4
The Role of the National Government
Regulatory quality is one of the World Bank’s six features of good governance, aggregate indicators of which are tracked over time to measure the institutions in a country and the way authority is exercised (Benson Wahlén, 2018; WBG, 2018b). The World Bank’s indicators of regulatory quality put an emphasis on openness and the rule making process, including its use of public consultations and formal impact assessment, and the accessibility of regulations (WBG, 2018a). Like other features of good governance, the functioning of the regulatory system is partly determined by the willingness and capacity of the government in a country to enact good policies (WBG, 2018b). The same way international action and global economic trends can influence national policy, a great deal of the regulatory agency’s capacity is determined at the levels above the agency itself.
Much of the regulatory agency’s effectiveness, efficiency, and independence are determined by a country’s political leaders, who are ultimately responsible for creating an environment conducive to product safety. This chapter discusses steps national governments can take to create an environment conducive to product safety. This includes the legal provisions that allow the regulatory system to function. It also considers different models for organizing such systems, including strategies for sharing work among countries.
Sometimes it is difficult to distinguish the role of the national government in facilitating an effective regulatory agency from the responsibility of the agency’s leadership to the same end. Cooperation with foreign regulators is a responsibility of the agency, for example, but one that can only be pursued with the support of the national political leaders. Given this
overlap, key concepts related to international cooperation are introduced in this chapter and revisited in Chapter 5. The value of sharing regulatory work among countries, for example, is introduced in this chapter; the steps agencies can take to operationalize such work sharing are discussed in the next chapter.
LEGAL PROTECTION
Providing a legal framework for product safety may be the most fundamental step national political leaders can take to protect food and medical products in their country. Legal provision defining the national regulatory system is the first indictor measured in the World Health Organization (WHO) Global Benchmarking Tool (WHO, 2018a). The legal provisions define what institutions are part of the regulatory system, the ways they are involved, and their ability to enforce rules (WHO, 2018a).
Momentum changing laws depends on political will, which can wax and wane over time (Ruger, 2007). Food safety in particular is not often high priority, especially in low-income countries (Jaffee et al., 2019; WHO, 2017). In such situations, convincing government of the health and economic benefits of investing in regulatory systems can help make food laws higher priority. Development partners also have role in encouraging political will. Actions such as those recommended in the previous chapter can help persuade leaders in low- and middle-income countries that investment in regulatory systems is a priority by “demonstrate[ing] that it is one” (Bollyky and Stergachis, 2013).
Assessing the level of public health protection provided by existing laws is usually the first step to passing food or medicines laws. Even when no national food or drug law exists, there are often pieces of other laws that touch on the topic (FAO, 2003; Fefer, 2012). The 2012 edition of the reference publication Managing the Drug Supply observed that when there are many laws on different aspects of the regulatory process, some are probably outdated, concluding, “because concepts of pharmaceutical policy are modern, legislation more than 20 years old may not be relevant; starting over may be simpler” (Fefer, 2012). It is not clear that there is a similar consensus regarding older food laws (Grace, 2015; WHO, 2017). A 2005 Food and Agriculture Organization of the United Nations (FAO) report commented that food laws in many countries “may not have been updated or may have been constantly amended creating a maze of rules which regulators, industry, and consumers find difficult to understand” (Vapnek and Spreij, 2005). Whether a country drafts new food and drug laws or revises old ones, provisions should be made for removing previous outdated laws to minimize confusion in the market (WHO, 2017).
The drafting of administrative laws is complicated, but can be eased by using model laws as examples (Fefer, 2012; WHO, 2017). The WHO has model medicines legislation, important elements of which are shown in Box 4-1. Box 4-2 shows sections of an FAO model food law for a single agency.1 Model laws can be used as tools in reviewing and amending existing food and drug laws (UNODC, n.d.). The points shown in Boxes 4-1 and 4-2, and the publications cited, can help guide legislators through the process of drafting laws. As their name suggests, model laws are examples; each country is responsible for tailoring revisions to the law to suit its own needs. For this reason, the FAO is somewhat circumspect about the usefulness of model food laws (FAO, 2019). While models are useful for countries pursuing regional harmonization programs, or for governing highly technical areas, much about food and agriculture laws depend on national history (FAO, 2019). Substantial adaptions to suit local context may be necessary.
In any case, a group of legal, health, and technical experts familiar with the subject and the local context is usually needed to write or revise laws. In cases where it is not possible to assemble such a group of experts from within a country, international organizations and donors can supply them (Fefer, 2012). Knowing how to access these supports and where to draw experts from remains a challenge, however. International meetings of regulatory authorities are a useful source for consultation (Fefer, 2012), another reason why the FAO and the WHO should convene such a meeting for food regulators, as recommended in Chapter 2.
In ensuring a legal mandate for regulatory systems, legislators should give special attention to three features that help determine an agency’s ability to function: independence, financial sustainability, and authority to cooperate internationally. Governments that provide these protections to their regulatory agencies do their part to foster safe, nutritious foods and good-quality medical products in their countries.
Independence
Independence is a conceptual cornerstone of a functional regulatory system designing food and drug laws. It is also necessary for consumer confidence in the system. For the purposes of running an effective regulatory system, independence refers to a lack of political interference in scientific decision making and in the hiring and firing of technical staff. As the 2012 Institute of Medicine’s report Ensuring Safe Foods and Medical Products Through Stronger Regulatory Systems Abroad explained, an
___________________
1 The cited document also has model laws for multiple agency systems and integrated systems.
agency’s independence relates directly to the trust consumers place in it (IOM, 2012). Independence also allows industry to function with confidence that the rules affecting their products will be applied in a predictable way (IOM, 2012).
Independence does not mean, however, that the agency works in a vacuum. Regulatory decisions have a social dimension, the interpretation of which makes it important for agencies to be accountable to national leaders. As one former U.S. Food and Drug Administration (FDA) commissioner observed, “to try to take politics out of FDA, first of all will not happen, and second, should not happen. Politics basically is good if handled properly because politics reflects the views of various people in society” (Adashi et al., 2019; Gordon, 2003). It is one thing, however, to have political appointees choose the relative priorities for an agency or make decisions regarding the manner in which work is conducted. It is something else to dismiss whole cadres of technical staff en masse for political reasons (IOM, 2012). The laws establishing regulatory agencies should protect against this kind of interference and shield technical staff from undue political influence (IOM, 2012).
