This chapter summarizes the presentations and panel discussion from the workshop session on brain–body interactions. The session focused on how the brain interacts with the body and the implications these interactions have for measuring and maximizing brain health and resilience. Evidence from research on brain–body interactions demonstrates the importance of this connection and suggests possible avenues for future research on brain health. Colleen McClung, professor of psychiatry and clinical and translational science at the University of Pittsburgh, described the effect of circadian rhythms on health across the life span. The relationship between early environmental risk factors and mental health disorders was explored by Elinor Sullivan, associate professor at the University of Oregon. Natalie Rasgon, professor of psychiatry and behavioral sciences at the Stanford University Medical Center, explained how insulin resistance serves as a link in brain–body interaction.
EFFECT OF CIRCADIAN RHYTHMS ON HEALTH ACROSS THE LIFE SPAN
McClung gave a presentation on the effect of circadian rhythms on brain and body health across the life span. Circadian rhythms change over the life span and contribute to different diseases at different stages of life, starting from early fetal development all the way through to old
age. Box 3-1 details how circadian rhythms are coordinated centrally in the brain by the suprachiasmatic nucleus (SCN). She explained that across a 24-hour day, circadian rhythms are prominent in every process in the body, including coordination, alertness, reaction time, cardiovascular activity, body temperature, and sleep. Disease symptoms and processes also appear to have circadian rhythms that revolve around the 24-hour cycle. For example, heart attacks are more common in the morning, while symptoms of restless leg syndrome typically occur in the evening; gout attacks occur mostly in the middle of the night, whereas stomach ulcers tend to occur in the middle of the day.
Evolution of Circadian Rhythms Over the Life Span
Circadian rhythms evolve and change across a person’s life span, noted McClung. These rhythms are shaped initially during the very early stages of development. At the beginning of the life span, a mother’s circadian rhythms influence the development of fetal circadian rhythms, including tissue homeostasis and neurodevelopment as well as the development and consolidation of feeding, metabolic, and sleep–wake rhythms. Evidence is emerging that disruptions to a mother’s circadian rhythms during pregnancy caused by shift work, for example, can have a long-term effect on the offspring (Logan and McClung, 2019).
Infants typically do not have a regular sleep–wake pattern—they usually sleep every 2 or 3 hours and eat whenever they are hungry. Patterns begin to coalesce in early childhood, and then a person’s circadian rhythms undergo substantial changes during adolescence and into adulthood. After entering puberty, adolescents tend to undergo a shift in their circadian rhythms from an early to a late chronotype1 (Roenneberg et al., 2004). For adolescents who must wake very early for school, this shift can create a sense of circadian misalignment and sleep loss that is similar to jet lag and puts stress on the adolescent brain. As people age, their rhythm gradually shifts back toward an earlier chronotype.
Melatonin secretion also changes over the life span (Grivas and Savvidou, 2007). Newborns have very little melatonin secretion, but it increases sharply and peaks during the early childhood and preteen years. Melatonin secretion begins to decline around puberty and continues to decrease through middle age to minimal secretion during old age. For older people, this loss of melatonin contributes to a loss of synchrony
___________________
1 “Chronotype” refers to how an individual’s circadian clock synchronizes or entrains to the 24-hour day (Roenneberg et al., 2004).
of circadian rhythms, and, because melatonin is an effective antioxidant, it may also contribute to neural degeneration later in life.
A person’s specific individual genotype also contributes to circadian rhythms. For example, most people shift back from the late-night phenotype after adolescence, others remain “late night” people for the rest of their lives. Many people have regular, normal sleep phases (roughly in the window of 10 p.m. to 8 a.m.), but others have delayed sleep phase (sleeping from 4 a.m. to 12 p.m.) or advanced sleep phase (5 p.m. to 3 a.m.). Still others have irregular sleep–wake patterns or a non-24-hour sleep–wake rhythm. The latter is experienced by people who cannot entrain to the environment—owing to blindness, dementia, cognitive impairment, or mental disorders, for example—such that their rhythms shift slightly each day.
In addition to genetic changes in circadian rhythms or differences in circadian rhythms, the modern lifestyle has markedly influenced our circadian clocks. Artificial lighting at night disrupts normal circadian rhythms, as do shift work, travel across time zones, eating late at night, and consuming caffeine, alcohol, and other drugs. Dim lighting in the morning is incompatible with the way human brains evolved based on sun exposure during the morning and sleeping in darkness at night. These lifestyle factors can have serious consequences for brain health and body health.
Circadian Desynchrony Contributes to Different Diseases at Different Stages of Life
Whether it is caused by genetic or environmental factors or both, circadian desynchrony contributes to a variety of conditions, including brain diseases that have circadian rhythms at their core, such as bipolar disorder, major depression, drug addiction, and schizophrenia (Kasper et al., 2018). Because every organ has a circadian rhythm, circadian disruption also affects metabolism, obesity, diabetes, cancer, and cardiac health.
Addiction
Childhood and adolescent sleep characteristics can predict later substance abuse. Studies have associated a variety of substance abuse outcomes in teenagers and young adults with different types of sleep and circadian disturbances, such as sleep quality, sleepiness, evening preference, and weekend delays in sleeping patterns (see Figure 3-1). Teenagers that experience a strong shift toward the evening chronotype during adolescence tend to have lower prefrontal cortical activation in response to reward, which correlates significantly with alcohol consumption (Hasler
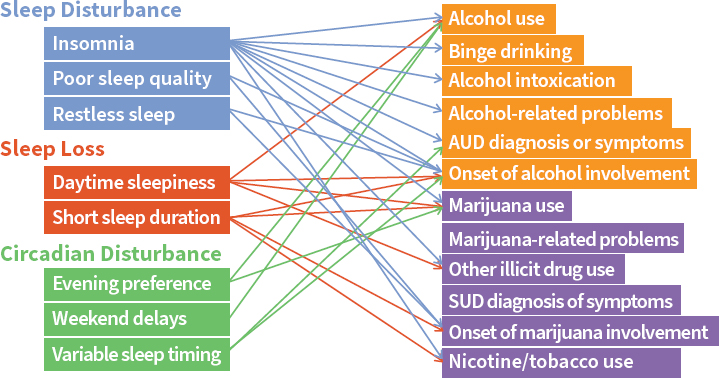
NOTE: AUD = alcohol use disorder; SUD = substance use disorder.
SOURCES: As presented by Colleen McClung at the workshop Brain Health Across the Life Span on September 24, 2019 (courtesy of Dr. Brant Hasler).
ncident Number for et al., 2013). This loss of top-down control tends to make them greater risk takers and increases the likelihood of impulsive activities, such as drug and alcohol consumption (Hasler et al., 2013). The ventral striatal response—the reward center of the brain—increases among people who are evening types, which is associated with greater alcohol dependence in young adults and teenagers. It has been posited that evening-type teenagers who are required to wake up very early in the morning are losing sleep because they cannot get to sleep at night, so they sleep very late on the weekends. This constant state of circadian misalignment and sleep deprivation contributes to increased risk for substance abuse.
