During the first workshop session, a diverse panel of speakers considered the inefficiencies of the current clinical trial enterprise; the boundaries of what might be considered a virtual clinical trial for medical product development; the opportunity of virtual clinical trials to expand access for participants; and regulatory questions regarding the remote collection of endpoints. Donna Cryer, president and chief executive officer (CEO) of the Global Liver Institute, offered a patient perspective on considerations necessary for designing clinical trials to meet the needs of trial participants. Craig Lipset, head of clinical innovation within global product development at Pfizer Inc., provided an industry perspective on the potential for virtual clinical trials to improve the efficiency of Phase 3 clinical research. Ray Dorsey, professor of neurology and director of the Center for Health and Technology at the University of Rochester, provided an academic perspective on the clinical trials landscape and how virtual clinical trials could increase participant access. The session was moderated by Linda Brady and Clay Johnston.
A PATIENT PERSPECTIVE
Donna Cryer, President and CEO, Global Liver Institute
Cryer explained that a quality clinical trial is one that generates the minimal amount of credible, replicable, and evaluable data needed to answer meaningful questions with the least time and cost burdens on participants. At the core of her definition is the hope that trials can generate and use data collected in the day-to-day course of a participant living his or her life or receiving care. In addition, Cryer emphasized the importance of being mindful of the divergence between research-setting and real-world effects. The lack of applicability or generalizability of clinical trial results can be disheartening to patients who did not meet trial inclusion criteria. This can be particularly problematic for patients who rely on treatments
developed from a study from which they would have been excluded, as inclusion criteria are listed on a drug label and can inform reimbursement decisions.
According to Cryer, virtual clinical trials should not be thought of as a separate type of trial, but as a way of thinking about trial design that meets her definition of a “quality clinical trial.” Merely addressing the challenges that new technologies can pose, such as computer literacy, would reduce a virtual trial to its underlying technology platform. A more important issue, Cryer proposed, is whether a virtual trial allows the study to operate well from the patients’ perspective. For example, scientific questions, as currently framed, may seek to understand if a treatment works. However, they may not address questions about whether the treatment will lead to a better lived experience with a disease. Cryer expressed hope that as more virtual trials are conducted and patient communities are engaged, the quality of endpoints and outcome measurements will be improved in a way that allows questions about a patient’s quality of life to be better addressed.
Cryer drew attention to the limited protocol flexibility of clinical trials, which often excludes large groups of patients from trials. Novel data mining techniques can shed light on which patient populations should be included in clinical trials, lead to more realistic inclusion/exclusion criteria, and result in better participant recruitment and retention. The consent process is also an issue, which can often be an “all-or-nothing” form filled with complex jargon. However, as Cryer noted of an Apple Research Kit demonstration, the consent process can be broken into easy to understand, digestible chunks of information, in which consent is serially provided as needed.
Cryer discussed issues related to the number, length, timing, and location of site visits. She agreed with Dorsey that technology can help lessen the burden on participants who have to travel to clinical trial sites, especially because 70 percent of potential trial participants live more than 2 hours away from a study center (Anderson, 2018). Given the availability of wearable technology and other home-based digital health technology, Cryer questioned the need for site-based visits to measure vitals given that these measurements could be collected passively using digital health technology.
The default model for clinical research, Cryer emphasized, should be a virtual trial because it offers an opportunity to foster ongoing relationships with participants, better understand clinical conditions longitudinally, and generate new and relevant research questions.
AN INDUSTRY PERSPECTIVE
Craig Lipset, Head of Clinical Innovation, Global Product Development, Pfizer Inc.
Advances in computing and technology that have expanded health care access for the community can also be applied to the conduct of clinical trials. When doing so, it will be important for industry to consider what aspects of a trial should be centralized (e.g., the investigator, coordinator, labs, or Institutional Review Boards [IRBs]) to achieve the benefits of trial decentralization, such as increased access, improved representation, and decreased burden of participation.
