1
Introduction and Overview1
Gene therapy is a technique that uses or modifies genes to prevent or treat disease. Gene therapy approaches are diverse and can include replacing a disease-causing gene with a correct copy, inactivating a gene that is functioning improperly, and introducing a new gene into the body to help fight disease, among other approaches (NLM, 2020). At the time of this workshop, the Food and Drug Administration (FDA) had approved four gene therapy products for use, including two genetically modified T-cell immunotherapies for different types of leukemia and lymphoma, one gene therapy for patients with mutation-associated retinal dystrophy, and one gene therapy for children less than 2 years old with spinal muscular atrophy.2 The design of clinical trials for gene therapy products is often complex and can present many translational, clinical, and ethical issues, including challenges with determining an optimal dosage, delivering the product effectively, and successfully recruiting patients and following them over the long term. Patients and clinicians may also face difficult decisions about enrolling in gene therapy trials because of uncertainty about
___________________
1 The planning committee’s role was limited to planning the workshop, and the Proceedings of a Workshop was prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and are not necessarily endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
2 See Approved Cellular and Gene Therapy Products at https://www.fda.gov/vaccines-bloodbiologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed January 12, 2020).
potentially long-lasting effects and concerns related to the future use of other therapeutic options, including different gene therapies.
One challenge, for example, is selecting an appropriate study population for a first-in-human clinical trial with a gene therapy whose greatest potential for clinical benefits is in very young children. For such therapies, it is important to identify ways to balance the potential clinical benefits with available safety data and to address when it would be appropriate to rely on data obtained from the preclinical program and natural history studies to support administering novel gene therapies to young children.
Another type of challenge faces clinical trials with gene therapies aimed at treating or curing rare genetic diseases, as the number of patients who are eligible to receive an experimental therapy during a clinical trial may be very limited. To address this, finding approaches that enable the effective use of data collected in natural history studies can further improve the efficiency of developing the gene therapies.
Yet another challenging set of issues in gene therapy trials involves dose selection and considerations for the possibility of repeat gene therapy administration. Gene therapy products often have long-lasting activity, and their administration may result in the formation of neutralizing antibodies or induce a pathologic immune response. A subsequent product administration to the same patient may result in lack of efficacy or severe toxicity that may preclude a repeat administration of the same therapy or a different therapy that targets the same gene, cell type, or tissue, or uses the same vector. Recognizing and managing immunogenicity in clinical trials, determining the appropriate product dosing and administration, and carefully monitoring for long-term effects of gene therapies are important tactics to employ when developing these novel treatments.
Those in the field have also found it challenging to measure the treatment benefits of gene therapies accurately. Changes in the expression and levels of transgene proteins (e.g., enzymes, blood clotting factors) following the administration of a gene therapy may not always be predictive of clinical benefits. Gaining a thorough understanding of how to optimally evaluate the clinical meaningfulness of blood and tissue measurements of transgene protein and creating reliable, functional assays may result in improved trial endpoints. Lastly, a clear understanding of the disease mechanism(s) and progression can be important for quantifying the clinical effectiveness of a gene therapy.
The scientific and translational issues described above are accompanied by myriad ethical issues, such as fairness in the selection of patients for trial participation, informed consent, and benevolence on the part of health care providers administering the experimental gene therapies. Developing improved educational tools to help patients and their providers understand the potential risks and benefits of specific gene therapies may help the
field move forward. Recognizing the potential design complexities and ethical issues associated with clinical trials for these types of therapies, the Forum on Regenerative Medicine held a 1-day workshop in Washington, DC, on November 13, 2019, to explore these issues with a variety of stakeholders in greater detail. Speakers at the workshop discussed patient recruitment and selection for gene-based clinical trials, explored how the safety of new therapies is assessed, reviewed the challenges involving dose escalation, and spoke about ethical issues such as informed consent and the role of clinicians in recommending trials as options to their patients. The workshop also included discussions of topics related to gene therapies in the context of other available and potentially curative treatments, such as bone marrow transplantation for hemoglobinopathies. The concept of repeat dosing and sensitization treatments was also explored by the broad array of stakeholders who took part in the workshop, including academic and industry researchers, regulatory officials, clinicians, bioethicists, individuals and patients, and representatives of patient advocacy groups. The workshop agenda is in Appendix A, biographical sketches of the speakers and moderators are in Appendix B, the Statement of Task for the workshop is in Appendix C, and the list of workshop attendees is in Appendix D.
