8
The Use of Compounded Bioidentical Hormone Therapy
In Chapter 1, the committee defined clinical utility as a multidimensional construct that not only reflects evidence about safety and effectiveness but also therapeutic need and patient preference.1 To address portions of the study’s overall charge, this chapter reviews the available evidence related to patients’ therapeutic need, preference, and overall use of compounded bioidentical hormone therapy (cBHT) preparations. To inform its work, the committee reviewed relevant data from the limited peer-reviewed literature; collected testimonies from patients, clinicians, and compounding pharmacists; and considered submitted data from key stakeholders. In its review, the committee focused on current patterns and trends of cBHT use and looked to identify factors that appear to influence patient interest. As a framework for this discussion, the chapter begins with a brief overview of the rise of cBHT and rationales for use, and it follows with a discussion on the assessed therapeutic need for cBHT, derived from evidence-based clinical guidance that inform practice.
As discussed within the chapter, cBHT medications are marketed to treat a broad spectrum of indications related to patient health and well-being. Given the limitations of the study’s scope, timeline, and resources, the chapter has a narrowed scope and predominant focus on the evidence related to the claims and the use of cBHT to treat menopausal symptoms. Limited discussions with respect to use in men or for other indications are included where relevant.
___________________
1 In the context of this report, therapeutic need relates to the treatment of menopausal and male hypogonadism symptoms.
THE WOMEN’S HEALTH INITIATIVE: IMPACT ON THE CURRENT USE OF HORMONE THERAPY
Findings from the Women’s Health Initiative
In the 1990s, the Women’s Health Initiative (WHI), a comprehensive prospective, multiethnic study, was launched to examine whether menopausal hormone therapy (MHT) might prevent the development of heart disease, as well as to assess overall health risks and benefits of select U.S. Food and Drug Administration (FDA)-approved hormone therapy (Hays et al., 2003; Stefanick et al., 2003). The MHT component of WHI was a long-term, randomized controlled trial (RCT) examining health outcomes that emerged after treating more than 27,000 healthy postmenopausal women either with placebo or the most commonly prescribed oral hormone replacement regimens at that time: Premarin (conjugated estrogens alone) or Prempro (conjugated estrogens combined with medroxyprogesterone acetate) (Rossouw et al., 2002; WHI, 2020).2,3
Since the launch of the study, research published more than 100 findings related to a broad spectrum of health risks and benefits for the use of hormone therapy in postmenopausal women (WHI, 2020). An interim analysis of the study results revealed an excess risk for coronary heart disease and stroke, breast cancer, and venous thromboembolic events for participants in the combined therapy (Prempro) arm (Rossouw et al., 2002).4 As a result, the RCT was terminated early. Although the estrogen-only treatment arm did not demonstrate an increased risk of breast cancer after 7 years, it did show a similar increase in stroke and a smaller increase in blood clots, particularly in the first 2 years of use. As a result, FDA issued requirements for all estrogen/progestin or estrogen-only hormone therapy products to contain a boxed warning on the potential serious adverse events associated with long-term administration (Stefanick, 2005). See Appendix H to review boxed warnings for bioidentical hormone therapy (BHT) products containing estrogens and testosterone.
After the termination of WHI, additional subgroup analyses were conducted showing that women in the youngest age group (50 to 59 years old) were at lowest risk for adverse outcomes. Indeed, the trial produced evidence of a potentially protective effect for those younger women
___________________
2 The WHI Hormone Therapy Trials included 27,347 women ages 50–79. The enrolled women were followed during active treatment versus placebo (5.6 years in the estrogen-plus-progestin trial, 7.2 years in the estrogen-alone trial), and for an extended period with no treatment. The total follow-up period for the study was 13 years.
3 Conjugated estrogens are a blend of estrogen sulfates purified from pregnant mares’ urine.
4 There were also treatment benefits of therapy reducing risk of fractures and colorectal cancer rates (Rossouw et al., 2002).
treated with conjugated estrogens alone, compared to those who received a placebo, for coronary heart disease, cancer, and all-cause mortalities in the 18-year cumulative follow-up analysis (Manson and Kaunitz, 2016; Manson et al., 2017). Given these modified findings demonstrated the greatest harm occurred in women over age 60 or 10 or more years beyond menopause, the leading menopause societies revised their practice guidelines.
The revised guidelines moved away from a one-size-fits-all recommendation that treatment should be for the shortest duration and at the lowest possible dose to one that allows for greater flexibility and individualized treatment (ACOG, 2014; de Villiers et al., 2016; NAMS, 2017). By 2016, the Endocrine Society’s revised statement on MHT reflected a more positive stance:
With updated data analysis suggesting the safety of MHT in younger postmenopausal women, most now agree that MHT is a highly effective and safe intervention to treat symptoms in the early menopause years. (Santoro et al., 2016)
Regarding long-term hormone therapy, the U.S. Preventive Services Task Force (USPSTF) recently reconfirmed its recommendation against using hormone therapy to prevent chronic medical conditions in postmenopausal women (USPSTF, 2019). Additionally, the online service UpToDate, a respected resource for evidence-based clinical guidance, recommends against the use of hormone therapy to treat menopause-associated osteoporosis, except in rare exceptions, or for more than 5 years in healthy women (Barbieri, 2019).
Effect of WHI on Perceptions and Treatment Options for Hormone Therapy
FDA-Approved Bioidentical Hormone Therapy
Early analyses and science communication efforts for WHI, coupled with FDA’s limited indications for use and requirements for boxed warnings of potential adverse effects, had a lasting effect on clinician- and patient-related concerns regarding the use of hormone therapy (Barlow, 2014; Thompson et al., 2017). The post-WHI era also served as the impetus for a “serious reevaluation” by pharmaceutical companies for alternative options to treat menopausal symptoms, including the use of “bioidentical”
hormones (Stefanick, 2005).5 As a result, the following years saw an expansion of FDA-approved bioidentical estrogen and progesterone products, in lower doses, and different routes of administration (FDA, 2020).
Despite the expanded selection of lower dosages and routes of administration of FDA-approved bioidentical hormone products made available following the release of the initial WHI findings, there were only modest gains in use rates for vaginal hormone preparations (Constantine et al., 2019a). In fact, there was a nationwide decline in filled prescriptions for either oral or transdermal FDA-approved bioidentical hormone products from 2002 to 2009, with an estimated 75 percent decrease for oral products and 25 percent decrease for transdermal products (Ettinger et al., 2012), as compared to the rates of use prior to the release of the WHI results. This decline in use led to suggestions that other competing factors were at play (Constantine et al., 2019b), including the parallel increase in the use of cBHT. An analysis of the Surveillance, Epidemiology, and End Results Program database from 1975 through 2014 suggested that the post-2002 rise in endometrial cancer rates in parallel with the decrease in use of approved estrogen-progestogen therapies was likely attributable to an increase in cBHT use, and to a lesser degree, the increasing prevalence of obesity and diabetes (Constantine et al., 2019b). Researchers noted that cBHT use is not systematically tracked and that the magnitude of its use was not definitively known.
cBHT
As discussed in Chapter 2, compounded medications are traditionally formulated to offer therapeutic alternatives for patients with unique medical needs that cannot be met by available FDA-approved BHT products (FDA, 2018; USP, 2015). Compounding can also fill gaps in cases of FDA-approved BHT product shortages and discontinuations (Glassgold, 2013). However, as reviewed in Chapter 5, FDA has reviewed and approved the sale of dozens of different BHT products, offering a selection of different doses and forms to address the diverse therapeutic needs of patient populations. Given the availability of FDA-approved bioidentical hormone products, the question remains, why do certain patients and providers use cBHT in lieu of available FDA-approved drug products?
___________________
5 A bioidentical hormone is a hormone that is chemically and structurally identical to one produced by the human body, with the implication that an identical structure translates to an identical physiologic response as endogenous hormones. Bioidentical hormones may be synthesized from plant or animal sources, or completely chemically synthesized, and are offered as FDA-approved drug products or as preparations that have not undergone FDA approval. See Chapter 4 for an additional discussion on this topic.
BIOIDENTICAL HORMONE THERAPY: INDICATIONS FOR USE
Informed by published guidance from the American Medical Association (2016), the committee created a figure that describes available options for “use” of FDA-approved BHT products and nonapproved cBHT preparations to treat menopausal symptoms. The committee structured its consideration of cBHT use with attention to evidence-based clinical guidance (see Figure 8-1).
Traditionally, patients see a physician prescriber (or nonphysician prescriber in certain situations) who evaluates their symptoms and medical history. Prescribers can then discuss treatment options from the perspectives of safety, efficacy, and medical need specific to the patient at hand. As illustrated in Box 1 of the top pathway in Figure 8-1, physicians can prescribe an FDA-approved drug product, with the prescriber informed by evidence-based labeled indications, safety and efficacy, and clinical practice guidance. The FDA-approved drug product label gives the physician prescriber and the patient information about indications, contraindications, warnings, side effects, dosing, and monitoring. In addition, boxed warnings are included for all FDA-approved drug products that include estrogen or testosterone (FDA, 2015; Stefanick, 2005).
As illustrated in Box 2 in Figure 8-1, clinicians can prescribe an FDA-approved BHT product for indications that are “off-label.”6 In this situation, the prescriber can be informed by clinical guidance and data on the safety and efficacy for the FDA-approved labeled indications. For the purpose of this report, off-label prescribing is either (1) for an indication that is not included in the product labeling, (2) at a dosage outside the recommended range, (3) uses a different route of administration, or (4) for a patient from a population not listed on the labeled recommendation (e.g., pediatric) (AMA, 2016). Approximately 10 to 20 percent of all prescriptions are for off-label use, with a much higher rate in some medical specialties (e.g., oncology, pediatrics, and rare diseases). Similar to medical guidance for cBHT prescribing, off-label prescribing should be limited to use that is supported by scientific evidence (AMA, 2016). As discussed in Chapter 7, the use of testosterone to treat female sexual dysfunction is a relevant example of off-label prescribing of an FDA-approved BHT product (AMA, 2016; Davis et al., 2019).
In other circumstances, many of which are not tracked, clinicians can prescribe non-FDA-approved cBHT for their patients. As noted in Box 3 of Figure 8-1, there are typically two reasons to prescribe cBHT: (1) to provide a medication in an alternate dose or form, or (2) to omit components of an
___________________
6 FDA does not regulate the practice of medicine and therefore does not regulate the use of off-label prescribing (21 U.S.C. § 396).
