5
Science of Compounded Topical Pain Creams
As discussed in Chapter 2, there are potential advantages for the use of topical medications to treat pain indications, including provision of local, regional, and systemic therapeutic effects, as well as the potential for better safety profiles than oral medications.1 Owing in part to their clinical potential, topical medications are one of the most commonly compounded preparations in the United States (HHS OIG, 2016; McPherson et al., 2016). The term topical refers to all preparations and products that are intended for application on the skin, mucous membranes, or external body cavities (e.g., mouth, nose, vagina).2 The term cream is used to designate any semisolid preparation (e.g., cream, ointment, gel, lotion), typically with a relatively soft and spreadable consistency, that is intended for external application to the skin.
To explore how the science of compounded topical pain creams affects their safety and effectiveness, this chapter begins with an overview of the art and science of compounding, which highlights the overall complexity involved in formulating safe and effective compounded preparations. This section is followed by a description of the dermal absorption of drugs, including the basic structure and properties of the skin that affect absorption and potential variation in skin absorption among individuals. Subsequent sections describe factors affecting drug delivery and dose, including
___________________
1 For a more detailed discussion on the use of topical creams in pain management, see Chapter 2.
2 Transdermal patches use a more complex topical delivery system that is designed to deliver drugs intended for systemic absorption; however, such systems are outside of the study’s scope.
properties of active ingredients, excipients, and penetration enhancers. Box 5-1 provides key definitions for this chapter.3 See Appendix D for a full glossary of terms.
___________________
3 This chapter draws on a paper commissioned by the Committee on the Assessment of the Available Scientific Data Regarding the Safety and Effectiveness of Ingredients Used in Compounded Topical Pain Creams titled “Topical Dosage Form Development and Evaluation,” by S. Narasimha Murthy (see Appendix C).
THE ART AND SCIENCE OF COMPOUNDING
Formulation science is critical in the development, manufacturing, and testing of chemical—including pharmaceutical—products and preparations. Individuals experienced in this area of expertise are able to make difficult determinations regarding the proper combination of active and inactive ingredients, in consideration of the quality, stability, and effectiveness of compounded preparations, including topical pain creams (American Chemical Society, 2020). It is important to note that compounding pharmacists often follow formulations provided by compounding supply companies or published in journals or textbooks (Birnie, 2004; Dooms and Carvalho, 2018); however, they are still individually responsible for assessing the quality, stability, and effectiveness of every compounded preparation they dispense. Of critical importance, a compounding pharmacist often does not have the same training or experience as a formulation scientist, nor access to the same data for evaluation and determination of quality, stability, and effectiveness. See Box 5-2 for an overview of selected considerations for formulation scientists.
Finally, given that compounding is an extemporaneous process, compounded topical pain creams are formulated based on unique prescriptions filled by individual pharmacies. As a result, compounded topical pain creams are more susceptible to modifications in process variables than manufactured drug products that are made by a single drug maker with detailed, U.S. Food and Drug Administration (FDA)-approved manufacturing protocols (Gudeman et al., 2013). Furthermore, the process by which a preparation is compounded is subject to interpharmacist and interpharmacy variations, so a patient may receive a compounded topical pain cream that
has distinct skin permeation and bioavailability profiles depending on who fills the prescription or where it is filled.
At the committee’s public meeting in May 2019, invited speaker Dr. S. Narasimha Murthy discussed how the process by which a formulation is compounded is critical to the attributes and performance of any drug. He concluded that changing even one variable in the compounding process—such as amount of time homogenizing the mixture, or the sequence in which drugs are added—will change the formulation microstructure, even if identical quantities of the same ingredients are used (Murthy, 2019). These changes in microstructure lead to notable differences in the characteristics and performance of the formulation and, ultimately, to differences in the absorption and bioavailability of the drug (Chang et al., 2013).
DERMAL ABSORPTION
Dermal absorption is a critical consideration in the science of compounded topical pain creams. The clinical benefits and potential harms of topically applied medications relate to the rate and extent of a drug’s absorption into the skin and beyond. Topically absorbed drugs first penetrate the outer barrier of dead skin cells (stratum corneum), then move through the viable layers of the epidermis to reach the vascularized dermis layer of the skin (see Figure 5-1). Skin cells (keratinocytes) are produced in the lower basal layer of the epidermis and then migrate upward, forming the epidermal skin. Specialized cells (melanocytes) in the epidermis produce melanin, which is taken up by keratinocytes to protect the skin from ultraviolet (UV) radiation; other cells have immunological functions (e.g., Langerhans cells, dendritic cells, memory T cells) (Richmond and Harris, 2014). Nerve endings extend from the dermis toward the epidermis like root fibers (Sewell et al., 2018).
In addition to having blood vessels, the dermis is primarily composed of extracellular matrix proteins that provide structure and elasticity, while also allowing free movement of immune cells (Richmond and Harris, 2014). The skin thus maintains high immunological surveillance and activity to ward off pathogenic and foreign substances. However, this process may also contribute to allergenic reactions to active ingredients or excipients.
Local, Regional, and Systemic Effects
Once a drug ingredient has crossed the outer layer of skin (stratum corneum), topical preparations such as pain creams can have local, regional, and/or systemic effects. Local effects of a drug ingredient occur primarily in the viable layers of skin, including the nerve endings in the epidermis and dermis (Ruela et al., 2016). Regional-area effects in muscles or joints occur
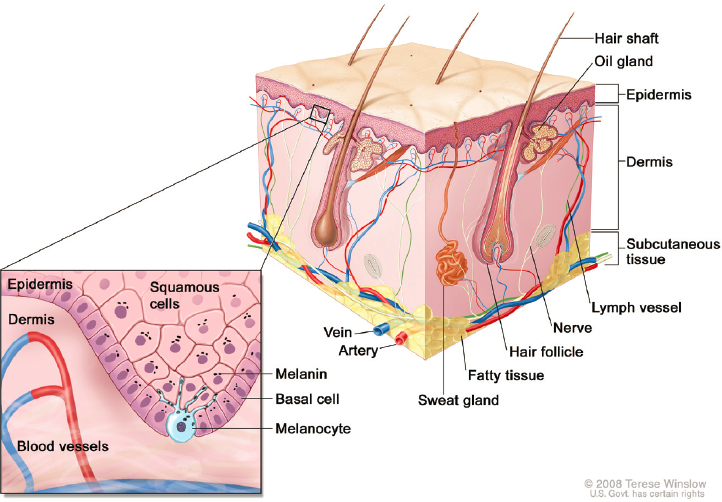
NOTES: The product or preparation must be formulated so the active drug is released from the cream or the patch into the skin. Additionally, the active drug must penetrate the outer protective barrier of the skin (stratum corneum) and reach the viable lower layers of the epidermis and dermis to effectively treat pain. Transdermal patches use a more complex topical delivery system to deliver drugs intended for systemic absorption; such systems are outside of the study’s scope.
SOURCE: Adapted from Winslow, 2008.
through subsequent diffusion of the drug ingredient through the skin and fatty layer—which also has nerve endings (Richmond and Harris, 2014)—to nearby tissues. Systemic effects occur throughout the body caused by uptake of the drug ingredient by blood or lymphatic vessels in the dermis; the ingredient ultimately travels to the central circulation (Ruela et al., 2016).
In theory, pharmaceuticals intended to treat local or regional pain act on nerves in the skin or in underlying muscles or joints by blocking nerve signals, reducing inflammation, relaxing muscle spasms, or potentiating the effects of other substances (Cline and Turrentine, 2016; Leppert et al., 2018). Distal responses to topical applications can also occur that, whether intended or not, can affect a medication’s safety and effectiveness profile. Systemic action is also an important consideration in reviewing potential drug–drug interactions. As an example, consider a patient who is prescribed the maximum oral amount of a nonsteroidal anti-inflammatory drug (NSAID) and then uses a topical gel formulated with another NSAID.
If the systemic contribution of the topically applied NSAID is sufficient, it will contribute to an excess of NSAIDs in the blood stream and create the potential for severe adverse reactions.
Today, most commercially manufactured FDA-approved creams are intended for local action, while patches are intended for systemic activity. However, there are exceptions in both cases. For example, nitroglycerin cream has long been used for its systemic effect (Fougera Pharmaceuticals, 2019), while lidocaine patches are generally used for regional analgesic effect in muscle tissue (Leppert et al., 2018). Of note, many compounded pain creams are marketed as transdermal (Swidan and Mohamed, 2016) and are intended to deliver the drug to tissues below the skin or into systemic circulation. For systemic activity, the transdermal patches or cream formulations deliver the drug into the systemic circulation through uptake in blood vessels in the dermis (Benson, 2005). (See Figure 5-2 for an illustrative example of the systemic absorption of a topical active ingredient.)
