2
Evaluation of the TSCA Systematic Review Approach
INTRODUCTION
As scientific evidence on the risks of chemicals has grown, both in scope and in the contributing disciplines, the need for evidence-based approaches to addressing risks to human and ecosystem health has been increasingly recognized. Methods first developed and used in clinical medicine and other areas for assembling and evaluating bodies of evidence have now been extended to assessing risks to human health and the environment. Evidence-based methods—with their transparency, objectivity, comprehensiveness, and reproducibility—serve as a foundation of modern clinical practice. Evidence-based methods include protocols and comprehensive documentation of assumptions and decisions in compiling evidence, which allows for tracing of every step of the evaluation. This transparency is one primary advantage of using these practices. The principal tool for evaluating the evidence base on a topic is systematic review. Consequently, over the past decade, several approaches to applying this methodology to assessing evidence on risks of environmental agents have been elaborated, such as by the European Food Safety Authority (EFSA), the Office of Health Assessment and Translation (OHAT) of the National Toxicology Program (NTP), the Texas Commission on Environmental Quality, and the Navigation Guide (Morgan et al. 2018; OHAT 2019; Schaefer and Meyers 2017; Woodruff and Sutton 2014). Additionally, the World Health Organization and the International Labour Organization have collaborated to develop a risk-of-bias tool for assessing data on prevalence of exposure. The newly developed methods are based on review of existing methods, influenced by consultation with experts, tested for validity and reliability, and published in the peer-reviewed literature (Pega et al. 2020). Methods have also been proposed for applying systematic review methods to risk evaluations for ecological receptors in a framework integrated with human health risk evaluations (Suter et al. 2020).
A chemical risk assessment includes an initial problem formulation as well as hazard assessment and dose-response assessment, exposure assessment, and risk characterization, as appropriate (NRC 2009). Figure 2-1 provides a schema for how systematic review is applied within chemical risk assessments. Prior to the conduct of a systematic review, planning and problem formulation should take place (see blue boxes in Figure 2-1). The planning and problem formulation should include stakeholder engagement and broad literature searching to find the evidence on the topic, and end with the identification of the most important questions and the best approach for answering such questions. The research questions and the approach should inform the first step of the systematic review—the development of the protocol. The planning and problem formulation should also determine if another evidence-based method could be applied to answer the research question required for the risk evaluation.
If a systematic review is the appropriate approach, development of the protocol is the first step of the review (see green boxes in Figure 2-1). Evidence identification, which includes searching and screening the literature and finding the reports as prescribed in the protocol, is the next step. Evidence evaluation follows. This step includes evaluation of the internal validity of the individual studies (i.e., Are the study results at risk of bias?), using the appropriate risk-of-bias tool for the type of study being reviewed. Synthesis consists of a qualitative evaluation of the evidence and can be complemented by a quantitative pooling—a meta-analysis.
This synthesis of the various specific streams of evidence is followed by hazard assessment (see orange box in Figure 2-1) with integration of the multiple evidence streams of human, animal and other
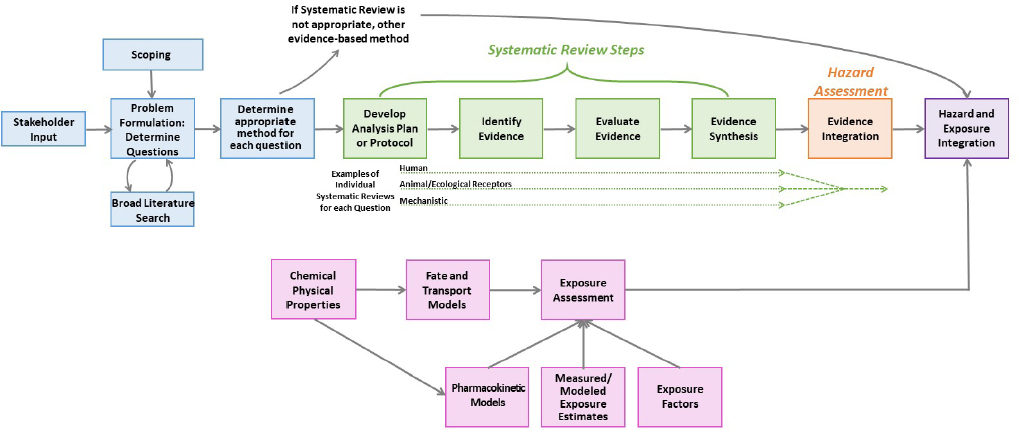
ecological receptors, and mechanistic findings. Gathering information on human and ecological exposures could also be approached with systematic review, but systematic review tools for those questions are not yet well developed (see pink boxes in Figure 2-1). For this reason, these steps are not shown as including the systematic review steps of protocol development, evidence identification, evaluation, and synthesis. Exposure and hazard data are integrated to characterize risk (see purple box in Figure 2-1).
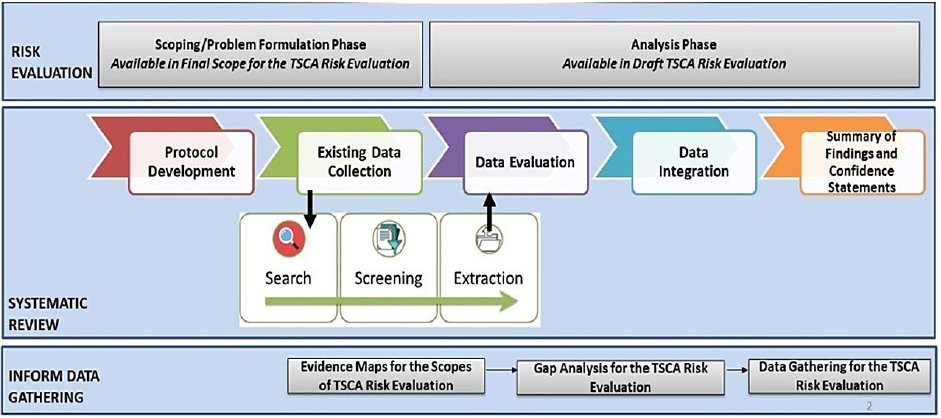
Figure 2-2 illustrates the Office of Pollution Prevention and Toxics’ (OPPT’s) approach to systematic review, which differs to an extent from the description in Figure 2-1 and includes systematic review as part of the broader process of risk evaluation. The committee has organized this chapter by the steps that are integral to systematic review as used generally (i.e., matching the current standards outlined by the Institute of Medicine [IOM] report Finding What Works in Health Care: Standards for Systematic Reviews [Bero et al. 2018; IOM 2011]) rather than following the specific steps outlined by OPPT. These steps, per the IOM report, are protocol development, evidence identification, evidence evaluation, and evidence synthesis. The systematic review protocol is informed by a problem formulation, which guides the development of a protocol. The evidence identified and evaluated for the systematic review is synthesized and then the evidence from the various streams is brought together for the data integration step to determine if a hazard exists. If the review conducted by OPPT is indeed a systematic review, then the committee expects to find each of the steps of a systematic review (even if not specifically called out as such). Additionally, the committee would expect that all systematic reviews should meet the definition and principles from the IOM (2011) report—explicit prespecified methods to identify, select, and synthesize the evidence from studies. When reviewing the methods and assessments used by OPPT, the committee compared all streams of evidence that OPPT specified as applying to the systematic review process to this definition. To determine the approach to systematic review being used within the Toxic Substances Control Act (TSCA) risk evaluations, the committee reviewed the Draft Risk Evaluation for Trichloroethylene (TCE) and the Risk Evaluation for 1-Bromopropane (n-Propyl Bromide) (1-BP) (EPA 2020a,c) and considered comments on the evaluations from the public and from peer-review evaluations (e.g., the Science Advisory Committee on Chemicals), as well as OPPT’s responses to the comments.
Systematic review approaches have been used widely and increasingly to assemble the evidence needed to assess human health and ecological receptors. The number of such systematic reviews focusing on environmental risks has doubled from 2016 to 2019 (Whaley et al. 2020). Yet, the use of systematic review to collect, evaluate, and synthesize the non-hazard evidence streams required for TSCA risk
evaluations, such as data on exposure, fate and transport, and chemical and physical properties, is not established and very little precedent exists for applying systematic review to these streams of evidence. Within the U.S. Environmental Protection Agency (EPA), the Guidelines for Human Exposure Assessment, the Guidelines for Ecological Risk Assessment, and the operating procedures for the use of the ECOTOXicology (ECOTOX) knowledgebase (EPA 1998, 2019b, 2020b) dictate how exposure, fate and transport, and physical chemical property data should be assembled for decisions about risks to human health and ecological receptors.
The agency is beginning to consider how to advance systematic review of these other streams of data. A recent paper from EPA’s Office of Research and Development (ORD) describes considerations required to advance systematic review for exposure science (Cohen Hubal et al. 2020). The authors describe how the tools for searching and organizing the literature can be applied to evaluating exposures. The article discusses Population (including animal or plant species), Exposure, Comparator, and Outcome (PECO) statements but stops short of addressing how PECO could be used to assemble exposure data. A checklist is provided for evaluating exposures, but, as written, it is proposed for evaluating exposures within the context of environmental epidemiology. The paper argues that in scientific literature, exposure information should be presented in a way that facilitates the determination of applicability to a PECO statement. Another paper authored by scientists in ORD proposes that systematic review and weight of evidence (WOE) are integral to ecological and human health assessments and provides an integrated framework, but the paper does not detail a process for how systematic review should be applied to all streams of evidence that are integrated to make a final risk determination (Suter et al. 2020).
The committee evaluated systematic review of the exposure and data on exposure, fate and transport, and chemical and physical properties similarly to how it evaluated other streams of evidence, such as whether there were explicit prespecified methods to identify, select, and synthesize the evidence from studies. The committee also considered whether OPPT followed the appropriate agency guidelines for these evidence streams (i.e., Guidelines for Human Exposure Assessment, the Guidelines for Ecological Risk Assessment, and the operating procedures for the use of the ECOTOX knowledgebase [EPA 1998, 2019b, 2020b]). The committee chose to use these agency sources as a reference because the Guidelines for Human Exposure Assessment are cited in the approach described for estimating consumer exposures in TSCA evaluations on the EPA website,1 and the Guidelines for Ecological Risk Assessment state that they are intended to be agency-wide.
___________________
1 See https://www.epa.gov/tsca-screening-tools/approaches-estimate-consumer-exposure-under-tsca, accessed November 13, 2020.
GENERAL FINDINGS
In planning its approach, the committee intended to review all systematic reviews (e.g., those focused on hazards and exposures) conducted within the Draft Risk Evaluation for Trichloroethylene and the Risk Evaluation for 1-Bromopropane (n-Propyl Bromide) with a tool used to assess bias in systematic reviews (AMSTAR-2) (Shea et al. 2017). Given that systematic review has been applied within the human health hazard assessment, the committee piloted this approach by reviewing the hazard evaluation of the TCE evaluation. The committee’s strategy was to test whether the use of the AMSTAR-2 instrument would be helpful and, if so, then the instrument would also be applied to assess the OPPT approach for the other streams of evidence in the TCE and the 1-BP evaluations. In this pilot, the committee found that the TCE evaluation was lacking adequate descriptions of many elements of a systematic review—the details about review methods that are needed to apply the AMSTAR-2 tool were either absent or provided in a disparate and varied set of documents, not all of which were referred to in the risk evaluation.
In this evaluation with the AMSTAR-2, four committee members reviewed the hazard stream in the TCE risk evaluation. In that pilot attempt only the element “Does this review contain the elements of a patient-intervention-comparison-outcome (PICO) statement?” received a positive evaluation by all reviewers. All reviewers gave a partial yes to the elements “Did the review authors use a comprehensive literature search strategy?” and “Did the review authors describe the included studies in adequate detail?” For all other elements of the AMSTAR-2 tool, reviewers gave a “no” response. The overall confidence in the results of the TCE hazard review would be considered “Critically Low,” indicating that the review “should not be relied on to provide an accurate and comprehensive summary of the available studies.” Given that the TCE risk evaluation was released in February 2020, about 1.5 years after the first risk evaluation under TSCA, the committee judged this document to be a reasonable exemplar of the program’s processes for the first 10 risk evaluations. Additionally, the committee also concluded that the quality of the systematic review was unlikely to be better for the exposure, chemical properties, and fate and transport streams of evidence, as systematic review is not as well developed in those areas. Consequently, the approaches to these evidence streams were given a general review and the AMSTAR-2 tool was not used.