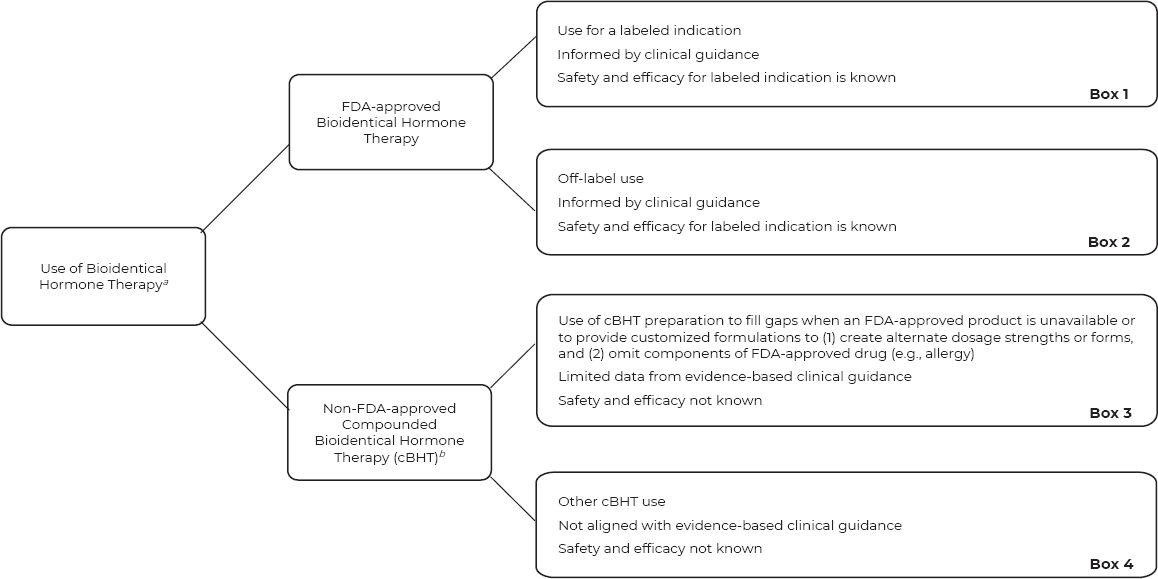
a This figure does not represent all possible uses of FDA-approved drug products or cBHT preparations.
b The use of non-FDA-approved cBHT is the prioritized focus of the current chapter.
SOURCE: Concept from AMA, 2016.
FDA-approved drug product (e.g., allergy). As noted in Chapter 7, there is insufficient evidence to establish whether cBHT preparations are safe or efficacious for their prescribed uses.
Box 4 of Figure 8-1 relates to “other use of cBHT” that goes beyond the historical rationale for compounded drug use. Similar to the situation in Box 3, there is insufficient evidence to establish whether cBHT preparations are safe or efficacious for their prescribed uses, but in addition, “other use of cBHT” does not align with evidence-based clinical guidance. (See the section “Professional Guidance and Clinical Practice Guidelines for the Use of cBHT” for an additional discussion.)
Purported Indications for the Use of cBHT
The vast majority of FDA-approved BHT products have labeled indications related to treating moderate to severe vasomotor symptoms and vulvovaginal atrophy of menopause in women and testosterone deficiency or hypogonadism in men (Crandall, 2019; NLM, 2020; Shifren et al., 2019; see Table 8-1 for a list of labeled indications).7 In direct contrast to the short list of indications for FDA-approved BHT products, there are a substantial number of purported indications for the use of cBHTs. Marketed claims for cBHT include, but are not limited to, the treatment of conditions related to antiaging (e.g., longer, fuller hair and smoother skin), sexual health, joint pain, general chronic pain, insomnia, cardiovascular diseases, and various mental health disorders.8 In addition, researchers have identified certain cBHT preparations marketed for long-term use that is not supported by the available safety evidence (Conaway, 2011). As noted in Chapters 3 and 7, cBHT preparations do not go through the FDA approval process, and they lack robust empirical evidence on their safety and efficacy to treated marketed indications.9 In general, the contraindications for cBHT might, at a minimum, be expected to be similar to those of similar FDA-approved BHT products. (See Table 8-1 for a list of labeled contraindications.) However, it is unknown whether, and if so how, these and other concerns are communicated to patients.
To better understand which special populations of patients use cBHT, in addition to or in lieu of FDA-approved drug products, the committee
___________________
7 Access to FDA drug labels is available on the National Library of Medicine’s (NLM’s) DailyMed online database at https://dailymed.nlm.nih.gov/dailymed/spl-resources-all-druglabels.cfm (accessed May 7, 2020).
8 Example cBHT indications for use were collected from submitted resources from stakeholders (see Appendix A); public statements from consumers, clinicians, and pharmacists (see Appendix A); and peer-reviewed literature (e.g., McPherson et al., 2019; Yuksel et al., 2017).
9 See Chapter 7 for a review of the safety and effectiveness of commonly formulated cBHT preparations.
| Bioidentical Hormone | Brand Name | Preparation | Label Indications |
|---|---|---|---|
| 17β-estradiol | Estrace | Pill | Moderate to severe vasomotor symptoms; moderate to severe symptoms of vulvar and vaginal atrophy; hypoestrogenism due to hypogonadism, castration, or primary ovarian failure; prevention of osteoporosis |
| 17β-estradiol | Alora | Patch | Moderate to severe vasomotor symptoms; moderate to severe symptoms of vulvar and vaginal atrophy; hypoestrogenism due to hypogonadism, castration, or primary ovarian failure; prevention of osteoporosis |
| 17β-estradiol | Climara | Patch | Moderate to severe vasomotor symptoms; moderate to severe symptoms of vulvar and vaginal atrophy; hypoestrogenism due to hypogonadism, castration, or primary ovarian failure; prevention of osteoporosis |
| 17β-estradiol | Vivelle-Dot | Patch | Moderate to severe vasomotor symptoms; moderate to severe symptoms of vulvar and vaginal atrophy; hypoestrogenism due to hypogonadism, castration, or primary ovarian failure; prevention of osteoporosis |
| 17β-estradiol | Minivelle | Patch | Moderate to severe vasomotor symptoms; prevention of osteoporosis |
| 17β-estradiol | Menostar | Patch | Prevention of osteoporosis |
| 17β-estradiol | Estrogel | Gel | Moderate to severe vasomotor symptoms; moderate to severe symptoms of vulvar and vaginal atrophy |
| Contraindications |
|---|
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; Active or recent (e.g., within the past year) arterial thromboembolic disease; Known sensitivity to FD&C Yellow No. 5 (tartrazine) |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; Active or recent (e.g., within the past year) arterial thromboembolic disease; Known hypersensitivity to product or its ingredients |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; Active or history of arterial thromboembolic disease; High risk of venous thrombosis or arterial thrombosis; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Known hypersensitivity to product or its ingredients |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Known hypersensitivity to product or its ingredients |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Anaphylactic reaction or angioedema with product |
| Bioidentical Hormone | Brand Name | Preparation | Label Indications |
|---|---|---|---|
| 17β-estradiol | Elestrin | Gel | Moderate to severe vasomotor symptoms |
| 17β-estradiol | Divigel | Gel | Moderate to severe vasomotor symptoms |
| 17β-estradiol | Estrace | Vaginal cream | Moderate to severe vulvar and vaginal atrophy due to menopause |
| 17β-estradiol | Estring | Vaginal ring | Moderate to severe vulvar and vaginal atrophy due to menopause |
| 17β-estradiol | Evamist | Spray | Moderate to severe vasomotor symptoms |
| 17β-estradiol | Imvexxy | Vaginal tablet | Moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause |
| Estradiol cypionate | Depo-estradiol | Injection | Moderate to severe vasomotor symptoms; hypoestrogenism due to hypogonadism |
| Contraindications |
|---|
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Anaphylactic reaction or angioedema with product |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Known hypersensitivity to product or its ingredients; Anaphylactic reaction or angioedema with product |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Anaphylactic reaction or angioedema with product |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Known hypersensitivity to product or its ingredients; Anaphylactic reaction or angioedema with product |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Anaphylactic reaction or angioedema with product |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Anaphylactic reaction or angioedema with product |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; Recent (e.g., within the past year) arterial thromboembolic disease; Known hypersensitivity to product or its ingredients |
| Bioidentical Hormone | Brand Name | Preparation | Label Indications |
|---|---|---|---|
| Micronized progesterone | Prometrium | Pill | Prevention of endometrial hyperplasia in nonhysterectomized postmenopausal women who are receiving conjugated estrogens tablets; also indicated for use in secondary amenorrhea |
| Micronized progesterone | Crinone | Vaginal gel | Supplementation or replacement as part of an assisted reproductive technology (ART) treatment for infertile women with progesterone deficiency (8%); secondary amenorrhea (4% and 8%) |
| Micronized progesterone | Prochieve | Vaginal gel | Supplementation or replacement as part of an assisted reproductive technology (ART) treatment for infertile women with progesterone deficiency (8%); secondary amenorrhea (4% and 8%) |
| Micronized progesterone | Endometrin | Ovules | To support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an assisted reproductive technology (ART) treatment program for infertile women |
| 17β-estradiol and micronized progesterone | Bijuva | Pill | Moderate to severe vasomotor symptoms due to menopause |
| Testosterone | Testim | Gel | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Testosterone | Vogelxo | Gel | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Testosterone | Androgel | Gel | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Contraindications |
|---|
| History of arterial thromboembolic disease; Undiagnosed abnormal genital bleeding; Known, suspected, or history of cancer of the breast; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease |
| Undiagnosed vaginal bleeding; Liver dysfunction or disease; Known or suspected malignancy of the breast or genital organs; Missed abortion; Known sensitivity or hypersensitivity to product or its ingredients |
| Undiagnosed vaginal bleeding; Liver dysfunction or disease; Known or suspected malignancy of the breast or genital organs; Missed abortion; Known sensitivity or hypersensitivity to product or its ingredients |
| History of arterial thromboembolic disease; Known allergic reaction; Undiagnosed vaginal bleeding; Liver dysfunction or disease; History of arterial thromboembolic disease; Known or suspected malignancy of the breast or genital organs; Ectopic pregnancy or missed abortion; Known allergic reactions |
| Undiagnosed vaginal bleeding; Known, suspected, or history of breast cancer; Known or suspected estrogen-dependent neoplasia; Active deep vein thrombosis, pulmonary embolism, or history of these conditions; Liver dysfunction or disease; Known or suspected pregnancy; History of arterial thromboembolic disease; Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders; Known hypersensitivity to product or its ingredients; Anaphylactic reaction or angioedema with product |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding; Pregnant women need to be aware of the potential for transfer of testosterone from men |
| Bioidentical Hormone | Brand Name | Preparation | Label Indications |
|---|---|---|---|
| Testosterone | Fortesta | Gel | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Testosterone | Natesto | Nasal gel | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Testosterone | Androderm | Patch | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Testosterone | Striant | Tablet (buccal system) | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Testosterone | Testopel | Pellet | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Testosterone cypionate | DepoTestosterone | Injection | Indicated for replacement therapy in males for conditions associated with symptoms of deficiency or absence of endogenous testosterone |
| Dehydroepiandrosterone (DHEA; also known as Prasterone) | Intrarosa | Vaginal insert | Indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause |
NOTE: This table is limited to products that include only those active ingredients identified in the committee’s Statement of Task at the time of publication.
