This chapter summarizes the workshop session on brain health in the social context, particularly with respect to both emotions and social disparities. Presenters and panelists looked at how an individual’s social context affects his or her brain health and resilience, how various social factors are important for understanding and predicting brain health, and how those factors are measured and validated. Stephanie Cacioppo, assistant professor of psychiatry and behavioral neuroscience and assistant professor at the Grossman Institute for Neuroscience at the University of Chicago, provided an overview of how the brain forms, maintains, and restores healthy relationships. Gregory Samanez-Larkin, assistant professor of psychology and neuroscience at Duke University, examined motivation, cognition, and decision making in everyday life. Life-course causes of later-life inequalities in brain health were explored by Jennifer Manly, professor of neuropsychology at Columbia University.
FROM ME TO WE: HOW THE BRAIN FORMS, MAINTAINS, AND RESTORES HEALTHY RELATIONSHIPS
Cacioppo described the improvement of brain health and social resilience as among the most important challenges facing contemporary science. When a 1978 report by the U.S. President’s Commission on Mental Health emphasized the importance of easing suffering from emotional distress syndromes such as loneliness, few anticipated that this issue would persist more than 40 years later. The issue is gaining an increasing amount of attention, with the number of scientific papers published per year on loneliness increasing by roughly tenfold (Cacioppo and Cacioppo, 2018a). Cacioppo described what the former Surgeon General of the United States, Vivek Murthy, described as the loneliness epidemic.
People are living longer than ever before, with the rise of the Internet transforming how people work, play, search, shop, study, communicate, and relate to one another. People are increasingly connected digitally, but social media do not necessarily protect them from loneliness or perceived social isolation—it depends on how they are used. These platforms may reduce loneliness in people who use them to connect, learn, or stay in touch with loved ones, but in people who use social media to the extent that they have only online connections with others, feelings of loneliness can increase (Cacioppo et al., 2015b). The prevalence of loneliness appears to be rising, from an estimated 11–17 percent of adults in the 1970s to more than 40 percent of adults middle-aged and older in recent years (Edmondson, 2010; Peplau et al., 1979; Perissinotto et al., 2012).
Defining and Measuring Loneliness
Loneliness is often discussed in conjunction with social resilience in publications. Social resilience is inherently a multilevel construct—revealed by capacities of individuals and also groups—to foster, engage in, and sustain positive social relationships and to endure and recover from social stressors and social isolation (Cacioppo et al., 2011). The premise underlying this work on loneliness and social resilience is that the brain is the main “social organ.” It is a key organ for forming, monitoring, and maintaining healthy connections with others as well as for regulating physiological processes relevant to morbidity and mortality. The brain helps organize a person’s social structures and social behaviors; it also regulates the social processes that determine health and longevity (Cacioppo and Cacioppo, 2018a).
People often think about humans as being unique compared to other species and think of themselves as unique and independent relative to those around them. Although individuals may appear to be distinct and independent, with no forces binding them together, people in
fact have more similarities than differences. Humans are a social species that is wired to form social connections and maintain those connections across the life span (Cacioppo and Cacioppo, 2012). The brain is primarily responsible for forming, monitoring, and maintaining those salutary connections with others. To illustrate the difference between objective and subjective isolation, Cacioppo used the example of how the same objective social interaction or relationship (e.g., with a sibling or a spouse) can be perceived either as caring and protective or as threatening and isolating. A person can feel “lonely in a crowd” when public speaking, for instance, while a person can feel extremely connected while completely alone just by thinking about loved ones. Thus, loneliness can be defined as perceived social isolation: a discrepancy between current and expected social relationships with a significant other.
Several different scales can be used to measure loneliness, but three main items have been evaluated as consistently reliable measures (Cacioppo and Cacioppo, 2018a).
- How often do you feel that you lack companionship?
- How often do you feel left out?
- How often do you feel isolated from others?
Effect of Loneliness on Physical and Mental Health
As the prevalence of loneliness rises, more evidence is accruing that loneliness is a major risk factor for poor physical and mental health outcomes (Cacioppo and Cacioppo, 2018a). For instance, the odds ratio for dying earlier from loneliness has been shown to be much higher (45 percent) than from excessive drinking (30 percent), obesity (20 percent), or air pollution (5 percent) (Holt-Lunstad et al., 2010). Studies in animals or humans demonstrate the effect of loneliness or lack of social connection on both physical and mental health, including the activation of the stress response, increases in inflammatory mechanisms, and increases in cell deaths in specific brain areas. A quantitative meta-analysis of functional imaging studies of social rejection found that when people feel rejected, a specific set of brain areas is activated, although this can be modulated as a function of whether the person is rejected by a stranger or a significant other. All the activated brain areas were in the specific networks involved in emotions and in expectations by relation (Cacioppo et al., 2013). When a person feels lonely, a different set of brain areas activates or deactivates in the social brain networks instead: areas that are important for empathy, compassion, perspective taking, and being in synchrony with others (Cacioppo et al., 2014).
Evidence from different types of social species may cast light on the
social dimension of the human brain. For instance, the locust shifts from solitary to social within a year; it has a brain that is about 30 percent larger when it is social than when it is nonsocial (Burrows et al., 2011; Ott and Rogers, 2010; Rogers and Ott, 2015). Studies using functional magnetic resonance imaging (fMRI) of people with different social network sizes have also revealed differences in sizes in various parts of the social brain (Cacioppo et al., 2014). Cacioppo emphasized that it is not the entire brain that increases in size, only the brain areas needed for social connections. When a locust is social, it only needs to communicate with olfactory senses or with touch, so the brain areas involved in motor sensory integration are bigger or more active. But when the locust is solitary, the visual cortex has greater activation because increased visual attention is needed to detect threats at a distance. Similarly, in humans, the visual cortex is also more activated in lonely individuals (Cacioppo et al., 2014). Cacioppo surmised that these individuals may have a hypervigilance to social threat or potential danger, and thus a hyperactivation in areas of the brain that are important for perspective taking, empathizing, or connecting with others.
