INTRODUCTION
Throughout the 20th and 21st centuries, biomedical scientific advances have provided numerous powerful tools to diagnose, prevent, and manage sexually transmitted infections (STIs). Even before modern antimicrobials became available, diagnostic testing (microscopy, early culture methods) was possible, and more recently, it has evolved at an accelerating pace. Over the past 40 years, technical advances in molecular biology and chemistry have led to newer tests and additional antibiotics and provided tools that can be applied to vaccine development.
Translating these advances into widely available products is often impacted by a time-consuming preclinical and clinical regulatory process, which may take years and require millions of dollars. Corporate decision making regarding developing new biomedical tools for STI management also may be impacted by cost-related factors, such as the potential for STI-related stigma to hinder vaccine acceptance, relatively less costly single- rather than multiple-dose therapy for many STIs, and the desire for low-cost, public health pricing. These factors have affected development of new antibiotics and vaccines more than diagnostic tests.
As the modern age of antimicrobial therapy dawned in the mid-1930s, the widespread availability of sulfonamide antibiotics made one of two widely recognized STIs (gonorrhea) treatable, fueling increased emphasis on accurate diagnosis as well. By the 1940s, when the “wonder drug” penicillin became available, effective treatment of the second major STI of concern, syphilis, became a reality and catalyzed expanded public health efforts to control both diseases (Hook and Kirkcaldy, 2018). These efforts became a governmental priority in the 1950s and 1960s and contributed to the ascendance of the Venereal Disease Research Laboratory1 (VDRL), a predecessor, in part, to today’s Centers for Disease Control and Prevention (CDC) as a leading public health agency. As recognized STIs have increased since the 1960s, VDRL/CDC expanded monitoring of STI prevalence, surveillance, and control efforts has been extended in varying degrees. Preferences for STI therapy in the United States have been
___________________
1 The Venereal Disease Research Laboratory was established in the 1920s as part of the U.S. Public Health Service’s efforts to study the etiology, prevention, and treatment of diseases such as syphilis and gonorrhea, especially in the military (Parascandola, 2001). Venereal diseases were mainly thought to be syphilis, gonorrhea, lymphogranuloma venereum, and chancroid. Since the 1970s, the field’s awareness of additional sexually transmitted pathogens has expanded, as has its understanding that the term “venereal” is stigmatizing and disparaging (Handsfield, 2015). Language has evolved again, from sexually transmitted “disease” to “infection” (Handsfield, 2015; Rietmeijer, 2015). Compared to “disease,” “infection” is a more medically accurate and holistic and less stigmatizing term (Bolan, 2019; Handsfield, 2015; Rietmeijer, 2015). See Chapter 1 for more information on stigma and language.
guided by CDC’s Sexually Transmitted Diseases Treatment Guidelines2 (Workowski and Bolan, 2015), a highly influential document with global impact. The guidelines also help shape areas of STI research emphasis. CDC also provides recommendations for STI testing and preferred test methods.
In the 1980s, the field of STIs experienced major changes. First, the HIV/AIDS pandemic led to increased interest in STIs, discussed in more detail in Chapter 5. Rising STI incidence, coupled with the substantial reproductive health morbidity associated with chlamydia infections, added additional urgency to CDC’s ongoing STI control efforts. Currently, CDC highlights three curable STIs (gonorrhea, chlamydia, and syphilis) for high-priority non-HIV STI surveillance and control. Surveillance for other common STIs, including trichomoniasis, herpes simplex virus (HSV), human papillomavirus (HPV), and HIV, and for less common infections, such as chancroid, ectoparasite infections, enteric infections, and reproductive tract syndromes whose pathogenesis is not yet entirely clear (e.g., nongonococcal urethritis [NGU]) and bacterial vaginosis [BV]), are carried out variably and inconsistently using various surveillance methods and elements of the public health agencies, as described in Chapter 2.
Emerging and reemerging conditions are also more difficult to detect, given a lack of screening and reporting consistency for many STIs, such as occurred with the recent reemergence of lymphogranuloma venereum among gay, bisexual, and other men who have sex with men (MSM) in the United States. Similarly, emerging global public health threats are now appreciated to be sexually transmissible, such as meningococcal, Ebola, and Zika infections (CDC, 2019c, 2021; Ladhani et al., 2020), but the magnitude of infections due to sexual transmission remains unknown.
In parallel to the evolution of STI control efforts at CDC, in the late 1970s, STI research grew as an academic discipline. Academic STI research, often in partnership with local public health agencies, is conducted with support from the National Institutes of Health (NIH), CDC, the pharmaceutical industry, and nonprofit agencies and foundations. Over the past 50 years, investigators have expanded the list of pathogens transmitted frequently through sexual contact to more than 30 bacterial, viral, and protozoan pathogens (see Appendix A for a partial list). The increased pathogen numbers, their biological variability, their ability to infect nongenital mucosal sites, and frequent coinfection with multiple
___________________
2 The next iteration (expected in 2021) will be the “Sexually Transmitted Infections Treatment Guidelines.” This report uses that language when referring to the guidelines anticipated in 2021 and the guidelines in general but the original “Sexually Transmitted Diseases Treatment Guidelines” title for the guidelines published in 2015 or earlier.
pathogens have added complexity to the challenge of STI control over and above the “traditional” challenges related to control of highly stigmatized medical conditions.
Further adding to this complexity is that STIs regularly and disproportionately burden marginalized groups (e.g., members of the lesbian, gay, bisexual, transgender, and queer [LGBTQ+] community and Black, Latino/a, American Indian/Alaska Native, and Native Hawaiian and Other Pacific Islander individuals) whose risk for infection is significantly impacted by access to health care and other social determinants of health (see Chapters 2, 3, and 9 for more information). Biomedical tools also do not exist in a vacuum—simply creating them will not ensure their uptake or proper use, so it is critical to think of them in the larger context—that is, successful STI prevention strategies require effectively integrating evidence-based biomedical, behavioral, and structural interventions across the life span (see Figure 1-3 in Chapter 1).
Since the 1980s, the “toolbox” of biomedical interventions for STI control has broadened beyond diagnosis, therapy, and barrier methods with the introduction of hepatitis B virus (HBV) vaccine, the first vaccine ever developed for an STI. In 2006, a second STI vaccine became available, first targeting HPV types 6, 11, 16, and 18, the causative agent of most cervical, anal, and oral mucosal cancers. An available nonavalent vaccine now expands coverage to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Similarly, in the 1990s, there was a diagnostic revolution as better, nonculture nucleic acid amplification tests (NAATs) became available, improving both test performance and ease of specimen collection (see below section on diagnostics). Box 7-1 describes advanced molecular technologies that have been applied to the STI field and are a promising area of research for future STI prevention and control.
Some biomedical tools for STIs, such as condoms, have generalizable benefits, including reducing risk for unplanned pregnancy, while others, such as vaccines, target specific pathogens. Other contraception methods also may modify STI risk, including intrauterine devices (IUDs), long-acting injectable hormonal contraceptives, oral contraceptives, and spermicides (Deese et al., 2018; McCarthy et al., 2019; Wilkinson et al., 2002). Furthermore, the potential role of antimicrobial agents has been expanded, and these now may be used for both treatment and prevention in exposed or at-risk persons. STIs such as gonorrhea have become increasingly resistant to standard antibiotic treatment; awareness of changing susceptibility and epidemiological surveillance are key to current control elements. All such biomedical tools need to be used in combination with positive sexual health messaging and behavioral and structural interventions (see Chapters 8, 9, and 12). This chapter is not intended to take the place of comprehensive texts or the vast literature on the topic but instead briefly summarizes the strengths, vulnerabilities, and potential opportunities
of biomedical tools to prevent, diagnose, and manage STIs to enhance sexual health.
TOOLS FOR STI DIAGNOSIS
Introduction
The large number of STIs, which vary in prevalence and associated morbidity, typically leads to the need to prioritize control efforts. Tests for STI pathogens are used in several different ways. Screening is routine testing as part of recommended health care in the absence of specific signs or symptoms; it is recommended for selected groups in whom asymptomatic STIs are relatively common (such as women under age 25 for chlamydia) or in whom infections may have particularly untoward effect (such as pregnant people and syphilis). As opposed to screening, “diagnostic” is the term characteristically used for testing patients who have symptoms to clarify the cause. Acceptable screening methods, such as self-collected specimens, and more highly reliable confirmatory diagnostic tests facilitate patient care and surveillance to more accurately delineate the prevalence, incidence, and morbidity associated with STIs.
Excellent diagnostic tests that offer the ability to help describe current STI epidemiology in the United States are the basis for reporting currently prioritized non-HIV STIs (gonorrhea, chlamydial infections, and syphilis) to CDC and are used to identify infections and guide treatment. Since 1997, highly reliable NAATs, which biochemically amplify microbial DNA for pathogens such as chlamydia, gonorrhea, trichomonas, Mycoplasma genitalium, HPV, and herpes simplex virus type 2 (HSV-2), have become widely available and, in general, become the preferred tests for STI detection in most settings. These tests provide improved accuracy (sensitivity/specificity), ease of specimen collection (noninvasive and self-collected), and easy, room-temperature transport requirements.
In contrast to other prioritized pathogens, the Food and Drug Administration (FDA) has not cleared a commercially available NAAT for syphilis, and its diagnosis has changed little in the past 80–100 years. The causative agent, Treponema pallidum, is cultured only in research laboratories. Serologic tests are most often used for syphilis screening and diagnosis. Specific screening and diagnostic recommendations for other STIs, such as trichomoniasis or Mycoplasma genitalium, are not prioritized as highly as surveillance and screening for HIV, gonorrhea, chlamydia, and syphilis. These diagnostic tools are all described below.
Approaches to STI Screening and Diagnosis
A variety of testing approaches exists for patient management; they reflect, in large part, the acuity of the problem. For persons seeking care for possible STI symptoms, rapid diagnosis and therapy initiation are important. As virtually all STIs may be asymptomatic or have symptoms that could be due to other causes, screening for asymptomatic infection also is a critical element for effective STI control. Thus, many settings have complementary roles for rapid diagnosis of symptomatic STIs and testing to screen for unsuspected STIs or clarify the causes of symptoms that may be due to STIs.
Syndromic STI Diagnosis
Syndromic management is often used to address genitourinary3 symptoms potentially due to STIs. It may be carried out with little or no laboratory support (e.g., Gram stain, saline, or KOH4 slide preparation and microscopy). By assessing patient symptoms on clinical evaluation, treatment may be initiated based on a World Health Organization or other algorithm (WHO, 2007). The advantages are that evaluations can be carried out rapidly and only modest resources are required to quickly alleviate symptoms, cure possible infections, and prevent spread.
Syndromic approaches, however, fail to detect asymptomatic STIs and require that symptoms are bothersome enough to drive someone to seek care. In addition, multiple STI pathogens may underlie the most common STI syndromes (genital ulceration, vaginal discharge in women, and urethral discharge in men). Even in carefully conducted research studies, a substantial minority (20–40 percent) of symptomatic patients do not have a demonstrable pathogen (Hylton-Kong et al., 2004). Despite these limitations, syndromic diagnosis is widely practiced in a number of settings (such as in emergency departments, urgent care clinics, and primary care settings). When resources and screening tests are unavailable, as with restricted health care access during the COVID-19 pandemic, syndromic management may be better than nothing.
Use of Tests for Screening and Diagnostic Testing for Chlamydia and Gonorrhea
NAATs are now preferred for gonorrhea and chlamydia screening, clarifying diagnosis in symptomatic persons, and guiding treatment to
___________________
3 Relating to the genital and urinary organs.
4 Potassium hydroxide.
prevent complications and transmission of those infections (Papp et al., 2014). Numerous commercially available assays are now FDA cleared for chlamydia and gonorrhea diagnosis. Many of these tests also may be performed using specimens collected by patients themselves. Recently, following recognition of the relatively high prevalence of rectal and oropharyngeal infections, FDA approved NAAT assays to detect chlamydia and gonorrhea in rectal and oropharyngeal samples (FDA, 2019). However, self-collection of rectal or oropharyngeal specimens for testing and sending of specimens to laboratories through the mail are not approved by FDA.
NAATs have completely replaced older types of diagnostic tests, such as cultures and enzyme immunoassays, except for specialized surveillance programs that use cultures to determine gonococcal antimicrobial susceptibility, such as CDC’s Gonococcal Isolate Surveillance Program (Kirkcaldy et al., 2016). Many cost-effectiveness studies and modeling studies have been published attesting to NAATs’ effectiveness in men and women for various types of screening programs and demonstrating them as an important and effective tool to prevent sequelae of STIs (Ronn et al., 2019). Some research and commercial NAAT assays are in development that simultaneously detect gonorrhea and validate its susceptibility to ciprofloxacin; in the context of progressive antimicrobial resistance, this would allow tailoring treatment options toward specific antibiotics (Allan-Blitz et al., 2017; Melendez et al., 2019).
Much laboratory testing is currently regulated under the Clinical Laboratory Improvement Amendments (CLIA); see Box 7-2 below for more information. Currently, most testing for STI pathogens is performed in licensed laboratories. Following careful evaluation, CLIA regulations for testing may be waived, allowing it to be performed in non-laboratory health care settings or even at home. Examples now include point-of-care (POC) tests for influenza and tests for noninfectious variables, such as blood glucose levels or pregnancy. Until the achievement of FDA-cleared, sensitive, and specific POC NAATs that achieve CLIA waiver, these often need to be performed in a laboratory as moderately or highly complex tests. For tests under development, a CLIA waiver would allow the test to be performed and interpreted outside of a laboratory by a “non-laboratorian,” such as a nurse or health care worker, thereby reducing the time to results and treatment and helping to reduce transmission.
Point-of-Care Tests
POC tests are diagnostic tests that are completed at or near the time and place of patient care. Multiple new, accurate POC or near-patient rapid tests for chlamydia and gonorrhea with performance characteristics
similar to those of laboratory-based NAATs (Gaydos and Melendez, 2020) are currently available or in late stages of development. For example, a 30-minute test to simultaneously detect chlamydia and gonorrhea recently received FDA clearance and is available for use (Van Der Pol et al., 2020). See Table 7-1 for more information on this test and others.
There are many advantages to POC tests. They allow for treating patients at the time of testing, thus shortening infection duration, preventing transmission, and simplifying follow-up testing to confirm a cure (Ronn et al., 2019). These tests also may lower the risk of complications and improve the patient experience (Tucker et al., 2013). For example, POC tests have already changed the course of the HIV epidemic because the immediate results allow providers to implement rapid antiretroviral therapy or pre-exposure prophylaxis (PrEP). Furthermore, it has been possible to nearly eliminate HIV transmission to newborns partially due to the availability of HIV-POC during pregnancy, labor, and delivery (Vrazo et al., 2018). POC allows for partner testing and couple testing in some settings (Boeras et al., 2011). The increasing availability of POC tests also can permit screening via the Internet or pharmacy recruitment (Gaydos et al., 2020) or even over-the-counter tests performed at home (see Box 7-3 for more information). Self-administered sample collection to screening for chlamydia and gonorrhea through at-home or at other non-clinic-based settings is particularly relevant for some American Indian/Alaska Native and rural communities, based on health care access and service use challenges (see Chapter 3 for more information). Modeling evidence shows that POC tests could reduce the prevalence of STI epidemics in the United States (Gaydos and Melendez, 2020; Ronn et al., 2019).
Implementing POC tests also faces barriers. For example, the time that patients are willing to wait is an important consideration for immediate treatment, although many POC tests for STIs produce results in 30 minutes or less (Gettinger et al., 2020; Widdice et al., 2018) (see Table 7-1). Additionally, single-use, individual POC test cartridges are substantially more costly than laboratory-based, large robotic platform assays (Drain et al., 2019). Often, test complexity and logistical issues can prevent the adoption of POC tests for routine use in or outside a clinic. These issues include the financial resources necessary for instruments and consumables, the need to obtain a CLIA certificate (if the assay is CLIA waived), validation of the new test, policies and staff training procedures, operator training, operator recertification and proficiency, getting results into the electronic medical records interface, space, clinic work flow disruption, and billing and reimbursement challenges (Gaydos and Melendez, 2020).