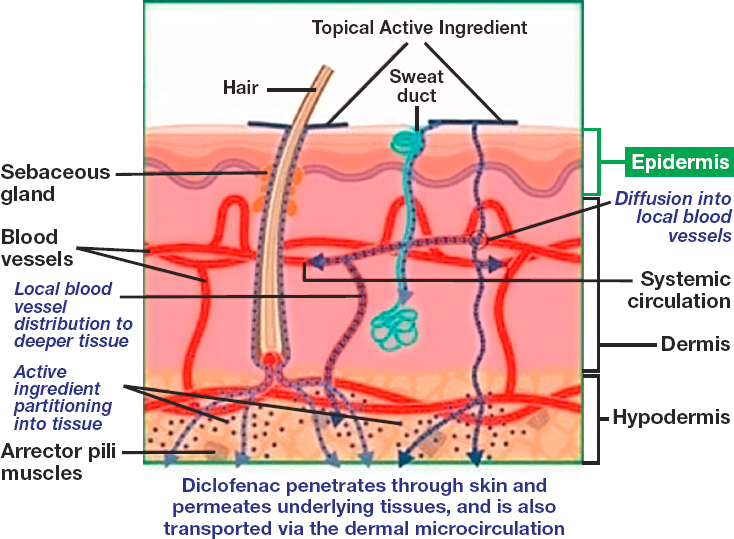
NOTES: Drugs that are applied topically can reach systemic circulation if the drug is able to penetrate to the blood vessels in the dermis. The figure was adapted from an illustration for topical diclofenac; for other active pharmaceutical ingredients, additionally absorption pathways may exist.
SOURCE: Adapted from Hagen and Baker, 2017.
FACTORS AFFECTING DERMAL ABSORPTION
The physical and chemical properties of skin are modified by factors relating to age, gender, and ethnicity, and these factors affect the dermal absorption of topical drugs. Physiochemical properties of active pharmaceutical ingredients (APIs) and features of the drug’s delivery mechanisms also contribute to the success of absorption through the skin (for more details, see Law et al., 2020). These considerations are discussed in greater detail in the sections below.
The Properties of Skin
When topical formulations are applied, the drug (e.g., the API) is released from the formulation and crosses several skin barriers—each with different physical and chemical properties that affect drug diffusion—to reach deeper sites for its pharmacological activity (Chang et al., 2013). The stratum corneum is the main barrier to external agents (Law et al., 2020). This layer is made up of tightly packed dead cells (corneocytes) in a lipid (fat) matrix, resulting in a relatively impermeable, mostly lipophilic layer (van Logtestijn et al., 2015) (see Figure 5-3). Moderately lipophilic (i.e., fat- or oil-soluble) drugs are able to pass the stratum corneum more readily than those that are hydrophilic (i.e., water soluble) or highly lipophilic. Skin also typically contains 10–20 percent water by weight, much of which is associated with hydrated corneocytes (Forslind, 1994; Singh and Morris, 2011). The lipid layer between corneocytes is composed of approximately equal molar amounts of free fatty acids, ceramide (waxy lipids), and cholesterol organized in a bilayer lamellar pattern, with more polar groups on the outer layers and long-chain fatty acids (waxlike) and cholesterol in the interior (Boncheva, 2014) (see Figure 5-3).
Pathways of Substances Through the Outer Barrier of Skin
Pathways of substances through the stratum corneum include diffusion through the lipid layer around skin cells (paracellular or intercellular), passage through the skin cells (transcellular), and entry through sweat or sebaceous glands or hair follicles (transappendageal) (Dabrowska et al., 2018) (see Figure 5-4). Substances that are soluble in oil or fats are able to penetrate the outer layer of skin though the lipid matrix around skin cells. Hydrating the skin, however, can increase the moisture content by 20-fold and may enhance the passage of substances that are more water soluble (Singh and Morris, 2011). Transcellular passage through skin cells is a more selective route that requires lipophilic and hydrophilic properties to cross the lipophilic cell membrane and the hydrophilic cellular contents.
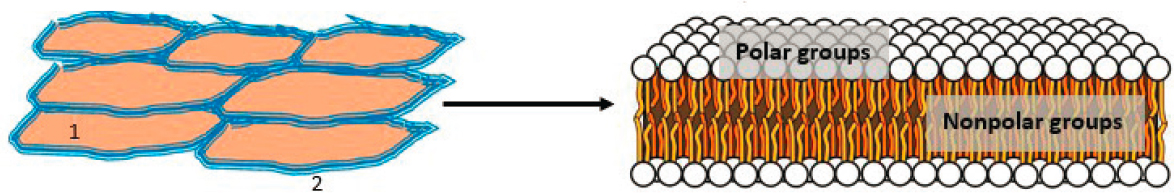
NOTE: (Left) Top view of the stratum corneum showing tightly packed skin cells (corneocytes in light brown) with intercellular space filled with lipids (blue). (Right) The intercellular lipids arranged in a bilayer lamellar pattern.
SOURCE: Phospholipids aqueous solution structures by Mariana Ruiz Villarreal, LadyofHats is licensed under Creative Commons CC0.
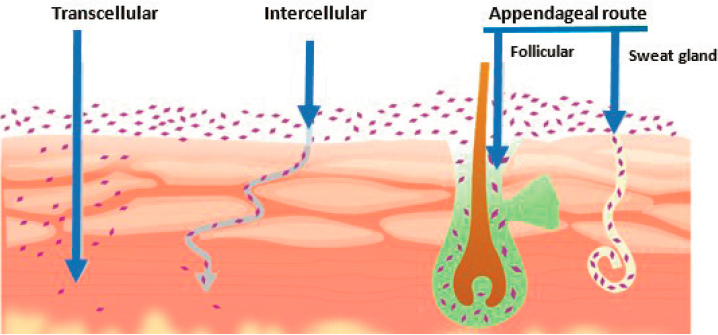
NOTE: Intracellular or transcellular transport through skin cells is seen on the left. Intercellular or paracellular transport around the cells is presented in the middle. Appendageal transport through hair follicles and pores is seen on the right.
SOURCE: Shaker et al., 2019.
Compared to the stratum corneum, the underlying viable epidermis and the dermis below that layer are more hydrophilic (i.e., more soluble in water) (Perrie et al., 2012), thereby favoring diffusion of hydrophilic substances. However, the lower fatty layer below the dermis favors diffusion of more lipophilic substances to adjacent tissues (see Figure 5-4).
The transappendageal pathway through skin pores and hair follicles is a means of entry for large or hydrophilic substances (Ruela et al., 2016). This could be considered a less important pathway, because pores represent only 0.1 percent of the surface area of the body (Dabrowska et al., 2018). In addition, although these pores extend below the skin layers into the dermis, substances must then penetrate the cellular barrier lining the pores. It has been reported that hair on the faces of men increases the permeability of the facial skin of men compared to women (Dabrowska et al., 2018).
Regional Differences in Skin Absorption on the Body
Regional differences in skin absorption on the body occur as a function of thickness of the outer stratum corneum layer, differences in skin lipid content (including those attributable to variation in sebaceous glands), hydration state, and amount of physical contact (Guzzo et al., 1996). Skin penetration is thus greater on the face, in the genital area, and in skin areas that contact or rub against each other; less penetration occurs on the trunk,
forearms, palms, and soles (Guzzo et al., 1996; Law et al., 2020; Prausnitz et al., 2012).4
Individual Differences in Metabolic Enzyme Activity
Individual differences in metabolic enzyme activity also play a role. Unlike the oral administration of drugs, the dermal route of exposure avoids the gastrointestinal tract and the first-pass metabolism process in the liver. Still, the skin is estimated to have about 10 percent of the capacity of the liver to metabolize drugs to either inactive or active forms, depending on the drug and enzymes (Singh and Morris, 2011). Therefore, individual differences in metabolic enzyme activity may also have an effect on topical dosing.5
Integrity of the Skin
Integrity of the skin is another important factor. Dermal penetration is increased in skin that is denuded or compromised (e.g., skin that is burned, abraded, or dry). Therefore, dermal penetration is affected by diseases that disrupt the skin—such as psoriasis or atopic dermatitis—or by health conditions such as hypothyroidism (Dabrowska et al., 2018).6 In general, enhancement of absorption through damaged or diseased skin is greater for substances that are more water soluble than for substances that are fat soluble (Law et al., 2020). Increased absorption through compromised skin could increase the effectiveness of topical treatments, but it may also enhance potential systemic absorption and increased the risk of toxicity (Law et al., 2020).
Effect of Age
Age can have effects on the skin that may alter dermal absorption.7 Preterm infants have a thinner epidermis and stratum corneum than adults,
___________________
4 For additional resources on the regional differences in skin absorption, see Bronaugh and Maibach, 1991; Feldmann and Maibach, 1967; and Rougier et al., 1986.
5 For drugs that reach viable layers and systemic circulation, elimination from the body occurs through urinary or biliary excretion routes, either metabolized or unmetabolized (Feldmann and Maibach, 1969). For more information on the metabolism of drugs in skin, see Bronaugh et al., 2005.
6 Note that for the purposes of this report, the committee maintained an explicit focus on the use of topical creams on intact skin. There remains a substantial literature base that reviews the safety, effectiveness, and use of topical pain creams on membrane and mucosal surfaces. However, this evidence was not explicitly reviewed or discussed in this report.
