5
Systems Approaches to Spur Innovations in Tackling Antimicrobial Resistance
The third session of the workshop focused on antimicrobial resistance (AMR) and examined systematic approaches to stimulate innovations in AMR. Speakers and discussants explored the elements of policy, regulatory, market, and funding environments that could foster innovation in the field to address AMR; novel strategies to facilitate the mitigation of the burden of AMR; and promising avenues for coordination across systems to advance innovation in AMR. The session was moderated by Cristina Cassetti, deputy division director, Division of Microbial and Infectious Diseases, National Institute of Allergy and Infectious Diseases. Christine Kreuder Johnson, professor of epidemiology and ecosystem health, University of California, Davis, shared lessons from the One Health approach used in Kathmandu, Nepal, to enhance animal and human surveillance systems to bolster innovation in AMR.
Wes Kim, senior officer, Antibiotic Resistance Project, The Pew Charitable Trusts, discussed the development and implementation of the Shared Platform for Antibiotic Research and Knowledge (SPARK) platform, which is spurring antibiotic discovery through data sharing. Daniel Berman, lead, Longitude Prize, shared how Nesta is incentivizing novel diagnostic tests to counter antibiotic resistance with the Longitude Prize. Jyoti Joshi, head of the South Asia Center for Disease Dynamics, Economics & Policy (CDDEP), discussed approaches to strengthening health systems in order to overcome market and regulatory barriers to innovation in AMR.
APPLYING LESSONS FROM ONE HEALTH SURVEILLANCE TO TACKLE ANTIMICROBIAL RESISTANCE
Christine Kreuder Johnson explored how using a One Health approach to enhance animal and human surveillance systems can bolster innovation in AMR. She described the typical surveillance design that was used across 27 countries in the U.S. Agency for International Development’s (USAID’s) PREDICT project, which engaged with countries to bolster animal and human surveillance within communities, and she provided a case presentation of this design that was implemented in a temporary settlement in Kathmandu, Nepal.
One Health Surveillance in Kathmandu, Nepal
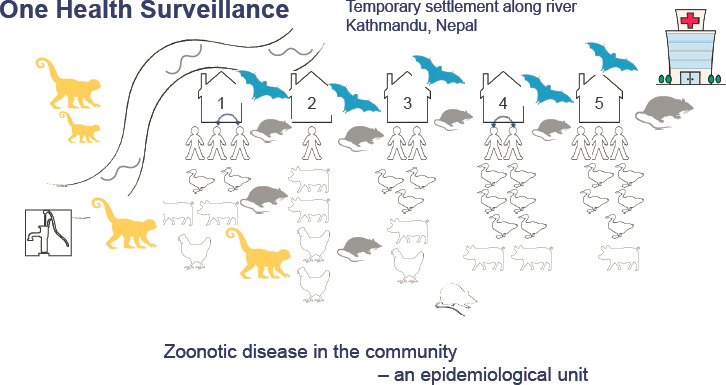
The PREDICT program developed an innovative approach informed by One Health called triangulation to conduct coordinated animal and human surveillance to identify zoonotic pathogens at high-risk animal–human interfaces, said Johnson. The approach involves (1) the concurrent sampling of people, livestock, and wildlife within their shared habitat at points of epidemiological contact; and (2) the incorporation of behavioral and social science through detailed questionnaires and surveys to investigate behavioral and social factors and inform risk mitigation and interventions. Central to the PREDICT project was engaging with communities to perform zoonotic disease surveillance within the community as a discrete epidemiological unit.
Johnson presented Figure 5-1, which depicts the temporary settlement community in Kathmandu where PREDICT’s surveillance model was used. While the PREDICT team was looking for viral threats in this community, they decided to take advantage of the opportunity to look for AMR as well. To pilot this innovation, they sampled across sectors using a One Health surveillance approach for 1 week to get a “snapshot” of AMR in the community.1 They engaged households in study enrollment and sampled several members of each household, sampled animals that were being reared, sampled wildlife active around homes or in nearby crops, and sampled the water from the nearby river. All work was conducted using appropriate biosafety practices for sampling. She added that they also looked for avian influenza in the community, because significant close contact between animals and humans in this setting presented many opportunities for sharing viral pathogens and AMR. The Center for Molecular Dynamics, a team fostering and implementing surveillance in Nepal, took on this work. This
___________________
1 Johnson noted that PREDICT also engaged with the local health care facility to conduct syndromic surveillance for viral threats, but microbial resistance was surveilled through only the community.
SOURCES: Johnson presentation, December 5, 2019; information from USAID.
team set up a laboratory in a house in this settlement and implemented the biosafety measures necessary to sample wildlife and other species.