An Evolutionary Theory of Loneliness
It has long been understood that brain health and survival depend on our collective abilities, not our individual might, said Cacioppo. Decades of research in social psychology shows that happy marriages have myriad beneficial effects on health through behavioral, cardiovascular, neuroendocrine, immune system, and cognitive pathways. People who are married have fewer physical problems, a better survival rate for some illnesses, and a lower mortality rate (Cacioppo, 2018; Goodwin et al., 1987; Lillard et al., 1995; Murphy et al., 1997; Waite and Lehrer, 2003). Throughout history, humans have survived and prospered by bonding together in couples, families, and tribes—to provide companionship, mutual protection, and aid. However, marital status is not the main factor associated with better health; rather, it is the quality of the relationship with a significant other that is crucial (Cacioppo, 2018).
Working toward a consistent definition of “significant other” is challenging, but it could be informed by studies of different species. For instance, adult female baboons (>5 years of age) who form stronger and more stable social bonds with other females live significantly longer than females who form weaker and less stable relationships (Silk et al., 2010). Another study tested two species, monogamous titi monkeys and nonmonogamous squirrel monkeys (Mendoza and Mason, 1986). When researchers removed one of the significant monkeys from a group of polygamous monkeys, they observed no increase in cortisol in the
remaining monkeys, presumably because the group could find another partner. When the researchers removed the offspring, however, they observed a large increase in plasma cortisol after 1 hour of separation. The opposite was found in the monogamous titi monkeys. When researchers removed a monkey from a monogamous pair, they saw a huge stress response after 1 hour, but not when they removed the offspring. This suggests that within the titi monkey’s social hierarchy, the partner is more significant than the offspring.
Cacioppo suggested that evolutionary heritage has shaped the human brain and biology to be inclined toward certain ways of feeling, thinking, and acting toward significant others. For instance, a variety of biological mechanisms have evolved that capitalize on aversive signals to motivate behaviors that increase the chances of short-term survival. Within this framework of evolutionary theory, loneliness is like a biological signal in that the aversiveness of loneliness serves as a biological warning signal analogous to hunger, thirst, and pain. It motivates attention and the repair or replacement of deficiencies in salutary relationships. In other words, it signals that something is wrong with a person’s “social body,” so the person needs to reconnect with others in order to survive (Cacioppo and Cacioppo, 2014, 2018b; Cacioppo and Patrick, 2008; Cacioppo et al., 2000, 2006).
Pathways of Loneliness
Multiple pathways link loneliness to morbidity and mortality, said Cacioppo. Although the deleterious effects of each pathway may be limited, their cumulative effects over time aggregate to produce significant damage to health and well-being. Interventions in these pathways have the potential to mitigate the deleterious effects of loneliness. She identified multiple pathways related to loneliness (Cacioppo and Cacioppo, 2018b; see Table 6-1). Loneliness causes, not just correlates with, increases in vascular resistance and blood pressure. When controlling for all standard predictors or stressors, loneliness can predict blood pressure increases in both older and younger adults (Hawkley et al., 2010b). Loneliness decreases sleep quality through micro-awakenings and poor sleep efficiency (Cacioppo et al., 2002a) and is associated with large increases in the hypothalamic–pituitary–adrenal (HPA) axis stress response; it can predict not only cortisone levels, but cortisone levels the next day (Adam et al., 2006).
Loneliness is associated with increases in depressive symptomatology1 as well as in prepotent responding, or impassivity. People who feel lonely
___________________
1 Loneliness is a different construct than depression: a person who is lonely feels not only sad, but in danger. Animal studies demonstrate that animals separated from their significant other for at least 2 weeks start showing signs of depressive symptomatology as well.
TABLE 6-1 Pathways Associated with Loneliness in Human and Animal Models
| Human Experimental and/or Longitudinal Research | Animal Models |
|---|---|
| Increased mortality (Luo et al., 2012) | Increased mortality (Karelina et al., 2009) |
| Increased sleep fragmentation (Cacioppo et al., 2002b; Hawkley et al., 2010a) | Decreased slow wave sleep and homeostatic rebound (Kaushal et al., 2012) |
| Elevated activation of the hypothalamic–pituitary–adrenocortical axis (Adam et al., 2006) | Elevated activation of the hypothalamic–pituitary–adrenocortical axis (Sapolsky et al., 1997) |
| Elevated vascular resistance and blood pressure (Hawkley et al., 2010b) | Elevated blood pressure (Coelho et al., 1991) |
| Up-regulation of gene expression for inflammatory biology and down-regulation of antiviral gene expression (Cole et al., 2007, 2011, 2015) | Up-regulation of gene expression for inflammatory biology and down-regulation of antiviral gene expression (Cole et al., 2015) |
| Decreased viral immunity (Pressman et al., 2005) | Decreased viral immunity (Cole et al., 2015) |
| Increased inflammation (e.g., peripheral IL-6 and IL-beta) (Jaremka et al., 2013) | Increased peripheral inflammation (e.g., peripheral IL-6) (Karelina et al., 2009) |
| Increased impulsive responding, hostility, and defensiveness | Increased prepotent responding and increased aggressiveness (Grippo et al., 2014; Matsumoto et al., 2012; Nin et al., 2011) |
| Increased depression, anxiety, and social withdrawal (Cacioppo et al., 2010) | Increased depression, anxiety, and social withdrawal (Matsumoto et al., 2012; Nin et al., 2011) |
NOTE: IL = interleukin.
SOURCE: Adapted from table presented by Stephanie Cacioppo at the workshop Brain Health Across the Life Span on September 25, 2019.
tend to gamble more, drink more, and consume more fat every day. In line with this impassivity, loneliness also increases suicide rates and ideation. Loneliness also increases defensiveness and self-centeredness. fMRI studies suggest that the latter is mostly due to self-preservation mechanisms and self-survival principles (Cacioppo et al., 2009).2 Lonely individuals tend to show deactivations of the reward systems in response to positive social versus positive nonsocial stimuli (Aron et al., 2005; Rilling et al., 2002).
