up. Infection Control and Hospital Epidemiology 24(8):580-583.
Hamilton, K. A., W. Ahmed, A. Palmer, K. Smith, S. Toze, and C. N. Haas. 2017. Seasonal assessment of opportunistic premise plumbing pathogens in roof-harvested rainwater tanks. Environmental Science and Technology 51(3):1742-1753.
Hamilton, K. A., M. T. Hamilton, W. Johnson, P. Jjemba, Z. Bukhari, M. LeChevallier, and C. N. Haas. 2018a. Health risks from exposure to Legionella in reclaimed water aerosols: Toilet flushing, spray irrigation, and cooling towers. Water Research 134:261-279.
Hamilton, K. A., K. Parrish, W. Ahmed, and C. N. Haas. 2018b. Assessment of water quality in roof-harvested rainwater barrels in greater Philadelphia. Water 10(2):92.
Heffelfinger, J. D., J. L. Kool, S. Fridkin, V. J. Fraser, J. Hageman, J. Carpenter, and C. G. Whitney. 2003. Risk of hospital-acquired Legionnaires’ disease in cities using monochloramine versus other water disinfectants. Infection Control and Hospital Epidemiology 24(8):569-574.
Hijnen, W. A. M., E. F. Beerendonk, and G. J. Medema. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Research 40:3-22.
Hines, S. A., D. J. Chappie, R. A. Lordo, B. D. Miller, R. J. Janke, H. A. Lindquist, K. R. Fox, H. S. Ernst, and S. C. Taft. 2014. Assessment of relative potential for Legionella species or surrogates inhalation exposure from common water uses. Water Research 56:203-13.
Hrubá, L. 2009. The colonization of hot water systems by Legionella. Annals of Agriculture and Environmental Medicine 16:115-119.
HSE (Health and Safety Executive). 2013a. Legionnaires’ disease: Technical guidance. Part 2: The control of Legionella bacteria in hot and cold water systems. London, United Kingdom: HSE Books.
HSE. 2013b. Legionnaires’ disease: Technical guidance, Part 1 L8: The control of Legionella bacteria in water systems. Fourth Edition. London, United Kingdom: HSE Books.
HSE. 2013c. HSG274 Part 3. Legionnaires’ disease: Technical guidance, Part 1 L8: The control of Legionella bacteria in other risk systems. London, United Kingdom: HSE Books.
Hunter, P. R. 2003. Epidemiological and risk assessment evidence of disease linked to HPC bacteria. In: Heterotrophic plate counts and drinking-water safety. J. Bartram, J. Cotruvo, M. Exner, C. Fricker, and A. Glasmacher (eds.). Published on behalf of the World Health Organization by IWA Publishing, London.
Iervolino, M., B. Mancini, and S. Cristino. 2017. Industrial cooling tower disinfection treatment to prevent Legionella spp. International Journal of Environmental Research and Public Health 14(10):1125. doi:10.3390/ijerph14101125.
Ji, P., J. Parks, M. A. Edwards, and A. Pruden. 2015. Impact of water chemistry, pipe material and stagnation on the building plumbing microbiome. PLoS ONE 10(10):e0141087.
Ji, P., W. J. Rhoads, M. A. Edwards, and A. Pruden. 2017. Impact of water heater temperature setting and water use frequency on the building plumbing microbiome. ISME Journal 11:1318-1330.
Ji, P., W. J. Rhoads, M. A. Edwards, and A. Pruden. 2018. Effect of heat shock on hot water plumbing microbiota and Legionella pneumophila control. Microbiome 6(30) doi:10.1186/s40168-018-0406-7.
Jjemba, P. K., L. A. Weinrich, W. Cheng, E. Giraldo, and M. W. LeChevallier. 2010. Re-growth of opportunistic pathogens and algae in reclaimed water distribution systems. Applied and Environmental Microbiology 76:4169-4178.
Jjemba, P., W. Johnson, Z. Bukhari, and M. LeChevallier. 2015. Develop best management practices to control potential health risks and aesthetic issues associated with reclaimed water storage and distribution. WRF11-03. Alexandria, VA: WateReuse Research Foundation.
Johansson P. J. H., K. Andersson, T. Wiebe, C. Schalén, and S. Bernander. 2006. Nosocomial transmission of Legionella pneumophila to a child from a hospital’s cold-water supply. Scandinavian Journal of Infectious Diseases 38(11-12):1023-1027.
Johnson, W. J., P. K. Jjemba, Z. Bukhari, and M. LeChevallier. 2018. Occurrence of Legionella in non-potable reclaimed water. Journal of the American Water Works Association 110:15-27.
June, S., and D. Dziewulski. 2018. Copper and silver biocidal mechanisms, resistance strategies, and efficacy for Legionella control. Journal of the American Water Works Association 10(12):E13-E35.
Karumathil, D. P., H.-B. Yin, A. Kollanoor-Johny, and K. Venkitanarayanan. 2014. Effect of chlorine exposure on the survival and antibiotic gene expression of multidrug resistant Acinetobacter baumannii in water. International Journal of Environmental Research and Public Health 11(2):1844-1854.
Khan, S., T. K. Beattie, and C. W. Knapp. 2016. Relationship between antibiotic- and disinfectant-resistance profiles in bacteria harvested from tap water. Chemosphere 152:132-141.
Kilvington, S., and J. Price. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. Journal of Applied Bacteriology 68:519-525.
Kim, B. R., J. E. Anderson, S. A. Mueller, W. A. Gaines, and A. M. Kendall. 2002. Literature review—Efficacy of various disinfectants against Legionella in water systems. Water Research 36:4433-4444.