SOURCE: NLM, 2020.
submitted a data request to the editor-in-chief of the International Journal of Pharmaceutical Compounding (IJPC), Loyd Allen, for a review of all cBHT-related articles published in the IJPC from 1997 to 2018. As summarized in a presentation to this committee on June 27, 2019, Allen noted that special populations using cBHT include patients who cannot tolerate certain components of FDA-approved drug products (e.g., lactose), need
| Contraindications |
|---|
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding |
| Carcinoma of the breast or known or suspected carcinoma of the prostate; Pregnancy or breastfeeding; Known hypersensitivity to the drug; serious cardiac, hepatic, or renal disease |
| Undiagnosed abnormal genital bleeding: Any postmenopausal woman with undiagnosed, persistent, or recurring genital bleeding should be evaluated to determine the cause of the bleeding before consideration of treatment |
alternate dosage strengths or forms (e.g., for those who have difficulty swallowing solids), or need preparations to support treatment compliance (e.g., in cases where convenience or personal preference is important). In the latter case, Allen cited the following reasons: compounds providing individualized flavors, ease of transporting and administration, combinations of drugs not otherwise obtainable, and those that are easily modifiable for
individual patients. Unfortunately, no specific examples were provided to better understand the applicability directly to cases regarding the use of cBHT to support a medical need. Allen did, however, note that children and the elderly would especially benefit from some of these important capabilities, though these are subpopulations not likely to be relevant for hormone therapy to treat menopausal symptoms. After initial research efforts, the committee identified a need to obtain additional clarity on evidence-based support for the use of cBHT to treat menopausal symptoms. To address this need, the committee reviewed available evidence-based clinical guidance issued by professional medical organizations.
PROFESSIONAL GUIDANCE AND CLINICAL PRACTICE GUIDELINES FOR THE USE OF CBHT
Overall Perspectives
As part of evidence-based medical practice, clinical guidance has a growing role for improving clinical decision making and potentially patient outcomes (IOM, 2011). In collaboration with the National Academies’ senior librarians, the committee conducted a search of large, global research databases and other online sources to identify published statements and professional guidance relevant to the committee’s charge. Databases searched included Embase, Medline, PubMed, Scopus, and Google. In this effort, the committee prioritized the findings of professional guidance issued by nonprofit medical societies, professional associations, or other evidence-based clinical resources.
In general, the collected statements expressed concerns regarding the quality, safety, and effectiveness of compounded preparations and cautioned against their use if FDA-approved BHT product options were available. In fact, in each of the professional guidance statements reviewed by the committee, FDA-approved BHT products were recommended as the first-line hormone therapy treatments (see Table 8-2 for relevant excerpts from clinical guidance).10
At the same time, many of the clinical guidance statements, such as the 2017 position statement on hormone therapy by the North American
___________________
10 Additional public statements issued by medical societies, professional associations, and women’s health advocacy organizations were reviewed but are not outlined in the chapter. Table 8-2 provides a non-comprehensive list of clinical guidance that represents the overall findings of the committee. For example, other statements, such as those issued by the American Association of Clinical Endocrinologists (aace.com), the National Women’s Health Network (nwhn.org), and Our Bodies Ourselves (ourbodiesourselves.org) also express concerns related to the safety and effectiveness of cBHT preparations (Cobin and Goodman, 2017; NWHN, 2020; Pearson, 2019).
TABLE 8-2 Professional Medical Guidance on the Use of cBHT
| Organization Name | Year | Concerns and Recommendations |
|---|---|---|
| American College of Obstetricians and Gynecologists (ACOG) | 2018 | “Patients should be counseled that menopausal hormonal therapies that are proved to be safe and effective by the FDA are more appropriate for their use than individual pharmacy-compounded preparations.” “Patients should be educated on the FDA approval status of compounded preparations and their risks and benefits, including the risks specific to compounding.” “Physicians should exercise caution in prescribing compounded hormones when FDA-approved alternatives exist.” (ACOG, 2018, p. 4) |
| American Medical Association (AMA) | 2016 |
“Some clinics that provide services for transgender individuals recommend compounded hormone therapy preparations made by compounding pharmacies such as topical testosterone and estradiol creams for cost-saving purposes, since many of the necessary drug therapies are not covered by insurance. There is no evidence that custom compounded hormone therapy products are safer or more effective than FDA-approved therapies.” (AMA, 2016, p. 9, lines 48–50, and p. 10, lines 1–2)
(CSAPH Report 4-I-16, Recommendation to amend Policy D-120.969) (AMA, 2016, p. 13) |
| Organization Name | Year | Concerns and Recommendations |
|---|---|---|
| Endocrine Society | 2019 | “Since the final hormone formulations of most compounding pharmacies are not subject to FDA monitoring for dose, purity, safety, or efficacy, there may be additional and at this point unknown risks associated with them.” “Nonetheless, compounded hormones are sometimes offered at a lower cost than FDA-approved preparations, and this can motivate patients to request them.” “The Society supports FDA regulation and oversight of all hormones regardless of chemical structure or method of manufacture. This should include, but not be limited to, the following: Surveys for purity and dosage accuracy; Mandatory reporting by drug manufacturers of adverse events; A registry of adverse events related to the use of hormone preparations; Inclusion of uniform information for patients, such as warnings and precautions, in packaging of hormone products.” (Endocrine Society, 2019, p. 2) |
| Global Consensus Position Statement on the Use of Testosterone Therapy for Women | 2019 | “Compounded ‘bioidentical’ testosterone therapy cannot be recommended for the treatment of hypoactive sexual drive disorder, due to the lack of evidence for efficacy and safety, unless an authorized equivalent preparation is not available (Expert opinion). In the absence of an available approved product, if a compounded product is needed, the compounding pharmacy should be compliant with purity of Active Pharmaceutical Ingredients (API) and Good Manufacturing Practice (GMP) to meet industry standards for quality and safety. Dosing should be limited to achieving testosterone concentrations in the physiologic premenopausal range.” (Davis et al., 2019, p. 4, Recommendation 12(d)) |
| International Menopause Society | 2016 | “Women requesting compounded BHT should be encouraged to consider regulated products containing hormones which are structurally identical to those produced in the body. These are available in a wide range of doses and delivery methods.” “Prescribing of compounded BHT is not recommended due to the lack of quality control and regulatory oversight associated with these products, together with lack of evidence of safety and efficacy.” (Baber et al., 2016, p. 134) |
| Organization Name | Year | Concerns and Recommendations |
|---|---|---|
| North American Menopause Society (NAMS) | 2017 | “Compounded bioidentical HT should be avoided, given concerns about safety, including the possibility of overdosing or underdosing, lack of efficacy and safety studies, and lack of a label providing risks.” “If compounded bioidentical HT is prescribed, concerns about safety should be discussed, and the indication for prescribing compounded rather than government-approved bioidentical HT should be documented (allergy, medical need for lower-than-available dose, different preparation).” (NAMS, 2017, p. 744, Recommendation III(a)) |
| UpToDate | 2019 | “Many women have turned to compounded ‘bioidentical’ hormone therapy as an alternative to conventional hormones for treating symptoms of menopause. ‘Bioidentical’ means that the hormones used for therapy are identical in molecular structure to the hormones produced by the ovaries. ‘Compounded’ means the preparation is mixed in a special compounding pharmacy in order to create a customized dose of hormones in the form of pills, creams, or vaginal suppositories.” “The quality of these custom compounded products is not regulated by the U.S. Food and Drug Administration (FDA), and the dose of hormones can vary from batch to batch. For these reasons, expert groups caution against using them.” (Barbieri, 2019) |
SOURCES: ACOG, 2018; AMA, 2016; Baber et al., 2016; Barbieri, 2019; Endocrine Society, 2019; Davis et al., 2019; NAMS, 2017.
Menopause Society, acknowledged that some patients with special medical needs, (e.g., allergies) may be unable to use certain FDA-approved BHT products, although specific examples of such clinical situations were not provided (NAMS, 2017). Throughout the clinical guidance statements, details regarding the avoidance of select components in FDA-approved BHT products (e.g., due to allergies) were minimal, and the committee could not identify any clinical guidance that outline a therapeutic need for a specific patient population to receive a specific cBHT formulations in lieu of FDA-approved BHT products. Furthermore, based on its review of peer-reviewed literature and clinical guidance statements, the committee was
unable to identify any specific life-threatening medical conditions requiring the patient’s use of cBHT preparations.11
The Use of cBHT: Specific Medical Needs
As discussed earlier in this report, compounding provides the option to omit components of FDA-approved drug products for patients with specific therapeutic needs that cannot be met by available FDA-approved drug products. Avoiding allergies is one of the most commonly cited historic rationales for compounding (Kelso, 2014; Swerlick and Campbell, 2013). Although there is evidence of allergies to active ingredients in FDA-approved drug products (Roby et al., 2006), the majority of the complaints are related to the inactive ingredients in these drug products (Abrantes et al., 2016).
Recent work characterized the role of inactive ingredients in FDA-approved oral drug products and their potential to trigger allergies in certain subpopulations of patients (Reker et al., 2019). For example, the drug label for Estrace (an FDA-approved estradiol tablet) includes the following warning:
ESTRACE should not be used in patients with known hypersensitivity to its ingredients. ESTRACE tablets 2 mg, contain FD&C Yellow No. 5 which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity. (NLM, 2018)
Such labels and warnings noting the potential for inducing allergy are not required for compounded preparations, including cBHT. See Chapter 5 for an additional discussion on the inadequate label requirements for cBHT preparations.
