Summary1
In response to the coronavirus disease 2019 (COVID-19) pandemic and the societal disruption it has brought, national governments and the international community have invested billions of dollars and immense amounts of human resources to develop a safe and effective vaccine in an unprecedented time frame. Vaccination against this novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), offers the possibility of significantly reducing severe morbidity and mortality and transmission when deployed alongside other public health strategies (e.g., non-pharmaceutical interventions and better diagnostic tests) and improved therapies. According to the World Health Organization (WHO), 149 COVID-19 vaccines are currently in pre-clinical development and 38 candidate vaccines are undergoing evaluation in clinical trials in the United States, Europe, and China. Domestically, the U.S. government has homed in on six COVID-19 vaccine candidates, with four currently in Phase 3 trials: the Johnson & Johnson JNJ-78436735, the Moderna/NIAID mRNA 1273, the University of Oxford/AstraZeneca AZD1222, and the Pfizer and BioNTech BNT162.
However, even if one or more safe and effective COVID-19 vaccines under development are authorized for use, they are very unlikely to be immediately available in amounts sufficient to vaccinate a large portion of the U.S. population, despite plans to begin large-scale production of promising vaccines even before trials are completed. Planning is urgently needed to
___________________
1 This Summary does not include references. Citations for the discussion presented in the Summary appear in the subsequent report chapters.
ensure equitable access to COVID-19 vaccine. To prepare for the inability to meet the anticipated high demand for COVID-19 vaccine in the early stages of availability, the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) asked the National Academies of Sciences, Engineering, and Medicine (the National Academies), in partnership with the National Academy of Medicine, to convene an ad hoc committee to develop an overarching framework for vaccine allocation to assist policy makers in the domestic and global health community. The full charge to the committee is presented in Chapter 1.
This report offers a framework for equitable allocation of COVID-19 vaccine. It is built on widely accepted foundational principles and recognizes the distinctive characteristics of COVID-19 disease, including its rates of infection, its modes of transmission, the groups and individuals most susceptible to infection, and varying rates of severe illness and death among those groups. This report’s recommendations address the institutional and administrative commitments needed to implement equitable allocation policies.
COVID-19 AND HEALTH EQUITY
Race and ethnicity and health equity are intertwined with the impact of COVID-19 and there are certain populations that are at increased risk of severe illness or death from COVID-19. In the United States and worldwide, the COVID-19 pandemic has shed light on the pervasive impacts of social and structural inequities in society. COVID-19 is having a disproportionate impact on people who are already disadvantaged by virtue of their race and ethnicity, age, health status, residence, occupation, socioeconomic condition, and/or other contributing factors. At a moment when racial inequality and discrimination are at the center of national conversations in the United States, and a well-established source of poor health outcomes as well as the legacy of medical experimentation, these considerations must be a critical component of COVID-19 vaccine allocation. The committee weighed these realities not only because of their moral and ethical implications, but also because, in our highly interconnected world, the challenges experienced by particular subpopulations have an effect on us all. If we have learned anything from this pandemic, it is that we are inevitably all in this together.
Current evidence has shown how COVID-19 disproportionately affects particular racial and ethnic minority groups, including Black, Hispanic or Latinx, American Indian and Alaska Native, and Native Hawaiian and Pacific Islander communities. Many of these groups disproportionately face social and structural factors and comorbid conditions that put them at higher risk of severe morbidity and mortality from COVID-19. Furthermore, historically, non-Hispanic Whites have had higher coverage for routine
immunizations compared to racial and ethnic minority groups. CDC has compiled data by race and ethnicity on the rates of COVID-19 cases, age-adjusted hospitalizations, and death. Compared to non-Hispanic Whites, American Indian and Alaska Native persons had a case rate that was 2.8 times higher, a hospitalization rate that was 4.6 times higher, and a death rate that was 1.4 times higher. Hispanic or Latinx persons had a case rate that was 2.8 times higher, a hospitalization rate that was 4.7 times higher, and a death rate that was 1.1 times higher. Black and African American persons had a case rate that was 2.6 times higher, a hospitalization rate that was 4.7 times higher, and a death rate that was 2.1 times higher.