Kirisits, M. J., J. J. Margolis, B. L. Purevdorj-Gage, B. Vaughan, D. L. Chopp, P. Stoodley, and M. R. Parsek. 2007. Influence of the hydrodynamic environment on quorum sensing in Pseudomonas aeruginosa biofilms. Journal of Bacteriology 189:8357-8360.
Kistemann, T., and F. Wasser. 2018. Big Data: Markante Erkenntnisse aus der Legionellen-Routineüberwachung. Sanitär und Heizungstechnik, 34-39.
Klein, G. 2018. The Intersection of Codes and Standards on Legionella in Premise Plumbing Systems. Presentation at the 4th meeting to the Committee on Legionella Management in Waters Systems. October 22, 2018. Washington, DC.
Knudson, G. B. 1985. Photoreactivation of UV-irradiated Legionella pneumophila and other Legionella species. Applied and Environmental Microbiology 49(4):975-980.
Kool, J. L., J. C. Carpenter, and B. S. Fields. 1999. Effect of monochloramine disinfection of municipal drinking water on risk of nosocomial Legionnaires’ disease. Lancet 353:272-277.
Kruse, E. B., A. Wehner, and H. Wisplinghoff. 2016. Prevalence and distribution of Legionella spp. in potable water systems in Germany, risk factors associated with contamination, and effectiveness of thermal disinfection. American Journal of Infection Control 44(4):470-474.
Kusnetsov, J., E. Iivanainen, N. Elomaa, O. Zacheus, and P. J. Martikainen. 2001. Copper and silver ions more effective against legionellae than against Mycobacteria in a hospital warm water system. Water Research 35(17):4217-4225.
Kusnetsov, J., L. K. Neuvonen, T. Korpio, S. A. Uldum, S. Mentula, T. Putus, N. N. Tran Minh, and K. P. Martimo. 2010. Two Legionnaires’ disease cases associated with industrial wastewater treatment plants: A case report. BMC Infectious Diseases 10:343. https://doi.org/10.1186/1471-2334-10-343.
Kwaik, Y. A., L.-Y. Gao, O. S. Harb, and B. J. Stone. 1997. Transcriptional regulation of the macrophage induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Molecular Microbiology 24:629-642.
Langlais, B., and D. Perrine. 1986. Action of ozone on trophozoites and free amoeba cysts, whether pathogenic or not. Ozone Science and Engineering 8:187-198.
Langlais, B., D. Recknow, and D. R. Brink. 1991. Ozone in water treatment: Applications and engineering. Chelsea, MI: Lewis Publishers.
Lautenschlager, K., N. Boon, Y. Wang, T. Egli, and F. Hammes. 2010. Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Research 44(17): 4868-4877.
Learbuch, K. L. G., M. C. Lut, G. Liu, H. Smidt, and P. W. J. J. van der Wielen. 2019. Legionella growth potential of drinking water produced by reverse osmosis. Water Research 157:55-63.
LeChevallier, M. W., C. D. Cawthon, and R. G. Lee. 1988. Inactivation of biofilm bacteria. Applied and Environmental Microbiology 54(10):2492-2499.
LeChevallier, M. W., W. Schulz, and R. G. Lee. 1991. Bacterial nutrients in drinking water. Applied and Environmental Microbiology 57(3):857-862.
LeChevallier, M. W., N. J. Welch, and D. B. Smith. 1996. Full-scale studies of factors related to coliform regrowth in drinking water. Applied and Environmental Microbiology 62(7):2201-2211.
LeChevallier, M. W., M. C. Besner, M. Friedman, and V. L. Speight. 2011. Microbiological quality control in distribution systems. In: Water quality & treatment: A handbook on drinking water. J. K. Edzwald (ed.). Denver, CO: American Water Works Association and McGraw-Hill, Inc.
Lecointe, D., E. Fagundez, P. Pierron, J. P. Musset, P. Brissé, D. Vollereau, D. Breton, C. Théodora, R. Beauvais, C. Malbrunot, L. Crine, C. Fèvre, et Groupe de Travail Eau et légionelles. 2010. Management of the Legionella-link risk in a multicentre area’s hospital: Lessons learned of a six-year experience. Pathologiebiologie 58:131–136.
Lecointe, D., R. Beauvais, N. Breton, R. Cailleret, and B. Pangon. 2018. Control of legionellae in a new healthcare facility following implementation of a thermal control strategy. Infectious Diseases 51(2):102-112.
Lee, J. Y., J. S. Yang, M. Han, and J. Choi. 2010. Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water resources. Science of the Total Environment 408(4):896-905.
Lee, W. H., D. G. Wahman, P. L. Bishop, and J. G. Pressman. 2011. Free chlorine and monochloramine application to nitrifying biofilm: comparison of biofilm penetration, activity and viability. Environmental Science and Technology 45:1412-1419.
Leoni, E., F. Catalani, S. Marini, and L. Dallolio. 2018. Legionellosis associated with recreational waters: A systematic review of cases and outbreaks in swimming pools, spa pools, and similar environments. International Journal of Environmental Research and Public Health 15(1612). doi:10.3390/ijerph15081612.
Leprat, R., V. Denizot, X. Bertr, D. Talon. 2003. Non-touch fittings in hospitals: a possible source of Pseudomonas aeruginosa and Legionella spp. Journal of Hospital Infection 53(1):77.
Lin, Y. E., R. D. Vidic, J. E. Stout, and V. L. Yu. 1996. Individual and combined effects of copper and silver ions on inactivation of Legionella pneumophila. Water Research 30(8):1905-1913.
Lin, Y. E., R. D. Vidic, J. E. Stout, and V. L. Yu. 2002. Negative effect of high pH on biocidal efficacy of copper and silver ions in controlling Legionella pneumophila. Applied and Environmental Microbiology 68(6):2711-2715.
Lin, Y. E., J. E. Stout, and V. L. Yu. 2011. Controlling Legionella in hospital drinking water: an evidence-based review of disinfection methods. Infection Control and Hospital Epidemiology 32(2):166-173.