In his introductory remarks to the workshop, Krishanu Saha, an associate professor and the Retina Research Foundation Kathryn and Latimer Murfee Chair in the Department of Biomedical Engineering at the University of Wisconsin–Madison, discussed the differences in how gene-based therapies move through clinical trials compared with most drugs in development. Typically, he explained, drug discovery and development entails screening thousands of compounds for the desired properties, conducting extensive preclinical studies, and enrolling hundreds if not thousands of patients in multiple clinical trials that can take 6 to 7 years to complete. From discovery to market, it is not uncommon for the drug development process to take 10 to 15 years (Janssen Pharmaceutica, 2020).
In contrast, gene-based therapy development starts with a few therapeutic candidates that developers test in 100 or fewer patients—or, in some cases, individual patients, Saha noted. In fact, the faces and names of these patients are often widely recognized and have been featured by major new outlets. The preclinical process, Saha said, which can take 3 to 6 years with traditional drug candidates, can be completed in 1 to 3 years with gene-based therapies. Another difference is that the therapeutic candidates themselves—cells, viruses, genome editors, antisense oligonucleotides, and others—are more complex than small-molecule drug candidates.
Given these differences, Saha said, there are challenging questions that require answering with regard to clinical trials for gene-based therapies. For example, he asked, what type of evidence is needed to bring a gene--
based therapy into human clinical trials, and how should that evidence be collected responsibly? What should an optimal starting dose be? What are the stopping criteria? How can delivery be optimized? How can researchers engage and communicate with all of the people involved in a clinical trial, including the patients? “Ultimately, what we are hoping to hear from the folks in the room [today] are ways to improve the design of these trials,” Saha said. The objectives of workshop can be found in Box 1-1.
As a way to clearly communicate the scope of this workshop during the planning process, the planning committee used the following definition of gene therapy from FDA3: “[t]he administration of genetic material to modify or manipulate the expression of a gene product or to alter the biological properties of living cells for therapeutic use.” For the purposes of workshop planning, the committee also considered any use of gene editing (including techniques such as CRISPR/Cas9 that allow for precise changes in the nucleic acids of a person, animal, or other living organism) to be a gene-based therapy.
ORGANIZATION OF THE WORKSHOP AND PROCEEDINGS
This Proceedings of a Workshop summarizes the presentations and discussions that took place at the workshop. The opening keynote lecture by Katherine High is covered later in this chapter, and Chapter 2 explores the early stages of development of gene-based therapies, including designing research questions and collecting preclinical data. Also included in
___________________
3 See What Is Gene Therapy? at https://www.fda.gov/vaccines-blood-biologics/cellular-genetherapy-products/what-gene-therapy (accessed January 12, 2020).
Chapter 2 is a discussion of challenges with transitioning to first-in-human clinical trials. Chapter 3 addresses ethical issues surrounding patient selection, enrollment, and consent for gene-based therapies and how these issues differ from those surrounding conventional clinical trials. The discussion in this section of the proceedings also touches on resources available to help patients and providers accurately understand the potential risks and benefits of participating in a gene-based clinical trial and explores communication strategies aimed at helping patients make informed decisions about participating in trials for gene-based therapies. Chapter 3 also includes a series of patient and family perspectives on these issues. Chapter 4 presents some lessons learned from the successes and challenges of accurately measuring clinical endpoints and outcomes for gene-based therapies and moving products through the translational pathway. Chapter 5 addresses the implications of the long-term follow-up and clinical management of patients who participate in gene-based clinical trials and discusses how data from a limited number of patients can be effectively used to determine if a gene-based therapy is safe and effective. Chapter 6 includes several stakeholder perspectives on possible approaches to supporting the clinical development of safe and effective gene-based therapies going forward. The final chapter also includes a summary of lessons learned and topics discussed throughout the workshop.
