3
A Framework for Equitable Allocation of COVID-19 Vaccine
Drawing from the lessons learned from other allocation frameworks, outlined in Chapter 2, the committee has derived foundational principles that inform its recommended coronavirus disease 2019 (COVID-19) vaccine allocation framework. Here, the committee describes the goal of its framework, the risk-based allocation criteria used to apply the principles, and the resulting allocation phases (see Figure 3-1). The chapter concludes with an in-depth description and discussion of the phases, including the rationale behind the inclusion of population groups listed in each phase.
The committee recognizes that decisions about COVID-19 vaccine allocation must be made under conditions of uncertainty. These unknowns include the safety and efficacy of the vaccines in specific populations (such as children, pregnant women, older adults, and individuals previously infected with COVID-19); the effectiveness of vaccines in tandem with existing preventive measures; public confidence in the vaccine; the possibility of ultra-cold storage requirements for the vaccine; the pharmacovigilance evidence; and many other unknowns.
Such unknowns require the framework to be adaptable to a variety of circumstances, including the state of the pandemic when a vaccine becomes available. Designing the framework to be adaptable to a range of possible circumstances means that the committee must consider how the framework would operate ethically and effectively in a range of plausible scenarios. Planning is crucial, but a rigid framework is unlikely to match the specific circumstances that actually emerge, and will likely change depending on the goal of the COVID-19 vaccination program, the state of the pandemic, the
state of the science, and the extent to which people are engaging in social distancing and other preventive measures. Chapter 4 describes several such scenarios and their implications for the framework.
Likewise, the framework must be implementable. To be able to guide policy makers in planning for vaccine allocation, it must be feasible to put the framework into operation. For example, it must be possible to accurately and quickly identify individuals or groups who have been prioritized to receive the vaccine.
One-third or more of the U.S. population may decline a free U.S. Food and Drug Administration (FDA)-approved vaccine for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Mullen O’Keefe, 2020). Concerns about inclusion and diversity in COVID-19 vaccine trials (Jaklevic, 2020) and unknowns like those previously noted compound the already significant doubts that some members of the public have about the vaccine. A mass vaccination program for public health will fail if there is widespread public mistrust. However, the committee believes its equitable allocation framework, if properly implemented and communicated, can secure public trust in the processes and outcomes of allocation by being based on foundational principles that are simple, clear, coherent, and consistent in their application. The hope is that this framework will gain public trust by fairly providing benefits to individuals and communities, thereby mitigating the damage that has been caused by the pandemic and aggravated by existing health inequities.
FOUNDATIONAL PRINCIPLES OF THE FRAMEWORK
The committee was charged with developing an overarching framework for the equitable allocation of COVID-19 vaccine. This framework is intended to assist and guide policy makers in planning for vaccine allocation under conditions of scarcity that will necessitate vaccinating people in phases over time. In presenting the sponsors’ charge at the committee’s first meeting on July 24, 2020, the director of the National Institutes of Health (NIH), Francis Collins, stressed that the overarching framework should include “foundational principles.” Such principles, which are summarized and explicated in the next section, informed the committee’s deliberations about allocation criteria.
The committee recognizes that its proposed framework must not only be equitable, but also be perceived as equitable by audiences who are socioeconomically, culturally, and educationally diverse, and who have distinct historical experiences with the health system. As a result, the presentation and communication of the framework must do justice to its scientific and ethical foundations. Therefore, the committee has designed the framework so that it:
- Can be easily and equally well understood by the diverse audiences whose concerns the vaccine allocation framework must address;
- Reflects widely accepted social and ethical principles;
- Can be reliably translated into operational terms;
- Distinguishes scientific and ethical judgments in their application; and
- Does not perpetuate discrimination and inequities.
Foundational Principles
The foundational principles for the equitable allocation framework for COVID-19 vaccine include ethical and procedural principles embedded in U.S. social institutions and culture (see Box 3-1). The committee recognized that the principles required for its deliberations had to be solid and broad enough to urgently address a pandemic of a magnitude not seen in a century with disastrous effects not only for persons with COVID-19 and their communities but also for the economy, education, and other central aspects of society.
The committee identified the principles in Box 3-1 as both necessary and sufficient for formulating vaccine allocation criteria and their implementation in phases of vaccine allocation. These principles do not reflect any specific ethical theory, but are both consonant with many ethical theories and grounded in U.S. social values and cultural discourse. The three substantive ethical principles have direct implications for allocation criteria and prioritization in different phases of allocation; the three procedural
principles are important for the development and implementation of allocation criteria and prioritizations that can be deemed equitable and legitimate and can thus be accepted by the public.
In its deliberations about allocation criteria, the committee quickly invoked a principle of maximum benefit that emphasizes maximizing societal benefit through the reduction of severe morbidity and mortality caused by the transmission of SARS-CoV-2. While spreading throughout the society, the virus has significantly harmed some populations more than others, particularly causing higher rates of infection, serious illness, hospitalization, and death among older adults in congregate settings and among people of color, the combination of which has been particularly lethal. This reality led the committee to formulate a principle of mitigation of health inequities to address the higher risks faced by such persons in work environments and living arrangements that pose higher risk of transmitting and acquiring infection and with a higher prevalence of health problems that make it more likely that they will suffer severe outcomes and even die from COVID-19. In difficult choices about vaccine allocation, the principle of equal concern directs attention to the equal worth and value of every person, protecting each person from discrimination. The procedural principle of fairness requires the engagement and participation of affected populations in setting allocation criteria and determining priority groups. Furthermore, the procedural principle of transparency ensures the disclosure of the principles, criteria, and priority groups that will determine people’s chances of getting a vaccine sooner rather than later. Finally, the framework cannot accomplish its goals unless all decisions are evidence-based.
Not unexpectedly, these principles overlap significantly with those in other frameworks for the allocation of scarce medical and public health resources, including vaccines for pandemic influenza (Williams and Dawson,
2020). Virtually every such framework has a principle like the committee’s with regard to maximum benefit. Most frameworks also include principles like the committee’s relating to equal concern and to equity and fairness (Emanuel et al., 2020; Nuffield Council on Bioethics, 2020; Persad et al., 2009; Toner et al., 2020; Williams and Dawson, 2020). These frameworks vary in how clusters of ethical considerations are combined into primary principles and in the weight assigned to those principles. The overlaps are evident in comparisons with the several COVID-19 vaccine allocation frameworks discussed in Chapter 2 (see Table 3-1). These frameworks are comparable to the committee’s framework in that they were also prepared by diverse multidisciplinary groups who aimed to produce practical frameworks that could be adopted and implemented.
TABLE 3-1 Comparison of Principles Across Different Frameworks for COVID-19 Vaccine Allocation
| Committee’s Foundational Principles | Johns Hopkins Interim Framework for COVID-19 Vaccine Allocation in the United States: Assisting Policy Maker, Stakeholder and Public Deliberation | WHO SAGE Values Framework for the Allocation and Prioritization of COVID-19 Vaccination | ACIP Proposed Ethics/Equity Framework |
|---|---|---|---|
| Maximum benefit | Promote public health and economic and social well-being | Human well-being | Maximize benefits and minimize harms |
| Equal concern | Equal respect | ||
| Mitigation of health inequities | Address inequities | Global and national equity | Equity |
| Give priority to the worse off | Justice | ||
| Fairness | Respect diversity of views in a pluralistic society | Legitimacy | Fairness |
| Transparency | Transparency | ||
| Evidence-based | Engage community members | ||
| Reciprocitya | Reciprocitya |
a Several frameworks for vaccine allocation include a principle of reciprocity, defined as rewarding people for their past contributions. It is important to recognize and honor people’s important and often risky contributions to help others, in part to encourage such actions in the future. However, there are ways of doing so without assigning priority for scarce resources such as vaccines. In the committee’s judgment, reciprocity should not be a criterion for priority in the allocation of a vaccine for COVID-19 in this pandemic. In this context, reciprocity is too broad and vague to clearly and impartially identify those particular individuals and or groups to whom it applies. However, in recruiting participants, sponsors of COVID-19 vaccine trials can promise or offer post-trial access to a safe and effective vaccine to those who receive a placebo or an ineffective vaccine.
Maximum Benefit
This principle encompasses the obligation to protect and promote the public’s health and its socioeconomic well-being in the short term and long term. Societal benefit is broadly understood in this context as the public’s health and socioeconomic well-being. While societal benefit includes the health and well-being of individuals, the committee recognizes that conflicts may emerge between societal and individual needs and risks that will require resolution. The framework the committee proposes seeks to combine them to the extent possible.
The vaccine allocation framework thus seeks to reduce the risks of severe morbidity and mortality caused by transmission due to SARS-CoV-2 for those (a) most at risk of infection and serious outcomes, for example, those in congregate living arrangements with comorbid conditions; (b) in roles considered to be essential for societal functioning; and (c) most at risk of transmitting SARS-CoV-2 to others. Individuals in the roles considered to be essential for societal functioning include those whose absence from their societal roles or work puts others and the society at risk of loss of needed goods and services if they become infected (e.g., physicians, nurses, other health care providers, first responders, workers employed in the food supply system, transportation workers, teachers, etc.).
Equal Concern
The government’s obligation to express equal concern or regard for its residents should both guide and constrain its allocation and distribution of goods, such as vaccines, and burdens, such as delays, in the provision of vaccines. This fundamental obligation requires that every person be considered and treated as having equal dignity, worth, and value. It presupposes basic equality: no one person is intrinsically more valuable or worthy of consideration than another. It entails the treatment of all as equals rather than, automatically, the provision of equal share (several versions of an egalitarian principle appear in Dworkin, 2011, which features a principle of equal concern and respect; Emanuel et al., 2020; Nuffield Council on Bioethics, 2020; Persad et al., 2009, 2020; Waldron, 2017).
The principle of equal concern retains its force even when it is necessary and ethically justifiable to ration vaccines and other health-related goods under conditions of scarcity. It requires allocation and distribution by criteria that are non-discriminatory in design and impact. It excludes rationing based solely on characteristics such as religion, race, ethnicity, national origin, disabilities, and others. The moral right to equal concern requires allocation of vaccine to proceed impartially according to fair criteria.
The principle of equal concern does not preclude consideration of people’s social roles in vaccine allocations. Some social roles are essential
in this pandemic to ensure the provision of necessary goods and services to the community and to individuals, including but not limited to medical care. This means that the people filling those roles (e.g., clinicians, emergency responders, food processors) may legitimately gain priority in those circumstances.
If the supply of vaccine is too limited to provide it to everyone in a particular priority group at the same time, and there are no further identifiable risk-based differences within that group, the principle of equal concern can support random selection (e.g., lottery) within that population group. It can also support a weighted lottery1 for vaccine allocation as it has for the allocation of COVID-19 therapies such as remdesivir (White et al., 2020).
Mitigation of Health Inequities
The obligation to mitigate health inequities and their effects has become particularly salient in this pandemic. SARS-CoV-2 infections and COVID-19 illnesses and deaths are strongly associated with race, ethnicity, occupation, and socioeconomic status. A significantly higher burden is experienced by Black, Hispanic or Latinx, American Indian and Alaska Native, and Native Hawaiian and Pacific Islander populations. This disproportionate burden largely reflects the impacts of systemic racism and socioeconomic factors that are associated with increased likelihood of acquiring the infection (e.g., frontline jobs that do not allow social distancing, crowded living conditions, lack of access to personal protective equipment [PPE], inability to work from home) and of having more severe disease when infected (as a result of a higher prevalence of comorbid conditions or other factors). The social groups at higher risk of COVID-19 also experience disproportionately large burdens of other adverse health conditions. Many factors contribute to these health inequities, defined as “systematic differences in the health status of different population groups” (WHO, 2017) (see Box 3-2). Fundamental health inequities in COVID-19 and in other health conditions are rooted in structural inequalities, racism, and residential segregation. Any vaccine allocation framework designed to reduce COVID-19 risk must explicitly address the higher burden of COVID-19 experienced by the populations affected most heavily, given their exposure and compounding health inequities. Mitigating those health inequities is, therefore, a moral imperative of an equitable vaccine allocation framework. In addition, any vaccine allocation plan implemented at the federal and state levels must respect the tribal sovereignty of American Indian and Alaska Native nations.
___________________
1 A weighted lottery system could be used to fairly allocate the scarce supply of vaccine with certain groups receiving heightened priority.
Thus, the vaccine allocation criteria should mitigate inequities in COVID-19 resulting from the factors just described. The committee’s allocation criteria do so in part by taking into account the “vulnerability” of
- People at increased risk of infection because of social conditions, such as crowded workplaces and multigenerational homes;2 and
- People at increased risk of severe outcomes because of comorbid conditions associated with social factors, limited access to health care, etc.
These allocation criteria identify people who are considered to be the most disadvantaged or the “worst off” because of conditions of ill health or social deprivation, or both, that could make them more susceptible to infection or severe illness or death. Such criteria are sometimes called “prioritarian” because of the primary place assigned to the “worst off” (Emanuel et al., 2020; Toner et al., 2020). A further way to mitigate the effects of health inequities is to incorporate a metric of social disadvantage, such as the Centers for Disease Control and Prevention’s (CDC’s) Social Vulner-
___________________
2 Multi-generational homes consist of more than two generations living under the same roof.
ability Index (SVI),3 the Area Deprivation Index (ADI),4 or the COVID-19 Community Vulnerability Index (CCVI),5 into the prioritization of vaccine recipients by making it an additional consideration (Schmidt, 2020).
The mitigation of health inequities also includes development and deployment of distribution systems that ensure that people who are allocated a vaccine actually receive it (e.g., by bringing it to them, if they cannot reach central distribution centers). Trusted community-based organizations, particularly those serving racial and ethnic populations most affected by COVID-19, should be involved in the implementation of the framework to ensure cultural and language proficiency and mitigate ongoing health inequities. This is discussed further in Chapter 6.
Fairness
Procedural fairness or justice is vitally important for the legitimacy and public acceptance of the allocation criteria and prioritizations based on these ethical principles (Daniels, 1996, 2007). The three substantive ethical principles must be interpreted in practical terms when applied in the vaccination program. These decisions about allocation, distribution, and access to vaccine should incorporate input from affected groups, especially those disproportionately affected by the pandemic. In developing its allocation phases, the committee benefited greatly from a public listening session and written public comments (described further in Appendix A). Chapters 5, 6, and 7 discuss the importance of further public engagement throughout this entire process.
Once informed by public input, decisions about whether a group has heightened risk and which individuals fall in that particular group should be data driven and made by impartial decision makers, such as public health officials. Ideally, affected individuals and communities should be
___________________
3 CDC’s SVI, which was developed for local preparedness for public health emergencies such as natural disasters and disease outbreaks, identifies geographic areas of vulnerability based on 15 U.S. Census variables. These variables capture many recognized social determinants of health, indicators of access, infection transmission, and increased risk of adverse COVID-19 outcomes (ATSDR, 2018).