Psychiatric Disorders
People with psychiatric disorders are profoundly influenced by changes in the circadian clock. Major disruption to the sleep and activity cycle is a common characteristic of disorders such as depression, bipolar disorder, schizophrenia, autism, and attention-deficit hyperactivity disorder (ADHD). In fact, bipolar disorder is becoming characterized as a circadian rhythm disorder, as schedule changes caused by international travel or night shift work can precipitate manic episodes, depressive episodes, or psychotic episodes. Depression is diurnal (i.e., worse in the morning), it is often seasonal, and it tends to occur more frequently in areas of the
world where there is little daylight for long periods of time. People with a preference toward “eveningness” are more susceptible to depression and make up the majority of people with bipolar disorder. Polymorphisms in several circadian genes associate with psychiatric disorders in humans and mice, and evidence has shown that circadian gene mutations have many phenotypes that resemble depression and bipolar disorder. Furthermore, evidence from genetic studies and animal studies suggests that circadian genes are directly involved in modulating mood and reward.
Animal Studies
Experimental shifting of animals’ light–dark cycles can lead to increased tumors. A mouse model of cancer has demonstrated that putting the animals on a shift-work cycle increases tumor growth, the number of tumors, and tumor aggressiveness (Logan et al., 2012). Mouse studies demonstrate that mutation in the core circadian genes leads to weight gain on a regular diet and to obesity on a high-fat diet, attributable to loss of circadian rhythm in the genes and peptides involved in metabolic control (Turek et al., 2005). Furthermore, a high-fat diet itself can disrupt behavioral and molecular circadian rhythms in mice, even in the absence of genetic mutation. A poor diet leads to irregular circadian rhythmicity in mice, especially in the fat and in the liver, which also contributes to weight gain. This is a vicious cycle of disrupting circadian rhythms with unhealthy food intake, with those circadian rhythms also influencing metabolic rates (Kohsaka et al., 2007). Recent research has focused on using timed restricted feeding to control this effect. Because mice are nocturnal, restricting their feeding to nighttime causes increased amplitude of rhythms and fewer problems with the metabolic system compared with mice who eat at various times of the day (Hatori et al., 2012).
Neurodegeneration
In older people with neurodegeneration, circadian rhythm disruption may increase the progression of neuronal loss, may lead to earlier loss of cognitive function, and may be related to accumulation of amyloid-beta and tau (Musiek, 2017). Figure 3-2 illustrates how this represents yet another vicious cycle: Alzheimer’s disease and other neurodegenerative diseases affect the circadian clock, while the circadian clock influences inflammation, oxidation, and endoplasmic reticulum (ER) stress, all of which worsen the brain disease and its progression (Chauhan et al., 2017).
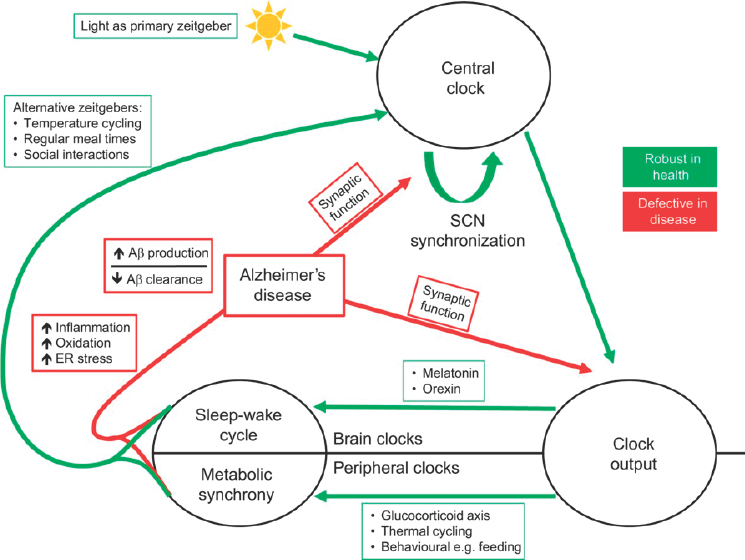
NOTES: “Zeitgeber” refers to a rhythmically occurring phenomenon that acts as a break in the regulation of circadian rhythm. Aβ = amyloid-beta; ER = endoplasmic reticulum; SCN = suprachiasmatic nucleus.
SOURCES: As presented by Colleen McClung at the workshop Brain Health Across the Life Span on September 24, 2019; Chauhan et al., 2017.
Monitoring Circadian Rhythms as a Diagnostic Tool
Circadian rhythms can easily be monitored as a diagnostic tool and might be helpful in determining who is at risk for certain diseases or for monitoring the progression of diseases such as bipolar disorder, said McClung. A person’s circadian rhythms can be understood by using a range of measures: activity and sleep patterns, cycling hormones (melatonin and cortisol), body temperature rhythms, peripheral circadian gene expression (blood, saliva, and buccal cells), and cycling metabolites (urine). Actigraphy is an easy-to-use technique for assessing a person’s sleep–wake patterns using a noninvasive wearable sensor, similar in size to a wristwatch, that automatically determines the person’s sleep–wakefulness state. This simple, low-cost solution can be used to gather valuable information and allow for long-term monitoring. For example, physicians can be trained to monitor the activity patterns of a person with bipolar
disorder or depression in order to recognize the onset of manic or depressive episodes and prompt a therapeutic intervention.
Strategies for Stabilizing Circadian Rhythms
McClung explained that circadian rhythms can be stabilized to both help and prevent disease in a number of ways, through either environmental or pharmacological stabilization. Natural strategies that people can use to stabilize their own rhythms include the following:
- Spending at least 20 to 30 minutes in natural morning sunlight
- Using a light therapy bulb or lamp if indoors all day
- Avoiding brightly lit screens for at least 1 hour before bed
- Going to bed at the same time every night and waking at same time each day
- Sleeping in complete darkness
- Restricting meals primarily to daytime (e.g., no late-night snacking)
The technology for measuring rhythms and for helping people with rhythm analysis and stabilization is improving. Mobile applications have been developed to track a person’s circadian rhythms, detect light, and recommend how much light the person needs (Wehr, 2018). Another application developed at the University of Michigan helps to entrain people before they travel overseas, by telling them when to take melatonin and when to get light. A technique called social rhythm therapy has been developed primarily for people with bipolar disorder by Ellen Frank and colleagues at the University of Pittsburgh, through which clinicians can intervene using a very strict sleep–wake schedule. In people with bipolar disorder who have disruptive locomotor rhythms, social rhythm therapy can improve their sleep–wake cycles and stabilize their moods. Daily bright light therapy between 12 p.m. and 2:30 p.m. has been shown to help people with bipolar depression (Sit et al., 2018).
Pharmacotherapies such as lithium and valproic acid—two first-line treatments for bipolar disorder—can enhance circadian rhythm amplitude (Johansson et al., 2011; Li et al., 2012). Pharmaceutical companies are working to develop medications that will target the circadian clock specifically to create the amplification of circadian rhythms but without the other numerous side effects that lithium and valproic acid can cause. This may represent the next wave of interventions to enhance circadian rhythms and prevent or treat diseases.