Allergies to inactive components in FDA-approved BHT products are commonly expressed rationales for the use of compounded preparations,
___________________
11 It should be noted that medical associations, societies, and other relevant health organizations that issue clinical guidance are often supported, in part, by the pharmaceutical industry. Furthermore, many of the co-authors of the issued guidance conduct medical research that may be funded, in part, by the pharmaceutical industry. All aspects of the pharmaceutical ecosystem (including FDA-approved and compounded drugs) should be extremely mindful of the great responsibility entrusted to them by the public to disclose all conflicts of interest (real and perceived) and to uphold esteemed medical and scientific ethics, values, and standards. After considering the disclosed conflicts of interest for the authors of the clinical guidance reviewed in this report, the study committee had sufficient confidence to allow the guidance to serve as an important piece of evidence used to inform its report conclusions.
in general (NAMS, 2017; Pinkerton, 2020).12 This stands in direct contrast to the very few published examples of allergic reactions to FDA-approved BHT products and the lack of clinical guidance on this issue. To collect additional insight on this topic, the committee turned to the Endocrine Society for nominations of practicing clinicians who would be able to speak to the medical conditions in which a cBHT preparation would be needed in lieu of FDA-approved treatments. The nominated clinicians, Drs. Cynthia Stuenkel and Nanette Santoro, stated that based on their experience, a small fraction of their patients may show an allergy to a component of FDA-approved BHT products (e.g., peanut oil). The clinicians noted, however, that in most cases there were alternative FDA-approved BHT products that would avoid those conditions (e.g., Bijuva), and given the safety concerns outlined by clinical guidance, only on rare occasions would they prescribe a compounded preparation as an alternative treatment (NASEM, 2019a).
Use of cBHT: Special Patient Populations
In addition to allergies, the committee tried to identify special conditions for which no FDA-approved BHT product exists and special populations requiring specific doses and forms of BHT that could not be treated with FDA-approved medications. Patients with female sexual dysfunction (FSD) and gender dysphoria are two indications for which there are no FDA-approved BHT medications.13,14 Both are treated off-label with FDA-approved BHT medications (AMA, 2016; Davis et al., 2019).
Female Sexual Dysfunction
Currently, there are no available FDA-approved BHT products to treat the diagnostic classifications of FSD.15 In addition, after a review of the evidence, the committee could not identify any professional medical organizations with evidence-based clinical guidance that recommend use of cBHT for treating FSD.
___________________
12 See also Senate Hearing. 110-129—Bioidentical Hormones: Sound Science or Bad Medicine, Hearing for the U.S. Senate Special Committee on Aging (https://www.govinfo.gov/app/details/CHRG-110shrg37150/CHRG-110shrg37150/summary [accessed April 13, 2020]).
13Female sexual dysfunction (FSD) is a complex condition associated with diagnostic classifications including hypoactive sexual desire, sexual arousal disorder, orgasmic disorder, or sexual pain disorder (Lightner, 2002).
14 A Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) recognized diagnosis that is “a noticeable incongruence between the gender the patient believes they are, and what society perceives them to be” (APA, 2013).
15 One FDA-approved drug product, Flibanserin, is approved to treat female sexual arousal disorder (FSAD) in premenopausal women, but is a nonhormonal product that includes mixed function serotonin agonist/antagonist (NLM, 2019).
Commonly used treatment options include nonpharmacologic approaches such as education, counseling, or psychotherapy (Clayton et al., 2018; FDA, 2015). For patients seeking hormone therapy options, particularly postmenopausal patients, clinicians often use off-label testosterone treatment regimens—at a dose that is approximately one-tenth of that given to a male—to address symptoms (Clayton et al., 2018).16 cBHT preparations containing testosterone appear to be an increasingly popular option as a result of the limited number, dosage forms, and dosing options of FDA-approved drug products containing testosterone currently on the market (AMA, 2016; Clayton et al., 2018).
A recent position statement endorsed by several medical societies concluded that the only evidence-based indication for testosterone therapy for women is for the treatment of hypoactive sexual desire disorder (HSDD),17,18 and that there is insufficient data to support the use of this treatment for any other symptom, clinical condition, or disease prevention (Davis et al., 2019). Regarding use of compounded testosterone therapy, the authors of this position statement concluded that cBHT is not recommended to treat symptoms of HSDD given the lack of evidence for efficacy and safety. The authors noted that an exception can be made if an “authorized equivalent preparation” is not available. In this case, the task force recommends that “the compounding pharmacy should be compliant with purity of active pharmaceutical ingredients and good manufacturing to meet industry standards for quality and safety,” and “dosing should be limited to achieving testosterone concentrations in the physiologic premenopausal range” (Davis et al., 2019, p. 4,664).
___________________
16 In addition to testosterone products to treat FSAD, evidence suggests the limited potential for a select few other off-label hormone therapies including, estrogens, ospemifene—a selective estrogen receptor modulator, and dehydroepiandrosterone (DHEA) (AMA, 2016).
17 This position statement has been endorsed by the International Menopause Society, the Endocrine Society, the European Menopause and Andropause Society, the International Society for Sexual Medicine, the International Society for the Study of Women’s Sexual Health, the North American Menopause Society, el Federación Latinoamericana de Sociedades de Climaterio y Menopausia, the Royal College of Obstetricians and Gynaecologists, the International Society of Endocrinology, the Endocrine Society of Australia, and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists.
18 Prior to the release of the DSM-5, the term hypoactive sexual desire disorder (HSDD) was used to describe all disorders pertaining to FSD. After the DSM-5, medical guidance merged the use of HSDD into the singular term female sexual interest/arousal disorder (FSIAD or FSAD). However, because HSDD and FSAD are widely considered to be two distinct FSD conditions, the sexual health community has not endorsed or adopted the merging of these terms. Thus, for the purposes of this report, the committee will use the term female sexual dysfunction, except when it is necessary to mirror terminology used in the referenced material (Clayton et al., 2018; Davis et al., 2019).
Gender Dysphoria
In recent years, medical researchers and professionals have found hormone therapy to be important and necessary for transgender or transitioning male and female adults and adolescents. Professional medical organizations including the Endocrine Society, the World Professional Association for Transgender Health, and other leaders in transgender health have developed clinical guidance describing off-label use of FDA-approved BHT for this patient population (AMA, 2016; Coleman et al., 2012; Fenway Health, 2015; UCSF Transgender Care, 2016).
Despite the availability of diverse FDA-approved BHT treatment options (e.g., various doses and forms), it is not uncommon for patients with gender dysphoria to be denied coverage for hormone therapy and other treatments under current health care policies (AMA, 2016). Consequently, certain health specialists prescribe cBHT preparations to this patient population (AMA, 2016; Coleman et al., 2012; Fenway Health, 2015; UCSF Transgender Care, 2016). However, the committee could not identify any professional medical organizations with evidence-based clinical guidance that recommend use of cBHT for gender dysphoria, in lieu of off-label use of FDA-approved BHT.
Considerations for Prescribers
There are important professional obligations for licensed physicians who prescribe hormone therapy. Based on the precautionary principle, physicians prescribing FDA-approved BHT and cBHT have a responsibility, where possible, to engage in practice informed by evidence-based clinical guidance. Similar to the guidance outlined by the Federation of
State Medical Boards for prescribing complementary and alternative medicines, providers need to respect patient autonomy—the right of patients to choose—while at the same time educating their patients to ensure that their decision making is based on evidence-based health information and is supported by techniques of shared decision making (Barry and Edgman-Levitan, 2012; Couët et al., 2015; FSMB, 2002; IOM, 2001).
Certain areas of potential liability exist for prescribers of cBHT, including the invalidation of malpractice insurance, personal liability, or possible criminal charges (Sellers and Utian, 2012). For example, at an open session meeting in May 2019, the committee heard testimony from the Tennessee Attorney General’s office that described instances in which physicians have lost their medical license and clinics have been closed owing to inappropriate claims regarding the benefits of cBHT without adequate support.19
THE USE OF CBHT: PATTERNS AND TRENDS
To identify patterns and trends in the use of cBHT, the committee reviewed available data from published national surveys; peer-reviewed literature; collected testimonies from patients, clinicians,20 and compounding pharmacists; and data submitted by FDA, the National Association of Boards of Pharmacy (NABP), and the Professional Compounding Centers of America. Insights from this review are presented below.
Insights from Surveys
There are limited peer-reviewed data on historical practices or current trends in the prescription and use of cBHT, and much of what is known comes from small surveys of patients, clinicians, and pharmacists (Constantine et al., 2016; Gass et al., 2015; Pinkerton and Constantine, 2016; Pinkerton and Santoro, 2015; Stuenkel and Manson, 2017). These surveys suggest that between 1.0 and 2.5 million U.S. women age 40 years or older use cBHT, accounting for some 26 million to 33 million prescriptions costing between $1 billion and $2 billion annually, which suggests the use of multiple cBHT prescriptions per individual (Constantine et al., 2016; Gass et al., 2015; Pinkerton and Constantine, 2016; Pinkerton and Santoro, 2015; Stuenkel and Manson, 2017). In a small study of 184
___________________
19 Office of Tennessee Attorney General. 2020. Email from B. Harrell to National Academies staff regarding State of Tennessee v. HRC Medical Centers, Inc. legal decision. April 29. Available through the National Academies Public Access File.
20 Testimony was collected from clinicians who self-identified as having a wide range of professional credentials (e.g., M.D., D.O., registered nurse, nurse practitioner, physician assistant) and specialties (e.g., internal medicine, cardiology, wellness, antiaging medicine, obstetrics and gynecology, family practice, urology, palliative care, pediatrician, emergency medicine).
patients, 77 percent of cBHT users believed it was safer than conventional hormone therapy (Iftikhar et al., 2011). A 2015 NAMS-sponsored online survey of 3,725 women revealed that of the 9 percent currently taking hormone therapy to treat menopausal symptoms, approximately one-third were using cBHT (Gass et al., 2015; see Figure 8-2).
A 2019 survey of compounding pharmacies, which then distributed questionnaires to their patients, produced 494 usable responses (putative response rate of 17.9 percent). From these responses, 50.1 percent of patients indicated their compounded prescriptions were for preparations designed as hormone therapies (McPherson et al., 2019). A survey conducted in Australia between October 2013 and March 2014 distributed questionnaires to 5,850 women identified through the Australian electoral roll. Analyzed results showed that of the 1,491 perimenopausal and postmenopausal women who responded, 1.1 percent used compounded estrogen and/or compounded progesterone and 0.9 percent used dehydroepiandrosterone (DHEA) and/or testosterone (Worsley et al., 2016).
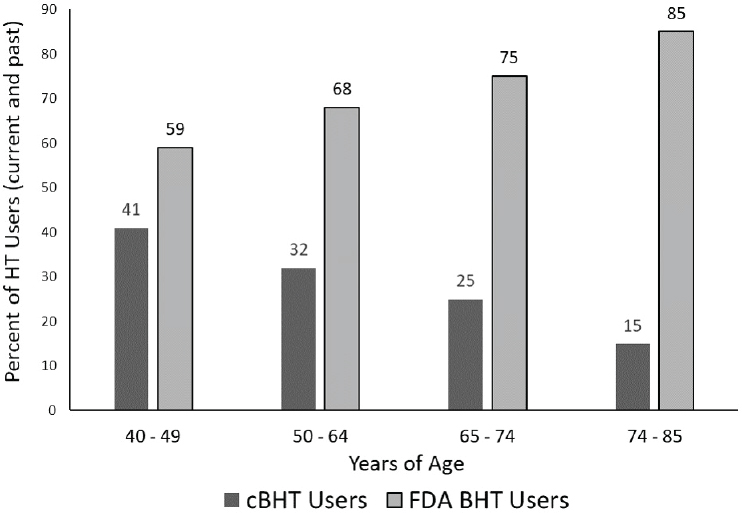
NOTE: BHT = bioidentical hormone therapy; cBHT = compounded bioidentical hormone therapy; FDA = U.S. Food and Drug Administration; HT = hormone therapy.
