4
Reproductive Steroid Hormones: Synthesis, Structure, and Biochemistry
Hormones are a diverse class of molecules that influence complex activities throughout the body. They are commonly categorized as peptides and proteins (e.g., insulin), amino acid-derived (e.g., melatonin), or steroids (e.g., estrogen) (Hiller-Sturmhöfel and Bartke, 1998) and are produced in the gonads and adrenal glands, as well as various other tissue and cell types, including those within the placenta and brain (Schiffer et al., 2019). Hormones can function as endocrine (uses the circulatory system to act on distant cells), paracrine (acts on adjacent cells), autocrine (acts at the surface of the cells in which they are produced), or intracrine (acts within the cell) agents (Rubinow, 2018).
Hormones influence different physiological responses by generating cellular signals through their interactions with ion channels on the surface of cells or by binding to and activating a class of proteins called receptors, which can be on the cell surface or inside the cell. A hormone molecule binding to its receptor triggers a cascade of signals in cells that either activates enzymes or causes changes in the expression of the cell’s genes. These actions can have a significant effect on biological function and physiological outcomes, including reproduction, sexual differentiation, development, growth, maintenance of the internal environment, organization of motivated behaviors, and regulation of metabolism and nutrient supply (Hiller-Sturmhöfel and Bartke, 1998; Mani et al., 2012; Melmed et al., 2016).
The remaining sections of the chapter will focus on steroid hormones, define what is meant by the term bioidentical steroid hormones, and provide a high-level overview of the structure and biochemistry of the hormones commonly used in compounded bioidentical hormone therapy (cBHT)
preparations (Cui et al., 2013; Kleine and Rossmanith, 2016; Kuhl, 2005; Melmed et al., 2016). The chapter will close with a discussion on the variability in the molecular signaling of steroid hormones and its implications on physiological responses.
REPRODUCTIVE STEROID HORMONES
Given the subject of this study, this chapter will focus on the actions and therapeutic effects of reproductive steroid hormones and prohormones,1 including estradiol (E2), estrone (E1), estradiol cypionate, estriol (E3), dehydroepiandrosterone (DHEA), pregnenolone, progesterone, testosterone, testosterone cypionate, and testosterone propionate.
Definition of Bioidentical Steroid Hormones
Steroid hormones can be classified into four groups: (1) natural hormones found in nature and formulated into drugs without undergoing chemical modifications; (2) hormones native to the body and synthesized from natural precursors; (3) hormones native to the body and synthesized from nonsteroidal precursors; and (4) synthetic and not native to the body (Bhavnani and Stanczyk, 2012). Irrespective of how they are created or synthesized, all steroid hormones are characterized by the various receptors to which they bind throughout the body.
A bioidentical hormone is a term that describes a hormone that is chemically and structurally identical to those produced by the human body, with the implication that an identical chemical structure translates to a physiologic response identical to that of the endogenous hormone. Bioidentical hormones may be synthesized from plant or animal sources, or completely chemically synthesized (American Chemical Society and Sociedad Química de México, 1999; Wang et al., 2011), and they are offered as U.S. Food and Drug Administration (FDA)-approved drug products or as preparations that have not undergone FDA approval (Cirigliano, 2007). What is important to remember is that it is the chemical structure of the molecule, and not the originating source, that determines whether a hormone is bioidentical.
Endogenous Source of Bioidentical Hormones
The human body produces all of the naturally occurring, or endogenous, steroid hormones from cholesterol obtained from food sources or produced internally by the body’s own cells. Cholesterol is metabolized in a cell’s
___________________
1 Prohormones are precursor molecules that the body can convert into the active hormone molecule.
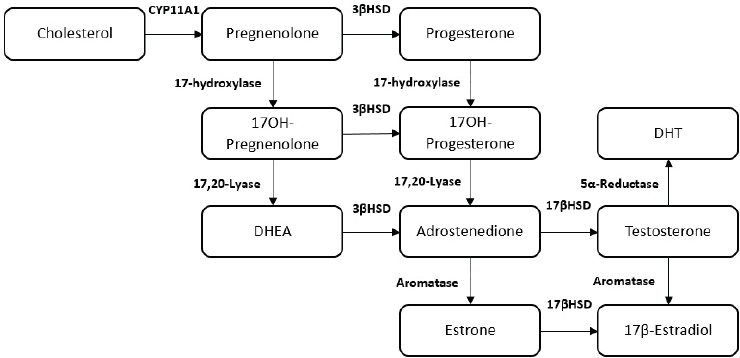
NOTES: Figure does not display all intermediate steroids, pathways, or enzymes related to the synthesis of steroid hormones. DHEA = dehydroepiandrosterone; DHT= dihydrotestosterone.
