4
Alternative Study Approaches
The objective for this session was to explore alternatives to the traditional RCT that could be used to improve participation of or relevance for older adults, said Robert Temple, deputy center director for clinical science in the Office of New Drugs at FDA’s CDER. Presenters in this session focused on several approaches for modifying the traditional RCT:
modifying the design to make trials more efficient and inclusive; using alternative settings for trials, including at home and in the care setting; and using computer modeling to supplement and improve clinical trials.
ADAPTIVE TRIAL DESIGN
An adaptive design, said Scott Berry, co-founder and president of Berry Consultants, is a design that has “prespecified dynamic aspects that are determined by the accruing information.” A trial using adaptive design is set up to have moving parts, and the path the trial takes is determined by the data collected. For example, he said, if accrued data show that the treatment in one arm is not as effective as the treatment in the other arm, randomization to the better arm might be increased. Otherwise, if data reveal that a subset of the participant population is at higher risk for adverse events, enrollment might be stopped for that population. These conditions are set up prospectively so that when the data meet a certain threshold, the trial is adapted and the “map” of the trial changes.
Adaptive design has two aspects: learning and changing. During the course of the trial, things are learned that, had they been known before the trial started, the design would have been different. Adaptive design allows for these changes to be made to the design during the trial itself. For this process to work, said Berry, important aspects, such as adverse events or differences in outcome by subpopulation, must be learned efficiently so that changes can be made. The hope of adaptive design is that trials are better, more efficient, and more effective at “getting the right answers and treating patients.”
Berry shared an example of an adaptive trial called the DAWN trial;1 DAWN used an enrichment design to compare thrombectomy to standard of care in stroke patients. The study was aimed at determining whether the thrombectomy device, which was approved for zero to 8 hours since stroke, was effective up to 24 hours. The enrichment design allowed the data being collected to change the enrollment criteria, said Berry. As patients were enrolled and data were collected on their outcomes, the criteria could be changed to enroll patients with smaller infarcted regions (in mL). Enrollment began at 50 mL and could go down to 45, 40, 35, or 30 based on the accruing data, and the sample size could also be adapted based on outcomes. Berry explained that this design was chosen based on an expectation that the efficacy of the device may depend on the size of the stroke. However, he said, the device was effective in all patients with all stroke sizes (Nogueira et al., 2017). Because of these results, the trial
___________________
1 See https://clinicaltrials.gov/ct2/show/NCT02142283 (accessed October 28, 2020).
stopped at 200 patients (the earliest possible time), but did not change the enrollment criteria.
Another adaptive trial, said Berry, is Influence of Cooling duration on Efficacy in Cardiac Arrest Patients (ICECAP), which was designed to examine whether putting patients into a hypothermic state after cardiac arrest could improve neurological outcomes.2 He noted that while this is a common treatment, and even considered standard of care in many hospitals, there are no large trials that have proven its efficacy or demonstrated optimal cooling time. ICECAP uses hypothermia as the standard of care for enrolled patients, and randomizes them to the length of time that they are kept in a hypothermic state (10 durations ranging from 6 to 72 hours). The first group of patients was randomized to 12, 24, or 48 hours. Response adaptive randomization is used every 50 patients, with the randomization probabilities set to put patients on the durations that are more likely to give better neurological outcomes, Berry said. It is an 1,800-patient trial, and adaptive randomization is conducted separately for two subgroups of patients (shockable and non-shockable). Due to the design, investigators may be able to determine the optimal duration of hypothermia for each subgroup, or could stop the trial early for futility or success within the subgroups. Another ongoing trial that uses response adaptive randomization, he said, is REMAP-CAP [Community-Acquired Pneumonia],3 which is examining various therapies for SARS-CoV-2.
Although Berry has never been involved in an adaptive trial where the goal was to improve the enrollment or treatment specifically for older adults, he said that there is potential to use the design for this purpose. For example, a trial could begin by enrolling younger adults, but with a predefined trigger for enrolling older adults if safety is demonstrated. A trial could use adaptive randomization across arms specifically for older adult populations, using randomization probabilities that are different for them. A trial could use an enrichment strategy, similar to the DAWN trial, that would allow for success or failure within older adults. Using these design strategies, said Berry, could remove some of the barriers that currently prevent older adults from being enrolled in trials, and could help build evidence on treatments that is specific to older adults.
