6
Evaluating Clinical Practice Guidelines for Prescribing Opioids for Acute Pain
In Chapter 4 the committee proposed an analytic framework that professional societies, state and federal policy makers, health care systems, payers, and key stakeholders could consider when developing evidence-based clinical practice guidelines (CPGs) for prescribing opioids for acute pain associated with surgical or medical indications. The analytic framework is for opioid prescribing strategies only and is based on the assumption that a clinician has already determined that opioids are needed for acute pain management. However, this framework does not exclude the consideration and use of nonopioid options, both pharmacologic and nonpharmacologic, with or without opioid analgesics.
In Chapter 5 the committee identified priority surgical and medical indications for which CPGs should be developed or improved. These indications are associated with acute pain episodes and were prioritized according to the prevalence of the indication—which was used as a proxy for the indication’s public health impact—and evidence that opioids play a role in acute pain management for these indications. In addition, the committee ascertained whether evidence-based CPGs were publicly available for any of the indications. If a CPG did not exist, other forms of guidance were considered.
In this chapter, the committee addresses its task of evaluating existing opioid prescribing guidelines for acute pain for selected indications from Chapter 5, against the analytic framework presented in Chapter 4. To do this, the committee identified seven indications—three surgical procedures and four medical conditions—that have public health impact, have some guidance and evidence regarding opioid prescribing, and were different in scope and context, to determine how the analytic framework might be applied to a range of indications that affect different populations. The three surgical procedures—cesarean and vaginal delivery, third molar (wisdom tooth) extractions, and total knee arthroplasty (TKA)—and the four medical conditions—renal stones, migraine headaches, low back pain, and sickle cell disease—differentially affect children, adolescents, adults, older populations, women of reproductive age, and minority populations. Evaluating any CPGs and other existing guidance chosen for each indication allowed the committee to identify opportunities for data optimization and research gaps for prescribing opioids.
The committee recognized that its task is predicated on the determination that opioids will be prescribed for acute pain for a given indication. However, in clinical practice the decision to use opioids
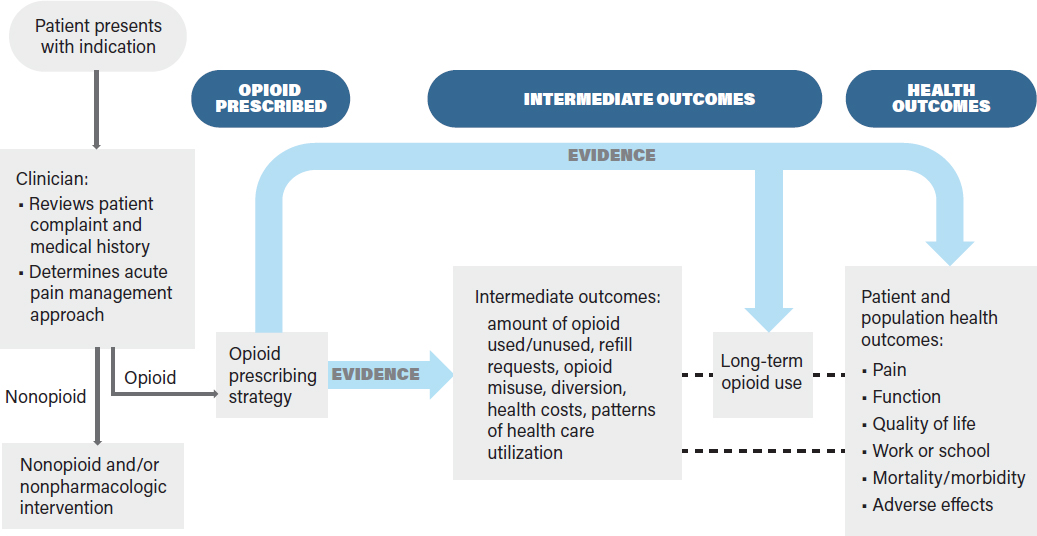
for acute pain often is made in the context of a comprehensive treatment plan tailored to an individual patient. Ideally, such a treatment plan considers the patient’s health status (obtained from a patient interview and review of the patient’s health record), including pre-existing conditions, comorbidities, prior reactions to opioids and other pharmaceuticals, treatment preferences, and the availability of and access to all recommended treatments. The comprehensive treatment plan for acute pain may include opioids alone or in conjunction with nonopioid and nonpharmacologic treatments prior to, concurrent with, or following the use of opioids. These other treatments may include heat, ice, physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), massage, and acupuncture, among others, depending on the specific indication and patient preferences. Patient education may also occur prior to prescribing opioids to ensure the patient understands his or her risks and benefits and is able to take the drug as prescribed. To acknowledge this need for a comprehensive treatment plan, the committee added the need for the clinician to consider the patient’s medical history and to develop an acute pain management approach to its analytic framework, as shown in Figure 6-1. A CPG would consider evidence for all aspects of Figure 6-1 in order to provide an accurate and effective recommendation on opioid use and dosing for the treatment of acute pain for the indication. Should the opioids not provide the expected pain relief or if unexpected adverse effects occur, the clinician may reevaluate the patient to determine if the diagnosis is correct and if other treatments are warranted.
APPLYING THE ANALYTIC FRAMEWORK TO SELECTED SURGICAL INDICATIONS
The committee selected three surgical procedures on which to apply its analytic framework: cesarean and vaginal delivery, third molar (wisdom tooth) dental extractions, and TKA. These indications were
selected because they represent varied patient populations (e.g., women, adolescents, and older individuals) and are performed in different settings (e.g., inpatient or outpatient care). Moreover, across these procedures the majority of patients undergoing them are prescribed opioids for immediate postsurgical pain. Access to care and prescribed opioids may also vary for each of these procedures, depending on the patient’s comorbidities, finances, health insurance, geography, and the care provider.
Cesarean and Vaginal Delivery
Childbirth is the most common reason for hospital admission and the most common procedure in the United States, with 3,855,500 births in 2017 (CDC, 2017a). Of these, approximately 32% (1,233,760) are cesarean deliveries and 68% are vaginal deliveries (2,621,740); of the latter, it is estimated that about 9% will have a severe perineal laceration (ACOG, 2016). In one study, opioids were prescribed for 86.7% of 3,288 women who delivered by cesarean delivery, with a median dose of 300 morphine milligram equivalents (MMEs) (interquartile range, 200–300); of the women who had a vaginal delivery, 30.4% were prescribed opioids at discharge with a median dose of 200 MMEs (interquartile range, 120–300) (Badreldin et al., 2018b). The amount of opioids prescribed for either delivery did not vary between women with a pain score of 0 of 10 and those with a pain score greater than 0 of 10 immediately prior to discharge. Bateman et al. (2017) found that among 720 women admitted to a hospital for cesarean delivery, 615 (85.4%) filled a discharge prescription for opioids. Mills et al. (2019) examined opioid prescribing data at discharge for women with uncomplicated vaginal delivery and found that almost 30% received opioids on the day of discharge; by contrast, Komatsu et al. (2018) found that fewer than 10% of women with vaginal deliveries used opioids after discharge. Compared with women in other countries, including Canada, Germany, and Sweden, patients in the United States were far more likely to receive opioid prescriptions after vaginal and cesarean delivery (Wong and Girard, 2018).
The committee chose childbirth as a priority procedure because of the prevalence of the procedure, the prevalence of opioid prescribing, the evidence of over-prescribing (Badreldin et al., 2018a), and the risk of persistent use of opioids after discharge (Peahl et al., 2019). The committee also notes that there is the potential for exposure of infants to opioids through breast milk (Ito, 2018). The committee applied its analytic framework to vaginal and cesarean deliveries to highlight how standardized methodology for CPG development may help identify the most effective opioid prescribing strategies along with the intermediate and health outcomes that may be associated with that prescribing.
Opioid Prescribing Guidelines
Although there is no evidence-based guidance that is labeled as a CPG and addresses opioid prescribing after vaginal or cesarean delivery, the American College of Obstetricians and Gynecologists’ (ACOG’s) Committee Opinion on Postpartum Pain Management makes a number of recommendations on the use of acetaminophen and NSAIDs, reserving opioid use for breakthrough pain (ACOG, 2018). The opinion recommends a shared decision-making model to optimize pain control and minimize unused opioid pills (ACOG, 2018).
The ACOG opinion paper (2018) provides a synopsis of the evidence that the authoring committee used to reach its recommendations, but this committee does not consider the opinion paper to be a CPG and recognizes that it is not intended to be. The ACOG committee collaborated with representatives of the American College of Nurse-Midwives and the American Academy of Family Physicians in developing its opinion paper. A conflict of interest statement is included.
In addition to the opinion paper, ACOG frequently publishes a number of practice bulletins, which are “evidence-based documents that summarize current information on techniques and clinical management issues.”1 To date, none of the bulletins apply to opioid prescribing at discharge after cesarean or vaginal delivery. The ACOG practice bulletins, unlike the committee opinions, address specific questions and have an in-depth presentation of supporting evidence for recommendations and conclusions. The recommendations are rated as Level A (good and consistent scientific evidence), Level B (limited or inconsistent scientific evidence), or Level C (primarily consensus and expert opinion). The practice bulletins also contain brief synopses of the literature search and discussions of how the subsequent articles were reviewed. For example, they were reviewed and evaluated for quality according to the method outlined by the U.S. Preventive Services Task Force (USPSTF) (see ACOG, 2016). In contrast, the recommendations in the committee opinions are not rated, nor is there any information as to how the evidence was reviewed or obtained. In the sections below, this committee considers what evidence gaps need to be addressed to develop evidence-based CPGs for vaginal and cesarean deliveries.
Patient Populations
Patients who undergo cesarean or vaginal delivery may experience a variety of types and intensity of pain during the early postpartum period. The AGOC committee opinion paper distinguishes pain management for vaginal versus cesarean deliveries. Special consideration is given to women who experience postpartum pain while breastfeeding, have opioid use disorder, have chronic pain, or are using other medications or substances that may increase sedation. Clinicians are referred to an AGOC committee opinion on opioid agonist pharmacotherapy for women with opioid use disorder, the Centers for Disease Control and Prevention (CDC) CPG on chronic pain (Dowell et al., 2016), and the National Academies report on pain management, which has information for women with chronic pain (NASEM, 2017). Considerations regarding opioid prescribing for other pre-existing or comorbid conditions are not discussed, although the ACOG committee recognizes that the range of health and socioeconomic statuses among women who give birth may require opioid prescribing be modified to address an individual’s physical and mental health, comorbidities, and home environment.
ACOG also has a committee opinion paper on Opioid Use and Opioid Use Disorder in Pregnancy (ACOG, 2017), which briefly discusses the use of opioids in postpartum women who used opioids during pregnancy, with a focus on breastfeeding. The opinion paper distinguishes among women who use opioids for medical reasons, who misuse opioids, and who have untreated opioid use disorder. ACOG also acknowledges that women who are ultra-rapid metabolizers of codeine may require close monitoring from their clinicians.
Most women who give birth are opioid naïve, having not filled an opioid prescription in the year prior to delivery, but they may have had varying degrees of opioid exposure prior to pregnancy. In addition, women may have various risk factors for prolonged opioid use following delivery, including pain disorders, mood disorders, and a history of substance use disorders (NIH, 2017; Osmundson et al., 2019; Sanmartin et al., 2019a,b).
Nonopioid Pain Management Strategies
The ACOG committee opinion paper recommends a stepwise nonopioid approach to postpartum pain management after vaginal or cesarean births, including both pharmacologic and nonpharmacologic
___________________
1 See https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins-List (accessed August 28, 2019).
therapies. For vaginal births, the first step is acetaminophen or an NSAID. ACOG states that the most common sources of acute pain following vaginal delivery are breast engorgement, uterine contractions, and perineal laceration, which may be treated with nonpharmacologic modalities and mild anti-inflammatory analgesics.
ACOG does not comment on the use of either acetaminophen or NSAIDs as a first step for cesarean births.
Opioid Prescribing Strategies
The 2018 ACOG committee opinion paper states that if analgesics are insufficient for pain management following a vaginal birth, then milder short-acting opioids in combination with acetaminophen may be an effective second step for pain control while the woman is in the hospital (ACOG, 2018). It further states that using an NSAID and acetaminophen simultaneously on a set schedule with milder opioids, if needed, is preferred over opioid/acetaminophen combinations (two pills containing a maximum dose of 325 mg acetaminophen, administered every 4–6 hours) for vaginal births and cesarean deliveries. Overall, oral opioids should be reserved for breakthrough pain.
With regard to opioid use for postoperative pain following cesarean delivery, the ACOG committee opinion recommends the use of neuraxial opioids supplemented by oral acetaminophen, NSAIDs, opioids, and opioids in combination with either acetaminophen or an NSAID, but it does not specify if this includes pain control at discharge. Oral opioids should be reserved for breakthrough pain (ACOG, 2018). ACOG does not identify the number of pills or duration of opioid treatment to be prescribed at discharge, although it acknowledges that over-prescribing has been documented. It cautions that under-prescribing and inadequate pain control are also of concern and are best approached on an individualized basis. Finally, the ACOG opinion paper recommends that if an opioid is prescribed for postpartum pain, the duration should be limited to the “shortest reasonable course expected for treating acute pain” (p. e39).
The committee notes that a number of opioid prescribing strategies have been recommended for acute pain following vaginal or cesarean birth. However, some of the studies were published after the ACOG opinion paper and thus could not be included in it. Some of these studies are briefly reviewed to highlight the types of evidence that might be considered and graded for an updated ACOG opinion paper or for the development of a practice bulletin on postpartum pain.
Mills et al. (2019) developed expert panel consensus guidelines for opioid prescribing following uncomplicated vaginal births. Using a Delphi approach, the panel recommended that the lowest dose of immediate-release opioids should be used for the shortest period of time for acute pain; however, the type, dosage, and duration of opioid were not specified. A Johns Hopkins expert panel also concluded that opioids should not be routinely prescribed following an uncomplicated vaginal birth (Overton et al., 2018). Of note, these were not evidence-based guidelines and did not assess patient-reported outcomes.
With regard to cesarean delivery, the Johns Hopkins expert panel recommended that opioid-naïve patients be prescribed 0–10 pills of 5 mg oxycodone at discharge. Prabhu et al. (2017) found that in using a shared decision approach to opioid prescribing at discharge, women undergoing cesarean sections preferred to have 20 5 mg oxycodone pills prescribed rather than the standard prescription of 40 pills. The women in this study had a median of four unused pills at 2 weeks postdischarge and 90% (45 of 50) of them reported being satisfied or very satisfied with their outpatient pain management. These results are similar to those obtained by Bateman et al. (2017).
When an intervention to reduce prescribing following cesarean delivery was implemented (no preordered opioids while hospitalized), the use of opioids in the hospital was reduced from 68% to 45% by optimizing NSAID and acetaminophen use; at discharge only 40% received an opioid prescription,
compared with the preintervention rate of 91% (Holland et al., 2019). It is important to note that the discharge opioid prescription was based on inpatient use and shared decision making between the patient and prescriber in which patients could choose the number of pills they were prescribed up to a defined limit. A limitation of this study is that women were not interviewed regarding pain scores after discharge.