7 For an in-depth review of the effects of age on skin absorption, refer to Roskos et al., 1989.
thus higher permeability is expected (Oranges et al., 2015). Before 30 weeks of gestation, infants have 100- to 1,000-fold greater skin permeability than term infants (Barker et al., 1987), while full-term infants have skin permeability that is 3-fold to 4-fold greater than adults (Fernandez et al., 2011). Higher permeability in neonates is thought to be related to incomplete maturation of the skin barrier (Singh and Morris, 2011). Full-term neonates have similar epidermal thickness and lipid composition as adults. However, in the first few months of life, surface pH decreases while sloughing of the outer layer of skin cells (desquamation) increases. In the first week after birth, sebum (lipid) secretion increases, and in the first 14–17 weeks after birth, capillary loops and cutaneous blood flow develop in the dermis (Ramos-e-Silva et al., 2012).
In adults, skin changes that occur with aging result in increasing dryness of the stratum corneum (Ramos-e-Silva et al., 2012) as well as thinning of the epidermis and dermis (Singh and Morris, 2011). Evidence suggests that drying of the stratum corneum with age is associated with less active sebaceous glands and lower surface lipid content—along with atrophy of the cutaneous capillaries—thereby reducing drug delivery through viable layers (Perrie et al., 2012; Ramos-e-Silva et al., 2012). Skin penetration by more hydrophilic drugs has been reported to decrease with age, whereas lipophilic drugs were not similarly affected (Perrie et al., 2012; Singh and Morris, 2011).
Compared to men, infants, children, and many women have larger surface areas relative to their body size and relatively larger volumes of drug distribution. As a result, they absorb a larger internal dose from an application to a proportionally similar skin area. For example, a study using stable isotopes of nanosized zinc oxide particles (19 nm) in sunscreen measured higher zinc isotope blood levels in women than in men, even though a smaller amount of sunscreen per area (g/cm2) was applied to the backs of women than to the backs of men (Gulson et al., 2010). The study was unable to distinguish the form of zinc absorbed, but it may have been soluble ionic zinc.
Difference Among Genders
Compared to women, men have longer keratinocytes, larger pores, more active sebaceous and sweat glands, and lower skin pH (Singh and Morris, 2011). However, the few studies that have examined gender differences in dermal absorption have found little difference in dermal absorption between men and women (Singh and Morris, 2011).
Racial and Ethnic Differences
Ethnic differences in skin properties that may potentially affect dermal absorption have been described in the literature, with some conflicting reports. Several studies report that compared to Caucasians, African Americans or Afro-Caribbeans have higher transepidermal water loss (see Muizzuddin et al., 2010), a thicker and more cohesive stratum corneum with more cell layers, and lower dermal penetration (Dabrowska et al., 2018; Muizzuddin et al., 2010; Singh and Morris, 2011). Higher levels of natural moisturizing factors in the stratum corneum have been reported in Chinese compared to Caucasians or African Americans; however, other studies report no significant differences in skin hydration among ethnic groups (Dabrowska et al., 2018).
A small experimental study compared dermal penetration of radio-labeled benzoic acid, caffeine, or acetyl-salicylic acid in Asian, African American, and Caucasian volunteers (6–9 per group). The vehicles (i.e., carrier substances) used for each of the three compounds to form the topical cream or gel were optimized for the compound’s properties; absorption was measured analyzing urine and skin tape strips. The study found no statistically significant differences among the three racial groups (Lotte et al., 1993). Another study measured percutaneous absorption using methyl nicotinate in four lipophilic vehicles in four ethnic groups (12 subjects per group; subjects aged 20–60 years). The study reported the order of increasing rate of absorption among the groups as Blacks < Asians < Caucasians < Hispanics (Leopold and Maibach, 1996). Considerable individual variation was observed within these groups, but the only statistically significant difference was lower skin absorption in Blacks compared to Hispanics (Leopold and Maibach, 1996). Absorption rates were more similar among racial groups than among vehicles, with vehicles showing consistent rank order of absorption rate for all four racial groups.
A larger study involving 73 African Americans, 119 Caucasians, and 149 East Asians reported the following racial differences in stratum corneum properties, in order of lowest to highest (Muizzuddin et al., 2010):
- Transepidermal water loss: African Americans < East Asians < Caucasians
- Skin barrier strength: East Asians < Caucasians < African Americans
- Stratum corneum cohesion (protein content): East Asians and Caucasians < African Americans
- Ceramides (lipid lamellae in the stratum corneum): African Americans < East Asians and Caucasians
- Maturation index: East Asians < Caucasians < African Americans
- Proteolytic enzymes8: African Americans < East Asians and Caucasians
Overall, the understanding of racial differences in skin properties and penetration of drugs is limited by the small number of studies, small numbers of study participants in most of those studies, and high levels of individual variation (Dabrowska et al., 2018). This work is further limited by the likelihood of great individual-level variability, which may be affected by diet and nutrition, health and diseases, genetic and environmental factors, socioeconomic status, and age. Furthermore, comparisons among skin types with different amounts of pigmentation may be confounded by greater UV damage in those with less pigmentation, particularly in older adults (Singh and Morris, 2011).
Physiochemical Properties of the Active Pharmaceutical Ingredient
Complex Considerations
The physiochemical properties of a drug’s active ingredients play a substantial role in the drug’s absorption. For example, a drug’s ability to be absorbed into the skin is affected by whether its substances are lipophilic (soluble in oil) or hydrophilic (soluble in water), as well as whether its substances are acidic or basic (i.e., pH). The surface of the skin is acidic in nature, while the inner layers have a more neutral physiological pH (Ohman and Vahlquist, 1994). The pH gradient across the different layers prevents microorganisms from penetrating into deeper layers of skin (Schmid-Wendtner and Korting, 2006), but it also poses challenges for drug absorption. In addition, the skin tissue contains enzymes to break down substances, which constitute a metabolic barrier (Pyo and Maibach, 2019). Therefore, drug properties that increase dermal absorption include
- low molecular weight, generally less than 400–500 Daltons;
- moderately lipophilic properties;
- a melting point below 200°C;
- both lipophilic and hydrophilic properties; and
- a high partition coefficient, so the drug will partition from the vehicle to the skin.
In addition to the above properties, a drug’s acid dissociation constant or acid ionization constant (pKa) is another important factor determining
___________________
8 Enzymes that help in the breakdown of skin protein as part of the turnover (sloughing) of the outer layer of skin.
dermal absorption. At an ambient pH that is equal to a drug’s pKa, half of the drug will be in the ionized form and half will be in the un-ionized form. At pH levels greater than the pKa, more of the drug will be in the ionized form. Therefore, a drug with a lower pKa will be in a more un-ionized state at skin pH (4–5) or neutral to basic pH. As noted in Appendix C, un-ionized forms are more extensively absorbed through nonpolar transdermal pathways through the skin than ionized forms are absorbed by polar pathways through the skin. In addition, oppositely charged drugs may form neutral ion pairs that, in turn, enhances their skin absorption (Hadgraft and Valenta, 2000). This complexity is critical to consider in selecting the appropriate APIs to compound together within a given formulation.
As an example of the considerations to be made, Table 5-1 provides a summary of select physiochemical properties of APIs commonly used in compounded topical pain creams. As outlined in the table, each ingredient, regardless of drug class, has unique considerations for the formulation process. Additional layers of complexity need to be considered in cases where multiple APIs (each with different pKAs) are combined with multiple ingredients within an excipient into a single formulation. See Box 5-3 for an illustrative example of considerations related to the absorption of compounded topical formulations with multiple APIs. For an additional discussion of these complex considerations, see Naik et al. (2000), Ng (2018), Prausnitz et al. (2012), Sewell et al. (2018), and Appendix C in this report.
Evidence to Evaluate Topical Absorption
There is limited clinical evidence available to evaluate the extent of topical absorption or potential transdermal penetration of ingredients commonly used in compounded topical pain creams. In vitro skin permeation testing using Franz diffusion cells is one method used by researchers to examine a drug’s potential permeability through animal or human skin (see Appendix C for an additional description of the assay). Research using these types of in vitro studies to examine the permeability of topical analgesics indicate that out of the reviewed drugs, ketamine has the highest flux, or rate of permeation over a specified area of human cadaver skin. In addition, there is a relatively high percent of drug absorbed for ketamine, diclofenac, and pentoxifylline with lower absorption for baclofen, bupivacaine, orphenadrine, clonidine, and gabapentin (Bassani and Banov, 2016; Wang and Black, 2013) (see Tables 5-2 and 5-3). However, these studies did not report the mass balance of applied and recovered drugs, an important quality-control measure for testing of percutaneous absorption (Kluxen et al., 2019). Therefore, it is not possible to thoroughly evaluate the methodological procedures used in the studies, nor is it possible to make conclusive statements about the drugs’ absorption.