HOME-BASED CLINICAL TRIALS
As discussed previously, one of the major barriers to including older adults in clinical trials is the issue of transportation. Requiring partici-
___________________
2 See https://clinicaltrials.gov/ct2/show/NCT04217551 (accessed October 28, 2020).
3 See https://clinicaltrials.gov/ct2/show/NCT02735707 (accessed October 28, 2020).
pants to travel to a trial site and incur the costs of gas, parking, and lost time can place a heavy burden on both trial participants and their caregivers. Steven R. Cummings, executive director at the San Francisco Coordinating Center, shared an alternative type of trial that can not only eliminate the barrier of travel but also has other advantages. Home-based trials, or virtual trials, are trials conducted without a clinical site. The center reaches out to participants in their own homes, so there are no geographic limitations on participation.
Home-based trials have six essential elements, said Cummings:
- Web-based home-based data collection.
- Web-based eConsent.
- Exams by telemedicine, mobile devices, or in-home nurses.
- Lab sample collection at home, or point-of-care testing.
- Delivery of study treatment to home (by overnight mail or research nurses).
- Patients report adverse events to a central site.
Cummings said that nearly all examinations, laboratory collection, and treatment can be performed in the participant’s home; much of this work is conducted by research nurses, who can reach about 90 percent of the U.S. population. Some assessments are performed via mobile phone or telemedicine, while some testing is conducted at the point of care. Safety monitoring relies on patients reporting adverse events to a central site that is overseen by a doctor 24 hours per day.
Cummings observed that this model has advantages and disadvantages for older adults. First, this model dramatically expands the feasibility for people to enroll in trials by eliminating travel concerns, and can increase the participation of those who have particular difficulty traveling, such as those with disabilities. The model draws on a larger population, allowing recruitment of either a large and diverse patient group, or enrollment of specific cohorts such as racial minorities or patients with certain conditions. A home-based model may be ideal for vaccine trials, said Cummings, because it can easily enroll enough older people to test the efficacy and safety in that population. However, the model has disadvantages as well. Not all older adults have access to the Internet, although the proportion of older adults with the Internet is increasing. Some older adults prefer face-to-face interactions rather than web-based communication, and some may need personal assistance to complete the tasks required. A few older adults do not want a research nurse visiting them in their homes.
Cummings gave two examples of home-based trials, one for a treatment for overactive bladder and another for Parkinson’s disease. The
