Proceedings of a Workshop
| IN BRIEF | |
|
September 2020 |
Integrating the Science of Aging and Environmental Health Research
Proceedings of a Workshop—in Brief
Understanding and ensuring healthy aging has become increasingly important as the human life span increases with each decade. The need to better understand the role of environmental exposures in the development of aging-related diseases, such as cardiovascular disease, cancer, and dementia, is now an important driver for research at the intersection of aging and environmental health. With that in mind, on June 9–10, 2020, the Standing Committee on the Use of Emerging Science for Environmental Health Decisions (ESEHD) of the National Academies of Sciences, Engineering, and Medicine (National Academies) held a 1.5-day workshop to explore how environmental exposures influence or mediate aging and how aging influences environmentally mediated health outcomes. The workshop brought together a multidisciplinary group of experts who described the current state of knowledge in the field as well as ideas for next steps including research opportunities and needs, enabling technologies and analytical tools, and mechanisms to anticipate and use new data to inform environmental health decisions. The workshop was sponsored by the National Institute of Environmental Health Sciences (NIEHS). This Proceedings of a Workshop in Brief summarizes the invited speakers’ presentations and the discussion periods that followed each session.
SETTING THE STAGE
Michelle Heacock of NIEHS opened the workshop by describing the institute’s overarching goals and how those aligned with the workshop. The first goal is to carry out basic biological research on the effects of the environment on biological systems. NIEHS is particularly interested in understanding the interplay between environmental exposures, developmental processes, and biological responses, with an emphasis on how the timing of exposures may affect responses. Second, NIEHS wants to understand individual susceptibility to environmental exposures and explore the roles of timing and duration of exposures as they interact with an individual’s characteristics such as genetic makeup, age, sex, and underlying health status. Beyond that, NIEHS wishes to understand the role played by the social determinants of health, such as age, gender, education, race, and income. She stated that together, these goals can help inform how an individual’s susceptibility and “cellular age” change as a function of exposure and how the status of the chronological cellular age contributes to biological response to an exposure. The workshop’s first three presenters then offered talks on aging and the environment to set the stage for more detailed presentations later.
Environmental Determinants of Aging: Why Not Measure Everything?
Gary Miller of Columbia University opened his presentation with the provocative question “If our phenotype is a result of the genes and environment… why do we spend a disproportionate amount of time and money and energy on genetics?” He stated that this is an imbalanced equation and described how research on the exposome is important. The concept of the exposome originally came from Chris Wild, a former director of the International Agency for Research on Cancer (IARC), who defined the exposome as all exposures from conception onwards, including those in the lifestyle, diet, and environment. But the concept did not come to the forefront until about five years later when the ESEHD organized a National Academies
![]()
workshop titled The Exposome: A Powerful Approach for Evaluating Environmental Exposures and Their Influences on Human Disease.1 Working with Dean Jones, also at IARC, Miller modified2 the definition of the exposome to “capture the cumulation of the exposures and the biological response throughout the life span. When we talk about the exposome and thinking about the external factors that are influencing our health, we think very commonly about the sort of environmental chemicals and contaminants we might see in our water or food. But we’re also concerned about things like odor and noise and ambient light, temperature and humidity, and even social factors around socioeconomic issues, issues of structural racism and access to care.” Ultimately researchers will want to learn how the body responds to these various exposures, but first having a clear idea of exactly what exposures humans experience throughout their lives is necessary emphasized Miller.
One promising approach is the use of high-resolution mass spectrometry to simultaneously measure thousands of environmental chemicals in biological samples, including detecting the chemicals themselves as well as their metabolites. In collaboration with the Mayo Clinic, Miller and his colleagues examined several hundred patients with a particular liver disorder, identified hundreds of environmental chemicals in their body tissues, and matched them up with altered downstream metabolic effects in the patients, such as changes in amino acid metabolism and in inflammation and fatty acid pathways. They have now moved on to studying over 2,000 patients with this condition in a “multi-omics” format to provide this exposome level approach.
In another collaboration with Richard Mayeux at Columbia, researchers of the Washington Heights–Inwood Community Aging Project3 conducted a longitudinal study of community residents in northern Manhattan. Potential participants were identified based on residence in area U.S. Census tracts and were white (32 percent), African American (28 percent), and Caribbean Hispanic (44 percent) participants 65 years of age or older and without dementia at the start of the study. Participants completed a baseline assessment and were followed up at 18- to 24-month intervals for up to 25 years. Using plasma samples collected from these participants, the researchers were able to compare the exposomes and metabolomes of those who later developed Alzheimer’s and a control group. They found that they could separate the ethnic groups by their exposomes and metabolomes, and the information on exposures for these ethnic groups may offer insights into the underlying causes of their differential vulnerabilities to Alzheimer’s disease.
Summing up, Miller said that “high resolution mass spectrometry has become the de facto machinery for exposome research, and works in biological and environmental matrices, not just plasma and tissue, but also in water and air samples… I think this provides a tool that facilitates this systematic, comprehensive, and unbiased approach to studying the environmental contributors to aging.”
Integrating the Science of Aging with Environmental Health Research Through the Perspective of Biomarkers
Luigi Ferrucci of the National Institute on Aging discussed biomarkers in integrating the science of aging with environmental health research. Ferrucci provided a “framework” presentation for how to conceptualize and think about aging. Although everyone ages, he said, people age at different rates and in different ways. Furthermore, there are different types of aging, which lead to different ways to measure it. The simplest type is chronological aging—how old a person is. Then there is biological aging, or how a person’s cells and molecules change over time; physiological or phenotypic aging, or how a person’s physical abilities change over time; and functional aging, or how a person’s ability to carry out specific tasks changes. Biological, phenotypic, and functional aging are not synchronized. Biological aging will not be reflected in phenotypic aging until the ability to cope with the accumulation of damage falls below a certain threshold.
For most people aging follows a typical trajectory, Ferrucci said. The first 15 or 20 years in humans are spent developing one’s capabilities. Next there is a period of relative stability, which is followed by periods of decline and then disability. These periods can be shorter or longer, depending on the individual.
Normally when people think about aging, they think about the period of decline and figuring out how to slow that decline. But the science shows that aging occurs well before that—in the period of stability and even in the developmental period. Aging is not initially apparent because of the presence of a variety of compensatory and resilience mechanisms. Over time, however, the body’s resilience shrinks, and the decline in functioning becomes more obvious. Researchers know a great deal about the reasons for the decline—cell damage and loss of function, the effects of disease, and so on—but little is known about resiliency, “which is really what you want to know when you study aging,” he said.
Ultimately the goal should be to understand the forces of resilience that oppose the forces of entropy that lead to the declines seen in aging, Ferrucci explained. Resilience is strongly influenced by genetics, but it also has a major environmental component. And, he added, we now have a powerful tool, which is the capacity to study epigenetics. Epigenetic factors
__________________
1 See http://nas-sites.org/emergingscience/files/2011/05/exposome-newsletter-final1.pdf.
2 Others have developed somewhat different definitions, see for example the one for the CDC in Dr. Julie Andersen’s presentation.