Outcomes of One Health Surveillance
Johnson provided an overview of the outcomes of PREDICT’s One Health Surveillance in the community in Kathmandu. The team screened a subset of samples for 88 AMR genes using quantitative polymerase chain reaction, and preliminary findings revealed that 69 of 88 AMR genes were detected in the community. Among the humans sampled, some predictive factors were identified. The burden of resistance increased with age, but having a dedicated location for trash, animal waste, and human waste each decreased the risk. Keeping animals increased the risk, with the highest burden of resistance found in households that kept swine. Households with animals had an average of 11.4 resistance genes, while those without animals had an average of 5.0 resistance genes. The fecal samples from humans, ducks, and chickens revealed a tremendous amount of sharing of AMR genes, she noted, and most humans in the community had most of the circulating AMR genes. She suggested that this was likely attributable to the close contact among animals and humans in the community.
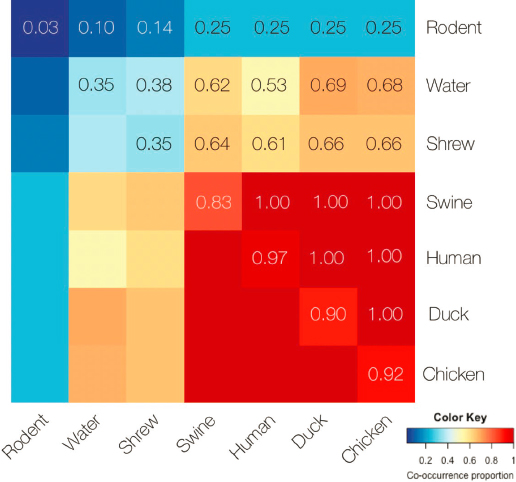
Johnson explained that they developed a novel way to visualize the community sharing of AMR genes by pairing samples from animal, human, and water sources, as shown in Figure 5-2. Wildlife had the fewest AMR genes overall, and their AMR genes had less in common with those in humans and
SOURCES: Johnson presentation, December 5, 2019; information from USAID.
livestock. Rodents had very little resistance but shrews that were trapped in people’s homes had greater levels of resistance. Swine, humans, ducks, and chickens had the highest levels of resistance overall. Among humans, ducks, and swine, every sample tested had at least one shared AMR gene. The PREDICT team also compared oral versus fecal sample types, she said. AMR genes were more similar in chicken and duck oral samples, while chicken, duck, and human fecal samples were the most similar. Although AMR was not found in the drinking water, it was found in water along the river.
Implications of AMR in the Environment
The preliminary findings from surveillance in the community in Kathmandu motivated hypotheses related to opportunities for transmission in the type of close-knit communities where there are dense livestock holdings and human communities, Johnson said. Even though the researchers did not find high prevalence in these particular samples, this setting had high potential for AMR in wildlife and possibly in other reservoirs such as waters, soil, and crops. Wildlife are underrecognized in terms of AMR, noted Johnson. Even minor amounts of AMR in wildlife can be an issue when methods for control and decreasing resistance begin to be implemented through antimicrobial
use, continued Johnson. If AMR genes are shared with wildlife through shared habitats with people, it could cause a problem for ongoing control. For instance, AMR in bats could be a potential threat to food safety, because they tend to live in dense areas and congregate in settings close to humans; their guano is also harvested and spread on crops as fertilizer. She highlighted this as an example of how resistance in wildlife—even among a species that is not typically thought of as sharing a habitat with humans—can spill back into at-risk human populations. This illustrates how much room there is for innovation in global surveillance for AMR, she said.
Challenges and Opportunities in Global Surveillance of Antimicrobial Resistance
Johnson touched on challenges and opportunities in global surveillance for AMR. Johnson said that the challenges to AMR surveillance are similar to the challenges faced for zoonotic disease surveillance in general. For instance, global disparities proliferate; low- and middle-income countries (LMICs) tend to have the greatest burdens of AMR but the most limited resources to improve surveillance. Gaps in the evidence base remain large, she added. As with zoonotic diseases, evidence is needed to fill these gaps to inform transmission-based interventions for disease control as the foundation for infectious disease control everywhere. She suggested that research could focus on prioritization of high-risk environments, movement of AMR from health care facilities to livestock holdings and the community, the importance of cross-species transmission, and directionality of transmission.
In a recent study, researchers used metagenomic analysis of untreated sewage to characterize the bacterial resistome across 60 different countries (Hendriksen et al., 2019). They found a strong correlation between abundance of AMR, sanitation, and health indices. These findings echo the findings of PREDICT in Kathmandu on a much broader scale, Johnson added.
Johnson highlighted USAID’s vision of tackling pandemic threats and training One Health workforces as a defining moment for countering microbial threats: there are opportunities for success in having human health and animal health coordinated in the field, being sampled and tested together. However, government engagement will be needed to facilitate policy change, and community engagement will be needed to facilitate behavior change. Supportive policies to tackle AMR could help to coordinate surveillance, convene data streams, and harmonize reporting frameworks to include humans, animals, and the environment. PREDICT has done this to standardize reporting across humans and animals.
