3
One-Carbon Metabolism Micronutrients
Micronutrients involved in one-carbon metabolism1 are critical to both maternal health and fetal development. Deficiencies of these nutrients have been linked to adverse pregnancy outcomes such as congenital birth defects, fetal growth disorders, and preterm birth. Over the past several decades, intake of one-carbon metabolism nutrients has changed. Folic acid fortification, for instance, makes the nutrient more pervasive in the food supply. While intake of certain one-carbon metabolism nutrients (e.g., synthetic folic acid) may be high in some subpopulation groups, intake of others (e.g., vitamin B12, choline) may be inadequate. The second session of the workshop, moderated by Tamera Hatfield, a maternal–fetal medicine specialist on faculty at the University of California, Irvine, reviewed evidence that has emerged on the roles and need for folate, folic acid, vitamin B12, and choline during pregnancy and lactation, and explored the potential misalignment of current recommendations with current intakes. Highlights from the session presentations are presented in Box 3-1.
DISCREPANCIES IN FOLATE AND VITAMIN B12 STATUS OF PREGNANT WOMEN ACROSS POPULATION GROUPS AND THE POSSIBLE IMPLICATIONS FOR CHILD OUTCOMES
In her remarks, Yvonne Lamers, associate professor in the Food, Nutrition and Health program at The University of British Columbia, provided
___________________
1 One-carbon metabolism encompasses interrelated biochemical reactions that involve the transfer of 1-carbon units (methyl groups). These reactions play key roles in various physiological processes.
an overview of the evidence that has emerged over the past several decades related to the roles and intake of folate, folic acid, and vitamin B12 during pregnancy. In addition to discussing the use of supplements and the status of pregnant women related to these nutrients, Lamers also identified open questions and knowledge gaps that currently exist.
Folic Acid Supplementation and Fortification
One of the biggest milestones in maternal nutrition that has taken place over the past 30 years is the recognition that periconceptional folic acid can prevent neural tube defects, said Lamers. First released in the early 1990s, recommendations for preconceptual folic acid supplementation were driven by results from two randomized controlled trials that found supplementing low-risk women reduced the incidence of neural tube defects by at least
90 percent (Berry et al., 1999; Czeizel, 2009). Current guidelines generally recommend that women of reproductive age who have a low risk of giving birth to a baby with neural tube defects take a 400 μg/day folic acid supplement at least 1 month prior to conception through 12 weeks gestation. However, there are some variations in recommendations internationally, noted Lamers. For instance, the New Zealand Ministry of Health recommends an 800 μg/day dose of folic acid and The Society of Obstetricians and Gynaecologists of Canada recommends that supplementation continues at least until 1 month postpartum or until the end of breastfeeding.
Despite recommendations, the prevalence of folic acid supplement use tends to be low. Estimates of preconceptual folic acid supplementation use in the United States and Canada range from 14 to 60 percent (Bailey et al., 2019; Chalmers et al., 2008; Masih et al., 2015) and 12 to 20 percent in Europe (McNulty et al., 2011; Nilsen et al., 2006). Women with planned pregnancies are more likely to be using folic acid supplements at preconception (Masih et al., 2015; Nilsen et al., 2006); however, approximately 45 percent of pregnancies in the United States are unplanned (Finer and Zolna, 2016), observed Lamers.
Folic acid fortification is a population-based strategy used to prevent neural tube defects. Mandatory folic acid fortification of foods (e.g., wheat flour, maize flour, rice) has been implemented in 71 countries. Mandatory folic acid fortification in the United States and Canada has been estimated to decrease neural tube defects by 31 and 46 percent, respectively (De Wals et al., 2007; Williams et al., 2002). Lamers commented that some countries—including New Zealand, the United Kingdom, and other European countries—have not adopted mandatory folic acid fortification programs owing to safety concerns.
Folate Status and Intake of Pregnant Women
Red blood cell (RBC) folate concentration is used to monitor folic acid supplementation programs. The World Health Organization (WHO) uses an RBC folate concentration of 906 nmol/L as a threshold for sufficiency and optimal neural tube defect prevention (Cordero et al., 2015). Approximately 20 percent of women in both the United States (Pfeiffer et al., 2019) and Canada (Colapinto et al., 2011) have RBC folate concentrations below this threshold, stated Lamers. She showed that although there are no differences by age groups, the prevalence of RBC folate concentrations falling below the WHO threshold tends to be higher among African American women and women who do not use supplements (Pfeiffer et al., 2019).
