Because I was HIV positive, I regularly tested my partners for HIV. But I didn’t understand that testing them for STDs like chlamydia, gonorrhea, and syphilis was just as important. I didn’t know that my partner was stepping out on me and had contracted chlamydia. They exposed me to chlamydia because I wasn’t testing for that, causing my viral load to increase and my CD4 to go down. That happens when someone who is living with HIV contracts an opportunistic infection.
—Participant, lived experience panel1
INTRODUCTION
In this chapter, the committee considers the interactions between HIV, the world’s most important sexually transmitted infection (STI), and “classical” STIs (especially the curable bacterial STIs) that lead to mucosal inflammation and genital ulcers. There is a large literature that examines the biological, epidemiological, and behavioral aspects of HIV–STI interactions. This chapter highlights aspects of these interactions that may affect the trajectory of the ongoing STI epidemics in the United States. The chapter also examines how prevention and care programs to control the distinct, yet intertwined HIV and “classical” STI epidemics diverged, with profound adverse effects on the spread of STIs. Considering this history emphasizes opportunities to develop more efficient programs going forward that recognize the “syndemic” relationship between HIV, STIs, and viral hepatitis that can and should be addressed in an integrated fashion.
HISTORY
HIV was first recognized in 1981, in Los Angeles and New York City where reports of gay, bisexual, and other men who have sex with men (MSM) who acquired pneumocystis pneumonia and/or Kaposi’s sarcoma secondary to immunodeficiency foreshadowed the AIDS pandemic (CDC, 1981; Gottlieb et al., 1981, 1982; Masur et al., 1981). Given the initial clustering of cases, this syndrome was initially termed “gay-related immune deficiency,” and later renamed “acquired immunodeficiency syndrome” (Marx, 1982). The appearance of the same disease in persons with hemophilia (CDC, 1983; Gottlieb et al., 1982; Marx, 1983), neonates (Marx,
___________________
1 The committee held virtual information-gathering meetings on September 9 and 14, 2020, to hear from individuals about their experiences with issues related to STIs. Quotes included throughout the report are from individuals who spoke to the committee during these meetings.
1983), injection drug using women and women sex workers (Harris et al., 1983; Masur et al., 1982), transfusion recipients (CDC, 1982), and children (Oleske et al., 1983; Rosner and Giron, 1983; Rubinstein et al., 1983) clarified the route(s) of transmission of the causal pathogen: sexual, blood-borne, and mother-to-child transmission during pregnancy, delivery, and breastfeeding (Royce et al., 1997).
In 1983 and 1984, the causative agent, a retrovirus named “human immunodeficiency virus,” was discovered and described, and antibody testing became available in 1985 (Gallo, 2002; Gallo and Montagnier, 2003). It was several years before it was accepted widely that HIV was primarily a sexually transmitted disease, however, and delays in prevention interventions contributed to the spread of the virus in both MSM and heterosexuals.
While HIV spread rapidly worldwide it attracted attention due to mounting morbidity and mortality in endemic regions and when it was shown to be indiscriminate in its potential to infect (e.g., children, women, blood product recipients, and celebrities). The aggressive and effective advocacy of lesbian, gay, bisexual, transgender, and queer (LGBTQ+) and other communities helped accelerate research, services, and policy changes (Shilts, 1987). The importance of advocacy in efforts to control the spread of HIV in the United States and worldwide is discussed later in this chapter.
HIV tests rendered the blood supply much safer (Weiss et al., 1985). Virus transmission remained an immense challenge nonetheless, through sexual, parenteral, and perinatal routes (Coates and Schechter, 1988; Royce et al., 1997; Vermund and Leigh-Brown, 2012). Although a worldwide pandemic ensued, the transmission of HIV per sexual encounter was recognized to be inefficient relative to other STIs. For example, gonorrhea transmission is very efficient, as it has a 20–53 percent chance of transmission from a woman infected with gonorrhea to a male partner through a single episode of vaginal intercourse (Hooper et al., 1978). By comparison, HIV transmission may occur just once in 300–1,000 sexual encounters involving someone with a chronic infection (Blower and Boe, 1993; Hollingsworth et al., 2008; Pinkerton et al., 2011; Powers et al., 2008; Røttingen and Garnett, 2002; Royce et al., 1997; Varghese et al., 2002).
As a consequence of transient very high viral loads, before the host immune system can respond, acutely infected persons have far higher transmission probabilities (Pilcher et al., 2004b; Pinkerton et al., 2011), closer to those seen with bacterial STIs. Viral load (blood and genital tract) in someone with HIV can predict sexual transmission risk to an HIV-negative partner (Baeten et al., 2011; Chakraborty et al., 2001; Cohen et al., 2011; Fideli et al., 2001; Quinn et al., 2000). While patients with acute
HIV infection have the highest viral load observed during the course of infection as the transmitted virus has rapid growth until restrained by evolving host defenses (Pilcher et al., 2004b), even chronically infected persons can be especially infectious if their viral loads are high, as with genital inflammation that recruits infected T-cells into the genital tract (Cohen et al., 2019; Passmore et al., 2016). Co-factors for the efficiency of sexual transmission continue to be elucidated (Council et al., 2020; Noël-Romas et al., 2020).
Blood-borne transmission among people who inject drugs is quite efficient because of the volume of infectious blood that can be derived from a person with untreated HIV (Degenhardt et al., 2010). The opioid crisis has contributed to a new wave of HIV in some parts of the United States, as illustrated by the Scott County, Indiana outbreak (Alpren et al., 2020; Crowley and Millett, 2017; Des Jarlais et al., 2020; Gonsalves and Crawford, 2018; Kishore et al., 2019; Peters et al., 2016).
While HIV spread is driven by the prevalence of unrecognized and untreated HIV infections (Cohen, 2006), co-factors and especially concomitant STIs enhance the efficiency of sexual HIV transmission, by increasing either the person’s infectiousness or the partner’s susceptibility (Baggaley and Hollingsworth, 2015; Blaser et al., 2014) (see Figure 5-1).
Sexual behaviors per se can affect the probability of transmission. For example, condomless anal intercourse leads to a transmission probability at least 10 times higher than penile-vaginal intercourse (Dosekun and Fox, 2010; Harman et al., 2013; Vermund and Leigh-Brown, 2012). It is generally believed that the density of lymphocytes and dendritic cells “defending” anal tissue (yet susceptible to HIV), and possibly the fragility of the anal and rectal mucosa and associated trauma, accounts for this risk (Harman et al., 2013; Kaul et al., 2008; Schneider et al., 1996).
Early in the pandemic, it was recognized that STIs (e.g., syphilis, herpes simplex virus type 2 [HSV-2], gonorrhea, and chlamydia) that were detected in people with HIV infection, might have amplified viral transmission through access provided by genital ulceration and mucosal inflammation (see Figure 5-1). Piot and Laga (1989, p. 624) focused on this idea in their early work in Zaire, stating that
Programs to control sexually transmitted diseases should be strengthened or initiated where they do not exist. Not only will this reduce the incidence of severe complications and sequelae of sexually transmitted disease, but it may also interfere with the spread of HIV. The two things not to do are to take the resources for AIDS prevention away from the general budget to control sexually transmitted diseases and to isolate the programs to prevent AIDS from those to control sexually transmitted diseases.
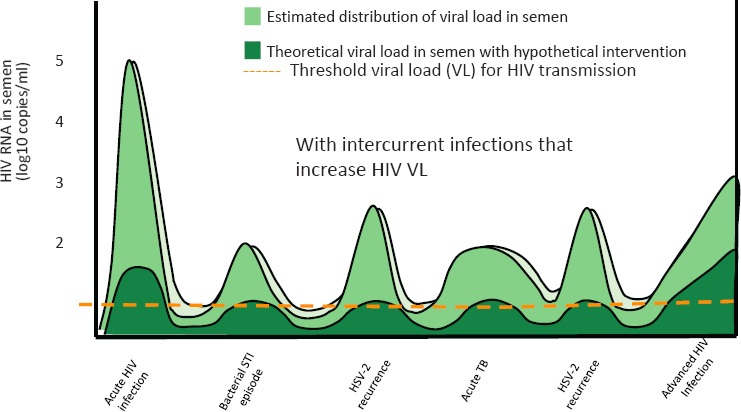
NOTE: HSV-2 = herpes simplex virus type 2; TB = tuberculosis.
SOURCE: Informed by Cohen and Pilcher, 2005.
The relationship between well-known STIs and HIV was described as “epidemiologic synergy” to emphasize how each might exacerbate the transmission or pathogenicity of the other (Wasserheit, 1992). The synergy concept highlighted key populations that might be affected by both HIV and other STIs, including the challenges in treating STIs in persons with advanced HIV who might be profoundly immunosuppressed. According to this idea, STIs made people more susceptible to HIV and rendered people with HIV more infectious as well.
Interest in the relationship between STIs and HIV led to an explosion of research, including study of the biological basis for epidemiologic synergy (Galvin and Cohen, 2004). When the HIV pandemic began, the virus only could be studied by growth in tissue culture, a laborious procedure that required intensive safety precautions. Developing a polymerase chain reaction (PCR) assay that amplified viral RNA allowed HIV to be quantitated as the number of copies in blood or a bodily secretion. Using this technique, Mellors et al. (1996) were able to show that the HIV concentration in blood was correlated with the rate of clinical decline in the MultiCenter AIDS Cohort Study. In remarkably consistent landmark articles from Uganda and Zambia, studies of serodiscordant heterosexual
couples (i.e., one partner with HIV and the other without) were able to correlate the blood concentration with the probability of heterosexual transmission (Fideli et al., 2001; Quinn et al., 2000). Subsequent field studies in Africa have helped define the concentration of HIV in mucosal secretions required for transmission (Baeten et al., 2011; Lingappa et al., 2010). Systematic reviews suggested the benefits of preventing coinfections, including STIs, in reducing HIV viral load and presumed HIV transmission risks (Modjarrad et al., 2008).
Just as PCR was a game changer for monitoring disease progression and treatment response, rapid, affordable, point-of-care (POC) diagnostics were a key to expanded HIV treatment and prevention (Manoto et al., 2018). The advent of these technologies for HIV diagnosis and management starkly contrasts with modern-day approaches to classical STIs, in which POC tests are only now emerging, and at a slow pace and high cost compared to POC tests for HIV (see Chapter 7).
Using PCR, investigators were able to demonstrate that STIs reversibly increased the concentration of HIV in genital secretions, supporting the theory that the higher viral concentrations evoked by STIs increased HIV transmission (Cohen et al., 1997; Johnson and Lewis, 2008). STIs also lowered the viral threshold for HIV transmission, with STI-induced denuded mucosa and epithelium (rich in inflammatory cells) increasing HIV acquisition (Galvin and Cohen, 2004; Mayer and Venkatesh, 2011). Furthermore, STIs increase the number of inflammatory cells available and the expression of critical receptors on such cells, further facilitating HIV infection (Galvin and Cohen, 2004; Sheffield et al., 2007). HIV variants less fit for growth were shown to persist as a cause of infection in the presence of an STI coinfection (Carlson et al., 2014). The emerging paradigm suggested that people with unrecognized or untreated STI–HIV coinfection might first transmit the more efficient “classical” STIs, causing enough inflammation in the sexual partner to set the stage for subsequent HIV transmission. In an effort to stem the global HIV pandemic, therapeutic studies of treatment of STIs to reduce HIV transmission efficiency were conducted.
Treatment of STIs to Prevent HIV: Before Antiretroviral Treatment
In the early days of the HIV epidemic, the principal tools to prevent sexual transmission were the “ABCs”—abstain from sex, be faithful in sexual partnerships, and use latex condoms consistently and correctly (AIDS Epidemic Sparks Campaign to Encourage Condom Use, 1985). While they are perhaps not always easy to use and are often not a popular option among prospective users, latex condoms are very effective at
preventing transmission of HIV and many other STIs (Fonner et al., 2014; Purcell et al., 2017; Shahmanesh et al., 2008; Weller and Davis, 2002).
Given the emphasis on “epidemiological synergy” (Fleming and Wasserheit, 1999) and data indicating STIs to be a risk factor for HIV transmission, it was hoped that treating one or more STIs could reduce the spread of HIV. Investigators attacked the STI/HIV problem in many ways, including increasing effort(s) to determine the most important STI coinfection (Abu-Raddad et al., 2008; Buchacz et al., 2004; Cohen, 1998; Fleming and Wasserheit, 1999; Freeman et al., 2006; Laga et al., 1993; Quinlivan et al., 2012; Sheffield et al., 2007; van de Wijgert et al., 2009) and ramp up diagnostic and treatment efforts against STIs. The basic idea was that the spread of HIV could be partially controlled through treatment of STIs and, perhaps, other coinfections (Modjarrad and Vermund, 2010).
As treatment of gonococcal infections significantly reduced HIV concentrations in semen (Cohen, 1998; Cohen et al., 1997), it was presumed that controlling gonorrhea, and perhaps chlamydia, would reduce HIV transmission efficiency. Similarly, treating trichomonas led to reduced HIV in vaginal secretions (Hobbs et al., 1999; Kissinger and Adamski, 2013; Price et al., 2003). HIV acquisition may have been reduced modestly after bacterial vaginosis treatment (Taha et al., 1998). Indeed, there has long been an interest in the effect of vaginal flora on HIV acquisition. The general idea was that replacing lactobacilli (normal vaginal flora) with anaerobic bacteria causes bacterial vaginosis, a disruption that facilitated HIV infection (Taha et al., 1998). Vaginal douching may be harmful for women by disturbing healthy vaginal microbiota (see Chapter 3 for more information on douching). More recently, the vaginal flora have been studied with molecular methods that carefully define bacterial species responsible for vaginal “dysbiosis” and inflammation, and this work has stimulated renewed interest in novel ways to prevent HIV (Masson et al., 2019; McClelland et al., 2018; Sabo et al., 2020).