Leveraging Circadian Rhythms to Optimize Treatment
Current treatments for a variety of diseases can take advantage of rhythms to optimize the time of day for greatest effect, said McClung. For example, a cluster-randomized trial found that influenza vaccination in the morning enhances antibody response more than afternoon vaccination because the immune system is primed in the morning (Long et al., 2016). Similarly, taking statins to treat heart disease at a particular time of day achieves a better effect, while birth control pills need to be taken at the same time of day every day to be highly effective. This technique is now being used in in chemotherapy because tumors tend to have a different circadian rhythm than the surrounding cells (Levi et al., 2007). By using a chemotherapy agent that attacks cells at a specific stage of the cell cycle, it is possible to maximize the effect on tumor cells while minimizing the effect on the surrounding cells if the treatment is delivered at the appropriate time of day.
Discussion
Huda Akil, codirector and research professor of the Molecular and Behavioral Neuroscience Institute and Quarton Professor of Neurosciences at the University of Michigan, asked McClung to elaborate on how early-life patterns can predict behavior much later in life. She replied that the studies are in the early stages, but mouse models indicate that disrupting the rhythms of pregnant mice with a protocol similar to shift work has an effect on the risk-taking and reward-related behavior later in life in the offspring mice. Although the underlying mechanism is not yet understood, females seem to be more susceptible than males, which indicates that it may be related to hormones in some way.
Rasgon commented that light therapy is useful when appropriately used in mood disorders and in patients with neurodegenerative disease, especially in dementia patients who exhibit sundowning syndrome2 or circadian-induced delirium. She asked if light therapy or some kind of sensory induction could promote a certain stabilization of the consciousness. McClung replied that there have not yet been many clinical studies to determine if changing circadian rhythms in these patients will improve their outcomes. However, research carried out in nursing home and hospital environments with variable light environments (e.g., dim levels of light that persist into the night) have found that exposure to very bright lights during the day and darkness during the night can improve
___________________
2 Sundowning refers to restlessness, aggression, anxiety, or other behavioral issues that can happen in the evening in patients with some forms of dementia, including Alzheimer’s disease.
cognition and perhaps even reduce sundowning, which is a common problem in dementia patients that can be a challenge to caregivers as well. Akil noted that there are certain wavelengths in sunrise and sunset that are important for setting the circadian rhythms, but those wavelengths are not completely captured by current lighting therapies.
A participant asked for clarification about how the decrease in melatonin may contribute to neurodegenerative diseases. McClung replied that data suggest that melatonin is protective in neurodegenerative disorders such as Huntington’s disease and amyotrophic lateral sclerosis. Melatonin is a signaling hormone in the mitochondria and acts as a potent antioxidant. Although the decline of melatonin over the life span is a natural biological process, the extent of the decrease varies by individual. Melatonin therapy also has the potential to help prevent or at least delay certain neurodegenerative diseases, she added.
EARLY ENVIRONMENTAL RISK FACTORS FOR MENTAL HEALTH DISORDERS
Elinor Sullivan explored how the early environment influences both brain health and the risk of mental health disorders later in life. Multiple prenatal factors are associated with risk for mental health disorders, she explained. These include known factors, such as toxicant exposure and teratogens, but maternal obesity, maternal depression, poor maternal nutrition, and increased maternal stress have also been linked to increased risk of mental health disorders for the offspring. Her presentation focused largely on the factors of maternal obesity and poor maternal nutrition. Maternal obesity has long been shown to be associated with increased risk for childhood and adult obesity as well as metabolic disease. More recent evidence indicates that maternal obesity is also associated with risk for children developing anxiety, autism spectrum disorder, ADHD, emotional difficulties, cognitive problems, and eating disorders.
Animal Model Studies of Early Environmental Exposures
Sullivan described how a nonhuman primate model has been used to help disentangle the comorbidity of these early environmental exposures by looking at how maternal obesity and Western-style diet (WSD) affect mental health–related behavior. The study design included two adult Japanese macaque breeding groups: one group remained on the control (CTR) diet and the other was placed on a high-fat WSD constructed to mimic the average American diet, which is high in saturated fat, high in caloric density, and high in sugar. Adult females were metabolically characterized in their nonpregnant and (third-trimester) pregnant states
to assess adiposity, glucose metabolism, and insulin response. To mimic what happens in humans, the adult female macaques were placed on a WSD about 2 years prior to pregnancy and kept on the diet during gestation and lactation.
As with humans, the animals who consumed the average American diet tended to be heavier, to have increases in adiposity in response to the diet, and to have increases in their insulin area under the curve, suggesting that they were less sensitive to insulin and had a slight impairment in glucose regulation. In the control group, most of the animals had a lean body fat of 10–15 percent, although some animals drifted up to body fat of 30–35 percent. When animals were placed on a WSD, the histogram shifted. Some animals remained at a healthy 10–15 percent body fat, but many more fell within the 30–35 percent range and others moved into the 40–45 percent fat for their adiposity. This allowed the investigators to look at the offspring based on the mother’s diet and her metabolic state as separate variables.
The offspring were weaned and subdivided into four groups:
- CTR/CTR offspring who stayed on the mother’s CTR diet
- WSD/WSD offspring who stayed on the mother’s WSD
- CTR/WSD offspring whose mothers ate the CTR diet, but the offspring were switched to the WSD
- WSD/CTR offspring whose mothers ate the WSD, but the offspring were switched to the CTR diet
The initial findings from this model pertain to anxiety-related behaviors in 4-month-old infants as manifested in their latency3 to explore three different novel objects of varying levels of potential threat (Sullivan et al., 2010). Males and females whose mothers ate the CTR diet would touch novel objects rapidly, as did male offspring of mothers on the WSD. However, female offspring of mothers who ate the WSD had increased latency to interact with all three novel objects. Thus, maternal WSD consumption leads to increased latency to explore novel objects in female offspring, suggesting that the offspring have increased anxiety compared to the other groups.
To characterize this behavior in more detail, the investigators looked at the effect of maternal WSD exposure on offspring anxiety at 11 months (Thompson et al., 2017). A novel object test using a human intruder was used to assess the offspring’s temperament relating to anxiety and depression. Among the offspring of mothers who consumed the WSD, increases
___________________
3 “Latency” refers to the time between a stimulus and a response, in this case the time between presentation of the objects and exploration of the objects.
were seen in both the number of occurrences and the percent of test time that the animals engaged in anxiety behavior. Placing those offspring on the CTR diet at weaning did not ameliorate the changes—in fact, it slightly intensified the differences in anxiety behavior in these animals. An increase in stress-related vocalization was also seen in animals exposed to the WSD prenatally and an increase in active forms of anxiety, such as trying to escape from the cage, both in the number of occurrences and in the percent of test time. Male offspring who consumed a WSD after weaning showed increases in stereotypy. Therefore, some effects are different primarily by the mother’s diet, while other behavioral effects seem to be driven by the offspring’s current diet.