| Drug | Molecular Weight (g/mol) | Log P (oct-water) | Melting Point (°C) | pKa |
|---|---|---|---|---|
| Amitriptyline | 277.40 | 4.92 | 197 | 9.4 |
| Baclofen* | 213.66 | 1.3 | 207 | 9.62 and 3.67 |
| Bupivacaine | 288.43 | 2.59 | 107 | 8.2 |
| Cannabidiol | 314.46 | — | 66 | 5.79 |
| Carbamazepine | 236.27 | 2.45 | 191 | 7 |
| Clonidine | 230.09 | 1.59 | 130 | 8.12 |
| Cyclobenzaprine* | 275.39 | 5.2 | 218 | 8.47 |
| Dexamethasone | 392.46 | 1.93 | 262 | 12.42 |
| Doxepin | 273.38 | –0.548 | 184 | 8.96 |
| Gabapentin | 171.24 | –1.1 | 166 | 3.68 and 10.70 |
| Ketamine | 237.73 | 3.12 | 92.5 | 7.5 |
| Lidocaine hydrochloride | 270.80 | < 0 | 77 | 7.9 |
| Meloxicam | 351.40 | 3.43 | 254 | 4.08 |
| Memantine | 179.31 | 3.28 | 258 | 10.27 |
| Naproxen | 230.26 | 2.79 | 153 | 4.15 |
| Nifedipine | 346.30 | 2.50 | 173 | 4.3 |
| Orphenadrine | 269.39 | 3.77 | 156 | 8.91 |
| Pentoxifylline | 278.31 | 0.38 | 105 | — |
| Topiramate | 339.36 | –0.5 | 125 | 8.6 |
| Tramadol | 263.38 | 1.34 | 181 | 9.4 |
NOTES: To add an additional layer of complexity of physiochemical properties that affect dermal absorption, certain drugs have chemical functional groups on the drug molecule with different pKa values, such as baclofen (pKa of 9.62 for the amino group and 3.67 for the carboxyl group) and gabapentin (pKa of 10.70 for the primary amine and 3.68 for carboxylic acid group). octwater = octanolwater partition coefficient; pKa = acid ionization constant.
* Zwitterionic in nature.
SOURCES: DrugBank, 2020; Expert Committee on Drug Dependence, 2017; Plumley et al., 2009; PubChem, 2020.
SOURCES: DrugBank, 2020; Expert Committee on Drug Dependence, 2017; Plumley et al., 2009; PubChem, 2020.
Given the limitations of in vitro data for many topical drugs, mathematical models are often used for predicting skin permeability. Skin permeability resulting in an absorbed dose can be modeled as a flux rate—a function of partition and diffusion coefficients over a length of the path to the target site, and the applied concentration—resulting in an amount of
drug reaching this site per time (Keurentjes and Maibach, 2019; Prausnitz et al., 2012) (see also Appendix C for additional discussion). Models of flux rate based on a compound’s octanol-water partition coefficient and size (molecular weight) have been reported to more accurately predict in vivo skin permeability than more complex models involving more parameters (Lian et al., 2008).
Nevertheless, a study recently evaluated three such simple models used to calculate fluxes of 17 drugs used in FDA-approved transdermal delivery systems. For more than two-thirds of these drugs, researchers found
| Active Pharmaceutical Ingredient | Percent of Applied Drug Dose Absorbed Across the Cadaver Skin | |
|---|---|---|
| Reference Cream | Versatile Cream | |
| Bupivacaine | 0.277 ± 0.108 | 0.441 ± 0.175 |
| Diclofenac | 0.846 ± 0.223 | 1.96 ± 0.896 |
| Gabapentin | 0.381 ± 0.429 | 0.30 ± 0.237 |
| Ketamine | 1.03 ± 0.317 | 1.45 ± 0.591 |
| Orphenadrine | 0.130 ± 0.0495 | 0.191 ± 0.0613 |
| Pentoxifylline | 1.51 ± 0.451 | 3.63 ± 1.78 |
NOTE: Versatile cream is a base commonly used in formulations for compounded topical creams.
SOURCE: Wang and Black, 2013.
| Active Pharmaceutical Ingredient | Percent of Applied Drug Dose Absorbed Across the Cadaver Skin | |
|---|---|---|
| Lipoderm | Lipoderm ActiveMax | |
| Baclofen | 0.27 ± 0.27 | 0.10 ± 0.08 |
| Clonidine | 3.955 ± 2.60 | 4.38 ± 0.95 |
| Gabapentin | 0.41 ± 0.34 | 0.19 ± 0.08 |
| Ketamine | 35.48 ± 9.03 | 45.52 ± 2.42 |
NOTE: Lipoderm and Lipoderm ActiveMax are two bases commonly used in formulations for compounded topical creams.
SOURCE: Bassani and Banov, 2016.
overestimation or underestimation by 10 to 100 times compared to experimental in vivo data. Although the model predictions were correlated with the in vivo results, the models underpredicted the rate flux for more than half of the drugs. There were several major limitations of the models: the models had uncertainties in their parameter databases, and the in vivo data were based on studies in the literature that used different study designs, sample sizes, and analytical methods (Keurentjes and Maibach, 2019). Overall, many factors limit the ability to predict skin absorption for many topically applied drugs.
CRITICAL FACTORS AFFECTING DRUG DELIVERY: ACTIVE INGREDIENTS AND EXCIPIENTS
Considerations for Active Ingredients
Not all drugs are absorbed in the skin to the same degree or at the same rate. In fact, some drugs may not be absorbed by the skin at all. Compounding pharmacists and other individuals who compound must consider not only physiochemical properties of active ingredients, but also how dose affects drug absorption, mechanisms of action of APIs, and selection of excipients. The sections below provide a brief overview of select critical factors that affect drug delivery through the skin.
Applied and Absorbed Dose of the Active Pharmaceutical Ingredient
Dose can refer the amount of drug administered to the individual (applied dose) or taken into the body (absorbed dose). For topical pain cream ingredients, the relevant dose for efficacy is the mass of drug delivered to the site of action. Key determinants of dose for a topical formulation involve (1) the concentration of the drug in the cream formulation, (2) the amount of cream applied, (3) the surface area of application on the body relative to body weight, and (4) the frequency of application (Law et al., 2020). Based on simple diffusion, the magnitude of the applied dose represents the driving force for dermal absorption (Prausnitz et al., 2012). Although the amount of drug applied to the skin is easily determined, the amount of absorption and the effective internal dose may vary depending on factors related to the drug or formulation, the application (including the vehicle), and properties of the skin and underlying tissues (Guzzo et al., 1996).
Furthermore, absorption through the skin is not necessarily constant for a given applied dose. For example, a large amount of cream applied to a smaller area versus a smaller amount of cream applied to a larger area may have the same applied dose. However, an excessive amount of cream over a small area may not allow all of the drug in the cream to contact the skin for absorption before it is rubbed off, adsorbed to clothing, or washed off.9
Similarly, the amount of drug that is solubilized in the vehicle is the available concentration for absorption, meaning that increasing the concentration of a drug in a cream will only increase the dose being delivered if the drug is solubilized (Prausnitz et al., 2012). Depending on drug properties and the vehicle, drugs may also penetrate the stratum corneum and either reside as a reservoir in skin layers or more readily reach systemic circulation
___________________
9 Of note, drug removal from the stratum corneum may also occur either by exfoliation (Law et al., 2020) or by washing, which is dependent on the nature of the drug and the solvent (Chan et al., 2013).
(Guzzo et al., 1996; Prausnitz et al., 2012). Another important consideration affecting the dose of a drug that penetrates the skin is frequency of application. Though data are sparse, multiple doses of topically applied drug have been shown to deliver more drug through the skin than single doses (Wester and Maibach, 2005). For example, 1 gram of cream applied twice daily may deliver more drug than 2 grams of cream applied once daily.
Insufficient dermal penetration for some drugs may reduce the internal dose to below the therapeutic range (Yamamoto et al., 2017). On the other hand, a slower rate of entry via skin absorption may achieve a more constant systemic dose rather than the peaks—which could result in toxicity for more sensitive individuals—and valleys that might occur by intravenous injection or oral ingestion (Brown et al., 2006; Prausnitz et al., 2012). For some substances that have low absorption by the oral route (e.g., CBD oil), dermal application with or without skin penetration enhancers has been reported to be an effective means of systemic drug delivery (Lodzki et al., 2003; Paudel et al., 2010a). This is particularly the case for substances that are greatly metabolized by the first-pass effect of the liver from the oral route of administration (Paudel et al., 2010b).
Mechanisms of Action of Active Ingredients
The local, regional, or systemic location where a drug is intended to exert its pharmacological effect is informed largely by the mechanism of action of that drug (Schenone et al., 2013). For example, a drug that blocks sodium channels would need to reach nerve cells, which contain sodium channels that mediate stimuli such as pain (Bhattacharya et al., 2009). Details on the mechanisms of actions for the drugs reviewed within this report can be found in Chapter 6.