4 The ADI is based on a measure created by the Health Resources and Services Administration to allow for rankings of neighborhoods by socioeconomic status disadvantage in a region of interest and includes factors for the domains of income, education, employment, and housing quality. It is primarily for county-level use but adapted and validated to the U.S. Census block group/neighborhood (University of Wisconsin School of Medicine and Public Health, 2020).
5 Developed by the Surgo Foundation, the CCVI combines indicators specific to COVID-19 with CDC’s SVI. These indicators are grouped into six themes: socioeconomic status, household composition and disability, minority status and language, housing type and transportation, epidemiologic factors, and health care system factors. An overall score is generated at the U.S. Census tract, and at the county and state levels (Surgo Foundation, 2020).
able to appeal decisions. The committee believes that the transparency of its principles will help adjudicate those deliberations.
Fairness should guide not only the formulation of allocation criteria, but also their application, which should be impartial and evenhanded, avoiding arbitrary exceptions and opportunities for gaming the system. Implementation should be as uniform as possible across the country, consistent with allowing discretion to state, tribal, local, and territorial (STLT) authorities to address specific patterns of SARS-CoV-2 transmission, extent of spread, and severity of outcomes. Unless clearly communicated and justified, extreme variation in applying the criteria can evoke charges of unfairness.
Transparency
The principle of transparency includes the obligation to communicate with the public openly, clearly, accurately, and straightforwardly about the vaccine allocation criteria and framework, as they are being developed and deployed. Central to this process is clear articulation and explanation of the allocation criteria. Those explanations must include the principles underlying these criteria, as grounded in widely accepted societal institutions and culture, as well as the procedures for ensuring their faithful implementation.
Sometimes governments present vaccine allocation criteria without explicitly or adequately explaining their grounding in principles. This is a mistake in at least two ways. First, the public has a legitimate reason to expect such a justification when criteria affect when they can receive a vaccination, especially when their government funds the vaccination program. Second, such communication is essential to generating and sustaining public trust in the vaccine allocation criteria and program.
Transparency should also extend to other aspects of procedural fairness. Individuals (or their trusted surrogates) must be able to observe, understand, and monitor how the program’s procedures are formulated and applied. That will require simple, clearly defined, and comprehensibly communicated rules. It will also require accessible documentation of how the allocation framework performs and how it responds to the unanticipated consequences inevitable with such a complex human enterprise. It also extends to any alterations of or departures from the allocation criteria and priority categories in practice along with the justification for doing so.
Without transparency regarding the allocation criteria, their ethical rationale, the deliberative process used to formulate them, and fair procedures, it will be difficult to generate and maintain the trust that is indispensable for the public’s cooperation with a mass vaccination program.
To achieve transparency, it is necessary to ensure that the allocation principles and processes are accessible and comprehensible to all those
affected by it. This cannot be done without empirically testing proposed communications in two essential ways: Can people find the allocation procedures and guiding principles easily, following their normal search patterns? Can they interpret them in ways that inform their evaluations regarding the allocation procedures’ legitimacy and their own vaccination choices? Chapter 6 discusses the science of risk communication, as applied to fulfilling this ethical principle.
Evidence-Based
Vaccination phases, specifying who receives the vaccine when, should be based on the best available scientific evidence, regarding risk of disease, transmission, and societal impact. The framework must be adaptive, capable of being changed as the understanding of the disease and its risk factors deepens and as vaccines become available, especially if some vaccines prove more useful for particular populations than others. If the criteria used to identify categories of individuals or groups for each phase evolve accordingly, those changes will need to be stated and applied clearly and in keeping with the framework’s foundational principles.
Using the Principles
Each pandemic has what Yale historian Frank Snowden calls its distinctive “personality” (Snowden, 2019), that is, its distinctive characteristics of disease and rates of infection, its modes of transmission, the groups and individuals most susceptible to infection, ages most affected, varying rates of severity and mortality, and so forth. Chapter 1 describes the current pandemic’s “personality” in detail. Determining the specific criteria for vaccine allocation will require attention to up-to-date scientific information about the pandemic, on the one hand, and to foundational principles, on the other. The ethical principles need to be specified and applied in the process of developing vaccine allocation criteria and phases to match the features of the pandemic, along with the characteristics, supply, safety, and efficacy of any available vaccines.
For example, applying the ethical principle of maximum benefit for vaccine allocation requires determining how best to protect and promote the public’s health and socioeconomic well-being, both immediate and long term, before the vaccine is available to everyone. That determination requires the best available scientific evidence, following the procedural principle of evidence based. Similar points apply to the ethical principles of mitigation of health inequities and equal concern, as well as to the procedural principles of fairness and transparency. The application of each allocation criterion and procedure must comply with each of these principles.
When conflicts arise, their resolution will require judicious balancing by trusted parties. These principles provide the foundation for the allocation criteria and the phases in vaccine allocation derived from them. The overall allocation framework reflects the committee’s best judgment about how to balance sometimes conflicting aims as the pandemic evolves and vaccine becomes incrementally available over time.
COVID-19 VACCINE ALLOCATION FRAMEWORK
Goal of the Framework
Previous proposals for allocation of scarce resources in pandemics and other settings articulate various overarching goals and also focus on reducing severe morbidity and mortality, reducing disease transmission, minimizing societal disruptions, maintaining national security, and mitigating health inequities. For example, the 2018 CDC guidance document Allocating and Targeting Pandemic Influenza Vaccine During an Influenza Pandemic states that its overarching goals are to reduce the impact of the pandemic on health and minimize the disruption to society and the economy (CDC, 2018).
Given the current state of the pandemic, the early phases of the committee’s proposed framework emphasize prevention of severe morbidity and mortality, particularly with regard to maintaining essential health and emergency services. The focus shifts toward reducing transmission6 in later phases. There are multiple reasons for this approach:
- Death is an irreversible outcome. There are legitimate claims for many groups (e.g., schoolchildren, “non-essential” workers) to be in earlier phases as negative societal impact could occur if these groups are not prioritized. For example, there might be a substantial impact on the economy if a primarily transmission-focused strategy is not employed from the outset. However, the non-trivial effects of an economic downturn or an online semester can at least be partially reversed.
- Preventing severe morbidity and mortality protects the health care system from being overwhelmed, contributing to the prevention of excess morbidity and mortality from other causes as well, with ripple effects on society and the economy.
- For vaccination to materially reduce transmission requires vaccinating a critical mass of individuals, much greater than will be possible in the early phases of vaccine deployment.
___________________
6 For clarification, the committee considered transmission in terms of transmitting infection to others and not acquiring infection.
- The ongoing COVID-19 vaccine trials are not designed to estimate the impact of the vaccine candidates on transmission and evidence of the vaccines’ actual impact on transmission might not be available for some time after FDA approval.
- While data on all aspects of COVID-19 are emerging, data on transmission risk groups (e.g., age, profession) are particularly limited.
A focus on preventing severe morbidity and mortality in the initial phases does not mean vaccinating only groups at a direct risk of these outcomes. Preventing transmission to groups at high risk of severe morbidity and mortality are also important. For example, vaccinating nursing home workers would protect the high-risk residents of these facilities—particularly if vaccine efficacy is lower among older adults compared to younger individuals. As more courses of vaccines become available, an increasing focus on reducing transmission, starting with high-transmission settings and moving to the general population, will ensure sustainable long-term control of COVID-19. Focusing on health care and emergency workers in the initial phases will mitigate the pandemic’s impact on severe morbidity and mortality due to disruptions in the health care system.
The committee considered years of life lost (YLL) averted, instead of number of deaths avoided, as an alternative metric for maximizing benefit. The committee favored the number of deaths avoided for the following reasons. First, the relative risk of COVID-19-related mortality is so high in older age groups (e.g., the mortality risk is 90 times higher among 65–74-year-olds compared to 18–29-year-olds) (CDC, 2020a) that from a pragmatic perspective, the YLL averted approach does not provide substantial additional advantage. This is not to say the YLL averted approach would be futile in all situations. For example, in a pandemic with a mortality pattern similar to seasonal influenza—in which the very young as well as older adults have disproportionately high mortality or that of the 1918 pandemic—young adults were also included in the high-mortality risk groups (in addition to older adults and the very young) (Dauer and Serfling, 1961). Second, YLL averted has not been widely used in policies for preventive interventions in pandemics and large outbreaks (with the exception of a few well-argued academic exercises) and there is little evidence of a social consensus around this approach in these situations, whereas reduction of number of deaths is a widely understood and accepted goal. Third, a YLL-focused approach is inconsistent with the committee’s principles of equal concern and mitigating health inequities and could be viewed as discriminating on the basis of age and not addressing the disproportionate impact on older adults.
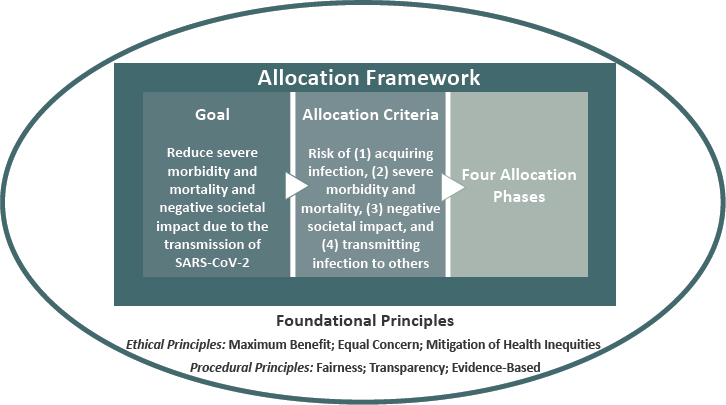
The goal of the committee’s framework for equitable allocation of COVID-19 vaccine is to:
Reduce severe morbidity and mortality and negative societal impact due to the transmission of SARS-CoV-2.
The framework pursues that goal while mitigating health inequities, showing equal concern for all, being fair and transparent, and building on the best available evidence. Ultimately, the U.S. COVID-19 vaccination program should aim to vaccinate all who choose to be vaccinated and are without medical contraindications to the vaccine.
Allocation Criteria
The principle of transparency, as well as the practical requirement of efficient, consistent administration of the framework have led the committee to develop risk-based criteria for operationalizing the foundational principles to achieve its goal (see Box 3-3). After presenting these criteria briefly, this section discusses their compatibility with the foundational principles, practical aspects of implementation, and likely implications for allocation as vaccine becomes increasingly available.
The committee notes that the fidelity of the allocation process to these foundational principles and criteria depends on the availability of data regarding vaccine safety, efficacy, and distribution. Achieving this goal requires comprehensive, consistent, real-time data collection that includes variables needed to assess the program’s success in mitigating health inequities, such as participants’ race and ethnicity, age, sex, and social status. The section provides operational definitions of these criteria, as suited to current and emerging evidence regarding the disease, the vaccine, and their impacts on society.
Risk of Acquiring Infection
Individuals have higher priority to the extent that they have a greater probability of being in settings where SARS-CoV-2 is circulating and of being exposed to a sufficient dose of the virus to become infected.
Risk of Severe Morbidity and Mortality
Individuals have higher priority to the extent that they have a greater probability of severe disease or death should they acquire infection.
Risk of Negative Societal Impact
Individuals have higher priority to the extent that societal function and other individuals’ lives and livelihood depend on them directly and would be imperiled if they fell ill. This risk is interpreted through the number of other people potentially affected. While no person is intrinsically more valuable than any other, some jobs are more valuable to society at this moment and under these extraordinary circumstances.
Risk of Transmitting Infection to Others
Individuals have higher priority to the extent that there is a higher probability of their transmitting the infection to others. This risk reflects individuals’ interactions with others, given their normal course of life and their material, physical, and social resources. It is important to note that there are limited data on differential transmissibility.
Compatibility of Allocation Criteria with Foundational Principles
Maximum Benefit
Each of these four types of risk reflects a threat to the public’s health, social, and economic well-being. Reducing each risk would bring both short- and long-term benefits. These risk-based criteria express the foundational principles in terms that are further specified in the allocation phases that follow.
Equal Concern
These criteria treat all people equally. They make no reference to who people are—only to their circumstances, what social roles they fill and what personal challenges they face (e.g., health). If more vaccine goes to members of one population group than another, it will not reflect who they are, but what they do, and what has happened in their lives.
Mitigation of Health Inequities
Although the criteria do not directly address health inequities, they do so indirectly. The first criterion addresses health inequities insofar as individuals subject to them are more likely to live and work in dense settings, where exposure to the virus is more likely. The second criterion addresses them indirectly insofar as those inequities have increased individuals’ risk of disease (e.g., social disadvantage is linked to having more disease and more severe disease). The third criterion addresses them indirectly insofar as workers who have been subject to health inequities play essential roles in jobs with greater societal impact (e.g., health and elder care).
Fairness
In applying the three substantive ethical principles to the development of allocation criteria, procedural fairness requires that we incorporate input from affected groups, especially those disproportionately affected by the pandemic. The committee’s deliberations benefited from its public listening session and written input. Its risk-based criteria focus solely on four forms of risk, with no explicit recognition of any other individual characteristics. The committee anticipates that the criteria will, in practice, tend to give higher priority to lower-income individuals (because they more frequently live in high-density settings, work in jobs that cannot be done without having personal contact with others, and have multiple comorbid conditions due to their circumstances and their relative lack of access to health care) and Black, Hispanic or Latinx, American Indian and Alaska Native, and Native Hawaiian and Pacific Islander communities, given the ways in which these risks disproportionately affect people in these groups.
Transparency
There are explicit, auditable procedures for defining risk and applying those definitions. The guidance provided by various reports of the National Academies of Sciences, Engineering, and Medicine can achieve transparency, including the procedural fairness that it requires (NRC, 1996).
Evidence-Based
These four risk-based criteria apply well-understood analytical procedures to the best available scientific evidence (NRC, 2009). The criteria should readily incorporate new evidence as it becomes available and characterize uncertainties in ways that can guide future data collection. Their application in the allocation phases reflects the committee’s assessment of the evidence regarding how vaccines can best maximize benefits to individu-
als and communities and the health inequities that must be mitigated in that process (NRC, 2009).
Allocation Phases
The committee has been tasked with considering the difficult choices that will need to be made for allocating a tightly constrained initial supply of vaccine (e.g., 10–15 million courses, enough to vaccinate approximately 3–5 percent of the U.S. population). The supply of vaccine will be incrementally phased in so that some people or groups receive it earlier than others. The committee here uses the term “phases,” suggesting successive deployments of a scare resource that is expected to be more broadly available over time. This approach applies the best available current evidence to implementing the framework’s foundational principles.