Intermediate Outcomes
There are robust data indicating that opioids are over-prescribed following childbirth. For example, approximately 75% of patients have unused opioids following cesarean delivery (Osmundson et al., 2017). On average, about 50% of opioids prescribed following cesarean delivery are unused, with 40 pills prescribed (various opioids) and 20 used (Bateman et al., 2017). Badreldin et al. (2018b) found that 45.7% of women after vaginal delivery and 18.5% of women after cesarean delivery who received an opioid prescription used 0 MME during the final hospital day. These data are in contrast to a small study by Osmundson et al. (2017) that found that 83% of women who had cesarean sections used opioids after discharge for a median of 8 days, and of the women who filled their prescriptions, 75% had unused pills (median per person 75 MME).
In a randomized controlled trial (RCT), Osmundson et al. (2018) found that individualized discharge opioid prescriptions based on an algorithm that correlated inpatient opioid use with postdischarge opioid use resulted in a greater than 50% reduction in the number of opioid pills prescribed at discharge after cesarean birth as compared with standard prescribing (average 14 pills versus 30 pills). Women in the individualized prescription group had 50% fewer unused pills and used only half the number of prescribed opioids than the standard group (8 pills versus 15 pills); patient-reported pain outcomes did not differ between the two groups. Prabhu et al. (2018) implemented a two-step strategy that decreased the usual discharge prescription following cesarean from 40 pills (5 mg oxycodone) to a maximum of 30 pills in the first patient education phase and to a maximum of 25 pills in the second phase, for an overall 35% reduction in the number of opioid pills prescribed, without an increase in refill requests (5–8%).
The ACOG opinion paper does not discuss intermediate outcomes such as unused pills, refill requests after discharge, or long-term opioid use. However, ACOG acknowledges that one of the reasons for making its recommendations is that 1 in 300 opioid-naïve patients exposed to opioids after cesarean birth will become a persistent opioid user (this estimate is taken from Bateman et al., 2016). Similar data are not given for vaginal births.
Health Outcomes
The ACOG opinion paper discusses health outcomes for the recommended analgesic therapies, including their effectiveness, possible adverse effects, and impacts on breastfeeding and comorbidities. In general, however, the health outcomes are not linked to specific opioid dosing. ACOG emphasizes that therapy should be individualized to each patient.
The committee notes that both short- and long-term health outcomes are concerns following discharge opioid prescribing. In a study of functional recovery following childbirth, pain- and opioid-free functional recovery occurred at a median of 20 days following vaginal delivery (opioid cessation occurred at a median of 0.5 days, with pain resolution at 15 days); on the other hand, following cesarean delivery, complete functional recovery did not occur until a median of 27 days (8 days for opioid cessation and 21 days for pain resolution) (Komatsu et al., 2018).
Of note, the risk of overdose in young children (median age 2 years) is markedly increased (odds ratio [OR]=2.41, 95% confidence interval [CI] 1.68–3.45) when the mother had received a prescription opioid in the preceding year (Finkelstein et al., 2017).2
Data Gaps and Research Needs
The committee identified several studies on specific opioid prescribing strategies used for vaginal or cesarean births and on the relationship of specific prescribing strategies with intermediate and patient outcomes. Areas where further research might be helpful for assessing long-term health outcomes include the use of opioids in patients with chronic opioid use, opioid use disorder, and indirect adverse effects on children in the home, including the effects on infants of mothers taking opioids while breastfeeding.
The committee found several studies of institution-specific quality improvement (QI) initiatives to reduce inappropriate postpartum opioid prescribing (Burgess et al., 2019; Holland et al., 2019; Prahbu et al., 2018). These studies documented a reduction in the opioid pills prescribed after the QI intervention and frequently, but not always, included data on patient-reported outcomes. Further information on opioid refills obtained outside the delivery hospital system and long-term outcomes would also be useful.
Third Molar Extraction
Opioid prescriptions for acute pain management after third molar extractions represent a significant proportion of opioid prescribing by dentists. It is estimated that 7–10 million third molar extractions are performed annually, making this procedure one of the most common procedures in dentistry associated with opioids for acute pain management (Friedman, 2007).
Baker et al. (2016) found that among a national sample of Medicaid patients (mean age 24.9 years) who underwent dental extraction between 2000 and 2010, 42% had filled an opioid prescription within 7 days of the procedure; hydrocodone was the most commonly prescribed opioid (78%). Early exposure to opioids in this population may increase the risk of persistent use and possible abuse, particularly in young females (Schroeder et al., 2019).
Dentists have traditionally managed postoperative pain after tooth extraction using NSAIDs, acetaminophen, and short-duration opioids (33–140 MMEs) (Gupta et al., 2018). The overall short-acting opioid prescribing rate of dentists since 2005 has been in the range of 12–18.5% (median of 16.5%) for all dental procedures according to nationwide studies (Gupta et al., 2018; Levy et al., 2015; Moore et al., 2006). In a study of opioid prescribing practices by dentists in South Carolina, however, the percentage of all initial opioid prescriptions after dental procedures was 45% (McCauley et al., 2016). Thus, regional variations exist for opioid prescribing practices by dentists.
There is evidence that a filled opioid prescription after third molar extractions increases the risk of persistent opioid use among opioid-naïve users aged 16–30 (Harbaugh et al., 2018). Furthermore, using 13- to 15-year-olds as a basis of comparison, the likelihood of persistent opioid use increased with increasing age (OR=1.39, 95% CI 1.01–1.91 for 16- to 18-year-olds; OR=2.13, 95% CI 1.55–2.92 for 19- to 24-year-olds; and OR=2.85, 95% CI 1.87–4.34 for 25- to 30-year-olds).
The committee chose third molar extraction as a priority surgical procedure for which a CPG might be developed because of the patient populations that are affected (e.g., adolescents and young adults), the high prevalence of the procedure, and the data that document the efficacy of nonopioid pain management strategies for this procedure.
___________________
2 This text has been revised since prepublication release.
Opioid Prescribing Guidelines
Currently, there are no evidence-based CPGs that specifically address opioid prescribing for the management of acute pain after third molar extractions. Both the American Dental Association (ADA) and the American Association for Oral and Maxillofacial Surgeons (AAOMS) provide some guidance for opioid prescribing, but they both defer the specific prescribing details to the best clinical judgment of the dental practitioner. The ADA Center for Evidence-Based Dentistry does not have any guidelines for pain control. However, the ADA website3 contains two statements that pertain directly to opioids: the 2018 Policy on Opioid Prescribing and the 2016 Statement on the Use of Opioids in the Treatment of Dental Pain. The statement recommends “consideration of nonsteroidal anti-inflammatory analgesics as the first-line therapy for acute pain management” but does not specify the type, release duration, or dosage of opioids to be considered for breakthrough pain (ADA, 2016). The statement also recommends that dentists follow and continually review CDC and state licensing board recommendations for safe opioid prescribing, as well as register with and make use of prescription drug monitoring programs. The policy states that “ADA supports statutory limits on opioid dosage and duration of no more than 7 days for the treatment of acute pain, consistent with CDC evidence-based guidelines” (ADA, 2018). There is no supporting documentation for any of these recommendations, nor is there a description on how the recommendations were derived. The committee did not consider these statements to meet the criteria for CPGs described by the 2011 Institute of Medicine (IOM) report Clinical Practice Guidelines We Can Trust. The committee notes that in 2015 ADA also developed The ADA Practical Guide to Substance Use Disorders and Safe Prescribing, and it has a number of webinars that provide more detailed information on specific aspects of opioid use in dentistry. Because the webinars are not CPGs, the committee did not consider them for this report.
The AAOMS white paper Opioid Prescribing: Acute and Postoperative Pain Management has a similar recommendation regarding NSAIDs as a “first-line analgesic therapy” and also states, “When indicated for acute breakthrough pain, consider short-acting opioid analgesics. If opioid analgesics are considered, start with the lowest possible effective dose and the shortest duration possible” (AAOMS, 2017).
The Center for Opioid Research and Education (CORE) Dental Opioid Guidelines, developed by a multidisciplinary consortium of dentists, periodontists, oral and maxillofacial surgeons, endodontists, and patients, used a modified Delphi approach to make recommendations for a stepped approach to treating acute pain in opioid-naïve patients undergoing any of 14 common dental procedures (CORE, 2018). For third molar extractions, CORE recommends that pain treatment begin with 1 g acetaminophen or 400 mg ibuprofen every 8 hours and, if needed, the maximum amount of opioids prescribed may be 15 5 mg oxycodone pills at discharge, based on the clinician’s assessment of the patient’s pain needs.
The committee selected ADA’s guidance on opioid prescribing as the basis for its evaluation of the analytic and evidence frameworks presented in Chapter 4 because ADA has a large membership whose members prescribe opioids and it has been engaged in the opioid overdose epidemic for several years.
Patient Populations
The ADA website contains little information on the patient populations that may be prescribed opioids. The 2016 ADA Statement on the Use of Opioids in the Treatment of Dental Pain recommends, “When considering prescribing opioids, dentists should conduct a medical and dental history to determine current medications, potential drug interactions, and history of substance abuse.” The ADA Practical Guide to
___________________
3 See https://www.ada.org/en/advocacy/current-policies/substance-use-disorders (accessed August 28, 2019).
Substance Use Disorders and Safe Prescribing has “techniques for managing dental pain for those who may be at risk for substance dependence” (ADA, 2015). However, the committee notes that this is a relatively small population compared with the number of people who have third molar extractions. In addition, the webinars on the ADA website have information regarding opioid prescribing in adolescents.
The lack of information on opioid prescribing for various patient populations undergoing third molar extractions is of concern because the patient population is predominantly between the ages of 15 and 25 years and those patients are typically opioid naïve.
Nonopioid Pain Management Strategies
The ADA Statement on the Use of Opioids in the Treatment of Dental Pain states that “dentists should consider nonsteroidal anti-inflammatory analgesics as the first-line therapy for acute pain management” and “recognize multimodal pain strategies for management for acute postoperative pain as a means for sparing the need for opioid analgesics” (ADA, 2016).
The committee notes that there is an abundance of strong evidence that NSAID/acetaminophen combination therapy is more efficacious than opioid therapy for acute pain after third molar extractions, with fewer side effects (Moore et al., 2018). Acute pain management after third molar extractions has been shown to respond to nonopioid medications, such as NSAIDs (e.g., ibuprofen or diclofenac) combined with acetaminophen, for pain relief equivalent to short-acting opioids. However, NSAIDs may be contraindicated in some patients, such as those with kidney or liver diseases. These nonopioids may be combined with physical modalities (ice/heat) and behavioral management for pain management (AAPD, 2018; Abdeshahi et al., 2013). Patients with breakthrough pain should be re-evaluated for other causes of pain such as infection or alveolar osteitis (dry socket). Excluding these causes, consideration of a short-acting opioid for a short duration may be indicated.
The committee recognizes that the adoption and incorporation of these alternatives to opioid prescribing in dentistry has been slow. The result has been dentists prescribing excess amounts of opioids after third molar extraction, resulting in some that are unconsumed (Mutlu et al., 2013), which allows for potential opioid diversion (Ashrafioun et al., 2014).
Opioid Prescribing Strategies
The 2018 ADA Policy on Opioid Prescribing states that “ADA supports statutory limits on opioid dosage and duration of no more than 7 days for the treatment of acute pain, consistent with CDC evidence-based guidelines.” Further information on why ADA supports this recommendation is not provided.
The committee finds that there is a paucity of prescribing strategies for the opioid management of acute pain after third molar extraction. Because third molar extraction pain typically lasts 3 to 5 days after the procedure, a prescription for 7 days of opioids may result in over-prescribing. Although short-acting, short-duration strategies for opioid dosing have been successful (Moore and Hersh, 2013), data on the specific dosing levels and duration have not been adequately evaluated.
Intermediate Outcomes
Neither the ADA Policy on Opioid Prescribing nor the ADA Statement on the Use of Opioids in the Treatment of Dental Pain provides information on any intermediate outcomes associated with opioid prescribing, such as the amount of opioids used for acute pain management.
A recent prospective study reported that patients used less than half of the prescribed opioids to manage their pain and reported more side effects when using opioids than when using nonopioid alternative medications (Maughan et al., 2016). However, most studies have been retrospective examinations of combined insurance and prescription databases on such outcome measures as the type of opioid and the strength and duration of prescriptions. The committee notes that one limitation to studies that use these data is that not all patients who have third molar extractions are represented because some patients may not have insurance that covers the procedure.
Health Outcomes
Neither the ADA Policy on Opioid Prescribing nor the ADA Statement on the Use of Opioids in the Treatment of Dental Pain provides information on any health outcomes associated with opioid prescribing for acute pain following third molar extraction.
The committee notes that the need for the long-term management of postextraction pain using opioids is minimal, a fact that is reflected in the lack of literature evaluating long-term health outcomes. Other sequalae subsequent to the extraction that may cause pain and require management include chronic infection and nerve injury. These sequalae require nonopioid, alternative approaches that are appropriate for managing these conditions (Bouloux et al., 2007).
Data Gaps and Research Needs
Opioids are commonly prescribed for third molar extractions, but the appropriate prescribing strategies for the typically young, opioid-naïve patients have not been studied. Additional research is needed to establish appropriate dosages because there is evidence that patients have different responses to pain due to, for example, their sex, age, history of substance use disorder, or history of persistent pain, and thus may require higher or lower doses to successfully manage their pain.
Health outcomes after short-term opioid use for third molar extraction pain have received minimal attention and represent another evidence gap. Further research is needed to identify the effects of opioids on such outcomes as the quality of life, the risk of substance use disorder, chronic opioid use, function, and mortality. This information would be useful in the acute pain management discussions between the prescriber and patient prior to third molar extractions.
Total Knee Arthroplasty
TKA, or knee replacement, is commonly performed in the United States for the treatment of symptomatic osteoarthritis, and opioids are typically prescribed for postoperative pain (Murphy and Helmick, 2012). The committee chose TKA as a priority surgical procedure for which a CPG might be developed because TKA is a relatively common procedure, procedure rates have increased in recent years, postoperative opioid prescribing is standard practice, opioid-naïve patients at the time of surgery are at risk for chronic opioid use, and a substantial proportion of patients undergoing TKA have current or recent opioid exposure.
In 2014 there were more than 752,900 knee arthroplasty procedures, making it the third most frequent operating room procedure (rate of 236.1 per 100,000 people) (McDermott et al., 2017). Moreover, given the aging population, TKA rates are expected to increase. Sloan et al. (2018) projected that the number of TKAs performed in the United States will grow by 85% to 1.26 million procedures by 2030, on the basis of 2000–2014 data from the Agency for Healthcare Research and Quality (AHRQ) and the National Inpatient Sample developed for the Healthcare Cost and Utilization Project.
Most patients undergoing TKA receive opioids for postoperative pain control. An analysis of health insurance administrative claims found that 72.0% of opioid-naïve patients undergoing TKA between 2007 and 2016 filled an opioid prescription, while 84.2% of sporadic opioid users and 95.9% of chronic opioid users filled an opioid prescription after surgery (Cook et al., 2019). Opioid prescribing varies considerably following TKA (Holte et al., 2019; Kahlenberg et al., 2019; Sabatino et al., 2018). For example, Sabatino et al. (2018) found that patients who underwent TKA were prescribed a median of 90 5 mg oxycodone equivalent opioid pills, with the number ranging from 10 to 200 pills. The extent to which opioids are under- or over-prescribed following TKA is unclear. Approximately 67% of patients received at least 1 refill, for a total mean number of pills prescribed of 176.4±108.0 (range, 10–480); the mean number of unused pills at 90 days was 29 (Sabatino et al. 2018).
