6
Prescription for Reducing Discarded Drugs
The study committee was charged with examining the federal health care costs, safety, and quality concerns associated with discarded drugs that result from weight-based dosing of medicines contained in single-dose vials. In this chapter, the committee summarizes its goals and recommendations for policy, practice, and research as they relate to the study’s charge.
Understanding the ways in which drug waste is conceptualized is an important part of any approach to address the costs associated with discarded excess drugs contained in single-dose vials. As discussed in previous chapters, on the surface it can seem extremely wasteful that health care providers discard significant portions of the drug in single-dose vials that can cost thousands of dollars. And, indeed, it is that appearance of significant waste that has led observers to attempt to calculate the total value of drugs “wasted” in this way each year and to look for ways to minimize this waste. However, as discussed in Chapter 5, the concept of waste is not the appropriate construct for assessing and addressing the issue of discarded drugs. Rather, the primary focus should be on the marginal costs of producing the drugs and the factors that go into determining their pricing and paying for them.
Any decision made by U.S. policy makers or regulators would have global implications because the United States is the biggest worldwide purchaser of drugs, and regulators in the rest of the world often follow U.S. Food and Drug Administration (FDA) guidance. Therefore, appropriate conceptualization of discarded drugs is vital for policy makers to
understand the trade-offs among different approaches for addressing the problem.
The fundamental issues addressed by this report can inform an overarching approach to understanding the causes and implications of the large amount of discarded drugs and to reducing certain inefficiencies in the system in which drugs are developed, administered, or paid for. This could set a precedent that the rest of the world may choose to adopt.
GOALS AND RECOMMENDATIONS
The committee’s analysis of the economics of weight-based drugs in single-dose vials (see Chapter 5) led them to a single profound conclusion:
Under the current system in which drugs are developed, administered, or paid for, when the drug is discarded, there is no money to recoup. Therefore, there is limited economic value to discarded injectable or infused drugs from single-dose vials.
While the committee asserts there is little recoverable value to discarded drugs, it does see several opportunities to reduce inefficiencies that could lead to cost savings and improve the quality of care for patients. Therefore, the committee’s overarching recommendation1 is:
RECOMMENDATION 5-1: Drug developers, health care providers, and payers should focus their efforts on reducing inefficiencies in drug development, delivery, and payment systems that lead to excess costs for both the health care system and for patients rather than on trying to recoup payments associated with the discarded drugs.
To lay the foundation for strategies to reduce certain inefficiencies that lead to discarded drugs while ensuring patient safety and quality care, the committee presents two broad goals and eight associated recommendations. The goals and proposed actions are oriented toward changing incentives and increasing efficiency in how these drugs are developed, administered, and paid for. The committee recognizes that successfully addressing the issue of discarded drugs will necessitate an implementation process that requires effective collaborations and efforts by all stakeholders in the biopharmaceutical supply chain. Therefore, in formulating its recommendations, the committee has considered the ways in which a proposed action could change if another is not carried out. In this sense,
___________________
1 The committee’s recommendations are numbered according to the chapter of the main report in which they first appear. Thus, Recommendation 5-1 is in Chapter 5.
the committee’s recommendations should ideally be viewed as a “package.” The committee’s recommendations, restated below, are supported by the findings and conclusions at the end of previous chapters.
Goal 1: Promote the Effective, Efficient, and Safe Use of Infused or Injectable Drugs
Weight-based dosing (which, for the purposes of this report, includes dosing based on body surface area [BSA]) is a common method for dose determination for infused and injectable drugs because of how early drug testing and clinical trials are currently conducted. The dosing for a drug is determined in the clinical trials that are performed, typically during the early stages of the development process, to demonstrate that the drug is safe and effective. As discussed in Chapter 2, the dosing that is ultimately shown to be safe and effective in pivotal trials is the one that regulators will approve for marketing. Pivotal trials are intended to provide the evidence and data that FDA ultimately uses to decide whether to approve a potential new medicine. Thus, if weight-based dosing is used in these pivotal trials, then a weight-based dosing regimen will be approved. Evidence from other dosing practices, such as dose banding and dose capping, suggests that alternatives to weight-based dosing might be just as safe and effective for many indications, but pivotal trials rarely compare weight-based dosing with other methods.
Empirical evidence supporting the use of weight-based dosing instead of fixed dosing for injectable or infused drugs is limited.
The use of trials considering different dosing strategies (e.g., weight based versus fixed dose) early in the drug development process could help to determine if dosing based on a patient’s body size (e.g., weight or body surface area) provides greater benefit than a fixed dose for a given therapeutic agent.
