2
Inclusion of Older Adults in Clinical Trials: An Evolving Landscape
Jerry Gurwitz, executive director of Meyers Primary Care Institute and division chief of geriatric medicine at the University of Massachusetts Medical School, introduced the first session of the workshop, which was designed to leave participants with “a healthy dose of reality about where we are and the future.” The objectives of the session were to review the
current landscape of R&D for older adults, consider medication issues such as adherence and polypharmacy, and examine barriers to conducting clinical research on older adults.
KNOWLEDGE GAPS AND ISSUES UNIQUE TO OLDER ADULTS
The “holy grail” of drug trials, said Rosanne M. Leipzig, professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, is the randomized controlled trial (RCT). The purpose of the RCT is to determine whether the drug is beneficial, and whether the benefits of the drug outweigh the risks. To study this question, RCTs have restrictive inclusion and exclusion criteria, as well as age criteria, with the aim of having a homogeneous population that differs only by study drug exposure (Zulman et al., 2011). If the results of the RCT are statistically significant, there may be no further trials, said Leipzig.
The result of this process, warned Leipzig, is that these drugs are approved and marketed for use in older adults, even if few older adults participated in the clinical trials that led to approval. The first question, then, is if RCT results can be extrapolated from young people to older people. Leipzig presented details from a trial on aspirin to demonstrate the risks of proceeding without sufficient evidence about how drugs work in older adults. Several large RCTs had demonstrated the efficacy of low-dose aspirin for secondary prevention of cardiovascular disease (i.e., prevention of heart attack and stroke in individuals at risk of recurrent clinical events), but the evidence about primary prevention (i.e., prevention of heart attack and stroke in individuals who have not yet developed cardiovascular disease) was less conclusive (McNeil et al., 2018). Despite the limited evidence, low-dose aspirin was widely used in older adults without a medical indication. The Aspirin in Reducing Events in the Elderly (ASPREE) trial1 was conducted to determine whether the daily use of aspirin by older adults would prolong healthy life. Community-dwelling individuals were enrolled in the trial—half of whom were between 65 and 73 years old, and half of whom were 74 years and older. Investigators combined the outcomes of death, dementia, and persistent physical disability into a composite endpoint of disability-free survival. On this endpoint, no difference was found between the group that took aspirin and the group that took a placebo (McNeil et al., 2018). In other words, said Leipzig, “there is absolutely no benefit” to taking aspirin. However, the group taking aspirin had a significantly higher occurrence of major hemorrhage. These results, said Leipzig, suggest that even for
___________________
1 See https://clinicaltrials.gov/ct2/show/NCT01038583 (accessed October 25, 2020).
healthy older adults, there would be potential for serious harm—with no benefits—in taking prophylactic aspirin.
A second question, said Leipzig, is whether trial results from some older adults can be extrapolated to all older adults. Even when older adults are included in clinical trials, she said, there is no guarantee that those individuals are representative of the wide heterogeneity that exists in older adults. For example, of three older adults, one may be in her 90s but may still be healthy, with the exception of high blood pressure; another may be in her 70s and also suffering from rheumatoid arthritis, diabetes, chronic obstructive pulmonary disease, and low vision; and another may have movement challenges due to Alzheimer’s and lumbar stenosis. Of these individuals, the 90-year-old with high blood pressure is the one most likely to be included in a trial. However, once the drug is approved, it can be prescribed to any of these individuals, despite important health status differences among them.
As an example of this issue, Leipzig shared data from the Systolic Blood Pressure Intervention Trial,2 which looked at the effects of treating to an intensive blood pressure–lowering target (less than 120 mm Hg) compared with treating to a standard blood pressure target (less than 140 mm Hg) in individuals 75 years and older (Williamson et al., 2016). The investigators examined a primary outcome of “a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, non-fatal stroke, non-fatal acute decompensated heart failure, and death from cardiovascular causes. All-cause mortality was a secondary outcome” (Williamson et al., 2016). The results showed significantly lower rates of both the primary and secondary outcomes in the intensive treatment group. However, when the data were stratified by different measures of frailty, said Leipzig, investigators found that frail adults generally benefited from lower blood pressure targets, while adults with lower baseline cognitive function did not experience these same benefits.
