3
Emerging Research on Associations Between Infectious and Noncommunicable Diseases
The workshop’s first session explored the current state of the science and emerging research on the convergence of infectious diseases and noncommunicable diseases (NCDs). Part A of the first session featured three case studies that focused on emerging and novel research on the associations between infections, microbial exposure, and NCDs. The presenters identified knowledge gaps and discussed overall implications for further research and practice. They also described cutting-edge study designs and tools being used to explore emerging associations between infectious diseases and NCDs, as well as the methods used to identify causal links and their temporal relationships.
The first part of the session was moderated by Julie Parsonnet, professor of medicine and of health research and policy, Stanford University. The first presenter, Casey Lynch, chief executive officer (CEO) and co-founder of Cortexyme, Inc., provided a case study on the role of infection in Alzheimer’s disease. The case study presented by John Harley, founding director of the Center for Autoimmune Genomics and Etiology at Cincinnati Children’s Hospital Medical Center, offered a new perspective on the Epstein-Barr virus (EBV) in autoimmune and inflammatory diseases. Finally, Cathryn Nagler, professor of pathology, medicine, and pediatrics at The University of Chicago, explored the role of the microbiome in food allergies in her case study.
ALZHEIMER’S DISEASE AND P. GINGIVALIS
Casey Lynch, CEO and co-founder of Cortexyme, Inc., explored the link between Alzheimer’s disease and the bacterium Porphyromonas gingivalis (P. gingivalis). Her company has developed a novel treatment for Alzheimer’s disease that is currently in late-stage clinical testing, and she discussed the data supporting the mechanism of action for this new therapy. Alzheimer’s disease is a major public health issue affecting 5.7 million people in the United States and 37 million people worldwide. According to 2018 data from the Alzheimer’s Association, the disease is associated with an estimated economic burden of $277 billion. Lynch said that P. gingivalis—the key pathogen in periodontal disease—is an instructive example of the difficulty establishing the role of infection in chronic disease because of the long latency period between infection and symptoms.
Neurobiology of Alzheimer’s Disease
Lynch explained that over the past 30 years, drug development for Alzheimer’s disease has focused primarily on the beta amyloid plaques in the brain of patients, a strategy that has not produced effective treatments (Soscia et al., 2010; Kolata, 2016; Kumar et al., 2016; Gosztyla et al., 2018). Research into the brains of patients with Alzheimer’s disease has also revealed neurofibrillary tangles of dystrophic neurons as well as chronic neuroinflammation, microglia l activation of the immune cells, complement cascade activation in the immune system, and inflammasome activation, which is part of the defense against pathogens (Kolev et al., 2009; Cully, 2018; Newcombe et al., 2018; Dionisio-Santos et al., 2019). She explained that these inflammatory processes indicate that there could be an infection upstream of the pathology.
Evidence is converging on the hypothesis that an infection in the brain is driving the pathology related to Alzheimer’s disease, said Lynch. The focus on eliminating beta amyloid plaques has shifted to investigating why that beta amyloid is being overproduced.1 Beta amyloid was found to behave like other antimicrobial peptides that are produced in response to infection. These antimicrobial peptides share many properties with beta amyloid, including their size and their ability to form oligomers. She said that although infections can cause a wide variety of neurological diseases (such as
___________________
1Amyloid is a general term for protein fragments that the body produces normally. Beta amyloid is a protein fragment snipped from an amyloid precursor protein. In a healthy brain, these protein fragments are broken down and eliminated. Amyloid plaques are hard, insoluble accumulations of beta amyloid proteins that clump together between the nerve cells (neurons) in the brains of Alzheimer’s disease patients (BrightFocus Foundation, 2017).
neurological Lyme disease, listeria, syphilis, and HIV), an infectious disease cause of Alzheimer’s has been elusive.
