1
Introduction and Workshop Overview1
The completion of the Human Genome Project in 2003 was followed by the emergence of direct-to-consumer (DTC) genetic testing in 2006 and 2007, as companies such as 23andMe, Navigenics, and deCODE Genetics were founded. Companies attempting to market their products as health products directly to consumers met regulatory and clinical challenges. During that time, DTC companies could perform genetic testing for anywhere from $400–$1,000, making the price out of reach for many consumers. Today, the prices for such services are much lower, making DNA sequencing more accessible to consumers than before and providing opportunities for consumer health and literacy engagement. Additionally, DTC testing has had implications for clinical care, research, and education.
Consumer genomics, which includes both DTC applications (i.e., genetic testing accessed by a consumer directly from a commercial company apart from a health care provider) and consumer-driven genetic testing (i.e., testing ordered by a health care provider in response to an informed patient request) has evolved considerably over the past decade. In that time, DTC genetic testing has moved from more personal utility-focused applications outside of traditional health care, such as exploring ancestry, to interfacing
___________________
1 The planning committee’s role was limited to planning the workshop, and the Proceedings of a Workshop was prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants and are not necessarily endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
with clinical care in non-traditional ways, such as collaborations between DTC companies and health systems.
As consumer genomics has increasingly intersected with clinical applications, discussions have arisen about the need to demonstrate clinical and analytical validity and clinical utility because of the potential for consumers to misinterpret the results of these tests and for ensuring the accuracy of the information for medical decision-making. Determining the clinical utility of consumer genomics entails examining the benefits and harms, which depend on various factors such as the availability of comparable risk assessment tools, costs of the intervention, and the personal value of the information (Khoury et al., 2009). In addition, the clinical readiness for and interest in this information have presented educational and training challenges for providers. Surveys of physicians have indicated that many of them are not confident in their ability to use genetic testing results in their patient care—a challenge for clinical genetic testing too (Owusu Obeng et al., 2018). At the same time, consumer genomics has emerged as a potentially innovative mechanism for thinking about health literacy and engaging participants in their own health and health care.
One of the reasons for using consumer genomics is its personal utility, or the usefulness that an individual derives from knowing his or her genetic information. If consumers are engaged and empowered to learn more about their health information and potential genetic risk factors, there could be opportunities for the health care system to learn effective strategies for engagement with consumers, including engaging populations that may not have adequate access to genetic testing. While the regulatory process for DTC genetic health risk tests involves the submission of a user comprehension study, there may still be questions about the extent to which consumer genomics companies are responsible for ensuring that consumers fully understand the information being presented to them so that they make informed decisions (Allyse et al., 2018).
The use of consumer genomics could also have implications for genetic research, given that many consumer genomics companies share participant data with external researchers for research and development purposes. To date, many of the individuals in genetic research studies and genomic databases are of European descent, meaning that data from underrepresented populations are lacking (Landry et al., 2018). If DTC genetic testing is able to reach traditionally underserved populations, data from consumer genomics companies may provide the opportunity to diversify datasets and help researchers gain more insights into the role that genetic differences play in individuals of different ancestry.
OVERVIEW OF THE WORKSHOP
To understand the complexity of the issues presented above more fully and to explore the current landscape of consumer genomics and the implications for how genetic test information is used or may be used in research and clinical care, the Roundtable on Genomics and Precision Health of the National Academies of Sciences, Engineering, and Medicine hosted a public workshop on October 29, 2019, in Washington, DC.2 Discussions included such topics as the diversity of participant populations, the impact of consumer genomics on health literacy and engagement, knowledge gaps related to the use of consumer genomics in clinical care, and regulatory and health policy issues such as data privacy and security. A broad array of stakeholders took part in the workshop, including genomics and consumer genomics experts, epidemiologists, health disparities researchers, clinicians, users of consumer genomics research applications, representatives from patient advocacy groups, payers, bioethicists, regulators, and policy makers.