3 See https://dss.niagads.org/cohorts/washington-heights-and-inwood-community-aging-project-whicap/, and https://dss.niagads.org/studies/sa000007.
change the expression of genes without changing the DNA sequence itself. This is due to epigenetic “marks,” such as methyl molecules, attaching to various places on the chromosomes that influence whether specific genes are turned on or off. He described the “epigenetic clock,” which is set at zero at birth. As life progresses, an individual may experience stressful conditions that influence their health, with malnutrition an extreme example. The body responds to such influences and these responses may be encoded in a new epigenome. This leads to an adaptive strategy that may be positive in the short term but could create problems later in life. Ferrucci offered that the life of an individual can be thought of “as a constant adaptation of the genome and gene expression to the environment and internal and external stresses that are affecting the epigenome.” He added that aging research should be examining not just the effects of the environment but also the body’s resulting compensatory mechanisms, which can be revealed in epigenetic changes decades after the environmental stresses that triggered them.
Contributions of Toxicants to the Development of Age-related Diseases
Julie Andersen of the Buck Institute provided an overview of how toxicants, such as chemicals or metals, contribute to the development of age related disease. Andersen noted that mapping the human genome has revealed a great deal about the effects of an individual’s genetics on his or her aging. However, according to recent research, genetic factors may account for as little as 7 percent of longevity. Thus, understanding environmental influences is critical in understanding aging.
Andersen reiterated that the exposome concept is crucial. The Centers for Disease Control and Prevention (CDC) defines the exposome as “all the exposures of an individual in a lifetime and how those exposures relate to health.” An individual can take in environmental toxicants in various ways, including inhalation, ingestion, and skin contact, and all of these routes may be important. The ultimate effects will depend on an individual’s genetic makeup, nutrition, lifestyle, and how they interact with the body and the metabolic responses that occur, which then constitute susceptibility risk factors and other health outcomes.
A great deal is already known about how environmental factors, including toxicants, affect aging-related diseases, Andersen said. For example, long term exposure to air pollution may interact with a person’s individual genetics and drive age related diseases, including Alzheimer’s disease. Exposure to the pesticide paraquat increases an individual’s risk of developing Parkinson’s disease. Andersen’s lab examines how exposures to metals can affect aging and age related neurodegenerative diseases. They have found, for instance, that mice given iron in the neonatal developmental stage, which corresponds to the first year of life in humans, will develop Parkinson’s-like symptoms when they are elderly (which is around 2 years for these mice). Exposing the worm Caenorhabditis elegans to iron also shortens its life span. In both experimental animals and humans, administering iron chelators reduces the neuronal deficits caused by iron.
Andersen summed up by stating that the key questions in this field include what underlies the effects of toxicants and other exposures on age related disease and how these exposures act to exacerbate aging processes. One important tool in answering these questions, she said, is the current ongoing development of databases documenting the role of toxicants in aging and age related disease.
EMERGING TRENDS AND TOOLS IN BIOLOGICAL AGING RESEARCH
Session 1 of the workshop focused on emerging trends and tools in biological aging research: new findings about the molecular pathways of aging; use of single-cell analysis to study biological aging; defining molecular “ageotypes”; and leveraging ‘omics profiling to estimate biological age.
Key Molecular Pathways of Aging
The first speaker, Rafael de Cabo of the NIH Intramural Research Program, discussed key molecular pathways of aging. De Cabo began by stating that genes, environment, and nutrition are key factors in how people age. Nonetheless, he emphasized that the biology of aging is incredibly complex. Aging is at the center of a huge network of processes, such as metabolism, each one of which is engulfed in multiple other processes that have profound effects at the molecular, cellular, organismal, and even population levels (Figure 1). De Cabo offered a brief overview of some of the key molecular pathways of aging.
At the cellular level, external inputs from the environment, nutrition, and anything that affects cellular responses, such as chemical exposures, produce signals. These signals set off a cascade of responses inside the cell along various molecular pathways. Over time the performance of these pathways will change in response to aging-related factors, including environmental toxicants, stress, and the programmed senescence of cells. At the cellular level, the hallmarks of aging include mitochondrial dysfunction, the loss of proteostasis, changes in gene stability, epigenetic alterations, and stem cell exhaustion. These produce changes at the tissue and organ levels, such as the onset and progression of various chronic diseases—cardiovascular diseases, cancer, diabetes, Alzheimer’s, and osteoporosis. These, in turn, lead to disability, mortality, and a whole collection of geriatric syndromes.
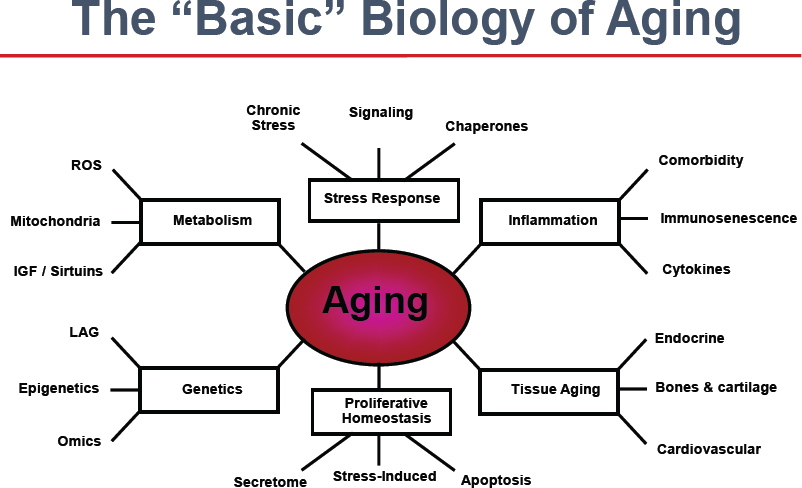
One goal of research in this field is finding ways to tweak these responses and reverse some of the effects of aging, thus helping to preserve health and improve survival. The molecular targets for interventions intended to promote healthy aging include sirtuin (SIRT), AMP-activated protein kinase (AMPK), and the mammalian target of rapamycin (mTOR) among other molecules. According to de Cabo, research has shown that altering these molecules through genetic manipulations or other approaches can have tremendous consequences in how the organism responds to environmental, genetic, and nutritional changes, potentially leading to increased lifespan. He also noted that increased longevity is often accompanied by improvement in or preservation of health and delayed onset of disease.
Use of Single Cell Analytic Technologies in Aging Research
Next, Murat Acar of Yale University described a series of aging experiments using baker’s yeast, Saccharomyces cerevisiae. Aging can be studied in many different organisms, Acar noted, with each having its own advantages and disadvantages. While yeast may be evolutionarily very distant from humans, aging in yeast does have similarities to human aging that can offer relevant insights. Yeast cells have the advantages of having a short lifespan, being easy to work with, and being relatively inexpensive compared with animals such as mice. He said that for understanding single-cell aging and single-cell analytical techniques, broadly speaking there are two approaches. The first is longitudinal tracking of a cell while it is aging in real time. The second is dynamic sampling or analysis of aging cells growing in batch cultures. His talk focused on the first approach.