Another crosscutting finding, discussed in detail in the evidence integration section of this chapter, relates to terminology and specifically to the interchangeable use of the terms “weight of evidence” and “systematic review.” The Procedures for Chemical Risk Evaluation under the Amended Toxic Substances Control Act, referred to as the “Risk Evaluation Rule” (40 CFR Part 702), specify that weight of the scientific evidence “means a systematic review method.” However, this definition may not be intended to mean a systematic review as defined by the IOM. Furthermore, the Draft Risk Evaluation for Trichloroethylene refers to a “weight of the evidence analysis” for an individual outcome involving successive determinations: first, considering individual lines of evidence; and second, integrating these disparate lines of evidence. The committee considers these two steps as data synthesis and data integration, respectively, and notes that the integration step is outside of systematic review. It is worth noting that a 2014 National Research Council (NRC) report found that the terms “systematic review” and “weight of evidence analysis” have been used interchangeably, leading to confusion. That report distinguished systematic review as including “protocol development, evidence identification, evidence evaluation, and an analytic summary of the evidence” while WOE analysis is a judgment-based process to infer causation that follows the systematic review (NRC 2014, p. 4). That report found “evidence integration to be more useful and more descriptive of the process that occurs after completion of systematic reviews” (NRC 2014, p. 4).
The remainder of this chapter is organized by systematic review step (problem formulation and protocol development, evidence identification, evidence evaluation, evidence synthesis, and evidence integration). For each step, the committee describes “the state of the practice,” or how the step is generally conducted; describes how the committee thinks the step is being conducted within TSCA risk evaluations; offers a critique of the approach being used in TSCA; and makes recommendations to improve
the approach to the step. The committee notes that describing what OPPT does in order to critique the process was challenged by the lack of documentation of the systematic review approaches used by OPPT.
PROBLEM FORMULATION AND PROTOCOL DEVELOPMENT
Planning the review and carrying out problem formulation generally precede the systematic review process, typically comprising a scoping review and engagement of stakeholders. Many groups consider planning the review and subsequent problem formulation as the most important steps in a review because a high-level view is taken at this stage that sets the approach and protocol for the review.
The product of problem formulation is a well-defined question, appropriately guiding the selection of the methodology during the planning stage (e.g., systematic scoping review, systematic map, or systematic review). If a systematic review, systematic map, or systematic scoping review is determined to be the appropriate method to address the research question, a protocol is then developed and registered. Systematic review protocols need to report the following: (1) the research question, (2) the sources that will be searched with a reproducible search strategy, (3) the explicit inclusion and exclusion criteria for study selection described in an unambiguous and replicable way, (4) the methods used to select primary studies, (5) tools for critical appraisals of the risk of bias or quality in the included primary studies, and (6) information about the approaches for evidence synthesis with sufficient clarity to support replication of these steps (Krnic Martinic et al. 2019).
A protocol makes the methods and the process of the review transparent, provides the opportunity for peer review of the methods, and stands as a record of the review process. Having a protocol minimizes the potential for bias in many steps throughout the systematic review, such as in evidence identification, by ensuring that inclusion of studies in the review does not depend on the findings of the studies (NRC 2014). The risk evaluation process is typically much broader than that of systematic review alone, and generally not every stream of evidence is evaluated using systematic review, while other evidenced-based approaches may be used. This section discusses the state of the practice for development of systematic review research questions and protocols and describes OPPT’s approach.
STATE OF THE PRACTICE
Planning the Review
Because minimizing bias is a guiding principle of systematic reviews, even the initial planning should be conducted as rigorously, objectively, and transparently as possible. This step may involve iterative consideration of sponsor and stakeholder needs, scoping of the topic—including considerations of feasibility—and input and participation from a multidisciplinary team sharing a variety of roles.
Once the plan to conduct a review assumes shape, a decision is made as to which type of review to perform. In some cases, a narrative approach may be chosen for any of a variety of reasons, including limited access to data or to express an expert opinion. However, if the goal is to provide an objective and comprehensive summary of how the evidence on a certain topic answers a specific research question, a systematic review should be conducted. The committee would like to emphasize that only a review conducted following all the steps in the framework is a systematic review.
Various motivations exist to conduct a systematic review in toxicology. In the frameworks that have been created by agencies, including NTP, EPA, and EFSA, the motivation for doing so is driven by the respective public health mandates and needs of the agency conducting the review (Morgan et al. 2018). Whether conducted by an agency or not, a systematic review may seek to clarify the human health or ecological effects of an evidence-rich chemical. In other cases, a systematic review may be undertaken
when evidence is scarce, to identify data gaps, or to assess the accuracy of a toxicological test method (NASEM 2017b).
While not necessarily required, scoping the literature on the topic is typically done to assess the need for a systematic review. This approach is particularly useful in fields in which little is known regarding the current state of the literature and when systematic reviews have not been performed previously. Scoping may range from a simple non-systematic search in one or two databases to a more formalized, resource-intensive scoping review (described in Levac et al. [2010] and Peters et al. [2015]). The findings of a scoping exercise may reveal that the question has already been adequately addressed or may confirm that better understanding of the evidence could provide clarity. A scoping search can inform the planning process by revealing important details such as the expertise required, the stakeholders interested in the topic, and the resources needed. Scoping may be conducted before or after a review team is formed, but the approach used should be transparent and objective. By the end of the planning stage, the decision as to whether or not to conduct the review will have been confirmed. The resources and the timeframe will have been established, and the review team and advisory group will be in place.
One major challenge in the planning phase of systematic reviews in toxicology, environmental health, and ecological toxicology and exposure is to compose a skilled review team, as experience among these disciplines in systematic review is still being built. Until sufficient systematic review capacity is built, clinical or preclinical systematic review experts may need to be engaged. Similarly, ecological risk assessment is still evolving. Consequently, expertise from those familiar with applying systematic review to human health should be brought in to develop systematic review protocols for data streams supporting ecological risk assessment. Following the planning stage and once the type of review needed is established, the stakeholder group begins the work on problem formulations.
Developing and Refining a Research Question
The ultimate goal of a systematic review is to address a specific question. The question should be developed a priori as it will shape many of the steps in the remainder of the review, giving form to the literature search and screening strategies.
In many contexts, narrowing the research question to the health or ecological outcomes that are of potential concern may be challenging, as there may be an array of outcomes, extending from markers of injury to specific diseases. In addition, traditional narrative literature reviews supporting risk assessments might include many different outcomes to determine the most sensitive endpoint on which to base the risk characterization (NRC 2009, 2014). Reviews that encompass large topics may benefit from an initially broad PECO statement to identify the body of literature and then multiple narrow and well-defined PECO statements to tease out the individual questions within the review. Scoping reviews can be extremely useful in identifying the reach and breadth of a systematic review prior to developing a research question. Assessing the breadth of the body of literature before defining the research question can allow for selection of specific endpoints, evidence streams, routes of exposure, or developmental stages. The problem formulation exercise, including scoping review results and input from stakeholders, can also be used to prioritize outcomes, study designs, and populations of interest. Moreover, scoping reviews can significantly improve the quality and usefulness of the inclusion and exclusion criteria for the literature search and selection. For example, which type of mechanistic evidence is relevant to the research question being addressed by a systematic review should be determined in advance based on an understanding of the pathogenesis for the outcome of concern. This advance determination will allow the creation of inclusion and exclusion criteria that eliminate mechanistic evidence not relevant to humans. If the reviewers have a baseline knowledge of the available literature database, it is easier to define and refine the criteria needed to exclude studies outside the interest of the review. NRC’s Review of EPA’s Integrated Risk Information System (IRIS) Process provides an approach for how to narrow a research question using a scoping review (NRC 2014).
For a health hazard assessment, the research question is typically focused on a PECO statement. This varies slightly from the health-based reviews which use a PICO statement, which were primarily focused on interventions (I) rather than exposures (E) (Rooney et al. 2014). The PECO statement is used to define the objective of the review and lay out the framework for the research question to be answered. Groups should enlist experts in various fields relevant to the review (i.e., epidemiologists, information specialists, and data analysts) and/or stakeholders with an interest in the outcome of the review to provide input in the PECO statement (OHAT 2019). Similarly, expertise within fields of importance to ecological risk assessment (e.g., ecotoxicology, ecology, environmental chemistry, and environmental engineering) should be engaged. For example, assessment endpoints, which clearly link to and support ecological protection goals, and associated measures of effect must be identified during problem formulation. Thus, an ecologically focused research question and PECO statement need to encompass these assessment endpoints. Once finalized, the PECO statement becomes the primary source of information used during the literature search and for the inclusion and exclusion criteria during literature screening.
While secondary PECO review question(s) may be necessary for complex risk-based assessments and listed as sub-questions, a clear single primary review question should drive the formulation of the review. Because this question will be the systematic review’s guiding element and principal goal, defining it precisely and appropriately is of crucial importance. A properly framed review question will facilitate all the review’s subsequent steps, including the definition of the eligibility criteria and the literature search, how the evidence will be collected, and how the results will be presented and synthesized. In particular, the question should help define the criteria for the inclusion and exclusion of research studies in a way that ensures that all relevant evidence is included to answer a particular question. For example, the review question could focus on a specific study type, such as chronic toxicity studies in animals, and would thus exclude any other study type, such as acute or sub-acute toxicity studies.
Determining the Appropriate Approach
The approach to answer the questions of interest is determined based on the results of the planning, question refinement, and scoping review processes. This decision should be based on the most appropriate methods to address the question as well as issues of transparency and efficiency.
Different approaches can be used for conducting the hazard assessment, including a systematic review, a narrative review with a protocol, an assessment by an authoritative body, or an update to a high-quality narrative or systematic review (NASEM 2019). Use of an existing systematic review may be an efficient and transparent way to address a question, particularly when the scoping review identified potentially relevant reviews. In the approach of using an existing systematic review, there is a search to identify current relevant reviews (i.e., those that match the PECO elements of the question to be addressed), the relevant review(s) are evaluated to identify a trustworthy review (i.e., using ROBIS or AMSTAR-2), and a bridge search (and, as needed, screening, risk-of-bias assessment, and synthesis) is conducted to update the search results. This process was recommended and demonstrated in a prior National Academies report, Using 21st Century Science to Improve Risk-Related Evaluations (NASEM 2017a).
Developing a Systematic Review Protocol
After the approach to answering the question is determined, the next step is to develop the protocol. The protocol is a detailed plan or set of steps that should describe the methods that will be used to conduct all the steps of the systematic review from evidence identification through evidence synthesis. Protocols are critical in de novo systematic reviews and can also be used in other evidenced-based literature searching methods, such as scoping reviews and narrative reviews. The PECO elements of the refined questions determine the search strategy and the eligibility criteria. These elements should be as
specific as possible, including listing specific outcomes of interest. Furthering transparency, protocols should be publicly registered, such as on PROSPERO or Open Science Framework (Booth et al. 2012; Foster and Deardorff 2017), or posted on a public website where they can be reviewed. Protocols should be posted for review prior to the start of the systematic review for public comment and peer review. Any revisions to the protocol should be as an amendment to the protocol. All versions of the protocol will remain available upon request, although the evaluation will usually proceed according to the most updated version of the protocol.
Standards of practice for applying evidenced-based methods for developing a research question, planning an approach, and developing a protocol are not well established for questions about exposures and ecological risks. The Guidelines for Human Exposure Assessment suggest that the problem formulation for human exposure assessment should include the identification of the individual, life stage(s), and group(s) or population(s) of concern; a conceptual model presenting the anticipated pathway(s) of the agent from the source(s) to receptor(s) of concern; and an analysis plan that charts the approach for conducting the assessment. Additionally, the Guidelines for Human Exposure Assessment suggest that aggregate exposures resulting from all potential uses of the compound should be calculated unless not needed per the specific research question (EPA 2019b).
The Guidelines for Ecological Risk Assessment state that the problem formulation for ecological risk assessments should depend on high-quality assessment endpoints and include conceptual models and an analysis plan. Assessment endpoints, and specific measures of effect, should be linked directly to the ecological protection or management goals, as framed within the conceptual models, along with exposure characteristics, ecosystems, and species of particular concern and at potential risk (EPA 1998).
Committee Description of the Approach in TSCA Risk Evaluations
OPPT is using a variety of software tools and approaches to conduct broad searching and to map the available evidence. The process has problem formulation and scoping occurring somewhat in parallel with the protocol development and data collection process (see Figure 2-2). Systematic review approaches use problem formulation to determine the protocol (see Step 1 in Figure 2-1). In advance of the risk evaluation, OPPT publishes scope documents that describe what it expects to consider in its risk evaluation pursuant to TSCA section 6(b). The TSCA website states the following:2
The scope of a risk evaluation will include the hazards, exposures, conditions of use, and the potentially exposed or susceptible subpopulations the Administrator expects to consider. The scope will also include:
- A Conceptual Model, which will describe the relationships between the chemical, under the conditions of use, and humans and the environment.
- An Analysis Plan, which will identify the approaches and methods EPA intends to use to assess exposures and hazards.
- “Conditions of use” under TSCA means “the circumstances, as determined by the Administrator, under which a chemical substance is intended, known, or reasonably foreseen to be manufactured, processed, distributed in commerce, used or disposed of.” For purposes of prioritization, the Administrator may determine that certain uses fall outside the definition of “conditions of use.” During the risk evaluation scoping process, EPA may decide to narrow the scope of the risk evaluation further, potentially excluding conditions of use that present low risk.
___________________
2 See https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-evaluations-existing-chemicals-under-tsca#determination, accessed November 13, 2020.