Link Between Periodontal Disease and Alzheimer’s Disease
Lynch explained that about a decade ago, epidemiological and twin studies began to emerge showing a link between periodontal and Alzheimer’s disease. Tooth loss, periodontal disease, and the bacterium P. gingivalis were identified as risk factors for Alzheimer’s disease, with prospective studies suggesting that having periodontal disease in one’s 40s or 50s increased the risk for developing Alzheimer’s disease later in life (Stein et al., 2007; Kaye et al., 2010; Sparks Stein et al., 2012; Kamer et al., 2015). These studies suggested that periodontal disease was happening first—that is, it was not just a case of people losing their memory and neglecting their oral health, she reported. The biology of periodontal disease has commonalities with Alzheimer’s disease. They are both chronic, slowly progressing, age-related, degenerative diseases, featuring low-grade chronic inflammation (Abbayya et al., 2015). Lynch emphasized that epidemiology cannot prove causation, so this evidence catalyzed international research efforts into testing the hypothesis of whether P. gingivalis, a keystone in periodontal disease, could be entering the brain and contributing to the development of Alzheimer’s disease.
P. gingivalis is a common infection in the mouth—half of elderly people have symptomatic periodontal disease and 80 to 90 percent are infected with this bacterium, Lynch reported. She then explained that this common gram-negative pathogen does not stay in the mouth if a person flosses and has bleeding gums—it can enter the circulatory system. A complex interaction of factors determines whether the bacterium then enters and infects the brain. Aging is a factor that can affect the immune system and the blood–brain barrier. Although genetic risk factors do exist, only a few hundred families have true familial disease, she stated. There are at least 25 genetic risk factors that predispose people to the disease, such as ApoE4 and immune system proteins, including TREM2, microglia l protein TLR4, and CR1 (Van Cauwenberghe et al., 2015). She said that this variability in the immune system might predispose certain people to brain infiltration and infection (Cao and Zheng, 2018). Traumatic brain injury, stroke, and microhemorrhage are also risk factors for Alzheimer’s disease (Ramos-Cejudo et al., 2018).
Bacterial Brain Infiltration Triggers Alzheimer’s Disease Pathology
The bacterial load of P. gingivalis in the brain is believed to directly cause neurodegeneration, said Lynch. P. gingivalis is an intracellular, asaccharolytic
bacterium that secretes enzymes to digest host, intracellular proteins. These enzymes gradually cause cell dysfunction and ultimately death. She added that the bacteria also cause downstream immune dysfunction, enhancing the organism’s ability to infect and propagate in the brain.
Lynch presented findings to suggest that P. gingivalis brain infiltration precedes and is correlated with Alzheimer’s disease symptoms and pathology. Gingipains are the putative virulence factor enzymes secreted by P. gingivalis (de Diego et al., 2013). In animal models, inhibition of gingipains has prevented neurodegeneration, inflammation, and other pathology related to Alzheimer’s disease. The presence of P. gingivalis in Alzheimer’s disease is supported by the identification of organism-specific DNA in cerebrospinal fluid and tissue gingipains through immunohistochemistry of Alzheimer’s-affected tissue in 95 percent of patients (Dominy et al., 2019). These findings were specifically presented by a study using the University of Auckland Brain Bank’s microarrays, which are tiny samples from many different brains. The study found that 95 percent of samples from Alzheimer’s patients were positive for gingipains in the region of the brain affected by Alzheimer’s disease (Dominy et al., 2019). Some of the control samples were also found positive with gingipains but asymptomatic, which is a helpful finding because it shows carriers of the bacteria prior to symptom manifestation, and establishes a timeline of infection. If only Alzheimer’s patients were infected and all of the controls were negative, it would suggest that this is a late-stage event, she explained. However, Alzheimer’s pathology is known to begin 10 to 20 years before symptoms emerge. In these asymptomatic people, the gingipain load continues to build up in their brains—along with their Tau pathology,2 which she noted is a good correlate to cognitive decline—until they later become symptomatic.
Evidence of Causation from Animal Models
Animal models have demonstrated that P. gingivalis infection infiltrates the brain and causes Alzheimer’s-like pathology, said Lynch. One study found that oral P. gingivalis infection in mice resulted in colonization and increased production of a component of amyloid plaques in their brains (Dominy et al., 2019). In another seminal study, wild-type mice were orally infected with P. gingivalis, inducing Alzheimer’s pathology after 22 weeks (see Figure 3-1).3 After entering the brain, the bacteria caused chronic, low-grade inflammation, which triggered beta amyloid plaques, caused activated microglia, and induced Tau tangle-like neurons. Approximately 50 percent of the neurons in the hippocampus, the memory center of the brain, died