Acar described how single yeast cells can be tracked in real time, and how phenotypes can be quantified as a function of time. Researchers take a variety of aging-related measurements from individual yeast cells, including gene expression, single-cell noise, variability in gene expression, cell cycle durations, and cell size variations across populations, and they input these data into quantitative models. Finally, they carry out genome wide single-cell DNA and RNA sequencing of sampled cells.
These experiments with yeast provide details on how aging affects yeast and offer insights into aging in other organisms, including humans. By using these techniques, Acar said he can gather cell population level statistics, the average age from wildtype yeast and from yeast missing specific genes or yeast growing in specific media. Survival dynamics of yeast populations and quantification of single cell aging phenotypes are also revealed. By using these technologies, Acar and others have quantified gene specific expression levels, genetic noise levels, division times, and cell size differences in the same population and how aging impacts these important phenotypes in real time.
Ageotypes: Molecular Pathways of Aging in People
Michael Snyder of Stanford University characterized his work as “trying to use big data to better manage people’s health, understand processes like aging, and actually try to improve those processes.” Snyder’s research involves “Longitudinal Personal Omics Profiling” with the goal of collecting very deep data on a group of human subjects from 10 different “omes”—genomes, proteomes, transcriptomes, microbiomes, exposomes, and more. The subjects provide blood, urine, and other samples; wear a variety of real time sensors; fill out stress questionnaires; and are given various clinical tests. Snyder was
his own first research subject, and has followed himself for 10 years, while the roughly 100 other people in the study have been followed for about 7.5 years. He noted that from just the first three years of data, they had uncovered 49 major health discoveries in people using these various technologies. No one technology found all of these things, some were found via the genomes, some from blood profiling, others were found with RNA, and so on.
Based on his work, Snyder stated that people have strong personal profiles that do not change much from year to year but do change slowly with age. The pattern of change also differs from person to person, although there are many commonalities. Based on their data, they have grouped their study subjects into general classes of agers, with four major ones: immune-agers, metabolic-agers, and liver and kidney agers. An individual can age faster in some classes and more slowly in others, and people can also age in more than one of the classes at once, for example in both metabolic and kidney function.
As part of the project, Snyder’s team is working to measure all the different environmental chemicals and microorganisms that a person is exposed to, including how much and when. Some of the people in the project wear an “exposometer” that keeps track of temperature and humidity and takes air samples so that chemicals and microorganisms in the air can be identified. He said that they have learned that people’s exposures are vast, consisting of hundreds of microbes, and they change from one sample to the next depending on the location of the person. He thinks that location is sometimes the number one factor, with some areas producing more bacterial exposures, others more fungal exposures, and so on. In addition, there are chemicals, such as plastics and Deet insect repellent, in every sample as well as certain carcinogens. Pesticides are strong in some areas, e.g. agricultural areas, but not so strong in others. Ultimately the goal is to combine all of this information and provide it to individuals as a “personal health dashboard” that can offer them and their physicians broad and deep information about their health, aging, and environments.
Leveraging Omics Profiling to Estimate Biological Age
Nathan Price of the Institute for Systems Biology next described another “omics” project to learn more about individuals’ health with a goal to estimate biological age independent of when a person was born but focused rather on molecular aspects of that person’s body to get an overall measure of a person’s health as they age. To do that Price and colleagues collected a huge amount of multidimensional longitudinal data on about 5,000 people over 4 years. Their data included whole-genome sequences, about 1,200 different analytes, including clinical chemistries, metabolites and proteins, and assayed their subjects’ gut microbiomes. The subjects also used wearable monitoring devices. The result was what Price called “personal dense dynamic data clouds,” or deep phenotyping.
Price’s team aimed to create a formula for “biological age” designed so that, on average, individuals’ biological age would increase by 1 year for each calendar year so that it was easy to compare biological age with a person’s chronological age. Price’s team found that having a disease increased a person’s biological age, but when a person did something to improve their health, such as starting a wellness program, biological age decreased, although there was considerable variation from person to person. Price’s conclusion was that multi-omics can yield an estimate of biological age that integrates information from many systems in the body, and when used to estimate biological age serves as a metric for wellness and a step toward understanding resilience and good health.
Panel Discussion 1
The Session 1 presentations on emerging trends and tools in biological aging research was followed by a panel discussion moderated by Donna L. Mendrick of the Food and Drug Administration (FDA). Ferrucci, Andersen, and Price were joined by Andrew Geller of the Environmental Protection Agency (EPA) and Caleb Finch of the University of Southern California.
Mendrick asked about the best way to apply new tools and trends in aging research to understand the interplay between environment and aging? Price answered that accumulating large amounts of longitudinal data, especially data presaging disease, will be particularly important and will have to span the academic and commercial spaces. The increasing use of wearable measuring devices will also make it possible to access much data not collected from controlled trials. Ferrucci agreed and said it will be important to design long term hybrid studies that include this sort of nontraditional data because “none of this technology that we have seen this morning by itself is enough.”
In response to a question about the possible reversibility of aging, Ferrucci said that while it is possible to help people reverse the functional effects of aging in various ways, those approaches are not reversing the biological effects of aging but instead are helping people compensate. Price added that biological age can be reduced, but everyone ultimately succumbs to aging and scientists need to be clear about what is irreversible and damages that cannot be dealt with.
Mendrick passed along a question from the audience about how one assesses environmental components in a study of aging. Price answered that with deep phenotyping data, it is relatively easy to do analyses over time to see what effects are related to various exposures. It is also possible to tease out the effects of exposure levels on different people. High exposures of certain chemicals are always bad no matter what an individual’s genetic makeup is, but at lower levels some people may be
more affected than others. Andersen agreed and said that she has observed similar results in her mouse models of Parkinson’s disease after iron exposure where mice with different genetic backgrounds have different susceptibilities.
Mendrick asked if there are existing databases that are publicly available with good data to which people could start applying Artificial Intelligence approaches. Price said yes, and mentioned multi-omics programs such as TOPMed, the UK Biobank, and the All of Us program.
In answer to a question, the EPA’s Geller spoke briefly about how the research described in the session might be useful to the EPA. “I think that there are certainly immediate impacts that it can have.” For example, most of EPA’s current actions related to lead are focused on children, but research incorporating lifespan exposures and impacts would potentially be useful for considering further actions mitigating lead exposures. He added that a variety of environmental exposures pose a risk of cognitive deficits, including air pollution, lead, phthalates, and pesticides, some of which are exacerbated by stress. He suggested that approaches that consider exposures both singly and cumulatively on cognitive deficits in older adults could be of use, especially for community-based actions addressing multiple stressors.
EXPLORING EMERGING AREAS OF INTEGRATION: EXPOSURES AND THE AGING PROCESS
The second session surveyed advancing areas of research at the intersection of environmental health and biological gaining. Specifically, the speakers described new findings on the influence of environmental exposures, including air pollution and chemical and heavy metal exposures on aging and age-related health outcomes.