Lipphaus, P., F. Hammes, S. Kotzsch, J. Green, S. Gillespie, and A. Nocker. 2014. Microbiological tap water profile of a medium-sized building and effect of water stagnation. Environmental Technology 35(5-8):620-628.
Liu, Z., J. E. Stout, L. Tedesco, M. Boldin, C. Hwang, W. F. Diven, and V. L. Yu. 1994. Controlled evaluation of copper–silver ionization in eradicating Legionella pneumophila from a hospital water distribution system. Infectious Diseases 169:919-922.
Liu, Z., J. E. Stout, M. Boldin, J. Rugh, W. Diven, and V. L. Yu. 1998. Intermittent use of copper-silver ionization for Legionella control in water distribution systems: A potential option in buildings housing individuals at low risk of infection. Clinical Infectious Diseases 26:138-140.
Liu, Z., Y. E. Lin, J. E. Stout, C. C. Hwang, R. D. Vidic, and V. L. Yu. 2006. Effect of flow regimes on the presence of Legionella within the biofilm of a model plumbing system. Journal of Applied Microbiology 101(2):437-442.
Liu, G., Y. Tao, Y. Zhang, M. Lut, W. J. Knibbe, P. van der Wielen, W. Liu, G. Medema, and W. van der Meer. 2017. Hotspots for selected metal elements and microbes accumulation and the corresponding water quality deterioration potential in an unchlorinated drinking water distribution system. Water Research 124:435-445.
Loenenbach, A. D., C. Beulens, S. M. Euser, J. P. G. van Leuken, B. Bom, W. van der Hoek, A. M. de Roda Husman, W. L. M. Ruijs, A. A. Bartels, A. Rietveld, J. W. den Boer, and P. S. Brandsema. 2018. Two community clusters of Legionnaires’ disease directly linked to a biologic wastewater treatment plant, The Netherlands. Emerging Infectious Diseases 24(10):1914-1918.
Loret, J. F., S. Robert, V. Thomas, A. J. Cooper, W. F. McCoy, and Y. Lévi. 2005. Comparison of disinfectants for biofilm, protozoa and Legionella control. IWA Journal of Water and Health 3(4):423-433.
Lu J., I. Struewing, S. Yelton, and N. Ashbolt. 2015. Molecular survey of occurrence and quantity of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in municipal drinking water storage tank sediments. Journal of Applied Microbiology 119(1):278-288.
Lukefar, J. L., and K. Ezekiel. 1994. Scalding water temperatures. Pediatrics 94(4): Letter to the Editor.
Maita, C., M. Matsushita, M. Miyoshi, T. Okubo, S. Nakamura, J. Matsuo, M. Takemura, M. Miyake, H. Nagai, and H. Yamaguchi. 2018. Amoebal endosymbiont Neochlamydia protects host amoebae against Legionella pneumophila infection by preventing Legionella entry. Microbes and Infection 20(4):236-244.
Mandel, A. S., M. A. Sprauer, D. H. Sniadack, and S. M. Ostroff. 1993. State regulation of hospital water temperature. Infection Control and Hospital Epidemiology 14(11):642-645.
Manuel, C. M., O. C. Nunes, and L. F. Melo. 2010. Unsteady state flow and stagnation in distribution systems affect the biological stability of drinking water. Biofouling 26(2):129-139.
Mao, G., Y. Song, M. Bartlam, and Y. Wang. 2018. Long-term effects of residual chlorine on Pseudomonas aeruginosa in simulated drinking water fed with low AOC medium. Frontiers in Microbiology 9:879. doi:10.3389/fmicb.2018.00879.
Marchesi, I., P. Marchegiano, A. Bargellini, S. Cencetti, G. Frezza, M. Miselli, and P. Borella. 2011. Effectiveness of different methods to control Legionella in the water supply: Ten-year experience in an Italian university hospital. Hospital Infection 77(1):47-51.
Marchesi, I., S. Cencetti, P. Marchegiano, G. Frezza, P. Borella, and A. Bargellini. 2012. Control of Legionella contamination in a hospital water distribution system by monochloramine. American Journal of Infection Control 40:279-281.
Marchesi, I., G. Ferranti, A. Bargellini, P. Marchegiano, G. Predieri, J. E. Stout, and P. Borella. 2013. Monochloramine and chlorine dioxide for controlling Legionella pneumophila contamination: Biocide levels and disinfection byproduct formation in hospital networks. IWA Journal of Water and Health 11(4):738-747.
Marrie, T., P. Green, S. Burbridge, G. Bezanson, S. Neale, P. S. Hoffman, and D. Haldane. 1994. Legionellaceae in the potable water of Nova Scotia hospitals and Halifax residences. Epidemiology and Infection 112:143-150.
Martínez, S. S., A. A. Gallegos, and E. Martínez. 2004. Electrolytically generated silver and copper ions to treat cooling water: an environmentally friendly novel alternative. International Journal of Hydrogen Energy 29(9):921-932.
Masters, S., J. Parks, A. Atassi, and M. A. Edwards. 2015. Distribution system water age can create premise plumbing corrosion hotspots. Environmental Monitoring and Assessment 187(9):559.
Mathys, W., J. Stanke, M. Harmuth, and E. Junge-Mathys. 2008. Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. International Journal of Hygiene and Environmental Health 211(1-2):179-185.
McClung, R. P., D. M. Roth, M. Vigar, V. A. Robers, A. M. Kahler, L. A. Coolet, E. D. Hilborn, T. J. Wade, K. E. Fullerton, J. S. Yoder, and V. R. Hill. 2017. Waterborne disease outbreaks associated with environmental and undetermined exposures to water—United States, 2013-2014. Morbidity and Mortality Weekly Report 66(44):1222-5.