The committee reviewed the scope documents that accompanied the TCE and 1-BP risk evaluations and found that the scoping and problem formulation documents merged the steps of problem formulation, protocol development, and the conduct of systematic review (see EPA 2017a,b). For these two assessments, the scope documents did not include PECO statements, but the problem formulation documents that were released after the evaluation was started did contain PECO statements. The committee notes that some of the more recently released scope documents contain more detailed information, such as the evidence identification methods and PECO statements3 (see Box 2-1). Additionally, the scope documents did not contain protocols as typically defined in systematic review, as the scope documents did not prespecify the approaches for conducting each step of the systematic review process.
Although the 1-BP evaluation states that a “preliminary review of the health effects literature” was conducted, it is difficult to determine how that review affected the PECO statement, other than in the outcomes considered. For the human health hazard assessment, health outcomes were limited to several specific organ systems, although there is a note stating that other outcomes may be assessed as necessary (see Table 2-1). For the population element, specific species could have been limited to those that were either relevant to the outcome or that were available in the literature. For the exposure element in human epidemiology studies, a very broad search was conducted including not only the chemical of interest but also any metabolites or mixtures and studies with only qualitative estimates of exposure, which would have limited use in a dose-response assessment.
In the exposure assessment, the problem formulation includes occupational and non-occupational scenarios, as well as the environmental releases. As with the problem formulations for hazard assessment, the problem formulation, scoping, and data collection processes are also merged for these streams. The determination of what exposures are relevant to the risk evaluation is dictated by the “conditions of use.” “For purposes of prioritization, the Administrator may determine that certain activities fall outside the definition of ‘conditions of use.’ During the risk evaluation scoping process, EPA may decide to narrow the scope of the risk evaluation further, potentially excluding conditions of use that present low risk.”4 For example in the TCE scope documents, all indoor studies of exposure are included and evaluated, but in the final risk evaluation, measurements of indoor home exposures were determined not to be related to a specific condition of use and thus were not included in determining exposures. In the search for data, sources that provide measured concentrations, as well as sources related to models, and all potential model inputs, are included. Additionally, in both the 1-BP and TCE evaluations if the manufacturer provided safety data sheets stipulating that workers will be provided personal protective equipment (PPE), EPA assumes that workers will be given proper PPE by their employer, that they will be trained to use it correctly, and that they will have no medical conditions precluding that use.
___________________
3 See, for example, https://www.epa.gov/sites/production/files/2020-09/documents/casrn_117-81-7_di-ethylhexyl_phthalate_final_scope.pdf, accessed November 13, 2020.
4 See https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-evaluations-existing-chemicals-under-tsca#determination, accessed November 13, 2020.
For the ecological risks, OPPT describes the environmental fate and transport, releases to and occurrence in the environment, and probable environmental hazards (ecotoxicology) that will be considered in the scope documents. In the analysis plan of the scope documents, OPPT identifies its objectives, the conditions of use, and data types associated with physical-chemical properties, environmental fate parameters, and ecotoxicology information. Conceptual models are used to account for exposure pathways.
TABLE 2-1 Health Hazard Assessment PECO Statement from the 1-BP Risk Evaluation
| PECO Element | Evidence Stream | Features Included |
|---|---|---|
| Population | Human |
Any population All life stages Study designs:
|
| Animal |
All non-human, whole-organism mammalian species
All life stages |
|
| Exposure | Human |
Exposure based on administered dose or concentration of 1-BP, biomonitoring data (e.g., urine, blood or other specimens), environmental or occupational-setting monitoring data (e.g., air, water levels), job title, or residence
Primary metabolites of interest as identified in biomonitoring studies Exposure identified as or presumed to be from oral, dermal, or inhalation routes Any number of exposure groups Quantitative, semi-quantitative or qualitative estimates of exposure Exposures to multiple chemicals/mixtures only if 1-BP or related metabolites were independently measured and analyzed |
| Animal |
A minimum of two quantitative dose or concentration levels of 1-BP plus a negative control group
Acute, subchronic, or chronic exposure from oral, dermal, or inhalation routes Exposure to 1-BP only (no chemical mixtures) Quantitative and/or qualitative relative/rank-order estimates of exposure |
|
| Comparator | Human | A comparison population (not exposed, exposed to lower levels, or exposed below detection) for endpoints other than death from acute exposure |
| Animal | Negative controls that are vehicle-only treatment and/or no treatment | |
| Outcome | Human |
Endpoints described in the 1-BP scope document:
Other endpoints |
| Animal |
SOURCE: EPA 2020c.
OPPT created PECO statements that guided three large literature searches to gather exposure data (under a PECO exposure statement); occupational exposure and information on industrial uses (under a Receptor, Exposure, Scenario/setting, and Outcome [RESO] statement); and information on chemical properties and factors related to environmental fate, transport, and ecological exposures and hazards (under a Pathways and Processes, Exposure, Setting or Scenario, and Outcome [PESO] statement) (see Box 2-1).
The very broad PECO-exposure (see Box 2-2) and RESO statements (see Box 2-3), one for consumer exposures and one for occupational exposures, have the goal of assembling all the potentially relevant literature to identify any measured levels of exposure in consumer or occupational settings. Additionally, the PECO statements include any models and model inputs necessary for modeling estimates of exposures. In addition to the actual PECO-exposure or RESO statements, OPPT also creates a list of all specific uses and scenarios for which there is a need to gather data, based on the knowledge obtained about the use of the chemical in prior steps.
Critique of the TSCA Approach
Looking at the core review elements of the Statement of Task, which is to address whether the TSCA approach to systematic review is “comprehensive, workable, objective, and transparent,” the committee finds that the approach to problem formulation and protocol development could be improved broadly to better meet these characteristics.
Comprehensive
The approach to problem formulation and protocol development is not comprehensive as it did not result in refined research questions or a documented approach to how the reviews required to support the risk evaluations should be conducted. The ill-defined questions within TSCA risk evaluations hinder the necessary prespecification of systematic review methods, notably the eligibility criteria for studies. Failing to adequately refine the focus of a systematic review leads to overly broad questions, in turn leading to the identification of heterogeneous studies, to more complicated analysis, and to challenges in integrating across evidence streams to draw conclusions.
With respect to the ecological assessments, the conceptual models are not consistently accounting for all exposure pathways. For example, within the environmental conceptual model in the TCE evaluation, land application of wastewater effluent is not considered, yet this practice from centralized and decentralized (e.g., advanced aerobic systems) wastewater treatment plants introduces chemical contaminants to soils. Similarly, though the range of instream dilution considerations for point source discharges is important for predicting exposure scenarios, the TCE evaluation uses 10- to 15-year-old Exposure and Fate Assessment Screening Tool (E-FAST) models in the TCE assessment. Consequently, there may be underestimation of surface water exposure levels in regions experiencing decreased flows due to climate change (e.g., prolonged droughts) and increased water extraction (see EPA 2020a, p. 98). Additionally, the documents do not prespecify all cut-off values for environmental fate parameters, such as those used for bioconcentration factors and bioaccumulation. As with the human health hazard assessments, the TCE evaluation does not include sufficient protocols that prespecify how the systematic review for ecological outcomes will be conducted.
For the more typical exposure factors, TSCA assessments do rely on the Exposure Factors Handbook, which is a well-documented and regularly updated source of information. However, there are some activities not covered in the handbook, and searches for those data streams should be included in the data-needs list in the problem formulation. Examples noted relevant to TCE consumer use would be activity pattern information on the frequency with which the average gun owner cleans his or her gun, other product-specific activities not included in the handbook, or air exchange rates associated with horse stables (EPA 2011). Additionally, in the TCE and the 1-BP evaluation, searches are needed on the actual use, type, and effectiveness of PPE for the different occupational uses of the products. The as-
sumption that PPE would be used consistently and by all workers is overly optimistic, a criticism that the committee noted in the public comments on the TCE and 1-BP risk evaluations. Additionally, breathing rates during occupational activities where the products of interest are used to estimate chemical intake, should also be included in the data needs list. Inclusion of these additional search terms in the product formulation would result in an exposure assessment that is more consistent with the Guidelines for Human Exposure Assessment.
Workable
The current approach taken to problem formulation and protocol development is adding to a laborious process for searching, screening, and evaluating the literature. Completing a scoping review prior to the development of the PECO statements could narrow the search to appropriate studies.
Objective
OPPT is using a variety of software tools and approaches to conduct broad searching and to map the available evidence; however, it is not using those approaches in an objective way to determine the research question. This may be because the TSCA process has problem formulation and scoping occurring somewhat in parallel with the protocol development and data collection process (see Figure 2-2).
Transparent
The process for problem formulation is not transparent. It is not well documented in any of the risk evaluations or related scope documents reviewed for this report, and procedures for problem formulation are not included in Application of Systematic Review in TSCA Risk Evaluations (herein 2018 guidance document). Moreover, the transparency of the entire risk evaluation is compromised because in addition to not developing clear questions for the systematic reviews, there are no protocols for the reviews or to guide the synthesis step. Consequently, the review process is not documented or prespecified from its start, and clarity is lacking when the review is finished and published.
Specifically, it is unclear how OPPT is determining the list of data needs that will inform the human exposure assessment. The TSCA webpage states that OPPT is utilizing EPA’s Guidelines for Human Exposure Assessment (EPA 2019b) for exposure assessments. If this is the case, the committee notes several inconsistencies with the guidelines. First, the guidelines specify that exposure calculations should be aggregate exposures, resulting from all potential uses of the compound (EPA 2019b). For example, a typical consumer’s exposure includes both day-to-day exposures occurring indoors as well as increased exposure resulting from product use, which may occur on a semi-regular basis. TSCA assessments do include indoor concentrations that result from aggregate exposure in the problem formulation statement for consumer exposure. However, later steps determine that the indoor exposures could not be linked to any individual consumer product, and those exposures are omitted from the final exposure assessment.
The data needs list does include many of the parameters needed to run the existing EPA models but does seem to exclude some necessary model inputs that should be included in the data search based on the guidelines. OPPT could improve these assessments and make them better align with agency guidelines if clear questions on frequency of use for consumer products that may contain the chemical of interest were identified in the problem formulation.
Recommendations
In order to improve these issues with TSCA’s approach to problem formulation and protocol development, the committee recommends the following:
- Scoping and mapping exercises and stakeholder engagement should be used to conduct a full problem formulation prior to the conduct of the systematic review.
- The results from problem formulation should include refined questions and an approach for each research question. A systematic review may not be required for every stream of evidence that is part of a risk evaluation. The full problem formulation and understanding of the literature base for an evaluation should allow OPPT to determine which research questions may be evaluated with a systematic review and which questions should be evaluated with a different evidenced-based approach. Ecological research questions should be linked to assessment endpoints, and ecological receptors of concern should be identified (e.g., algae, aquatic macrophytes, invertebrates, fish, amphibians, and threatened and endangered species). When chemicals are identified as bioaccumulative, other receptors (e.g., birds and mammals) should also be included. When there is no adequate literature base to answer questions, OPPT should be transparent about its alternative approaches. Regardless of the approach taken, OPPT should ensure that the reviews are comprehensive, objective, transparent, and consistent. OPPT should also highlight and explain areas in which deviations from a systematic review process occur.
- Evidence streams should be clearly defined to facilitate the determination of which evidence-based methods should be followed for each stream. Such a definition is especially critical for the exposure and non-hazardous streams.
- Potential redundancies should be reduced by explicitly considering appropriate methods to address questions that may include updating an existing and adequate systematic review rather than conducting a de novo review.
- A systematic review protocol that details the prespecified methods, including eligibility and critical appraisal criteria, and that is peer-reviewed and publicly posted before the review commences should be prepared. Ideally, this would be one document or, if multiple documents are needed, there should be clear crosswalks between documents.
- The problem formulation for the exposure assessment should more closely follow the Guidelines for Human Exposure Assessment and include inputs on frequency of product use, exposure factors related to specific uses, breathing rates, and use of PPE.
EVIDENCE IDENTIFICATION
Evidence identification is the next step in the systematic review process (see Figure 2-1). This step includes searching for the evidence related to the particular question and strategies for selecting both the evidence to be considered and the evidence to be excluded from consideration. As noted in Finding What Works in Health Care: Standards for Systematic Reviews (IOM 2011), this step presents the first opportunity for bias to enter the review. Without a comprehensive search to identify evidence informing the PECO statement, the resulting systematic review “will reflect and possibly exacerbate existing distortions in the biomedical literature” (IOM 2011, p. 81). Therefore, it is critical to have pilot search strategies and to have quality assurance (QA) and quality control (QC) measures during the evidence identification step of the systematic review process (IOM 2011).
STATE OF THE PRACTICE
Searching for the Evidence
Searching for the evidence starts with the design of a search plan that is aligned with the PECO question(s). This plan needs to be sensitive enough that it does not inadvertently exclude evidence relevant to the review question, without returning an unmanageably large amount of irrelevant information. The search plan should be specified in protocol, include databases to be searched and search
strategies (for at least one database), discuss gray literature that will be searched, and may also include other methods of search such as snowball searching, scanning references of included studies, and using existing systematic reviews. The date of the searches that are planned for updating should also be documented. A comprehensive search strategy should be guided by the PECO question in the selection of search terms, be in line with the pertinent inclusion criteria (publication date or language(s) to be considered), strike a balance between sensitivity (the ability to identify relevant evidence) and specificity (the ability to exclude irrelevant information), and be appropriately documented in the protocol and made publicly available.