COVID-19 has also disproportionately affected members of other groups (see Table S-1). In particular, older adults are extremely vulnerable to severe outcomes and death due to COVID-19; people aged 65 and older represent 8 out of every 10 reported deaths due to COVID-19 in the United States.
TABLE S-1 Key Data on the Impact of COVID-19 on Certain Populations
| Population | Key Impact Data |
|---|---|
| Black |
|
| Hispanic/Latinx |
|
| American Indian and Alaska Native |
|
| Native Hawaiian and Pacific Islander |
|
| Older adults (≥65 years) |
|
| Older adults (>80 years) |
|
| Population | Key Impact Data |
|---|---|
| People with underlying or comorbid conditions |
|
| People who live and/or work in congregate settings |
|
| Sex |
|
| Children |
|
| People who are pregnant or breastfeeding |
|
NOTES: This table is included in Chapter 1 with references. The following groups are omitted from the table due to a lack of COVID-specific epidemiological data: people who are undocumented, people with mental and physical disabilities, and people experiencing homelessness.
LESSONS LEARNED FROM OTHER ALLOCATION EFFORTS
This is not the first time the nation, or the world, has faced the issue of allocating what is likely to be an early scarcity of resources in the midst of a public health emergency. Plans drawing on those experiences are beginning to emerge for ensuring equitable allocation of vaccines and therapeutics for COVID-19. The committee began its work by reviewing lessons learned from previous mass vaccination efforts in the United States and globally, including from the 2009 H1N1 influenza vaccination campaign and the 2013–2016 vaccination efforts during the Ebola outbreak in West Africa. These lessons are described in Box S-1.
The committee also reviewed and synthesized relevant elements of principles, goals, and prioritization strategies proposed in other frameworks recently developed for allocating scarce resources during the COVID-19 pandemic. Some of these frameworks are vaccine specific (including an interim framework developed by a group at Johns Hopkins University, forthcoming efforts from CDC, and a values framework developed by WHO), some focused on inpatient treatments (like remdesivir), and others address the overall allocation of scarce medical resources. These frameworks are discussed in detail in Chapter 2.
A FRAMEWORK FOR EQUITABLE ALLOCATION OF COVID-19 VACCINE
Foundational Principles, Goal, and Allocation Criteria
The committee based its framework for equitable allocation of COVID-19 vaccine on current evidence, recognizing its uncertainties and the need for flexibility as evidence emerges and medical realities change. The framework’s foundational principles guide its goal, allocation criteria, and allocation phases (see Figure S-1).
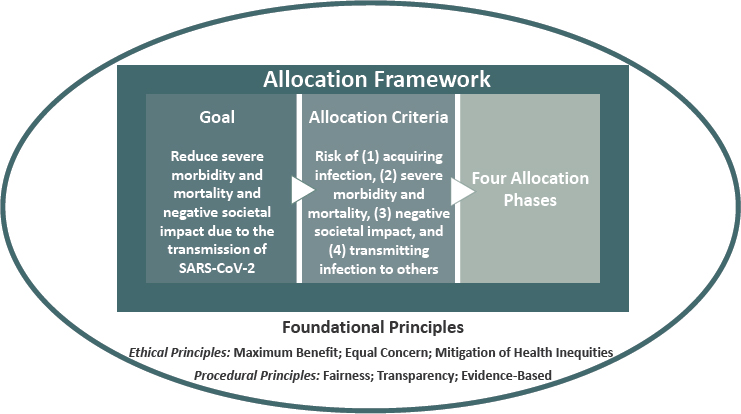
To ensure that the allocation framework is equitable and can be seen as equitable, the committee designed its framework so that it (1) can be easily and equally understood by diverse audiences, (2) reflects widely accepted social and ethical principles, (3) can be reliably translated into operational terms, (4) distinguishes scientific and ethical judgments in its application, and (5) does not perpetuate discrimination and inequities. The foundational principles consist of ethical and procedural principles that reflect this line of thinking:
- Ethical Principles
- Maximum benefit encompasses the obligation to protect and ° promote the public’s health and its socioeconomic well-being in the short and long term.