The trajectory of opioid use following TKA varies by patient factors and prior opioid exposure. In a study of insurance claims (Bedard et al., 2017), the percentage of patients filling an opioid prescription fell from 69.3% patients in the first month after TKA to 24.9% at 3 months and to 14.9–16.3% at 6–12 months after surgery. Among opioid-naïve patients, 10.2%, 4.0%, and 3.3% were using opioids at 3, 6, and 12 months after surgery. In contrast, patients who were using opioids at the time of surgery were more likely to continue to fill prescriptions in the months following surgery, with 50.4%, 38.3%, and 33.2%, doing so at 3, 6, and 12 months after surgery. In addition, patients younger than 50 years and female patients were more likely to continue to fill prescriptions. Patients with anxiety, depression, low back pain, myalgia, and drug or alcohol dependence or who used tobacco were also more likely to continue filling prescriptions. Finally, Goesling et al. (2016) noted that among opioid-naïve patients, continued opioid use at 6 months was correlated with greater overall body pain, greater joint pain, and catastrophizing reported by patients on the day of surgery.
Importantly, there is growing evidence that opioid prescribing after TKA can be reduced without compromising pain control. In an RCT, 304 arthroplasty patients received either 30 or 90 5 mg oxycodone pills at discharge. The lower dose arm had fewer unused pills at 30 days postoperatively, with no difference in pain score. There was also no difference in patient-reported outcomes at 6 weeks. At 90 days, the lower dose arm also had lower mean MMEs prescribed, with no difference in the number of MMEs consumed (Hannon et al., 2019). Two single-institution QI initiatives also suggested that opioids are currently over-prescribed after TKA. Holte et al. (2019) found that implementing a strict opioid prescribing guideline after TKA resulted in a decline in the initial opioid prescription, the number of refills, and the total postoperative dose. Kahlenberg et al. (2019) reported that after implementation of a new opioid prescribing guideline, which set a limit of 70 pills after total joint replacement, the mean number of pills prescribed decreased from 91±26.6 pills to 65±16.3 pills, and the number of postoperative telephone encounters also decreased (the authors noted that most postoperative calls are typically to nurse practitioners for prescription refills). Neither of these QI reports contacted patients postoperatively about pain or unused pills or opioids prescribed beyond the surgical providers.
Opioid Prescribing Guidelines
In 2015 the American Academy of Orthopaedic Surgeons (AAOS) published Surgical Management of Osteoarthritis of the Knee: Evidence-Based Clinical Practice Guideline, which was endorsed by several professional societies, including the American Association of Hip and Knee Surgeons. This CPG does not specifically mention opioid use for acute pain after TKA, but it does make strong recommendations for the inpatient use of the pain management techniques of peri-articular local anesthetic infiltration, peri-articular nerve blockade, and neuraxial anesthesia to decrease opioid use when performing orthopedic procedures (Weber et al., 2016). The CPG contains both inclusion and exclusion criteria
for the evidence base, and it follows a pre-established protocol for CPG development that tracks closely with the procedures recommended in the 2011 IOM report Clinical Practice Guidelines We Can Trust and the rationale of the USPSTF for making expert opinion-based recommendations.
Additionally, in 2015 AAOS also published an information statement supporting the standardization of opioid prescription protocols and policies in all settings to control and limit opioid prescription use and dose (AAOS, 2015). The information statement recommends the following strategies for ensuring safe opioid prescribing:
- Establish ranges for acceptable amounts and durations of opioids to treat postprocedural pain, tailored to the intensity of the procedure (small, moderate, and large procedures);
- Avoid prescriptions from multiple providers, and coordinate prescribing with primary care or the usual prescribers for patients currently on opioids;
- Review prescription drug monitoring programs prior to prescribing;
- Avoid prescriptions for the treatment of chronic pain; and
- Have a strict limit of opioid prescription size that is expected to be appropriate to the pain.
Patient Populations
As discussed previously, approximately 30% of patients undergoing TKA are exposed to opioids at the time of surgery, and opioid requirements for opioid-exposed patients are often higher than for opioid-naïve patients (Bedard et al., 2017; Cook et al., 2019; Goesling et al., 2016). In addition, mental health conditions, overall body and surgical site pain, medical comorbidities, and tobacco and other substance use are correlated with greater opioid use following surgery (Bedard et al., 2017; Cook et al., 2019; Goesling et al., 2016). The AAOS CPG provides evidence and recommendations on risk factors that may affect postoperative outcomes, including the rates of complications, revision, function, and patient-reported outcomes. These risk factors include body mass index, comorbidities (e.g., diabetes, liver disease, chronic pain), compliance with preoperative therapy, and depression and anxiety. However, the CPG examines only chronic pain as a risk factor for outcomes following surgery and does not specifically address preoperative opioid use.
Nonopioid Pain Management Strategies
The AAOS CPG for TKA identifies peripheral nerve blockage and peri-articular local anesthetic infiltration as best practices to enhance postoperative pain control, and it cites the supporting evidence and specifies its quality. For example, the CPG notes strong evidence (defined as two or more high-quality studies) to support the use of peripheral nerve block versus parenteral opioids alone to reduce postoperative opioid consumption, minimize opioid-related side effects, improve postoperative range of motion, and enhance patient-reported outcomes in the immediate postoperative period. Similarly, there is strong evidence to support the use of peri-articular infiltration of local anesthesia for postoperative pain control, as it was superior to a placebo in enhancing postoperative function, reducing opioid use, improving patient-reported pain, and increasing patient-reported satisfaction following TKA.
Concerning pharmacologic opioid alternatives, a recent systematic review indicates that NSAIDs offer similar relief to opioids for knee osteoarthritis (Smith et al., 2016). One meta-analysis examined the use of alternative therapies after TKA to reduce pain and opioid use (Tedesco et al., 2017). In that study, electrotherapy and acupuncture after TKA were associated with reduced and delayed opioid consumption, but continuous passive motion, preoperative exercise, and cryotherapy were not.
Opioid Prescribing Strategies
Opioids are routinely prescribed following TKA (Bedard et al., 2017). As noted earlier, numerous studies have examined opioid dosing for TKA and provide an evidence base to be considered when creating guidelines for postoperative opioid prescribing following TKA. The AAOS CPG does not specifically address opioid prescribing following TKA, although it does consider opioid use and pain control as outcomes by which other best practices should be examined. Although enhancing pain control and reducing opioid use are identified as optimal outcomes, the best practices in opioid prescribing are not described (e.g., use, dosing, and timing of opioid alternatives alongside opioid analgesics and the identification of patients at risk for poor pain- and opioid-related outcomes), and no information is given on the type of opioid, dosing, or duration that should be followed in the postoperative period.
The AAOS statements highlight the importance of clinician–patient discussions about pain—including the use of a pain relief toolkit to facilitate those discussions—and behavioral interventions to address “depression, post-traumatic stress disorder, and ineffective coping strategies” (AAOS, 2015), but no further guidance on the latter intervention is given. This information is not included in the AAOS CPG.
Intermediate Outcomes
The committee identified several studies that have examined the intermediate outcomes following opioid use, including the amount of opioids prescribed and refilled and health care use for follow-up pain management such as telephone calls, emergency visits, and rehospitalizations. In addition to the previously mentioned 2019 studies by Hannon et al., Holt et al., Huang and Copp, and Kahlenberg et al., all of which found that opioids were over-prescribed after TKA surgery, long-term opioid use of up to 1 year following TKA surgery has also been described (Bedard et al., 2017). These studies typically obtained data from databases such as electronic medical records and administrative databases from health insurers. The results include, for example, the finding that opioid refills declined in the months following TKA, from 69% in the first month after surgery to 15–16% at 6–12 months after surgery (Bedard et al., 2017). Overall, approximately 8% of opioid-naïve patients continue to use opioids at 6 months, compared with roughly 53% of opioid-exposed patients (Goesling, 2016). Importantly, patients with preoperative opioid exposure also reported less pain relief following TKA (Smith et al., 2017). Approximately 60% of patients require refills of opioids, and the refill rates are lower among patients with optimal pain control in the hospital prior to discharge (Wilke et al., 2019).
The AAOS CPG does not address any intermediate outcomes for opioid dosing, although it does state that the use of its recommended pain management techniques may reduce opioid use.
Health Outcomes
Many of the studies described previously did not analyze patient-reported health outcomes such as pain reduction, function, or return to work or other activities. A few studies, however, have interviewed patients at varying times after surgery to ascertain pain status (Huang and Copp, 2019).
Studies using only administrative data do not capture patient-reported outcomes such as pain, opioid use, and satisfaction; however, recent studies have examined the effect of reductions in opioid prescribing on patient outcomes following TKA. For example, in an RCT Hannon et al. (2019) compared 30 or 90 pills of oxycodone following TKA and total hip arthroplasty and found that smaller opioid prescriptions reduced the number of unused pills but made no difference in patient-reported pain (Hannon et al., 2019). Similarly, Huang and Copp (2019) examined 51 consecutive patients undergoing TKA and noted over-prescribing by 34% compared with the amount used and the pain reported.
The 2015 AAOS CPG does address best practices specific to postoperative outcomes including complications, readmissions, revision rates, functional outcomes, and patient-reported outcomes, which indirectly address pain and opioid use following surgery. The guidelines include such statements as “Moderate evidence supports that patients with select chronic pain conditions have less improvement in patient reported outcomes with TKA.” However, the AAOS CPG has no recommendations on opioid prescribing at discharge. It does have strong recommendations regarding perioperative interventions and immediate postoperative outcomes. For example, the CPG states, “Strong evidence supports that peripheral nerve blockade for TKA decreases postoperative pain and opioid requirements.” Four studies are cited that compared opioids with nerve block in terms of patient outcomes; the assessments were made on the first postoperative day, and long-term opioid use and pain outcomes were not well characterized.
Data Gaps and Research Needs
The committee considered the available evidence and guidelines, including expert testimony at the committee’s public sessions, and identified the following evidence gaps. First, it has been suggested that there is a need for multicenter prospective studies using common definitions of key terms and data elements and a standardization of multimodal perioperative pain programs (David Jevsevar, Dartmouth, personal communication, July 9, 2019). In addition, it will be critical to ensure appropriate and uniform risk adjustment for baseline predictor variables (e.g., prior opioid exposure, medical comorbidities, mental health conditions, and social determinants of health). Jevsevar also suggested the greater use of quality improvement registries and longitudinal databases of large vertically integrated health systems that have high retention rates. Furthermore, Jevsevar called attention to patients who have additional types of pain and to polypharmacy in frail elderly patients as well as to the importance of the settings of care, including site of surgery and discharge location. The committee concurs with these ideas and notes that other research gaps include risk stratification for complex pain and interventions to treat these individuals.
The committee also identified evidence gaps in intermediate outcomes (e.g., opioid use disorder) and patient-reported health outcomes (e.g., function, quality of life). As noted above, the committee found evidence gaps regarding nonpharmacological pain treatments, including patient education and behavioral therapy (Tedesco et al., 2017). In light of the high prevalence of opioid use among patients before TKA, the committee found evidence gaps regarding co-management strategies among orthopedic surgeons, pain specialists, and primary care clinicians, particularly for opioid-exposed patients. Addiction medicine specialists may also be important to include for patients with opioid use or substance use disorders who are undergoing TKA surgery. Notably, the majority of opioid prescribers for patients with knee arthritis undergoing TKA may not be orthopedic surgeons; primary care and internal medicine physicians have been found to be the highest opioid prescribers before and after total joint arthroplasty (Namba et al., 2018). Moreover, nurse practitioners and physician assistants in orthopedic departments may also prescribe opioids postoperatively. This suggests that a collaborative effort to develop guidelines for opioid prescribing after TKA that includes input from these other prescribers would enhance the reach and impact of such a guideline and improve prescribing practices.
APPLYING THE ANALYTIC FRAMEWORK TO SELECTED MEDICAL INDICATIONS
The acute pain associated with surgical procedures is usually assumed to be time limited as the patient recovers from the surgery or procedure. However, the acute pain associated with many medical
conditions is much more variable and depends on the nature of the indication. For example, the acute pain associated with renal stones is typically limited to the time required for the stone to move from the kidney or ureter to outside the body. Once the renal stones are eliminated, the acute pain is expected to subside. The acute pain resulting from a sprained joint, broken bone, or strained muscle may also be expected to ease once the joint, bone, or muscle heals. Preventing acute pain from becoming chronic is an important consideration in pain management.
The committee chose four medical indications that are known to have acute pain episodes with which to assess its analytic framework for opioid prescribing: renal stones, migraine headache, low back pain, and sickle cell disease. Assessing these indications allowed the committee to determine whether the guidance available for each indication addressed issues such as acute versus chronic pain, specific opioid prescribing, other treatment modalities, population variations, and intermediate or health outcomes. Renal stones generally occur in a mature population and often result in emergency department (ED) visits. Migraine headaches may occur in both children and adults and are frequently treated in EDs, but may also be treated in primary care and specialty clinics. Acute low back pain is relatively common, can be debilitating, and may have a variety of causes that are difficult to diagnose. Sickle cell disease may also occur in both adults and children and affects predominantly black and, to a lesser extent, Hispanic populations. These indications provided a range of medical conditions and varying levels of clinician guidance to help the committee determine whether its analytic framework is broadly applicable to medical conditions.
Renal Stones
Renal stones are a common cause of acute pain. The terms renal stones, kidney stones, renal colic, and nephrolithiasis are used interchangeably to refer to the underlying obstruction in the urinary system that causes the pain. Stones may be composed of a variety of compounds, most commonly calcium oxalate and calcium phosphate (Türk et al., 2016). Based on data from the 2007–2010 National Health and Nutrition Examination Survey (NHANES) of the U.S. population, the overall prevalence of kidney stones was calculated to be 8.8% (95% CI 8.1–9.5), 10.6% (95% CI 9.4–11.9) among men, and 7.1% (95% CI 6.4–7.8) among women. Compared with whites, blacks and Hispanics were less likely to report a renal stone (OR=0.37, 95% CI 0.28–0.49 and OR=0.60, 95% CI 0.49–0.73, respectively) (Scales et al., 2012). Almost 1 in 11 people in the United States experience renal stones at some point in their lives (Pearle et al., 2014).
Opioids are frequently prescribed for acute pain caused by renal stones. In the 2016 National Hospital Ambulatory Medical Care Survey (NHAMCS), opioids were found to have been prescribed for more than 300,000 ED visits for patients diagnosed with renal calculus. Renal stones accounted for 2.1% of all ED visits at which opioids were prescribed at discharge (Schappert and Rui, 2019). In the 2010 NHAMCS, the diagnosis with the highest proportion of discharge opioid prescriptions was nephrolithiasis, with 62.1% of patients receiving an opioid prescription (Kea et al., 2016). In primary care clinics, Mundkur et al. (2018) found that renal stones were the eighth leading cause of opioid prescribing for acute pain, with 15.3% of patients receiving a prescription for opioids at the initial visit.