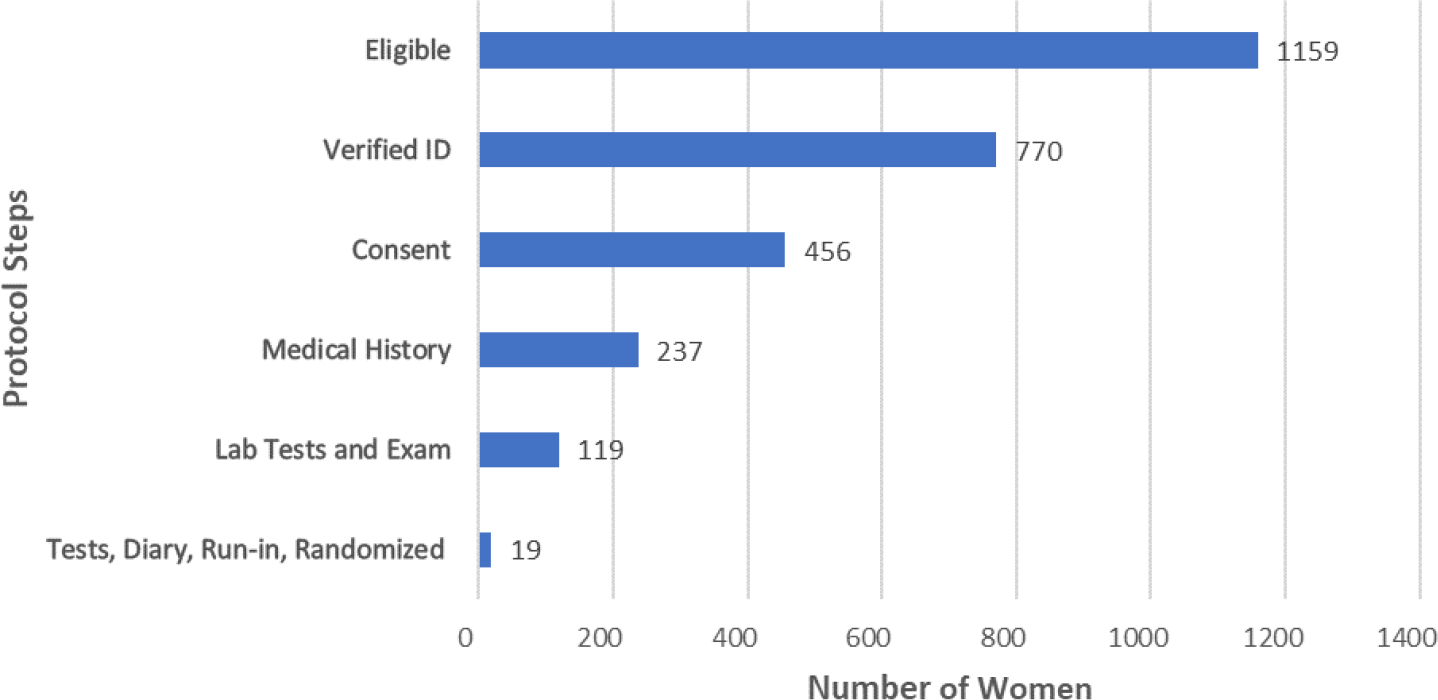
first, called REMOTE, is a trial funded by Pfizer to study Detrol versus a placebo for the treatment of overactive bladder in women. The goal of the trial, which was approved by FDA under an Investigational New Drug Application, was to mimic a clinic site-based trial. The protocol was complex, he said, involving many steps and an average of more than 90 interactions per participant. At each step, between 44 and 84 percent of interested and eligible women dropped out (see Figure 4-1). By the end, only 1.6 percent of the women made it through to the randomization process. The investigators, said Cummings, were “trying to translate a standard protocol, not take advantage of the event of the simplicity you could have from home,” and the complexity and number of the steps “seemed to wither potential participants.” While FDA was very supportive and the electronic methods worked well, “complexity killed the trial.”
Another example of an at-home trial, said Cummings, is the TOPAZ trial, which is examining whether a drug called Zoledronate can reduce fracture risk in Parkinson’s patients. The risk of bone fracture is high in patients with Parkinson’s, and it is unknown whether any of the approved fracture drugs work in this population. Zoledronate, which has been shown to increase bone strength, is delivered via infusion. The TOPAZ trial, funded by NIA, began recruiting patients in 2020 with a goal of enrolling 3,500 participants 65 and older. The participant population includes patients with disabilities, and explicitly includes patients with dementia who need a representative to provide consent, he said. Recruitment is conducted in three ways: via neurologists in practice, through electronic medical record (EMR) searches, and through social media. Potential participants are referred to a website with an electronic consent
SOURCE: As presented by Steven R. Cummings, August 5, 2020.
form, and are then asked to fill out a short questionnaire. If eligible, they have a telemedicine visit with a neurologist to confirm the diagnosis of Parkinson’s and collect additional information; patients who are referred by a neurologist can skip this step. Next, a nurse visits the patient’s home to perform a finger stick blood test for glomerular filtration rate level, and a mouth exam is performed to make sure that they have no lesions that would be contraindicated. Finally, the nurse gives an intravenous dose of either Zoledronate or placebo. Outcomes are assessed through follow-up phone calls and emails every 4 months, with confirmation of fractures through the patients’ EMRs. Adverse events are reported to a central phone line with physician oversight. A similar trial called Adaptable is under way to test outcomes for different doses of aspirin for 15,000 patients with cardiovascular disease, said Cummings.