- Equal concern requires that every person be considered and ° treated as having equal dignity, worth, and value.
- Mitigation of health inequities includes the obligation to explicitly address the higher burden of COVID-19 experienced by the populations affected most heavily, given their exposure and compounding health inequities.
- Procedural Principles
- Fairness requires engagement with the public, particularly ° those most affected by the pandemic, and impartial decision making about and even-handed application of allocation criteria and priority categories.
- Transparency includes the obligation to communicate with the public openly, clearly, accurately, and straightforwardly about the allocation framework as it is being developed, deployed, and modified.
- Evidence-based expresses the requirement to base the allocation framework, including its goal, criteria, and phases, on the best available and constantly updated scientific information and data.
Guided by these foundational principles, the goal of the committee’s framework for equitable allocation of COVID-19 vaccine is to:
Reduce severe morbidity and mortality and negative societal impact due to the transmission of SARS-CoV-2.
The framework pursues that goal while mitigating health inequities, showing equal concern for all, being fair and transparent, and building on the best available evidence. Given the current state of the pandemic, the early phases of the committee’s proposed framework emphasize prevention of severe morbidity and mortality, particularly with regard to maintaining essential health and emergency services. The focus shifts toward reducing transmission in later phases. There are multiple reasons for this approach:
- Death is an irreversible outcome. There are legitimate claims for many groups (e.g., schoolchildren, “non-essential” workers) to be in earlier phases as negative societal impact could occur if these groups are not prioritized. For example, there might be a substantial impact on the economy if a primarily transmission-focused strategy is not employed from the outset. However, the non-trivial effects of an economic downturn or an online semester can at least be partially reversed.
- Preventing severe morbidity and mortality protects the health care system from being overwhelmed, contributing to the prevention of excess morbidity and mortality from other causes as well, with ripple effects on society and the economy.
- For vaccination to materially reduce transmission requires vaccinating a critical mass of individuals, much greater than will be possible in the early phases of vaccine deployment.
- The ongoing COVID-19 vaccine trials are not designed to estimate the impact of the vaccine candidates on transmission and evidence of the vaccines’ actual impact on transmission might not be available for some time after U.S. Food and Drug Administration approval.
- While data on all aspects of COVID-19 are emerging, data on transmission risk groups (e.g., age, profession) are particularly limited.
To operationalize its foundational principles, the committee developed four risk-based criteria that were then used to set general priorities among population groups.
- Risk of acquiring infection: Individuals have higher priority to the extent that they have a greater probability of being in settings where SARS-CoV-2 is circulating and of being exposed to a sufficient dose of the virus.
- Risk of severe morbidity and mortality: Individuals have higher priority to the extent that they have a greater probability of severe disease or death if they acquire infection.
- Risk of negative societal impact: Individuals have higher priority to the extent that societal function and other individuals’ lives and livelihood depend on them directly and would be imperiled if they fell ill.
- Risk of transmitting infection to others: Individuals have higher priority to the extent that there is a higher probability of their transmitting the infection to others.
The committee recognizes that decisions about COVID-19 vaccine allocation must be made under conditions of uncertainty. These unknowns include the safety and efficacy of the vaccines in specific populations (such as children, pregnant women, older adults, and individuals previously infected with COVID-19), the effectiveness of vaccines in tandem with existing preventive measures, public confidence in the vaccine, the possibility of ultra-cold storage requirements for the vaccine, the pharmacovigilance evidence, and many other unknowns. Chapter 4 describes how the allocation process can adapt to plausible scenarios involving these factors.