Opioid Prescribing Guidelines
Practice guidelines exist for acute pain caused by renal stones. In particular, the European Association of Urology (EAU) issued comprehensive evidence-based guidelines for the diagnosis and management of renal stones in 2019.4 The committee also found that EAU developed its evidence-based guidelines for
___________________
4 See https://uroweb.org/guideline/urolithiasis (accessed June 27, 2019).
renal colic using a methodology that is consistent with the analytic and evidence frameworks described in Chapter 4. The EAU evidence summary for the management of renal colic declares, “Non-steroidal anti-inflammatory drugs are very effective in treating renal colic and are superior to opioids (Section 3.4.1.1), with the level of evidence rated as 1b.” The EAU guidelines made a strong recommendation to “offer a non-steroidal anti-inflammatory as the first drug of choice (Section 3.4.1.1).” The guidelines made a weak recommendation to “offer opiates … as a second choice,” with specific mentions of hydromorphone, pentazocine, or tramadol. EAU also issued guidelines on medical therapy to expel stones and on active stone removal through shock wave lithotripsy, ureteroscopy, and percutaneous nephrolithotomy.
Nevertheless, Europe and the United States differ with regard to clinical practice, the scale of opioid misuse, and attitudes toward pain relief with opioids. Moreover, the committee noted that several RCTs described in the EAU guideline for acute renal colic were carried out in other countries (e.g., Bansal et al., 2016; Ener et al., 2009) where clinical practice and cultural expectations regarding pain and relief of pain may differ from those in the United States. Thus, the committee did not consider the EAU guidelines to be appropriate for assessing its framework for opioid prescribing for acute pain from renal stones. A systematic review of studies on the prevention of renal stones in adults was performed for the American College of Physicians CPG, but it does not deal with treatment of pain caused by renal stones (Fink et al., 2013).
The American Urological Association (AUA) issued evidence-based guidelines for the medical management of renal stones in 2014 (Perle et al., 2014) and also for the surgical management of renal stones.5 The committee found that these guidelines were based on a systematic review of evidence and that the methodology of the evidence review and standards for guideline recommendations were consistent with the committee’s guideline development process and the evidence framework. However, these AUA guidelines did not consider the management of acute pain due to renal colic or the specific use of opioids. Moreover, the literature review for the medical management guideline was only through 2011, and key studies considered by the EUA guidelines were published after this date. Still, these guidelines demonstrate that AUA has a standardized process in place for developing evidence-based CPGs.
Patient Populations
Renal stones are more common in certain U.S. populations (Scales et al., 2012; Shoag et al., 2015). Between 1994 and 2010 the prevalence of renal stones increased and in 2010 was found to be 10.6% in men (95% CI 9.4–11.9) and 7.1% in women (95% CI 6.4–7.8). Blacks and Hispanics have a lower prevalence of renal stones than whites, although the prevalence among blacks rose by more than 150% during this period (Scales et al., 2012). The prevalence of renal stones has also risen among children and adolescents, and stones are more frequent among girls than boys, unlike the situation in adults (Shoag et al., 2015). Renal stones are more common among people with obesity, diabetes, and metabolic syndrome; the increase in these conditions may be driving the increased prevalence of stones (Scales et al., 2012). Renal stones are also more common in people with a lower intake of fluids and dietary calcium.
Both of the AUA guidelines address the occurrence of renal stones in adults and children. Both guidelines also offer recommendations on the diagnosis and treatment of renal stones based on the stone type and size. No other patient considerations are given in the guidelines.
___________________
5 See https://www.auanet.org/guidelines/kidney-stones-surgical-management-guideline (accessed June 27, 2019).
Nonopioid Pain Management Strategies
Because the management of acute pain is not considered in the AUA guidelines for the medical and surgical management of renal stones, and because consideration of other management strategies is outside the scope of the committee’s task, the committee was unable to assess the use of opioids for acute pain from renal stones. Nevertheless, the committee found numerous studies that have assessed the pharmacologic treatment of pain due to renal stones. Some of this evidence is briefly described below. These studies might provide a foundation for assessing pain management in future guidelines. The committee also notes, as described previously, that the EAU recommends NSAIDs to treat renal stones.
There is considerable evidence to support the use of nonopioid pharmacotherapies for renal stones. Pathan et al. (2018) carried out a systematic review and meta-analysis of 36 RCTs on the efficacy of NSAIDs, opioids, and paracetamol (acetaminophen) in the treatment of acute renal colic. In these trials, pharmaceuticals were generally administered intravenously or intramuscularly with a pain assessment conducted about 30 minutes later. The analysis concluded that, compared with opioids, NSAIDs had a marginal benefit for initial pain reduction at 30 minutes, required fewer rescue treatments, and had lower vomiting rates. NSAIDs and paracetamol did not differ in pain relief at 30 minutes, but NSAIDs required fewer rescue treatments. The review concluded that NSAIDs should be the preferred analgesic option for patients presenting to the ED with renal stones, despite heterogeneity among the included studies and the overall quality of evidence. The committee notes that the clinical outcome in these trials was pain relief at 30 minutes and not at discharge. The trials did not study patient-reported outcomes such as longer-term pain relief, function, ability to work, or quality of life.
The 2018 review by Pathan et al. included a large 2016 placebo-controlled RCT whose active arms were 75 mg of intramuscular diclofenac, 0.1 mg/kg of intravenous morphine, and 1 gram of paracetamol (Pathan et al., 2016). In the primary endpoint, reduction of initial pain by 50% or more at 30 minutes, diclofenac was statistically superior to morphine, and paracetamol approached statistical superiority over morphine. Diclofenac had a statistically significant lower frequency of rescue analgesia and persistent pain than the other arms. The study concluded that “intramuscular non-steroidal anti-inflammatory drugs can be safely used as the first-line treatment and offer the fastest, most effective, and sustained relief from renal colic presentations in the emergency setting” (p. 2000). The study has been criticized for using a fixed single dose of morphine rather than titrating the dose (Riou and Aubrun, 2016). Of note, the study was conducted in Qatar, and the median age of participants was 34.7 years. Hence, the committee notes that the findings may not be generalizable to the population of the United States. Pathan et al. (2018) reached conclusions similar to those in a 2005 Cochrane review (Holdgate and Pollock, 2005).
In addition, there is some evidence suggesting that nonpharmacological pain modalities may be effective in relieving acute pain from renal colic, and thus, might be part of a nonopioid and nonpharmacologic approach that could reduce the need for opioids (e.g., Ayan et al., 2013; Beltaief et al., 2018; Kaynar et al., 2015).
Opioid Prescribing Strategies
The AUA evidence-based guidelines for the medical management of renal stones (Perle et al., 2014) do not address acute pain management, with or without opioids.
The EAU guidelines for renal stones recommend clinicians “offer opiates (hydromorphine, pentazocine, or tramadol) as a second choice”; this recommendation is based on weak evidence. There is a further recommendation that pethidine be avoided for patients with renal stones. No further opioid dosing information is provided.
An important limitation of the clinical trials analyzed in evidence-based reviews is that opioids were generally used at a single fixed parenteral dose in EDs. This prescribing protocol is outside the scope of this report, which is focused on opioid prescribing for outpatients or at discharge.
Intermediate Outcomes
The AUA evidence-based guidelines for the medical management of renal stones (Perle et al., 2014) do not address intermediate outcomes that may be associated with prescribing opioids for acute pain management. The committee found no studies that address intermediate outcomes of opioids prescribed for acute renal colic, such as the number of pills used and the number left over or relief of pain several days after treatment.
Health Outcomes
The AUA evidence-based guidelines for the medical management of renal stones (Perle et al., 2014) do not address the health outcomes that may be associated with prescribing opioids for acute pain from renal stones. The committee found no reports of functional status, quality of life, or the ability to work or go to school after treatment with opioids or nonopioid interventions for acute pain from renal stones.
Shoag et al. (2019) analyzed NHANES data and found that patients reporting a greater number of passed stones were also more likely to report current opioid use. This relationship persisted when smoking and arthritis, which are known to be associated with opioid use, were taken into account in a multivariable analysis. The authors acknowledged the limitations of the cross-section survey design and their inability to verify the patient-reported history of renal stones.
Data Gaps and Research Needs
Single-dose NSAIDs are effective for the short-term relief of acute pain due to renal colic and are marginally more effective than parenteral opioids in fixed doses, and they have fewer adverse effects. Evidence is lacking from RCTs regarding prescribing opioids or other medications for renal colic. Additional research on QI initiatives to reduce opioid over-prescribing for acute pain from renal colic that assess pain relief several days after discharge from the ED, the dosage of unused opioid pills, or the need for opioid refills would also be helpful (e.g., Motov et al., 2018).
Migraine Headache
Migraine headaches can cause severe pain with significant disability. They are one of the top five pain conditions among 18–44-year-olds that are treated in EDs according to a 2011 national survey (Weiss et al., 2014),6 although the 2016 National Hospital Ambulatory Care Survey did not include migraine headaches among the top 20 conditions for which opioids are prescribed in the ED (Schappert and Rui, 2019). Headaches are one of the top 10 conditions treated with opioids in primary care settings (Mundkur et al., 2018), but this categorization also included general headaches.
Migraine headache is common among people presenting for care for acute pain. The 1-year period prevalence of migraines is about 18% in women and 6% in men, with the prevalence peaking between the ages of 25 and 55 years (AHS, 2019). Migraines also occur in children and adolescents, with their prevalence increasing with age (1–3% in 3- to 7-year-olds, 4–11% in 7- to 11-year-olds, and 8–23% by
___________________
6 This text has been revised since prepublication release.
age 15 years) (Oskoui et al., 2019). In adults, migraines may be either episodic (fewer than 15 migraine or headache days in 1 month) or chronic (at least 15 monthly headache days with at least 8 monthly migraine days) (AHS, 2019). Diagnostic criteria for pediatric migraines include at least five headaches over the past year that lasted 2–72 hours when untreated, with requirements for additional features and associated symptoms (Oskoui et al., 2019).
A majority of migraine sufferers (approximately 52%) are seen in primary care settings, while 17% are treated in the ED (Burch et al., 2015). Acute migraine causes 1.2 million visits to EDs annually (Orr et al., 2016). The management of migraine headaches consists of preventive approaches using a wide variety of nonopioid medications and interventions (Silberstein et al., 2012).
The committee selected migraines for its assessment of its analytic framework because they are common, opioids are prescribed for them, and the diagnosis is sufficiently narrow to enable research to fill in data gaps. Despite the fact that opioids are not recommended for migraines as first-line therapy, they are frequently prescribed to patients presenting in emergency outpatient settings. Furthermore, headaches are one of the key conditions associated with a large rise in opioid prescribing in EDs (Dodson et al., 2018; Minen et al., 2018).
Opioid Prescribing Guidelines
Guidelines and supporting documentation for the pharmacological management of acute migraines have been published and updated by the American Headache Society (AHS)7 and the American Academy of Neurology (AAN) (Marmura et al., 2015). These guidelines promote the initial prescribing of a variety of nonnarcotic medications prior to prescribing opioids such as butorphanol for regular use. However, the adoption of these guidelines has been variable in clinical practice.
The 2000 report Practice Parameter: Evidence-Based Guidelines for Migraine Headache (an Evidence-Based Review): Report of the Quality Standards Subcommittee of the American Academy of Neurology (Silberstein, 2000) was based on four evidence-based reviews performed by Duke University and sponsored by AHRQ. Two of the reviews covered self-administered drug treatment for migraine and parenteral drug treatment for acute migraine. A technical review by AHRQ contains details of the methodology and grading of the evidence considered for the guideline; the technical review considered both the effect on headache pain and the tolerability of self-administered drug treatments for acute migraine headache compared to placebo, alternative drug treatments, and non-drug therapies in controlled trials (Gray, 1999). Efficacy and adverse events are also reported.
In 2018, the AHS Position Statement on Integrating New Migraine Treatments into Clinical Practice was released. Marmura et al. (2015) reviewed the evidence on which the AHS document and the Silberstein (2000) conclusions are based. Marmura et al. (2015) outlined their review process and how they rated the evidence. Their paper also states that the authors’ approach is “consistent” with that in the 2011 IOM report Clinical Practice Guidelines We Can Trust.
The AHS paper builds on AAN’s CPG (Silberstein, 2000). For the AAN guideline, seven organizations formed the U.S. Headache Consortium, which developed the document. Members of the consortium were identified, levels of evidence were graded, and the strength and quality of the evidence, scientific effect measures, and clinical impression of effect were defined. According to the AHS (2019, p. 3) position statement, “Input was … elicited from multiple stakeholders, including health insurance providers, employers, pharmacy benefit service companies, device manufacturers, pharmaceutical and
___________________
7 See https://americanheadachesociety.org/resources/guidelines/guidelines-position-statements-evidence-assessments-andconsensus-opinions (accessed August 28, 2019).
biotechnology companies, patients, patient advocates, and experts in headache medicine from North America and Europe.”
It is important to note that this guideline is specific to adults with migraines. However, a study using 2007–2008 commercial claims data of adolescents aged 13–17 years with two or more claims for a headache found that nearly half (46%) of the adolescents had received an opioid prescription (DeVries et al., 2014). On the date of the prescription, 24% had been diagnosed with a migraine, and nearly one-third (29%) received three or more opioid prescriptions during the study’s observation period. The Practice Guideline Update Summary: Acute Treatment of Migraine in Children and Adolescents: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society (Oskoui et al., 2019) recommends both preventive medications and nonopioid analgesics as first-line management in children. However, the guideline also notes that treatment strategies will depend on the exact diagnosis as well as patient characteristics. The pediatric guidelines for migraine state, “There is no evidence to support the use of opioids in children with migraine” (Oskoui et al., 2019, p. 10).
A Canadian primary care group adapted six high-quality guidelines published through 2011 to develop a CPG for the management of adult headaches, Primary Care Management of Headache in Adults, Clinical Practice Guideline, September 2016, 2nd Edition (see Becker et al., 2015). In that guideline, opioids are listed as a fourth-line treatment for migraines.
Patient Populations
The 2019 AHS position statement notes that “the severity, frequency, and characteristics of migraine vary among persons and, often, within individuals over time, and symptom profiles or biomarkers that predict efficacy and side effects for individuals have not yet been identified” (p. 2). The statement goes on to say that treatment plans need to be individualized based on the patient’s preferences and health status, the course of the patient’s migraine episodes (e.g., presence, type, and severity of associated symptoms and attack-related disability), contraindications (e.g., cardiovascular disease), and the use of concomitant medications (AHS, 2019). The position statement also encourages clinicians to pay specific attention to women who may be or wish to become pregnant, as preventive medications may be teratogenic.