In summary, said Cummings, home-based trials are feasible, less expensive, and supported by FDA, and they may be particularly valuable for older adults. While many clinical trials require a site because of the need for specialized exams such as MRIs, even these trials can incorporate some elements of at-home trials, such as home delivery of drugs, in-home exams, and telehealth visits. One lesson that has been learned in the conduct of at-home trials, said Cummings, is that “relationships are very important.” Patients are more likely to participate if they are referred by their physician, and having one-on-one calls and virtual check-ins are important to encourage participation and retention. Nurse home visits can also help build a relationship between the participants and the clinical trial staff.
REAL-WORLD CLINICAL TRIALS
“What are some real-world opportunities for research involving older adults with concomitant illness and polypharmacy?” asked Steven Chen, associate dean for clinical affairs at the University of Southern California (USC) School of Pharmacy. By working “where the seniors are,” he said, researchers can align their efforts and produce evidence that is generalizable in the real world. Chen presented examples of real-world trials in two areas: Programs for All-inclusive Care for the Elderly (PACE) and community pharmacies.
PACE is a program that serves mostly dual-eligible older adults (those who qualify for Medicare and Medicaid), and provides medical services and supports for everyday living needs through an interdisciplinary team. Services provided include home care, physical therapy, primary care, social services, transportation, and prescription drugs. Chen and colleagues conducted a clinical trial several years ago that integrated clinical pharmacy teams into the largest private Medicaid provider in the nation. The trial included 10 teams, each with a pharmacist, a resident, and a clinical phar-
macy technician, and had a telehealth component. Outcomes including health care quality, safety, costs, patient and provider satisfaction, and patient access were measured. The trial showed that clinical pharmacy teams providing comprehensive medication management improved health care quality and medication safety while driving down emergency room visit rates and hospitalization rates for high-risk patients.
Community pharmacies are another avenue for real-world trials. Chen noted that older individuals visit pharmacies about twice as often as they visit physicians (Berenbrock et al., 2020), making pharmacies a highly convenient option for a clinical trial. Older adults, said Chen, are more likely to use in-person pharmacies rather than automated pharmaceutical services; they often need assistance with medication use and managing or monitoring for medication safety; they may find visiting pharmacies socially fulfilling; and there are multiple services offered at pharmacies (e.g., health screenings, immunizations, smoking cessation, and other preventative services). In addition to these benefits, pharmacies can collect and aggregate robust data, particularly when it is combined with health plan and/or health system data. Studies have shown that community pharmacies can have a marked impact on medication adherence (NCPA, 2020; Torres-Robles et al., 2018; Wright et al., 2016). Chen shared an example of a study that examined a pharmacy embedded within mental health clinics across the United States. The pharmacy offers a variety of services to improve medication adherence, and the study found that this model results in excellent adherence rates and reduced hospitalization rates (Wright et al., 2016). A program founded and directed by Chen, the California Right Meds Collaborative,4 partners with health plans to offer Comprehensive Medication Management conveniently through pharmacists in select community pharmacies. Health plans stratify patients from high to low risk, and the high-risk patients are sent to these community pharmacies that are trained by USC using the Institute for Healthcare Improvement Breakthrough Series for collaborative training.5 Participating health plans provide a value-based payment for services. At the time of the workshop, the project was in the implementation phase, said Chen, having since transitioned to full roll-out in early 2021.
Chen briefly mentioned a real-world study that was conducted in Black barbershops; the study examined the use of pharmacists to manage hypertension (Victor et al., 2018). The most remarkable thing about this
___________________
4 See http://www.calrightmeds.org (accessed January 15, 2021).
5 For more information on the Institute for Healthcare Improvement Collaborative Model for Achieving Breakthrough Improvement, see http://www.ihi.org/resources/Pages/IHIWhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchievingBreak throughImprovement.aspx (accessed December 15, 2020).
study, he said, was the fact that participating patrons visited barbershops an average of every 2 weeks, and had been visiting the same barbershop for an average of more than 10 years. This amount of face time is far more than any health clinic, he said. The study was in partnership with primary care physicians and had the ability to provide point-of-care testing onsite for basic chemistry panel tests, said Chen. Results showed that nearly 90 percent of Black men with hypertension who worked with a pharmacist were able to reach goal blood pressure, versus 32 percent with usual care. (See Chapter 5 for more details on this study.)