Acceptability and decision making by all stakeholders in a clinical situation will be important for POC tests for STIs to be widely adopted (Garfield et al., 2016). A strong movement has recently begun toward
TABLE 7-1 Point-of-Care Tests Available and in Development for STIs
| Assay | Io CT/NG | GeneXpert CT/NG | Medical Sexual Health Test | ResistancePlus GC | Syphilis Antibody Rapid Test | DPP POC Test for HIV and Syphilis Serology | OSOM Rapid POC TV Antigen Test |
|---|---|---|---|---|---|---|---|
| Company | Binx Health | Cepheid | Visby | SpeeDx | Syphilis Health Check | Chembio | Sekisui |
| Platform | Table Top Integrated | Table Top Integrated | None required Integrated | Table Top PCR machine | Lateral flow immunochromagraphic | Lateral flow | Immunochromagraphic |
| Technology | NAAT Small molecule chemistry | Real-time PCR | Real-time PCR | PlexPCR | Antigen-antibody serology | Antigen-antibody serology | Trichomonas vaginalis membrane proteins Mouse antibodies |
| Sample Type | Self- and clinician-collected vaginal swabs; male urine | Swabs (cervical, self-collected vaginal); male and female urine | Self- and clinician-collected vaginal swabs | Swabs (cervical, vaginal, pharyngeal, and ocular); male and female urine | Blood, plasma, serum | Blood, plasma, serum | Vaginal swabs |
| Procedure | ~4 steps | ~4 steps | ~2 steps | ~4 steps | ~2 steps | ~3 steps | ~3 steps |
| Assay | Io CT/NG | GeneXpert CT/NG | Medical Sexual Health Test | ResistancePlus GC | Syphilis Antibody Rapid Test | DPP POC Test for HIV and Syphilis Serology | OSOM Rapid POC TV Antigen Test |
|---|---|---|---|---|---|---|---|
| Result Time | 30 min. | 90 min. | 20 min. | 50 min. | 10 min. | 15 min. | 10 min. |
| Regulatory | FDA, CE-IVD | FDA, CE-IVD | FDA pending | CE-IVD FDA pending | FDA, CLIA waived | FDA approved | FDA, CLIA waived |
NOTE: CE-IVD = European conformity investigational device; CLIA = Clinical Laboratory Improvement Amendments; CT = Chlamydia trachomatis; DPP = Dual Path Platform; FDA = Food and Drug Administration; NAAT = nucleic acid amplification test; NG = Neisseria gonorrhoeae; PCR = polymerase chain reaction; POC = point of care; TV = Trichomonas vaginalis.
SOURCE: Table adapted from Gaydos and Melendez, 2020.
broad stakeholder input, value propositions, and comprehensive evaluation of devices beyond clinical performance and cost (Korte et al., 2020). Given the growth of STI rates in the United States, especially among underserved and/or marginalized populations, reliable and affordable POC testing could be a higher programmatic priority of the federal government, in both expanding its availability substantially and educating health care providers about the indications for screening or diagnosis. The STI National Strategic Plan: 2021–2025 highlights POC tests as innovative
tools for preventing and controlling STIs (HHS, 2020). The committee concurs with the plan’s assessment that the development and implementation of accessible, effective, and affordable POC tests promise to enhance rapid STI diagnosis and treatment (see Chapter 12 for more information).
Syphilis Diagnosis
Syphilis is primarily diagnosed by serological methods initially developed more than a century ago. The current algorithm requires positive results from two tests, performed sequentially: treponemal and nontreponemal tests. Treponemal tests are qualitative and detect antibodies to synthetic treponemal proteins; if positive, the person usually remains positive for life, despite treatment. Nontreponemal tests, such as the rapid plasma reagin (RPR) and VDRL tests, detect nonspecific antibodies to cardiolipinlecithin-cholesterol antigens. When the nontreponemal test is positive, it requires performing a titer, which can be used to confirm treatment success if follow-up titers decrease over time. The nontreponemal tests are important to confirm and manage positive treponemal tests, but they are labor intensive, subjective, and affected by environmental conditions (Hamill et al., 2018). Both types of test may have false-positive results, may not be positive in persons at the earliest stages of infection, and cannot readily distinguish treated from untreated infections.
Better assays for syphilis diagnosis are urgently needed. NAATs are only available in research settings, for genital ulcer specimens, and have been multiplexed to differentiate syphilis from HSV, chancroid, and lymphogranuloma venereum chlamydia. Their further development and deployment would be a concrete step toward reducing syphilis in the United States by making diagnosis more reliable and accessible (Theel et al., 2020).
Automated and even new POC tests have been developed for the treponemal antibody, which can be performed outside of the laboratory with CLIA waiver using finger-stick blood. POC syphilis serological tests appear to be performing well in patients with RPR-positive (more likely to be active) syphilis and show promise for the United States in outreach and antenatal care settings (Obafemi et al., 2019), where they are sorely needed to help reduce congenital syphilis rates (Rogozińska et al., 2017; Tinajeros et al., 2006). Dried blood spots also can be collected at home and mailed in to a laboratory for treponemal syphilis testing, if laboratory validation studies are performed. No test, however, is currently able to replace the RPR assay. Developing a highly sensitive and specific serological syphilis test that reflects disease activity but avoids the pitfalls of the current nontreponemal tests would be of great benefit to the field.
Diagnostics for Other STIs of Public Health Importance
Trichomoniasis, HPV, HSV, and Mycoplasma genitalium are among the additional STIs of concern. Each infection, in addition to vaginitis/vaginosis syndromes, can now be diagnosed using NAAT assays and even POC tests (Gaydos et al., 2017a,b; Schwebke et al., 2019). Combination vaginal discharge/vaginitis assays include diagnostic targets for BV, trichomonas, and yeast. Older, nonmolecular assays/algorithms for BV, such as the Amsel and Nugent methods, are widely used but have been demonstrated to be highly subjective and less sensitive and less specific than molecular amplification NAAT assays that actually detect and quantify the presence of contributing organisms (Marrazzo et al., 2010).
As explained in Chapter 2, however, none of these infections are currently reportable to CDC. Several are highly prevalent, but no good surveillance data or recommendations for screening of asymptomatic persons are available. HPV infection has been previously inferred with an abnormal Pap smear or anal scrape/swab to find squamous intraepithelial lesions, but this is now augmented by HPV molecular screening. Additionally, antibody tests exist for or HSV-1 and -2 diagnosis, but they have substantial problems with the timeliness of the serological response to infection, sensitivity, and specificity; routine screening for HSV is not currently recommended. Mycoplasma genitalium has garnered much interest recently. Few antibiotics are available to treat it, and increasing resistance to azithromycin is a concern (CDC, 2019a; van der Schalk et al., 2020). Some NAAT assays in development include assays to evaluate antimicrobial susceptibility to azithromycin; this would allow precision treatment decisions regarding specific antibiotics (Gaydos and Melendez, 2020; Gaydos et al., 2019). More data are needed regarding the importance of M. genitalium as a major STI causing public health problems and its appropriate management (see Chapter 2).
Multiplex Diagnostic Opportunities
The large number of STI pathogens and their potential to cause coinfections remain challenges to clinicians and those prioritizing which pathogens are most pressing. A multiplex assay tests for multiple organisms at one time. As explained above, current commercial NAATs detect both chlamydia and gonorrhea, which came about as a market response to the recommendation of major professional organizations to screen for both of these curable infections. A multiplex assay for the differential diagnosis of genital ulcer disease outside of the research setting also would be useful, especially to differentiate syphilitic from herpetic lesions. As tests improve, it may be increasingly possible to detect additional STI organisms in one assay. Less common STIs might not be amenable to screening
if insurance will not pay (see Chapters 4 and 10 for more information on insurance coverage). To multiplex or not requires careful thought from developers and clinicians, as well as patients, who may not need or desire screening for more than chlamydia and gonorrhea. As screening requirements for new or different STIs change over time, considerations for optimal strategies for development of multiplex tests for multiple STIs, notably syphilis, HIV, and hepatitis B, need to be considered.
Gaps and Opportunities in STI Diagnosis
In summary, NAATs are now commercially available for diagnostic and screening tests for most STIs, except syphilis. Multiple organizations have recommended expanded screening with NAATs, including CDC (Workowski and Bolan, 2015), the United States Preventive Services Task Force (Bibbins-Domingo et al., 2016; LeFevre, 2014; USPSTF, 2016), Infectious Diseases Society of America’s HIV Medical Association (Aberg et al., 2014), and others (Committee on Adolescence and Society for Adolescent Health and Medicine, 2014). Barriers to adopting highly accurate POC rapid tests are that too few clinics, practitioners, or insurance companies promulgate them because of perceived financial and logistical barriers (Gaydos and Melendez, 2020). These barriers can be overcome, however, and these tests show promise as STI control strategies as they become more widely available (Gaydos and Melendez, 2020). For highly sensitive and specific NAAT assays and new POC tests to have an impact on the STI epidemic, they must be prioritized for development (Eisinger et al., 2020) and used far more widely. Therefore, several questions that call for expanded research remain, including
- Can test usage be increased substantially, targeting highest zip code venues and selected subpopulations at highest risk?
- Can health care providers be engaged to include STI screening as a fundamental part of their clinical practice?
- Can multiplex testing be optimized so that multiple infections are detected in one assay?
- Can implementation studies help expand the development and clinical use of POC tests?
- Can self-testing kits that could be used for home testing or via online distribution systems be established?
- Can a better assay for syphilis diagnosis be developed?
- How can these diagnostics be used most judiciously in confronting progressive antimicrobial resistance?
ANTIMICROBIAL TOOLS FOR STI TREATMENT
Desired characteristics for antimicrobial therapies for STI management include high degrees of efficacy, safety, and tolerability; ease of administration; and widespread availability at a low cost (Unemo et al., 2017). When possible, single-dose, oral therapy is preferred in order to ensure adherence and enhance therapeutic efficacy. In addition, unlike other infections commonly encountered in clinical practice in which bacteria are isolated and tested for antimicrobial susceptibility to guide therapeutic decision making, current practice assumes that recommended STI therapy will be highly effective when used without such testing.
Barriers to Therapeutic Innovation
Unfortunately, while most STIs are treatable, no individual antimicrobial agents are effective for more than a few STIs, and the “pipeline” for new drug development has slowed. Overall, only 12 companies won FDA approval for new antibiotics over the past decade; 2 of those have gone out of business. Other pharmaceutical companies have filed for bankruptcy in 2019 and 2020 or are being sold for a fraction of their previous market value (Perros, 2019; Solman, 2020). This trend is partly related to the commercial disadvantages of short-course medications for public health use compared to the market advantages of drugs that patients may take for years (e.g., antihypertensives, lipid-lowering medications) or have the appeal of “lifestyle” enhancements (i.e., erectile dysfunction treatments, antidepressants) (Solman, 2020). The pharmaceutical industry has generally disengaged from antibiotic development because of the poor return on investment and challenges in funding for-profit research of antibiotics (Solman, 2020). The antibiotic pipeline has contracted at a time when development of antimicrobial resistance by Neisseria gonorrhoeae, one of the most common multiple drug-resistant pathogens, is threatening the continued reliability of therapy for this prioritized and widespread infection. Development of new antimicrobials for other STIs has also slowed and is limited; Table 7-2 lists examples of current clinical trials for STI antibiotics.
Decisions related to STI treatment are profoundly impacted by treatment guidelines issued by public health agencies. These guidelines vary from nation to nation. In the United States, the CDC STI Treatment Guidelines are among the organization’s most widely accessed publications and the primary source of guidance for STI treatment (Barrow et al., 2020). Clinicians use these guidelines and expect that once an infection has been diagnosed, the recommended treatment will be more than 95 percent effective. The CDC STI Treatment Guidelines are updated at approximately 5-year intervals through a relatively complex process of literature review and expert consultation to generate treatment recommendations
that clinicians can be confident of. The Guidelines published in 2015 are under revision with an anticipated publication date of 2021; treatment recommendations are also updated occasionally in Morbidity and Mortality Weekly Report publications. Each revision contains numerous changes, reflecting in part the accrual of information in the interval since the last revision. Given the rapidly changing field, however, more frequent reviews and revisions are highly desirable. Given the state of technology, particularly the possibility of rapid online publication, disseminating updated guidelines could be a continuous rather than an episodic process.
Antimicrobial Therapy for Bacterial STIs
In the United States, STI control surveillance and public health management strategies have focused on the three most common and widely recognized STIs (gonorrhea, chlamydia, and syphilis) and their complications. Other STIs and related syndromes (e.g., trichomoniasis and STI syndromes, such as NGU, BV, genital ulcer disease, and pelvic inflammatory disease [PID]) are common and present different therapeutic challenges.
Gonorrhea
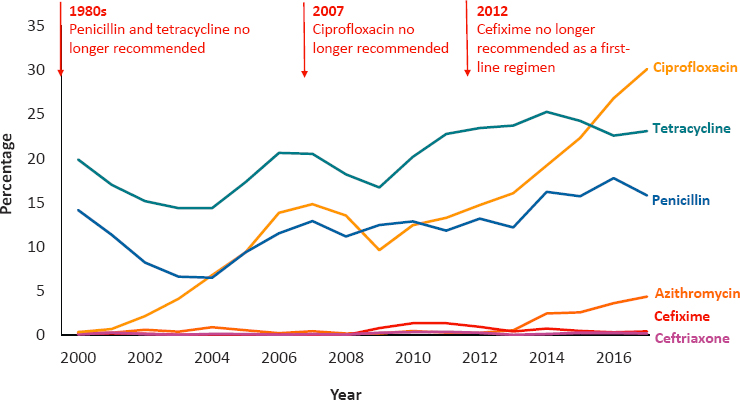
Since antimicrobial therapy was introduced for gonorrhea in the 1930s, it has progressively developed resistance to each drug (CDC, 2019a; Młynarczyk-Bonikowska et al., 2020), as shown in Figure 7-1. In recent years, this tendency and the slow development of new antimicrobials have become a recognized public health challenge, as highlighted in the 2020–2025 National Action Plan for Combating Antibiotic-Resistant Bacteria, and CDC’s including it as an urgent threat in the 2019 report on antibiotic resistance in the United States (CDC, 2019a; Federal Task Force on Combating Antibiotic-Resistant Bacteria, 2020).