Selecting the Evidence
Literature searches can yield thousands of records. In order to prevent subjectivity and reduce bias in the evidence selection, systematic reviews prespecify eligibility criteria based on the PECO question in the study protocol. The protocol should also specify how the quality of the selection is controlled, usually by independent duplicate review (i.e., requiring that two screeners, or one screener and an artificial intelligence [AI] tool, independently carry out the selection, with a procedure to resolve disagreements). In addition, it should provide instruction on how the selection is documented to allow its replication by others.
The selection is usually carried out in two stages: (1) title and abstract and (2) full text. At the first stage, all identified records are screened on the basis of the title and abstract in order to exclude the records that are obviously beyond the scope of the review. Studies rejected at this stage of the process will either be completely off-topic or fail to meet one or more eligibility criteria.
The second stage of the selection is a full-text review, during which reasons for the exclusion of each study need to be documented. Detailed documentation of the decision(s) made in the selection process is essential for the transparency of the review. Screeners’ assessments should be captured, as well as the solutions in case of disagreements. All full texts retrieved should be kept in a database for the systematic review.
A widely accepted tool for summarizing the selection process is the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses5) flow diagram presented in Moher et al. (2009). Koustas et al. (2014) provide a practical example of its use for a toxicological systematic review.
In order to handle the vast amount of identified records and appropriately document the selection process, various software applications are now available that allow reviewers to implement an efficient and transparent record management process. Van der Mierden et al. (2019) provided an overview of informatics solutions to support various processes of a systematic review, and the systematic review toolbox contains more than 100 software tools for a broad range of systematic review tasks.6
The committee notes that there are no standards of practice to search for the evidence in the streams that are typically included in the exposure assessment (see Figure 2-1). The Guidelines for Human Exposure Assessment and the Guidelines for Ecological Risk Assessment do provide guidelines as to what should be included in an exposure assessment (EPA 1998, 2019b), and the approach described for searching for evidence for other streams is similar to the hazard assessment.
COMMITTEE DESCRIPTION OF THE APPROACH IN TSCA RISK EVALUATIONS
Searching for the Evidence
OPPT uses exhaustive search strategies that include major scientific databases, backward searching for studies in previous chemical risk assessments, additional gray literature sources, studies submit-
___________________
5 See www.prisma-statement.org, accessed November 13, 2020.
6 See http://systematicreviewtools.com/advancedsearch.php, accessed November 13, 2020.
ted under TSCA, and studies identified in peer review (see Figure 2-3). The terms used and databases searched were found to be exhaustive (see Table 2-2).
In the TCE evaluation, the searches conducted are wide-ranging as a result of the broadly framed questions. For example, to support the review of the occupational data, OPPT uses a grouped data acquisition strategy. Rather than conducting a separate search for each exposure to be determined, exposures are grouped into three data acquisition strategies. Specifically, one search looks for information that can be used to assess consumer exposures; a second assesses environmental release data for occupational exposures; and a third gathers physical chemical property data that can be used to run the models to calculate exposure. The search for the needed chemical properties was included in the ecological search, but data were used for all types of models. This approach improves the efficiency of the data search but makes following and evaluating the process difficult. However, it is evident that the process includes areas such as monitoring data, models, and completed exposure assessments. Addi-
TABLE 2-2 Search Strategies and Terms in TSCA Risk Evaluations
| Physical/Chemical Properties | Conditions of Use | Fate, Engineering, Exposure, Human Health | Environmental Health | |
|---|---|---|---|---|
| Search terms | CAS RN Chemical name Chemical structure |
CAS RN Chemical name Synonyms Trade names Common misspellings |
CAS RN Chemical name Synonyms AND Use terms OR Exposure, Engineering and Fate terms OR Health Effect Terms |
CAS RN Chemical name Synonyms as identified by STN International and Pesticide Action Network |
| Sources | STN REAXYS ChemSpider |
Information reported to EPA Trade publications Open literature reports Citations in other assessments Safety data sheets EPA’s Chemical and Product Categories NIH’s household product database Company websites |
Existing assessments Peer-reviewed sources (Pub Med, Web of Science, Toxline) Gray literature
|
ECOTOX Knowledgebase approach:
|
NOTE: ATSDR, Agency for Toxic Substances and Disease Registry; CAS RN, CAS Registry Number; ECOTOX, ECOTOXicology; EPA, U.S. Environmental Protection Agency; NHANES, National Health and Nutrition Examination Survey; NIH, National Institutes of Health; NIOSH, National Institute for Occupational Safety and Health.
SOURCE: U.S. Environmental Protection Agency, presentation to the committee, June 19, 2020.
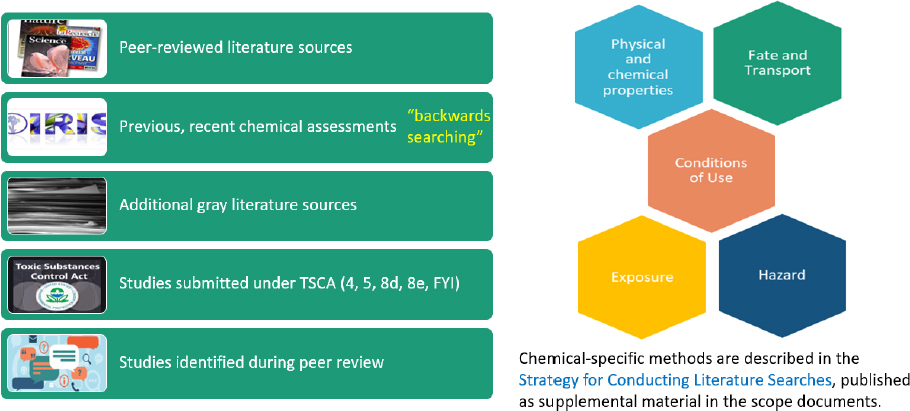
tionally, the OPPT process is relatively thorough in using a wide range of search terms and considering both the published literature and the gray literature. Gray literature searching included the first 100 sites on Google, web scraping (e.g., Agency for Toxic Disease Registry and National Institute for Occupational Safety and Health documents), and databases (e.g., ChemView). The process allows for the inclusion of other data that are submitted during peer review. It was noted in the TCE evaluation that additional reports were suggested in the public comments; those reports were screened and in some cases included. These additional materials may be government reports or other gray literature, which can be difficult to search for in a comprehensive fashion.
OPPT collects information on physical-chemical properties and environmental fate parameters. These routine physical-chemical properties (e.g., water solubility, vapor pressure, log Kow, and Henry’s Law Constant) provide indications of environmental compartments (e.g., surface water, groundwater, sediment, and air) where exposure may occur and are required to support environmental modeling efforts. OPPT conducts literature searches to populate a database for further review. Similarly, OPPT also reviews the literature and the U.S. Geological Survey, EPA, and the U.S. Department of Agriculture databases for ambient surface water exposure data from the United States and other countries. In addition to identifying empirical datasets, OPPT uses the Estimation Programs Interface (EPI) Suite modeling for physical-chemical property and environmental fate information. Such practices are not surprising. As there are approximately 350,000 chemicals and chemical mixtures registered for commercial use around the world (Wang et al. 2020), empirical data on physical-chemical properties are not consistently available, and environmental fate parameters are relatively limited. Environmental fate modeling is thus necessary, though it remains challenging to cover the range of environmental exposure scenarios and compartments with the existing tools. More recent information is available through the National Hydrography Dataset. Its use would be advantageous to improve dilution expectations and thus aquatic exposure predictions, particularly since stream flow datasets within E-FAST 2014 are reported to be 15 to 30 years old (Card et al. 2017).
OPPT also relies on the Ecological Structure Activity Relationships (ECOSAR) program to estimate ecotoxicity data when empirical data are limited. It is not clear whether or how ECOSAR is consistently being used to predict acute toxicity information for fish, aquatic and terrestrial invertebrates, and algae during each risk evaluation. Similarly, it is not clear whether physical chemical property information is being evaluated a priori to ensure it is captured within ECOSAR applicability domains. When empirical ecotoxicology data are lacking, another ORD tool, the Web-based Interspecies Correlation Estimation model, is available to support cross-species predictions. In addition, EPA ORD has advanced adverse outcome pathway (AOP) conceptual models to support mechanistic ecotoxicology data integration within risk evaluations. It has developed Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS), another innovative tool that presents bioinformatic opportunities to advance toxicity extrapolation efforts across species. However, it does not appear that these models have been identified during systematic reviews.
Many databases support the human exposure assessments, but the process for searching for these data is unclear. For example, in the TCE risk evaluation, OPPT relied on a consumer product use database that is more than 30 years old and may not reflect current usage patterns. However, a process could not be identified for obtaining more relevant and recent data. Information is not readily available on what chemicals are in particular products, as databases with such information are lacking both in quantity and quality.
It is also unclear whether the specific search statements are intended to identify factors that may be important for the exposure calculations for the conditions of use. For example, one pathway considered for TCE was related to hoof polish for horses. It was assumed that the duration of use and the mass of polish used was the same as for shoe polish. It was assumed that the barn where the product was being applied was the same size as a garage but that the air exchange rate was higher, with reference to a sin-
gle report from Pennsylvania State University supporting that value.7 It is unclear if a systematic search was conducted to obtain data for this parameter or if the search was limited to finding a source of data.
Selecting the Evidence
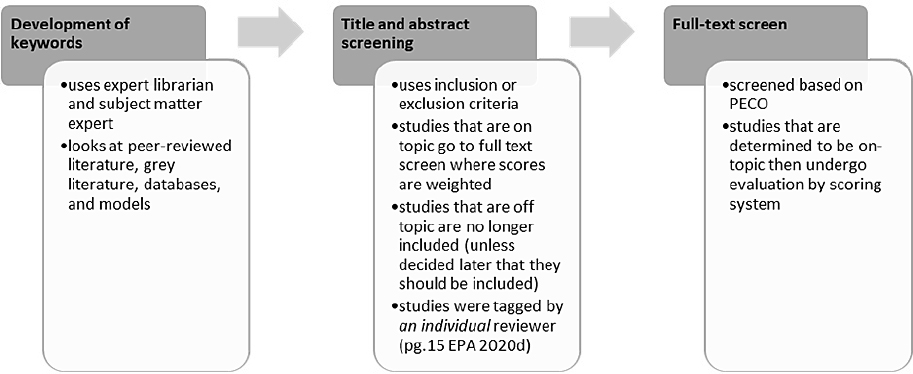
To select evidence, OPPT then screens the titles and abstracts against a list of needs for the evaluation. However, the committee was provided conflicting information on how this step was conducted. The 2018 guidance document states that OPPT uses PECO at title and abstract screening; the Strategy for Conducting Literature Searches for TCE: Supplemental Document to the TSCA Scope Document states that OPPT uses a list of data needs. Next, a full-text screening of the papers is conducted. As noted in the problem formulation section, OPPT uses PECO or PECO-like statements to compare articles to determine eligibility. For occupational data, there is a RESO statement to gather information on potential occupational exposures, and for the TCE and 1-BP evaluations such statements are used to inform the full-text screening. This process, as carried out for the evaluations of TCE and 1-BP, is illustrated in Figure 2-4. The broad PECO/PECO-like statements lead to unclear and shifting eligibility criteria and to an unclear or questionable selection process (i.e., changes in the process are allowed and reasons are not specified). In more recent evaluations, OPPT is using a number of AI-based tools to help with the large number of references (Kellie Fay, poster presentation to committee, June 19, 2020). These tools aim to make the screening of articles more efficient by automatically prioritizing articles by using user feedback to push the most relevant articles to the top of the list (Howard et al. 2016).
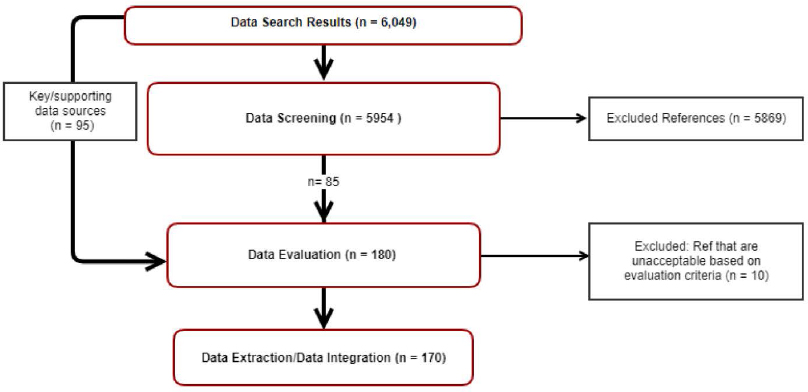
The literature flow diagram for the TCE human health hazard assessment shows that more than 6,000 studies were identified from the initial search using key words, 5,954 studies went through title and abstract screening, and 180 studies were evaluated during the full-text screen (including 95 key studies from previous assessment activities) (see Figure 2-5). Of the 180 studies identified for human health hazard assessment data evaluation, 119 studies were selected for full-text evaluation for animal and mechanistic data (see Risk Evaluation for TCE Systematic Review Supplemental file: Data Quality Evaluation of Human Health Hazard Studies—Animal and Mechanistic Data). No information was supplied in the risk evaluation documents identifying how these studies were selected. While it appears that the studies were selected from the general human health hazard assessment pool, the lack of details on how the agency arrived at the particular subset of animal and mechanistic studies makes it impossible to determine the process by which the studies were identified. Additionally, no information is supplied related to the excluded references (e.g., which studies were excluded and why).