Leipzig said this trial demonstrates the importance of measuring and analyzing by constructs that vary among older adults, including adverse effects. For example, researchers could potentially use measures of frailty, cognitive impairment, functional ability, and history of falls, she said. However, this approach presents challenges, including a lack of clarity on constructs and tools to measure, the need to use more than one tool, and the potential need for direct observation of these constructs. In addition, said Leipzig, researchers should aim to measure outcomes that matter for older adults. While mortality is an often used outcome in studies, some older adults may be more concerned about other outcomes, such
___________________
2 See https://clinicaltrials.gov/ct2/show/NCT01206062 (accessed October 25, 2020).
as functional ability, cognition, falls, and frailty. Another consideration is the reproducibility of constructs. In the case of disability, it is not a static condition that can be measured, but rather a function of the social environment. For example, if a person with an impairment is able to access assistive devices, he or she may not be considered disabled. Similarly, nursing home placement is dependent on factors such as the number of nursing home beds available in the area, and whether the person is financially able to “age in place” by hiring assistants and retrofitting his or her home.
In summary, said Leipzig, five issues should be addressed moving forward: (1) clinical trials should enroll greater numbers of older adults; (2) clinical trials should enroll older adults with greater heterogeneity; (3) investigators should analyze outcomes with this heterogeneity in mind; (4) investigators should use outcomes that are both reproducible and meaningful to patients; and (5) constructs and tools used to capture heterogeneity should be improved, and whether these constructs and tools should depend on the disease and/or treatment being tested should also be explored.
AGE-RELATED CHANGES THAT IMPACT DRUG METABOLISM
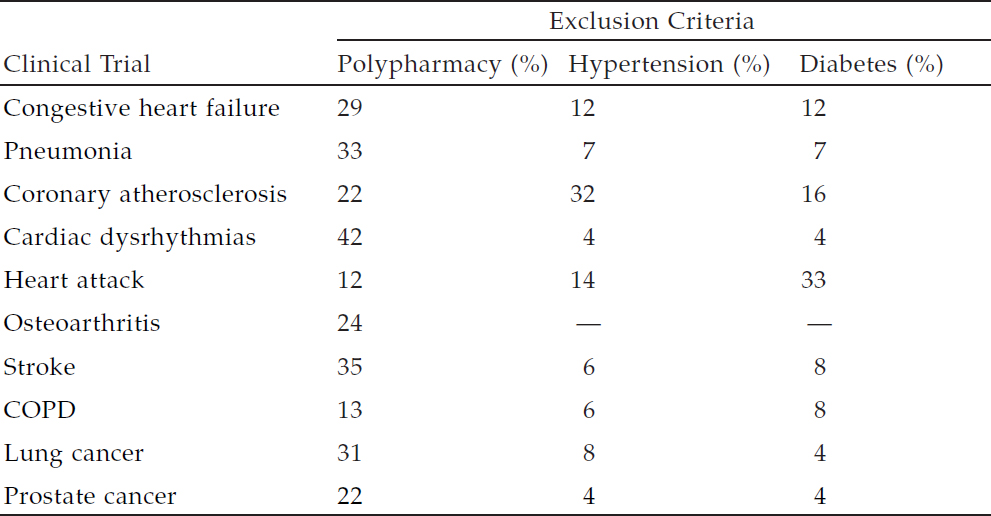
One of the reasons why drugs may affect older adults differently from younger people, said George A. Kuchel, Travelers Chair in Geriatrics and Gerontology and professor of medicine at the University of Connecticut Center on Aging, is that aging changes the process of metabolizing drugs (Mangoni and Jackson, 2004). Many different physiological systems impact drug metabolism, as shown in Figure 2-1. In the digestive system, drugs may interact with food in the stomach; before the drug ever moves on to the liver and the bloodstream, gut absorption issues may develop, and the microbiome may affect metabolism. If a drug is absorbed via the skin or sublingually, it moves directly to the bloodstream, where it interacts with blood proteins, tissues, and receptors. Aging impacts these systems and, in turn, drug metabolism in a number of known ways.