Lipset introduced a 2011 study conducted by Pfizer, REMOTE,1 which was designed to validate available virtual technologies by repeating a standard brick-and-mortar clinical trial that Pfizer had conducted for Detrol, a drug used to treat overactive bladder. The trial’s components (e.g., recruiting patients online and capturing patient-reported outcomes electronically) were not novel at the time. However, the linkage between different components of the study and the introduction of unique components, such as delivering the investigational drug directly to the participant, were new approaches. REMOTE was eventually discontinued because it failed to recruit enough women with a disease severity matching those who participated in the original trial (additional details on challenges leading to early termination can be found on p. 27). However, it did successfully demonstrate the ability to screen and acquire consent from participants, monitor safety, and capture required data to indicate both safety and efficacy.
According to Lipset, the REMOTE trial did not operate at the available limit of technology when it was conducted in 2010, nor did it require any new legislation, safe harbor, or guidance from regulators. As a result, new regulatory or technical frameworks may not be necessary to successfully launch virtual trials. However, Lipset mentioned that even though virtual trials are not limited by U.S. regulatory policies, there is state-by-state variability in regulations regarding telemedicine and Internet prescribing for domestic trials, as well as regulatory variability by country.
Lipset noted that since REMOTE, and mostly over the past 2 to 3 years, the industry has evolved, with well-capitalized companies and contract research organizations entering the virtual space. What seems to be missing, he noted, is movement beyond pilot programs and so-called hybrid protocols that dictate specific “visits” be done remotely to let the participant choose how, when, and where they want to participate. Lipset asserted that the ultimate goal should be protocols that allow participants
___________________
1 Additional information can be found in Appendix D.
to decide whether they want to participate at a site or remotely—what he calls a “location variable” trial.
Accommodating patient desires would require flexible processes based on different patient needs and preferences, but doing so is not impossible. Most patients already have a diagnosis, course of treatment, and a treating physician overseeing their care. Virtual trials could take advantage of this paradigm and complement the clinical position and practice of the treating physician without requiring them to become a clinical investigator. However, more effective engagement between the research and clinical practice communities would depend on the development of endpoints that are more resilient and agnostic to location than those relied on today. A major rate limiter for virtual trials to be conducted and for these benefits to be realized will be a lack of will and culture. Additionally, it is likely, Lipset noted, that cost savings from virtual trials would occur in the long term.
AN ACADEMIC PERSPECTIVE
Ray Dorsey, Director, Center for Health and Technology, University of Rochester
Current Model for Clinical Trials
Clinical trials as currently conducted are expensive, inefficient, and inaccessible. Citing a study conducted by DiMasi and colleagues (2016), Dorsey stated that the cost of drug development has doubled every 12 years, from $200 million in 1979 to $2.6 billion in 2016. At the same time, pharmaceutical industry productivity, in terms of new molecular entities developed, has declined for the past 50 years (Scannell et al., 2012).
Furthermore, Dorsey noted that clinical trials fail to adequately represent the patient population (with trial participation as low as 5 percent for patients with certain conditions2) and fail to be participant centered.3 Participants, who are often sick, have financial and time burdens placed on them to travel to research sites where they volunteer to be exposed to known and unknown risks. This all occurs, Dorsey continued, on the investigator’s terms, not the participants’ terms.
Dorsey described drug development as “long, inefficient, and likely to fail” (see Figure 2-1). For example, drug development for neurological
___________________
2 Trial participation for patients with cancer typically does not exceed 5 percent (Sacristan et al., 2016).
3 Participant centered: If a clinical study is participant centered, the burden of participation, such as time spent in travel and in clinics, financial costs associated with travel and missed work, and complications to a person’s routine due to additional examinations and procedures, is minimized (Holloway, 2018).