Part of the value of model laws, such as those presented in Boxes 4-1 and 4-2, is in creating a template to allow the agency some authority over topics that are too technical or too complicated to legislate. Provisions for independence are valuable in those areas. For example, the regulation of complex biologic medicines and their analogous generics, called biosimilars, is one of the challenges facing regulators today, including many in low- and middle-income countries (Garcia and Araujo, 2016; Godman et al., 2018; Hodkinson and Blockman, 2018). Access to these medicines may be impeded if laws affecting the approval and substitution of biosimilars or the regulator’s ability to educate prescribers are overly restrictive (Falit et al., 2015). Independent regulators should be allowed to make these assessments based on scientific evidence. Biologic markets may be in particular need of greater competition. About half of the world’s 100 million diabetics do not have reliable access to insulin, for example, a problem blamed in part on lack of competition among the three companies that manufacture the vast majority of the world’s insulin supply (99 percent by value, 96 percent by volume) (Gotham et al., 2018; HAI, 2015).
Beyond the legal protections for regulatory agencies, a culture of openness is an investment in the agency’s success. For this reason, the Organisation for Economic Co-operation and Development’s (OECD’s) guidance on regulatory independence, like the World Bank’s indicators of regulatory governance, gives considerable attention to transparency and public consultation (OECD, 2014b; World Bank, n.d.-a). As the World Bank guidance explains, “Where citizens know the rules that govern their society and have a role in shaping them, they are more likely to comply with those rules.
Corruption is lower and the quality of regulation is higher” (World Bank, n.d.-b). Publishing the scientific basis for rules and giving the public open and advertised opportunity to comment on these rules is part of regulatory transparency, as are the terms for vetting staff for conflicts of interest (see Box 4-3).
The independence of the regulatory agency was the topic of a recent white paper from the Aspen Institute and a companion commentary in Health Affairs wherein seven former FDA commissioners called for a restructuring of the agency, removing it from the U.S. Department of Health and Human Services’ (HHS’s) authority (Aspen Institute, 2019; Califf et al., 2019). A comment on such reorganization is not within the scope of this report, nor is there evidence that an agency’s relative place in the government organization predicts its successful functioning. OECD guidance on this topic emphasizes that, while independence of the regulatory agency is difficult to measure, it goes beyond its place in a government organizational chart (OECD, 2014b). A 2016 analysis of 78 medicines regulatory agencies found no relationship between an agency’s structural autonomy and regulatory quality, concluding, “both good and bad regulatory systems can have semi-autonomous” structures (Pezzola and Sweet, 2016). The authors went on to note that an agency’s autonomy is not necessarily related to its funding, concluding, “in developing countries guaranteed funding is much more difficult [than structural independence] to achieve” (Pezzola and Sweet, 2016).
Financial concerns were also at the center of the former FDA commissioners’ recent call for reorganization (Aspen Institute, 2019). Separating
from the HHS would, they reasoned, reduce the intermediary steps in appropriations review, ultimately guaranteeing the agency more stable and readily available funding (Aspen Institute, 2019). The budgetary barriers the former commissioners are trying to overcome may be unique to the United States, but the larger question of sustainable financing is not. The regulatory agency’s independence is partly dependent on its financing, underscoring the importance of a stable and diversified revenue stream to its effective functioning (OECD, 2016b). This funding is particularly vulnerable in low- and middle-income countries (Roth et al., 2018).
Sustainable Financing
Government leaders tend to view the regulatory agency as a necessary cost center, meaning that its staffing and work are an expense with little perceived economic or political return, at least until something goes wrong. In any case, such perception is not accurate; quality, effective regulation encourages economic growth (Jalilian et al., 2007). The nature of the relationship between the agency and the economy depends on the agency’s charge and the national economy. In Mexico and Brazil, the food and drug regulatory agency oversees products that account for 10 to 25 percent (respectively) of the gross domestic product (Arriola Penalosa et al., 2017; Moscou et al., 2016). In poorer countries, where essential items such as food and medicine account for a greater share of household spending, the regulatory agency may be even more prominent in the national economy (Whitmore Schanzenbach et al., 2016).
An interest in guaranteeing the financial viability of the regulatory authority should therefore be a central government priority: The agency’s functioning has implications for both health and economic growth. And, because its funding at least partially determines how the agency operates, governments should give careful attention to the financing streams they authorize. Both the WHO Global Benchmarking Tool and the FAO and the WHO Food Control System Assessment Tool evaluate the financial resources that governments provide to agencies (Caipo, 2019; WHO, 2018a). Neither assessment is overly prescriptive regarding the nature of the funding streams, implicitly recognizing that there are multiple effective means to do so. Broadly speaking, most governments have a short list of sources from which to fund regulatory agencies: taxes, fees on regulated industry, earmarks, or some combination thereof. (Income in the form of fines and penalties, though revenue for the regulatory agency, are excluded from this discussion as they are not a predictable or substantial source of revenue, and because reliance on fines can create perverse incentives.)
Budgetary Subvention
Direct budgetary subvention may be the simplest way to fund the regulatory agency. It is also the means recommended by OECD, mostly because it avoids any conflict of interest that can arise from making the regulatory agency dependent on user fees (OECD, 2014b). But subvention depends on tax collection, and tax collection is challenging in low- and middle-income countries (Glenday et al., 2019; IMF, 2011). The tax base in these countries is, by definition, constrained. Still, global prosperity is increasing. As Figure 4-1 illustrates, between 2000 and 2015 the share of the world’s population living in a high-income or upper-middle-income country has gone from about a quarter to slightly more than half, while the percentage living in a low-income country has shrunk by 32 percentage points (Gill et al., 2019). In theory this means the taxable income in these countries has grown. But the tax base in low- and middle-income countries can be difficult to measure, making cost-effective tax collection difficult even as governments in these countries are under pressure to provide more public services (Gill et al., 2019).