SOURCE: Gass et al., 2015
It is important to remember there are limited regulatory and statutory requirements for cBHT labeling and package insert information for patients. Consequently, a portion of cBHT patients may not be sufficiently informed as to whether their medication is compounded, FDA-approved, or safe and effective to treat their symptoms. This is an important context to be mindful of when reviewing self-reported survey data on cBHT use.
Insights from the Nurses’ Health Study 2
Because the committee was unable to identify publicly available information to conduct an analysis of formulations, frequency, or trends of use of cBHT preparations in the United States, it submitted a request to review unpublished national data from the Nurses’ Health Study 2 (NHS2). NHS2 is an ongoing, large, prospective cohort study collecting comprehensive information on the lifestyles and health status of U.S. women in an effort to identify risk factors for major chronic diseases. Since 1976, participants of NHS2 have completed questionnaires every 2 years to update exposures and ascertain outcomes,21 and every questionnaire cycle has asked women about their use of hormone therapies. In 2015, 87,677 participants were specifically asked for the first time about their use of “bioidentical estrogen,” “bioidentical progesterone,” and testosterone. In coordination with NHS2 investigators, the committee was able to access data regarding the frequency of use of these bioidentical hormones in this study population (NHS, 2019).
Based on the questionnaire administered in 2015, when the average respondent was age 60 and approximately 10 years postmenopausal, 16 percent of NHS2 participants reported they used some kind of hormone therapy (see Table 8-3). Of those reporting they used hormone therapy, 11 percent reported use of a “bioidentical” product. The most commonly reported bioidentical hormones were a combination of estrogen and progesterone (26 percent), a combination of estrogen, progesterone, and testosterone (25 percent), and testosterone alone (22 percent) (see Table 8-4). The questionnaire did not specifically refer to “compounded” BHT, a common oversight that impedes accurate surveillance of cBHT use.22,23
___________________
21 For access to current and past questionnaires, see https://www.nurseshealthstudy.org/participants/questionnaires (accessed January 20, 2019).
22 In addition, although these data provide some quantitative data as to the frequency of use of bioidentical hormones in the general U.S. population, they may not provide full insight into the potential patient population. The respondents to the NHS2 questionnaire are primarily white, and to what extent similar use patterns exist in nonmedically trained individuals or women of color is not known.
23 Bijuva (estradiol and progesterone capsules) is the first only FDA-approved combination BHT product. However, reported use of bioidentical estrogen and progesterone medications does not clarify whether the medication is compounded or FDA approved. Other BHT combinations are likely compounded for use.
TABLE 8-3 Data from the 2015 NHS2 Questionnaire on Hormone Therapy Use (n = 87,677)
| Noncurrent Users of HT | Current Users of Conventional HT | Current Users of Bioidentical HT | |
|---|---|---|---|
| n = 73,793 | n = 12,558 | n = 1,534 | |
| Age, mean (SD) | 61 (5%) | 60 (5%) | 61 (5%) |
| BMI, Mean (SD) | 27.7 (6.4%) | 27.9 (6.5%) | 28.3 (6.7%) |
| Age at menopause (SD) | 50.0 (4.5%) | 49.8 (4.5%) | 49.8 (4.7%) |
| Previous breast cancer (%) | 5,102 (6.9%) | 1,022 (8.1%) | 126 (8.2%) |
| Postmenopausal (%) | 60,900 (82.5%) | 10,360 (82.5%) | 1,245 (81.2%) |
NOTE: BMI = body mass index; HT = hormone therapy; SD = standard deviation.
SOURCE: NHS, 2019.
TABLE 8-4 Frequency of Types of Hormones Among Women Reporting Use of BHT (n = 1,534)
| Any bioidentical estrogen | 248 (16.2%) |
| Any bioidentical progesterone | 951 (62.0%) |
| Any testosterone | 335 (21.8%) |
| Bioidentical estrogen only | 140 (9.1%) |
| Bioidentical progesterone | 121 (7.9%) |
| Bioidentical testosterone only | 335 (21.8%) |
| Bioidentical estrogen + testosterone | 108 (7.0%) |
| Bioidentical estrogen + progesterone | 397 (25.9%) |
| Bioidentical progesterone + testosterone | 45 (2.9%) |
| Bioidentical estrogen + progesterone + testosterone | 388 (25.3%) |
SOURCE: NHS, 2019.
cBHT: Types and Amounts of Use
To examine the common types and amounts of cBHT preparations used by patients, the committee gathered descriptive data on dispensed drugs purchased from 503B outsourcing facilities, as well as from information from NABP. In addition, qualitative testimonies by patient, clinician, or pharmacist groups, and responses from a committee stakeholder questionnaire, were reviewed and discussed during committee deliberations.24
___________________
24 See Appendix A for additional details regarding the National Academies Committee Stakeholder Questionnaire.
FDA-Submitted Data from 503B Outsourcing Facilities
In May 2019, the committee submitted a data request to FDA for a compiled list of the most commonly dispensed cBHT preparations from registered 503B outsourcing facilities during 2017–2018. These data showed that 503B outsourcing facilities produced 3,777,663 individually packaged compounded hormone products in 2017 and 4,215,899 in 2018, an increase of 11.6 percent. Over this period, testosterone was the most frequently prepared compounded hormone by 503B outsourcing facilities, followed by estradiol, testosterone cypionate, progesterone, and estriol. Estrone, pregnenolone, and DHEA were compounded in small quantities, and estradiol cypionate was not formulated by any of the outsourcing facilities over this 2-year period.25
Only three hormones—estradiol, testosterone, and testosterone cypionate—saw an increase in their use in compounded preparations manufactured by outsourcing facilities over the period of 2017–2018 (see Table 8-5). Estriol and progesterone were all used in fewer compounded products coming from 503B facilities in 2018 compared to 2017, with compounding of progesterone-containing products by 503B facilities falling more than 58 percent (see Table 8-5).
The most frequent dosage form depended on the type of hormone contained in the formulation and whether the preparation contained a single ingredient or multiple ingredients. For example, progesterone and testosterone were most commonly compounded as capsules and pellets, respectively. Estradiol was most frequently compounded in pellet form as a stand-alone active product ingredient, but when made into a multi-ingredient product, capsules were the most frequent dosage form produced (see Chapter 5 for more information on the diversity of available cBHT formulations and dosage forms).
Data on cBHT from NABP
In June 2019, the committee submitted a data request to NABP to gather information on the most common formulations dispensed to patients. From its 2016 to 2018 collection of pharmacy inspection application requests, NABP submitted a compiled list of the five most dispensed
___________________
25 FDA 2019 email from G. Cosel to National Academies staff regarding aggregated volume output of products containing ingredients of interest to compounded bioidentical hormone therapy study. May 30, 2020. Available through the National Academies Public Access File. Data were aggregated across all outsourcing facilities in order to keep each outsourcing facilities’ production volume confidential. Additional outsourcing facility preparation reports can be found at https://www.fda.gov/drugs/human-drug-compounding/information-outsourcing-facilities (accessed April 13, 2020).
formulations from both 503A compounding pharmacies and 503B outsourcing facilities. In general, the 503A compounding pharmacy data suggested that progesterone in capsules and testosterone in creams were the two most commonly dispensed cBHT preparations, followed by estradiol/estriol and estradiol cream formulations. From NABP’s 503B outsourcing facilities data, it appears that progesterone capsules, testosterone pellets, and testosterone cypionate injections were the most commonly dispensed preparations.
Although these data provided a snapshot of the formulations in demand over the past few years, there are substantial limitations. The majority of these reports came from pharmacies that voluntarily approached NABP requesting an inspection (though some inspections were mandated because of state disciplinary action), so they cannot be viewed as representative of the dispensing trends of all 503A compounding pharmacies or 503B outsourcing facilities. In addition, there were instances in which the pharmacists filling out the applications failed to note whether their top hormone therapy medications were FDA approved or compounded, and incompleteness of the submitted data limited their usefulness.26 Data from 503A compounding pharmacies suggest increased use of progesterone, whereas the data provided by the 503B data outsourcing facilities suggest reduced demand for progesterone. Given the limitations of these data, the committee is unable to reconcile the two submitted data sources (NABP, 2019).
Submitted Testimonials
In addition to reviewing data from clinical guidance, peer-reviewed literature, national surveys, and federal and state databases, the committee also reviewed submitted statements and testimonies from patients, clinicians, researchers, and compounding pharmacists. While these were helpful in verifying the broad spectrum of available cBHT formulations, the information was anecdotal or derived from small or nonrepresentative samples. Additional evidence is needed to provide quantifiable and verifiable evidence to examine trends or determine future projections regarding use.
___________________
26 Although the application form specifically asked pharmacists to list their top compounded preparations, there were certain instances where it was not clear whether pharmacists accidently listed FDA-approved drug products. To address this issue in the analysis of the data, if the complete formulation listed was not found in FDA’s list of available hormone therapy products, then formulation was counted in the total tally for compounded preparations. If the formulation listed was also found to be available as an FDA-approved drug product, to avoid false positives, this entry was not incorporated into the final data set.
| Year of Production | Estriol | Estradiol | Progesterone | |||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | |
| Single-ingredient formulation | 3,822 | 2,882 | 619,571 | 692,879 | 205,539 | 82,653 |
| Multi-ingredient (combination) formulation | 16,600 | 13,382 | 36,839 | 18,918 | 15,351 | 8,912 |
| Total quantity compounded | 20,422 | 16,264 | 656,410 | 711,797 | 220,890 | 91,565 |
| Percentage change in quantity compounded from 2017 to 2018 | –20.4 | 8.4 | –58.6 | |||
NOTE: Production levels were measured as individual packages.
SOURCE: NASEM, 2019b.