Currently, only injectable ceftriaxone is recommended globally as first-line therapy for gonorrhea, and development of newer drugs has been limited (St. Cyr et al., 2020). In the past two decades, only four new medications have been evaluated in the United States. Two evaluations of newer antimicrobials (delafloxacin and solithromycin) have been stopped due to less than predicted efficacy (see Hook et al., 2019, for example), and evaluation of just two additional new antimicrobials (zoliflodacin and gepotidacin) has only recently begun. Both of the newer antimicrobials currently entering Phase III trials have been developed with partial
TABLE 7-2 Examples of Clinical Trials of STI Antibiotics
| Study Title [ClinicalTrials. gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| Gonorrhea Trials | ||||||||
| A Phase III, Randomized, Multi-Center, Open-Label Study in Adolescent and Adult Participants Comparing the Efficacy and Safety of Gepotidacin to Ceftriaxone Plus Azithromycin in the Treatment of Uncomplicated Urogenital Gonorrhea Caused by Neisseria gonorrhoeae [NCT04010539] | Recruiting | Phase 3 | Gepotidacin and ceftriaxone plus azithromycin | Randomized, parallel assignment | Estimated 600 12+-year-old men and women with urogenital gonococcal infection |
|
Sponsor: United Kingdom: GlaxoSmith-Kline Locations: USA: California (4 sites); Florida (2 sites); Georgia (2 sites); Indiana; Louisiana; Massachusetts; North Carolina (4 sites); Ohio; Tennessee; Texas (2 sites) Australia: Sydney (5 sites); Queensland (2 sites); Victoria (4 sites); Western Australia Germany: Berlin (2 sites); Frankfurt (2 sites); Hamburg; Munich (2 sites) Mexico: Guadalajara (2 sites) Spain: Alicante; Barcelona (4 sites); Madrid (3 sites); Seville United Kingdom: Birmingham; Brighton; Leeds; London (5 sites); Manchester; St. Helens |
September 8, 2023 |
| Study Title [ClinicalTrials. gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| A Multi-Center, Randomized, Open-Label, Non Inferiority Trial to Evaluate the Efficacy and Safety of a Single, Oral Dose of Zoliflodacin Compared to a Combination of a Single Intramuscular Dose of Ceftriaxone and a Single Oral Dose of Azithromycin in the Treatment of Patients with Uncomplicated Gonorrhoea [NCT03959527] | Recruiting | Phase 3 | Zoliflodacin 3 g PO and ceftriaxone 500 mg IM plus azithromycin 1 g PO | Randomized, parallel assignment; single dose of zoliflodacin or comparators combination in single dose: ceftriaxone and azithromycin | Estimated 1,092 12+-year-old men and women with uncomplicated gonorrhea |
|
Sponsor: Switzerland: Global Antibiotics Research and Development Partnership Locations: USA: Alabama; California; Indiana; Louisiana; Ohio; Washington Netherlands: Amsterdam South Africa: Bothas Hill; Johannesburg; Tongaat Thailand: Bangkok (4 sites) |
August 2021 |
| Chlamydia Trial | ||||||||
| Randomized, Open-Label, Multi-Center Study of Azithromycin Compared With Doxycycline for Treating Anorectal Chlamydia trachomatis Infection Concomitant to a Vaginal Infection [NCT03532464] | Unknown | Phase 4 | Azithromycin and doxycycline | Randomized, parallel assignment | Estimated 460 18+-year-old women with C. trachomatispositive test |
|
Sponsor: France: University Hospital, Bordeaux Locations: France: Marseille; Bordeaux (2 sites); Nantes; Paris; Roubaix; Tours |
December 1, 2019 |
| Syphilis Trials | ||||||||
| Trial Evaluating the Clinical Efficacy of Cefixime for Treatment of Early Syphilis in Non-Pregnant Women [NCT03752112] | Recruiting | Phase 1/2 | Cefixime 400 mg PO and benzathine penicillin 2.4 MU | Randomized, parallel assignment (2:1) | Estimated 180 18+-year-old women with positive syphilis test |
|
Sponsor: Switzerland: World Health Organization Location: Brazil: Fortaleza |
September 30, 2021 |
| Study Title [ClinicalTrials. gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| Clinical Trial Evaluating the Clinical Efficacy of Cefixime for Treatment of Early Syphilis [NCT03660488] | Recruiting | Phase 2 | Cefixime 400 mg oral capsule [Suprax] and benzathine penicillin G | Randomized, parallel assignment | Estimated 100 18+-year-old men and women with primary, secondary, or early latent syphilis |
|
Sponsor: USA: University of California, Los Angeles Locations: USA: California (9 sites); Nevada |
October 2021 |
| One Dose Versus Three Weekly Doses of Benzathine Penicillin G for Patients with Early Syphilis [NCT02857959] | Unknown | Phase 4 | Benzathine penicillin G | Randomized, parallel assignment | Estimated 150 18–60-year-old men and women with confirmed early symptomatic syphilis (primary or secondary) or high-titer latent syphilis |
|
Sponsor: China: Peking Union Medical College Hospital Location: China: Beijing |
August 2020 (Estimated) |
| A Phase 4 Comparative Trial of Benzathine Penicillin G 2.4 Million Units Administered as a Single Dose Versus Three Successive Weekly Doses for Treatment of Early Syphilis in Subjects with or Without HIV Infection [NCT03637660] | Recruiting | Phase 4 | Benzathine penicillin G 2.4 MU IM | Randomized, parallel assignment | Estimated 560 18+-year-old men and women with untreated primary, secondary, or early latent syphilis |
|
Sponsor: USA: National Institute of Allergy and Infectious Diseases Locations: USA: Alabama; Georgia; Indiana; Louisiana; Maryland; Massachusetts; North Carolina; Pennsylvania; Washington |
March 1, 2022 |
| Study Title [ClinicalTrials. gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| Randomized, Clinical Trial to Compare the Serological Response Rates of Serofast Early Syphilis Cases Retreated with Three Doses Benzathine Penicillin and Absence of Any Retreatment [NCT02884115] | Unknown | Phase 4 | Benzathine penicillin | Randomized, parallel assignment | Estimated 150 18–60-year-old men and women; early syphilis cases determined to be serofast at 6 months after initial treatment |
|
Sponsor and Location: China: Peking Union Medical College Hospital | September 2020 (Estimated) |
NOTES: ClinicalTrials.gov search and study recruitment status are as of September 4, 2020. DSMB = Data and Safety Monitoring Board; IM = intramuscular; mg = milligram; MSM = men who have sex with men; MU = million units; NAAT = nucleic acid amplification test; PO = per os; RPR = rapid plasma regain; USA = United States of America.
SOURCE: Bolan, 2019.
support from the federal government (BARDA5 and National Institute of Allergy and Infectious Diseases [NIAID]), nongovernmental agencies, and pharmaceutical companies (see Table 7-2). Oral cefixime, penicillin, ciprofloxacin, and azithromycin all have clinically unacceptable levels of resistance, and the need for effective oral drugs is acute. Even with ceftriaxone, different doses and recommendations to use it in combination with other antibiotics vary from region to region (Fifer et al., 2020; WHO, 2016; Workowski and Bolan, 2015). Increasing appreciation of the relatively high frequency of infection at nongenital sites (i.e., the oropharynx and rectum) and that therapy effective for genital infection may not be effective at other sites has further complicated recommendations for gonorrhea therapy. With these challenges in mind, expanded research in antibiotic development and evaluation for gonorrhea treatment is urgently needed, especially given the limited number of new antimicrobials in the pipeline and that the results of the few ongoing studies are not anticipated for several years.
___________________
5 “Biomedical Advanced Research and Development Authority (BARDA), part of the HHS Office of the Assistant Secretary for Preparedness and Response, was established to aid in securing our nation from chemical, biological, radiological, and nuclear (CBRN) threats, as well as from pandemic influenza (PI) and emerging infectious diseases (EID).” For more information, see phe.gov/about/barda/Pages/default.aspx (accessed June 11, 2020).
Chlamydia
The current treatment recommendation for chlamydia infections is a single dose of azithromycin or multi-day course of doxycycline (Workowski and Bolan, 2015). Doxycycline is not recommended for use in pregnancy, however, and may be less effective in persons with poor medication adherence (Marrazzo and Suchland, 2014). Declining cure rates may change treatment recommendations. Unlike gonorrhea, antimicrobial resistance remains rare for chlamydia, but like gonorrhea, the efficacy of recommended therapy varies when genital infections are compared to other sites (see Table 7-2). Furthermore, the heavy reliance on nonculture methods to detect chlamydia and possible persistence of chlamydial nucleic acids for weeks following effective therapy have complicated assessing therapies (both established and emerging) and cures.
Syphilis
Although substantially less common than either gonococcal or chlamydial infections, syphilis is closely linked to increased risk for HIV acquisition, is an important preventable cause of serious congenital infections, and is increasing, both in the United States and globally. Benzathine penicillin G, whose precursor is available from a single non-U.S. source, remains the globally recommended first-line therapy; its use is complicated by approximately 10 percent of the U.S. population reporting a penicillin allergy (although the true prevalence is likely to be much lower) (Blumenthal et al., 2019) and repeated instances of global shortage of it. The continuing rise in congenital syphilis is not from penicillin failure or allergy, but rather is associated with inadequate screening and treatment during pregnancy due to health system failures, which is indicative of the effect of social determinants on STI health outcomes (Trivedi et al., 2019).
Few alternatives to penicillin are available. Azithromycin, a macrolide antibiotic, was considered promising for single-dose therapy; however, a substantial prevalence of macrolide antibiotic resistance mutations and reports of treatment failure have tempered enthusiasm. Multiple-dose therapy with doxycycline remains the sole accepted alternative. Given its oral administration, it may be used more frequently, while parenteral administration becomes more complicated with limitations in nonurgent clinical services in the COVID-19 era. Finally, as described earlier, syphilis diagnosis to guide therapy continues to rely primarily on serological tests based on methods from more than 50 years ago, which are still compromised by false-positive results and slow and difficult-to-interpret therapy response. Newer, well-tolerated alternatives to penicillin are badly needed for improved syphilis control (TAG, 2019).
STI Syndromes
Managing STI syndromes presents yet another set of challenges for control. As discussed in the diagnostics section above, syndromic management can be time efficient and cost effective for the most common syndromes, while preventing transmission. For example, BV is the most common cause of vaginal discharge. Current research suggests that this syndrome is a polymicrobial dysbiosis for which sexual partners play an important contributing role in pathogenesis, response to treatment, and risk for recurrence (see Chapter 2 and Appendix A). Several new therapeutic agents have recently been or are under investigation for therapy and prevention; however, current treatment response is unsatisfactory (20–30 percent failure rate) and recurrences are common (Workowski and Bolan, 2015). Genital ulcer disease is not always diagnosed properly and may represent Haemophilus ducreyi, syphilis, or HSV-1 or HSV-2. Treatment for chancroid and syphilis differ, and antiviral suppression of HSV requires yet a third approach. The limitations of syndromic management, however, may result in all-too-common mistreatment and unnecessary exposure to antibiotics.
Antimicrobial Therapy for Viral STIs
Chronic, non-HIV viral infections, such as due to HSV and HPV, require a different array of antimicrobial agents. Though antimicrobial therapy does not cure these infections, it may hasten resolution of acute signs and symptoms and reduce the probability of sexual transmission. Behavioral and structural interventions, then, become even more important in preventing and controlling viral STIs (see Chapters 8 and 9).
Antimicrobials that halt herpes virus replication have been available for over three decades but are underused because approximately 80 percent of persons with genital herpes are not diagnosed. After success with acyclovir and valacyclovir to suppress HSV-2 viral shedding, new compounds have been explored, many of natural origin (Akram et al., 2018; Shiraki, 2018; Vere Hodge and Field, 2013). After decades of use of these drugs, emerging resistance is an increasing concern. Research priorities for future HSV management might include evaluation of new medicinal therapies and pharmacologic modification of existing drug classes (primarily viral thymidine kinase inhibitors) to provide easier-to-take, long-acting antiviral activity. Finding a drug to cure HSV-2 has been elusive, as is also true of other herpes viruses that are so well adapted to living actively or quiescently in a human host for a lifetime.
HPV, too, has been a difficult target for antiviral drug development, though far less investment has been made, given successes in vaccine
development (Archambault and Melendy, 2013; Cherry et al., 2013; Kanwar et al., 2011). HPV is treated with cellular ablation, as by freezing or heating cells (cold coagulation or thermal ablation). For smaller lesions, a variety of techniques are used. More extensive (and traumatic) laser ablations and loop electrosurgical excision are deployed for larger or more serious lesions; cancers are treated surgically.
Antimicrobial Therapy for Protozoan STIs
Trichomoniasis, while not reportable, is among the most prevalent vaginal STIs. It infects more women than gonorrhea or chlamydia, and, despite clearly being an STI, is rarely tested for in men. The 5-nitroimid-azole drugs are the only available treatments, by oral and less often parenteral routes (Bouchemal et al., 2017). Only metronidazole and tinidazole are available and FDA approved for trichomoniasis. The 5-nitrothiazolyl derivative nitazonxanide is also effective to varying degrees, as are drugs that can be used when a patient has a hypersensitivity to 5-nitroimid-azoles: disulfiram and nithiamide. More research is needed for therapeutics in the face of growing drug resistance (O’Donoghue et al., 2019).
Gaps and Opportunities in STI Treatment
Currently, no single or even two or three antimicrobials are sufficient to control bacterial and protozoan STIs. Rather, the substantial menu of pathogens requires an equally large variety of antimicrobial agents. Management of common STIs has been variously challenged by, among other things, progressive antimicrobial resistance (gonorrhea, trichomoniasis), limited screening despite widely accepted recommendations and clinical guidelines (chlamydia), and underdiagnosis (especially among marginalized populations with suboptimal access to health care and/or health insurance) and therapeutic challenges associated with chronic, incurable viral infections (e.g., HSV, HPV). The long timelines and high costs to develop new STI antimicrobials further reduce the incentives for pharmaceutical companies to prioritize developing new drugs (CDC, 2019a; Perros, 2019; Solman, 2020).
Antimicrobial agents are also now used to address goals other than curing a specific, recognized pathogen, such as syndromic diagnosis being widely used to accelerate the time to treatment and as a cost-saving measure. For bacterial and protozoan STIs, therapy is also frequently recommended for persons exposed to infected sex partners (STI “contacts”) before or even without proven infection.
For viral STIs, therapy is not curative and is largely directed at controlling acute manifestations and possibly reducing transmission risk.
In recent years, community-wide antibacterial therapy has been used to intervene in or halt widespread STI outbreaks.
TOOLS FOR STI PREVENTION
Condoms and Barrier Method Contraceptives
Condoms have played a major role as part of both STI and pregnancy prevention efforts for thousands of years, predating modern antimicrobial therapy. They are in widespread use (more than 450 million sold annually in the United States) (Planned Parenthood, 2018) by more than 33 million Americans in 2020 (Statista, 2020), though a majority of coital episodes are condomless (Copen, 2017). Among tools for STI prevention, condoms (when used correctly and consistently) have the distinct advantage of reducing risk for both acquiring and transmitting virtually all STIs and preventing unintended pregnancy.
Condoms, however, also have limitations (D’Anna et al., 2012). Persons may use them incorrectly by failing to put them on before sex, and they break about 1–2 percent of the time and may slip off during intercourse (Macaluso et al., 1999). Condoms may vary greatly in quality globally, though in the United States, manufacturing standards ensure quality in approved products. In addition, decisions regarding condom use also must occur with each sexual encounter, and they may be perceived to reduce sensation of the penis and sometimes their partners. Usually, promotion and use has focused on the latex or polyurethane external condom, although limited research indicates that the internal condom is also effective for STIs and pregnancy. Condom promotion and interventions to encourage correct and consistent use have been the specified outcome of many STI- and HIV-risk reduction interventions, and distribution has been a prominent component of many public health measures to reduce STIs.
It is difficult, if not impossible, to determine the magnitude of benefit of condom use on preventing the spread of STIs; it is clear, however, that condom promotion alone has not been sufficient. Furthermore, condom use has been declining more recently in a key demographic (MSM) (DiClemente et al., 2002; Hess et al., 2017; Paz-Bailey et al., 2016), possibly related to expanded antiretroviral therapy for HIV and post-exposure prophylaxis, reducing anxieties about HIV, and strong preferences for condomless sex. Behavioral and marketing research to enhance condoms’ attractiveness, uptake, and successful use has suggested that increasing use is possible in a wide variety of settings and among specific groups (i.e., adolescents, see Chapter 8 for more information) (Malekinejad et al., 2017; Pérez et al., 2018; Wang et al., 2018).
Data are more limited regarding diaphragm use; the data available suggest reduced risk for STI acquisition, though concurrent spermicide use makes it difficult to assess their relative contributions (de Bruyn et al., 2011; Padian et al., 2007).
Other Contraceptive Measures
Another available contraceptive measure is spermicidal preparations (short-action foams, creams, jellies, film, and suppositories applied at each instance of intercourse and currently designed mainly to prevent pregnancy). Detergent-based spermicides are toxic for some STI pathogens. Clinical trials in which high-risk populations used nonoxynol-9 detergent spermicides, however, demonstrate no clear, significant protective effect for bacterial STIs (Roddy et al., 1998, 2002; Wilkinson et al., 2002) and were associated with an increased risk of HIV in one trial (Van Damme et al., 2002), perhaps due to irritation and inflammation from the chemicals.
The opportunity to use reversible contraceptives has greatly benefited women’s health; at the same time, the interplay between STIs and contraceptives is complex and has been reviewed in detail (Deese et al., 2018; McCarthy et al., 2019). Research on hormonal contraceptives (i.e., to prevent pregnancy) and related risk for STI acquisition is the subject of ongoing research. Hormonal contraceptives (most specifically progestins) may increase the risk of exposed cisgender women to acquiring chlamydial and gonococcal infections (Pettifor et al., 2009); these changes, however, may be modified by which hormones are used and their dosage. Hormonal contraceptives’ role in the risk for viral STIs, such as HSV and HPV, and trichomoniasis is unclear (Deese et al., 2018; McCarthy et al., 2019). Epidemiologic studies in the 1980s suggested that IUDs were associated with risk for pelvic inflammatory disease (PID) in cisgender women with an infection, such as gonorrhea or chlamydia, but not for acquisition of infection. IUDs have evolved substantially, and modern IUDs no longer have this association (Deese et al., 2018; Jatlaoui et al., 2016), although insertion may carry a risk for upper genital tract infection.