LESSONS FROM THE DEVELOPMENT OF A GENE THERAPY TO TREAT CHILDREN AND ADULTS WITH INHERITED VISION LOSS
There are several challenges that gene-based therapy developers face in bringing a product to market, according to Katherine High, the president and head of research and development at Spark Therapeutics. During her keynote lecture High shared examples of these challenges and spoke about her experience with obtaining FDA approval for the first gene therapy for a genetic disease, in this case a rare inherited retinal dystrophy that goes by several names, including Leber congenital amaurosis and retinitis pigmentosa (Russell et al., 2017).
In the United States, 1,000 to 2,000 individuals have this disease, which is caused by an autosomal recessive mutation in a gene called retinal pigment epithelium 65 kilodalton protein (RPE65), High explained. It is characterized by the early onset of retinal degeneration and nyctalopia or “night blindness.” High said that many of these patients are identified during infancy by parents who notice their infant cannot visually track or follow an object or that the infant experiences involuntary eye movements (nystagmus). By the second decade of life, nearly everyone with this disorder
has significant visual impairment and over time, most people with the condition will progress to blindness (Chung et al., 2019).
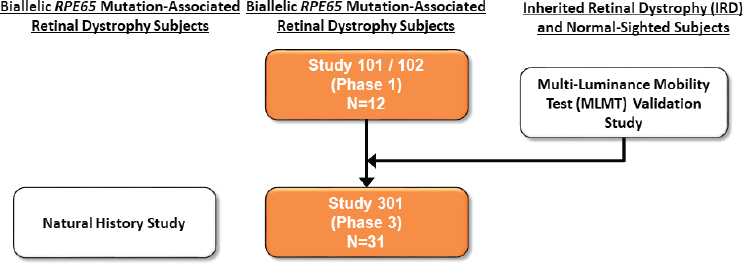
When the team at Spark Therapeutics first started this project, High said, there was no treatment for any inherited retinal dystrophy, but there was compelling evidence that subretinal injection of an adeno-associated virus (AAV) vector expressing the correct form of RPE65 could restore vision in a naturally occurring dog model of the disease (Acland et al., 2001; Bennicelli et al., 2008). In 2007 the Spark team initiated dose-escalation Phase 1 trials in the eye with the worst function in 12 adult and pediatric patients (see Figure 1-1). This study showed that even the highest dose of the RPE65-carrying viral vector was safe, and the higher dose was then injected into the opposite (previously untreated with this viral vector) eyes of the 12 test subjects.
Using Natural History Data in Clinical Trials
In addition to the clinical trials of AAV2-RPE65, FDA encouraged the company to conduct a natural history study in patients with biallelic RPE65 mutation-associated retinal dystrophy; the data from such studies can be useful in interpreting safety and efficacy data generated from a trial. High said that natural history data, if robust, can be used as a control group for a clinical trial, although in the case of retinitis pigmentosa the available natural history data were mainly from single-institution case reports and therefore were not convincing enough to be used in this way. The research team attempted to overcome this challenge by conducting a natural history study in parallel to the Phase 3 trial. The natural history study involved a retrospective chart review of patients who had a genetically confirmed diagnosis and at least two office visits. By working with seven referral centers on three continents,
SOURCES: Katherine High workshop presentation, November 13, 2019. Originally from https://www.fda.gov/media/108680/download (accessed March 30, 2020).
High and her colleagues developed a database of 70 individuals ranging in age from 1 to 43 who met the inclusion criteria, from which they were able to construct curves describing the loss of individuals’ visual field and visual acuity over time (Chung et al., 2019; Holladay, 1997).
Understanding Important Characteristics of the Study Population
In studies of genetic diseases, High said, it is important that the study design call for verifying the genetic diagnosis in each individual and for being aware of any genotype or phenotype correlations that may affect safety or efficacy. For a progressively degenerative disease such as this one, it is important to stratify the Phase 3 randomization process based on disease stage and severity. For the Phase 3 trial of AAV2-RPE65, the two arms were balanced in terms of the number of subjects less than 10 years old and those 10 years of age and older (as a proxy for disease progression/severity) and also in the subjects’ ability to pass the primary endpoint, the mobility test, above or below a defined level of illumination at baseline (Russell et al., 2017).