___________________
2 Lonely individuals showed hyperactivation of the visual cortex in response to negative social stimuli versus nonsocial stimuli, as well as hyperactivations of temporoparietal junctions on both sides of the brain.
Brain Dynamics of Loneliness
Cacioppo presented data from studies on the brain dynamics of loneliness that looked at when and how fast the associated brain regions are activated (Cacioppo et al., 2015a, 2016). For example, lonely participants were able to differentiate a social threat from a nonsocial threat about twice as fast as nonlonely participants. Converging evidence suggests that the brain network for alertness is hyperconnected in lonely individuals based on connectivity analysis of resting-state fMRI data. In lonely individuals, investigators observed hyperactivation in the network of alertness (the cingulo-opercular network) and hyperconnectedness in the supramarginal gyrus network, which is associated with taking perspective of other relationships.
Together, the behavioral, neuroimaging, and electroencephalographic (EEG) data suggest that there is a paradoxical element to loneliness. Lonely people feel isolated and receive the biological signal that they need to approach and connect with others to survive. They have this huge motivation to connect, but at the same time, their brains are hyperalert for potential threats to the extent that they see more foes than friends. They tend to see more cues confirming their hypothesis, such as misinterpreted facial expressions of people they are interacting with, then behavioral confirmation processes lead to social withdrawal. Going forward, the temporal dynamics for the operation of loneliness and for each specific pathway need to be better understood. Another research question is to look at whether loneliness is associated with many or all of those pathways in everyone, or if it is associated with different pathways or subsets of pathways across people and social contexts.
Discussion
Lis Nielsen, chief of the Individual Behavioral Processes Branch of the Division of Behavioral and Social Research at the National Institute on Aging (NIA), remarked that research on loneliness is garnering increased public attention and asked about the level of evidence that would be needed to investigate loneliness targets experimentally. Cacioppo replied that according to an analysis of existing loneliness interventions, one-way social support does not necessarily help as much as other interventions. This is in line with social evolutionary theories that survival depends on mutual aid and protection that is a two-way street of exchanging information and support. Group interventions that bring together people who are lonely are also not very effective owing to the paradox of loneliness. Participants want to go to the meetings, but when they do, they find foes rather than friends. They misinterpret what people are saying, they feel defensiveness, and then they play the blame game. Participants often
return home even more distressed because they found confirmation in the behaviors of others. This could be compounded by the tendency of depressed or lonely individuals to remember more negative memories than positive ones, she added. Cognitive behavioral therapeutic interventions tend to be more oriented toward addressing the functions of the social brain network.
Deanna Barch, chair and professor of psychological and brain sciences, professor of radiology, and Gregory B. Couch Professor of Psychiatry at Washington University in St. Louis, asked Cacioppo to elaborate on the nature of loneliness—for example, whether it is a trait-level characteristic. Cacioppo said that we all have the potential to feel lonely at some point in time. It is not an on-off symptom, but a modulation of certain brain areas depending on mind state or mood. Neurotic people tend to be less responsive to some loneliness interventions and less open to being helped with their loneliness. However, she suggested that loneliness is more like a state than a trait, given that a person can go in and out of it so easily.
Damien Fair, associate professor of behavioral neuroscience, associate professor of psychiatry, and associate scientist at the Advanced Imaging Research Center at the Oregon Health & Science University, commented that it is difficult to define brain health without having a target outcome. He suggested that some of the factors Cacioppo discussed in the context of loneliness might be potential targets for brain health writ large, such as quality of life, life expectancy, and the development of psychopathologies. After defining loneliness as a risk factor, the researchers identified brain changes in the animal models, cortisol changes, and behavioral changes related to this risk factor. Fair suggested that the next question is to differentiate between (1) the changes that are related specifically to loneliness or causative of loneliness, and (2) the changes that make one resilient to the risk factor of loneliness. Cacioppo said that longitudinal studies have controlled for the other factors, including genetic expressions, and found that sensitivity to social rejection (not loneliness per se) is heritable 30–35 percent of the time. This sensitivity can be a trigger to all of the other factors. Evidence also shows that constellations of factors like cortisol, sleep salubrity, and the HPA axis drive increases in the effect of loneliness.
Fair asked about individual-level variability in measuring a target such as loneliness, particularly in the temporal domain for prediction purposes. Cacioppo responded that chronic loneliness lasts for at least 2 to 4 weeks; studies should be conducted to understand the individual-level dynamics of loneliness at play during this period. She remarked that loneliness does not discriminate—it touches every gender, every ethnicity, and every context. It has been posited that there are three different types of connections in an individual: intimate connections, relational connections,
and collective connections. Further study is warranted to understand the relationships between those connections (or lack thereof) and loneliness. Early research suggests that the collective connections do not move as fast as the intimate ones or the relational ones; collective connections tend to be protective of personal loneliness. Cacioppo said:
If you have a sense that you belong to someone even if you feel lonely every other day, the fact that you belong to a group that is bigger than yourself—and you have a bigger purpose in life—that would be really helpful for you to feel less lonely on a daily basis.
MOTIVATION, COGNITION, AND DECISION MAKING IN EVERYDAY LIFE
Samanez-Larkin focused on potential ways that emotional health actually improves with age. Attention is often focused on the relatively linear declines associated with aging in terms of fluid cognitive deficits, attention, inhibiting interference, memory, and so forth. However, evidence that older adults experience more positive emotions and fewer negative emotions in daily life—and report being better able to control their emotions—suggest that emotional health may improve with age in some respects.
Longitudinal evidence from positron emission tomography (PET) and fMRI studies provides insight into the functional and structural changes in the brain that account for age-related impairments in cognition. For example, this evidence suggests that changes in episodic memory, which would typically be ascribed to the medial temporal lobes, seem to be related more strongly to gradual structural and functional decline causing gross losses in the frontal cortex. However, the neurobiological bases of motivational and emotional health improvements are not yet well understood. Even today, some researchers maintain a dualistic position with respect to biology and motivation—meaning, that findings about age differences in cognition are either attributable to biological changes or to motivational changes, as if motivational changes are not biological. This highlights the need for research on how motivational systems may change with age in ways that maintain the stability of emotional health and may even drive improvements.