Additional Concerns
In addition to the limited regulation and oversight of producing and dispensing cBHT medications, there is a lack of standardized data collection and meaningful surveillance of patient use of cBHT preparations. A constantly evolving compounding landscape, including the emergence of large, mail-order compounding pharmacies and recent changes in insurance coverage (McPherson et al., 2019) contribute to the limited data sources. In recent years, payers have drastically reduced their coverage of compounded medications (CMS, 2018; DHA, 2017), but if insurance companies that cover cBHT are tracking prescriptions for compounded medications, these data have not been published widely nor analyzed by researchers. Furthermore, no data source exists to capture the use of cBHT by patients who pay out of pocket. Quantifiable data are needed to adequately inform understanding of use and trends, policy decisions related to the clinical utility of cBHT, and other influencing factors, such as financial incentives.27
___________________
27 See Chapter 3 for more information on cBHT formulations and conflicts of interest.
| Testosterone | Testosterone Propionate | Testosterone Cypionate | |||
|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 |
| 2,691,848 | 3,165,230 | 2,330 | 2,013 | 139,013 | 163,765 |
| 34,625 | 52,844 | 6,197 | 4,954 | 5,928 | 7,467 |
| 2,726,473 | 3,218,074 | 8,527 | 6,967 | 144,941 | 171,232 |
| 18.0 | –18.3 | 18.1 | |||
FACTORS DRIVING THE USE OF CBHT
While there is limited evidence-based support for the use of cBHT to treat menopausal symptoms, the available data suggest millions of patients use thousands of different cBHT formulations every day. Given this fact, the committee deemed it critical in its examination of clinical utility to explore potential factors influencing interest and use of these non-FDA-approved preparations. To address these points, the committee reviewed the potential influence of cBHT marketing; physician practices and perspectives; patient mistrust in the health care industry and commercial pharmaceutical industry; patient interests in the “natural” movement; and prescription costs. Information related to factors influencing use of cBHT came from a few qualitative studies and several hundred testimonials; however, the
committee could not identify any large-scale descriptive surveys of cBHT users to inform its understanding of various influencers.
The Role of Marketing
Media Influence
Published surveys suggest that a substantial number of patients rely on media outlets such as social media, books, Internet marketing, celebrities, and television commercials to educate themselves about cBHT (McPherson et al., 2019; Pinkerton and Santoro, 2015). Much of this information seems to originate from direct-to-consumer marketing by compounding pharmacies and commercial wellness clinics (AMA, 2016; see Table 8-6 for excerpts from select published statements and professional guidance). For example, a 2017 study of 100 websites promoting or offering cBHT services or products identified through a Google search found that nearly
| Organization Name | Year | Concerns and Recommendations |
|---|---|---|
| American Medical Association | 2016 | “There have been some ethical and conflict of interest issues associated with commercial wellness clinics and compounding pharmacies that prescribe and dispense CHT. Some compounding pharmacies that sell CHT also market the products to the public by providing listings of their offerings and offer referrals to providers who can prescribe the CHT. Some proprietors of commercial wellness clinics have published peer-reviewed journal articles that have been viewed as misleading and questionable rhetorical approaches may be used to appeal to those lacking scientific literacy, for example, failing to distinguish between ‘cutting edge medicine’ and ‘untested or unproven therapies.’” (AMA, 2016, p. 6) |
| British Menopause Society | 2019 | “The Advertising Standards Association (ASA) ruled in 2017 against the ‘misleading’ promotion of cBHRT when a prescribing dermatherapy cosmetic clinic in Stratford upon Avon was reported. This test case led to a ruling being passed that these clinics and prescribers of cBHRT should not claim greater safety and efficacy as there was no evidence from clinical trials for these products. The ASA also advised that there was insufficient evidence that multiple serum and saliva tests could be used to precisely individualize therapy. The public should be cautious of marketing that can give rise to false securities and should avoid purchasing cBHT products over the Internet.” (Panay et al., 2019, p. 62) |
SOURCES: AMA, 2016; Panay et al., 2019.
half originated from medical clinics (Yuksel et al., 2017). These medical clinics often promoted cBHT preparations as less risky than conventional hormone therapies, with 65 percent of their websites marketing cBHT as having either a lower risk of causing breast cancer risk or even being protective against breast cancer (Yuksel et al., 2017). For further discussion on the use and oversight of marketing, see Chapter 3.
Although there are no specific data to inform conclusions about the effects of celebrity endorsements, there have been a number of highly visible and influential celebrity accounts and endorsements of cBHT over the past 15 years (Pinkerton, 2015). In reviewing the 3,397 patient responses to the committee’s stakeholder questionnaire question, “How did you first learn about cBHT products,” 202 patients reported online media sources, 94 noted social media discussions, and 93 responded using the “other” option, many noting they got their information from books that were often authored by high-profile celebrities.28
Claims of Customization
In contrast to the historic rationales for compounding, advocates of cBHT often promote the view that cBHT medications are superior to FDA-approved BHT because they offer individualized hormone preparations and can provide a wider range of optional doses and dosage forms throughout the course of treatment (AMA, 2016). The prospect of personalized medications and avoiding perceived one-size-fits-all treatments appears to have a substantial appeal to patients (McPherson et al., 2019; Pinkerton and Santoro, 2015; Yuksel et al., 2017). This may be influenced, in part, by popular discourse in both social media and popular press (Marcon et al., 2018).
In contrast to the growing interest in customized and personalized medications, compounders often offer preprinted prescription pads for cBHT with a checklist of popular ingredient combinations, concentrations, and dosage forms (NASEM, 2019c; see Figure 8-3). These are often used as a marketing tool to increase the requests for and sale of cBHT, and they may appeal to certain clinicians by making the compounding prescription process “quick and easy.” However, there is no available evidence to suggest the sample menus of formulations, and associated doses and dosage forms, are supported by empirical data related to their safety and efficacy. As a result, there is concern that the goal of treatment customization through the use of compounded medications is being replaced by a list of check boxes without adequate assessment or evaluation of the individual patient’s complex needs (NASEM, 2019c).
___________________
28 See Appendix A for additional details regarding the National Academies Committee Stakeholder Questionnaire.

SOURCE: NASEM, 2019c.
Patient Perspectives
The existing data on motivations for use come primarily from a few qualitative studies exploring women’s reported reasons for seeking cBHT to treat their menopausal symptoms (Fishman et al., 2015; Thompson et al., 2017). Women taking cBHT seemed to be simultaneously “pulled toward” cBHT and “pushed away” from FDA-approved BHT
by conflicting psychosocial forces (Thompson et al., 2017). Motivations “pulling” patients toward cBHT are beliefs in safer and more “natural” hormone therapy alternatives, beliefs often reinforced by their clinicians. Women were “pushed away” from conventional hormone therapies by an overarching distrust of the medical system, their concerns with conflicts of interest with the pharmaceutical industry, and their fears about the safety of FDA-approved hormone therapy products (Thompson et al., 2017). In addition, some women who turned to cBHT did so out of frustration with clinicians who were unable or unwilling to take their symptoms seriously or provide effective treatments (Fishman et al., 2015).
Beliefs in Safer, More “Natural” Hormone Therapy Alternatives
Patients using cBHT have reported that being “natural” makes cBHT safer than conventional hormone therapy, safe when taken long term, and considered safe for use even for breast cancer survivors (Fishman et al., 2015). Those taking cBHT, however, seemed unaware that there were inadequate safety data supporting cBHT use or that there are bioidentical FDA-approved drug products (Fishman et al., 2015). They also believed the products they were taking were natural rather than synthetic, despite the fact that the hormones used to create certain cBHT medications may be synthesized to become bioidentical. These findings are supported by surveys demonstrating that patients using cBHT hold strong beliefs or report preferences for “natural,” customizable medications that they believe have a greater safety profile than FDA-approved hormone therapy products (AMA, 2016; Fishman et al., 2015; Huntley, 2011; Iftikhar et al., 2011; McPherson et al., 2019; Pinkerton and Santoro, 2015; Thompson et al., 2017). These appear to be common misconceptions, and even when these misconceptions are pointed out, some patients continue to believe cBHT preparations are safer than FDA-approved hormone therapies (AMA, 2016; Thompson et al., 2017).
In addition, there are data to suggest patients and even prescribing physicians are uncertain as to whether cBHT preparations are FDA-approved medications (Constantine et al., 2016; Pinkerton and Santoro, 2015). For example, Pinkerton and Santoro (2015) found that out of 801 survey respondents (women ages 45–60), 86 percent did not know whether cBHT was an FDA-approved medication. Misconceptions and inaccuracies by both patients and prescribers regarding the safety and efficacy of compounded preparations are a concern. The inadequate labeling requirements (e.g., unstandardized package inserts for patients, no requirement for boxed warnings) potentially contribute to the lack of informed use.
Physician Input
Consultations with physicians appear to influence patients’ interest in and use of cBHT. The committee could identify little about the proportion of clinicians who prescribe cBHT, their specialties, or their rates of prescribing. There are, however, a few small descriptive surveys suggesting that a segment of the medical community considers cBHT preparations an appropriate alternative to FDA-approved therapies.
In a 2019 survey of 494 patients, 78.9 percent said their prescriber was the first person to suggest the option of cBHT (McPherson et al., 2019). In addition, in a nonrepresentative sample of 3,397 cBHT users from the National Academies committee’s stakeholder questionnaire, 55.4 percent reported first learning about cBHT from their health care provider.29 Another small Internet-based survey of 128 physicians reported that, as compared to 56.9 percent of family physicians, only 37.7 percent of obstetricians-gynecologists agreed with the statement, “Patients should be counseled that conventional menopausal hormone therapy is more appropriate than compounded preparations” (Dubaut, 2018). Although there is limited available evidence for the committee to consider, in general, it appears that physicians’ beliefs affect their patients’ potential use of cBHT.
Mistrust in Health Care Institutions
There are additional influences that “push” patients away from FDA-approved drug products. Over the past four decades, Gallup polling revealed that confidence in almost all U.S. institutions, such as Congress and the news media, has deteriorated, but the most dramatic decline has occurred in “confidence in the medical system,” which fell from 80 percent in 1975 to 37 percent in 2015 (Baron and Berinsky, 2019). This apparent public mistrust does not appear to be interpersonal, or directed toward individual health care providers, but rather appears to be institutional, or directed toward medical systems as a whole (Baron and Wolfson, 2019; Pearson and Raeke, 2000).
One factor that may be fueling growing mistrust of health care institutions is the relative deemphasis in developing personal relationships between clinicians and patients that has evolved over the years as the health care system attempts to reduce the costs of care (IOM, 2001). To satisfy the desire for a more personal relationship, some patients show interests in pursuing the services of boutique cBHT compounding pharmacies, all-inclusive wellness centers, or specialized health care providers. In fact, in qualitative studies on menopausal decision making, most interviewed
___________________
29 See Appendix A for additional details regarding the National Academies Committee Stakeholder Questionnaire.
patients who reported receiving cBHT prescriptions described their clinical care experience as superior to their conventional medical care. They were satisfied with the individualized care that they received, time spent at each appointment, and follow-up (Fishman et al., 2015; Thompson et al., 2017).