Possible Mechanisms for Behavioral Differences
Sullivan said her group is exploring a set of mechanisms that may underlie the behavioral differences observed in the animal studies, including the following:
- Increased inflammation
- Maternal diet versus the maternal metabolic state
- Changes in the development of neurotransmitter systems critical in behavioral regulation
- Alterations in stress sensitivity as characterized by the hypothalamic–pituitary–adrenal axis
- Alterations in the early postnatal environment, such as maternal–infant behavior and attachment
A primary mechanism is increased inflammation. Chronic elevations in adiposity are associated with an increase in peripheral markers of inflammation. In their nonhuman primate model, the investigators were able to show that maternal WSD consumption resulted in increased developmental exposure to inflammatory cytokines. Increased microglial inflammation in the fetal hypothalamus suggests that the mother’s inflammation affects the fetal environment and increases the offspring’s exposure to inflammation. This is transmitted to the fetal brain, increasing neural inflammation, or at least microglial activation. The investigators believe that this inflammatory process in the brain affects the development of critical neurotransmitter systems.
Evidence suggests that maternal obesity and WSD consumption also influence the development of neural pathways that regulate behavior because of alterations in the serotonergic and dopamine systems. In the serotonergic system, for example, Sullivan and colleagues found that offspring exposed to a WSD during the prenatal and early postnatal periods
showed a reduction in tryptophan-hydroxylase 2 (TPH2)4 expression, suggesting that they were producing less serotonin (Sullivan et al., 2010). This hypothesis was supported by further research that revealed that the offspring from mothers who consumed a WSD had reduced amounts of serotonin in their cerebral spinal fluid (Thompson et al., 2017). By then characterizing the serotonin projection systems, the study found alterations in the frontopolar cortex (a region associated with complex, higher-order behavior). Similar studies are being conducted to explore the relationships between obesity, diet, serotonin, and serotonergic projections to the amygdala.
Effect of Maternal Diet on Offspring Behavior
Sullivan provided an overview of her 2010 study’s conclusions about the effect of a maternal WSD on offspring behavior. In nonhuman primates, maternal WSD and obesity impair offspring brain development and behavior, with offspring from WSD mothers at increased risk for developing behavioral disorders. Increased anxiety is seen in both male and female offspring, with earlier onset in females. Males in particular exhibited increased repetitive behaviors, with those increases driven by the mother’s diet but also, more profoundly, by the offspring’s current diet consumption. Male offspring also displayed increased aggression toward novel peers. When examining the offspring’s social behaviors across settings, investigators saw signs of social withdrawal and impairments in social behavior among both male and female animals whose mothers consumed a WSD.
Sullivan outlined the potential programming mechanisms underpinning these behavioral alterations in WSD offspring. The WSD offspring have increased stress sensitivity, as evidenced by elevated cortisol across developmental time points that is almost double the levels found in the control offspring. WSD offspring have increased exposures to inflammation during development, as well as altered development of the serotonin system and changes in dopamine innervation of the prefrontal cortex. Anatomical and functional magnetic resonance imaging scanning studies have also revealed alterations in cortical–subcortical connectivity among this group. Changes in maternal behavior also contribute, with the WSD mothers nursing their infants more and grooming them less during the early stages of development; they also tend to retrieve their infants less frequently from dangerous situations.
To explore the effects of maternal diet versus metabolic state, researchers looked at specific behaviors and found that most behaviors were programmed by the mother’s diet and nutrition (see Figure 3-3). However,
___________________
4 TPH2 is the rate-limiting enzyme for serotonin synthesis expression in the dorsal assay.
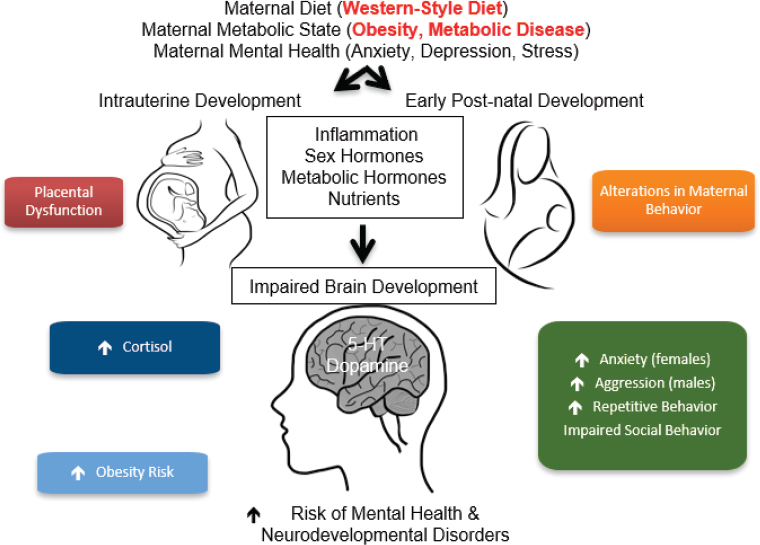
SOURCES: As presented by Elinor Sullivan at the workshop Brain Health Across the Life Span on September 24, 2019; adapted from Rivera et al., 2015.
certain behaviors appear to be more strongly influenced by the maternal metabolic state. The investigators posit that both in nonhuman primates and in humans, the mother’s diet, metabolic state, and mental health directly influence intrauterine development. The nonhuman primate model provides evidence for placental dysfunction and inflammation in the placenta, as indicated by less blood flow through the placenta of WSD mothers. Alterations in maternal behavior and direct effects on the early postnatal development are believed to occur through pathways such as inflammation and alterations in sex hormones, metabolic hormones, and nutrients. These factors come together to influence the way the brain is developing, Sullivan said. Thus far, investigators have found differences in the serotonin system and dopamine system, as well as changes in the melanocortin system that directly controls energy balance regulation. The primary behavioral outputs of anxiety, aggression, repetitive behaviors, and impaired social behavior are thought to be behavioral indicators of increased risk for mental health and neurodevelopmental disorders in humans, she added.
Effect of Maternal Diet and Obesity on Offspring Risk for Neurodevelopmental Disorders
Sullivan explained that the investigators’ next steps were to translate the findings from nonhuman primates into humans. Over the past decade, they have been characterizing mothers’ diets and obesity and looking at their offspring’s risk for neurodevelopmental disorders. Investigators began with a small pilot study looking at 68 mother–infant pairs and recently obtained National Institutes of Health (NIH) funding to recruit 300 participants; Sullivan presented preliminary data from the pilot study. The major study goals were to characterize the changes in the in utero environment associated with maternal obesity, poor nutrition, maternal stress, and maternal depression to determine which factors are the strongest predictors of alterations in infants’ and toddlers’ behaviors associated with ADHD and other neurodevelopmental disorders. Investigators are currently characterizing the infants and toddlers up to 3 years of age, and if their hypotheses are confirmed, they hope to develop new ideas for prevention and intervention to reduce neurodevelopmental disorders.
One of their first findings was that negative emotions are elevated in infants from families with ADHD. By looking at infants’ affect at 6 months of age, investigators found that infants with a familial risk of ADHD (i.e., either one of their parents or a sibling was diagnosed with ADHD) showed early differences in how they interact in laboratory behavioral assessment tasks (Sullivan et al., 2015). Infants with a familial history of ADHD showed increased negative vocalizations during arm restraints and decreased attention-seeking regulatory behaviors during still-face paradigms. The same children are now 7 and 8 years of age, and the early 6-months-old measure strongly predicts the risk for ADHD, suggesting that this is an early biomarker for risk for ADHD.