When conducting real-world clinical trials for the older adult population, said Chen, there are a few things to keep in mind. First, it is important to consider how the trials align with the lifestyles of older adults, and if they are convenient to where patients are already going. For example, community pharmacies are accessible and offer support, socialization, and other services that older adults use. Second, researchers should consider where and how data will be collected in these real-world settings. Finally, researchers need to avoid “disrupting culture.” For example, he said, the lead researcher on the barbershop study, Ronald Victor, feared that sending pharmacists into Black barbershops would “change that social environment that is so important to the Black community.” While it did not end up doing so, said Chen, it is an important consideration when research is conducted in real-world settings.
QUANTITATIVE SYSTEMS PHARMACOLOGY MODELS
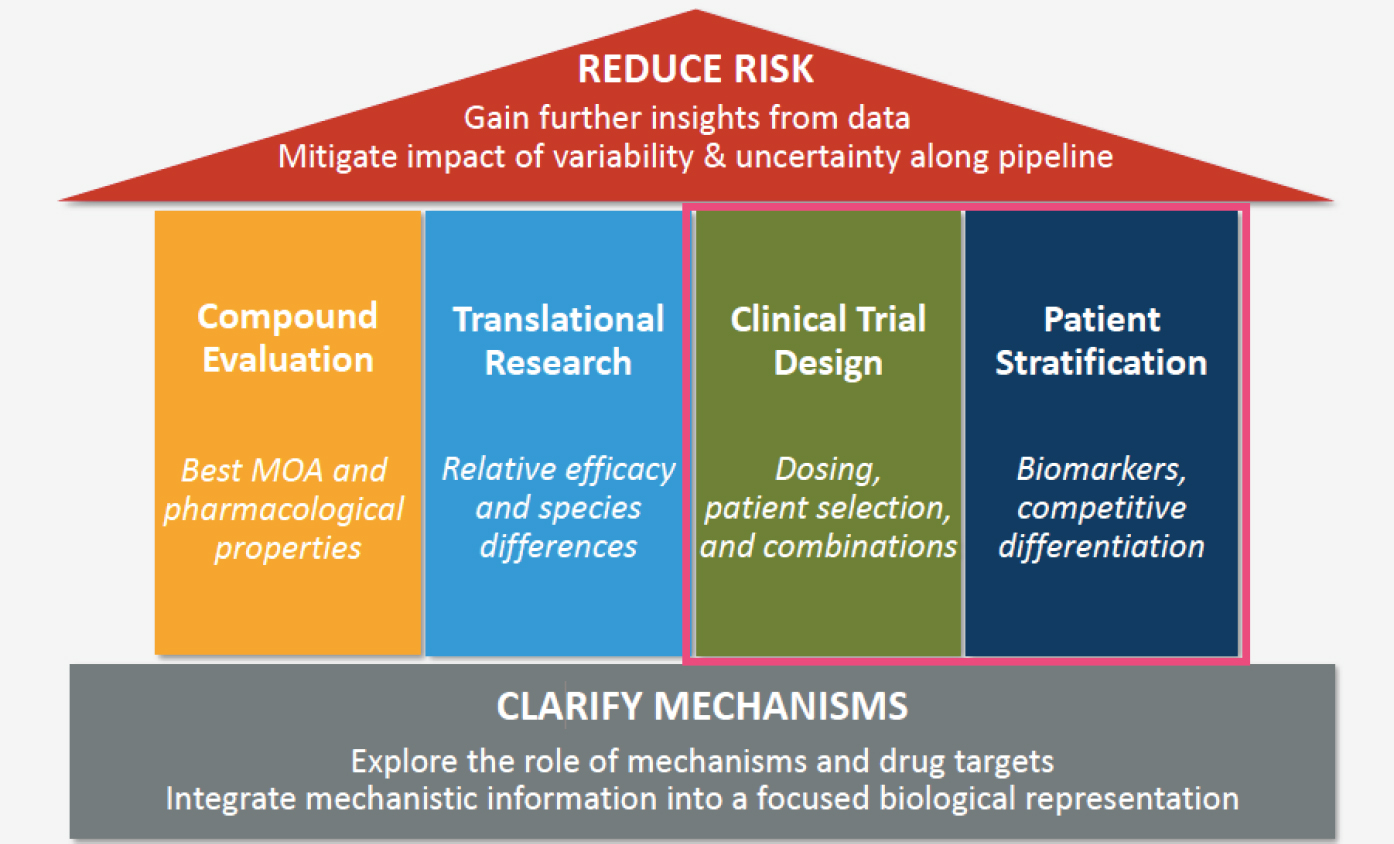
Mechanistic modeling, said Christina Friedrich, chief engineer at Rosa & Co. LLC, uses biological data and understanding to explore disease and drug mechanisms. There are three main subtypes of mechanistic modeling, she explained. Quantitative systems pharmacology (QSP) uses mathematical models that integrate preclinical and clinical information to explore drug mechanisms of action and disease processes (see Figure 4-2). Quantitative systems toxicology approaches can be used to examine and predict the toxicity of drugs, while physiologically-based pharmacokinetic modeling can be used to explore how physiological properties and drug properties interact to produce exposure profiles in tissues of interest. While Friedrich focused on QSP, she said that all types of mechanistic modeling could play a role in supporting geriatric drug development.
When developing new drugs, Friedrich said, the question is whether a compound will have a beneficial clinical outcome in patients. In vitro, preclinical, and clinical data are collected to better understand the mechanism of the compound (e.g., it inhibits a pathway), as well as the process of the disease. This mechanistic understanding can help researchers understand how the drug may affect the pathophysiological processes
NOTE: MOA = mode of action.
SOURCE: As presented by Christina Friedrich, August 5, 2020.