___________________
7 See https://extension.psu.edu/horse-stable-ventilation, accessed November 13, 2020.
Using the inclusion/exclusion criteria based on the PECO statement for TCE (see Table 2-3), the populations identified in the PECO statement include any population and all life stages, and although fetal cardiotoxicity was an important outcome for the TCE evaluation, no justification was given for limiting outcomes of animal and in vitro studies to developmental toxicity.
Critique of the TSCA Approach
Looking at the core review elements of the Statement of Task, which address whether the TSCA approach to systematic review is “comprehensive, workable, objective, and transparent,” the committee finds that the TSCA approach to searching for evidence identification could be improved on all of these characteristics.
Comprehensive
TSCA risk evaluations include searches for evidence in most major scientific databases, backward searching for studies in previous chemical risk assessments, additional gray literature sources, studies submitted under TSCA, and studies identified in peer review (see Figure 2-3). The terms used and databases searched were found to be exhaustive. The ecological assessment and the human health exposure assessment rely often on databases that support the models for those evaluations. The committee found that the process for searching for these types of data may not be comprehensive. For example, the hydrology data used in the TCE evaluation were not the most recent, and the product use information was more than 30 years old.
Workable
The TSCA approach could be more efficient, as the broad PECO statements led to inclusion and exclusion criteria that allowed inclusion of studies that may not be relevant. OPPT is using a number of AI-based tools to help make the process of screening hundreds of references more efficient. Many of these tools have been validated on dozens of systematic reviews, and the committee is supportive of their use (Gartlehner et al. 2019; Howard et al. 2020; Van der Mierden et al. 2019). However, these tools will only work well when precise and explicit inclusion and exclusion criteria are used consistently by all screeners.
TABLE 2-3 Inclusion and Exclusion Criteria for the TCE Risk Evaluation
| PECO Element | Evidence Stream | Papers/Features Included | Papers/Features Excluded |
|---|---|---|---|
| Population | Animal |
|
|
| Mechanistic/Alternative Methods |
|
||
| Exposure | Animal |
|
|
| Mechanistic/Alternative Methods |
|
|
|
| Comparator | Animal |
|
|
| Mechanistic/Alternative Methods |
|
|
|
| Outcome | Animal | ||
| Mechanistic/Alternative Methods |
|
|
|
| General Considerations | Papers/Features Included | Papers/Features Excluded | |
|
|||
a Some of the studies that are excluded based on the PECO statement may be considered later during the systematic review process. For TCE, EPA will evaluate studies related to susceptibility and may evaluate, toxicokinetics and physiologically based pharmacokinetic models after other data (e.g., human and animal data identifying adverse health outcomes) are reviewed. EPA may also review other data as needed (e.g., animal studies using one concentration, review papers).
b EPA will review key and supporting studies in the IRIS assessment that were considered in the dose-response assessment for non-cancer and cancer endpoints as well as studies published after the IRIS assessment.
c EPA may screen for hazards other than those listed in the scope document if they were identified in the updated literature search that accompanied the scope document.
d EPA may translate studies as needed.
SOURCE: EPA 2018b.
Objective
The process for searching and selecting the evidence lacked objectivity, because the inclusion and exclusion criteria were broad and thus less objective. A benefit of systematic review is that clear, predefined inclusion or exclusion criteria increase objectivity of the process for selecting the evidence.
Transparent
Overall, the committee found that the lack of information about the specific processes used for the identification of evidence reduced confidence in the findings and were inconsistent with systematic review practices. Information about the search process was scattered across multiple documents within the docket for TCE, making the identification of details laborious and time consuming. The committee recommends organizing the information in one main document with clear references to supporting documents.
In the TSCA evaluation process, eligibility criteria are not predefined in the protocols and shift during the systematic review process. Outcomes specified are frequently too broad for true systematic review and would have been focused in scoping exercises. These shifts in inclusion and exclusion criteria are particularly problematic when used in building machine learning models as the shifting exclusion criteria may mislead and confuse the algorithm, resulting in exclusion of relevant studies. The committee also noted that the outcomes to be assessed are not specifically outlined in the protocol. If not the case, the systematic review and its conclusions are at risk of bias from incomplete reporting.
Recommendations
In order to improve these issues with OPPT’s approach to evidence identification, the committee recommends the following:
- Registering the protocol for each risk evaluation is important: That protocol should include an explicit search strategy, and search strategies for each database should be consistently listed in the appendix to the risk evaluation.
- OPPT could improve the evidence identification process by requesting information from manufacturers, such as ingredients for products, and from organizations that have provided data previously during the peer-review stage. Such requests made earlier in the process could lead to more complete data gathering. TSCA provides OPPT with authority to collect information on chemical manufacturing, processing, and use, which could be used to collect information in advance of the risk evaluation (TSCA section 8(a) Reporting Requirements, 15 U.S.C. 2607).
- Machine learning and AI-based tools should be used for searching and screening, especially if the tools are validated by the developer and users or there are publications available that document this validation.
- Eligibility criteria need to be based on PECO statements that are formulated in a standard way and need to be predefined in the protocol. The eligibility of outcomes needs to be carefully considered a priori to prevent a systematic exclusion of outcomes that could bias the results, such as excluding studies that have findings counter to those anticipated for the included outcomes.
- Documentation of all studies identified in searches should be more clear with the provision of a list of included studies, detailed evidence tables of included studies, and documentation of excluded studies with reasons for exclusion.
- OPPT should specify the methods by which the screening will be conducted. Examples include the number of reviewers (e.g., two screeners or one screener and an AI tool) and how disagreements are handled.
EVALUATION OF THE EVIDENCE
Following evidence identification, the next step in the systematic review process is evaluation of the evidence (see Figure 2-1). In a systematic review, the individual studies are critically appraised using predefined criteria and then the body of evidence (i.e., all of the included studies for a particular question and outcome) is synthesized (qualitatively and/or quantitatively) and evaluated to draw a conclusion and specify a level of confidence in that conclusion. The systematic review should assess the strengths and limitations of the evidence so that decision makers and stakeholders can judge whether the data and results of the included studies are valid (IOM 2011).
State of the Practice
Individual human, animal and other ecological receptors, and mechanistic studies are assessed for internal validity (commonly referred to as “risk of bias” in systematic review) by considering aspects relevant to the type of study (OHAT 2019). Bias is a systematic error that leads to study results that differ from the truth. Bias can lead to an observed effect when in truth there is not one, or to no observed effect when there is a true effect. Risk of bias is the appropriate term, as a study may be unbiased despite a methodological flaw. The risk-of-bias assessment differs from an assessment of study quality, which is the appraisal of included studies to evaluate the extent to which study authors conducted their research to the highest possible standards (Higgins et al. 2011). Some tools assess risk of bias and study quality separately because the risk of bias addresses how valid the individual studies are; a study can be of high quality and still have a high risk of bias. Many markers of a high-quality study (e.g., whether a study’s investigator has performed a sample size calculation and whether the study is reported adequately or has received appropriate ethical approvals) are unlikely to have any direct implication for the potential for a study to be affected by bias.
There are many tools for assessing risk of bias, such as those used by the Navigation Guide, OHAT, and the IRIS Program, and there is no consensus on the best tool for risk-of-bias analysis. However, there are best practices. For example, tools are preferred that rely on the evaluation of individual domains rather than the creation of overall quality scores (Eick et al. 2020). Such tools provide a structured framework within which to make qualitative decisions on the overall quality of studies and to identify potential sources of bias. Overall quality scores may not adequately distinguish between studies with high and low risk of bias in meta-analyses (Herbison et al. 2006). Importantly, there is also a lack of empirical evidence on the use of quality scores (Jüni et al. 1999).
While there is inevitably variation in the internal validity and risk of bias across individual studies, it is standard practice to include all studies, even the studies with a high risk of bias into the evidence synthesis. The most appropriate method to exclude studies from evidence synthesis is based on predefined exclusion criteria that should preclude an irrelevant study from being evaluated.
Although there is not a specific standard of practice for evaluating exposure data, the agency Guidelines for Human Exposure Assessment discuss the importance of critically reviewing data for use in an exposure assessment. To address the quality of analytical methods, the Guidelines for Human Exposure Assessment suggest a series of questions that should be asked when reviewing data for use in an exposure assessment: Has an authoritative body adopted these (and other considerations about whether the exposure data are useful for the research question being addressed in the exposure assessment)? Were the study objectives and designs suitable for the purpose of the exposure assessment? When evaluating the study data, consideration needs to be given to potential bias in the exposure data, which may be selective for high or low exposures; for example, some occupational monitoring data focus on the most highly exposed workers (EPA 2019b). For data on human exposures that are generated by mathematical models, the Guidelines for Human Exposure Assessment also discuss methods for model evaluation to test whether the analytical results from the model are of sufficient quality to serve as a basis for decisions.
To complete a model evaluation, the model operation and results are verified both qualitatively and quantitatively through calibration, or the process of adjusting selected model parameters within an expected range until the differences between model predictions and field observations meet selected criteria. Then, important sources of uncertainty, including measurement error, statistical sampling error, non-representativeness of data, and structural uncertainties in scenarios and formulations of models, are checked. Sensitivity analysis may also be conducted to determine the extent to which estimates are dependent on variability and uncertainty in the parameters within the model. The guidelines for model evaluation apply to several different types of models included in exposure assessment, such as physiologically based pharmacokinetic (PBPK) modeling and fate and transport models (EPA 2019b).
As yet, there is not a complementary tool matching the NTP’s OHAT Risk-of-Bias tool for application to ecotoxicology studies. One increasingly used method for assessing the quality of ecotoxicity studies is the Criteria for Reporting and Evaluating Ecotoxicity Data (CRED) (Moermond et al. 2016). CRED, which was built from Klimisch et al. (1997), presents a comprehensive and state-of-the-practice approach for evaluation of ecotoxicological information. CRED includes four reliability categories: reliable without restrictions, reliable with restrictions, not reliable, and not assignable. These are used for 20 reliability criteria falling into the categories of test set-up, test compound, test organisms, exposure conditions, and statistical design and biological response. CRED was developed, in part, to ensure that high-quality ecotoxicology information, including mechanistic evidence, is not excluded a priori from regulatory assessment processes simply because a study was not performed according to a standardized protocol using a standardized model species. The reliability of CRED as an assessment approach was determined from a ring trial that compared it to the Klimisch method (Kase et al. 2016). Results showed that the CRED evaluation method was a more detailed and transparent evaluation of reliability and relevance than the Klimisch method. Ring test participants perceived it to be less dependent on expert judgment, more accurate and consistent, and practical regarding the use of criteria and time needed for performing an evaluation.
Committee Description of the Approach in TSCA Risk Evaluations
OPPT has developed an extensive de novo critical appraisal tool, termed TSCA’s “fit-for-purpose evaluation framework,” which is applied to human, animal, ecological receptors, mechanistic, exposure, fate, and physical chemical property studies. OPPT has stated that the evaluation strategies were developed after review of various qualitative and quantitative scoring systems. OPPT considered items such as NTP’s OHAT Risk-of-Bias tool, CRED, and EPA ORD’s draft IRIS handbook. These tools were not adopted because they do not encompass the entirety of TSCA’s scope and specifically do not include either exposure assessment or fate and transport assessment (Francesca Branch, presentation to the committee, July 23, 2020).