The next focus of the study found that obesity was associated with increased inflammation during pregnancy in humans. As observed in the nonhuman primate study and reported in other literature, study participants who were obese had elevations in a series of cytokines, including interleukin-6 (IL-6), tumor necrosis-factor alpha, and monocytechemoattractant protein-1 (Gustafsson et al., 2019). Furthermore, maternal prepregnancy body mass index (BMI) and inflammatory profile were associated with higher negative emotionality among infants at 6 months of age. In addition to being related to familial risk of ADHD, offspring from obese mothers have also shown increased negative affect during the still-face paradigm, in which an adult expresses a neutral and unresponsive face toward an infant (Gustafsson et al., 2019). By probing this relationship further, investigators found that the relationship between maternal prepregnancy BMI and the 6-month-old infant’s negative affect goes through inflammation—specifically, trimester IL-6 (Gustafsson et al., 2019). They
believe that this inflammatory pathway, which was also observed in the nonhuman primates, is an important driver and represents another potential biomarker for increased risk for neurodevelopmental disorders.
When investigators added maternal nutrition into this relationship, they found that maternal prepregnancy BMI and fatty acid levels influence the child’s negative affect. By looking at negative behaviors during the still-face paradigm and their relationship with prepregnancy BMI, negative behaviors increase as the mother’s BMI increases (Gustafsson et al., 2019). If the mother’s omega-3 fatty acid levels were one standard deviation below the mean of omega-3 fatty acids, this relationship was further exacerbated, with those offspring showing higher levels of negative behaviors. However, this relationship no longer held among mothers who were just one standard deviation above the mean of omega-3 fatty acids. Offspring whose mothers had an elevated prepregnancy body mass but were consuming higher levels of omega-3 fatty acid were actually protected from this effect. Sullivan emphasized that this is an optimistic finding, suggesting that some healthy foods can ameliorate some of the behavioral changes programmed by maternal obesity.
The investigators posit that inflammation is the common pathway to ADHD and other neurodevelopmental disorders. Omega-3 fatty acid was negatively associated with inflammation, maternal distress and depression were positively associated with inflammation, and maternal BMI was strongly associated with increased maternal inflammation. These factors were able to predict child ADHD symptoms at 4 to 6 years of age through the pathway of maternal inflammation.
Future Research Directions
In addition to expanding the pilot study to validate findings in a larger cohort, the group’s other ongoing studies will further examine inflammation as a mechanism in the breakdown of self-regulation and psychopathology. They will also explore the mechanisms for maternal nutrition-induced behavioral programming by looking at epigenetics, the microbiome, neuroimaging measures, and cell isolation and stimulation studies from the umbilical cord. The group’s long-term goals are to develop clinical biomarkers of risks for neurodevelopmental disorders in order to develop early interventions. These may include dietary interventions (e.g., reduction in fat content and alterations in fat composition), exercise interventions, antioxidant treatments (e.g., resveratrol), and supplementation with critical amino acids (e.g., tryptophan). Thus far, the most promising finding is that omega-3 fatty acid consumption or
supplementation could be an effective intervention. Ultimately, Sullivan’s group aims to design effective prevention strategies as well.
Discussion
Akil asked if features other than omega-3 fatty acid shortages or deficits contribute to the inflammatory process. Sullivan replied that all unrefined carbohydrates—but specifically sugar—and saturated fats are the two major drivers of this inflammation. Rather than total fat intake, it is likely the type of fat that is the concern. Omega-3 fatty acids are known to be protective, whereas elevated omega-6 fatty acids are helping to drive inflammation to some degree. With respect to unrefined carbohydrates, detailed nutrition measures in humans are already available that could be used to inform direct testing of those relationships in nonhuman primate models. When asked about any interaction between familial ADHD vulnerability and maternal obesity, Sullivan said that interaction was not observed in the small pilot study, but other evidence suggests that both children and adults with ADHD are more at risk for obesity.
Sullivan was also asked to comment on the harmonization of measures or other common elements in related major projects that are under way, such as the Environmental Influences on Child Health Outcomes (ECHO) studies, the Healthy Brain Initiative, and the Healthy Brain and Child Development studies. She said that her group is working with ECHO investigators to try to align their efforts toward common inflammatory and imaging measures in order to expand the sample size, bring more diversity to the study populations, and validate similar measures across sites. Damien Fair, associate professor of behavioral neuroscience, associate professor of psychiatry, and associate scientist at the Advanced Imaging Research Center at the Oregon Health & Science University, added that across those three major projects, researchers are attempting to coordinate brain imaging measurement and processing. Akil remarked that coordinating and standardizing measurements for better reliability as well as validity and cross-comparisons will be important moving forward.
INSULIN RESISTANCE: A LINK IN BRAIN–BODY INTERACTIONS
In her presentation, Rasgon described how insulin resistance is a link in brain–body interactions. She described the purpose of using insulin as one of the linking agents—both peripherally and centrally—in body and brain connections and explored a conceptual framework for understanding brain health versus brain disease.
Insulin Resistance and the Link Between Metabolic Dysfunction and Brain Diseases
About 19 years ago, a specific link was postulated between metabolic dysfunction and brain diseases such as affective disorders, mood disorders, and Alzheimer’s disease. This metabolic dysfunction was manifested behaviorally through cognitive impairment, giving rise to the idea that the cognitive impairment may be in part attributable to metabolic dysfunction underpinned by the effect of impaired glucose utilization in the brain. As illustrated in Figure 3-4, the multiple mediators and neurotransmitters involved include serotonin and melatonin as part of the serotonin cascade as well as cortisol and estrogen (McIntyre et al., 2009; Rasgon and Jarvik, 2004). The latter is a pivotal hormone responsible for the sex-specific differences in various illnesses, both of the brain and of the body. In this context, the brain–body link relates to the notion of insulin resistance.
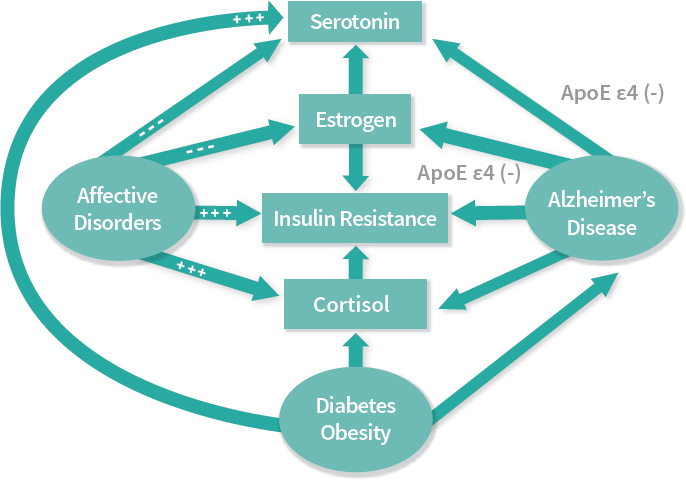
NOTES: Apolipoprotein E (ApoE) is a protein involved in metabolism of fats in the body and highly implicated in the development of Alzheimer’s and cardiovascular diseases. ϵ4 is a particular allele of the gene that produces apolipoprotein E, and is the allele most associated with the development of dementia.
SOURCES: As presented by Natalie Rasgon at the workshop Brain Health Across the Life Span on September 24, 2019; adapted from Rasgon and McEwen, 2016.