Dietary folate and folic acid data reveal both inadequate and possibly excessive intake in the population. Describing a recent analysis of National Health and Nutrition Examination Survey (NHANES) data (Bailey et al.,
2019), Lamers noted that 16 percent of all pregnant women in the United States have a total dietary folate intake below the Estimated Average Requirement (EAR), indicative of inadequacy. This finding suggests that, despite the folic acid fortification efforts in the United States, supplements are still needed. However, the NHANES analysis also revealed that 48 percent of pregnant supplement users exceed the Tolerable Upper Intake Level (UL) for folic acid of 1,000 μg/day. Excessive intake is attributed to supplement use rather than fortified food sources, said Lamers. Explaining that similar findings have been reported in Canada, she remarked that prenatal supplements often contain 800–1,000 μg folic acid and that Canadian women are encouraged to continue supplementation throughout the duration of their pregnancy. Lamers pointed to a recent workshop (Lamers et al., 2018) that explored the current state of inadequate and excessive folic acid intake as an impetus to align folic acid content in prenatal supplements to the current recommendations of 400 μg/day folic acid.
Birth and Child Developmental Outcomes Related to Prenatal Folic Acid Intake
Studies have investigated relationships between prenatal folic acid intake and a variety of birth and developmental outcomes. There appears to be no association between folic acid supplementation and birth weight, risk of preterm birth, or stillbirths and neonatal deaths (Lassi et al., 2013). Lamers explained that there is no association between folic acid intake in the first trimester and risk of asthma in children, but the evidence for a relationship in the second and third trimesters is conflicting (Crider et al., 2013). There are inconsistent relationships with risk of autism, obesity, and insulin resistance in the offspring (Gao et al., 2016; Xie et al., 2016).
In exploring the relationships between outcomes and high folic acid intake, Lamers cautioned that there are important limitations to consider. She noted that relationships reported in the literature are associations and face issues of biases and confounding. Furthermore, there is no standard definition for what constitutes high or excessive folic acid intake, and the available evidence does not lend itself to determining dose–response relationships. Lamers described the existing evidence on these relationships as inconsistent and equivocal. She put forth the concept of the precautionary principle in the face of scientific uncertainty, which she captured by saying that “400 micrograms was shown to be effective in lowering neural tube defects, so why more?”
It is biologically plausible that folate, as a key methyl donor, leads to epigenetic alterations (e.g., DNA methylation, histone modification) and changes to gene expression, suggested Lamers. She continued, stating that
given the Developmental Origins of Health and Disease (DOHaD) theory, it is possible that folate plays a role in disease outcomes.
In addition to its role in the closure of the neural tube in the first weeks of pregnancy, folate is a key nutrient for brain function. Observational studies have reported either positive or no associations between maternal folate status in early or late pregnancy and child behavioral outcomes, reported Lamers. Two randomized trials have been conducted to further explore this relationship. A multicenter, randomized controlled trial used a two-by-two factorial design to assess the effects of 400 μg/day of natural folate called 5-methyltetrahydrofolate (5-MTHF), fish oil, and placebo during the second half of pregnancy. Children of women who received the 5-MTHF supplement showed higher executive function at 8.5 years of age (Catena et al., 2016). Another trial, conducted in Northern Ireland, randomized 119 women to receive either 400 μg/day folic acid or placebo during the second and third trimesters of their pregnancies. At 7 years of age, children of the women who received the folic acid supplement had improved developmental outcomes (McNulty et al., 2019). Investigators also found significantly lower DNA methylation of genes related to brain development in the cord blood of neonates born to women who continued folic acid supplementation in their second and third trimesters (Caffrey et al., 2018). “Whether those epigenetic alterations are related to the brain outcome status has not been looked at yet. These data have just been recently released,” advised Lamers.
Research Needs Related to Folate and Folic Acid
Lamers highlighted three key questions that have emerged since the release of the periconceptional folic acid supplementation recommendations in the early 1990s. First, she stated that a need exists to identify women who are at risk for insufficient folate intake. For some women, the barrier may be a matter of access. As evidenced by the 20 percent of reproductive-aged women with RBC folate below the WHO threshold for sufficiency, which was independent of socioeconomic status, another barrier may be knowledge transfer, suggested Lamers. She also thought that changes in dietary patterns in the population (e.g., promotion of plant-based diets and avoidance of gluten-containing foods) merit monitoring of folic acid and folate intake. Second, questions have been raised as to whether more folate or folic acid is needed among women who have the methylenetetrahydrofolate reductase (MTHFR) 677C>T genetic variant. Third, Lamers emphasized the need for trimester-specific, dose–response studies to assess the effects of prolonged folic acid supplementation.
