5
Dietary Supplements1
During pregnancy and lactation, there are specific nutritional needs that support and enhance the growth and development of the fetus and infant. With substantial changes to the landscape of dietary supplements and dietary intakes over the past several decades, large variations now exist in how those needs can be met through a combination of dietary intake and supplements. The fourth session of the workshop, which was moderated by Tamera Hatfield of University of California, Irvine, explored the role of optimal supplement use and bioavailability, with consideration of the patterns of use and necessity of supplements; differences between prescription and nonprescription formulations were also discussed. Highlights from the session presentations are presented in Box 5-1.
DIETARY SUPPLEMENT USE AND ITS MICRONUTRIENT CONTRIBUTION DURING PREGNANCY AND LACTATION IN THE UNITED STATES
Regan Bailey, professor in the Department of Nutrition Science at Purdue University and director of the Indiana Clinical and Translational Sciences Institute at the Purdue Nutrition Assessment Center, described findings from two National Health and Nutrition Examination Survey (NHANES) analyses (Bailey et al., 2019; Jun et al., 2020). To provide context, Bailey provided an overview of how the pertinent data were collected.
___________________
1 In the workshop agenda, this session was called “Nutritional Supplements.” The session heading has been changed herein to align with terminology used by session speakers.
NHANES interviewers conduct a dietary supplement inventory in participants’ homes and also collect demographic and background information. Clinical assessments are performed in a medical exam center and include a 24-hour dietary recall; a second 24-hour dietary recall is subsequently conducted by telephone. From the analyses, Bailey highlighted findings related to patterns of dietary supplement use and the content and contributions of dietary supplements.
Patterns of Dietary Supplement Use
Use of dietary supplements is common among pregnant and lactating women, although it differs by subpopulation groups. Prevalence of dietary supplement use is higher among pregnant and lactating women (77 and 70 percent, respectively) than it is for nonpregnant, nonlactating women (45 percent). Any supplement use was more common among pregnant women 35–44 years of age, compared to pregnant women 20–34 years
of age (88.1 versus 75.2 percent) (Jun et al., 2020). Use of any dietary supplement and use of a prenatal supplement was more prevalent among pregnant women with higher family incomes and most prevalent among non-Hispanic whites (Jun et al., 2020).
Most pregnant and lactating women use dietary supplements considered multivitamin mineral products (72.9 and 64.4 percent, respectively). The analysis defined such products as containing at least three vitamins and one or more minerals, explained Bailey. The cross-sectional data revealed that dietary supplement use is higher as pregnancies progress (see Figure 5-1). Bailey found this result troubling, given the critical importance of folic acid in early pregnancy. The analysis also revealed that only a small portion of women in all stages of pregnancy used an iodine-containing dietary supplement.
Bailey noted that, beginning in 2007, NHANES started collecting information about participants’ motivation to use certain products. Pregnant and lactating women commonly reported they were using a dietary supplement because a health care provider told them to do so. In contrast, nonpregnant, nonlactating women commonly reported that they used a dietary supplement based on their own motivation.
Content and Contributions of Dietary Supplements
The content of dietary supplements used by pregnant and lactating women vary widely, remarked Bailey. Most of the dietary supplements contained key vitamins. For instance, approximately 70 percent of pregnant and lactating women consumed a dietary supplement containing thiamin, riboflavin, niacin, vitamin B6, folic acid, vitamin B12, vitamin C, vitamin D, and vitamin E (Jun et al., 2020). However, far fewer pregnant and lactating women used dietary supplements containing vitamin A (approximately 40 percent) or choline (less than 8 percent). Mineral content of the dietary supplements was more variable. Only about 5 percent of pregnant and lactating women used a dietary supplement containing phosphorous, whereas more than 60 percent used a product containing calcium. Bailey cautioned that this assessment says nothing about the amount of vitamins and minerals found in the dietary supplement products.