Rasgon discussed the extent to which insulin resistance may be responsible for the brain–body miscommunication and how it could potentially be used as a target for intervention. The functional effects of insulin have been well established. In the periphery, insulin is specifically responsible for glucose use; in the brain, insulin has a significant adaptive plasticity role and a neuroprotective role. The pleiotropic and pleomorphic representation of the function of insulin makes it an interesting agent to consider as a link between brain and body disorders. Insulin resistance is a condition in which tissue responsiveness to the normal action of insulin is impaired, which may or may not be a consequence of weight gain. It is manifested by decreased insulin receptor sensitivity to the circulating levels of insulin, which can eventually lead to hyperglycemia. Insulin resistance forms a mechanistic foundation for a number of illnesses (Rasgon and Jarvik, 2004; Rasgon et al., 2002). The duration of the lack of tissue responsiveness to insulin and, therefore, the condition of insulin resistance, can last for decades. It does not necessarily result in diabetes in all cases, but it may lead independently to cardiovascular disease, mood disorders, and dementia. In the periphery, the metabolic dysfunction of insulin resistance has distinct endpoints in somatic illness and in central nervous system (CNS) illnesses, including obesity, depression, hypertension, metabolic syndrome, atherosclerosis, diabetes mellitus, microalbuminuria, endothelial dysfunction, and polycystic ovary syndrome.
Insulin Resistance and Changes in Hippocampal Structure and Function
Rasgon presented data on insulin resistance and changes in hippocampal structure and function to illustrate how peripheral insulin resistance has correlates in the brain. A number of brain imaging studies have been carried out in subjects aged 45–65 years who were at genetic risk for Alzheimer’s disease but did not yet have any identifiable appreciable cognitive impairment. The studies show (1) a direct linear correlation between decreased hippocampal volume and increased insulin resistance in the periphery; (2) decreased connectivity between the hippocampus and prefrontal cortex as insulin resistance increases; and (3) very distinct metabolism impairment in the medial prefrontal cortex attributable to ensuing insulin resistance, according to fluorodeoxyglucose-positron emission tomography (Kenna et al., 2013; Rasgon et al., 2011, 2014). Taken together, these methods for assessing brain correlates of peripheral insulin resistance suggest that there is actually a CNS representation.
Treating Insulin Resistance in Patients with Mood Disorders
Next, Rasgon considered the “chicken and egg” scenario in the relationship between insulin resistance and mood disorders: which precedes the other, and what can be achieved by modifying peripheral insulin resistance?
Epigenetic Modulation of Metabolic Subtypes of Depression
Rasgon described a study that found a link between insulin resistance and telomere length in antidepressant response to a peroxisome proliferator-activated receptor gamma (PPARG) agonist, suggesting that there are different metabolic subtypes of depression (Lin et al., 2015). The study looked at the effects of the PPARG agonist pioglitazone, which is an anti-diabetic drug used in a placebo-controlled design as adjuvant treatment in patients with unremitted major depression. The subjects’ usual treatment for depression was supplemented with pioglitazone or placebo for 3 months. In addition to finding that pioglitazone was effective in reducing depression versus placebo, investigators were able to identify a number of predictors of that treatment response, some of which were specifically related to peripheral insulin resistance. Improvement in depression associated with improvement in glucose metabolism in insulin-resistant subjects and response to pioglitazone was stronger in younger patients. Subjects with longer telomeres, which are known biomarkers of inflammation and allostatic load, exhibited greater declines in depression severity in the active arm, but not in the placebo arm. Based on these data, the investigators posited that there is a metabolic subtype of depression in which depressive disorder is associated with a distinct metabolic signature in the periphery. Furthermore, people with this metabolic subtype may have a different biology and response to treatment with medications typically used for diabetes.
The pioglitazone study spurred further investigation into the epigenetic modulation of the metabolic subtype of depression. Work by the National Institute on Aging (NIA) reversibility research network on the effect of early childhood adversity on cognitive performance in midlife has revealed that early childhood adversity is a pivotal moment—it is both a window of vulnerability and a window of opportunity. In looking at biological processes, there is evidence that emotional abuse is associated with multiple biological and neurobiological correlates to depression. A number of biomarkers of allostatic load and stress are predicted by childhood trauma and are related to peripheral insulin resistance. This allows for deeper endophenotyping of the metabolic type of depression: it is associated with childhood trauma, it is insulin resistant in the periphery, and it is manifested by multiple distinct molecules as
predictors of decreased emotion regulation and cognitive regulation in the brain (Bigio et al., 2016; Nasca et al., 2018). One of those molecules is acetylcarnitine, an epigenetic glutamatergic modulator, which has subsequent downstream effects in the brain.
Novel Mechanisms of Brain Plasticity in Mood and Cognition
Work on acetylcarnitine indicates that in the cross talk between the brain and the body, deficient plasticity has a significant number of mediators, said Rasgon. Although it is not yet clear which mediators precede the others, the data suggest that the combination of sex differences driven by estrogen that make women more vulnerable to childhood trauma is compounded by subsequent metabolic dysfunction, in which glutamatergic changes can trigger the cascade of changes in neuronal plasticity (Nasca et al., 2017). It is already understood how these peripheral events and central events collate because of our understanding of diabetes prevalence and comorbidities and multimorbidities between the various illnesses represented by these deficiencies.
Studies of acetylcarnitine deficiency in subjects with major depressive disorder and treatment-resistant depression have also illustrated the changes in the glutamatergic modulator system in the brains of those patients. Compared to healthy controls, the patients with severe depression have a nearly linear decline in glutamatergic plasticity (Nasca et al., 2018). This same agent is also a mediator of insulin resistance—this time in the brain. Looking at central insulin resistance reveals that there could potentially be cross talk between the body and the brain in the molecular signature of those regulatory factors, but it could also be two completely distinct processes unrelated to each other. Data are emerging from studies of in vivo brain insulin resistance in patients with major depressive disorder and characterized by acetylcarnitine deficiency, suggesting that there is an in vivo nanotechnology method that can be used to assess actual central insulin resistance by measuring the same biomarkers of insulin function among others in exosomes, which are the peripherally circulating baggage from the central nervous system. By enriching for the brain-derived exosomes and looking at the specific insulin receptor substrate concentration in those brain-derived exosomes, investigators are finding that healthy controls have significantly less turnover of the insulin-resistant substrate.5
___________________
5 Rasgon, N. Workshop PowerPoint presentation—Insulin Resistance: A Link in Brain–Body Interactions. Available at http://www.nationalacademies.org/hmd/Activities/Aging/BrainHealthAcrossTheLifeSpanWorkshop/2019-JUN-26.aspx (accessed March 12, 2020).
Future Research Directions
Rasgon concluded by outlining future directions based on the findings of the mechanistic studies she described. Figure 3-5 illustrates potential trajectories over the life span from health to risk, then to poor health and disease. Early-life adversity can be associated with metabolic phenotypes, with future studies needed to further elucidate the biochemical pathways involved and to identify potential targets for intervention. This conceptualization situates pregnancy-related metabolic and psychological events as the starting points that can trigger trajectories at different stages, which are mediated along the way by a range of normal and pathological, environmental, and internal conditions. The trajectories begin with convergence at early life but then diverge with various life events.