Ethical Considerations with Pediatric Subjects
Because the preclinical studies in dogs had demonstrated that the earlier the intervention, the better the eventual outcome, the research team wanted to include children in the clinical trials from the start. However, High said, if including children in an interventional trial involves more than minimal risk,4 the trial has to offer the prospect of direct clinical benefit for every child enrolled. The Children’s Hospital of Philadelphia’s institutional review board (IRB) deemed that because the administration procedure involved general anesthesia, removal of some or all of the fluid inside the eye, and subretinal injection, the trial clearly involved more than minimal risk. As a result, the Phase 1/2 trial design called for starting with a dose at which the majority of affected eyes in the dog study recovered vision and then escalating from there. Federal regulatory bodies agreed.
Developing and Validating Efficacy Endpoints
Regulators at FDA asked High and her team to also conduct a validation study in normal subjects and in those with inherited retinal dystrophies in order to assess the performance characteristics of the multi-luminance
___________________
4 Federal regulations define minimal risks based on the risks “ordinarily encountered in daily life or during routine physical or psychological examinations or tests” (Wendler et al., 2005, p. 827).
mobility test (MLMT) (the primary endpoint in Phase 3) that they had devised in close dialogue with FDA. (Additional details on the development of the multi-luminance mobility test are discussed by Albert Maguire in Chapter 4.) High noted that FDA’s 2018 draft guidance document on human gene therapy trials for rare diseases emphasizes the importance of discussing primary efficacy endpoints with FDA because well-established, disease-specific efficacy endpoints are not available for many rare diseases.
The MLMT, a mobility test conducted at a series of light levels, served as a novel primary efficacy endpoint for inherited retinal dystrophy caused by RPE65 mutations. In the separate validation study, which compared performance in both sighted participants and those with inherited retinal dystrophies, the investigators found that no subjects with an inherited retinal dystrophy improved over the course of 1 year without treatment and that in 28 percent of these subjects the condition worsened.
Points to Consider with Gene Therapy Trial Design
A randomized controlled crossover trial design was used for the Phase 3 clinical trial of AAV2-RPE65. A two-to-one randomization was used so that people would sign up knowing that they had a two-thirds chance of being part of the intervention, High noted. Using such a design would be more difficult for diseases that are fatal, she said. In those cases, alternative clinical trial designs are needed, with the exact trial design depending on if there are alternative treatments available and if the disease is currently treated by a complex medical regimen but one with decades of clinical experiences, as is the case with hemophilia.
However, by day 30 of the trial it was clear that those in the intervention group were seeing marked improvement in their ability to maneuver quickly and accurately in dim light compared with the control subjects. As the study progressed, that improvement persisted for 1 year, and members of the control group became eligible to receive the intervention as part of the crossover clinical trial design. Those in the control group that crossed over to receive the intervention experienced the same benefits as the original intervention group. Members of both groups also experienced marked improvement in their visual fields after receiving the intervention. When asked if patients receiving the lowest dose were allowed to receive a second, higher dose, High replied that large animal studies suggest that re-administration should be safe. However, she reminded the audience that studies of other gene therapies have found that animal studies are poor predictors of human immune response to the viral vector used to deliver the gene. “I think that represents a risk that would have to have, in my opinion, a strongly worded consent form to re-administer to the same eye,”
she said. She also noted that there is a dose-dependent risk of triggering an inflammatory response in the eye.
In closing, High said that understanding the pathophysiology and natural history of the disease played a critical role in developing the clinical endpoint because it led to measuring mobility at different levels of illumination, something that existing mobility tests did not do. High noted, too, that collecting clinical measurements repeatedly over time can yield important information, which is why the Spark clinical trial collected data at baseline, 30 days, 90 days, 180 days, and 365 days.
This page intentionally left blank.