First, Caleb Finch of the University of Southern California provided an overview on ways in which exposure science and research on age-related disease can be integrated in molecular and population-level research. As an example of this integration, Finch described work on the effect of two airborne sources of accelerated aging, air pollution of traffic origin and cigarettes, on age-related neurogenerative disease. He referred to these pollutants as “gerogens,” or factors that accelerate aging.
Research has shown that genetics plays a surprisingly modest role in aging and influencing an individual’s lifespan, Finch said. Environmental factors have much more influence. But even with identical genetic and environmental background, researchers see differences in aging trajectories, which are the result of developmental variations that start early in life.
Traffic-related air pollution and cigarette smoke share commonalities as gerogens. Both involve fine particles that reach deep into the lungs and are essentially incompletely burned organic materials. And they have various degrees of overlapping toxic chemicals, with carcinogens, polyaromatic hydrocarbons, and toxic metals of particular interest. They both shorten lifespan by 5 to 10 years, depending on the dose, and they accelerate the same diseases of aging: heart attacks and strokes, Alzheimer’s and brain atrophy, and various cancers, including lung and kidney. They also work via similar mechanisms—oxidative stress and inflammation that go down to the subcellular level in the mitochondria.
These gerogens affect the brain in different ways, and there is evidence that the three most studied components of air pollution—particulate matter 2.5 (PM2.5), or particles 2.5 microns or less in diameter; nitric oxides; and ozone—may interact with different brain systems. Smoking has been shown to accelerate cerebral cortex atrophy during aging in a dose-dependent manner. High levels of PM2.5 have been shown to cause a loss of white matter volume in the brain that is equivalent to the loss seen over 1 to 2 years of aging, and women with certain variants of the Alzheimer’s risk gene ApoE4 are more susceptible to this effect.
Understanding the mechanisms by which these gerogens produce their effects has suggested various treatments, and Finch’s group is examining the potential of anti-Alzheimer’s drugs to protect against the brain effects of air pollution. Research in mice has already shown promise, he said.
Air Pollution Exposures
Jamaji Nwanaji-Enwerem of Harvard University discussed findings from the Veterans Affairs Normative Aging Study on the effect of particulate matter exposure on biomarkers of aging. One of the major themes that emerged during the workshop was the search for appropriate measures of aging. Nwanaji-Enwerem described several biomarkers that capture parts of the aging process and showed how those biomarkers were affected by exposure to air pollution. Although there are a number of such molecular hallmarks, ranging from mitochondrial dysfunction to stem cell exhaustion, Nwanaji-Enwerem chose to focus his research on epigenetic alterations.
One specific epigenetic process is DNA methylation, which involves the attachment of a methyl molecule to a cytosine base in the genome. Methylation is particularly important because it can affect gene expression, including the ability to silence expression. As Ferrucci noted with his mention of the “epigenetic clock,” researchers have used methylation “marks” to develop novel metrics for biological aging, referred to as “DNA methylation age,” explained Nwanaji-Enwerem. The measure has been shown to be closely related to such things as all-cause mortality risk and various diseases. “You can have two people who are both age 46 but may have absolutely different methylation ages,” he said.
Three such methylation age measures are DNAm-Age, which is calculated from 353 specific sites in an individual’s genome; Pheno-Age, which takes into account various health-related variables such as white blood cell count; and Grim-Age, which takes into account plasma proteins and, for smokers, cigarette-pack years. Nwanaji-Enwerem noted that Grim-Age outperforms Pheno-Age, which outperforms DNAm-Age in estimating methylation age.
Using a Normative Aging Study that was begun in the 1960s with a group of healthy young men, Nwanaji-Enwerem and his colleagues demonstrated a relationship between methylation age and exposure to both fine-particle air pollution and metals. Every increase of 1 microgram per cubic meter of fine particulate mass was associated with an increase of 0.64 year in methylation age. They also found a striking correlation between the presence of manganese in urine and methylation age—a 1 nanogram per milliliter increase in manganese was associated with a 9.93-year increase in Pheno-Age.
Summing up, Nwanaji-Enwerem said that although much work remains to be done to further understand why certain DNA methylation age metrics are sensitive to certain exposures, but not to others, the use of methylation age provides important insights into how environment affects aging and health.
Joel Kaufman of the University of Washington addressed the question of how air pollution affects human health, particularly heart and lung health. He presented data to support the notion that PM pollution—in particular from traffic—is associated with several major diseases in older adults, focusing on atherosclerosis and clinical changes in the lung consistent with emphysema. Kaufman has paired state-of-the-art pollution exposure measures with an ongoing cohort study, the multicity Multiethnic Study of Atherosclerosis (MESA)4 research study to observe how pollution is linked to the progression of cardiovascular disease. The study had a cohort of about 7,000 participants who were 45 to 84 years of age and were free of cardiovascular disease at the start.
The first part of the effort was an extensive campaign in major cities all over the U.S. to collect outdoor concentrations of PM2.5, ozone, oxides of nitrogen, and black carbon, producing about two million total hours of active monitoring at places relevant to the participants, e.g., the locations of their homes. These data were then integrated in a model with geographical information that included traffic data, land use, population density, distance to fixed locations such as coastlines, a vegetation index, and other variables.
A great deal of information was also gathered on the MESA participants, including genetic information, physical activity, individual risk factors for cardiovascular disease, and many physical measurements. Using CT scans, Kaufman found a strong association between the levels of PM2.5 and nitrogen oxides in an individual’s environment and how quickly coronary artery calcium accumulated in the participants, which is highly predictive of future cardiovascular disease, especially atherosclerosis. In other participants they saw a substantial increase over time in the progression rate of emphysema strongly associated with ozone exposures.
Kaufman closed with some comments on how his data should be interpreted. He noted that their results showed primarily subclinical disease. For example, what they saw was not yet emphysema, but changes in emphysema-like lung characteristics over time. Similarly, the long-term concentration gradients they found were associated with an increased risk of cardiovascular disease, but there are a lot of mechanisms that play into the development of atherosclerosis and cardiovascular disease. He acknowledged that much further work on the mechanisms underlying his team’s epidemiological data is needed.
Integrating Environmental Exposures and Other Stressors – Racial Disparities
Uchechi Mitchell of the University of Illinois at Chicago described her work to examine racial disparities in cardiometabolic risk with age. Mitchell took a population and public health perspective to this topic. She stated that population health and the field of public health have come far because of the integration of biomarkers into large scale national and population surveys. This permits a more nuanced examination and modeling of how change in human physiological systems leads to morbidity and mortality and allows the investigation of how social and structural determinants of health, living and working conditions, and individual life experiences may contribute to poor health.