Vitamin B12 Needs and Status During Pregnancy
Lamers showed the Dietary Reference Intake values that have been established for adequate vitamin B12 intake among women. For women of reproductive age, the EAR and Recommended Dietary Allowance (RDA) are set at 2.0 and 2.4 μg/day, respectively, based on hematologic indicators. The values for pregnant women increase to 2.2 μg/day for the EAR and 2.6 μg/day for the RDA, to account for fetal deposition and increases in absorption, and further increase to 2.3 and 2.8 μg/day, respectively, for lactating women.
Vitamin B12 is found in animal sources and fortified foods, noted Lamers. Given this, there are various population groups at risk for inadequate B12 intake, including certain ethnic groups and those who follow dietary patterns that limit or exclude animal sources (e.g., vegetarian, vegan). Studies of vitamin B12 intake using dietary data indicate that women across trimesters of pregnancy are consuming above the levels recommended for adequacy (Blumfield et al., 2013), with median intake estimated to be approximately 5 μg/day in supplemented women (Wu et al., 2013).
Lamers contrasted the vitamin B12 dietary intake findings with biomarker data. Over the course of pregnancy, total B12 concentrations decrease (Schroder et al., 2017; Visentin et al., 2016), likely owing to hemodilution and other pregnancy-related physiologic changes, said Lamers. She suggested recently derived pregnancy-specific cutoffs for vitamin B12 biomarkers (Schroder et al., 2019) should be validated. To validate such work, the relationship between low vitamin B12 status and a host of perinatal outcomes has been evaluated (e.g., low birth weight, preterm birth, small-for-gestational age). From this work, it has been estimated that 34 percent of neural tube defects in Canada may be related to low vitamin B12 status, reported Lamers.
Adequate intake of vitamin B12 during pregnancy may have implications not only for birth outcomes but also for the infant’s vitamin B12 status. Fetal vitamin B12 stores develop in utero. Evidence suggests that at 12 months of age, an infant’s vitamin B12 status is associated with maternal serum vitamin B12 status during pregnancy rather than breast milk concentrations (Deegan et al., 2012).
Research Needs Related to Vitamin B12
Lamers listed some key knowledge gaps related to vitamin B12. Recognizing low vitamin B12 status is an independent risk factor for neural tube defect, she suggested vitamin B12 trials using different doses were needed to determine a dose–response relationship. Noting that the dietary intake data suggest many pregnant women meet or exceed existing intake recommendations, she emphasized the need to identify women at risk of inadequate
intakes and questioned whether the current recommendations are too low. Finally, she thought that additional research is needed to explore the change in vitamin B12 biomarker concentrations over the course of pregnancy and its relationships to functional outcomes.
THE GROWING SCIENCE ON THE BENEFITS OF CHOLINE FOR MOTHERS AND INFANTS
Marie Caudill, professor in the Division of Nutritional Sciences at Cornell University, described the role of choline in fetal development. Caudill also provided an overview of the changes to choline metabolism during pregnancy, reviewed evidence on the relationship between choline intake during pregnancy and a range of outcomes, presented on choline intake levels among pregnant women in the United States, and discussed postnatal demands for choline.
Role of Choline in Fetal Development
Large amounts of choline are needed for fetal growth. Phosphatidylcholine, a derivative of choline, is important for cell membranes and cell division. It is also needed for the synthesis of very low-density lipoproteins necessary for lipid transport from the maternal liver. Other choline derivatives play key roles in the development of the central nervous system. For instance, the developing hippocampus requires acetylcholine and the developing myelin sheath uses the choline derivative sphingomyelin. Choline is also needed for cellular methylation reactions. There is a wide range of these types of reactions, including DNA methylation (an epigenetic modification) using the choline derivative betaine. Epigenetic modification of the fetal and placental genomes “can influence gene expression, protein synthesis, cellular function, and ultimately have lasting effects on the health of the offspring,” said Caudill.