Neurobiology of Age Differences in Decision Making
To help address this research gap, Samanez-Larkin’s group looks at how individual and age differences in motivation and cognition influence decision making across the life span. Decision making is a capacity that
recruits a broad range of interacting psychological processes and neurobiological systems. Their early research found that older adults tend to perform the same or better as younger adults in some decision tasks—such as intertemporal choice tasks—but they tend to perform worse than younger adults in certain types of tasks, such as reinforcement learning.3 He noted that the older adults still learned in these tasks, it just took more time.
Reinforcement learning tasks require learning quickly from experience. Early fMRI studies suggested that the age difference in reinforcement learning tasks was related, at least in part, to reduced representation of prediction errors in the medial prefrontal cortex in older adults. He described this as a weaker teaching signal in the ventral medial prefrontal regions. These brain regions contain many dopamine receptors, so it was assumed that this age-related difference in decision making was likely related to a dopaminergic deficit with age. In fact, subsequent studies showed that giving participants the dopamine precursor levodopa could improve reinforcement learning in older adults and normalize that value-based signal in the prefrontal cortex. This highlights the important role of dopamine in reinforcement learning and suggests that declines in reinforcement learning are caused by decreasing levels of dopamine with age.
Neuroimaging evidence also sheds some light on why older adults seem to perform the same, if not better, on decision-making tasks involving intertemporal choice. In some studies, older adults were more likely to wait for a larger reward than younger adults, who tended to choose a smaller reward that was immediately available. Evidence from fMRI studies shows that older people were primarily representing the reward magnitude in the medial prefrontal cortex. This suggests that the older adults were less likely to factor the delay into their decision making and that the value signal seen in older adults is mostly a function of the reward’s magnitude; behaviorally, the time delay does not appear to matter as much. Evidence from different laboratories showed the same pattern, with the clusters associated with age differences occurring in almost exactly the same places, but the researchers’ conclusions based on those similar findings were very different.
Samanez-Larkin’s group maintained that the findings are evidence for preservation with age. They speculated that older adults have more lived experience and thus understand that a delayed large reward will feel just as good or better than an immediate small reward. Another research group came to the opposite conclusion, that these findings are evidence for decline with age. They suggested that older adults have a motivational deficit in that they cannot muster as much excitement
___________________
3 Typically, reinforcement learning tasks requiring the participant to make a choice, receive feedback, and then make a new choice based on the evidence provided thus far.
about an early or immediate reward as younger people can, which is likely related to reduced motivational dopaminergic signaling in older adults. Samanez-Larkin’s group hypothesized that older adults tend to perform worse on reinforcement learning tasks and the same or better on choice-based decision tasks for the same reason: they are more willing to tolerate delays due to decline of dopamine with age and a consequent global motivational deficit. In other words, older people find it harder to get excited and motivated. This hypothesis is counter to findings about emotional experience and age-related social preferences conducted by Carstensen and other social-psychology-oriented aging labs, which suggest that motivation changes with age, but it does not go away.
Time Horizons and Goals Shift with Age
Socioemotional selectivity theory holds that time horizons change with age. As people age, the awareness that time is limited influences their goals and changes their motivation. Some of the early evidence about age-related changes in motivation came from simple social partner preference studies, in which participants are asked to choose a person with whom to spend 30 minutes of free time—with the author of a book the person recently read, with a recent acquaintance with whom the person seems to have much in common, or with a close friend or family member (Carstensen and Fredrickson, 1998; Fredrickson and Carstensen, 1990; Fung et al., 1999).
Younger people are more likely to choose the author or recent acquaintance and are somewhat indifferent in their preference for the three options. Older adults tend to be less indifferent than younger people and demonstrate a strong preference for the close social partner, who is associated with known usefulness and positive value. Based on this evidence, investigators hypothesized that the age effects on behavior, and potentially on brain function, depend on the goal relevance of the rewards. The early work on reward processing in the aging brain from his lab had used monetary rewards, so it is possible that the older adult participants tended to be more financially comfortable, making the small monetary reward less motivating.
Samanez-Larkin’s group has explored age-related differences in how the type of reward relates to motivation. One study hypothesized that age effects on behavior and frontostriatal function would depend on the goal relevance of rewards. The study used three versions of the intertemporal choice task (Seaman et al., 2016): (1) a standard version of the task, in which participants chose between an immediate smaller monetary reward and a delayed larger monetary reward; (2) a social version of the task, in which the reward magnitude was the length of time spent with a close
social partner; and (3) a health version of the task, in which the reward magnitude was the dosage of a hypothetical drug that would improve organ function as well as cognitive and mental health. Figure 6-1 shows that in the monetary reward task, older people are statistically as likely to choose the immediate option as younger people. However, in the social and health reward tasks, older adults are more likely to take the smaller immediate reward than younger people.4
These findings warrant a behavioral explanation, because if low dopamine levels reduce the motivation for immediate reward in older people, they can apparently still become excited for immediate rewards behaviorally. Another study looked at subjective value signals during three decision-making tasks.
Each participant’s individual preferences were taken into account based on their behavioral choices, in order to look at the representation of subjective usefulness and subjective value. In this study, age differences were not observed in the medial prefrontal cortex; the adults of all ages similarly represented subjective value. This suggests that this basic value signal or usefulness signal in the brain seems to be stable across adulthood (Seaman et al., 2018).