RECOMMENDATION 2-1: The U.S. Food and Drug Administration should require sponsors of pivotal trials for new or extended therapeutic indications to use fixed dosing for a given clinical indication unless safety and efficacy would be compromised. Manufacturers of products already in the market should consider conducting additional studies after approval so that these drugs can, when indicated, be converted to fixed dosing.
Drugs contained in a single-dose vial are intended to be used a single time for a single patient. Single-dose vials usually do not contain
antimicrobial preservatives that help prevent contamination from bacterial growth, which is why the remainder is typically discarded immediately after a dose is drawn. Multiple-dose vials, however, are intended to contain more than one dose. They have preservatives to stop bacterial growth but can be contaminated if safe injection practices are not followed. To complicate matters, current federal policies, research, and practice relevant to the costs associated with discarded drugs contained in single-dose vials are fragmented across a variety of agencies and professional organizations.
The committee has identified significant conflicts among the safety concerns that motivate the regulatory guidance on the repackaging of single-dose drugs—that is, dividing the content of a single-dose vial into multiple individual doses—by pertinent federal agencies. For example, according to FDA, using multiple single-dose vials to treat a single patient can lead to medication errors and sharing a single vial among multiple patients can lead to contamination. However, the Centers for Medicare & Medicaid Services (CMS) permits health care providers to administer repackaged doses to multiple patients, provided that each repackaged dose is used for a single patient. In contrast, guidance from the Centers for Disease Control and Prevention (CDC) states that vials labeled by the manufacturer as single dose or single use should be used only for one patient and repackaging is not acceptable.
A number of other Organisation for Economic Co-operation and Development (OECD) countries have well-established clinical and operational approaches that allow for delivering medication to multiple patients from a single vial through vial sharing. Several others have promoted the use of standardized doses. For example, dose banding, a clinical practice in which drug doses that fall within predefined ranges established through clinical calculations are rounded up or down for patients, is widely used in the United Kingdom and other parts of Europe. Such upward or downward rounding is typically designed to allow use of a complete vial, without discarding any medication.
Nonetheless, efforts to standardize the delivery of medication to multiple patients from a single-dose vial, when necessary and allowed, currently entail practical operation costs and potential regulatory hurdles, whereas the marginal costs of manufacturing are low. As discussed in previous chapters, increasing the number of different vial sizes would come at a cost. Each size is a different product from the manufacturer’s perspective and raises the overall cost of production.
Regulatory guidance governing vial sharing varies among the U.S. Food and Drug Administration, the Centers for Medicare & Medicaid Services, the Centers for Disease Control and Prevention, and the
United States Pharmacopeia, and thus creates ambiguity and confusion for clinicians and health care administrators, although there is limited information about the safety of vial sharing.
Several technologies and clinical strategies show promise for reducing discarded drugs from single-dose vials but are not encouraged by current policies or are not feasible without reducing current practice-based constraints.
Vial sharing and other clinical practices such as dose banding and dose rounding are possible mechanisms for reducing discarded drug. For small clinics and hospitals, vial sharing may be challenging to coordinate due to limited patient volume as well as the need to obtain special equipment to allow prolonged storage for safe drug use.
RECOMMENDATION 2-2: The Secretary of the U.S. Department of Health and Human Services should direct the Centers for Medicare & Medicaid Services, the U.S. Food and Drug Administration, and the Centers for Disease Control and Prevention to work with the United States Pharmacopeia and other nonfederal partners, pharmacists, and infectious disease experts to review and harmonize existing policies and guidelines on drug administration and repackaging. These policies and guidelines should be informed by the successful experiences from other industrialized countries in reducing the amounts of discarded drugs.
RECOMMENDATION 4-1: The Secretary of the U.S. Department of Health and Human Services should direct the Centers for Medicare & Medicaid Services, the U.S. Food and Drug Administration, and the Centers for Disease Control and Prevention to initiate a partnership with other agencies, including the U.S. Department of Defense and the U.S. Department of Veterans Affairs, to work with health care and other organizations with expertise in areas such as industrial design and systems engineering to identify and implement technological systems that allow single-dose vials to be used safely across multiple patients.
RECOMMENDATION 4-2: The Secretary of the U.S. Department of Health and Human Services (HHS) should develop and implement policies that require drug manufacturers to produce injectable and infused drugs in multiple-dose vials when it is safe to do so. The Secretary of HHS should routinely review and evaluate the impact of such policies.