Allocation Phases
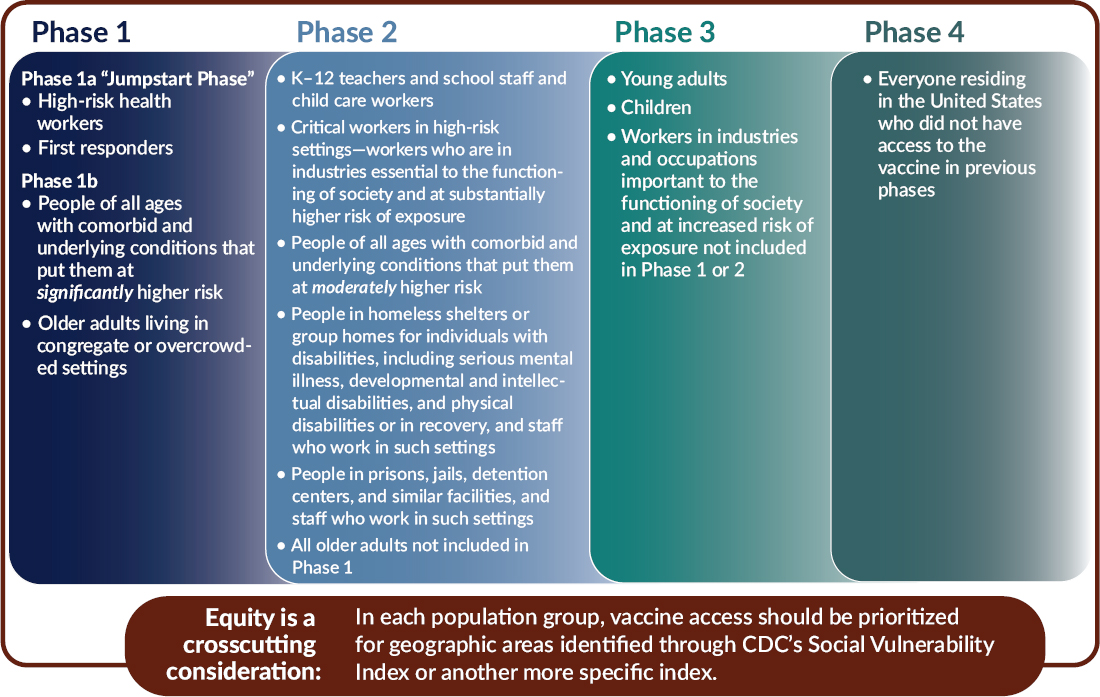
In light of the foundational principles, goal, and allocation criteria, the committee recommends a four-phased approach to equitable COVID-19 vaccine allocation (see Figure S-2 and described in detail in Chapter 3). The committee uses the term “phases,” suggesting successive deployments, rather than the hierarchical term “tiers.” Within each phase, all groups have equal priority. This approach applies the best available current evidence to implementing the framework’s foundational principles. It cannot be emphasized enough that the dynamic nature of the COVID-19 pandemic means that features of the pandemic will change over time, as will collective understanding of its effects.
For each population group, the committee recommends prioritizing for areas identified as vulnerable through CDC’s Social Vulnerability Index
(SVI) or another more specific index such as the COVID-19 Community Vulnerability Index (CCVI). The evidence clearly shows that people of color—specifically Black, Hispanic or Latinx, American Indian and Alaska Native, and Native Hawaiian and Pacific Islander—have been disproportionately impacted by COVID-19, with higher rates of severe morbidity, mortality, and transmission. This disproportionate burden largely reflects the impacts of systemic racism and socioeconomic factors that are associated with increased likelihood of acquiring the infection (e.g., frontline jobs that do not allow social distancing, crowded living conditions, lack of access to personal protective equipment, inability to work from home) and of having more severe disease when infected (as a result of a higher prevalence of comorbid conditions or other factors). Use of a vulnerability index, like SVI or CCVI, represents an attempt to incorporate the variables that the committee believes are most linked to the disproportionate impact of COVID-19 on people of color. A vulnerability index allows the efficient focus of resources on these needs instead of on discrete racial and ethnic categories. The committee does not propose an approach in which, within each phase, all vaccine is first given to people in high-SVI areas. Rather the committee proposes that state, tribal, local, and territorial (STLT) authorities ensure that special efforts are made to deliver vaccine to residents of high-vulnerability areas (defined as 25 percent highest in the state).