SOURCE: Rubinow, 2018.
mitochondria to generate the steroid hormone pregnenolone. Further metabolism of pregnenolone through a series of enzymatic steps gives rise to all of the biologically active steroid hormones (Melmed et al., 2016) (see Figure 4-1).
Exogenous Sources of Bioidentical Hormones
Exogenous source material, including plants, must be processed chemically to create bioactive hormone products that the human body’s endogenous hormone receptors can recognize. Plants, for example, do not produce cholesterol. Rather, they produce related chemicals known as plant sterols that can be converted chemically through laboratory-based enzymatic processing into steroid hormone products (see Figure 4-2).
Regardless of whether the bioidentical steroid hormone is produced endogenously or exogenously, it is the structure of the hormone and the way in which it is metabolized that determines its biological action. This point is critical to understanding the effects of steroid hormones.
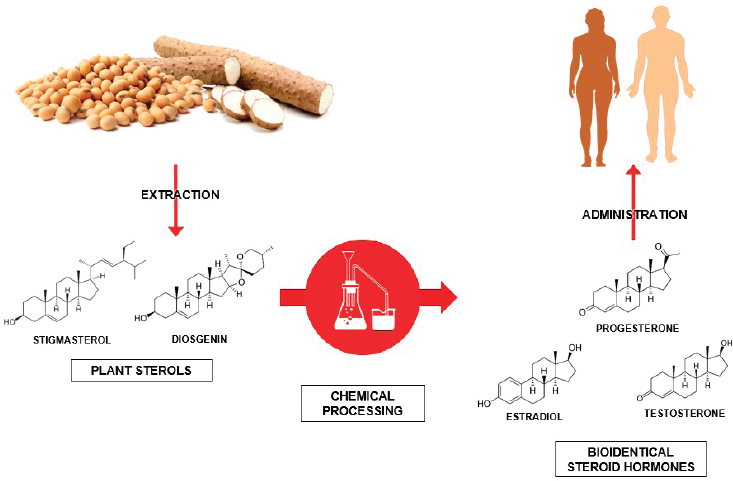
SOURCES: American Chemical Society and Sociedad Química de México, 1999; MaskaRad, 2018, Stock Illustration ID: 957444020; Popovaphoto, 2015, Stock Illustration ID: 498946090; YuanruLi, 2018, Stock Illustration ID: 916334012; Wang et al., 2011.
STRUCTURE AND BIOCHEMISTRY OF HORMONES COMMONLY FOUND IN COMPOUNDED BIOIDENTICAL HORMONE PREPARATIONS
The structure and biochemistry of steroid hormones commonly used in compounded preparations are described below.
Estrogens
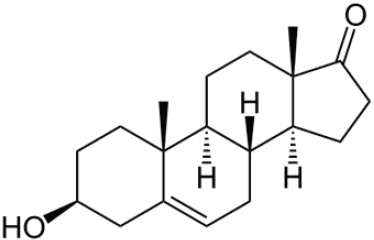
Estrogens—estrone (E1), estradiol (E2), and estriol (E3)—are the predominant female sex hormones, produced primarily in developing follicles in the ovaries. E1 can be converted to E2, E2 can be converted to E1, and within the placenta of a pregnant woman, both E1 and E2 can be converted to E3. Estrogen production increases through puberty and the reproductive years and diminishes as ovarian follicles decrease with age, ultimately falling to low levels during the menopause transition and postmenopausal period. Estrogen production can also occur in male testes and act to regulate gonadal development and spermatogenesis. See Figures 4-3, 4-4, and 4-5 for the chemical structures of estrone, estradiol, and estriol.