In the gastrointestinal (GI) system, older adults secrete less gastric acid, and this decrease is sometimes augmented by the use of proton-pump inhibitors and antacids (Bai et al., 2016). The lower level of acid can potentially interfere with drug ionization; impair the absorption of certain medications; impair reduction of ferric iron to more absorbable ferrous iron; and impair the liberation of vitamin B12 from food (Lam et al., 2013; Mitra and Kesisoglou, 2013). As a result of these changes, older individuals may suffer from iron and B12 deficiencies, said Kuchel. The metabolism of other drugs may be enhanced due to age-related changes in the GI tract. For example, due to lower dopamine decarboxylase activ-
SOURCES: As presented by George Kuchel, August 5, 2020. Originally from Geneva Hargis, Ph.D., and Christopher Bonin, Ph.D., University of Connecticut School of Medicine.
ity in the mucosa of the GI tract, the absorption of L-DOPA (used for Parkinson’s disease) is enhanced. The role of age-related changes in the microbiome is still being studied, but it is becoming clear that it can have an important effect on drug metabolism. For example, the microbiome is known to activate or reactivate certain medications (e.g., digoxin and irinotecan), and can convert drugs (e.g., nitrazepam) to a teratogenic form.
Aging tends to cause an increase in body fat and a decrease in muscle mass, as well as a decrease in total body water (Westbury et al., 2020). As a result, fat-soluble drug metabolism may be enhanced, while water-soluble drug metabolism may be impaired (Mangoni and Jackson, 2004). However, Kuchel noted, much of the data about body composition in aging comes from cross-sectional rather than longitudinal studies. Kuchel added that there is a need for more information about how and when these changes occur and how they vary due to factors such as sex.
Age-related changes in the blood, liver, and kidneys can also impact drug metabolism. The bottom line, said Kuchel, is that there is a great deal of heterogeneity in the impact of aging on drug metabolism. While aging increases the metabolism of some drugs, it decreases the metabolism of others. Even within the same class of drug, aging may affect drug metabolism differently (Hilmer, 2019). There is heterogeneity among individuals,
as health status, chronic diseases, frailty, and other factors can impact metabolization of a drug. In addition, there is a need to further understand the roles that nutrition, alcohol, lifestyle, and the microbiome play in drug metabolism. All of the heterogeneity and unknown factors make it difficult to know how an individual will respond to a drug, said Kuchel. What is ultimately needed is a way to predict a specific individual’s drug metabolism to better guide clinical decision making at the patient bedside.
BARRIERS TO CONDUCTING CLINICAL TRIALS THAT INCLUDE OLDER ADULTS
National Institutes of Health Perspective
In 2016, the 21st Century Cures Act was passed by Congress. This act led the National Institutes of Health (NIH) to conduct a workshop considering barriers and opportunities for the inclusion of individuals in clinical studies based on age.3 While the examination of this issue was largely motivated by a concern that children were being inadequately studied in clinical trials, NIH “saw a problem with inclusion on both ends of the age spectrum,” said Marie Bernard, deputy director of the National Institute on Aging (NIA) at NIH. In addition to the workshop, the law required NIH to revisit its policy with regard to inclusion and reporting by age.
In preparation for the workshop, Bernard conducted an analysis of NIH-funded Phase 3 clinical trials, focusing on the top 10 causes of hospitalization and/or impacts on disability-adjusted life years in older adults. Bernard said that one would “expect a fairly representative population” in Phase 3 trials because they are large trials that test effectiveness as well as efficacy. The analysis looked at the mean age and age range, inclusion and exclusion criteria, and any age requirements of the trials.