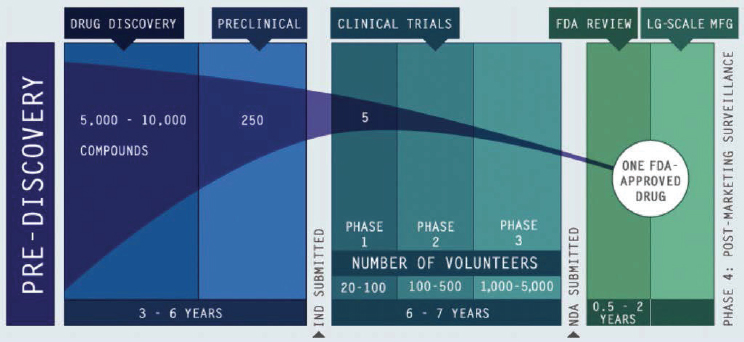
NOTE: FDA = U.S. Food and Drug Administration; IND = Investigational New Drug; NDA = New Drug Application.
SOURCES: As presented by Ray Dorsey, November 28, 2018; PhRMA, 2012. An updated figure is available at https://www.phrma.org/graphic/the-biopharmaceutical-research-and-development-process (accessed June 25, 2019).
disorders, which are the leading cause of disability in the world (Collins, 2017), is marked by failure,4 emphasized Dorsey. The mismatch between the burden of neurological disorders and the success rates of drug development indicates there is a need for new tools to be used by industry, noted Dorsey.
New Models for Clinical Trials
The pharmaceutical industry has already begun to leverage emerging digital health technologies to make clinical research accessible, convenient, and less costly. For example, Pfizer’s REMOTE study conducted all aspects of the clinical trial remotely via Web-based approaches (ClinicalTrials.gov, 2013; Orri et al., 2014). This trial, Dorsey added, laid the groundwork for how virtual clinical trials may be conducted going forward. As depicted in Table 2-1, most aspects of a clinical trial take place at individual trial sites. However, Dorsey envisions that in the near term, clinical trials could be conducted using a mix of venues, including centrally (at one trial site), at multiple individual trial sites, and/or remotely (via digital health technolo-
___________________
4 From 2002 to 2012, the failure rate of drug development for Alzheimer’s disease was more than 99 percent (Cummings et al., 2014). Drug development for Parkinson’s disease has also been unsuccessful. The most effective drug to provide symptomatic relief for Parkinson’s, Levadopa, was discovered as a therapeutic agent in 1967 (Hornykiewicz, 2010).
TABLE 2-1 Current and Future Models for Clinical Trials, Categorized by Stages in Clinical Trials
| Recruitment | Pre-Screening | Enrollment | Interim Assessments | Final Assessments | Longitudinal Follow-Up | |
|---|---|---|---|---|---|---|
| Current | Site | |||||
| Future | Centrally and remotely | Site(s) | Remotely | Site(s) | Centrally and remotely | |
SOURCE: As presented by Ray Dorsey, November 28, 2018.
gies), depending on the type of data needed. For example, pre-screening could occur centrally at one trial site, biopsies and medical imaging could be conducted at multiple trial sites, and interim assessments could be conducted remotely through digital health technologies, noted Dorsey.
Dorsey presented a diagram (see Figure 2-2) created by Andrea Coravos, CEO of Elektra Labs, that categorizes clinical trials based on where and how the data are captured (Coravos, 2018). According to Figure 2-2, decentralized trials have deceased reliance on an intermediary (e.g., a member of the study team) and physical trial site location. The REMOTE trial mentioned earlier, said Dorsey, was unique in that remote and fully virtual methods were used to capture data. However, Dorsey continued, there are likely to be more clinical trials that incorporate both traditional and decentralized models.
Examples of Virtual Clinical Trials
Dorsey provided three examples that illustrate how virtual trials can increase participant access and geographic representation, improve the participant experience, and enhance recruitment for patient subpopulations: (1) a Michael J. Fox Foundation virtual study, (2) AT-HOME PD,5 and (3) a 23andMe LRRK2 (leucine-rich repeat kinase gene) study.
