3
Observational Emulation of the Target Trials and Practical Considerations
The previous chapter described an approach to addressing the research questions through the specification of the protocol of hypothetical target trials for both opioid initiation and tapering. The next step in investigating comparative effectiveness and safety questions would be to conduct the target trials, or, if this is not possible, to attempt to emulate the target trials using analyses of observational data. This chapter outlines a general procedure for emulation of the opioid initiation and tapering target trials described in Chapter 2, using Department of Veterans Affairs (VA) databases. While this chapter does not define a specific analysis strategy, as the committee was not charged with conducting the actual analyses and did not review the VA’s databases, it does lay out considerations of how to emulate each of the components of the protocol of the target trial: eligibility criteria, treatment strategies, treatment assignment, follow-up, outcomes, causal contrasts, and statistical analysis plan.
Before the committee describes the emulation procedures for each component of the target trials, it is important to note that the target trials described in the previous chapter (see Table 2-3) were chosen among several trials that could reasonably be considered to address the research questions. For instance, variations of the target trials’ eligibility criteria or treatment strategies could be justifiable or even necessary because the available VA datasets might not include all information needed to emulate the target trials previously described. Additionally, although the focus of the research questions is concomitant opioid and benzodiazepine prescription, variations could also include asking the same research questions among
individuals who had been prescribed opioids only, without benzodiazepines; however, this was outside the committee’s scope.
The process of specifying and emulating the target trial is an iterative process guided by data constraints, with the final analysis being a compromise that is likely to differ from the originally proposed target trial. Therefore, this and the previous chapter should be viewed as guidance on how to structure the specifications of a relevant target trial and how to describe the observational data analyses to carry out the emulation of that trial. The committee begins by briefly describing possible data sources.
DATA SOURCES
Potential sources for data key to the observational emulation of the target trials from 2010 through 2017 are the VA Corporate Data Warehouse (CDW), the outpatient prescription data from the VA’s Pharmacy Benefits Management Services, and the Centers for Disease Control and Prevention’s National Death Index (NDI). The CDW data can be used to identify those veterans receiving services who have relevant health conditions, such as pain conditions and opioid use disorders, noted during medical encounters. These data include treatment use, demographic characteristics, and clinical diagnoses for all patients seen at VA facilities. The NDI contains information on individuals’ date and cause of death, such as the underlying cause of death and detailed information about the death, as well as identifiers such as name and social security number (Ilgen et al., 2016). Those data originate from state vital statistics offices and are based on the results of medical examiner or coroner investigations. The VA purchases mortality data from the NDI so that the date of death and cause of death can be known for VA patients and linked to medical records data (Lin et al., 2019). Other possibly useful data sources include the Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn rosters, which can be used to obtain service information and fill in missing race and ethnicity data, and the VA Suicide Prevention Applications Network database, for use in identifying non-fatal suicide events (attempts, serious suicidal ideation).
OVERVIEW OF THE EMULATION PROCEDURE
In this section, the committee describes a procedure for the emulation of the two target trials (see Chapter 2) that will estimate the effect of opioid initiation and tapering strategies on patient outcomes.
The emulation procedure starts by identifying a cohort of patients in the VA who are eligible for the initiation and tapering studies at some time point during the period of interest. That time point then defines the start of follow-up time (baseline). It is important to note that, in the observational
data, patients might be eligible for both studies, although with different baseline times; this situation is illustrated in Figure 3-1. A patient on benzodiazepines but not opioids could be initially eligible for the initiation emulation (left side of Figure 3-1) and then, after initiating and remaining on opioids for at least 90 days, for the tapering study (right side of Figure 3-1). Dotted lines on Figure 3-1 depict other possible trajectories. In cases where a single patient is eligible at multiple times for the same study, one of the multiple eligibility time points could be chosen at random to serve as baseline for that patient, or, alternatively, multiple eligible time points could be included in the emulation.1
___________________
1 See Hernán and Robins (2016) for further discussion.
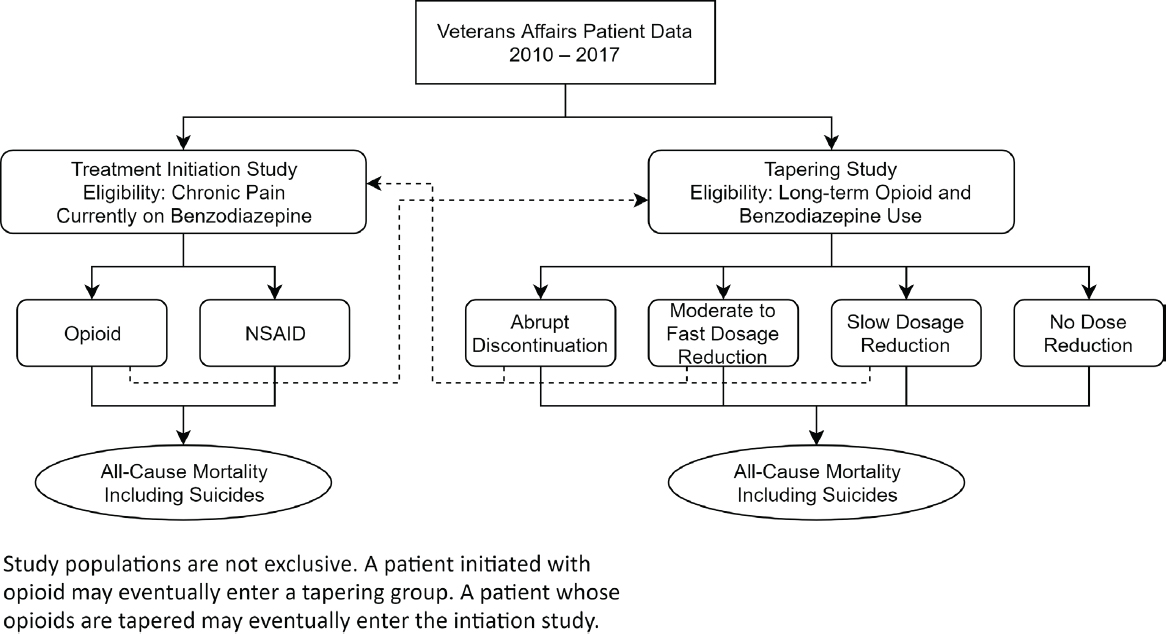
NOTE: NSAID = nonsteroidal anti-inflammatory drug.
The next step is to assign the eligible patients to the treatment strategies that are consistent with their baseline data. Because VA patients were not randomly assigned to these treatment strategies, the analysis should include adjustments for the potential confounding factors—prognostic factors that are imbalanced across treatment strategies—to approximate the estimates that would arise through randomization as closely as possible. The availability of information on these factors is essential for the success of the emulation procedure. Finally, the outcome distribution is compared between groups defined by their treatment strategies, after appropriate adjustment for pre- and post-baseline confounding factors.