Johnson noted that the World Health Organization (WHO) is also doing this for humans, but including animal and environmental data in WHO reporting going forward would be key in standardizing reporting frameworks.
USAID’s vision of engaging scientific colleagues around the world in the One Health strategy could support global AMR efforts, she suggested. The One Health workforce consists of more than 6,200 people working in more than 60 laboratories in 30 countries; they have already sampled more than 145,000 animals and humans as part of the PREDICT project, helping to minimize the spillover of zoonotic disease threats from animals into human populations (Cima, 2020). They have detected more than 1,100 unique viruses, including zoonotic diseases of public health concern, such as Bombali Ebola virus, Zaire Ebola virus, Marburg virus, and MERS- and SARS-like coronaviruses2 (Cima, 2020). She emphasized the need to have people working in the field in advance of outbreaks who have the skills for ethical sampling of both humans and animals. The PREDICT project in Kathmandu demonstrated that the PREDICT strategy could be applied to AMR and yield informative findings, she noted.
SPARKING ANTIBIOTIC DISCOVERY THROUGH DATA SHARING AND SCIENTIFIC COLLABORATION
Wes Kim presented on an innovative avenue for data sharing and scientific collaboration to spur antibiotic discovery. SPARK is an online data discovery tool being provided to the Bio-X discovery community to help catalyze the discovery and development of new antibiotics.
Pipeline Analysis of Antibiotic Candidates in Development
Pew’s recent pipeline analysis demonstrated continued insufficient candidates in development, said Kim. For the past 5 years, Pew has been tracking the global pipeline for clinical candidates, including both small molecules and “nontraditionals,” which tend to be biologics, but Kim focused on the small molecules. As of Pew’s last report, there were 37 small molecules in clinical development, 4 in new drug application review, and 1 that had received a complete response. He noted that year after year, the total number of candidates in clinical development has been steady—ranging between the upper 30s and lower 40s—but most of those candidates will never be approved, and the 42 current candidates will decline sharply as they go through clinical development. Of the 42 current candidates, 17 target the gram-negative bacteria among the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens.
The ESKAPE pathogens are considered superbugs because of their higher affinity for developing resistance, so these 17 candidates could potentially fulfill an unmet public health need (The Pew Charitable Trusts,
___________________
2 MERS is Middle East respiratory syndrome and SARS is severe acute respiratory syndrome.
2019a). Pew also tracks the novelty of drug candidates, because clinicians and infectious disease doctors need drugs that provide either novel scaffolds or novel mechanisms of action that potentially help delay the development of resistance. Pew’s evaluation found that only 1 of the 17 candidates in the clinical pipeline that target gram-negative ESKAPE pathogens represents a novel class candidate. Unfortunately, that candidate has since been discontinued because of toxicity issues. Kim said that although the three approvals that occurred in 2019 are cause for optimism, it is important to stay adamant in pushing for the development of new antibiotics.
Development of the Shared Platform for Antibiotic Research and Knowledge
Kim traced the development of Pew’s SPARK tool. Between 2014 and 2015, Pew convened leaders in the field of antibiotics discovery to catalyze the development of antibiotics by creating a Scientific Roadmap for Antibiotic Discovery (Talkington et al., 2016). They considered the scientific gaps that are contributing to the lack of novel discoveries and new antibiotics and identified three overarching priorities: guidelines for drug discovery, new starting material and chemical scaffold, and a platform for knowledge exchange. New starting material might include three to five physical and chemical properties of small molecules that allow them to penetrate and remain in the pathogens. A knowledge-sharing platform is needed to ensure that work does not just sit on the shelf.
The leaders also expressed concern about market instability for the development of new antibiotics. Together, these three priorities underscored the need for a platform for collaborating and sharing results from studies to answer gram-negative efflux and permeation questions and to collate lessons learned to minimize unnecessary redundancy in future research endeavors. Kim explained that based on those three priorities, Pew designed and launched the SPARK platform. Box 5-1 provides more details about SPARK. The SPARK database has already received contributions from industry, including Novartis and Achaogen (The Pew Charitable Trusts, 2018, 2019b,c), and they are currently in discussions with other pharmaceutical and biotechnology companies that are interested in sharing data. SPARK also collects data from academic nonprofit organizations who screen compounds for their contractor; once those data are released, they are added to SPARK. The platform was launched in 2018 and currently has more than 600 registrants.
Challenges to Implementing a Data-Sharing Platform
Kim remarked that operationalizing SPARK was challenging in several respects. He shared some of the challenges that Pew encountered and
explained how they are working to mitigate those challenges. The implementation of SPARK raised concerns about intellectual property. He noted that Pew does not expect lead assets to be shared; rather, they hope that stakeholders will consider sharing inactive programs or completed programs that are sitting on the shelf. For instance, one company that has patented compound structures but has not released the biological activity data was comfortable sharing chemical structure data. Pew is working to further mitigate challenges related to intellectual property concerns and make organizations comfortable sharing their data, he added.