Excipients (“Inactive” Ingredients)
A wide range of bases, vehicles, and solvents are used to formulate topical pain creams. These would generally be classified as excipients, which constitute a wide range of materials that influence the quality attributes of topical products, physicochemical characteristics of the drug, and sensorial characteristics (i.e., taste, smell, texture) of the formulation. Excipients are frequently called “inactive” ingredients. However, it is important to note that excipients will ultimately affect the final performance of the compounded preparation by affecting properties such as solubility, stability, release of the active ingredient, and skin penetration. In some cases, patients are allergic to certain excipients; this requires preparations to be specially compounded without these allergens. Specific bases, vehicles, and solvents may be used in compounding to avoid certain allergenic excipients used in
commercial products (e.g., peanut oil) or to support patient compliance by adding flavoring to a medication for a young child (McBane et al., 2019). Excipients are critical components of a topical pain cream formulation and play a number of roles, including
- enhancing the solubility of the active drug,
- modifying the viscosity of the cream,
- emulsifying the formulation,
- enhancing drug penetration,
- stabilizing the formulation,
- extending the shelf life of a cream, and
- modifying the sensorial properties of the cream.
The United States Pharmacopeia (USP) provides a list of excipients as well as instructions to verify their identity and purity. USP <795> advises that all excipients in compounded preparations should meet compendial standards or otherwise be evaluated for safety and purity (USP, 2018). Table 5-4 contains a partial list of excipients found within published monographs from the USP-National Formulary, which outline quality standards for each listing. A second list of more than 1,700 unique excipients are published online in FDA’s inactive ingredient database, which also describes the maximum potencies of excipients in FDA-approved products (FDA, 2019). These lists were developed over years of study to identify inactive ingredients that have been shown to be generally safe for inclusion in compounded preparations and commercial drug products. As such, they serve
TABLE 5-4
Compendial Excipients from USPNational Formulary 19
| Functional Categories of Excipients (number) | Listing of Excipients |
|---|---|
| Emollient (35) | Alkyl (c1215) benzoate; almond oil; aluminum monostearate; canola oil; castor oil; cetostearyl alcohol; cholesterol; coconut oil; cyclomethicone dimethicone; ethylene glycol stearates; glycerin; glyceryl monooleate; glyceryl monostearate; hydrogenated lanolin; isopropyl isostearate; isopropyl myristate; isopropyl palmitate; isostearyl isostearate; lecithin; mineral oil; mineral oil, light; myristyl alcohol; octyldodecanol; oleyl alcohol; oleyl oleate; petrolatum; polydecene, hydrogenated; propylene glycol dilaurate; propylene glycol monolaurate; safflower oil; soybean oil, hydrogenated; sunflower oil; wax, cetyl esters; xylitol; zinc acetate |
| Functional Categories of Excipients (number) | Listing of Excipients |
|---|---|
| Ointment base (24) | Caprylocaproyl polyoxylglycerides; coconut oil; diethylene glycol monoethyl ether; lanolin; lanolin, hydrogenated; lanolin alcohols; lauroyl polyoxylglycerides; linoleoyl polyoxylglycerides; ointment, hydrophilic; ointment, white; ointment, yellow; oleoyl polyoxylglycerides; paraffin; petrolatum; petrolatum, hydrophilic; petrolatum, white; polydecene, hydrogenated; polyethylene glycol; polyethylene glycol 3350; polyethylene glycol monomethyl ether; polyglyceryl 3 diisostearate; rose water ointment; squalane; stearoyl polyoxylglycerides; vegetable oil, hydrogenated, type II; vitamin E polyethylene glycol succinate |
| Stiffening agent (18) | Alphalactalbumin; castor oil, hydrogenated; cetostearyl alcohol; cetyl alcohol; cetyl palmitate; dextrin; hard fat; paraffin; paraffin, synthetic; rapeseed oil, fully hydrogenated; rapeseed oil, superglycerinated fully hydrogenated; sodium stearate; stearyl alcohol; wax, cetyl esters; wax, emulsifying; wax, microcrystalline; wax, white; wax, yellow |
| Suppository base (6) | Agar; cocoa butter; hard fat; palm kernel oil; polyethylene glycol; polyethylene glycol 3350 |
| Suspending and/or viscosityincreasing agent (89) | Acacia; agar; alamic acid; alginic acid; alphalactalbumin; aluminum monostearate; attapulgite, activated; attapulgite, colloidal activated; bentonite; bentonite, purified; bentonite magma; carbomer 910; carbomer 934; carbomer 934p; carbomer 940; carbomer 941; carbomer 1342; carbomer copolymer; carbomer homopolymer; carbomer interpolymer; carboxymethylcellulose calcium; carboxymethylcellulose sodium; carboxymethylcellulose sodium 12; carboxymethylcellulose sodium, enzymatically hydrolyzed; carmellose; carrageenan; cellulose, microcrystalline; cellulose, microcrystalline, and carboxymethylcellulose sodium; cellulose, powdered; cetostearyl alcohol; chitosan; corn starch, pregelatinized hydroxypropyl corn; corn syrup; corn syrup solids; cyclomethicone; dextrin; egg phospholipids; ethylcellulose; gelatin; gellan gum; glyceryl behenate; glyceryl dibehenate; guar gum; hydroxyethyl cellulose; hydroxypropyl cellulose; hypromellose; isomalt; kaolin; magnesium aluminum silicate; maltitol solution; maltodextrin; mediumchain triglycerides methylcellulose; pectin; polycarbophil polydextrose; polydextrose, hydrogenated; polyethylene oxide; polysorbate 20; polysorbate 40; polysorbate 60; polysorbate 80; polyvinyl alcohol; potassium alginate; povidone; propylene glycol alginate; pullulan; silica, dentaltype; silica, hydrophobic colloidal; silicon dioxide; silicon dioxide, colloidal; sodium alginate; sorbitan monolaurate; sorbitan monooleate; sorbitan monopalmitate; sorbitan monostearate; sorbitan sesquioleate; sorbitan trioleate starch, corn; starch, hydroxypropyl; starch, hydroxypropyl pea; starch, hydroxypropyl potato; starch, pea; starch, potato; starch, pregelatinized hydroxypropyl pea; starch, pregelatinized hydroxypropyl potato; starch, tapioca; starch, wheat; sucrose; sucrose palmitatel tragacanth; vitamin E polyethylene glycol succinate; xanthan gum |
SOURCE: USP, 1999.
as important resources to support the selection and use of excipients in compounded preparations (Osterberg et al., 2011).10
Of particular concern for compounded topical formulations is the marketing and use of excipients that do not have quality standards established by USP or have not been evaluated by FDA. Consequently, those excipients have limited (if any) available evidence of quality, safety, and functionality for their use in compounded preparations. Even in the case of excipients that have been reviewed for use in one commercial topical product, their effect on dermal delivery and absorption may not be the same when the excipients are used for another formulation. Understanding the effects of individual ingredients is further confounded when multiple inactive and active ingredients are incorporated into formulations.
Composition and Safety Profiles of Ingredients Used in Excipients
A review of topical formulations in the Journal of Pharmaceutical Sciences explains that individuals who compound should consider “intended dosage form, route of administration, safety profile, manufacturing process, and regulatory aspects” when selecting excipients (Simoes et al., 2018). Of course, excipients that cause degradation of the API are undesirable, thus part of the formulation development process involves verifying that excipients do not cause API degradation. Changing ratios of components or substituting excipients can dramatically affect product quality or alter product performance.
Additionally, when designing a formulation, it is important to understand which excipients have the potential to interact with each other by way of synergistic or opposing excipient–excipient interactions (Beraldode-Araújo et al., 2019; Karande and Mitragotri, 2009). For example, an excipient used to decrease viscosity may affect the performance of a permeation enhancer, thus inadvertently increasing the absorbed dose of drug delivered to the tissues below (Osborne and Musakhanian, 2018). On the other hand, if inappropriately formulated, the effects of excipients may have actions that oppose one another (Karande and Mitragotri, 2009). For example, the inclusion of certain preservatives may decrease the effect of an emulsifying agent. As an important consequence, the use of proprietary bases that do not disclose the composition makes it difficult for even the most experienced formulation scientist to evaluate how the ingredients may affect product quality and performance.
In a search for publically available formulations of compounded topical pain creams, the committee identified three examples that listed excipients
___________________
10 Additional reference lists are available in international pharmacopeia, such as the European Pharmacopeia (see https://www.edqm.eu/en/databases [accessed March 2, 2020]).
| Select Excipients | Active Pharmaceutical Ingredients in Select Compounded Topical Pain Creams | Listed Excipients |
|---|---|---|
| Sample 1 |
Amitriptyline HCl 2%
Baclofen 2% Ketamine HCl 5% Ketoprofen 10% |
Ethyl alcohol |
| Ethoxy diglycol reagent | ||
| Emulsifix | ||
| Lipoderm | ||
| Sample 2 |
Baclofen 2%
Clonidine HCl 0.2% Gabapentin 10% Ketamine HCl 5% |
Propylene glycol |
| Lipoderm | ||
| Sample 3 |
Amitriptyline HCl 2%,
Clonidine HCl 0.01% |
Glycerine USP |
| Lipoderm |
SOURCE: FDA, 2018.
(see Table 5-5). It is important to note that after reviewing these excipients, the committee determined the following:
- The rationale for the selection of the chemicals in the formulations is unclear.
- It is unclear whether the excipients selected for these pain creams were selected based on guidance from the FDA and USP lists.
- It is unclear whether any of the ingredients that are not on the FDA or USP lists could be unsafe individually or in combination.