Nonopioid Pain Management Strategies
Silberstein (2000) and the AHS (2019) conclude that prevention is critical to migraine management and that both pharmacological and nonpharmacological methods may be effective in preventing migraines. Silberstein (2000) states that nonpharmacologic treatment may be used prior to or during a migraine. Nonpharmacologic treatments include behavioral treatments, categorized as relaxation, biofeedback therapy, and cognitive–behavioral training, and physical treatments such as acupuncture, cervical manipulation, and mobilization. The AHS (2019) recommends the use of NSAIDs or nonopioid analgesics and an acetaminophen and caffeinated analgesic combination for adult patients with mild to moderate migraine episodes and recommends triptans or dihydroergotamine for moderate to severe episodes. For pediatric migraine, the AHS (2019) recommends the use of nonopioid analgesics, such as ibuprofen, as an initial treatment option. The AHS emphasizes the importance of preventive pharmacologic therapies including triptans, but notes that they are underused, are not always effective and may have side effects. The AHS (2019) notes that only 3–13% of patients with migraine use preventive treatments and estimates that approximately 40% of patients with episodic migraine and almost all of those with chronic migraine could benefit from preventive treatment.
Opioid Prescribing Strategies
Overall, there is little evidence about the best opioid prescribing strategies other than that opioid use should be avoided when possible. A gap in the literature needed for CPG development is an indication of which prescribing strategies minimize adverse outcomes if and when opioids are used. Silberstein (2000) grades the pharmacologic treatments for acute migraine as follows:
- butorphanol nasal spray (Grade A quality evidence, strong scientific and clinical effect, frequent adverse effects; consensus role: moderate to severe migraine; rescue therapy, limit use); and
- oral combinations of acetaminophen and codeine (Grade A quality evidence, less strong scientific and clinical effect, occasional adverse effects; consensus role: moderate to severe migraine; rescue therapy, limit use).
The recommended adult prescribing strategies in Silberstein (2000) include the following:
- Butorphanol nasal spray is a treatment option for some patients with migraine (Grade A); and
- Butorphanol may be considered when other medications cannot be used or as a rescue medication when significant sedation would not jeopardize the patient (Grade C).
The AHS (2019) specifically indicates that while there is established evidence that the opioid butorphanol is effective for migraine, it is not recommended for use (p. 10); there is no citation to explain the reason for this recommendation. Codeine/acetaminophen and tramadol/acetaminophen combinations are listed as probably effective for migraines with auras; specific references for the ratings are not given. Marmura et al. (2015) reviewed studies of tramadol alone and of tramadol in combination with acetaminophen and found both to be effective in reducing migraine pain, but not eliminating pain. Pringsheim et al. (2016) stated that in migraine patients for whom initial treatments for acute pain relief have failed,
opioids or acetaminophen in combination with codeine or tramadol can be considered, provided they are used infrequently. While butorphanol nasal spray has received a Level A recommendation, and codeine/acetaminophen and tramadol/acetaminophen have received Level B recommendations in the AHS acute treatment guidelines, these medications are not recommended for routine use because of concerns about dependence, addiction, and the development of medication overuse headache. (p. 1198)
According to the AHS (2019), for acute pediatric migraine treatment there is evidence to support the efficacy of ibuprofen and acetaminophen for children and adolescents and of triptans primarily for adolescents. Additional recommendations focus on early treatment for acute migraine episodes and counseling on lifestyle factors that can exacerbate migraine, including avoiding triggers and medication overuse.
Intermediate Outcomes
Intermediate outcomes were not addressed in either Silberstein (2000) or the AHS (2019).
Health Outcomes
All of the studies included in Silberstein (2000) or the AHS (2019) focused on pain relief and in some cases on adverse effects from the use of opioids such as butorphanol. No other health outcomes or harms were reported.
Data Gaps and Research Needs
There is a paucity of information on the long-term health outcomes associated with the prescribing of opioids for migraine headaches. Lipton et al. (2019) recently examined unmet treatment needs of acute migraine patients using oral medications, including opioids, and found that 96% of respondents to the Migraine in American Symptoms and Treatment survey had one or more unmet treatment needs, such as inadequate freedom of pain after 2 hours (48%), recurrence within 24 hours of initial relief (38%), or delay of treatment secondary to fear of side effects (21%). Among those reporting unmet needs, 8.1% had opioid or barbiturate overuse (defined as use during 10 or more days per month). This suggests the need for further evaluation of opioid misuse and of opioid’s lack of effectiveness for migraines.
The AHS position paper (2019) stated that symptom profiles or biomarkers that predict efficacy and side effects for individuals have not yet been identified (AHS, 2019). The committee concludes that such research would be helpful in refining and individualizing the use of opioids and nonpharmacologic treatments for migraines.
Low Back Pain
Low back pain is a common diagnosis in EDs (Kea et al., 2016; Schappert and Rui, 2019) and primary care clinics (Ashman et al., 2018; Mundkur et al., 2018). In 2010, low back pain was the leading indicator for years lived with disability (U.S. Burden of Disease Collaborators, 2013). As shown in Chapter 5, Table 5-3, there is evidence that opioids are frequently prescribed for low back pain in EDs (Rui et al., 2016) and primary care (Deyo et al., 2011; Mundkur et al., 2018). Opioid prescribing practices for back pain are not uniform and may vary by geographic region (Webster et al., 2009), patient age (Pierce et al., 2019), and clinician adherence to prescribing guidelines (Hanley et al., 2017). There are many pharmacologic and nonpharmacologic treatment options for acute pain associated with low back pain, and data show that opioid prescribing for acute low back pain is significantly associated with long-term continued opioid use (Sanger et al., 2019).
In this section, the committee focuses on adults with acute low back pain episodes for whom pain management may include opioids. Given its frequency and impact, the management of low back pain has been the subject of extensive research, systematic reviews, and CPGs.
Opioid Prescribing Guidelines
Numerous organizations have developed guidance documents for the management of low back pain. The most recent is the 2017 CPG on acute, subacute, and chronic low back pain from the American College of Physicians (discussed in more detail below). Other organizations that have developed guidance for the treatment of back pain include the U.S. Department of Veterans Affairs/U.S. Department of Defense (VA/DoD, 2014), the Institute for Clinical Systems Improvement (ICSI, 2018), Kaiser Permanente (2017), the American Physical Therapy Association (Delitto et al., 2012), the American College of Occupational and Environmental Medicine (Hegmann et al., 2014), and the joint CPG from the American Pain Society and the American College of Physicians (Chou and Huffman, 2007). International organizations with multiple member countries, including Australia, Brazil, Canada, the Netherlands, and the United Kingdom, as well as countries in Africa, have also developed CPGs for the diagnosis and treatment of low back pain (Oliveira et al., 2018).
The committee focused on a recent, comprehensive evidence-based CPG on low back pain, which was published in 2017 as Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline from the American College of Physicians (ACP CPG) (Qaseem et al., 2017), along with a supporting systematic review (Chou et al., 2017b), to evaluate the evidence within the context of the analytic framework described in Chapter 4. The 2017 guideline is a partial update of the 2007 American College of Physicians guideline addressing management of acute, subacute, and chronic low back pain; acute back pain was defined as lasting less than 4 weeks. The 2017 guideline also described radicular low back pain as resulting in lower extremity pain, paresthesia, or weakness from nerve root impingement.
The ACP CPG is an evidence-based guideline that meets many of the criteria for a CPG discussed in Chapter 4. It identifies the authors of the guideline, the methodology for the ACP CPG development process, and the grading system used for evidence review. The CPG was based on “two background evidence reviews and a systematic review sponsored by the Agency for Healthcare Research and Quality” (Chou et al., 2016, 2017a,b). The strength of recommendations (“strong” or “weak”) and the quality of evidence (“high,” “moderate,” or “low”) were graded according to an established system (Qaseem et al., 2010). The CPG and the underlying evidence reviews were subjected to peer review and published in a medical journal (Qaseem et al., 2017). Disclosures of conflicting interests for the CPG authors are available online. Key questions are provided in an appendix along with the literature search strategy. Thus, the committee finds that the ACP CPG development process aligns with the process described in Chapter 4 and in the 2011 IOM report Clinical Practice Guidelines We Can Trust.
Patient Populations
The ACP CPG states, “The target audience for this guideline includes all clinicians, and the target patient population includes adults with acute, subacute, or chronic low back pain” (Qaseem et al., 2017, p. 1). For example, patients could have radicular or nonradicular low back pain or symptomatic spinal stenosis.
Nonopioid Pain Management Strategies
The CPG addresses numerous pharmacologic and nonpharmacologic interventions for low back pain. Studies that were assessed include those comparing different interventions versus placebo or against one another. The ACP CPG recommends that acute pain management of low back pain begin with nonpharmacologic treatments because they are effective at improving pain and function and have fewer side effects than pharmacologic therapy (Qaseem et al., 2017). The CPG notes that most acute pain is self-limited, improving “over time regardless of treatment”; thus, treatment is mainly for short-term symptomatic relief. Superficial heat is recommended, based on moderate-quality evidence. Other recommended interventions, albeit with low-quality evidence, are massage, acupuncture, and spinal manipulation. If pharmacologic interventions are used, the ACP CPG recommends NSAIDs or skeletal muscle relaxants, based on moderate-quality evidence indicating improved function and pain versus placebo.
Opioid Prescribing Strategies
The ACP CPG concluded that there is insufficient evidence to determine the effect of opioids versus placebos for acute low back pain. Based on one RCT included in the ACP systematic review, naproxen with a combination of oxycodone and acetaminophen did not improve acute low back pain or functional outcomes at 1-week follow-up compared with naproxen plus placebo, naproxen plus acetaminophen, or
naproxen plus cyclobenzaprine (Friedman et al., 2015). Moreover, the group randomized to oxycodone/acetaminophen had a higher rate of adverse effects than the group randomized to placebo, including drowsiness, dizziness, and nausea or vomiting. The number of harms with opioid use was approximately equal to the number of benefits. Of note, this trial excluded patients with radicular symptoms. Therefore, the ACP CPG does not list opioids as a recommended pharmacologic treatment for acute low back pain, and opioid prescribing strategies in persons with acute low back pain are not addressed. The ACP CPG recommends that opioids only be considered as a treatment option in patients who fail first-line therapies such as NSAIDs, duloxetine, or tramadol, and in patients for whom benefits are likely to outweigh risks. (Note: Tramadol is an opioid.)
There is some evidence that opioids continue to be frequently prescribed for acute low back pain and that there is substantial variation among providers. In an urban ED, for instance, clinicians’ opioid prescribing rates varied from 12.0% to 78.2% (Hoppe et al., 2017).
Intermediate Outcomes
The ACP CPG focuses on the clinical benefits and harms of opioids and does not address intermediate outcomes such as opioid over-prescribing, the number of pills prescribed, or refills. However, in the RCT of a combination of oxycodone and acetaminophen versus placebo in addition to naproxen, Friedman et al. (2015) found that 31% of patients in the oxycodone/acetaminophen arm took the medication only once or not at all within 1 week, as compared with 23% of the placebo group, suggesting that there may be leftover opioid pills. However, these intermediate outcomes do not need to be addressed in the CPG for acute low back pain as there is a strong recommendation, based on moderate-quality evidence, that pharmacologic treatment with NSAIDs or skeletal muscle relaxants is preferred to the use of opioids.
Health Outcomes
As noted previously, an RCT found that naproxen plus oxycodone/acetaminophen did not improve functional outcomes compared with naproxen plus placebo or naproxen plus cyclobenzaprine (Friedman et al., 2015). This RCT was cited in the ACP CPG for back pain. There are also observational studies on the use of opioids for low back pain such as the studies by Franklin et al. (2008) and Webster et al. (2007), but they are not cited in either the ACP CPG or in the AHRQ review (Chou et al., 2017a) on which the CPG is based.
There are some studies showing that early use of opioids for acute low back pain is associated with worse outcomes regarding worker’s compensation for disability (Franklin et al., 2008; Webster et al., 2007). An earlier review found that “opioids do not seem to expedite return to work in injured workers or improve functional outcomes of acute back pain in primary care” (Deyo et al., 2015, p. 1). However, a more recent systematic review of studies that examined possible adverse outcomes from the use of opioids for acute low back pain, Sanger et al. (2019) found that initial opioid prescribing was associated with long-term opioid use (an intermediate outcome), but not associated with the duration of unemployment as a result of back pain.
Data Gaps and Research Needs
The committee finds that opioid dosing may not be the primary issue regarding the development or revision of a CPG for acute low back pain because of the lack of evidence that opioids are more effective for this indication. Therefore, more research is needed to determine whether opioids are effective for
treating acute low back pain and which patients may be more responsive to opioids and under what circumstances (e.g., in case of a lack of response to other treatments, particularly NSAIDs).
The one RCT by Friedman et al. (2015) and the earlier studies showing long-term adverse outcomes in workers call into question the assumption of most health care providers, particularly ED clinicians, that early administration of opioids is effective for treating acute low back pain. Thus, it should be a priority to develop evidence to more closely examine the short- and long-term effectiveness of opioids for low back pain with respect to several patient-centered outcomes. Evidence can be gathered from RCTs or from cohort studies; the latter may be particularly useful for studying longer-term outcomes such as disability and opioid misuse or the development of chronic back pain. Such studies might also identify populations where opioids are more or less effective, such as those with different pain severity, back pain with or without the presence of radiculopathy, or prior response to opioids. The committee notes that if there is evidence that opioids are effective in some populations with acute low back pain, then additional research that focuses on optimal prescribing strategies in those populations would be warranted, but until effectiveness is established, there will be no advantage in comparing different doses.
Earlier studies on the long-term adverse effects of early opioid prescribing among workers with low back pain, including the Franklin et al. (2008) and Webster et al. (2007) studies described earlier and new studies by Cifuentes et al. (2010) and Furlan and Carnide (2010), found that opioid use was associated with more adverse effects (e.g., more disability at 1 year, higher medical costs, an increased risk of surgery, and long-term opioid use), but these studies did not find the results to be definitive and recommended more research. However, these studies were carried out before the peak of opioid morbidity and mortality in the United States and before the appreciation of serious public health risks linked to excessive opioid prescribing by clinicians. The ACP evidence review concluded that there is moderate evidence from a well-designed RCT that nonpharmacologic treatments are as effective for acute low back pain as opioids. Other pharmacologic treatments have fewer adverse effects than opioids for patients and no serious and widespread public health risks. In light of the ongoing opioid overdose epidemic and the availability of effective and safer drugs, the committee does not prioritize further studies to assess the long-term harms of opioids in patients with low back pain.
The committee recognizes that opioids are currently prescribed for acute back pain, thus, it is reasonable to assess how current prescribing practices—duration and dose—for opioids might affect both intermediate and clinical outcomes, including long-term opioid use, the number of unused opioid pills, and long-term health outcomes. Such studies are most easily conducted as retrospective or prospective observational studies or as QI initiatives conducted within a health care institution or clinical department. In Chapter 7, the committee discusses the methodological challenges with such observational or QI studies.
Sickle Cell Disease
Sickle cell disease (SCD) is an autosomal recessive hemoglobinopathy that affects both children and adults. It is characterized by such phenotypic features as vaso-occlusive crisis (VOC); nociceptive, ischemic, and inflammatory responses; and acute and chronic pain; and it is multi-focal. According to CDC (2017b) there are about 100,000 cases of SCD in the United States, and about 60% are adults (Brousseau et al., 2010). SCD occurs in about 1 out of every 365 black or African-American births and 1 out of every 16,300 Hispanic-American births; about 1 in 13 black or African-American babies is born with the sickle cell trait (CDC, 2017b).
There are about 700,000 ED visits and nearly as many hospital admissions annually for SCD crisis, and 22% of the deaths in patients with SCD occur during acute painful crisis. People with SCD who have higher rates of pain also have increased mortality rates (NHLBI, 2014). Although most children with SCD live to be adults, in general their lifespans are shortened by 20–30 years (NHLBI, 2014).
The Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) is a public−private partnership with the U.S. Food and Drug Administration (FDA), the American Pain Society (APS), and the American Academy of Pain Medicine (AAPM). This collaboration developed the ACTTION−APS−AAPM Pain Taxonomy (AAAPT) (Field et al., 2019). The AAAPT defines acute SCD pain (crisis) as lasting for at least 2 hours, and having had its onset within the past 10 days; must exhibit at least one physical sign (i.e., tenderness to palpation, pain on movement, or decreased range of motion); is not explained by a SCD complication, with or without a painful comorbidity; and occurs with or without chronic SCD pain. Acute SCD pain occurs more frequently than chronic SCD pain.
Opioid Prescribing Guidelines
CPGs have been developed to treat pain associated with SCD. In 1999 the APS produced the Guideline for the Management of Acute and Chronic Pain in Sickle Cell Disease (Benjamin et al., 1999). This was the first comprehensive evidence-based guideline to address treatment of the pain of SCD. In 2014 the National Heart, Lung, and Blood Institute (NHLBI) produced the Evidence-Based Management of Sickle Cell Disease Expert Panel Report, 2014, which may be considered an evidence-based CPG that addresses the comprehensive management of SCD, including acute pain episodes (e.g., VOC) and other acute symptoms of the disease such as renal failure, hepatobiliary complications, and fever (see also Adams-Graves and Bronte-Jordan, 2016; Yawn et al., 2014). The CPG offers guidance to primary care and emergency medicine providers for the appropriate care of adults, infants, children, and adolescents with SCD, including the management of acute complications. Public comments from outside stakeholders, including medical societies, patient advocacy organizations, and industry were considered in developing the report, and the report was endorsed by a number of professional organizations involved in SCD management.
The process for developing the CPG is explained at some length and follows the recommendations of the IOM (2011) and USPSTF. Specifically, the scope of the expert panel was defined, key questions were developed, and a literature search was conducted using a population–intervention or exposure–comparator–outcome–setting methodology (see NHLBI, 2014, Exhibit 2). The CPG is based on 549 studies, including RCTs. The report authors note that only a few RCTs and large prospective cohort studies have been conducted in the management of SCD. Where evidence was lacking or inadequate, the panel relied on member expertise to provide practical guidance (NHLBI, 2014). Evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. The framework was also used to rate the strength of the recommendations, although the panel modified GRADE to include a moderate strength/evidence category. Systematic reviews and CPGs from other organizations were reviewed and included if they were applicable to the SCD population, even if they did not deal directly with patients with SCD, such as guidelines on pain management for other pain-related indications. Panel recommendations for managing acute pain in SCD were adapted from other professional societies, specifically the APS’s 1999 guideline for managing the pain of SCD (Benjamin et al., 1999). With regard to managing VOC acute pain, the panel stated, “Many specific recommendations for acute VOC management are included in this section that address treatment beyond what is listed in
the Key Question. The expert panel felt it was important to include current practices that have not yet been validated by evidence, but are currently being used” (p. 32).
Patient Populations
The NHLBI CPG recommendations are intended to be for all settings where patients present with VOC. The manifestation and diagnosis of VOC are discussed, including genotype variations in presentation. The CPG notes that there are no biomarkers or imaging studies to assess VOC pain severity. The NHBLI CPG states, “Emergency Severity Index (ESI) Version 4 triage system, which is used by more than half of emergency departments in the United States, suggests that persons with SCD be triaged as ESI level 2, a very high priority, and rapid placement be facilitated” (p. 32). Pain management both in the ED and at home are considered.
The acute pain management algorithm in the NHLBI report is applicable to any health care setting where a patient with SCD and with VOC may present for care. The recommendations for treating VOC are applicable to both adults and children. Treatment considerations for VOC in subpopulations of patients (e.g., elderly patients or patients with comorbidities) are not included.
Nonopioid Pain Management Strategies
The NHBLI CPG states that the primary management of VOC is analgesia, typically with opioids. Concurrent therapies, including heat, hydration, and nonpharmacologic therapy, are recommended to help with pain control. Antihistamines may also be used to treat itching secondary to opioid administration in the acute VOC management phase.
Opioid Prescribing Strategies
The NHBLI CPG (2014) does provide some recommendations on opioid dosing, but they are almost exclusively meant for an ED or hospital inpatient setting. It recommends treating severe VOC pain with parenteral opioids. The dose is to be “based on total daily short-acting opioid dose currently being taken at home to manage the VOC” (p. 34). The duration of dosing is dependent on the severity of the pain, and, if opioids are used, then they should be administered every 15–30 minutes until the patient reports that his or her pain is under control. Doses may be maintained or escalated by 25% until the pain is controlled, and pain relief and side effects should be assessed after each opioid dose.
For patients taking long-acting opioids at home for chronic pain, the guideline recommends that the decision to continue long-acting opioids in the setting of acute pain be made on an individual basis. In most circumstances, clinicians are advised to continue oral long-acting opioids to avoid a break in coverage and prevent withdrawal. The CPG does not provide specifics on the opioids that may be most effective and for whom, nor does it provide explicit information on the dose or duration of prescribing. The CPG does state that meperidine is not to be used, unless it is the only effective opioid for an individual patient.
The NHLBI CPG makes several recommendations regarding opioid dosing during the time the patient presents for care in the ED or a hospital setting that is outside the scope of this report. However, the CPG makes the following recommendations on the use of opioids for patients at discharge from either setting:
- At discharge, evaluate inpatient analgesic requirements, wean parenteral opioids prior to conversion to oral opioids, and adjust home dose of long- and short-acting opioid prescriptions to prevent opioid withdrawal after discharge. (Consensus–Panel Expertise)
- In adults and children with SCD and a VOC, do not use meperidine unless it is the only effective opioid for an individual patient. (Consensus–Adapted)
Intermediate Outcomes
Because acute pain in SCD is typically episodic and recurrent, the committee recognizes that it may be difficult to apply the analytic framework and assess the impact of different opioid prescribing strategies on long-term health outcomes. However, it may be possible to assess the effect of different prescribing strategies on intermediate outcomes such as the number of refills requested and the number of pills used and unused. The committee acknowledges that such information may not be indicative of opioid use because some patients with SCD may also be taking opioids for chronic SCD pain and not all VOCs are managed in the ED. Nevertheless, it may be possible to link opioid prescribing strategies to refill requests and fewer adverse effects or other health outcomes.
The NHBLI report does not discuss any intermediate effects from the use of opioids for acute pain for VOC.
Health Outcomes
The NHBLI CPG cites evidence (two RCTs and two observational studies) that opioids are effective for treating acute VOC pain (Benjamin et al., 1999; NHLBI, 2014). However, it also bases the recommendation on “indirect, high-quality evidence from populations without SCD” (p. 33). In particular, the NHLBI report cites the 2009 APS review of studies on chronic noncancer pain (Chou et al., 2009).
The NHBLI CPG does not present evidence that opioids may cause adverse health effects in either children or adults, although it does recommend adjusting the home dose of opioids to prevent withdrawal after discharge. There are no studies linking opioid doses to pain relief outside of the hospital.
Data Gaps and Research Needs
SCD is a relatively rare disease, which makes it difficult to study, particularly using RCTs to assess the effectiveness of various opioid prescribing strategies. A priority research need is to further research the management of VOCs in patients on chronic opioids or with chronic pain, possibly in combination with an opioid use disorder. Such research would require a multidisciplinary QI initiative.
The NHLBI report indicates that more research is needed to “better describe the clinical course of the occurrence and treatment results of all the acute and chronic complications of SCD; comparative effectiveness studies to provide clear outcomes on best approaches to SCD and its complications” (p. 93).
Because SCD affects largely communities of color, there is also a need for any prescribing guideline to consider that patients with SCD may also suffer health disparities due to socioeconomic factors, bias, discrimination, and a lack of doctor–patient communication (Meints et al., 2019). Patients of color may be disproportionately labeled as “drug-seeking,” and because they are disproportionately represented among SCD patients, they may be at special risk of undertreatment for pain (Elander et al., 2004). For example, one earlier study of opioid prescribing across all conditions found that white patients were
significantly more likely to receive opioid prescriptions in EDs than black patients (Pletcher et al., 2008). Another study found evidence of provider bias specifically in treating black patients with SCD who were classified as having an opioid addiction (Elander et al., 2004). In addition to disparities in prescribing, there may be limited access to pharmacies that stock opioids in communities where SCD patients reside (Morrison et al., 2000).
There is some evidence that suggests that a home-based acute pain management setting for SCD is conducive to greater nonopioid medication use (e.g., NSAIDs and antidepressants) (Smith and Scherer, 2010).
As noted in the NHLBI report, analgesics other than opioids may be used to treat acute VOC pain. In efforts to reduce the risk of opioid use disorder and over-prescribing to treat SCD, one study found accumulating evidence that the use of N-methyl-D-aspartate receptor antagonists such as ketamine and lidocaine may decrease opioid consumption during SCD acute pain episodes (Puri et al., 2019). Ketamine added to morphine has also been shown to achieve better pain control and decrease the number of repeated doses of opioids (Alshahrani et al., 2019). Moreover, the FDA approval of L-glutamine oral powder in July 2017 (the second FDA-approved treatment for SCD) is projected to alter patients’ incidence and management of SCD pain episodes.8 The extent to which these new agents will result in less opioid prescribing without worsening pain control is unknown (Puri et al., 2019). More research on alternatives to opioid analgesics may lead to reduced use.
Finally, the CPG does not address opioid prescribing for acute VOC pain in specific patient populations such as patients with comorbidities and mobility issues. As these patient factors may influence their response and access to opioids, further research on such factors is warranted. Specifically, it would be helpful to expand studies that attempt to characterize SCD pain genotypes and phenotypes—including ischemic nociceptive, neurological, inflammatory, biobehavioral, and psychosocial factors—as they relate to developing nonopioid pain strategies.
REFERENCES
AAOMS (American Association of Oral and Maxillofacial Surgeons). 2017. Opioid prescribing: Acute and postoperative pain management. https://www.aaoms.org/docs/govt_affairs/advocacy_white_papers/opioid_prescribing.pdf (accessed April 12, 2019).
AAOS (American Academy of Orthopaedic Surgeons). 2015. Information statement: Opioid use, misuse, and abuse in orthopaedic practice. https://www.aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1045%20Opioid%20Use,%20Misuse,%20and%20Abuse%20in%20Practice.pdf (accessed August 28, 2019).
AAPD (American Academy of Pediatric Dentistry). 2018. Pain management in infants, children, adolescents, and individuals with special health care needs. Pediatric Dentistry 40(6):321–329.
Abdeshahi, S.K., M.A. Hashemipour, V. Mesgarzadeh, A. Shahidi Payam, and A. Halaj Monfared. 2013. Effect of hypnosis on induction of local anaesthesia, pain perception, control of haemorrhage and anxiety during extraction of third molars: A case-control study. Journal of Cranio-Maxillo-Facial Surgery 41(4):310–315.
ACOG (American College of Obstetricians and Gynecologists). 2016. Prevention and management of obstetric lacerations at vaginal delivery. Practice Bulletin 128(1):e1–e15.
ACOG. 2017. ACOG committee opinion no. 711: Opioid use and opioid use disorder in pregnancy. Obstetrics & Gynecology 130:e81–e94.
ACOG. 2018. ACOG committee opinion no. 742: Postpartum pain management. Obstetrics & Gynecology 132(1):e35–e43.
ADA (American Dental Association). 2015. The ADA practical guide to substance use disorders and safe prescribing. O’Neil, M., ed. https://onlinelibrary.wiley.com/doi/10.1002/9781119062738.fmatter (accessed May 12, 2019).
___________________
8 See https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approved-l-glutamine-powder-treatment-sicklecell-disease (accessed August 29, 2019).
ADA. 2016. Statement on the use of opioids in the treatment of dental pain. https://www.ada.org/en/advocacy/current-policies/substance-use-disorders (accessed August 29, 2019).
ADA. 2018. Policy on Opioid Prescribing. https://www.ada.org/en/advocacy/current-policies/substance-use-disorders (accessed November 4, 2019).
Adams-Graves, P., and L. Bronte-Jordan. 2016. Recent treatment guidelines for managing adult patients with sickle cell disease: Challenges in access to care, social issues, and adherence. Expert Review of Hematology 9(6):541–552.
AHS (American Headache Society). 2019. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 59(1):1–18.
Alshahrani, M.S., L.P. Asonto, M.M. El Tahan, A.H. Al Sulaibikh, S.Z. Al Faraj, A.A. Al Mulhim, M.F. Al Abbad, S.A. Al Nahhash, M.N. Aldarweesh, A.M. Mahmoud, N. Almaghraby, M.A. Al Jumaan, T.O. Al Junaid, F.M. Al Hawaj, S. Al Kenany, O.F. El Sayed, H.M. Abdelwahab, M.M. Moussa, B.K. Alossaimi, S.K. Alotaibi, T.M. Al Mutairi, D.A. Al Sulaiman, S.D. Al Shahrani, D. Alfaraj, and W. Alhazzani. 2019. Study protocol for a randomized, blinded, controlled trial of ketamine for acute painful crisis of sickle cell disease. Trials 20(1):286.
Ashman, J.J., P. Rui, and T. Okeyode. 2018. Characteristics of office-based physician visits, 2015. National Center for Health Statistics Data Brief June (310):1–8.
Ashrafioun, L., P.C. Edwards, A.S. Bohnert, and M.A. Ilgen. 2014. Nonmedical use of pain medications in dental patients. American Journal of Drug and Alcohol Abuse 40(4):312–316.
Ayan, M., U. Tas, E. Sogut, M. Suren, L. Gurbuzler, and F. Koyuncu. 2013. Investigating the effect of aromatherapy in patients with renal colic. The Journal of Alternative and Complementary Medicine 19(4):329–333.
Badreldin, N., W.A. Grobman, K.T. Chang, and L.M. Yee. 2018a. Opioid prescribing patterns among postpartum women. American Journal of Obstetrics and Gynecology 219(1):e101–e103.
Badreldin, N., W.A. Grobman, K.T. Chang, and L.M. Yee. 2018b. Patient and health care provider factors associated with prescription of opioids after delivery. Obstetrics & Gynecology 132(4):929–936.
Baker, J.A., J. Avorn, R. Levin, and B.T. Bateman. 2016. Opioid prescribing after surgical extraction of teeth in Medicaid patients, 2000–2010. JAMA 315(15):1653–1654.
Bansal, A., M. Kumar, S. Sankhwar, S. Goel, M. Patodia, R. Aeron, and V. Bhaskar. 2016. Prospective randomized comparison of three endoscopic modalities used in treatment of bladder stones. Urologia 83:87–92.
Bateman, B.T., J.M. Franklin, K. Bykov, J. Avorn, W.H. Shrank, T.A. Brennan, J.E. Landon, J.P. Rathmell, K.F. Huybrechts, M.A. Fischer, and N.K. Choudhry. 2016. Persistent opioid use following cesarean delivery: Patterns and predictors among opioid-naïve women. American Journal of Obstetrics and Gynecology 215(353):e1–e18.
