1
Introduction1
On November 18 and 19, 2019, the National Academies of Sciences, Engineering, and Medicine (the National Academies) hosted a public workshop in Washington, DC, titled Sharing Clinical Trial Data: Challenges and a Way Forward. The workshop followed the release of the 2015 Institute of Medicine (IOM) consensus study report Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk, and was designed to examine the current state of clinical trial data sharing and reuse and to consider ways in which policy, technology, incentives, and governance could be leveraged to further encourage and enhance data sharing. The workshop planning committee responded to a Statement of Task (see Box 1-1) to develop the workshop agenda and identify speakers to invite.2 The workshop was sponsored and convened jointly by the Forum on Drug Discovery, Development, and Translation; the Forum on Neuroscience and Nervous System Disorders; the National Cancer Policy Forum; and the Roundtable on Genomics and Precision Health, with additional support from the Wellcome Trust.
___________________
1 This workshop was organized by an independent planning committee whose role was limited to identification of topics and speakers. This Proceedings of a Workshop was prepared by the rapporteurs as a factual summary of the presentations and discussion that took place at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants and are not endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
2 The workshop agenda can be found in Appendix B. Archived webcast videos and speakers’ presentations are available on the National Academies website. See http://nationalacademies.org/hmd/Activities/Research/DrugForum/2019-Nov-18.aspx (accessed February 10, 2020).
This workshop also builds on a body of related activities, including the IOM workshop summaries Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020 (IOM, 2012a) and Sharing Clinical Research Data (IOM, 2013) and other National Academies publications discussing the sharing and reuse of clinical trial data.3
___________________
3 A collection of National Academies publications on clinical trial data sharing is available at https://www.nap.edu/collection/90/clinical-trial-data-sharing (accessed March 2, 2020).
REVISITING THE 2015 INSTITUTE OF MEDICINE CONSENSUS STUDY REPORT
SHARING CLINICAL TRIAL DATA: MAXIMIZING BENEFITS, MINIMIZING RISK
Joanne Waldstreicher, chief medical officer at Johnson & Johnson and co-chair of the workshop, highlighted that the 2015 study outlined guiding principles for the sharing of individual participant data (IPD) from clinical trials and issued recommendations regarding the roles and responsibilities of key stakeholders, the timeframe in which data should be shared, and considerations for access to and governance of shared clinical trial data (IOM, 2015). The 2015 IOM study did not address the sharing of real-world data, such as the information contained in patient electronic health records or administrative data from health insurance claims, she added. Jeffrey M. Drazen, New England Journal of Medicine group editor and co-chair of the workshop, clarified that the 2015 study focused on data from clinical trials for two reasons: (1) data obtained from clinical trials are of high quality, and (2) individuals put themselves at risk and relinquish control of aspects of their clinical care when they participate in a clinical study. He emphasized the difficulty of enrolling participants who satisfy inclusion criteria for the study, and who are available and willing to participate.
Bernard Lo, president and chief executive officer of The Greenwall Foundation and chair of the 2015 IOM study, provided a brief overview of the report’s recommendations (see Box 1-2 for the full text). The first recommendation called for establishing a culture of data sharing, which could include, Lo summarized, considering data sharing for academic promotion and requiring data-sharing provisions in research grant proposals. The second recommendation addressed the types of clinical trial data to be shared and the timeline for sharing. The third recommendation provided guidance on operational strategies to facilitate clinical trial data sharing, such as the needs for independent review panels to assess requests for access to data and transparency surrounding the availability and accessibility of clinical trial data. The fourth recommendation called for an ongoing, multi-stakeholder process to address the structural and logistical elements of data sharing.
PROGRESS MADE IN CLINICAL TRIAL DATA SHARING SINCE THE 2015 INSTITUTE OF MEDICINE CONSENSUS STUDY REPORT
To reflect on progress made in clinical trial data sharing since the release of the 2015 IOM consensus study report Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk, Drazen said that data collected during randomized controlled clinical trials were originally considered to
be the property of those who conducted the study. As data science and technology have evolved to better enable researchers to analyze large volumes of data, discussions regarding data ownership and the need for data sharing have continued to advance. Today, Drazen said, the focus is on finding ways to share clinical trial data for the benefit of key stakeholder groups: the clinical trial participants who put themselves at risk, the skilled clinical trialists who gather the high-quality data, and the data scientists who mine and analyze the data for new insights. The task, he said, is to define the value of shared data and align incentives for sharing for the benefit of human health.