___________________
2 Tau is a protein needed for normal neuronal function.
3 These are regular mice, not transgenic mice that overexpress proteins.
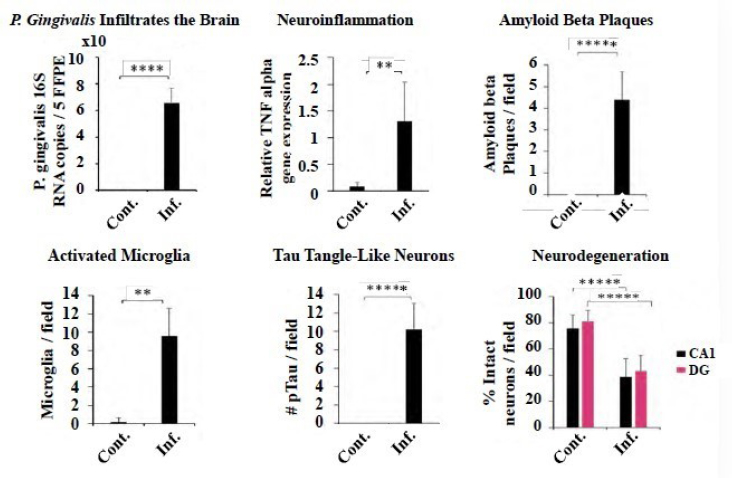
NOTES: Cont. = control; Inf. = infected; * = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001; ***** = p < 0.00001; 5FFPE = formalin fixed paraffin embedded; CA1 = carbonic anhydrase 1; DG = dentate gyrus; #pTau = Tau protein; TNF = tumor necrosis factor.
SOURCES: Lynch presentation, June 11, 2019; Ilievski et al., 2018.
(Ilievski et al., 2018). Lynch said that it is unprecedented for an animal model to mimic the characteristic pathology of Alzheimer’s disease so closely. In addition to addressing causation, the mouse model may also provide a new model for drug development that will be more translatable to human studies.
Initial Clinical Studies on Gingipain Inhibition
Based on these breakthroughs, Lynch’s company is developing a new drug, COR388, to treat patients with mild to moderate Alzheimer’s disease. COR388 is the first gingipain virulence factor inhibitor. Available data support the key role of P. gingivalis in the disease’s progression, she said. In a Cortexyme phase 1B clinical study for COR388, there were no serious adverse effects, with 20 percent of the placebo and 14 percent of the COR388 treated subjects experiencing drug-related adverse effects. She also noted that nine out of nine patients with mild to moderate Alzheimer’s disease were found to have P. gingivalis DNA in their cerebral spinal fluid
(Dominy et al., 2019). She reported that a separate observational study found that all 50 patients with mild to moderate disease were also positive for P. gingivalis DNA fragments. Lynch said that these studies reinforce the prevalent immunohistochemistry data that P. gingivalis is highly prevalent in a well-diagnosed Alzheimer’s population.
Lynch presented more data suggesting that gingipain inhibition shows beneficial effects upstream of neurodegeneration, inflammation, and other pathology. As part of the drug development process, researchers are developing biomarkers to track chronic P. gingivalis infection in the central nervous system and understand the effect of treatment. Cortexyme’s clinical study found decreases in inflammatory biomarkers in the blood after a 28-day treatment with the COR388 molecule, as well as changes to fragmentation of important proteins in the cerebral spinal fluid (Dominy et al., 2019). The hope is that the clinical drug COR388 could reduce the fragmentation of proteins in the brains of Alzheimer’s patients, thus preserving the integrity of the brain proteins.
She said preliminary exploratory cognitive testing of a small group of patients treated with COR388 for 28 days has suggested a trend to benefit or significant cognitive benefit. Although the ultimate goal of the intervention was to level off decline by blocking any further neurodegeneration caused by the bacteria, the investigators found potential improvement that is consistent with the mouse studies, in which COR388 reduced inflammation quickly. Neurons cannot be revived after they are dead, she noted, but the immune response is adaptable to any infection, including P. gingivalis. The mouse and clinical studies suggest that treating the infection can potentially make existing but dysfunctional neurons become functional again. She said that an ongoing international phase II/III study is currently enrolling, with top-line data expected by the end of 2021.