The critical appraisals for different types of studies use different domains (see Table 2-4), but in general the method of study evaluation is the same. The data quality domains are based on a variety of sources, but they were not directly adopted from any one source for any domain. For example, the domains for ecological hazards are based on CRED and criteria from the ECOTOX knowledgebase. Within each domain there are several metrics or questions. For the epidemiologic studies there are 7 domains, and within each domain there are between 2 and 7 metrics for a total of 22 metrics. For each metric, options include “high” (a quantitative score of 1), “medium” (score of 2), “low” (score of 3), and “unacceptable” (score of 4). The metrics generally include items that assess elements of study quality, risk of bias, reporting quality, and relevance. For example, statistical power is included within the test organism data quality domain used to assess the ecological hazard and toxicity studies and the study participation quality domain of the epidemiologic studies. These metrics are weighted with another numeric value (1 or 2), and then a multi-metric numeric value is identified, based on the sum of the weighted metric scores divided by the total number of metric weighting factors. This final study quality score is then cat-
TABLE 2-4 TSCA Data Quality Domains by Data Stream
| Data Stream | Data Sources or Types of Studies | Data Quality Domain |
|---|---|---|
| Physical chemical property data | Not listed | Representativeness, appropriateness, evaluation/review, reliability/unbiased (method objectivity), reliability/analytic method |
| Fate data | Experimental data, field studies, modeling data, monitoring data | Test substance, test design, test conditions, test organisms, outcome assessment, confounding/variable control, data presentation and analysis |
| Occupational exposure and environmental release data | Monitoring data, environmental release data, published models for exposures or releases, completed exposure or risk assessments, reports for data or information other than exposure or release data | Reliability, representativeness, accessibility/clarity, variability and uncertainty |
| Consumer, general population, and environmental exposure data | Monitoring data, modeling data, survey-based data, epidemiological data, experimental data, completed exposure assessments and risk characterizations, database sources not unique to a chemical | Reliability, representativeness, accessibility/clarity, variability and uncertainty |
| Ecological hazard | Acute and chronic toxicity to aquatic invertebrates and fish (e.g., freshwater, saltwater, and sediment-based exposures); toxicity to algae, Cyanobacteria, and other microorganisms; toxicity to terrestrial invertebrates; acute oral toxicity to birds; toxicity to reproduction of birds; toxicity to terrestrial plants; toxicity to mammalian wildlife | Test substance, test design, exposure characterization, test organism, outcome assessment, confounding/variable control, and data presentation and analysis |
| Animal and in vitro toxicity studies | Animal: oral, dermal, and inhalation routes; lethality, irritation, sensitization, reproduction, fertility, developmental, neurotoxicity, carcinogenicity, systemic toxicity, metabolism, pharmacokinetics, absorption, immunotoxicity, genotoxicity, mutagenicity, endocrine disruption In vitro: irritation, corrosion, sensitization, genotoxicity, dermal absorption, phototoxicity, ligand binding, steroidogenesis, developmental, organ toxicity, mechanisms, high throughput, immunotoxicity | Test substance, test design, exposure characterization, test organism/test model, outcome assessment, confounding/variable control, and data presentation and analysis |
| Epidemiological studies | Controlled exposure, cohort, case-control, cross-sectional, case-crossover | Study participation, exposure characterization, outcome assessment, potential confounding/variability control, analysis |
SOURCE: Derived from EPA 2018a.
egorized as high (≥1 to <1.7), medium (≥1.7 to <2.3), low (≥2.3 to ≤3), or unacceptable (4). It is worth noting that if any of the metrics are scored as “unacceptable” then the final study quality score is also deemed unacceptable. Strengths and limitations are considered when assigning a quality rating for each relevant metric. With proper justification, a reviewer may adjust the overall quality rating to capture professional judgment not originally included in the metric criteria (Francesca Branch, poster presentation to the committee, August 24, 2020). Unacceptable studies are then excluded from further analysis. The details of TSCA’s “fit-for-purpose evaluation framework” are included as appendices to the 2018 guidance document. Generally, the approach is tiered with the following steps:
- A check for relevance of data;
- A quality evaluation that considers all reasonably available data deemed potentially relevant to the risk evaluation;
- Reporting quality and risk-of-bias elements integrated in the review process;
- Elimination of unacceptable studies from further consideration;
- Weighting of criteria for key elements, in some cases; and
- The planning, execution, and QA/QC assessment activities supporting the data evaluation.
Critique of the TSCA Approach
Looking at the core review elements of the Statement of Task, which address whether the OPPT approach to systematic review is “comprehensive, workable, objective, and transparent,” the committee finds the TSCA “fit-for-purpose evaluation framework” could improve on all these elements.
Comprehensive
TSCA’s “fit-for-purpose evaluation framework” is comprehensive in that it contains many domains and each domain has many metrics. Yet, the committee found that although the 2018 guidance document discusses the use of PBPK models in risk assessments, and that OPPT will use evaluation strategies for animal and in vitro toxicity data to assess the quality of the data supporting the model, the document does not give guidance as to how these models will be evaluated. The committee could not find evidence of this practice being followed in the draft TCE risk evaluation document and supplemental materials.
Similarly, exposure models are scored only based on reliability, representativeness, accessibility or clarity, and variability or uncertainty, but little seems to be done to actually evaluate the model. For example, a model could score “high” in the reliability category simply for being published in a peer-reviewed journal, when in fact the reliability of the model may never have been evaluated.
Workable
The committee notes that TSCA’s “fit-for-purpose evaluation framework” may not produce the desired results. It includes items that do not assess risk of bias, such as relevance and incomplete reporting. In systematic review, study relevance should be addressed with predefined eligibility criteria, which are used during screening to select relevant studies and exclude irrelevant studies from the evaluation step. For example, if the systematic review is focused on a certain life stage, then studies not including that life stage would be excluded in ether title-abstract or full-text screening. Incomplete reporting can be a challenge in evaluating a study, but it is not a marker of the validity of the study findings.
The reliance on numeric quality scores is problematic because scores do not distinguish between high- and low-quality studies, and the relationship between quality scores and an association or effect is inconsistent and unpredictable (Greenland and O’Rouke 2001; Herbison et al. 2006; Jüni et al. 1999).
More generally, the use of numerical scoring in critical appraisal does not follow standards for the conduct of systematic reviews. Additionally, there was no justification provided for the weighting of specific metrics within the domains to create the overall quality score, making it difficult to determine if the weights are appropriate. The committee notes that many public comments also discussed these problems with using numeric scores to evaluate studies.
The committee notes that completing the detailed evaluations of each study that may be included with risk evaluation is time consuming. In a study comparing the risk-of-bias assessments for epidemiologic studies from OHAT, the IRIS Program, and TSCA, the authors found that the TSCA evaluation tool took the most time to complete with a mean of 40 minutes per study, compared to 32 minutes (IRIS) and 20 minutes (OHAT) (Eick et al. 2020). While a mean increase of 8 minutes of review time per study may not seem that laborious, it is potentially severely burdensome for reviews with many studies.
Objective
All evaluations of studies have an inherent subjectivity, which is not overcome with numeric scoring. No data were shared with the committee showing that the TSCA evaluation framework and numeric scoring schema had been validated or tested for reliability. Allowing a reviewer to override the score after it has been applied is another threat to objectivity.
Another problematic element of TSCA’s “fit-for-purpose evaluation framework” is that the unacceptable studies are excluded from further analyses. Any fatal flaws in the methodology or conduct should be included in the exclusion criteria applied during the screening process. Once a study is determined to be eligible, the study could be included in the synthesis and the risk-of-bias assessment and its limitations accounted for in any qualitative or quantitative synthesis. Given the large number of metrics scored for these data types, the possibility that a single unsatisfactory rating could nullify the use of a particular study from synthesis is problematic, as it may lead to a biased review. In the synthesis step, low-quality studies may be excluded as a sensitivity analysis, but it is inappropriate to leave them out of synthesis completely.
Statistical power and statistical significance are not markers of risk of bias or quality. Statistical significance is not a measure of association or strength of association and should not be used to evaluate studies. In fact, combining multiple small, low-powered but similar studies in a synthesis is one of the potential benefits of systematic review. The committee notes that this critique was also shared in many public comments reviewed and was published in a commentary in the American Journal of Public Health (Singla 2019).
Transparent
The committee found that several facets of TSCA’s “fit-for-purpose evaluation framework” lacked transparency. The use of numeric scores prevents users of the reviews from making their own determinations about important strengths and limitations in study methods based on results presented in the review. The process of critical appraisal of the studies is not documented. OPPT reported to the committee that it generally follows standard practice by using two independent reviewers, but it was unclear how discrepancies between the two reviewers are handled.
The committee also found that the documentation of the study scores was hard to follow. In the TCE evaluation, the environmental releases and occupational exposure study scores are tracked in one document and the data extraction is tracked in another. There were studies listed as having been extracted that were not included in the tables of extracted studies. One study was excluded because it only had occupational exposure data, but there was no indication as to whether it was then evaluated as a source of occupational data. There were studies for which the score changed from the scoring document to the extraction document without documentation as to why.
Recommendations
In order to improve these issues with TSCA’s “fit-for-purpose evaluation framework,” the committee recommends the following:
- Do not use numeric scores to evaluate studies; replace them with domain-based scoring as is done in the tools used in the Navigation Guide and OHAT.
- Use established tools for assessing risk of bias and study quality such as those developed for use by OHAT or the Navigation Guide, or, at a minimum, remove inappropriate appraisal criteria from the current tools.
- Do not exclude studies based on risk of bias, study quality, or reporting quality.
- The CRED approach is robust and should continue to be employed during TSCA risk evaluations for ecotoxicology information. Clarify this interface with the ECOTOX knowledgebase. A risk-of-bias instrument should be developed for ecotoxicology studies, building from the OHAT Risk-of-Bias approach.
- Develop a method for clearly tracking how articles are handled through the steps of extraction and evidence evaluation, so those reading the risk evaluation can follow how articles are handled in each step.
- Improve documentation of how the evaluation is applied, including at what points in the process quality determinations may be changed and on what basis.
EVIDENCE SYNTHESIS
Evidence synthesis represents the next step in a systematic review. It involves a qualitative analysis as well as a quantitative analysis, when feasible and appropriate, of the results of the individual studies within a stream of evidence (e.g., fetal cardiac effects of TCE in animal models).
State of the Practice
According to the Cochrane Handbook for Systematic Reviews of Interventions, evidence synthesis is a process of bringing together data from a set of included studies with the aim of drawing conclusions about a body of evidence (Higgins et al. 2019). The process consists of summarizing study characteristics, quality, and effects, and combining results and exploring differences among the studies (e.g., variability of findings and uncertainties), using qualitative and/or quantitative methods. The choice of methods for data synthesis depends on data completeness and the hypothesis to be addressed. Whereas statistical methods, such as meta-analysis, are often preferred for combining results quantitatively, other methods, including graphic displays, may be used when a statistical method is not feasible or appropriate due to incomplete or incompatible data extracted from different studies. To ensure transparency and consistency of a systematic review, approaches and methods for evidence synthesis should be prespecified in the protocol. Following evidence synthesis, evidence integration across multiple evidence streams—an essential step in the TSCA risk evaluation process—is done but is not a part of the traditional systematic review process.
There is much research and a growing consensus on how certainty in a body of evidence should be determined in the field of toxicology. For systematic reviews for environmental health assessments, Rooney et al. (2014) proposed a system for rating certainty in the overall body of evidence across outcomes based on GRADE (Grading of Recommendations Assessment, Development and Evaluation) (Balshem et al. 2011; Guyatt et al. 2011). The GRADE environmental health working group is adapting the original GRADE approach to environmental health. For each outcome, the body of evidence
is assessed to determine a final certainty in evidence by rating high certainty, moderate certainty, low certainty, or very low certainty. Conventionally, bodies of evidence for outcomes informed by randomized controlled trials (RCTs) start at high initial certainty and non-randomized studies start at low initial certainty to account for the lack of a prognostic balance (present in well-done RCTs). This initial level of certainty may be decreased or increased if certain attributes are present in the studies. The criteria that can lower the certainty are as follows: (1) limitations of detailed design and execution (overall risk of bias), (2) inconsistency (or heterogeneity), (3) indirectness to PECO and applicability to main PECO, (4) imprecision (number of events and confidence intervals), and (5) publication bias. It is necessary to be transparent about considerations that informed this judgment, and the ranking is normally done by two reviewers with a conflict resolution process (defined in the protocol) in place. This certainty rating is considered helpful as it increases the transparency of the final conclusions.
The approach developed by Morgan et al. (2016) and the approach used by the Navigation Guide (Woodruff and Sutton 2014) are similar to the original GRADE approach, but they use slightly different criteria for setting initial levels of certainty and for upgrading and downgrading. For example, in the Navigation Guide observational studies start at a moderate rather than a low level of confidence, and the NTP uses consistency across species as an extra upgrading criterion. These examples illustrate that there is not yet consensus in the toxicology field regarding evidence synthesis, but there is some convergence on common baseline methods, such as the GRADE approach, to bring consistency. Moreover, the committee would like to emphasize that all of the above mentioned methods have undergone pilot testing, stakeholder vetting, and peer review and have been made public.
Within the agency, the Guidelines for Human Exposure Assessment, the Guidelines for Ecological Risk Assessment, and the operating procedures for the use of the ECOTOX knowledgebase dictate how exposure, fate and transport, and physical chemical property data should be synthesized for decisions about risks to human health and ecological receptors. The Guidelines for Human Exposure Assessment indicate that aggregate exposures integrated across sources and routes of exposure should be estimated and that probabilistic exposure models are preferred to those that are based on a single data point, such as measure of central tendency. Sensitivity analyses are also suggested to account for the most important sources of uncertainty in the model (EPA 1998, 2019b, 2020b). The standard of practice for species sensitivity distributions (SSDs) in risk evaluations is the SSD Toolbox.8
Committee Description of the Approach in TSCA Risk Evaluations
Section 3.4 of the 2018 guidance document does not separate evidence synthesis from evidence integration and instead combines the two into a single step of data integration (EPA 2018a, p. 26):
Data integration is the stage where the analysis, synthesis and integration of data/information takes place by considering quality, consistency, relevancy, coherence and biological plausibility. It is in this stage where the weight of the scientific evidence approach is applied to evaluate and synthetize multiple evidence streams in order to support the chemical risk evaluation.