The Michael J. Fox Foundation Virtual Study
Individuals with and without Parkinson’s disease were enrolled using The Michael J. Fox Foundation’s tool, Fox Trial Finder,6 a clinical trial
___________________
5 Available at https://clinicaltrials.gov/ct2/show/record/NCT03538262?term=AT+HOME+PD&rank=1 (accessed April 29, 2019).
6 Fox Trial Finder was created to help increase the flow of willing participants into clinical trials, thereby accelerating the development of drugs for Parkinson’s disease. Fox Trial Finder lists ongoing Parkinson’s disease clinical trials and matches participants to trials for which they are best suited (The Michael J. Fox Foundation, 2019a).
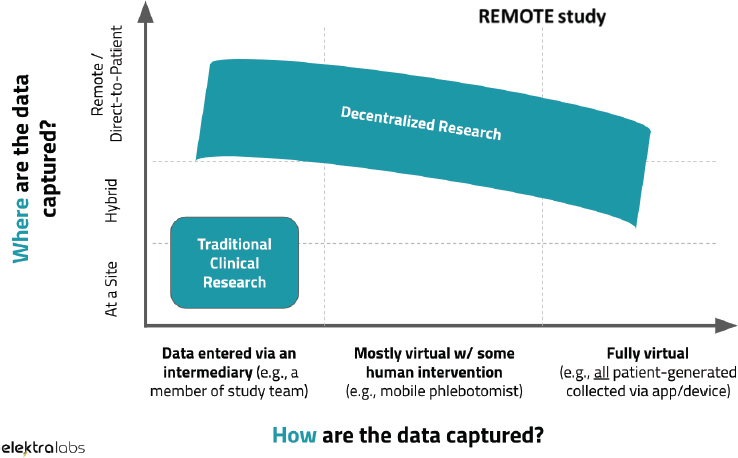
SOURCES: As presented by Ray Dorsey, November 28, 2018; Coravos, 2018. Published on https://blog.andreacoravos.com/decentralized-clinical-trials-e9dbde90ea95 (accessed March 20, 2019).
matching tool (Dorsey et al., 2015). More than 160 participants from 39 sites spread across the country were enrolled in the study. Parkinson’s disease is typically visually diagnosed. The virtual platform used in the study allowed investigators to visually examine Parkinson’s disease status remotely via videoconferencing, without requiring participants to leave their homes. Furthermore, it allowed for wide geographic representation and enabled participation for those who previously had no means of doing so. In a follow-up evaluation of participants’ experience, 90 percent of participants reported satisfaction with the trial, 80 percent reported they were more willing to participate in a similarly designed trial, and 85 percent reported they would be more able to participate if they could do so remotely (Dorsey et al., 2015). Similar research, said Dorsey, has shown this to be the case for Alzheimer’s disease.
AT-HOME PD
This study will follow-up with participants from two large, multicentered Phase 3 Parkinson’s disease studies (Steady PD III7 and Sure PD38) remotely via an annual virtual visit using Web-based video conferencing. AT-HOME PD participants will also provide self-reported outcomes quarterly through Fox Insight9 (an online clinical study) in addition to providing monthly assessment data on tremor, gait, voice, and balance collected via a smartphone.
23andMe LRRK2 Study
This study will investigate the linkage between the leucine-rich repeated kinase (LRRK2) genetic mutation10 and Parkinson’s disease by recruiting a national cohort of carriers. Given LRRK2’s rarity in the general population, a traditional study would require establishing multiple sites around the world. However, by leveraging Fox Insight’s online platform, this study was able to recruit a cohort of 300 participants—50 of whom have Parkinson’s disease. This study will follow participants remotely, with annual virtual Parkinson’s disease examinations (University of Rochester–Udall Center, n.d.).