EPSTEIN-BARR VIRUS IN AUTOIMMUNE AND INFLAMMATORY DISEASES
John Harley, founding director of the Center for Autoimmune Genomics and Etiology at Cincinnati Children’s Hospital Medical Center, provided a genomic perspective on EBV in autoimmune and inflammatory diseases. EBV infects more than 95 percent of the adult human population (Cohen, 2000).4 EBV is a gamma-herpes virus that is transmitted through saliva and breastfeeding, infects the tonsils, and then disseminates to other parts of the body (Moss et al., 2007). He explained that in low- and middle-income countries, the virus tends to be acquired during the first 2 years of life. In middle- and
___________________
4 This has been established most convincingly in children, who have a far lower rate of the virus than adults.
upper-income countries, approximately 70 percent of people acquire the virus during adolescence (Balfour et al., 2013). In the latent phase, the virus avoids and suppresses immune responses. Harley said that everyone who is infected with EBV is suppressing cancer—an EBV-transformed B cell that is in latency III of the virus (Saha and Robertson, 2019).5
Diseases Attributed to Epstein-Barr Virus
Many diseases have been attributed to EBV, said Harley. For instance, lupus, an idiopathic inflammatory disease affects an estimated 200,000 people in the United States and millions of people worldwide (Rees et al., 2017).6 The molecular mimicry process that causes lupus may originate from humoral responses to EBV nuclear antigen 1 (Harley and James, 2006). Virtually all patients with lupus are infected with EBV, Harley said. However, most adults infected by EBV are asymptomatic or only have upper respiratory illness (Cohen, 2000). Approximately 3 percent of people develop mononucleosis from the virus—typically during adolescence. The virus lives in the memory B cells and then comes out of those memory B cells slowly during life (Tracy et al., 2012). EBV is associated with nearly all cases of multiple sclerosis, although the mechanism is yet unknown (Guan et al., 2019). It is also linked to almost all cases of nasopharyngeal cancer, which is highly prevalent in Southeast Asia (Cohen, 2000). Approximately one-third of cases of Hodgkin’s lymphoma are attributable to EBV (Massini et al., 2009). EBV is also associated with carcinoma of the stomach, diffuse large B cell lymphoma activated cell type, immunoblastoid B cell lymphoma (particularly in people who are immunocompromised), leiomyosarcomas, T-cell and nasal natural killer lymphomas, and Burkitt’s lymphoma (Okano and Gross, 2012).
Genomic Perspective on the Origin of Lupus
Harley explained that research is ongoing into the origins of lupus, from both environmental and genetic perspectives. Genetics provides an indication, or a tag, for mechanisms that operate to create disorders such as lupus, he stated. The most powerful tool for this work is a genomewide association study (GWAS), which compares large numbers of cases and controls to identify hundreds of thousands of markers across the human
___________________
5 Depending on the viral gene expression pattern, primary EBV infection establishes three types of latent infection statuses. Type III latency occurs when most latent genes are expressed (Kang and Kieff, 2015).
6 Women are 10 times more likely to have lupus than men; it is 3.5 times more frequent among people who are African American and of African ancestry.
genome (Welter et al., 2014). A concept in which genes related to disease susceptibility are localized or identified, called linkage disequilibrium blocks, are used to identify causal associations among many possible candidates (Robinson, 1998; Wangler et al., 2017). An estimated 40,000 of these blocks have been identified for almost 2,000 phenotypes in the human population, which are available in the online GWAS catalog, Harley stated. Researchers studying lupus have spent two decades working on these genes, he added. Meta-analyses of lupus genetic associations have determined that there are dozens of individual genetic mechanisms in the genome that alter risk for lupus (Langefeld et al., 2017).