- No information is available about whether these ingredients cause instability in the API or interact unfavorably with each other.
Among the listed excipients in Table 5-5, Lipoderm is listed twice and Emulsifix is listed once. Unfortunately, neither Emulsifix nor Lipoderm provide a publicly available list of excipients to inform the decision-making process of the pharmacists, prescribing clinician, or patient. This is also the case with several other proprietary bases used in compounded preparations.
One exception is Lipoderm ActiveMax, a patented base that is similar to Lipoderm and Emulsifix in that it contains preformulated excipients for topical application. The composition of Lipoderm ActiveMax is available on a publicly available patent (Ray and Hodge, 2013) and is detailed below in Table 5-6. In reviewing the composition of this base, half of the listed excipients are not included either in the USP-National Formulary 19 compendium or in the FDA inactive ingredient database. In addition, given the long list of excipients included, it is difficult to evaluate the potential
TABLE 5-6
Listed Components in Lipoderm ActiveMax from Patent US2013/0085171
| Excipient | Listed on FDA-Approved Inactive Ingredient Database (Y/N) | Listed on USP List of Excipients | Listed on the Homœopathic Pharmacopœia of the United States |
|---|---|---|---|
| Water | n/a | Y | n/a |
| Cetearyl alcohol | Y | N | N |
| Piukenetia volubilis seed oil | N | N | N |
| Isopropyl myristate | Y | Y | N |
| Propylheptyl caprylate | N | N | N |
| Sodium stearoyl glutamate | N | N | N |
| PEG8/SMDI copolymer | N | N | N |
| PEG100 stearate | N | N (includes other forms of PEGs) |
N |
| Glyceryl stearate | Y | Y | N |
| Glycerin | Y | Y | N |
| Tocopheryl acetate | N | Y (in other forms) |
N |
| Lecithin | Y | Y | N |
| Hydrogenated lecithin | N | N | N |
| Populus tremuloides bark extract | N | N | Y |
| Lonicera japonica (honeysuckle) flower extract | N | N | N |
| Lonicera caprifolium (honeysuckle) flower extract | N | N | N |
| Leuconostoc radish root ferment filtrate | N | N | N |
| Pentaclethra macroioba seed oil | N | N | N |
| Butyrospermum parkii (shea butter) | N | N | N |
| Carthamus tinctorius (safflower) seed oil | N | Y | N |
| Cocos nucifera (coconut) oil | Y | Y | N |
| Tocopherol | Y | Y | N |
| Excipient | Listed on FDA-Approved Inactive Ingredient Database (Y/N) | Listed on USP List of Excipients | Listed on the Homœopathic Pharmacopœia of the United States |
|---|---|---|---|
| Ascorbyl palmitate | Y | N (includes other forms of palmitate) |
N |
| Squalane | Y | Y | N |
| Ceramide 3 | N | N | N |
| Alcohol | Y | Y (in other forms) |
N |
| Caprylic capric triglyceride | N | Y (medium chain) | N |
| Xanthan gum | Y | Y | N |
| Gluconolactone | Y | N | N |
| Sodium dehydroacetate | N | N | N |
| Disodium edetate (EDTA) | Y | Y | N |
| Butylated hydroxytoluene | Y | N | N |
NOTE: EDTA = ethylenediaminetetraacetic acid; FDA = U.S. Food and Drug Administration; PEG = polyethylene glycol; SMDI = saturated methylenediphenyldiisocyanate; USP = United States Pharmacopeia.
SOURCES: FDA, 2019; HPUS, 2020; Ray and Hodge, 2013; USPNF, 2019.
interactions of these excipients with each other or with APIs included in the pain cream; the patent provides minimal information to this regard.11 Furthermore, it is doubtful that each of those 30 or more ingredients actually contribute a unique advantage to the formulation, because formulations should be designed to be as simple as possible (Chang et al., 2013). This casts a cloud of doubt on the necessity of all the listed excipients in optimizing the safety and the effectiveness of the preparation.
___________________
11 The available patent information generally describes how these excipients enhance penetration. For example, paragraph 0060 states “In particular, the absorption profiles indicate a rapid penetration to a peak flux for gabapentin and baclofen occurring approximately 1 hour after dose application, and between approximately 4 to approximately 10 hours after dose application for ketamine” (Ray and Hodge, 2013).
Classes of Excipients: Penetration Enhancers
Penetration enhancers are a class of excipients of additional importance. This type of excipient enhances penetration of a drug into the inner tissues and ultimately into the blood stream (Prausnitz and Langer, 2008).12 Many chemicals can enhance penetration through the outer skin barrier by disrupting the highly ordered structure of lipid bilayers. However, this increased transdermal penetration is also associated with increased potential for irritation (Prausnitz and Langer, 2008). Many preformulated bases sold by such companies as the Professional Compounding Centers of America and Medisca are marketed as transdermal bases and touted for their ability to penetrate deeper below the skin (Medisca, 2020; PCCA, 2013). In addition, even products intended to act near the skin surface, such as sunscreen agents, are actually absorbed into the systemic circulation (Matta et al., 2019). Clearly, safety is an issue if large doses of a topically applied drug are absorbed systemically, especially if it is assumed that the drug is limited to local action or a drug has a narrow therapeutic window.
The 11 commonly accepted categories of permeation enhancers, classified by chemical structure, are water; hydrocarbons; alcohols; acids; amines; amides; esters; surfactants; terpenes, terpenoids, and essential oils; sulfoxides; and lipids (Karande and Mitragotri, 2009). Between and within these classes are numerous chemicals with different properties that affect their effectiveness as enhancers. A recent analysis of skin permeation studied more than 40 different enhancers in hairless mouse skin or human epidermal membrane. The analysis found that molecules within the same class and those with similar structures can have drastically different enhancer potencies. For example, 2-nonanol exhibited an enhancer potency parameter over four times greater than 2-octanol (Li and Chantasart, 2019). Obviously, factors such as potency and concentration need to be considered by individuals who compound when they choose to add permeation enhancers to their compounded preparations.
Dimethyl sulfoxide (DMSO) is a penetration enhancer used in compounded topical pain creams that has repeatedly occasioned concern among the committee. DMSO appears to be used in compounded topical pain creams as both a penetration enhancer and as an active ingredient (Kopsky and Keppel Hesselink, 2011; Russo and Santarelli, 2016). While the evidence is far from conclusive, some studies suggest that DMSO may help treat osteoarthritis pain and complex regional pain syndrome (Brien et al., 2008; NHS, 2020). In fact, DMSO is the primary inactive ingredient in PENNSAID, an FDA-approved topical solution for osteoarthritis pain
___________________
12 For additional information on penetration enhancers and their various effects, see Dragičević and Maibach, 2017.
(Nuvo Research, 2016). However, the primary concern surrounding DMSO is its effect as a potent penetration enhancer.
The material safety data sheet for DMSO states “DMSO readily penetrates skin and may significantly enhance the absorption of numerous chemicals. Increased absorption of these other chemicals could lead to their increased toxicity” (Fisher Scientific, 2007). The instructions for use for PENNSAID caution patients to apply the medication to clean skin and to avoid the application of other drugs or cosmetics to the area (Nuvo Research, 2016). There is concern that the addition of DMSO to other ingredients in topical pain creams may affect toxicity and safety of the preparation. Furthermore, patients using compounded DMSO preparations may not be provided with adequate instructions and warnings to ensure safe use.
Given the potential complexity of compounding, the Federal Food, Drug, and Cosmetic Act (FDCA) requires that to qualify for exemptions from new drug approval and other, drugs compounded at 503A or 503B facilities must not “present demonstrable difficulties for compounding” and directed FDA to provide guidance on this matter.13 Accordingly, in 2016, FDA developed a “Difficult to Compound” list that will preclude the use of listed drugs in compounded preparations. Many examples of drug preparations and categories have been nominated to the Difficult to Compound List. However, no final determinations on which drugs will be included have been made to date.14 See Box 5-4 for FDA’s criteria for a drug that is difficult to compound.
___________________
13 Federal Food, Drug, and Cosmetic Act. 21 U.S. Code Chapter 9.
14 See Section 503A Bulks List Final Rule Questions and Answers; Guidance for Industry; Small Entity Compliance Guide; Availability. 84 FR 24027 (May 24, 2019) (FDA announces development of criteria to evaluate drugs as demonstrably difficult to compound under 503A and 503B).
REFERENCES
American Chemical Society. Formulation chemistry. https://www.acs.org/content/acs/en/careers/college-to-career/chemistry-careers/formulation-chemistry.html (accessed March 6, 2020).
Barker, N., J. Hadgraft, and N. Rutter. 1987. Skin permeability in the newborn. Journal of Investigative Dermatology 88(4):409–411.
Bassani, A. S., and D. Banov. 2016. Evaluation of the percutaneous absorption of ketamine HCL, gabapentin, clonidine HCL, and baclofen, in compounded transdermal pain formulations, using the Franz finite dose model. Pain Medicine 17(2):230–238.
Benson, H. A. 2005. Transdermal drug delivery: Penetration enhancement techniques. Current Drug Delivery 2(1):23–33.