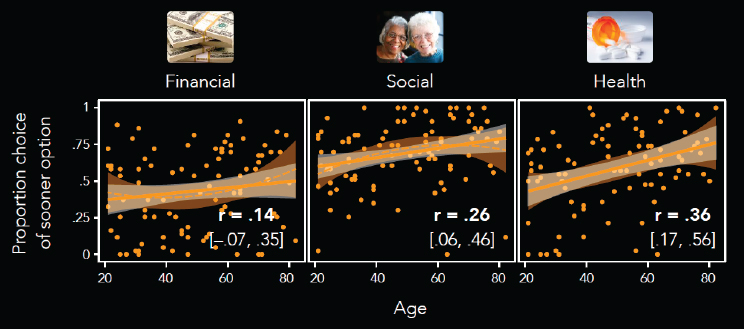
SOURCES: As presented by Gregory Samanez-Larkin at the workshop Brain Health Across the Life Span on September 25, 2019; adapted from Seaman et al., 2016.
___________________
4 Samanez-Larkin noted that in other studies, the positive effect is generally not seen in the financial reward task. He posited that because the financial, social, and health tasks were intermixed in this study, perhaps it oriented the older adults’ future thinking in such a way that they are more focused on the present in the financial task than they might normally be.
Age Differences in the Dopamine System
To further investigate the functional sequelae of the well-established, age-related dopamine decline, Samanez-Larkin’s group conducted a meta-analysis of different components of the dopamine system. After analyzing three decades of PET and single-photon emission computed tomography (SPECT) imaging studies of adult age differences in the dopamine system, researchers found very strong declines in dopamine transporters across both classes of dopamine receptors (i.e., D1-like and D2-like receptors). However, they did not find significant age difference in dopamine synthesis capacity, which is a measure of how well dopamine can be packaged and prepared for release (Karrer et al., 2017). This suggests that older adults are able to produce dopamine and package it relatively well, but the receptor differences limit the extent to which that dopamine can affect signaling. Signal transmission may be limited because there are fewer sites to act postsynaptically. The age-related decline in dopamine transporters may actually be helpful, he noted, because the transporters are located on the presynaptic cells that pull dopamine back in. When there are fewer transporters, there is more dopamine present that can potentially act postsynaptically.
Evidence for Motivational Brain Health
This evidence for the health of the aging dopamine system was interesting, but the underlying processes were still unclear, given the very strong age correlations with D2-like and D1-like receptors. The researchers posited that perhaps the motivational effects and functional value signals in the medial prefrontal cortex—a brain region that has significantly more glutamate than dopamine receptors—functionally shift away from the dopamine system as people age. In the meta-analysis, the reported statistics yielded very large regions of interest, including the prefrontal cortex and all of the striatum. To address this, Samanez-Larkin’s group used higher-resolution data from 132 adults ranging in age from 20 to 85 years to look more closely at the striatum and certain cortical regions (Seaman et al., 2019). They parsed up frontal cortex into all the sub gyri, the striatum into all the striatal subregions, and the mediotemporal lobe into subregions, and then plotted the percentage differences per decade in D2-like-receptor availability. The analysis showed substantial regional variation in the decline of D2-like receptors with aging (see Figure 6-2). Certain regions show a strong percentage decline per decade estimated from these cross-sectional data, while other regions show no evidence of an age effect.5 The strongest declines were seen in the lateral frontal
___________________
5 All of the data are available at http://bit.ly/agingdopamine (accessed November 13, 2019).
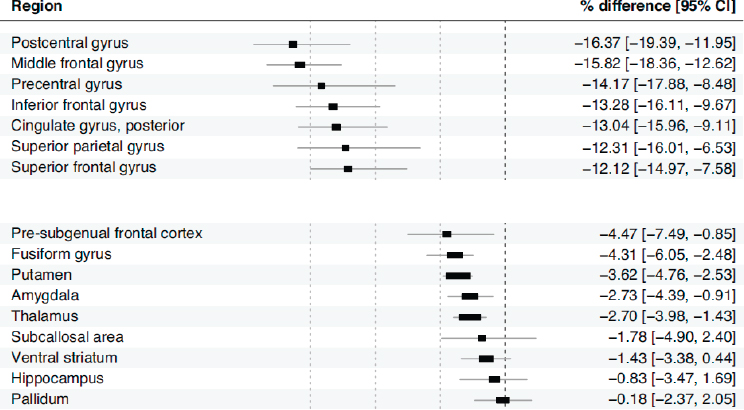
NOTE: CI = confidence interval.
SOURCES: As presented by Gregory Samanez-Larkin at the workshop Brain Health Across the Life Span on September 25, 2019; adapted from Seaman et al., 2019.
cortex, while the weakest effects (or no effects) were seen in the ventral striatum and pallidum.
Samanez-Larkin emphasized that this evidence does not support the idea of global motivational decline. Even though the declines in the dopamine system are well documented, the meta-analysis revealed that synthesis capacities are relatively preserved; D2-like receptors are also preserved in certain regions of the brain. It appears that dopamine production, postsynaptic action, and signal transmission are maintained with age in certain subcomponents of these circuits. Perhaps some subparts of the circuits are actually working relatively well and their functions are dopamine mediated, which would affect fMRI evidence for preserved signaling. This could also be caused by the preservation of dopamine in those circuits. He suggested that evidence for motivational brain health—rather than decline—is the meaningful evidence that is emerging from this fMRI, behavioral, and PET research.
Future Research Directions
Samanez-Larkin concluded by describing some of his laboratory’s future research directions. They have ongoing fMRI studies on anticipation and the experience of social versus health rewards, as well as plans
for PET studies to look at how different types of rewards elicit dopamine release in different parts of the dopamine system as people age. Although his team is using somewhat more naturalistic stimuli, the paradigms are still relatively basic—positive social rewards, negative social rewards, or social incentives (Holland et al., 2019). They are also looking at how these reward-type differences influence function, as well as how those factors are related to differences in dopamine levels.
Discussion
Barch asked about age-related changes in neurotransmitter systems other than dopamine. Samanez-Larkin replied that his laboratory recently completed a meta-analysis on the age effects of serotonin; there appears to be no preservation of the serotonin system and a relatively clear decline with age. Nielsen asked if there is any evidence that individual differences in the dopamine system are a function of life-course individual differences in impulsivity or other traits, or any evidence about individual differences in loneliness or social isolation in terms of the response to social reward tasks. Samanez-Larkin replied that this type of social engagement information is not collected very well in this arena. In general, however, his group’s work shows between-subject variability at every age, with very strong individual differences, even though the age effects are relatively consistent; more work remains to be done to explain that variance.