Genetic and environmental factors converge in working toward a unified understanding of the etiology of lupus, said Harley. The aim is to find a parsimonious way to unify what is known about lupus into a conceptual construct that can be used to develop better diagnostics, therapeutics, prevention, and prognosis. A primary research aim is to identify the mechanism through which EBV causes lupus, based on the assumption that both genes and the environment contribute to the origins of disease. Population-level genetic factors, such as natural selection, have been suggested to change lupus allelic frequency, but there has not yet been insight into that mechanism (Ramos et al., 2014). GWAS studies are consistent in showing that an estimated 90 percent of the polymorphisms associated with lupus are regulatory, so research is looking for a regulatory type of explanation in the phenotypes that have been studied (Deng and Tsao, 2014). It has also been observed that EBV makes its own transcription factors, which are regulators of gene expression (Harley et al., 2018).
Harley and colleagues predicted that the transcription factors made by EBV are concentrated in the lupus genetic loci. He said that about 136 genes have now been identified using a procedure called chromatin immunoprecipitation7 followed by next-generation sequencing (ChIP-Seq) (Szalkowski and Schmid, 2011). This procedure involves creating an antibody against the transcription factor and analyzing it after it immunoprecipitates (i.e., isolating the antigen using a specific antibody that binds to it) to determine the loci where the transcription factor shares a variant with the disease. Looking at 1,544 human datasets, they found that the co-factor for transcription EBV nuclear antigen 2 (EBNA-2) binds to half of the lupus loci (Harley et al., 2018). They also identified a regulatory cluster that is hypothesized to be EBV related, suggesting that the EBV-infected B cell is the only cell type in which this binding happens—this is not observed in B cells that are not EBV-infected or in any other cell type. Therefore, they concluded that the evidence suggests a substantial proportion of the EBV risk for lupus comes from the EBV-infected B cells that live in the patient.
___________________
7 A technique used to identify physical interactions between proteins and DNA in cells.
Applying the Genomic Approach to Other Conditions
Harley explained that genome informatics approaches also apply to other conditions attributed to EBV, including multiple sclerosis, rheumatoid arthritis, and type 1 diabetes. Based on evidence linking these diseases to EBV, it is now possible to look at all diseases that qualify for the analysis against the transcription factors with ChIP-Seq datasets. Analysis suggests that chronic lymphocytic leukemia has an EBV story, with EBV nuclear antigen 3-C LP and EBNA-2 all clustering with chronic lymphocytic leukemia genes (Kim et al., 2017). He said that vitiligo has also been recently added to this group; in some instances, it shows allelic specificity consistent with the possibility that this is actually a genetic mechanism (Harley et al., 2018). Similar work can be done on diseases that are not EBV related, but have a similar regulatory story, such as breast cancer and coronary artery disease. He said that research is continuing to add a substantial number of disorders that are potentially related to EBV infection.
ROLE OF THE MICROBIOME IN FOOD ALLERGIES
Cathryn Nagler, professor of pathology, medicine, and pediatrics at The University of Chicago, focused her presentation on the role of the microbiome in food allergies. She opened by describing the scope of the food allergy epidemic and the dramatic increase in prevalence of food allergies (Gupta et al., 2018, 2019). An estimated 32 million people in the United States now have food allergies, including 1 in 10 adults and 1 in 13 children (Gupta et al., 2018, 2019; FARE, 2019). More than half of adults and more than 40 percent of children with food allergies have experienced a severe reaction (Gupta et al., 2018, 2019; FARE, 2019). Between 2007 and 2016, insurance claims with diagnoses of anaphylactic food reactions increased by 377 percent (FAIR Health, 2017; FARE, 2019). She said that to explain such a dramatic generational increase in food allergies, researchers turned to the microbiome. She defined the microbiome as the microbes that populate a person’s skin and mucosal surfaces, which can have beneficial functions for health. Nagler explained that while the microbiome consists of all classifications of micro-organisms, bacteria tend to receive greater focus because they are understood most clearly at this point.