It should be noted that the guidance offered through the committee’s allocation framework is intended to inform the work of the federal government, the Advisory Committee on Immunization Practices (ACIP), STLT authorities, and potentially other countries in their COVID-19 vaccine allocation planning. Certain communities (such as the U.S. military) may handle vaccine allocation separately from this proposed framework. If the federal government were to provide states with an allotment of COVID-19 vaccine, in the interest of speed and workability, that allocation could be based on these jurisdictions’ population size.7 While there is obviously variation among STLT communities in disease burden and demographic features, these differences are not large enough to justify the delay and deliberation that would be required to decide on customized allocations to locations. Speed is essential because many difficult choices need to be made at the state and local levels.
One exception to a straightforward population-based approach to the allocation of vaccine would be to withhold a percentage (e.g., 10 percent) of available vaccine supply at the federal level as a reserve for deployment by CDC for use in areas of special need (identified through a vulnerability index, such as the SVI or the CCVI) or epidemiological “hot spots.”8 Transparency of deployment will be important. If by the time a COVID-19 vaccine becomes available, the United States has achieved the success seen in other countries in stopping widespread community transmission with non-
___________________
7 There remains uncertainty as to whether private entities, such as health care systems or businesses, will be able to access allotments of COVID-19 vaccines outside of a federal-to-state allotment system.
8 Planning for whether an epidemiological “hot spot” reserve would be valuable and make a difference also depends on the characteristics of the vaccine (e.g., how long it takes for immunity to develop, etc.).
pharmaceutical (behavioral) interventions, including test, trace, isolate, and quarantine approaches, a more focused outbreak response may be feasible.
It is important to acknowledge that the federal government will allocate vaccine to Indian Health Service (IHS), tribal, and urban Indian facilities directly through existing IHS system mechanisms. Federal trust responsibility for delivery of health care to citizens of federally recognized tribes mandates that. To do so successfully, IHS allocation will require additional funding and external oversight. While separate from state allocation, it may also be in states’ best interest to supplement the IHS, tribal, and urban Indian allocation with a portion of their own supply of vaccine, in order to protect the public’s health. However, even in this scenario, in order to ensure tribal sovereignty, states would not oversee how tribal governments allocate vaccine.
Operationalizing the Criteria to Determine Allocation Phases
Data will not be available to characterize each individual in terms of the framework’s risk-based criteria. Even were such data available, an allocation scheme based on individual priority scores would be technically impractical for expeditiously delivering millions of courses of vaccine to geographically distributed individuals. To determine the population groups that comprise each allocation phase, the committee operationalized the criteria by characterizing certain population groups in terms of the risks faced by their typical members and the ability of a vaccine to reduce those risks (see Table 3-2). In applying the risk-based criteria and determining priorities, the committee also considered the roles of mitigating factors such as access to PPE and the ability to social distance and isolate or telework. The committee recognizes that each of the four risks depends on what mitigation strategies are possible and employed. Its analyses reflect typical current mitigation actions for each group. Thus, it does not consider whether the individuals involved, their employers, regulators, and others could do more to mitigate the risks.
Table 3-2 summarizes the committee’s assessments. It shows risk levels, relative to the general population, for typical members of specific major population groups, for each of the four risk-based criteria (see Box 3-3). Where risks depend on the behavior of institutions (e.g., providing PPE) or individuals (e.g., hand washing), the risk rating assumes current practices when this report was written. Actual risk levels will depend on actual practices, with the right-hand column noting some critical mitigating factors. Those practices are one source of the heterogeneity in the risks faced by members of each group. STLT authorities will need local knowledge to understand which members of each group, in their community, face higher and lower risk of each type.
There is no simple way to aggregate the four risk-based criteria, which interact in different ways in different settings. Criteria 1 (risk of acquiring infection) and 2 (risk of severe morbidity and mortality) combine to determine individuals’ health risk (the probability that they will get the disease and become very ill, or die, if they do). Those two criteria combine with Criterion 3 (risk of negative societal impact) to determine the risk to vital social functions. Criteria 1 (risk of acquiring infection) and 4 (risk of transmitting infection to others) combine to determine how fast the infection spreads (what is the chance of someone getting the infection and giving it to others). Those two risks are often, but not always, related. For example, some people with high risk of acquiring infection may circulate little after being exposed. Because of these interactions among the criteria, the committee deliberately proposes no weighting scheme. Rather, the committee has set priorities by the groups’ risk profiles, treating those within each phase equally and relying on the dedication and good judgment of STLT authorities to work out the details in keeping with the framework’s guiding principles and the best available evidence.
Discussion of the Allocation Phases
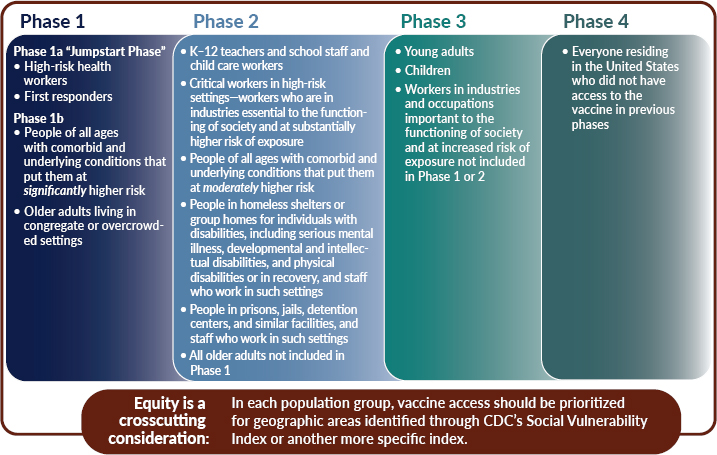
The committee recommends a four-phased approach to COVID-19 vaccine allocation. For each population group, the committee recommends prioritizing for areas identified as vulnerable through CDC’s SVI or by another more specific index such as the CCVI. This issue is discussed further later in this chapter. Within each phase, all groups have equal priority.
The first phase includes a “jumpstart” phase: Phase 1a. Included in Phase 1a would be “frontline” health workers—health professionals who are involved in direct patient care, as well as those working in transport, environmental services, or other health care facility services—who risk exposure to bodily fluids or aerosols. Under conditions of such scarcity, access should not be defined by professional title, but rather by an individual’s actual risk of exposure to COVID-19. The rationale for including “frontline” health workers in the first phase is manifold: their contact with patients with SARS-CoV-2 (despite the use of PPE, which can be limited in some settings); the fact that they work in an essential industry, but may be precluded from performing their professional duties if they are exposed or infected; and the reality that many such workers are potentially important nodes in onward transmission networks, given that many who are in low-wage jobs may also contribute to further transmission due to living in crowded, often multi-generational living situations where social distancing is unrealistic. The latter is especially true for many individuals who work in nursing homes, assisted living facilities, group homes, and as home health aides. In addition to frontline health care workers, first responders are included
TABLE 3-2 Applying the Allocation Criteria to Specific Population Groups
| Phase | Population Group | Criterion 1: Risk of Acquiring Infection | Criterion 2: Risk of Severe Morbidity and Mortality | Criterion 3: Risk of Negative Societal Impact | Criterion 4: Risk of Transmitting Infection to Others | Mitigating Factors for Consideration |
|---|---|---|---|---|---|---|
| 1a | High-risk health workers | H | M | H | H | Adequate access to personal protective equipment. Workplace management of exposure. |
| 1a | First responders | H | M | H | H | Adequate access to personal protective equipment. Workplace management of exposure. |
| 1b | People with significant comorbid conditions (defined as having two or more) | M | H | M | M | Ability to maintain social distance and isolate. |
| 1b | Older adults in congregate or overcrowded settings | H | H | L | M | Effective institutional management of exposure. |
| 2 | K–12 teachers and school staff and child care workers | H | M | H | H | Online schooling, especially for lower grades, recognizing educational and social impacts. |
| 2 | Critical workers in high-risk settings | H | M | H | M | Adequate access to personal protective equipment. Workplace management of exposure. |
| 2 | People with moderate comorbid conditions | M | M | M | M | Ability to maintain social distance and isolate. |
| 2 | People in homeless shelters or group homes and staff | H | H | L | H | Adequate access to personal protective equipment. Effective institutional/workplace management of exposure. |
| 2 | Incarcerated/detained people and staff | H | M | L | H | Adequate access to personal protective equipment. Effective institutional/workplace management of exposure. |
| 2 | All older adults | M | H | L | L | Ability to maintain social distance and isolate. |
| 3 | Young adults | H | L | M | H | Ability to maintain social distance and isolate. Closure of congregate settings (e.g., bars). |
| 3 | Children | M | L | M | H | Ability to participate in online schooling. |
| 3 | Workers in industries important to the functioning of society | M | M | M | M | Adequate access to personal protective equipment. Effective institutional/workplace management of exposure. |
NOTES: Cell entries are for a typical member of each group. H = high risk; L = low risk; M = medium risk. All groups are heterogeneous, and ratings indicate the median risk. All cell entries are relative to risks in the overall population, not measures of absolute risk, and are based on the committee’s expert judgment of the evidence and the unknowns at the time of the report’s writing. There is no weighting of these different criteria and no aggregation. Within each phase, the population groups are of similar priority, and authorities have the flexibility to adapt the priority population groups to their specific conditions. Lastly, the committee has elected not to use the designation “essential worker.” Instead, the committee refer to these workers as critical workers in high-risk settings as they are both working in industries vital to the functioning of society and in occupations where they cannot avoid exposure risk by, for example, teleworking. This is described in additional detail later in this chapter.
as well. The “jumpstart” phase is followed by Phase 1b, which includes those older adults living in congregate settings—such as nursing homes or skilled nursing facilities—and other similar settings. Last, individuals with select high-risk comorbid and underlying conditions are included in Phase 1. Knowledge of the relative risks stemming from specific underlying risk factors is evolving quickly and will be better known by the time vaccines become available. This would allow decision makers to target for vaccination, more effectively than is possible today, those individuals at greatest risk of severe morbidity and mortality.
Recognizing the importance of education and child development, K–12 teachers and school staff are included in Phase 2. It is important to include this group relatively early to restart in-person education. The first cohort of critical workers who are in industries essential to the functioning of society and at higher risk of exposure are included in Phase 2. The expansion of vaccine supply would allow for the immunization of another cohort of individuals with comorbid and underlying conditions that put them at increased risk, as well as all older adults not already included in Phase 1. People who are incarcerated or detained and people who live in group homes and homeless shelters—congregate settings—are also included in Phase 2, along with the staff who work in such settings. With respect to these groups, the committee stressed the importance of recognizing their reduced autonomy and the difficulty of preventing spread in such settings should COVID-19 be introduced. Last, all older adults not included in Phase 1 would be included.
In Phase 3, vaccine supply will become even more widely available and allow the broader immunization of workers important to restoring full economic activity. In this phase, many workers will still be able to safely work from home and thus would be prioritized for later access to the vaccine. The broad immunization of children and young adults is included in this phase, given emerging evidence of the role they may play in asymptomatic transmission, especially in intrafamilial situations. An important caveat here is that broad immunization of children will depend on whether COVID-19 vaccines have been adequately tested for safety and efficacy in these age groups—similar issues also apply to pregnant women. Most initial trials are testing vaccines among older age groups, who are known to suffer more severe morbidity and mortality.
Finally, once vaccine supply becomes more broadly available (Phase 4), vaccines would be made available to individuals who are interested in receiving the vaccine for personal protection. Ideally, these individuals would be willing to participate in an egalitarian process (such as a lottery) if there are persistent local or regional shortages in this phase.
It is important to acknowledge that unknowns about the COVID-19 vaccine and the nature of the pandemic itself persist, but the committee ap-
proached its framework under the best available evidence today. Under the context described, the committee’s allocation approach is shown in Figure 3-2 and is further described in greater detail—first as a description of the various phases, followed by discussion of ensuring equity across all phases. The proposed approach assumes a poorly controlled outbreak in which the relative distribution of severe morbidity and mortality burden is similar to what exists today. Given the epidemiological features of COVID-19 so far, it is reasonable to assume these conditions will hold around the anticipated start of the U.S. COVID-19 vaccination program. However, it is possible that the United States will be able to substantially control the outbreak, as in countries such as New Zealand. In that case, a prioritization approach that initially emphasizes reducing transmission over direct protection from severe morbidity and mortality could be considered.
Overlap and Size of the Allocation Phases
The committee acknowledges that the population groups included in each phase overlap to a certain extent. A population may fit into multiple phases; for example, a group of critical workers in high-risk settings may also belong to a population with significant comorbid conditions, and an older adult may live in a congregate multi-generational setting. When individuals within a group fall into multiple phases, the higher phase should take precedent. STLT authorities must consider the cumulative effect of populations belonging to multiple groups and adhere to the stated foundational principles and apply the risk-based criteria to ensure that the implementation of the allocation phases meets the goal to reduce severe morbidity, mortality, and negative societal impact due to the transmission of the SARS-CoV-2.
The committee’s estimates of group size do not consider either the heterogeneity of the groups nor their overlap. The effective size of each group will be smaller to the extent that some of its members have lower risks or are in an earlier phase. The committee has not attempted to estimate that heterogeneity and overlap. As a result, the group sizes presented here are upper bounds that are very unlikely to be reached. For example, some health care facilities will be in regions with very low disease prevalence or will have the resources and management needed for stringent mitigation strategies, thereby reducing the number of high-risk workers in Phase 1a. Some K–12 teachers and staff will have more than one significant comorbid condition, putting them in Phase 1b, reducing the group size in Phase 2. Some members of each group may refuse the vaccine when their group’s time comes, reducing its initial size, perhaps delaying demand until field experience satisfies those individuals’ need for demonstrations of safety and effectiveness.
Phase 1
Phase 1 includes the following groups:
- High-risk health workers;
- First responders;
- People of all ages with comorbid and underlying conditions that put them at significantly higher risk; and
- Older adults living in congregate or overcrowded settings.
In a limited supply scenario, high-risk and high-exposure workers in health care facilities and first responders should constitute an initial “jumpstart” Phase 1a. This would be followed by Phase 1b, comprised of people with comorbid and underlying conditions that put them at significantly higher risk and older adults living in congregate or overcrowded settings.
Phase 1a would cover approximately 5 percent of the U.S. population, and in its entirety, Phase 1 would cover an estimated 15 percent. Such a structure could help kick off initial vaccine administration, while STLT authorities prepare distribution procedures for the next phases.
Phase 1a
Population: High-Risk Health Workers
This group includes frontline health care workers (who are in hospitals, nursing homes, or providing home care) who either (1) work in situations where the risk of SARS-CoV-2 transmission is higher, or (2) are at an elevated risk of transmitting the infection to patients at higher risk of mortality and severe morbidity. These individuals—who are themselves unable to avoid exposure to the virus—play a critical role in ensuring that the health system can care for COVID-19 patients.