“Spirited disagreement” persists among data generators and data users regarding how, when, and whether to share clinical trial data, Lo said. Data generators (i.e., clinical trialists), for example, expend great effort in planning and executing a trial and might believe that they are due long-term usage rights. On the other hand, Lo continued, data users
are eager to use clinical trial data for secondary analyses to answer current questions. The challenge, he emphasized, is to arrive at data-sharing terms that recognize both perspectives and meet the ultimate goal of providing benefit to patients.
Lo suggested that debates about data sharing can be evidence driven and that metrics are needed for measuring benefits and values. An objective of data sharing is to generate and operationalize important new knowledge, he added. However, it is unclear whether secondary analyses of clinical trial data do, in fact, generate new knowledge. Therefore, metrics that reflect whether sharing of clinical trial data leads to improved health and clinical outcomes could be considered. But there is still a question as to whether all clinical trials should have equal priority for data sharing, he continued. Some trials might provide greater value or benefit from data sharing than others. For example, sharing data from a large-scale, multi-center, pivotal clinical trial that was designed to impact clinical practice could be of high value. There are also opportunity costs that should be recognized and quantified, Lo concluded (e.g., external parties may beat investigators to conducting planned secondary analyses as a result of trial data being simultaneously released with publication, and may be a disincentive in clinical research).
Sharing Individual Participant Data
Deborah Zarin, director of the Program for Advancement of the Clinical Trial Enterprise at Brigham and Women’s Hospital and Harvard, illustrated the current clinical Trial Reporting System (TRS) conceptually as a pyramid (see Figure 1-1). The foundation of a TRS is the prospective registration of trials in a public database (e.g., ClinicalTrials.gov) that provides a searchable record of ongoing and completed clinical trials. Registry entries include key protocol details, which enable users to search for trials in a research area of interest and to track the progress of a study. The next level includes summary results reporting, in which a minimum set of aggregate data from trials is reported in a structured and searchable format in a trial registry, with links to peer-reviewed journal articles. At the top of the pyramid is the sharing of IPD.
Zarin noted the need for clarity regarding what is meant by “data” and “sharing” when discussing IPD sharing. The value of IPD varies as it is transformed from raw data (e.g., case report forms) to aggregated and coded datasets. The type, quality, and usability of the metadata (e.g., definition of variables) are also important. Sharing is generally considered to be the process of making the data available for independent use by another research team, Zarin said. Zarin continued that opinions vary, however, regarding the extent to which the original research team
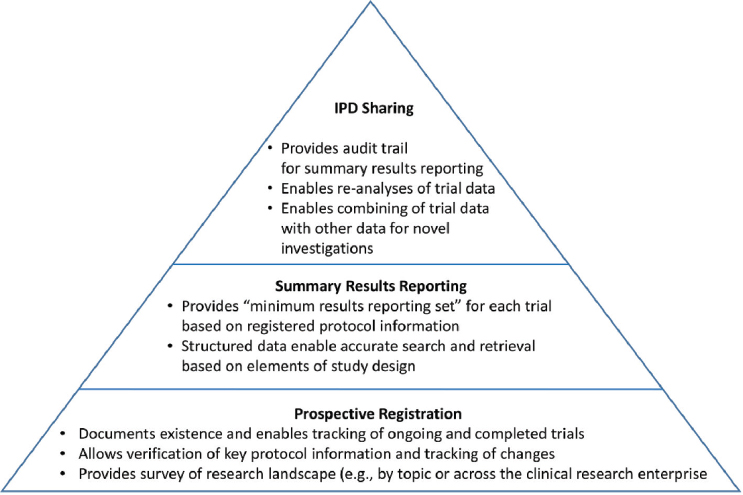
NOTE: IPD = individual participant data.