EMULATING THE INITIATION AND TAPERING TARGET TRIALS
Tables 3-1 and 3-2 reiterate the specification of the components of the target initiation and tapering target trials, already described in Table 2-3, and delineate the emulation procedures using available observational data. That is, the target trial columns of Tables 3-1 and 3-2 replicate the relevant columns in Table 2-3, and the last column in each of the tables outlines the proposed observational analyses to emulate the eligibility criteria, treatment strategies, treatment assignment, start and end of follow-up, outcomes, causal contrasts, and statistical analysis of the target trials. Table 3-1 addresses the opioid initiation trial emulation, while Table 3-2 addresses the opioid tapering trial emulation.
TABLE 3-1 Opioid Initiation Target Trial Emulation
| Study Component | Target Trial | Emulation Using Observational Data |
|---|---|---|
| Eligibility criteria | Chronic pain diagnosis No prescriptions for opioids or non-aspirin NSAIDS in the past 90 days Long-term benzodiazepine therapy (defined based on pilot data) Exclude: Individuals with serious illnessa Individuals prescribed opioids used for treatment of opioid use disorder Individuals with surgery or acute painful injury within the past 90 daysb |
Data to determine the use of analgesics during the past 90 days and the use of benzodiazepines will come from pharmacy fills and will require information specifically on fill dates, dose, and supply duration. Data for the diagnoses of chronic pain, serious illnesses, surgeries, or acute painful injuries will come from the medical visit records. Opioid use for opioid use disorder treatment will be measured through a combination of pharmacy fills (for buprenorphine) and clinic codes for opioid treatment programs. |
| Treatment strategies |
|
Patients will be assigned to the strategies consistent with their pharmacy fill data, based on initiation at baseline with an opioid or NSAID. Adherence to the strategy is defined by continued fills during the year after baseline. An example of non-adherence would be if a patient could not tolerate over-sedation from opioid use and discontinued as a result. |
| Treatment assignment | Individual randomization |
Assumed to be random conditional on baseline confounders, including, but not limited to
Diagnoses associated with clinical visits in VA medical records will be used to define these variables. |
| Study Component | Target Trial | Emulation Using Observational Data |
|---|---|---|
| Start and end of follow-up | Start of follow-up (baseline): time of assignment to a treatment strategy End of follow-up: the earliest of 18 months, death, or the administrative end of follow-up (end of the study) |
Start of follow-up (baseline): time of assignment to a treatment strategy. End of follow-up: the earliest of 18 months, the date of death based on National Death Index records, or the administrative end of follow-up. |
| Outcomes |
|
Deaths ascertained from National Death Index data, with all-cause mortality measured as a death record with a date of death and suicide deaths as those records with underlying cause of death recorded as ICD-10 codes X60–X84, Y87.0, *U03.c |
| Causal contrast |
|
Observational analog of the intention-to-treat effect: this effect may be close to null and therefore relatively uninformative because adherence to the assigned treatment strategies is expected to be low in the observational data. Observational analog of the per-protocol effect. |
| Study Component | Target Trial | Emulation Using Observational Data |
|---|---|---|
| Statistical analysis | Intention-to-treat analysis: check for balance on key variables, e.g., mental health diagnoses and SUDs. Per-protocol analysis: patients will be censored at the time they deviate from their assigned strategy. To adjust for the potential selection bias induced by censoring, inverse probability weighting will be used. The weights will be a function of the baseline and post-baseline (time-varying) confounders. Both analyses may require further adjustment for selection bias due to loss to follow-up. Pre-specified sub-groups to be examined for potential effect modification include, e.g., those with pain severity, history of overdose, history of suicide attempt. |
Intention-to-treat analysis: same as in target trial, except that an individual may have multiple eligibility points, and adjustment for baseline confounders is required. Per-protocol analysis: same, except that a single subject may have multiple eligibility points. All variables will be obtained from medical records, including clinic visit information, diagnoses, and pharmacy records. |
a Serious illness is defined by Kelley and Bollens-Lund (2018) as a health condition that carries a high risk of mortality and negatively affects a person’s daily functioning. The committee recommends operationalizing this as any of the following conditions: cancer, chronic obstructive pulmonary disease, congestive heart failure, dementia, or severe neurologic disorder (e.g., amyotrophic lateral sclerosis, multiple sclerosis).
b 90 days was chosen to minimize the likelihood of opioids being prescribed for acute rather than chronic pain conditions. However, the committee acknowledges that the choice of 90 as opposed to 30 or 60 is arbitrary.
c The researchers who perform the study should determine whether this definition is sufficiently accurate for their purposes.
TABLE 3-2 Opioid Tapering Target Trial Emulation
| Study Component | Target Trial | Emulation Using Observational Data |
|---|---|---|
| Eligibility criteria | Long-term opioid therapy defined as 3+ opioid fills ≥21 days apart in a ≥84-day period for ≥84-day supply (Larochelle et al., 2016) Average opioid MMEa/day is ≥30 over the prior 84 daysb Long-term benzodiazepine therapy (defined based on pilot data) Exclude: Individuals with serious illnessc Individuals prescribed opioids for the treatment of opioid use disorder Individuals with surgery or acute painful injury within the 90 days prior to baseline |
Data to determine opioid use will come from pharmacy fills and will require information specifically on fill dates, dose, and supply duration. Data for diagnoses qualifying as serious illnesses will come from the medical visit records. Opioid use for opioid use disorder treatment will be measured through a combination of pharmacy fills (for buprenorphine) and clinic codes for opioid treatment programs. |
| Treatment strategies |
Participants who cannot tolerate their assigned dosage change will be excused from following their assigned strategy. Percentage of taper is relative to opioid dose at baseline and is calculated over the next 3 months. After that period, dosage is left to the physician’s discretion. |
The treatment strategy to which a participant is assigned is determined by the average change in opioid dose during the 3-month period after baseline. This will minimize the impact of changes that are due to non-clinical reasons, as those changes should be followed by a correction (e.g., early prescription fill due to patient vacation, followed by a late fill). Tapering treatment strategies are defined the same as in the target trial. An example of non-adherence would be if a patient’s pain worsened and functioning declined and the patient returned to the original dosage after starting a taper. |
| Study Component | Target Trial | Emulation Using Observational Data |
|---|---|---|
| Treatment assignment | Individual randomization |
Assumed to be random conditional on the baseline confounders, including
Diagnoses associated with clinical visits in VA medical records will be used to define these variables. |
| Start and end of follow-up | Start of follow-up (baseline): time of assignment to a treatment strategy End of follow-up: the earliest of 6 months, death, or the administrative end of follow-up (end of the study) |
Start of follow-up (baseline): time of assignment to a treatment strategy. End of follow-up: the earliest of 6 months, the date of death based on National Death Index records, or the administrative end of follow-up. |
| Outcomes |
|
Deaths will be ascertained from National Death Index data, with all-cause mortality measured as a death record with a date of death and suicide deaths as those records with underlying cause of death recorded as ICD-10 codes X60–X84, Y87.0, *U03.e |
| Causal contrast |
|
Observational analog of the intention-to-treat effect: this effect may be close to null and therefore relatively uninformative because adherence to the assigned treatment strategies is expected to be low in the observational data. Observational analog of the per-protocol effect. |
| Study Component | Target Trial | Emulation Using Observational Data |
|---|---|---|
| Statistical analysis | Intention-to-treat analysis: check for balance on key variables, e.g., mental health diagnoses and SUDs. Per-protocol analysis: patients will be censored at the time they deviate from their assigned strategy. To adjust for the potential selection bias induced by censoring, inverse probability weighting will be used. The weights will be a function of the baseline and post-baseline (time-varying) confounders. Both analyses may require further adjustment for selection bias due to loss to follow-up. Pre-specified sub-groups to be examined for potential effect modification include, e.g., patients with pain severity, history of overdose, or history of suicide attempt. |
Intention-to-treat analysis: N/A Per-protocol analysis: same as in target trial, except that a single subject may contribute two clones. All variables will be obtained from medical records, including clinic visit information, diagnoses, and pharmacy records. |
a MME = morphine milligram equivalent.