The idea for this workshop, explained Cathy Wicklund, the director of the graduate program in genetic counseling, an associate professor at the Feinberg School of Medicine’s Center for Genetic Medicine at Northwestern University, and the workshop planning committee co-chair, grew out of a 2018 workshop on disparities in access to genomic medicine which raised the issue of whether some populations were missing the potential benefits of genomic medicine (NASEM, 2018). One of the things that the roundtable had looked at during those discussions was access to genetic services including genetic testing, and DTC genetic testing was seen as an area where individuals were accessing genetic services outside of the traditional public health or health care system model. This, Wicklund said, prompted the roundtable to explore how well companies are reaching diverse or underserved populations and if the opportunity exists to work with DTC service providers to decrease inequities and disparities in genomic databases and their applicability to underserved populations. Excluding newborn screening, it is possible that more people have had some form of genomic testing outside of the traditional health care model than within health care, said Greg Feero, a professor in the Department of Community and Family Medicine at the Geisel School of Medicine, a faculty member with the Maine Dartmouth Family Medical Residency Program, an associate editor for JAMA, and the workshop planning committee co-chair. As of 2018, consumer genomics industry estimates indicated that more than 12 million individuals had submitted samples for DTC genetic testing; by early 2019,
___________________
2 The workshop agenda, speaker biographical sketches, Statement of Task, and registered attendees can be found in Appendixes A, B, C, and D, respectively.
MIT Technology Review estimated that 26 million people had contributed their DNA sequencing information to one of the large consumer genomics databases and that AncestryDNA and 23andMe were among the largest in terms of participants (Regalado, 2019).
The age of DTC genomics began 1 year before the first iPhone appeared, noted Geoffrey Ginsburg, the director of the Duke Center for Applied Genomics & Precision Medicine; a professor of medicine, pathology, and biomedical engineering at the Duke University Medical Center; and the roundtable co-chair. In the intervening 13 years, the concept of consumer genomics and DTC genomic testing has evolved considerably. While many have applauded the growing use of DTC genomics as an approach to thinking about how to engage the public in health literacy and their own health and health care, that sentiment is not universal, Ginsburg said. The clinical provider community, he continued, has worried about the day when patients start coming to their appointments with their genomic data and asking what the results mean.
In addition, there are some concerns that consumers are availing themselves of these tests without a clear picture of what information they provide and what other purposes their data may be used for, an issue that the workshop would examine in one of the panel sessions. Over the course of the workshop, Ginsburg said, “we are going to have an opportunity to look at the landscape of this continually evolving field and think about the implications for research, for clinical care, for reaching and giving access to the underserved and underrepresented communities.” In addition, the workshop focused on health-related information coming from DTC testing, rather than ancestry insights, and considered the seminal question of how to integrate data from consumer genomics tests into health care.
SETTING THE STAGE: THE EVOLUTION OF DIRECT-TO-CONSUMER GENETIC TESTING
DTC genetic testing encompasses four major areas, explained Robert Nussbaum, the chief medical officer at Invitae and the opening keynote speaker at the workshop: ancestry; personal traits; multifactorial genetic risk scores for diseases such as type 2 diabetes, rheumatoid arthritis, and Crohn’s disease; and testing for Mendelian disorders such as cardiomyopathy and hereditary breast and ovarian cancer. The use of DTC genomic tests by individuals to obtain information about their ancestry and personal traits can serve as a gateway to involving these people in research, though there is concern about the clinical utility of multifactorial genetic risk scores. For example, one study of relatives of people with Crohn’s disease found that providing them with genetic test results had no effect on their smoking behavior, even for those relatives with elevated genetic
risk scores for Crohn’s disease (Hollands et al., 2012; Whitwell et al., 2011). Another review found that communicating genetic-based risk estimates had a similar lack of effect on health behavior changes for different multifactorial conditions where lifestyle modifications could be indicated (Hollands et al., 2016).
For Mendelian disorders, the value of DTC genomic testing depends to some extent on what type of analysis has been performed on an individual’s genome. One type of analysis, for example, looks at individual genomic variants. Data from one unpublished study at Invitae that Nussbaum described showed that a 24-variant screen for familial hypercholesterolemia missed up to two-thirds of individuals whose whole-genome sequence identified a mutation in the low-density lipoprotein receptor gene involved in familial hypercholesterolemia. If someone is having DTC testing done for a different reason, such as ancestry, adding a medically relevant test in that setting can identify some healthy people who are not aware they have a potentially deleterious mutation, Nussbaum said.