and ultimately clinical outcomes, she said. QSP turns this mechanistic understanding into an explicit mathematical model of disease and drug effects that can be tested on virtual patients and virtual disease states. For example, the model could be used to see what would happen if one pathway instead of another is inhibited, or how inhibiting a pathway might affect the clinical outcomes for younger patients versus older patients. Once this model is built, said Friedrich, it can be used to inform every step of drug development, from compound evaluation to postmarketing studies. At any of these steps, the goal of using QSP is to reduce risk: “How do you get more information or more insights out of the data that you already have so you can mitigate the impact of variability and uncertainty all along the pipeline?”
For drug development for older adults, Friedrich said, QSP would be particularly useful in supporting different clinical trial designs. For example, it could be used to make different dosing decisions for the geriatric population, to look at potential interactions between the drug and other drugs that are commonly used, or to examine the potential effects of comorbidities. QSP could also be enormously helpful with patient stratification; different types of virtual patients could be built to test the drug on subtypes of geriatric patients. To build this geriatric QSP model, said Friedrich, the first step would be to create an initial QSP model of the general population using data from in vitro, preclinical, and clinical
studies. Next, the model would be refined to more specifically represent the geriatric population. As other speakers discussed, there are a number of differences between older and younger adults, including differences in drug absorption and metabolism, liver and kidney function, and comorbidities and polypharmacy. These factors can be represented mechanistically in a QSP model to better anticipate clinical outcomes, said Friedrich.
Friedrich presented an example of the use of QSP in a pediatric population; the process would be similar for the geriatric population. Amgen developed a Bispecific T-cell Engager (BiTE) artificial antibody to treat B-cell acute lymphoblastic leukemia. Amgen was interested in determining the optimal dosing regimen for adults, children, and infants; however, data were only available for the adult population. A QSP model was built using the adult data to represent the disease progression and the mechanism of action of the BiTE antibody. A variety of virtual adult patients were built to match the data that had already been collected. Next, the model was refined using known immunological and physiological differences between adults and children (e.g., bone marrow volume); inputting these factors resulted in a pediatric version of the QSP model. Friedrich said this model increased confidence for moving ahead in pediatric populations and also helped Amgen to identify dosing strategies for subpopulations.