The results of the analysis (see Table 2-1) show that the mean age for trial participants skews young for these conditions, said Bernard. The analysts compared the mean age of the trial participants to the mean age of manifestation of the conditions, and found that only trials on prostate cancer came close to matching the age of the affected population. NIH concluded that “participants in trials may not represent real-world populations with these diseases of older adults,” said Bernard. In fact, the analysis found that older adults were often excluded from trials altogether; 27 percent of the studies had arbitrary upper age caps, and many studies had exclusion criteria that would indirectly exclude many older
___________________
3 Notice of Intent to Revise the NIH Policy and Guidelines on the Inclusion of Children as Participants in Research Involving Human Subjects. See https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-008.html (accessed November 11, 2020).
adults. For example, many older adults have hypertension (65 percent), diabetes (21 percent), or take five or more medications (39 percent). Yet, many trials excluded participants for these same criteria (see Table 2-2). Specifically, 12 to 42 percent of studies excluded individuals who took multiple medications; 4 to 32 percent excluded on the basis of hypertension; and 4 to 33 percent excluded individuals with diabetes.
TABLE 2-1 Mean Age of Trial Participants for Select Types of Studies
| Number of Studies | Mean Age (years) | |
|---|---|---|
| Congestive heart failure | 45 | 61.0 ± 9.2 |
| Cardiac dysrhythmias | 24 | 58.0 ± 7.5 |
| Coronary atherosclerosis | 106 | 59.2 ± 6.5 |
| Heart attack | 76 | 58.8 ± 7.0 |
| Stroke | 113 | 53.3 ± 8.3 |
| COPD | 14 | 58.4 ± 7.1 |
| Pneumonia | 48 | 53.4 ± 7.5 |
| Lung cancer | 117 | 52.4 ± 6.0 |
| Prostate cancer | 65 | 65.4 ± 4.6 |
| Osteoarthritis | 15 | 64.6 ± 6.5 |
NOTE: COPD = chronic obstructive pulmonary disease.
SOURCE: As presented by Marie Bernard, August 5, 2020.
NOTE: COPD = chronic obstructive pulmonary disease.
SOURCE: As presented by Marie Bernard, August 5, 2020.
With these data in hand, NIH held a workshop titled Inclusion Across the Lifespan in June 2017;4 workshop participants delved into the challenges and barriers to including children and older adults in clinical research, and sought to identify strategies to produce more age-inclusive clinical studies. Based on the conversations at the workshop, along with other deliberations across NIH, NIH announced a new policy in December 2017. The policy requires that clinical study applications submitted to NIH must include a plan for enrolling individuals across the lifespan.5 The policy outlined that if the investigators plan to exclude participants based on age, then a rationale and justification must be provided. It may not be appropriate, for example, to include children in an Alzheimer’s disease study or older adults in a measles study. In addition, the policy requires investigators to provide progress reports with anonymized, individual-level data about enrollees’ age, sex/gender, and race/ethnicity.
The policy has been in place for a year and a half, said Bernard, and NIH held a follow-up workshop in September 2020.6 This workshop was intended to provide evidence-based and practical advice to investigators. Bernard said that although many trials do not have an official upper or lower age limit, investigators are still “not meaningfully including older adults and children.” The workshop helped investigators think about appropriate recruitment policies, inclusion and exclusion criteria, creative ways of configuring study design, and analysis strategies that include all groups. In addition to focusing on age, the workshop emphasized the inclusion of other groups who are traditionally underrepresented in clinical trials, including minority groups, women, and individuals in rural settings. Bernard added that to ensure compliance with the “spirit as well as the letter of the policy,” NIH will monitor progress to see if investigators are meeting their goals for recruitment, and will make demographic data from trials publicly available.
Industry Perspective
One of the major obstacles to enrolling older adults in clinical trials, said Katherine Dawson, senior vice president of the Therapeutics Development Group at Biogen, is the fact that the public is largely unfamiliar with the clinical research process. An online survey conducted in 2017
___________________
4 The workshop summary is available at https://report.nih.gov/UploadDocs/NIH%20Inclusion%20Across%20the%20Lifespan%20Workshop%20Summary%20Report.pdf (accessed October 26, 2020).
5 Revision: NIH Policy and Guidelines on the Inclusion of Individuals Across the Lifespan as Participants in Research Involving Human Subjects. See https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html (accessed November 11, 2020).