Rasgon highlighted the factors of allostatic load and resilience as main points of interest for trying to identify important milestones and windows for intervention. She added that within this framework of bilateral communication, reverse translation is as important as direct translation.
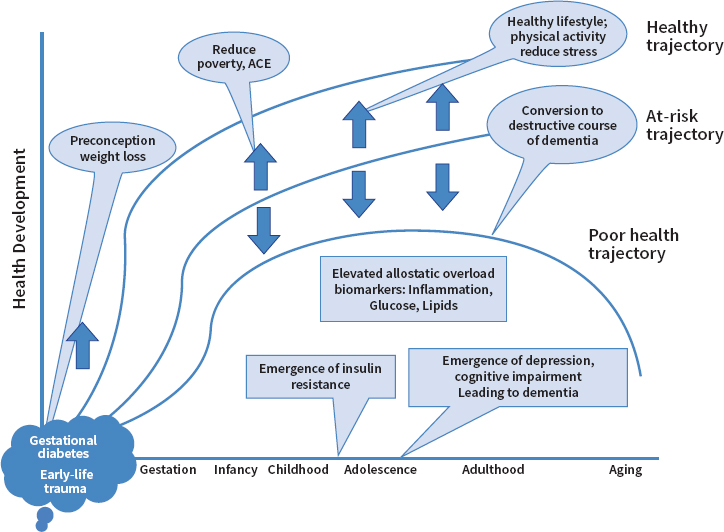
NOTE: ACE = adverse childhood experience.
SOURCES: As presented by Natalie Rasgon at the workshop Brain Health Across the Life Span on September 24, 2019; Watson et al., 2018.
Therefore, an important future research direction is to identify phenotypic presentations in cohorts of people with illness, then to return to animal studies to try to understand mechanisms that underlie those presentations. This would allow for testing potential interventions in large cohorts. This should also include multifaceted interventions that involve not only the biological interventions but also various psychosocial strategies for augmenting and improving the delivery of those biological interventions.
Discussion
Rasgon was asked to elaborate on the correlation between insulin resistance, corticoids, and other component factors of stress. She explained that insulin receptors are highly collinear with the cortisol receptors in the hippocampus. They are expressed in the same regions, and they have a mutually potentiating effect of insulin and cortisol toxicity. Models of major depression, for example, show that people with major depression have an overproduction of cortisol and increased insulin resistance in the brain, specifically in the hippocampus. The same also holds in the periphery, she said. People with Cushing’s syndrome, for instance, have primary hypercortisolemia, which is a well-known model for insulin resistance and for mood disorder, so there is a strong correlation between them. Stress as a concept is a very multinomial construct, she added. Studies tend to focus only on stress that leads to negative outcomes, with the notion of positive stress often overlooked in research. She suggested that this is a research gap to be addressed—perhaps it may be even more stressful to have a healthy brain and joyful experience. She surmised that positive stress could have a bigger imprint in the brain than having a negative effect, because people may be more used to dealing with adversity than to dealing with joy.
Lis Nielsen, chief of the Individual Behavioral Processes Branch of the Division of Behavioral and Social Research at NIA, asked about potential nonpharmacological lifestyle interventions along the insulin resistance pathway. Rasgon said that work is ongoing in both humans and animals. New animal data are emerging on the transmission of behavioral and biological imprints from biological mothers to offspring. This epigenetic modulation relates not only to trauma and upbringing but also relates to diet and to the level and extent of physical activity (not necessarily rigorous and nonrigorous exercise). Because these factors contribute to pathophysiology, interventions that target food intake, food composition, and caloric intake could have a positive effect related to the effect of insulin resistance on the metabolic endophenotype of depression and subsequent cognitive diseases. Going forward, research efforts should focus on a combination of psychopharmacological, behavioral, and environmental interventions, Rasgon said.
PANEL DISCUSSION ON BRAIN–BODY INTERACTIONS
To begin the discussion, Bruce McEwen, Alfred E. Mirsky Professor at The Rockefeller University, commented on the positive functions of circadian rhythms and metabolic hormones. Ultradian (rhythms longer than 1 hour but less than 1 day) and circadian variation of cortisol facilitate the turnover of synapses in many parts of the brain, enabling motor learning. If the circadian variation is disrupted and cortisol is elevated at the wrong time, or disturbs the sleep–wake cycle, then these effects are impaired such that the brain is not able to adapt as efficiently. In the context of metabolic hormones, McEwen noted the idea that the insulin-like growth factor 1 (IGF1) hormone from the liver is required for exercise to stimulate neurogenesis in the dentate gyrus of the hippocampus; if IGF1 is blocked in animal studies, exercise no longer stimulates neurogenesis. In fact, a number of other hormonal factors from muscle and bone, and perhaps other parts of the body, also facilitate this and other processes of plasticity. Another surprising facet of metabolic hormones is that both lectin and growth hormone are made in the brain, especially in the hippocampus, but they can also come from outside the brain and have complementary effects for neuroprotection to enhance cognitive and other functions. The same is true for prolactin and ghrelin; hippocampal studies show that they do not appear to be made in the brain, but they can access the brain and have generally neuroprotective effects. However, problems occur when the brain becomes resistant to those hormones.
Need for Basic Neuroscience to Adopt a Holistic Perspective
Akil remarked that basic neuroscientists should focus on nonneuronal cell types and take a more holistic perspective on the brain as part of the entire body—meaning, thinking also about the ways in which the brain is a target of the body, rather than thinking about neurocircuitry and the brain in isolation. She noted that a question that commonly arises in discussions about the burden of brain disorders is whether the apparent increase in prevalence of disorders such as autism and Alzheimer’s disease is attributable to people’s brains becoming less healthy or to an increase in the willingness to talk about, diagnose, and report brain disorders. Similarly, brain disorders related to aging are often framed as an inevitable consequence of people living to older ages, without sufficient consideration as to how brain health is influenced by a person’s body, their eating and sleeping habits, fulfillment of their basic needs, and how the person interacts with the external physical world.
Continuum of Brain Health and Disease
To help clarify where health ends and disease begins, said Rasgon, a continuum may be more useful than a more parsimonious illness-versus-health construct. Unlike infectious diseases, brain disorders and somatic disorders such as diabetes have lengthy and continuing effects; for example, prediabetes can last for decades before it becomes diabetes; cognitive impairment can precede dementia but may never get to the end stage. Brain diseases—as with many “body” diseases—are not discrete events in time. They may develop as a continuum and accumulation, mirroring the relationship between stress and allostatic load. It is now understood that neurodegeneration can begin very early in life. If neonates and children have genetic risk factor APOE4 for Alzheimer’s disease, they tend to have smaller hippocampi. Although this does not mean they will necessarily get Alzheimer’s disease, they already have certain changes in the brain. This underscores the difficulty in drawing a distinct line between what constitutes illness versus health.