The implementation of SPARK also encountered challenges because of disparate formats and sources of data, said Kim. For example, a gap in the field of antibiotics discovery is the development of an assay to measure the extent to which a compound gets into a pathogen, where it gets in, and where it accumulates. Researchers currently use a range of different methodologies for this work, which makes it difficult to compare data across laboratories. SPARK is working with their discovery experts to collate these data by
identifying certain assays that provide sufficient data and congruence across multiple laboratories. That will allow SPARK to provide collated, curated data to the community and thus enable the community to work on new ways to address antibiotic permeation and accumulation.
Finally, Kim highlighted the challenge of growing critical mass and ensuring sustained usefulness for SPARK. They are creating strategies to build awareness and maintain interest in the platform. For example, they announce data donations periodically and are developing an ambassador program of researchers who use SPARK regularly. These ambassadors are working in the field, speaking at conferences, publishing, and publicly talking about how they use SPARK in their own research. Kim said that ultimately, SPARK is not for Pew—it was designed by scientists for scientists. Their aim is to grow the database and provide data that will be used for in vitro data analysis, which will then be channeled back into SPARK to facilitate the discovery of novel antibiotics.
INCENTIVIZING NOVEL DIAGNOSTIC TESTS TO COUNTER ANTIBIOTIC RESISTANCE
Daniel Berman presented on incentivizing novel diagnostic tests to counter AMR. His organization, Nesta, is an innovation foundation with a focus on implementation rather than the act of innovating; they seek out projects that bring together technology and innovation to address social objectives. The mission of Nesta Challenges is to stimulate and speed up problem-solving activity on the most difficult challenges society faces, especially ones that are being overlooked. Nesta Challenges uses innovation competitions to excite and engage the broadest community of problem solvers and create solutions that are high quality, sustainable, and impactful. Unlike traditional grants or other methodologies, Nesta has people compete with each other against a defined objective.3
Nesta’s Longitude Prize
Berman described one of Nesta Challenges’s initiatives, the Longitude Prize. It is a £10 million prize fund with an £8 million prize payout to one winner that will reward a transformative, rapid, accurate, and affordable point-of-care diagnostic test that can significantly reduce antibiotic misuse or overuse, anywhere in the world. The first applicant to “pass the post” wins the entire prize. To register for the prize, applicants must prove their vision matches the vision of the Longitude Prize project. He explained that
___________________
3 More information about the Nesta Challenges methodology is available from https://challenges.org/impact/reports/nesta-challenges-practice-guide-2019 (accessed February 6, 2020).
the second step, registering to win, is much more difficult—only 20 applicants had registered to win the prize as of December 2019. To win the prize, competitors must meet the following eight specific criteria with a rapid, point-of-care diagnostic test that reduces inappropriate antibiotic prescribing and is accurate, affordable, and impactful globally:
- Needed: The test must improve the antibiotic treatment decisions related to this globally occurring problem.
- Accurate: The test must eliminate harmful treatment decisions and give confidence to the user.
- Affordable: The test must be affordable for purchase and use everywhere that it is needed.
- Rapid: The test must deliver a result in less than 30 minutes from sample collection.
- Easy to use: The test can be used and interpreted anywhere in the world without advanced medical resources.4
- Scalable: It is an original idea with a plan for full-scale manufacture and distribution.
- Safe: The benefits of using the test far outweigh any risks associated with it.
- Connected: The test has built-in data recording and transmission capability.
By definition, these types of prizes are designed to be difficult challenges, Berman remarked. Typically, test designers would make trade-offs among these criteria, but the Longitude Prize requires all eight criteria to be met. In addition to the eight criteria, the test should also be environmentally stable and easily carried, and it should not require a cold chain, household electricity, or a laboratory technician to deliver results. In terms of accuracy, the prize rules do not specify a necessary degree of specificity or sensitivity, because those factors are context specific, but the winning test must be accurate enough to be clinically useful.
Overview of the Ongoing Competition
Berman said that as of December 2019, 55 teams were in the competition, including start-up companies, academic groups, and midsized diagnostic companies.5 The teams come from Australia, Belgium, Canada, France, India,
___________________
4 Berman noted that it must truly be a point-of-care test; for example, the commonly used Cepheid test would not be considered a point-of-care test.
5 More information on competing teams can be found at https://longitudeprize.org/teams (accessed March 3, 2020).
Israel, Malaysia, the Netherlands, Sweden, Turkey, and the United Kingdom. Teams are working on a range of different test types, including bacterial versus viral differentiation, pathogen identification, antibiotic susceptibility testing, urinary tract infections, and tests for blood infections such as sepsis. Novel projects involving microfluidics, lateral flow, biosensor/biowire, and polymerase chain reaction are some of the technologies being deployed, he said, with many teams are utilizing multiple technologies. Some teams competing for the prize have developed projects that are quite novel, he added.