Given the high prevalence of genital HSV-2 (Weiss, 2004), Celum and colleagues studied the ability of acyclovir (an antiviral drug that suppresses herpes virus infection activity) to reduce HIV infectiousness (Celum et al., 2008, 2010) and acquisition in serodiscordant couples (Celum et al., 2008). Acyclovir, however, was not able to prevent HIV transmission; the authors and others have postulated that this inability to reduce HIV infections may have been due to inadequate dosing and/or to persistent inflammation that treating HSV-2 did not eliminate (Zhu et al., 2009). Acyclovir for genital ulcers only modestly reduced the HIV blood viral burden (Celum et al., 2010).
Looking back, a far more ambitious plan was mass treatment of STIs to reduce HIV incidence. Nine STI/HIV prevention trials were conducted between 1995 and 2010 (Celum et al., 2008, 2010; Ghys et al., 2001;
Gregson et al., 2007; Grosskurth et al., 1995; Hayes et al., 2010; Kamali et al., 2003; Kaul et al., 2004; Watson-Jones et al., 2008; Wawer et al., 1998, 1999; Wetmore et al., 2010). These trials were launched at a time when HIV was spreading rapidly, and no other biological prevention strategies were available in low-resource settings. STI mass treatment trials were primarily conducted outside of the United States, where the intersecting high prevalence of HIV and STIs allowed assessment of this hypothesis.
In a community-randomized trial conducted in the Mwanza district of Tanzania, HIV incidence was reduced by 40 percent by prompt syndromic treatment of STIs (Grosskurth et al., 1995). Other similar trials, however, did not realize the same benefit, and the causes for this discrepancy have been the subject of extensive consideration: issues relating to the stage of the epidemic in which the intervention occurred, the exact nature of the approach to STI control, populations affected, and other factors (Hayes et al., 2010; White et al., 2008). The results of these studies were disappointing and suggested that while STI control was important, it was not a robust strategy, apart from other measures, for substantial HIV prevention (Abuelezam et al., 2016; Vermund and Hayes, 2013). The STI outcomes in many of these studies have defined innovative strategies to reduce STI incidence, an outcome quite important to this report. Ultimately, development of potent combinations of antiretroviral agents led the HIV research community away from further and more intensive consideration of STI control as a major pillar of HIV prevention (Heaton et al., 2015).
Management of HIV, Antiretroviral Treatment, and STIs
In 1988, azidothymidine (AZT or zidovudine [ZDV]) was recognized to reduce replication of HIV and death from HIV/AIDS (Fischl et al., 1987) and the transmission of HIV to the child during and after pregnancy (Connor et al., 1994). By 1996, triple-drug, multi-class ZDV had become the standard of HIV care (Carpenter et al., 1996), and by 2006, antiretroviral treatment (ART) treatment was typically reduced to one pill per day for treatment-naïve individuals.
Observational studies of HIV serodiscordant couples (Fideli et al., 2001; Quinn et al., 2000) found no sexual transmissions when the person living with HIV had a low viral load. Similarly, antiretroviral drugs prevented HIV transmission in pregnancy by reducing viral loads, particularly in parts of sub-Saharan Africa where young, reproductive-age women bore a disproportionate burden of disease (Guay et al., 1999; Stringer et al., 2003). In 2011, the HPTN 052 trial (Cohen et al., 2011) proved that ART virtually eliminated HIV transmission in heterosexual couples once viremia is suppressed, putting the “treatment as prevention” (TasP) prospect at the forefront of HIV control policies (Cohen et
al., 2011, 2012, 2016). Newer studies in MSM engaged in condomless anal intercourse confirmed the remarkable relationship between suppressing HIV replication and eliminating transmission (Rodger et al., 2016).
These studies ultimately led to considering ART-based treatment as a tool for prevention—TasP. This strategy seeks to maximize the proportion of persons living with HIV who are successfully diagnosed, treated, and virally suppressed, such that they are far less likely to transmit the virus. Recognizing the potential of effective treatment to stop the spread of HIV, the “Swiss Statement” (Vernazza and Bernard, 2016; Vernazza et al., 2008) outlined the potential for unprotected intercourse in people with HIV with effective and sustained suppression of viral replication. The Swiss Statement presaged the very popular global U=U campaign (undetectable viral load equals untransmittable) (Cohen et al., 2012; Hasse et al., 2010; Persson, 2010; The Lancet HIV, 2017). U=U seeks to simplify HIV prevention strategies, though there are challenges with this unitary TasP message (Grace et al., 2020; Rendina et al., 2020). While not undermining its significance, TasP alone is not likely to be sufficient to end the AIDS epidemic. Recent community-based trials of ART failed to show the desired population-level reduction of HIV anticipated, suggesting the need for more robust combination prevention strategies (Abdool Karim, 2019; Havlir et al., 2019; Hayes et al., 2019; Makhema et al., 2019). (See Chapter 7 for a discussion of STI PrEP.) Advances in HIV treatment, and efforts to increase HIV diagnosis, continue to transform the appreciation of the relationship between STIs and HIV. Since HIV was recognized as a sexually transmitted pathogen, several classical STIs were appropriately identified as potential markers of higher HIV exposure risk and amplified acquisition (Cohen et al., 2019; Kalichman et al., 2010; Melo et al., 2019) (see Figure 5-1). HIV is frequently detected in STI clinic patients, so STI clinics are important venues at which to screen and identify people with highly infectious acute, early, and/or untreated HIV infection (Fiscus et al., 2007; Kojima et al., 2009; Pilcher et al., 2004a; Powers et al., 2007; Rutstein et al., 2016; Wolpaw et al., 2011). Detecting STIs in this setting allows for effective treatment. Since ART takes many weeks to reduce viral burden, the immediate treatment of STIs in people with newly detected HIV infection has an important public health benefit, as demonstrated for the treatment of gonorrhea (Cohen et al., 1997).
Unfortunately, HIV testing is often overlooked when STIs are detected, especially in MSM (Klein et al., 2014; Millett et al., 2011). Conversely, STI screening is performed very inconsistently among persons infected with HIV (Landovitz et al., 2018a; Li et al., 2019a). Smith et al. (2019) noted that people newly diagnosed with HIV had frequently sought care in the previous year prior to diagnosis, but did not receive an HIV test even though STIs were often the presenting complaint. This represents a critical
“missed opportunity” for HIV diagnosis when another STI is suspected and/or screened for (Peterman et al., 2015). For example, an MSM with an STI in New York City had a substantial chance to acquire HIV in the subsequent year (Pathela et al., 2015). Models suggest that targeting persons with gonorrhea or syphilis would substantially improve the impact of pre-exposure prophylaxis (PrEP) programs for HIV (described later in this chapter) (Kasaie et al., 2019). In 2019, researchers at the Centers for Disease Control and Prevention (CDC) and the University of Albany published a modeling study of U.S. MSM that found that 10.2 percent of HIV infections were attributable to infection by chlamydia and/or gonorrhea in one or both of the partners (Jones et al., 2019). While the focus of this report is preventing and treating STIs, the additional critical public health benefits related to HIV and viral hepatitis cannot be overlooked. And, in turn, screening for STIs among persons living with HIV or being monitored for high HIV risk is vital to reduce STIs in the United States (Dresser et al., 2020; Kennedy et al., 2017). Metrics and programmatic integration in the United Kingdom may serve as a model (see, e.g., Michael et al., 2017; Molloy et al., 2017).
In a recent multi-jurisdictional study, Norkin et al. (2021) found that previously diagnosed HIV infection was common among persons diagnosed with early syphilis, latent syphilis, and gonorrhea, yet that over a quarter of these patients were out of care and/or had unsuppressed HIV viral loads at the time of STI diagnosis. This result indicates that a greater integration of HIV and STI control efforts would improve the highly interrelated landscapes of HIV and STI care and transmission (Norkin et al., 2021).
The management of HIV has critical lessons for STI prevention and care. Missed opportunities for HIV detection and care have been addressed with policy changes, including (1) removing barriers to broader clinic-based screening by eliminating written consent for an HIV test and adopting verbal consent with a chart notation (Wing, 2009); (2) increasing resources for clinic and community testing, including expanded testing tailored to specific high-prevalence communities (Chou et al., 2019); and (3) following United States Preventive Services Task Force guidelines for broad population-based screening designed to test more persons who do not perceive themselves to be at risk or whose clinical providers do not appreciate their risk. Strategies to provide convenient and routine testing for persons at risk apply just as well to STIs as they do for HIV (Owens et al., 2019).
By some estimates, HIV detection rates in the United States have risen to 86 percent of all persons living with HIV; still, one in seven such persons is unaware of their HIV status. This pool of individuals likely represents a highly marginalized subset and may be disenfranchised in
a variety of ways from mainstream testing, public health, and medical infrastructures. As recently as 2015, the median time from HIV infection to diagnosis was estimated to be 3 years, and one in four people had been living with the virus for 7 or more years before diagnosis. Delayed detection of HIV is most notable in sexual and racial minorities, especially MSM of color (CDC, 2010; Gamarel et al., 2018; Millett et al., 2011). Furthermore, use of UNAIDS 90-90-90 (2020 goal) and 95-95-95 (2030 goal) metrics may overestimate true coverage in the neediest communities (Haber et al., 2020). Such a failure to test and link to care is mimicked in the STI field, where treatment for STIs is often delayed or overlooked altogether, typically in marginalized populations that do not receive optimized health care screening or care.
HIV diagnosis and linkage to care through programs like the Ryan White HIV/AIDS Program (discussed below) should facilitate targeted and regular STI testing. Kalichman et al. (2011) reviewed STI incidence and prevalence in persons living with HIV or AIDS, noting a 19 percent point prevalence of one or more STIs. Accordingly, current CDC (Workowski and Bolan, 2015) and World Health Organization (WHO, 2016) guidelines call for routinely testing for and treating STIs in people living with HIV. About half of all U.S. people with HIV receive services from the Ryan White HIV/AIDS Program (HRSA, 2020).
The rising incidence of STIs in persons living with HIV or AIDS after the availability of ART launched a discussion of “risk compensation” or “behavioral disinhibition.” This public health paradox theorizes that reduced fear of transmitting or acquiring HIV due to ART may have led to reduced use of condoms, more partners, and higher STI rates (DiClemente et al., 2002; Eaton and Kalichman, 2007; Moskowitz and Roloff, 2010; Pinkerton, 2001). Reports on this topic in both heterosexual men (Apondi et al., 2011; Crepaz et al., 2004; Westercamp et al., 2014) and MSM have varying opinions and conclusions (Cassell et al., 2006; Delva and Helleringer, 2016; Dukers et al., 2001; Katz et al., 2002; Rietmeijer et al., 2003; Stolte et al., 2001; Venkatesh et al., 2011). Qualitative studies suggest that MSM self-report less condom use with less fear of HIV lethality; it is not as apparent that this loss of fear has affected the sexual behavior of heterosexual people (Flagg et al., 2015; Harawa et al., 2008; Vanable et al., 2012; Wade Taylor et al., 2013; Woods et al., 2013).
Another issue is the lack of perceived harm from the consequences of STIs in persons living with HIV or AIDS. MSM may not see untreated STIs and HIV as serious consequences within their communities, given that the principal damage of STIs is to fertility in people assigned female at birth or their neonates (Marston et al., 2017; Ross, 2001; Tsevat et al., 2017; Wiesenfeld et al., 2012). It is not widely appreciated that without effective ART, STIs can have more pathogenic consequences, as sometimes
demonstrated by severe or unusual manifestations of syphilis, HSV-2, and chancroid (Courjon et al., 2015; Hanson et al., 2014; Mena Lora et al., 2017; Oette et al., 2005; Peeling and Hook, 2006; Rompalo et al., 2001a,b; Tran et al., 2005; Wang et al., 2012).
Antiretroviral Agents as Pre-Exposure Prophylaxis (PrEP) for HIV
Beyond TasP, another biological solution for the prevention of an infection is pre- and post-exposure prophylaxis (PrEP and PEP). PrEP, used as designed, represents a theoretically powerful tool for disrupting the trajectory of the HIV epidemic and has received great attention. Trials of tenofovir/emtricitabine (TDF-FTC), and more recently tenofovir alafenamide (TAF)-FTC, demonstrated remarkable HIV protection in men (Grant et al., 2010; Mayer et al., 2020; McCormack et al., 2016; Molina et al., 2017) and likely comparable effects in women (although not as well documented) (Janes et al., 2018). Despite its efficacy, HIV PrEP effectiveness has been limited by slow and incomplete uptake in populations most at risk for HIV; mathematical modeling suggests high PrEP use rates are needed to have an impact on the HIV epidemic (Buchanan et al., 2020; Gomez et al., 2012; Marshall et al., 2018). Encouragingly, a report linked PrEP prescriptions to reduced HIV incidence in some U.S. communities (Jenness et al., 2019). Several important health care providers and industry partners have assured support for the Ending the HIV Epidemic: A Plan for America campaign (discussed below) (HHS, n.d.). This plan focuses on HIV detection, TasP, and PrEP (Fauci et al., 2019).
In addition, a long-acting injectable agent, cabotegravir, prevented HIV in MSM better than oral TDF-FTC (HPTN 083) (HPTN, 2020; Landovitz et al., 2018b). A trial of this same agent in women (HPTN 084) demonstrated that cabotegravir was safe and superior to daily oral FTC/TDF for HIV prevention among cisgender women in sub-Saharan Africa (Delany-Moretlwe et al., 2020). The antiretroviral agent dapivirine, embedded in vaginal rings, led to modest reduction in HIV acquisition, depending on reliable and proper use of the devices (Baeten et al., 2016; Rosenberg, 2017). Recently, the European Medicines Agency and WHO have looked favorably on distribution of dapivirine antiretroviral microbicide rings to help reduce acquisition of HIV (Baeten et al., 2016; Rosenberg, 2017; WHO, 2020, 2021). All of these HIV PrEP strategies may lend themselves to further consideration of STI PrEP (discussed in Chapter 7). In addition, multi-purpose rings to prevent HIV or STIs or pregnancy concomitantly are in development (Achilles et al., 2018; Dallal Bashi et al., 2019; Li et al., 2019b; Young Holt et al., 2020).