A TSCA risk evaluation involves multiple research questions, which are combined into three core elements—hazard assessment, exposure assessment, and dose-response assessment—which are then integrated for risk characterizations. To determine whether and how a systematic review approach can be followed for evidence synthesis, the specific stream of evidence that needs to be synthesized must first be identified. As noted throughout this chapter, the goals and objectives of the systematic reviews conducted within TSCA risk evaluations are unclear, resulting in lack of clarity on what should be synthesized.
___________________
8 See https://www.epa.gov/chemical-research/species-sensitivity-distribution-ssd-toolbox, accessed November 13, 2020.
For risk evaluation of TCE, OPPT developed a problem formulation and scoping document (EPA 2018b), which indicates four categories of evidence: physical and chemical properties, conditions of use, exposures, and hazards. Each of these categories may consist of multiple evidence streams. For example, exposures may be divided into environmental release, fate and transport, environmental exposure, and human exposure (occupational versus consumers). Evidences in environmental release and fate and transport are upstream events to environmental and human exposure. Hazards can be divided into human health hazards and environmental hazards. OPPT developed a PESO statement for environmental release and fate, a RESO statement for engineering and occupational exposure, a PECO statement for consumers and ecological receptors, and a PECO statement for human hazards.
The PECO statement for human hazards indicates three parallel streams of evidence: human, animal, and mechanistic/alternative methods. Taking into consideration species, route of exposure, type of health outcome (e.g., cancer versus non-cancer), and organ or body system, there could be a large number of research questions that could be evaluated with a systematic review and ultimately synthesized. The committee recognizes that in the context of TSCA risk evaluation, defining a sub-stream of evidence is not always trivial and may not be unique but is a prerequisite. It finds that, in the absence of an explicit definition of an evidence stream, it is difficult to assess whether OPPT should follow a systematic review approach for evidence synthesis and, if so, how evidence synthesis should be conducted. Making that judgment is further complicated by OPPT’s merger of evidence synthesis within a stream and the step of evidence integration across streams. The following are a few examples from the committee’s review of the draft TCE evaluation.
Environmental release and aquatic exposure are two components that are important for evaluating questions about ecological risks. OPPT assessed aquatic exposure based on monitoring data, but where monitoring data were unavailable, it used model-predicted surface water concentrations with environmental release data as input to the E-FAST Version 2014 model, but it did not provide a plan for assessing sensitivity and validity of such a model-based synthesis. The draft TCE evaluation stated that OPPT followed a systematic review process for the monitoring data stream, although the problem formulation document failed to indicate so. Evidence synthesis for both environmental release and monitoring data could be made more quantitative for each scenario because the data appear adequate for analysis of range and distribution.
For the human exposure stream, OPPT calculated the central tendency and a high-end estimate of exposure for each occupational or consumer exposure scenario, using both model-based estimates and, when available, measured data. However, the committee could find no information presented in the draft TCE evaluation on how to combine data from multiple datasets. When evaluating how this defined process is applied in the case of concentrations in dry cleaners, for example, the committee found that documentation was not transparent. Concentrations are not presented for the individual studies selected, and there is no mention as to how they were combined.
In synthesizing the environmental hazard stream, OPPT adopted statistical methods to derive an SSD for aquatic species (Etterson 2020). SSDs, which are routinely used by the agency to derive water quality criteria for protection of aquatic life and to assess ecological risks of pesticides (Posthuma et al. 2001), are more advantageous than deterministic approaches, including hazard quotients, when data are adequate. For example, SSDs fit common toxicity values (e.g., no observable effect concentration, EC50) from all included aquatic studies along a probabilistic distribution, from which the 5-percentile or HC05 can be selected to quantify hazard on the basis of the entire stream of evidence. The SSDs are innovative because the distribution, hence HC05, effectively accounts for variability (e.g., species sensitivity at different trophic position) and uncertainty (e.g., between-experiment variation). NRC made a recommendation in relation to the 2010 IRIS assessment of TCE to encourage the agency to fit a probabilistic distribution for any comparable values of the point of departure (POD), such as benchmark dose level (NRC 2010). SSDs are common probabilistic hazard assessment tools utilized elsewhere by EPA (e.g., Office of Water and Office of Pesticide Programs) that when coupled with probabilistic environmental
exposure distributions (EEDs) of measured or predicted chemical concentrations, provide robust inputs for performing probabilistic ecological risk assessments. Thus, these EEDs and SSDs represent useful implementation opportunities for meta-analysis within the context of risk assessment.
OPPT followed a systematic review approach within evidence streams of human, animal and other ecological receptors, and mechanistic models, respectively, as indicated in the problem formulation document (EPA 2018b). It is less clear whether OPPT considered all non-cancer endpoints as a single research question or developed more refined questions for each organ or body system. Evidence synthesis for non-cancer endpoints was qualitative, in which the key study or studies were chosen to advance to later steps. This approach can be improved by using more quantitative methods such as fitting a probabilistic distribution for associated PODs, as illustrated and recommended by NRC (2010). The use of SSDs for environmental hazards also illustrates such approaches. For cancer endpoints, the TCE risk evaluation included a meta-analysis to synthesize evidence, combining three cancers—non-Hodgkin lymphoma, kidney cancer, and liver cancer—in human studies. Although OPPT followed a systematic review approach for evidence from mechanistic models, the number of mechanistic studies was limited and the evidence synthesis was narrative, concluding that a genotoxic mode of action is highly plausible for kidney cancer.
The committee notes that OPPT’s risk evaluation has consistently discussed sources of uncertainty, occasionally described the range of an uncertainty factor, but rarely quantified the impact of uncertainties on downstream events along the risk assessment process. For example, distributional properties such as percentiles can be derived for aquatic concentrations of TCE within an occupational or consumer exposure scenario or across scenarios. The application of SSDs for environmental hazard represents a good example of using probabilistic distributions to quantify uncertainty and variability, but they are dependent on sufficient data availability. Probabilistic distribution–based approaches for acute and chronic datasets should be utilized when feasible and appropriate. The committee also notes that in the 2018 guidance document (EPA 2018a), OPPT does not provide guidance to identify specific research questions for which data should be synthesized.
OPPT conducted a dose-response assessment for human cancer and non-cancer endpoints in its evaluations of 1-BP and TCE. For non-cancer endpoints, OPPT included the studies appropriate for the dose-response assessment and organized them by exposure route, chronic or acute exposure, and target organ. OPPT then estimated a POD using either benchmark dose software (BMDS), no observed adverse effect level, or lowest observed adverse effect level for every included study after fitting every dose-response model implemented in the BMDS (EPA 2012a), and converted the PODs to human equivalent concentrations (HECs) applying relevant uncertainty factors. Finally, OPPT synthesized these studies by selecting the most sensitive POD and associated HEC both within and across the organ or exposure groups. The committee also observed that in the evaluation of 1,4-Dioxane (EPA 2019a), OPPT chose the POD associated with the best statistically fit dose-response model (EDF 2020).
OPPT conducted a similar dose-response assessment for cancer endpoints. However, the dose-response assessment of cancer endpoints was inconsistent across different evaluations. In the evaluation of TCE, for example, OPPT used three models for each cancer endpoint: a multistage model; an average (frequentist) model over multistage, log-probit, and Weibull models; and a Bayes’ average model, included in the BMDS. OPPT then chose the most sensitive POD and associated risk estimate. In the evaluation of 1,4-Dioxane, however, OPPT used a different approach to synthesize across multiple cancers by looking at cancer risk at any sites in addition to cancer at an individual site. This approach was implemented in the statistical model MS-Combo in BMDS, which assumes that cancer risk at one site is independent of another. This composite cancer risk approach has the statistical property of being more sensitive than estimating the risk of any individual cancer. As a result, the risk estimate from the MS-Combo becomes the default outcome if the objective is to determine the most conservative risk estimate. There is a lack of consistency in current TSCA practice with respect to cancer dose-response modeling of 1,4-Dioxane and TCE. The model-average or MS-Combo approach is not yet state of the practice in risk
assessment. The systematic review protocol in these evaluations did not include choice and justification of adopting these approaches.
Risk assessment in general, and evidence synthesis and integration in particular, can could benefit from emerging methodologies such as key characteristic approaches andt the the framework of Adverse Outcome Pathways (AOP). The key characteristics approaches are designed to facilitate the organization and utilization of mechanistic information in hazard identification (Arzuga et al. 2019; La Merrill et al. 2020; Smith et al. 2016). An AOP pieces together key chemical exposure induced biological events, from molecular and cellular level activities to tissues and organ level effects and to population health along plausible pathways to inform relevance and causality of adverse outcomes (Villeneuve et al. 2014a,b). As reviewed in the 2017 National Academies report Using 21st Century Science to Improve Risk-Related Evaluations (NASEM 2017), the AOP framework is still evolving and has not become the state-of-practice for risk-based evaluation. The committee encourages OPPT to track these and other evolving methodologies.
Critique of the TSCA Approach
OPPT failed to clearly identify evidence streams, especially sub-streams, for which it determined to follow a systematic review approach for evaluation. Looking at the core review elements of the Statement of Task, which address whether the TSCA approach to systematic review is “comprehensive, workable, objective, and transparent,” the committee finds that the TSCA approach to evidence synthesis could be improved with regard to each of these four desired characteristics.
Comprehensive
The OPPT approach for evidence synthesis is not comprehensive, as it does not contain elements that are important to addressing the research question. OPPT failed in the TCE draft evaluation to include all eligible datasets, after a critical appraisal, to describe the variability and uncertainty when data were adequate and relevant. OPPT could have addressed the level of confidence in the body of evidence more comprehensively and more quantitatively if taking a more probabilistic and quantitative approach. The lack of definition of evidence streams that require a data synthesis also makes a judgment of comprehensiveness difficult.
Workable
Without a clear definition of evidence streams or documented approaches to evidence synthesis within each evidence stream, it is difficult to assess and reproduce the results of evidence synthesis. Concurrent implementation of evidence synthesis and evidence integration further makes evidence synthesis approaches of TSCA risk evaluations less workable.
Objective
The committee did not find a consistent pattern in documenting the objectives or methods of choice for synthesis in the draft risk evaluation of TCE or the support documents. Characterizing variability and uncertainty within the body of evidence is an essential objective of data synthesis. In situations in which data appeared adequate to support a more quantitative discussion of uncertainty and variability analysis, OPPT offered only a qualitative discussion of the sources of uncertainty. Examples include surface water concentration estimates and human hazards of non-cancer endpoints. When adequate and appropriate, a quantitative (e.g., a probabilistic distribution such as SSD) approach for evidence synthesis, especially in view of variability and uncertainty, is preferred. This approach also applies to the syn-
thesis of PODs after dose-response assessment using the benchmark dose approach in contrast to the current approach that chooses either the most sensitive risk value, or the best fitted statistical model, or average. This preference holds whether or not OPPT determines to adopt a systematic review approach for an evidence stream.
Transparent
The absence of a well-documented protocol reduces transparency and consistency of evidence synthesis. Lack of documentation and justification for the use of average modeling or composite risk modeling for cancer dose-response assessment in the evaluation of 1,4-Dioxane and TCE raises concern about consistency in TSCA risk evaluations. While the committee recognizes the value of exploring new methodologies in this case, careful examination and full documentation of the underlying assumptions and requirements of these methods are necessary to ensure transparency and consistency. The MS-Combo, for example, assumes that cancers at different sites are independent, an assumption that needs justification (EPA 2012b). Violation of this assumption likely leads to a downward bias in risk estimation. For the model-averaging approach, including every model offered in the BMDS as OPPT did in the 1-BP evaluation for the Bayes average cancer model requires strong justification. OPPT did not document inclusion criteria or provide justification regarding selection of the underlying model components. Evaluation of evidence synthesis was also complicated and made less transparent and consistent by the fact that OPPT carried out evidence synthesis (within an evidence stream) and evidence integration (across multiple evidence streams) concurrently. Public comments concurred with the committee’s observation of a lack of transparency, as this step was not noted within the TSCA risk evaluations or the 2018 guidance document.
Recommendations
OPPT’s adaptation of systematic review to support its chemical risk evaluation creates both challenges and flexibilities. In order to improve these issues with TSCA’s process for evidence synthesis, the committee recommends the following:
- Document the synthesis methods and data requirements in the study protocol for each evidence stream. This should be done for every evidence stream whether or not a systematic review approach will be taken.
- Separate evidence synthesis from evidence integration. Evidence synthesis deals with more homogeneous data within a single stream, and evidence integration deals with more heterogeneous data from multiple streams.
- Develop guidance for evidence synthesis, considering objectives, data requirements, strengths, and limitations. Seek stakeholders’ input, incorporate feedback, document changes, and amend the 2018 guidance document. Refer to this amended guidance document in the protocol(s).