Mitchell stated that population health research focuses on the phenotypic manifestations of the decline associated with aging by examining cumulative measures of biological risk, which is referred to as “allostatic load.” She defined allostasis as the ability of physiological systems to adapt to external stimuli, such as stress, while allostatic load is the cumulative effect—the “wear and tear”—that stress imposes on an individual’s body. Over time repeated exposures to external stressors can tax an individual’s physiological systems and lead to dysregulation, damaging that person’s ability to adapt and come back to baseline when faced with future exposures. In large-scale population studies allostatic load is captured with indirect measures, such as the levels of various biomarkers (blood pressure, cholesterol levels, etc.) and represented as an overall risk level, such as cardiometabolic risk. Mitchell referenced work by Arline Geronimus who first proposed the “weathering hypothesis” that African American women’s health deteriorates in early adulthood as a result of their cumulative exposure to
__________________
socioeconomic disadvantages.5 Subsequent work6 by Geronimus and colleagues found that at equivalent chronological ages, black Americans had higher allostatic load and functioned worse than whites, who did not reach the same level of risk for high allostatic load compared to blacks until almost ten years later.
Working with data from the Health and Retirement Study, a national survey of adults ages 51 and older, Mitchell examined racial and ethnic differences in the levels of and changes in cardiometabolic risk using blood-based biomarker data that was updated every 4 years. At baseline they found risk levels to be highest among older blacks, followed by Hispanics, and then whites. After 4 years those risk levels had, on average, decreased among whites and Hispanics but increased among blacks. The primary reason for the overall decline in the first two groups, Mitchell found, was improvements in their lipid profiles and inflammatory levels, while the risk increase in the older black adults was due to worsening blood pressures and glucose levels.
A likely explanation for the difference, Mitchell said, was found in treatment levels for chronic conditions. Older blacks were much more likely than whites to have skipped taking their medications because of cost or financial hardship. This is an example of how social and economic factors can drive health differences, Mitchell said. Over the course of their lives, individuals also encounter different circumstances that can increase their risk for disease, including poverty and environmental exposures. She showed maps of the city of Chicago, where there is substantial overlap between areas of high environmental exposures and residents who identify as black. The same overlap is seen for predominantly Hispanic neighborhoods in the city. She stated that these groups of residents also have differential access to various protective factors, such as education and high-quality health care. And she noted that despite the clear evidence of health impacts from exposure to heavily polluting industrial facilities, decisions on siting such facilities, such as scrap metal shredding plants in areas predominantly occupied by blacks and Hispanics, are still being made today. “Racism is the primary toxin that is underlying these disparities,” emphasized Mitchell. For older individuals, health impacts may be greater because those individuals from racial and ethnic minorities are less likely to be able to move out of disadvantaged neighborhoods that have greater exposure to environmental pollutants. Her final comment was that “to really address these racial and ethnic disparities in health requires an ecological approach that comprehensively addresses the exposome while increasing the attention to how exposures within the environment are distributed systematically across subgroups of the population.”
Integrating Environmental Exposure and Other Stressors – Maternal Stress, Particulate Matter Exposure, and Development
Rosalind Wright of Mount Sinai is a pulmonary critical-care physician who deals with people at the end stages of life. To truly understand aging, she said, it is necessary to go all the way back to the beginning of life and examine the uterine environment in which a baby develops. For this, she said, we need to know what a pregnant woman’s cumulative environmental exposures have been and how those shape her own underlying physiology and impact the programing of the infant.
She focused her presentation on her research on the effects of fetal exposures in utero and how these affect the length of a newborn’s telomeres, which are thought to influence how the body’s cells age. Telomeres are DNA-protein complexes that sit at the ends of each chromosome and protect it from damage. The length of the telomere decreases each time a cell divides so that over time the telomere provides less protection and a cell’s DNA becomes more vulnerable to damage. Shorter telomere lengths have been linked to a variety of health and aging issues.
Wright works with the Programming of Intergenerational Stress Mechanisms (PRISM) study, which was designed to acquire as much information as possible about the various factors that affect pregnant women—and thus their babies—and to try to understand how those factors affected the babies’ health after birth. “We look at physical factors, home allergens, tobacco smoke exposure, air pollution exposure,” she said. “We also look at chemical exposures, and we are also interested in nutrition, perhaps as a protective factor, as well as the source of the exposure to environment toxins.” They take extensive urine, blood, saliva, hair, and other samples from pregnant women, as well as placental and cord blood samples after the birth. Finally, they consider psychological stress.
So newborn telomere length is used as a potential “biomarker of aging” due to the effects of the maternal environment on a fetus during development. Babies born with shorter telomeres could be expected to age more poorly, perhaps have poorer health as they age, and even have shorter lives. Previous studies had found that smoking by mothers during pregnancy is associated with shorter telomere length in their children at birth, as is a higher maternal body mass index and whether the mother experienced high levels or stress or depression during pregnancy. Wright’s research found that mothers exposed to high levels of PM2.5 tended to give birth to babies with shorter telomeres, but the effect was only significant for
__________________
5 Geronimus, A. T. 1992. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity & Disease. 2(3):207-221.
6 Geronimus, A. T., M. Hicken; D. Keene, J. Bound. 2011. Weathering” and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States. American Journal of Public Health 96 (5): 826-833.
male babies, and pregnant women were able to avoid this affect if they had high levels of antioxidants in their diet during pregnancy. She finished by stating that because aging begins in the womb, there is a need to take an intergenerational life course approach to understand it.
Panel Discussion 2
Following the second set of presentations, Jiu-Chiuan (JC) Chen of the University of Southern California, one of the workshop organizers, led a panel discussion with the afternoon’s speakers—Finch, Nwanaji-Enwerem, Kaufman, Mitchell, and Wright. Chen began by asking in what area of research on aging and the environment is there sufficient knowledge to guide interventions? Kaufman answered that although there are still many missing pieces, there are some areas in which there is enough known for policy makers to take action. “We know that there’s no such thing as a safe level of lead. We know that there’s no such thing as a safe level of inhaled particulates.” Mitchell added that the existing science also supports policies to protect communities that are disproportionately exposed to both environmental and social stressors.
Nwanaji-Enwerem offered some suggestions for research that could be useful for policy development and implementation. “We have all these markers and measures, but at the end of the day we don’t really know what they mean,” he said. It would be helpful to know what changes people can make in their lives to help counteract or avoid some of the negative effects associated with those measures.
Wright agreed that there is not yet enough known about the ideal interventions to be able to move forward and added that it is not enough simply to study the risk factors; more needs to be known about potential resiliency factors as well. “Things like nutrition, things like social networks—there is evidence that these can mitigate effects of psychological stress and also mitigate effects of some of these chemical toxins we look at.” And, she added, it would be helpful to start getting data as early in life as possible, even while a child is still in utero, since it is now known that that period plays a role in one’s health and aging as an adult.
Finch agreed and added there is evidence that exposure to smoking and lead in utero have an effect not only on that child but on that child’s children. The environment can produce multigenerational effects, so research is needed on how to recognize who might be at risk and what can be done to reverse or ameliorate that risk. “There’s a huge potential to examine existing lifestyles, diets, and existing drug use to look for potential protection,” he said, and research into that area is already ongoing in human populations.
Mitchell said that both approaches to healthy aging—the individual, physician-directed approach, and the collective, public health approach—will be important and that it is necessary to think about the proper balance between them. For instance, identifying and encouraging resilience factors in individuals may be much harder and not have as great a payoff as working to understand and improve community-level resources that can improve individual resilience or limit exposure to environmental stressors.