McNeill, L. S., and M. A. Edwards. 2001. Iron pipe corrosion in drinking water distribution systems. Journal of the American Water Works Association 93(7):88-100.
Miller, J., and G. D. Simpson. 1999. Chemical control of Legionella. AWT Annual Meeting, Palm Springs, CA. October 26–30, 1999. http://www.ibrarian.net/navon/paper/Chemical_Control_of_Legionella.pdf?paperid=9579048.
Miyamoto, M., Y. Yamaguchi, and M. Sastsu. 2000. Disinfectant effects of hot water, ultraviolet light, silver ions and chlorine on stains of Legionella and nontuberculous mycobacteria. Microbios 101(398):7-13.
Mòdol, J., M. Sabria, E. Reynaga, M. L. Pedro-Botet, N. Sopena, P. Tudela, I. Casas, and C. Rey-Joly. 2007. Hospital-acquired Legionnaires’ disease in a university hospital: impact of the copper-silver ionization system. Clinical Infectious Diseases 44:263-265.
Moore, G., and J. Walker. 2014. Presence and control of Legionella pneumophila and Pseudomonas aeruginosa biofilms in hospital water systems. Chapter 17 In: Biofilms in infection prevention and control. A Healthcare Handbook. Academic Press.
Moore, G., M. Hewitt, D. Stevenson, J. T. Walker, and A. M. Bennett. 2015. Aerosolization of respirable droplets from a domestic spa pool and the use of MS-2 coliphage and Pseudomonas aeruginosa as markers for Legionella pneumophila. Applied and Environmental Microbiology 81(2)555-561.
Moore, M., and S. Shelton. 2014. Updated guidelines for the control of Legionella in Western Pennsylvania. Allegheny County Health Department Pittsburgh Regional Health Initiative. https://www.rand.org/content/dam/rand/pubs/external_publications/EP60000/EP66197/RAND_EP66197.pdf.
Moore, M. R., M. Pryor, B. Fields, C. Lucas, M. Phelan, and R. E. Besser. 2006. Introduction of monochloramine into a municipal water system: impact on colonization of buildings by Legionella spp. Applied and Environmental Microbiology 72:378-383.
Moritz, A. R., and F. C. Henriques. 1947. Studies of thermal injury: II. The relative importance of time and surface temperature in the causation of cutaneous burns. American Journal of Pathology 123:695-720.
Mouchtouri, V., E. Velonakis, A. Tsakalof, C. Kapoula, G. Goutziana, A. Vatopoulos, J. Kremastinou, and C. Hadjichristodoulou. 2007. Risk factors for contamination of hotel water distribution systems by Legionella species. Applied and Environmental Microbiology 73(5):1489-1492.
Mouchtouri, V., G. Goutziana, J. Kremastinou, and C. Hadjichristodoulou. 2010. Legionella species colonization in cooling towers: risk factors and assessment of control measures. American Journal of Infection Control 38(1):50-55.
Muraca, P., J. E. Stout, and V. L. Yu. 1987. Comparative assessment of chorine, heat, ozone and UV light for killing Legionella pneumophila within a model plumbing system. Applied and Environmental Microbiology 53(2):447-453.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Microbiomes of the built environment: a research agenda for indoor microbiology, human health, and buildings. Washington, DC: National Academies Press.
Nguyen, C., C. Elfland, and M. A. Edwards. 2012. Impact of advanced water conservation features and new copper pipe on rapid chloramine decay and microbial regrowth. Water Research 46(3):611-621.
Niedeveld C. J., F. M. Pet, and P. L. Meenhorst. 1986. Effect of rubbers and their constituents on proliferation of Legionella pneumophila in naturally contaminated hot water. Lancet 328(8500):180-184.
Nogueira, R., K. U. Utecht, M. Exner, W. Verstraete, and K. H. Rosenwinkel. 2016. Strategies for the reduction of Legionella in biological treatment systems. Water Science and Technology 74(4):816-823.
Norton, C. D., and M. W. LeChevallier. 2000. A pilot study of bacteriological population changes through potable treatment and distribution. Applied and Environmental Microbiology 66(1):268-276.
NRC (National Research Council). 2006. Drinking water distribution systems: Assessing and reducing risks. Washington, DC: National Academies Press.
NRC. 2012. Water reuse: Potential for expanding the nation’s water supply through reuse of municipal wastewater. Washington, DC: National Academies Press.
Oguma, K., H. Katayama, and S. Ohgaki. 2004. Photoreactivation of Legionella pneumophila after inactivation by low- or medium-pressure ultraviolet lamp. Water Research 38(11):2757-2763.
Oh, J. L., R. Noga, V., Shanov, H. Ryu, H. Chandra, B. Yadav, J. Yadav, and S. Chae. 2019. Electrically heatable carbon nanotube point-of-use filters for effective separation and in-situ inactivation of Legionella pneumophila. Chemical Engineering Journal 366:21-26.
Okubo, T., M. Matsushita, S. Nakamura, J. Matsuo, H. Nagai, and H. Yamaguchi. 2018. Acanthamoeba S13WT relies on its bacterial endosymbiont to backpack human pathogenic bacteria and resist Legionella infection on solid media. Environmental Microbiology Reports 10(3):344-354.
Olsen, J. S., T. Aarskaug, I. Thrane, C. Pourcel, E. Ask, G. Johansen, V. Waagen, and J. M. Blatny. 2010. Alternative routes for dissemination of Legionella pneumophila causing three outbreaks in Norway. Environmental Science and Technology 44:8712-8717.
Paranjape, K., É. Bédard, L. G. Whyte, J. Ronholm, M. Prévost, and S. P. Faucher. 2019. Presence of Legionella spp. in cooling towers: The role of microbial diversity, Pseudomonas, and continuous chlorine application. bioRxiv. doi: https://doi.org/10.1101/540302.