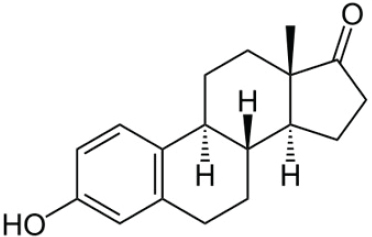
Estrone (E1)
Ovaries are the main source of E1. E1 is a minor hormone in the female reproductive cycle, though it plays a larger physiological role following menopause. Estradiol biosynthesis can occur through a reduction of E1 by 17-beta-hydroxysteroid dehydrogenase (HSD) enzymes.
Estradiol (E2)
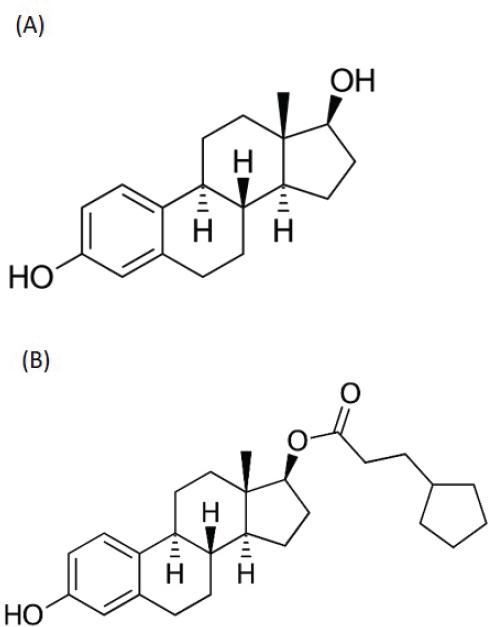
Ovaries are the main source of E2. It is also formed by aromatization of testosterone or synthesized by estrone’s reduction by 17β-hydroxysteroid dehydrogenase enzymes in tissues outside of the gonads. E2 is a potent estrogen that plays a dominant role in nonpregnant and premenopausal women’s development and the regulation of reproductive cycle. The synthesis of E2 is controlled by enzymes called aromatases, which are expressed widely throughout the body and are either activated or inhibited by other hormones or local tissue factors, adding a layer of complexity to the control of E2 production.
Estradiol cypionate is a naturally occurring hormone that is the ester form of E2. In its activated complex, it promotes the transcription of genes involved in the female reproduction system and in producing secondary sex characteristics. Estradiol cypionate can also serve as a prohormone that the body converts readily into the endogenous form, estradiol (NLM, 2020a).
Estriol (E3)
Estriol (E3) is derived from E1 and E2 and is primarily synthesized within the placenta during pregnancy. It is a far less potent estrogen than E2, and with little circulating in nonpregnant women, it serves as a minor female sex hormone (Cui et al., 2013; Keller, 1974). Though considered to be a relatively weak estrogen, E3 is often included in some of the most commonly compounded formulations of estrogens (e.g., Bi-est, Tri-est).
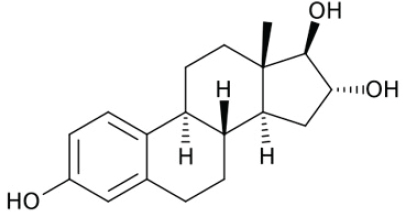
Pregnenolone
Pregnenolone (P5), is a major precursor of most steroid hormones, including the androgens, estrogens, progestogens, and adrenal steroids (see Figure 4-6). P5 is a neurosteroid, meaning that it is expressed in the brain and can act locally to acutely regulate neuronal function (Ratner et al., 2019).
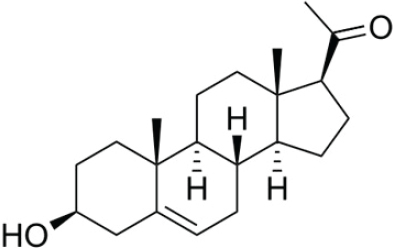
Progesterone
Progesterone, similar to pregnenolone, is a neurosteroid and precursor for steroid hormones. Progesterone’s major source of production comes from the corpus luteum cells that form after ovulation, but it is also secreted by the adrenal glands and placenta. Following menopause, progesterone levels are low. Because of its ability to prevent excessive growth of the uterine lining—a precursor to endometrial cancer—progesterone is often formulated into hormone preparations to biochemically balance the effects of estrogen therapy in postmenopausal women with an intact uterus. (See Figure 4-7 for the chemical structure of progesterone.)