In conclusion, said Friedrich, QSP modeling “connects the dots between mechanisms and outcomes.” A QSP model of non-geriatric adult physiology can serve as a foundation for a geriatric model, and mechanistic impacts of aging, comorbidities, and polypharmacy can all be incorporated. Researchers can use a geriatric QSP model with a range of virtual patients to anticipate systemic outcomes, reduce risks, and customize treatment for older adults.
CLINICAL TRIAL SIMULATION
The goal of clinical trial simulation, said N. Seth Berry, senior director of the Decision Sciences Group at IQVIA, is to optimize the design of a clinical trial. Many elements influence the outcomes of a clinical trial, including protocol design, inclusion/exclusion criteria, and patient adherence patterns. These elements can be modeled, then various scenarios simulated to test what would happen given specific design choices, and this information used to plan a real-life clinical trial. Simulating a clinical trial begins with generating virtual patients, said Berry. This can be done by resampling from an existing general database, such as the National Health and Nutrition Examination Survey6 or the National Health and
___________________
6 For more information on the National Health and Nutrition Examination Survey, see https://www.cdc.gov/nchs/nhanes/index.htm (accessed December 17, 2020).
Aging Trends Study.7 Depending on the therapeutic area and clinical trial endpoints, more tailored sources of data can be used, such as data from the National Institute of Neurological Disorders and Stroke or from disease-specific associations. An additional element to creating virtual patients, said Berry, is to develop a disease progression model that represents a patient’s disease over time. This is particularly important in the older adult population, said Berry, where the disease may progress faster or have different attributes. Disease progression models have been developed for diseases including Parkinson’s and Alzheimer’s. These models are a fundamental element to performing a clinical trial simulation, Berry said, especially in the older adult population.
Another element of a simulated trial is developing the drug model, or a PK–PD model. As Friedrich discussed, there are a variety of benefits to modeling how a drug will work prior to a clinical trial. The primary rationale for incorporating a PK and/or PD model into a simulation, Berry said, is to identify the potential sources of variability, and to understand the impact of that variability. As Kuchel mentioned, many age-related changes can affect PK and PD, such as body composition, metabolism, and elimination.
Simulated trials can also help plan for adherence issues in clinical trials. Berry said that “one of the biggest challenges” in clinical trials is adherence, a particularly large challenge in the geriatric population. Adherence in this group is “across the board, fairly low” and correlated with education level, significance of health-related problems, dosing frequency, and polypharmacy. Adherence can affect trial outcomes; low adherence can drop concentration levels below the therapeutic threshold, while non-compliance (e.g., double dosing) can lead to adverse events. Determining an appropriate dosing schedule can be a challenge, Berry said, because more frequent dosing can provide forgiveness for missed doses, but less frequent dosing can be more convenient for participants.
Berry shared a case study of clinical trial simulations that were conducted to determine whether weight-based dosing adjustments were needed for the use of the antibiotic Piperacillin/Tazobactam (PTZ) in the obese population. Researchers performed Monte Carlo simulations using a previously developed PTZ population PK model and found that no such adjustments were required. Furthermore, the simulation also validated the use of extended-infusion regimens for both normal weight and obese individuals.
Precision dosing applications, said Berry, can be integrated into simulated clinical trials and also have real-world use. These applications have
___________________
7 For more information on the National Health and Aging Trends Study, see https://nhats. org (accessed December 17, 2020).
the ability to individualize dosing to optimize a patient’s exposure and corresponding efficacy and safety response, especially for narrow therapeutic index drugs. In the real world, the applications can be connected to electronic health records (EHRs) as well as wearable sensors to better inform patients and providers on dosing and adherence-related issues. There is a huge opportunity, said Berry, to pair precision dosing applications with machine learning and clinical trial or real-world simulations to better understand the impact of polypharmacy and potential drug interactions in the older adult population.
DISCUSSION
Simplifying Home-Based Trials
Temple asked Cummings to reflect on lessons learned from the REMOTE trial, in which many interested and eligible participants dropped out at each step of the trial before randomization. Cummings responded that the “best way to help people get through steps is to eliminate them.” Simplifying the trial to capitalize on the benefits of the at-home model rather than duplicating a traditional trial, he said, is the surest way to be successful. Temple added that when participants do get stuck getting through the steps, having an established relationship with a person they can call is enormously helpful, particularly for older participants.