6 About Inclusion Across the Lifespan-II. See https://www.nia.nih.gov/Inclusion-Across-Lifespan-2020#About (accessed November 11, 2020).
(Anderson et al., 2018) found that although 84 percent of people believe clinical research is important and 90 percent consider participating in research to be safe, a majority did not know where studies are conducted or what agency oversees clinical research. Forty-five percent of respondents rarely discussed clinical trial participation with their providers, and 39 percent underestimated how long drug development takes. Of the 18 percent of respondents who had previously participated in clinical research, 49 percent reported that it disrupted their daily routine. The respondents ranked the factors that were most important to their potential participation in a clinical trial; the top two factors were “potential risks and benefits” and “purpose of the clinical research study” (see Figure 2-2).
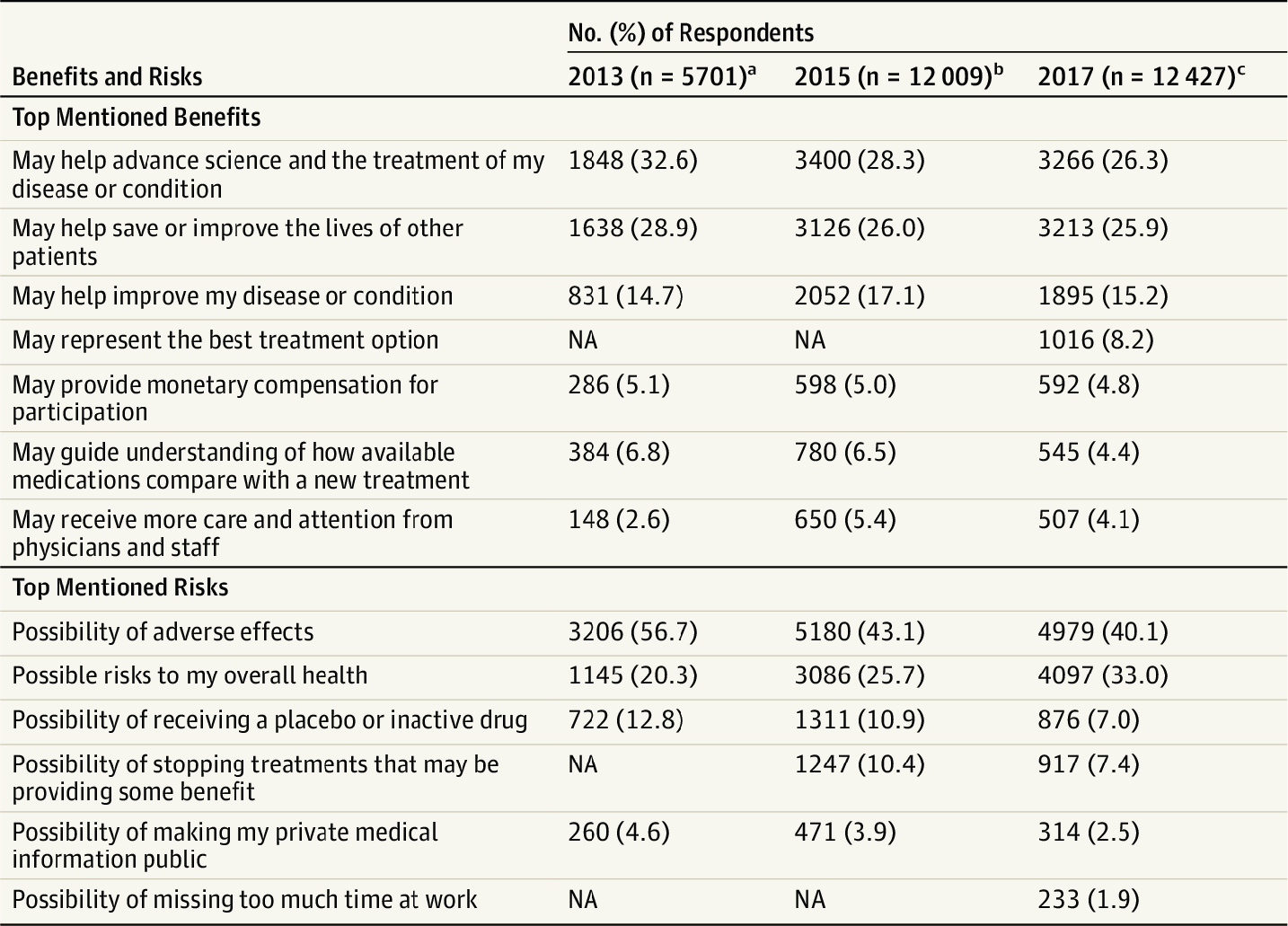
NOTE: NA = not asked (in 2013 and/or 2015 studies).