b This threshold was used because labeling for OxyContin extended release defines opioid tolerant as consuming 30 MME/day. Researchers might consider a lower dose threshold if the purpose is to include anyone who could be considered for a taper.
c Serious illness is defined by Kelley and Bollens-Lund (2018) as a health condition that carries a high risk of mortality and negatively affects a person’s daily functioning. The committee recommends operationalizing this as any of the following conditions: cancer, chronic obstructive pulmonary disease, congestive heart failure, dementia, or severe neurologic disorder (e.g., amyotrophic lateral sclerosis, multiple sclerosis).
d Speed of tapering: there is no generally accepted rate of tapering speed (i.e., rate of dosage decrease per week/month); options would need to be explored using pilot data. The tapering speeds proposed for this trial should not be considered medical guidance.
e The researchers who perform the study should determine whether this definition is sufficiently accurate for their purposes.
Tables 3-1 and 3-2 describe an initial emulation strategy that has not been evaluated and would likely require modification after an initial review of the available data. It is important to remember that the process of specifying a target trial is necessarily iterative: after the initial set of design choices for the target trial are made (as shown in Chapter 2), an examination of the available observational data may indicate that the components of the target trial need to be modified. For example, a proposed definition of the eligibility criteria might initially include a characteristic that is found to be unavailable in the observational data. This process could then be repeated until the specified components of the target trial and the available data are consistent with each other or until it is determined that the only target trials that could be emulated using the available data are of little interest. Importantly, this process should not involve any evaluation of the outcomes of interest.
TREATMENT STRATEGIES
Emulating the initiation and tapering target trials requires using information from VA pharmacy data to determine the treatment strategy. The pharmacy data contain information on medications filled and dispensed to VA patients, either in VA facilities or through mail order, with data fields such as the date dispensed, days supplied, number of units, and dose per unit. This section outlines the major considerations related to measurement treatment strategies from pharmacy records.
The patterns of medication use that can be derived from these data may be thought of as either (a) the prescribed course of treatment, if the goal is to draw inferences about the effects of assignment to treatment strategies (intention-to-treat), or (b) patients’ actual medication consumption, if the goal is to draw causal inferences about the effects of the medication strategy (per-protocol). Measurement choices must address the fact that some patients will have highly variable medication use patterns, which may not appear consistent with any specific treatment strategy because of changes made to the treatment plan over time. Furthermore, even some relatively stable patterns of prescribing and medication use may appear variable because of the reliance on dispensing data, for example, if patients choose to fill their prescriptions on different days each month.
For the initiation study, one can assume that the date of initiation is the date that an opioid or nonsteroidal anti-inflammatory drug (NSAID) medication was first dispensed. Prescriptions that are written and never filled by the patient are not included in the pharmacy data, which is a limitation if one seeks to study the deviations from assignment that would occur early after randomization in the target trial.
The challenges of measuring treatment strategies are even greater for the tapering study, which relies on calculating medication dosages in order
to differentiate between strategies. Calculating monthly averages2 of daily opioid dosages, rather than calculating dosages that vary from day to day, should reduce some non-informative variability. Figure 3-2 illustrates multiple hypothetical trajectories of opioid use patterns over time that are relevant to this study, with the vertical axis representing dose and the horizontal axis representing time. The top several trajectories depict monthly averaged dosages over the observation period from which the intended course of treatment is relatively easy to infer and that are consistent with protocols for the tapering target trial. The last trajectory demonstrates the type of variability from month to month that is often present in opioid dispensing–based measurement and from which the intended course of treatment is difficult to infer. There are likely many other trajectories than those shown, especially taking into account concurrent benzodiazepine use. The trajectories might also be stratified, for example, by pain severity over time.
___________________
2 The committee intended that treatment months would be the unit of measurement, meaning for each patient, time would be measured as 30-day periods, starting at the point of the initial medication fill relevant to the study. Of note, the most common number of days supplied of opioid fills in VA pharmacy records is 30 days.
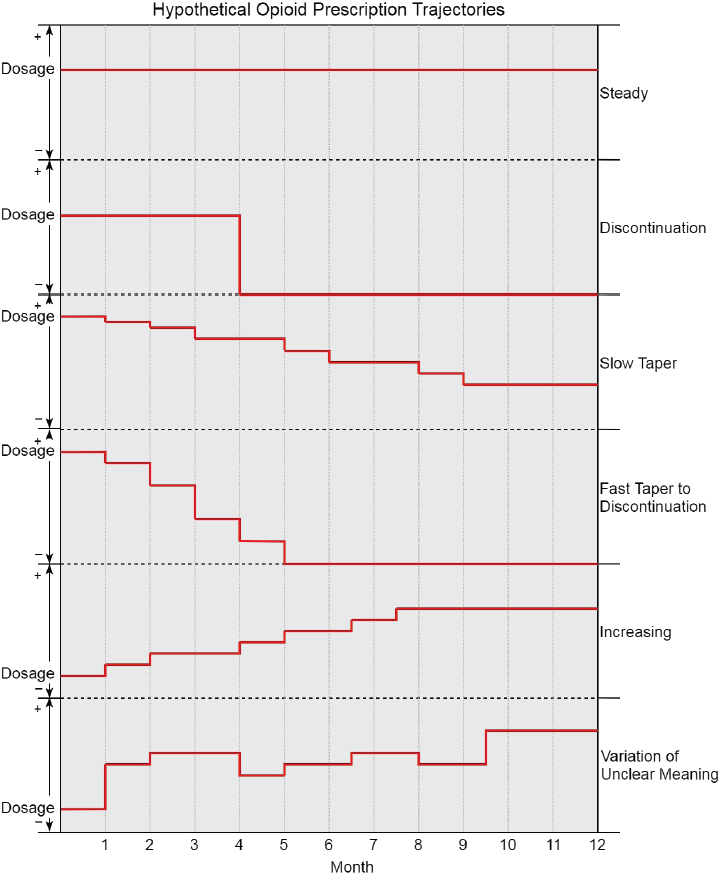
The specification of the treatment strategies for the target trial should be mindful of the various combinations of treatment strategies that a single patient might experience over time and must be realistic in order to avoid comparisons that are not relevant for clinical decision making or that are hopelessly confounded. Sensitivity analyses can assess the impact of measurement decisions on the study conclusions. For example, alternative measurements of treatment strategy may assume that medications prescribed to be taken “as needed” are taken by the patient at a faster (e.g., maximum
allowed under the prescription instructions) or slower (e.g., half as often as allowed) pace.