Credibility of Models
Several workshop participants had questions about mechanistic models. First, a planning committee member asked about how well a model can account for synergistic effects of multimorbidity, beyond the summative effects of individual conditions. Friedrich responded that this is one of the benefits of mechanistic models, and that they are “very well suited” to looking at complex relationships. She agreed that “biology is not linear,” so models must incorporate aggregated effects as well as downstream feedback loops and interactions. Friedrich noted that using only clinical data makes it very difficult to tease apart synergistic effects, so a mechanistic model can help add more information.
Temple asked Friedrich for clarification on how a pharmacodynamic model can help design a clinical trial. She responded that the PD model “comes from the entirety of the existing literature,” and represents the overall mechanism of the drug as well as the perturbations that the drug is expected to affect. A PD model starts with a representation of the actual disease pathophysiology, including pathways that are known or hypothesized to be involved. Next, drug effects on the various pathways are
introduced, using existing literature that demonstrates the mechanism of action and effects on different biomarkers. Finally, the new drug is introduced using data about its mechanism of action on a pathway. The model will “simulate out the ripple effects from interfering with that particular pathway all the way down to the clinical endpoint that we are trying to predict,” she said. There are multiple models, each a “virtual patient” with different underlying biology that could change their response to the drug.
Another participant observed that while the data on PD for older adults are scant, the models require the input of assumptions about PD. Friedrich commented that “it is important to set appropriate expectations for what the model can do;” she noted that when data are poor, the model will not give a “precise prediction of exactly what will happen.” However, she said, “you will always have a better understanding of the issues with a model than without the model.” The model can help researchers anticipate aspects of variability, and identify what is known and what is not known. For example, a model could be used to determine which additional experiments or biomarker collection would be most useful to reduce the uncertainty. Friedrich said that the point of a model is not necessarily “trying to predict all the way to the final endpoint,” but rather helping to make more informed steps along the way.
Friedrich added that “the beauty of the mechanistic model” is that the researcher can use it to dig deeper into understanding what is happening in the clinical trial. The model is not one piece of data, but rather data that should be seen in the context of the overall biological picture.
Engaging Pharmacists in Adaptive Trials
A participant asked Chen and Scott Berry about the possibility of using pharmacy in the design of adaptive trials. Berry responded that this could “absolutely” be done, and that trials could use random probabilities for drug doses that are based on safety and efficacy for different populations. He noted that there are potentially two types of adaptive randomization that could be used, one in which doses are adjusted based on how previous patients respond to a given treatment, and one in which doses are adjusted for a particular patient based on how that individual has responded to treatment. Chen covered the “nuts and bolts” of engaging pharmacists in adaptive design trial. He noted that a standard of care that pharmacists provide is Comprehensive Medication Management8—an approach that takes into account each patient’s condition,
___________________
8 For more information on Comprehensive Medication Management, see https://www.accp.com/docs/positions/misc/CMM%20Brief.pdf (accessed December 17, 2020).
medical history, comorbidities, and medications. The frequency of contact between pharmacists and patients allows pharmacists to detect adverse drug reactions or potential problems early on. He suggested that engaging pharmacists in adaptive trials would be beneficial because of “that extra layer of monitoring.” Chen said that pharmacists are required to log adverse drug reactions and that there are platforms available to track and standardize all drug-related problems that are identified. This frequent monitoring, he said, could help prevent dropouts in a trial because drug reactions are caught and managed early, before the participant suffers a serious adverse event.
Randomized, Embedded, Multifactorial Adaptive Platform in Clinical Settings
A workshop participant asked Scott Berry for more information about the REMAP design. Berry gave an example of a REMAP trial that is studying community-acquired pneumonia. He said that this trial embeds multiple therapies within a health care system, randomizes the therapies, and continuously provides updates on which therapies are providing better outcomes, safety, and efficacy. The trial has been expanded to study COVID-19 and is investigating six different domains of therapy, with the therapies randomized within the health care system. He noted that this model is particularly useful during the pandemic.