Fair remarked that two items can cause the type of variance that is seen in these data: one is the real signal—that is, the real variability with regard to a given measurement—and the other is noise. The two may need to be teased apart to understand what it means to be resilient. Samanez-Larkin responded by acknowledging the limitations of these cross-sectional data. Very little longitudinal PET data are available, and some of the changes they are investigating take decades to become apparent. It might be possible to identify lifestyle factors and create retrospective measures, but there is no earlier time point to relate the data to; the participants could simply have wildly different intercepts and the same rates of age-related receptor loss. In terms of noisy data in general, Samanez-Larkin’s group is interested in brain signal variability. They have analyzed functional neural signal variability in fMRI data to ascertain how volatile the brain signal is, how that variability changes with age, and how it is related to decision making; they are also looking at whether fMRI signal variability is related to differences in dopamine receptors.
LIFE-COURSE CAUSES OF LATER-LIFE INEQUALITIES IN BRAIN HEALTH
Manly presented on life-course causes of inequalities and disparities in brain health later in life. She began with a review of challenges faced in researching brain health disparities. First, this research cannot be conducted using convenience samples, which proliferates in research on older adult brain health. The second challenge is that brain health disparities research must measure life-course individual or contextual factors, such as social determinants of health or the social exposome, sufficiently well to determine the relative contributions of bio-psycho-behavioral-social factors to disease or interactions. Experts in quantifying brain health have not traditionally focused on social determinants of health across the life course. As a result, measurements of social determinants are often lacking even in studies that have exquisite measurements of brain health outcomes. This has hampered discovery and acceleration in the field of brain health disparities.
A third challenge is the difficulty in determining the degree of bias in estimates of early-life or life-course factors and how they relate to brain health. Observational research will not have value unless it is used to develop targeted interventions using accurate estimates. However, it is difficult to determine bias if the target or reference population has not been defined, thus threatening external and internal validity. The fourth challenge is to develop harmonized measures,6 both of risk factors and of brain health outcomes; these are critical for combining cohorts, synthesizing research, and accelerating knowledge.
Evidence for Disparities in Later-Life Brain Health and Resilience
Manly provided an overview of available evidence for disparities in brain health and in aging, as well as some of the methodological challenges in research and some of the identified mechanisms that could help to explain these disparities in later-life brain health. The Washington Heights–Inwood Columbia Aging Project (WHICAP) longitudinal study in Northern Manhattan found that African Americans and Caribbean Hispanics are more likely to develop incident Alzheimer’s disease over time (Tang et al., 2001). These disparities persist even after adjusting for years of education, occupation, income, or history of stroke, hypertension,
___________________
6 Harmonized measures are measures that are standard across research groups and fields. Harmonized measures may avoid the problem of duplicative or overlapping research, as well as allowing larger studies to be conducted with greater power to observe subtle phenomena related to brain health and resilience.
and diabetes. Furthermore, the interventions that would be expected to mediate these differences do not seem to have a substantial effect on these disparities.
Evidence of racial and ethnic disparities was also found in the Kaiser Permanente Health Study in Northern California, which followed people in the health care system over many years. African Americans, American Indians, and Alaskan Natives were found to have the highest risk of developing dementia, while Asian Americans were at lower risk. Although researchers were not able to look at some of the social factors, such as education, that might explain these disparities in incident dementia, they were able to look at cerebrovascular and cardiovascular disease, but found that these did not explain the disparities (Mayeda et al., 2016).
Manly noted that there is also a geographic dimension to these disparities in risk for Alzheimer’s disease and dementia. An analysis of the Centers for Disease Control and Prevention death records found that both African Americans and whites have a higher risk of dying of all-cause dementia if they were born in a “stroke belt” state; even if they migrated to the north and eventually died there, they brought this risk with them from the South (Glymour et al., 2011). Racial disparities in stroke are well known—with African Americans at higher risk—but it is less commonly known that whites aged 85 years and older are at higher risk of having stroke than African Americans, according to a national longitudinal study of stroke disparities (Howard et al., 2011).
Methodological Challenges in Brain Health Disparities Research
Manly highlighted the influence of selection bias in this field. The studies she presented in the previous section are population-representative, community-based longitudinal studies. Selection bias is prevalent in studies that use clinic-based or convenience samples, in which participants are recruited from clinics that specialize in memory disorders, for example. Mistrust and stigma are direct causes of the problem of selection bias. She noted that a long history of stigma and mistrust persists to this day, widening the gap between the people who have cognitive impairments and the people who go to a doctor with those complaints. This is a consequence of historical medical abuses, including use of IQ tests to support racist policies, as well as the lack of evidence on the benefits of medical research for underserved communities facing intractable disparities. Ongoing experiences of discrimination in the medical setting are common, and health care systems broadly lack the necessary cultural and linguistic competencies.
The Minority Aging Research Study in Chicago found that African Americans who were diagnosed with clinical Alzheimer’s disease dementia were more likely to have mixed neuropathology than whites who had the same diagnosis (Barnes et al., 2015). However, the number of whites that participated in autopsy far exceeds the number of African Americans. Thus, the findings do not necessarily indicate that Alzheimer’s disease has more mixed pathology in African Americans versus whites—it is possible that the African Americans who volunteered for the study were more likely to get diagnosed with Alzheimer’s disease. African Americans who are formally diagnosed with Alzheimer’s disease tend to have more psychiatric symptoms of irritability, agitation, paranoia, and behavioral issues than whites who are formally diagnosed.