Park, S., K. Lee, E. J. Lee, S. Y. Lee, K. H. In, H.-K. Kim, and M.-S. Kang. 2014. Humidifier disinfectant-associated children’s interstitial lung disease. American Journal of Respiratory and Critical Care Medicine 189(1):48-56.
Park, C. L., Y. S. Kim, and H. J. Yang. 2017. Analysis of incidence and prevalence trend of pediatric asthma before and after stopping sales of humidifier disinfectant. Seoul: Asian Medical Center; 2017. Pp. 4-5.
Pedro-Botet, M., J. Stout, and V. Yu. 2002. Legionnaires’ disease contracted from patient homes: The coming of the third plague? European Journal of Clinical Microbiology and Infectious Diseases 21(10):699-670.
Perinel, S. V., Forest, M. Landraud, J. Pourchez, F. Girardot, S. Riffard, M. Stauffert, J.-M. Vergnon, and S. Allegra. 2018. Deposition pattern of aerosolized Legionella using an ex vivo human-porcine respiratory model. International Journal of Hygiene and Public Health 221(2):252-259.
Peter, A., and E. Routledge. 2018. Present-day monitoring underestimates the risk of exposure to pathogenic bacteria from cold water storage tanks. PLoS ONE 13(4): e0195635.
Pickering, C. A. C. 2014. Humidifiers: The use of biocides and lung disease. Thorax 69:692-693.
Pinto, A. J., J. Schroeder, M. Lunn, W. Sloan, and L. Raskin. 2014. Spatial-temporal survey and occupancy-abundance modeling to predict bacterial community dynamics in the drinking water microbiome. mBio 5(3):e01135-14.
Plouffe, J. F., L. R. Webster, and B. Hackman. 1983. Relationship between colonization of hospital building with Legionella pneumophila and hot water temperatures. Applied and Environmental Microbiology 46(3):769-770.
Pourchez, J., L. Leclerc, F. Girardot, S. Riffard, N. Prevot, and S. Allegra. 2017. Experimental human-like model to assess the part of viable Legionella reaching the thoracic region after nebulization. PLoS ONE 12(10):e0186042.
Pressman, J. G., W. H. Lee, P. L. Bishop, and D. G. Wahman. 2012. Effect of free ammonia concentration on monochloramine penetration within a nitrifying biofilm and its effect on activity, viability and recovery. Water Research 46(3):882-894.
Prest, E. I., F. Hammes, S. Kotzsch, M. C. M. van Loosdrecht, and J. S. Vrouwenvelder. 2016a. A systematic approach for the assessment of bacterial growth-controlling factors linked to biological stability of drinking water in distribution systems. Water Science and Technology: Water Supply 16(4):865-880.
Prest, E. I., F. Hammes, M. C. M. van Loosdrecht, and J. S. Vrouwenvelder. 2016. Biological stability of drinking water: Controlling factors, methods, and challenges. Frontiers in Microbiology 7:45.
Prévost, M., A. Rompré, H. Baribeau, J. Coallier, and P. Lafrance. 1997. Service lines: Their effect on microbiological quality. Journal of the American Water Works Association 89(7):78-92.
Prévost, M., M. Doberva, S. Allegra, S. Faucher and E. Bédard. 2017. Impact of temperature, copper and chlorine exposure on the viability and recovery of clinical and environmental strains of Legionella pneumophila. The 9th International Conference on Legionella. Rome, Italy.
Proctor, C. R., M. Gächter, S. Kötzsch, F. Rölli, R. Sigrist, J.-C. Walser, and F. Hammes. 2016. Biofilms in shower hoses—Choice of pipe material influences bacterial growth and communities. Environmental Science: Water Research and Technology 2:670-682.
Proctor, C. R., M. Reimann, B. Vriens, and F. Hammes. 2018. Biofilms in shower hoses. Water Research 131:274-286.
Pruden, A., M. A. Edwards, J. O. Falkinham III, M. Arduino, J. Bird, R. Birdnow, E. Bédard, A. Camper, J. Clancy, E. Hilborn, V. Hill, A. Martin, S. Masters, N. R. Pace, M. Prévost, A. Rosenblatt, W. Rhoads, J. E. Stout, and Y. Zhang. 2013. Research needs for opportunistic pathogens in premise plumbing: methodology, microbial ecology, and epidemiology. Water Research Foundation Project 4379 Final Report. Denver, CO: Water Research Foundation.
Prussin, A. J., D. O. Schwake, and L. C. Marr. 2017. Ten questions concerning the aerosolization and transmission of Legionella in the built environment. Building and Environment 123:684-695.
Pryor, M., S. Springthorpe, S. Riffard, T. Brooks, Y. Huo, G. Davis, S. A. Sattar. 2004. Investigation of opportunistic pathogens in municipal drinking water under different supply and treatment regimes. Water Science and Technology 50:83-90.
Public Health Ontario (Ontario Agency for Health Protection and Promotion). 2017. Evidence brief: Humidifier use in health care. Toronto, ON: Queen’s Printer for Ontario.
PWGSC (Public Works and Government Services Canada). 2013. Control of Legionella in mechanical systems. MD15161. Ottawa, Canada.
Rhoads, W. J., A. Pruden, and M. A. Edwards. 2014. Anticipating challenges with in-building disinfection for control of opportunistic pathogens. Water Environment Research 86(6):540-549.
Rhoads, W. J., P. Ji., A. Pruden, and M. A. Edwards. 2015a. Water heater temperature set point and water use patterns influence Legionella pneumophila and associated microorganisms at the tap. Microbiome 3:67 doi:10.1186/s40168-015-0134-1.