Fair and efficient tax collection is a precursor to meeting many of the Sustainable Development Goals (UN, 2018). The International Monetary Fund has long been involved in supporting governments to collect taxes (IMF, 2011). A 2011 analysis of the Fund’s experience in this area emphasized the importance of political will for making progress, and the need to communicate the value of public spending to taxpayers (IMF, 2011). The value of the regulatory agency for health and consumer protection is clear and should be relatively straightforward to communicate to the public, a topic expanded on in Chapter 5. Regardless, there are many factors influencing how governments balance competing priorities in budget allocation. Even when subvention provides agencies with reliable funding, questions regarding the sufficiency of such funding may make other funding sources attractive.
User Fees
Fees “assessed to users for goods or services provided by the federal government,” called user fees, are an important source of funding for many regulatory agencies (GAO, 2015). Agencies may charge for services such as application review or inspection, or tasks, such as testing samples in a laboratory. User fees are meant to apply in circumstances where the recipient benefits “beyond what is normally available to the public” (Austin, 2019). Drug companies, for example, profit from the market access regulatory approval provides beyond the general benefit to society from the access to any company’s products. By the same token, charging for tasks in the
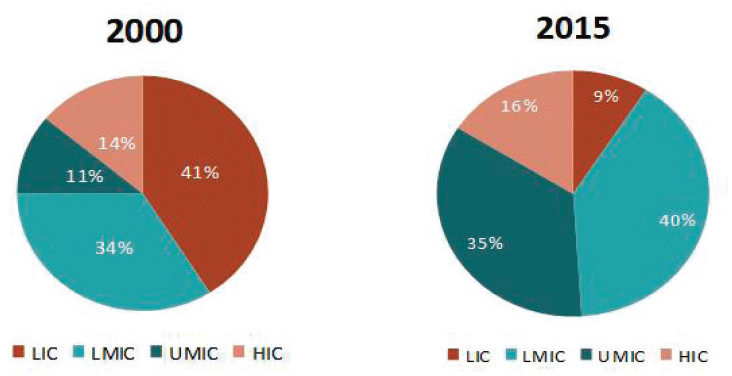
NOTE: HIC = high-income country; LIC = low-income country; LMIC = lower-middle-income country; UMIC = upper-middle-income country.
SOURCE: Gill et al., 2019.
public interest is considered undesirable because of the risk that the charge deters use of the service. Salmonella testing, for example, has public benefit beyond the benefit to the requester of the test, so charging for such services may be counterproductive (MacDonald et al., 1999).
Relying on user fees to fund regulatory work is controversial, partly because it is difficult to disentangle the relative public and private benefits accrued (Austin, 2019; GAO, 2015). Even when the commercial benefit is clear and the regulatory burden easy to measure, as in the case of a drug company seeking market approval for a new molecule, user fees can alter the relationship between the regulator and industry (GAO, 1997). Almost two-thirds of the FDA’s human drugs program is funded by user fees, as are about half of its total program costs (FDA, 2018). The use of fees to fund reviews has reduced the time required for market authorization of a standard new drug by about 18 months (Dabrowska and Thaul, 2018). At the same time, it has raised concerns that industry funding has influenced the agency’s priorities in favor of new drug review and away from other work that does not generate revenue (Austin, 2019).
Funding the regulatory agency, especially its basic functions, from general revenues may therefore be desirable, but it is not always an option, especially in low- and lower-middle-income countries (Roth et al., 2018). A recent report on medicines regulation in Africa found that only 9 of 26 countries were funded by government (with any user fee revenue paid directly to the government treasury), but that the majority subsist on charges for market authorization and retention (Ndomondo-Sigonda et al., 2017). Drug regulatory authorities in Kenya, Malawi, Tanzania, Uganda, Zambia, and Zimbabwe receive minimal or no budgetary support from subvention (Ndomondo-Sigonda et al., 2017). A recent analysis of 95 countries’ medicines regulatory agencies found that all charged some fee for registering new medicines, and that the fee charged was roughly proportional to market size (Morgan et al., 2017).
Given the pervasiveness of user fees, governments need to pay special attention to the way the system is designed, specifically to “the authority to collect and spend the revenues” (MacDonald et al., 1999). Because revenues from user fees are not always credited to the collecting agency, some may be used to fund the fee-generating program, or to offset other expenses; others are paid to the national treasury (Austin, 2019; GAO, 1997; MacDonald et al., 1999). Most agencies would have a clear preference for systems that allow them control over the fee, but legislators may dislike this, seeing an attempt to circumvent their funding authority (Austin, 2019).
Furthermore, for user fees to be acceptable to industry, charges must be a justifiable, accurate reflection of the cost of the service (GAO, 1997; MacDonald et al., 1999). Matching the incremental cost of the activity to the fee can be challenging, adjusting the fee to reflect the public benefit of
the service even harder (Austin, 2019). Fees may be inaccurate if inflation increases the cost of the service, or if a new technology allows it to be done more cheaply (MacDonald et al., 1999). Underestimates on the fee schedule, even slight ones, can accumulate as an activity expands, gradually pushing an agency into deficit (MacDonald et al., 1999). It is therefore preferable that the legislature review the fee schedule every few years, as adjustments may be necessary.
Dependence on user fees can also make the agency vulnerable to market shifts. Demand for the fee-generating service may dry up, making a financial safety net in the form of government appropriations even more important (Austin, 2019; GAO, 1997). When budgetary subvention cannot provide that safety net, earmarks may be a viable stopgap.
Earmarked Revenues
In this discussion, earmarking refers to the flagging of a tax or other revenue for a specific purpose (Cashin et al., 2017; Porter and Walsh, 2006). Earmarked revenues sometimes fund programs that relate to the revenue source (e.g., using a tobacco tax to fund smoking cessation), but they may be unrelated (Porter and Walsh, 2006). Countries can also earmark a percentage of expenditures, mandating what share of the budget should be spent on health, for example. These expenditure earmarks are usually used to enforce government priorities (Cashin et al., 2017).
Earmarked taxes are a common revenue stream in low- and middle-income countries (Boakye, 2016). In places where the tax collection system is not developed, taxes on tobacco, alcohol, or unhealthy foods are seen as an effective way to fund the government’s health spending (Creese, 2011).