The problem of selection bias even affects studies that are looking to characterize neuropathology in vivo. A study that has been recruiting African Americans in St. Louis for cerebrospinal fluid lumbar puncture collection and PET amyloid imaging recently concluded that there are race-dependent biological mechanisms for these expressions of Alzheimer’s disease (Morris et al., 2019). Researchers found that the African Americans in their study had less cerebrospinal fluid t-tau. However, the African Americans were matched to the whites in the cohort for years of education, which Manly suggested is not representative of the community in St. Louis or anywhere in the country. Furthermore, the African Americans in their cohort did not have any more cerebrovascular-disease-like white-matter hyperintensities than did the whites in the cohorts, which signals that the African Americans in their study are unusual.
Potential Mechanisms for Disparities
An analysis of the Midlife in the United States (MIDUS) and WHICAP studies found a narrowing of the disparity in memory performance between African Americans and whites in the older age group. This is evidence for an age-as-leveler effect, said Manly. People from minority groups who survive longer tend to be a heartier cohort than white people who survive into midlife, as a function of survival bias (Zahodne et al., 2016). This survivor effect helps to explain the crossover of stroke prevalence across race as people age that was described in the previous section (Howard et al., 2011).
Another mechanism that has been focused on in explaining brain health disparities is cerebrovascular disease. There are disparities in the burden of white-matter hyperintensities across race (Brickman et al., 2008). Whites have a lower overall burden of white-matter hyperintensities compared to African Americans and Hispanics, with no apparent interaction with age, but the scanning began when people were around 70 years of
age. Interaction or acceleration must have occurred at some point earlier among the African Americans and Hispanics. This highlights another challenge: understanding the neuropathological mechanisms underlying brain health disparities will require studying people earlier in life.
A more recent study found a tighter link between white-matter hyperintensity burden and cognition in African Americans than in whites (Zahodne et al., 2015). This contrasts with the same study’s findings about the relationship between cognitive trajectory and hippocampal volume. Whites with low hippocampal volume were at higher risk for developing Alzheimer’s disease than whites with high hippocampal volume, but hippocampal volume was not related to risk of developing Alzheimer’s disease among the non-Hispanic African Americans in the study. This suggests that there may be different pathways to cognitive decline across race and ethnicity.
Many researchers have been looking at genetic research to try to explain some of the disparities in brain health. However, these ancestry differences can generally be explained by social factors. One study compared African Americans with Alzheimer’s disease to matched controls, finding that higher levels of African ancestry (both at the whole genome level and at specific Alzheimer’s disease–related genetic loci, like ABCA7) are associated with an increased risk for Alzheimer’s disease (Hohman et al., 2016). However, Manly cautioned that social factors correlate with African ancestry and could confound the relationships with cognitive outcomes—in other words, African ancestry may be a very strong marker for experiences of discrimination and other social factors. Higher African ancestry has been associated with having a lower education level, having parents with fewer years of schooling, receiving no inheritance from one’s parents, having a lower income, and having less wealth. Ancestry does not biologically mediate or influence these factors, but African ancestry is a marker for social experiences of individuals, parents, and grandparents. In other diseases associated with genetic ancestral markers, such as diabetes, these types of social factors account for the relationship between ancestry and disease (Marden et al., 2016).
Manly’s group is working on a study based on the WHICAP cohort dataset looking at cognitive outcomes, racial self-identification, and African ancestry among Caribbean Latino older adults who were followed longitudinally.7 Those people in the lowest quartile of African ancestry had higher cognitive test scores compared to people with a higher degree of African ancestry, but these differences are explained entirely by the quality and quantity of the person’s educational experience and by the
___________________
7 Available at http://www.nationalacademies.org/hmd/Activities/Aging/BrainHealthAcrossTheLifeSpanWorkshop/2019-JUN-26.aspx (accessed March 12, 2020).
person’s early-life socioeconomic status, which is mainly driven by the degree of the person’s education.
Forthcoming Research on Brain Health Disparities
Manly described forthcoming research based on the dataset from the ongoing Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, which has followed a large and geographically diverse cohort of older adults in the United States to look at vascular contributions to cognitive impairment and dementia. An advantage of the REGARDS dataset is that it collects data about every place each participant has ever lived.
Effect of Historical Investments in Quality of Schooling on Cognition Later in Life
Economists have been using the administrative data from schools for many years to predict human capital outcomes and show differences across race in those outcomes. Manly’s group used administrative data to explore the relationship between school quality and race across time and location. Specifically, they looked at the effect of historical investments in quality of schooling on cognition later in life. For instance, the length of the academic year for schools in the South, particularly for those with African American students, was much shorter than in the North. If an African American person born in the 1930s reports having gone to school for 8 years in certain states, it is likely that the school was only open about half of the year. Similarly, the student–teacher ratio for African American children in some states was very high, which has an effect on later-life cognition. After controlling for early-life confounds, such as family socioeconomic status, as well as for state-level or state-fixed effects, Manly’s team found that people across races who attended schools with longer term lengths had improved cognition compared to people who attended schools in states or counties that had shorter term lengths.8
Based on these data, Manly’s team developed an overall score for school quality based on these administrative records. For every 1-year increase of policy-predicted years of education, people in the REGARDS study were at 40 percent lower odds of having cognitive impairment at baseline. Adding confounds such as age, sex, gender, state fixed effects, and parent education reveals an interaction in which white women, white
___________________
8 Manly, J. Workshop presentation—Life-Course Causes of Later-Life Inequalities in Brain Health. Available at http://www.nationalacademies.org/hmd/Activities/Aging/BrainHealthAcrossTheLifeSpanWorkshop/2019-JUN-26.aspx (accessed March 12, 2020).
men, and African American women have a payoff for going to higher-quality schools, but not African American men.
Discrimination and Cognitive Function Among Older Non-Hispanic African Americans
Manly’s group is also working on studies of discrimination and cognitive function. Preliminary findings suggest that in general, African Americans with more education report experiencing more discrimination. Furthermore, there is interaction between sex, years of education, and discrimination on cognitive function. The trend is that self-reported discrimination among African American men with graduate degrees is negatively related to cognition; this is not the case for African American men who have high school or college degrees.