In another unpublished study of more than 270,000 patients referred by health care providers for gene testing based on personal or family history of cancer, Nussbaum and his colleagues found that when they stratified the data by self-reported ethnicity, the test for one particular gene associated with a higher incidence of colorectal cancer, MUTYH, was 100 percent incomplete for Asians, which means, he said, the existing allele-specific DTC test for MUTYH mutations is not designed to test for any of the cancer-associated MUTYH variants found in Asians. Similarly, he said, the current test is 75 percent incomplete for African Americans, 46 percent incomplete for Latinos, and 33 percent incomplete for Caucasians. “Variant-specific DTC [panels], depending on what gene and what variant you’re talking about, can have very different yields, depending on the ethnic background of the people involved,” Nussbaum said.
The routes to obtaining genetic testing include the traditional health care provider-initiated service, DTC with no physician involvement, and hybrid models that are consumer-driven, but with physician involvement (Phillips et al., 2019) (see Figure 1-1). A hybrid model may be useful if there are roadblocks to accessing clinically valid genetic testing, Nussbaum said; such potential roadblocks include a scarcity of genetic counselors that leads to long wait times, the discomfort that non-genetics specialists may have in ordering a test, out-of-date testing guidelines, the cost of testing, the reluctance of insurers to pay for testing, and logistical barriers that DTC companies have been good at lowering. The hybrid model, he continued, engages with and does not ignore providers, and it limits the gatekeeping role of payers because consumers can order and pay for these tests directly. Nussbaum noted, though, that genetics specialists may still not like the hybrid model.
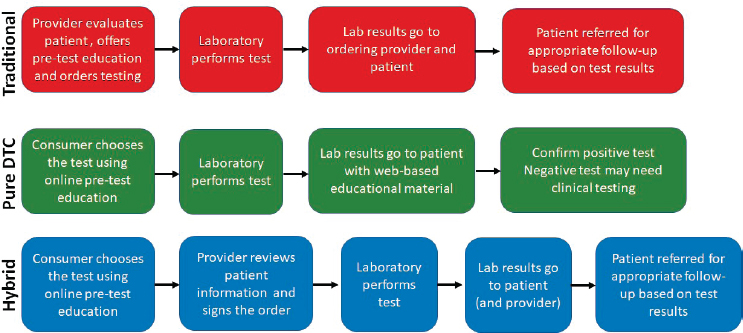
SOURCE: Robert Nussbaum workshop presentation, October 29, 2019.
A survey of consumer attitudes toward the pure DTC and hybrid models that Nussbaum and his colleagues conducted found that diagnostic testing was the number one reason for having a test; 52 percent of the respondents gave this reason, compared with 49 percent who said they had a test to obtain ancestry and heritage information. Other common responses were pre-symptomatic and predictive reasons, proactive health testing, carrier testing, and non-health-related self-exploration. Consumers do have “paradoxical concerns” regarding genomic risk screening by this hybrid model, Nussbaum said, which makes it different from the pure DTC model. For example, respondents raised privacy concerns related to physician involvement and about the resulting clinical grade assigned to the results, which for many comes with a heightened sense that getting the test is a serious action. As a result, consumers have misperceptions about actionability and reservations about taking this kind of genetic test that stem from worries about the psychological burden of obtaining distressing information, he said.
Many consumers are also skeptical about the credibility and quality of the results—something Nussbaum calls the “Theranos effect”—given that payers will not cover these tests. At the same time, some consumers view keeping their genomic information private from insurers as a potential benefit of this model compared with the traditional physician-ordered model.
Consumers also voice real fears about several recurring issues, Nussbaum said. These include
- a general fear of having their genetic information existing “out there” in the ether;
- worries about identifying preexisting conditions their insurance will not cover or that will cause their premiums to increase;
- concerns that the testing company will sell their genetic data to unauthorized third parties;
- worries about government or law enforcement agencies gaining access to their data; and
- concerns about “bad actors” using their data for nefarious purposes.
In conclusion, Nussbaum said, a number of interesting paradoxes are found at the intersection among hybrid testing, the pure DTC, and the medical care system. “There is obviously a thirst for this information, yet people are not quite sure how best to get it,” he said. “You would think they would be more reliant on their own physicians for it, and yet, I think there is some concern.” In his experience, he said, consumers have a wide range of opinions and attitudes about genomic testing, so this should be taken into account when discussing what the “consumer” wants from or thinks about DTC testing.
This page intentionally left blank.