These groups include not only clinicians (e.g., nurses, physicians, respiratory technicians, dentists and hygienists) but also other workers in health care settings who meet the Phase 1a risk criteria (e.g., nursing assistants, environmental services staff, assisted living facility staff, long-term care facility staff, group home staff, and home caregivers). The health care settings employing these workers who are at increased risk of exposure to the virus may also include ambulatory and urgent care clinics; dialysis centers; blood, organ, and tissue donation facilities; and other non-hospital health care facilities. Finally, there are community and family settings where care for infected patients occurs. Not all the workers in these settings are paid for their labor, but, while they are caring for infected people, they all need to be protected from the virus.
Situations associated with higher risk of transmission include caring for COVID-19 patients, cleaning areas where COVID-19 patients are admitted, treated, and housed, and performing procedures with higher risk of aerosolization such as endotracheal intubation, bronchoscopy, suctioning, turning the patient to the prone position, disconnecting the patient from the ventilator, invasive dental procedures and exams, invasive specimen collection, and cardiopulmonary resuscitation. In addition, there are other frontline health care workers who, if they have uncontrolled exposure to the patients or the public in the course of their work, should be in this initial phase. This group includes those individuals distributing or administering the vaccine—especially in areas of higher community transmission—such as pharmacists, plasma and blood donation workers, public health nurses, and other public health and emergency preparedness workers. The committee also includes morticians, funeral home workers, and other death care professionals involved in handling bodies as part of this high-risk group.
Rationale
Frontline health workers are particularly important in stemming the pandemic and preventing death and severe illness. From the beginning of the pandemic, many frontline workers have worked in environments where they have been exposed to the virus, often without adequate PPE. These individuals are critical to providing essential care, especially to older adults who are at the greatest risk of COVID-19 disease or death. Vaccinating these individuals not only enables them to provide these services, but also reduces the risk that they will spread the infection as they work in hospitals, nursing homes, assisted living facilities, home care, and group homes, and when they return to their own homes and communities.
Frontline health workers are at significantly higher risk of becoming infected with SARS-CoV-2 compared to members of the general public. A recent cohort study using data from the United States and the United Kingdom found that frontline health care workers had nearly 12 times the risk of the general population of testing positive for COVID-19 (Nguyen et al., 2020). This risk is exacerbated by the ongoing shortage of PPE especially in nursing homes and, in a study of health care personnel at 13 academic medical centers, workers who reported inadequate access to PPE had a higher rate of detectable SARS-CoV-2 antibodies than did those who did not report a PPE shortage (McGarry et al., 2020; Self et al., 2020). Protecting health care workers will have a great impact on protecting older individuals, who receive a large share of health services and have borne a large share of the disease burden from COVID-19.
In the first months of the pandemic, some hospitals were unprepared for the large number of COVID-19 cases. Exposure of hospital workers
was often poorly controlled, and many workers had inadequate PPE. Tens of thousands of hospital workers have been infected, and many hundreds have died, although there are no accurate data on these cases. While there is still a severe national PPE shortage, it appears that many hospitals are now better able to protect members of their workforce who directly work with COVID-19 patients. However, this is not true uniformly across the country, and, even better-equipped hospitals still leave some workers exposed. Nursing homes have struggled with having adequate PPE since the beginning of the pandemic and some continue to do so (Clark, 2020; McGarry et al., 2020). Individuals who provide home care or work in hospitals, nursing homes, and assisted living (or similar) facilities—who are also at higher risk for severe illness and death because of comorbid conditions and age—should be among the first to receive the vaccine.
Vaccination is not a substitute for non-medical preventive policies and equipment. All exposed workers should, for example, be provided an adequate supply of appropriate PPE. It is vitally important that the prospect of vaccination not supplant efforts to either ensure adequate supplies of PPE or continue mitigation strategies after vaccination.
In considering those health care workers who are at an elevated risk of transmitting the infection to patients at higher risk of mortality and severe morbidity, it is also important to note that nursing home residents and staff have been at the center of the pandemic since the first reported cases. Nearly 80 percent of all COVID-19 deaths in the United States have occurred in people over the age of 65 (CDC, 2020g). As of September 8, 2020, there were 331,864 confirmed or suspected COVID-19 cases and 51,700 deaths among nursing home residents, according to the Centers for Medicare & Medicaid Services (CMS, 2020a), and these numbers are likely to be underreported (Ouslander and Grabowski, 2020). Nursing home workers are at increased risk themselves—CMS also reports that nearly 800 nursing home staff in the United States have died from COVID-19—and play a role in spreading infection within and between institutions (CMS, 2020b). Asymptomatic spread by nursing home workers is well established (Lee et al., 2020) and vaccinating this group could have a significant impact on the incidence of infection in this setting. Nursing home and home care employment is low paying, with many workers holding jobs at more than one nursing home or home care setting. Many of these workers take public transportation and live in multi-generational housing, increasing the likelihood of exposure and of exposing others. In addition to their occupational and community exposures, these workers are statistically at a higher risk of contracting COVID-19 and experiencing severe health effects because they come from populations with higher rates of comorbid conditions (Silver et al., 2020). A notable proportion of nursing home workers are Black (27.8 percent), as are home care workers (Black: 29.7 percent and Latinx: 17.5
percent) (McCormack et al., 2020). A sizable proportion of such workers are over 65 as well (Black: 9.1 percent and Latinx: 11.3 percent).
Estimated Group Size9
According to the best currently available estimates for the United States, among health care practitioners and technical staff, 6,728,000 are exposed to COVID-19 more than once per week; among health care support staff, 3,160,000 are exposed to COVID-19 more than once per week. There are also approximately 1,500,000 full-time nursing home employees, 432,000 health care practitioners who work in skilled nursing facilities, and 3,162,000 home health care workers (Baker et al., 2020; BLS, 2019d). There are approximately 291,000 public health workers in the United States (Beck et al., 2014), including 41,000 public health nurses in state and local health departments and 59,000 community health workers (Beck et al., 2014; BLS, 2019c). There are also 621,000 pharmacists and pharmacy staff (BLS, 2019e), as well as 200,000 dentists in the United States (ADA, 2020). The number of morticians, undertakers, and funeral directors in the United States is estimated to be approximately 25,000 (Statista, 2020).
Population: First Responders
This group includes emergency medical services (EMS) personnel, police, and firefighters (including volunteer firefighters). Like health workers, many first responders have been working in situations in which exposure to infected individuals is sometimes unavoidable. However, first responders in some jobs and in some communities may not be at an increased risk of exposure, and inclusion in this category should reflect occupational risk. First responders who are not at higher risk of exposure need not be prioritized. Given their public serving role, first responders who become ill can transmit infection to their families and to the broader community. Although data on exposure risk for first responders are limited, initial estimates indicate higher infection rates among first responders in higher COVID-19 transmission settings.
Rationale
First responders are central to society’s overall functioning, to its response to the virus, and to ensuring that others with medical emergencies receive necessary immediate care. When emergency medical personnel and
___________________
9 Estimated group sizes across phases are not intended to be entirely cumulative, and the committee acknowledges there is overlap between the group estimates provided. Please see the discussion of limitations at the end of this chapter for additional discussion of data.
firefighters are unable to work, because of illness or when isolating because of exposure to the virus, their ability to provide badly needed medical, rescue, and firefighting services, is impaired. First responders who are at higher risk of exposure and who are also at higher risk for severe illness and death because of comorbid conditions and age should be among the first receiving the vaccine in this group.
Many of the reasons for protecting health care workers also apply to first responders. These include the social value of maintaining emergency services, reciprocity for the assumption of additional risk by these groups, and—in some cases—higher risk of acquiring infection and, potentially, transmitting the virus. Similarly, until substantial and sustained suppression of SARS-CoV-2 transmission is achieved, first responders are likely to need PPE for performing their responsibilities.
Estimated Group Size
An estimated 2.1 million first responders are included in this population group, comprising 262,000 EMS personnel, 701,000 police, and 1,100,000 firefighters (approximately 300,000 of whom are paid, with the rest serving in a volunteer capacity, and a subset of whom provide emergency medical services) (BJS, 2019; BLS, 2019f, 2020c; Evarts and Stein, 2020).
Phase 1b
Population: People of All Ages with Comorbid and Underlying Conditions That Put Them at Significantly Higher Risk
It remains unclear precisely which comorbid and underlying conditions put individuals at a significantly higher risk of severe COVID-19 disease or death. CDC continues to gather evidence on this topic, and lists the following as factors associated with an increased risk of severe COVID-19 disease: cancer, chronic kidney disease, chronic obstructive pulmonary disease, immunocompromised state from solid organ transplant, obesity (body mass index [BMI] ≥30), serious heart conditions (e.g., heart failure, coronary artery disease, cardiomyopathies), sickle cell disease, and type 2 diabetes mellitus (CDC, 2020d). Vaccinating all individuals with these comorbid conditions in Phase 1b is not possible, because the group includes hundreds of millions of people in the United States. In a highly constrained vaccine scenario, the initial group of recipients with comorbid and underlying conditions could focus specifically on individuals with two or more of these designated conditions.
It should be noted that as the relationship between severe COVID-19 disease and certain comorbid conditions becomes clearer, this list should
evolve. The committee acknowledges that there are uncertainties about the feasibility of identifying and administering vaccine to this group, but expect that a spectrum of approaches may be employed to identify these high-risk individuals in the initial phase. ACIP and CDC will play a key role in assessing relevant evidence on this topic, and in the process of prioritization, it will be critical to recognize that not all comorbid conditions are equal when it comes to their placement in an allocation framework.
Rationale
According to data recently published through the COVID-19 Associated Hospitalization Surveillance Network (COVID-NET) from March 1 through August 15, 2020, approximately 75 percent of adults hospitalized for COVID-19 in the United States had at least two comorbid conditions. More than 60 percent of hospitalized adults had three or more underlying conditions (McClung, 2020).10
Multiple studies have explored a range of comorbid and underlying conditions as potential risk factors for severe COVID-19 disease. According to CDC’s surveillance data for March 2020, people with COVID-19 who had underlying health conditions—most commonly hypertension, obesity, cardiovascular disease, diabetes mellitus, and chronic lung disease—were six times as likely to be hospitalized and 12 times as likely to die from the disease as those without underlying health conditions. A study from a large health care system in New York City found that individuals below age 60 with a BMI of 30 or higher were more likely to be admitted to acute and critical care than patients in the same age categories with a BMI below 30 (Lighter et al., 2020). Another recent study suggests that, in particular, those with chronic heart failure, kidney disease, and a BMI of 40 or higher are at particularly higher risk (Petrilli et al., 2020). Ultimately, given the higher risk of adverse outcomes in individuals with select comorbid conditions and the evolving evidence on this topic, it will be critical to monitor how the nature and number of comorbid conditions affect severe morbidity and mortality at the individual level.
Estimated Group Size
There are currently no clear data from which to accurately estimate the size of the population group with multiple select comorbid conditions,
___________________
10 The list of comorbid conditions assessed in COVID-NET differs slightly from CDC’s current list of conditions that put individuals at “increased risk” of severe illness from COVID-19 disease. The COVID-NET list includes hypertension, obesity, diabetes, cardiovascular disease, neurologic disease, chronic lung disease, renal disease, asthma, immune suppression, gastrointestinal/liver disease, and autoimmune disease.
which the committee acknowledges as a key limitation and something that could benefit from future research. A recent modeling study by Clark et al. (2020) may provide a general range of the size of this population group. In the study, the authors highlighted a “high-risk” group defined as individuals who would require hospitalization if infected with COVID-19, calculated using age-specific infection–hospitalization ratios for COVID-19. The study estimated that 19–20 million people in the United States fall into this category. Given that approximately 75 percent of those hospitalized for COVID-19 based on the COVID-NET data had multiple comorbid conditions, the committee estimates that the value of 19–20 million may approximate the number of individuals with multiple comorbid conditions (from the preceding CDC list).
Population: Older Adults Living in Congregate or Overcrowded Settings
This group includes older individuals living in congregate and overcrowded situations (e.g., long-term care facilities, homeless shelters or group homes, prisons, and jails) that increase their risk of SARS-CoV-2 infection and resultant morbidity and mortality. The scientific community’s understanding of age-specific COVID-19 mortality is still emerging, and there are concerns, based on the lower efficacy of other vaccines (such as influenza vaccine) among the elderly, that COVID-19 vaccines will have a lower efficacy among older adults. For these reasons, ACIP should determine age guidelines as health and vaccine efficacy data become more available.
Rationale
According to CDC, the case fatality proportion for COVID-19 is substantially higher among older adults in the United States. As previously mentioned, approximately 80 percent of all deaths have occurred in adults 65 and older (CDC, 2020g). Similarly, the risk of hospitalization from COVID-19 increases with age, with rates per 100,000 persons being significantly higher for adults aged 65 and older (~199 per 100,000 for 65–74-year-old individuals, ~329 per 100,000 for 75–84-year-old individuals, and ~513 per 100,000 for individuals 85 and older) (CDC, 2020c). A significant proportion of COVID-19 deaths occurred in individuals living in long-term care facilities (CMS, 2020a). Data from Canada and other countries, as well as investigative reporting in the United States, suggest that the percentage of COVID-19 deaths occurring in residents of long-term care facilities may be higher than indicated by CDC’s database (CIHI, 2020; NYT, 2020).
Whatever the precise numbers, it is clear that directly protecting older adults—particularly those living in congregate or overcrowded settings—-
will have a substantial impact on COVID-19-related severe outcomes. Although there is some uncertainty regarding how well the vaccine will work in older individuals, models find that prioritizing older adults will have a substantial impact on mortality, even if the vaccine is up to 50 percent less effective among people aged 60 or older compared to people younger than 60 (Lipsitch, 2020). In addition, adjuvanted vaccines, such as the recombinant zoster vaccine (Shingrix), have been demonstrated to provide efficacy to older adults across the age spectrum (Bastidas et al., 2019; Dagnew et al., 2020).
The committee suspects that many older adults living in overcrowded settings may live in multigenerational households. Historically, in virtually every society, people have lived together in households comprised of three and even four generations (Miller and Nebeker-Adams, 2017). Although such households are less common overall in the United States today, they are still often found in lower-income communities. Such households typically have relatively few bedrooms and bathrooms, with crowded sleeping arrangements and reduced opportunities for practicing social distancing. Because many individuals living in multi-generational households in the United States also work in jobs that put them at an elevated risk of exposure to COVID-19 it is important to vaccinate the older adults in those households, to protect them from acquiring COVID-19.
The combination of the risk of severe disease due to advanced age and the higher risk of acquiring infection and transmission among older adults included in this population group makes it among the highest priority groups for receiving the COVID-19 vaccine.