Bateman, B.T., N.M. Cole, A. Maeda, S.M. Burns, T.T. Houle, K.F. Huybrechts, C.R. Clancy, S.B. Hopp, J.L. Ecker, H. Ende, K. Grewe, B. Raposo Corradini, R.E. Schoenfeld, K. Sankar, L.J. Day, L. Harris, J.L. Booth, P. Flood, M.E. Bauer, L.C. Tsen, R. Landau, and L.R. Leffert. 2017. Patterns of opioid prescription and use after cesarean delivery. Obstetrics & Gynecology 130(1):29–35.
Becker, W.J., T. Findlay, C. Moga, N.A. Scott, C. Harstall, and P. Taenzer. 2015. Guideline for primary care management of headache in adults. Canadian Family Physician 61(8):670–679.
Bedard, N.A., A.J. Pugely, S.B. Dowdle, K.R. Duchman, N.A. Glass, and J.J. Callaghan. 2017. Opioid use following total hip arthroplasty: Trends and risk factors for prolonged use. Journal of Arthroplasty 32(12):3675–3679.
Beltaief, K., M.H. Grissa, M.A. Msolli, N. Bzeouich, N. Fredj, A. Sakma, H. Boubaker, W. Bouida, R. Boukef, and S. Nouira. 2018. Acupuncture versus titrated morphine in acute renal colic: A randomized controlled trial. Journal of Pain Research 11:335–341.
Benjamin, L.J., C.D. Dampier, A. Jacox, V. Odesina, D. Phoenix, and B.S. Shapiro. 1999. Guideline for the management of acute and chronic pain in sickle-cell disease. Glenville, IL: American Pain Society Clinical Practice Guideline Series, No. 1.
Bouloux, G.F., M.B. Steed, and V.J. Perciaccante. 2007. Complications of third molar surgery. Oral and Maxillofacial Surgery Clinics of North America 19(1):117–128.
Brousseau, D.C., J.A. Panepinto, M. Nimmer, and R.G. Hoffmann. 2010. The number of people with sickle-cell disease in the United States: National and state estimates. American Journal of Hematology 85(1):77–78.
Burch, R.C., S. Loder, E. Loder, and T.A. Smitherman. 2015. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache 55(1):21–34.
Burgess, A., A. Harris, J. Wheeling, and R. Dermo. 2019. A quality improvement initiative to reduce opioid consumption after cesarean birth. American Journal of Maternal/Child Nursing 44(5):250–259.
CDC (Centers for Disease Control and Prevention). 2017a. Births and nationality. https://www.cdc.gov/nchs/fastats/births.htm (accessed April 17, 2019).
CDC. 2017b. Data & statistics on sickle cell disease. https://www.cdc.gov/ncbddd/sicklecell/data.html (accessed August 28, 2019).
Chou, R., and L.H. Huffman. 2007. Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Annals of Internal Medicine 147(7):505–514.
Chou, R., G.J. Fanciullo, P.G. Fine, J.A. Adler, J.C. Ballantyne, P. Davies, M.I. Donovan, D.A. Fishbain, K.M. Foley, J. Fudin, A.M. Gilson, A. Kelter, A. Mauskop, P.G. O’Connor, S.D. Passik, G.W. Pasternak, R.K. Portenoy, B.A. Rich, R.G. Roberts, K.H. Todd, C. Miaskowski, and American Pain Society–American Academy of Pain Medicine Opioids Guidelines. 2009. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain 10(2):113–130.
Chou, R., D.B. Gordon, O.A. de Leon-Casasola, J.M. Rosenberg, S. Bickler, T. Brennan, T. Carter, C.L. Cassidy, E.H. Chittenden, E. Degenhardt, S. Griffith, R. Manworren, B. McCarberg, R. Montgomery, J. Murphy, M.F. Perkal, S. Suresh, K. Sluka, S. Strassels, R. Thirlby, E. Viscusi, G.A. Walco, L. Warner, S.J. Weisman, and C.L. Wu. 2016. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. The Journal of Pain 17(2):131–157.
Chou, R., R. Deyo, J. Friedly, A. Skelly, R. Hashimoto, M. Weimer, R. Fu, T. Dana, P. Kraegel, J. Griffin, S. Grusing, and E.D. Brodt. 2017a. Nonpharmacologic therapies for low back pain: A systematic review for an American College of Physicians clinical practice guideline. Annals of Internal Medicine 166(7):493–505.
Chou, R., R. Deyo, J. Friedly, A. Skelly, M. Weimer, R. Fu, T. Dana, P. Kraegel, J. Griffin, and S. Grusing. 2017b. Systemic pharmacologic therapies for low back pain: A systematic review for an American College of Physicians clinical practice guideline. Annals of Internal Medicine 166(7):480–492.
Cifuentes, M., B. Webster, S. Genevay, and G. Pransky. 2010. The course of opioid prescribing for a new episode of disabling low back pain: Opioid features and dose escalation. Pain 151(1):22–29.
Cook, D.J., S.W. Kaskovich, S.C. Pirkle, M.A.C. Mica, L.L. Shi, and M.J. Lee. 2019. Benchmarks of duration and magnitude of opioid consumption after total hip and knee arthroplasty: A database analysis of 69,368 patients. Journal of Arthroplasty 34(4):638–644.
CORE (Center for Opioid Research and Education). 2018. Dental opioid guidelines. https://www.solvethecrisis.org/dental-guidelines (accessed May 12, 2019).
Delitto, A., S.Z. George, L. Van Dillen, J.M. Whitman, G. Sowa, P. Shekelle, T.R. Denninger, and J.J. Godges. 2012. Low back pain. Journal of Orthopaedic & Sports Physical Therapy 42(4):A1–A57.
DeVries, A., T. Koch, E. Wall, T. Getchius, W. Chi, and A. Rosenberg. 2014. Opioid use among adolescent patients treated for headache. Journal of Adolescent Health 55(1):128–133.
Deyo, R.A., D.H.M. Smith, E.S. Johnson, M. Donovan, C.J. Tillotson, X. Yang, A.F. Petrik, and S.K. Dobscha. 2011. Opioids for back pain patients: Primary care prescribing patterns and use of services. Journal of the American Board of Family Medicine 24(6):717–727.
Deyo, R.A., M. Von Korff, and D. Duhrkoop. 2015. Opioids for low back pain. British Medical Journal 350:g6380.
Dodson, H., J. Bhula, S. Eriksson, and K. Nguyen. 2018. Migraine treatment in the emergency department: Alternatives to opioids and their effectiveness in relieving migraines and reducing treatment times. Cureus 10(4):e2439.
Dowell, D., T.M. Haegerich, and R. Chou. 2016. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 315(15):1624–1645.
Elander, J., J. Lusher, D. Bevan, P. Telfer, and B. Burton. 2004. Understanding the causes of problematic pain management in sickle cell disease: Evidence that pseudoaddiction plays a more important role than genuine analgesic dependence. Journal of Pain and Symptom Management 27(2):156–169.
Ener, K., K. Agras, M. Aldemir, E. Okulu, and O. Kayigil. 2009. The randomized comparison of two different endoscopic techniques in the management of large bladder stones: Transurethral use of nephroscope or cystoscope? Journal of Endourology 23:1151–1155.
Field, J.J., S.K. Ballas, C.M. Campbell, L.E. Crosby, C. Dampier, D.S. Darbari, D.K. McClish, W.R. Smith, and W.T. Zempsky. 2019. AAAPT diagnostic criteria for acute sickle cell disease pain. The Journal of Pain 20(7):746–759.
Fink, H.A., T.J. Wilt, K.E. Eidman, P.S. Garimella, R. MacDonald, I.R. Rutks, M. Brasure, R.L. Kane, J. Ouellette, and M. Monga. 2013. Medical management to prevent recurrent nephrolithiasis in adults: A systematic review for an American College of Physicians clinical guideline. Annals of Internal Medicine 158:535–543.
Finkelstein, Y., E.M. Macdonald, A. Gonzalez, M.L.A. Sivilotti, M.M. Mamdani, D.N. Juurlink, and Canadian Drug and Effectiveness Research. 2017. Overdose risk in young children of women prescribed opioids. Pediatrics 139(3):e20162887.
Franklin, G.M., B.D. Stover, J.A. Turner, D. Fulton-Kehoe, T.M. Wickizer, and Disability Risk Identification Study. 2008. Early opioid prescription and subsequent disability among workers with back injuries: The disability risk identification study cohort. Spine 33(2):199–204.
Friedman, B.W., A.A. Dym, M. Davitt, L. Holden, C. Solorzano, D. Esses, P.E. Bijur, and E.J. Gallagher. 2015. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: A randomized clinical trial. JAMA 314(15):1572–1580.
Friedman, J.W. 2007. The prophylactic extraction of third molars: A public health hazard. American Journal of Public Health 97:1554–1559.
Furlan, A.D., and N. Carnide. 2010. Opioids for workers with an acute episode of low-back pain. Pain 151(1):1–2.
Goesling, J., S.E. Moser, B. Zaidi, A.L. Hassett, P. Hilliard, B. Hallstrom, D.J. Clauw, and C.M. Brummett. 2016. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain 157(6):1259–1265.
Gray, R.N. 1999. Self-administered drug treatments for acute migraine headache. Agency for Health Care Policy and Research (US). https://www.ncbi.nlm.nih.gov/books/NBK45465 (accessed August 28, 2019).
Gupta, N., M. Vujicic, and A. Blatz. 2018. Opioid prescribing practices from 2010 through 2015 among dentists in the United States: What do claims data tell us? Journal of the American Dental Association 149(4):237–245.
Hanley, K., S. Zabar, L. Altshuler, H. Lee, J. Ross, N. Rivera, C. Marvilli, and C. Gillespie. 2017. Opioid vs. nonopioid prescribers: Variations in care for a standardized acute back pain case. Substance Abuse 38(3):324–329.
Hannon, C.P., T.E. Calkins, J. Li, C. Culvern, B. Darrith, D. Nam, T.L. Gerlinger, A. Buvanendran, and C.J. Della Valle. 2019. The James A. Rand Young Investigator’s Award: Large opioid prescriptions are unnecessary after total joint arthroplasty: A randomized controlled trial. Journal of Arthroplasty 34(7 Suppl):S4–S10.
Harbaugh, C.M., R.P. Nalliah, H.M. Hu, M.J. Englesbe, J.F. Waljee, and C.M. Brummett. 2018. Persistent opioid use after wisdom tooth extraction. JAMA 320(5):504–506.
Hegmann, K.T., M.S. Weiss, K. Bowden, F. Branco, K. DuBrueler, C. Els, S. Mandel, D.W. McKinney, R. Miguel, K.L. Mueller, R.J. Nadig, M.I. Schaffer, L. Studt, J.B. Talmage, R.L. Travis, T. Winters, M.S. Thiese, and J.S. Harris. 2014. ACOEM practice guidelines: Opioids for treatment of acute, subacute, chronic, and postoperative pain. Journal of Occupational and Environmental Medicine 56(12):e143–e159.
Holdgate, A., and T. Pollock. 2005. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database of Systematic Reviews(1):CD004137.
Holland, E., B.T. Bateman, N. Cole, A. Taggart, L.A. Robinson, R. Sugrue, X. Xu, and J.N. Robinson. 2019. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstetetrics & Gynecology 133(1):91–97.
Holte, A.J., C.N. Carender, N.O. Noiseux, J.E. Otero, and T.S. Brown. 2019. Restrictive opioid prescribing protocols following total hip arthroplasty and total knee arthroplasty are safe and effective. Journal of Arthroplasty 34(7S):S135–S139.
Hoppe, J.A., C. McStay, B.C. Sun, and R. Capp. 2017. Emergency department attending physician variation in opioid prescribing in low acuity back pain. Western Journal of Emergency Medicine 18(6):1135–1142.
Huang, P.S., and S.N. Copp. 2019. Oral opioids are overprescribed in the opiate-naïve patient undergoing total joint arthroplasty. Journal of the American Academy of Orthopaedic Surgeons 27(15):e702–e708.
ICSI (Institute for Clinical Systems Improvement). 2018. Health care guideline: Adult acute and subacute low back pain. https://www.icsi.org/wp-content/uploads/2019/08/March-2018-LBP-Interactive2.pdf (accessed August 29, 2019).
Ito, S. 2018. Opioids in breast milk: Pharmacokinetic principles and clinical implications. The Journal of Clinical Pharmacology S8(S10):S151–S163.
Kahlenberg, C.A., J.G. Stepan, A. Premkumar, F.D. Lovecchio, and M.B. Cross. 2019. Institutional guidelines can decrease the amount of opioids prescribed after total joint replacement. HSS Journal 15(1):27–30.
Kaiser Permanente. 2017. Non-specific back pain guideline. https://wa.kaiserpermanente.org/static/pdf/public/guidelines/backpain.pdf (accessed August 28, 2019).
Kaynar, M., E. Tekinarslan, S. Keskin, I. Buldu, M.G. Sönmez, T. Karatag, and M.O. Istanbulluoglu. 2015. Effective radiation exposure evaluation during a one year follow-up of urolithiasis patients after extracorporeal shock wave lithotripsy. Central European Journal of Urology 68(3):348–352.
Kea, B., R. Fu, R.A. Lowe, and B.C. Sun. 2016. Interpreting the National Hospital Ambulatory Medical Care Survey: United States emergency department opioid prescribing, 2006–2010. Academic Emergency Medicine 23(2):159–165.
Komatsu, R., B. Carvalho, and P. Flood. 2018. Prediction of outliers in pain, analgesia requirement, and recovery of function after childbirth: A prospective observational cohort study. British Journal of Anaesthesia 121(2):417–426.
Levy, B., L. Paulozzi, K.A. Mack, and C.M. Jones. 2015. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007–2012. American Journal of Preventive Medicine 49(3):409–413.
Lipton, R.B., S. Munjal, D.C. Buse, A. Alam, K.M. Fanning, M.L. Reed, T.J. Schwedt, and D.W. Dodick. 2019. Unmet acute treatment needs from the 2017 Migraine in America Symptoms and Treatment Study. Headache 59(8):1310–1323.
Maughan, B.C., E.V. Hersh, F.S. Shofer, K.J. Wanner, E. Archer, L.R. Carrasco, and K.V. Rhodes. 2016. Unused opioid analgesics and drug disposal following outpatient dental surgery: A randomized controlled trial. Drug and Alcohol Dependence 168:328–334.
Marmura, M.J., S.D. Silberstein, and T.J. Schwedt. 2015. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 55(1):3–20.
McCauley, J.L., J.M. Hyer, V.R. Ramakrishnan, R. Leite, C.L. Melvin, R.B. Fillingim, C. Frick, and K.T. Brady. 2016. Dental opioid prescribing and multiple opioid prescriptions among dental patients: Administrative data from the South Carolina prescription drug monitoring program. Journal of the American Dental Association 147(7):537–544.
McDermott, K.W., W.J. Freeman, and A. Elixhauser. 2017. Overview of operating room procedures during inpatient stays in U.S. hospitals, 2014. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb233-Operating-Room-Procedures-United-States-2014.jsp?utm_source=ahrq&utm_medium=en-1&utm_term=&utm_content=1&utm_campaign=ahrq_en3_20_2018 (accessed August 27, 2019).