a Data are from the 2013 Center for Information & Study on Clinical Research Participation Perceptions & Insights Study (all respondents). Percentages not all based on 5,701 because of missing responses to some survey items (n = 5,669 in the benefits sample and 5,650 in the risks sample).
b Data are from the 2015 Center for Information & Study on Clinical Research Participation Perceptions & Insights Study (all respondents).
c Data are from the 2017 Center for Information & Study on Clinical Research Participation Perceptions & Insights Study (all respondents).
SOURCES: As presented by Katherine Dawson, August 5, 2020. Originally from Anderson et al., 2018.
Another study that looked more specifically at older adults, said Dawson, found that older adults had a variety of reasons for not participating in a trial even if they would benefit from participation (Bloch and Charasz, 2014). Only 44 percent would agree to participate if they believed that they would have a personal benefit, and only 21 percent would participate if they believed that they would have potentially no benefit, said Dawson. Reasons respondents gave for not participating included “I think I am too old for this type of experiment,” “I already use a lot of drugs,” and “I am afraid for my well-being.”
Dawson discussed a number of challenges to enrolling older adults in trials in the areas of recruitment, retention, and safety. With respect to recruitment, factors that may encourage participation include access to a potential new treatment, access to a study physician with high expertise, and altruism. Factors that may deter participation include the complexity and length of a trial, misperceptions and fear of the unknown, mistrust of pharmaceutical companies, the possibility of a placebo, and caregiver concerns about safety and side effects. One interesting deterrent for participation, said Dawson, is the type of procedures that would be required. In recruiting for trials, Biogen found that nearly 30 percent of potential participants would not participate if a trial required a lumbar puncture, and up to 50 percent would not participate if it required magnetic resonance imaging (MRI). Retaining older adults in clinical trials can also be challenging, said Dawson. Retention difficulties increase with longer studies, particularly if it is difficult for participants to reach the site, or if the burdens on their caregivers are heavy. Participants may also drop out due to unrelated changes in health, or if adhering to the drug protocol is difficult. Finally, safety can be a big challenge with older adults in clinical trials. There are risks of interactions and adverse drug reactions, and physiological changes related to aging can affect drug metabolism; additionally, participants may also have unrelated underlying diseases that can progress.
Dawson offered suggestions for how industry could improve the recruitment, retention, and safety of older adults in clinical trials. First, industry should forge relationships with patient advocacy and community leaders to ensure that patients understand the process and the need for patients to enroll. Second, industry should partner with the “recruitment gatekeepers” at clinical trial sites to ensure that eligible patients—such as older adults—are not excluded from trials. Third, patient and caregiver feedback should be incorporated into the development of protocol and patient-facing materials to make the trial more accessible and understandable. Fourth, there is a need to address common issues, such as transportation and study updates and reminders. Finally, clinical trial developers need to carefully balance inclusion and exclusion criteria and
ensure that participants are representative of the population and appropriate for the treatment under investigation. Gurwitz asked Dawson if the pharmaceutical industry would respond to a geriatric incentive program similar to the U.S. Food and Drug Administration’s (FDA’s) pediatric exclusivity provision,7 which provides 6 months of additional exclusivity in return for conducting pediatric studies. She responded that the pediatric exclusivity provision, an incentive that was introduced within the field of pediatrics, has been “a good carrot,” and that an exclusivity increase for geriatric products may be similarly helpful.