Rhoads, W. J., A. Pearce, A. Pruden, and M. A. Edwards. 2015b. Anticipating the effects of green buildings on water quality and infrastructure. Journal of the American Water Works Association 107(4):50-61.
Rhoads, W. J., A. Pruden, and M. A. Edwards. 2016a. Survey of green building water systems reveals elevated water age and water quality concerns. Environmental Science: Water Research and Technology 2:164-173.
Rhoads, W. J., A. Pruden, and M. A. Edwards. 2016b. Convective mixing in distal pipes exacerbates L. pneumophila growth in hot water plumbing. Pathogens 5(1):E29.
Rhoads, W. J., E .D. Garner, P. Ji, N. Zhu, J. Parks, D. O. Schwake, A. Pruden, and M. A. Edwards. 2017a. Distribution system operational deficiencies coincide with reported Legionnaires’ disease clusters in Flint, MI. Environmental Science and Technology 51(20):11986-11995.
Rhoads, W. J., A. Pruden, and M. A. Edwards. 2017b. Interactive effects of corrosion, copper, and chloramines on Legionella and mycobacteria in hot water plumbing. Environmental Science and Technology 51(12):7065-7075.
Rhoads, W. J., M. S. Spencer, and M. A. Edwards. 2018. Investigation of continued Legionella pneumophila positivity at the Illinois Veteran’s Home in Quincy, IL. Final Report on Initial Phase of Work Submitted to submitted to Michael Hoffman, aid to Governor of Illinois, Oct. 3 2018.
Riffard, S., S. Douglass, T. Brooks, S. Springthorpe, L. G. Filion, S. A. Sattar. 2001. Occurrence of Legionella in groundwater: an ecological study. Water Science and Technology 43(12):99-102.
Rohr, U., M. Senger, F. Selenka, R. Turley, and M. Wilhelm. 1999. Four years of experience with silver-copper ionization for control of Legionella in a German University Hospital hot water plumbing system. Clinical Infectious Diseases 29(6):1507-1511.
Rossman, J. 2003. Non-chemical alternatives to cooling tower disinfection. Water Quality Products, March 27, 2003. https://www.wqpmag.com/nonchemical-alternatives-cooling-tower-disinfection.
Rossoni, E. M. M., and C. C. Gaylarde. 2000. Comparison of sodium hypochlorite and peracetic acid as sanitizing agents for stainless steel food processing surfaces using epifluorescence microscopy. International Journal of Food Microbiology 61:81-85.
Saby, S., A. Vidal, and H. Suty. 2005. Resistance of Legionella to disinfection in hot water distribution systems. Water Science and Technology 152:15-28.
Salehi, M., M. Abouali, M. Wang, Z. Zhou, A. P. Nejadhashemi, J. Mitchell, S. Caskey, and A. J. Whelton. 2018. Case study: fixture water use and drinking water quality in a new residential green building. Chemosphere 195:80-89.
Schulze-Röbbecke R., and K. Buchholtz. 1992. Heat susceptibility of aquatic mycobacteria. Applied and Environmental Microbiology 58:1869-1873.
Serrano-Suárez, A., J. Dellundé, H. Salvadó, S. Cervero-Aragó, J. Méndez, O. Canals, S. Blanco, A. Arcas, and R. Araujo. 2013. Microbial and physicochemical parameters associated with Legionella contamination in hot water recirculation systems. Environmental Science and Pollution Research 20:5534-5544.
Shah, P., A. Barskey, A. Binder, C. Edens, S. Lee, J. Smith, S. Schrag, C. Whitney, and L. Cooley. 2018. Legionnaires’ disease surveillance summary report, United States: 2014–2015. Atlanta, GA: CDC Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases.
Shaheen, M., C. Scott, and N. J. Ashbolt. 2019. Long-term persistence of infectious Legionella with free-living amoebae in drinking water biofilms. International Journal of Hygiene and Environmental Health 10.1016/j. ijheh.2019.04.007.
Sheffer, P. J., J. E. Stout, M. M. Wagener, and R. R. Muder. 2005. Efficacy of new point-of-use water filter for preventing exposure to Legionella and waterborne bacteria. American Journal of Infection Control 33(5):S20-S25.
Shen, Y., G. L. Monroy, N. Derlon, D. Janjaroen, C. H. Huang, E. Morgenroth, S. A. Boppart, N. J. Ashbolt, W. T. Liu, and T. H. Nguyen. 2015. Role of biofilm roughness and hydrodynamic conditions in Legionella pneumophila adhesion to and detachment from simulated drinking water biofilms. Environmental Science and Technology 49(7):4274-4282.
Shi, P., S. Jia, X.-X. Zhang, T. Zhang, S. Cheng, and A. Li. 2013. Metagenomic insights into chlorination effects on microbial antibiotic resistance in drinking water. Water Research 47(1):111-120.
Shrivastava, R., R. K. Upreti, S. R. Jain, K. N. Prasad, P. K. Seth, and U. C. Chaturvedi. 2004. Suboptimal chlorine treatment of drinking water leads to selection of multidrug-resistant Pseudomonas aeruginosa. Ecotoxicology and Environmental Safety 58(2):277-283.
SPX Cooling Technologies. 2009. Cooling towers fundamentals. Second edition. Overland Park, KS: SPX Cooling Technologies.
Srinivasan, A., G. Bova, T. Ross, K. Mackie, N. Paquette, W. Merz, and T. M. Perl. 2003. A 17-month evaluation of a chlorine dioxide water treatment system to control Legionella species in a hospital water supply. Infection Control and Hospital Epidemiology 24:575-579.
State of California Energy Commission Staff. 2004. Cooling Water Management Program Guidelines for Wet and Hybrid Cooling Towers at Power Plants. https://www.energy.ca.gov/2005publications/CEC-700-2005-025/CEC-700-2005-025.PDF.