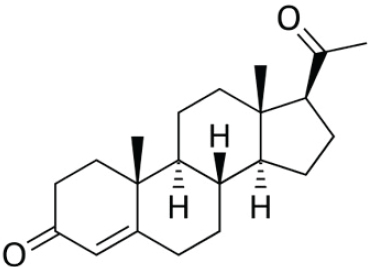
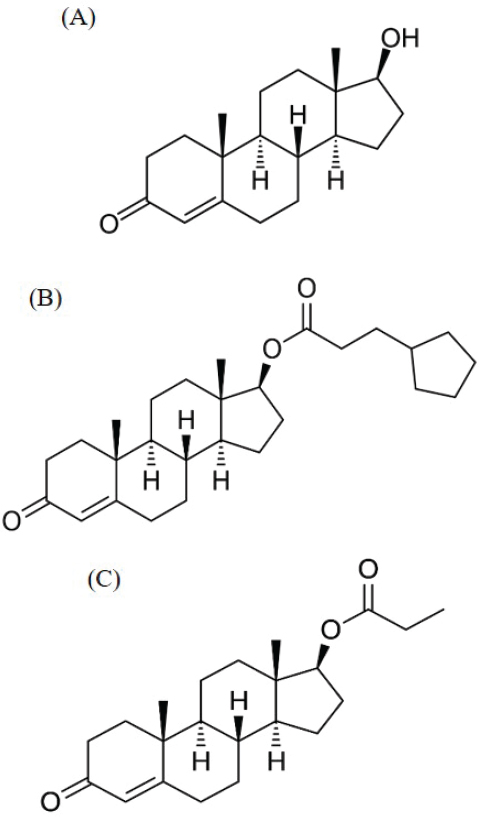
Testosterone
Testosterone, Testosterone Cypionate, and Testosterone Propionate
In men, testosterone is an androgen that is synthesized predominantly in the testis from cholesterol through the intermediate DHEA. In premenopausal women, the adrenals and ovaries are the key sources that contribute to circulating levels of plasma testosterone. In postmenopausal women, testosterone is almost exclusively derived from nonovarian tissues.
Testosterone is a potent androgen, and it plays a major role in the development of the male reproductive system and the sexual health of both men and women. Other forms of testosterone, such as testosterone cypionate and testosterone propionate, are also used in compounding. Testosterone cypionate and testosterone propionate are prohormones that the body converts to testosterone (NLM, 2020b). These two forms of testosterone may be useful in compounding because of their differing solubilities. (See Figure 4-8 for the chemical structures of testosterone, testosterone cypionate, and testosterone propionate.)
Dehydroepiandrosterone
DHEA is an endogenous hormone produced in the adrenal glands and brain. In addition to its own role as a bioactive hormone and neurosteroid, circulating DHEA serves as an important precursor for estrogens and testosterone in postmenopausal women (see Figure 4-9).
STEROID HORMONE SIGNALING
A hormone’s unique chemical structure has direct implications for its resultant biologic activity. This activity, including biological functions and physiological outcomes, results from complex signaling processes. The sections below provide a brief and limited discussion of select aspects of this complexity.
Types of Signaling Mechanisms
There are two general types of steroid hormone signaling, classical and rapid (Belfiore and LeRoith, 2018; Kampa et al., 2008; Kleine and Rossmanith, 2016; Schwartz et al., 2016). In classical mechanisms of hormone action, hormones cross through the plasma membrane and directly bind to and act through intracellular nuclear receptors to regulate transcription of thousands of genes.2 Rapid steroid hormone signaling occurs through steroid receptors residing in the cell membrane, which when activated by hormones, can initiate downstream signaling to stimulate enzymes, amplify effects of other activated receptors, or directly influence the transcription of various genes. Steroids can also activate cell signaling by binding to nonsteroid membrane receptors, such as the epidermal growth factor receptor, or by binding to ion channels, which include calcium and potassium channels.
___________________
2 Ligand-independent activation of steroid hormone receptors can also occur. Certain steroid hormone receptors can tether to other proteins/transcription factors to bind indirectly to DNA at hormone response elements to affect gene expression (Rubinow and Schmidt, 2018).