A 2017 analysis of the use of earmarks for health emphasized that most of the arguments for and against earmarking are theoretical because, “despite vast country experience with the policy instrument—at least 80 countries earmark for health—little empirical evidence has been introduced into the debate” (Cashin et al., 2017). For these reasons, it is still not clear what circumstances are best suited to earmarking. Earmarks may work well, however, when the link between a country’s stated priorities and its budget are weak because the earmark protects some revenue from competing interests (Cashin et al., 2017). Earmarks may, therefore, be a good funding option for regulatory agencies, as food and drug safety is usually a nominal priority for governments, but one that does not always translate into the budget making process.
Matching resources to priorities is a fundamental challenge of budgeting. There are administrative and economic tradeoffs to all funding options discussed in this chapter. The executive and legislative branches of government need therefore to revisit the regulatory agency’s funding
at regular intervals, to ensure both its sufficiency and that it is managed with efficiency and openness. Ideally, the funding for the regulatory agency should be arranged for multiple years at a time to encourage stability and prevent sudden cuts (OECD, 2014b), though in practice, almost 90 percent of regulatory agencies have single-year budgets (OECD, 2014b).
The balance of revenues that any government should use to fund its regulatory agency depends, however, on local context. Even though all countries struggle with the tradeoffs, and many with the same budgetary constraints, there is surprisingly little evidence as to what contexts are best suited to different financing strategies (Cashin et al., 2017; Ndomondo-Sigonda et al., 2017). This would be an important area for the Centers of Excellence in Regulatory Science to research. The optimal percentage of revenue regulatory agencies should draw from user fees or the conditions conducive to reliance on earmarks are the types of questions the centers might consider. Table 4-1 lists some advantages and disadvantages of various funding sources and might be a starting point for the Centers of Excellence in their research.
International Cooperation
It is important for national laws to guarantee sustainable funding to regulatory agencies because all agencies, even those with considerable resources, are facing the limits of their resources. Work sharing with foreign regulatory agencies allows countries to make more efficient use of their staff and funding. (Box 4-4 explains some important terms relevant to regulatory cooperation.) The World Health Assembly’s 2014 resolution on strengthening regulatory systems encouraged member states “to engage in global, regional, and sub-regional networks of national regulatory authorities as appropriate, recognizing the importance of collaboration to pool regulatory capacities” (WHO, 2014). Like sustainable financing, a mandate for international cooperation is so central to the work of modern regulatory agencies that the WHO and the FAO benchmarking tools both assess it in their benchmarking evaluations (Caipo, 2019; WHO, 2018a). For this reason, governments should support their regulatory agencies to participate in international regulatory cooperation and harmonization programs. The drafting or revision of a country’s food and drug laws is an important time to ensure these protections are in place.
When done well, regulatory harmonization serves the goal of making markets for food and medical products safer and more competitive (Pombo et al., 2016). If regulators can expand their information base through secure electronic databases, for example, the chances of an unsafe product permeating multiple markets is reduced (Allchurch et al., 2016). There are also obvious benefits to efficiency. Through sharing inspection resources,
TABLE 4-1 Pros and Cons of Different Financing Streams
| Pros | Cons | |
|---|---|---|
| Budgetary Subvention |
|
|
| User Fees |
|
|
| Earmarked Taxes |
|
|
SOURCES: Austin, 2019; Cashin et al., 2017; GAO, 2015.
for example, regulators can substantially improve their reach. Another important benefit for heads of government to realize is that participation in regional regulatory programs strengthens national capacity (Kaddu et al., 2018). For example, the U.S. Agency for International Development and U.S. Pharmacopeia Promoting the Quality of Medicines program support joint dossier review for the East African Community (Kaddu et al., 2018). These joint reviews have clear benefits to manufacturers seeking a more streamlined process for market entry, and they advance the goal of regulatory convergence (Kaddu et al., 2018). They also involve participants from the various regulatory agencies in the region, who return to their home offices with stronger technical skills and professional relationships with colleagues abroad.
The presence of trade secret information in inspection reports can be a barrier to information sharing. There are strategies to address this barrier, however. Confidentiality agreements among agencies can enable information sharing. In 2017, for example, confidentiality agreements between the European Medicines Agency (EMA) and the FDA allowed the agencies to share inspection reports from medicines manufacturers in their respective
jurisdictions and beyond, including manufacturing centers like India and China (FDAMap, 2017). Such agreements can be useful, though there are legally simpler tools, such as unilateral reliance, discussed later in this chapter, that may work better for less advanced agencies.
Low- and middle-income countries are increasingly working toward regional harmonization. Because of the economic implications for trade, regional economic communities have been leaders in harmonization, these include the East African Community, the Economic Community of West African States, the Association of Southeast Asian Nations (ASEAN), and the Asia-Pacific Economic Cooperation (Kamwanja et al., 2011; Pombo et al., 2016).
Regulatory cooperation programs are so common that even understanding the scope and goals of various and sometimes overlapping programs can be challenging. To complicate the matter, these efforts are often not well documented, especially the ones involving most low- and middle-income countries (Roth et al., 2018). Some lessons can be garnered, however, from analysis of some long-standing regulatory cooperatives in Europe and Latin America.
Examples of Regional Cooperation
The EMA, a regulatory network for European Union countries, has existed since 1995, but the legal framework supporting its work was put into place about 30 years earlier (Allchurch et al., 2016). Market authorization was the impetus for collaboration, driven by the European Community’s common market (Allchurch et al., 2016). Today, EMA members rely on each other’s inspection reports, clinical trials, and market surveillance data, and have pooled resources to develop electronic systems for data sharing (Allchurch et al., 2016). Fully harmonized drug laws are still a goal for EMA members, a reminder that significant meaningful cooperation is possible even without perfect equivalence among systems (Allchurch et al., 2016).
Regional regulatory harmonization and work-sharing programs in Latin American and Southeast Asia have a roughly 20-year history (Pombo et al., 2016; Teo, 2016). The Pan American Network for Drug Regulatory Harmonization and the ASEAN Pharmaceutical Product Working Groups have both made good progress producing harmonized technical guidelines for their members (Pombo et al., 2016; Teo, 2016). Both programs have also identified similar barriers to implementing these guidelines, including lack of resources and capacity (Pombo et al., 2016; Teo, 2016). If the national regulatory agency does not have enough staff, or staff with suitable training, even relatively simple guidelines can be aspirational. Other times, agencies lack the resources to follow technical guidelines. For example,
guidelines on regulatory action against falsified and substandard medicines may reference detection technologies that not all agencies have (Pombo et al., 2016). Full implementation of technical guidance can also be challenging, especially when the regional guidance is intended for countries at widely different maturity levels (Pombo et al., 2016; Teo, 2016).