Estimated Group Size
There are approximately 1,347,000 nursing home residents in the United States and 811,000 individuals living in residential care facilities. In addition, 4,700,000 adults over the age of 65 live below the poverty line, meaning the individuals included in this group total more than 6.8 million people (CDC, 2020b,f,h; Cubanski et al., 2018). In addition, according to 2016 estimates, 21 percent of adults aged 65 and older in the United States lived in multi-generational households (out of approximately 49.2 million total), with a disproportionate number from communities of color (Cohn and Passel, 2018; Rieger, 2017; Roberts et al., 2018).
Phase 2
Phase 2 includes the following groups:
- K–12 teachers and school staff and child care workers;
- Critical workers in high-risk settings—workers who are in industries essential to the functioning of society and at substantially higher risk of exposure;
- People of all ages with comorbid and underlying conditions that put them at moderately higher risk;
- People in homeless shelters or group homes for individuals with disabilities, including serious mental illness, developmental and intellectual disabilities, and physical disabilities or who are in recovery, and staff who work in such settings;
- People in prisons, jails, detention centers, and similar facilities, and staff who work in such settings; and
- All older adults not included in Phase 1.
It is important to note the changing vaccine supply levels at various points during the allocation process, and as vaccine supply increases, efforts should be expanded to the additional populations listed in Phase 2. Phase 2 would cover an estimated 30–35 percent of the U.S. population; combined with Phase 1, the groups included across both phases would total approximately 45–50 percent of the population. Moving to Phase 2, it is important to note the overlap issue discussed earlier in this chapter. Individuals who fall within population groups in this phase may also be high-risk health workers or first responders, may have comorbid and underlying conditions that put them at significantly higher risk, or may be older and living in congregate or overcrowded settings and therefore should be vaccinated in Phase 1.
Population: K–12 Teachers and School Staff and Child Care Workers
This group includes K–12 school staff and child care workers (such as nursery school staff), including teachers, administrators, environmental services staff, maintenance workers, and school bus drivers.
Rationale
Across the nation, states and localities are placing a high priority on reopening schools and expanding child care programs to promote children’s educational and social development and facilitate parents’ employment. Exposure is very difficult to control in these institutions, especially those providing care or education to young children. All workers in these facilities are among those who need to be protected from the virus during Phase 2. Due to the nature of their work, teachers and school staff who return to work in schools are at higher risk of SARS-CoV-2 infection and serve an important societal role in ensuring that students’ educational needs are met. One could also argue that vaccinating teachers and school staff could help
to reduce SARS-CoV-2 transmission, with these teachers and staff serving as connections between schools and the community.
Furthermore, the importance of re-opening schools, especially for elementary-aged children, cannot be overstated. Reestablishing a sense of normalcy for students and their families through in-person education will help to achieve long-term health benefits for children and will facilitate important social development for them as well.
As some states and localities choose to begin re-opening schools, it is also important to consider the direct impact of COVID-19 on teachers and staff. A recent study found that 39.8 percent of teachers had “definite” and 50.6 percent had “definite or possible” risk factors for severe COVID-19 disease (with similar results for other school staff), emphasizing the vaccine’s potential importance in protecting teachers and promoting in-person education safely (Gaffney et al., 2020). Therefore, it is likely that many teachers at highest risk would be vaccinated in Phase 1b.
Estimated Group Size
Across the United States, there are 8,605,000 teachers and staff at elementary and secondary schools; there are also approximately 463,000 people who provide child care services (BLS, 2019f, 2020e).
Population: Critical Workers in High-Risk Settings—Workers Who Are in Industries Essential to the Functioning of Society and at Substantially Higher Risk of Exposure
Another group included in Phase 2 is comprised of people whose work is vital to the functioning of society and the economy, and whose work causes them to have a higher level of exposure to persons with SARS-CoV-2 infection. The U.S. Department of Homeland Security (DHS) has identified categories of Essential Critical Infrastructure Workers11 whose functioning “is imperative during the response to the COVID-19 emergency for both public health and safety as well as community well-being” (Krebs, 2020, p. 3).
The list of categories of workers designated by DHS includes many groups of workers who are at higher risk of exposure. Others designated by DHS, however, are either able to telework or are otherwise isolated and not at higher risk of exposure. Recent work has found that 37 percent of jobs in the U.S. economy are teleworkable. Many of these jobs are in occupations in essential industries, but they also represent “white collar” positions in industries that are generally considered “blue collar” (Dingel and Neiman, 2020). Thus,
___________________
11 See https://www.cisa.gov/publication/guidance-essential-critical-infrastructure-workforce (accessed September 15, 2020).
while performing “essential work,” these employees are able to avoid the exposure risk while doing vital work. For this reason, the committee has elected not to use the designation “essential worker” in the allocation framework. Instead, the committee refers to these workers as critical workers in high-risk settings because they are both working in industries vital to the functioning of society and in occupations where they cannot avoid exposure risk.
The industries in which these critical workers are employed are essential to keeping society and the economy functioning. Since the beginning of the pandemic, millions of people have been going to work and risking exposure to the virus to ensure that markets have food; drug stores have pharmaceutical products; public safety and order are maintained; mail and packages are delivered; and buses, trains, and planes are operating. This group also includes other health workers who are not already accounted for in Phase 1a. Importantly, only those whose jobs or occupations in these essential industries where the workers cannot avoid a high risk of exposure qualify as critical workers in this group.
There is no single complete list of all workers who should be in the Phase 2 prioritization. The designation of critical worker in a high-risk setting should be a function of the likelihood of uncontrolled exposure and will undoubtedly vary by state and situation. It may be, for example, that coal miners, many of whom already have occupational lung disease, are not provided adequate protection. If this is the case, they would be included in this category. Similarly, there are likely to be government workers who have uncontrolled exposure; for example, workplace inspectors or meat and poultry inspectors employed by the U.S. Department of Agriculture are likely to fit into this category.
It would be useful if public health agencies, including CDC, the Occupational Safety and Health Administration, the Mine Safety and Health Administration, and state and local public health agencies, provided additional guidance in the designation of jobs or tasks involved as well as occupational code or job title in this group.
Rationale
Large numbers of these workers whose work is vital to the function of society and the economy have been infected with COVID-19 while on the job, although precise counts are not available (The Lancet, 2020; Michaels and Wagner, 2020). Those members of these sectors who are at higher risk for exposure and infection should be given priority. These workers are more likely to be Black or Latinx than workers who are able to work safely from home (Hawkins, 2020; McCormack et al., 2020). Many of them work without adequate protection while in close proximity with coworkers and members of the public. Groups of critical risk workers who are at higher risk
of exposure (Waltenburg et al., 2020) include workers in the U.S. food supply system who plant, harvest, and package crops; slaughter and process meat; and deliver food to stores and stock shelves and staff checkout lines. In many food system workplaces, adequate protections have not been provided. There are many reasons why food system workers are at increased risk of infection and disease, including prolonged close workplace contact with coworkers, frequent community contact with fellow workers, mobility of the workforce (i.e., migrant workers), shared transportation to and from the workplace, lack of paid sick leave, and congregate housing situations (including living in employer-furnished housing and shared living quarters, and living in crowded and multi-generational homes) (Oliver, 2020). For economic and legal reasons, these low-paid workers may be less likely to attempt to use the health care system for care. Workers in other sectors are at increased risk as well, including workers employed in public transportation (such as buses, trains, car services or planes), especially in localities or situations where passengers are not required to wear masks. Included in this population group are postal workers and workers in warehouses and fulfillment centers. In addition, there are many other workers whose jobs are vital to society’s functioning and who therefore are required to work when other workers are home. Members of these groups whose work entails being in close proximity to other potentially infected people, and who are not able to be adequately protected from exposure, are in this phase as well. Not all critical risk workers are U.S. citizens or green card holders; some may have come to the United States as refugees or may be undocumented. All workers in this population group need to be provided the vaccine, and special efforts must be made to reach these workers in ways that encourage them to be vaccinated.
Echoing what was stated in Phase 1, it is important to note that while community transmission of SARS-CoV-2 continues, vaccination is not a substitute for providing other interventions to mitigate exposure risk, such as engineering and administrative controls, paid sick leave for potentially infectious workers, and providing adequate PPE (OSHA, 2020).
Estimated Group Size
Workers from numerous essential industries are included in this group, such as workers in food and beverage production (1,700,000), cashiers and food store workers (865,000), workers in the utilities sector (e.g., electric, water, telecommunications; 539,000), postal service workers (497,000), delivery workers (e.g., truck drivers; 1,506,000), passenger vehicle drivers (1,077,000), construction workers (7,214,000), and public transit workers (179,000). There are more than 15 million health care workers in the United States, though a large percentage of them are already covered in Phase 1a (BLS, 2019d,f, 2020a,b,d,f; USDA, 2020; USPS, 2020; Walten-
burg et al., 2020). Ideally, 20 percent of workers from those in industries deemed to be essential would be covered in this initial group.
Population: People of All Ages with Comorbid and Underlying Conditions That Put Them at Moderately Higher Risk
Drawing on CDC’s list of comorbid conditions discussed in Phase 1b, this population group would include anyone with one of the previously mentioned conditions. Phase 1b includes individuals with two or more comorbid conditions from among those listed.
Other comorbid conditions and potentially rare diseases should be considered for inclusion in this phase as evidence emerges. In addition to CDC’s list of comorbid conditions that put individuals at increased risk, CDC has also compiled a list of comorbid conditions that might put individuals at increased risk. This list includes asthma (moderate to severe); cerebrovascular disease; cystic fibrosis; hypertension; immunocompromised state from blood or bone marrow transplant, immune deficiencies, HIV/AIDS, use of corticosteroids, or use of other immunosuppressive medicines; neurologic conditions; liver disease; pregnancy; pulmonary fibrosis; smoking; thalassemia; and type 1 diabetes mellitus (CDC, 2020d).
Rationale
As per to the discussion of Phase 1b, the rationale for prioritizing persons with such conditions is that the vaccine may have a greater impact among those with increased likelihood of severe illness (hospitalization and intensive care unit admission) and death than in persons without these conditions, resulting in a decreased burden on the health care system and more lives being saved from all conditions. Based on the aforementioned COVID-NET data, approximately 12 percent of adults hospitalized for COVID-19 in the United States between March 1 and August 15, 2020, had one of the listed comorbid or underlying conditions.12
Estimated Group Size
Without accounting for those with multiple comorbid conditions in Phase 1b, the committee is not currently in a position to accurately estimate the number of individuals in this population group. Furthermore, it
___________________
12 The list of comorbid conditions assessed in COVID-NET differs slightly from CDC’s current list of conditions that put individuals at “increased risk” of severe illness from COVID-19 disease. The COVID-NET list includes hypertension, obesity, diabetes, cardiovascular disease, neurologic disease, chronic lung disease, renal disease, asthma, immune suppression, gastrointestinal/liver disease, and autoimmune disease.
remains possible that additional comorbid conditions will be included in this category as evidence emerges, but this population group would likely include tens of millions of people.
Population: People in Homeless Shelters or Group Homes for Individuals with Disabilities, Including Serious Mental Illness, Developmental and Intellectual Disabilities, and Physical Disabilities or Who Are in Recovery, and Staff Who Work in Such Settings
This group includes people who live in homeless shelters or group homes for people with disabilities that include serious mental illness, developmental and intellectual disabilities, and physical disabilities or who live in recovery in group homes, as well as the staff in these facilities.
Rationale
Many of these people are at risk because of their underlying diseases, chronic health care needs, limited access to health care, and because of their living setting (Landes et al., 2020).
Among people who experience homelessness, many are at higher risk of acquiring and transmitting infection, given the frequency of time spent in public places or in congregate settings such as shelters and due to other challenges (e.g., not receiving proper communication about the pandemic). Individuals living in congregate settings face increased risk of exposure to COVID-19 if they have limited or shared bathroom facilities, share utensils and other personal items, and have limited ability to practice social distancing or hygiene, including frequent hand washing. In addition, staff at these facilities are at increased risk of exposure and are more likely to transmit SARS-CoV-2 if infected. Many people who experience homelessness may suffer from one or more underlying health conditions that may put them at higher risk and therefore they are included in vaccination in the first phase.
People with disabilities, including serious mental illness, developmental and intellectual disabilities, and physical disabilities or who are in recovery in group homes may also have conditions that increase their risk of severe COVID-19 outcomes, and their autonomy is reduced by living in a group home setting, putting them at risk of acquiring infection and transmission. Additionally, group homes may differ from more institutionalized settings in that residents often have the right and ability to access their local communities, work outside the residence, and have visitors from outside the home, which increases the risk of exposure and transmission (NCD, 2020).
Estimated Group Size
It is estimated that 469,000 people live in group homes and 575,000 people experience homelessness across the United States (Culhane et al., 2020; Williams et al., 2013); staff at such facilities should be included here as well.
Population: People in Prisons, Jails, Detention Centers, and Similar Facilities, and Staff Who Work in Such Settings
Another group to be included in Phase 2 are staff members and people in prisons, jails, and detention centers, including immigration detention facilities. A prisoner is defined as anyone who is deprived of personal liberty against his or her will following conviction of a crime. Although not afforded all the rights of a free person, a prisoner is assured certain rights by the U.S. Constitution and the moral standards of the community. Detainees are individuals who are kept in jail or some other holding facility even though they have not been convicted of a crime. A majority of detainees in jails are individuals who cannot obtain sufficient funds to post bail and who are not released from jail pending a trial on the criminal charges. Others may be in detention centers after entering the country without documentation and are now awaiting resolution of their asylum or other claims in immigration detention facilities.
Rationale
Data show that persons in state and federal prisons have a 5.5-fold greater risk of contracting COVID-19 compared to the general U.S. population (Saloner et al., 2020). These people, as well as those in jails and detention centers, have reduced autonomy and cannot physically distance themselves from others in their congregate living setting and thus need additional protection (Page et al., 2020). As a result, the risk of their both acquiring and transmitting SARS-CoV-2 infection to others is higher.
Vaccination in Phase 2 of those in detention centers after entering the country without documentation is important because other controls, such as maintaining 6-foot distancing, are difficult or impossible to achieve. Most of these people are housed in one of the more than 250 public and private facilities under contract with the federal government, but with varying levels of care because they are not always subject to federal standards.
Outbreaks of seasonal influenza demonstrate the inadequate nature of the medical system in these facilities (Page et al., 2020). Furthermore, as has been described in studies of seasonal influenza vaccine, vaccinating individuals held in immigration detention facilities can help to prevent outbreaks of infectious disease both within these facilities and between
facilities and the rest of society (Omer, 2019; Sunderji et al., 2020). This is an especially important consideration for staff in these facilities, as they serve as the conduit between the two.