Finch said that there is good evidence of synergy between air pollution and tobacco smoke in terms of increasing cancer risk and affecting cognitive function. However, there is less known about the details of that—exactly what compounds are interacting synergistically, for instance, or the magnitude of the synergistic effect—and that should be put on the agenda of the environmental toxicology field.
To close out the workshop’s first day, Mary Ann Ottinger of the University of Houston first offered a recap of the day’s presentations and then identified several unifying themes. One was the many examples of environmental factors that adversely affect the rate of aging in individuals. Another was the novel metrics that are emerging to assess these interrelationships. The exposome concept encompasses all the different aspects of an individual’s environment that may affect health and aging. There is a wide variety of data being brought to bear on studies of the environment and aging, including data from the various omics, the microbiome, and measures from external devices, such as exposure meters. “It is really important to coalesce this information and make it available to everyone,” she said.
Ottinger went on to say that the interrelationships among functional, phenotypic, and biological aspects of aging and the role of stressors and their impact on molecular, cellular, or whole organism responses need to be understood. This brings up the fundamental question of what model is best to study a certain kind of response or stressor or target. Finally, she said, multidisciplinary and transdisciplinary approaches and the integration of human cell and animal models all offer opportunities for accelerating research in this area.
Chemical Exposures
At the beginning of the workshop’s second day, Michelle Heacock from NIEHS spoke briefly to welcome the participants and to acknowledge the national conversation taking place at that time over racial injustice. She said that NIEHS has been at the forefront of efforts to recognize and understand the health impacts that environmental hazards have on disadvantaged and diverse communities. “NIEHS remains committed to uncovering the social burdens that combine with other social
determinants of health such as age, gender, education, race, and income to create health disparities as well as to ensure environmental justice.”
With that, the first day’s session on Exposures and the Aging Process began with presentations on the effect of chemicals and heavy metal exposures on aging and age-related health outcomes.
The first speaker, Beate Ritz, discussed her research on pesticide exposures and the development of neurodegenerative disorders. Ritz began her talk with the statement that Parkinson’s disease is the second most common neurodegenerative disease after Alzheimer’s disease. It is known for its motor symptoms, such as tremors, rigidity, and akinesia, which eventually end up trapping a person in an unresponsive body. But it is also a chronic disease that has a number of non-motor symptoms, including cognitive decline and dementia, depression, sleep disorders, and peripheral nervous system disorders. It is caused by a loss of certain cells, called dopaminergic cells, in the substantia nigra of the brain.
Ritz discussed the role that environmental exposures to pesticides might play in the risk of development of Parkinson’s disease. Age is actually the strongest risk factor for Parkinson’s, and that may be in part due to an accumulation of exposures to pesticides, many of which are known neurotoxicants. So, she set out to study the precise role that pesticides play in the development of this debilitating disease.
In her Parkinson’s, Environment, and Genes (PEG) study, Ritz enrolled about 850 patients with Parkinson’s disease plus 1,000 controls and took a detailed look at the pesticides that they had been exposed to over their lifetimes using the pesticide application reports that must be filed in California any time that pesticides are applied. The study found that people who were exposed to the pesticide paraquat and the fungicide maneb had an 80 percent increased risk for developing Parkinson’s and those who were exposed to just paraquat had about a 25–30 percent increase in risk.
Looking more closely, Ritz found that only certain people were at increased risk, depending on the variant they had of a dopaminergic transporter gene. Those with a gene variant that allowed them to quickly metabolize the pesticide were not at increased risk of Parkinson’s after exposure to paraquat, while the slow metabolizers had a two- or threefold increased risk if they were exposed. In short, she said, it requires both exposure to the pesticide and genetic susceptibility to cause the increased risk of Parkinson’s that has been observed.
John Meeker of the University of Michigan discussed the influence of chemical exposures of human development. Meeker noted, there are almost countless types of chemicals found in consumer products: fire retardants, chemicals to strengthen products, colorants, chemicals to produce water resistance, the list goes on and on. Since the end of the second world war, there has been a vast increase in chemical production and there are now an estimated 80,000 to 100,000 chemicals on the market. The use of consumer products is a major source of exposures to chemicals in wood, metals, and plastics.
Research has found an almost equally large number of ways that chemicals affect human development. They affect the timing of puberty and increase the chances of developing obesity and diabetes. They have been linked to congenital hypothyroidism. They affect sperm quality and increase the risk of infertility and preterm birth. One common theme is that environmental chemicals tend to affect endocrine function in various ways, such as modifying the production of hormones and affecting their transport and binding.
As a specific example, Meeker described some of the research on phthalates, a well-studied class of chemicals used in a wide variety of products, including polyvinyl chloride, which is used to make flexible pipe, as well as fragrances and personal care and household products. He and his colleagues studied the effects of phthalates on birth outcomes in an area of Puerto Rico that has a history of environmental contamination. By measuring the level of phthalate metabolites in the urine of pregnant women, they showed an association between exposure to phthalates and preterm births. This is an important health outcome, he noted since preterm birth is associated both with increased infant mortality and the occurrence of many diseases and disorders later in life and on into adulthood. Preterm birth is a huge public health problem worldwide, including in the U.S. Another study of the effects of phthalates on pregnant women in Mexico City found that girls born to these exposed women entered puberty at earlier than average ages but took longer than usual to complete the process. For boys, however, the effect was the opposite.
Such studies are difficult to do, Meeker said, because they require many years to complete, while research funding tends to cover only three to five-year time blocks. But they are important because the potential health effects can stay with people throughout their lives. He also said that future research needs include methods to study chemical mixtures, effects on development, study designs, understanding mechanisms more deeply, long-term consequences, such as effects on aging, and risk assessment and management issues.
Heavy Metal Exposures
Brandon Pierce of the University of Chicago discussed his work on the effect of heavy metal exposures on aging. Pierce returned to the topic of telomeres, which appear to be damaged by exposure to arsenic and certain other metals. As previously noted, each time a cell divides, bits of its telomeres, the protective DNA-protein complexes at the ends of each chromosome, are lost. Over time a telomere thus loses its ability to protect a cell’s DNA, eventually triggering senescence. An
enzyme called telomerase acts to maintain the length of the telomere in stem cells and certain other cells, such as skin cells, that need to be able to divide over and over again.
Studies in model organisms have shown that suppressing the action of the telomerase leads to shortened telomeres and accelerated aging, Pierce said. Furthermore, human studies have found that people with shorter telomeres appear to be at higher risk for coronary heart disease and early death. Those studies do not prove causality, Pierce noted—it is possible that poorer health might lead to shorter telomeres, for instance—so some researchers have studied genetically-based variations in telomere length to avoid the possibility that the differences in length are due to illness. These genetic studies have provided evidence that shorter telomeres do indeed increase the risk of cardiovascular disease as well as Alzheimer’s disease.