Beraldo-de-Araújo, V. L., A. Beraldo-de-Araújo, J. S. R. Costa, A. C. M. Pelegrine, L. N. M. Ribeiro, E. d. Paula, and L. Oliveira-Nascimento. 2019. Excipient-excipient interactions in the development of nanocarriers: An innovative statistical approach for formulation decisions. Scientific Reports 9(1):10738.
Bhattacharya, A., A. D. Wickenden, and S. R. Chaplan. 2009. Sodium channel blockers for the treatment of neuropathic pain. Journal of the American Society for Experimental NeuroTherapeutics 6(4):663–678.
Birnie, C. 2004. Resources for today’s compounding pharmacist. Journal of the American Pharmacists Association 44(4):526.
Boncheva, M. 2014. The physical chemistry of the stratum corneum lipids. International Journal of Cosmetic Science 36(6):505–515.
Brien, S., P. Prescott, N. Bashir, H. Lewith, and G. Lewith. 2008. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthritis and Cartilage 16(11):1277–1288.
Bronaugh, R., and H. Maibach. 1991. In vitro percutaneous absorption: Principles, fundamentals, and applications, 1st ed. Boca Raton, FL: CRC Press.
Bronaugh, R., M. Kraeling, and J. Yourick. 2005. Skin metabolism during in vitro percutaneous absorption. In Percutaneous absorption: Drugs, cosmetics, mechanisms, methods, 4th ed., edited by R. Bronaugh, N. Dragičević, and H. I. Maibach. Boca Raton, FL: Taylor & Francis Group.
Brown, M. B., G. P. Martin, S. A. Jones, and F. K. Akomeah. 2006. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Delivery 13(3):175–187.
Chan, H. P., H. Zhai, X. Hui, and H. I. Maibach. 2013. Skin decontamination: Principles and perspectives. Toxicology & Industrial Health 29(10):955–968.
Chang, R. K., A. Raw, R. Lionberger, and L. Yu. 2013. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS Journal 15(1):41–52.
Cline, A. E., and J. E. Turrentine. 2016. Compounded topical analgesics for chronic pain. Dermatitis 27(5):263–271.
Dabrowska, A. K., F. Spano, S. Derler, C. Adlhart, N. D. Spencer, and R. M. Rossi. 2018. The relationship between skin function, barrier properties, and body-dependent factors. Skin Research and Technology 24(2):165–174.
Dooms, M., and M. Carvalho. 2018. Compounded medication for patients with rare diseases. Orphanet Journal of Rare Diseases 13(1):1.
Dragičević, N., and H. I. Maibach. 2017. Percutaneous penetration enhancers: Physical methods in penetration enhancement. Berlin, Germany: Springer-Verlag.
DrugBank. 2020. DrugBank database. https://www.drugbank.ca (accessed April 6, 2020).
Expert Committee on Drug Dependence. 2017. Cannabidiol (CBD). Geneva, Switzerland: World Health Organization Technical Report Series. https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf (accessed April 6, 2020).
FDA (U.S. Food and Drug Administration). 2018. Examples of topically applied pain medication formulas. Available through the Public Access File of the National Academies of Sciences, Engineering, and Medicine. https://www8.nationalacademies.org/pa/managerequest.aspx?key=HMD-HSP-18-18 (accessed April 6, 2020).
FDA. 2019. Inactive ingredient search for approved drug products. https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed March 6, 2020).
Feldmann, R. J., and H. I. Maibach. 1967. Regional variation in percutaneous penetration of 14c cortisol in man. Journal of Investigative Dermatology 48(2):181–183.
Fernandez, E., R. Perez, A. Hernandez, P. Tejada, M. Arteta, and J. T. Ramos. 2011. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics 3(1):53–72.
Fisher Scientific. 2007. Material safety data sheet dimethyl sulfoxide. https://fscimage.fishersci.com/msds/07770.htm (accessed March 6, 2020).
Forslind, B. 1994. A domain mosaic model of the skin barrier. Acta Dermato-Venereologica 74(1):1–6.
Fougera Pharmaceuticals. 2019. Nitro-bid-nitroglycerin ointment, label. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e464e9bb-48e8-4b9f-9fff-e220cfbac0c5 (accessed March 6, 2020).
Gudeman, J., M. Jozwiakowski, J. Chollet, and M. Randell. 2013. Potential risks of pharmacy compounding. Drugs in R&D 13(1):1–8.
Gulson, B., M. McCall, M. Korsch, L. Gomez, P. Casey, Y. Oytam, A. Taylor, M. McCulloch, J. Trotter, L. Kinsley, and G. Greenoak. 2010. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicological Sciences 118(1):140–149.
Guzzo, C. A., G. S. Lazurus, and V. P. Werth. 1996. Dermatological pharmacology. In Goodman & Gilmans’s the pharmacological basis of therapeutics, 9th ed., edited by J. G. Hardman, L. E. Limbird, P. B. Molinoff, R. W. Ruddon, and A. Goodmn Gilman. New York: McGraw-Hill Medical.
Hadgraft, J., and C. Valenta. 2000. pH, PKA and dermal delivery. International Journal of Pharmaceutics 200(2):243–247.
Hagen, M., and M. Baker. 2017. Skin penetration and tissue permeation after topical administration of diclofenac. Current Medical Research and Opinion 33(9):1623–1634.
HHS OIG (U.S. Department of Health and Human Services Office of Inspector General). 2016. High Part D spending on opioids and substantial growth in compounded drugs raise concerns. https://oig.hhs.gov/oei/reports/oei-02-16-00290.asp (accessed March 3, 2020).
HPUS (Homœopathic Pharmacopœia of the United States). 2020. Homœopathic pharmacopœia database. Subscription required to view. http://www.hpus.com (accessed April 6, 2020).
Karande, P., and S. Mitragotri. 2009. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochimica et Biophysica Acta 1788(11):2362–2373.
Keurentjes, A. J., and H. I. Maibach. 2019. Percutaneous penetration of drugs applied in transdermal delivery systems: An in vivo based approach for evaluating computer generated penetration models. Regulatory Toxicology and Pharmacology 108:104428.
Kluxen, F. M., S. Grégoire, A. Schepky, N. J. Hewitt, M. Klaric, J. Y. Domoradzki, E. Felkers, J. Fernandes, P. Fisher, S. F. McEuen, R. Parr-Dobrzanski, and C. Wiemann. 2019. Dermal absorption study OECD TG 428 mass balance recommendations based on the EFSA database. Regulatory Toxicology and Pharmacology 108:104475.
Kopsky, D. J., and J. M. Keppel Hesselink. 2011. Multimodal stepped care approach involving topical analgesics for severe intractable neuropathic pain in CRPS type I: A case report. Case Reports in Medicine 2011:319750.
Law, R. M., M. A. Ngo, and H. I. Maibach. 2020. Twenty clinically pertinent factors/observations for percutaneous absorption in humans. American Journal of Clinical Dermatology 21(1):85–95.
Leopold, C. S., and H. I. Maibach. 1996. Effect of lipophilic vehicles on in vivo skin penetration of methyl nicotinate in different races. International Journal of Pharmaceutics 139(1):161–167.
Leppert, W., M. Malec-Milewska, R. Zajaczkowska, and J. Wordliczek. 2018. Transdermal and topical drug administration in the treatment of pain. Molecules 23(3).
Li, S. K., and D. Chantasart. 2019. Skin permeation enhancement in aqueous solution: Correlation with equilibrium enhancer concentration and octanol/water partition coefficient. Journal of Pharmaceutical Sciences 108(1):350–357.
Lian, G., L. Chen, and L. Han. 2008. An evaluation of mathematical models for predicting skin permeability. Journal of Pharmaceutical Sciences 97(1):584–598.
Lodzki, M., B. Godin, L. Rakou, R. Mechoulam, R. Gallily, and E. Touitou. 2003. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. Journal of Controlled Release 93(3):377–387.
Lotte, C., R. C. Wester, A. Rougier, and H. I. Maibach. 1993. Racial differences in the in vivo percutaneous absorption of some organic compounds: A comparison between black, Caucasian and Asian subjects. Archives of Dermatological Research 284(8):456–459.
Matta, M. K., R. Zusterzeel, N. R. Pilli, V. Patel, D. A. Volpe, J. Florian, L. Oh, E. Bashaw, I. Zineh, C. Sanabria, S. Kemp, A. Godfrey, S. Adah, S. Coelho, J. Wang, L. A. Furlong, C. Ganley, T. Michele, and D. G. Strauss. 2019. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: A randomized clinical trial. JAMA 321(21):2082–2091.
McBane, S. E., S. A. Coon, K. C. Anderson, K. E. Bertch, M. Cox, C. Kain, J. LaRochelle, D. R. Neumann, and A. M. Philbrick. 2019. Rational and irrational use of nonsterile compounded medications. Journal of the American College of Clinical Pharmacy 2(2):189–197.
McPherson, T., P. Fontane, R. Iyengar, and R. Henderson. 2016. Utilization and costs of compounded medications for commercially insured patients, 2012-2013. Journal of Managed Care & Specialty Pharmacy 22(2):172–181.