DISCUSSION
Involving Older Adults in Phase 1 and 2 Trials
There is a growing consensus, said Gurwitz, that new therapies, including vaccines, should be tested for safety, efficacy, and effectiveness in individuals who are most likely to need those therapies or vaccines. Yet, many Phase 1 and 2 trials do not enroll older adults, said Gurwitz. Kuchel responded that this reality is largely driven by a concern about exposing older adults—especially those with comorbidities—to the potential risks of newly discovered drugs. Dawson agreed, noting that Phase 1 trials often enroll healthy volunteers rather than affected patients. However, said Kuchel, excluding older adults results in many missed opportunities with regard to drug validation and proving efficacy. For example, he said, it has become clear that the way older adults respond to the influenza vaccine is quite different from the way younger adults respond, and because of differences in cell-mediated immunity (e.g., activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen) versus humoral immunity (immunity that is mediated by macromolecules, such as antibodies, complement proteins, and certain antimicrobial peptides, found in extracellular fluids), the outcome markers that involve antibody titers do not work well in older adults. Because of these types of differences, Kuchel concluded, it is “absolutely critical” that older adults be included at even the earliest stages of clinical trials. Leipzig added that including older adults at earlier stages would also be beneficial for determining the appropriate dose of the drug, which can vary by age.
___________________
7 For more information on FDA’s Pediatric Exclusivity Provision, see https://www.fda.gov/science-research/pediatrics/pediatric-exclusivity-provision (accessed December 15, 2020).
Enrolling Heterogeneous Older Adult Populations
When thinking about increasing heterogeneity in clinical trials, said Gurwitz, people generally think about characteristics such as age, sex, and race. However, Leipzig’s presentation emphasized the importance of enrolling for heterogeneity on characteristics such as functional status, cognition, frailty, and multimorbidity. Gurwitz asked Leipzig how such characteristics might be prioritized in clinical trials. Leipzig responded that the first question that should be asked is whether the exclusion criteria are appropriate and whether they impact the interpretation of results. For example, excluding participants for multimorbidity or polypharmacy may not be appropriate in a trial of a drug that is likely to be used in a population with a high rate of multimorbidity and polypharmacy. Second, there is a need to better define frailty in older adults, as existing indexes use different combinations of physical, cognitive, and observational information that are confounded. Ideally, there would be a “minimal panel” for measuring frailty to determine whether that population differs (e.g., whether that population is unlikely to benefit, whether that population is likely to experience adverse effects). Leipzig noted, however, that the definition of frailty may change depending on the drug, disease, or treatment under investigation.
Making Participation in Clinical Trials Easier
Gurwitz asked the presenters to consider ways to make it easier for older adults to participate in clinical trials. For example, he said, investigators might consider meeting participants where they are, such as in nursing homes, rather than requiring participants to travel to the clinical trial investigation site. Dawson noted that at the current moment, with the COVID-19 pandemic, entering nursing homes is not feasible. However, she said, the pandemic is serving as a catalyst for thinking differently about how to conduct trials, such as performing assessments remotely. Leipzig added that studies have shown that individuals are more likely to participate if the investigators come to them because it eliminates participant concerns about travel, taking time off from work, and weather. Kuchel countered, however, that this preference may depend on the individual. During the worst days of the COVID-19 pandemic (e.g., Kuchel saw trial participants in their 80s who wanted to come to the trial site and continue to participate). He added that recruitment is also easier when the trial offers some benefit to the participants. For example, a trial that offers an exercise intervention may have less difficulty recruiting than a trial that is focused on studying mechanisms and does not offer personal benefit to the participant. Gurwitz noted that meeting subjects where they are may
cost more money than using a traditional trial site. Bernard added that this approach needs to be “properly funded and properly powered to be able to answer the questions that are being asked.” Some analyses have demonstrated that lower cost studies often do not yield outcomes that are as significant as larger, higher cost studies. Spending more to enhance recruitment and retention of an appropriate study population “may well be more expensive, but it is the right thing to do,” Bernard concluded.
This page intentionally left blank.