Meints, S.M., A. Cortes, C.A. Morais, and R.R. Edwards. 2019. Racial and ethnic differences in the experience and treatment of noncancer pain. Pain Management 9(3):317–334.
Mills, J.R., M.M. Huizinga, S.B. Robinson, L. Lamprecht, A. Handler, M. Petros, T. Davis, and K. Chan. 2019. Draft opioid-prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstetrics & Gynecology 133(1):81–90.
Minen, M.T., E. Ortega, R.B. Lipton, and R. Cowan. 2018. American Headache Society survey about urgent and emergency management of headache patients. Headache 58(9):1389–1396.
Moore, P.A., and E.V. Hersh. 2013. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: Translating clinical research to dental practice. Journal of the American Dental Association 144(8):898–908.
Moore, P.A., H.S. Nahouraii, J.G. Zovko, and S.R. Wisniewski. 2006. Dental therapeutic practice patterns in the U.S. II. Analgesics, corticosteroids, and antibiotics. General Dentistry 54(3):201–207.
Moore, P.A., K.M. Ziegler, R.D. Lipman, A. Aminoshariae, A. Carrasco-Labra, and A. Mariotti. 2018. Benefits and harms associated with analgesic medications used in the management of acute dental pain: An overview of systematic reviews. Journal of the American Dental Association 149(4):256–265.
Morrison, R.S., S. Wallenstein, D.K. Natale, R.S. Senzel, and L.-L. Huang. 2000. “We don’t carry that”—Failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. New England Journal of Medicine 342(14):1023–1026.
Motov, S., J. Drapkin, M. Butt, A. Thorson, A. Likourezos, P. Flom, and J. Marshall. 2018. Analgesic administration for patients with renal colic in the emergency department before and after implementation of an opioid reduction initiative. Western Journal of Emergency Medicine 19(6):1028–1035.
Mundkur, M.L., K. Rough, K.F. Huybrechts, R. Levin, J.J. Gagne, R.J. Desai, E. Patorno, N.K. Choudhry, and B.T. Bateman. 2018. Patterns of opioid initiation at first visits for pain in United States primary care settings. Pharmacoepidemiology and Drug Safety 27(5):495–503.
Murphy, L., and C.G. Helmick. 2012. The impact of osteoarthritis in the United States: A population-health perspective. The American Journal of Nursing 112(3):S13–S19.
Mutlu, I., A.O. Abubaker, and D.M. Laskin. 2013. Narcotic prescribing habits and other methods of pain control by oral and maxillofacial surgeons after impacted third molar removal. Journal of Oral and Maxillofacial Surgery 71(9):1500–1503.
Namba, R.S., E.W. Paxton, and M.C. Inacio. 2018. Opioid prescribers to total joint arthroplasty patients before and after surgery: The majority are not orthopedists. Journal of Arthroplasty 33(10):3118–3124.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Pain management and the opioid epidemic: Balancing societal and individual benefits and risks of prescription opioid use. Washington, DC: The National Academies Press.
NHLBI (National Heart, Lung, and Blood Institute). 2014. Evidence-based management of sickle cell disease: Expert panel report, 2014: Evidence report. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute.
NIH (National Institutes of Health). 2017. Any mood disorder. https://www.nimh.nih.gov/health/statistics/any-mood-disorder.shtml (accessed August 28, 2019).
Oliveira, C.B., C.G. Maher, R.Z. Pinto, A.C. Traeger, C.-W.C. Lin, J.-F. Chenot, M. van Tulder, and B.W. Koes. 2018. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. European Spine Journal 27(11):2791–2803.
Orr, S.L., B.W. Friedman, S. Christie, M.T. Minen, C. Bamford, N.E. Kelley, and D. Tepper. 2016. Management of adults with acute migraine in the emergency department: The American Headache society evidence assessment of parenteral pharmacotherapies. Headache 56(6):911–940.
Oskoui, M., T. Pringsheim, L. Billinghurst, S. Potrebic, E.M. Gersz, D. Gloss, Y. Holler-Managan, E. Leininger, N. Licking, K. Mack, S.W. Powers, M. Sowell, M.C. Victorio, M. Yonker, H. Zanitsch, and A.D. Hershey. 2019. Practice guideline update summary: Pharmacologic treatment for pediatric migraine prevention: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 93(11):500–509.
Osmundson, S.S., L.A. Schornack, J.L. Grasch, L.C. Zuckerwise, J.L. Young, and M.G. Richardson. 2017. Postdischarge opioid use after cesarean delivery. Obstetrics & Gynecology 130(1):36–41.
Osmundson, S.S., B.L. Raymond, B.T. Kook, L. Lam, E.B. Thompson, L.A. Schornack, C.E. Voorhees, and M.G. Richardson. 2018. Individualized compared with standard postdischarge oxycodone prescribing after cesarean birth: A randomized controlled trial. Obstetrics & Gynecology 132(3):624–630.
Osmundson, S.S., A.D. Wiese, J.Y. Min, R.E. Hawley, S.W. Patrick, M.R. Griffin, and C.G. Grijalva. 2019. Delivery type, opioid prescribing, and the risk of persistent opioid use after delivery. American Journal of Obstetrics and Gynecology 220(4):405–407.
Overton, H.N., M.N. Hanna, W.E. Bruhn, S. Hutfless, M.C. Bicket, M.A. Makary, B. Matlaga, C. Johnson, J. Sheffield, R. Shechter, H. Nguyen, G. Osgood, C. Walsh, R. Burkhart, A. Blair, W. Ludwig, S. Nesbit, P. Wang, S. Morgan, C. Jones, L. Kodadek, J. Taylor, Z. Enumah, R. Gilmore, M. Habibi, K. Williams, J. Russell, K. Wang, J. Etra, S. Broderick, and T. Zavadsky. 2018. Opioid-prescribing guidelines for common surgical procedures: An expert panel consensus. Journal of the American College of Surgeons 227(4):411–418.
Pathan, S.A., B. Mitra, L.D. Straney, M.S. Afzal, S. Anjum, D. Shukla, K. Morley, S.A.A. Hilli, K.A. Rumaihi, S.H. Thomas, and P.A. Cameron. 2016. Delivering safe and effective analgesia for management of renal colic in the emergency department: A double-blind, multigroup, randomised controlled trial. The Lancet 387(10032):1999–2007.
Pathan, S.A., B. Mitra, and P.A. Cameron. 2018. A systematic review and meta-analysis comparing the efficacy of nonsteroidal anti-inflammatory drugs, opioids, and paracetamol in the treatment of acute renal colic. European Urology 73(4):583–595.
Peahl, A.F., V.K. Dalton, J.R. Montgomery, Y.-L. Lai, H.M. Hu, and J.F. Waljee. 2019. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Network Open 2(7):e197863.
Pearle, M.S., D.S. Goldfarb, D.G. Assimos, G. Curhan, C.J. Denu-Ciocca, B.R. Matlaga, M. Monga, K.L. Penniston, G.M. Preminger, T.M.T. Turk, and J.R. White. 2014. Medical management of kidney stones: AUA guideline. Journal of Urology 192(2):316–324.
Pierce, D.P.R., B. Pierce, C.-I. Cheng, and J. Perzhinsky. 2019. Assessing treatment and monitoring of musculoskeletal conditions using opioid versus nonopioid therapy: A cross-sectional study. Medicine 98(15):e15128.
Pletcher, M.J., S.G. Kertesz, M.A. Kohn, and R. Gonzales. 2008. Trends in opioid prescribing by race/ethnicity for patients seeking care in U.S. emergency departments. JAMA 299(1):70–78.
Prabhu, M., H. Dubois, K. James, L.R. Leffert, L.E. Riley, B.T. Bateman, and M. Henderson. 2018. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstetrics & Gynecology 132(3):631–636.
Pringsheim, T., W.J. Davenport, M.J. Marmura, T.J. Schwedt, and S. Silberstein. 2016. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache 56(7):1194–1200.
Puri, L., K.J. Morgan, and D.L. Anghelescu. 2019. Ketamine and lidocaine infusions decrease opioid consumption during vaso-occlusive crisis in adolescents with sickle cell disease. Current Opinion in Supportive and Palliative Care 13(4):402–407.
Qaseem, A., V. Snow, D.K. Owens, P. Shekelle, and Clinical Guidelines Committee of the American College of Physicians. 2010. The development of clinical practice guidelines and guidance statements of the American College of Physicians: Summary of methods. Annals of Internal Medicine 153(3):194–199.
Qaseem, A., T.J. Wilt, R.M. McLean, M.A. Forciea, and Clinical Guidelines Committee of the American College of Physicians. 2017. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 166(7):514–530.
Riou, B., and F. Aubrun. 2016. Titrated doses are optimal for opioids in pain trials. The Lancet 388(10048):961.
Rui, P., K. Kang, and J.J. Ashman. 2016. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2016_ed_web_tables.pdf (accessed April 19, 2019).
Sabatino, M.J., S.T. Kunkel, D.B. Ramkumar, B.J. Keeney, and D.S. Jevsevar. 2018. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. Journal of Bone and Joint Surgery 100(3):180–188.
Sanger, N., M. Bhatt, N. Singhal, K. Ramsden, N. Baptist-Mohseni, B. Panesar, H. Shahid, A. Hillmer, A. D Elia, C. Luo, V. Rogers, A. Arunan, L. Baker-Beal, S. Haber, J. Henni, M. Puckering, S. Sun, K. Ng, S. Sanger, N. Mouravaska, M.C. Samaan, R. de Souza, L. Thabane, and Z. Samaan. 2019. Adverse outcomes associated with prescription opioids for acute low back pain: A systematic review and meta-analysis. Pain Physician 22(2):119–138.
Sanmartin, M.X., M.M. Ali, J. Chen, and D.S. Dwyer. 2019a. Prescription opioid misuse, sources of opioids, and reasons for opioid misuse among reproductive-aged parenting women with major depressive episode. Addictive Behaviors 98:106057.
Sanmartin, M.X., M.M. Ali, P. Novak, and J. Chen. 2019b. Sources and main motivations for prescription opioid misuse among reproductive-aged parenting women in the United States. Substance Use & Misuse 54(8):1332–1336.
Scales, C.D., Jr., A.C. Smith, J.M. Hanley, C.S. Saigal, and Urologic Diseases in America Project. 2012. Prevalence of kidney stones in the United States. European Urology 62(1):160–165.
Schappert, S., and P. Rui. 2019. Estimated number and percent distribution of hospital emergency department visits at which opioids were prescribed at discharge, for selected diagnosis groups: United States, 2016. Special Topics. https://www.cdc.gov/nchs/ahcd/ahcd_products.htm (accessed September 10, 2019).
Schroeder, A.R., M. Dehghan, T.B. Newman, J.P. Bentley, and K.T. Park. 2019. Association of opioid prescriptions from dental clinicians for U.S. adolescents and young adults with subsequent opioid use and abuse. JAMA Internal Medicine 179(2):145–152.
Shoag, J., G.E. Tasian, D.S. Goldfarb, and B.H. Eisner. 2015. The new epidemiology of nephrolithiasis. Advances in Chronic Kidney Disease 22(4):273–278.
Shoag, J.E., N. Patel, L. Posada, J.A. Halpern, T. Stark, J.C. Hu, and B.H. Eisner. 2019. Kidney stones and risk of narcotic use. Journal of Urology 202(1):114–118.
Silberstein, S.D. 2000. Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 55(6):754–762.
Silberstein, S.D., S. Holland, F. Freitag, D.W. Dodick, C. Argoff, and E. Ashman. 2012. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults. Neurology 78(17):1337–1345.
Sloan, M., A. Premkumar, and N.P. Sheth. 2018. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. Journal of Bone and Joint Surgery 100(17):1455–1460.
Smith, S.R., B.R. Deshpande, J.E. Collins, J.N. Katz, and E. Losina. 2016. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: Systematic analytic review. Osteoarthritis and Cartilage 24(6):962–972.
Smith, S.R., J. Bido, J.E. Collins, H. Yang, J.N. Katz, and E. Losina. 2017. Impact of preoperative opioid use on total knee arthroplasty outcomes. Journal of Bone and Joint Surgery 99(10):803–808.
Smith, W.R., and M. Scherer. 2010. Sickle-cell pain: Advances in epidemiology and etiology. ASH Education Program Book 2010(1):409–415.
Tedesco, D., D. Gori, K.R. Desai, S. Asch, I.R. Carroll, C. Curtin, K.M. McDonald, M.P. Fantini, and T. Hernandez-Boussard. 2017. Drug-free interventions to reduce pain or opioid consumption after total knee arthroplasty: A systematic review and meta-analysis. JAMA Surgery 152(10):e172872.
Türk, C., A. Petřík, K. Sarica, C. Seitz, A. Skolarikos, M. Straub, and T. Knoll. 2016. EAU guidelines on interventional treatment for urolithiasis. European Urology 69(3):475–482.
U.S. Burden of Disease Collaborators. 2013. The state of U.S. health, 1990–2010. JAMA 310(6):591–606.
VA/DoD (U.S. Department of Veterans Affairs/U.S. Department of Defense). 2014. VA/DoD clinical practice guideline for the non-surgical management of hip and knee osteoarthritis: Guideline summary. https://www.healthquality.va.gov/guidelines/CD/OA/OASUM6FIA.pdf (accessed August 19, 2019).
Weber, K.L., D.S. Jevsevar, and B.J. McGrory. 2016. AAOS clinical practice guideline: Surgical management of osteoarthritis of the knee: Evidence-based guideline. Journal of the American Academy of Orthopaedic Surgeons 24(8):e94–e96.
Webster, B.S., S.K. Verma, and R.J. Gatchel. 2007. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery, and late opioid use. Spine 32(19):2127–2132.
Webster, B.S., M. Cifuentes, S. Verma, and G. Pransky. 2009. Geographic variation in opioid prescribing for acute, work-related, low back pain and associated factors: A multilevel analysis. American Journal of Industrial Medicine 52(2):162–171.
Weiss, A.J., L.M. Wier, C. Stocks, and J. Blanchard. 2014. Statistical brief #174: Overview of emergency department visits in the United States, 2011. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf (accessed November 3, 2019).
Wilke, B.K., D.T. Foster, C.A. Roberts, G.G. Shi, E.R. Lesser, M.G. Heckman, J.L. Whalen, and S.R. Clendenen. 2019. Early in-hospital pain control is a stronger predictor for patients requiring a refill of narcotic pain medication compared to the amount of narcotics given at discharge. Journal of Arthroplasty 34(7):1354–1358.
Wong, C.A., and T. Girard. 2018. Undertreated or overtreated? Opioids for postdelivery analgesia. British Journal of Anaesthesia 121(2):339–342.
Yawn, B.P., G.R. Buchanan, A.N. Afenyi-Annan, S.K. Ballas, K.L. Hassell, A.H. James, L. Jordan, S.M. Lanzkron, R. Lottenberg, W.J. Savage, P.J. Tanabe, R.E. Ware, M.H. Murad, J.C. Goldsmith, E. Ortiz, R. Fulwood, A. Horton, and J. John-Sowah. 2014. Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA 312(10):1033–1048.
This page intentionally left blank.




