CONFOUNDING VARIABLES
In designing the analyses of observational data, it is important to consider confounders that may alter the impact of the intervention—opioid tapering or initiation—on the primary outcomes of all-cause mortality and suicide. Those confounders can be thought of as factors that are predictive of the receipt of a particular treatment strategy and also risk factors for one of the outcomes (Kyriacou and Lewis, 2016). Confounding, if not adjusted for, can bias or even reverse the direction of the estimate of the treatment effect. For example, if an analysis of the association between patient outcomes and treatment strategy was conducted using a dataset in which there was confounding by indication, and if the analysis failed to include a good measurement for the indication for treatment as a covariate in the analyses or to control for this confounding in some other way (e.g., limiting the analysis to individuals with the indication), the estimate of the association would be biased and possibly qualitatively incorrect. Because the proposed studies use time-dependent exposure classification (i.e., allow treatment status to vary over time), the measurement of the relevant confounders has to be considered not only at baseline but also at each visit during follow-up (see Statistical Analysis in Tables 3-1 and 3-2).
Confounders may be either observed (measured) or unobserved (unmeasured). Furthermore, a confounder may be measured with error, which then results in residual confounding. For example, a physician’s impression that a patient is misusing his or her opioids—an impression that is likely to affect the choice of treatment strategy and may be correlated with other patient characteristics that affect outcome—would be an unmeasured confounder if the physician impression is not recorded in the medical record or is otherwise unavailable to the investigator. It would be partially measured if it were recorded in some medical records but not others or if only indirect indications of the physician’s impression were available. It would be measured inaccurately, at best, because the physician’s impression about misuse is unlikely to be a fully accurate measure of whether a patient is truly misusing the opioids. Common statistical models can only adjust for measured confounders, and the adjustment will be highly dependent on the quality and consistency of the measurement of the confounder. Under strong assumptions, other statistical methods (e.g., instrumental variable analysis) may be able to adjust for unmeasured confounding, but these methods are not generally applicable to the comparison of treatment strategies sustained in time, like the ones under consideration here.
Below, a number of potential confounders are provided, both those likely to be measured and those unlikely to be measured consistently or at all. While the goal was to make this list as comprehensive as possible, the committee acknowledges that there are likely to be unanticipated confounders. For each example provided below, the committee considers how it might relate to opioid initiation and tapering and to outcomes of suicide and all-cause mortality.
Examples of Measured Confounders
Medical Illnesses
Co-existing medical illness may affect the opioid treatment approach in several ways. First, such medical illnesses as end-stage renal disease and cirrhosis can limit the safety of other analgesic options, such as NSAIDs and acetaminophen, making the use of opioids potentially more appropriate than some alternatives (Chandok and Watt, 2010; Hartmann et al., 2010; Klinge et al., 2018; O’Connor and Corcoran, 2012). Such medical illnesses as cancer, dementia, congestive heart failure, and chronic obstructive pulmonary disease may also limit patients’ prognoses, making the long-term risk of opioids a less important consideration. Therefore, medical illness is more likely to be associated with opioid initiation and less likely to be associated with opioid tapering (Platt, 2010). Medical illness is also associated with increased all-cause mortality and with suicide (Ilgen et al., 2016).
Psychiatric Illnesses
Psychiatric illnesses, including depression, are associated with a substantially increased risk of suicide in veterans (Ilgen et al., 2010). Coexisting psychiatric illness, particularly in patients with poorly controlled mood symptoms or a history of suicide attempt, increases the risk of opioid-related harms (Maloney et al., 2007). However, there is a well-documented phenomenon of adverse selection where patients with mental health and substance use comorbidities are more likely to be prescribed opioids, most likely because of clinicians’ desire to treat emotional, in addition to physical, pain (Quinn et al., 2017; Sullivan and Howe, 2013; Sullivan et al., 2005). It is also possible that psychiatric illness influences the likelihood of opioid tapering. Clinicians may be more likely to initiate tapering in response to uncontrolled psychiatric symptoms in some cases or, in other cases, less likely to initiate tapering because of the challenge of managing pain in patients with psychiatric comorbidities. Psychiatric illness is associated with both increased all-cause mortality and increased suicide risk (Chesney et al., 2014; Ilgen et al., 2016).
Opioid Misuse Behaviors
Opioid misuse behaviors include such things as running out of opioids early, missing appointments, and taking opioids for symptoms other than pain. Even if the patient does not have a diagnosable opioid use disorder, the risk of continuing opioids in the setting of those behaviors may be greater than the benefits. Some opioid misuse behaviors are able to be identified from medical chart data (Kim et al., 2012). For example, the risk may exceed the benefit if a patient has multiple episodes of lost or stolen medication, which may appear in the medical records as “early” or redundant refills (Sehgel et al., 2012). Therefore, patients with opioid misuse might be more likely to be withdrawn from opioids, and many clinics have policies that recommend tapering in those situations (Kahan et al., 2011). Due to other comorbidities such as a psychiatric illness that may contribute to opioid misuse, these patients might also have increased all-cause mortality and risk of suicide.
Substance Use Disorders
Substance use disorders (SUDs) increase the risk of opioid-related harms, particularly in patients who are of concern for active substance use (including opioid use disorder and other SUDs (Mark and Parish, 2019). However, as described above, “adverse selection” may mean that those patients are more likely to be initiated on opioids and less likely to be tapered (Merrill et al., 2012; Sullivan and Howe, 2013). These patients also have increased all-cause mortality and risk of suicide (Bohnert and Ilgen, 2019; Bohnert et al., 2010, 2017). Although SUD diagnoses are included in medical records data, it is likely that this method of measurement both misses cases and incorrectly classifies individuals as having a SUD who would not meet the diagnostic criteria for such a disorder.
History of Overdose
Whether intentional or unintentional, opioid overdose has been associated with a higher risk of future opioid overdose (Boscarino et al., 2016). It is also likely associated with tapering (Chang et al., 2018), although recent studies suggest that most patients who have had an overdose with a prescribed opioid are re-started on that same opioid (NASEM, 2017). Given the comorbidities that are more prevalent in patients with a history of prescribed opioid overdose (psychiatric illness, opioid misuse, substance use), this group likely has higher all-cause mortality and suicide risk than patients with no history of overdose. Overdoses treated outside of the VA system are unlikely to be recorded and available for measurement.
Examples of Unobserved or Poorly Measured Confounders
Clinician Perception of Pain Etiology
Although guidelines caution against this approach (Hooten et al., 2017), the perception that a patient has a “legitimate” reason to have pain (e.g., cancer, severe injury) versus pain conditions perceived as having a less clear etiology (e.g., back pain, fibromyalgia) might influence the decision to choose one treatment instead of another (e.g., opioids instead of NSAIDS and cognitive behavioral therapy) (Collett, 2001; Sluka and Clauw, 2016). Pain etiology could also relate to suicide and all-cause mortality. For example, Ilgen et al. (2013) showed that psychogenic pain, which could be viewed as non-legitimate, was associated with suicide after adjusting for other psychiatric diagnoses. Diabetes, which could be viewed as a “legitimate” cause of neuropathic pain (Schreiber, 2015), is associated with increased all-cause mortality (Cheng et al., 2016).