Goal 2: Implement an Efficient and Effective Reimbursement System for Clinician Administration of Infused or Injected Drugs
The biopharmaceutical supply chain, especially in the United States, contains numerous intermediaries who negotiate drug prices, establish the lists of drugs covered by insurance, and act as drug distributors, prescribers, and patients. For Medicare Part B, prescribers largely determine which drugs are to be purchased, and patient cost-sharing is specified by insurance plans. Medicare and private payers reimburse health care providers for the drugs they administer based on the drug’s average sales price (ASP) plus a percentage add-on for administering the drug to account for costs incurred in drug delivery, mixing, and storage. Because the add-on is based on a percentage, the dollar amount is larger when the drug price is high. Thus, the reimbursement for the drug and the add-on may incentivize the selection and administration of higher cost drugs. Uncoupling the payments from the drug ASP, and focusing the payment for clinical administration on CMS’s assessment of the time and complexity of treatment administration and safety monitoring would minimize incentives for clinicians’ selection and administration of more expensive drugs when alternatives are available.
Accordingly, the concern about discarded drugs is partly due to the particular approach to paying—and reimbursing health care providers—for these drugs. Currently, under Medicare Part B, a clinician or health care provider (clinic, physician office, or hospital) typically buys drugs directly from manufacturers or from intermediaries, such as wholesalers or specialty pharmacies, stores them, and administers them to a patient as needed. The health care provider then submits a claim to the patient’s health insurer for reimbursement, including for any drug that is discarded. The committee recognizes the importance of how changes to the current reimbursement for clinician-administered drugs would have a substantial impact, not only on health care providers but also on Medicare, private insurance payers, and patients, and that any actions toward that end may require modifications to existing law.
Under the current system in which drugs are developed, administered, or paid for, health care providers do not have incentives that encourage the efficient use of infused or injectable drugs in single-dose vials. They receive payment for up to the amount of drug indicated on the label of a single-dose vial, including that which is discarded. This lack of incentives could discourage efforts to reduce the amount of discarded drugs.
RECOMMENDATION 2-3: The Secretary of the U.S. Department of Health and Human Services should require the Centers for Medicare & Medicaid Services (CMS) to uncouple add-on payments to clinicians for infused or injected drugs under Medicare Part B from the drug average sales price, focusing the add-on payment instead on CMS’s assessment of the time and complexity of drug management and safety monitoring.2
RECOMMENDATION 2-4: The Secretary of the U.S. Department of Health and Human Services should require the Center for Medicare & Medicaid Innovation to design and evaluate new payment models that reimburse health care providers by treatment episode, rather than by the volume or cost of a drug vial. Findings from the demonstrations should be disseminated widely to other relevant federal agencies, private payers, purchasers, clinicians, and consumers.
Currently, the JW modifier is the mechanism through which the discarded amount of drug from a single-dose vial is documented and reimbursed. As discussed in previous chapters, the very nature of administering single-dose drugs makes them prone to some portions being discarded. Medicare reimburses health care providers the amount of drug indicated on the vial or package label of a single-dose product, including what is discarded. The requirement for coding with the JW modifier appears to be an administrative burden for health care providers because the level of compliance is highly variable. Not all health care providers use the code, and those who do use it do so inconsistently. Some observers have argued that enforcing the requirement for using the JW modifier would lead to increased transparency regarding discarded drug payments. Others, however, argue that the JW modifier provides an economic incentive for discarding drugs and hinders efficient ways to distribute or administer drugs because it allows health care providers to be reimbursed regardless of whether all of the drug is actually used.
The committee recognizes that several analyses using data from the JW modifier have reported that the discarded drugs may be costing CMS billions of dollars annually. The estimates do not accurately capture the economic value of discarded drugs because they are based on the assumption that every ounce in a vial has an equal value and that the value of an ounce administered to treat a patient is equal to the value of an ounce of drug that is discarded, which, in reality, is not the case. As explained in
___________________
2 The text in this recommendation was modified since the prepublication release of this report to improve technical accuracy of the language around payments to clinicians for infused or injected drugs under Medicare Part B.
previous chapters, it is easy enough to multiply the per-milliliter cost of a drug by the number of milliliters discarded each year to get a putative value for how much is “wasted” and then add up all such drugs to get a total, but interpreting that number as the value of the discarded drugs is problematic, given the decisions that go into pricing and reimbursing for these drugs in this country. The price of a drug is typically based not on how much is used but on the willingness to pay for the drug’s therapeutic benefit. Put differently, because manufacturing costs do not drive drug prices, the quantity of a drug that a patient receives also does not drive pricing. Because of this, payment strategies used in many other countries provide a uniform reimbursement for a given treatment no matter how much of the drug was required for a specific patient.