Estimated Group Size
There are currently an estimated 2.3 million incarcerated or detained individuals in the United States, in addition to 423,000 correctional officers, jailers, and support staff, bringing the total to more than 2.7 million people in this group (Akiyama et al., 2020; BLS, 2019f).
Population: All Other Older Adults Not Included in Phase 1
Beyond the older adult group already discussed in Phase 1b (those older adults living in congregate or overcrowded settings), this group includes all older adults residing in the United States. As discussed earlier, the committee defers to ACIP to determine specific age guidelines as health and vaccine efficacy data become more available.
Rationale
As discussed in the rational for a subset of older adults in Phase 1b, the case fatality proportion for COVID-19 is substantially higher among older adults in the United States, and the rate of hospitalization for COVID-19 increases with age. Ultimately, one could argue that age is itself an underlying condition for COVID-19, given the higher risk of severe disease and death due to COVID-19 among older adults.
Estimated Group Size
It is estimated there are more than 49.2 million older adults (people 65 and older) living in the United States (Roberts et al., 2018). Accounting for some overlap with the previous groups, it is estimated that there are 13.2 million older adults in the United States without comorbid or underlying conditions.
Phase 3
Phase 3 includes the following groups:
- Young adults;
- Children; and
- Workers in industries and occupations important to the functioning of society and at increased risk of exposure not included in Phases 1 or 2.
Phase 3 would cover approximately 40–45 percent of the U.S. population. Cumulatively, Phases 1–3 would then cover 85–95 percent of the U.S. population.
Population: Young Adults
This group includes all young adults aged 18–30 residing in the United States.
Rationale
In Phase 3, vaccine supply will become more widely available and allow for broader immunization of the U.S. population, which is essential to stem transmission and restore full social and economic activity. While both the case fatality proportion and the hospitalization rate for COVID-19 are substantially lower in young adults aged 18–30, there is increasing evidence that this group may be disproportionately fueling asymptomatic and/or presymptomatic transmission (Moghadas et al., 2020; Souchery, 2020). Studies have shown that adults under the age of 30 report significantly higher levels of social contacts and broader social networks than adults in any other age group (Bruine de Bruin et al., 2020), thus potentially putting them at heightened risk of both acquiring infection and transmission.
In addition, this group includes college-aged individuals, who are more likely to be living in congregate settings—such as college dormitories, house shares, and other communal living facilities—and thus face increased risk of contracting SARS-CoV-2 infections. Numerous outbreaks of COVID-19 are already occurring in such settings in the United States. Furthermore, SARS-CoV-2 infections in college-aged adults can threaten the health of faculty and other university staff, many of whom are older or have underlying illnesses that put them at risk of severe COVID-19. Similarly, the 2019 U.S. Census data show that approximately one in two young adults currently live in parental homes, and thus are at higher risk of transmitting the infection to their family members, who may also be at increased risk of severe disease and death due to age or comorbidity (U.S. Census, 2019b).
Given the emerging evidence of the role of pre-symptomatic and asymptomatic transmission in intrafamilial situations and/or congregate settings, the committee deemed it critical to include this group in Phase 3.
Estimated Group Size
According the 2019 U.S. Census Bureau data, there are approximately 58 million young adults between the ages of 18 and 30 (U.S. Census, 2019a). Accounting for the potential overlap with other groups across other
phases, the committee estimates that approximately 46.5 million young adults would be included in this phase.
Population: Children
This group includes all children—including schoolchildren who attend preschool, elementary school, middle school, and high school.
Rationale
While the proportion of children who become infected with SARS-CoV-2 and who become severely ill is much smaller than that in adults, severe cases of COVID-19 do occur in children, and the long-term effects of such illnesses are not yet understood. Children also can play a role in COVID-19 disease transmission (Gaffney et al., 2020). Furthermore, when SARS-CoV-2 infections are documented in children, they can cause major disruptions of educational activities (e.g., school closings, quarantine, and isolation) for children, staff, and families. They can threaten the health of teachers and staff, many of whom are older or have underlying illnesses that put them at risk of severe COVID-19, as well as members of their extended families. These disruptions can also reduce parents’ and guardians’ ability to work. Vaccination and the resultant immunity to SARS-CoV-2 infection among children will allow schools of all types and sizes to safely re-open and remain open, which will, in turn, allow parents and guardians to return to the workforce. At the same time, the other important benefits to children being back in school (e.g., provision of nutritious meals, emotional well-being, detection of and response to possible child abuse or neglect, and so on) can be realized.
It is important to note that clinical trials of COVID-19 vaccine have not started in children in the United States. It will be critical to conduct trials to gain a better understanding of the safety and efficacy of COVID-19 vaccine among children before they receive the vaccine.
Estimated Group Size
There are approximately 74 million children (infant to 17 years of age) in the United States (Federal Interagency Forum on Child and Family Statistics, 2020).
Population: Workers in Industries and Occupations Important to the Functioning of Society and at Increased Risk of Exposure Not Included in Phases 1 or 2
The inclusion of workers in this category represents a social choice: beyond the workers designated in Phases 1 and 2, which jobs and industries
are important or desirable to maintaining the normal functioning of society? Examples of such occupational groups include university professors and staff, workers in restaurants, hotels, and the entertainment industry; those in banks and libraries; and those in hair and nail salons, barber shops, and exercise facilities, or in factories or other goods-producing facilities. Many of these workers are among the DHS designated categories of Essential Critical Infrastructure Workers and include workers whose jobs are of economic importance and who have continued to work from outside their homes since the beginning of the pandemic. However, their risk of exposure or severe illness is lower than that of individuals in Phase 2. The jobs of some of these workers are primarily in settings where distancing and other protective measures can be implemented without great difficulty, but who may still be at increased risk. There are others in this population group, like those employed in theater, sports, and other aspects of entertainment, who cannot easily socially distance or use PPE, but whose industry was not considered to be as essential to societal functioning and was therefore suspended at the beginning of the pandemic.
Rationale
These workers play important roles in society; are central to the return of commerce and normalcy; and are often exposed to large numbers of individuals in the performance of their jobs. Their safe return to work is important as society re-opens and, comparing this cohort of workers to those discussed in Phase 2, their inclusion in Phase 3 focuses more on prevention of transmission of the virus. In comparison to workers included in Phase 2, workers in Phase 3 are likely to have lower exposure risk to SARS-CoV-2 through their occupation, or hold a role that is considered less central to economic and social recovery, or both. Including this group in Phase 3 will support social and economic recovery and restoration as access to the vaccine becomes more widespread.
Estimated Group Size
The workers included here cover a wide variety of industries that are important to societal function and re-opening. Among the occupations that could potentially be included here are college and university faculty and staff (~3,089,000); factory workers in production and non-supervisory roles (~8,400,000); restaurant wait staff (nearly 2.6 million); hotel cleaning and management staff (nearly 1.2 million); bank tellers (~442,000); librarians (~136,000); barbers, hair stylists, and cosmetologists (~406,000); and exercise instructors (~326,000) (BLS, 2019a,b,f). Ideally, Phase 3 would vaccinate the remaining 80 percent of workers from industries deemed to be essential.
Phase 4
Shifting to a more routine vaccination strategy, Phase 4 includes every person residing in the United States who did not have access to the vaccine in previous phases (and for whom the vaccine is not medically contraindicated, although no contraindications are known at this time). In a pandemic caused by a new pathogen, most—if not all—individuals are at risk of being infected by the pathogen. Estimates of the percent of the population with immunity vary for COVID-19 and the efficacy of COVID-19 vaccines is yet to be determined (Britton et al., 2020). Therefore, precise estimates of target vaccination coverage are not available. Nevertheless, the resumption of social functions will require high vaccination coverage in the general population. The United States should ensure that all U.S.-based individuals who did not have access to the vaccine in previous phases (and for whom the vaccine is not medically contraindicated) have access to the vaccine.
Ensuring Equity
As discussed earlier in this chapter, the principles and allocation criteria underlying these phases explicitly avoid perpetuating health inequities, while implicitly valuing the essential social roles played by individuals in groups that have faced discrimination, as well as their greater risks due to health conditions reflecting inequities (Karaca-Mandic et al., 2020). In defining each priority group, the committee has considered their equity implications. For example, it has included all health staff at risk of exposure, not just those who are paid or are better paid (e.g., physicians, nurses). Each phase gives equal priority to all individuals in a group, facing similar exposure and with similar vulnerability. Nonetheless, when applying these criteria, vaccine distribution systems must actively ensure equity.
Social Vulnerability Index
The evidence clearly shows that people of color—specifically Black, Hispanic or Latinx, American Indian and Alaska Native, and Native Hawaiian and Pacific Islander—have been disproportionately impacted by COVID-19, with higher rates of morbidity, mortality, and transmission. As previously mentioned, this largely reflects the impacts of systemic racism and socioeconomic adversity that are associated with higher exposures to the virus and greater severity of disease among those infected, due to comorbid conditions, lack of access to health care or other public health services, and other factors such as inadequate housing and lack of access to clean water and nutrition.
The committee’s allocation framework focuses on these underlying causes by recommending the application of a vulnerability index, and spe-
cifically CDC’s SVI or another more specific index such as the CCVI, within its framework instead of focusing on discrete racial and ethnic categories. The committee considered multiple options for addressing inequities before deciding on a vulnerability index-based approach. For example, a purely race/ethnicity-based prioritization approach is likely to increase mistrust in communities of color, because they may suspect a lack of ethical and safety oversight for a new vaccine given a long history of mistreatment by the medical community in the name of research. Second, a purely race/ethnicity-based allocation may omit other important social determinants of health. Third, such an allocation could be legally challenged.
Among vulnerability-based indices, the committee also considered the ADI for identifying vulnerable areas. While the ADI captures several important sociodemographic factors, several variables relevant to COVID-19, such as crowding and the proportion of the population over age 65, are not a part of the ADI.
Use of CDC’s SVI in the committee’s framework represents an attempt to incorporate the variables that the committee believes are most linked to the disproportionate impact of COVID-19 on people of color and other vulnerable populations. CDC’s SVI was developed for local preparedness for public health emergencies such as natural disasters and disease outbreaks, and it identifies geographic areas of vulnerability based on 15 U.S. Census variables (ATSDR, 2018). These variables capture many recognized social determinants of health (e.g., income or race and ethnicity), indicators of access (e.g., transportation), infection transmission factors (e.g., crowding), and increased risk of adverse COVID-19 outcomes (e.g., proportion aged 65 or older). This index can be calculated at the U.S. Census tract level—enabling immunization programs to better identify areas of vulnerability. Where relevant data are available, vaccine programs could also consider SVI-derived indices such as the CCVI—an index that includes SVI variables as well as COVID-19-specific ones: (1) indicators of known COVID-19 comorbidities, and (2) health system factors, which account for access to health care resources in a community that are relevant to this pandemic (e.g., intensive care unit beds).
Operationalizing the Social Vulnerability Index
The committee does not propose an approach in which, within each phase, all vaccine is first given to people in high-SVI areas. Rather the committee proposes that the SVI be used in two ways. First as previously noted, a reserved 10 percent portion of the total federal allocation of COVID-19 vaccine may be reserved to target areas with a high-SVI (defined as the top 25 percent of the SVI distribution within the state). Second, the committee proposes that STLT authorities ensure that special efforts are made to
deliver vaccine to residents of high-vulnerability areas (defined as the 25 percent highest in the state).
While other equity considerations, such as disability status and age, are partially addressed in the criteria underlying the phases, there are additional concerns that need to be addressed. For example, the ability of frail or disabled individuals to access vaccination locations must be taken into account while operationalizing vaccine access.
Costs Associated with Vaccination
Several COVID-19 vaccines under development have received considerable taxpayer support. Therefore, it is essential that COVID-19 vaccines be delivered through a central mechanism that ensures the availability of vaccines to all individuals, whatever their social and economic resources, employment, immigration, or insurance status. This can best be achieved if this federal mechanism makes vaccines available at no cost to public health and health care sectors. To ensure equity and to decrease vaccine hesitancy, there should be no out-of-pocket costs for those being vaccinated and this includes covering fees for the administration of vaccine. Chapter 6 and Recommendation 3 discuss further the issue of costs associated with vaccination.
Legal Status
All individuals in the United States and its territories should receive the vaccine in the appropriate phase, irrespective of their legal status, and individuals whose legal status is uncertain should be reassured by federal and state authorities that their coming forward to receive the vaccine will not lead to deportation or be used against them in immigration proceedings, or be considered use of a public benefit under the “Public Charge” rule and therefore be potentially detrimental to attaining citizenship (Page et al., 2020). In addition to considerations of equity and fairness, including all individuals in the immunization program is appropriate from a disease control perspective. If there are pockets of susceptibility among those who do not receive the vaccine, the risk of outbreaks is likely to increase for everyone—including those who are legally present in the United States—because no vaccine is 100 percent effective.
Considerations for Pregnant Women
Although data are uncertain regarding the risk of adverse outcomes associated with COVID-19 in pregnancy, current evidence suggests that pregnant women are more likely to be hospitalized with COVID-19 than are non-pregnant women, and infants born to women infected with SARS-CoV-2 during pregnancy appear to have increased risk for preterm birth and admission to
a neonatal intensive care unit (Allotey et al., 2020; CDC, 2020e). Therefore, it is concerning that most, if not all, of the current Phase 2 and 3 vaccine trials are excluding pregnant women, thus putting them at a disadvantage for protecting themselves against SARS-CoV-2. Operation Warp Speed, NIH, and CDC should prioritize an assessment of vaccine efficacy, effectiveness, and safety among pregnant and lactating women in their pre-clinical and clinical development and post-marketing surveillance plans. These data and the characteristics of the approved vaccines will enable ACIP to develop recommendations for vaccinating pregnant women against SAS-CoV-2.
Vaccine Allocation for the Military13
The U.S. military, which is tasked with protecting the United States from foreign threats, currently comprises approximately 1.2 million active duty troops, 781,000 reservists, and 728,000 civilian employees working for the U.S. Department of Defense (DoD, 2020). The U.S. military has its own health care system, which serves active duty troops and their dependents, who live in diverse settings inside and outside the United States, ranging from onboard ships to military bases to civilian communities. Among active duty troops and their dependents are individuals with varying levels of risk for infection and life-threatening complications of COVID-19. These include frontline health care providers, those living in congregate settings or in tightly confined spaces (e.g., outbreaks have occurred on U.S. naval ships), and those with underlying comorbid conditions associated with an increased risk of severe COVID-19, among others. While the U.S. military has separate advisory groups (e.g., the Armed Forces Epidemiology Board) and decision-making processes with regard to health care, disease prevention, and public health, in the absence of a separate allotment of COVID-19 vaccine to the U.S. military, the committee recommends that priority setting for the use of COVID-19 vaccine among active duty troops and their dependents, as well as reservists, follow the principles and criteria set forth for use in the civilian population. Civilian employees working for DoD should be considered for COVID-19 vaccination, as appropriate, through programs established to provide vaccine to other civilian populations.