Berman said that the tests that are being developed for the Longitude Prize fall into two categories: (1) tests that are designed for the U.S. and European markets that are finding venture capital and national government support, and (2) tests that are designed for LMICs that are struggling to find financing, investment, and joint-venture-type collaborations. He pointed out that there are many tests in the latter category that would have significant impact if successful, but many of these tests are stalled in development owing to lack of investment.
Teams are required to self-fund for the most part, although they are also supported through grants, investment funding, and technical support. Nesta estimates that teams will need at least £30 million to bring a diagnostic test to market. Berman pointed out that diagnostics do not have the same quick uptake once they are brought to market, which is an additional challenge. He reported that 29 teams were awarded Discovery Awards of £25,000, 3 Longitude Prize teams were awarded boost grants of up to £100,000 by the Biotechnology Industry Research Assistance Council, and three initial investments will be made from a £3 million impact investment fund established by an anonymous donor. Some of the teams from India and the United Kingdom have been hosted by accelerators or incubators. He added that to foster collaboration and provide access to experts, Nesta Challenges convenes workshops on commercial plans, intellectual property, and regulatory filing.
Opportunities to Improve the Longitude Prize
Berman discussed some aspects of the Longitude Prize that, in hindsight, Nesta would do differently. For instance, Nesta would have collaborated with more national partners, academic medical centers, laboratories, and accelerators. In India, Nesta is working with the Department of Biotechnology, which has an ecosystem of accelerators; the Longitude Prize would have benefited from participating in more arrangements like that, he said. It would also have been helpful to increase funding support through prototype development and validation, especially considering LMIC usability objectives, he added.
Berman suggested that the Longitude Prize would have benefited from being linked to a Carb-X initiative for maximum impact. Nesta has now established links with payers, but earlier involvement of potential payers and provider institutions could have been useful in steering developers toward
priorities and deepening their understanding of clinical pathways. He added that the Longitude Prize would also have benefited from more advocacy to support increased biological testing in AMR.
Potential Antimicrobial Resistance Investment Fund
Berman concluded by challenging the current thinking about funding mechanisms. He said that the efforts to address the AMR crisis will fail unless a new or existing funding organization or mechanism can be effectively deployed. To that end, Nesta is encouraging the development of an AMR investment fund to catalyze innovation and develop a feasible market mechanism through a multistakeholder investment fund. Such a fund would call for new commitments from governments and institutions leading the AMR fight to facilitate innovation and overcome the failed market for antibiotics and diagnostics. Products could be funded based on WHO and member country priorities. Leadership by governments with support from development banks could be used to explore different mechanisms to finance the fund. Based on the antimicrobial investment fund’s initial success, scale up could be achieved through G20 or other multilateral institutions, he added.
STRENGTHENING SYSTEMS TO OVERCOME BARRIERS TO INNOVATION
Jyoti Joshi explored how AMR efforts can be integrated into existing interventions and initiatives, and she discussed strategies to strengthen health systems and overcome market and regulatory barriers to innovation on AMR. She drew on her work with CDDEP, which is a small nonprofit think tank that works to bridge the gap between academia and implementation, including efforts to help address the complex challenge of AMR.
Global and National Action Plans for Antimicrobial Resistance
Joshi described the five pillars of WHO’s Global Action Plan on Antimicrobial Resistance:
- Developing awareness and understanding through communication, education, and training;
- Building knowledge and evidence through AMR surveillance, laboratory science, and operational research;
- Infection prevention and control in health care, animal health, food, and the community;
- Optimizing use through regulation, antimicrobial stewardship, animal health, and agriculture; and
- Investment, research, and development of new medicines and innovations (WHO, 2015).
She noted that the action plan is a policy document that does not yet have an integrated implementation footprint; many of the interventions under way are isolated, particularly in LMICs. CDDEP supports countries in tackling AMR by helping them to develop national action plans for AMR (NAP-AMR).
Joshi described a NAP-AMR as a “plan of plans” that is developed in collaboration with many country-level stakeholders, including government ministries, donors, nongovernmental organizations, and civil society. The planning process also involves consumers who take antibiotics, feed them to their animals, and ultimately ingest antibiotics that end up in water and sanitation systems. NAP-AMR plans are multisectoral and relate to numerous ministries in LMICs, including animal husbandry, health, population and family welfare, finance, human resources development, agriculture and farmers’ welfare, and education and development.