Based on this assessment, as previously noted, the committee concludes that a significant amount of money cannot be recouped from discarded drugs to be used to offset federal health care costs. In essence, compliance with the JW requirement would enable CMS to track the amount of drug that is discarded from single-dose vials but does not offer practical ways to reduce the amount of discarded drugs.
Currently, attempting to estimate a single value for the cost of discarded drugs from single-dose vials to the Medicare program using the JW modifier would be incomplete and underestimated because the modifier is underused by health care providers and the resulting data may not be representative. Also, information on discarded drugs from Medicare Advantage plans and other commercial insurance plans are currently not available.
The use of the JW modifier as a basis for data collection and measurement of the amount of discarded drugs from single-dose vials has no current practical application to addressing the issue of discarded drugs under the current system in which drugs are developed, administered, or paid for.
RECOMMENDATION 3-1: While the current Medicare Part B drug reimbursement system is in place, the Centers for Medicare & Medicaid Services should discontinue the use of the JW modifier.
Several versions of draft congressional legislation focused on discarded drugs from single-dose drug vials have proposed rebates from manufacturers to health care providers and payers for discarded drug amounts. However, most bills do not propose refunds directed to patients, who would continue to have cost-sharing responsibilities for the cost of discarded drugs that were not used in their care.
Legislative initiatives proposing manufacturer rebates to providers and or payers for discarded drugs ignore the fact that a patient often would have been required to pay part of the cost of this discarded drug.
Based on the committee’s assessment, it is not feasible to recoup significant funds via rebates for discarded drugs that could be spent on other health care services, so manufacturer rebates for unused drug are unlikely to achieve the intended aim. Also, given the variety of ways that drugs are priced and reimbursed, a rebate system for clinician-administered drugs would likely be quite complex. Because the committee’s assessment shows that there is limited economic value to discarded drugs from single-dose vials under the current system in which drugs are developed, administered or paid for, a rebate strategy seems unlikely to achieve the intended goals. However, if the rebates that have been proposed in legislation are indeed implemented, the committee asserts that patients need to be included in those rebates.
RECOMMENDATION 4-3: In the event that legislation is enacted or regulatory action is taken to require rebates from manufacturers for discarded drugs, the U.S. Congress should require that rebates be directed first to cover the patient’s out-of-pocket expense for the discarded drug and thereafter to health care providers and payers.
INTERDEPENDENCIES OF RECOMMENDATIONS
The nation’s biopharmaceutical sector—including the researchers who develop and test drugs, drug manufacturers and distributors, pharmacies, the hospitals and health care providers who administer the drugs, the payers (such as Medicare and private health plans), and the various agencies that regulate drug development, manufacture, use, and reimbursement—is a complex, interconnected system. Changes in one part of the system inevitably affect other parts, often in unanticipated or even undesirable ways. Those looking to implement the recommendations in the report should keep this in mind and be aware of how a proposed change in one area may also affect other areas.