One analysis compared median nutrient intakes from dietary supplements to their Recommended Dietary Allowances (RDAs) (Jun et al., 2020). Bailey explained that the data for nutrient intake from dietary supplements were skewed, necessitating use of the median. She further noted that the Estimated Average Requirement (EAR) is typically used to assess the proportion of a population group at risk of inadequacy; the RDA, which is two standard deviations above the EAR, is a higher intake level for comparison. Among pregnant women, median intake from dietary supplements contrib-
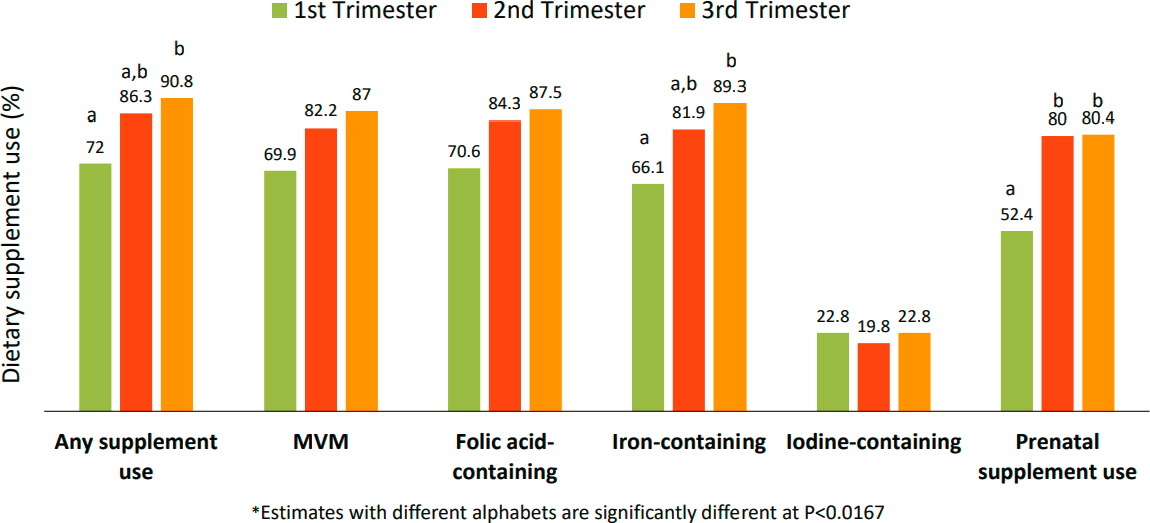
NOTES: Within each group, bars with different letters are significantly different from each other. MVM = multivitamin and mineral.
SOURCES: Presented by Regan Bailey. Created from data presented in Jun et al., 2020.
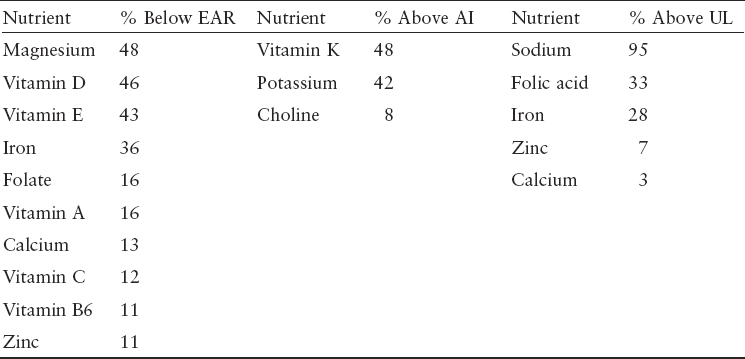
Bailey shifted her remarks to describe an analysis that assessed total nutrient intake, which includes contributions from foods, beverages, and supplements (Bailey et al., 2019). Substantial proportions of pregnant women who use supplements had total usual intakes below the EAR or less than the AI for several nutrients (see Table 5-1). She noted that despite the recently updated potassium AI being lower than the previous value, only 42 percent of pregnant women had total usual intakes above the AI. Echoing a comment made by Caudill during the second session, Bailey also noted that only 8 percent of pregnant women have total usual choline intakes above the AI. Total usual intakes of some nutrients exceeded the
uted to less than 100 percent of the RDAs for magnesium, phosphorous, calcium, selenium, iodine, and vitamin D. For choline, dietary supplements contributed approximately 2 percent of the Adequate Intake level. Pregnant women consumed more than the RDA from dietary supplements for several nutrients. For instance, dietary supplements provided 218 and nearly 296 percent of the pregnancy RDAs for folic acid and vitamin B12, respectively. A similar analysis was conducted for lactating women, although Bailey indicated that less is known about their supplement use. Lactating women appear to be using prenatal vitamins for a period of time postpartum, as evidenced by the median intake of iron dietary supplements contributing to nearly 300 percent of the RDA.