A high-profile clinical trial in Africa, the Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial, found no difference in risk of HIV acquisition with three different contraceptives: intramuscular depot medroxyprogesterone acetate, a copper IUD, or a levonorgestrel implant (ECHO Trial Consortium, 2019); additional research on the effect of these contraception approaches on non-HIV STIs is ongoing.
Multipurpose Prevention Technologies
Interventions can be combined to prevent several unwanted consequences of sex. A single biomedical tool to prevent any combination of STI infections, HIV, and/or pregnancy is highly desirable, as consumer demand will be considerable for multipurpose prevention technologies (MPTs) (Anderson et al., 2020; Li et al., 2019; Smith et al., 2017). Of course, internal and external condoms are precisely such a technology (Beksinska et al., 2020). Microbicides used intravaginally were thought to be highly promising, as when the CAPRISA 004 trials showed modest topical tenofovir effects to prevent both HIV and HSV infection in South African women (Abdool Karim et al., 2015; Karim et al., 2014). A number of antibiotics are effective against multiple STI pathogens and may be considered MPTs. Vaginal rings may offer reliable contraception and some degree of HIV prevention (Dallal Bashi et al., 2019; Derby et al., 2017). Other than condoms, no products are designed to prevent one or more classical STIs and pregnancy. While studies of the acceptability and desirable characteristics of MPTs have outlined their theoretical promise, proven and acceptable products lag, and no MPTs are yet available with public health impact (Guilamo-Ramos et al., 2018; Hunter et al., 2018; Hynes et al., 2018, 2019; Vargas et al., 2019).
Antibiotics for STI Prevention
Effective STI treatment prevents transmission. Initially recommended by Surgeon General Thomas Parran in 1938, testing and preventative treatment of persons sexually exposed to partners with STIs has been an essential element of control efforts (Hook, 2013). Partners may be treated through expedited partner therapy, in which infected persons are given sufficient medication to treat themselves and their sexual partners, without clinically assessing the partners. Clinicians or public health workers, such as disease intervention specialists, can offer expedited partner therapy. It therefore provides timely treatment for recent partners, especially for male partners of women with chlamydia or gonorrhea. See Chapter 10 for more information.
STI PrEP
An alternative strategy to reduce STIs is PrEP, perhaps first explored in the U.S. Navy on a large scale (Rasnake et al., 2005). In one experiment, Harrison et al. (1979) attempted to reduce gonorrhea infections with minocycline, which proved effective only against highly susceptible organisms; the trial demonstrated the hazards of selecting resistant mutant bacteria with antibiotic prophylaxis.
The relatively high incidence of STIs in MSM has led to particular interest in doxycycline PrEP (Bolan et al., 2015; Molina et al., 2018). In a small trial conducted in MSM in France, doxycycline reduced syphilis and chlamydia acquisition by 73 and 70 percent, respectively (Molina et al., 2018). Gonorrhea acquisition was not affected, given that it is now generally resistant to doxycycline. Enthusiasm over this potential intervention has led to a number of ongoing investigations to determine both the benefits and challenges (e.g., cost, durability, side effects, promotion of antimicrobial resistance, vulnerability to other STIs, and potential undesirable changes in the microbiome) of antibiotic PrEP. An analogous concept is the chronic use of acyclovir or valacyclovir to reduce transmission of HSV-2 to sexual partners.
Mass antimicrobial therapy for STI control has been attempted on several occasions. Yaws, a nonvenereal treponemal disease, was controlled with mass administration of penicillin, and a side effect in parts of Africa and China was reduced syphilis infection (Marks et al., 2014; Mitjà et al., 2013). In fact, it reduced syphilis rates in China to close to zero, until the liberalization of international travel and sexual mores led to reintroduction and spread. More recent efforts of mass therapy as part of efforts to curtail large, sustained syphilis outbreaks in Vancouver, Canada, have not been successful. Currently, the utility of this method for STI treatment and prophylaxis remains a research question rather than an evidence-based public health tool. Effects of STI mass treatment campaigns to control HIV are discussed in Chapter 5.
Vaccines
Optimal prevention of most infectious diseases requires vaccines with exceptional efficacy and safety (Doherty et al., 2016). Notable global successes related to vaccine development include the eradication of smallpox, the near-eradication of polio, and control of respiratory infections, such as measles (Minor, 2015; Plotkin, 2014). The magnitude and gravity of the STI epidemic has garnered considerable interest in vaccine strategies. However, STIs represent a highly diverse group of viral, bacterial, and protozoal organisms that have little in common except the mode of transmission and, in some but not all cases, the mucosal or epithelial tissues infected. Accordingly, each potential vaccine requires substantial individual investment in knowledge related to the biology of transmission, infection pathogenesis, and credible ideas about protective immune responses (“correlates of protection”). The “lumping” of STIs in discussion of vaccine development leads to unrealistic expectations. In addition, if STI vaccine development is successful, strategies required for optimal deployment will be complicated, recognizing the uneven distribution of
certain diseases and general vaccine challenges in the current health care system. However, mRNA-based technology is a potential new tool in the development of prophylactic vaccines; this promising field is progressing rapidly, with potential application to STI vaccines, such as HIV and HSV (Abbasi, 2020; Egan et al., 2020; Maruggi et al., 2019; Pardi et al., 2018).
Two STI vaccines (hepatitis B and HPV) exist and have led to substantial reductions in prevalence. Several other STIs have received substantial research and investment, including HIV, gonorrhea, and genital herpes. The NIAID recently created and funded six new STI vaccine cooperative research centers, whose efforts target syphilis, chlamydia, and gonorrhea (Eisinger et al., 2020; NIAID, 2019). A classical vaccine development approach was successful for hepatitis B: identify the correlate of protective immunity (hepatitis B surface antibody) and engineer a vaccine to mount this precise response to a vaccine-induced antigenic challenge (Das et al., 2019; Gerlich, 2013). In developing the HPV vaccine, an empirical approach using type-specific, virus-like particles was highly successful, even in the absence of a clear correlate of immunity (Wang and Roden, 2013). In contrast, despite decades of intensive investigation, the correlates of protective immunity to strive for in vaccine development and response still are not fully elucidated for HIV, gonorrhea, and herpes (Haynes et al., 2012).
Developing STI vaccines requires consideration of target populations at risk, including adolescents and young adults. Vaccines have been characterized as either essential to achieve global control or merely helpful for public health interventions (Hawkes et al., 2014). Many childhood vaccines, including hepatitis B, are termed essential to protect public health and legally mandated for school attendance throughout the United States. The hepatitis B vaccine was introduced in acknowledgment that adult coverage rates were abysmal and that integrating this long-acting vaccine into the childhood series would be programmatically more successful.
Unfortunately, HPV vaccines are more often seen as optional and voluntary, limiting full coverage and their ultimate impact on prevention of genital and oral cancers and genital warts (Baezconde-Garbanati et al., 2017; Holman et al., 2014). Opt-in (not mandated) vaccines have lower coverage rates than opt-out (mandated) vaccines. The HPV vaccines protect against genital HPV infection, high-grade cervical lesions, invasive cervical cancer, anogenital warts, and some head and neck cancers (Her-per, 2020; Kobayashi et al., 2018; Lei et al., 2020). Broader HPV vaccine coverage, however, is essential for full public health benefits (Baezconde-Garbanati et al., 2017; Holman et al., 2014). When practitioners see the vaccine as optional or guess who might need it, coverage levels can be compromised (Kashani et al., 2019; North and Niccolai, 2016).
A Brief Summary of STI Vaccine Progress
Different vaccines work in a variety of ways, but among the most common is stimulating an anamnestic response to a challenge antigen (i.e., the body has seen the antigen before [in the vaccine] and therefore mounts a rapid, protective, usually antibody response to the challenge [the infectious agent]). Vaccines may prevent infection, blunt infection such that no illness is noted after exposure, reduce the severity of illness, or prevent mortality, even if incidence is not affected. Some vaccines produce lifelong immunity after a single dose, while others require periodic boosters. For the hepatitis B vaccine, three doses produce lifelong immunity in 95 percent of recipients. Possible lifelong immunity to the viral types in the vaccine is expected in most recipients of just two doses of HPV vaccine.
The diversity of organisms that cause STIs will involve a more complex and elusive immune response that may require the vaccine to induce both humoral and cellular immunity. The field to date has been unsuccessful with HSV-2 viral vaccines. Bacterial and parasitic STI vaccines against syphilis, gonorrhea, chlamydia, or trichomoniasis, to name a few, have been similarly unsuccessful, but clinical trials continue. Table 7-3 lists examples of current STI vaccine trials (this does not include trials studying the existing HPV vaccines).
Neisseria gonorrhoeae
Gonococcal infections evoke an intense granulocytic inflammatory response but virtually no protection from recurrent infection(s) (Lovett and Duncan, 2018). Using a mouse model of vaginal infection, Liu et al. (2017) demonstrated some protection with a prototype outer membrane vesicle vaccine. This observation may be particularly relevant because of cross-immunity between Neisseria meningitides and N. gonorrhoeae, noted with the use of commercially available meningococcal vaccine (Bexsero) (TAG, 2019). The N. meningitides vaccine contains outer membrane vesicles; meningococcal and gonococcal vesicles share considerable homology. In a retrospective cohort study, the effectiveness of a unique New Zealand meningococcal B vaccine (MeNZB, similar to Bexsero) against gonorrhea-associated hospitalization was assessed in 935,496 individuals born from 1984 to 1999 and eligible for meningococcal B vaccination from 2004 to 2008 (Paynter et al., 2019). After adjustment (sex, ethnicity, and economic deprivation), vaccine effectiveness against hospitalization caused by gonorrhea was estimated to be 24 percent (95% confidence interval: 1–42%) (Paynter et al., 2019). In follow-up to this observation, several randomized clinical trials in various phases have been initiated to evaluate Bexsero for gonorrhea prevention (see Table 7-3); however, results are unlikely to be available for several years (Petousis-Harris and Radcliff, 2019; Russell et al., 2019). A successful
TABLE 7-3 Examples of Clinical Trials of STI Vaccines
| Study Title [ClinicalTrials.gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| Gonorrhea Trials | ||||||||
| Immunisation for Adolescents Against Serious Communicable Diseases [NCT04398849] |
Not yet recruiting | Observational study | 4CMenB vaccine (Bexsero) | Prospective cohort | All consenting 14–19-year-olds residing in the Northern Territory, Australia in 2020–2021 (Estimated 7,100) |
|
Sponsor: Australia: University of Adelaide Locations: not listed |
December 31, 2024 |
| Study Title [ClinicalTrials.gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| Use of Bexsero Immunisation to Detect Cross Reactive Antigens and Anti-Gonococcal Antibodies in Key Populations in Kenya [NCT04297436] |
Not yet recruiting | N/A | 4CMenB vaccine (Bexsero) | To assess if immunisation of individuals at risk for gonococcal infection with 4CMenB (Bexsero) elicits humoral and T cell cross-reactive responses against Neisseria gonorrhoeae | Estimated 50 18–25-year-old men or women |
|
Sponsor: United Kingdom: University of Oxford Location: Kenya: Kilifi |
April 30, 2022 |
| A Phase II Randomized, Observer-Blind, Placebo-Controlled Study, to Assess the Efficacy of Meningococcal Group B Vaccine rMenB+OMV NZ (Bexsero) in Preventing Gonococcal Infection [NCT04350138] |
Recruiting | Phase 2 | 4CMenB vaccine (Bexsero) and placebo | Randomized, parallel assignment | Estimated 2,200 18–50-year-old men and women |
|
Sponsor: USA: National Institute of Allergy and Infectious Diseases Locations: USA: Alabama; Georgia (2 sites); Louisiana; Maryland Thailand: Bangkok (2 sites) |
August 1, 2023 |
| A Multi-Centre Randomised Controlled Trial Evaluating the Efficacy of the Four-Component Meningococcal B Vaccine, 4CMenB (Bexsero), in the Prevention of Neisseria Gonorrhoeae Infection in Gay and Bisexual Men [NCT04415424] |
Not yet recruiting | Phase 3 | 4CMenB (Bexsero) and placebo | Randomized, parallel assignment (1:1) | Estimated 730 18–40-year-old high-risk men (cis and trans), trans women, and nonbinary people who have sex with men |
|
Sponsor: Australia: Kirby Institute Locations: Australia: Sydney (2 sites); Queensland; Melbourne |
February 2024 |
| Study Title [ClinicalTrials.gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| Institute for Global Health and Infectious Diseases 11911 - Cross-Reactive N. gonorrhoeae Immune Responses Induced by a N. meningitidis Vaccine [NCT04094883] |
Recruiting | Phase 4 | 4CMenB vaccine (Bexsero) | Single group assignment | Estimated 15 18–25-year-old men or women |
|
Sponsor: USA: University of North Carolina at Chapel Hill Location: USA: North Carolina |
February 2021 |
| Chlamydia Trial | ||||||||
| A Phase I First in Human, Double-Blind, Parallel, Randomised and Placebo Controlled Clinical Trial of the Safety of SSI’s Adjuvanted Chlamydia Vaccine CTH522 in Healthy Women Aged 18 to 45 Years [NCT02787109] |
Completeda | Phase 1 | CTH522-CAF01, CTH522Al(OH)3, and placebo | Randomized assignment (3:3:1) | 35 18–45-year-old women |
|
Sponsor: Denmark: Statens Serum Institut Location: United Kingdom: London |
Completed July 31, 2017 |
| Study Title [ClinicalTrials.gov Identifier] | Recruitment Status | Status/Phase | Intervention(s) | Intervention Model Description | Participants | Primary Outcome Measure(s) | Trial Sponsor and Trial Location(s) | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| Herpes Simplex Virus Trials | ||||||||
| Safety and Efficacy of 4 Investigational HSV 2 Vaccines Administered by Intramuscular Route in Adults With Recurrent Genital Herpes Caused by HSV 2 [NCT04222985] |
Active, not recruiting | Phase 1 and 2 | Six HSV 2 formulations | Randomized, sequential assignment | Estimated 381 18–55-year-old, HSV-2 seropositive men and women |
|
Sponsor: France: Sanofi Pasteur Locations: USA: Florida; Massachusetts; North Carolina; Washington |
May 2021 |
| A Randomized, Placebo-Controlled, Double-Blind Study to Assess the Efficacy and Safety of a Maintenance Dose of GEN-003 in Subjects with Genital Herpes Infection [NCT03146403] |
Terminatedb | Phase 2 | GEN-003 with Matrix-M2 adjuvant and placebo | Randomized, parallel assignment (1:1) | Estimated 33 participants who completed the GEN-003-003 study |
|
Sponsor: USA: Genocea Biosciences, Inc. Locations: USA: Alabama; California (2 sites); North Carolina; Ohio; Oregon; Texas; Washington |
N/A |
a Results published. See Abraham et al., 2019.
b Terminated in 2017 because of a business decision to cease spending on the GEN-003 vaccine project (https://clinicaltrials.gov/ct2/show/NCT03146403 [accessed September 15, 2020]).
NOTES: ClinicalTrials.gov search and study recruitment status are as of September 2, 2020. AESI = adverse events of special interest; HSV-2 = herpes simplex virus type 2; IgA = Immunoglobulin A; IgG = Immunoglobulin G; IgM = Immunoglobulin M; MAAE = medically attended adverse event; SAE = serious adverse event; USA = United States of America.
N. meningitides vaccine for N. gonorrhoeae has obvious design implications for a more specific and effective gonorrhea vaccine. In addition to these clinical trials, researchers have identified nine new potential proteins as vaccine candidates (TAG, 2019).
Chlamydia trachomatis
The feasibility of vaccines to prevent chlamydia is supported by limited data indicating that persons with recent infections are at somewhat reduced risk for reinfection (Brunham and Rey-Ladino, 2005; Phillips et al., 2019; Poston and Darville, 2018), although reinfections certainly do occur (Geisler et al., 2013). A vaccine might prevent infection or sequelae of infection, including PID and female factor infertility (Phillips et al., 2019). However, PID is difficult to diagnose with accuracy, complicating assessment of vaccine benefits (Workowski and Bolan, 2015).