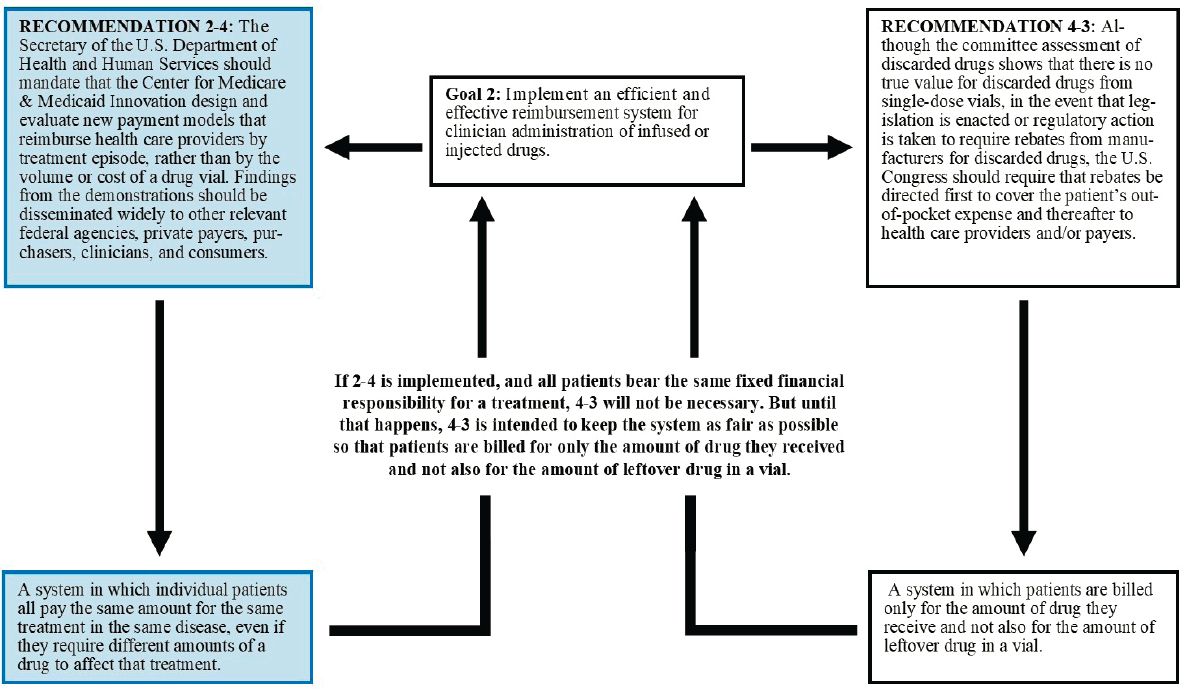
Policy makers should also note that this report’s recommendations are interdependent, although their interrelationships vary. In some cases, two or more recommendations are designed to have similar effects, such as the multiple recommendations intended to move the system away from the current standard practice of discarding whatever drug is left in a vial after a single patient has been treated rather than sharing the vial among patients. In other cases, recommendations may focus on different issues. The ultimate goal of Recommendation 2-4, for instance, is to create a more
equitable payment system. The committee recognizes that different people may have different goals in achieving an equitable payment system, including all patients paying the same amount for the same treatment or payment based on need, ability to pay, or to ensure that the needs of the most vulnerable are first addressed. If this goal is met, Recommendation 4-3 may not be necessary. But until that payment system is achieved, the recommendation to cover patients’ out-of-pocket expenses with manufacturer rebates is intended to keep the system as fair as possible, so that patients are billed only for the amount of drug they receive and not also for the amount of leftover drug in a vial—the current system charges patients based on a factor totally unrelated to the patient (the size of the vial in which the manufacturer has chosen to package the drug) (see Figure 6-1).
Also, consider Recommendations 2-1 and 4-2. Both strive to promote the effective use of infused or injectable drugs in efficient and safe ways but feature different approaches. The ultimate goal of Recommendation 2-1 is to reduce the proportions of the content of vials that are discarded. Fixed doses reduce the proportion of drugs that are discarded but would not completely eliminate leftover drug. For example, in the case where different amounts of fixed doses are used for different indications of a drug that is available only in a vial that is sized for one of those indications, some drug may still be discarded. However, weight-based dosing results in a wide range in doses and amount of drug needed per patient. Consequently, it is almost certain that there will be remaining drug in the vial. The goal of Recommendation 4-2 is to reduce the proportions of drugs discarded by allowing the content of a vial to be used for more than one patient. If this recommendation is implemented, Recommendation 2-1 may not be necessary.
Policy makers need to be aware of and prepared for the possibility of unintended consequences of implementing new policies. For example, as long as drug manufacturers have near-total freedom to set drug prices, they will likely react to policies that reduce their profits. Thus if Recommendation 4-3 is adopted, and rebates from manufacturers for discarded drugs are directed to patients to cover their out-of-pocket expense, manufacturers may respond by increasing the price of individual vials so that the profit margins they realize from a particular drug remain approximately the same. Another potential unintended consequence is that the widespread use of multiple-dose vials envisioned in Recommendation 4-2 could give an advantage to larger medical centers, where there are more likely to be multiple patients who need the same drug at approximately the same time and thus can jointly receive the contents of a multi-dose vial. Smaller centers would be less likely to benefit due to vials’ expiration between recipients.
While the committee’s recommendations aim to achieve greater efficiencies in the manufacturing, distribution, and use of injected or infused
drugs, it is not possible to predict how much, if any, financial savings might be associated with implementing those recommendations in the absence of additional data for economic analyses. The case for these recommendations is based mainly on nonfinancial considerations, such as safety and equity and overall efficiency.