Pain Treatment History
Long-term opioid treatment is typically reserved for patients who have tried and failed with multiple other pain treatments (HHS, 2016). However, the “and failed” is often subjective and is not systematically captured in the medical record (Palmer et al., 2015).
Opioid Misuse Behaviors
Although some opioid misuse behaviors are easily found in the medical record (e.g., having gone to multiple clinicians for opioid prescriptions, missed appointments, illicit substance use), others are not consistently documented (angry or aggressive behavior related to opioids, asking for a specific opioid by name) (Ives et al., 2006; Palmer et al., 2015). Opioid misuse, whether documented in the patient record or not, may lead clinicians to withhold or taper opioids, and is also associated with an increased risk of overdose-related mortality (Fields, 2011).
Patient Treatment Preferences
Although participation in the proposed target trial, and thus opioid dosage reduction, would be voluntary, it is unknown what proportion of VA patients who experienced opioid tapering during the years of the proposed observational study did so voluntarily. It is important to note that tapering a patient off opioids because he or she wants to reduce the dose or discontinue opioids is likely different from tapering a patient for other
reasons, such as clinic-specific policies or prescriber concerns that a patient is diverting medications or experiencing harms from opioid use (Frank et al., 2016; Glajchen, 2001; Hadlandsmyth et al., 2018). Patient willingness to participate might be related to potential adverse events of the treatment strategies, possibly due to a correlation with other factors such as opioid misuse and mental health conditions.
STATISTICAL ANALYSIS
At baseline, patients will be assigned to the treatment strategy that is compatible with their observed data. Then the observational analog of the intention-to-treat analysis will be similar to that of the intention-to-treat analysis of the target trial (if the trial were actually conducted); that is, it will be done by comparing the outcome distributions between groups defined by the treatment strategy assigned at baseline. The main difference between how the target trial and observational analog would be analyzed is that the observational analysis will require an adjustment for baseline covariates that are imbalanced across the different treatment strategies. However, if adherence to the treatment strategy assigned at baseline is low, which often is the case with patients prescribed opioids and benzodiazepines, the intention-to-treat and per-protocol effects are likely to be very different, and the preferred estimate will depend on the goal of the analysis. As discussed in Chapter 2, clinicians and patients may find the per-protocol estimates to be more relevant in informing their individual clinical decision making (Hernán and Robins, 2017). Thus, while the intention-to-treat estimate is potentially important in informing recommendations for treatment strategies, the per-protocol effect is likely to be a more practical inferential target. The observational analog of the per-protocol analysis would be essentially identical to a non-naive per-protocol analysis of the target trial (if the trial were actually conducted). Such a per-protocol analysis will require adequate adjustment for both baseline and post-baseline (time-varying) confounders. This adjustment could be carried out via g-methods, such as the g-formula (see Keil et al., 2014; Robins et al., 2007) or inverse probability (IP) weighting (see Hernán et al., 2006; Robins et al., 2000).
If IP weighting is used, patients will be censored if and when they deviated from their initial treatment strategy. For example, consider a patient in the initiation trial who is assigned to opioid initiation at time 0 but discontinues treatment after 4 months. The patient would be then censored at month 4, whereas uncensored individuals at that time will be weighted by the inverse of their probability of remaining uncensored. Because the definition of censoring depends deterministically on the observed treatment, the denominator of the IP weights is defined by
the time-varying probability of treatment, which needs to be estimated as a function of baseline and post-baseline confounders at prior times (Robins et al., 2000).
Censored patients could become eligible once again for the target trial at a later point. A statistically efficient way to handle multiple eligibility periods in the analysis is to include a copy of a patient’s data in the analysis for each instance of multiple eligibility, assigning a different “time 0” and treatment strategy to each record that aligns with an eligibility event. This approach requires that the estimation of standard errors adjust for correlations due to repeated measures within patients (Hernán and Robins, 2016). While the committee cannot give a definitive recommendation about the final analytic strategy without pilot data, it is quite likely that this will be the preferable approach.
In the tapering trial, a patient’s data at baseline may be compatible with more than one treatment strategy. To handle this problem, a copy (clone) of a patient’s data for each possible treatment strategy compatible with the patient’s data at a given time point would be included in the analysis. Clones are then censored when their data stop being compatible with their particular treatment strategy.3
Sample Size
Assessing the magnitude of an effect that would be detectable at a given level of significance and statistical power provides context for how conclusive one might expect the analysis to be before conducting it, and it provides context for the interpretation of results. Key inputs for evaluating the adequacy of the sample size for conducting an informative analysis include the number of eligible patients per analysis, the comparability of patients across the treatment strategy groups, the expected value of the outcome, the size of a clinically meaningful effect of one treatment strategy versus another on the outcome, and the variation in the outcome. That assessment of the magnitude of an effect is illustrated by considering all-cause mortality in the context of the dosage reduction study. Krebs et al. (2011) report an all-cause mortality rate of 0.066 per patient-year in a cohort of patients with pain who were prescribed methadone or morphine and whose estimated conditional probabilities of receiving methadone versus morphine equaled or exceeded 0.10. Given that rate, the committee provides an illustration of the role of outcome prevalence on the sample size required to detect a clinically meaningful effect. Consider a simple comparison of two independent groups where the power is estimated using an independent two-sample test of proportions with continuity correction (Fleiss et al.,
___________________
3 See Hernán and Robins (2016) for further discussion.
2003). To illustrate, assume the clinically meaningful difference to detect is a difference in mortality relative risk of 1.2 between two treatment strategies. However, prior to emulating the target trial, investigators should form their own assessment of the most clinically meaningful difference in relative risks upon which to base a power calculation. To detect a relative risk of mortality of 1.2 for one group versus another with 80 percent power at alpha = 0.05 (two-sided test) would require 15,110 patients across both groups. If this sample size of patients is not available for analysis, then the emulation will be under-powered and the result inconclusive.
In such a case, one might consider whether the outcome should instead be treated as a secondary outcome. However, when the outcome is rare, large sample sizes are required to detect clinically meaningful effects. That is an even greater concern for outcomes such as suicide. Ilgen et al. (2016) found a suicide rate of 44 per 100,000 person-years in a cohort followed after an initial opioid prescription fill. Given this rate, consider again a simple independent two-group comparison where group assignment is assigned randomly and the groups are of equal size. To detect a relative risk of mortality of 1.2 for one group versus another with 80 percent power at alpha = 0.05 (two-sided test), about 2.2 million patients across both groups would be required. However, the number of patients required is likely to be larger than that, given that the treatment strategies to be compared are unlikely to be of equal size. Vanderlip et al. (2014) found 7.5 percent of patients with chronic pain who initiated long-term opioid therapy had discontinued by 90 days later. If those under the strategy of discontinuation were compared with all others, the sample sizes required would be 570,000 for the discontinuation group and 7.6 million for the group of all others. The scenario for which the committee illustrates sample size considerations is simplified relative to the proposed emulation, and considering additional factors might increase or decrease sample size requirements relative to the illustration provided here. Nevertheless, the calculations demonstrate the challenge posed by examining rare outcomes.