A vaccine that takes advantage of the immunogenicity of the chlamydial major outer membrane protein has been tested in a human Phase 1 clinical trial (Abraham et al., 2019; Tifrea et al., 2020). The genital C. trachomatis vaccine candidate, CTH522, contains engineered heterologous immunorepeats from segments of the chlamydial major outer membrane protein. Vaccines manufactured with either liposomal CAF01 or aluminum hydroxide were administered three times via intramuscular injection, followed by two intranasal inoculations (without adjuvant). This historic trial indicated that both vaccines (with different adjuvants) were well tolerated and immunogenic (Abraham et al., 2019) (see Table 7-3). CTH522:CAF01 induced more consistent cell-mediated interferon-γ responses and higher serum antibody titers, so it is preferred. Phase 2 trials will follow, and a marketable vaccine could be available in several years, if results and market forces are favorable. Research into other potential vaccine candidates continues as well (Bulir et al., 2016; TAG, 2019).
Herpes simplex virus type 2 (HSV-2)
HSV-2 infection is incurable and recurrent (CDC, 2017). Recurrences can be painful and correlate with increased probability of infecting sexual partners (Corey, 1982; Schiffer and Corey, 2009) but can be suppressed or shortened with antiviral treatment, which also reduces transmission (Workowski and Bolan, 2015). Much current vaccine research is focused on “therapeutic vaccination”: enhancing host immunity to better manage existing infections (Hofstetter et al., 2014). These vaccines appear to reduce recurrences and genital tract shedding of the virus. T cell and antibody responses have been demonstrated to last up to 12 months (Dropulic and Cohen, 2012). However, the commercial development of a suppressive HSV-2 vaccine remains uncertain (WHO, 2019). Despite several encouraging phase 2 trials, only a single HSV-2 vaccine is in clinical trials (see Table 7-3).
Little progress has been made on a vaccine to prevent HSV-2 infection (Kim and Lee, 2020). Several previous large NIH-led trials of preventative vaccines have failed (Belshe et al., 2012). Most recently, a live-attenuated vaccine candidate has harnessed antibody-mediated cell toxicity, a promising approach; safety is ensured by the deletion of amino acid sequences in key HSV proteins such that sustainable infection cannot occur (Görander et al., 2014).
Treponema pallidum
This microorganism is a spirochete, a spiral bacterium that causes syphilis. It theoretically could be controlled with a vigorous immune response (Lithgow and Cameron, 2017). Ideally, a syphilis vaccine would prevent infection, the primary manifestation (primary infection) represented by the cutaneous chancre (ulcer). Failing this, substantially reducing the dissemination of spirochetes, persistence (latency and sequelae), and reinfection would all be helpful (McIntosh, 2020). The biology and pathogenesis of syphilis infection, however, make vaccine development challenging for many reasons (Duan et al., 2020). The T. pallidum surface has a very limited array of outer membrane proteins and an atypical lipid content; this bacterial surface seems to contribute to the inability of the immune system to protect against infection.
While the exact correlates of protective immunity are unknown, a rabbit experiment with irradiated T. pallidum published in 1973 has been touted as a test of concept, suggesting the feasibility of a human vaccine targeting treponemal surface antigens on the outer membranes (Miller, 1973). A reduction or delay in chancre formation was noted, along with reduced recovery of spirochetes from other tissues. No active clinical trials for syphilis vaccines exist; however, current research on vaccine development focuses on a better understanding of surface-exposed treponemal proteins and a better determination of the best correlates of immunity (NIAID, 2019). For example, Lithgow et al. (2017) have focused on treponemal proteins Tp0751 and TPrK. There remains, however, some debate about the physical location of these proteins. A paper by Luthra et al. (2020) found no protection against local or disseminated infection following challenge with T. pallidum in rabbits immunized with Tp0751, suggesting Tp0751 is in fact a subsurface protein. Results such as these challenge the probability that these proteins can credibly serve as reliable immunogens (Luthra et al., 2020; Radolf et al., 2016).
Preventing “vertical transmission” to the fetus during pregnancy is an especially important goal, given the number of people who do not get tested and/or treated and the severity of congenital syphilis in the newborn (Kimball et al., 2020) (see Chapters 2 and 3). Congenital syphilis during pregnancy is a leading, preventable cause of adverse pregnancy
outcomes (miscarriage, spontaneous abortion, congenital infections causing lifelong sequelae) and is increasing in the United States (CDC, 2019b), a true failure of public health and health care.
Stakeholders in STI Vaccine Development
NIH and CDC
NIH has the most visible U.S. investment in STI vaccine development. As mentioned above, the 2019 cooperative research programs launched by NIAID fund vaccine development for chlamydia, syphilis, and gonorrhea (Eisinger et al., 2020; NIAID, 2019), with the grants totaling many millions of dollars over 5 years. This is focused largely on immunology, animal models, and early clinical trials (NIAID, 2019; TAG, 2019).
CDC plays a critical and unique policy and scientific advisory role in deployment once vaccines are available. The Advisory Committee on Immunization Practices comprises medical and public health experts who develop recommendations on the use of proven vaccines in the civilian population of the United States (CDC, 2020). The recommendations stand as public health guidance for safe use of vaccines and related biological products (CDC, 2020). In addition, the committee is charged to undertake economic analysis of vaccine policy decisions and whether the right vaccines are deployed for the right populations (CDC, 2020).
CDC also plays a critical role in vaccine education and messaging. Accordingly, the success of any STI vaccine will depend to a great extent on CDC activities and successful collaboration between CDC and vaccine developers. A good example of this relationship can be seen with the HPV vaccine. Use grew exponentially since its introduction, but uptake has plateaued in recent years; only about half of adolescents aged 13–17 had completed the vaccine series in 2018 (Walker et al., 2019). The vaccine has been “marketed,” however, more as preventing cancer rather than STIs, to evade community sensitivities related to STI-associated stigma (Klein and Luedtke, 2008). The economic potential for preventive STI vaccine(s) remains uncertain, compromising investment and development strategies from the private sector.
Industry and other partners
Industry involvement is crucial for successful vaccine development and bringing products to market. Vaccines are not nearly as profitable compared to pharmaceuticals and biologics for therapy. Hence, companies will critically assess the market forces before they decide to partner for moving promising candidates forward in the developmental pathway. Whether consumers (community members), health care providers, and governments engage productively will
depend on conservative considerations of safety, cost, and benefit. Will consumers and health care workers flock to an STI vaccine? HPV and HBV vaccines prevent cancer, so there is less stigma for them, but a vaccine clearly focused on an STI may be shunned by consumers afraid of social and familial opprobrium.
The role of the Bill & Melinda Gates Foundation; Gavi, The Vaccine Alliance; WHO; governments; and perhaps other foundations may be critical here. Industry may embrace a product if it can mitigate risk and maximize income. As was done to bring relatively expensive vaccines—Haemophilus influenzae type b and pneumococcus—to children in low-income nations, bulk purchase guarantees in exchange for more affordable pricing may work to promulgate STI vaccines, too. See the section in Chapter 12 on Public–Private Partnerships for STI Prevention and Control for more information.
STI Vaccines and Risk Compensation
Increasing access to antiretroviral therapy for people living with HIV and to HIV PrEP for vulnerable, HIV-seronegative populations has been associated with rising STI incidence in MSM in some studies (Ramchandani and Golden, 2019). Other research has not found a relationship with antiretroviral therapy and rising STI rates (Freeborn and Portillo, 2018; Liu and Buchbinder, 2017). Many experts believe that increasing rates of STIs reflect, in part, “risk compensation” that result from reduced fear of HIV infection, especially in MSM (Rojas Castro et al., 2019). It seems likely that an STI vaccine might inspire similar changes in behavior, influencing persons at risk to see themselves as protected from STI risk. Nonetheless, such developments do not negate the benefits from reduced HIV risk in the context of personal choices about which risk reduction strategies individuals select for themselves.
On the other hand, the HPV vaccine has not been associated with increased sexual risk taking (Hansen, 2016; Wu et al., 2019). No matter the efficacy of any novel STI vaccine, incident infections with other organisms could well compromise its deployment and relative benefits, potentially more serious if the vaccine were not particularly efficacious. The HIV PrEP field may prove helpful here, as assertive counseling has helped mitigate risk compensation in a number of populations (i.e., some or most consumers can be educated not to overvalue STI vaccines and to appreciate the need for continued risk reduction).
Gaps and Opportunities in STI Prevention
Human sexual behavior is a fundamental and powerful biological and social drive and often also motivated by the wish to have children. Given broad nonadherence with or unavailability of condoms, STIs are unlikely to be properly controlled without a vaccine (Ortayli et al., 2014) and other biological interventions (Eisinger et al., 2020). This section has highlighted, however, the daunting biological challenges of mucosally mediated infections, the social stigmas of biomedical interventions, and investment obstacles for the private sector, which may make more money from other products. STI vaccine development remains insecure, but the committee applauds funding cooperative research centers for this goal. Likewise, research continues into doxycycline to prevent bacterial STIs. Although this is a promising approach to controlling STIs, better data are needed on aspects such as target populations, doses and regimens, and the overall risk–benefit analysis of this strategy (Grant et al., 2020).
Critical policy and deployment strategies present additional substantial challenges, as discussed above. Deploying the hepatitis B and HPV vaccines has not been without challenges and lessons learned (Hsieh et al., 2015). The challenges have included misinformation related to safety, reduced acceptance related to the stigma of STIs and misperceptions of risk, and a limited infrastructure for adolescent and adult vaccine deployment and costs. For example, in the United States, major advances in population-wide hepatitis B vaccine coverage beyond health care workers did not occur until vaccination was recommended beginning at birth. Additionally, the relatively high cost of HPV vaccines is also a barrier that may apply to new STI vaccines as they emerge. Adolescents below 18 years of age may require parental consent in some venues, which introduces yet another barrier that will likely require further consideration by policy makers and public health officials (Hawkes et al., 2014). The challenges in developing, implementing, and ensuring widespread use of STI prevention tools highlights the need for accurate and available diagnostic and treatment tools, as well as concurrent behavioral and structural interventions.
CONCLUSIONS AND RECOMMENDATION
Infections from a large number of bacteria, viruses, and protozoans, each with its own biological characteristics, are classified as STIs. These organisms share sexual and often perinatal transmission as fundamental characteristics, but they require different therapies and ancillary control measures. Accurate and timely diagnosis is crucial, yet many of these infections are commonly asymptomatic and so may go unrecognized, creating a long window for transmission and causing a variety of serious
complications. The consequences of many STIs may become apparent only years later. Additional progress needs to be made in developing and implementing biomedical tools to successfully combat the STI epidemic (Eisinger et al., 2020).
Conclusion 7-1: Recent scientific advances provide the means for improving STI diagnosis, but existing tools are currently underused and results may not be available in a timely fashion, permitting further transmission. Increasing antimicrobial resistance has threatened the continued efficacy and ease of STI treatment and the pipeline for development of both new antimicrobials and vaccines for STI prevention and treatment has been constrained by an unfavorable business case that includes an expensive and time-consuming regulatory pathway to approval and widespread availability.
Conclusion 7-2: Syndromic diagnosis and treatment for persons with genitourinary symptoms remain common. The efficacy of this approach to prevent STI transmission to others is limited because it fails to address the frequent occurrence of asymptomatic or otherwise unrecognized infections and is contrary to principles of antimicrobial stewardship to slow the evolution of antimicrobial resistance.
Therefore, the committee recommends:
Recommendation 7-1: To improve the efficacy and reach of tools for STI management and prevention, the National Institutes of Health should prioritize development of point-of-care (POC) diagnostic tests; development of diagnostic tests for active syphilis; promotion of public–private partnerships (PPPs) to develop new antimicrobials; and expedited development of vaccines.
- POC diagnostics: Prioritize development of POC diagnostic tests to reduce the interval between testing and treatment. Use of these POC tests should be promoted to reduce opportunities for transmission. Optimally, POC tests should be inexpensive, rapid, and receive a Clinical Laboratory Improvement Amendments waiver to permit increased testing at sites providing health care or at home.
- New diagnostics for syphilis: Promote development of new, innovative diagnostic tests for active syphilis that distinguish untreated, active syphilis from previously treated infection, which is required to effectively control syphilis.
- Antimicrobials and vaccines for STI treatment and prevention: Subsidize and encourage PPPs with the goals of developing new,
- readily accessible antimicrobials for STI treatment and expediting development of vaccines for prevention of high-priority STIs, such as chlamydia, gonorrhea, syphilis, and herpes.
Much needs to be accomplished to expand and improve the tools used for prevention and management of STIs (Eisinger et al., 2020). Federally initiated public–private partnerships (PPPs) will help to address a pace of development of new tools often hindered by a business case that makes investment in development less attractive than for medications taken more often and already promoted as contributing to health maintenance (see Chapter 12 for more information on PPPs). Ultimately, however, the goal of STI prevention will be most effectively accomplished by development and widespread use of safe and effective vaccines (Eisinger et al., 2020). Ongoing trials of vaccines for gonorrhea promise to provide tools for one of the world’s most common STIs. A recent initiative (Operation Warp Speed) to rapidly develop and test vaccines for SARS COVID-19 provides a model to be emulated for other prioritized STI pathogens, such as C. trachomatis (chlamydia), Treponema pallidum (syphilis), and HSV.
CONCLUDING OBSERVATIONS
In the interval since The Hidden Epidemic, technological advances have expanded the tools available to contribute to control of STIs and provided new methods with enormous potential for further additions to the intervention “toolbox.” NAATs have supplanted less sensitive, less specific, and more cumbersome diagnostic tests and are now the tests of choice for chlamydia, gonorrhea, and trichomoniasis. POC NAATs and additional molecular tests for emerging STIs, such as BV and Mycoplasma genitalium, are becoming available as well and promise to expand the availability and timeliness of STI testing for both diagnosis and screening.
In contrast, despite the technical advances with the potential to facilitate development, relatively few new antimicrobials have been studied for STI treatment in the past 20 years. Losing the antibiotic pipeline infrastructure means losing the ability to innovate (Solman, 2020). More recently, stimulated in part by the threat posed by progressive antimicrobial resistance, several newer antimicrobials for gonorrhea have entered clinical trials (delafloxacin, solithromycin, zoliflodacin, gepotidacin), but after unacceptable failure rates, delafloxacin and solithromycin are no longer being evaluated. Similarly, other than the noteworthy exception of vaccines for HPV, which have been marketed for cancer rather than STI prevention, STI vaccine research and other prevention tools (e.g., antibiotic PrEP, expedited partner therapy), have moved forward in limited fashion, also due to the difficult development process and significant
time and resources needed. Research on potential gonococcal vaccines has been recently stimulated by the observed benefit of type B meningococcal vaccines on gonorrhea incidence and NIAID investment in basic research aimed at developing prototype vaccines for syphilis, gonorrhea, and chlamydia. Developing these drugs and vaccines for STI management and prevention has been, in part, constrained by the impact of perceived STI-related stigma on assessment of potential markets and a costly and time-consuming regulatory pathway. New and existing biomedical tools will need to be integrated with behavioral and structural interventions across the life span to have the greatest effect on the STI epidemic.
REFERENCES
Abbasi, J. 2020. COVID-19 and mRNA vaccines—first large test for a new approach. JAMA 324(12):1125-1127.
Abdool Karim, S. S., Q. Abdool Karim, A. B. Kharsany, C. Baxter, A. C. Grobler, L. Werner, A. Kashuba, L. E. Mansoor, N. Samsunder, A. Mindel, and T. N. Gengiah. 2015. Tenofovir gel for the prevention of herpes simplex virus type 2 infection. New England Journal of Medicine 373(6):530-539.
Aberg, J. A., J. E. Gallant, K. G. Ghanem, P. Emmanuel, B. S. Zingman, and M. A. Horberg. 2014. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases 58(1):e1-e34.
Abraham, S., H. B. Juel, P. Bang, H. M. Cheeseman, R. B. Dohn, T. Cole, M. P. Kristiansen, K. S. Korsholm, D. Lewis, A. W. Olsen, L. R. McFarlane, S. Day, S. Knudsen, K. Moen, M. Ruhwald, I. Kromann, P. Andersen, R. J. Shattock, and F. Follmann. 2019. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. The Lancet Infectious Diseases 19(10):1091-1100.
Akram, M., I. M. Tahir, S. M. A. Shah, Z. Mahmood, A. Altaf, K. Ahmad, N. Munir, M. Daniyal, S. Nasir, and H. Mehboob. 2018. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytotherapy Research 32(5):811-822.