Mistrust in the Pharmaceutical Industry
Similar to public attitudes toward health care institutions, many people in the United States do not hold drug companies in high regard. A 2012 Harris Poll revealed that only 12 percent of U.S. adults believed pharmaceutical companies to be “generally honest and trustworthy” (Miller, 2013). This mistrust may be rooted in public concerns surrounding drug safety, as was suggested in a 2006 survey (Olsen and Whalen, 2009). The survey included 1,726 respondents and evaluated the U.S. public’s perception of FDA, the pharmaceutical industry, and Congress (Olsen and Whalen, 2009). Overall, 96 percent of respondents indicated having some degree of concern about prescription drug adverse reactions, even when these drugs are taken as prescribed. Moreover, 42 percent of respondents said that they were either “somewhat distrusting” or “strongly distrusting” of pharmaceutical companies, in general.
Cost Considerations for cBHT
The overall cost of hormone therapy for managing menopause-related vasomotor symptoms are substantial and commonly include routine physician consultations, laboratory testing, and management of adverse events (Utian, 2005). According to a 2016 survey, 26 to 33 million cBHT prescriptions were filled annually with total sales estimated at $1.3 billion to $1.6 billion (Pinkerton and Constantine, 2016). With annual costs of conventional FDA-approved hormone therapies ranging from $430 to $830, patients experiencing menopausal symptoms may seek alternative treatments, including cBHT, that are often marketed as safer and less expensive (PCCA, 2019; Williams-Frame and Carpenter, 2009).30
Although recent studies have highlighted the increasing cost of compounded medications, no studies have specifically examined the cost of cBHTs. Based on results from a national survey, McPherson et al. reported that overall out-of-pocket costs for cBHTs may be higher than those of noncompounded prescriptions. The average out-of-pocket cost reported for cBHTs was $88 in a 2017 survey, whereas the average price of FDA-approved postmenopausal hormone therapy prescriptions was $49
___________________
30 A 2019 PCCA national survey of 14 within-network pharmacies concluded that the median sales price of select cBHT was lower than the median sales prices of select FDA-approved BHT products (available through the National Academies Public Access File).
(McPherson et al., 2019). As an illustrative example of costs, in a testimony submitted to the committee by a prescriber of cBHT, the cost of topical cBHT preparations (Bi-est/Tri-est, progesterone, testosterone, and DHEA) was estimated to range from $40 to $60 per prescription per month depending on the compounding pharmacy, liquid base used, and dispensing method (NASEM, 2019d). The total cost per patient can amount to $80 to $120 per month initially; however, total cost may increase to $160 to $220 per month by 3 years into menopause. Additional testing associated with treatment can cost $285 to $360 for hormone testing and $180 out-of-pocket for ancillary testing (mammography, bone density, and blood tests covered by insurance). Annual consultation and office visit fees can be estimated from $800 to $3,000 (NASEM, 2019d).
Overall, true cost comparisons between cBHT and FDA-approved versions are complicated, given that an individual patient’s out-of-pocket costs will vary greatly based on his or her insurance status and other medication use. With the volume of prescribed cBHTs increasing, additional studies investigating the cost and the relationship between cost and use of cBHT and FDA-approved treatments for menopause- and male hypogonadism–related symptoms are needed (Pinkerton and Constantine, 2016). In the meantime, patient preference for costs should be discussed in the context of safety and effectiveness; however, these cost considerations cannot supersede those related to the medication’s safety and effectiveness.
CONCLUDING STATEMENTS
In summary, after considering the paucity of evidence, including, but not limited to peer-reviewed literature, clinical guidance issued by professional medical organizations, and oral and written public statements from patients, prescribers, and pharmacists, the committee has concerns regarding the current volume and scope of use of cBHT. Reviewed clinical guidance indicated there may be a small population of patients with a therapeutic need for cBHT, including those with an allergy or intolerance to an ingredient within an FDA-approved BHT product. However, data suggest there may be millions of patients using cBHT, implying cBHT preparations are being prescribed for reasons outside of recognized therapeutic need. As noted in a 2018 National Academies report, research results that lack clear clinical utility may still have personal meaning to a patient or participant. Nevertheless, existing frameworks tend to prioritize clinical utility over personal meaning or preference when assessing the value of prescribing a particular drug (NASEM, 2018).
Throughout the course of this study, prescribers, patients, and advocates of cBHT submitted written statements and public testimony sharing their experiences with cBHT. It is clear from these communications that many clinicians, compounding pharmacists, and patients using cBHT hold minimum, if any, concerns regarding the medications’ safety and effectiveness. The evidence suggests that confounding factors, including unsubstantiated marketing claims, general misinformation, a mistrust of the pharmaceutical and health care industries, and cost may influence patient perspectives on overall clinical utility of cBHT. This implies that there are opportunities for new or expanded continuing education efforts for clinicians, as well as science communication and health literacy initiatives for patients regarding the effectiveness and safety of both FDA-approved BHT and cBHT.
REFERENCES
Abrantes, C. G., D. Duarte, and C. P. Reis. 2016. An overview of pharmaceutical excipients: Safe or not safe? Journal of Pharmaceutical Sciences 105(7):2019–2026.
ACOG (American College of Obstetricians and Gynecologists). 2014. Practice Bulletin No. 141: Management of menopausal symptoms. Obstetrics & Gynecology 123(1):202–216.
ACOG. 2018. Committee opinion: Compounded bioidentical menopausal hormone therapy. Washington, DC: American College of Obstetricians and Gynecologists.
AMA (American Medical Association). 2016. Hormone therapies: Off-label uses and unapproved formulations (resolution 512-a-15). Chicago, IL: American Medical Association.
APA (American Psychiatric Association). 2013. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Philadelphia, PA: American Psychiatric Publications.
Baber, R. J., N. Panay, A. Fenton, and the IMS Writing Group. 2016. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 19(2):109–150.
Barbieri, R. 2019. Patient education: Menopausal hormone therapy (beyond the basics). https://www.uptodate.com/contents/menopausal-hormone-therapy-beyond-the-basics/print (accessed February 12, 2020).
Barlow, D. H. 2014. Understanding the impact of the women’s health initiative. Menopause 21(7):683–684.
Baron, R. J., and A. J. Berinsky. 2019. Mistrust in science—a threat to the patient–physician relationship. New England Journal of Medicine 381(2):182–185.
Baron, R. J., and D. B. Wolfson. 2019. Building trust in the profession: What can we learn from choosing wisely? American Journal of Medicine 132(5):550–551.
Barry, M. J., and S. Edgman-Levitan. 2012. Shared decision making—pinnacle of patient-centered care. New England Journal of Medicine 366(9):780–781.
Clayton, A. H., I. Goldstein, N. N. Kim, S. E. Althof, S. S. Faubion, B. M. Faught, S. J. Parish, J. A. Simon, L. Vignozzi, K. Christiansen, S. R. Davis, M. A. Freedman, S. A. Kingsberg, P.-S. Kirana, L. Larkin, M. McCabe, and R. Sadovsky. 2018. The International Society for the Study of Women’s Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clinic Proceedings 93(4):467–487.
CMS (Centers for Medicare & Medicaid Services). 2018. Medicare part D coverage of multi-ingredient compounds. Washington, DC: U.S. Department of Health and Human Services.
Coleman, E., W. Bockting, M. Botzer, P. Cohen-Kettenis, G. Cuypere, J. Feldman, L. Fraser, J. Green, G. Knudson, W. Meyer, S. Monstrey, R. Adler, G. Brown, A. Devor, R. Ehrbar, R. Ettner, E. Eyler, R. Garofalo, D. Karasic, and K. Zucker. 2012. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. International Journal of Transgenderism 13:165–232.
Conaway, E. 2011. Bioidentical hormones: An evidence-based review for primary care providers. Journal of the American Osteopathic Association 111(3):153–164.
Constantine, G. D., D. F. Archer, S. Graham, B. A. Bernick, and S. Mirkin. 2016. Prescribing of FDA-approved and compounded hormone therapy differs by specialty. Menopause 23(10):1075–1082.
Constantine, G. D., S. Graham, K. Lapane, K. Ohleth, B. Bernick, J. Liu, and S. Mirkin. 2019a. Endometrial safety of low-dose vaginal estrogens in menopausal women: A systematic evidence review. Menopause (New York) 26(7):800–807.
Constantine, G. D., G. Kessler, S. Graham, and S. R. Goldstein. 2019b. Increased incidence of endometrial cancer following the Women’s Health Initiative: An assessment of risk factors. Journal of Women’s Health (2002) 28(2):237–243.
Couët, N., S. Desroches, H. Robitaille, H. Vaillancourt, A. Leblanc, S. Turcotte, G. Elwyn, and F. Légaré. 2015. Assessments of the extent to which health-care providers involve patients in decision making: A systematic review of studies using the option instrument. Health Expectations 18(4):542–561.
Crandall, C. J. 2019. Treatment of vulvovaginal atrophy. JAMA 322(19):1910–1911.
Davis, S. R., R. Baber, N. Panay, J. Bitzer, S. C. Perez, R. M. Islam, A. M. Kaunitz, S. A. Kingsberg, I. Lambrinoudaki, J. Liu, S. J. Parish, J. Pinkerton, J. Rymer, J. A. Simon, L. Vignozzi, and M. E. Wierman. 2019. Global consensus position statement on the use of testosterone therapy for women. The Journal of Clinical Endocrinology & Metabolism 104(10):4660–4666.
de Villiers, T. J., J. E. Hall, J. V. Pinkerton, S. C. Pérez, M. Rees, C. Yang, and D. D. Pierroz. 2016. Revised global consensus statement on menopausal hormone therapy. Maturitas 91:153–155.
DHA (Defense Health Agency). 2017. Defense Health Agency controls over high-risk pharmaceutical payments. DODIG-2018-033. Department of Defense. https://www.dodig.mil/DesktopModules/ArticleCS/Print.aspx?PortalId=48&ModuleId=2973&Article=1376792 (accessed June 4, 2020).
Dubaut, J. P., F. Dong, B. L. Tjaden, D. A. Grainger, J. Duong, and L. L. Tatpati. 2018. Prescribing bioidentical menopausal hormone therapy: A survey of physician views and practices. Journal of Women’s Health (2002) 27(7):859–866.
Endocrine Society. 2019. Position statement: Compounded bioidentical hormone therapy. Washington, DC: Endocrine Society.
Ettinger, B., S. M. Wang, R. S. Leslie, B. V. Patel, M. J. Boulware, M. E. Mann, and M. McBride. 2012. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause 19(6):610–615.
FDA (U.S. Food and Drug Administration). 2015. The voice of the patient: Female sexual dysfunction. Silver Spring, MD: U.S. Food and Drug Administration.
FDA. 2018. Compounding and the FDA: Questions and answers. https://www.fda.gov/drugs/human-drug-compounding/compounding-and-fda-questions-and-answers (accessed February 11, 2020).