Effect of Modern Lifestyle Factors on the Microbiota
Nagler presented the hypothesis that modern industrialized lifestyle factors trigger shifts in the composition of our commensal8 microbiota in ways
___________________
8Commensalism refers to the association between two organisms in which one benefits and the other is neither harmed nor benefits.
that are detrimental to human health (Iweala and Nagler, 2019). There is some evidence linking NCDs such as obesity, diabetes, autism, inflammatory bowel disease, and many other diseases to changes in the microbiome (West et al., 2015). Multiple lifestyle factors contribute to altered commensal microbial biodiversity, she said. Antibiotic use, including both prescribed antibiotics and antibiotics in our food and water supply, is one of the biggest lifestyle culprits (Langdon et al., 2016). The prevalent high-fat, low-fiber diet is also a significant factor in shaping the microbiome (West et al., 2015). Humans and our microbiota have coevolved over millennia, Nagler said, and human ancestors consumed a diet that was high in fiber, low in processed foods, and low in sugar. “Our bacteria eat what we eat, and we’ve changed their food source,” she said. Another factor she suggested is the transition from rural and suburban living, in which people were in close contact with bacteria from the environment, to living in sealed houses in urban settings. Vaccine-induced immunity and reduced exposure to infection is a modern lifestyle factor that may help explain why NCDs have increased as the prevalence of infectious disease has decreased, according to Nagler. Yet another factor Nagler suggested is the eradication of previously common enteropathogens, like Helicobacter pylori and gastrointestinal helminths. She proposed that in a setting of genetic susceptibility, these lifestyle factors are driving up the prevalence of food allergies.
Colonizing Healthy Microbiota to Protect Against Allergic Response
To address the increasing prevalence of food allergies, Nagler and her colleagues have collaborated with a group at the University of Naples that provided fecal samples from 20 healthy infants and 20 infants with cow’s milk allergy (CMA) (Berni Canani et al., 2016).9 At 4 to 5 months of age, samples from the healthy infants showed the expected type of bacteria, which were dominated by yogurt-type bacteria—conventional probiotics, Lactobacillales, and Bifidobacteria. In the CMA infants, those types of bacteria were depleted; instead, they had an adult-type microbiota, suggesting that the normal ecological succession that populates microbiota from birth through the first few years of life had been accelerated (Berni Canani et al., 2016).
To study the interactions of the bacteria with the host, Nagler and colleagues created a germ-free mouse model to investigate whether mice colonized with a healthy microbiota would be protected against food allergy.10 She explained that this is a powerful methodology because it allows investigators to identify specific populations of bacteria, then precisely control
___________________
9 She noted that this study population was a much more homogeneous population than would be seen in a clinic in the United States in terms of racial, ethnic, and dietary diversity.
10 In a germ-free mice facility, mice are housed in flexible film isolators to ensure sterility.
and introduce them into groups of mice. They collected fecal samples from four healthy donors and four CMA donors. All of the donors were 6 months of age and were controlled for all identified demographic variables. Each donor’s sample was contained in its own isolator to keep the microbiomes of each donor intact. The first experiment involved transferring fecal samples from breastfed infants, but the translation of the fecal material from the humans into the mice was imperfect because of the presence of the breast milk–dominated bacteria, Bifidobacteria. To improve the transfer of human bacteria into mice, they used formula-fed human donors and fed the mice the same formula the infants were consuming. The results from 100 gnotobiotic mice demonstrate that the mice colonized with the healthy infant microbiota were protected from an allergic response to food. The mice that received microbiota from CMA donors were susceptible to anaphylaxis, as were the germ-free mice to a lesser extent. The anaphylaxis correlated with the induction of an antigen specific to bovine lactoglobulin-specific immunoglobulin E (IgE), bovine lactoglobulin-specific IgG1, and mucosal mast cell protease-1 response (Feehley et al., 2019).
The research team then identified the bacterial populations and discovered that differentially abundant operational taxonomic units (OTUs), or bacterial sequences, can distinguish mice colonized by the healthy versus CMA donors (Feehley et al., 2019). Nagler said that nine OTUs significantly correlated with genes upregulated in the ileum of healthy or CMA-colonized mice (Feehley et al., 2019). Three were from the family Lachnospiraceae, which had been previously identified as allergy protective. The closest match for the protective Lachnospiraceae OTUs was Anaerostipes caccae (A. caccae), which produces a butyrate found in the human infant gut.
Mice monocolonized with A. caccae were protected against an allergic response to food using the same parameters they had previously measured. Another study demonstrated that another species of bacterium from the Clostridia class (which contains the Lachnospiraceae) helps to protect against colonization with bacterial pathogens early in life (Velasquez-Manoff, 2015). The picture that emerges from these studies is that bacterial populations operate like the gut’s “peacekeepers” (see Figure 3-2). Nagler explained that bacteria such as Anaerostipes and Faecalibacterium prausnitzii ferment dietary fiber to make short-chain fatty acids, including butyrate, which contribute to maintaining the healthy epithelial barrier function. Specifically, the epithelial barrier needs an intact mucous layer to keep opportunistic microbes and toxins in food from gaining access to the rest of the body and causing disease.