Donor support for harmonization, including that recommended in the previous chapter, tends to focus on removing technical barriers. Providing a secure, electronic platform for information sharing, for example, is necessary for harmonization and something donors can provide (Allchurch et al., 2016; Luigetti et al., 2016). The non-technical barriers to harmonization are more the purview of national governments. Lack of political will or trust among countries, for example, is an important non-technical barrier, and one that takes time to overcome (Luigetti et al., 2016; Rägo, 2013). There may also be legal barriers preventing regulatory authorities from participating in harmonization, if there is no legal guarantee for confidentiality, for example (Luigetti et al., 2016).
Some forms of regulatory cooperation take longer to establish then others, though none will get far without leadership from the national government. Much of the responsibility for empowering regulators to collaborate with other countries and international organizations lies with the national government. Given the increasingly global nature of food and drug markets, regulatory cooperation is crucial, especially for regulators in small or less developed countries. If done properly, regulatory processes can be harmonized across whole groups of countries, making it easier to ensure compliance with requirements, and also controlling the regulatory burden on industry.
The safety of food and medical products is one of a range of public health matters that transcend national boundaries and have political, social, and economic dimensions. The term “global health diplomacy” is used to describe such matters and the negotiations at many levels that influence health policy (Kickbusch et al., 2007). Global health diplomacy serves the goals of both protecting health and building relationships among countries (Brown et al., 2018). Attention to these relationships is an investment in solving problems that require action at both national and international levels (WHO EMRO, n.d.). Supporting its regulatory agencies is part of government’s responsibility to its citizens. Political leaders can take a meaningful step to meeting that responsibility through attention to the laws that protect the regulatory agency.
Recommendation 4-1: National governments should guarantee in legislation that national regulatory agencies be independent and financially viable, with statutes that encourage cooperation with other agencies and require a scientific basis for decision making.
TABLE 4-2 Structure and Funding of a Sample of Regulatory Agencies
| Country (Regulatory Agency) | Structure | Funding Source(s) | Comments |
|---|---|---|---|
| Argentina (Administración Nacional de Medicamentos Alimentos y Tecnología) | Autonomous | Subvention | Administratively and financially independent but user fees go to central funding; decision making is independent |
| Australia (Therapeutic Goods Administration) | Autonomous | User fees | |
| Ethiopia (Food and Medicine and Health Care Administration and Control Authority) | Semi-autonomous | Subvention User fees | Under Department of Health but reports to parliament |
| Ghana (Food and Drugs Authority) | Operationally autonomous (not financially) | Subvention User fees | Does not sit under the Ministry of Health; independent agency that reports to the minister |
| India (Drug Controller General of India, Drug Control Authority) | Semi-autonomous, under Ministry of Health | Subvention | Minimal user fees, which are unsustainable and provided to the ministry |
| Indonesia (Badan Pengawas Obat dan Makanan) | Ministry-level institution | Subvention | Minimal user fees; head of Badan Pengawas Obat dan Makanan is a minister-level position, reports to president |
| Lao People’s Democratic Republic (Food and Drug Department) | Not autonomous, under Ministry of Health | Subvention Donors |
| Netherlands (Medicines Evaluation Board) | Autonomous | User fees | Regulatory decisions are independent of ministry |
| Pakistan (Drug Regulatory Authority Pakistan) | Autonomous | Subvention User fees | Under Ministry of Health but independent in its decision making; minimal government funding (~2 percent) |
| Papua New Guinea (Pharmaceutical Service Standards Branch) | Not autonomous, under Department of Health | Subvention | Fees are returned to treasury |
| Singapore (Health Sciences Authoritya) | Autonomous, under Ministry of Health | Subvention User fees | Statutory board under Ministry of Health, autonomy in decision making |
| South Africa (South African Health Products Regulatory Authority) | Autonomous, under the Department of Health | Subvention User fees | Independent public entity that retains revenue generated, employs its own staff, and is accountable to parliament |
| United States of America (U.S. Food and Drug Administration [FDA]) | Autonomous, under Department of Health and Human Services | Subvention User fees | Fee proportions vary by centers; decisions made almost exclusively by civil servants in the FDA (delegated decision making by law and regulation) |
| Zimbabwe (Medicines Control Authority of Zimbabwe) | Autonomous, not under Ministry of Health | User fees | Not under Ministry of Health, but minister is responsible for actions |
a Health Products Regulation Group.
SOURCE: Roth et al., 2018.
In evaluating the support governments provide to their regulatory agencies, it easy to become overly focused on institutional form, if the agency operates as an autonomous or semi-autonomous division of the ministry of health, for example, or as a separate entity. There are multiple effective structures regulatory agencies can take (see Table 4-2); the best match for a particular country depends mostly on national history and context. Attention to the agencies’ authority, accountability, capacity to monitor the market and enforce rules, and ability to protect public health should be the priority for legislators designing or updating a regulatory authority.
OECD guidance on regulatory policy gives some attention to the role of core policies—strategic statements from the government defining the underlying principles and governing of the regulatory agency (OECD, 2016a). The guidance emphasizes the role of the executive branch in providing
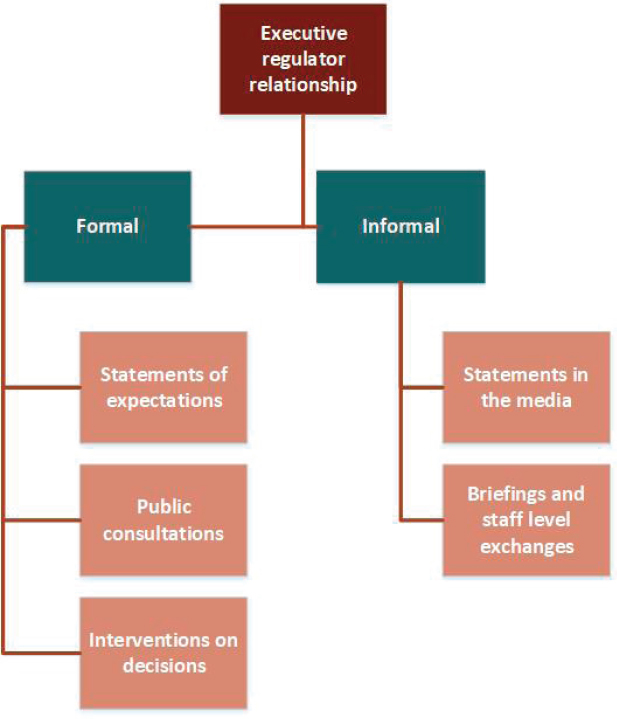
SOURCE: OECD, 2016b.