Medisca. 2020. Cream base reference chart. https://www.medisca.com/Files/ReferenceCharts/Cream%20&%20Gel%20Bases%20Reference%20Chart%20-%20MUS.pdf (accessed March 6, 2020).
Muizzuddin, N., L. Hellemans, L. Van Overloop, H. Corstjens, L. Declercq, and D. Maes. 2010. Structural and functional differences in barrier properties of African American, Caucasian and East Asian skin. Journal of Dermatological Science 59(2):123–128.
Murthy, S. 2019. Topical formulations for dermal and transdermal drug delviery. Paper commissioned by the Committee on the Assessment of the Available Scientific Data Regarding the Safety and Effectiveness of Ingredients Used in Compounded Topical Pain Creams (see Appendix C).
Naik, A., Y. N. Kalia, and R. H. Guy. 2000. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharmaceutical Science and Technology Today 3(9):318–326.
Ng, K. W. 2018. Penetration enhancement of topical formulations. Pharmaceutics 10(2):51.
NHS (National Health Service, United Kingdom). 2020. DMSO cream (50%) for complex regional pain syndrome. https://www.iow.nhs.uk/Downloads/Chronic%20Pain/DMSO%20CRPS%20patient%20leaflet.pdf (accessed March 6, 2020).
Nuvo Research. 2016. Pennsaid (diclofenac sodium) topical solution label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020947s010s011lbl.pdf (accessed March 6, 2020).
Ohman, H., and A. Vahlquist. 1994. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Dermato-Venereologica 74(5):375–379.
Oranges, T., V. Dini, and M. Romanelli. 2015. Skin physiology of the neonate and infant: Clinical implications. Advances in Wound Care 4(10):587–595.
Osborne, D. W., and J. Musakhanian. 2018. Skin penetration and permeation properties of transcutol(r)-neat or diluted mixtures. AAPS PharmSciTech 19(8):3512–3533.
Osterberg, R. E., C. C. Demerlis, D. W. Hobson, and T. J. McGovern. 2011. Trends in excipient safety evaluation. International Journal of Toxicology 30(6):600–610.
Paudel, K. S., D. C. Hammell, R. U. Agu, S. Valiveti, and A. L. Stinchcomb. 2010a. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Development and Industrial Pharmacy 36(9):1088–1097.
Paudel, K. S., M. Milewski, C. L. Swadley, N. K. Brogden, P. Ghosh, and A. L. Stinchcomb. 2010b. Challenges and opportunities in dermal/transdermal delivery. Therapeutic Delivery 1(1):109–131.
PCCA (Professsional Compounding Centers of America). 2013. PCCA’s quick reference base guide. http://www.tachepharmacy.com/wp-content/uploads/2017/03/PCCAs-Quick-Reference-Base-Guide.pdf (accessed March 13, 2020).
Perrie, Y., R. K. Badhan, D. J. Kirby, D. Lowry, A. R. Mohammed, and D. Ouyang. 2012. The impact of ageing on the barriers to drug delivery. Journal of Controlled Release 161(2):389–398.
Plumley, C., E. M. Gorman, N. El-Gendy, C. R. Bybee, E. J. Munson, and C. Berkland. 2009. Nifedipine nanoparticle agglomeration as a dry powder aerosol formulation strategy. International Journal of Pharmaceutics 369(1–2):136–143.
Prausnitz, M. R., and R. Langer. 2008. Transdermal drug delivery. Nature Biotechnology 26(11):1261–1268.
Prausnitz, M. R., P. M. Elias, T. J. Franz, M. Schmuth, J.-C. Tsai, G. K. Menon, W. M. Holleran, and K. R. Feingold. 2012. Skin barrier and transdermal drug delivery. In Dermatology, 3rd ed., edited by J. Bolognia, J. Jorizzo, and J. Schaffer. Philadelphia, PA: Elsevier.
PubChem. 2020. PubChem: Explore chemistry search engine. https://pubchem.ncbi.nlm.nih.gov (accessed April 6, 2020).
Pyo, S. M., and H. I. Maibach. 2019. Skin metabolism: Relevance of skin enzymes for rational drug design. Skin Pharmacology and Physiology 32(5):283–294.
Ramos-e-Silva, M., J. C. Boza, and T. F. Cestari. 2012. Effects of age (neonates and elderly) on skin barrier function. Clinics in Dermatology 30(3):274–276.
Ray, J. R. I., and C. D. Hodge. 2013. Compounded transdermal pain management. U.S. Patent US9724315B2, filed December 16, 2011, and issued April 2013.
Richmond, J. M., and J. E. Harris. 2014. Immunology and skin in health and disease. Cold Spring Harbor Perspectives in Medicine 4(12):a015339.
Roskos, K. V., H. I. Maibach, and R. H. Guy. 1989. The effect of aging on percutaneous absorption in man. Journal of Pharmacokinetics and Biopharmaceutics 17(6):617–630.
Rougier, A., D. Dupuis, C. Lotte, R. Roguet, R. C. Wester, and H. I. Maibach. 1986. Regional variation in percutaneous absorption in man: Measurement by the stripping method. Archives of Dermatological Research 278(6):465–469.
Ruela, A. L. M., A. G. Perissinato, M. E. d. S. Lino, P. S. Mudrik, and G. R. Pereira. 2016. Evaluation of skin absorption of drugs from topical and transdermal formulations. Brazilian Journal of Pharmaceutical Sciences 52:527–544.
Russo, M. A., and D. M. Santarelli. 2016. A novel compound analgesic cream (ketamine, pentoxifylline, clonidine, DMSO) for complex regional pain syndrome patients. Pain Practice 16(1):E14–E20.
Schenone, M., V. Dančík, B. K. Wagner, and P. A. Clemons. 2013. Target identification and mechanism of action in chemical biology and drug discovery. Nature Chemical Biology 9(4):232–240.
Schmid-Wendtner, M. H., and H. C. Korting. 2006. The pH of the skin surface and its impact on the barrier function. Skin Pharmacology and Physiology 19(6):296–302.
Sewell, M. J., C. N. Burkhart, and D. S. Morrel. 2018. Dermatological pharmacology. In Goodman & Gilman’s the pharmacological basis of therapeutics, 13th ed., edited by L. L. Brunton, R. Hilal-Dandan, and B. C. Knollman. New York: McGraw-Hill Education.
Shaker, D. S., R. A. H. Ishak, A. Ghoneim, and M. A. Elhuoni. 2019. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Scientia Pharmaceutica 87(3):17.
Simoes, A., F. Veiga, C. Vitorino, and A. Figueiras. 2018. A tutorial for developing a topical cream formulation based on the quality by design approach. Journal of Pharmaceutical Sciences 107(10):2653–2662.
Singh, I., and A. P. Morris. 2011. Performance of transdermal therapeutic systems: Effects of biological factors. International Journal of Pharmaceutical Investigation 1(1):4–9.
Swidan, S. Z., and H. A. Mohamed. 2016. Use of topical pain medications in the treatment of various pain syndromes. Topics in Pain Management 31(7):1–8.
USP (United States Pharmacopeia). 1999. The United States Pharmacopeia national formulary (USP24 NF19). Philadelphia, PA: National Publishing.
USP. 2018. <795> pharmaceutical compounding—Nonsterile preparations. In The United States Pharmacopeial Convention, edited by USP. Rockville, MD: USP.
USP-NF (United States Pharmacopeia and National Formulary). 2020. The United States Pharmacopeia (USP) and the National Formulary (NF) compendia of monographs. Subscription required to view. https://www.uspnf.com (accessed April 6, 2020).
van Logtestijn, M. D., E. Dominguez-Huttinger, G. N. Stamatas, and R. J. Tanaka. 2015. Resistance to water diffusion in the stratum corneum is depth-dependent. PLoS ONE 10(2):e0117292.
Wang, X., and L. Black. 2013. Ex vivo percutaneous absorption of ketamine, bupivacaine, diclofenac, gabapentin, orphenadrine, and pentoxifylline: Comparison of versatile cream vs. reference cream. International Journal of Pharmaceutical Compounding 17(6):520–525.
Wester, R. C., and H. I. Maibach. 2005. Effect of single vs multiple dosing in percutaneous absorption. In Percutanous absorption: Drugs, cosmetics, mechanisms, methodology. 4th ed., edited by R. Boronaugh and H. I. Maibach. Boca Raton, FL: Taylor & Francis.
Winslow, T. 2008. Skin with melanocyte anatomy. PDQ® Screening and Prevention Editorial Board. PDQ Skin Cancer Screening. Bethesda, MD: National Cancer Institute. Updated March 27, 2020. https://www.cancer.gov/types/skin/patient/skin-screening-pdq (accessed April 7, 2020).
Yamamoto, S., M. Karashima, Y. Arai, K. Tohyama, and N. Amano. 2017. Prediction of human pharmacokinetic profile after transdermal drug application using excised human skin. Journal of Pharmaceutical Sciences 106(9):2787–2794.


