Potential Microbiome-Modulating Therapeutics
Microbiome-modulating therapeutics could help to prevent and treat allergic or inflammatory disease, said Nagler. She is part of an academic
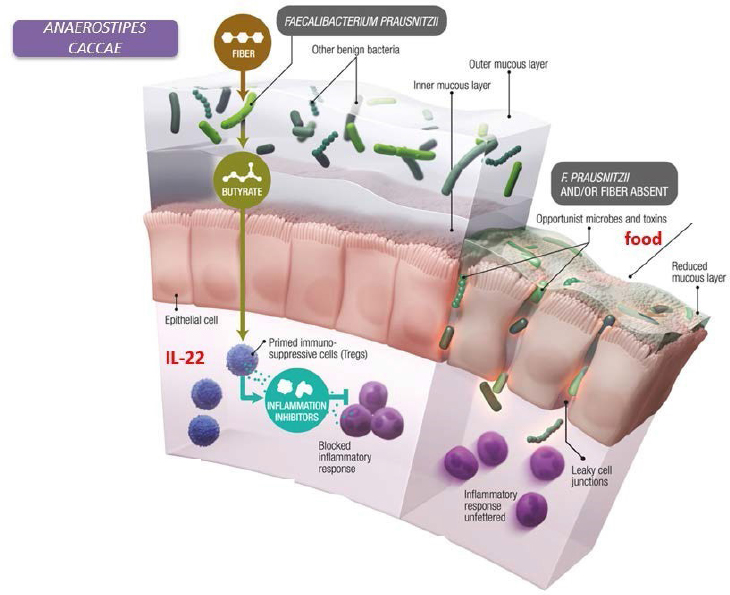
NOTE: IL-22 = Interleukin 22.
SOURCES: Nagler presentation, June 11, 2019; Bollrath and Powrie, 2013; modified from Velasquez-Manoff, 2015.
startup (ClostraBio) that is working to engineer synthetic drugs to mimic the protective function of healthy bacteria. The first approach is to make synthetic polymers to confer stability, solubility, and control of active ingredient release. To this end, ClostraBio is developing polymers that have conjugated microbial metabolites. The first candidate is butyrate; however, they are also considering live biotherapeutic approaches and prebiotic dietary fibers to present bacteria with their substrate and all of the components they need to be able to optimally produce the metabolites.
DISCUSSION
Julie Parsonnet, professor of medicine and of health research and policy, Stanford University, thanked the presenters and opened the floor for questions. The discussion further delved into issues specifically related to P. gingivalis and Alzheimer’s disease, and food allergies and the microbiome.
P. gingivalis Infection and Alzheimer’s Disease
Srinath Reddy, president of the Public Health Foundation of India, noted that the process of amyloid formation and Tau tangles are generally understood to be protective mechanisms that evolve against multiple microbial threats, not specific to P. gingivalis. For instance, studies have suggested that people treated for herpes virus are better protected against dementia than people who are exposed to herpes virus but not treated. He asked Lynch if antimicrobial peptide response could be an evolutionary protective mechanism. Lynch replied that this response certainly is a protective mechanism and not specific to one pathogen. She noted that the constellation of data around P. gingivalis is compelling with respect to causation, but more work is needed around other pathogens, and such hypotheses need to be tested in human clinical studies to progress beyond basic science and make a difference for patients.