Summary of the Population Groups Within Each Allocation Phase
As summarized here and described more fully in Chapter 3, the committee based its specific proposals on broad estimates of the number of individuals covered across each phase of the allocation framework, a practice also used by WHO. Importantly, the committee acknowledges that the population groups included in each allocation phase overlap to a certain extent, and there are assuredly individuals who fit into multiple categorizations. When individuals within a group fall into multiple phases, the higher phase should take precedent. It also recognizes the heterogeneity within each group, with some members facing less risk and having greater ability to protect themselves and others. The framework provides guidance to the STLT authorities administering the program in adapting its risk-based criteria to these realities in ways consistent with its foundational principles.
Phase 1
Phase 1 of the allocation framework has two subsections: a “Jumpstart” Phase 1a that covers approximately 5 percent of the U.S. population, and a Phase 1b covering an additional 10 percent.
Phase 1a includes high-risk health workers (e.g., in hospitals or nursing homes, or providing home care). These health professionals are involved in direct patient care. Also included are workers who provide transportation, environmental services, and other health care facility services and who risk exposure to bodily fluids or aerosols. This group is included in Phase 1a for multiple reasons: their critical role in maintaining health care system functionality, their high risk of exposure to patients exhibiting symptoms of COVID-19, and their risk of then transmitting the virus to others, including family members. This is of particular concern for those workers who are members of communities that have been disproportionately impacted by COVID-19. First responders whose jobs put them at high risk of exposure to COVID-19 are also included in Phase 1a (although depending on the jurisdiction and outbreak context, this may not include all first responders). Like frontline health workers, first responders play vital roles in both the response to COVID-19 and society’s overall functioning.
Phase 1b focuses attention on two groups that are particularly vulnerable to severe morbidity and mortality due to COVID-19: (1) people of all ages with comorbid and underlying conditions that put them at significantly higher risk and (2) older adults living in congregate or overcrowded settings. CDC currently lists the following comorbid conditions as associated with increased risk of severe COVID-19 disease: cancer, chronic kidney disease, chronic obstructive pulmonary disease, immunocompromised state from solid organ transplant, obesity (body mass index ≥30), serious heart conditions (e.g., heart failure, coronary artery disease, cardiomyopathies), sickle cell disease, and type 2 diabetes mellitus. Recognizing the limited initial vaccine supply, Phase 1b proposes setting a priority on individuals with two or more of these conditions, recognizing that these priorities can be refined as better evidence emerges. Based on data from the COVID-19 Associated Hospitalization Surveillance Network (COVID-NET), adults with two or more comorbid conditions make up the large majority of those hospitalized for COVID-19 in the United States.
Phase 1b also includes older adults living in congregate or overcrowded settings—including nursing homes, long-term care facilities, homeless shelters, group homes, prisons, or jails. As a group, they face the joint risk factors of severe disease and reduced resilience associated with advanced age and of acquisition and transmission due to their living settings. A significant proportion of COVID-19 deaths in the United States have occurred among individuals living in nursing homes and long-term care facilities, highlighting the critical need to protect individuals in this group.
Phase 2
Moving to Phase 2 and beyond, it is important to note the overlap issue discussed earlier. Individuals who fall within population groups in this
phase may also be high-risk health workers or first responders, have comorbid and underlying conditions that put them at significantly higher risk, or be older and living in congregate or overcrowded settings, and therefore should be vaccinated in Phase 1.
Phase 2 of the allocation framework would cover approximately 30–35 percent of the U.S. population, bringing the total coverage across Phases 1 and 2 to an estimated 45–50 percent of the total population. K–12 teachers, school staff, and child care workers are included in Phase 2. This category includes administrators, environmental services staff, maintenance workers, and school bus drivers, all of whom are essential to education and face disease exposure. Vaccinating these individuals supports their vital societal role in providing children’s education and development, while reducing their role in transmission between schools and the community and protecting their own health risks from exposure in these settings. Phase 2 also includes critical workers in high-risk settings—a group of individuals whose occupations are in essential industries and who cannot avoid a high risk of exposure to COVID-19. They include workers in the food supply system, public transit, and other vital services. It would be useful for public health agencies, including CDC, the Occupational Safety and Health Administration, the Mine Safety and Health Administration, and state and local public health agencies, to provide additional guidance in the designation of jobs or tasks involved as well as occupational codes or job titles in this group.