Integrating Antimicrobial Resistance into Existing Interventions and Initiatives
Joshi explained that an AMR lens needs to be applied to existing interventions in order to address the AMR problem (WHO, 2018b, 2019g). AMR-specific interventions are those that specifically address the transmission of resistance through hospitals, humans, or animal–human interaction. These interventions strengthen components of health systems, agricultural systems, and environmental management of antibiotic use as well as introducing AMR and antimicrobial use surveillance and stewardship. AMR-sensitive interventions are primarily aimed at objectives other than AMR, but they indirectly help AMR containment. She noted that AMR-sensitive interventions that already exist in vertical programs can be intertwined. In this way, the AMR perspective can be used to identify and address gaps in funding for AMR-sensitive interventions. For example, AMR-sensitive interventions can increase capacity in health care, schools, households, and agriculture; they can contribute to scale up of infection prevention measures, such as improving UNICEF’s6 water, sanitation, and hygiene practices and extending vaccination of people and animals.
Joshi highlighted multiple existing entry points for AMR in existing initiatives and interventions. For instance, universal health coverage can provide a minimum services package for social insurance for all or select at-risk groups. Maternal and child health programs and the community-based
___________________
6 Officially the United Nations Children’s Fund, known as UNICEF.
Integrated Management of Childhood Illness provide opportunities to build awareness about AMR and stewardship in practice. Hospital-based quality-of-care programs and disease-specific programs (e.g., tuberculosis, malaria, HIV, and emerging zoonotic diseases) can also be used to build antibiotic stewardship and implement infection prevention and control activities to tackle AMR. Pooled procurement and generic drugs can help to ensure continuous access to quality-assured antimicrobials when needed, she added.
Barriers to Accessing Antibiotics
CDDEP published a report that identified three barriers to access to antibiotics based on scenarios in high-, middle-, and low-income settings, said Joshi (Frost et al., 2019). The first barrier is that weak drug discovery, difficulties in market entry, and poor stewardship lead to irrational selection and use of antibiotics. The second barrier is that antibiotics are not affordable for many in LMICs, and government funding for health is low. She pointed out that increasingly, AMR is a major cause of death in LMICs, yet more people in LMICs die from lack of access to antibiotics than from AMR, which creates further complications. The third barrier is that weak health systems, unreliable supply chains, and poor-quality control practices fail to deliver antibiotics to patients in need. The siloed nature of health systems and the poor management of supply chains make it difficult to ensure access to antibiotics in settings where they are needed, she added.
Joshi provided several findings from the report that exemplify these access barriers to antibiotics (Frost et al., 2019). Despite the availability of the vaccine, people die of Streptococcus-related meningitis or pneumonia around the world. The burden of gonococcal isolates with resistance to ciprofloxacin is greatest in countries that cannot afford to diagnose or treat people. Antibiotic consumption has risen in LMICs, even though the per capita consumption remains higher in the United States. For instance, the increase in per-capita antibiotic consumption in India is 300 times greater than in the United States. She noted that antibiotic prescription practices and the antibiotics prescribed vary worldwide; some countries have good-quality accreditation programs, but many LMICs do not. Additional challenges rise from the availability of antibiotics to consumers without prescription, she said. Although regulations require consumers to have prescriptions to obtain antibiotics, enforcement of these regulations has been a challenge. As a result, new chemical entities are not entering the market in places where they are most urgently needed.
Challenges in Harmonizing Antimicrobial Resistance Efforts
AMR control efforts are in different phases of evolution and management worldwide, Joshi noted. Harmonizing those efforts under the umbrella
of One Health is a valuable goal, albeit one that will be difficult to achieve. Providing additional examples from the CDDEP report (Frost et al., 2019), Joshi noted that catastrophic health spending is a major challenge. Access to health care is among the primary limitations to containing AMR, yet most people in LMICs access health care through out-of-pocket expenditure. Unless good-quality, accredited, benchmarked care is provided through universal health coverage, access to health care will remain as a limitation for AMR efforts. Cost of care is another barrier, she said. Health care costs are immense in both LMICs and high-income countries. In Germany for example, the costs of second- and third-line antibiotics are much greater than the cost of first-line antibiotics, so uncomplicated infections are easier to treat.
Addressing Regulatory and Systemic Barriers to Foster Antimicrobial Resistance Innovation
Joshi discussed some of the regulatory barriers to innovation on AMR. The siloed approach to AMR interventions in human, animal, food, feed, and environmental sectors is a barrier to innovation, she remarked. As a result, there is a dearth of data connecting AMR with other types of interventions, such as the introduction of conjugate vaccines, that may have an effect on AMR. More data are also needed on the transmission of AMR through food chains, animals, and the environment, she added. More funding is needed for One Health sectors to undertake AMR interventions. Funding for drug discovery and stewardship is siloed and typically limited to funding research. Extending funds to implementation will require the appropriate plans, governance, and systems to be in place, however.
The regulatory capacity for monitoring animal and human antibiotic use, particularly the quality of antibiotic drugs, is another barrier to fostering innovation on addressing AMR. New drugs cannot be tested without sufficient funding, human resources, and laboratory capacity. She suggested that regulation and policy innovations that provide room for experimentation are needed to overcome these regulatory barriers.