Limitations
The approach described in this chapter, namely the emulation of particular initiation and tapering target trials using observational data and analyses, comes with limitations that relate to the risk of bias in non-randomized studies of interventions (Sterne et al., 2016). The domains of potential bias include bias due to confounding, bias in the selection of participants into the study, bias in the classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of the reported results. Here, the committee focuses discussion on bias from measurement
(i.e., the misclassification of exposures/interventions and outcomes) and bias from confounding factors.
Measurement Bias
Misclassification of treatment strategy The classification of intervention status in both the initiation and the tapering studies is based on dispensing information from the VA’s Pharmacy Benefits Management Services. Because for most veterans co-payments are low or zero, veterans have strong financial incentives to obtain outpatient prescriptions through the VA system. The dispensing information is highly accurate (Aspinall et al., 2016) and is a step closer to ingestion than prescribing information. Thus, the exposure information, in this type of observational data, is often better than that in a randomized controlled trial, where all that is typically known is the prescriber’s intent. Nonetheless, automated prescription records indicate medications dispensed, not ingested, which may result in non-differential overestimation of ingestion, especially for symptomatic treatments, and thus a dilution of findings. In addition, the misclassification of opioid exposure due to access to illicit sources of opioids is of concern. Over-the-counter NSAIDs could also be a confounding variable, one that is more likely in the opioid exposure group.
For the tapering study specifically, the appropriate classification of the intervention categories represents a specific challenge as it requires inference of the intended treatment strategy (e.g., continuation, slow taper, fast taper, etc.) from multiple consecutive prescription fills (see Table 3-2).
Misclassification of outcomes (primary) All-cause mortality is ascertainable in the data with great accuracy. The NDI is regarded as the most authoritative source of death information for the U.S. population and includes more than 99 percent of all deaths in the United States (Lash and Silliman, 2001; Patterson and Bilgrad, 1986; Rich-Edwards et al., 1994; Sathiakumar et al., 1998). Suicide mortality, however, is likely subject to misclassification due to the misattribution of the intent of the injury causing death (Rockett et al., 2018; Stone et al., 2017).
Confounding
Prescribers and patients make decisions regarding opioid initiation and tapering strategies in light of the patient’s characteristics and preferences and the prescriber’s clinical experience. When such factors are also risk factors for the study outcomes (i.e., confounders), they might bias the results. A hypothetical example would be psychiatric conditions, which could affect the likelihood of treatment with opioids and benzodiazepines
and also the likelihood of suicide. The observational study will adjust for baseline and post-baseline confounders via IP weighting as described above. Residual confounding remains a threat to the validity of results to the extent that confounders are inaccurately measured or unmeasured (see above for a discussion of key confounding factors, including those that are likely unmeasured in the VA data). To address residual confounding, the committee proposes using quantitative sensitivity analysis for unmeasured or poorly measured confounding factors (e.g., opioid misuse behaviors) to estimate the strength of residual confounding that would be necessary to explain the observed association (Schneeweiss, 2006).
Limited Statistical Power
As illustrated above, the sample size required to conduct a test with sufficient statistical power to compare treatment strategies is very large for a low-prevalence outcome such as suicide. One must assess a priori whether there would be a sufficient number of patients in the database to detect a clinically meaningful effect when emulating the target trial.
Generalizability
The interpretation of findings from the emulation of the target trial using observational data must take into account how generalizable the study’s findings would be. For example, the proposed trial emulation would be applied to data from 2010 to 2017, so trends in opioid use and prescribing practices along with changing responses to opioid misuse and prescribing practices might reduce the generalizability of findings to future time periods. Furthermore, the study would use data from the VA population, so findings might not be generalizable beyond the VA because there are major demographic differences between VA users and other populations, with the VA population being more male and older on average than the broader population (NCVAS, 2019). Steps could be taken to enhance generalizability, such as by examining the treatment effects of interest within subgroups (e.g., by gender) or by weighting to match the demographics of the broader population (Stuart et al., 2015). Such steps are useful for enhancing generalizability with respect to the observable differences between the study population and the broader population, but they will not address unobservable differences.
CONCLUSIONS
Randomized trials are the preferred method for quantifying the causal effects of treatments on clinical outcomes. However, trials often are not
possible for a variety of reasons, such as cost, ethics, and logistics, and the results from the trials that are feasible might have limited applicability in routine clinical settings. In those cases, analyses of existing observational data can be conducted to emulate the “target trial” of interest. An explicit emulation of the target trial—the hypothetical pragmatic randomized trial that would estimate the effect of interest, if conducted—mitigates or clarifies some of the limitations of studies using observational data. The committee described the rationale, advantages, and limitations of such an approach in Chapter 2.
The committee chose to describe two hypothetical target trials and sample emulation procedures for investigating the effects of (1) opioid initiation and (2) opioid dosage tapering in patients receiving chronic benzodiazepine treatment, and the committee developed protocols and analytic strategies for those trials, while recognizing that many other studies are of potential interest. The object of the trials is to determine preferred approaches for opioid initiation or tapering strategies for patients participating in the trials, while taking into account the potential limitations of the available observational dataset.
The committee emphasizes that the examples of studies in this report are only two of the possible target trials, chosen because they not only most directly address the Statement of Task, but also are the minimum number of studies needed to address the task. Adjustments to the proposed studies would likely be necessary after examining the observational data and determining how best to approach the studies and analyze the data. Many other studies would also be of interest beyond the outcomes of mortality and suicide in the population of veterans treated with opioids and benzodiazepines. For example, standardized self-report measures of pain, social and emotional functioning, depression, anxiety, and co-prescription of other central nervous system depressant medications could be examined to determine the effects of these factors on patient functioning over time. Examining the clinical and functional outcomes of veterans prescribed only opioids or only benzodiazepines would also be informative. The VA medical record contains a wealth of clinical information that could be analyzed to determine the potential benefits, as well as risks, to patients with a wide variety of characteristics who are prescribed opioids and benzodiazepines.
The committee views the proposed analysis plans and any related investigations as an excellent opportunity to use the rich VA clinical databases to elucidate the connections among important clinical conditions, treatment outcomes, and changes in opioid and benzodiazepine prescribing practices over the years 2010–2017. Significant changes in prescribing practice occurred over that period, so comparisons of the outcomes of different treatment strategies could yield important insights into best treatment practices. For example, because of understandable concerns about
high-dosage opioid treatment, many practitioners in the United States have dramatically curtailed opioid prescribing in recent years in response to increasing rates of opioid use disorder (NASEM, 2017), yet that leaves many patients struggling to cope with chronic pain problems for which they had previously relied on opioid medication. The proposed observational studies has the potential to reveal important insights that could help health care providers improve chronic pain treatment by beginning to understand the most appropriate role of opioid treatment in a comprehensive program of chronic pain management.