The Pervasive Issue of Drug Pricing
As discussed in previous chapters, the issue of discarded weight-based drugs from single-dose vials takes place in the larger context of concerns about skyrocketing drug prices in the country. One of the clearest lessons from this investigation into weight-based drugs is the burden that the cost of such drugs places on many patients who rely on them to extend or save their lives. Regardless of whether some portion of a drug vial is discarded, many of these drugs are very expensive—thousands of dollars per treatment is not unusual—and traditional fee-for-service Medicare beneficiaries pay up to 20 percent of the billed amount for their medical care, without a cap on annual expenses. Supplemental private insurance and, in some cases, Medicaid can reduce out-of-pocket costs significantly, although that burden may not be totally eliminated.
Concerns about reducing discarded drug take place in the larger context of concerns about drug costs, and particularly the cost to patients, in the United States.
Under the current system in which drugs are developed, administered or paid for, approaches to reduce the discarding of drugs from single-dose vials could have unintended consequences. Successful efforts may eventually trigger drug companies to raise the prices to derive constant or increased revenue for their products. Approaches to reducing discarded drugs from single-dose vials would need to leverage other strategies aimed at lowering drug prices, including allowing the Secretary of the U.S. Department of Health and Human Services to negotiate directly with manufacturers regarding the price of drugs.
It is important to emphasize the connection between discarded drugs from single-dose vials and the resulting economic hardship for many Americans due to the prices of these expensive drugs. The high costs of U.S. prescription drugs are particularly burdensome for older Americans in the Medicare population—individuals who often have multiple chronic conditions, such as cancer, rheumatoid arthritis, and other immune disorders—and for individuals who are unfortunate enough to develop a disease whose treatment requires more expensive drugs. Among Medicare
beneficiaries, those without supplemental insurance incur substantially higher out-of-pocket costs than their counterparts with private or other supplemental coverage. Even for those individuals with health insurance, most have deductibles, copayments, or coinsurance to pay.
The 2018 National Academies of Sciences, Engineering, and Medicine report Making Medicines Affordable: A National Imperative put forward a range of proposals to help prevent drug expenditures from spiraling out of control without discouraging continued innovation in drug development. This committee endorses the proposals in that report. In Chapter 5, the committee described how allowing the Secretary of the U.S. Department of Health and Human Services to negotiate directly for drugs could reduce the overall spending on Part B drugs for Medicare and for patients. This committee recognizes the importance of that proposal to reduce drug prices and to guard against unintended consequences in efforts to reduce discarded drugs and the associated costs, and it also recognizes that allowing the secretary to negotiate directly would require modifications to the Medicare Modernization Act of 2003 prohibiting the Medicare program from negotiating the price of drugs with manufacturers. In short, the set of recommendations offered in this report would yield significant results if they are leveraged with these other broader strategies aimed at lowering drug prices in the United States.
A PIVOTAL TIME FOR ACTION
Ideally, implementing these recommendations would lead to a future in which the development, use, and payment for weight-based drugs look different in a number of ways. First, fewer drugs would be administered based on weight. While there may always be certain drugs that are best administered in doses based on weight, BSA, or some related patient characteristic, many of the weight-based drugs being used today would likely work just as well with fixed doses, eliminating the need to calculate the dose by a patient’s weight. The general assumption in oncology that drugs should be administered according to weight needs to be challenged, so that only those drugs that truly require it are developed and studied in trials as weight-based drugs.
For those drugs that do require weight-based dosing, the ideal future will be one in which multiple-use vials are much more common, minimizing the amounts of these expensive drugs that must be discarded. To the extent that there still is a need for vials smaller than today’s single-dose vials, ideally, these should also be designed and manufactured, or managed using new technologies (Recommendation 4-1), so that they can be used by more than one patient.
Finally, in the ideal future, payments for weight-based drugs will be more fair than they are today and drugs more affordable for all patients who need them. Moving away from payments based on the amount of drug used and toward episode-based payments will make the system more fair and more manageable, but the recommendations here can do relatively little to make drugs more affordable. Uncoupling physician payments for administration from drug price (Recommendation 2-3) will remove one incentive for physicians to use more expensive drugs. Ultimately, ensuring drug affordability will require a much broader and more sustained effort, of the sort described in the Making Medicines Affordable report, which is why Chapter 5 of this report reiterates some of the recommendations from that study.
The United States needs to act now more than ever due to an aging society and other factors, including the rising cost of health care.