Allan-Blitz, L. T., R. M. Humphries, P. Hemarajata, A. Bhatti, M. W. Pandori, M. J. Siedner, and J. D. Klausner. 2017. Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of Neisseria gonorrhoeae in a large health system. Clinical Infectious Diseases 64(9):1268-1270.
Anderson, D. J., J. A. Politch, R. A. Cone, L. Zeitlin, S. K. Lai, P. J. Santangelo, T. R. Moench, and K. J. Whaley. 2020. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies. Biology of Reproduction 103(2):275-285.
Archambault, J., and T. Melendy. 2013. Targeting human papillomavirus genome replication for antiviral drug discovery. Antiviral Therapy 18(3):271-283.
Baezconde-Garbanati, L., B. A. Lienemann, M. Robles, E. Johnson, K. Sanchez, R. Singhal, J. Steinberg, J. M. Jaque, M. A. Pentz, and S. Gruber. 2017. Implementation of HPV vaccination guidelines in a diverse population in Los Angeles: Results from an environmental scan of local HPV resources and needs. Vaccine 35(37):4930-4935.
Barrow, R. Y., F. Ahmed, G. A. Bolan, and K. A. Workowski. 2020. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recommendations and Reports 68(5):1-20.
Beksinska, M., R. Wong, and J. Smit. 2020. Male and female condoms: Their key role in pregnancy and STI/HIV prevention. Best Practice & Research Clinical Obstetrics & Gynaecology 66:55-67.
Belshe, R. B., P. A. Leone, D. I. Bernstein, A. Wald, M. J. Levin, J. T. Stapleton, I. Gorfinkel, R. L. A. Morrow, M. G. Ewell, A. Stokes-Riner, G. Dubin, T. C. Heineman, J. M. Schulte, and C. D. Deal. 2012. Efficacy results of a trial of a herpes simplex vaccine. New England Journal of Medicine 366(1):34-43.
Bibbins-Domingo, K., D. C. Grossman, S. J. Curry, K. W. Davidson, J. W. Epling, Jr., F. A. García, A. R. Kemper, A. H. Krist, A. E. Kurth, C. S. Landefeld, C. M. Mangione, W. R. Phillips, M. G. Phipps, M. P. Pignone, M. Silverstein, and C. W. Tseng. 2016. Serologic screening for genital herpes infection: U.S. Preventive Services Task Force recommendation statement. JAMA 316(23):2525-2530.
Blumenthal, K. G., J. G. Peter, J. A. Trubiano, and E. J. Phillips. 2019. Antibiotic allergy. Lancet 393(10167):183-198.
Boeras, D. I., N. Luisi, E. Karita, S. McKinney, T. Sharkey, M. Keeling, E. Chomba, C. Kraft, K. Wall, J. Bizimana, W. Kilembe, A. Tichacek, A. M. Caliendo, E. Hunter, and S. Allen. 2011. Indeterminate and discrepant rapid HIV test results in couples’ HIV testing and counselling centres in Africa. Journal of the International AIDS Society 14:18.
Bolan, G. 2019. Division of STD prevention. Presentation to the Committee on Sexually Transmitted Infections in the United States, Meeting 1 (August 26). https://www.nationalacademies.org/event/08-26-2019/docs/D3423E23A3A4A9B4447C205483C8F840EF1481A1EEE2 (accessed February 17, 2021).
Bolan, R. K., M. R. Beymer, R. E. Weiss, R. P. Flynn, A. A. Leibowitz, and J. D. Klausner. 2015. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: A randomized, controlled pilot study. Sexually Transmitted Diseases 42(2):98-103.
Bouchemal, K., C. Bories, and P. M. Loiseau. 2017. Strategies for prevention and treatment of Trichomonas vaginalis infections. Clinical Microbiology Reviews 30(3):811-825.
Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nature Reviews Immunology 5(2):149-161.
Buckley, C., B. M. Forde, E. Trembizki, M. M. Lahra, S. A. Beatson, and D. M. Whiley. 2018. Use of whole genome sequencing to investigate an increase in Neisseria gonorrhoeae infection among women in urban areas of Australia. Scientific Reports 8(1):1503.
Bulir, D. C., S. Liang, A. Lee, S. Chong, E. Simms, C. Stone, C. Kaushic, A. Ashkar, and J. B. Mahony. 2016. Immunization with chlamydial type III secretion antigens reduces vaginal shedding and prevents fallopian tube pathology following live C. muridarum challenge. Vaccine 34(34):3979-3985.
Carnevale, C., P. Richards, R. Cohall, J. Choe, J. Zitaner, N. Hall, A. Cohall, S. Whittier, D. A. Green, M. E. Sobieszczyk, P. Gordon, and J. Zucker. 2021. At home testing for sexually transmitted infections during the COVID-19 pandemic. Sexually Transmitted Diseases 48(1):e11-e14.
CDC (Centers for Disease Control and Prevention). 2017. Genital herpes—CDC fact sheet (detailed). https://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm (accessed November 9, 2020).
CDC. 2018. Test complexity categorization. https://www.cdc.gov/clia/test-complexities.html (accessed November 2, 2020).
CDC. 2019a. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: Centers for Disease Control and Prevention.
CDC. 2019b. Sexually transmitted disease surveillance 2018. Atlanta, GA: Department of Health and Human Services.
CDC. 2019c. Zika virus: Sexual transmission and prevention. https://www.cdc.gov/zika/prevention/sexual-transmission-prevention.html (accessed January 19, 2021).
CDC. 2020. Advisory Committee on Immunization Practices (ACIP): General committee-related information. https://www.cdc.gov/vaccines/acip/committee/index.html (accessed November 18, 2020).
CDC. 2021. Ebola (Ebola virus disease): Transmission. https://www.cdc.gov/vhf/ebola/transmission/index.html (accessed January 19, 2021).
Cherry, J. J., A. Rietz, A. Malinkevich, Y. Liu, M. Xie, M. Bartolowits, V. J. Davisson, J. D. Baleja, and E. J. Androphy. 2013. Structure based identification and characterization of flavonoids that disrupt human papillomavirus-16 E6 function. PLoS One 8(12):e84506.
CMS (Centers for Medicare & Medicaid Services). 2020. Clinical laboratory improvement amendments (CLIA). https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA (accessed November 18, 2020).
CMS. n.d. Tests granted waived status under CLIA. https://www.cms.gov/Regulations-andGuidance/Legislation/CLIA/Downloads/waivetbl.pdf (accessed November 18, 2020).
Coltart, C. E. M., A. Hoppe, M. Parker, L. Dawson, J. J. Amon, M. Simwinga, G. Geller, G. Henderson, O. Laeyendecker, J. D. Tucker, P. Eba, V. Novitsky, A. M. Vandamme, J. Seeley, G. Dallabetta, G. Harling, M. K. Grabowski, P. Godfrey-Faussett, C. Fraser, M. S. Cohen, and D. Pillay. 2018. Ethical considerations in global HIV phylogenetic research. The Lancet HIV 5(11):e656-e666.
Committee on Adolescence and Society for Adolescent Health and Medicine. 2014. Screening for nonviral sexually transmitted infections in adolescents and young adults. Pediatrics 134(1):e302-e311.
Copen, C. E. 2017. Condom use during sexual intercourse among women and men aged 15-44 in the United States: 2011-2015 National Survey of Family Growth. National Health Statistics Reports (105):1-18.
Corey, L. 1982. The diagnosis and treatment of genital herpes. JAMA 248(9):1041-1049.
Dallal Bashi, Y. H., C. F. McCoy, D. J. Murphy, P. Boyd, P. Spence, K. Kleinbeck, B. Devlin, and R. K. Malcolm. 2019. Towards a dapivirine and levonorgestrel multipurpose vaginal ring: Investigations into the reaction between levonorgestrel and addition-cure silicone elastomers. International Journal of Pharmaceutics 569:118574.
D’Anna, L. H., A. D. Margolis, L. Warner, O. A. Korosteleva, L. O’Donnell, C. A. Rietmeijer, J. D. Klausner, W. Nomura, and C. K. Malotte. 2012. Condom use problems during anal sex among men who have sex with men (MSM): Findings from the Safe in the City study. AIDS Care 24(8):1028-1038.
Das, S., K. Ramakrishnan, S. K. Behera, M. Ganesapandian, A. S. Xavier, and S. Selvarajan. 2019. Hepatitis B vaccine and immunoglobulin: Key concepts. Journal of Clinical and Translational Hepatology 7(2):165-171.
de Bruyn, G., S. Shiboski, A. van der Straten, K. Blanchard, T. Chipato, G. Ramjee, E. Montgomery, and N. Padian. 2011. The effect of the vaginal diaphragm and lubricant gel on acquisition of HSV-2. Sexually Transmitted Infections 87(4):301-305.
Deese, J., S. Pradhan, H. Goetz, and C. Morrison. 2018. Contraceptive use and the risk of sexually transmitted infection: Systematic review and current perspectives. Open Access Journal of Contraception 9:91-112.
Dennis, A. M., J. T. Herbeck, A. L. Brown, P. Kellam, T. de Oliveira, D. Pillay, C. Fraser, and M. S. Cohen. 2014. Phylogenetic studies of transmission dynamics in generalized HIV epidemics: An essential tool where the burden is greatest? Journal of Acquired Immune Deficiency Syndromes 67(2):181-195.
Derby, N., M. Aravantinou, J. Kenney, S. R. Ugaonkar, A. Wesenberg, J. Wilk, L. Kizima, A. Rodriguez, S. Zhang, O. Mizenina, K. Levendosky, M. L. Cooney, S. Seidor, A. Get-tie, B. Grasperge, J. Blanchard, M. Piatak, Jr., J. D. Lifson, J. Fernández-Romero, T. M. Zydowsky, and M. Robbiani. 2017. An intravaginal ring that releases three antiviral agents and a contraceptive blocks SHIV-RT infection, reduces HSV-2 shedding, and suppresses hormonal cycling in rhesus macaques. Drug Delivery and Translational Research 7(6):840-858.
DiClemente, R. J., E. Funkhouser, G. Wingood, H. Fawal, S. D. Holmberg, and S. H. Vermund. 2002. Protease inhibitor combination therapy and decreased condom use among gay men. Southern Medical Journal 95(4):421-425.
Doherty, M., P. Buchy, B. Standaert, C. Giaquinto, and D. Prado-Cohrs. 2016. Vaccine impact: Benefits for human health. Vaccine 34(52):6707-6714.
Doudna, J. A. 2020. The promise and challenge of therapeutic genome editing. Nature 578(7794):229-236.
Drain, P. K., J. Dorward, A. Bender, L. Lillis, F. Marinucci, J. Sacks, A. Bershteyn, D. S. Boyle, J. D. Posner, and N. Garrett. 2019. Point-of-care HIV viral load testing: An essential tool for a sustainable global HIV/AIDS response. Clinical Microbiology Reviews 32(3).
Dropulic, L. K., and J. I. Cohen. 2012. The challenge of developing a herpes simplex virus 2 vaccine. Expert Review Vaccines 11(12):1429-1440.
Duan, J., Y. Zhao, X. Zhang, H. Jiang, B. Xie, T. Zhao, and F. Zhao. 2020. Research status and perspectives for pathogenic spirochete vaccines. Clinica Chemica Acta 507:117-124.
ECHO (Evidence for Contraceptive Options and HIV Outcomes) Trial Consortium. 2019. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. The Lancet 394(10195):303-313.
Egan, K. P., L. M. Hook, A. Naughton, N. Pardi, S. Awasthi, G. H. Cohen, D. Weissman, and H. M. Friedman. 2020. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins GC, GD, and GE protects mice against HSV-1 genital lesions and latent infection. PLoS Pathogens 16(7):e1008795.
Eisinger, R. W., E. Erbelding, and A. S. Fauci. 2020. Refocusing research on sexually transmitted infections. Journal of Infectious Diseases 222(9):1432-1434.
FDA (Food and Drug Administration). 2017. Administrative procedures for CLIA categorization. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/administrative-procedures-clia-categorization (accessed February 17, 2021).
FDA. 2019. FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea (accessed November 2, 2020).
FDA. 2020a. CLIA categorizations. https://www.fda.gov/node/365445#scorecard (accessed November 18, 2020).
FDA. 2020b. CLIA waiver by application. https://www.fda.gov/medical-devices/ivdregulatory-assistance/clia-waiver-application (accessed November 2, 2020).
Federal Task Force on Combating Antibiotic-Resistant Bacteria. 2020. National action plan for combating antibiotic-resistant bacteria, 2020-2025. Washington, DC: Department of Health and Human Services, Department of Agriculture, and Department of Defense.
Fifer, H., J. Saunders, S. Soni, S. T. Sadiq, and M. FitzGerald. 2020. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. International Journal of STD & AIDS 31(1):4-15.
Freeborn, K., and C. J. Portillo. 2018. Does pre-exposure prophylaxis for HIV prevention in men who have sex with men change risk behaviour? A systematic review. Journal of Clinical Nursing 27(17-18):3254-3265.
Garfield, S., J. Polisena, S. S. D, A. Postulka, Y. L. C, S. K. Tiwana, E. Faulkner, N. Poulios, V. Zah, and M. Longacre. 2016. Health technology assessment for molecular diagnostics: Practices, challenges, and recommendations from the medical devices and diagnostics special interest group. Value Health 19(5):577-587.
Gaydos, C. A., and J. H. Melendez. 2020. Point-by-point progress: Gonorrhea point of care tests. Expert Review of Molecular Diagnostics 1-11.
Gaydos, C. A., K. Dwyer, M. Barnes, P. A. Rizzo-Price, B. J. Wood, T. Flemming, and M. T. Hogan. 2006. Internet-based screening for Chlamydia trachomatis to reach non-clinic populations with mailed self-administered vaginal swabs. Sexually Transmitted Diseases 33(7):451-457.
Gaydos, C. A., M. Barnes, B. Aumakhan, N. Quinn, P. Agreda, P. Whittle, and T. Hogan. 2009. Can e-technology through the Internet be used as a new tool to address the Chlamydia trachomatis epidemic by home sampling and vaginal swabs? Sexually Transmitted Diseases 36(9):577-580.
Gaydos, C. A., Y. H. Hsieh, L. Harvey, A. Burah, H. Won, M. Jett-Goheen, M. Barnes, P. Agreda, N. Arora, and R. E. Rothman. 2011. Will patients “opt in” to perform their own rapid HIV test in the emergency department? Annals of Emergency Medicine 58(1):S74-S78.
Gaydos, C. A., M. Jett-Goheen, M. Barnes, L. Dize, P. Barnes, and Y. H. Hsieh. 2016. Use of a risk quiz to predict infection for sexually transmitted infections: A retrospective analysis of acceptability and positivity. Sexually Transmitted Infections 92(1):44-48.
Gaydos, C. A., S. Beqaj, J. R. Schwebke, J. Lebed, B. Smith, T. E. Davis, K. H. Fife, P. Nyirjesy, T. Spurrell, D. Furgerson, J. Coleman, S. Paradis, and C. K. Cooper. 2017a. Clinical validation of a test for the diagnosis of vaginitis. Obstetrics and Gynecology 130(1):181-189.
Gaydos, C. A., J. D. Klausner, N. P. Pai, H. Kelly, C. Coltart, and R. W. Peeling. 2017b. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sexually Transmitted Infections 93(S4):S31-S35.
Gaydos, C. A., L. E. Manhart, S. N. Taylor, R. A. Lillis, E. W. Hook, 3rd, J. D. Klausner, C. V. Remillard, M. Love, B. McKinney, and D. K. Getman. 2019. Molecular testing for Mycoplasma genitalium in the United States: Results from the AMES prospective multicenter clinical study. Journal of Clinical Microbiology 57(11).
Gaydos, C. A., M. Barnes, J. Holden, B. Silver, R. Smith, J. Hardick, and T. C. Quinn. 2020. Acceptability and feasibility of recruiting women to collect a self-administered vaginal swab at a pharmacy clinic for sexually transmissible infection screening. Sex Health 17(4):392-394.
Geisler, W. M., S. Y. Lensing, C. G. Press, and E. W. Hook, 3rd. 2013. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. Journal of Infectious Diseases 207(12):1850-1856.