Tolerable Upper Intake Level (UL) (see Table 5-1). Sodium, for instance, was consumed in excess, with 95 percent of pregnant women with usual intakes above the UL. As sodium is rarely included in supplements, this level of intake comes exclusively from foods and beverages. Prevalence of intakes below the EAR and above the UL for iron and folic acid differed by supplement use (see Figure 5-2). Bailey remarked that supplement use was driving intakes above the UL for these nutrients.
TABLE 5-1 Comparison of Total Usual Intakes of Pregnant Women in the United States to Dietary Reference Intake Values
NOTES: With the exception of sodium, the estimates are of only pregnant supplement users. AI = Adequate Intake; EAR = Estimated Average Requirement; UL = Tolerable Upper Intake Level.
SOURCES: Presented by Regan Bailey. Reprinted from Bailey et al., 2019, used under CC BY 4.0.
Caveats to the Analyses
Bailey noted some of the limitation to the NHANES analyses she presented. First, she acknowledged that multiple cycles of NHANES data were combined in order to have a sufficient sample size of pregnant women and that dietary intakes over that time may have changed. Furthermore, dietary intake data are based on 24-hour dietary recall, which are accompanied by their own set limitations. Emphasizing a point made by Pearce in session 3, Bailey stated that there is no database for iodine content in foods, which prevented its inclusion in the analyses. Finally, she indicated that
these analyses did not include omega-3 fatty acid use, as others had recently published on the topic (Thompson et al., 2019).
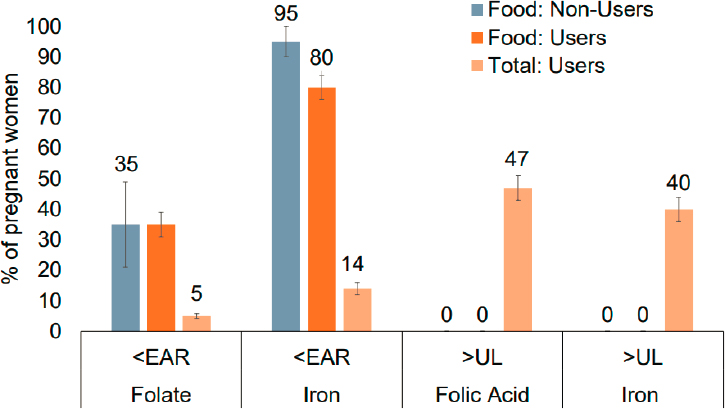
NOTE: EAR = Estimated Average Requirement; UL = Tolerable Upper Intake Level.
SOURCES: Presented by Regan Bailey. Reprinted from Bailey et al., 2019, used under CC BY 4.0.
PRENATAL SUPPLEMENT FORMULATIONS
Laura Borgelt, associate dean of administration and operations at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences and professor in the Departments of Clinical Pharmacy and Family Medicine, began her remarks by highlighting challenges consumers face when trying to compare prenatal multivitamin and mineral (PMVM) products. To demonstrate this, Borgelt showed how the Supplement Facts labels from two PMVM products listed different units for the same nutrient, presented different Daily Values for the same amount of a nutrient, and used different forms of a nutrient (e.g., ferrous fumarate versus ferrous bisglycinate and iron protein succinylate). She underscored the need to translate the information into something that is meaningful for the consumer.
Borgelt provided a broad overview of PMVM formulations. Over the course of her presentation, she touched on variations in formulations and labeling, bioavailability, characteristics indicative of high-quality products, and differences in sources of the nutrients.
Variations in PMVM Formulations and Labeling
Regulation of prescription and nonprescription formulations are slightly different and could have important implications for what a patient actually receives, Borgelt said. Using two public databases with supplement label information, her group evaluated the contents of 82 nonprescription and 132 prescription PMVM products (Saldanha et al., 2017). Overall, nonprescription formulations had a greater number of vitamins and minerals per product and higher vitamin A, vitamin D, iodine, and calcium content. Prescription formulations, by contrast, had higher folic acid content. Approximately one-third of nonprescription PMVM products evaluated included botanical ingredients and 8 percent included probiotics. In a separate study, Borgelt’s group found higher calcium, iodine, vitamin D, and choline content among nonprescription PMVM products and higher folic acid and iron content among the prescription formulations (DeSalvo et al., 2018). She suggested the prescription formulations may correct deficit intakes of iron and folate but may not fill the dietary gap for other nutrients.