Sources of Variance in a Steroid Signal
Multiple Forms and Locations of Steroid Receptors
Steroid receptors exist as multiple isoforms—structurally varied products of the same gene. For example, there are two isoforms of the progesterone receptor for progesterone (Singhal et al., 2017); two isoforms of the androgen receptor (Liegibel et al., 2003); and two forms of the estrogen receptor—ERα and ERβ—are coded for by two separate genes, and owing to alternative splicing events, yield multiple associated isoforms (Yaşar et al., 2017). Steroid receptor isoforms, when bound to their respective steroid hormones, can produce different tissue-specific gene expression patterns and often mediate distinct functional effects. Adding to this complexity, additional diversity can be introduced through posttranslational modifications of receptor isoforms (Faus and Haendler, 2006).
Active Metabolites
The metabolism of steroids can produce other active hormone products with additional mechanisms of action. For example, progesterone is metabolized to produce the neurosteroid allopregnenolone, which acts to modulate the activity of the γ-amino butyric acid (GABA) receptor in the central nervous system. Other active metabolites of progesterone are also found in the brain and produce different effects on excitatory and inhibitory neurotransmitter receptors. In addition, because most enzymes involved in steroid hormone metabolism have been located in the brain, it is suggested that many “parent” steroid hormones (e.g., progesterone, estradiol, and DHEA) may also function as neurosteroids (Porcu et al., 2016).
Coregulators
There is substantial variability in the biological actions and outcomes of steroid hormones. This variability is best illustrated by their interactions with a broad spectrum of protein partners, known as hormone coregulators (Wang et al., 2016), to produce biological effects. For example, there are roughly 350 different coregulatory proteins—coactivators and coinhibitors—that bind to steroid receptors to determine whether genes are turned on or off (Lonard and O’Malley, 2012). Adding complexity to the situation, these coregulatory proteins often combine in groups, and the effect of each individual coregulator within that group is determined by specific chemical modifications to that protein (e.g., phosphorylation or methylation) (Lonard and O’Malley, 2007).
In addition, coregulatory proteins exist in a tissue-specific fashion and are highly context dependent. These features enable estrogen-like compounds called selective estrogen receptor modulators (SERMs) to act as an estrogen agonist (activating biological responses) in some tissues, such as in bone and the heart, and an antagonist (blocking biological responses) in others, including the uterus and breast, depending on the coregulator profile in the cells (Wardell et al., 2014).
Implications of Signaling Variance for Physiological Responses
One of the confounding factors that makes it difficult to predict how a given mixture of hormones will affect various physiological processes in the body is that the affinity of a hormone for its receptor—the strength with which it binds to that receptor—does not necessarily determine either the potency of the effect or even the direction of its action (Salahudeen and Nishtala, 2017). Further complicating the assessment of receptor affinity is the extraordinary variability of reported findings based on the source (endogenous versus exogenous) (Fuentes and Silvera, 2019) and the tissue or the cell line used to measure receptor binding (Yaşar et al., 2017). In addition, a given hormone’s binding partners, particularly the coregulators (as noted above), exist in different concentrations in different tissues, producing tissue-specific effects (Smith and O’Malley, 2004). Similarly, the exact mix of steroid hormones present in a given tissue or cell can affect the activity of the individual hormones in that tissue or cell (Uht et al., 1997). A major source of variability in hormone signaling is the genomic context, resulting in variation in the structure and activity of hormone receptors and the enzymes involved in biosynthesis of the steroid hormones themselves (Fuentes and Silvera, 2019). Therefore, the resulting differences in levels of steroid hormones and receptor actions must also be considered in determining the potential benefits and drawbacks of a particular mix of hormones (Ferrell et al., 2005).
It is also important to recognize that hormones within a given class are not going to produce similar effects on all tissues. For example, medroxyprogesterone acetate is a progestogen but has biological effects that diverge from those of progesterone (Frye, 2013; Nilsen and Brinton, 2003). These differences might translate into different effects on brain and estrogen-sensitive tissues such as breast and uterus. In a similar manner, the genomic effects of conjugated equine estrogens—hormone ingredients in FDA-approved drug products—are not identical to those of E2 (Wroolie et al., 2011). Finally, hormone levels tell only part of the story, meaning, they do not permit inferences about tissue levels based on local metabolism nor about cellular effects of those levels in a given tissue (Fuentes and Silvera, 2019; Smith and O’Malley, 2004).