A number of environmental exposures are also thought to affect telomere length, Pierce said. There is good evidence that cigarette smoke leads to shortened telomere length, for example, but most other hypothesized relationships are still unproven. Pierce and his colleagues have looked for evidence that environmental exposures to various metals, including arsenic, cadmium, and lead, are associated with decreased telomere length in humans. Focusing on arsenic, he said that the results of the studies that have been done to date have been mixed, with studies in different groups finding different telomere effects. So, the answer to whether arsenic exposure affects telomere length will have to wait for the results of more definitive experiments.
Next Andres Cardenas of the University of California, Berkeley spoke about the effects of prenatal exposure to metals on the epigenome. Epigenetics, Cardenas explains, refers to the way in which gene expression is controlled independent of the DNA sequence. “You’re changing the expression of genes without actually changing the sequence itself.” This is done with epigenetic “marks” attached to the chromosomes at various places that influence whether specific genes are turned on or off. These epigenetic marks are tissue-specific, so different tissues in an individual’s body can have different epigenomes. They can also be passed along as cells divide and can even be passed from parent to child.
It is believed that exposure of an embryo or early-stage fetus in utero to certain environmental toxicants can disrupt the normal epigenetic programming of cells during critical early stages of development. This may, in turn, result in the development of various diseases later in life, including metabolic disorders, cancers, and cardiovascular disease. Cardenas described research on a prospective birth cohort in Bangladesh that was designed to explore this possibility.
The mothers in this cohort had been exposed while pregnant to a drinking water source that was contaminated with arsenic, thus exposing their babies in utero. At birth the researchers collected the placentas, umbilical cords, umbilical arteries, and umbilical vein epithelial cells and performed epigenome-wide association studies. What they found was that the arsenic appeared to disrupt epigenetic programming in a tissue-specific manner, with some tissues, such as arterial tissue, much more sensitive than others. This in turn indicated that the arsenic-induced epigenetic disruptions might play a role in insulin signaling pathways and the development of basal cell carcinoma.
Another study, Project Viva, was later conducted in Boston. In this case, levels of mercury, a neurotoxin that can produce cognitive defects in children, were determined in pregnant mothers. These were followed up by measurements made in the children at birth and in early and mid-childhood to see if the epigenetic changes associated with mercury exposure were persistent. The epigenetic changes associated with mercury exposure were present in the newborns and again in early childhood (age 3), but then attenuated in later childhood. So, Cardenas said, some epigenetic changes are persistent while others are more malleable. This suggests some exciting possibilities for interventions, Cardenas said. “Diseases like cardiovascular disease and cancer might have an early life origin and might be hiding in the epigenome, which would provide an opportunity for prevention.”
Panel Discussion 3
Kristen Malecki next led a panel discussion with the morning’s speakers—Ritz, Meeker, Pierce, and Cardenas—as well as Emma Lavoie of the EPA and Suzanne Fitzpatrick of the FDA. Malecki asked Ritz to expand on a comment she made toward the end of her talk about the role of the microbiome on aging. The dementia associated with Parkinson’s and Alzheimer’s diseases arises because of abnormal deposits of a protein, alpha-synuclein, in the brain. The clumps of protein, called Lewy bodies, are also found in the gut, Ritz said, and she has hypothesized that the Lewy bodies move through the autonomic nervous system to the brain, where they stimulate the aggregation of the proteins associated with the dementia. Ritz’s team is interested in what is different about some people’s guts that may lead the Lewy bodies to migrate to the brain and whether the microbiome may be playing a role. The immune system in the gut is very much in contact with the microbiome, she said. If there is an interaction between the two that leads to protein aggregation and movement, what factors make the microbiome either good or bad for a person, which is a question she hopes to tackle in the next five years.
Next Malecki turned to Meeker to ask how certain it is that the developmental markers in use for children are really biomarkers of aging and do they track with normal aging? Meeker responded that there is mounting evidence for a range of developmental end points some of which are associated with aging-related diseases later in life. But, he said, what is missing
is what ties all this together. “Are there underlying biological things going on that tie them together, or are they separate events?” That is still not clear.
Turning to Pierce, Malecki passed on an audience question about whether animal studies might be able to advance research on the effects of arsenic on telomeres. Pierce answered that research on animals could be very important in exploring some of the basic science issues in the field as well as addressing some of the inconsistent findings seen in humans.
Malecki asked Cardenas two epigenetics question: How stable is the epigenome over time? And what is known about the effects of environmental exposures on the epigenome over time and are the effects cumulative? There is no general rule about the epigenome’s stability, Cardenas said. The stability is very dependent on which gene is affected and on when the exposure occurs. But some changes are very stable.
Malecki next asked the EPA’s Lavoie about whether the research presented at the workshop could help inform the work of environmental risk assessment and policy making, particularly regarding children’s environmental health. Given the evidence showing that children have unique sensitivities or vulnerabilities to chemicals or stressors, Lavoie said, this must be taken into account in carrying out risk assessments.
Of the final panel member, the FDA’s Fitzgerald, Malecki asked how she saw the multi-omics tools discussed in the workshop being used in environmental decision making. “I think all of these tools really are important as FDA looks at how it regulates these different compounds,” Fitzgerald said. Noting that there are significant amounts of metals in children’s food, she said that the new tools will be helpful in determining the effects of cumulative and aggregate exposures, particularly in vulnerable populations such as children and older adults.
Malecki then opened the discussion to the audience with a general question about critical research needs. Fitzgerald suggested that it will be important to gather data on mixtures and learn how to do cumulative and aggregate assessments. Responding to Malecki’s question about protective factors against Parkinson’s disease, Ritz said that is a very complex issue. There is some indication that having folate or certain antioxidants in the diet could protect against some environmental exposures, and there is also evidence that physical activities may have a protective effect. Recent research found that diabetes is a risk factor for both Alzheimer’s and Parkinson’s disease, so metabolism also seems to be involved in increasing risk. One needs to think about how to make the various body systems, such as the metabolism and the immune system, more resilient.
CONSIDERATIONS FOR DECISION MAKING: CONCURRENT BREAKOUT SESSIONS
For the breakout sessions, Katherine James of the University of Colorado–Anschutz Medical Campus explained that the workshop participants would be divided into two groups for discussions that would then be summarized in a final plenary session. The overall topics of the breakout sessions were to be A, research priorities in aging science and environmental health and B, how best to implement the science when creating policy for clinical and public health practices. Group A would focus on the science and B on the translation of the science into public policy. Gina Solomon of the University of California, San Francisco and a member of the Standing Committee and Marianne Ottinger led Group A and Katherine James of the planning committee and Gary Ginsburg from the Standing Committee led Group B.
Group A: Critical Research Priorities to Advance the Science
Joel Kaufman summarized the Group A discussion. The Group agreed that one of the key issues around which to develop a research agenda is the degree to which the health disparities seen in aging are explained by environmental exposures. A second key area to explore is how environmental exposures influence biological aging, as reflected by such measures as epigenetics or telomere length. These measures could then be linked to the standard hallmarks of aging in humans to explore the relationships between environmental agents and aging processes. The third priority area would be to try to understand the exposome in the elderly, which should probably include social factors. A fourth area concerned translating research from animal and experimental models to humans in population studies and vice versa. The goal would be to have rapid movement of information in both directions to inform research in both areas. Other issues mentioned included the influences of environmental exposures on specific disease processes with aging, the role of the microbiome, understanding mixtures in cumulative exposures, and developing improved animal models of aging.