A study of 24 prescription PMVM products, representing 61 percent of the market share, revealed both variations in formulations and deviations from the label contents (Andrews et al., 2019) (see Box 5-2). In contrast to these results, Borgelt’s group analytically assessed the contents of 20
nonprescription and 16 prescription products and found nutrient contents were generally lower than the amount listed on the label. Based on the contents listed on the labels, most of the prescription and nonprescription PMVM products evaluated in this analysis could help a pregnant woman meet her daily recommended intake of nutrients assuming average dietary intake (95 and 88 percent of the prescription and nonprescription products, respectively). However, fewer products could fill the dietary gap when the analysis used measured content of the products (79 and 82 percent of the prescription and nonprescription products, respectively).
Borgelt remarked that the content and labeling of some key nutrients during pregnancy are particularly variable across PMVM products. For instance, there is a lack of clear guidance on what units should be used to label the quantity of folic acid contained in the product (e.g., dietary folate equivalents, micrograms folic acid). Labeled folic acid content in the vast majority of prescription and nonprescription PMVM products tends to be high (Saldanha et al., 2019a) and exceeds the daily intake recommended by the U.S. Preventive Services Task Force. Borgelt indicated that there is a need for “consistent and clear recommendations for expression of folate and folic acid including labeling, daily value, upper limits, criteria for making health claims, and national recommendations.” With respect to iron, she noted that supplements are made with different forms (e.g., ferrous sulfate, fumarate, gluconate), which has implications for the quantity of
iron absorbed. Echoing a comment made by O’Brien in session 3, Borgelt stated that the form of iron commonly used in clinical trials (ferrous sulfate) is not the form of iron available to consumers. She suggested that consideration should be given to consistency of the chemical form of iron used. Borgelt said that the differences between prescription and nonprescription formulations may not be clinically significant in a healthy population, and she suggested that other factors such as cost may guide selection of products particularly when there are issues of access.
Tools exist to help estimate the contents of PMVM products. Borgelt described one such resource—the Dietary Supplement Ingredient Database (NIH ODS, 2020). The database created national estimates of 20 ingredients (10 vitamin and 10 minerals) across multiple lots of 71 different nonprescription PMVM products. These content estimates can be used to approximate the contents of a given nonprescription PMVM product.
Bioavailability of PMVM Formulations
There is no standard definition for a PMVM product. Accordingly, products vary with respect to ingredients, doses, and salt forms, and have changed over time (e.g., addition of botanicals, probiotics, docosahexaenoic acid [DHA], eicosapentaenoic acid [EPA]). Borgelt explained that these compositional differences, together with homeostatic mechanisms and the size of the ingested load, affect bioavailability. Obesity may also be a factor. Levels of fat-soluble vitamins are lower among women with obesity, owing to volumetric dilution and hepatic signaling, which has implications for bioavailability. “Higher bioavailability is not necessarily better. It may mean we absorb more, but that may or may not be a good thing if you are leading into excess,” Borgelt cautioned.
Bioavailability is measured through dissolution, or how long a tablet or capsule takes to dissolve in solution. Different chemical forms of nutrients can affect their bioavailability. The different inorganic forms of iron and zinc, for instance, can have different bioavailability. Bioavailability can also be affected by nutrient interaction. High iron intake can restrict the bioavailability of zinc, whereas vitamin C, magnesium, and calcium can affect the bioavailability of iron. Inactive ingredients can also affect bioavailability. The risk for these types of interactions to occur is elevated when a PMVM product contains multiple ingredients. The United States Pharmacopeia (USP), a supplement quality assurance company, has dissolution standards, but Borgelt noted that these standards are voluntary.
It is difficult to systematically evaluate the potential for interactions with PMVM products. The Micromedex database (Micromedex Solutions, 2020) includes 171 drug–drug interactions for prenatal vitamins, with 23 classified as major and 112 classified as moderate. For example, niacin,
which is found in prenatal formulations, can have major interactions with statins. Not all cataloged interactions were with prescription medications. Iron and zinc, for instance, can interact with each other if included in the same formulation, said Borgelt. Given the potential for interactions, questions arise; for example, should supplements contain only a single nutrient to prevent interactions, or does the convenience of a single pill outweigh the potential for interactions?