In summary, the overall effects of steroids are highly context dependent, and they are affected by an individual’s genetic background, current steroid milieu, and prior exposure. The diverse and dynamic signaling produced by steroid hormones emphasizes the need for preclinical and clinical evaluation that would identify potential beneficial and adverse health effects using the specific mixture of hormones that is to be used to treat patients.
REFERENCES
American Chemical Society and Sociedad Química de México. 1999. An international historic chemical landmark: The “marker degreadation” and creation of the Mexican steroid industry 1938–1945. Washington, DC: American Chemical Society.
Belfiore, A., and D. LeRoith. 2018. Principles of Endocrinology and Hormone Action. New York: Springer.
Bhavnani, B. R., and F. Z. Stanczyk. 2012. Misconception and concerns about bioidentical hormones used for custom-compounded hormone therapy. The Journal of Clinical Endocrinology & Metabolism 97(3):756–759.
Cirigliano, M. 2007. Bioidentical hormone therapy: A review of the evidence. Journal of Women’s Health 16(5):600–631.
Cui, J., Y. Shen, and R. Li. 2013. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends in Molecular Medicine 19(3):197–209.
Faus, H., and B. Haendler. 2006. Post-translational modifications of steroid receptors. Bio-medicine and Pharmacotherapy 60(9):520–528.
Ferrell, R. J., K. A. O’Connor, G. Rodríguez, T. Gorrindo, D. J. Holman, E. Brindle, R. C. Miller, D. E. Schechter, L. Korshalla, J. A. Simon, P. K. Mansfield, J. W. Wood, and M. Weinstein. 2005. Monitoring reproductive aging in a 5-year prospective study: Aggregate and individual changes in steroid hormones and menstrual cycle lengths with age. Menopause 12(5):567–577.
Frye, C. A., C. J. Koonce, and A. A. Walf. 2013. Progesterone, compared to medroxyprogesterone acetate, to c57bl/6, but not 5α-reductase mutant, mice enhances object recognition and placement memory and is associated with higher bdnf levels in the hippocampus and cortex. Neuroscience Letters 551:53–57.
Fuentes, N., and P. Silveyra. 2019. Estrogen receptor signaling mechanisms. Advances in Protein Chemistry and Structural Biology 116:135–170.
Hiller-Sturmhöfel, S., and A. Bartke. 1998. The endocrine system: An overview. Alcohol Health and Research World 22(3):153–164.
Kampa, M., V. Pelekanou, and E. Castanas. 2008. Membrane-initiated steroid action in breast and prostate cancer. Steroids 73(9-10):953–960.
Keller, P. 1974. Pregnancy. In Clinical endocrinology: Theory and practice. 2nd ed, edited by A. Labhart. Berlin, Germany: Springer-Verlag. Pp. 667–706.
Kleine, B., and W. G. Rossmanith. 2016. Hormones and the endocrine system. 1st ed. New York: Springer.
Kuhl, H. 2005. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 8(Suppl 1):3–63.
Liegibel, U. M., U. Sommer, I. Boercsoek, U. Hilscher, A. Bierhaus, H. U. Schweikert, P. Nawroth, and C. Kasperk. 2003. Androgen receptor isoforms ar-a and ar-b display functional differences in cultured human bone cells and genital skin fibroblasts. Steroids 68(14):1179–1187.
Lonard, D. M., and B. W. O’Malley. 2007. Nuclear receptor coregulators: Judges, juries, and executioners of cellular regulation. Molecular Cell 27(5):691–700.
Lonard, D. M., and B. W. O’Malley. 2012. Nuclear receptor coregulators: Modulators of pathology and therapeutic targets. Nature Reviews Endocrinology 8(10):598–604.
Mani, S. K., P. G. Mermelstein, M. J. Tetel, and G. Anesetti. 2012. Convergence of multiple mechanisms of steroid hormone action. Hormone and Metabolic Research 44(8):569–576.
Melmed, S., K. Polonsky, P. Larsen, and H. Kronenberg. 2016. Williams textbook of endocrinology. 13th ed. Philadelphia, PA: Elsevier.