clarity regarding the role and responsibilities of the regulatory agency, and in setting up a system protected from undue political interference (OECD, 2014b). Beyond the setting of expectations, the relationship between the president or parliament of a country and its regulatory agency includes various formal and informal interactions, shown in Figure 4-2 (OECD, 2016b).
OECD guidance also encourages countries to provide clear authority for coordination among regulators (OECD, 2016a). This includes coordination between the national regulatory agency and its counterparts at sub-national and local levels, as well as coordination with its counterpart agencies abroad (OECD, 2016a). The ability of regulators to work effectively across jurisdictions is central to the functioning of a modern regulatory agency.
Food and medical product regulatory decisions are made at the local, national, regional, and global levels. Some jobs, the inspection of restaurants and pharmacies, for example, must be a local responsibility, while WHO prequalification and Codex standards for international food trade are necessarily global. In determining which jobs to assign to local authorities and which ones are candidates for international collaborative action, it is important to consider possible economies of scale. Work that depends on specialized information and skills is more suitable to regional or global cooperative arrangements. For example, a local health department has an obvious responsibility for food safety in its jurisdiction, the ideas of sharing such responsibility with foreign authorities is not appropriate. Not all distinctions are so clear, and some degree of overlap, particularly between national and shared regional or global responsibilities, is inevitable (OECD, 2014b). Both ASEAN and Pan American Network for Drug Regulatory Harmonization regulators have reported problems balancing subtly competing technical guidelines from their regional regulatory collaborative and
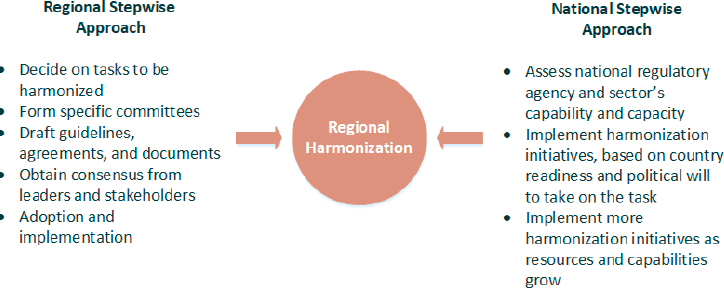
SOURCE: Teo, 2016.
global organizations such as the International Conference on Harmonization (Pombo et al., 2016; Teo, 2016). National laws can help clarify these gray areas, giving guidance on how to resolve possible conflicts (OECD, 2014b). Figure 4-3 shows steps that both national and regional authorities can take to ease the process of regulatory harmonization.
REFERENCES
Adashi, E. Y., R. S. Rajan, and I. G. Cohen. 2019. When science and politics collide: Enhancing the FDA. Science 364(6441):628–631.
Allchurch, M. H., D. B. Barbano, M. H. Pinheiro, and J. Lazdin-Helds. 2016. Fifty years of the European Medicines regulatory network: Reflections for strengthening intra-regional cooperation in the region of the Americas. Revista Panamericana de Salud Pública 39(5):288–293.
Arriola Penalosa, M. A., R. Cavazos Cepeda, M. Alanis Garza, and M. M. Lumpkin. 2017. Optimized medical product regulation in Mexico: A win-win for public and economic health. Therapeutic Innovation and Regulatory Science 51(6):744–750.
Aspen Institute. 2019. Former commissioners call for the FDA to be made into an independent federal agency. https://www.aspeninstitute.org/news/press-release/independent-fda (accessed August 9, 2019).
Austin, A. D. 2019. Economics of federal user fees. Washington, DC: Congressional Research Center.
Benson Wahlén, C. 2018. World Bank updates governance indicators data set. https://sdg.iisd.org/news/world-bank-updates-governance-indicators (accessed July 25, 2019).
Boakye, S. 2016. Revenue earmarking in Ghana: Management and performance issues. Occasional Paper Series (Institute for Fiscal Studies, Accra, Ghana).
Bollyky, T. J., and A. Stergachis. 2013. A report of the safety and surveillance working group. Bill & Melinda Gates Foundation. http://apps.who.int/medicinedocs/en/m/abstract/Js21347en (accessed December 8, 2019).
Brown, M. D., J. N. Bergmann, T. E. Novotny, and T. K. Mackey. 2018. Applied global health diplomacy: Profile of health diplomats accredited to the United States and foreign governments. Global Health 14(1):2.
Caipo, M. 2019. FAO/WHO Food Control System Assessment Tool. Paper presented at Committee on Stronger Food and Drug Regulatory Systems Abroad, February 25, San Jose, Costa Rica.
Califf, R. M., M. Hamburg, J. E. Henney, D. A. Kessler, M. McClellan, A. C. von Eschenbach, and F. Young. 2019. Seven former FDA commissioners: The FDA should be an independent federal agency. Health Affairs 38(1):84–86.
Cashin, C., S. Sparkes, and D. Bloom. 2017. Earmarking for health: From theory to practice. In WHO/HIS/HGF/HFWorkingPaper/17.5. https://www.who.int/health_financing/documents/earmarking-for-health/en (accessed February 13, 2020).
Creese, A. 2011. WHO/HAI project on medicine prices and availability. Geneva, Switzerland, and Amsterdam, Netherlands: WHO and HAI.
Dabrowska, A., and S. Thaul. 2018. Prescription Drug User Fee Act (PDUFA): 2017 reauthorization as PDUFA VI.