Leveraging Expanded Datasets
Moving the field of disparities in brain health forward will require taking advantage of studies that start at an earlier age, said Manly. Her group has identified a number of cohorts that began when participants were in high school, such as the Project TALENT dataset and the High School and Beyond dataset. These studies conducted cognitive testing when participants were adolescents, which allows for tracking survival (and potentially for the survival effect) as well as for looking at early-life predictors of later-life cognition. Project TALENT is being linked to Medicare, which has been used to show that lower levels of cognitive function as an adolescent predicted a formal diagnosis of Alzheimer’s disease later in life (Huang et al., 2018).
Ways Forward to Improve Research on Brain Health Disparities
Manly concluded by offering strategies for improving research on brain health disparities going forward. Racism should be measured in Alzheimer’s disease and related dementia studies, because systemic racism becomes embodied in the biology of racialized groups. This is how race becomes a risk factor for later-life problems or inequalities in brain health. Measuring racism will require designing population-based longitudinal studies that bridge the gap between (1) biology and genetics and (2) the life course and social exposome. Population-feasible biomarkers for neuropathology will need to be developed and included in these studies; lumbar puncture or PET will not be feasible, so blood tests will probably need to be developed in diverse cohorts. Only with all of
those factors measured in the same cohort will it be possible to calculate population-attributable factors. This would allow researchers to explore how intervening in certain social factors could have an effect across the life course.
Regardless of their size, highly selected samples are not useful for disparities research (Keyes and Westreich, 2019). Researchers looking at brain health disparities need more clarity about the limits of convenience or volunteer samples, how to control for the confounds of sampling, and how to discuss these confounds in their work. Social forces such as racism and discrimination, educational quality and increasing school segregation, and neighborhood inequalities should be acknowledged explicitly in national plans to reduce the effect or burden of neuropathology and Alzheimer’s disease and related dementia on the population. Doing so will require unequivocally making the case that early-life economic and social policy is tantamount to brain health policy, perhaps through Alzheimer’s disease national plans, Alzheimer’s disease summits, and accountability measures.
Discussion
Bruce McEwen, Alfred E. Mirsky Professor at The Rockefeller University, remarked that the Safe American Family (SAF) study has looked at how building bonds between adolescents and their parents or caregivers, as well as mitigating bullying and racial discrimination, can improve physical and mental health outcomes 10 to 15 years later, including brain volume and type 2 diabetes. Manly replied that it is critical to understand how these types of interventions have effects throughout the life course; the role of inflammation in metabolic syndromes and Alzheimer’s disease, for example, warrants further investigation. McEwen added that insulin resistance is a known pathway toward dementia that can be exacerbated—especially in people with certain genotypes—by experiences such as abuse, neglect, and poverty. Understanding the roles of inflammation and overactivity of glutamatergic systems in the brain that drive the amyloid-beta hypothesis about Alzheimer’s disease could be further enriched by studying attempts to intervene early, as in the SAF study.
Lis Nielsen asked about how to enhance the value of datasets for studying health disparities, for example, by adding retrospective measures of social exposome variables to studies that focused on older age or by designing earlier life-course studies to incorporate those types of measures, so they will be valuable for long-term studies of aging and brain health. In terms of retrospective measures that could be added, Manly emphasized the value of retrospectively collecting and geocoding
location data from older adult participants about every place they have ever lived. These data have great value in analyzing the effects of childhood exposures and administrative policies in educational systems on the trajectory of brain health. For example, they can be used to look at factors such as walkability, green spaces, business, and crime.
Studies are looking at how the policing and punishment policies in individual schools may relate to stress and outcomes in children as well as to later-life outcomes. In terms of designing early-life studies to add value to later-life research, Manly suggested that cognitive function is important for understanding the real effects of interventions. At the age of 65 years, a person is at an intercept that is strongly related to family history, young life cognition, and exposures after that age. Much research on Alzheimer’s disease and related dementias is focused on slope, distinguishing the trajectories with respect to slope over time, and its relationship to hippocampal volume, diabetes, and other biomarkers. The biggest effect to be had in maintaining brain health is on the intercept with which a person enters older age. Thus, early-life studies and measures of cognition earlier in life are critical for determining the potential effect of interventions.
PANEL DISCUSSION ON BRAIN HEALTH IN THE SOCIAL CONTEXT
Fair asked the panelists to discuss how the definition of brain health affects how its outcomes should be measured—for instance, quality of life and stress are reflected in various types of outcomes depending on the population being described. Barch replied that brain health can be thought about as (1) a person’s accumulative reserve, which sets the intercept or starting point, and (2) the degree to which a person is affected by adversities, such as stress or the onset of illness. These adversities are likely to be related and difficult to tease apart unless they are measured from an early stage—from birth or even in utero would be ideal. Social determinants are being set in utero, and exposures could potentially be setting up a proinflammatory phenotype very early in life that has effects later. More practically, it would be helpful to focus on intermediate outcomes that are already known to be predictive and to look at whether their genesis is even earlier than assumed.
Manly suggested that in the context of aging brain health, it would be useful to be explicit that the trajectories of cognitive decline start much earlier in life, before they have their greatest effect on function and impose the greatest burden and cost to society. Manly also highlighted the challenge of how to measure brain health across the entire life course. She suggested linking and triangulating among studies focusing on different
time periods during the life course. Even if the studies do not use the same cognitive measures or focus on the same outcomes, this strategy may be useful as a starting point for exploring potential targets and identifying critical periods across the life course. Fair noted the importance of bringing context to bear in prospectively trying to coordinate and design new studies and new research. Ted Satterthwaite, assistant professor in the Department of Psychiatry at the University of Pennsylvania School of Medicine, remarked that investment will be needed to harmonize, chain back, and link those data sources. A relatively small, inexpensive study could harmonize different measures and outcomes across large-scale expensive studies, so they can be linked in different populations of interest and provide additional return on that investment.
This page intentionally left blank.


