In Dorsey’s opinion, virtual trials offer numerous advantages compared with traditional studies (see Table 2-2); perhaps most importantly, they enable studies to be more participant centered. The geographic reach of a virtual trial, Dorsey noted, will not be determined by where someone lives, but by whether they have Internet access. Additionally, virtual trials can offer benefits such as comfort, convenience, and confidentiality for participants. Virtual trials can also reduce the time to initiate study,11 allow
___________________
7 Available at https://clinicaltrials.gov/ct2/show/NCT02168842?term=Steady+PD+III&rank=1 (accessed April 29, 2019).
8 Available at https://clinicaltrials.gov/ct2/show/NCT02642393 (accessed April 29, 2019).
9 Fox Insight is an online study that seeks to build a large, diverse cohort of participants that is representative of Parkinson’s patients. Once enrolled and every 90 days thereafter, participants are asked to enter health and disease information. Fox Insight is meant to complement in-person research and curated data are made available to researchers worldwide in real time. Launched as a beta in March 2015, more than 5,000 participants (80 percent of whom have a Parkinson’s disease diagnosis) contributed data before the study’s formal launch in April 2017 (The Michael J. Fox Foundation, 2019b).
10 The majority of Parkinson’s disease cases are idiopathic (meaning there is not a known cause). However, for approximately 10 percent of cases there is a genetic linkage. Of this subset of Parkinson’s disease cases, a mutation in the LRRK2 gene is the most common cause and represents up to 2 percent of all Parkinson’s disease cases (The Michael J. Fox Foundation, n.d.).
11 The AT-HOME PD trial, which used a virtual platform, was able to enroll its first participants in less than 6 months after receiving funding.
TABLE 2-2 The Many Advantages of Virtual Clinical Trials
| Characteristic | Traditional Study | Virtual Study |
|---|---|---|
| Focus | Participants, investigator, sites | Participants |
| Geographic reach | Sites | Internet access |
| Sites | Many | One |
| Institutional Review Boards | Many | One |
| Time to initiate study | Long | Medium |
| Investigators | Many | Handful |
| Assessments | Episodic | Frequent |
| Variance | High | Low |
| Comfort | Low | High |
| Convenience | Low | High |
| Confidentiality | Low | High |
| Cost | High | Moderate |
SOURCE: As presented by Ray Dorsey, November 28, 2018.
for more frequent participant assessments, and simplify the complexity of dealing with multiple IRBs.
Dorsey envisions that virtual trials could reduce costs in the long term. While the tools to conduct virtual trials already exist, the main barriers preventing industry from applying these tools may be creativity and will, which Dorsey hopes can be increased and galvanized.
DISCUSSION
Johnston opened the discussion by noting that like the U.S. health care system, clinical trials are not designed based on the needs of the patient (participant), but rather the needs of the investigator. Cryer acknowledged this deficiency and reiterated the importance of the clinical trial infrastructure to be a complement for treating physicians’ clinical practices to enable seamless access to and participation in clinical trials—especially for minority populations for whom treating physicians may not have an adequate set-up for traditional clinical trials. Technologies used to drive health care transformation could be leveraged to better access those physicians and patients, Cryer emphasized. Dorsey echoed this sentiment, stating that the availability of digital health technology has extended health care beyond institutions and into the community and patients’ homes.
Steven Cummings, director of the San Francisco Coordinating Center, provided a different perspective that resulted from analyzing 12 clinical
trials at Genentech-Roche for efficiency opportunities, including transformation to siteless trials. Given the necessity of intensive examinations and monitoring of participants, in Cummings’s opinion, none of these trials could be converted to completely siteless studies. However, through the course of the reviews, it was discovered that the number of assessments could have been reduced by 20 to 80 percent, with potential reductions in the number of visits to clinical sites. Simplifying protocols, Cummings emphasized, would make trials more participant centered.