Group B: Critical Translational Principles to Inform Decision Making
John Vandenberg summarized Group B’s discussion. One of the major themes that emerged, he said, was that aging research would include the entire life span, from the prenatal to the elderly, and research to be conducted across that entire spectrum. A major area of focus in moving from research to implementation that the group discussed was communication. One aspect is finding ways to improve how researchers communicate science to policy makers to inform their decision making, which is challenging and something most scientists are not trained in. It is also important to communicate to the public the benefits to society of research on aging and the environment. A second major area the group identified had to do with taking advantage
of the large amount of data that has already been accumulated in this field. The idea would be to explore which parts of that data might be particularly useful for informing policy decisions.
Final Panel Discussion
Katherine James moderated a final panel discussion with Luigi Ferrucci, Joel Kaufman, John Vandenberg, Kristi Pullen Fedinick of the Natural Resources Defense Council, and Sandra Howard. James began the discussion with a question about how to use the current knowledge in the field and what next steps could address critical gaps in knowledge.
Ferrucci answered that it will be important to develop a better understanding of how to measure aging and to define its phenotypic manifestations. It will also be crucial to understand the underlying mechanisms of aging and how those mechanisms at the cellular and molecular level are affected by genetic, behavioral, and environmental factors. The goal will be to create an environment that helps slow down aging and prevents disease and disability in old age.
Kaufman then spoke about the importance of interdisciplinary research groups. It is important to be able to move back and forth among things like environmental health studies, medical research, and basic science because knowledge in each of these areas raises questions and informs research in the others. Ferrucci supported Kaufman’s comment by speaking of his own experience in bringing together molecular biologists, epidemiologists, and environmental scientists. At first, they were uncomfortable and did not know how to talk with one another, but after a few months the synergy was fantastic.
Howard said that it is important for researchers to involve the public from the very beginning, communicating with community members about the goals of a project, how it will be carried out, and the possible potential benefits. Pullen Fedinick agreed and said it is necessary to build trust with a community. As part of that, researchers should not see science in a vacuum, but in the context of the people whose lives are affected and understand what is happening in those communities in order to inform the science and make it as relevant as possible.
James next asked Vandenberg how best to improve the environment over time. Vandenberg said that it is crucial to have a clear understanding of exactly what in the environment is affecting health. The idea of the exposome can play a key role here by providing insights into the various factors that are important to public health. A tremendous amount of data has been accumulated about environmental exposures, he said, but there are still big gaps for many chemicals. The knowledge developed through exposome studies could help fill those gaps and provide insights into how exposures interact with other factors, such as nutrition and behavior.
Concerning how to incorporate research on aging and the environment into clinical practice, Kaufman said that while there are some well developed areas in which clinical practice can be informed by the science, in most cases the science is still too immature. Ferrucci agreed, but he described his “dream” of a future when doctors are proactive in dealing with aging issues and helping patients improve their resilience rather than simply responding when symptoms of a disease appear.
CLOSING THOUGHTS
In her closing remarks Kristen Malecki offered her perspective on major unifying themes that she thought had emerged from the workshop. “We need a good definition of what aging is, and we also need to understand that aging is a trajectory,” she began. The importance of the life-course approach to environmental research was a major theme—working not just with early-life cohorts but middle- and later-life cohorts as well. Another theme is that bridging research on aging and environmental health is a “logical next step” given that many common hallmarks of gaining are linked to systemic outputs, such as chronic disease and geriatric health outcomes, that are also influenced by environment and nutrition. A third major theme was the need to consider multiple biomarkers and how they change with aging over time and to consider multiple exposures and how they may interact. Reflecting on the impact of environmental exposures on aging, she noted the need to “think about the environment in a larger context” and how the exposome—the totality of environmental exposures throughout the life span—is one way to do that. Research is also needed on whether and how environmental factors influence health disparities. Moving forward, environmental research will also benefit from multi-disciplinary team approaches in acknowledgement “that aging is a systemic process that begins at pre-conception.” Malecki said. The role of resilience is not well understood and requires further research. Finally, Malecki emphasized the need for a greater focus on the science of exposures. Attention is really needed on the population susceptibility and vulnerability to environmental exposures that are underlying health disparities in aging an age-related disease to inform and advance public health policy, she concluded.
DISCLAIMER: This Proceedings of a Workshop—in Brief was prepared by Robert Poole, Fran Sharples, and Keegan Sawyer as a factual summary of what occurred at the workshop. The planning committee’s role was limited to planning the workshop. The statements made are those of the rapporteurs or individual meeting participants and do not necessarily represent the views of all meeting participants, the planning committee, or the National Academies of Sciences, Engineering, and Medicine.
ORGANIZING COMMITTEE ON INTEGRATING THE SCIENCE OF AGING AND ENVIROMENTAL HEALTH RESEARCH: This workshop was organized by the following experts: Murat Acar, Yale University; Jiu-Chiuan Chen, Keck School of Medicine; Katherine James, University of Colorado-Anschutz Medical Campus; Kristen Malecki, University of Wisconsin-Madison; Donna L. Mendrick, National Center for Toxicology Research, U.S. FDA; Gary Miller, Columbia University; and Mary Ann Ottinger, University of Houston.
Reviewers: The Proceedings of a Workshop—in Brief was reviewed in draft form by Andrew Geller, U. S. Environmental Protection Agency; Jaclyn Goodrich, University of Michigan; and Kristen Malecki, University of Wisconsin to ensure that it meets institutional standards for quality and objectivity. The review comments and draft manuscript remain confidential to protect the integrity of the process.
Sponsor: This workshop was supported by the National Institute of Environmental Health Sciences. For more information, contact the Board on Life Sciences at (202) 334-3947 or visit https://www.nationalacademies.org/bls/board-on-life-sciences.
About the Standing Committee on Emerging Science for Environmental Health Decisions
The Standing Committee on Emerging Science for Environmental Health Decisions is sponsored by the National Institute of Environmental Health Sciences to examine, explore, and consider issues on the use of emerging science for environmental health decisions. The Standing Committee’s workshops provide a public venue for communication among government, industry, environmental groups, and the academic community about scientific advances in methods and approaches that can be used in the identification, quantification, and control of environmental impacts on human health.
Presentations and proceedings such as this one are made broadly available, including at https://www.nationalacademies.org/our-work/standing-committee-on-the-use-of-emerging-science-for-environmental-health-decisions.
Suggested citation: National Academies of Sciences, Engineering, and Medicine. 2020. Integrating the Science of Aging and Environmental Health Research: Proceedings of a Workshop—in Brief. Washington, DC: The National Academies Press. https://doi.org/10.17226/25908.
Division on Earth and Life Studies

Copyright 2020 by the National Academy of Sciences. All rights reserved.