Mirroring the debate regarding risk compensation or behavioral disinhibition as contributing to high rates of STIs after scale-up of ART, PrEP
has also sparked intense scrutiny of sex behaviors leading to greater STI acquisition (Blumenthal and Haubrich, 2014; Cassell et al., 2006; Grov et al., 2015; Guest et al., 2008; Marcus et al., 2013, 2019; Milam et al., 2019; Rojas Castro et al., 2019). While STI rates were clearly on the rise before TDF-FTC as PrEP (Marrazzo et al., 2018), the increase has continued. Of 88 articles that examined STI prevalence at the time of PrEP initiation, a systematic review found that in studies that enabled a composite outcome of chlamydia, gonorrhea, and early syphilis, the pooled prevalence was 23.9 percent (95% confidence interval: 18.6%–29.6%) at baseline (Ong et al., 2019).
There are multiple explanations for concurrent rise of STIs and PrEP. These include increased detection due to more frequent testing and improved diagnostics of STIs, as mandated by many PrEP implementation guidelines, and behavioral risk taking among PrEP users who eschew condoms and are therefore de facto at greater risk for STIs (Ramchandani and Golden, 2019; Scott and Klausner, 2016; Stenger et al., 2017). Despite what is clearly a complex relationship of STI rates in the setting of PrEP, one thing should not be ignored: in the context of a population with a more generalized HIV epidemic, presenting with a new STI should prompt a provider to discuss PrEP. These missed opportunities are just one of the reasons that PrEP uptake has fallen short of the level needed to make the greatest impact. (See Chapter 7 for a discussion of PrEP for STIs apart from HIV.)
Substance Use, Alcohol Use, and STI/HIV Risk
The syndemic of STI and HIV risk is linked with substance use and/or alcohol use in at least three contexts. First, persons using drugs such as opioids, cocaine, or methamphetamines may support their cravings by selling sex or exchanging sex for drugs. Second, sexual activity may be linked to use of alcohol or party or club drugs/chemsex (to enhance or facilitate greater pleasure or extended sexual activity), including MDMA (3,4-methylenedioxy-methamphetamine, often known as “ecstasy”), GHB (gamma hydroxybutyrate, also known as a “date rape” drug), Rohypnol (flunitrazepam, also a “date rape” drug), ketamine, methamphetamine, LSD (acid), and cocaine/crack cocaine, to name a few of the most popular drugs. Third, some MSM have drugs that for decades have been used in concert with sex, including alcohol, erectile dysfunction drugs, such as sildenafil, volatile nitrates (poppers), and the aforementioned club drugs (McCarty-Caplan et al., 2014). When persons who wish to have sex are using alcohol and/or drugs, risk taking is more likely and condom use is far less likely (Bonar et al., 2014; Pellowski et al., 2018; Reynolds and Fisher, 2019; Santelli et al., 2001; Storholm et al., 2017).
CONSEQUENCES OF STIs IN PEOPLE LIVING WITH HIV
STIs among people living with HIV represent a significant risk for reproductive tract sequelae for themselves and their sexual partners. While STIs may cause fewer reproductive complications in MSM, bridging STIs to the heterosexual male population and to women has been demonstrated (Williamson et al., 2019). Additionally, the rise in syphilis among women has led to a dramatic rise in congenital syphilis, both in the United States and worldwide (Liew et al., 2021; Schmidt et al., 2019). Currently, CDC (Barrow et al., 2020) and WHO (2016) treatment and prevention guidelines recommend frequent testing for and treatment of STIs, but HIV—given its potential lethality, incurability, and high prevalence—remains a far greater funding and programmatic priority from governments and foundations alike (Barrow et al., 2020).
STIs are used to represent markers of sexual behavior. A history of an STI is often required to enroll in HIV prevention trials and to justify PrEP. HIV prevention trials rarely target both HIV and STIs, focusing on HIV as the most serious of all the STIs. While an STI may indicate higher-than-average HIV risk, the trends of STIs and HIV may not be in sync. For example, as ART and PrEP use expands, HIV incidence has declined, perhaps a “treatment as prevention” effect. At the same time, STI rates continue to rise.
Recently, the White House has launched Ending the HIV Epidemic: A Plan for America, with four pillars (Fauci et al., 2019):
- Diagnose all individuals with HIV as early as possible after infection;
- Treat HIV infection rapidly and effectively to achieve sustained viral suppression;
- Prevent at-risk individuals from acquiring HIV, including the use of PrEP and syringe exchange programs; and
- Rapidly detect and respond to emerging clusters of HIV infection to reduce new transmissions.
While treatment of HIV/AIDS is a key pillar, STI control is not explicitly included in this strategy, which may represent a missed opportunity for better integration of management of HIV, STIs, and viral hepatitis. Importantly, before leaving office, the Trump administration’s Office of the Assistant Secretary for Health at the Department of Health and Human Services (HHS) published an HIV National Strategic Plan (HIV-NSP) in January 2021 (HHS, 2020a). Additionally, President Biden, in December 2020, committed his administration to updating the National HIV/AIDS Strategy for the United States (the most recent iteration covered
2015–2020) (KFF, 2020), which may presumably start with a review of the HIV-NSP. The HIV-NSP, however, is focused on the 48 U.S. counties responsible for half of all HIV diagnoses, along with the District of Columbia and San Juan, Puerto Rico, and 7 rural states with HIV risks derived from the opioid epidemic and other concerns. The Office of the Assistant Secretary for Health also updated the National Viral Hepatitis Action Plan (HHS, 2020c) and recently released the first-ever federal STI National Strategic Plan (STI-NSP, see Chapter 12 for more information) (HHS, 2020b). The STI-NSP emphasizes coordination with Ending the HIV Epidemic (EHE) (HHS, 2020b) (see Box 5-1 and additional discussion later in this chapter).
IMPORTANT LESSONS FROM THE HIV PANDEMIC
The worsening spread of STIs motivated the request for this report and is linked to the novel STI-NSP (HHS, 2020a). The committee believes there are important lessons to be learned from historical and ongoing attempts to control the spread of HIV. First, successes in treating and preventing HIV reflect unique, sustained, and powerful advocacy by
affected communities. The rise of the Gay Men’s Health Crisis and other community-based organizations across the country, along with establishment of local and national activist and advocacy organizations, including the AIDS Coalition to Unleash Power, the National Association of People with AIDS, NMAC (formerly the National Minority AIDS Council), the Latino Commission on AIDS, the Black AIDS Institute, and the Treatment Action Group in the 1980s and 1990s, books and dramatic works, such as And the Band Played On (book and movie), The Normal Heart and Angels in America (plays), Philadelphia (movie), and celebrity disclosure of HIV infection (e.g., Rock Hudson, Liberace, Arthur Ashe, Magic Johnson, Greg Louganis, Freddie Mercury, and Charlie Sheen) inspired massive commitments to treating and preventing HIV. The late Surgeon General C. Everett Koop’s “Understanding AIDS” mailing to all U.S. households by CDC in 1986 acknowledged HIV as a major public health problem and urged Americans to seek testing (Koop, 1986). This was a remarkable moment: the Surgeon General with CDC, independent of the White House, acknowledged an STI, urged all Americans to get tested, and advocated for healthier sexual behaviors to the entire U.S. population.
Effective advocacy ultimately led to resources for HIV treatment and infection at a level that has been unavailable for STIs and many other infectious diseases. In 1990, the Ryan White Comprehensive AIDS Resources Emergency Act was enacted, named for a courageous young boy with hemophilia who died from HIV and whose family was ostracized; his family were tireless in their search to access government resources and showed understanding and compassion for people with HIV. This critical and remarkably successful legislation was the first attempt to mount a nationwide response to the care and treatment needs of people with HIV by expanding funding for high-prevalence cities and metropolitan areas and providing funding to states and HIV primary care clinics in areas with lower prevalence, which has allowed for high-quality care for more than half of the people living with HIV in the United States.
In 2003, President Bush developed the President’s Emergency Plan for AIDS Relief with broad bipartisan support, providing resources to address global HIV prevention and treatment. These substantial funding commitments, nationally and globally, resulted in advances in diagnostics and therapies that fundamentally shifted outcomes, notably in Africa.
Moreover, the rise in the number of persons living with HIV or AIDS that accompanied breakthroughs in treatment has sustained a level of advocacy and attention to HIV that is perhaps unparalleled relative to other health conditions, at least before the COVID-19 pandemic. Public health messaging centers on positive messages from diverse individuals
living with HIV, including celebrities and prominent people from all walks of life and HIV-negative allies among civic leaders, faith leaders, elected officials, athletes, and celebrities.
These observations need to be compared with historical efforts at STI control; classical STIs have simply never received the same funding or national awareness. There has been no parallel movement or awareness, with rare literary exceptions like the 1882 Henrik Ibsen play Ghosts (shocking and reviled at the time of its release) and the little-known 1993 play In the Clap Shack by well-known novelist William Styron. While an Internet search will find many celebrities diagnosed with HSV-2, chlamydia, gonorrhea, syphilis, and other STIs, none speak out in advocacy for prevention and control as is seen for HIV. Advocacy organizations exist2 but are not anywhere near as successful in defining STIs as an important cause worthy of public attention as HIV community-based and policy-oriented groups have been. In fact, comedy routines discussing STIs may actually have an opposing effect: leading people to be less concerned about STI severity and prevention. For example, Saturday Night Live’s Weekend Update on October 12, 2019, parodied that STIs have a relatively low negative impact on quality of life compared to other infectious diseases, such as influenza (SNL, 2019). Although such content is designed for entertainment, trivializing the harms of STIs likely also influences viewers’ attitudes/beliefs and behaviors. The STI epidemic’s growth reflects many social, biological, and behavioral forces; limited resources for public awareness and risk reduction education, and limited investments in surveillance, screening, and treatment, also contribute to STI spread.
CONCLUSIONS
This chapter has sought to provide historical information about HIV and emphasize the inextricable linkage between HIV, classical STIs, and viral hepatitis. Ultimately, HIV and many types of viral hepatitis are most importantly STIs. While the committee was not charged with providing policy recommendations related to HIV, it offers a series of conclusions (see Box 5-2 for examples of potential synergies between HIV and STI prevention, treatment, and control):
Conclusion 5-1: STIs and HIV are inextricably linked. Over the past 40 years, however, they frequently have been addressed in silos by the U.S. public health system even though they are very often detected concomitantly.
___________________
2 See https://www.ncsddc.org/national-sti-coaltion-members (accessed October 16, 2020).
Conclusion 5-2: There are opportunities for greater synergies for HIV and STIs that would improve prevention, treatment, and linkage to care for both. STI and HIV service integration at the client level and program integration at the federal, state, and local levels will enhance the prevention and care of both. Furthermore, increased education for HIV providers regarding treatment and prevention of STIs is needed.
Conclusion 5-3: Significant federal investments have been made toward screening individuals for HIV, yet when such screening is not integrated with screening for bacterial STIs, it represents a missed opportunity. Conversely, it is equally important to assess HIV status among those diagnosed with a bacterial STI; and for those with HIV infection, screening for other STIs should be elevated as a priority within routine clinical management of HIV.
Conclusion 5-4: Ending the HIV Epidemic (EHE) has the potential to drive significant improvements in the health of communities affected by STIs; therefore, targets for STI reduction could be added to the EHE Plan in partnership with CDC. While this initiative is supporting STI clinics, the absence of more central consideration of STIs in the EHE has the potential to limit the achievements of EHE goals and sustain ongoing transmission of these infections.
In past decades, STIs and HIV have mostly been addressed by the U.S. public health system in “silos” even though they are very often detected in combination. Advances in HIV can be ascribed to funding and growth of a massive community of researchers, clinical and community-based services providers, and people living with HIV and their advocates. In addition, the rapidity of scientific progress for new diagnostic, therapeutic, and preventative tools contrasts to comparatively indolent progress for other STIs, despite their clinical recognition for centuries and their microbiological characterization for many decades. Progress with HIV underscores what can be done if a problem is confronted and prioritized; programmatic successes with HIV serve to emphasize the promise for substantially improved STI control. Yet, public health support for STIs has lagged the demands of the rising incidence in the United States, contributing to the rise of STIs and a relative dearth of vaccine research, innovative diagnostics, and improved treatments. Unfortunately, success in preventing and treating HIV has likely contributed to STI spread in some populations, as concern around HIV infection has diminished. STIs cannot be seen as simply an annoyance or inevitability, given the seriousness of STI long-term sequelae, including infertility. STI and HIV service integration will enhance the prevention and care of both.
REFERENCES
Abdool Karim, S. S. 2019. HIV-1 epidemic control—insights from test-and-treat trials. New England Journal of Medicine 381(3):286-288.
Abu-Raddad, L. J., A. S. Magaret, C. Celum, A. Wald, I. M. Longini, Jr., S. G. Self, and L. Corey. 2008. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 3(5):e2230.
Abuelezam, N. N., A. W. McCormick, T. Fussell, A. N. Afriyie, R. Wood, V. DeGruttola, K. A. Freedberg, M. Lipsitch, and G. R. Seage, 3rd. 2016. Can the heterosexual HIV epidemic be eliminated in South Africa using combination prevention? A modeling analysis. American Journal of Epidemiology 184(3):239-248.