Characteristics That Indicate High-Quality PMVM Products
Borgelt offered some ideas of characteristics that could be indicative of high-quality PMVM products. Quality assurance is one such feature. She identified Consumer Lab, NSF, and USP as three of the top quality assurance companies that test and certify supplements. Although there are variations across the companies, in general they verify that a supplement contains what is listed on its label, that it does not contain specified contaminants, and that it is made according to good manufacturing practices. Borgelt stated that prescription and nonprescription PMVM products are regulated differently and suggested being cautious with products containing health claims.
Supplements can potentially contain contaminants. A recent study from Canada found that nearly half of the 26 PMVM products they evaluated exceeded lead toxicity standards (Schwalfenberg et al., 2018). Other heavy metals (e.g., aluminum, nickel, titanium, thallium) were detected in all the samples. The level of contamination was not correlated with cost of the product. Borgelt described some of her recommendations related to minimizing exposure to contaminants in PMVM products, including testing for toxic elements, establishing maximal acceptable levels for exposure to toxic agents, ensuring high-quality products are used, and adding additional regulations, oversight, and accountability.
Differences in Sources of Nutrients Supplemented in PMVM Products
Borgelt discussed the sources of nutrients contained within PMVM products, using omega-3 fatty acids and folate as examples. The omega-3 fatty acids DHA and EPA are found in fish oils, whereas alpha-linolenic acid (which the body can convert to DHA and EPA) is found in plant sources. Cold-water fish and shellfish are among the best sources of DHA and EPA, although concerns have been raised about mercury contamination and exposure from such sources. Borgelt suggested that the safest sources of these nutrients may be purified fish oil or fortified foods.
For folate, the form provided depends on the source. Folate (vitamin B9) naturally occurs in a range of foods (e.g., leafy vegetables, eggs, liver, milk), whereas folic acid is found in supplements and fortified foods. In the body, both are converted to a metabolically active form called L-5-methyltetrahydrofolate (L-5-MTHF). Acknowledging that diet is the preferred source of folate, Borgelt noted that folate, folic acid, and L-5-MTHF each increases plasma and red blood cell folate concentrations. Some individuals have been found to have a genetic polymorphism that can affect the conversion of folate and folic acid to L-5-MTHF. Accordingly, some companies favor using L-5-MTHF in their supplements. Borgelt suggested that genetics are important to consider and can affect how information is communicated to patients.
DISCUSSION
Following the presentations, Bailey and Borgelt responded to questions from the audience. Topics covered included prescription prenatal formulations, preparations and labeling of supplements, 5-MTHF, and data on and considerations related to supplement use and total nutrient intakes.
Prenatal Prescription Formulations
Hatfield opened the discussion by asking Borgelt what is known about patients who use prenatal prescription formulations. Borgelt responded that cost is often a driving factor, and that women on public insurance typically are the ones using prescription formulations. Adding a personal anecdote, Bailey said that for two of her pregnancies her health care provider would not see her until she was 8 weeks pregnant. She thought this practice is a missed opportunity, especially for prevention of neural tube defects, as nearly half of all pregnancies in the United States are unplanned.
An unidentified audience member wondered what differentiates a prenatal formulation that is available by prescription only from its nonprescription counterpart. Bailey explained that dietary supplements are regulated through the Dietary Supplement Health and Education Act, known as DSHEA, whereas prescription formulations have a different set of regulations. Agreeing with Bailey, Borgelt suggested that there may also be differences in manufacturing and testing processes. Drawing on points made during Borgelt’s presentation, Bailey mentioned that prenatal prescription formulation contents can also differ, such has having higher levels of folic acid.
Preparations and Labeling of Supplements
With respect to preparation of supplements, Johanna Dwyer of the National Institutes of Health’s Office of Dietary Supplements asked if there are issues of compaction with prenatal formulations. Borgelt said that the dissolution of prenatal products is highly variable across different supplement preparation (e.g., capsule, chewable, tablet) and appears to be more contingent on excipients used in the product. Building on this comment, Cindy Turner-Maffei of the Healthy Children Project asked if there is additional information on other preparations, such as orally administrated sprays. Sublingual administration is likely not appropriate for all vitamins and minerals, and bioavailability will depend, in part, on the excipients used in the products, noted Borgelt. With an explosion of new products on the market, including different preparations, she raised concern about maintaining quality and purity.