Phase 2 includes people of all ages with comorbid and underlying conditions that put them at moderately higher risk, which the committee defined as having one of the previously mentioned conditions and potentially some rare diseases as well.
Phase 2 also includes people in homeless shelters or group homes, and staff who work in those settings. Group home populations include people with disabilities—such as serious mental illness, developmental and intellectual disabilities, and physical disabilities—as well as those in recovery. Many of these individuals have chronic health care needs and challenging living settings that increase potential exposure. Phase 2 includes people in prisons, jails, detention centers, and similar facilities, and staff who work in those settings, with the expectation that they have limited opportunity to follow public health measures such as maintaining physical distance, putting them at significant risk of acquiring and transmitting COVID-19.
All older adults not included in Phase 1b are included in Phase 2, because advanced age is in itself a risk factor for severe disease and death due to COVID-19.
Phase 3
Phase 3, which assumes wider availability of COVID-19 vaccine, focuses on preventing transmission of COVID-19 and restoring social and
economic activity. This phase would cover an estimated 40–45 percent of the U.S. population, bringing the total to 85–95 percent vaccination coverage across Phases 1–3. Phase 3 includes young adults, children, and workers in industries that are both important to the functioning of society and pose moderately high risk of exposure. Young adults between the ages of 18 and 30 typically have broader social networks than older adults, increasing their risks of infection and transmission, but are less likely to become severely ill or die due to COVID-19, making them targets for transmission prevention. Children, too, are much less likely than adults to experience severe outcomes due to COVID-19, but can play a role in transmission. However, it is important to note that clinical trials of COVID-19 vaccine have not started in children in the United States. Workers in this category are important to the functioning of society and are at moderately high risk of exposure. Representative industries may include universities, entertainment, and goods-producing industries, whose occupational risk of transmission is lower than those in Phase 2 because they work in settings where protective measures are likely to be implemented without great difficulty.
Phase 4
Finally, Phase 4 includes everyone residing in the United States who did not have access to the vaccine in prior phases.
While the committee’s phased allocation approach is limited by imperfect data, information unknowns, and potential unintended consequences, it is intended to be adapted by STLT partners based on their needs, and should rely on mid-course corrections and real-time updates based on the science about effectiveness of different vaccines in different populations.
IMPLEMENTATION CONSIDERATIONS
In Chapters 5 and 6, the report also discusses the administration, monitoring, data collection, communication, community engagement, health promotion, and evaluation activities needed to implement an effective, equitable national COVID-19 vaccination program, including the roles of federal and STLT authorities and their partners. CDC traditionally holds a leadership role in vaccination program coordination, working with federal partners such as the Office of the Assistant Secretary for Preparedness and Response, the U.S. Food and Drug Administration, NIH, the Health Resources and Services Administration, and the Centers for Medicare & Medicaid Services. Secure vaccine storage, transport, and safe, efficient, and equitable vaccine distribution are critical to a successful national COVID-19 vaccination program, especially given the potential vaccine ultra-cold chain requirements and a multi-dose vaccine regimen. Successfully establishing a coordinated approach to COVID-19 vaccination will require leveraging existing systems, along with strong and real-time rapid monitoring and evaluation procedures, including assessment of the program’s penetrance among key populations.
Several COVID-19 vaccines under development have received considerable taxpayer support. Therefore, it is essential that COVID-19 vaccines be delivered through a central mechanism that ensures availability of vaccines to all individuals, regardless of their social and economic resources or their employment, immigration, or insurance status. This can best be achieved if this federal mechanism makes vaccines available at no cost to the public health and health care sectors. To ensure equity and to decrease vaccine hesitancy, there should be no out-of-pocket costs for those being vaccinated and this includes covering fees for administration of the vaccine.