Achilles, S., C. W. Kelly, D. L. Blithe, J. Long, B. A. Richardson, B. Devlin, C. W. Hendrix, S. M. Poloyac, M. A. Marzinke, D. Singh, J. M. Piper, J. Steytler, and B. A. Chen. 2018. Safety and pharmacokinetics of dapivirine and levonorgestrel vaginal rings for multipurpose prevention of HIV and pregnancy (abstract OA12.02LB). Madrid, Spain: HIV Research for Prevention (HIVR4P), October 21-25.
AIDS Epidemic Sparks Campaign to Encourage Condom Use. 1985. Contraceptive Technology Update 6(12):161-163.
Alpren, C., E. L. Dawson, B. John, K. Cranston, N. Panneer, H. D. Fukuda, K. Roosevelt, R. M. Klevens, J. Bryant, P. J. Peters, S. B. Lyss, W. M. Switzer, A. Burrage, A. Murray, C. Agnew-Brune, T. Stiles, P. McClung, E. M. Campbell, C. Breen, L. M. Randall, S. Dasgupta, S. Onofrey, D. Bixler, K. Hampton, J. L. Jaeger, K. K. Hsu, W. Adih, B. Callis, L. R. Goldman, S. P. Danner, H. Jia, M. Tumpney, A. Board, C. Brown, A. DeMaria, Jr., and K. Buchacz. 2020. Opioid use fueling HIV transmission in an urban setting: An outbreak of HIV infection among people who inject drugs—Massachusetts, 2015-2018. American Journal of Public Health 110(1):37-44.
Apondi, R., R. Bunnell, J. P. Ekwaru, D. Moore, S. Bechange, K. Khana, R. King, J. Campbell, J. Tappero, and J. Mermin. 2011. Sexual behavior and HIV transmission risk of Ugandan adults taking antiretroviral therapy: 3 year follow-up. AIDS 25(10):1317-1327.
Baeten, J. M., E. Kahle, J. R. Lingappa, R. W. Coombs, S. Delany-Moretlwe, E. Nakku-Joloba, N. R. Mugo, A. Wald, L. Corey, D. Donnell, M. S. Campbell, J. I. Mullins, and C. Celum. 2011. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Science Translational Medicine 3(77):77ra29.
Baeten, J. M., T. Palanee-Phillips, E. R. Brown, K. Schwartz, L. E. Soto-Torres, V. Govender, N. M. Mgodi, F. Matovu Kiweewa, G. Nair, F. Mhlanga, S. Siva, L. G. Bekker, N. Jeenarain, Z. Gaffoor, F. Martinson, B. Makanani, A. Pather, L. Naidoo, M. Husnik, B. A. Richardson, U. M. Parikh, J. W. Mellors, M. A. Marzinke, C. W. Hendrix, A. van der Straten, G. Ramjee, Z. M. Chirenje, C. Nakabiito, T. E. Taha, J. Jones, A. Mayo, R. Scheckter, J. Berthiaume, E. Livant, C. Jacobson, P. Ndase, R. White, K. Patterson, D. Germuga, B. Galaska, K. Bunge, D. Singh, D. W. Szydlo, E. T. Montgomery, B. S. Mensch, K. Torjesen, C. I. Grossman, N. Chakhtoura, A. Nel, Z. Rosenberg, I. McGowan, and S. Hillier. 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New England Journal of Medicine 375(22):2121-2132.
Baggaley, R. F., and T. D. Hollingsworth. 2015. Brief report: HIV-1 transmissions during asymptomatic infection: Exploring the impact of changes in HIV-1 viral load due to coinfections. Journal of Acquired Immune Deficiency Syndromes 68(5):594-598.
Barrow, R. Y., F. Ahmed, G. A. Bolan, and K. A. Workowski. 2020. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recommendations and Reports 68(5):1-20.
Blaser, N., C. Wettstein, J. Estill, L. S. Vizcaya, G. Wandeler, M. Egger, and O. Keiser. 2014. Impact of viral load and the duration of primary infection on HIV transmission: Systematic review and meta-analysis. AIDS (London, England) 28(7):1021-1029.
Blower, S. M., and C. Boe. 1993. Sex acts, sex partners, and sex budgets: Implications for risk factor analysis and estimation of HIV transmission probabilities. Journal of Acquired Immune Deficiency Syndromes (1988) 6(12):1347-1352.
Blumenthal, J., and R. H. Haubrich. 2014. Will risk compensation accompany pre-exposure prophylaxis for HIV? Virtual Mentor 16(11):909-915.
Bonar, E. E., R. M. Cunningham, S. T. Chermack, F. C. Blow, K. L. Barry, B. M. Booth, and M. A. Walton. 2014. Prescription drug misuse and sexual risk behaviors among adolescents and emerging adults. Journal of Studies on Alcohol and Drugs 75(2):259-268.
Buchacz, K., P. Patel, M. Taylor, P. R. Kerndt, R. H. Byers, S. D. Holmberg, and J. D. Klausner. 2004. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS 18(15):2075-2079.
Buchanan, A. L., S. Bessey, W. C. Goedel, M. King, E. J. Murray, S. Friedman, M. E. Halloran, and B. D. L. Marshall. 2020. Disseminated effects in agent based models: A potential outcomes framework and application to inform pre-exposure prophylaxis coverage levels for HIV prevention. American Journal of Epidemiology. doi: 10.1093/aje/kwaa239.
Carlson, J. M., M. Schaefer, D. C. Monaco, R. Batorsky, D. T. Claiborne, J. Prince, M. J. Deymier, Z. S. Ende, N. R. Klatt, C. E. DeZiel, T. H. Lin, J. Peng, A. M. Seese, R. Shapiro, J. Frater, T. Ndung’u, J. Tang, P. Goepfert, J. Gilmour, M. A. Price, W. Kilembe, D. Heckerman, P. J. Goulder, T. M. Allen, S. Allen, and E. Hunter. 2014. HIV transmission. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science 345(6193):1254031.
Carpenter, C. C., M. A. Fischl, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 1996. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society—USA. JAMA 276(2):146-154.
Cassell, M. M., D. T. Halperin, J. D. Shelton, and D. Stanton. 2006. Risk compensation: The Achilles’ heel of innovations in HIV prevention? BMJ 332(7541):605-607.
CDC (Centers for Disease Control and Prevention). 1981. Kaposi’s sarcoma and pneumocystis pneumonia among homosexual men—New York City and California. Morbidity and Mortality Weekly Report 30(25):305-308.
CDC. 1982. Possible transfusion-associated acquired immune deficiency syndrome (AIDS)—California. Morbidity and Mortality Weekly Report 31(48):652-654.
CDC. 1983. Leads from the MMWR. Acquired immunodeficiency syndrome among patients with hemophilia. JAMA 250(24):3277-3278.
CDC. 2010. Prevalence and awareness of HIV infection among men who have sex with men—21 cities, United States, 2008. Morbidity and Mortality Weekly Report 59(37):1201-1207.
Celum, C., A. Wald, J. Hughes, J. Sanchez, S. Reid, S. Delany-Moretlwe, F. Cowan, M. Casapia, A. Ortiz, J. Fuchs, S. Buchbinder, B. Koblin, S. Zwerski, S. Rose, J. Wang, and L. Corey. 2008. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: A randomised, double-blind, placebo-controlled trial. Lancet 371(9630):2109-2119.
Celum, C., A. Wald, J. R. Lingappa, A. S. Magaret, R. S. Wang, N. Mugo, A. Mujugira, J. M. Baeten, J. I. Mullins, J. P. Hughes, E. A. Bukusi, C. R. Cohen, E. Katabira, A. Ronald, J. Kiarie, C. Farquhar, G. J. Stewart, J. Makhema, M. Essex, E. Were, K. H. Fife, G. de Bruyn, G. E. Gray, J. A. McIntyre, R. Manongi, S. Kapiga, D. Coetzee, S. Allen, M. Inambao, K. Kayitenkore, E. Karita, W. Kanweka, S. Delany, H. Rees, B. Vwalika, W. Stevens, M. S. Campbell, K. K. Thomas, R. W. Coombs, R. Morrow, W. L. Whittington, M. J. McElrath, L. Barnes, R. Ridzon, and L. Corey. 2010. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. New England Journal of Medicine 362(5):427-439.
Chakraborty, H., P. K. Sen, R. W. Helms, P. L. Vernazza, S. A. Fiscus, J. J. Eron, B. K. Patterson, R. W. Coombs, J. N. Krieger, and M. S. Cohen. 2001. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: A probabilistic empiric model. AIDS 15(5):621-627.
Chou, R., T. Dana, S. Grusing, and C. Bougatsos. 2019. Screening for HIV infection in asymptomatic, nonpregnant adolescents and adults: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 321(23):2337-2348.
Coates, R. A., and M. T. Schechter. 1988. Sexual modes of transmission of the human immunodeficiency virus (HIV). Annals of Sex Research 1:115-137.
Cohen, M. S. 1998. Sexually transmitted diseases enhance HIV transmission: No longer a hypothesis. Lancet 351(Suppl 3):5-7.
Cohen, M. 2006. Amplified transmission of HIV-1: New clues to the AIDS pandemic. Transactions of the American Clinical and Climatological Association (117):213-225.
Cohen, M. S., and C. D. Pilcher. 2005. Amplified HIV transmission and new approaches to HIV prevention. The Journal of Infectious Diseases 191(9):1391-1393.
Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, and J. J. Eron, Jr. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: Implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi research group. Lancet 349(9069):1868-1873.
Cohen, M. S., Y. Q. Chen, M. McCauley, T. Gamble, M. C. Hosseinipour, N. Kumarasamy, J. G. Hakim, J. Kumwenda, B. Grinsztejn, J. H. Pilotto, S. V. Godbole, S. Mehendale, S. Chariyalertsak, B. R. Santos, K. H. Mayer, I. F. Hoffman, S. H. Eshleman, E. Piwowar-Manning, L. Wang, J. Makhema, L. A. Mills, G. de Bruyn, I. Sanne, J. Eron, J. Gallant, D. Havlir, S. Swindells, H. Ribaudo, V. Elharrar, D. Burns, T. E. Taha, K. Nielsen-Saines, D. Celentano, M. Essex, and T. R. Fleming. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine 365(6):493-505.
Cohen, M. S., C. Holmes, N. Padian, M. Wolf, G. Hirnschall, Y. R. Lo, and E. Goosby. 2012. HIV treatment as prevention: How scientific discovery occurred and translated rapidly into policy for the global response. Health Affairs 31(7):1439-1449.
Cohen, M. S., Y. Q. Chen, M. McCauley, T. Gamble, M. C. Hosseinipour, N. Kumarasamy, J. G. Hakim, J. Kumwenda, B. Grinsztejn, J. H. S. Pilotto, S. V. Godbole, S. Chariyalertsak, B. R. Santos, K. H. Mayer, I. F. Hoffman, S. H. Eshleman, E. Piwowar-Manning, L. Cottle, X. C. Zhang, J. Makhema, L. A. Mills, R. Panchia, S. Faesen, J. Eron, J. Gallant, D. Havlir, S. Swindells, V. Elharrar, D. Burns, T. E. Taha, K. Nielsen-Saines, D. D. Celentano, M. Essex, S. E. Hudelson, A. D. Redd, and T. R. Fleming. 2016. Antiretroviral therapy for the prevention of HIV-1 transmission. New England Journal of Medicine 375(9):830-839.
Cohen, M. S., O. D. Council, and J. S. Chen. 2019. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: The biologic basis for epidemiologic synergy. Journal of the International AIDS Society 22:e25355.
Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O’Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 study group. New England Journal of Medicine 331(18):1173-1180.
Council, O. D., S. Zhou, C. D. McCann, I. Hoffman, G. Tegha, D. Kamwendo, M. Matoga, S. L. Kosakovsky Pond, M. S. Cohen, and R. Swanstrom. 2020. Deep sequencing reveals compartmentalized HIV-1 in the semen of men with and without sexually transmitted infection-associated urethritis. Journal of Virology 94(12):e00151-20.
Courjon, J., T. Hubiche, N. Dupin, P. A. Grange, and P. Del Giudice. 2015. Clinical aspects of syphilis reinfection in HIV-infected patients. Dermatology 230(4):302-307.
Crepaz, N., T. A. Hart, and G. Marks. 2004. Highly active antiretroviral therapy and sexual risk behavior: A meta-analytic review. JAMA 292(2):224-236.
Crowley, J. S., and G. A. Millett. 2017. Preventing HIV and hepatitis infections among people who inject drugs: Leveraging an Indiana outbreak response to break the impasse. AIDS and Behavior 21(4):968-972.
Dallal Bashi, Y. H., C. F. McCoy, D. J. Murphy, P. Boyd, P. Spence, K. Kleinbeck, B. Devlin, and R. K. Malcolm. 2019. Towards a dapivirine and levonorgestrel multipurpose vaginal ring: Investigations into the reaction between levonorgestrel and addition-cure silicone elastomers. International Journal of Pharmaceutics 569:118574.
Degenhardt, L., B. Mathers, P. Vickerman, T. Rhodes, C. Latkin, and M. Hickman. 2010. Prevention of HIV infection for people who inject drugs: Why individual, structural, and combination approaches are needed. Lancet 376(9737):285-301.
Delany-Moretlwe, S., J. P. Hughes, P. Bock, S. Gurrion, P. Hunidzarira, D. Kalonji, N. Kayange, J. Makhema, P. Mandima, C. Mathew, M. Mokgoro, J. Mpendo, P. Mukwekwerere, N. Mgodi, P. Nahirya Ntege, G. Nair, C. Nakabiito, H. Nuwagaba-Biribonwoha, R. Panchia, N. Singh, B. Siziba, J. Farrior, S. Rose, R. Berhanu, P. Anderson, Y. Agyei, S. H. Eshleman, M. Marzinke, E. Piwowar-Manning, A. Asmelash, F. Conradie, M. Moorhouse, P. Richardson, S. Beigel-Orme, L. Emel, K. Bokoch, R. White, S. Hosek, B. Tolley, N. Sista, K. Shin, A. Adeyeye, J. Rooney, A. Rinehart, K. Smith, B. Hanscom, M. Cohen, and M. Hosseinipour. 2020. Long acting injectable cabotegravir is safe and effective in preventing HIV infection in cisgender women: Interim results from HPTN 084. HIV Research for Prevention conference (virtual): LB1479.