Kent Kester, vice president and head of translational science and biomarkers at Sanofi Pasteur, asked whether antibiotics would be an effective treatment for Alzheimer’s disease as related to intracellular infections by P. gingivalis. Lynch replied that it has been well studied in the dental field that P. gingivalis cannot be eradicated with broad-spectrum antibiotics because it is persistent, often resistant to antibiotics, exists inside biofilms inside cells, and is gram-negative. As result, it is a chronic recurring infection. Virulence factor inhibitors work against P. gingivalis by blocking the toxicity of gingipains. Even though the immune response to amyloid-beta characteristic of Alzheimer’s disease is probably not specific to P. gingivalis, Lynch and her research team believe that the constellation of pathology it produces is tied to P. gingivalis and the activity of these gingipains. Therefore, they believe that the fragmentation of intracellular proteins in neurons is a gingipain-dependent effect. Blocking the gingipain toxicity renders the bacteria completely benign and staves off the P. gingivalis bacteria. This strategy represents a new type of approach to infection that has novel benefits in terms of both adverse events and antibiotic resistance.
Jay Varma, senior advisor at the Africa Centres for Disease Control and Prevention, asked if there are any epidemiologic associations between populations with different levels of dental hygiene and the occurrence of Alzheimer’s disease or if it is so persistently difficult to eradicate that it may not even correlate to dental caries or other issues. Lynch responded that such an analysis is not very feasible, given the large number of variable factors related to having periodontal disease and Alzheimer’s. She clarified that the contention is not that periodontal disease causes Alzheimer’s—people can get Alzheimer’s without having ever had symptomatic periodontal disease—but it is the same infection in two different organs.
Lynch emphasized that it is not yet known if treating periodontal disease will reduce the risk of Alzheimer’s disease, because it is difficult to treat
the infection and inflammation well enough to test the hypothesis. Once an infection is established, the treatment itself could also cause translocation of the bacteria; the effect of reducing the bacterial load in the mouth versus moving it around the body is not yet understood. However, because the existing epidemiology suggests that a high bacterial load in the mouth is one of the risk factors for Alzheimer’s disease (Harding et al., 2017), good oral health and preventive oral care beginning in childhood are likely to be beneficial.
Harley asked Lynch if their drug has been tested for periodontal disease. Lynch replied that it has been tested in a mouse model and a naturally occurring dog model that found efficacy in periodontal disease. They are carrying out a sub-study of the Alzheimer’s study to assess efficacy in periodontal endpoints. There is potential for FDA approval for periodontal disease, because it is a chronic recurring disease with an unmet medical need.
Food Allergies and the Microbiome
On the topic of microbiome, Kester asked if repopulating the gut with butyrate-producing bacteria would be a feasible approach. Nagler replied that it is probably not possible, because repopulation is difficult. A patient with a food allergy has a dysbiotic—but complete—microbiome, so there is no niche in which to introduce new bacteria—hence her team’s microbial metabolite approach. They have found that one of their drugs under development induces antimicrobial peptides in the small intestine, which may have a microbiome-modulating capability. Starting to modulate the microbiome with fiber and perhaps live therapeutics could begin to create a foothold that would allow it to repopulate to a certain extent, she said.
Varma asked if the so-called gut peacekeepers are exclusively acquired in the birth canal or if they can be acquired at some point during infancy. Nagler explained that Clostridia are obligate anaerobes that are acquired from the environment; they are highly oxygen sensitive and are predominantly spore formers. There is an association with mode of birth and susceptibility to disease, with higher rates of disease in children that are born by cesarean section (Neu and Rushing, 2011). However, the bacteria acquired at birth are Lactobacilli from the mother’s vaginal tract, while babies born by cesarean section have skin-derived bacteria as their founder bacteria. How long that founder effect persists is still a matter of debate, she said.
Finally, Parsonnet asked if presenters anticipate that patterns of food allergies, lupus, or Alzheimer’s disease will begin to affect low- or middle-income countries as they have higher-income countries. Nagler replied that the prevalence of food allergies in China and Japan has been increasing rapidly over the past decade or so, possibly caused by the lifestyle factors of diet and overuse of antimicrobials (Loh and Tang, 2018). She noted that in
the United States, the age of introduction of allergenic foods contributes to driving the increase, which may not be affecting other populations. Harley responded that in the case of multiple sclerosis, the age of infection is a contributor. People who get a serious EBV infection during their teenage years have an increased risk of between two- and seven-fold of multiple sclerosis (Endriz et al., 2017). Humans’ primitive ancestors tended to acquire the virus in the first few years of life, which appears to be more of a T-cell response than the humoral response that tends to happen later in life.
This page intentionally left blank.
