Engaging with communities will be a critical task for STLT authorities to ensure equity and develop effective, localized COVID-19 vaccination plans (further discussed in Chapters 5 and 6). Community-based organizations and other partner organizations—including hospitals, pharmacies, faith-based organizations, community centers, and schools and universities—can support community outreach and foster accountability. Employers and unions could support improved access by providing work-site clinics and by covering costs for employees.
As part of community engagement, the ethical principles, implementation processes, expected outcomes, and how well the program has achieved equitable allocation of safe and effective COVID-19 vaccine actual performance must be transparently communicated. Communication must be accessible and available for a diverse audience, and should pay attention to disease processes that can be misunderstood unless properly explained, equity in the vaccination program’s procedures and performance, empirical testing, and appropriate tailoring. Effective communication requires cultural competence, establishment of a trusted authority, special consideration for unfamiliar material, and approaches to address different users’ needs, including engagement with a variety of partners. The communication workforce must reflect the diversity of the communities being vaccinated, and must rely on the scientific foundations of risk communication and community engagement, as well as collect the evidence needed to serve the public effectively.
Achieving Acceptance of COVID-19 Vaccine
Recent polling data suggest that approximately one-third of U.S. residents would not accept a COVID-19 vaccine if offered today, with skepticism even higher among certain populations, including Black and Hispanic communities. Histories of medical research exploitation, such as during the Tuskegee syphilis study, fuel skepticism in minority communities. Beyond this understandable distrust, vaccine hesitancy is increasingly common in the United States, and influential anti-vaccine groups have been particularly effective in spreading their views online. Concerns about the development and approval of COVID-19 vaccines, including the unprecedented speed of testing for safety and efficacy in clinical trials, and significant concerns of political considerations affecting evaluation of the data from those trials, create a more challenging environment for vaccine hesitancy and reduced acceptance.
WHO’s Measuring Behavioral and Social Drivers of Vaccination (BeSD) Increasing Vaccination Model offers one tool for investigating people’s motivations toward becoming vaccinated. It considers people’s thoughts and feelings, as well as the social processes that affect their motivation. Multiple reviews of the evidence have found that there is not a “one-size-fits-all” so-
lution to vaccine hesitancy. Rather, addressing this issue requires a combination of interventions, including the engagement of community leaders, mass media campaigns, and health care professional training. People-centered and dialogue-based solutions, including those based on social marketing tactics, will be key to promoting acceptance of COVID-19 vaccine. Those guiding and implementing these programs must represent the communities they are trying to reach. In Chapter 7, the committee reviews the complex and dynamic landscape of vaccine hesitancy and discusses its specific application and relevance to COVID-19 vaccination.
ENSURING EQUITY IN COVID-19 VACCINE ALLOCATION GLOBALLY
Entities outside of the United States are also working to ensure COVID-19 vaccine access and equitable allocation worldwide. The Access to COVID-19 Tools Accelerator was established by a diverse range of development partners and its vaccine pillar—referred to as COVAX—is convened by the Coalition for Epidemic Preparedness Innovations and Gavi, the Vaccine Alliance. Gavi, the Vaccine Alliance’s financing approach for COVAX is designed to provide all countries with the opportunity to
participate in securing an initial supply of vaccine for 20 percent of their population. The COVAX Facility provides a pooling mechanism for procurement. A total of 156 economies, representing more than two-thirds of the global population, are now either committed to or eligible for the COVAX Facility—with more to expected to follow. Although the United States is not currently among those countries, the report discusses the reasons favoring its participation, including COVAX serving as an insurance policy to OWS, should the vaccine that it is supporting prove less effective or less available than hoped; the recognition that infectious disease threats do not respect international boundaries; the need for domestic preparedness and national security; and the moral duty to support it.
CONCLUDING REMARKS
SARS-CoV-2 will continue to spread around the world until a vaccine is developed and widely distributed and administered. Ultimately, in these uncertain and challenging times, the integrity of the COVID-19 vaccine development, allocation, and distribution processes will be critical to ensuring widespread access to vaccines that are safe and effective, and convincingly so for the public. The committee hopes that the evidence-based deliberations and policy recommendations set forth in this report and summarized in Box S-2 contribute to society’s ability to respond to and recover from the COVID-19 pandemic.