Delva, W., and S. Helleringer. 2016. Beyond risk compensation: Clusters of antiretroviral treatment (ART) users in sexual networks can modify the impact of ART on HIV incidence. PLoS One 11(9):e0163159.
Des Jarlais, D. C., V. Sypsa, J. Feelemyer, A. O. Abagiu, V. Arendt, D. Broz, D. Chemtob, C. Seguin-Devaux, J. M. Duwve, M. Fitzgerald, D. J. Goldberg, A. Hatzakis, R. E. Jipa, E. Katchman, E. Keenan, I. Khan, S. Konrad, A. McAuley, S. Skinner, and L. Wiessing. 2020. HIV outbreaks among people who inject drugs in Europe, North America, and Israel. The Lancet HIV 7(6):e434-e442.
DiClemente, R. J., E. Funkhouser, G. Wingood, H. Fawal, S. D. Holmberg, and S. H. Vermund. 2002. Protease inhibitor combination therapy and decreased condom use among gay men. Southern Medical Journal 95(4):421-425.
Dosekun, O., and J. Fox. 2010. An overview of the relative risks of different sexual behaviours on HIV transmission. Current Opinion in HIV and AIDS 5(4):291-297.
Dresser, M., J. Hussey, and N. Premchand. 2020. Kicking the habit—how can we improve the routine care for patients who are well controlled living with HIV? International Journal of STD & AIDS 31(13):1315-1319.
Dukers, N. H., J. Goudsmit, J. B. de Wit, M. Prins, G. J. Weverling, and R. A. Coutinho. 2001. Sexual risk behaviour relates to the virological and immunological improvements during highly active antiretroviral therapy in HIV-1 infection. AIDS 15(3):369-378.
Eaton, L. A., and S. Kalichman. 2007. Risk compensation in HIV prevention: Implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Current HIV/AIDS Reports 4(4):165-172.
Fauci, A. S., R. R. Redfield, G. Sigounas, M. D. Weahkee, and B. P. Giroir. 2019. Ending the HIV epidemic: A plan for the United States. JAMA 321(9):844-845.
Fideli, U. S., S. A. Allen, R. Musonda, S. Trask, B. H. Hahn, H. Weiss, J. Mulenga, F. Kasolo, S. H. Vermund, and G. M. Aldrovandi. 2001. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Research and Human Retroviruses 17(10):901-910.
Fischl, M. A., D. D. Richman, M. H. Grieco, M. S. Gottlieb, P. A. Volberding, O. L. Laskin, J. M. Leedom, J. E. Groopman, D. Mildvan, R. T. Schooley, et al. 1987. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. New England Journal of Medicine 317(4):185-191.
Fiscus, S. A., C. D. Pilcher, W. C. Miller, K. A. Powers, I. F. Hoffman, M. Price, D. A. Chilongozi, C. Mapanje, R. Krysiak, S. Gama, F. E. Martinson, and M. S. Cohen. 2007. Rapid, real-time detection of acute HIV infection in patients in Africa. The Journal of Infectious Diseases 195(3):416-424.
Flagg, E. W., H. S. Weinstock, E. L. Frazier, E. E. Valverde, J. D. Heffelfinger, and J. Skarbinski. 2015. Bacterial sexually transmitted infections among HIV-infected patients in the United States: Estimates from the medical monitoring project. Sexually Transmitted Diseases 42(4):171-179.
Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually Transmitted Infections 75(1):3-17.
Fonner, V. A., C. E. Kennedy, K. R. O’Reilly, and M. D. Sweat. 2014. Systematic assessment of condom use measurement in evaluation of HIV prevention interventions: Need for standardization of measures. AIDS and Behavior 18(12):2374-2386.
Freeman, E. E., H. A. Weiss, J. R. Glynn, P. L. Cross, J. A. Whitworth, and R. J. Hayes. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS 20(1):73-83.
Gallo, R. C. 2002. Historical essay. The early years of HIV/AIDS. Science 298(5599):1728-1730.
Gallo, R. C., and L. Montagnier. 2003. The discovery of HIV as the cause of AIDS. New England Journal of Medicine 349(24):2283-2285.
Galvin, S. R., and M. S. Cohen. 2004. The role of sexually transmitted diseases in HIV transmission. Nature Reviews Microbiology 2(1):33-42.
Gamarel, K. E., K. M. Nelson, R. Stephenson, O. J. Santiago Rivera, D. Chiaramonte, and R. L. Miller. 2018. Anticipated HIV stigma and delays in regular HIV testing behaviors among sexually-active young gay, bisexual, and other men who have sex with men and transgender women. AIDS and Behavior 22(2):522-530.
Ghys, P. D., M. O. Diallo, V. Ettiegne-Traore, G. A. Satten, C. K. Anoma, C. Maurice, J. C. Kadjo, I. M. Coulibaly, S. Z. Wiktor, A. E. Greenberg, and M. Laga. 2001. Effect of interventions to control sexually transmitted disease on the incidence of HIV infection in female sex workers. AIDS 15(11):1421-1431.
Gomez, G. B., A. Borquez, C. F. Caceres, E. R. Segura, R. M. Grant, G. P. Garnett, and T. B. Hallett. 2012. The potential impact of pre-exposure prophylaxis for HIV prevention among men who have sex with men and transwomen in Lima, Peru: A mathematical modelling study. PLoS Medicine 9(10):e1001323.
Gonsalves, G. S., and F. W. Crawford. 2018. Dynamics of the HIV outbreak and response in Scott County, IN, USA, 2011-15: A modelling study. The Lancet HIV 5(10):e569-e577.
Gottlieb, M. S., H. M. Schanker, P. T. Fan, A. Saxon, and J. D. Weisman. 1981. Centers for Disease Control (CDC). Pneumocystis pneumonia—Los Angeles. Morbidity and Mortality Weekly Report 30(21):250-252.
Gottlieb, M. S., H. M. Schanker, P. T. Fan, A. Saxon, and J. D. Weisman. 1982. Pneumocystis carinii pneumonia among persons with hemophilia A. Morbidity and Mortality Weekly Report 31(27):365-367.
Grace, D., R. Nath, R. Parry, J. Connell, J. Wong, and T. Grennan. 2020. “… if U equals U what does the second U mean?”: Sexual minority men’s accounts of HIV undetectability and untransmittable scepticism. Culture, Health & Sexuality 1-17.
Grant, R. M., J. R. Lama, P. L. Anderson, V. McMahan, A. Y. Liu, L. Vargas, P. Goicochea, M. Casapia, J. V. Guanira-Carranza, M. E. Ramirez-Cardich, O. Montoya-Herrera, T. Fernandez, V. G. Veloso, S. P. Buchbinder, S. Chariyalertsak, M. Schechter, L. G. Bekker, K. H. Mayer, E. G. Kallas, K. R. Amico, K. Mulligan, L. R. Bushman, R. J. Hance, C. Ganoza, P. Defechereux, B. Postle, F. Wang, J. J. McConnell, J. H. Zheng, J. Lee, J. F. Rooney, H. S. Jaffe, A. I. Martinez, D. N. Burns, and D. V. Glidden. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New England Journal of Medicine 363(27):2587-2599.
Gregson, S., S. Adamson, S. Papaya, J. Mundondo, C. A. Nyamukapa, P. R. Mason, G. P. Garnett, S. K. Chandiwana, G. Foster, and R. M. Anderson. 2007. Impact and process evaluation of integrated community and clinic-based HIV-1 control: A cluster-randomised trial in eastern Zimbabwe. PLoS Medicine 4(3):e102.
Grosskurth, H., F. Mosha, J. Todd, E. Mwijarubi, A. Klokke, K. Senkoro, P. Mayaud, J. Changalucha, A. Nicoll, G. ka-Gina, et al. 1995. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: Randomised controlled trial. Lancet 346(8974):530-536.
Grov, C., T. H. Whitfield, H. J. Rendina, A. Ventuneac, and J. T. Parsons. 2015. Willingness to take PrEP and potential for risk compensation among highly sexually active gay and bisexual men. AIDS and Behavior 19(12):2234-2244.
Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, C. Ducar, M. Deseyve, L. Emel, M. Mirochnick, M. G. Fowler, L. Mofenson, P. Miotti, K. Dransfield, D. Bray, F. Mmiro, and J. B. Jackson. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354(9181):795-802.
Guest, G., D. Shattuck, L. Johnson, B. Akumatey, E. E. Clarke, P. L. Chen, and K. M. MacQueen. 2008. Changes in sexual risk behavior among participants in a PrEP HIV prevention trial. Sexually Transmitted Diseases 35(12):1002-1008.
Haber, N. A., C. R. Lesko, M. P. Fox, K. A. Powers, G. Harling, J. K. Edwards, J. A. Salomon, S. A. Lippman, J. Bor, A. Y. Chang, A. Anglemyer, and A. Pettifor. 2020. Limitations of the UNAIDS 90-90-90 metrics: A simulation-based comparison of cross-sectional and longitudinal metrics for the HIV care continuum. AIDS (London, England) 34(7):1047-1055.
Hanson, C., R. Fischer, G. Fraga, A. Rajpara, D. Hinthorn, D. Aires, and D. Liu. 2014. Lues maligna praecox: An important consideration in HIV-positive patients with ulceronodular skin lesions. Dermatology Online Journal 21(3).
Harawa, N. T., J. K. Williams, H. C. Ramamurthi, C. Manago, S. Avina, and M. Jones. 2008. Sexual behavior, sexual identity, and substance abuse among low-income bisexual and non-gay-identifying African American men who have sex with men. Archives of Sexual Behavior 37(5):748-762.
Harman, A. N., M. Kim, N. Nasr, K. J. Sandgren, and P. U. Cameron. 2013. Tissue dendritic cells as portals for HIV entry. Reviews in Medical Virology 23(5):319-333.
Harris, C., C. B. Small, R. S. Klein, G. H. Friedland, B. Moll, E. E. Emeson, I. Spigland, and N. H. Steigbigel. 1983. Immunodeficiency in female sexual partners of men with the acquired immunodeficiency syndrome. New England Journal of Medicine 308(20):1181-1184.
Hasse, B., B. Ledergerber, B. Hirschel, P. Vernazza, T. R. Glass, A. Jeannin, J. M. Evison, L. Elzi, M. Cavassini, E. Bernasconi, D. Nicca, and R. Weber. 2010. Frequency and determinants of unprotected sex among HIV-infected persons: The Swiss HIV cohort study. Clinical Infectious Diseases 51(11):1314-1322.
Havlir, D. V., L. B. Balzer, E. D. Charlebois, T. D. Clark, D. Kwarisiima, J. Ayieko, J. Kabami, N. Sang, T. Liegler, G. Chamie, C. S. Camlin, V. Jain, K. Kadede, M. Atukunda, T. Ruel, S. B. Shade, E. Ssemmondo, D. M. Byonanebye, F. Mwangwa, A. Owaraganise, W. Olilo, D. Black, K. Snyman, R. Burger, M. Getahun, J. Achando, B. Awuonda, H. Nakato, J. Kironde, S. Okiror, H. Thirumurthy, C. Koss, L. Brown, C. Marquez, J. Schwab, G. Lavoy, A. Plenty, E. Mugoma Wafula, P. Omanya, Y.-H. Chen, J. F. Rooney, M. Bacon, M. van der Laan, C. R. Cohen, E. Bukusi, M. R. Kamya, and M. Petersen. 2019. HIV testing and treatment with the use of a community health approach in rural Africa. New England Journal of Medicine 381(3):219-229.
Hayes, R., D. Watson-Jones, C. Celum, J. van de Wijgert, and J. Wasserheit. 2010. Treatment of sexually transmitted infections for HIV prevention: End of the road or new beginning? AIDS 24(Suppl 4):S15-S26.
Hayes, R. J., D. Donnell, S. Floyd, N. Mandla, J. Bwalya, K. Sabapathy, B. Yang, M. Phiri, A. Schaap, S. H. Eshleman, E. Piwowar-Manning, B. Kosloff, A. James, T. Skalland, E. Wilson, L. Emel, D. Macleod, R. Dunbar, M. Simwinga, N. Makola, V. Bond, G. Hoddinott, A. Moore, S. Griffith, N. Deshmane Sista, S. H. Vermund, W. El-Sadr, D. N. Burns, J. R. Hargreaves, K. Hauck, C. Fraser, K. Shanaube, P. Bock, N. Beyers, H. Ayles, and S. Fidler. 2019. Effect of universal testing and treatment on HIV incidence—HPTN 071 (POPART). New England Journal of Medicine 381(3):207-218.
Heaton, L. M., P. D. Bouey, J. Fu, J. Stover, T. B. Fowler, R. Lyerla, and M. Mahy. 2015. Estimating the impact of the US President’s Emergency Plan for AIDS Relief on HIV treatment and prevention programmes in Africa. Sexually Transmitted Infections 91(8):615-620.
HHS (Department of Health and Human Services). 2020a. National Strategic Plan: A roadmap to end the epidemic for the United States 2021-2025. Washington, DC: Department of Health and Human Services.
HHS. 2020b. Sexually transmitted infections National Strategic Plan for the United States: 2021-2025. Washington, DC: Department of Health and Human Services.
HHS. 2020c. Viral hepatitis National Strategic Plan for the United States: A roadmap to elimination (2021-2025). Washington, DC: Department of Health and Human Services.