States, S., J. Kuchta, W. Young, L. Conley, J. Ge, M. Costeloa, J. Dowling, and R. Wadowsky. 1998. Controlling Legionella using copper–silver ionization. Journal of the American Water Works Association 90(9):122-129.
Stodlka, J., and R. Vitkovi. 2016. Estimation of the drift eliminator efficiency using numerical and experimental methods. EPJ Web of Conferences. Volume 114, EFM15 – Experimental Fluid Mechanics 2015. Article No. 02111. https://doi.org/10.1051/epjconf/201611402111.
Stojek, N. M. and J. Dutkiewicz. 2011. Co-existence of Legionella and other Gram-negative bacteria in potable water from various rural and urban sources. Annals of Agricultural and Environmental Medicine 18(2):330-334.
Stout, J. E., and V. L. Yu. 2003. Experiences of the first 16 hospitals using copper-silver ionization for Legionella control: implications for the evaluation of other disinfection modalities. Infection Control and Hospital Epidemiology 24:563-568.
Stout, J. E., V. U. Yu, Y. C. Yee, S. Vaccarella, W. Diven, and T. C. Lee. 1992. Legionella pneumophila in residential water supplies: environmental surveillance, with clinical assessment for Legionnaires’ disease. Epidemiology and Infection 109:49-57.
Sullivan, E. 2018. Cool: Antimicrobial Option Reduces Legionella Risks. HPAC Engineering. June 15, 2018. https://www.hpac.com/managing-facilities/cool-anti-microbial-option-reduces-legionnella-risks.
Sydnor, E. R. M., G. Bova, A. Gimburg, S. E. Cosgrove, T. M. Perl, and L. L. Maragakis. 2012. Electronic-eye faucets: Legionella species contamination in healthcare settings. Infection Control and Hospital Epidemiology 33(3):235-240.
Symons, J. M. 1978. Ozone, chlorine dioxide and chloramines as alternatives to chlorine for disinfection of drinking water. Cincinnati, Ohio: U.S. Environmental Protection Agency.
Temmerman, R., H. Vervaeren, B. Noseda, N. Boon, W. Verstraete. 2006. Necrotrophic growth of Legionella pneumophila. Applied and Environmental Microbiology 72(6):4323-4328.
Thomas, J. M., and N. J. Ashbolt. 2011. Do free-living amoebae in treated drinking water systems present an emerging health risk? Environmental Science and Technology 45:860-869.
Totaro, M., P. Valentini, A.L. Costa, S. Giorgi, B. Casini, A. Baggiani. 2018. Rate of Legionella pneumophila colonization in hospital hot water network after time flow taps installation. Journal of Hospital Infection 98:60-63.
Triantafyllidou, S., D. Lytle, C. Muhlen, and J. Swertfeger. 2016. Copper–silver ionization at a U.S. hospital: interaction of treated drinking water with plumbing materials, aesthetics and other considerations. Water Research 102:1-10.
Trussell, R. R., R. S. Trussell, A. Salveson, E. Steinle-Darling, C. He, S. Snyder, and D. Gerrity. 2015. Equivalency of advanced treatment trains for potable reuse, user manual for treatment train toolbox. Final report for Water Environment and Reuse Foundation Project 11-02.
Tsagkari, E., and W. T. Sloan. 2018. Turbulence accelerates the growth of drinking water biofilms. Bioprocess and Biosystems Engineering 41(6):757-770.
Tsvetanova, Z. G., and E. J. Hoekstra. 2012. Assessment of microbial growth potential of PVC flexible tubing in contact with drinking water. Water Science and Technology: Water Supply 12(4):489-495.
Tung, K. Y., M. L. Chen, H. J. Wang, G. S. Chen, M. Peck, J. Yang, and C. C.-H. Liu. 2005. A seven-year epidemiology study of 12,381 admitted burn patients in Taiwan—using the Internet registration system of the Childhood Burn Foundation. Burns 31(1):S12–17.
Türetgen, I., and A. Cotuk. 2007. Monitoring of biofilm-associated Legionella pneumophila on different substrata in model cooling tower system. Environmental Monitoring and Assessment 125(1-3):271-279.
USGBC (U.S. Green Building Council). 2015. Green Building Economic Impact Study. Prepared by Booz Allen Hamilton.
USGBC. 2016a. LEED v4 Water Efficiency Credits. http://www.usgbc.org/credits/healthcare/v4/water-efficiency.
USGBC. 2016b. LEED v4 Energy and Atmosphere Credits. https://www.usgbc.org/credits/healthcare/v4/energy-%26-atmosphere (Accessed 01 Jan 2019).
van Amerongen G., J. V. Lee, and J. M. Suter. 2013. Legionella and solar water heaters. http://solarheateurope.eu/2017/10/31/legionella-solar-water-heaters.
van der Kooij, D., J. S. Vrouwenvelder, and H. R. Veenendaal. 2003. Elucidation and control of biofilm formation processes in water treatment and distribution using the Unified Biofilm Approach. Water Science and Technology 47(5):83-90.
van der Kooij, D., and P. W. J. J. van der Wielen. 2014. Microbial growth in drinking-water supplies. Problems, causes, control and research needs. London, UK: IWA Publishing.
van der Kooij, D., G. L. Bakker, R. Italiaander, H. R. Veenendaal, and B. A. Wullings. 2017. Biofilm composition and threshold concentration for growth of Legionella pneumophila on surfaces exposed to flowing warm tap water without disinfectant. Applied and Environmental Microbiology 83(5):e02737-16.
van der Kooij, D., H. R. Veenendaal, R. Italiaander, E. J. van der Mark, and M. Dignum. 2018. Primary colonizing Betaproteobacteriales play a key role in the growth of Legionella pneumophila in biofilms on surfaces exposed to drinking water treated by slow sand filtration. Applied and Environmental Microbiology 84(24):e01732-18.
van der Lugt, W., S. M. Euser, J. P. Bruin, J. W. Den Boer, J. T. Walker, and S. Crespi. 2017. Growth of Legionella anisa in a model drinking water system to evaluate different shower outlets and the impact of cast iron rust. International Journal of Hygiene and Environmental Health 220(8):1295-1308.
van Hoof, J., L. M. Hornstra, E. van der Blom, O. W. Nuijten, and P. van der Wielen. 2014. The presence and growth of Legionella species in thermostatic shower mixer taps: an exploratory field study. Building Services Engineering Research and Technology 35(6):600-612.