Adrian Hernandez from Duke University posed the question of what incentives participants might need to remain engaged in a virtual trial over the long term. Dorsey responded by suggesting that many individuals with Parkinson’s disease, for example, want to participate in research and contribute to the development of knowledge. However, for continued engagement, the study design and virtual interface of a study platform is important, such as providing participants with real-time data on their health, or use of data analytics to project long-term health outcomes. Regardless of the purpose, the basic aim should be to provide value to the participants, Dorsey emphasized.
Lipset and Cryer both made distinctions on the appropriateness of providing feedback depending on study type and patient population. Lipset agreed that providing real-time feedback to participants is a good idea in observational trials. However, it would involve significant planning to determine what data could be returned and when in blinded, randomized clinical trials. Cryer, on the other hand, distinguished between the trial design considerations for participants who need to consciously manage conditions with high-burden symptoms versus those with low-burden symptoms. Cryer proposed that the former could be provided feedback with health management strategies, while the latter may need a creative approach to inspire engagement with their health data. Human-centered design,12 Cryer emphasized, should be leveraged to make retention more fulfilling for participants.
Nitin Desai, chief medical officer at Health Wizz, asked the speakers to comment on any legal or ethical barriers that may prevent engagement with potential trial participants. Lipset acknowledged this issue, but noted that a more significant upstream challenge is the lack of awareness by treating physicians about trials in which their patients could be enrolled. Virtual trials can make trials more accessible, but should be accompanied by other channels to improve awareness of trial participation, such as social media,
___________________
12 Human-centered design is a design and management framework that seeks to develop solutions made for the people at the core of the problem. By building deep empathy for whom the trial is being designed, innovative solutions will be more likely to fit seamlessly into people’s lives and address their needs (Design Kit, n.d.).
said Lipset. Cryer and Johnston also commented on repurposing ClinicalTrials.gov to allow people to more optimally identify, learn, and engage with trials in a user-friendly way.
Sally Okun, vice president of policy and ethics at PatientsLikeMe, noted the regulatory challenges associated with using real-world data (e.g., claims data, electronic health record [EHR] data, and data emerging from digital health technologies). According to Okun, the use of such technology is not prohibited by regulation, but the use of data generated from these technologies does face regulatory hurdles. Related to Okun’s comment, Lipset emphasized that there is an opportunity for using real-world data in prospective virtual trials, in which participants provide their own EHR data—a process that does not require special regulatory approval. The proliferation of tools such as AppleHealth provides potential for patients to bring their own data into studies. Dorsey suggested that EHR data may not necessarily be real-world data because they are sporadically collected. For example, he might see a patient with Parkinson’s disease four times per year, which means that he has a limited idea of how that patient is dealing with his or her disease day to day. The use of digital health technologies can help illuminate the patient’s daily experience. For example, based on data collected from these technologies, Dorsey and his colleagues observed that Huntington’s disease patients were lying down for about half the day (Adams et al., 2017). In Dorsey’s opinion, regulators might like to see more digital health technologies being leveraged to capture new and useful data.
Lipset emphasized a core problem regarding the lack of reliable and stable digital biomarkers, regardless of whether they are sourced from medical grade devices or consumer grade devices. He emphasized the importance of investing in validation of new biomarkers in early research phases so they are ready for use during Phase 3 of clinical trials, while at the same time being mindful of substitution of prior measures.
Emily Butler, a statistician from GlaxoSmithKline, commented that a not-insignificant proportion of data collected during a clinical trial is not examined, and asked the speakers if there should be a balance between optimal data collection upfront and collecting data that are valuable. According to Dorsey, overemphasizing data collection is not necessarily a bad thing, citing examples of medical discoveries such as nocturnal hyperglycemia and sleep disorders, which resulted from more intensive measurements. On the other hand, Cryer emphasized the importance of including the patient in developing the data collection plan. Citing her own experiences as a patient with multiple conditions, she highlighted how a large number of measurements could be consolidated by involving the patient. According to Lipset, the burden and complexity of collected data may be used to justify the necessity of an in-person visit. However, the fear of missing something is more likely to explain this trend, Lipset added.