HHS. n.d. Ending the HIV Epidemic: A plan for America (overview). https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview (accessed October 12, 2020).
Hobbs, M. M., P. Kazembe, A. W. Reed, W. C. Miller, E. Nkata, D. Zimba, C. C. Daly, H. Chakraborty, M. S. Cohen, and I. Hoffman. 1999. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sexually Transmitted Diseases 26(7):381-387.
Hollingsworth, T. D., R. M. Anderson, and C. Fraser. 2008. HIV-1 transmission, by stage of infection. The Journal of Infectious Diseases 198(5):687-693.
Hooper, R. R., G. H. Reynolds, O. G. Jones, A. Zaidi, P. J. Wiesner, K. P. Latimer, A. Lester, A. F. Campbell, W. O. Harrison, W. W. Karney, and K. K. Holmes. 1978. Cohort study of venereal disease. I: The risk of gonorrhea transmission from infected women to men. American Journal of Epidemiology 108(2):136-144.
HPTN (HIV Prevention Trials Network). 2020. HPTN 083. https://www.hptn.org/research/studies/hptn083 (accessed January 30, 2021).
HRSA (Health Resources and Services Administration). 2020. About the Ryan White HIV/AIDS program. https://hab.hrsa.gov/about-ryan-white-hivaids-program/about-ryan-white-hivaids-program (accessed June 23, 2020).
Janes, H., L. Corey, G. Ramjee, L. N. Carpp, C. Lombard, M. S. Cohen, P. B. Gilbert, and G. E. Gray. 2018. Weighing the evidence of efficacy of oral PrEP for HIV prevention in women in southern Africa. AIDS Research and Human Retroviruses 34(8):645-656.
Jenness, S. M., K. M. Maloney, D. K. Smith, K. W. Hoover, S. M. Goodreau, E. S. Rosenberg, K. M. Weiss, A. Y. Liu, D. W. Rao, and P. S. Sullivan. 2019. Addressing gaps in HIV preexposure prophylaxis care to reduce racial disparities in HIV incidence in the United States. American Journal of Epidemiology 188(4):743-752.
Johnson, L. F., and D. A. Lewis. 2008. The effect of genital tract infections on HIV-1 shedding in the genital tract: A systematic review and meta-analysis. Sexually Transmitted Diseases 35(11):946-959.
Jones, J., K. Weiss, J. Mermin, P. Dietz, E. S. Rosenberg, T. L. Gift, H. Chesson, P. S. Sullivan, C. Lyles, K. T. Bernstein, and S. M. Jenness. 2019. Proportion of incident human immunodeficiency virus cases among men who have sex with men attributable to gonorrhea and chlamydia: A modeling analysis. Sexually Transmitted Diseases 46(6):357-363.
Kalichman, S. C., L. Eaton, and C. Cherry. 2010. Sexually transmitted infections and infectiousness beliefs among people living with HIV/AIDS: Implications for HIV treatment as prevention. HIV Medicine 11(8):502-509.
Kalichman, S. C., J. Pellowski, and C. Turner. 2011. Prevalence of sexually transmitted coinfections in people living with HIV/AIDS: Systematic review with implications for using HIV treatments for prevention. Sexually Transmitted Infections 87(3):183-190.
Kamali, A., M. Quigley, J. Nakiyingi, J. Kinsman, J. Kengeya-Kayondo, R. Gopal, A. Ojwiya, P. Hughes, L. M. Carpenter, and J. Whitworth. 2003. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: A community randomised trial. Lancet 361(9358):645-652.
Kasaie, P., C. M. Schumacher, J. M. Jennings, S. A. Berry, S. A. Tuddenham, M. S. Shah, E. S. Rosenberg, K. W. Hoover, T. L. Gift, H. Chesson, D. German, and D. W. Dowdy. 2019. Gonorrhoea and chlamydia diagnosis as an entry point for HIV pre-exposure prophylaxis: A modelling study. BMJ Open 9(3):e023453.
Katz, M. H., S. K. Schwarcz, T. A. Kellogg, J. D. Klausner, J. W. Dilley, S. Gibson, and W. McFarland. 2002. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. American Journal of Public Health 92(3):388-394.
Kaul, R., J. Kimani, N. J. Nagelkerke, K. Fonck, E. N. Ngugi, F. Keli, K. S. MacDonald, I. W. Maclean, J. J. Bwayo, M. Temmerman, A. R. Ronald, and S. Moses. 2004. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: A randomized controlled trial. JAMA 291(21):2555-2562.
Kaul, R., C. Pettengell, P. M. Sheth, S. Sunderji, A. Biringer, K. MacDonald, S. Walmsley, and A. Rebbapragada. 2008. The genital tract immune milieu: An important determinant of HIV susceptibility and secondary transmission. Journal of Reproductive Immunology 77(1):32-40.
Kennedy, C. E., S. A. Haberlen, and M. Narasimhan. 2017. Integration of sexually transmitted infection (STI) services into HIV care and treatment services for women living with HIV: A systematic review. BMJ Open 7(6):e015310.
KFF (Kaiser Family Foundation). 2020. President-elect Biden releases statement on World AIDS Day, calls for expanded support for PEPFAR, Global Fund. https://www.kff.org/news-summary/president-elect-biden-releases-statement-on-world-aids-day-calls-forexpanded-support-for-pepfar-global-fund (accessed February 2, 2021).
Kishore, S., M. Hayden, and J. Rich. 2019. Lessons from Scott County—progress or paralysis on harm reduction? New England Journal of Medicine 380(21):1988-1990.
Kissinger, P., and A. Adamski. 2013. Trichomoniasis and HIV interactions: A review. Sexually Transmitted Infections 89(6):426-433.
Klein, P. W., I. B. K. Martin, E. B. Quinlivan, C. L. Gay, and P. A. Leone. 2014. Missed opportunities for concurrent HIV-STD testing in an academic emergency department. Public Health Reports 129(Suppl 1):12-20.
Kojima, Y., T. Kawahata, and H. Mori. 2009. Cases of HIV type 1 acute infection at STI-related clinics in Osaka. AIDS Research and Human Retroviruses 25(7):717-719.
Koop, C. E. 1986. Surgeon general’s report on acquired deficiency syndrome. https://www.nlm.nih.gov/exhibition/survivingandthriving/education/Activity-Surgeon-General/Surgeon-Generals-Report-on-AIDS.pdf (accessed November 15, 2020).
Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, M. Alary, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: Results from a cohort study. AIDS 7(1):95-102.
Landovitz, R. J., J. L. Gildner, and A. A. Leibowitz. 2018a. Sexually transmitted infection testing of HIV-positive Medicare and Medicaid enrollees falls short of guidelines. Sexually Transmitted Diseases 45(1):8-13.
Landovitz, R. J., S. Li, B. Grinsztejn, H. Dawood, A. Y. Liu, M. Magnus, M. C. Hosseinipour, R. Panchia, L. Cottle, G. Chau, P. Richardson, M. A. Marzinke, C. W. Hendrix, S. H. Eshleman, Y. Zhang, E. Tolley, J. Sugarman, R. Kofron, A. Adeyeye, D. Burns, A. R. Rinehart, D. Margolis, W. R. Spreen, M. S. Cohen, M. McCauley, and J. J. Eron. 2018b. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Medicine 15(11):e1002690.
Li, J., C. Armon, F. J. Palella, Jr., R. M. Novak, D. Ward, S. Purinton, M. Durham, and K. Buchacz. 2019a. Chlamydia and gonorrhea incidence and testing among patients in the Human Immunodeficiency Virus Outpatient Study (HOPS), 2007−2017. Clinical Infectious Diseases 71(8):1824-1835.
Li, J., G. Regev, S. K. Patel, D. Patton, Y. Sweeney, P. Graebing, S. Grab, L. Wang, V. Sant, and L. C. Rohan. 2019b. Rational design of a multipurpose bioadhesive vaginal film for co-delivery of dapivirine and levonorgestrel. Pharmaceutics 12(1).
Liew, Z. Q., V. Ly, and C. Olson-Chen. 2021. An old disease on the rise: New approaches to syphilis in pregnancy. Current Opinion in Obstetrics and Gynecology 33(2):78-85.
Lingappa, J. R., J. P. Hughes, R. S. Wang, J. M. Baeten, C. Celum, G. E. Gray, W. S. Stevens, D. Donnell, M. S. Campbell, C. Farquhar, M. Essex, J. I. Mullins, R. W. Coombs, H. Rees, L. Corey, and A. Wald. 2010. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One 5(9):e12598.
Makhema, J., K. E. Wirth, M. Pretorius Holme, T. Gaolathe, M. Mmalane, E. Kadima, U. Chakalisa, K. Bennett, J. Leidner, K. Manyake, A. M. Mbikiwa, S. V. Simon, R. Letlhogile, K. Mukokomani, E. van Widenfelt, S. Moyo, R. Lebelonyane, M. G. Alwano, K. M. Powis, S. L. Dryden-Peterson, C. Kgathi, V. Novitsky, J. Moore, P. Bachanas, W. Abrams, L. Block, S. El-Halabi, T. Marukutira, L. A. Mills, C. Sexton, E. Raizes, S. Gaseitsiwe, H. Bussmann, L. Okui, O. John, R. L. Shapiro, S. Pals, H. Michael, M. Roland, V. DeGruttola, Q. Lei, R. Wang, E. Tchetgen Tchetgen, M. Essex, and S. Lockman. 2019. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. New England Journal of Medicine 381(3):230-242.
Manoto, S. L., M. Lugongolo, U. Govender, and P. Mthunzi-Kufa. 2018. Point of care diagnostics for HIV in resource limited settings: An overview. Medicina (Kaunas) 54(1).
Marcus, J. L., D. V. Glidden, K. H. Mayer, A. Y. Liu, S. P. Buchbinder, K. R. Amico, V. McMahan, E. G. Kallas, O. Montoya-Herrera, J. Pilotto, and R. M. Grant. 2013. No evidence of sexual risk compensation in the Iprex trial of daily oral HIV preexposure prophylaxis. PLoS One 8(12):e81997.
Marcus, J. L., K. A. Katz, D. S. Krakower, and S. K. Calabrese. 2019. Risk compensation and clinical decision making—the case of HIV preexposure prophylaxis. New England Journal of Medicine 380(6):510-512.
Marrazzo, J. M., J. C. Dombrowski, and K. H. Mayer. 2018. Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement. PLoS Medicine 15(1):e1002485.
Marshall, B. D. L., W. C. Goedel, M. R. F. King, A. Singleton, D. P. Durham, P. A. Chan, J. P. Townsend, and A. P. Galvani. 2018. Potential effectiveness of long-acting injectable preexposure prophylaxis for HIV prevention in men who have sex with men: A modelling study. The Lancet HIV 5(9):e498-e505.
Marston, M., J. Nakiyingi-Miiro, S. Kusemererwa, M. Urassa, D. Michael, C. Nyamukapa, S. Gregson, B. Zaba, and J. W. Eaton. 2017. The effects of HIV on fertility by infection duration: Evidence from African population cohorts before antiretroviral treatment availability. AIDS 31(Suppl 1):S69-S76.
Marx, J. L. 1982. New disease baffles medical community. Science 217(4560):618-621.
Marx, J. L. 1983. Spread of AIDS sparks new health concern. Science 219(4580):42-43.
Masson, L., S. Barnabas, J. Deese, K. Lennard, S. Dabee, H. Gamieldien, S. Z. Jaumdally, A. L. Williamson, F. Little, L. Van Damme, K. Ahmed, T. Crucitti, S. Abdellati, L. G. Bekker, G. Gray, J. Dietrich, H. Jaspan, and J. S. Passmore. 2019. Inflammatory cytokine biomarkers of asymptomatic sexually transmitted infections and vaginal dysbiosis: A multicentre validation study. Sexually Transmitted Infections 95(1):5-12.
Masur, H., M. A. Michelis, J. B. Greene, I. Onorato, R. A. Stouwe, R. S. Holzman, G. Wormser, L. Brettman, M. Lange, H. W. Murray, and S. Cunningham-Rundles. 1981. An outbreak of community-acquired Pneumocystis carinii pneumonia: Initial manifestation of cellular immune dysfunction. New England Journal of Medicine 305(24):1431-1438.
Masur, H., M. A. Michelis, G. P. Wormser, S. Lewin, J. Gold, M. L. Tapper, J. Giron, C. W. Lerner, D. Armstrong, U. Setia, J. A. Sender, R. S. Siebken, P. Nicholas, Z. Arlen, S. Maayan, J. A. Ernst, F. P. Siegal, and S. Cunningham-Rundles. 1982. Opportunistic infection in previously healthy women. Initial manifestations of a community-acquired cellular immunodeficiency. Annals of Internal Medicine 97(4):533-539.
Mayer, K. H., and K. K. Venkatesh. 2011. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. American Journal of Reproductive Immunology 65(3):308-316.
Mayer, K. H., J.-M. Molina, M. A. Thompson, P. L. Anderson, K. C. Mounzer, J. J. De Wet, E. DeJesus, H. Jessen, R. M. Grant, P. J. Ruane, P. Wong, R. Ebrahimi, L. Zhong, A. Mathias, C. Callebaut, S. E. Collins, M. Das, S. McCallister, D. M. Brainard, C. Brinson, A. Clarke, P. Coll, F. A. Post, and C. B. Hare. 2020. Emtricitabine and tenofovir alafenamide vs. emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. The Lancet 396(10246):239-254.
McCarty-Caplan, D., I. Jantz, and J. Swartz. 2014. MSM and drug use: A latent class analysis of drug use and related sexual risk behaviors. AIDS and Behavior 18(7):1339-1351.