Choline Metabolism and Needs During Pregnancy
Phosphatidylcholine and free choline are the two main forms of dietary choline. In presenting Figure 3-1, Caudill described the different pathways for these compounds. Both forms of dietary choline can enter circulation and be provided to the fetus. Additionally, free choline in the maternal liver will be converted to phosphatidylcholine by one of two pathways:
- Most free choline will go through the cytidine diphosphate–choline pathway (“CDP–choline pathway”). The resulting phosphatidylcholine is enriched with a shorter-chain saturated fatty acid.
- Some of the free choline will be routed through the phosphatidylethanolamine N-methyltransferase pathway (“PEMT pathway”), where the choline-derived methyl groups are used to methylate phosphatidylethanolamine. The resulting phosphatidylcholine will be enriched with long-chain polyunsaturated fatty acids, like docosahexaenoic acid (DHA). Tracer studies conducted in pregnant women show that phosphatidylcholine molecules produced by the PEMT pathway progressively increase from maternal circulation to the placenta to cord blood. “What this suggests is that there is a unique requirement for PEMT-phosphatidylcholine by the developing fetus, and it is mostly likely related to its enrichment in DHA and other long-chain unsaturated fatty acids,” offered Caudill.
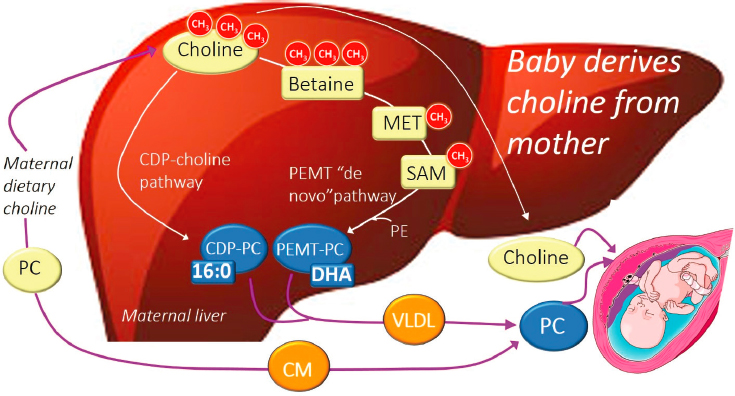
NOTE: 16:0 = palmitic acid; CDP = cytidine diphosphate; CH3 = methyl group; CM = chylomicron; DHA = docosahexaenoic acid; MET = methionine; PC = phosphatidylcholine; PE = phosphatidylethanolamine; PEMT = phosphatidylethanolamine N-methyltransferase; SAM = S-adenosylmethionine; VLDL = very low-density lipoprotein.
SOURCES: Presented by Marie Caudill. Reprinted from Korsmo et al., 2019, used under CC BY 4.0.
Circulating choline-derived methyl donors are depleted during pregnancy. Although folate can serve as a methyl donor, high folate intake during pregnancy cannot compensate for the decreases in choline-derived methyl donors, stated Caudill. Tracer isotope studies have revealed two reasons for this depletion. First, pregnant women spare free choline for
the CDP-choline pathway, rather than converting it to betaine. Second, pregnant women use more of the betaine and dimethylglycine (both choline-derived methyl donors) that are produced for cellular methylation and other one-carbon reactions. Conversely, concentrations of circulating choline and phosphatidylcholine increase during pregnancy. Pregnant women upregulate both the CDP-choline and PEMT pathways to generate more phosphatidylcholine than nonpregnant women and convert more of the endogenously made phosphatidylcholine back to choline for transfer to the fetus. Infants are born with choline concentrations three to five times that of their mothers, noted Caudill.
Evidence from studies of nonpregnant women of reproductive age indicates that choline and DHA have a synergistic relationship. As choline intake increases, the PEMT-choline pathway is upregulated and hepatic DHA export increases (West et al., 2013). Caudill mentioned that determining if this upregulation can increase delivery of DHA to the fetus is a current area of investigation.
Effects of Higher Choline Intake During Pregnancy
Relationships between high maternal choline intake and various metabolic responses and epigenetic measures have been investigated, observed Caudill. Higher choline intake during pregnancy has been found to increase circulating betaine and dimethylglycine concentrations (Yan et al., 2012, 2013) and enhance placental DNA methylation (Jiang et al., 2012). Some of the metabolic disturbances stemming from variants in choline- and folate-metabolizing genes can also be overcome by higher choline intake during pregnancy (Ganz et al., 2016, 2017).