Falit, B. P., S. C. Singh, and T. A. Brennan. 2015. Biosimilar competition in the United States: Statutory incentives, payers, and pharmacy benefit managers. Health Affairs (Millwood) 34(2):294–301.
FAO (Food and Agricultural Organization of the United Nations). 2003. Annex 6. Guidelines for developing a national food law.www.fao.org/3/a-y8705e.pdf (accessed July 27, 2019).
FAO. 2019. Development law—Issue #1 of 2019. http://www.fao.org/legal/development-law/magazine-1-2019/ir/#second (accessed August 8, 2019).
FDA (U.S. Food and Drug Administration). 2018. Fact sheet: FDA at a glance. https://www.fda.gov/about-fda/fda-basics/fact-sheet-fda-glance (accessed April 29, 2019).
FDA. 2019. Regulatory harmonization and convergence. https://www.fda.gov/vaccines-blood-biologics/international-activities/regulatory-harmonization-and-convergence (accessed November 13, 2019).
FDAMap. 2017. The practical implications of FDA and EMA sharing confidential GMP information. https://www.fdamap.com/the-practical-implications-of-fda-and-ema-sharing-confidential-gmp-information.html (accessed September 30, 2019).
Fefer, E. 2012. Pharmaceutical legislation and regulation. In MDS-3: Managing Access to Medicines and Health Technologies. Arlington, VA: Management Sciences for Health.
GAO (U.S. Government Accountability Office). 1997. Federal user fees: Budgetary treatment, status, and emerging management issues. Washington, DC: U.S. Government Accountability Office.
GAO. 2015. Federal user fees: Key considerations for designing and implementing regulatory fees. Washington, DC: U.S. Government Accountability Office.
Garcia, R., and D. V. Araujo. 2016. The regulation of biosimilars in Latin America. Current Rheumatology Reports 18(3):16.
Gill, I., I. Bharali, and G. Glenday. 2019. Domestic revenue mobilization: Estimating the gaps between ability and effort (April 2019). http://centerforpolicyimpact.org/wp-content/uploads/sites/18/2019/04/CPIGH-Policy-brief_Domestic-Revenue-Mobilization__April-2019_FINAL.pdf (accessed August 5, 2019).
Glenday, G., I. Bharali, and Z. Wang. 2019. Enhancing domestic revenues: Constraints and opportunities—a cross country comparative study of tax capacity, effort and gaps. Durham, NC: The Center for Policy Impact in Global Health and Duke University Center for International Development.
Godman, B., A. Bucsics, P. Vella Bonanno, W. Oortwijn, C. C. Rothe, A. Ferrario, S. Bosselli, A. Hill, A. P. Martin, S. Simoens, A. Kurdi, M. Gad, J. Gulbinovic, A. Timoney, T. Bochenek, A. Salem, I. Hoxha, R. Sauermann, A. Massele, A. A. Guerra, Jr., G. Petrova, Z. Mitkova, G. Achniotou, O. Laius, C. Sermet, G. Selke, V. Kourafalos, J. Yfantopoulos, E. Magnusson, R. Joppi, M. Oluka, H. Y. Kwon, A. Jakupi, F. Kalemeera, J. O. Fadare, O. Melien, M. Pomorski, M. Wladysiuk, V. Markovic-Pekovic, I. Mardare, D. Meshkov, T. Novakovic, J. Furst, D. Tomek, C. Zara, E. Diogene, J. C. Meyer, R. Malmstrom, B. Wettermark, Z. Matsebula, S. Campbell, and A. Haycox. 2018. Barriers for access to new medicines: Searching for the balance between rising costs and limited budgets. Frontiers in Public Health 6:328.
Gordon, A. 2003. The delicate dance of immersion and insulation: The politicization of the FDA commissioner. Cambridge, MA: Harvard University.
Gotham, D., M. J. Barber, and A. Hill. 2018. Production costs and potential prices for biosimilars of human insulin and insulin analogues. BMJ Global Health 3(5):e000850.
Grace, D. 2015. Food safety in low and middle income countries. International Journal of Environmental Research and Public Health 12(9):10490–10507.
HAI (Health Action International). 2015. Inequities and inefficiencies in the global insulin market. Fact Sheet 1. Amsterdam, Netherlands: HAI.
Hodkinson, B., and M. Blockman. 2018. Strategies and ethics to ensure equitable access to biological medicines in the treatment of autoimmune inflammatory diseases. Current Allergy & Clinical Immunology 31(4).
IMF (International Monetary Fund). 2011. Revenue mobilization in developing countries. Washington, DC: International Monetary Fund Fiscal Affairs Department.
IOM (Institute of Medicine). 2012. Ensuring safe foods and medical products through stronger regulatory systems abroad. Washington, DC: The National Academies Press.
Jaffee, S., S. Henson, L. Unnevehr, D. Grace, and E. Cassou. 2019. The safe food imperative: Accelerating progress in low- and middle-income countries. Agriculture and food series. Washington, DC: World Bank.
Jalilian, H., C. Kirkpatrick, and D. Parker. 2007. The impact of regulation on economic growth in developing countries: A cross-country analysis. World Development 35(1):87–103.
Kaddu, G., E. D’Amore, A. Clark, and P. Nkansah. 2018. Strengthening regulatory systems to improve medical product quality in low- and middle-income countries. Rockville, MD: Promoting the Quality of Medicines (PQM) program. U.S. Pharmacopeial Convention.
Kamwanja, L. A., J. Saka, A. Awotedu, I. Fute, and M. Ndomondo-Sigonda. 2011. Situation analysis study on medicines registration harmonisation in Africa—Final report for the economic community of West African states (ECOWAS). Midrand, South Africa: NEPAD.
Kickbusch, I., G. Silberschmidt, and P. Buss. 2007. Global health diplomacy: The need for new perspectives, strategic approaches and skills in global health. Bulletin of the World Health Organizaton 85(3):230–232.
Luigetti, R., P. Bachmann, E. Cooke, and T. Salmonson. 2016. Collaboration, not competition: Developing new reliance models—regulatory collaboration. Exchange of assessment reports (ARS) with regulators outside the European Union (EU). WHO Drug Information 30(4):9.
MacDonald, J., F. Kuchler, J. Buzby, F. Lee, and L. Aldrich. 1999. User fees in federal agencies. In User-fee financing of USDA meat and poultry inspection. Washington, DC: U.S. Department of Agriculture.