McClelland, R. S., J. R. Lingappa, S. Srinivasan, J. Kinuthia, G. C. John-Stewart, W. Jaoko, B. A. Richardson, K. Yuhas, T. L. Fiedler, K. N. Mandaliya, M. M. Munch, N. R. Mugo, C. R. Cohen, J. M. Baeten, C. Celum, J. Overbaugh, and D. N. Fredricks. 2018. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: A nested case-control study. Lancet Infectious Diseases 18(5):554-564.
McCormack, S., D. T. Dunn, M. Desai, D. I. Dolling, M. Gafos, R. Gilson, A. K. Sullivan, A. Clarke, I. Reeves, G. Schembri, N. Mackie, C. Bowman, C. J. Lacey, V. Apea, M. Brady, J. Fox, S. Taylor, S. Antonucci, S. H. Khoo, J. Rooney, A. Nardone, M. Fisher, A. McOwan, A. N. Phillips, A. M. Johnson, B. Gazzard, and O. N. Gill. 2016. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 387(10013):53-60.
Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272(5265):1167-1170.
Melo, M. G., E. Sprinz, P. M. Gorbach, B. Santos, T. M. Rocha, M. Simon, M. Almeida, R. Lira, M. C. Chaves, T. Kerin, I. Varella, and K. Nielsen-Saines. 2019. HIV-1 heterosexual transmission and association with sexually transmitted infections in the era of treatment as prevention. International Journal of Infectious Diseases 87:128-134.
Mena Lora, A. J., M. Braniecki, A. Nasir, and M. Brito. 2017. The great impostor: Lues maligna in an HIV-infected male. SAGE Open Medical Case Reports 5:2050313x17731050.
Michael, S., M. Gompels, C. Sabin, H. Curtis, and M. T. May. 2017. Benchmarked performance charts using principal components analysis to improve the effectiveness of feedback for audit data in HIV care. BMC Health Services Research 17(1):506.
Milam, J., S. Jain, M. P. Dube, E. S. Daar, X. Sun, K. Corado, E. Ellorin, J. Blumenthal, R. Haubrich, D. J. Moore, and S. R. Morris. 2019. Sexual risk compensation in a preexposure prophylaxis demonstration study among individuals at risk of HIV. Journal of Acquired Immune Deficiency Syndromes 80(1):e9-e13.
Millett, G. A., H. Ding, G. Marks, W. L. Jeffries 4th, T. Bingham, J. Lauby, C. Murrill, S. Flores, and A. Stueve. 2011. Mistaken assumptions and missed opportunities: Correlates of undiagnosed HIV infection among black and Latino men who have sex with men. Journal of Acquired Immune Deficiency Syndromes 58(1):64-71.
Modjarrad, K., and S. H. Vermund. 2010. Effect of treating co-infections on HIV-1 viral load: A systematic review. Lancet Infectious Diseases 10(7):455-463.
Modjarrad, K., E. Chamot, and S. H. Vermund. 2008. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS 22(16):2179-2185.
Molina, J. M., I. Charreau, B. Spire, L. Cotte, J. Chas, C. Capitant, C. Tremblay, D. Rojas-Castro, E. Cua, A. Pasquet, C. Bernaud, C. Pintado, C. Delaugerre, L. Sagaon-Teyssier, S. L. Mestre, C. Chidiac, G. Pialoux, D. Ponscarme, J. Fonsart, D. Thompson, M. A. Wainberg, V. Dore, and L. Meyer. 2017. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: An observational cohort study. Lancet HIV 4(9):e402-e410.
Molloy, A., H. Curtis, F. Burns, and A. Freedman. 2017. Routine monitoring and assessment of adults living with HIV: Results of the British HIV Association (BHIVA) national audit 2015. BMC Infectious Diseases 17(1):619.
Moskowitz, D. A., and M. E. Roloff. 2010. Moderators of sexual behavior in gay men. Archives of Sexual Behavior 39(4):950-958.
Noël-Romas, L., M. Perner, R. Molatlhegi, C. Farr Zuend, A. Mabhula, S. Hoger, A. Lamont, K. Birse, A. Berard, S. McCorrister, G. Westmacott, A. Leslie, V. Poliquin, R. Heffron, L. R. McKinnon, and A. D. Burgener. 2020. Vaginal microbiome-hormonal contraceptive interactions associate with the mucosal proteome and HIV acquisition. PLOS Pathogens 16(12):e1009097.
Norkin, S. K., S. Benson, A. M. Civitarese, A. Reich, M. Chomsky Albright, A. Convery, I. M. Kasarskis, H. Cassidy-Stewart, K. Howe, X. Wang, M. R. Golden, C. M. Khosropour, S. N. Glick, and R. P. Kerani. 2021. Inadequate engagement in HIV care among people with HIV newly diagnosed with an STD: A multi-jurisdictional analysis. Sexually Transmitted Diseases. doi: 10.1097/OLQ.0000000000001381.
Oette, M., J. Hemker, T. Feldt, A. Sagir, J. Best, and D. Häussinger. 2005. Acute syphilitic blindness in an HIV-positive patient. AIDS Patient Care and STDs 19(4):209-211.
Oleske, J., A. Minnefor, R. Cooper, Jr., K. Thomas, A. dela Cruz, H. Ahdieh, I. Guerrero, V. V. Joshi, and F. Desposito. 1983. Immune deficiency syndrome in children. JAMA 249(17):2345-2349.
Ong, J. J., R. C. Baggaley, T. E. Wi, J. D. Tucker, H. Fu, M. K. Smith, S. Rafael, V. Anglade, J. Falconer, R. Ofori-Asenso, F. Terris-Prestholt, I. Hodges-Mameletzis, and P. Mayaud. 2019. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: A systematic review and meta-analysis. JAMA Network Open 2(12):e1917134.
Owens, D. K., K. W. Davidson, A. H. Krist, M. J. Barry, M. Cabana, A. B. Caughey, S. J. Curry, C. A. Doubeni, J. W. Epling, Jr., M. Kubik, C. S. Landefeld, C. M. Mangione, L. Pbert, M. Silverstein, M. A. Simon, C. W. Tseng, and J. B. Wong. 2019. Screening for HIV infection: U.S. Preventive Services Task Force recommendation statement. JAMA 321(23):2326-2336.
Passmore, J. A., H. B. Jaspan, and L. Masson. 2016. Genital inflammation, immune activation and risk of sexual HIV acquisition. Current Opinion in HIV and AIDS 11(2):156-162.
Pathela, P., S. L. Braunstein, S. Blank, C. Shepard, and J. A. Schillinger. 2015. The high risk of an HIV diagnosis following a diagnosis of syphilis: A population-level analysis of New York City men. Clinical Infectious Diseases 61(2):281-287.
Peeling, R. W., and E. W. Hook, 3rd. 2006. The pathogenesis of syphilis: The great mimicker, revisited. Journal of Pathology 208(2):224-232.
Pellowski, J. A., T. B. Huedo-Medina, and S. C. Kalichman. 2018. Food insecurity, substance use, and sexual transmission risk behavior among people living with HIV: A daily level analysis. Archives of Sexual Behavior 47(7):1899-1907.
Persson, A. 2010. Reflections on the Swiss consensus statement in the context of qualitative interviews with heterosexuals living with HIV. AIDS Care 22(12):1487-1492.
Peterman, T. A., D. R. Newman, L. Maddox, K. Schmitt, and S. Shiver. 2015. Risk for HIV following a diagnosis of syphilis, gonorrhoea or chlamydia: 328,456 women in Florida, 2000-2011. International Journal of STD & AIDS 26(2):113-119.
Peters, P. J., P. Pontones, K. W. Hoover, M. R. Patel, R. R. Galang, J. Shields, S. J. Blosser, M. W. Spiller, B. Combs, W. M. Switzer, C. Conrad, J. Gentry, Y. Khudyakov, D. Waterhouse, S. M. Owen, E. Chapman, J. C. Roseberry, V. McCants, P. J. Weidle, D. Broz, T. Samandari, J. Mermin, J. Walthall, J. T. Brooks, and J. M. Duwve. 2016. HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. New England Journal of Medicine 375(3):229-239.
Pilcher, C. D., M. A. Price, I. F. Hoffman, S. Galvin, F. E. Martinson, P. N. Kazembe, J. J. Eron, W. C. Miller, S. A. Fiscus, and M. S. Cohen. 2004a. Frequent detection of acute primary HIV infection in men in Malawi. AIDS 18(3):517-524.
Pilcher, C. D., H. C. Tien, J. J. Eron, Jr., P. L. Vernazza, S. Y. Leu, P. W. Stewart, L. E. Goh, and M. S. Cohen. 2004b. Brief but efficient: Acute HIV infection and the sexual transmission of HIV. The Journal of Infectious Diseases 189(10):1785-1792.
Pinkerton, S. D. 2001. Sexual risk compensation and HIV/STD transmission: Empirical evidence and theoretical considerations. Risk Analysis 21(4):727-736.
Pinkerton, S. D., H. W. Chesson, R. A. Crosby, and P. M. Layde. 2011. Linearity and nonlinearity in HIV/STI transmission: Implications for the evaluation of sexual risk reduction interventions. Evaluation Review 35(5):550-565.
Piot, P., and M. Laga. 1989. Genital ulcers, other sexually transmitted diseases, and the sexual transmission of HIV. BMJ 298(6674):623-624.
Powers, K. A., W. C. Miller, C. D. Pilcher, C. Mapanje, F. E. Martinson, S. A. Fiscus, D. A. Chilongozi, D. Namakhwa, M. A. Price, S. R. Galvin, I. F. Hoffman, and M. S. Cohen. 2007. Improved detection of acute HIV-1 infection in sub-Saharan Africa: Development of a risk score algorithm. AIDS 21(16):2237-2242.
Powers, K. A., C. Poole, A. E. Pettifor, and M. S. Cohen. 2008. Rethinking the heterosexual infectivity of HIV-1: A systematic review and meta-analysis. Lancet Infectious Diseases 8(9):553-563.
Price, M. A., D. Zimba, I. F. Hoffman, S. C. Kaydos-Daniels, W. C. Miller, F. Martinson, D. Chilongozi, E. Kip, E. Msowoya, M. M. Hobbs, P. Kazembe, and M. S. Cohen. 2003. Addition of treatment for trichomoniasis to syndromic management of urethritis in malawi: A randomized clinical trial. Sexually Transmitted Diseases 30(6):516-522.
Purcell, D. W., D. Higa, Y. Mizuno, and C. Lyles. 2017. Quantifying the harms and benefits from serosorting among HIV-negative gay and bisexual men: A systematic review and meta-analysis. AIDS and Behavior 21(10):2835-2843.
Quinlivan, E. B., S. N. Patel, C. A. Grodensky, C. E. Golin, H. C. Tien, and M. M. Hobbs. 2012. Modeling the impact of Trichomonas vaginalis infection on HIV transmission in HIV-infected individuals in medical care. Sexually Transmitted Diseases 39(9):671-677.
Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, and R. H. Gray. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. New England Journal of Medicine 342(13):921-929.
Ramchandani, M. S., and M. R. Golden. 2019. Confronting rising STIs in the era of PrEP and treatment as prevention. Current HIV/AIDS Reports 16(3):244-256.
Rendina, H. J., J. Cienfuegos-Szalay, A. Talan, S. S. Jones, and R. H. Jimenez. 2020. Growing acceptability of undetectable = untransmittable but widespread misunderstanding of transmission risk: Findings from a very large sample of sexual minority men in the United States. Journal of Acquired Immune Deficiency Syndromes 83(3):215-222.
Reynolds, G. L., and D. G. Fisher. 2019. A latent class analysis of alcohol and drug use immediately before or during sex among women. American Journal of Drug and Alcohol Abuse 45(2):179-188.
Rietmeijer, C. A., J. L. Patnaik, F. N. Judson, and J. M. Douglas, Jr. 2003. Increases in gonorrhea and sexual risk behaviors among men who have sex with men: A 12-year trend analysis at the Denver metro health clinic. Sexually Transmitted Diseases 30(7):562-567.
Rodger, A. J., V. Cambiano, T. Bruun, P. Vernazza, S. Collins, J. van Lunzen, G. M. Corbelli, V. Estrada, A. M. Geretti, A. Beloukas, D. Asboe, P. Viciana, F. Gutiérrez, B. Clotet, C. Pradier, J. Gerstoft, R. Weber, K. Westling, G. Wandeler, J. M. Prins, A. Rieger, M. Stoeckle, T. Kümmerle, T. Bini, A. Ammassari, R. Gilson, I. Krznaric, M. Ristola, R. Zangerle, P. Handberg, A. Antela, S. Allan, A. N. Phillips, J. Lundgren, and Partner Study Group. 2016. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 316(2):171-181.
Rojas Castro, D., R. M. Delabre, and J. M. Molina. 2019. Give PrEP a chance: Moving on from the “risk compensation” concept. Journal of the International AIDS Society 22(Suppl 6):e25351.
Rompalo, A. M., M. R. Joesoef, J. A. O’Donnell, M. Augenbraun, W. Brady, J. D. Radolf, R. Johnson, and R. T. Rolfs. 2001a. Clinical manifestations of early syphilis by HIV status and gender: Results of the Syphilis and HIV study. Sexually Transmitted Diseases 28(3):158-165.
Rompalo, A. M., J. Lawlor, P. Seaman, T. C. Quinn, J. M. Zenilman, and E. W. I. Hook. 2001b. Modification of syphilitic genital ulcer manifestations by coexistent HIV infection. Sexually Transmitted Diseases 28(8):448-454.
Rosenberg, Z. 2017. Pooled efficacy analysis of two phase III trials of dapivirine vaginal ring (DVR) for the reduction of HIV-1 infection risk in HIV uninfected women in sub-Saharan Africa. http://programme.ias2017.org/Abstract/Abstract/5704 (accessed January 29, 2021).
Rosner, F., and J. A. Giron. 1983. Immune deficiency syndrome in children. JAMA 250(22):3046.
Ross, J. 2001. Pelvic inflammatory disease. BMJ 322(7287):658-659.