FDA. 2020. Orange Book: Approved drug products with therapeutic equivalence evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (accessed March 26, 2020).
Fenway Health. 2015. The medical care of transgender persons. Boston, MA: The Fenway Institute.
Fishman, J. R., M. A. Flatt, and R. A. Settersten, Jr. 2015. Bioidentical hormones, menopausal women, and the lure of the “natural” in U.S. anti-aging medicine. Social Science & Medicine (1982) 132:79–87.
FSMB (Federation of State Medical Boards). 2002. Model guidelines for the use of complementary and alternative therapies in medical practice. Washington, DC: Federation of State Medical Boards.
Gass, M. L. S., C. A. Stuenkel, W. H. Utian, A. LaCroix, J. H. Liu, J. L. Shifren, and North American Menopause Society Advisory Panel. 2015. Use of compounded hormone therapy in the United States: Report of the North American Menopause Society survey. Menopause (New York) 22(12):1276–1284.
Glassgold, J. M. 2013. Compounded drugs. Congressional Research Service. http://fas.org/sgp/crs/misc/R43082.pdf (accessed June 4, 2020).
Hays, J., J. R. Hunt, F. A. Hubbell, G. L. Anderson, M. Limacher, C. Allen, and J. E. Rossouw. 2003. The Women’s Health Initiative recruitment methods and results. Annals of Epidemiology 13(9 Suppl):S18–S77.
Huntley, A. L. 2011. Compounded or confused? Bioidentical hormones and menopausal health. Menopause International 17(1):16–18.
Iftikhar, S., L. T. Shuster, R. E. Johnson, S. M. Jenkins, and D. L. Wahner-Roedler. 2011. Use of bioidentical compounded hormones for menopausal concerns: Cross-sectional survey in an academic menopause center. Journal of Women’s Health (2002) 20(4):559–565.
IMS (International Menopause Society). 2020. Mission and vision of IMS. https://www.imsociety.org/aims_of_ims.php (accessed March 25, 2020).
IOM (Institute of Medicine). 2001. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press.
IOM. 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
Kelso, J. M. 2014. Potential food allergens in medications. Journal of Allergy and Clinical Immunology 133(6):1509–1520.
Lightner, D. J. 2002. Female sexual dysfunction. Mayo Clinic Proceedings 77(7):698–702.
Manson, J. E., and A. M. Kaunitz. 2016. Menopause management—getting clinical care back on track. New England Journal of Medicine 374(9):803–806.
Manson, J. E., A. K. Aragaki, J. E. Rossouw, G. L. Anderson, R. L. Prentice, A. Z. LaCroix, R. T. Chlebowski, B. V. Howard, C. A. Thomson, K. L. Margolis, C. E. Lewis, M. L. Stefanick, R. D. Jackson, K. C. Johnson, L. W. Martin, S. A. Shumaker, M. A. Espeland, J. Wactawski-Wende, and WHI Investigators. 2017. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The Women’s Health Initiative randomized trials. JAMA 318(10):927–938.
Marcon, A. R., M. Bieber, and T. Caulfield. 2018. Representing a “revolution”: How the popular press has portrayed personalized medicine. Genetics in Medicine 20(9):950–956.
McPherson, T., P. Fontane, and R. Bilger. 2019. Patient experiences with compounded medications. Journal of the American Pharmacists Association 59(5):670–677.
Miller, J. E. 2013. From bad pharma to good pharma: Aligning market forces with good and trustworthy practices through accreditation, certification, and rating. Journal of Law, Medicine & Ethics 41(3):601–610.
NABP (National Association of Boards of Pharmacy). 2019. Compiled list of the five most dispensed formulations from 503A compounding pharmacies and 503B outsourcing facilities from 2016 to 2018. NABP response to inquiry regarding common formulations dispensed to patients (available through the National Academies Public Access File).
NAMS (North American Menopause Society). 2017. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause (New York) 24(7):728–753.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2018. Returning individual research results to participants: Guidance for a new research paradigm. Washington, DC: The National Academies Press. https://doi.org/10.17226/25094.
NASEM. 2019a. Endocrine Society Nominees: Drs. Cynthia Stuenkel and Nanette Santoro; September 19, 2019 (available through the National Academies Public Access File).
NASEM. 2019b. FDA submitted volume output of 503B facility products containing ingredients of interest to compounded bioidentical hormone therapy study. May 30, 2020 (available through the National Academies Public Access File).
NASEM. 2019c. PowerPoint presented by R. Ju and L. Furlong on behalf of the FDA to the Committee during the open session of Meeting One. March 5, 2019 (available through the National Academies Public Access File).
NASEM. 2019d. Daved Rosensweet, MD, presentation of responses to questions posed by the Committee as follow-up to Dr. Rosensweet’s May 22, 2019, open session testimony. August 22, 2019 (available through the National Academies Public Access File).
National Women’s Health Network. 2020. Menopause and natural hormones. https://nwhn.org/natural-hormones-at-menopause (accessed May 1, 2020).
NHS (Nurses’ Health Study). 2019. Nurses’ Health Studies prospective investigations. https://www.nurseshealthstudy.org/about-nhs (accessed June 9, 2020).
NLM (National Library of Medicine). 2018. Label: Estrace-estradiol tablet. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a3803ba3-4eee-4e2e-ac8c-821a4e6720cc (accessed February 11, 2020).
NLM. 2019. Label: ADDYI-flibanserin tablet, film coated. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394 (accessed February 11, 2020).
NLM. 2020. DailyMed. https://dailymed.nlm.nih.gov/dailymed (accessed February 11, 2020).
Olsen, A. K., and M. D. Whalen. 2009. Public perceptions of the pharmaceutical industry and drug safety: Implications for the pharmacovigilance professional and the culture of safety. Drug Safety 32(10):805–810.
Panay, N., and Medical Advisory Council of the British Menopause Society. 2019. BMS—consensus statement: Bioidentical HRT. Post Reproductive Health 25(2):61–63.
PCCA (Professional Compounding Centers of America). 2019. Report of PCCA Regulatory Opinion Survey submitted to the Committee for review. November 14, 2019 (available through the National Academies Public Access File).
Pearson, C. 2019. Comments presented to the Committee at the second open session meeting held in Washington, DC. May 6, 2019 (available through the National Academies Public Access File).
Pearson, S. D., and L. H. Raeke. 2000. Patients’ trust in physicians: Many theories, few measures, and little data. Journal of General Internal Medicine 15(7):509–513.
Pinkerton, J. V. 2020. Hormone therapy for postmenopausal women. New England Journal of Medicine 382(5):446–455.
Pinkerton, J. V., and G. D. Constantine. 2016. Compounded non-FDA-approved menopausal hormone therapy prescriptions have increased: Results of a pharmacy survey. Menopause (New York) 23(4):359–367.
Pinkerton, J. V., and N. Santoro. 2015. Compounded bioidentical hormone therapy: Identifying use trends and knowledge gaps among US women. Menopause (New York) 22(9):926–936.
Reker, D., S. M. Blum, C. Steiger, K. E. Anger, J. M. Sommer, J. Fanikos, and G. Traverso. 2019. “Inactive” ingredients in oral medications. Science Translational Medicine 11(483):eaau6753.
Roby, R. R., R. H. Richardson, and A. Vojdani. 2006. Hormone allergy. American Journal of Reproductive Immunology 55(4):307–313.
Rossouw, J. E., G. L. Anderson, R. L. Prentice, A. Z. LaCroix, C. Kooperberg, M. L. Stefanick, R. D. Jackson, S. A. A. Beresford, B. V. Howard, K. C. Johnson, J. M. Kotchen, J. Ockene, and Writing Group for the Women’s Health Initiative Investigators. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333.
Santoro, N., G. D. Braunstein, C. L. Butts, K. A. Martin, M. McDermott, and J. V. Pinkerton. 2016. Compounded bioidentical hormones in endocrinology practice: An Endocrine Society scientific statement. The Journal of Clinical Endocrinology & Metabolism 101(4):1318–1343.
Sellers, S., and W. H. Utian. 2012. Pharmacy compounding primer for physicians. Drugs 72(16):2043–2050.
Shifren, J. L., C. J. Crandall, and J. E. Manson. 2019. Menopausal hormone therapy. JAMA 321(24):2458–2459.
Stefanick, M. L. 2005. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. American Journal of Medicine 118(Suppl 12B):64–73.
Stefanick, M. L., B. B. Cochrane, J. Hsia, D. H. Barad, J. H. Liu, and S. R. Johnson. 2003. The Women’s Health Initiative postmenopausal hormone trials: Overview and baseline characteristics of participants. Annals of Epidemiology 13(9 Suppl):S78–S86.
Stuenkel, C. A., and J. E. Manson. 2017. Compounded bioidentical hormone therapy: Does the regulatory double standard harm women? JAMA Internal Medicine 177(12):1719–1720.
Swerlick, R. A., and C. F. Campbell. 2013. Medication dyes as a source of drug allergy. Journal of Drugs in Dermatology 12(1):99–102.
Thompson, J. J., C. Ritenbaugh, and M. Nichter. 2017. Why women choose compounded bioidentical hormone therapy: Lessons from a qualitative study of menopausal decision-making. BMC Women’s Health 17(1):97.
UCSF (University of California, San Francisco) Transgender Care. 2016. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. San Francisco, CA: Department of Family and Community Medicine, University of California, San Francisco.
U.S. Senate Special Committee on Aging. 2007. Bioidentical hormones: Sound science or bad medicine. April 19, 2007.
USP (United States Pharmacopeia). 2015. USP global public policy position: Ensuring patient safety in compounding medicines. Rockville, MD: United States Pharmacopeia.
USPSTF (U.S. Preventive Services Task Force). 2019. Screening for pancreatic cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA 322(5):438–444.
Utian, W. H. 2005. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health and Quality of Life Outcomes 3:47.
WHI (Women’s Health Initiative). 2020. WHI publications and paper proposals (Excel). https://www.whi.org/papers (accessed February 11, 2020).
Williams-Frame, A., and J. S. Carpenter. 2009. Costs of hormonal and nonhormonal prescription medications for hot flashes. Women’s Health (London, England) 5(5):497–502.
Worsley, R., R. J. Bell, P. Gartoulla, and S. R. Davis. 2016. Low use of effective and safe therapies for moderate to severe menopausal symptoms: A cross-sectional community study of Australian women. Menopause 23(1):11–17.
Yuksel, N., L. Treseng, B. Malik, and U. Ogbogu. 2017. Promotion and marketing of bioidentical hormone therapy on the Internet: A content analysis of websites. Menopause (New York) 24(10):1129–1135.












