- Strive for more quantitative analysis of uncertainty and variability by using a probabilistic distribution approach (e.g., SSDs) or at a minimum describing distributional properties as recommended by NRC (2010).
- Strengthen analysis of uncertainty and variability by focusing on the propagation and aggregation of uncertainty and variability from upstream to downstream of the risk assessment process. For example, it is important to understand how uncertainty and variability in environmental release of TCE affect the uncertainty and variability of model-predicted concentrations in surface water and how estimated concentrations affect environmental or human hazards.
- Develop a clear approach for extracting data from occupational exposure studies and clearly document values extracted from each study. Then, clearly document how values from various
- studies are synthesized into a single estimate of the central tendency and high-end exposure estimates.
- Dose-response assessment is amenable to systematic reviews of hazard assessment. The process of dose-response assessment should be planned, justified, and prespecified in the systematic review protocol, including eligibility criteria for study and endpoint selection (e.g., number of dose levels, group size), model selection (e.g., biological plausibility, statistical appropriateness), and methods for synthesis (e.g., probabilistic distribution of PODs and the most conservative risk estimate). Upon completion of the dose-response assessment and associated evidence synthesis, results should be reported in accordance to the criteria specified in the protocol.
EVIDENCE INTEGRATION
State of the Practice
Evidence integration is typically considered outside of the systematic review process itself (see Figure 2-1) and, in the context of risk evaluations, only employed when an evaluation reviews different evidence streams that have to be reconciled and, as the name suggests, integrated. The outcome of the integration step is an overall conclusion that is based on the holistic consideration of the various evidence streams. Risk evaluations that only contain data from a single stream of evidence (e.g., a systematic review on hypospadias and dibutyl phthalate in rats) would not have other evidence streams to integrate and could therefore be finalized after the evidence synthesis or move straight into hazard and exposure integration (see Figure 2-1). For evaluations with multiple streams of evidence (i.e., human and animal data), the highest level of evidence conclusions for a specific health outcome are integrated to inform a hazard assessment (OHAT 2019). This applies whether or not the data support the health effect conclusion or provide no evidence of effect. A qualitative descriptor may be applied to each stream of evidence based on a specific health effect or group of effects (e.g., whether there is sufficient evidence or suggestive evidence of an effect of anti-androgenic toxicity due to dibutyl phthalate exposure in rats), and then an overall conclusion is drawn from consideration of the streams together (i.e., the integration). As demonstrated in Figure 2-1, there is a process for integrating across evidence streams to reach the exposure assessment (see pink boxes) and a process for the hazard assessment; these two parts are then also integrated to inform the risk characterization.
Expert judgment is typically used to assign descriptor categories that describe a final conclusion, although there are various guidelines for bringing evidence together from the different streams (e.g., GRADE, adapted for animal studies based on the historical Bradford-Hill considerations (Hill 1965; Hooijmans et al. 2018; OHAT 2019; Woodruff and Sutton 2014). Although these examples are more in line for hazard identification, similar principles might also apply when integrating other types of evidence, such as exposure data (NRC 2014). In the end, the evidence integration step is generally tasked with answering the question of causation (NRC 2014). This should be done for each health effect that the data support through a narrative, qualitative assessment of all evidence streams (NRC 2014).
Committee Description of the Approach in TSCA Risk Evaluations
According to Table 3-1 in the 2018 guidance document, the evidence integration step has three phases. The first phase (planning) involves the development of a strategy for analyzing and summarizing data across studies within each evidence stream (discussed previously by the committee as synthesis) and a strategy for weighing and integrating evidence across those streams. Phase two (execution) involves the implementation of the strategies developed in the planning phase as well as the development of WOE conclusions. The table notes that documented professional judgments may be invoked in some
of these analyses. The third phase involves a check on the quality of the data used and discussion of uncertainties.
During phase two, OPPT conducts a WOE evaluation, as the term is generally used, because it is here that EPA makes judgment-based decisions to infer causation. The results of the evidence integration are applied to support the risk evaluation that is the basis for decision making. The Risk Evaluation Rule requires TSCA risk decisions to be based on such WOE evaluations (EPA 2018a, pp. 26–27 and Table 3-1). However, the committee notes some confusion around the terminology used to describe the steps, as detailed in Box 2-4.
In the 2018 guidance document, the descriptions of evidence integration lack details and specificity. As noted previously, the committee examined TSCA risk evaluations for 1-BP and TCE to develop opinions on the clarity and appropriateness of EPA’s approach, and OPPT provided the committee with several presentations and posters to elaborate on the processes. In those presentations, OPPT presented the evidence integration step as comprised of an evaluation of individual evidence streams by summarizing the strength of the evidence for both hazard and exposure information separately (see Figure 2-6a) and examining the coherence across the bodies of evidence by making inference across evidence streams. These lead to the evidence integration summary (see Figure 2-6b). While a specific process was not described, the presentation did provide some considerations that went into the evaluation and inference-making and pointed to some examples where OPPT had conducted evidence integration for the committee to further consider.
While the WOE/evidence integration details are scant for most areas of the 1-BP evaluation, the TCE evaluation document did provide some methodological details on the WOE evaluation for a single hazard endpoint—congenital heart defects. This evaluation presents three levels of WOE determinations “made in succession, first for subsets of a line of evidence, then for the full lines of evidence, and then for the overall database, each building on the assessments that came before” (EPA 2020a, p. 612). The process detailed in Appendix G-2 of the TCE evaluation is an adapted version of the EPA Risk Assessment Forum 2016 Weight of Evidence in Ecological Assessment document (EPA 2016) that utilizes symbols (ranging from ---, --, -, 0, +, ++, +++ depending on the specific area [i.e., reliability, outcome/strength, and relevance] for the purposes of scoring at each level of the assessment [i.e., individual studies, across studies within one stream/line of evidence, and across lines of evidence]). Using these metrics and steps, the TCE evaluation concluded that the epidemiological studies provided “suggestive evidence” (+). Various animal studies were judged to be “weakly positive” (0/+) and “positive” (for TCE metabolites) (+), and the inhalation studies were judged to be “negative evidence” (-). The mechanistic studies were described as providing “strong and consistent supporting information” (++). When combined to a summary score of (+), these scores are said to represent “positive overall evidence that TCE may produce cardiac defects in humans” (EPA 2020a, p. 621). A mathematical average was used to integrate evidence areas for all studies that appears to be a translation of the symbols into numbers with the examples equating ++ to a value of 2 and 0/- scores to a value of -0.5; however, how this mathematical average is used is unclear.
With regard to exposures, TSCA risk evaluations include calculations of occupational exposures, exposures from the use of consumer products, ecological exposures, and exposures to the general population as part of the systematic review process using either measured data or through models (with models requiring chemical properties, release data both to the environment and within the context of specific occupational activities or consumer uses, and exposure factors). Calculating these exposures is clearly a required step in making a risk determination yet is not part of a traditional systematic review. One could argue that in a systematic review, the next step is to integrate data from multiple sources and determine the most likely exposure value. These resulting exposures are then combined with the hazard numbers to make a risk determination (see purple box in Figure 2-1). For an exposure assessment, this activity would most logically combine measured distributions to develop an overall distribution of exposure and compare that to estimates from multiple available models, considering sources of uncertainty and variability. However, the two data streams were not integrated in the assessments reviewed by the committee. Additionally, there was no integration of occupational exposure through inhalation and dermal exposure. All exposures were separately evaluated in the risk assessment.
Critique of the TSCA Approach
The committee has not found the 2018 TSCA systematic review document, the several presentations made to the committee by OPPT, or the 1-BP and TCE evaluations sufficiently detailed to provide the information needed to assess the methodology and appropriateness of the framework for evidence integration. Therefore, when considering the core review elements of the Statement of Task, “comprehensive, workable, objective, and transparent,” the committee finds that the evidence integration step is lacking in all respects. Where possible, the committee elaborates on processes that specifically impact each of the core review elements.
Comprehensive
Given that the WOE determination or evidence evaluation for heart defects in the TCE evaluation was the only detailed example provided, it is uncertain as to whether this process represents a limited use for a specific endpoint for TCE or represents a method that will be used (in its current form) for future risk assessments. The committee finds that OPPT has conflated aspects of three important systematic review elements: evaluating individual studies, a body of evidence (i.e., strength or certainty of evidence for a conclusion), and a level of confidence in a recommendation or determination of causation. Furthermore, the condensation of synthesis, evidence integration, and integration of hazard and exposure into one step makes it difficult to assess the extent to which the procedures used are comprehensive.
Workable
Without examples of how well the WOE process described in the TCE evaluation could be applied for other endpoints or evidence streams it is difficult to judge if it is sufficiently robust to be applied broadly and provide the desired outcome.
Objective
The lack of a true protocol, determined at the start of the evaluation, or a documented standard for evidence integration calls into question the objectivity of the process. There was also confusion relat-
ed to the apparent repetition of data quality evaluations at this stage of the process when evaluation of individual study quality and relevance should have been dealt with during the data evaluation stage. It is unclear if this sequencing impacts the objectivity of the process.
Transparent
The committee has the most concern with regard to the transparency of the evidence integration steps, much of which stems from confusion related to the terminology used in the various documents (and sometimes even the variations in use within one document), the lack of information presented to describe the process, and the lack of documentation to explain deviations from the little process that was documented. This confusion was also manifest within the public comments to TSCA assessments as the approach to integration of the evidence was not included in a protocol and also was not described in the 2018 guidance document.
With regard to the terminology, terms such as “strength of evidence,” “sufficient and insufficient evidence,” “confidence” in the evidence, and a number of other descriptors such as “summary scores” and “positive overall evidence” serve only to create confusion because some of these terms have not been well defined or are used differently throughout different assessment documents, within the presentations, or within the 2018 guidance document.
When comparing the work done in the 1-BP and the TCE evaluations, very little consistency can be observed between the evidence integration steps other than the lack of transparency as to what methods were used and how decisions were made. Very little information is provided about the process used to integrate the available data, and although qualitative statements are provided, the data do not appear to actually be integrated. The term “data integration” is mentioned in several of the other evidence streams as well, including exposure and environmental fate, but it is not clear as to how the data were selected or integrated into the assessment. Significant improvement in transparency and consistency is needed to fully understand EPA’s process of evidence integration within this assessment.
The lack of documented process and lack of documentation to explain deviations greatly impacts the transparency of the evidence integration. The committee notes here a number of examples in which a lack of explanation was problematic:
- The evaluation of heart defects in the TCE evaluation followed a new, “fit-for-purpose” modification of the EPA Risk Assessment Forum 2016 Weight of Evidence in Ecological Assessment document (EPA 2016). It is unclear from the documentation provided in the risk assessment docket, and from the presentations by the agency, why this deviation existed and why it was needed.
- The use of two different methods within the evidence integration step (i.e., true mathematical average versus semi-qualitative grouping) is troubling, particularly due to the lack of rationale provided for this divergence. This runs completely counter to the guidance provided by the risk assessment forum in the Weight of Evidence in Ecological Assessment document which states,
Symbols are preferable to numerical scores because their use implies that they cannot be numerically combined. Two strongly supporting laboratory tests (++ and ++) are not equal to four somewhat supporting field tests (+, +, +, +). For a test result, a − score for study design and a + score for replication of the test do not sum to 0, because they are not commensurable. They simply signify different results for the different qualitative properties. Adding numerical scores generates a number with no units that signifies no quantity in particular. (EPA 2016, p. 29)
- In reviewing the supplemental data table for congenital heart defects in the TCE evaluation, the method of data integration across subsets did not seem consistent with the true average method discussed in the risk assessment document. There were some instances (e.g., toxicological [in vivo animal]—inhalation and toxicological [in vivo animal]—other) in which the overall subset value was greater than the sum of the contributing factors. While there is no issue with upgrading overall quality, the reasons for these deviations from the stated protocol were unclear.
Recommendations
In order to improve these issues with TSCA’s process for evidence integration, the committee recommends the following:
- Although the integration of different evidence streams is not part of the systematic review process, it is desirable that this process is conducted in a structured, transparent, and prespecified way.
- Develop guidance for evidence integration of multiple streams. Currently, the guidance within the agency for integrating evidence from different streams is lacking and needs to be developed. The guidance should include frameworks for how to best consider different lines of evidence in many types of information that informs the evaluation, such as the PBPK models that integrate different streams of hazard evidence (i.e., human, animal, and mechanistic) and exposure models that integrate across sources and routes of exposure. The guidance should also include processes for documenting decisions and engaging stakeholders.
- As has been noted in the committee’s reviews of the completed TCE and 1-BP risk evaluations, there is significant lack of clarity in the language used to describe the integration process, for both hazard and exposure, and the lack of clarity is not limited to the evidence descriptors. It is difficult to understand why and how certain steps were taken, particularly regarding the inclusions and exclusions of certain data.
- Integration methods should be documented a priori and applied consistently for each assessment unless a strong argument for a deviation exists, and then a detailed justification should be included at each step.






