Gerlich, W. H. 2013. Medical virology of hepatitis B: How it began and where we are now. Virology Journal 10:239.
Gettinger, J., N. Van Wagoner, B. Daniels, A. Boutwell, and B. Van Der Pol. 2020. Patients are willing to wait for rapid sexually transmitted infection results in a university student health clinic. Sexually Transmitted Diseases 47(1):67-69.
Görander, S., M. Ekblad, T. Bergström, and J. Liljeqvist. 2014. Anti-glycoprotein G antibodies of herpes simplex virus 2 contribute to complete protection after vaccination in mice and induce antibody-dependent cellular cytotoxicity and complement-mediated cytolysis. Viruses 6(11):4358-4372.
Grant, J. S., C. Stafylis, C. Celum, T. Grennan, B. Haire, J. Kaldor, A. F. Luetkemeyer, J. M. Saunders, J. M. Molina, and J. D. Klausner. 2020. Doxycycline prophylaxis for bacterial sexually transmitted infections. Clinical Infectious Diseases 70(6):1247-1253.
Guilamo-Ramos, V., M. Reading, A. S. Bowman, D. C. Perlman, and S. Barrett. 2018. Multipurpose prevention technologies: A global sexual and reproductive health priority. The Journal of the Association of Nurses in AIDS Care 29(1):6-9.
Hamill, M. M., K. J. Mbazira, A. N. Kiragga, C. A. Gaydos, M. Jett-Goheen, R. Parkes-Ratanshi, Y. C. Manabe, E. Nakku-Joloba, and A. Rompalo. 2018. Challenges of rapid plasma reagin interpretation in syphilis screening in Uganda: Variability in nontreponemal results between different laboratories. Sexually Transmitted Diseases 45(12):829-833.
Handsfield, H. H. 2015. Sexually transmitted diseases, infections, and disorders: What’s in a name? Sexually Transmitted Diseases 42(4).
Hansen, B. T. 2016. No evidence that HPV vaccination leads to sexual risk compensation. Human Vaccines & Immunotherapeutics 12(6):1451-1453.
Harrison, W. O., R. R. Hooper, P. J. Wiesner, A. F. Campbell, W. W. Karney, G. H. Reynolds, O. G. Jones, and K. K. Holmes. 1979. A trial of minocycline given after exposure to prevent gonorrhea. New England Journal of Medicine 300(19):1074-1078.
Hawkes, S., E. Kismodi, H. Larson, and K. Buse. 2014. Vaccines to promote and protect sexual health: Policy challenges and opportunities. Vaccine 32(14):1610-1615.
Haynes, B. F., P. B. Gilbert, M. J. McElrath, S. Zolla-Pazner, G. D. Tomaras, S. M. Alam, D. T. Evans, D. C. Montefiori, C. Karnasuta, R. Sutthent, H.-X. Liao, A. L. DeVico, G. K. Lewis, C. Williams, A. Pinter, Y. Fong, H. Janes, A. DeCamp, Y. Huang, M. Rao, E. Billings, N. Karasavvas, M. L. Robb, V. Ngauy, M. S. de Souza, R. Paris, G. Ferrari, R. T. Bailer, K. A. Soderberg, C. Andrews, P. W. Berman, N. Frahm, S. C. De Rosa, M. D. Alpert, N. L. Yates, X. Shen, R. A. Koup, P. Pitisuttithum, J. Kaewkungwal, S. Nitayaphan, S. Rerks-Ngarm, N. L. Michael, and J. H. Kim. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. New England Journal of Medicine 366(14):1275-1286.
Herper, M. 2020. FDA approves Gardasil 9, the HPV vaccine, to prevent head-and-neck cancer. https://www.statnews.com/2020/06/12/fda-approves-gardasil-9-the-hpv-vaccine-to-prevent-head-and-neck-cancer (accessed January 21, 2021).
Hess, K. L., N. Crepaz, C. Rose, D. Purcell, and G. Paz-Bailey. 2017. Trends in sexual behavior among men who have sex with men (MSM) in high-income countries, 1990-2013: A systematic review. AIDS and Behavior 21(10):2811-2834.
HHS (Department of Health and Human Services). 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington, DC: Department of Health and Human Services.
Hofstetter, A. M., S. L. Rosenthal, and L. R. Stanberry. 2014. Current thinking on genital herpes. Current Opinion in Infectious Diseases 27(1):75-83.
Hogenson, E., M. Jett-Goheen, and C. A. Gaydos. 2019. An analysis of user survey data for an Internet program for testing for sexually transmitted infections, I Want the Kit, in Maryland and Washington, DC. Sexually Transmitted Diseases 46(12):768-770.
Holman, D. M., V. Benard, K. B. Roland, M. Watson, N. Liddon, and S. Stokley. 2014. Barriers to human papillomavirus vaccination among U.S. adolescents: A systematic review of the literature. JAMA Pediatrics 168(1):76-82.
Hook, E. W., 3rd. 2013. Remembering Thomas Parran, his contributions and missteps going forward: History informs us. Sexually Transmitted Diseases 40(4):281-282.
Hook, E. W., 3rd, and R. D. Kirkcaldy. 2018. A brief history of evolving diagnostics and therapy for gonorrhea: Lessons learned. Clinical Infectious Diseases 67(8):1294-1299.
Hook, E. W., 3rd, M. R. Golden, S. N. Taylor, E. Henry, C. Tseng, K. A. Workowski, J. Swerdlow, A. Nenninger, and S. Cammarata. 2019. Efficacy and safety of single-dose oral delafloxacin compared with intramuscular ceftriaxone for uncomplicated gonorrhea treatment: An open-label, noninferiority, phase 3, multicenter, randomized study. Sexually Transmitted Diseases 46(5):279-286.
Hsieh, M. H., J. M. Brotherton, and A. A. Siddiqui. 2015. Hepatitis B vaccines and HPV vaccines have been hailed as major public health achievements in preventing cancer—could a schistosomiasis vaccine be the third? PLoS Neglected Tropical Diseases 9(5):e0003598.
Hunter, L. A., L. Nelson, J. M. Chow, B. Y. Holt, and H. M. Bauer. 2018. Contraceptive method use and chlamydia positivity among California family planning clients: The case for new multipurpose prevention technologies. Journal of Women’s Health (2002) 27(6):768-774.
Hylton-Kong, T., A. R. Brathwaite, G. R. Del Rosario, S. Kristensen, P. Kamara, P. E. Jolly, E. W. Hook, 3rd, J. P. Figueroa, and S. H. Vermund. 2004. Marginal validity of syndromic management for reproductive tract infections among pregnant women in Jamaica. International Journal of STD & AIDS 15(6):371-375.
Hynes, J. S., J. M. Sales, A. N. Sheth, E. Lathrop, and L. B. Haddad. 2018. Interest in multipurpose prevention technologies to prevent HIV/STIs and unintended pregnancy among young women in the United States. Contraception 97(3):277-284.
Hynes, J. S., A. N. Sheth, E. Lathrop, J. M. Sales, and L. B. Haddad. 2019. Preferred product attributes of potential multipurpose prevention technologies for unintended pregnancy and sexually transmitted infections or HIV among U.S. women. Journal of Women’s Health (2002) 28(5):665-672.
Jatlaoui, T. C., K. B. Simmons, and K. M. Curtis. 2016. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections. Contraception 94(6):701-712.
Kanwar, R. K., N. Singh, S. Gurudevan, and J. R. Kanwar. 2011. Targeting hepatitis B virus and human papillomavirus induced carcinogenesis: Novel patented therapeutics. Recent Patents on Anti-Infective Drug Discovery 6(2):158-174.
Karim, Q. A., C. Baxter, and S. A. Karim. 2014. Microbicides and their potential as a catalyst for multipurpose sexual and reproductive health technologies. BJOG 121(Suppl 5):53-61.
Kashani, B. M., M. Tibbits, R. C. Potter, R. Gofin, L. Westman, and S. Watanabe-Galloway. 2019. Human papillomavirus vaccination trends, barriers, and promotion methods among American Indian/Alaska Native and non-Hispanic white adolescents in Michigan 2006-2015. Journal of Community Health 44(3):436-443.
Kim, H. C., and H. K. Lee. 2020. Vaccines against genital herpes: Where are we? Vaccines (Basel) 8(3).
Kimball, A., E. Torrone, K. Miele, L. Bachmann, P. Thorpe, H. Weinstock, and V. Bowen. 2020. Missed opportunities for prevention of congenital syphilis—United States, 2018. Morbity and Mortality Weekly Report 69:661-665.
Kirkcaldy, R. D., A. Harvey, J. R. Papp, C. Del Rio, O. O. Soge, K. K. Holmes, E. W. Hook, 3rd, G. Kubin, S. Riedel, J. Zenilman, K. Pettus, T. Sanders, S. Sharpe, and E. Torrone. 2016. Neisseria gonorrhoeae antimicrobial susceptibility—the Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveillance Summary 65(7):1-19.
Klein, K., and S. Luedtke. 2008. Human papillomavirus vaccination: A case for mandatory immunization? The Journal of Pediatric Pharmacology and Therapeutics 13(1):47-50.
Kobayashi, K., K. Hisamatsu, N. Suzui, A. Hara, H. Tomita, and T. Miyazaki. 2018. A review of HPV-related head and neck cancer. Journal of Clinical Medicine 7(9).
Korte, B. J., A. Rompalo, Y. C. Manabe, and C. A. Gaydos. 2020. Overcoming challenges with the adoption of point-of-care testing: From technology push and clinical needs to value propositions. Point of Care 19(3):77-83.
Ladhani, S. N., J. Lucidarme, S. R. Parikh, H. Campbell, R. Borrow, and M. E. Ramsay. 2020. Meningococcal disease and sexual transmission: Urogenital and anorectal infections and invasive disease due to Neisseria meningitidis. Lancet 395(10240):1865-1877.
LeFevre, M. L. 2014. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 161(12):902-910.
Lei, J., A. Ploner, K. M. Elfström, J. Wang, A. Roth, F. Fang, K. Sundström, J. Dillner, and P. Sparén. 2020. HPV vaccination and the risk of invasive cervical cancer. New England Journal of Medicine 383(14):1340-1348.
Li, J., G. Regev, S. K. Patel, D. Patton, Y. Sweeney, P. Graebing, S. Grab, L. Wang, V. Sant, and L. C. Rohan. 2019. Rational design of a multipurpose bioadhesive vaginal film for co-delivery of dapivirine and levonorgestrel. Pharmaceutics 12(1).
Lithgow, K. V., and C. E. Cameron. 2017. Vaccine development for syphilis. Expert Review of Vaccines 16(1):37-44.
Lithgow, K. V., R. Hof, C. Wetherell, D. Phillips, S. Houston, and C. E. Cameron. 2017. A defined syphilis vaccine candidate inhibits dissemination of Treponema pallidum subspecies pallidum. Nature Communications 8:14273.
Liu, A. Y., and S. P. Buchbinder. 2017. CROI 2017: HIV epidemic trends and advances in prevention. Topics in Antiviral Medicine 25(2):35-50.
Liu, Y., L. A. Hammer, W. Liu, M. M. Hobbs, R. A. Zielke, A. E. Sikora, A. E. Jerse, N. K. Egilmez, and M. W. Russell. 2017. Experimental vaccine induces TH1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunology 10(6):1594-1608.
Lovett, A., and J. A. Duncan. 2018. Human immune responses and the natural history of Neisseria gonorrhoeae infection. Frontiers in Immunology 9:3187.
Luthra, A., J. M. Montezuma-Rusca, C. J. La Vake, M. LeDoyt, K. N. Delgado, T. C. Davenport, M. Fiel-Gan, M. J. Caimano, J. D. Radolf, and K. L. Hawley. 2020. Evidence that immunization with TP0751, a bipartite Treponema pallidum lipoprotein with an intrinsically disordered region and lipocalin fold, fails to protect in the rabbit model of experimental syphilis. PLoS Pathogens 16(9):e1008871.
Ma, B., M. T. France, J. Crabtree, J. B. Holm, M. S. Humphrys, R. M. Brotman, and J. Ravel. 2020. A comprehensive non-redundant gene catalog reveals extensive within-community intraspecies diversity in the human vagina. Nature Communications 11(1):940.
Macaluso, M., J. Kelaghan, L. Artz, H. Austin, M. Fleenor, E. W. Hook, 3rd, and T. Valappil. 1999. Mechanical failure of the latex condom in a cohort of women at high STD risk. Sexually Transmitted Diseases 26(8):450-458.
Malekinejad, M., A. Parriott, J. C. Blodgett, H. Horvath, R. K. Shrestha, A. B. Hutchinson, P. Volberding, and J. G. Kahn. 2017. Effectiveness of community-based condom distribution interventions to prevent HIV in the United States: A systematic review and meta-analysis. PLoS One 12(8):e0180718.
Marks, M., A. W. Solomon, and D. C. Mabey. 2014. Endemic treponemal diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene 108(10):601-607.
Marrazzo, J., and R. Suchland. 2014. Recent advances in understanding and managing Chlamydia trachomatis infections. F1000Prime Reports 6:120.
Marrazzo, J. M., D. H. Martin, D. H. Watts, J. Schulte, J. D. Sobel, S. L. Hillier, C. Deal, and D. N. Fredricks. 2010. Bacterial vaginosis: Identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sexually Transmitted Diseases 37(12):732-744.
Maruggi, G., C. Zhang, J. Li, J. B. Ulmer, and D. Yu. 2019. mRNA as a transformative technology for vaccine development to control infectious diseases. Molecular Therapy 27(4):757-772.
McCarthy, K. J., E. L. Gollub, L. Ralph, J. van de Wijgert, and H. E. Jones. 2019. Hormonal contraceptives and the acquisition of sexually transmitted infections: An updated systematic review. Sexually Transmitted Diseases 46(5):290-296.
McIntosh, E. D. G. 2020. Development of vaccines against the sexually transmitted infections gonorrhoea, syphilis, chlamydia, herpes simplex virus, human immunodeficiency virus and Zika virus. Therapeutic Advances in Vaccines and Immunotherapy 8:2515135520923887.
Melendez, J. H., J. Hardick, M. Barnes, K. R. Page, and C. A. Gaydos. 2018. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates in Baltimore, Maryland, 2016: The importance of sentinel surveillance in the era of multi-drug-resistant gonorrhea. Antibiotics (Basel) 7(3).
Melendez, J. H., Y. H. Hsieh, M. Barnes, J. Hardick, E. A. Gilliams, and C. A. Gaydos. 2019. Can ciprofloxacin be used for precision treatment of gonorrhea in public STD clinics? Assessment of ciprofloxacin susceptibility and an opportunity for point-of-care testing. Pathogens 8(4).
Melendez, J. H., M. M. Hamill, G. Armington, C. A. Gaydos, and Y. C. Manabe. 2020. Home-based testing for sexually transmitted infections: Leveraging online resources during the COVID-19 pandemic. Sexually Transmitted Diseases 48(1):e11-e14.
Miller, J. N. 1973. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by γ-irradiation. Journal of Immunology 110(5):1206-1215.
Minor, P. D. 2015. Live attenuated vaccines: Historical successes and current challenges. Virology 479-480:379-392.
Mitjà, O., K. Asiedu, and D. Mabey. 2013. Yaws. Lancet 381(9868):763-773.
Młynarczyk-Bonikowska, B., A. Majewska, M. Malejczyk, G. Młynarczyk, and S. Majewski. 2020. Multiresistant Neisseria gonorrhoeae: A new threat in second decade of the XXI century. Medical Microbiolgy and Immunology 209(2):95-108.
Molina, J. M., I. Charreau, C. Chidiac, G. Pialoux, E. Cua, C. Delaugerre, C. Capitant, D. Rojas-Castro, J. Fonsart, B. Bercot, C. Bebear, L. Cotte, O. Robineau, F. Raffi, P. Charbonneau, A. Aslan, J. Chas, L. Niedbalski, B. Spire, L. Sagaon-Teyssier, D. Carette, S. L. Mestre, V. Dore, and L. Meyer. 2018. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: An open-label randomised substudy of the ANRS IPERGAY trial. The Lancet Infectious Diseases 18(3):308-317.
NIAID (National Institute of Allergy and Infectious Diseases). 2019. NIH awards will advance development of vaccines for sexually transmitted infections. https://www.niaid.nih.gov/news-events/nih-awards-will-advance-development-vaccines-sexually-transmittedinfections (accessed November 2, 2020).