REFERENCES
Aspinall, S. L., M. M. Sales, C. B. Good, V. Calabrese, P. A. Glassman, M. Burk, V. R. Moore, M. M. Neuhauser, L. Golterman, H. Ourth, M. A. Valentino, and F. E. Cunningham. 2016. Pharmacy benefits management in the Veterans Health Administration revisited: A decade of advancements, 2004–2014. Journal of Managed Care Specialty Pharmacy 22(9):1058–1063.
Bohnert, A. S. B., and M. A. Ilgen. 2019. Understanding links among opioid use, overdose, and suicide. New England Journal of Medicine 380(1):71–79.
Bohnert, A. S. B., K. Roeder, and M. A. Ilgen. 2010. Unintentional overdose and suicide among substance users: A review of overlap and risk factors. Drug and Alcohol Dependence 110(3):183–192.
Bohnert, K. M., M. A. Ilgen, S. Louzon, J. F. McCarthy, and I. R. Katz. 2017. Substance use disorders and the risk of suicide mortality among men and women in the U.S. Veterans Health Administration. Addiction 112(7):1193–1201.
Boscarino, J. A., H. L. Kirchner, J. M. Pitcavage, V. R. Nadipelli, N. A. Ronquest, M. H. Fitzpatrick, and J. J. Han. 2016. Factors associated with opioid overdose: A 10-year retrospective study of patients in a large integrated health care system. Substance Abuse and Rehabilitation 7:131–141.
Chandok, N., and K. D. S. Watt. 2010. Pain management in the cirrhotic patient: The clinical challenge. Mayo Clinic Proceedings 85(5):451–458.
Chang, D. C., J. Klimas, E. Wood, and N. Fairbairn. 2018. A case of opioid overdose and subsequent death after medically supervised withdrawal: The problematic role of rapid tapers for opioid use disorder. Journal of Addiction Medicine 12(1):80–83.
Cheng, Y. J., E. W. Gregg, D. B. Rolka, and T. J. Thompson. 2016. Using multi-year national survey cohorts for period estimates: An application of weighted discrete Poisson regression for assessing annual national mortality in U.S. adults with and without diabetes, 2000–2006. Population Health Metrics 14:48.
Chesney, E., G. M. Goodwin, and S. Fazel. 2014. Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry 13(2):153–160.
Collett, B. J. 2001. Chronic opioid therapy for non-cancer pain. British Journal of Anaesthesiology 87(1):133–143.
Fields, H. L. 2011. The doctor’s dilemma: Opiate analgesics and chronic pain. Neuron 69(4):591–594.
Fleiss, J. L., B. Levin, and M. C. Paik. 2013. Statistical methods for rates and proportions. New York: Wiley.
Frank, J. W., C. Levy, D. D. Matlock, S. L. Calcaterra, S. R. Mueller, S. Koester, and I. A. Binswanger. 2016. Patients’ perspectives on tapering of chronic opioid therapy: A qualitative study. Pain Medicine 17(10):1838–1847.
Glajchen, M. 2001. Chronic pain: Treatment barriers and strategies for clinical practice. Journal of the American Board of Family Medicine 14(3):211–218.
Hadlandsmyth, K., K. R. Stewart, M. B. Paez, M. Steffen, M. Meth, H. S. Reisinger, and H. J. Mosher. 2018. Patient perspectives on opioids: Views of inpatient veterans with chronic pain. Pain Medicine. doi: 10.1093/pm/pny136. [Epub ahead of print].
Hartmann, B., D. Czock, and F. Keller. 2010. Drug therapy in patients with chronic renal failure. Deutsches Arzteblatt International 107(37):647–656.
Hernán, M. A., and J. M. Robins. 2016. Using big data to emulate a target trial when a randomized trial is not available. American Journal of Epidemiology 183(8):758–764.
Hernán, M. A., and J. M. Robins. 2017. Per-protocol analyses of pragmatic trials. New England Journal of Medicine 377(14):1391–1398.
Hernán, M. A., E. Lanoy, D. Costagliola, and J. M. Robins. 2006. Comparison of dynamic treatment regimes via inverse probability weighting. Basic & Clinical Pharmacology & Toxicology 98(3):237–242.
HHS (Department of Health and Human Services). 2016. Draft report on pain management best practices: Updates, gaps, inconsistencies, and recommendations. https://www.hhs.gov/ash/advisory-committees/pain/reports/2018-12-draft-report-on-updates-gapsinconsistencies-recommendations/index.html (accessed July 11, 2019).
Hooten, M., D. Thorson, J. Bianco, B. Bonte, A. J. Clavel, J. Hora, C. Johnson, E. Kirksson, M. P. Noonan, C. Reznikoff, K. Schweim, J. Wainio, and N. Walker. 2017. Pain: Assessment, non-opioid treatment approaches, and opioid management. Institute for Clinical Systems Improvement health care guideline. https://www.icsi.org/wp-content/uploads/2019/01/Pain.pdf (accessed July 11, 2019).
Ilgen, M. A., A. S. Bohnert, R. V. Ignacio, J. F. McCarthy, M. M. Valenstein, H. M. Kim, and F. C. Blow. 2010. Psychiatric diagnoses and risk of suicide in veterans. Archives of General Psychiatry 67(11):1152–1158.
Ilgen, M. A., F. Kleinberg, R. V. Ignacio, A. S. Bohnert, M. Valenstein, J. F. McCarthy, F. C. Blow, and I. R. Katz. 2013. Noncancer pain conditions and risk of suicide. JAMA Psychiatry 70(7):692–697.
Ilgen, M. A., A. S. B. Bohnert, D. Ganoczy, M. J. Bair, J. F. McCarthy, and F. C. Blow. 2016. Opioid dose and risk of suicide. Pain 157(5):1079–1084.
Ives, T. J., P. R. Chelminski, C. A. Hammett-Stabler, R. M. Malone, J. S. Perhac, N. M. Potisek, B. B. Shilliday, D. A. DeWalt, and M. P. Pignone. 2006. Predictors of opioid misuse in patients with chronic pain: A prospective cohort study. BMC Health Services Research 6(1):46.
Kahan, M., L. Wilson, A. Mailis-Gagnon, and A. Srivastava. 2011. Canadian guideline for safe and effective use of opioids for chronic noncancer pain. Clinical summary for family physicians, part 2: Special populations, Canadian Family Physician 57(11):1269–1276.
Keil, A. P., J. K. Edwards, D. B. Richardson, A. I. Naimi, and S. R. Cole. 2014. The parametric g-formula for time-to-event data: Intuition and a worked example. Epidemiology 25(6):889–897.
Kelley, A. S., and E. Bollens-Lund. 2018. Identifying the population with serious illness: The “denominator” challenge. Journal of Palliative Medicine 21(S2):S7–S16.
Kim, H. M., E. G. Smith, D. Ganoczy, H. Walters, C. M. Stano, M. A. Ilgen, A. S. B. Bohnert, and M. Valenstein. 2012. Predictors of suicide in patient charts among patients with depression in the Veterans Health Administration health system: Importance of prescription drug and alcohol abuse. Journal of Clinical Psychiatry 73(10):e1269–e1275.
Klinge, M., T. Coppler, J. M. Liebschutz, M. Dugum, A. Wassan, A. DiMartini, and S. Rogal. 2018. The assessment and management of pain in cirrhosis. Current Hepatology Reports 17(1):42–51.