Systems will need to be improved to reduce the barriers to research and development of novel antibiotics, said Joshi. Strategies for strengthening systems include innovative funding mechanisms for novel antibiotics and support for the registration of newly discovered molecules, as needed. In developing new antibiotics, registration should be factored into the planning and cost in addition to the discovery, research, and development phase. The antibiotic registration process should be aligned across national regulatory authorities to make it faster and simpler, she suggested. Mechanisms to monitor the quality of antibiotics are also needed. However, this will require strengthening drug regulatory capacity in LMICs, given that antibiotics can
now be ordered online from countries around the world that may not have stringent regulatory and monitoring processes in place.
Health systems in LMICs will need to be strengthened to improve access and ensure appropriate use of antibiotics, said Joshi. This warrants a two-pronged approach to ensure that new molecules are accessible where they are needed and that the functions of existing molecules are retained and preserved. To achieve both of these aims, substantial investment will be needed to strengthen systems and foster collaboration across stakeholders (Gandra et al., 2017). Innovation of new molecules and preservation of current antibiotics will require collaboration across a broad range of stakeholders, including governments, national regulatory agencies, international agencies and donors, pharmaceutical industries and associations, clinical research organizations, distributors, retailers and pharmacists, logistics and supply chains, academia, and civil society.
Joshi added that funding will also be required to ensure that these systems are integrated, equitable, and sustainable in providing access to antibiotics, facilitating stewardship, and enforcing antibiotic prescription restrictions. She suggested that the AMR lens that is already being used in One Health approaches can also help to ensure that the appropriate balance is struck between access to effective treatment and protection from the overuse of antibiotics that promotes AMR.
DISCUSSION
Cristina Cassetti opened the discussion with her reflections on the panel presentations. She remarked that Johnson’s presentation highlighted the importance of conducting surveillance in communities where people are in close contact with farm animals and wildlife. She commented that Kim’s presentation revealed that there is much room for strengthening the development pipeline, and she noted Berman’s acknowledgment of the need for earlier engagement with partners, payers, and downstream funders. Joshi’s presentation identified the key barriers to innovation in AMR, highlighting the weak drug discovery pipeline, the difficulty of entering the markets, and poor stewardship of antibiotics, said Cassetti.
Peter Daszak asked Berman about the philosophy behind awarding the Longitude Prize to just a single winner and asked why the prize model is gaining traction over the more traditional grant-based method. Berman pointed out that grants tend to create an ecosystem in which it is possible to predict who will apply for them; competitions tend to bring in actors who otherwise would not have been involved. Berman suggested that this difference is a likely reason for the recent shift toward prize models. He acknowledged that the prize-based model leads to unhappy nonwinning teams; however, Nesta has distributed grants of as little as £25,000 to many
of the competing teams. He also asserted that the true value of winning is not in the prize itself. Being the winner of a prize, such as the Longitude Prize, brings prestige and marketability that is a prize in and of itself. Furthermore, the judging panel and committee for the Longitude Prize consist of key actors in the space; thus, all teams benefit from having participated in terms of marketing and networking around highly specific global health objectives.
Greg Armstrong commented that the lack of investment in infection control is an additional barrier to fostering innovations on addressing AMR. Infection control is an orphaned problem in global health, and it is difficult to engage funders or governments in infection control, according to Armstrong; yet, infection control exacerbates AMR and puts patients and health care workers at serious risk for blood-borne and other pathogens. Joshi agreed and pointed out that there are limited data on the transmission dynamics of AMR in LMICs and a lack of investment in infrastructure elements, such as water and sanitation. The value of such investments for infection control is underappreciated because of the lack of data and research, but programs and governments do not have the ability to fund research on transmission dynamics of AMR. However, she noted that research is catching up in programs that promote quality of care and are the recipients of government investment.
John Gardinier, retired, National Center for Health Statistics, remarked on the inattention to negative results across scientific research. Kim reiterated that when Pew engages with potential data contributors, they do not request data from active programs; rather, they target programs that have been discontinued for any reason. This approach was informed by Pew’s engagement in the community, which revealed that the same mistakes were being made repeatedly in antibiotics discovery research. The resources being directed into the antibiotic discovery field are diminishing, so there is a growing need to be more efficient with the approach to scientific discovery and to avoid repeating mistakes. In collecting data for SPARK from discontinued programs, Pew hopes to prevent labs from repeating mistakes that have already been made elsewhere.
Rick Bright, director, Biomedical Advanced Research and Development Authority, commented that like other major public health threats, AMR will require end-to-end innovation to work toward solutions. He maintained that there is no such thing as negative data. The innovation taking place within consortiums, incubators, accelerators, hubs, and other partnerships around the world have a responsibility to collect and share all data, because what seems like unsatisfactory endpoints for one particular goal might be the panacea for another. He noted the unique challenges associated with sharing data. Berman agreed that the issue of sharing data is of special concern in the field of antibiotic development. He mentioned the REVIVE project, developed by the Global Antibiotic Research and Development Partnership,
which is intended to revive the area of antimicrobial discovery. He suggested that governments should take a proactive approach to creating paradigms for sharing data. For example, governments could create projects to fund milestones toward antibiotic development and then manage antibiotic development projects.