Regarding supplement labels, Anna Maria Siega-Riz of the University of Massachusetts Amherst wanted to know who serves as the reference group for the Daily Values on the Supplement Facts panel. Borgelt thought this was an important consideration, particularly for prenatal formulations. From the audience, Kathleen Koehler, a consultant in Washington, DC, raised the point that some of the differences in Daily Values may reflect the transition to new values for updated labels. Agreeing that the units for labeling folate is an important issue to raise, she said there has been a transition toward using dietary folate equivalents, but noted that the special recommendation during pregnancy has been in the units of micrograms of preformed folic acid.
5-MTHF
A webcast audience member asked Borgelt to expand on the comments she made during her presentation regarding 5-MTHF. Borgelt said that folate, folic acid, and 5-MTHF each appears to increase relevant biomarkers, and suggested that differences between the forms are likely not clinically meaningful among women with healthy diets, despite statistically significant differences being found. Yvonne Lamers of The University of British Columbia, who presented on folate in session 2, added that the methylenetetrahydrofolate reductase (MTHFR) genetic variant is common in the population. In the United States and Canada, approximately 10 percent of the population is homozygous, and 40 percent heterozygous for the MTHFR polymorphism. Lamers indicated that, in countries without folic acid fortification, homozygous women had significantly lower red blood cell folate concentrations and that the MTHFR genotype was associated with higher risk of neural tube defects. These relationships are not seen in the United States or Canada because women are folate replete. She added that
although 5-MTHF appears to increase folate status more rapidly among those with low baseline status, this does not appear to be a significant issue in replete populations.
Regarding the use of 5-MTHF in supplements, Dwyer inquired about its dissolution properties. Borgelt said that its ability to dissolve appears to be acceptable, but she was not clear on its stability.
Data on Supplement Use and Total Nutrient Intakes
Siega-Riz asked Bailey to clarify how NHANES collects the prenatal supplement use data. Bailey explained NHANES gathers supplement use data two ways. During the in-home interview, the interviewer asks to see the supplement bottle and asks participants how often in the previous 30 days they consumed the product. During the 24-hour dietary recalls, participants are also asked about supplement use. Analyses suggest participants have a digit preference when responding to the in-home interview (e.g., 30, 25, 10, 5 days) and that approximately 80 percent of those who indicated they took a supplement every day during the in-home interview went on to report it in their dietary recall. Bailey acknowledged that there could be changes in product use, omissions, insertions, and recall bias, which can affect the estimates. Siega-Riz suggested that other data have shown that pregnant women tend to overreport their supplement use, and she wondered if we have a clear understanding of patterns of use.
Angela Odoms-Young of the University of Illinois at Chicago asked if the NHANES data can be used to tell which health care providers are motivating pregnant women to take supplements, particularly if any of the information is coming from the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). Bailey noted that the question simply states “any health care provider.” She suggested the data could be stratified by WIC participation status, but stated such an analysis has yet to be conducted.
Considerations Related to Total Nutrient Intake
Thinking about evidence on total intakes, Siega-Riz wondered if there were ways to optimize the collection of data on nutrient intakes. Bailey thought that technology is advancing the collection of data on supplement use. As one example, she noted that, rather than conducting in-home interviews, some studies ask participants to take pictures of their supplements. Bailey also remarked that, despite concerns about the misreporting of supplement use, population distributions of serum and red blood cell folate are nearly superimposable with distributions of usual total folate intake, aside from some deviations at the tails.
Carolyn Hightower of the Vitamix Foundation asked Bailey to build on the concept of total nutrient intake and remark on intakes from food. Bailey stated that the population does not follow the Dietary Guidelines for Americans. “Pregnancy is not very different from nonpregnant populations in that intakes of fruits, vegetables, and whole grains remain very low relative to recommendations. Sodium, saturated fat, and added sugars remain high,” she said. Recognizing that a host of improvements could be made across the population, Bailey noted that there are some nutrients that are difficult to meet intake requirements from food alone.
REFLECTIONS ON THE DAY
To end the first day of the workshop, Siega-Riz offered her reflections on some key topics that emerged from the presentations and discussions. Referring to evidence presented in session 1 that suggested that the Dietary Reference Intakes for protein adequacy may be underestimated, Siega-Riz showed NHANES data indicating that the usual protein intake of pregnant women is generally within the range of the higher estimates of needs. She thought the new evidence on choline was important to consider. Regarding iodine, Siega-Riz wondered about the implications of shifts in dietary patterns and iodized salt use on women’s health. Finally, she underscored the importance of understanding the context of nutrient intakes from foods and noted that several presentations emphasized the importance of the quality of foods consumed.
__________________