Røttingen, J.-A., and G. P. Garnett. 2002. The epidemiological and control implications of HIV transmission probabilities within partnerships. Sexually Transmitted Diseases 29(12):818-827.
Royce, R. A., A. Sena, W. Cates, Jr., and M. S. Cohen. 1997. Sexual transmission of HIV. New England Journal of Medicine 336(15):1072-1078.
Rubinstein, A., M. Sicklick, A. Gupta, L. Bernstein, N. Klein, E. Rubinstein, I. Spigland, L. Fruchter, N. Litman, H. Lee, and M. Hollander. 1983. Acquired immunodeficiency with reversed t4/t8 ratios in infants born to promiscuous and drug-addicted mothers. JAMA 249(17):2350-2356.
Rutstein, S. E., A. E. Pettifor, S. Phiri, G. Kamanga, I. F. Hoffman, M. C. Hosseinipour, N. E. Rosenberg, D. Nsona, D. Pasquale, G. Tegha, K. A. Powers, M. Phiri, B. Tembo, W. Chege, and W. C. Miller. 2016. Incorporating acute HIV screening into routine HIV testing at sexually transmitted infection clinics, and HIV testing and counseling centers in Lilongwe, Malawi. Journal of Acquired Immune Deficiency Syndromes 71(3):272-280.
Sabo, M. C., D. A. Lehman, B. Wang, B. A. Richardson, S. Srinivasan, L. Osborn, D. Matemo, J. Kinuthia, T. L. Fiedler, M. M. Munch, A. L. Drake, D. N. Fredricks, J. Overbaugh, G. John-Stewart, R. S. McClelland, and S. M. Graham. 2020. Associations between vaginal bacteria implicated in HIV acquisition risk and proinflammatory cytokines and chemo-kines. Sexually Transmitted Infections 96(1):3-9.
Santelli, J. S., L. Robin, N. D. Brener, and R. Lowry. 2001. Timing of alcohol and other drug use and sexual risk behaviors among unmarried adolescents and young adults. Family Planning Perspectives 33(5):200-205.
Schmidt, R., P. J. Carson, and R. J. Jansen. 2019. Resurgence of syphilis in the United States: An assessment of contributing factors. Infectious Diseases 12:1178633719883282.
Schneider, T., R. Ullrich, and M. Zeitz. 1996. The immunologic aspects of human immunodeficiency virus infection in the gastrointestinal tract. Seminars in Gastrointestinal Disease 7(1):19-29.
Scott, H. M., and J. D. Klausner. 2016. Sexually transmitted infections and pre-exposure prophylaxis: Challenges and opportunities among men who have sex with men in the US. AIDS Research and Therapy 13:5.
Shahmanesh, M., V. Patel, D. Mabey, and F. Cowan. 2008. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: A systematic review. Tropical Medicine & International Health 13(5):659-679.
Sheffield, J. S., G. D. Wendel, Jr., D. D. McIntire, and M. V. Norgard. 2007. Effect of genital ulcer disease on HIV-1 coreceptor expression in the female genital tract. The Journal of Infectious Diseases 196(10):1509-1516.
Shilts, R. 1987. And the band played on: Politics, people and the AIDS epidemic. New York: St. Martin’s Press.
Smith, D. K., M. H. Chang, W. A. Duffus, S. Okoye, and S. Weissman. 2019. Missed opportunities to prescribe preexposure prophylaxis in South Carolina, 2013-2016. Clinical Infectious Diseases 68(1):37-42.
SNL (Saturday Night Live). 2019. Weekend update: Pete Davidson on sexually transmitted diseases. https://snltranscripts.jt.org/2019/weekend-update-pete-davidson-on-sexuallytransmitted-diseases.phtml#:~:text=Weekend%20Update%20Pete%20Davidson%20on%20Sexually%20Transmitted%20Diseases,-0&text=Colin%20Jost%3A%20According%20to%20the,highs%20because%20of%20dating%20apps.&text=Pete%20Davidson%3A%20I%20don’t,can%20cure%20with%20a%20shot (accessed January 30, 2021).
Stenger, M. R., S. Baral, S. Stahlman, D. Wohlfeiler, J. E. Barton, and T. Peterman. 2017. As through a glass, darkly: The future of sexually transmissible infections among gay, bisexual and other men who have sex with men. Sexual Health 14(1):18-27.
Stolte, I. G., N. H. Dukers, J. B. de Wit, J. S. Fennema, and R. A. Coutinho. 2001. Increase in sexually transmitted infections among homosexual men in Amsterdam in relation to HAART. Sexually Transmitted Infections 77(3):184-186.
Storholm, E. D., J. E. Volk, J. L. Marcus, M. J. Silverberg, and D. D. Satre. 2017. Risk perception, sexual behaviors, and PrEP adherence among substance-using men who have sex with men: A qualitative study. Prevention Science 18(6):737-747.
Stringer, E. M., M. Sinkala, J. S. Stringer, E. Mzyece, I. Makuka, R. L. Goldenberg, P. Kwape, M. Chilufya, and S. H. Vermund. 2003. Prevention of mother-to-child transmission of HIV in Africa: Successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS 17(9):1377-1382.
Taha, T. E., D. R. Hoover, G. A. Dallabetta, N. I. Kumwenda, L. A. Mtimavalye, L. P. Yang, G. N. Liomba, R. L. Broadhead, J. D. Chiphangwi, and P. G. Miotti. 1998. Bacterial vaginosis and disturbances of vaginal flora: Association with increased acquisition of HIV. AIDS 12(13):1699-1706.
The Lancet HIV. 2017. U=U taking off in 2017. The Lancet HIV 4(11):e475.
Tran, T. H., N. Cassoux, B. Bodaghi, C. Fardeau, E. Caumes, and P. Lehoang. 2005. Syphilitic uveitis in patients infected with human immunodeficiency virus. Graefe’s Archive for Clinical and Experimental Ophthalmology 243(9):863-869.
Tsevat, D. G., H. C. Wiesenfeld, C. Parks, and J. F. Peipert. 2017. Sexually transmitted diseases and infertility. American Journal of Obstetrics and Gynecology 216(1):1-9.
van de Wijgert, J. H., C. S. Morrison, J. Brown, C. Kwok, B. Van Der Pol, T. Chipato, J. K. Byamugisha, N. Padian, and R. A. Salata. 2009. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sexually Transmitted Diseases 36(6):357-364.
Vanable, P. A., M. P. Carey, J. L. Brown, R. A. Littlewood, R. Bostwick, and D. Blair. 2012. What HIV-positive MSM want from sexual risk reduction interventions: Findings from a qualitative study. AIDS and Behavior 16(3):554-563.
Varghese, B., J. E. Maher, T. A. Peterman, B. M. Branson, and R. W. Steketee. 2002. Reducing the risk of sexual HIV transmission: Quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sexually Transmitted Diseases 29(1):38-43.
Venkatesh, K. K., T. P. Flanigan, and K. H. Mayer. 2011. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS 25(16):1939-1949.
Vermund, S. H., and R. J. Hayes. 2013. Combination prevention: New hope for stopping the epidemic. Current HIV/AIDS Reports 10(2):169-186.
Vermund, S. H., and A. J. Leigh-Brown. 2012. The HIV epidemic: High-income countries. Cold Spring Harbor Perspectives in Medicine 2(5):a007195.
Vernazza, P., and E. J. Bernard. 2016. HIV is not transmitted under fully suppressive therapy: The Swiss statement—eight years later. Swiss Medical Weekly 146:w14246.
Vernazza, P., B. Hirschel, E. Bernasconi, and M. Flepp, pour le Commission fédérale pour les problèmes liés au sida (CFS) et le Commission d’experts clinique et thérapie VIH et sida de l’Office fédéral de la santé publique (OFSP). 2008. Les personnes séropositives ne souffrant d’aucune autre mst et suivant un traitement antirétroviral efficace ne transmettent pas le vih par voie sexuelle. Bulletin des Dédecins Suisses 89(5):165-169.
Wade Taylor, S., C. O’Cleirigh, K. H. Mayer, and S. A. Safren. 2013. HIV-infected men who have sex with men who engage in very high levels of transmission risk behaviors: Establishing a context for novel prevention interventions. Psychology, Health & Medicine 18(5):576-587.
Wang, H., X. Wang, and S. Li. 2012. A case of lues maligna in an AIDS patient. International Journal of STD & AIDS 23(8):599-600.
Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sexually Transmitted Diseases 19(2):61-77.
Watson-Jones, D., H. A. Weiss, M. Rusizoka, J. Changalucha, K. Baisley, K. Mugeye, C. Tanton, D. Ross, D. Everett, T. Clayton, R. Balira, L. Knight, I. Hambleton, J. Le Goff, L. Belec, and R. Hayes. 2008. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. New England Journal of Medicine 358(15):1560-1571.
Wawer, M. J., R. H. Gray, N. K. Sewankambo, D. Serwadda, L. Paxton, S. Berkley, D. McNairn, F. Wabwire-Mangen, C. Li, F. Nalugoda, N. Kiwanuka, T. Lutalo, R. Brookmeyer, R. Kelly, and T. C. Quinn. 1998. A randomized, community trial of intensive sexually transmitted disease control for AIDS prevention, Rakai, Uganda. AIDS 12(10):1211-1225.
Wawer, M. J., N. K. Sewankambo, D. Serwadda, T. C. Quinn, L. A. Paxton, N. Kiwanuka, F. Wabwire-Mangen, C. Li, T. Lutalo, F. Nalugoda, C. A. Gaydos, L. H. Moulton, M. O. Meehan, S. Ahmed, and R. H. Gray. 1999. Control of sexually transmitted diseases for AIDS prevention in Uganda: A randomised community trial. Rakai project study group. Lancet 353(9152):525-535.
Weiss, H. 2004. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes 11(Suppl 1):24A-35A.
Weiss, S. H., J. J. Goedert, M. G. Sarngadharan, A. J. Bodner, R. C. Gallo, and W. A. Blattner. 1985. Screening test for HTLV-III (AIDS agent) antibodies. Specificity, sensitivity, and applications. JAMA 253(2):221-225.
Weller, S., and K. Davis. 2002. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database of Systematic Reviews (1):CD003255.
Westercamp, N., K. Agot, W. Jaoko, and R. C. Bailey. 2014. Risk compensation following male circumcision: Results from a two-year prospective cohort study of recently circumcised and uncircumcised men in Nyanza province, Kenya. AIDS and Behavior 18(9):1764-1775.
Wetmore, C. M., L. E. Manhart, and J. N. Wasserheit. 2010. Randomized controlled trials of interventions to prevent sexually transmitted infections: Learning from the past to plan for the future. Epidemiologic Reviews 32:121-136.
White, R. G., K. K. Orroth, J. R. Glynn, E. E. Freeman, R. Bakker, J. D. Habbema, F. Terris-Prestholt, L. Kumaranayake, A. Buve, and R. J. Hayes. 2008. Treating curable sexually transmitted infections to prevent HIV in Africa: Still an effective control strategy? Journal of Acquired Immune Deficiency Syndromes 47(3):346-353.
WHO (World Health Organization). 2016. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland: World Health Organization.
WHO. 2020. European medicines agency (EMA) approval of the dapivirine ring for HIV prevention for women in high HIV burden settings. https://www.who.int/news/item/24-07-2020-european-medicines-agency-(ema)-approval-of-the-dapivirine-ring-for-hiv-prevention-for-women-in-high-hiv-burden-settings (accessed January 25, 2021).
WHO. 2021. WHO recommends the dapivirine vaginal ring as a new choice for HIV prevention for women at substantial risk of HIV infection. https://www.who.int/news/item/26-01-2021-who-recommends-the-dapivirine-vaginal-ring-as-a-new-choice-for-hiv-prevention-for-women-at-substantial-risk-of-hiv-infection (accessed January 29, 2021).
Wiesenfeld, H. C., S. L. Hillier, L. A. Meyn, A. J. Amortegui, and R. L. Sweet. 2012. Subclinical pelvic inflammatory disease and infertility. Obstetrics and Gynocology 120(1):37-43.
Williamson, D. A., E. P. F. Chow, C. L. Gorrie, T. Seemann, D. J. Ingle, N. Higgins, M. Easton, G. Taiaroa, Y. H. Grad, J. C. Kwong, C. K. Fairley, M. Y. Chen, and B. P. Howden. 2019. Bridging of Neisseria gonorrhoeae lineages across sexual networks in the HIV preexposure prophylaxis era. Nature Communications 10(1):3988.
Wing, C. 2009. Effects of written informed consent requirements on HIV testing rates: Evidence from a natural experiment. American Journal of Public Health 99(6):1087-1092.
Wolpaw, B. J., C. Mathews, M. Chopra, D. Hardie, M. N. Lurie, and K. Jennings. 2011. Diagnosis and counselling of patients with acute HIV infection in South Africa. Sexually Transmitted Infections 87(1):71-72.
Woods, W. J., N. Sheon, J. A. Morris, and D. Binson. 2013. Gay bathhouse HIV prevention: The use of staff monitoring of patron sexual behavior. Sexuality Research and Social Policy 10(2):77-86.
Workowski, K. A., and G. A. Bolan. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 64(RR-03):1-137.
Young Holt, B., J. Kiarie, G. S. Kopf, K. Nanda, A. Hemmerling, and S. L. Achilles. 2020. Bridging the gap: Advancing multipurpose prevention technologies from the lab into the hands of women. Biology of Reproduction 103(2):286-288.
Zhu, J., F. Hladik, A. Woodward, A. Klock, T. Peng, C. Johnston, M. Remington, A. Magaret, D. M. Koelle, A. Wald, and L. Corey. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature Medicine 15(8):886-892.
This page intentionally left blank.




