Akil agreed about the need to shift the perspective away from the cause-and-cure binary. She added that the field of medicine more broadly is often biased toward focusing on conditions such as infections or injuries that have clear triggers, treatments, and endpoints. This is evident in early global health efforts that focused on infectious diseases. Similarly, in the field of genetics, there is a tendency to identify a single faulty gene as the source of the problem. However, probabilistic thinking then shifts bias and risk over a longer time frame, which reveals cumulative effects. Many brain disorders have patterns that are not discrete—they are slower, longer, and cumulative, which gives rise to questions about how to measure the disorder and the boundaries of the potential windows for intervention (e.g., when is too early, when is too late).
A participant noted that a state of balance can be attained after a brain disorder diagnosis such as obsessive–compulsive disorder (OCD) or bipolar disorder, for example, and asked if a person in that state of balance would not experience symptoms. Rasgon said that OCD and bipolar disorder are treatable, but not curable; other brain disorders such as Alzheimer’s disease are not yet even treatable. Although it is possible to contain the presentation of these types of illnesses, it does not mean that the illness is gone. McClung added that there is no consensus about the starting point of bipolar disorder. Some people believe that it occurs after a discrete break during adolescence—meaning, there is no childhood bipolar disorder. Others believe that the disorder begins much earlier and that certain factors can predict which children are at risk of developing the full-blown disease. Circadian rhythm patterns of the sleep–wake cycle in children can predict worse outcomes in terms of bipolar disorder
and schizophrenia. Researchers are also looking at sleep patterns related to schizophrenia. For example, people with schizophrenia have disease-specific disrupted sleep spindles that also occur prepsychosis in at-risk subjects. She agreed that the field of brain health seems to be shifting its focus to understanding the early stages of diseases, noting that early intervention to try to prevent diseases is a better strategy than trying to treat them once they have progressed.
Akil suggested that some brain disorders do not have a discrete beginning and ending. If a person with a seizure disorder, for example, receives the optimal combination of treatments and has not had a seizure for decades, then that person has a higher risk of seizure, but has achieved a state of health in general. Furthermore, the seeds of brain disorders are multifactorial, spanning the biology of the brain, genetics, maternal context, and the early-life uterine environment, but also a host of other factors and life events that are less understood but contribute to the manifestation of a disorder. She added that a brain disorder itself is another important agent—for instance, being bipolar is itself a stressor and a burden—which contributes to a vicious cycle. Breaking those cycles is an important part of resetting the trajectory of illness.
Toward a Preventive, Life-Course Approach to Brain Health
Discussing brain health in the context of disease is an appropriate starting point, said Nielsen. However, decisions about how to define the presence of disease can be avoided by moving toward a preventive, long-term, life-course perspective about avoiding disease through resilience, restorative processes, and the potential to achieve positive health by mitigating risk factors or early exposures. Rasgon said that preventive interventions should focus on implementing education and behavioral paradigms where they are needed the most, such as underserved populations and communities with limited access to health care.
Researchers in the field are consistently finding that familial transmission and upbringing are dimensions in which education can modify potential risk and potentially limit children’s exposure to vulnerabilities that lead to risk factors for disease. Educating parents who are living with an illness about how their own lifestyles are modeled by their children—both positively and negatively—could help to identify earlier windows of opportunity for interventions to ensure that the next generation does not become a vulnerable group as well. Akil remarked that relatively simple public health interventions related to lifestyle factors (e.g., diet, sleep, exercise) that promote general health are the foundation of brain health (NRC and IOM, 2000). McEwen pointed out that in terms of interventions, one size does not fit all. It is important to look at a person’s early-life
history to identify windows of opportunity for change at different periods in time, not only during infancy and childhood but also extending into adolescence and young adulthood (NASEM, 2019).
Rasgon offered a vignette based on the clear transmission of the risk for ADHD with a high-fat diet. She suggested that if having ADHD and obesity negatively affects a person’s parenting style, then some children of those parents may respond to emotional distress by self-medicating with unhealthy food. This in itself is a primary preceding metabolic dysfunction that may lead to brain illness. Because it is already widely understood that somatic illnesses such as obesity are detrimental to overall health, she suggested that a simple way to operationalize this complexity in public messaging is to highlight the brain health outcomes of those illnesses as well as their physical health outcomes.
McClung commented that an opportunity for change is to dispel widespread misperceptions about the amount of sleep that children and adolescents actually need and about the consequences of sleep deprivation. Typical school start times are a directly modifiable factor that would benefit adolescents broadly, but especially among those at risk. Children or adolescents with a family history of psychiatric disease should be on a preventive schedule that allows them to have the appropriate amount of sleep within a structured circadian cycle. Advocacy efforts are already under way to push back school start times, but they are generally met with resistance.
Measurement Strategies
Akil highlighted the task of defining what needs to be measured. McClung described how measuring circadian rhythms can be used to predict “crashes” in people with bipolar or other disorders in which circadian rhythmicity is part of the symptomatology, as well as how measuring rhythm can be used to administer drugs at the appropriate time. Rasgon discussed how measurement can be used to distinguish between different types of depression to determine whether regular antidepressant treatment should be augmented with anti-insulin resistance treatment in certain people. Simple early childhood measurements can also be used to predict the child’s risk of developing ADHD later on in life. She asked participants to discuss the extent to which whether measuring physiological markers such as circadian rhythm, metabolic levels, and insulin resistance is truly relevant to brain health, or whether they are just low-hanging fruit. Nielsen replied that the NIH Science of Behavior Change initiative is concentrating on promoting brain health by persuading people to adopt and maintain lifelong healthy behavioral patterns—with adherence to those patterns being the linchpin. Work in the field of behavior change
is focused on identifying and tailoring interventions to specific malleable psychological and behavioral targets, but the challenge ahead will be to address the behavioral phenotypes that characterize people’s amenability to various forms of intervention, such as public health messaging, in order to effect real long-term changes in people’s lifestyles. For instance, some people may need specific assessment of their behavioral plasticity and ability to be intervened upon—captured via some psychological or psychobiological measure—that will provide guidance about how to intervene and support maintenance and adherence.
McClung pointed out several studies that could be instructive about how measurements can be used to determine the optimal type of treatment for a patient, rather than relying on the trial-and-error approach common in current psychiatric practice. Studies on both depression and bipolar disorder have used measurements to predict treatment response. One study used actigraphy watches in people with depression to measure their circadian rhythms over a period of time, before receiving an acute dose of ketamine. Investigators were able to predict which people responded to treatment based on their previous circadian rhythms and—because ketamine enhances circadian rhythms in certain people—they were able to predict the people in which ketamine would have a lasting effect. Studies have also found that although people with bipolar disorder all have circadian rhythms that are disrupted, some have longer rhythms, and some have shorter. People who have a shorter rhythm respond better to lithium, because lithium lengthens the rhythm, while people who have a longer rhythm respond better to valproic acid.
Akil asked how to ensure that this type of knowledge can be translated more broadly into actions that can empower people who want to achieve greater health. As huge amounts of data become available at our fingertips from actigraphy and other rapid, easy measurements, it will likely become clear that some people benefit from ongoing feedback about their own data and use it to drive improvement in behavior, while other people may be much less interested. The field of brain health should seek to provide tools that match a range of styles and personalities, which will require another level of analysis. In addition to providing people with their own data, social supports will be needed to inform preventive measures and promote healthier lifestyles.




