Kim added that Pew has engaged in discussions with government-funded programs in which the argument has been made that data from tax-funded research should be shared publicly. He suggested that a balance must be struck between the value proposition of intellectual property as a competitive advantage and the value of sharing data.
Marcos Espinal commented that he agreed with the panelists who called for the development for new products and compounds. However, he was concerned about how to protect new compounds as they are developed. AMR is a problem that has persisted since the advent of antibiotics, he noted, but the United Nations high-level meeting raised the stakes for AMR in a way that has driven interest and action. However, until governments take the initiative, the issues of AMR will continue to go unaddressed, and new antibiotics will be lost to resistance.
Espinal highlighted progress in the tripartite agreement among WHO, the Food and Agriculture Organization of the United Nations, and the World Organisation for Animal Health, as well as global and national action plans, but he noted that the action plans must be followed through to implementation to have an impact. He noted that in Latin America, countries have rules and laws that prohibit the sale of antibiotics without prescriptions, but these rules and laws are not enforced. Given the poor enforcement of policies regarding human health, he raised the question of how these concerns are being managed in animal health. He lauded the creation of multipartner trust funds but reiterated that the greatest challenge is protecting new products from AMR.
Berman suggested that the issues at the heart of Espinal’s remarks are stewardship and demand, which are distinct from the issues and concerns around innovation. He agreed with Espinal’s sentiment that the preferred approach to managing AMR is reducing demand and promoting stewardship and the rational use of antibiotics; however, it is also necessary to put focus on innovation, especially considering the amount of time required to develop new antibiotics. He agreed that there are development banks that are helping countries create stewardship programs and properly implement One Health, but innovation requires both push and pull funding that require separate financing streams.
Joshi added that the biggest challenge to the preservation of antibiotics is awareness, which is contingent on sociocultural norms that vary widely across settings. For example, in LMICs, it is not uncommon for a person to purchase antibiotics after 2 or 3 days of unresolved sickness without ever
consulting a doctor, owing to costs of care and other cultural practices. Behavior change is required because the laws to prevent this activity are already in place. Best practices for implementing the necessary behavioral and enforcement changes have not been established, however. She suggested that those best practices need to be developed, tested, and scaled up as interventions in order to change the behaviors of consumers, practitioners, and dispensaries.
People need to understand that accessing antibiotics without a prescription is unethical and causes harm by accelerating resistance; this message needs to be appropriately packaged and delivered to the public. She also commented on the use of action plans. CDDEP worked with WHO to develop a guidance document on how to implement national plans, because many countries are not aware of how to do so. Implementing national AMR plans also helps in seeking funding, she added. For example, countries implementing vaccines need to have a plan for monitoring changes in transmission and resistance so their vaccination programs can be sustainable; these data can also be used to pitch for AMR funding.
Rafael Obregón asked whether PREDICT’s social behavior dimension goes beyond the design and innovation development process and into implementation. Johnson explained that they made great efforts to bring social science and behavioral science expertise and approaches into their surveillance activities in each county where PREDICT worked. They investigated detailed quantitative questions related to human and animal activities in each setting, but they also collected qualitative social science data about cultural issues, such as the thoughts and beliefs of the community about interactions with animals, which were used to understand the communities’ lens of zoonotic disease and inform interventions. PREDICT’s findings have now been packaged around the human activities that potentially increase microbial threats. Obregón remarked that Joshi’s recommendations for strengthening health systems did not include a recommendation regarding engaging communities and working around behavioral issues. He pointed out that the Lancet Commission Report on Quality Health Systems identified empowering consumers as a critical element, calling for an assessment of consumer levels of satisfaction and the assurance that consumers understand what quality means (Kruk et al., 2018). He noted that these issues also pertain to AMR. He asked how our understanding of how people engage with the ecosystem around antibiotics could be integrated into the overall approach to strengthening health systems in the context of AMR.
Joshi highlighted the importance of advocacy through communities, government regulations that leave room for experimentation, and policies that engage all stakeholders, including consumers, health care providers, and civil society. Health systems need to have resilience and preparedness built in, she explained. The challenges of AMR cannot be addressed through a
one-time peak in awareness, like an “antibiotic awareness week.” Instead, AMR needs to be incorporated into systematic, routine activities, such as infection prevention and control. She emphasized the importance of messaging around the consumption of antibiotics, which must be uniform across primary health care physicians and specialists. Health systems need to work toward co-creating clear, impactful messaging that is consistent and understood by children, parents, and health care providers alike. She asserted that appropriately addressing these issues is dependent on how activities are planned, funded, and implemented.




