North, A. L., and L. M. Niccolai. 2016. Human papillomavirus vaccination requirements in U.S. schools: Recommendations for moving forward. American Journal of Public Health 106(10):1765-1770.
Nour, S., Y. H. Hsieh, R. E. Rothman, M. Jett-Goheen, O. Langhorne, L. Wu, S. Peterson, and C. A. Gaydos. 2012. Patients can accurately perform their own rapid HIV point-of-care test in the emergency department. Point of Care 11(4):176-179.
Obafemi, O. A., K. A. Wendel, T. S. Anderson, T. E. Scott, S. E. Rowan, E. A. Travanty, and C. A. Rietmeijer. 2019. Rapid syphilis testing for men who have sex with men in outreach settings: Evaluation of test performance and impact on time to treatment. Sexually Transmitted Diseases 46(3):191-195.
O’Donoghue, A. J., B. Bibo-Verdugo, Y. Miyamoto, S. C. Wang, J. Z. Yang, D. E. Zuill, S. Matsuka, Z. Jiang, J. Almaliti, C. R. Caffrey, W. H. Gerwick, and L. Eckmann. 2019. 20S proteasome as a drug target in Trichomonas vaginalis. Antimicrobial Agents and Chemotherapy 63(11).
Ortayli, N., K. Ringheim, L. Collins, and T. Sladden. 2014. Sexually transmitted infections: Progress and challenges since the 1994 International Conference on Population and Development (ICPD). Contraception 90(6 Suppl):S22-S31.
Padian, N. S., A. van der Straten, G. Ramjee, T. Chipato, G. de Bruyn, K. Blanchard, S. Shiboski, E. T. Montgomery, H. Fancher, H. Cheng, M. Rosenblum, M. van der Laan, N. Jewell, and J. McIntyre. 2007. Diaphragm and lubricant gel for prevention of HIV acquisition in Southern African women: A randomised controlled trial. The Lancet 370(9583):251-261.
Papp, J. R., J. Schachter, C. A. Gaydos, and B. Van Der Pol. 2014. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recommendations and Reports 63(RR-02):1-19.
Parascandola, J. 2001. John Mahoney and the introduction of penicillin to treat syphilis. Pharmacy in History 43(1):3-13.
Pardi, N., M. J. Hogan, F. W. Porter, and D. Weissman. 2018. mRNA vaccines—a new era in vaccinology. Nature Reviews Drug Discovery 17(4):261-279.
Patel, A. V., C. A. Gaydos, M. Jett-Goheen, M. Barnes, L. Dize, P. Barnes, and Y. H. Hsieh. 2018. Assessing association between I Want the Kit risk quiz tool and sexually transmitted infection positivity in male users for sexually transmitted infection screening. International Journal of STD & AIDS 29(2):122-127.
Paynter, J., F. Goodyear-Smith, J. Morgan, P. Saxton, S. Black, and H. Petousis-Harris. 2019. Effectiveness of a group B outer membrane vesicle meningococcal vaccine in preventing hospitalization from gonorrhea in New Zealand: A retrospective cohort study. Vaccines 7(1):5.
Paz-Bailey, G., M. Noble, K. Salo, and S. J. Tregear. 2016. Prevalence of HIV among U.S. female sex workers: Systematic review and meta-analysis. AIDS and Behavior 20(10):2318-2331.
Pérez, A., E. K. Santamaria, and D. Operario. 2018. A systematic review of behavioral interventions to reduce condomless sex and increase HIV testing for Latino MSM. Journal of Immigrant and Minority Health 20(5):1261-1276.
Perros, M. 2019. Spoken remarks: Presented at Meeting 3 of the Committee on Prevention and Control of Sexually Transmitted Infections in the United States. Recording available at https://www.nationalacademies.org/event/10-27-2019/prevention-and-control-of-sexually-transmitted-infections-in-the-united-states (accessed February 17, 2021).
Petousis-Harris, H., and F. J. Radcliff. 2019. Exploitation of Neisseria meningitidis group B OMV vaccines against N. gonorrhoeae to inform the development and deployment of effective gonorrhea vaccines. Frontiers in Immunology 10:683.
Pettifor, A., S. Delany, I. Kleinschmidt, W. C. Miller, J. Atashili, and H. Rees. 2009. Use of injectable progestin contraception and risk of STI among South African women. Contraception 80(6):555-560.
Phillips, S., B. L. Quigley, and P. Timms. 2019. Seventy years of chlamydia vaccine research—limitations of the past and directions for the future. Frontiers in Microbiology 10:70.
Planned Parenthood. 2018. Fun facts about condoms!, edited by Planned Parenthood of the Pacific Southwest.
Plotkin, S. 2014. History of vaccination. Proceedings of the National Academy of Sciences 111(34):12283-12287.
Poston, T. B., and T. Darville. 2018. Chlamydia trachomatis: Protective adaptive responses and prospects for a vaccine. Current Topics in Microbiology and Immunology 412:217-237.
Radolf, J. D., R. K. Deka, A. Anand, D. Smajs, M. V. Norgard, and X. F. Yang. 2016. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nature Reviews Microbiology 14(12):744-759.
Ramchandani, M. S., and M. R. Golden. 2019. Confronting rising STIs in the era of PrEP and treatment as prevention. Current HIV/AIDS Reports 16(3):244-256.
Rasnake, M. S., N. G. Conger, K. McAllister, K. K. Holmes, and E. C. Tramont. 2005. History of U.S. military contributions to the study of sexually transmitted diseases. Military Medicine 170(4 Suppl):61-65.
Rietmeijer, C. A. 2015. You say STD…. Sexually Transmitted Diseases 42(9).
Roddy, R. E., L. Zekeng, K. A. Ryan, U. Tamoufé, S. S. Weir, and E. L. Wong. 1998. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. New England Journal of Medicine 339(8):504-510.
Roddy, R. E., L. Zekeng, K. A. Ryan, U. Tamoufé, and K. G. Tweedy. 2002. Effect of nonoxynol-9 gel on urogenital gonorrhea and chlamydial infection: A randomized controlled trial. JAMA 287(9):1117-1122.
Rogozińska, E., L. Kara-Newton, J. R. Zamora, and K. S. Khan. 2017. On-site test to detect syphilis in pregnancy: A systematic review of test accuracy studies. BJOG 124(5):734-741.
Rojas Castro, D., R. M. Delabre, and J. M. Molina. 2019. Give PrEP a chance: Moving on from the “risk compensation” concept. Journal of the International AIDS Society 22(Suppl 6):e25351.
Ronn, M. M., N. A. Menzies, T. L. Gift, H. W. Chesson, T. A. Trikalinos, M. Bellerose, Y. Malyuta, A. Berruti, C. A. Gaydos, K. K. Hsu, and J. A. Salomon. 2019. Potential for point-of-care tests to reduce chlamydia-associated burden in the United States: A mathematical modeling analysis. Clinical Infectious Diseases 70(9):1816-1823.
Russell, M. W., A. E. Jerse, and S. D. Gray-Owen. 2019. Progress toward a gonococcal vaccine: The way forward. Frontiers in Immunology 10:2417.
Schiffer, J. T., and L. Corey. 2009. New concepts in understanding genital herpes. Current Infectious Disease Reports 11(6):457-464.
Schwebke, J., S. Taylor, R. Ackerman, R. Schlaberg, N. Quigley, C. Gaydos, S. Chavoustie, P. Nyirjesy, C. Remillard, P. Estes, B. McKinney, D. Getman, and C. Clark. 2019. Clinical validation of the Aptima bacterial vaginosis and Aptima candida/trichomonas vaginitis assays: Results from a prospective multicenter clinical study. Journal of Clinical Microbiology 58.
Shiraki, K. 2018. Antiviral drugs against alphaherpesvirus. Advances in Experimental Medicine and Biology 1045:103-122.
Smith, J. M., J. A. Moss, P. Srinivasan, I. Butkyavichene, M. Gunawardana, R. Fanter, C. S. Miller, D. Sanchez, F. Yang, S. Ellis, J. Zhang, M. A. Marzinke, C. W. Hendrix, A. Kapoor, and M. M. Baum. 2017. Novel multipurpose pod-intravaginal ring for the prevention of HIV, HSV, and unintended pregnancy: Pharmacokinetic evaluation in a macaque model. PLoS One 12(10):e0185946.
Solman, P. 2020. How a crumbling antibiotics infrastructure could yield “catastrophe.” PBS NewsHour. https://www.pbs.org/newshour/show/how-a-crumbling-antibiotics-infrastructure-could-yield-catastrophe (accessed November 16, 2020).
Srinivasan, S., M. T. Morgan, T. L. Fiedler, D. Djukovic, N. G. Hoffman, D. Raftery, J. M. Marrazzo, and D. N. Fredricks. 2015. Metabolic signatures of bacterial vaginosis. mBio 6(2):e00204-00215.
St. Cyr, S., L. Barbee, K. A. Workowski, L. H. Bachmann, C. Pham, K. Schlanger, E. Torrone, H. Weinstock, E. N. Kersh, and P. Thorpe. 2020. Update to CDC’s treatment guidelines for gonococcal infection, 2020. Morbidity and Mortality Weekly Report 69(50):1911-1916.
Statista. 2020. Usage of condoms in housholds in the U.S. 2020. https://www.statista.com/statistics/275455/us-households-usage-of-condoms (accessed November 18, 2020).
TAG (Treatment Action Group). 2019. Gonorrhea, chlamydia, and syphilis pipline report. https://www.ncsddc.org/wp-content/uploads/2019/03/TAG_Pipeline_STI_2019_draft.pdf (accessed October 25, 2020).
Theel, E. S., S. S. Katz, and A. Pillay. 2020. Molecular and direct detection tests for Treponema pallidum subspecies pallidum: A review of the literature, 1964-2017. Clinical Infectious Diseases 71(Suppl 1):S4-S12.
Tifrea, D. F., S. Pal, J. Fairman, P. Massari, and L. M. de la Maza. 2020. Protection against a chlamydial respiratory challenge by a chimeric vaccine formulated with the Chlamydia muridarum major outer membrane protein variable domains using the Neisseria lactamica porin B as a scaffold. NPJ Vaccines 5(1):37.
Tinajeros, F., D. Grossman, K. Richmond, M. Steele, S. G. Garcia, L. Zegarra, and R. Revollo. 2006. Diagnostic accuracy of a point-of-care syphilis test when used among pregnant women in Bolivia. Sexually Transmitted Infections 82(Suppl 5):v17-v21.
Trivedi, S., C. Williams, E. Torrone, and S. Kidd. 2019. National trends and reported risk factors among pregnant women with syphilis in the United States, 2012-2016. Obstetrics and Gynecology 133(1):27-32.
Tucker, J. D., C. H. Bien, and R. W. Peeling. 2013. Point-of-care testing for sexually transmitted infections: Recent advances and implications for disease control. Current Opinion in Infectious Diseases 26(1):73-79.
Unemo, M., C. S. Bradshaw, J. S. Hocking, H. J. C. de Vries, S. C. Francis, D. Mabey, J. M. Marrazzo, G. J. B. Sonder, J. R. Schwebke, E. Hoornenborg, R. W. Peeling, S. S. Philip, N. Low, and C. K. Fairley. 2017. Sexually transmitted infections: Challenges ahead. The Lancet Infectious Diseases 17(8):e235-e279.
USPSTF (United States Preventive Services Task Force). 2016. Final recommendation statement: Syphilis infection in nonpregnant adults and adolescents: Screening. JAMA 315(21):2321-2327.
Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. M. Tshibaka, V. Ettiègne-Traoré, C. Uaheowitchai, S. S. A. Karim, B. Mâsse, J. Perriëns, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. The Lancet 360(9338):971-977.
van den Munckhof, E. H. A., R. L. van Sitter, K. E. Boers, R. F. Lamont, R. Te Witt, S. le Cessie, C. W. Knetsch, L. J. van Doorn, W. G. V. Quint, A. Molijn, and M. A. Leversteinvan Hall. 2019. Comparison of Amsel criteria, Nugent score, culture and two CE-IVD marked quantitative real-time PCRs with microbiota analysis for the diagnosis of bacterial vaginosis. European Journal of Clinical Microbiology & Infectious Diseases 38(5):959-966.
Van Der Pol, B., S. N. Taylor, L. Mena, J. Lebed, C. J. McNeil, L. Crane, A. Ermel, A. SukhijaCohen, and C. A. Gaydos. 2020. Evaluation of the performance of a point-of-care test for chlamydia and gonorrhea. JAMA Network Open 3(5):e204819.
van der Schalk, T. E., J. F. Braam, and J. G. Kusters. 2020. Molecular basis of antimicrobial resistance in Mycoplasma genitalium. International Journal of Antimicrobial Agents 55(4):105911.
Vargas, S. E., M. M. Midoun, M. Guillen, M. L. Getz, K. Underhill, C. Kuo, and K. M. Guthrie. 2019. A qualitative systematic review of women’s experiences using contraceptive vaginal rings: Implications for new technologies. Perspectives on Sexual and Reproductive Health 51(2):71-80.
Vere Hodge, R. A., and H. J. Field. 2013. Antiviral agents for herpes simplex virus. Advances in Pharmacology 67:1-38.
Vrazo, A. C., D. Sullivan, and B. Ryan Phelps. 2018. Eliminating mother-to-child transmission of HIV by 2030: 5 strategies to ensure continued progress. Global Health, Science and Practice 6(2):249-256.
Walensky, R. P., and I. V. Bassett. 2011. HIV self-testing and the missing linkage. PLoS Medicine 8(10):e1001101.
Walker, T. Y., L. D. Elam-Evans, D. Yankey, L. E. Markowitz, C. L. Williams, B. Fredua, J. A. Singleton, and S. Stokley. 2019. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2018. Morbidity and Mortality Weekly Report 68(33):718-723.
Wang, J. W., and R. B. Roden. 2013. Virus-like particles for the prevention of human papillomavirus-associated malignancies. Expert Review of Vaccines 12(2):129-141.
Wang, T., M. Lurie, D. Govindasamy, and C. Mathews. 2018. The effects of school-based condom availability programs (CAPs) on condom acquisition, use and sexual behavior: A systematic review. AIDS and Behavior 22(1):308-320.
WHO (World Health Organization). 2007. Training modules for the syndromic management of sexually transmitted infections. 2nd ed. Geneva, Switzerland: WHO Press.
WHO. 2016. WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva, Switzerland: World Health Organization.
WHO. 2019. WHO preferred product characteristics for herpes simplex virus vaccines. Geneva, Switzerland: World Health Organization.
Widdice, L. E., Y. H. Hsieh, B. Silver, M. Barnes, P. Barnes, and C. A. Gaydos. 2018. Performance of the Atlas Genetics rapid test for Chlamydia trachomatis and women’s attitudes toward point-of-care testing. Sexually Transmitted Diseases 45(11):723-727.
Wilkinson, D., M. Tholandi, G. Ramjee, and G. W. Rutherford. 2002. Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: Systematic review and meta-analysis of randomised controlled trials including more than 5000 women. The Lancet Infectious Diseases 2(10):613-617.
Workowski, K. A., and G. A. Bolan. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 64(RR-03):1-137.
Wu, T., S. Qu, Y. Fang, M. Ip, and Z. Wang. 2019. Behavioral intention to perform risk compensation behaviors after receiving HPV vaccination among men who have sex with men in China. Human Vaccines & Immunotherapeutics 15(7-8):1737-1744.
Zhang, Y., C. Wymant, O. Laeyendecker, M. K. Grabowski, M. Hall, S. Hudelson, E. Piwowar-Manning, M. McCauley, T. Gamble, M. C. Hosseinipour, N. Kumarasamy, J. G. Hakim, J. Kumwenda, L. A. Mills, B. R. Santos, B. Grinsztejn, J. H. Pilotto, S. Chariyalertsak, J. Makhema, Y. Q. Chen, M. S. Cohen, C. Fraser, and S. H. Eshleman. 2021. Evaluation of phylogenetic methods for inferring the direction of HIV transmission: HPTN 052. Clinical Infectious Diseases 72(1):30-37.
This page intentionally left blank.






























