Additional Considerations of the Framework
The committee notes the following considerations as STLT authorities adapt the allocation framework to their local conditions.
___________________
13 This specific discussion is focused on active duty troops and their dependents. Veterans would be considered in the phases previously described.
STLT Flexibility with Transitioning Between and Within Phases
It is important to reiterate that the COVID-19 vaccination phases identify population groups of similar priority. Within phases, STLT authorities have the flexibility to adapt the priority population groups to their specific conditions. For example, some counties have no tertiary hospitals and are served by neighboring counties, and others may have chicken and pork production facilities or universities. Some areas may have no evidence of virus spread and be given a lower geographic priority as compared to other areas of a state. Furthermore, populations in each phase, especially in Phases 1a and 1b, may well exceed the amount of vaccine available, or Phases 1 and 2 may even become merged or overlapping. Currently, some individual-level data to properly classify individuals in specific categories may be difficult to collect or ascertain. It is important that the allocation process does not obstruct or slow down vaccination—the ability to move quickly to vaccinate priority groups does matter. Also, as previously mentioned many unknowns remain regarding the safety and efficacy of the vaccines in certain populations (such as children, pregnant women, older adults, and individuals previously infected with COVID-19). STLT authorities will have to make final decisions on refining and applying the suggested priorities previously listed, and should plan for situations when prioritization has to be adapted mid-process. In doing so, they should refer to the principles and allocation criteria that guided the formulation of the phases.
Unintended Consequences
The committee acknowledges the risk of potential unintended consequences of the allocation framework and the need to assess prioritization based on operational and supply realities. For example, immunizing older adults early on, and the resulting perception of their security, could affect one of the key reasons used to encourage younger people to follow guidance on preventive measures currently being encouraged to prevent the spread of COVID-19. This argument could apply to everyone who receives the vaccine and chooses not to be careful in following key preventive measures. As a result, the committee acknowledges that STLT authorities and other decision makers need to remain vigilant of these realities and other public health interventions being implemented in tandem with the vaccine allocation and distribution.
Demographic Data Limitations
It is critical to acknowledge the limitations around the use of demographic data across phases in this chapter. The task of accurately describing the total number of individuals included in each priority group and phase was challenging because of the near-certain—and as of yet uncaptured—overlap between individuals counted across phases. For example, there is
likely significant overlap between those counted in the nursing home population and the population of older adults living in overcrowded settings, and significant overlap between members of multi-generational families and other categories listed for vaccination during earlier phases, such as occupational groups. As a result, the committee acknowledges that the population estimates provided serve as a guidepost for the general size of key priority groups discussed, but do not reflect a wholly accurate and nuanced analysis of phase population size in relation to one another. Population values for each group will be improved as the program is under way.
CONCLUDING REMARKS
This equitable COVID-19 vaccination allocation framework will be dynamic and, it is hoped, ever-improving. Features of the COVID-19 pandemic will change over time, as will collective understanding of its effects (e.g., the list of comorbid conditions that put individuals at higher risk of severe disease or death due to COVID-19 infection). The committee recognizes the current uncertainty regarding COVID-19, its spread, the availability of treatments, and the possibility that new evidence may change the risks and, with them, the priorities. Additional adjustments in response to new evidence and data should be made as necessary. For example, it is important to consider new information on key vaccine characteristics emerging from vaccine trials and other sources such as the number of vaccine courses to be made available, considerations for special populations (e.g., pregnant women or individuals previously infected with COVID-19), anticipated vaccine efficacy, and anticipated vaccine safety and pharmacovigilance planning as it becomes available. Making mid-course corrections will be the rule rather than the exception and will be dependent on real-time surveillance of all aspects of the program.
REFERENCES
ADA (American Dental Association). 2020. Workforce. https://www.ada.org/en/science-research/health-policy-institute/dental-statistics/workforce (accessed September 22, 2020).
Akiyama, M. J., A. C. Spaulding, and J. D. Rich. 2020. Flattening the curve for incarcerated populations—COVID-19 in jails and prisons. The New England Journal of Medicine 382:2075–2077. doi: 10.1056/NEJMp2005687.
Allotey, J., E. Stallings, M. Bonet, M. Yap, S. Chatterjee, T. Kew, L. Debenham, A. C. Llavall, A. Dixit, D. Zhou, R. Balaji, S. I. Lee, X. Qiu, M. Yuan, D. Coomar, M. van Wely, E. van Leeuwen, E. Kostova, H. Kunst, A. Khalil, S. Tiberi, V. Brizuela, N. Broutet, E. Kara, C. R. Kim, A. Thorson, O. T. Oladapo, L. Mofenson, J. Zamora, and S. Thangaratinam. 2020. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 370:m3320. doi: 10.1136/bmj.m3320.
ATSDR (Agency for Toxic Substances and Disease Registry). 2018. CDC’s Social Vulnerability Index. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (accessed September 22, 2020).
Baker, M. G., T. K. Peckham, and N. S. Seixas. 2020. Estimating the burden of United States workers exposed to infection or disease: A key factor in containing risk of COVID-19 infection. PLOS ONE 15(4):e0232452. doi: 10.1371/journal.pone.0232452.
Bastidas, A., G. Catteau, S. Volpe, T. Mrkvan, A. Enemuo, J. Smetana, T. Schwarz, L. Rombo, K. Pauksens, E. Berengier, C. Hervé, L. Oostvogels, and A. Schuind. 2019. Long-term immunological persistence of the adjuvanted recombinant zoster vaccine: Clinical data and mathematical modeling. Open Forum Infectious Diseases 6(Suppl 2):S84–S85. doi: 10.1093/ofid/ofz359.183.
Beck, A. J., M. L. Boulton, and F. Coronado. 2014. Enumeration of the governmental public health workforce, 2014. American Journal of Preventive Medicine 47(5 Suppl 3):S306–S313. doi: 10.1016/j.amepre.2014.07.018.
BJS (U.S. Bureau of Justice Statistics). 2019. Local police departments, 2016: Personnel summary. Washington, DC: U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/lpd16p_sum.pdf (accessed August 28, 2020).
BLS (U.S. Bureau of Labor Statistics). 2019a. Manufacturing: NAICS 31-33. https://www.bls.gov/iag/tgs/iag31-33.htm (accessed September 15, 2020).
BLS. 2019b. May 2019 national industry-specific occupational employment and wage estimates: NAICS 611300—colleges, universities, and professional schools. https://www.bls.gov/oes/current/naics4_611300.htm (accessed September 15, 2020).
BLS. 2019c. Occupational employment and wages, May 2019: 21-1094 community health workers. https://www.bls.gov/oes/current/oes211094.htm (accessed September 15, 2020).
BLS. 2019d. Occupational employment and wages, May 2019: 29-0000 healthcare practitioners and technical occupations (major group). https://www.bls.gov/oes/current/oes290000.htm#nat (accessed September 22, 2020).
BLS. 2019e. Occupational employment statistics: May 2018 national industry-specific occupational employment and wage estimates: NAICS 446110-pharmacies and drug stores. https://www.bls.gov/oes/2018/may/oessrci.htm (accessed September 22, 2020).
BLS. 2019f. Occupational employment statistics: May 2019 occupational profiles. https://www.bls.gov/oes/current/oes_stru.htm (accessed August 28, 2020).
BLS. 2020a. Construction: NAICS 23. https://www.bls.gov/iag/tgs/iag23.htm (accessed September 15, 2020).
BLS. 2020b. Delivery truck drivers and driver/sales workers. https://www.bls.gov/ooh/transportation-and-material-moving/delivery-truck-drivers-and-driver-sales-workers.htm (accessed September 15, 2020).
BLS. 2020c. EMTs and paramedics. https://www.bls.gov/ooh/healthcare/emts-and-paramedics.htm (accessed August 28, 2020).
BLS. 2020d. Industries at a glance: Utilities: NAICS 22. https://www.bls.gov/iag/tgs/iag22.htm (accessed September 15, 2020).
BLS. 2020e. Occupational employment statistics: May 2019 national industry-specific occupational employment and wage estimates-NAICS 611100-elementary and secondary schools. https://www.bls.gov/oes/current/oessrci.htm (accessed September 22, 2020).
BLS. 2020f. Occupational employment statistics-occupational employment and wages, May 2019-53-3052-bus drivers, transit and intercity. https://www.bls.gov/oes/current/oes533052.htm (accessed September 22, 2020).
Braveman, P. 2006. Health disparities and health equity: Concepts and measurement. Annual Review of Public Health 27:167–194. doi: https://doi.org/10.1146/annurev.publhealth.27.021405.102103.
Britton, T., F. Ball, and P. Trapman. 2020. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science 369(6505):846–849. doi: 10.1126/science.abc6810.
Bruine de Bruin, W., A. M. Parker, and J. Strough. 2020. Age differences in reported social networks and well-being. Psychology and Aging 35(2):159–168. doi: 10.1037/pag0000415.
CDC (Centers for Disease Control and Prevention). 2018. Interim updated planning guidance on allocating and targeting pandemic influenza vaccine during an influenza pandemic. Atlanta, GA: CDC. https://www.cdc.gov/flu/pandemic-resources/pdf/2018-InfluenzaGuidance.pdf (accessed September 22, 2020).
CDC. 2020a. About social determinants of health (SDOH). Atlanta, GA: CDC. https://www.cdc.gov/socialdeterminants/about.html (accessed September 21, 2020).
CDC. 2020b. COVID-19 hospitalization and death by age. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html (accessed September 22, 2020).
CDC. 2020c. COVID-19: Older adults. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed September 22, 2020).
CDC. 2020d. COVID-19: People with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019ncov%2Fneed-extra-precautions%2Fgroups-at-higher-risk.html (accessed August 28, 2020).
CDC. 2020e. COVID-19: Pregnancy Data. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19.html (accessed September 22, 2020).
CDC. 2020f. Nursing home care. https://www.cdc.gov/nchs/fastats/nursing-home-care.htm (accessed August 28, 2020).
CDC. 2020g. Provisional COVID-19 death counts by sex, age, and state. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku (accessed September 22, 2020).
CDC. 2020h. Residential care communities. https://www.cdc.gov/nchs/fastats/residential-care-communities.htm (accessed August 28, 2020).
CIHI (Canadian Institute for Health Information). 2020. Pandemic experience in the long-term care sector: How does Canada compare with other countries? Ottowa, ON: CIHI. https://www.cihi.ca/sites/default/files/document/covid-19-rapid-response-long-term-caresnapshot-en.pdf (accessed September 22, 2020).
Clark, A., M. Jit, C. Warren-Gash, B. Guthrie, H. H. X. Wang, S. W. Mercer, C. Sanderson, M. McKee, C. Troeger, K. L. Ong, F. Checchi, P. Perel, S. Joseph, H. P. Gibbs, A. Banerjee, R. M. Eggo, E. S. Nightingale, K. O’Reilly, T. Jombart, W. J. Edmunds, A. Rosello, F. Y. Sun, K. E. Atkins, N. I. Bosse, S. Clifford, T. W. Russell, A. K. Deol, Y. Liu, S. R. Procter, Q. J. Leclerc, G. Medley, G. Knight, J. D. Munday, A. J. Kucharski, C. A. B. Pearson, P. Klepac, K. Prem, R. M. G. J. Houben, A. Endo, S. Flasche, N. G. Davies, C. Diamond, K. van Zandvoort, S. Funk, M. Auzenbergs, E. M. Rees, D. C. Tully, J. C. Emery, B. J. Quilty, S. Abbott, C. J. Villabona-Arenas, S. Hué, J. Hellewell, A. Gimma, and C. I. Jarvis. 2020. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. The Lancet Global Health 8(8):e1003–e1017. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(20)30264-3/fulltext (accessed September 22, 2020).
Clark, C. A. 2020. AHCA/NCAL: “COVID-19 testing positivity rates and PPE supply shortage for nursing homes across nation.” Los Alamos Daily Post, August 7, 2020. https://ladailypost.com/ahca-ncal-covid-19-testing-positivity-rates-ppe-supply-shortages-for-nursing-homes (accessed September 22, 2020).
CMS (Centers for Medicare & Medicaid Services). 2020a. COVID-19 nursing home data. https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg (accessed September 22, 2020).
CMS. 2020b. COVID-19 nursing home dataset-archived data-week ending 7/12/20. https://data.cms.gov/Special-Programs-Initiatives-COVID-19-Nursing-Home/COVID-19-Nursing-Home-Dataset-Archived-Data-Week-E/e9t9-t34c (accessed September 22, 2020).
Cohn, D., and J. S. Passel. 2018. A record 64 million Americans live in multigenerenational households. Pew Research Center FactTank: News in the Numbers, April 5, 2018. https://www.pewresearch.org/fact-tank/2018/04/05/a-record-64-million-americans-live-in-multigenerational-households (accessed September 22, 2020).
Cubanski, J., W. Koma, A. Damico, and T. Neuman. 2018. How many seniors live in poverty? Kaiser Family Foundation. https://www.kff.org/medicare/issue-brief/how-many-seniors-live-in-poverty (accessed September 22, 2020).
Culhane, D., D. Treglia, K. Steif, R. Kuhn, and T. Byrne. 2020. White Paper: Estimated emergency and observational/quarantine capacity need for the US homeless population related to COVID-19 exposure by county; projected hospitalizations, intensive care units and mortality. http://www.bu.edu/ssw/files/2020/03/COVID-paper_0325_1127am.pdf (accessed September 22, 2020).
Dagnew, A. F., N. P. Klein, C. Hervé, G. Kalema, E. Di Paolo, J. Peterson, B. Salaun, and A. Schuind. 2020. The adjuvanted recombinant zoster vaccine in adults aged ≥65 years previously vaccinated with a live-attenuated herpes zoster vaccine. The Journal of Infectious Diseases jiaa083. doi: org/10.1093/infdis/jiaa083.
Daniels, N. 1996. Justice and justification: Reflective equilibrium in theory and practice. New York: Cambridge University Press.
Daniels, N. 2007. Just health: Meeting health needs fairly. New York: Cambridge University Press.
Dauer, C. C., and R. E. Serfling. 1961. Mortality from influenza. American Review of Respiratory Disease 83(2P2):15–28. https://www.atsjournals.org/doi/abs/10.1164/arrd.1961.83.2P2.15 (accessed September 22, 2020).
Dingel, J., and B. Neiman. 2020. White Paper: How many jobs can be done at home? Chicago, IL: Becker Friedman Institute for Economics at the University of Chicago.