Verhoef, L. P., E. P. F. Yzerman, J. P. Bruin, and J. W. Den Boer. 2004. Domestic exposure to legionellae for Dutch Legionnaires’ disease patients. Archives of Environmental Health 59:597-603.
VisTEch. 2019. How drift eliminators help combat Legionella. https://www.vistechcooling.co.uk/articles/how-drifteliminators-help-combat-legionella.
Volk, C. J., and M. W. LeChevallier. 2000. Assessing biodegradable organic matter. Journal of the American Water Works Association 92(5):64-76.
Vonberg, R. P., T. Eckmanns, J. Bruderek, H. Rüden, and P. Gastmeiera. 2005. Use of terminal tap water filter systems for prevention of nosocomial legionellosis. Journal of Hospital Infection 60(2):159-162.
Walker, J. T., C. W. Mackerness, D. Mallon, T. Makin, T. Williets, and C. W. Keevil. 1995. Control of Legionella pneumophila in a hospital water system by chlorine dioxide. Journal of Industrial Microbiology 15:384-390.
Wallet, F., C. Emery, E. Briand, and P.-A. Cabanes. 2016. Prevalence of Legionella in the production and distribution of domestic hot water. Environment, Risques, and Santé 15(1):29-38.
Walraven, N., W. Pool, and C. Chapman. 2016. Efficacy of copper-silver ionization in controlling Legionella in complex water distribution systems and a cooling tower. Journal of Water Process Engineering 13:196-205.
Wang, H., M. A. Edwards, J. O. Falkinham, and A. Pruden. 2013a. Probiotic approach to pathogen control in premise plumbing systems: A review. Environmental Science and Technology 47(18):10117-10128.
Wang, H., M. Pryor, M. A. Edwards, J. O. I. Falkinham, and A. Pruden. 2013b. Effect of GAC pre-treatment and disinfectant on microbial community structure and opportunistic pathogen occurrence. Water Research 47(15):5760-5772.
Water Research Foundation. 2018. Blending requirements for water from direct potable reuse treatment facilities. PI: Andrew Salveson, Carollo. WRF Project 4536. Denver, CO: WRF.
Wickramanayake, G. B., A. J. Rubin, and O. J. Sproul. 1984. Inactivation of Naegleria and Giardia cysts in water by ozonation. Journal of the Water Pollution Control Federation 56:983–988.
Williams, K., A. Pruden, J. Falkinham, and M. Edwards. 2015. Relationship between organic carbon and opportunistic pathogens in simulated glass water heaters. Pathogens 4:355-372.
Yapicioglu, H., T. G. Gokemen, D. Yidizdas, F. Koksal, F. Ozlu, E. Kale-Cekinmez, and A. Candevir. 2011. Pseudomonas aeruginosa infections due to electronic faucets in a neonatal intensive care unit. Journal of Pediatrics and Child Health 48(5):430-434.
Yiallouros, P. K., T. Papadouri, C. Karaoli, E. Papamichael, M. Zeniou, D. Pieridou-Bagatzouni, G. T. Papageorgiou, N. Pissarides, T. G. Harrison, and A. Hadjidemetriou. 2013. First outbreak of nosocomial Legionella infection in term neonates caused by a cold mist ultrasonic humidifier. Clinical Infectious Diseases 57(1):48-56.
Zahran, S., S. P. McElmurry, P. E. Kilgore, D. Mushinski, J. Press, N. G. Love, R. C. Sadler, and M. S. Swanson. 2018. Assessment of the Legionnaires’ disease outbreak in Flint, Michigan. Proceedings of the National Academy of Sciences 115:E1730-E1739.
Zhang, Y., and M. Edwards. 2009. Accelerated chloramine decay and microbial growth by nitrification in premise plumbing. Journal of the American Water Works Association 101(11):51-62.
Zhang, Y., A. Griffin, and M. Edwards. 2010. Effect of nitrification on corrosion of galvanized iron, copper, and concrete. Journal of the American Water Works Association 102(4):83-93.
Zhang, Z., C. McCann, J. Hanrahan, A. Jencson, D. Joyce, S. Fyffe, S. Piesczynski, R. Hawks, J. E. Stout, V. L. Yu, and R. D. Vidic. 2009. Legionella control by chlorine dioxide in hospital water systems. Journal of the American Water Works Association 101(5):117-127.
Zhou, Z. Y., B. J. Hu, L. Qin, Y. E. Lin, H. Watanabe, Q. Zhou, and X. D. Gao. 2014. Removal of waterborne pathogens from liver transplant unit water taps in prevention of healthcare-associated infections: a proposal for a cost-effective, proactive infection control strategy. Clinical Microbiology and Infection 20:310-314.
Zobrist, J., S. R. Müller, A. Ammann, T. D. Bucheli, V. Mottier, M. Ochs, R. Schoenenberger, J. Eugster, and M. Boller. 2000. Quality of roof runoff for groundwater infiltration. Water Research 34(5):1455-1462.




































