NABP (National Association of Boards of Pharmacy). 2019. Compiled list of the five most dispensed formulations from 503A compounding pharmacies and 503B outsourcing facilities from 2016 to 2018. NABP response to inquiry regarding common formulations dispensed to patients (available through the National Academies Public Access File).
Nilsen, J., and R. D. Brinton. 2003. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proceedings of the National Academy of Sciences 100(18):10506–10511.
NLM (National Library of Medicine). 2020a. Estradiol cypionate. https://pubchem.ncbi.nlm.nih.gov/compound/9403 (accessed May 15, 2020).
NLM. 2020b. Testosterone propionate. https://pubchem.ncbi.nlm.nih.gov/compound/5995 (accessed May 15, 2020).
Porcu, P., A. M. Barron, C. A. Frye, A. A. Walf, S. Y. Yang, X. Y. He, A. L. Morrow, G. C. Panzica, and R. C. Melcangi. 2016. Neurosteroidogenesis today: Novel targets for neuroactive steroid synthesis and action and their relevance for translational research. Journal of Neuroendocrinology 28(2).
Ratner, M. H., V. Kumaresan, and D. H. Farb. 2019. Neurosteroid actions in memory and neurologic/neuropsychiatric disorders. Frontiers in Endocrinology 10(169).
Rubinow, D. R., and P. J. Schmidt. 2018. Is there a role for reproductive steroids in the etiology and treatment of affective disorders? Dialogues in Clinical Neuroscience 20(3):187–196.
Rubinow, K. B. 2018. An intracrine view of sex steroids, immunity, and metabolic regulation. Molecular Metabolism 15:92–103.
Salahudeen, M. S., and P. S. Nishtala. 2017. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharmaceutical Journal 25(2):165–175.
Schiffer, L., L. Barnard, E. S. Baranowski, L. C. Gilligan, A. E. Taylor, W. Arlt, C. H. L. Shackleton, and K.-H. Storbeck. 2019. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. Journal of Steroid Biochemistry and Molecular Biology 194:105439.
Schwartz, N., A. Verma, C. B. Bivens, Z. Schwartz, and B. D. Boyan. 2016. Rapid steroid hormone actions via membrane receptors. Biochimica Biophysica Acta 1863(9):2289–2298.
Singhal, H., M. E. Greene, A. L. Zarnke, M. Laine, R. Al Abosy, Y.-F. Chang, A. G. Dembo, K. Schoenfelt, R. Vadhi, X. Qiu, P. Rao, B. Santhamma, H. B. Nair, K. J. Nickisch, H. W. Long, L. Becker, M. Brown, and G. L. Greene. 2017. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget 9(4):4282–4300.
Smith, C. L., and B. W. O’Malley. 2004. Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocrinology Review 25(1):45–71.
Uht, R. M., C. M. Anderson, P. Webb, and P. J. Kushner. 1997. Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the ap-1 response element. Endocrinology 138(7):2900–2908.
Wang, F.-Q., K. Yao, and D. Wei. 2011. From soybean phytosterols to steroid hormones. In Soybean and health, edited by H. El-Shemy. Rijeka, Croatia: Intech Language.
Wang, L., D. M. Lonard, and B. W. O’Malley. 2016. The role of steroid receptor coactivators in hormone dependent cancers and their potential as therapeutic targets. Hormones and Cancer 7(4):229–235.
Wardell, S. E., E. R. Nelson, and D. P. McDonnell. 2014. From empirical to mechanism-based discovery of clinically useful selective estrogen receptor modulators (SERMs). Steroids 90:30–38.
Wroolie, T. E., H. A. Kenna, K. E. Williams, B. N. Powers, M. Holcomb, A. Khaylis, and N. L. Rasgon. 2011. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17β-estradiol versus conjugated equine estrogens. American Journal of Geriatric Psychiatry 19(9):792–802.
Yaşar, P., G. Ayaz, S. D. User, G. Güpür, and M. Muyan. 2017. Molecular mechanism of estrogen-estrogen receptor signaling. Reproductive Medicine and Biology 16(1):4–20.
This page intentionally left blank.
