Pregnancy and infant outcomes have also been assessed in relation to high choline intake. One study reported that women who consumed more than 498 mg/day of choline had a 50 percent reduction in neural tube defects as compared to women consuming less than 290 mg/day of choline, independent of total folate intake (Shaw et al., 2004). In another study, plasma cortisol concentrations were 33 percent lower among infants whose mothers consumed 930 mg/day of choline, as compared to infants whose mothers consumed 480 mg/day of choline (Jiang et al., 2012). Caudill explained that the reduction in infant stress response may be mediated through choline exerting epigenetic modifications; reductions in stress reactivity, in turn, may have long-term implications, reducing risk for certain chronic disease (e.g., depression, diabetes, hypertension, obesity) and improving learning outcomes (e.g., attention, learning, memory). High choline intake during pregnancy may also reduce the expression of the preeclampsia risk factor called soluble fms-like tyrosine kinase 1 (sFlt-1) (Jiang et al., 2013).
Investigators have also explored the relationship between maternal choline intake and offspring’s cognitive function. In rodent studies, offspring whose mothers were supplemented in the prenatal period had significantly fewer errors on a 12-arm radial maze over the course of their life span and did not experience age-related increases in errors over time (Meck and Williams, 2003). High maternal choline intake in rodent models have also been shown to be protective against a range of neural insults in the offspring (e.g., Alzheimer’s disease, autism, Down syndrome, early-life iron deficiency, fetal alcohol syndrome) (Korsmo et al., 2019).
Caudill characterized the evidence in humans as mixed, but noted that there is a growing body of evidence to suggest that higher maternal choline intake during pregnancy may improve certain aspects of children’s cognitive function. For instance, infants of mothers who consumed 930 mg/day of choline throughout the third trimester appear to have faster information processing speeds at 1 year of age, as compared to infants whose mothers consumed 480 mg/day of choline (Caudill et al., 2018). Preliminary evidence suggests differences between these two groups persist; at 7 years of age, children whose mothers were supplemented had superior attention, visual memory, and problem solving (Bahnfleth et al., 2019). Acknowledging the small sample size in this study, Caudill added that these findings are consistent with what has been reported in animal studies, a large prospective cohort study, and other randomized controlled trials.
Choline Intake Levels
Only 8 percent of pregnant women have intakes that meet the choline Adequate Intake (AI) of 450 mg/day. Rather, average choline intake is approximately 322 mg/day, and it is lower among those consuming a vegetarian diet, as predominant sources of choline are animal sources. There are common genetic variants that can interfere with either folate or choline metabolism, which can further increase a woman’s gap between intake and need, reported Caudill.
Most of the leading prenatal vitamins do not contain choline, or if they do, it is in small amounts (25–50 mg). Caudill stated that companies are increasing the amount choline in prenatal vitamins, a change that is supported by the American Medical Association. The American Academy of Pediatrics has recently called on health care providers to ensure adequate choline is consumed during the first 1,000 days to support neurodevelopment.2
___________________
2 The first 1,000 days refers to the period from conception through a child’s second birthday.
Postnatal Demands for Choline
Infants have high demands for choline in the postnatal period, noted Caudill. Circulating choline concentrations are elevated in the first year of life. There is evidence to suggest concentrations above 14 μmol/L facilitate the transfer of choline across the blood–brain barrier. Lactating women have higher circulating choline concentrations than women who are not breastfeeding, which is thought to facilitate transfer of choline to breast milk (Ilcol et al., 2005). Building on this, Caudill showed that breast milk choline concentrations are approximately 15 times higher than circulating concentrations in the maternal blood (Ilcol et al., 2005). High maternal choline intake can increase the choline content of breast milk (Davenport et al., 2015).
DISCUSSION
After Caudill’s presentation, she and Lamers responded to questions from the audience. Topics explored included clinical considerations related to choline, recommendations for choline intake and supplementation, dose and formulation of folic acid supplementation, and considerations of folic acid in the food supply.