DoD (U.S. Department of Defense). 2020. DoD Personnel, Workforce Reports & Publications. https://www.dmdc.osd.mil/appj/dwp/dwp_reports.jsp (accessed August 28, 2020).
Dworkin, R. 2011. Justice for hedgehogs. Cambridge, MA: Belknap Press.
Emanuel, E. J., G. Persad, R. Upshur, B. Thome, M. Parker, A. Glickman, C. Zhang, C. Boyle, M. Smith, and J. P. Phillips. 2020. Fair allocation of scarce medical resources in the time of COVID-19. The New England Journal of Medicine 382(21):2049–2055. doi: 10.1056/NEJMsb2005114.
Evarts, B., and G. P. Stein. 2020. U.S. fire department profile 2018. NFPA Research. https://www.nfpa.org/-/media/Files/News-and-Research/Fire-statistics-and-reports/Emergency-responders/osfdprofile.pdf (accessed September 22, 2020).
Federal Interagency Forum on Child and Family Statistics. 2020. America’s children in brief: Key national indicators of well-being, 2020. Washington, DC: U.S. Government Printing Office. https://www.childstats.gov/americaschildren/index.asp (accessed October 29, 2020).
Gaffney, A. W., D. Himmelstein, and S. Woolhandler. 2020. Risk for severe COVID-19 illness among teachers and adults living with school-aged children. Annals of Internal Medicine. doi: 10.7326/M20-5413.
Hawkins, D. 2020. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. American Journal of Industrial Medicine 63(9):817–820. doi: 10.1002/ajim.23145.
Jaklevic, M. C. 2020. Researchers strive to recruit hard-hit minorities into COVID-19 vaccine trials. JAMA 324(9):826–828. doi: 10.1001/jama.2020.11244.
Karaca-Mandic, P., A. Georgiou, and S. Sen. 2020. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Internal Medicine. Published online August 17, 2020. doi: 10.1001/jamainternmed.2020.3857.
Krebs, C. C. 2020. Advisory memorandum on identifications of essential critical infrastructure workers during COVID-19 response. Washington, DC: U.S. Department of Homeland Security. https://www.cisa.gov/sites/default/files/publications/Version_3.1_CISA_Guidance_on_Essential_Critical_Infrastructure_Workers_0.pdf (accessed August 28, 2020).
The Lancet. 2020. Editorial: The plight of essential workers during the COVID-19 pandemic. The Lancet 395(10237):1587. doi: 10.1016/S0140-6736(20)31200-9.
Landes, S. D., M. A. Turk, M. K. Formica, K. E. McDonald, and J. D. Stevens. 2020. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disability and Health Journal 100969. doi: 10.1016/j.dhjo.2020.100969.
Lee, S., P. Meyler, M. Mozel, T. Tauh, and R. Merchant. 2020. Asymptomatic carriage and transmission of SARS-CoV-2: What do we know? Canadian Journal of Anaesthesia 67:1424–1430. doi: 10.1007/s12630-020-01729-x.
Lighter, J., M. Phillips, S. Hochman, S. Sterling, D. Johnson, F. Francois, and A. Stachel. 2020. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clinical Infectious Diseases 71(15):896–897. doi: 10.1093/cid/ciaa415.
Lipsitch, M. 2020. Using models of SARS-CoV-2 dynamics to inform vaccine prioritization. Paper read at Committee on Equitable Allocation of Vaccine for the Novel Coronavirus Public Workshop.
McClung, N. 2020. Epidemiology of individuals at increased risk of COVID-19 disease. Presented at the August 26, 2020, Meeting of the Advisory Committee on Immunization Practices.
McCormack, G., C. Avery, A. K.-L. Spitzer, and A. Chandra. 2020. Economic vulnerability of households with essential workers. JAMA 324(4):388–390. doi: 10.1001/jama.2020.11366.
McGarry, B. E., D. C. Grabowski, and M. L. Barnett. 2020. Severe staffing and personal protective equipment shortages faced by nursing homes during the COVID-19 pandemic. Health Affairs. doi: 10.1377/hlthaff.2020.01269.
Michaels, D., and G. R. Wagner. 2020. Occupational Safety and Health Administration (OSHA) and worker safety during the COVID-19 pandemic. JAMA. doi: 10.1001/jama.2020.16343.
Miller, R. B., and C. A. Nebeker-Adams. 2017. Multigenerational households. In Encyclopedia of couple and family therapy, edited by J. Lebow, A. Chambers, and D. C. Breunlin. Cham, Switzerland: Springer International Publishing. Pp. 1–3.
Moghadas, S. M., M. C. Fitzpatrick, P. Sah, A. Pandey, A. Shoukat, B. H. Singer, and A. P. Galvani. 2020. The implications of silent transmission for the control of COVID-19 outbreaks. Proceedings of the National Academy of Sciences 117(30):17513–17515. doi: 10.1073/pnas.2008373117.
Mullen O’Keefe, S. 2020. One in three Americans would not get COVID-19 vaccine. Gallup News. https://news.gallup.com/poll/317018/one-three-americans-not-covid-vaccine.aspx (accessed September 22, 2020).
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Communities in action: Pathways to health equity. Washington, DC: The National Academies Press.
NCD (National Council on Disability). 2020. NCD comments to National Academies on preliminary COVID-19 vaccine allocation framework. https://ncd.gov/publications/2020/ncd-comments-national-academies-preliminary-covid-19-vaccine-allocation-framework (accessed September 9, 2020).
Nguyen, L. H., D. A. Drew, M. S. Graham, A. D. Joshi, C.-G. Guo, W. Ma, R. S. Mehta, E. T. Warner, D. R. Sikavi, C.-H. Lo, S. Kwon, M. Song, L. A. Mucci, M. J. Stampfer, W. C. Willett, A. H. Eliassen, J. E. Hart, J. E. Chavarro, J. W. Rich-Edwards, R. Davies, J. Capdevila, K. A. Lee, M. N. Lochlainn, T. Varsavsky, C. H. Sudre, M. J. Cardoso, J. Wolf, T. D. Spector, S. Ourselin, C. J. Steves, A. T. Chan, on behalf of the Coronavirus Pandemic Epidemiology Consortium. 2020. Risk of COVID-19 among front-line healthcare workers and the general community: A prospective cohort study. The Lancet Public Health 5(9):E475–E483. doi: 10.1016/S2468-2667(20)30164-X.
NRC (National Research Council). 1996. Understanding risk: Informing decisions in a democratic society. Washington, DC: National Academy Press.
NRC. 2009. Science and decisions: Advancing risk assessment. Washington, DC: The National Academies Press.
Nuffield Council on Bioethics. 2020. Rapid Policy Briefing: Fair and equitable access to COVID-19 treatments and vaccines. https://www.nuffieldbioethics.org/assets/pdfs/Fair-and-equitable-access-to-COVID-19-treatments-and-vaccines.pdf (accessed September 22, 2020).
NYT (The New York Times). 2020. More than 40% of U.S. coronavirus deaths are linked to nursing homes. https://www.nytimes.com/interactive/2020/us/coronavirus-nursing-homes.html#:~:text=More%20Than%2040%25%20of%20U.S.%20Coronavirus%20Deaths%20Are%20Linked%20to%20Nursing%20Homes,-By%20The%20New&text=At%20least%2068%2C000%20residents%20and,a%20New%20York%20Times%20database (accessed August 28, 2020).
Oliver, S. 2020. Epidemiology of COVID-19 in essential workers, including healthcare personnel. Paper read at ACIP July 2020 Meeting.
Omer, S. 2019. Withholding flu vaccine from migrants isn’t just cruel. It’s dangerous. The Washington Post, August 26, 2019. https://www.washingtonpost.com/outlook/2019/08/26/withholding-flu-shots-detained-migrants-isnt-just-cruel-its-dangerous (accessed September 22, 2020).
OSHA (Occupational Safety and Health Administration). 2020. Guidance on preparing workplaces for COVID-19. Washington, DC: OSHA. https://www.osha.gov/Publications/OSHA3990.pdf (accessed August 28, 2020).
Ouslander, J. G., and D. C. Grabowski. 2020. COVID-19 in nursing homes: Calming the perfect storm. Journal of the American Geriatrics Society. doi: 10.1111/jgs.16784.
Page, K. R., M. Venkataramani, C. Beyrer, and S. Polk. 2020. Undocumented U.S. immigrants and COVID-19. The New England Journal of Medicine 382(21):e62. doi: 10.1056/NEJMp2005953.
Persad, G., A. Wertheimer, and E. J. Emanuel. 2009. Principles for allocation of scarce medical interventions. The Lancet 373(9661):423–431. doi: 10.1016/S0140-6736(09)60137-9.
Persad, G., M. E. Peek, and E. J. Emanuel. 2020. Fairly prioritizing groups for access to COVID-19 vaccines. JAMA. Published online September 10, 2020. doi: 10.1001/jama.2020.18513.
Petrilli, C. M., S. A. Jones, J. Yang, H. Rajagopalan, L. O’Donnell, Y. Chernyak, K. A. Tobin, R. J. Cerfolio, F. Francois, and L. I. Horwitz. 2020. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 369:m1966. doi: 10.1136/bmj.m1966.
Rieger, S. 2017. The continued growth of multigenerational living. Joint Center for Housing Studies of Harvard University. March 9, 2017. https://www.jchs.harvard.edu/blog/the-continued-growth-of-multigenerational-living (accessed September 15, 2020).
Roberts, A. W., S. U. Ogunwole, L. Blakeslee, and M. A. Rabe. 2018. The population 65 years and older in the United States: 2016—American Community Survey Reports. ACS-38 Issued October 2018. https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf (accessed September 22, 2020).
Saloner, B., K. Parish, J. A. Ward, G. DiLaura, and S. Dolovich. 2020. COVID-19 cases and deaths in federal and state prisons. JAMA 324(6):602–603. doi: 10.1001/jama.2020.12528.
Schmidt, H. 2020. Vaccine rationing and the urgency of social justice in the COVID-19 response. Hastings Center Report 50(3):46–49. doi: 10.1002/hast.1113.
Self, W. H., M. W. Tenforde, and W. B. Stubblefield. 2020. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network—13 academic medical centers, April–June 2020. Morbidity Mortality Weekly Report 69:1221–1226. doi: 10.15585/mmwr.mm6935e2.
Silver, S. R., J. Li, W. L. Boal, T. L. Shockey, and M. R. Groenewold. 2020. Prevalence of underlying medical conditions among selected essential critical infrastructure workers—behavioral risk factor surveillance system, 31 states, 2017–2018. Morbidity and Mortality Weekly Report 69:1244–1249. doi: 10.15585/mmwr.mm6936a3.
Snowden, F. M. 2019. Epidemics and society: From the black death to the present. New Haven, CT: Yale University Press.
Souchery, S. 2020. COVID-19 cases among US young adults spike. CIDRAP News, June 26, 2020. https://www.cidrap.umn.edu/news-perspective/2020/06/covid-19-cases-among-us-young-adults-spike (accessed September 22, 2020).
Statista. 2020. Number of morticians, undertakers, and funeral directors in the United States from 2012 to 2019. Statista. https://www.statista.com/statistics/752908/number-of-morticians-undertakers-and-funeral-directors-us (accessed September 22, 2020).
Sunderji, A., K. N. Mena, J. Winickoff, J. Melinek, and J. Sharfstein. 2020. Influenza vaccination and migration at the US southern border. American Journal of Public Health 110(4):466–467. doi: 10.2105/AJPH.2019.305547.
Surgo Foundation. 2020. The COVID-19 community vulnerability index (CCVI). https://precisionforcovid.org/ccvi (accessed September 15, 2020).
Toner, E., A. Barnill, C. Krubiner, J. Bernstein, L. Privor-Dumm, M. Watson, E. Martin, C. Potter, D. Hosangadi, N. Connell, C. Watson, M. Schoch-Spana, T. Goodwin Veenema, D. Meyer, E. L. Daugherty Biddison, Al Regenberg, T. Inglesby, and A. Cicero. 2020. Interim framework for COVID-19 vaccine allocation and distribution in the United States. Baltimore, MD: Johns Hopkins Center for Health Security. https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2020/200819-vaccine-allocation.pdf (accessed September 22, 2020).
University of Wisconsin School of Medicine and Public Health. 2020. Neighborhood atlas: Area deprivation index. https://www.neighborhoodatlas.medicine.wisc.edu (accessed September 15, 2020).
U.S. Census. 2019a. National population by characteristics: 2010–2019. https://www.census.gov/data/datasets/time-series/demo/popest/2010s-national-detail.html (accessed September 22, 2020).
U.S. Census. 2019b. Historical living arrangements of adults. https://www.census.gov/data/tables/time-series/demo/families/adults.html (accessed September 22, 2020).
USDA (U.S. Department of Agriculture). 2020. Ag and food sectors and the economy. https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/ag-and-food-sectors-and-the-economy/#:~:text=In%202018%2C%20the%20U.S.%20food,of%20all%20U.S.%20nonfarm%20employment (accessed August 28, 2020).
USPS (U.S. Postal Service). 2020. Number of postal employees since 1926. https://about.usps.com/who-we-are/postal-history/employees-since-1926.pdf (accessed September 15, 2020).
Waldron, J. 2017. One another’s equals: The basis of human equality. Cambridge, MA: The Belknap Press of Harvard University Press.
Waltenburg, M. A., T. Victoroff, C. E. Rose, et al. 2020. Update: COVID-19 among workers in meat and poultry processing facilities—United States, April–May 2020. Morbidity and Mortality Weekly Report 69:887–892. doi: 10.15585/mmwr.mm6927e2.
White, D. B., M. Schmidhofer, E. McCreary, R. Bariola, G. M. Snyder, R. Sackrowitz, N. N. Jonassaint, J. Daley, D. M. Yealy, T. Sonmez, M. U. Unver, P. Pathak, G. Persad, and R. Truog. 2020. A model hospital policy for fair allocation of scarce medications to treat COVID-19. University of Pittsburgh Department of Critical Care Medicine. https://www.ccm.pitt.edu/?q=content/model-hospital-policy-allocating-scarce-critical-care-resources-available-online-now (accessed September 22, 2020).
WHO (World Health Organization). 2017. 10 facts on health inequities and their causes. https://www.who.int/features/factfiles/health_inequities/en (accessed August 28, 2020).
Williams, J. H., and A. Dawson. 2020. Prioritising access to pandemic influenza vaccine: A review of the ethics literature. BMC Medical Ethics 21(1):40. doi: 10.1186/s12910-020-00477-3.
Williams, J., D. De Vos, and D. Russell 2013. 2010 Census Group quarters enumeration assessment report. Washington, DC: U.S. Census Bureau. https://www.census.gov/content/dam/Census/library/publications/2013/dec/2010_cpex_243.pdf (accessed September 22, 2020).