SOURCES: As presented by Deborah Zarin, November 18, 2019; Zarin and Tse, 2016.
that collected the data should remain involved. The value of data can be impacted by whether data sharing is active (i.e., proactively made available) or latent (i.e., whether the data generator expects that others will ever look at their data). It is human nature, she said, to behave differently when someone might be observing. For example, Zarin explained, the pharmaceutical industry expects that all clinical data it generates will be reviewed by regulatory authorities, and data are collected, documented, and analyzed accordingly. In contrast, researchers in academia generally do not expect that level of external review of their data. Given that all data might not be of equal priority for sharing, Zarin suggested it may be useful to think about what balance of sharing (active versus latent) would be appropriate. One question she posed for consideration is whether making all trials at risk for audit would enhance value.
To put IPD sharing in the TRS in context, Zarin shared recent statistics from the ClinicalTrials.gov database. Most clinical trials appear to be registered, she said, although about one-third of these trials were registered late (more than 3 months after the start of the trial). Zarin stated that ideally, every trial that is registered should have summary results reported on ClinicalTrials.gov. However, not all registered trials are con-
ducted or completed, so the number of registered trials and the number of summary results reported are not expected to be equal, she explained. Currently, an estimate of only about 50 percent of trials report summary results (directly in the registry or via peer-reviewed publication). Reporting varies by sector and is more common for trials subject to regulation. Few trials registered in ClinicalTrials.gov have links to IPD, Zarin said. For example, out of 4,700 clinical trials completed in 2016 (with at least one U.S. site), only 5 have links to IPD; and only 2 out of about 2,000 drug efficacy studies (Phase 2 and beyond) have links to IPD. In fact, only 653 studies had links to IPD out of 155,000 completed studies in ClinicalTrials.gov, Zarin noted. The International Committee of Medical Journal Editors (ICMJE) instituted a policy that, starting in 2019, all trials must provide a data-sharing plan registering to be considered for publication in an ICMJE journal.4 Looking at the 7,309 clinical trials launched in 2019 (registered in ClinicalTrials.gov with a site in the United States), Zarin found that 1,120 (15 percent) have declared the intention to share IPD (3,749 declared they would not share IPD, and 2,440 were undecided or did not respond).
In summary, Zarin said that about half of the trials registered in ClinicalTrials.gov report summary results, and a very small fraction report IPD. She emphasized that, although IPD sharing is important, trial registration and summary results reporting form the foundation of the evidence base. In addition, failure to share IPD via a searchable system that is linked to trial registration and summary results can lead to reporting bias. She prompted workshop participants to consider what the goals of IPD sharing should be. Is a low burden of posting and using IPD desirable? Should there be a target percentage of trials with IPD available, and if so, what would the target be? Should all trials be at risk of audit? Is the ultimate goal of sharing IPD to facilitate high-impact discoveries? Finally, what indicators might suggest that a change of strategy for sharing of IPD is warranted?
ORGANIZATION OF THE WORKSHOP AND PROCEEDINGS
This Proceedings of a Workshop summarizes the presentations and discussions that took place at the workshop. Following the introductory remarks (Chapter 1), the first three workshop panels considered the current landscape for clinical trial data sharing and reuse. Panelists shared their perspectives on current data-sharing policies in practice (Chapter 2), described case examples of data-sharing platforms (Chapter 3), and discussed balancing the value and benefits of sharing data with the risks
___________________
4 See http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html#two (accessed February 10, 2020).
and costs (Chapter 4). The next three panels considered some of the key challenges to clinical trial data sharing and reuse. Panelists discussed data interoperability and platform usability (Chapter 5) and infrastructure sustainability (Chapter 6) and examined challenges and disincentives for sharing in the context of statistical replication (Chapter 7). The final two panels of the workshop focused on moving data sharing forward. Panelists explored opportunities for overcoming the technical and sustainability challenges discussed (Chapter 8) and considered steps to incentivize and promote data sharing and reuse (Chapter 9).