Krebs, E. E., W. C. Becker, J. Zerzan, M. J. Bair, K. McCoy, and S. Hui. 2011. Comparative mortality among Department of Veterans Affairs patients prescribed methadone or long-acting morphine for chronic pain. Pain 152(8):1789–1795.
Kyriacou, D. N., and R. J. Lewis. 2016. Confounding by indication in clinical research. JAMA 316(17):1818–1819.
Larochelle, M. R., J. M. Liebschutz, F. Zhang, D. Ross-Degnan, and J. F. Wharam. 2016. Opioid prescribing after nonfatal overdose and association with repeated overdose: A cohort study. Annals of Internal Medicine 164(1):1–9.
Lash, T. L., and R. A. Silliman. 2001. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology 12(2):259–261.
Lin, L., T. Peltzman, J. F. McCarthy, E. M. Oliva, J. A. Trafton, and A. S. B. Bohnert. 2019. Changing trends in opioid overdose deaths and prescription opioid receipt among veterans. American Journal of Preventive Medicine 57(1):106–110.
Maloney, E., L. Degenhardt, S. Darke, R. P. Mattick, and E. Nelson. 2007. Suicidal behaviour and associated risk factors among opioid-dependent individuals: A case-control study. Addiction 102(12):1933–1941.
Mark, T. L., and W. Parish. 2019. Opioid medication discontinuation and risk of adverse opioid-related health care events. Journal of Substance Abuse Treatment 103:58–63.
Merrill, J. O., M. Von Korff, C. J. Banta-Green, M. D. Sullivan, K. W. Saunders, C. I. Campbell, and C. Weisner. 2012. Prescribed opioid difficulties, depression and opioid dose among chronic opioid therapy patients. General Hospital Psychiatry 34(6):581–587.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Pain management and the opioid epidemic: Balancing societal and individual benefits and risks of prescription opioid use. Washington, DC: The National Academies Press.
NCVAS (National Center for Veterans Analysis and Statistics). 2019. Profile of veterans: 2017. Department of Veterans Affairs. https://www.va.gov/vetdata/docs/Quickfacts/2017_Veterans_Profile_Fact_Sheet.pdf (accessed July 18, 2019).
O’Connor, N. R., and A. M. Corcoran. 2012. End-stage renal disease: Symptom management and advance care planning. American Family Physician 85(7):705–710.
Palmer, R. E., D. S. Carrell, D. Cronkite, K. Saunders, D. E. Gross, E. Masters, S. Donevan, T. R. Hylan, and M. Von Kroff. 2015. The prevalence of problem opioid use in patients receiving chronic opioid therapy: Computer-assisted review of electronic health record clinical notes. Pain 156(7):1208–1214.
Patterson, B. H., and R. Bilgrad. 1986. Use of the National Death Index in cancer studies. Controlled Clinical Trials 7(3):249.
Platt, M. 2010. Pain challenges at the end of life—Pain and palliative care collaboration. Reviews in Pain 4(2):18–23.
Quinn, P. D., K. Hur, Z. Chang, E. E. Krebs, M. J. Bair, E. L. Scott, M. E. Rickert, R. D. Gibbons, K. Kroenke, and B. M. D’Onofrio. 2017. Incident and long-term opioid therapy among patients with psychiatric conditions and medications: A national study of commercial health care claims. Pain 158(1):140–148.
Rich-Edwards, J. W., K. A. Corsano, and M. J. Stampfer. 1994. Test of the National Death Index and Equifax nationwide death search. American Journal of Epidemiology 140(11):1016–1019.
Robins, J. M., M. A. Hernán, and B. Brumback. 2000. Marginal structural models and causal inference in epidemiology. Epidemiology 11(5):550–560.
Robins, J. M., M. A. Hernán, and A. Rotnitzky. 2007. Effect modification by time-varying covariates. American Journal of Epidemiology 166(9):994–1002; discussion 1003–1004.
Rockett, I. R. H., E. D. Caine, H. S. Connery, G. D’Onofrio, D. J. Gunnell, T. R. Miller, K. B. Nolte, M. S. Kaplan, N. D. Kapusta, C. L. Lilly, L. S. Nelson, S. L. Putnam, S. Stack, P. Värnik, L. R. Webster, and H. Jia. 2018. Discerning suicide in drug intoxication deaths: Paucity and primacy of suicide notes and psychiatric history. PLOS ONE 13(1):e0190200.
Sathiakumar, N., E. Delzell, and O. Abdalla. 1998. Using the National Death Index to obtain underlying cause of death codes. Journal of Occupational and Environmental Medicine 40(9):808–813.
Schneeweiss, S. 2006. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiology and Drug Safety 15(5):291–303.
Schreiber, A. K., C. F. Nones, R. C. Reis, J. G. Chichorro, and J. M. Cunha. 2015. Diabetic neuropathic pain: Physiopathology and treatment. World Journal of Diabetes 6(3):432–444.
Sehgal, N., L. Manchikanti, and H. S. Smith. 2012. Prescription opioid abuse in chronic pain: A review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 15(3 Suppl):ES67–ES92.
Sluka, K. A., and D. J. Clauw. 2016. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 338:114–129.
Sterne, J. A., M. A. Hernán, B. C. Reeves, J. Savović, N. D. Berkman, M. Viswanathan, D. Henry, D. G. Altman, M. T. Ansari, I. Boutron, J. R. Carpenter, A. W. Chan, R. Churchill, J. J. Deeks, A. Hróbjartsson, J. Kirkham, P. Jüni, Y. K. Loke, T. D. Pigott, C. R. Ramsay, D. Regidor, H. R. Rothstein, L. Sandhu, P. L. Santaguida, H. J. Schünemann, B. Shea, I. Shrier, P. Tugwell, L. Turner, J. C. Valentine, H. Waddington, E. Waters, G. A. Wells, P. F. Whiting, and J. P. Higgins. 2016. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Online) 355:i4919.
Stone, D. M., K. M. Holland, B. Bartholow, J. E. Logan, W. LiKamWa McIntosh, A. Trudeau, and I. R. H. Rockett. 2017. Deciphering suicide and other manners of death associated with drug intoxication: A Centers for Disease Control and Prevention consultation meeting summary. American Journal of Public Health 107(8):1233–1239.
Stuart, E. A., C. P. Bradshaw, and P. J. Leaf. 2015. Assessing the generalizability of randomized trial results to target populations. Prevention Science 16(3):475–485.
Sullivan, M. D., and C. Q. Howe. 2013. Opioid therapy for chronic pain in the United States: Promises and perils. Pain 154:S94–S100.
Sullivan, M. D., M. J. Edlund, D. Steffick, and J. Unützer. 2005. Regular use of prescribed opioids: Association with common psychiatric disorders. Pain 119(1):95–103.
Vanderlip, E. R., M. D. Sullivan, M. J. Edlund, B. C. Martin, J. Fortney, M. Austen, J. S. Williams, and T. Hudson. 2014. National study of discontinuation of long-term opioid therapy among veterans. Pain 155(12):2673–2679.




