Morgan, S. G., B. Yau, and M. M. Lumpkin. 2017. The cost of entry: An analysis of pharmaceutical registration fees in low-, middle-, and high-income countries. PLoS One 12(8):e0182742.
Moscou, K., J. C. Kohler, and A. MaGahan. 2016. Governance and pharmacovigilance in Brazil: A scoping review. Journal of Pharmaceutical Policy and Practice 9:3.
Ndomondo-Sigonda, M., J. Miot, S. Naidoo, A. Dodoo, and E. Kaale. 2017. Medicines regulation in Africa: Current state and opportunities. Pharmaceutical Medicine 31(6):383–397.
OECD (Organisation for Economic Co-operation and Development). 2014a. Being an independent regulator. In The governance of regulators. https://www.oecd-ilibrary.org/content/publication/9789264209015-en (accessed August 9, 2019).
OECD. 2014b. The governance of regulators, OECD best practice principles for regulatory policy: Paris, France: OECD Publishing.
OECD. 2016a. Governance of regulators’ practices: Accountability, transparency and coordination. Paris, France: OECD Publishing. https://www.oecd-ilibrary.org/governance/governance-of-regulators-practices_9789264255388-en (accessed August 9, 2019).
OECD. 2016b. How does independence work in practice?: Key trends and evidence. In Being an independent regulator. Paris, France: OECD Publishing.
OECD. n.d. Overview of methodologies. https://www.oecd.org/site/schoolingfortomorrowknowledgebase/futuresthinking/overviewofmethodologies.htm (accessed October 3, 2019).
Pezzola, A., and C. M. Sweet. 2016. Global pharmaceutical regulation: The challenge of integration for developing states. Global Health 12(1):85.
Pombo, M. L., A. Porras, P. C. Saidon, and S. M. Cascio. 2016. Regulatory convergence and harmonization: Barriers to effective use and adoption of common standards. Revista Panamericana de Salud Pública 39(5):217–225.
Porter, R., and S. Walsh. 2006. Earmarks in the federal budget process. Cambridge, MA: Harvard Law School.
Rägo, L. 2013. Reflections on the experiences of the World Health Organization. Washington, DC: National Academies of Sciences, Engineering, and Medicine. http://www.nationalacademies.org/hmd/Activities/Research/DrugForum/2013-FEB-13/Day2/Session4/28-Rago-Video.aspx (accessed September 12, 2019).
Roth, L., D. Bempong, J. B. Babigumira, S. Banoo, E. Cooke, D. Jeffreys, L. Kasonde, H. G. M. Leufkens, J. C. W. Lim, M. Lumpkin, G. Mahlangu, R. W. Peeling, H. Rees, M. Ndomondo-Sigonda, A. Stergachis, M. Ward, and J. Nwokike. 2018. Expanding global access to essential medicines: Investment priorities for sustainably strengthening medical product regulatory systems. Global Health 14(1):102.
Ruger, J. P. 2007. Global health governance and the World Bank. The Lancet 370 (9597):1471–1474.
Teo, H. S. 2016. Medicines regulatory systems and scope for regulatory harmonization in Southeast Asia. Washington, DC: The World Bank Group.
UN (United Nations). 2018. Countries urged to strengthen tax systems to promote inclusive economic growth. New York: United Nations Department of Economic and Social Affairs. https://www.un.org/development/desa/en/news/financing/tax4dev.html (accessed July 30, 2019).
UNODC (United Nations Office on Drugs and Crime). n.d. Model laws and treaties. https://www.unodc.org/unodc/en/legal-tools/model-treaties-and-laws.html (accessed November 13, 2019).
Vapnek, J., and M. Spreij. 2005. Perspectives and guidelines on food legislation, with a new model food law. Rome, Italy: Development Law Service FAO Legal Office.
WBG (The World Bank Group). 2018a. Global indicators of regulatory governance full data 2018. Washington, DC: The World Bank Group.
WBG. 2018b. Worldwide governance indicators. https://info.worldbank.org/governance/wgi/#home (accessed July 26, 2019).
Whitmore Schanzenbach, D., R. Nunn, L. Bauer, and M. Mumford. 2016. Where does all the money go: Shifts in household spending over the past 30 years. Washington, DC: The Hamilton Project.
WHO (World Health Organization). 1999. Marketing authorization of pharmaceutical products with special reference to multisource (generic) products: A manual for drug regulatory authorities.http://apps.who.int/medicinedocs/en/d/Js2273e/12.html (accessed February 13, 2020).
WHO. 1999. Annex 8: National drug regulatory legislation: Guiding principles for small drug regulatory authorities. Geneva, Switzerland: WHO.
WHO. 2014. Regulatory system strengthening for medical products. http://apps.who.int/medicinedocs/documents/s21456en/s21456en.pdf (accessed April 29, 2019).
WHO. 2017. Food safety and nutrition food law guidelines. Brazzaville, Congo: WHO Regional Office for Africa. https://www.afro.who.int/publications/food-safety-and-nutrition-food-law-guidelines (accessed August 8, 2019).
WHO. 2018a. WHO Global Benchmarking Tool (GBT) for evaluation of national regulatory system of medical products. In National Regulatory System (RS): Indicators and Fact Sheets. Geneva, Switzerland: WHO. https://www.who.int/medicines/areas/regulation/01_GBT_RS_RevVI.pdf?ua=1 (accessed July 26, 2019).
WHO. 2018b. WHO Global Benchmarking Tool (GBT) for evaluation of national regulatory system of medical products: Glossary and definitions revision VI version 1. Geneva, Switzerland: WHO.
WHO EMRO (Eastern Mediterraean Regional Office). n.d. Global health needs global health diplomacy. http://www.emro.who.int/health-topics/health-diplomacy/about-health-diplomacy.html (accessed November 7, 2019).
World Bank. n.d.-a. Global indicators of regulatory governance. https://rulemaking.worldbank.org (accessed September 9, 2019).
World Bank. n.d.-b. Transparency of rulemaking. https://rulemaking.worldbank.org/en/data/comparedata/transparency (accessed September 9, 2019).


