Clinical Considerations Related to Choline
Hatfield opened the discussion by admitting that choline was not a nutrient she assesses in her obstetric patients and wondered if choline should be included in routine prenatal labs. Agreeing that choline is an underappreciated essential nutrient, Caudill explained that there is no good biomarker for choline status. She suggested that knowing whether a woman consumes foods from animal sources could serve as a proxy. Following up on this comment, Emily Oken of the Harvard Medical School wanted to know which foods in particular contain choline. Identifying egg yolks as a good source, Caudill noted that animal flesh foods provide approximately 100 mg/serving whereas cruciferous vegetables and legumes provide approximately 30–40 mg/serving. Oken also asked if conditions that affect the liver, such as fatty liver disorder, affect choline metabolism. Caudill indicated that it is possible, but that no studies have been conducted that show a higher need in this clinical population.
Recommendations for Choline Intake and Supplementation
Jessi Silverman of the Center for Science in the Public Interest wondered if the choline AI was appropriate. Caudill suggested that if optimization
of specific endpoints guides the selection of an AI, then there is evidence that 930 mg/day of choline is better than 480 mg/day, suggesting the current AI is too low. With the majority of pregnant women having a choline intake below the AI, raising the intake recommendation would elevate the importance of supplementation, Caudill offered.
Given that prenatal formulations are including DHA, Johanna Dwyer of the National Institutes of Health’s Office of Dietary Supplements asked what dose of choline should be used in prenatal supplements. Noting that the recommended intake of choline is in milligrams per day, whereas other nutrients are in micrograms per day, Caudill explained that space ultimately limits the amount of choline that can be included in a prenatal formulation. She mentioned that some companies are now looking to package choline with DHA in a single capsule. Caudill suggested that the dose of choline for a once-per-day capsule would likely need to be around 350–500 mg. She went on to describe concerns about excessive choline intake. Set at 3,500 mg/day, the choline UL is based on evidence that unabsorbed choline can be metabolized by colonic bacteria, producing a fishy body odor. She further noted that concerns have been raised regarding high choline intake promoting hypo-tension and worsening cardiovascular outcomes.
With respect to the elevated choline needs of infants, Carolyn Hightower of the Vitamix Foundation asked how infant formula choline content compares to breast milk concentrations. Caudill explained that the choline content depends on whether the infant formula is cow milk based (resulting in choline content similar to breast milk) or soy based (resulting in choline concentrations less than breast milk). She noted, however, that the form of choline in infant formulas is different from that found in breast milk, and there is evidence to suggest that it is less bioavailable.
Dose and Formulation of Folic Acid Supplementation
With a sizable portion of pregnant women exceeding the periconceptional recommendations and the UL for folic acid, Hatfield questioned why prenatal supplements contain such a high dose of folic acid. Lamers thought that the 800 μg dose found in U.S. and Canadian prenatal supplements is historical. Hatfield also wondered what dose of folic acid supplementation should be given to a woman who has had neural tube defects in prior pregnancies. Lamers explained that a trial from the United Kingdom showed that a periconceptional 4 mg/day dose of folic acid prevented recurrent neural tube defects. The selected dose of folic acid used in the trial was a matter of convenience, as it was available in the hospital conducting the study. Given the significant findings, it is now not ethical to test a lower dose, so there is a lack of dose–response data for this outcome. She suggested that observational studies may eventually point to a lower dose.
Recognizing that some prenatal supplement companies are using methylfolate in their products rather than folic acid, Caudill wondered if there are any concerns with respect to the prevention of neural tube defects. Acknowledging that the two forms are biologically different, Lamers noted that 5-methyltetrahydrofolate (5-MTHF) increases red blood cell folate concentrations more than an equimolar amount of folic acid. She continued by stating that neural tube defects have been shown to be prevented by 400 μg/day folic acid, so it is unethical to recommend a different folate form, as the mechanisms underlying its protective effects are not well understood.
Considerations of Folic Acid in the Food Supply
A member of the webcast viewing audience questioned why the United States and Canada had differences in reductions in neural tube defects with the introduction of folic acid fortification of foods. Lamers admitted that the cause of the difference is not known but thought it may be caused in part by different data collection approaches. Another member of the webcast audience wanted to know if folic acid intake is affected if a woman limits or avoids gluten. Lamers stated that foods that contain gluten (e.g., wheat flour and wheat-based products) are often the ones that are fortified with folic acid, so it could affect intake. She noted, however, that fortified foods only account for approximately 150 μg/day folic acid intake and underscored the need for periconceptional folic acid supplementation in all women.
__________________
This page intentionally left blank.














